- Department of Pathobiology and Population Sciences, Royal Veterinary College, Hatfield, United Kingdom
Due to concerns about the welfare of fast-growing (FG) strains of broiler chicken, animal welfare organisations have advocated the use of certain slower-growing (SG) strains that meet key welfare targets under test conditions. However, a widespread transition to SG strains could negatively affect sustainability because these birds tend to have higher feed conversion ratios and longer production cycles. It is important therefore to review the extent and limits of SG welfare improvements under test conditions and on commercial farms, to support the best policy decisions. Following a systematic literature search, 63 source papers were identified. Most reported comparative welfare outcomes for at least one SG strain with at least one FG counterpart, whilst a minority examined the suitability of various SG strains for niche production. The literature review considered different types of study design and accounted for confounding factors such rearing environment and diet. Additionally, a quantitative analysis of effect size for mortality, gait score and contact dermatitis was conducted across studies that had compared birds under similar rearing conditions and that had used SG strains with an average daily growth rate of at least 40g/day. Modern, commercial SG strains performed better on most relevant welfare traits than FG strains. This was the case even when the ‘fairest’ comparisons were made at equivalent bodyweights (when SG birds were older), under matched-environmental conditions, experimentally or on farm. The quantitative analysis found that FG strains had a higher estimated incidence rate ratio for mortality (risk of death over a given period of time) of between 1.69 and 2.16, contact dermatitis affecting 15-25% more birds, and a mean gait score 0.65 points higher than SG strains. FG strains were also less active but other differences in behaviour were inconsistent with some behaviours (e.g. dustbathing) often absent altogether. Growth rate was generally, but not always, predictive of welfare problems. Alternative strategies, such as the slaughter of birds prior to the onset of any welfare decline, could be evaluated in future in terms of welfare improvement and efficiency of production.
1 Introduction and methodologies
1.1 Background
Global per capita consumption of chicken meat has increased rapidly in the past 30 years, and FAO projections suggest that this trend will continue, albeit at slower acceleration, over at least the next 10 years (OECD-FAO, 2021). Advances in genetics and nutrition combined with increasingly efficient management practices have lowered the costs of broiler production, making chicken an affordable source of animal protein for many consumers. Chicken is also a relatively sustainable choice for an animal-derived food. The environmental cost per consumed calorie is lower for chicken compared to other forms of meat when considering resource usage (land and water) and harmful emissions (greenhouse gas and nitrogen) (e.g. Eshel et al., 2014). It is not possible to be precise on exact environmental costs as there are limitations in many studies published to date. For example, most evaluations have not extended beyond the farm gate to consider the impact of rejects or downgrades (Constantini et al., 2021). As methodologies evolve, more precise cost comparisons will become available.
The intensification of chicken production is accompanied by ongoing concerns about the welfare of broiler birds, an aspect that is rarely considered in life cycle assessment models (Constantini et al., 2021). Scientific studies report high prevalences of metabolic disorders, poor leg health and skin lesions in broiler birds as well as a diminished behavioural repertoire (Julian, 2005; Bessei, 2006; Knowles et al., 2008; Shepherd and Fairchild, 2010; Hartcher and Lum, 2020).
The incidence, prevalence and severity of these disorders and restrictions is influenced by management decisions relating to stocking density (Evans et al., 2023; Shynkaruk et al., 2023), feeding strategy and lighting practices (e.g. Fidan et al., 2017; Kang et al., 2023). In turn these practices affect air and litter quality, which have strong proximate effects on birds (Dawkins et al., 2004; Bessei, 2006). However, recently, a panel of scientific experts concluded that the genetic composition of modern commercial strains was associated with more negative welfare consequences (including bone lesions, locomotor disorders, soft tissue lesions and inability to perform certain behaviours) than any other factor (EFSA AHAW Panel et al., 2023). Selection for a rapid growth rate, with a disproportionate focus on increased breast muscle composition to increase production efficiency, has fundamentally altered the birds’ morphology (Caplen et al., 2012; Bennett et al., 2018), and the direct influence of genetics on the musculo-skeletal, cardiovascular and behavioural integrity of the broiler chicken has long been recognised (Kestin et al., 1999; Chen et al., 2011; Kappell et al., 2012). A revision of breeding goals to give a greater weight to welfare-related traits, could (fully or partially) solve these problems (e.g. Dawkins and Layton, 2012). However, to date, the incorporation of health and welfare traits in breeding programmes for fast-growing commercial broiler strains has had only a limited effect in reducing the prevalence and severity of welfare problems (Hartcher and Lum, 2020).
In response to the issues identified and resultant public concern (Clark et al., 2016; Mulder and Zomer, 2017; Yang and Hong, 2019; Heinola et al., 2023)animal welfare organisations across Europe, the UK and the USA have developed a set of standards for broiler welfare bracketed under the banner of the European Chicken Commitment (ECC) or in the UK and USA, the Better Chicken Commitment (BCC)). Retailers who take on this pledge must ensure that birds have been reared at a maximum stocking density of 30kg/m2 (6lbs/square foot in USA) and provided with natural light, perches and pecking substrates. Crucially, only approved strains of broiler chicken that have demonstrated better welfare outcomes in small experimental trials can be used (Section 1.4). The approved strains so far have all had slower-growth rates than the commonly used commercial strains.
The aim of the current article is to review the scientific evidence concerning the welfare of slower-growing (SG) strains to address the following considerations which may help to progress real world improvements in broiler welfare. Improved welfare when housed in small pens in experimental facilities, may or may not be sustained when birds are housed under larger-scale commercial production conditions. Also, the ECC/BCC approval assessments, along with many research programs and on-farm welfare assessments, take place when birds reach a slaughter weight of approximately 2.2kg and so welfare problems that appear in early life and then resolve or remain hidden (e.g. some forms of contact dermatitis, post-hatch mortality) could be missed. Current SG strains are diverse, some may have better welfare outcomes than others, and further scrutiny could clarify the extent to which welfare outcomes are directly associated with growth potential rather than other genetic attributes or management practices.
Finally, characterising and, where possible quantifying, any relative improvement in welfare outcomes of SG vs faster-growing conventional (FG) strains is important to help balance wider concerns around broiler production. Because SG strains have higher feed conversion ratios and longer production cycles, the economic and environmental costs of production will differ from those of FG strains. There may therefore be efficiency and sustainability trade-offs if producers transition to SG strains. If the welfare of SG strains is substantially better than that of FG strains then these trade-offs will need to be balanced and addressed. However, if welfare improvements are small, or inapparent under commercial conditions, then other approaches to improve bird welfare may need to be considered.
1.2 Search strategy
A search strategy was employed to ensure all relevant papers published within the past 20 years comparing strains of different growth rate were included in the review. The primary focus was to find manuscripts reporting welfare-relevant data for both fast- and slow-growing strains using the same methodologies and observation techniques. Papers that compared welfare-relevant data for between different slow-growing strains were also included.
Web of Science was selected as the primary database, with tests showing that all papers detected in other databases were also detected in WoS, but that WoS identified additional relevant papers.
Primary searches were conducted initially using the term “broiler” alongside “slow*-grow*” to capture papers that might include the following terms: slow-growing; slow-growth; slower-growing; slower-growth. The search was repeated using the term “chicken” alongside “slow*-grow*”. The results were refined to seek additional papers published in the English language that had used terms such as “welfare” or welfare-relevant performance metrics such as “mortality” or ‘livability”. Checks showed that papers were not missed because of the inclusion/exclusion of a hyphen in the terms slow-grow or slower-grow. The searches were conducted in February, 2023 and repeated in September, 2023, with the number of hits shown for September, 2023 below.
Search 1: Broiler and “slow*-grow*” 626 hits; refined by “welfare or livability or mort*” = 205 hits
Search 2: Chicken and “slow*-grow*” 749 results; refined by “welfare or livability or mort*” = 199 hits
Search 3: Broiler and “medium-grow” 72 hits refined by “welfare or livability or mort*” = 20 hits
Search 4: Chicken and “medium-grow” 72 hits refined by “welfare or livability or mort*” = 20 hits
These results were scrutinised individually.
There was considerable (but not total) redundancy between Search 1 and 2; and between Search 3 and 4. Papers were excluded for reasons including: reviews with no new data; no welfare-relevant information contained e.g. concerned primarily with carcass composition, hatching or slaughter practices; no comparison between strains e.g. single strain diet studies or observations of behaviour on free-range; studies of broiler breeders; or studies conducted outdoors under tropical or sub-tropical conditions.
Overall 56 papers were retained from search 1; one additional paper from search 2 and two additional papers from search 3. No additional papers were retained from Search 4, however four papers were subsequently added after following cited links, providing 63 source papers to start the review (see Appendix 1).
1.3 Structure of narrative review and source material
The first part of the review considers studies that compared at least one SG strain directly against at least one FG strain. Most welfare problems (musculoskeletal, locomotor, cardiovascular) increase with age and bodyweight (e.g. Forseth et al., 2023). Because conventional FG strains have been selected for high feed efficiency they reach slaughter weight at an early age. Thus, when comparisons are made at the same age the welfare of SG strains is likely to appear favourably because they will be of lighter weight. Such comparisons are thus unlikely to capture weight-associated declines in welfare in SG strains. Particularly if FG are kept for study purposes beyond normal slaughter age to match slaughter dates for SG strains, over-estimation of FG welfare problems is likely. Comparisons taken when strains are at the same bodyweight are thus, generally considered to be ‘fairer’. The approval of strains for the ECC or BCC assurance standards requires for example that strains are examined when the flock reaches an average bodyweight of 2.2kg (RSPCA, 2017). However, this means that early-life welfare problems may be missed and it implicitly assumes that welfare outcomes recorded at a single time-point are the culmination of a linear increase over time. If instead welfare problems increase with age or bodyweight in a non-linear way, then this cross-sectional approach will not capture differences in the duration of time that birds have experienced welfare problems. To address these issues taking repeated welfare measurements to capture both age and weight-related changes may be the fairest approach, but such demanding studies remain a minority in the literature.
In recognition of these complexities, the narrative review is structured in Sections to distinguish studies that (as their main focus) compare FG and SG strains in indoor housing: at the same age (Section 2.1); at the same bodyweight (Section 2.2); or at ‘commercial’ slaughter weight and age, noting that slaughter weight will be both lower and reached at an older age in SG than in FG strains (Section 2.3). In Section 2.4 a small number of studies that have taken repeated measures over time and evaluated both age and bodyweight effects are considered. Section 2.1 additionally includes information from studies that compared different SG strains for their suitability for higher welfare production systems as the majority of these compared strains at the same age. Within each section, small-scale studies conducted under experimental or laboratory conditions are differentiated from those conducted on commercial farms. Studies conducted on commercial flocks have the greatest applicability and generality to the real-world situation but it can often be difficult to achieve tight experimental control of contextual factors, or to achieve sufficient replication at the flock level.
Quantitative analyses comparing results from a subset of studies that compared FG and SG strains with relatively high average daily growth rates in indoor housing are presented in Section 3. These analyses were conducted on the most commonly utilised welfare indicators, mortality (Section 3.1), gait score (Section 3.2), footpad dermatitis FPD (Section 3.3) and hockburn HB (Section 3.4).
Sample sizes varied from small experimental studies with welfare measures taken on 5 or 6 individuals per strain to studies conducted at commercial abattoirs where measures were taken on 30 million or more individuals (e.g. Forseth et al., 2023). Confounding factors further complicate any simple aggregation of studies. To take diet as one example, some studies provide the same diet to all strains, either a non-limiting diet designed to provide a high level of energy and other nutrients to support the rapid growth of FG strains, or a lower-energy diet designed primarily for SG strains. However, in other studies birds are fed their own strain-appropriate diets, or the timing of a switch between starter and growing, or growing and finishing diets is adjusted (Supplementary Table 1). There is also wide variation in stocking densities across studies, reported either as birds/m2 or kg/m2 (Supplementary Table 1). In some cases, stocking densities varied not only as birds grew but also due to experimental changes in bird numbers. For example in some studies birds were sequentially killed to assess post-mortem welfare indicators, resulting in sharp declines in group size and stocking density as each study progressed (e.g. Williams et al., 2013; Sarica et al., 2014; Akyuz et al., 2022). Declines in stocking density during a trial can also occur if there is high bird mortality with no replacement. These contextual factors are discussed where relevant within each Section.
1.4 Strain characteristics
The terms Breed, Strain and Genotype are used variably, sometimes interchangeably, and often inconsistently within the agricultural and scientific literature. Here we recognise that Genotype refers to the genetic composition of a broiler bird but we use the term Strain throughout. A strain is a population of animals that can be distinguished from other populations based on certain characteristics. Since commercial broiler chickens are selected and marketed with such distinction in mind it is appropriate to refer to them as strains, whether they are pure breeds or crosses between breeds.
There is no universal definition of a fast- or a slow-growing broiler. Historically, FG birds were described as those with average daily (live-weight) gains (ADGs) of over 50g/day. However, due to continued selection (e.g. Zuidhof et al., 2014), the fastest growing and most commonly used commercial strains, Ross 308 and Cobb 500, now have cumulative ADGs of over 60g/day (see Table 1). The average is calculated over the entire rearing period and naturally it changes with bird age. A Ross 308 bird gains an average of 24g/day in week 1 (resulting in a 4.8 fold increase in weight during that week) and an average of 100g/day in week 6 (resulting in a 1.3 fold increase in weight during that week) (Aviagen, 2022).
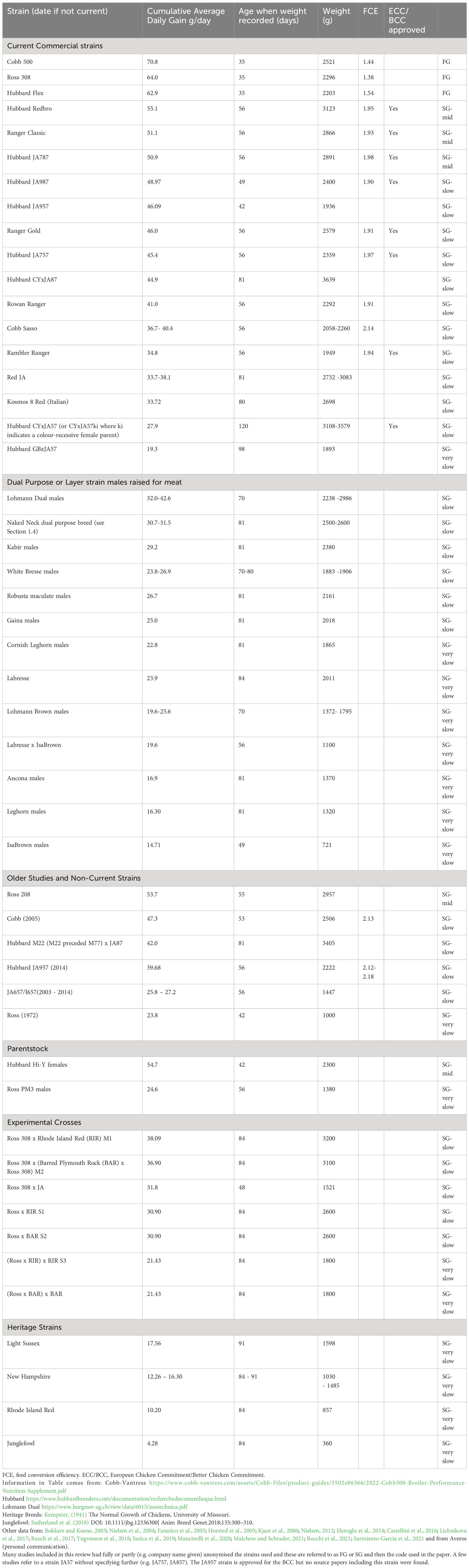
Table 1 The names and performance characteristics of strains that have been included in comparative studies.
In contrast, SG strains show immense variability (Table 1). General conclusions about SG birds are therefore not possible, but have to be qualified by referring to the ADGs categories shown in Table 1. Supplementary Table 1 assists by providing a resource to establish quickly which strains were used in any particular study. SG strains are now often ‘designed’ for specific markets such as ECC, free-range with slaughter at either 8 or 12 weeks, organic or other traditional production methods (e.g Label Rouge). Conventional male parents such as the Hubbard M99, M77, grey-barred (GB) or Colouryield (CY) are mated with female parent-stock, such as the Hubbard JA57, JA87 or Redbro to produce the broilers raised for meat. Sometimes the parents are indicated in the designation of the meat chicken strain e.g. CYJA57 generally indicates a male Colouryield crossed with a female JA57 but sometimes the female parent is indicated first, or in other ways e.g. JACY57. Sometimes some letters are omitted e.g. CY57. If the male parent is the conventional M99 or M77 then the broiler strain is usually designated with the additional number derived from that parent e.g. M77 x JA57 as JA757. Increasingly, even these designation are being replaced by market-friendly names that do not indicate parentage e.g. the meat chicken that derives from male M77 and Redbro female parents was originally designated as the 7Redbro, but is now known simply as the Hubbard REDBRO. The Redbro male parent can also be mated with various female parents to produce slower-growing broilers for specialist markets e.g. with P6N females = Norfolk black chickens, with JA87 females = RedJA87, or with JA57 females = RedJA. -
In the egg-laying sector, male chicks are usually killed after hatching but there is growing interest from an ethical perspective in raising them for meat consumption. Strains that can perform a ‘dual purpose’ (egg and meat) function are therefore of interest. The Naked Neck strain is dual-purpose breed in its own right, although the trait for a naked (featherless) neck is also a readily available option for many other SG strains. Table 1 summarises the growth characteristics for the strains included in the source studies of this review, in descending order of expected ADG, and indicates whether they have been approved for production under ECC/BCC standards.
In the narrative review different categories of growth are captured thus: FG = a strain with an ADG of ≥60g/day; SG-mid = ADG of 50-59.9g/day; SG-slow = ADG of 25-49.9g/day and SG-very slow = ADG of < 25g/day). The ongoing selection for growth makes it hard to compare studies across time. Strains that were considered FG 15 or more years ago, had growth rates that would now be characteristic of modern SG-mid or even SG-slow strains (Table 1).
1.5 Welfare indicators in source papers
The most frequent welfare indicator in the source papers was mortality (34 studies) although only a handful of studies separated early vs. later mortality, or culls vs. birds found dead, and even fewer provided information on causes of mortality. Other relatively frequent welfare indicators were, walking ability (22 studies) and contact dermatitis, most often FPD (35 studies) and HB (28 studies).
Mortality is considered an essential indicator of welfare because broiler chickens are likely to have experienced severe reductions in health before dying, often of metabolic conditions characterised by ascites, pulmonary hypertension or heart disease, or from complications relating to leg health (Julian, 2005). In some cases, even where death is sudden for the individual (e.g. due to predation) the welfare of other flock members may be reduced due to increased fearfulness.
Leg health is also highly relevant because it is often associated with pain (Caplen et al., 2013; Caplen et al., 2014). Poor leg health also reduces the capacity of broilers to perform behaviours such as walking, foraging and perching (Weeks et al., 2000; Abeyesinghe et al., 2021) and this may increase frustration (Evans et al., 2023). Leg health is most often measured using a gait scoring method (Kestin et al., 1992; Garner et al., 2002) whereby birds are encouraged to walk a short distance in a straight line and trained assessors record difficulties in movement on a 6-point scale and 18 source papers used this method. Another method of assessing leg health includes latency-to-lie tests, whereby birds are placed in a very shallow bath of water at room temperature and the time they remain standing is recorded. For ethical reasons, as soon as a bird sits it is removed from the water (Weeks et al., 2002). A third method is the modified rotarod test (Malchow et al., 2019a) whereby an individual bird perches on a slowly rotating rod and, as the velocity of rotation increases the latency to leave (by hopping off) is recorded. Both of these latency measures show generally strong correlations with gait score (Weeks et al., 2002; Malchow et al., 2019a) which is most often used at a commercial scale.
Contact dermatitis reflects poor skin health, with conditions such as FPD, HB and breast blisters caused by prolonged contact with wet litter, especially under cold/hot temperatures. These conditions are characterised by painful, inflamed or necrotic lesions and reduced bird activity (Freeman et al., 2020). However, in contrast to the standardised gait score used in most research studies, scoring systems for contact dermatitis vary greatly. Many protocols use ordinal rating scales with between 2 and 6 points, but some have up to 8 points and others just report a binary presence/absence. It is not easy to convert between scales as the distances between points may not be equal (Souza et al., 2022). Indeed the scale used in RSPCA assessments (RSPCA, 2017) runs from 0 (no lesions) to 2 (extensive lesions) scale, with points at intervals of 0.5. However, there is an additional category 0+ which represents the appearance of pre-lesion conditions such as swelling or discolouration of the skin. Such variation in recording methods makes it challenging to perform a quantitative overview. Even attempting a binary classification between studies is not easy because birds with minor lesions may be counted as negative in some studies, but positive in studies using a 0+ point. The form of quantitative analysis conducted in Section 3 had to be adjusted for welfare indicators where standard scoring systems had, or had not, been employed.
Measures of stress, behaviour, fearfulness and post-mortem indicators were either employed in too few studies or were recorded using such diverse methods (particularly so for behaviour) that they could only be considered in the narrative and not the quantitative part of the current review.
2 Narrative review by study type
2.1 Studies comparing FG and SG strains: birds kept to the same age
Comparing FG and SG strains at the same age and hence usually the same date has a number of advantages. It reduces the influence of extraneous variables such as season or weather and allows birds to be assessed at approximately the same stage of neural development. Most of the studies reviewed in this section were conducted in controlled indoor experimental facilities with the further potential advantage of being able to mark and follow individual birds over time. Birds were therefore generally kept in small group pens, usually of around 20 to 50 individuals, but as low as 6 and as high as 225 (Supplementary Table 1). A handful of studies provided small outdoor runs adjacent to indoor pens for some or all of the experimental groups (Comert et al., 2016; Lichnikova et al., 2017; Abdourhamane and Petek, 2022, Abdourhamane and Petek, 2023). Three studies reviewed in this section were conducted on commercial farms (enriched indoor with natural light, Baxter et al., 2021; or organic, Castellini et al., 2016; Lindholm et al., 2017).
Some of the studies provided both FG and SG strains with an identical but relatively high energy diet (e.g. Bokkers and Koene, 2003; Cavusoglu and Petek, 2019; Steenfeldt et al., 2019; Baxter et al., 2021; Abdourhamane and Petek, 2022, Abdourhamane and Petek, 2023), likely containing nutrient levels beyond those needed by SG birds and not fully representing the commercial situation. A different strategy was to provide an intermediate diet (Malchow et al., 2019b, Malchow et al., 2019c) or non-identical diets, so that FG birds received higher energy/nutrient diets than SG birds for part or all of the growing period (Comert et al., 2016; Akyuz et al., 2022) (Supplementary Table 1). If there is a positive interaction between genetic susceptibility to poor welfare outcomes and dietary energy level then the greatest difference in welfare outcomes between strains would be expected in these studies. However, the majority of studies reviewed in this section provided both FG and SG strains a relatively low-energy diet in either experimental facilities (e.g. Nielsen, 2012; Sarica et al., 2014; Wallenbeck et al., 2016; Lichnikova et al., 2017; Rezaei et al., 2018; Ghayas et al., 2020, Ghayas et al., 2021; Snyder et al., 2022; Wilhelmsson et al., 2019; Yngvesson et al., 2018) or on commercial farms (Castellini et al., 2016 (Supplementary Table 1)). In one study, the energy intake for the FG birds was even lower than that provided for SG birds (Lindholm et al., 2017). Restricting the nutrient intake of the FG Ross 308 birds reduced their ADG substantially (35g/day, Lindholm et al., 2016; 40g/day, Rezaei et al., 2018) compared to the expected potential ADG of over 60g/day (Table 1).
Feed restriction was in most cases intended as a counter-measure to compensate for keeping FG birds to older ages than they would be commercially. With two exceptions (Steenfeldt et al., 2019; Baxter et al., 2021) who made a final comparison between SG and FG birds at 42 days of age, most other studies kept both SG and FG strains to at least 56 days of age (Bokkers and Koene, 2003; Nielsen, 2012; Sarica et al., 2014; Lindholm et al., 2017; Wallenbeck et al., 2016; Rezaei et al., 2018; Cavusoglu and Petek, 2019; Ghayas et al., 2020, Ghayas et al., 2021; Akyuz et al., 2022; Wilhelmsson et al., 2019) and sometimes to as long as 84 or 91 days of age (Lichnikova et al., 2017), well beyond the normal FG slaughter age. Without feed restriction, data could have derived from excessively heavy FG birds that would not be found under commercial conditions. But dietary restriction was either not fully effective, with FG birds reaching excessive weights of over 5.5kg (Lichnikova et al., 2017) or it did effectively restrict weight gain but was associated with signs of, sometimes extreme, hunger (Lindholm et al., 2017), reminiscent of the situation for feed restricted broiler parent stock (e.g. Arrazola et al., 2020; Dixon et al., 2022).
Thus, despite certain advantages, assessing the welfare of strains with very different growth rates at the same age raises questions about the fairness of the comparison (see Section 1.3). Without feed restriction the welfare outcomes for FG birds tended to be worse than for SG-slow or SG-very slow birds in terms of health problems such as heart abnormalities, tendon degeneration, scoliosis and rotated tibia (Bokkers and Koene, 2003), reduced walking ability (Cavusoglu and Petek, 2019; Steenfeldt et al., 2019) or other aspects of leg health (Abdourhamane and Petek, 2022); contact dermatitis (Cavusoglu and Petek, 2019; Steenfeldt et al., 2019; Abdourhamane and Petek, 2022; Akyuz et al., 2022) and susceptibility to heat stress (Brugaletta et al., 2022). Plumage and skin were dirtier (Cavusoglu and Petek, 2019) and active behaviours such as locomotion and perching were reduced (Bokkers and Koene, 2003; Abdourhamane and Petek, 2022, Abdourhamane and Petek, 2023). The walking ability and feather cover of FG birds was also worse than that of an SG-mid strain when compared at 6 weeks of age, and active behaviours were reduced (Baxter et al., 2021).
The welfare outcomes of FG birds provided with a limiting diet and kept beyond normal slaughter age did not appear to be greatly improved. Mortality exceeded that of SG-slow and SG-very slow strains (Rezaei et al., 2018; Sarica et al., 2019; Ghayas et al., 2020) with leg culls highlighted as a particular cause (Rezaei et al., 2018). Feed restricted FG birds also experienced more severe contact dermatitis (Sarica et al., 2014; Castellini et al., 2016; Wilhelmsson et al., 2019), greater susceptibility to heat stress (Nielsen, 2012; Wilhelmsson et al., 2019) and a less-active behavioural repertoire (Nielsen et al., 2004; Nielsen, 2012; Castellini et al., 2016; Wallenbeck et al., 2016; Lichnikova et al., 2017; Yngvesson et al., 2018; Ghayas et al., 2021; Snyder et al., 2022) than SG birds. For some indicators, FG strains appeared to fare worse than SG strains regardless of the diet provided, e.g. > 60% of FG birds vs <7% of SG birds showed signs of hock-burn regardless of whether energy was relatively low (Wilhelmsson et al., 2019) or high (Cavusoglu and Petek, 2019; Akyuz et al., 2022). It is however hard to draw conclusions due to the competing influences of variable feed restriction and increased age for the FG birds.
There were a few welfare indicators which did not show such clear differences between FG and SG strains or where FG birds fared better. Whereas FG birds were generally less active than SG birds, they continued to perform some highly-motivated activities such as comfort behaviour at similar levels to many SG-slowstrains (Bokkers and Koene, 2003; Wallenbeck et al., 2016; Abdourhamane and Petek, 2023) and SG-mid strains (Baxter et al., 2021). And while FG birds were less likely to perch (Abeyesinghe et al., 2021) or more specifically to sleep or rest on perches (Yngvesson et al., 2018), the amount of sleeping and resting when all resting locations were considered together did not differ between strains of different growth potential (Wallenbeck et al., 2016; Yngvesson et al., 2018). However, other studies observed very low levels of dustbathing (e.g. compared with levels seen in laying hens or junglefowl) in all broiler strains examined (Bokkers and Koene, 2003; Wallenbeck et al., 2016; Baxter et al., 2021) and some also found low levels of foraging (Baxter et al., 2021). For a handful of welfare indicators, poorer outcomes in SG strains were reported in age-matched comparisons, including the presence of deviated keel bones (Bokkers and Koene, 2003). The biological significance of this finding is uncertain, despite incidental reports that keel bone deviations (in laying hens) may be associated with pain or restricted movement (Riber et al., 2018), because the likely cause was increased perching which would generally be viewed as positive, and because this problem should be solvable via improved perch design. There is no consensus as to whether fearfulness differs between FG and SG strains, with some studies finding no difference (e.g. Abdourhamane and Petek, 2022) but others reporting greater fearfulness in SG-slow strains using both human avoidance and tonic immobility tests (Cavusoglu and Petek, 2019; for the same strain), or in tonic immobility tests but not in tendency to use an outdoor area (Lindholm et al., 2017; different strain). The tonic immobility test may be the better test to assess fearfulness in this context as it is not confounded by the generally greater locomotor ability or potentially differing motivations of SG strains.
Although a handful of studies recorded welfare-relevant information only at the final slaughter age (e.g Brugaletta et al., 2022), most took repeated measurements over time, providing a useful overview of how broiler welfare changes with bird age. A universal finding was that welfare decreased with bird age, and almost always at a faster rate for the FG strains than for SG strains across the span of growth potential (e.g. activity and perching behaviour, Bokkers and Koene, 2003; Baxter et al., 2021; contact dermatitis, Akyuz et al., 2022; Wilhelmsson et al., 2019; walking ability, Baxter et al., 2021; Wilhelmsson et al., 2019). As a specific example, the percentage of birds with no walking difficulties decreased more slowly when measured weekly between weeks 3 and 6 for SG-mid Redbro birds (86%, 84% 61% and 58%) than for FG Ross 308 birds (76%, 55%, 38% and 16%) (Baxter et al., 2021). Malchow et al. (2019b); Malchow et al. (2019c) monitored bird activity continuously using transponders for FG, SG-slow and SG-very slow strains (Supplementary Table 1, Table 1). The use of elevated structures (grids) for perching increased for all strains to approximately 3 weeks of age but then decreased for the FG birds after this time, whilst increasing further for the SG strains. Early provision of elevated structures was not sufficient to increase the locomotor activity of the FG birds to levels seen in the SG birds (Malchow et al., 2019b). The greatest use of the grids (17.1%) was observed in the FG chickens at week 4 during the light period, whereas use of grids continued to increase in the SG-slow birds, such that by 70 days of age time spent on the grids was approximately 67% at night and approximately 45% during the light period (Malchow and Schrader, 2021). Taking repeated measurements also enabled comparisons between strains across different time periods rather than at equivalent ages, but these have not provided consistent results and highlight our concerns about single cross-sectional assessments (Section 1.3). For example, the gait and HB scores for FG birds at 42 days of age were found to be better than those of SG-slow birds at 56 days (Cavusoglu and Petek, 2019) but worse than those of SG-slow birds at 63 days (Wilhelmsson et al., 2019). Similarly contradictory effects were also found for FPD (Cavusoglu and Petek, 2019; Wilhelmsson et al., 2019).
Keeping broilers in small groups under controlled environmental conditions facilitated the collection of data on physiology and immune function in a small number of studies, although a general hypothesis that selection for rapid growth might have negatively affected immune function was not supported by rather mixed results (Comert et al., 2016; Akyuz et al., 2022; Snyder et al., 2022). Montoro-Dasi et al. (2020) found lower anti-microbial resistance in SG strains. Ghayas et al. (2020) found a higher respiration rate (RR) for FG birds but also (strangely) found heart-rate inversely related to RR. Decreased expression of relevant brain proteins in FG broilers compared with SG-slow or SG very slow strains and junglefowl, and high oxygen requirements for growth, were suggested as reasons why FG birds are less able to withstand high ambient temperatures (Brugaletta et al., 2022). Williams et al. (2013) found no strain differences in the colonization or infection of organs by Campylobacter jejeuni but this infection exacerbated the higher prevalences of contact dermatitis seen in FG birds.
Studies evaluating the suitability of different SG-slow and SG-very slow strains for higher-welfare farming conditions also tend to be conducted when birds are at the same age. Rauch et al. (2017) evaluated different SG-slow strains (Supplementary Table 1, Table 1) and reported a positive correlation between bodyweight and reduced leg health with one exception, as heavier JA987 birds had better gait scores than Rowan Ranger chickens (Rauch et al., 2017). Despite this, the JA987 birds had dirtier plumage and more skin scratches, and did not meet the German scheme requirements (Rauch et al., 2017). Louton et al. (2019) similarly found gait score, plumage soiling and scratches to be positively correlated with strain ADG (Supplementary Table 1, Table 1) again with one exception, as heavier Ranger Gold birds had lower HB scores than Rowan Rangers. Other diverse studies have examined the suitability of various SG-slow and SG very-slow strains for extensive or organic rearing production (Almeida et al., 2012; Eleroglu et al., 2015; Castellini et al., 2016; Mancinelli et al., 2020; Sarmiento-Garcia et al., 2021), with observations continuing until slaughter at between 81 and 120 days of age. Castellini et al. (2016) compared the performance of seven SG strains (Supplementary Table 1) and found that an “adaptability index”, calculated from 49 separate indicators, was negatively correlated with growth rate. Mancinelli et al. (2020) obtained similar results when comparing six SG strains (Supplementary Table 1). In both studies, the very slowest-growing strains spent more time outdoors, performed more comfort behaviour, and had lower levels or severities of fearfulness, FPD, breast blisters, inflammation markers, and stress levels. As above, there were occasional exceptions with Ranger Classic and Ranger Gold birds generally having better welfare outcomes than two slightly slower-growing JA87 or JA57 crosses (Mancinelli et al., 2020). A few studies found no differences between strains in fearfulness (Eleroglu et al., 2015), HB or FPD (Nielsen et al., 2004) or mortality (Sarmiento-Garcia et al., 2021). But whether or not differences between strains are detected will depend on study power (studies are not always replicated at the flock level, Supplementary Table 1), and on how similar the SG strains are in terms of origin and growth rate. If welfare indicator values are particularly low (e.g. perfect plumage and dermatitis scores were found by Nielsen et al. (2004) and Horsted et al. (2005)) or very high (e.g. mortality, Sarmiento-Garcia et al., 2021), this could also mask subtle strain effects.
2.2 Studies comparing FG and SG strains: measurements taken at the same bodyweight
2.2.1 Strain comparisons in experimental facilities
The assessment of birds at the same bodyweight is often considered a ‘fairer’ way of comparing strains and this is the foundation of the Broiler Breed Welfare Assessment Protocol (BBWAP), used to approve strains for RSPCA Standards, Better Chicken and European Chicken Commitments (RSPCA, 2017). Approval is contingent on reaching various criteria including a total mortality of < 3%, more than 95% of birds having gait scores <3, and no birds with FPD or HB scores of 1 or more. Birds must be fed a freely-available non-limiting diet and welfare-relevant measures taken when an average weight of 2.2kg is reached. This latter requirement is useful but it can present practical scheduling difficulties. Essentially, the date at which each strain is expected to reach a certain target weight has to be predicted in advance based on published breed performance guidelines, from practical experience or from the very early performance of the birds under test. But however carefully this is approached, on the day of testing birds may have either exceeded or fallen short of their predicted weights. For example, van der Eijk et al. (2023) planned to assess a FG and SG-mid strain on days when they were expected to have reached 2.3kg. However, the actual average bodyweight of their respective test days was 2.2kg for the SG strain, and 2.4kg for the FG strain. Abeyesinghe et al. (2021) also found that birds did not quite meet weight by age predictions but, in this case unlike van der Eijk et al. (2023), both of two strains of SG-slow birds slightly exceeded, rather than fell short, of their predicted weights on their planned test days. Despite at least 10 strains being approved for Better Chicken and European Chicken Commitments (see Table 1), in most studies the identities of the strains were anonymised due to commercial considerations. Only two studies have openly reported the outcomes of strain comparisons using this protocol, with birds housed in replicated groups of 50 in indoor pens at a stocking density of 8.5 birds/m2. These are described next.
Dixon (2020) compared three FG strains, Ross 308, Cobb 500 and Hubbard Flex with the SG-slow Hubbard JA757. The required welfare outcomes were assessed when birds approached 2.2kg and again at 2.5kg, when welfare had generally deteriorated. The BBWAP does not specify in detail how bird behaviour should be assessed but Dixon (2020) took weekly observations. The JA757 birds, older at the time of assessment, had better gait score, HB, plumage cover and plumage cleanliness scores. FPD was infrequent with no differences between strains. The JA757 birds spent less time feeding, drinking and sitting than the FG strains and more time standing, moving, foraging, preening, dustbathing and perching. Perching in particular occurred very rarely for the conventional strains but accounted for 10-12% of the time budget of the JA757 birds between 16 and 37 days of age. Locomotion decreased sharply with age, whilst preening increased. As reported for other recent studies (Section 2.1) dustbathing was an infrequent behaviour for all strains, occupying a maximum of 1% of the time budget for JA 757 birds, and 0.05% for conventional strains. The overall conclusion was that the welfare of the slower-growing JA757 was better than that of any of the FG strains, but mortality exceeded the RSPCA threshold even for this strain.
A second study using the BBWAP compared the health outcomes of one FG strain with two SG-slow strains when birds were as close to the 2.2kg target as possible (Abeyesinghe et al., 2021; Supplementary Table). This study additionally assessed bird behaviour when birds were 29 days (FG and both SG) and 43 days of age (SG strains only) and looked specifically at associations between behaviour and health outcomes, whilst recognising that the time elapsed between behaviour and health measures varied between strains. Higher levels of valgus leg deformity, hock burn and higher (worse) gait scores were found for the FG birds compared with one of the SG strains, with the other SG strain intermediate between these two. The median gait score for the FG birds was above 2, indicating that many birds had problems walking as they approached slaughter age. No differences were found between strains when considering the prevalence of footpad dermatitis, which was very low for all birds, perhaps reflecting the high standards of environmental control and resultant good litter condition in this laboratory facility. The paper provided detailed contextual information on hatching rates, parental characteristics, feed conversion and growth rate, and also examined associations between health outcomes measured at equivalent bird weight, and behaviour assessed at equivalent bird age. Time spent on the perch was strongly indicative of better health, whilst the behaviour of side-lying (with legs extended to the side rather than tucked under the body) was strongly indicative of poorer health. Because of the strong associations between behaviour and health measures, the authors suggested that non-intrusive monitoring of behaviour (e.g. via automated visual analysis) might effectively replace future hands-on assessments which may be stressful or uncomfortable for live birds. Behavioural measures can be sensitive ways of detecting early signs of declining health, as has been found in other contexts (e.g. Littin et al., 2008; Mandel et al., 2017).
Other strain assessments conducted in experimental facilities have examined stocking density alongside strain. Weimer et al. (2020) compared a conventional Aviagen FG strain with a Hubbard SG strain at stocking densities of 29kg/m2 and 37 kg/m2 with non-limiting diets. Mortality and FPD did not differ between strains and, whilst more HB lesions were detected on the FG birds, toe damage was more frequent in SG birds. The lower stocking density was associated with reduced HB but greater toe damage in the SG birds, both effects possibly due to greater activity. van der Eijk et al. (2022) kept FG and SG-mid birdsin large enriched pens of between 517 and 903 birds/pen at stocking densities varying between 24 to 42kg/m2. Again, non-limiting diets were provided. Birds from the SG-mid strain performed less feeding but more locomotion, standing, comfort behaviour and foraging and they made greater use of enrichments provided. The SG-mid strain also showed lower fearfulness (contrary to some results reported in Section 2.1). Gait, FPD and skin lesions were all substantially lower in the SG-mid strain (van der Eijk et al., 2023), although there were no strain effects on HB or breast cleanliness. Contrary to the authors’ hypothesis that the SG-mid strain might benefit most from housing at a lower stocking density both strains performed more positive behaviours (comfort, play, foraging) and benefitted at least equally with reduced skin lesions and improved gait scores at the lower stocking densities (van der Eijk et al., 2022, van der Eijk et al., 2023). One study reported greater tibial strength in FG than SG-slow or SG-very slow birds, particularly those provided with outdoor access (Fanatico et al., 2005) and Singh et al. (2021) reported that FG birds showed a stronger antigen response, though argued that this may be an artefact of fast growth rate and that a pathogen challenge test would be more conclusive.
2.2.2 Strain comparisons on commercial farms
Rayner et al. (2020) compared a conventional FG strain with an expected ADG of 63g/day (strain C), with two commercial SG-slow strains with expected ADGs of 49g/day (strain A) or 45g/day (strain B). In this study, the SG strains were fed a lower-energy diet than the FG strain. Strain A and some strain B flocks were housed at a predicted stocking density of 30kg/m2 by the end of the rearing period, while strain C and other strain B flocks were housed at a predicted stocking density of 34kg/m2. In actuality, growth was slightly lower than predicted for all strains, which were slaughtered at day 49 (strain A); 45 (strain B) or 35 (strain C). Health assessments were made just prior to slaughter when chickens had reached roughly equivalent weights. The authors’ primary conclusion was that chickens from the FG strain had reduced welfare in comparison to the other two strains, with three-fold higher mortality, significantly higher scores for gait, HB, FPD, pre-processing culls and a behavioural profile that indicated less capacity for positive experience. The FG birds were not observed using the straw bales provided, and they showed far lower levels of play, exploration and foraging behaviour.
2.3 Studies comparing FG and SG strains: measurements taken at commercial slaughter age and weight
Studies that compare strains at commercial slaughter-weights include experimental and epidemiological investigations and, in both cases, the SG birds are likely to be both older and less heavy than FG birds. Here, we first review studies where the FG and SG birds have been raised under similar environmental and management conditions so confounding effects are limited to the age and weight differences just mentioned. We then turn to studies where SG birds have been reared under different environmental conditions, generally at lower stocking densities, with environmental enrichment (which may include foraging and pecking resources, and elevated platforms or perches), and sometimes with access to a veranda or outdoor range. In these studies, age and weight differences are further confounded by major management differences and so the comparison is really between “systems” or “concepts” rather than strains but, when conducted on commercial farms, these may be the most representative of current business practices.
2.3.1 Reared in similar environments
Kjaer et al. (2006) found that a SG-very slow strain (Lohmann Brown) remained free of FPD throughout the growing period, whereas FG birds showed signs of FPD by 14 days of age, and of HB by 28 days of age, with prevalence and severity increasing with time, so that nearly 90% were affected by the time of slaughter at 42 days. Fanatico et al. (2008) had intended to compare strains at equivalent bodyweights but despite both strains being fed a low nutrient diet, the Cobb (old strain, see Table 1) birds grew faster, meaning that both age and bodyweight differed at the time of assessment. In their first experiment using female birds and an outdoor treatment condition, mortality and gait score were both substantially and significantly higher for the Cobb strain. In a second experiment with male birds, the welfare outcomes for FG birds that received a conventional diet rather than a low nutrient diet were particularly poor. However, this work was conducted over 15 years ago and the FG outcomes for mortality and gait score appear to be substantially worse than would be expected for current FG strains.
Bergmann et al. (2017) compared two Ross 308 flocks reared at the same lower stocking density (28.7kg/m2, Supplementary Table 1) and provided with the same enrichment as six SG-slow Cobb Sasso flocks. The Ross flocks were slaughtered at 32 days at an average liveweight of 1.74kg, while the Cobb Sasso flocks were slaughtered at 40 days at an average liveweight of 1.88kg. On day 30, the behavioural profile of the two strains was very similar, including their use of straw bales and perches. The Cobb Sasso birds performed slightly more foraging and locomotion at this time, but as they approached their own slaughter age, their foraging level was very similar to that of the slaughter-age Ross birds, and the time they spent lying was actually greater. Incidentally, it seemed that enrichment benefitted the Ross 308 birds, as the same study found that Ross 308 birds housed in non-enriched flocks showed greatly reduced activity.
A longitudinal study conducted between, 2015 and, 2021 provides a particularly valuable insight into strain effects under commercial conditions because it followed flocks from the same farms during a period when farmers were switching from using FG Ross 308 birds to SG-mid Hubbard 787 birds (Forseth et al., 2023). The conditions on the farms were not substantially altered and so the impact of many of the usual confounding factors was greatly reduced, although statistical models still examined the potential influence of season and slight variations in stocking density. The authors were able to assess the post-mortem prevalence of ascites, hepatitis, small size and discolouration in birds from, 4271 flocks; and the prevalence of skin lesions in birds from, 3879 flocks, with data from over 63 million birds included in total. They found an overall significantly higher prevalence of condemned carcasses from the Ross 308 strain (2.17%) compared with the Hubbard JA787 (0.65%). The causes of condemnation also varied, with ascites the most common reason for condemnation in the Ross 308 birds, and discolouration the most common reason in the JA787 birds. Because of the study design and careful statistical analysis, the authors were firm in their conclusion that strain had a major role in bird welfare and sustainability of production.
2.3.2 Reared in differing environments
A number of studies have focused on comparing different “systems”, with strain (usually SG-slow or SG-very slow) included as just one of number of features differentiating conventional from alternative (‘high welfare’ e.g. Dutch Retail broiler, or Better Life schemes; or free-range or organic) production (Tuyttens et al., 2008; Allain et al., 2009; Bergmann et al., 2016; Gocsik et al., 2016; Rocchi et al., 2021; Averos et al., 2022; de Jong et al., 2022). Factors such as the nutrient quality of the diet, stocking density, provision of natural light and environmental enrichment will differ between systems. For most of these studies it is not possible to conclude more than that the welfare outcomes of the alternative systems are generally better as measured by overall welfare index (Tuyttens et al., 2008; de Jong et al., 2022), lower numbers of sick or wounded birds (Tuyttens et al., 2008; Averos et al., 2022), improved walking ability (Tuyttens et al., 2008; Bergmann et al., 2016), lower levels of ascites, hepatitis, skin lesions, or discolouration (Allain et al., 2009; Bergmann et al., 2016; Rocchi et al., 2021), improved behavioural repertoire (Averos et al., 2022), or reduced fearfulness (Averos et al., 2022, but not Tuyttens et al., 2008). Sometimes the differences appear substantial. For example, Allain et al. (2009) estimated the prevalence of all skin lesions to be 63% in FG flocks but just 29% in SG flocks, Bergmann et al. (2016) estimated a 9-fold increase in risk of footpad hyperkeratosis for Ross birds in conventional systems vs Cobb Sasso birds in alternative systems, and Allen et al. (2023), in a study of over 17,000 trailer-loads of broilers transported to the abattoir, found that dead on arrival rates were 73% lower in Hubbard JA strains (not specified further) than in Ross 308 or Cobb 500 flocks. However, the reasons for these effects cannot be ascribed solely to strain. They could relate partially, equally or even more strongly to the lower stocking densities, outdoor access, natural light and enrichment that are typical features of alternative systems. Occasionally, key welfare measures such as mortality do not differ between “systems” or are actually better for FG strains kept indoors than for SG strains in organic systems (e.g. Rocchi et al., 2021).
Although it is not possible to eliminate confounding factors when studying commercial flocks, sometimes there is sufficient variation within each system to draw conclusions about the relative influence of strain vs other management factors. For example, Gocsik et al. (2016) compared data obtained during the Welfare Quality ™ project between, 2008 and, 2011 for 168 flocks kept in conventional systems using FG strains and four alternative systems using SG strains. Prior literature was used to establish how system attributes (including strain) were linked with each of the welfare measures used in the WQ protocol. Where multiple attributes influenced a welfare measure then the relative importance of each was estimated. Overall, it was concluded that strain had a greater influence on the welfare of birds (29% to 36%) than any other single environmental or management variable. However, stocking density (18% to 24%) and length of the dark period (17% to 24%) were also strong influences. The mortality of the FG strains kept in standard conditions was slightly greater than for the SG strains in alternative systems. Data obtained for each flock were combined into integrated welfare scores, leading the authors to conclude that the overall welfare of broilers was best in the so-called ‘middle market’ Volwaard and Puur & Eerlijk systems that used SG strains, a moderate stocking density of 25 to 31 kg/m2, and which provided access to a veranda but not to an outdoor range.
2.4 Studies comparing FG and SG strains: measurements taken at both equivalent ages and at equivalent target weights
2.4.1 Canada studies – indoor pens
An ambitious series of studies was conducted at a research facility in Canada, to compare conventional and SG strains of broiler chickens reared under standardised conditions (Dawson et al., 2021; Santos et al., 2022a, Santos et al., 2022b; Torrey et al., 2021). Overall, 16 anonymised strains were assessed, three conventional and 13 SG. One conventional strain (A) and a very slow-growing strain (T) were not fully replicated and their data were not included in most of the resulting publications. The remaining strains were compared both at the same ages, with conventional strains leaving the study early when they reached slaughter weight, and at two target weights (TWs) based on breeder estimates. TW1 was 2.1kg which was expected to be reached at 34d for FG strains, and at approximately 48d for other strains. TW2 was 3.2kg which was expected to be reached at approximately 48 d for FG strains, and at approximately 62d for other strains. The authors’ classification of strain based on ADG varies slightly from the scheme used in this review, but both FG strains had ADGs > 60g/day; eight of strains fell into our SG-mid category with ADGs between 50 and 60g/day (the authors classified strains F, G, I and M at the top end of this range and strains E, H, O and S at the lower end), and four strains fell into our SG-slow category, with ADGs between 43 to 48g/day (Supplementary Table 1).
Overall, in this study mortality was lower than generally found under commercial conditions (Torrey et al., 2021) and differences between strains related primarily to the proportion of birds culled. Strains B (FG) and F (SG-mid) accounted for nearly half of the culling required because of lameness. Dawson et al. (2021) evaluated the behaviour of birds from all of these strains, using both direct observations and by fitting 2 birds/pen with accelerometers, while Santos et al. (2022a) reported outcomes related to tibial bone health and strength and Santos et al. (2022b) reported outcomes related to walking ability (measured by latency-to-lie and group obstacle tests rather than the more common gait scoring method) and contact dermatitis. The results were reported both at equivalent ages and at equivalent bodyweights.
By Age: When compared at 48 days, birds from FG strains stood for a shorter time than SG strains, and had the lowest frequency of barrier crossings in a group-obstacle test. FG birds also had a greater total prevalence of FPD, severe FPD and total HB than SG strains. Severe HB was rare but greater for FG (4.16%) than other strains (0.01 to 1.27%). Accelerometry revealed increasing inactivity with age. At 28 days the FG birds were inactive for approximately 17h/day, nearly 1h/day more than the SG-mid (top-end) strains and nearly 2h/day more than the other SG strains. As inactivity increased with age, the differences between strains became less apparent. Direct behaviour observations confirmed these findings, with greater inactivity shown by FG birds. All strains spent similar amounts of time preening, drinking and eating. FG birds were less likely to use enrichments, particularly elevated platforms in comparison with other strains at both 28 and 42 days of age. Observations of foraging, dustbathing and comfort activities were too infrequent to analyse, although the morning/early afternoon timing of direct observations may have underestimated the occurrence of these behaviours.
By Target Weight: At TW1, there were no strain differences in tibial bone strength but by TW2 the bone strength of FG and SG-fast strains was greater than for SG-mid or SG-slow strains, even when adjusted for bodyweight (Santos et al., 2022a). In addition, the FG birds had a lower prevalence of tibial dyschondroplasia than the SG-mid strains. These beneficial outcomes for FG birds were attributed to recent efforts by breeding companies to improve skeletal integrity. At TW1 the FG birds fared relatively well in the walking ability tests, standing for longer than the SG-fast chickens in the shallow water bath, but by TW2 this situation had reversed. At this later assessment period, the FG birds had shorter legs, less dry bone matter and heavier bodies and they were less able to stand. They were also less active in group obstacle tests than all of the SG strains. Additionally, the FG birds had higher total rates of FPD than SG-mid strains at TW1, and higher rates of HB, FPD, including severe FPD, than SG-mid and SG-slow strains at TW2 (Santos et al., 2022b). At TW1 (but not TW2) inactivity was greater for the SG strains. This was considered a surprising result but the SG birds were of course, older by this time, and perhaps less motivated to perform juvenile activities including play. However, the SG-slow birds were most likely to use the enrichments, particularly the elevated platforms at both TWs.
2.4.2 Netherlands studies – indoor pens
Two studies assessed FG Ross 308 and SG-slow JA757 birds raised in standard pens with a single perch, or under enriched conditions with dustbathing substrate, elevated platforms with ramps and live soldier flies (the feed was adjusted for the standard pens to provide same overall nutrient level) (de Jong et al., 2021 and Guz et al., 2021).
By Age: In both studies there were no significant effects of strain or interactions of strain with housing type for mortality or gait score, whilst de Jong et al. (2021) also found no effects on HB or FPD measures. Direct observations showed the JA757 birds were more active (average of all scan periods 18.5% vs 14%; focal observations 14% vs 10%) and less likely to show idle sitting behaviour. Idle behaviour occupied >50% time by 22 days (Ross) or 30 days (JA757). There were no differences between strains for comfort behaviour but the JA757 birds spent more time foraging (Guz et al., 2021). Providing enrichment increased the activity and perching behaviour of JA57 birds, without improving health scores, but had no effect on Ross birds. For the birds housed in enriched pens, observations of dustbathing were very infrequent and did not differ between strains
By Target Weight: There were no significant effects of strain or interactions of strain with housing type for mortality, gait score, HB or FPD measures (de Jong et al., 2021). However, Guz et al. (2021) found that JA57 birds had improved markers of tibial bone development and reduced levels of leg deviation, when compared with Ross birds at equivalent weights of approximately 2.2kg. Pen enrichment improved bone development further in both strains. In both studies, when assessed at the same target weight, with older JA57 birds compared with younger Ross broilers, JA57 birds showed more standing idle, less time spent feeding and more activity and were more likely to be seen on the perches and less likely to be found on the ground underneath the elevated platforms or ramps (de Jong et al., 2021; Guz et al., 2021).
3 Quantitative overview of FG vs SG comparisons
3.1 Methods
The narrative above compared some of the advantages and disadvantages of different approaches to strain welfare comparisons, highlighting the potential confounding influences of bird age and diet. A qualitative summary of major results was presented without attempting to quantify effect sizes. This was partly because of high between-study variation in welfare outcomes recorded and, in the methods, and scales of measurement. In addition, the sheer number of different SG strains included in research to date (often with just one or two studies per strain) also made it difficult to draw quantitative conclusions.
However, it was feasible to conduct a quantitative analysis for four of the most commonly included welfare indicators: mortality, gait score, footpad dermatitis and hockburn. For the analysis to be relevant to the majority of current commercial broiler production, strict inclusion and exclusion criteria were employed.
Within-study data were included only when the SG strains in the study had an ADG of >40g/day and were used in current indoor commercial production. Other inclusion criteria were that all strains within the same study had to be housed and managed under similar conditions, using indoor housing (with or without enrichment, elevated structures and natural light), although stocking density could not be standardised as generally varied within studies as a function of bird weight. Independent treatments (e.g. stocking density) within studies were included as separate datapoints. Free-range and organic systems were not included because of their niche status and because welfare outcomes in these systems vary more due to externalities such as predation, weather and disease.
A substantial proportion of studies (or treatments within studies) reviewed in Section 2 were also excluded from the quantitative comparison for any of the following additional reasons:
● FG birds were kept for longer than 62 days of age (to increase relevance to commercial practice);
● Number of birds per treatment was <50 (to avoid excessively small sample sizes);
● Study published prior to, 2013 (to ensure relevance given ongoing changes in breeding goals);
● Strains were reared in different housing conditions (to avoid undue influence of externalities)
● Strains were mixed together (to ensure independence of findings).
If strains had been compared at multiple time points or bird weights, then data that had been taken at around normal slaughter age/weight e.g. around 42 to 56 days or 2.2kg were preferentially included compared with data taken at earlier or later stages.
Incidence rate ratios (IRR) of mortality for FG and SG breeds were calculated for each study. Because some studies had no FPD or HB presence among SG breeds, risk difference between FG and SG breeds was calculated for each study and used in the meta-analysis. For gait score analysis, means and standard deviations were calculated for FG and SG breeds in each study. Some studies had a single FG and multiple SG breeds, a single SG and multiple FG breeds or multiple FG and SG breeds. To avoid “double-counting” of the number of birds in the meta analysis, we split the number of birds by the number of comparisons in the analysis while keeping the mortality, FPD, HB or gait score the same in the multiple comparison. For example, if 100 FG birds were compared to two different SG breeds, n=50 was used for the FG in each of the comparison; the mortality, FPD, HB or gait score for the FG in the multiple comparison remained the same. A three-level random effect meta-analysis was carried out to estimate the pooled IRR or pooled risk difference for FPD or HB while accounting for the between and within study variation using the Inverse variance method. This approach would further account for the dependency in the effect size due to the identical mortality (FPD, HB or gait score) figures used in the multiple comparison. Similarly for the gait score analysis, a three-level random effect mean difference model using inverse variance weighting approach was used for pooling. Higgin’s & Thompson’s I² heterogeneity statistic was used to quantify the percentage of variation across studies. We also performed sensitivity analysis to assess the impact of sample size for mortality in the commercial studies on the pooled estimates by excluding those commercial studies.
All analysis was done using R version 4.3.1 and the <meta> package.
3.2 Mortality
The incidence rate ratios (IRR) for total mortality reported for 26 independent treatments from 11 eligible studies are shown in Figure 1. The pooled IRR was 1.69 (1.38-2.06), demonstrating that the risk of new deaths arising over a given period of time is approximately 1.69 times greater for FG strains compared with commercial SG strains housed indoors. Thus, if SG mortality were 2%, the FG birds would have 2% x 1.69 mortality = 3.38%.
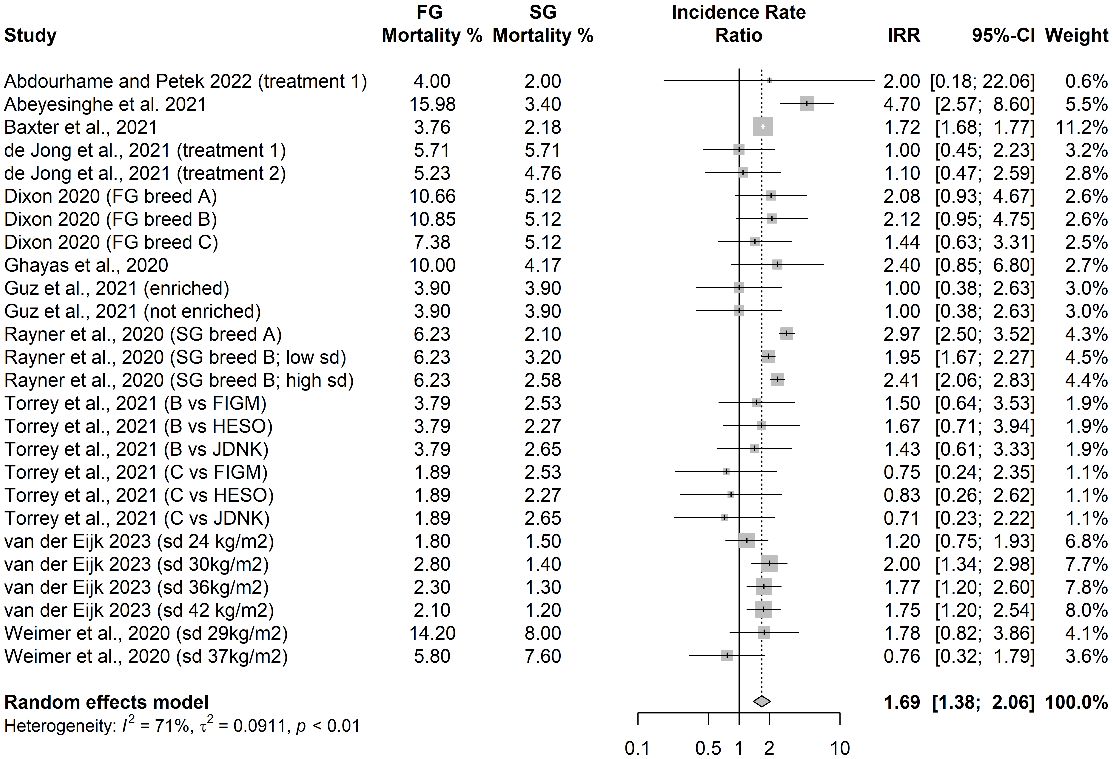
Figure 1 Incidence rate ratios for broiler mortality of fast-growing (FG) and slow-growing (SG) strains.
SG birds were often kept for a greater period of time than FG birds, until they reached slaughter weight, so it is important to also consider what is captured in an average daily mortality analysis. Most studies did not provide the timing of death for individual birds but the total mortality analysis presented above implicitly assumes that all birds that died did so on the first day of the study. We therefore modelled the other extreme scenario whereby all birds that died did so on the last day of the study. This resulted in a pooled IRR of 2.16 (1.74-2.67), i.e the risk is more than doubled.
3.3 Gait score
All eligible studies recorded gait on a scale from 0 (best) to 5 (worse) towards the end of the rearing period. Overall, when 16 independent treatments across these 7 studies were included in the analysis, the pooled mean gait score of FG strains was 0.65 points greater than that of commercial SG strains housed indoors (Figure 2).
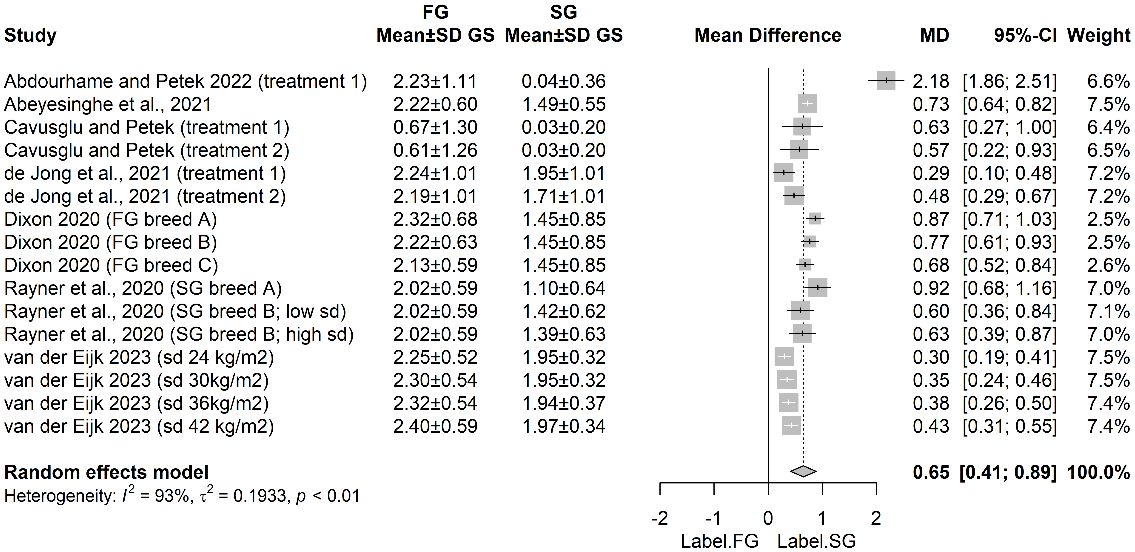
Figure 2 Mean difference in gait score (GS) for fast-growing (FG) and slow-growing (SG) strains. SD, standard deviation.
3.4 Footpad dermatitis
Overall, across 21 independent treatments from 10 independent studies there was an absolute increase in % FPD (score above 0 in any of the various scoring systems used) in FG strains. On average, 15% more birds/flock experienced some degree of FPD in FG strains than in commercial SG strains housed indoors (Figure 3).
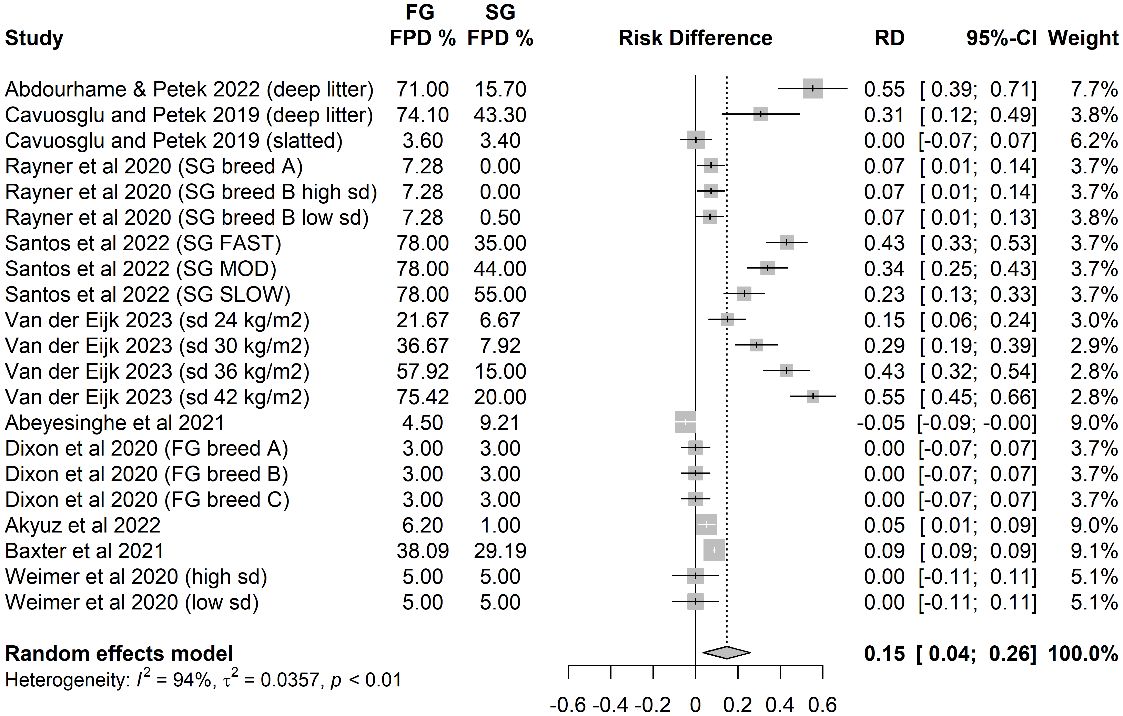
Figure 3 The risk difference (probability of FPD in fast-growing (FG) strains minus the probability of FPD in slow-growing (SG) strains).
3.5 Hockburn
Overall, across 21 independent treatments from 10 independent studies there was an absolute increase in % HB (score above 0 in any of the various scoring systems used) in FG strains. On average, 25% more birds/flock experienced some degree of HB in FG strains than in commercial SG strains housed indoors (Figure 4).
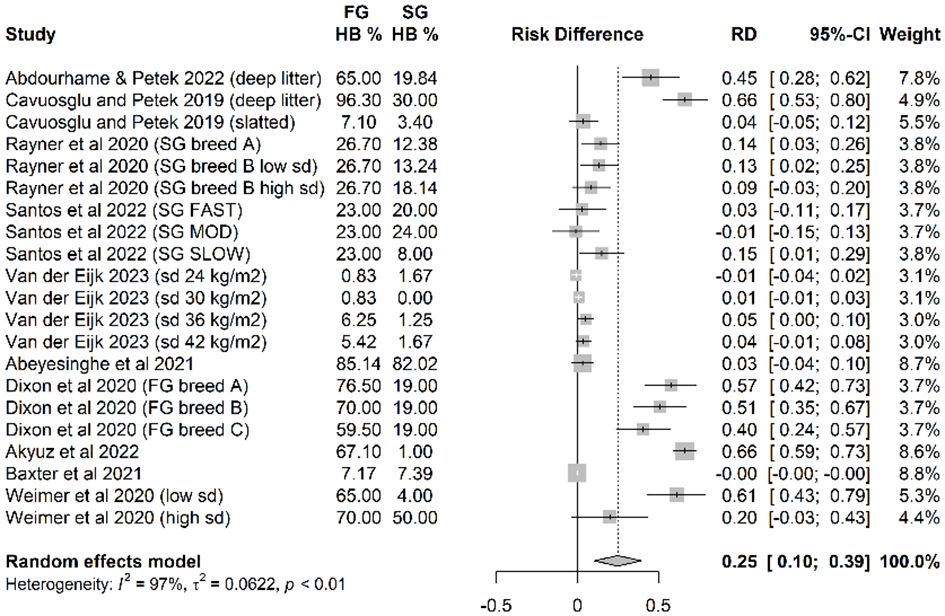
Figure 4 The risk difference (probability of HB in fast-growing (FG) strains minus the probability of HB in slow-growing (SG) strains).
4 Discussion
One of the aims of this review was to assess whether the welfare of SG strains is relatively and substantially better than that of FG strains to support decisions on future directions of broiler production that acknowledge both welfare and sustainability. One reason why this comparison is difficult is because of a shifting baseline. Despite ongoing increases in ADG in FG strains, the incorporation of certain welfare traits in breeding goals has reportedly led to a sharp reduction in some welfare problems, particularly ascites (Neeteson et al., 2023). Some aspects of leg health have also steadily improved since, 2000 (Kappell et al., 2012; Santos et al., 2022a). Continued progress means that the Aviagen breeding company expects 96 - 98% of Ross 308 birds to have no leg health defects by, 2023 and, 2025 respectively (Neeteson et al., 2023). Independent confirmation of achievement of these goals would be a major step forward given that the most recent studies, whilst acknowledging a lag, suggest average gait scores remain between 2 and 3. Overall, Aviagen breeding goals now encompass 40 different traits covering health, welfare, robustness, environmental impact, reproduction and production (Neeteson et al., 2023). The phenotypes of commercial SG strains are also in flux, with some possessing ADGs that exceed those of the fastest growing strains from just a few years ago. Due to ongoing changes in commercial strains, we treat comparative results from older studies with great caution. However, 51/63 papers were conducted in the past 10 years and 37/63 in the past 5 years (Supplementary Table 1). The results of the most recent papers will be most relevant to the current commercial context.
The predominant conclusion arising from both our narrative and quantitative review is that, despite more emphasis on welfare in breeding goals, FG strains perform more poorly on many relevant physical and behavioural traits than modern, commercial SG birds with relatively high growth rates. This is the case even when the ‘fairest’ comparisons are made at equivalent bodyweights (when SG birds are older) and when conducted under matched-environmental conditions, experimentally or on farm. From the most recent studies SG strains showed better walking ability (Dixon, 2020; Abeyesinghe et al., 2021; van der Eijk et al., 2023); leg health (Guz et al., 2021), skin conditions (Dixon, 2020; Abeyesinghe et al., 2021; Forseth et al., 2023; van der Eijk et al., 2023; Weimer et al., 2020) and spent more time standing or walking (de Jong et al., 2021; Guz et al., 2021; van der Eijk et al., 2023). The quantitative analyses confirmed this impression with SG strains having an estimated mean gait score 0.65 points lower than FG strains, the latter primarily scoring midway between 2 (definite and identifiable gait abnormality that does not affect ability to move) and 3 (obvious gait defect that affects its ability to move); 15% fewer birds/flock with FPD and 25% fewer birds/flock with HB. Walking ability in particular seems to show a much steeper late-rearing period decline in FG birds than SG birds (Santos et al., 2022b). The associations between gait score, underlying causation or pathology, and pain are complex. Research has shown that whilst birds with gait score 2 show little improvement in walking speed when given an appropriate analgesic drug (Tahamtani et al., 2021) birds with gait score 3, do respond to analgesic drugs with faster walking speeds, suggesting that pain relief improves mobility in these more seriously affected animals (Caplen et al., 2013).
The mortality of FG strains was also higher than that of SG strains with ADGs of >40g/day, with an estimated incidence rate ratio (risk of death over a given time period) of between 1.69 and 2.16 depending on whether deaths were expected to occur at the start or the end of the rearing period. In reality, deaths occur throughout rearing, though generally not linearly (Baxter et al., 2021) found mortality was higher at days 3 and 7, than at days 14, 21 or 28 in both FG and SG strains, Similarly, Dixon (2020) found mortality to be higher in the first 14 days compared with later periods. Dixon (2020) noted yolk sac infections as one cause (Abeyesinghe et al., 2021; Figure 2) reported the timing of mortality from day 7 onwards and found a steep increase in FG mortality from around day 28. The birds studied by Torrey et al. (2021, additional data accessed with permission) also showed higher mortality to day 7 than during other weeks of the rearing period, with increased mortality later in rearing (e.g. from day 42 to 49) for both the FG strains but only for a few of the SG-mid strains (e.g. F and H). Therefore, there is some evidence that mortality is higher early in rear, then tends to occur relatively evenly throughout the rearing period for both FG and SG strains, with a sharp increase in late-rearing period mortality and culls restricted to specific strains (Dixon, 2020; Baxter et al., 2021). Based on these considerations, the ‘real’ increase in mortality risk for FG strains will lie somewhere between the two estimates provided by the scenarios in Section 3.1. Only a few studies provided information on causes of mortality. FG birds were culled primarily because of leg problems (e.g. Rayner et al., 2020; Abeyesinghe et al., 2021; Baxter et al., 2021) but also due to small bird size or unexplained weakness. Birds might be found dead due to ascites, hepatitis and heart attacks (Dixon, 2020; Abeyesinghe et al., 2021; Wilhelmsson et al., 2019), with FG birds more susceptible to infections (e.g. Fanatico et al., 2008). In free-range systems, predation rather than disease accounted for most mortality (e.g. Horsted et al. (2005).
Without downplaying the strong conclusion outlined above, there are nuances. For example, FG and SG strains did not differ consistently in the performance of relatively sedentary behaviours such as preening (Dawson et al., 2021), resting or sleeping (Wallenbeck et al., 2016; Yngvesson et al., 2018). FG strains generally made less use of enrichments, especially elevated structures such as raised platforms or perches (Malchow and Schrader, 2021). This will be associated with decreased welfare if birds possess a desire for elevation that they cannot achieve but it is also possible that heavy birds re-align their expectations and motivations and do not experience frustration. This is an important point that relates not only to FG birds to but most of the SG strains studied. Only the very slowest growing birds (e.g. males of layer strains) appear to show ‘natural’ chicken behaviour as demonstrated by heritage strains or ancestral junglefowl (for a thought experiment exploring the concept of “natural” in a welfare context, and the use of ancestral strains as a baseline, see Yeates, 2018). Generally perching, dustbathing and even, in some studies foraging, occurred at very low levels in all strains studied (e.g. Castellini et al., 2016; Baxter et al., 2021; Dawson et al., 2021; Guz et al., 2021). It is possible that the motivation of broilers to do these behaviours has reduced alongside their physical capacity but a number of studies suggest that this is not the case. For example, when provided with elevated structures that are easier to access than traditional perches, broilers show high usage levels (Norring et al., 2016; Malchow et al., 2019c).
FG birds appeared to have a higher susceptibility to heat stress than SG-slow or SG-very slow strains (Nielsen, 2012; Brugaletta et al., 2022; Wilhelmsson et al., 2019). Heat stress is a cause of significant harm to birds during heat waves in Europe and throughout the year in many warmer regions of the world (Saeed et al., 2019). However, there is little information on the susceptibility of SG-mid strains for comparative purposes. For a handful of measures, the SG birds had poorer welfare outcomes than FG strains: toe pecking (Weimer et al., 2020), antigen response (Singh et al., 2021), breast blisters (Allain et al., 2009), skin scratches and damage (de Jong et al., 2022). Occasionally, SG strains experienced surprisingly high first-week mortality (Nielsen et al., 2010; (Abeyesinghe et al., 2021, SG strain SGN, additional data accessed). There was also inconsistency as to whether SG strains were more (Cavusoglu and Petek, 2019), equally (Tuyttens et al., 2008; Eleroglu et al., 2015; Abdourhamane and Petek, 2022), variably depending on test (Lindholm et al., 2017) or less (Averos et al., 2022; van der Eijk et al., 2022) fearful than FG strains. This might reflect SG strain differences and/or differences in measurement methods, whereby locomotor measures are confounded with differences in activity. However, the lack of transparency in strains observed in many of the studies, as well as the limited replication of those identified strains means that it is currently impossible to draw reliable conclusions about which SG strains may have superior welfare outcomes compared with other SG strains.
Even so, generally, growth rate remains an extremely good proxy marker of welfare outcomes. In studies of multiple strains, very strong correlations between ADG and welfare outcomes have been reported for mortality, leg health, skin condition and behaviour (Dawson et al., 2021; Rauch et al., 2017; Louton et al., 2019; Sarica et al., 2019; Mancinelli et al., 2020). However, there have been a few minor exceptions. For example, Rauch et al. (2017) found JA957 birds had better gait than a lower ADG strain, Louton et al. (2019) found strain differences in HB were not totally predicted by ADG, Mancinelli et al. (2020) found that some Ranger strains had better welfare outcomes than two slightly slower-growing JA crosses, Bergmann et al. (2017) reported welfare outcomes for Cobb Sasso strain were not substantially better than for an FG strain tested under the same conditions, whilst Torrey et al. (2021) found one FG strain had lower leg culls than predicted by ADG, and Druyan et al. (2008) found no direct association between ADG and ascites. A dissociation of a very different kind is demonstrated by the fact that reducing the ADG of FG strains by restrictive feeding only partially resolves welfare problems (whilst itself causing hunger) e.g. Wilhelmsson et al. (2019). These dissociations show there is at least further potential to breed for better welfare, even in FG strains, as argued by Dawkins and Layton (2012) who proposed that the generally close associations between ADG and welfare might simply reflect historically narrow, production-focussed breeding goals.
Steps towards breeding FG birds with better welfare have already been partially (Neeteson et al., 2023), but not fully, successful. If further dissociation between growth rate and welfare can be achieved it raises the possibility of FG strains with excellent welfare. But this aspiration requires closer scrutiny and it is not clear whether it is in the interests of the two global companies to move further in this direction. Information about how welfare goals are balanced against traditional production outcomes is commercially sensitive. This raises ethical questions, similar to those arising in the laying sector where, again, breeding is controlled by a very small number of commercial operators (Fernyhough et al., 2019). Fernyhough et al. (2019) argued that, even if breeding goals remain hidden, welfare information about the product (the birds sold to farms) should be publicly available alongside performance objectives. This would reduce the burden on charities and NGOs to devise their own welfare-assessment protocols for strain approval, and would allow for benchmarking and tracking of welfare changes over time. The need for openly available welfare information and consistent assessment methods may become even more pressing with the advent of precision breeding techniques (Haskell et al., 2023). In the interests of transparency, it would also be useful if breeding companies could provide more information on the genetics of each strain, their origins and relationships to other strains.
Rather than developing slower-growing strains of broiler chicken, an alternative approach to improving broiler welfare might be to slaughter birds (of any strain) at the age where welfare problems start to become apparent, although as discussed later, there are sustainability implications of this suggestion as more birds would need to be reared to satisfy the same level of demand Apart from early-life mortality, the prevalence and severity of most welfare problems increases with age and weight (e.g. activity and perching behaviour, Bokkers and Koene, 2003; Baxter et al., 2021; Dawson et al., 2021); contact dermatitis (Akyuz et al., 2022; Wilhelmsson et al., 2019; walking ability, Baxter et al., 2021; Wilhelmsson et al., 2019). As a specific example, the percentage of birds with no walking difficulties (GS zero) between week 3 and week 6 was 86%, 84%, 61% and 58% for Redbro birds, compared with 76%, 55%, 38% and 16% for Ross 308 (Baxter et al., 2021). An analogy could be drawn with the concept of humane endpoints in laboratory animal science (Hendricksen et al., 2010). Indeed, humane culling was adopted in some of the research studies reviewed here (Rezaei et al., 2018). A modelling exercise conducted by Knowles et al. (2008) suggested that reducing slaughter age from 40 to 28 days of age would have a greater effect in reducing gait score than switching breed, lowering stocking density to 18.5kg/m2, increasing hours of darkness or increasing antibiotic usage. Since SG birds require more land use, feed and other resources it would be timely to consider the comparative economics, environmental impact, welfare, ethics and consumer acceptability of welfare-based endpoints vs use of SG strains. Ethical concerns have been raised about the very short lives of male layer chicks and dairy calves, but the short lifespan of broiler chickens has not to date been a focus of concern. Further shortening of lifespan to avoid poor welfare outcomes might therefore be an acceptable solution.
Welfare outcomes for both FG and SG strains depend not only on genetics but also on the rearing environment. van der Eijk et al. (2022); van der Eijk et al. (2023) found that both FG and SG strains benefitted from lower stocking density, which resulted in improved litter quality and additional space for birds to move. Bergmann et al. (2017) found that FG broilers greatly benefitted from provision of enrichment, showing much improved activity. But enrichment for FG strains is often not effective, particularly beyond the two or three weeks of life, when FG birds tend to stay underneath elevated platforms rather than using them (Dawson et al., 2021; Malchow et al., 2019a, Malchow et al., 2019b; de Jong et al., 2021; Guz et al., 2021; Malchow and Schrader, 2021). Good environments matter to all strains, however, certain environmental improvements will be effective only in strains with the genetic potential to benefit.
The current review has highlighted the great diversity of methods employed in assessing the welfare of broiler strains which has limited the welfare data we could quantify. To facilitate future overviews or even meta-analysis of data, voluntary steps should be taken by researchers (and breeding companies) to standardise methods across studies. At the very least, any study comparing the welfare of different broiler strains should take repeated measures and report data at both equivalent ages and bodyweights. A better approach would characterise change in outcomes over time to capture non-linear temporal change and represent lifetime welfare. Reporting the timing and causation of mortality is important for both welfare and sustainability evaluations. FG strains should not be feed restricted and, unless there are reasons not to do so, the most common scoring systems for gait, FPD and HB should be used. Finally, it should be noted that welfare is a multidimensional concept that cannot be assessed with just one or two measures (Mason and Mendl, 1993). Despite guidance from long-standing frameworks such as the Five Freedoms or Five Domains model (Mellor, 2017; Webster, 2016) many source papers used only one or two indicators, generally relating to physical health problems known to affect broilers such as contact dermatitis or leg health. Even when considering a single indicator such as contact dermatitis, wide variation in measurement scales makes comparisons difficult. In particular, the 0+ point on the RSPCA (2017) scale which measures pre-lesion conditions such as swelling or discolouration requires further consideration. If future research establishes that conditions scored at 0+ are either painful or strongly predictive of worsening condition, then measurement scales should be adjusted accordingly.
A rather small number of studies e.g. Castellini et al. (2016), recorded many indicators, but some, such as back and wing feather condition, may have been redundant (essentially measuring the same trait) (Nicol et al., 2011) and an agreed indicator profile for future research would be constructive. Bird affect is a key component of broiler welfare which can be accessed via behavioural assessment (Nicol et al., 2011; Abeyesinghe et al., 2021) but has often been neglected. Behavioural recording was approached in many different ways by those source studies that employed it in this review. Different sampling and recording approaches are better suited to capture of event and state behaviours, but ethograms and observation and behaviour test protocols to capture specific behaviours of key importance would benefit from standardisation across studies for comparability. These should consider scalability between experimental and commercial studies and incorporate observer blinding to treatments/strains and integrated testing of intra and inter-observer reliability wherever possible.
In summary, improving the welfare of broiler chickens is an important priority but the current demand for chicken meat and the potential trade-offs between higher welfare production sustainability and efficiency do not facilitate an easy commercially applicable solution. In an attempt to address both issues animal welfare and environmental organisations often promote an “eat less, eat better” message in relation to the consumption of animal-derived foods. Our review indicates genetic variation provides scope to improve welfare of all strains through selection, but welfare improvements in FG strains to date have not fully resolved key concerns, for example around leg health. The extent to which welfare is prioritised in breeding goals also remains unclear. In situations where welfare traits compete with production goals, breeding companies have difficult decisions to make. The diversity and changing nature of SG strains available for different market sectors (Supplementary Table 1; Table 1) means that data are very sparse for many SG strains. Greater dialogue between breeding companies and independent scientists could help to focus research efforts where they are most needed. A more immediate solution could be to kill FG birds for meat at an earlier age, hence lower live-weight, as several welfare indicators worsen in the last weeks of production, although clarity on temporal progression of others is needed. To meet the current demand for chicken meat, this would necessitate more birds and a higher turnover, so economic modelling would be required to compare these costs with those of using SG strains. We have demonstrated that slower growing strains with ADGs of >40g/day show substantial improvements in many welfare outcomes compared to conventional fast-growing strains: mortality risk (0.46-0.59 times lower) l), contact dermatitis (FPD 15%, HB 25% fewer birds) and improvement in walking ability (0.65 lower mean gait score).
These welfare improvements warrant greater consideration in production strategies as they can offset some of the costs associated with poorer FCR and greater land use for SG strains. The use of SG strains may also reduce the need for feed restriction in broiler breeders (Arrazola and Torrey, 2021). However, comprehensive assessment of welfare differences between SG and FG is limited by the inconsistency of methods used and uncertainty over some aspects of welfare, such as fearfulness. Crucially, greater transparency in welfare assessment of specific strains (ideally incorporating information provided by breeding companies) is needed to allow realistic incorporation of welfare data into economic life cycle analyses and to identify strains with true potential to close the gap to sustainability goals without welfare compromise.
Author contributions
CN: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization. SA: Writing – review & editing, Project administration, Funding acquisition. Y-MC: Writing – review & editing, Formal analysis, Data curation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Animal Welfare Foundation (AWF) and the British Poultry Council. AWF is a fundraising and grant giving charity (charity number, 1192203) directed by veterinary and animal welfare professionals, which uses scientific knowledge to improve the welfare of animals through research, education and debate.
Acknowledgments
More information can be found at www.animalwelfarefoundation.org.uk. The authors would like to thank James Bentley (Hubbard breeding company, part of the Aviagen Group) for advice on strain characteristics. We also acknowledge gratefully the help of Dr Ingrid de Jong, Stephanie Torrey, and Jerine van de Eijk who provided supplementary raw data from their publications that enabled our quantitative analyses and Inma Estevez and Xavier Averos who provided data on the growth of a Ross 308 x Hubbard JA cross. Natalie Chancellor is also thanked for her contributions to data collation on dietary energy, included in Supplementary Table 1 and Daniel Parker of Slate Hall Veterinary Practice for comments on an earlier draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2024.1374609/full#supplementary-material
References
Abdourhamane I. M., Petek M. (2022). Health-based welfare indicators and fear reaction of slower growing broiler compared to faster growing broiler housed in free range and conventional deep litter housing systems. J. Appl. Anim. Welfare Sci. doi: 10.1080/10888705.2022.2100221
Abdourhamane I. M., Petek M. (2023). Effects of range access on behavioral-based welfare indicators and foot health condition of slow- and fast-growing broiler. Acta Veterinaria Eurasia 49, 75–81. doi: 10.5152/actavet.2023.22094
Abeyesinghe S., Chancellor N. M., Hernandez Moore D., Chang Y.-M., Pearce J., Demmers T., et al. (2021). Associations between behaviour and health outcomes in conventional and slow-growing breeds of broiler chicken. Animal 15, e100261. doi: 10.1016/j.animal.2021.100261
Akyuz H. C., Onbasilar E. E., Bayraktaroglu A. G., Ceylan A. (2022). Age and sex related changes in fattening performance, dermatitis, intestinal histomorphology, and serum IgG level of slow- and fast-growing broilers under the intensive system. Trop. Anim. Health Prod. 54, e312. doi: 10.1007/s11250-022-03315-3
Allain V., Mirabito L., Arnould C., Colas M., Le Bouquin S., Lupo C., et al. (2009). Skin lesions in broiler chickens measured at the slaughterhouse: relationships between lesions and between their prevalence and rearing factors. Br. Poult. Sci. 50, 407–417. doi: 10.1080/00071660903110901
Allen S. E., Parker C. D., Verheyen K. L. P., Nicol C. J., Chang Y. M. (2023). Effects of external ambient temperature at loading, journey duration and flock characteristics on the dead-on-arrival rate in broiler chickens transported to slaughter in Great Britain. Poult. Sci. 102, e102634. doi: 10.1016/j.psj.2023.102634
Almeida G. F. D., Hinrichsen L. K., Horsted K., Thamsborg S. M., Hermansen J. E. (2012). Feed intake and activity level of two broiler genotypes foraging different types of vegetation in the finishing period. Poult. Sci. 91, 2105–2113. doi: 10.3382/ps.2012-02187
Arrazola A., Torrey S. (2021). Welfare and performance of slower growing broiler breeders during rearing. Poult. Sci. 100, e101434. doi: 10.1016/j.psj.2021.101434
Arrazola A., Widowski T. M., Guerin M. T., Kirie E. G., Torrey S. (2020). The effect of alternative feeding strategies on the feeding motivation of broiler breeder pullets. Animal 14, 2150–2158. doi: 10.1017/S1751731120000993
Averos X., Nazar F. N., Estevez I. (2022). Animal welfare assessment: Quantifying differences among commercial medium and fast growth broiler flocks. Front. Anim. Sci. 3, e868851. doi: 10.3389/fanim.2022.868851
Aviagen (2022) Ross 308 performance objectives. Available online at: https://aviagen.com/assets/Tech_Center/Ross_Broiler/RossxRoss308-BroilerPerformanceObjectives2022-EN.pdf (Accessed 9.1.24).
Baxter M., Richmond A., Lavery U., O'Connell N. E. (2021). A comparison of fast growing broiler chickens with a slower-growing breed type reared on Higher Welfare commercial farms. PLoS One 16, e0259333. doi: 10.1371/journal.pone.0259333
Bennett C. E., Thomas R., Williams M., Zalasiewicz J., Edgeworth M., Miller H., et al. (2018). The broiler chicken as a signal of a human reconfigured biosphere. R. Soc. Open Sci. 5, e180325. doi: 10.1098/rsos.180325
Bergmann S., Louton H., Westermaier C., Wilutzky K., Bender A., Bachmeier J., et al. (2016). Field trial on animal-based measures for animal welfare in slow growing broilers reared under an alternative concept suitable for the German market. Berliner und Munchener Tierarztliche Wochenschrift 129, 453–461. doi: 10.2376/0005-9366-16035
Bergmann S., Schwarzer A., Wilutzky K., Louton H., Bachmeier J., Schmidt P., et al. (2017). Behavior as welfare indicator for the rearing of broilers in an enriched husbandry environment- A field study. J. Vet. Behav. - Clin. Appl. Res. 19, 90–101. doi: 10.1016/j.jveb.2017.03.003
Bessei W. (2006). Welfare of broilers: a review. World’s Poult. Sci. J. 62, 455–466. doi: 10.1079/WPS2005108
Bokkers E. A. M., Koene P. (2003). Behaviour of fast- and slow growing broilers to 12 weeks of age and the physical consequences. Appl. Anim. Behav. Sci. 81, 59–72. doi: 10.1016/S0168-1591(02)00251-4
Brugaletta G., Greene E., Ramser A., Maynard C. W., Tabler T. W., Sirri F., et al. (2022). Effect of cyclic heat stress on hypothalamic oxygen homeostasis and inflammatory state in the jungle fowl and three broiler-based research lines. Front. Vet. Sci. 9, e905225. doi: 10.3389/fvets.2022.905225
Caplen G., Colborne G. R., Hothersall B., Nicol C. J., Waterman-Pearson A. E., Weeks C. A., et al. (2013). Lame broiler chickens respond to non-steroidal anti-inflammatory drugs with objective changes in gait function: A controlled clinical trial. Vet. J. 196, 477–482. doi: 10.1016/j.tvjl.2012.12.007
Caplen G., Hothersall B., Murrell J. C., Nicol C. J., Waterman-Pearson A. E., Weeks C. A., et al. (2012). Kinematic analysis quantifies gait abnormalities associated with lameness in broiler chickens and identifies evolutionary gait differences. PLoS One 7, e40800. doi: 10.1371/journal.pone.0040800
Caplen G., Hothersall B., Nicol C. J., Parker R. M. A., Waterman-Pearson A. E., Weeks C. A., et al. (2014). Lameness is consistently better at predicting broiler chicken performance in mobility tests than other broiler characteristics. Anim. Welfare 23, 179–187. doi: 10.7120/09627286.23.2.179
Castellini C., Mugnai C., Moscati L., Mattioli S., Amato M. G., Mancinelli A. C., et al. (2016). Adaptation to organic rearing system of eight different chicken genotypes: behaviour, welfare and performance. Ital. J. Anim. Sci. 15, 37–46. doi: 10.1080/1828051X.2015.1131893
Cavusoglu E., Petek M. (2019). Effects of different floor materials on the welfare and behaviour of slow- and fast-growing broilers. Arch. Anim. Breed. 62, 335–344. doi: 10.5194/aab-62-335-2019
Chen C. Y., Misztal I., Aguilar I., Tsuruta S., Meuwissen T. H. E., Aggrey S. E., et al. (2011). Genome-wide marker-assisted selection combining all pedigree phenotypic information with genotypic data in one step: An example using broiler chickens. J. Anim. Sci. 89, 23–28. doi: 10.2527/jas.2010-3071
Clark B., Stewart G. B., Panzone L. A., Kyriazakis I., Frewer L. J. (2016). A systematic review of public attitudes, perceptions and behaviours towards production diseases associated with farm animal welfare. J. Agric. Environ. Ethics 29, 455–478. doi: 10.1007/s10806-016-9615-x
Comert M., Sayan Y., Kirkpinar F., Bayraktar O. H., Mert S. (2016). Comparison of carcass characteristics, meat quality, and blood parameters of slow and fast grown female broiler chickens raised in organic or conventional production system. Asian-Australas J. Anim. Sci. 29 (7), 987-97. doi: 10.5713/aja/15/0812
Constantini M., Ferrante V., Guarino M., Bacenetti J. (2021). Environmental sustainability assessment of poultry productions through life cycle approaches: a critical review. Trends Food Sci. Technol. 110, 201–212. doi: 10.1016/j.tifs.2021.01.086
Dawkins M. S., Donnelly C. A., Jones T. A. (2004). Chicken welfare is influenced more by housing conditions than by stocking density. Nature 427, 342–344. doi: 10.1038/nature02226
Dawkins M. S., Layton R. (2012). Breeding for better welfare: genetic goals for broiler chickens and their parents. Anim. Welfare 21, 147–155. doi: 10.7120/09627286.21.2.147
Dawson L. C., Widowski T. M., Liu Z. Z., Edwards A. M., Torrey S. (2021). In pursuit of a better broiler: a comparison of the inactivity, behavior, and enrichment use of fast- and slower growing broiler chickens. Poult. Sci. 100, e101451. doi: 10.1016/j.psj.2021.101451
de Jong I. C., Blaauw X. E., van der Eijk J. A. J., da Silva C. S., van Krimpen M. M., Molenaar R., et al. (2021). Providing environmental enrichments affects activity and performance, but not leg health in fast- and slower-growing broiler chickens. Appl. Anim. Behav. Sci. 241, e105375. doi: 10.1016/j.applanim.2021.105375
de Jong I. C., Bos B., van Harn J., Mostert P., te Beest D. (2022). Differences and variation in welfare performance of broiler flocks in three production systems. Poult. Sci. 101, e101933. doi: 10.1016/j.psj.2022.101933
Dixon L. M. (2020). Slow and steady wins the race: The behaviour and welfare of commercial faster growing broiler breeds compared to a commercial slower growing breed. PLoS One 15, e0231006. doi: 10.1371/journal.pone.0231006
Dixon L. M., Dunn I. C., Brocklehurst S., Baker L., Boswell T., Caughey S. D., et al. (2022). The effects of feed restriction, time of day, and time since feeding on behavioral and physiological indicators of hunger in broiler breeder hens. Poult. Sci. 101, e101838. doi: 10.1016/j.psj.2022.101838
Druyan S., Hadad Y., Cahaner A. (2008). Growth rate of ascites-resistant versus ascites-susceptible broilers in commercial and experimental lines. Poult. Sci. 87, 904–911. doi: 10.3382/ps.2008-00003
EFSA AHAW Panel, Nielsen S. S., Alvarez J., Bicout D. J., Calistri P., Canali E., et al. (2023). EFSA Scientific Opinion: Welfare of broilers on farm. Efsa J. 21, 7788. doi: 10.2903/jefsa.2023.7788
Eleroglu H., Yildirim A., Duman M., Sekeroglu A. (2015). The welfare of slow growing broiler genotypes reared in organic system. Emirates J. Food And Agric. 27, 454–459. doi: 10.9755/ejfa.2015.04.026
Eleroglu H., Yildirim A., Sekeroglu A., Duman M. (2014). Comparison of the growth performance and carcass characteristics of two slow-growing broiler genotypes fed diets supplemented with dry oregano (origanum vulgare l.) or lemon balm (melissa officinalis l.) leaves under the organic system. Kafkas Universitesi Veteriner Fakultesi Dergesi 20, 49–58. doi: 10.9775/kvfd.2013.9444
Eshel G., Shepon A., Makov T., Milo R. (2014). Land, irrigation water, greenhouse gas and reactive nitrogen burdens of meat, eggs and dairy production in the United States. PNAS 111, 11996–12001. doi: 10.1073/pnas.1402183111
Evans L., Brooks G. C., Anderson M. G., Campbell A. M., Jacobs L. (2023). Environmental complexity and reduced stocking density promote positive behavioral outcomes in broiler chickens. Animals 13, e2074. doi: 10.3390/ani13132074
Fanatico A. C., Pillai P. B., Cavitt L. C., Owens C. M., Emmert J. L. (2005). Evaluation of slower-growing broiler genotypes grown with and without outdoor access: growth performance and carcass yield. Poult. Sci. 84, 1321–1326. doi: 10.1093/ps/84.8.1321
Fanatico A. C., Pillai P. B., Hester P. Y., Falcone C., Mench J. A., Owens C. M., et al. (2008). Performance, livability, and carcass yield of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 87, 1012–1021. doi: 10.3382/ps.2006-00424
Fernyhough M., Nicol C. J., van de Braak T., Toscano M. J., Tønnessen T. (2019). The ethics of laying hen genetics. J. Agric. Environ. Ethics. 33, 15–36. doi: 10.1007/s10806-019-09810-2
Fidan E. D., Nazligul A., Turkylmaz M. K., Aypak S. U., Kilimci F. S., Karaaslan S., et al. (2017). Effect of photoperiod length and light intensity on some welfare criteria, carcass and meat quality characteristics in broilers. Rev. Bras. Zootecnia- Braz. J. Anim. Sci. 46, 202–210. doi: 10.1590/S1806-92902017000300004
Forseth M., Moe R. O., Kittelsen K., Skjerve E., Toftaker I. (2023). Comparison of carcass condemnation causes in two broiler hybrids differing in growth rates. Sci. Rep. 13, e4195. doi: 10.1038/s41598-023-31422-0
Freeman N., Tuyttens F. A. M., Johnson A., Marshall V., Garmyn A., Jacobs L. (2020). Remedying contact dermatitis in broiler chickens with novel flooring treatments. Animals 10, e1761. doi: 10.3390/ani10101761
Garner J. P., Falcone C., Wakenell P., Martin M., Mench J. A. (2002). Reliability and validity of a modified gait scoring system and its use in assessing tibial dyschondroplasia in broilers. Br. Poult. Sci. 43, 355–363. doi: 10.1080/00071660120103620
Ghayas A., Hussain J., Mahmud A., Jaspal M. H. (2020). Evaluation of three fast- and slow-growing chicken strains reared in two production environments. South Afr. J. Of Anim. Sci. 50, 378–388. doi: 10.4314/sajas.v50i3.4
Ghayas A., Hussain J., Mahmud A., Jaspal M. H., Ishaq H. M., Hussain A. (2021). Behaviour, welfare, and tibia traits of fast- and slow-growing chickens reared in intensive and free range systems. South Afr. J. Anim. Sci. 51, 22–32. doi: 10.4314/sajas.v51i1.3
Gocsik E., Brooshooft S. D., de Jong I. C., Saatkamp H. W. (2016). Cost-efficiency of animal welfare in broiler production systems: A pilot study using the Welfare Quality (R) assessment protocol. Agric. Syst. 146, 55–69. doi: 10.1016/j.agsy.2016.04.001
Guz B. C., de Jong I. C., Da Silva C. S., Veldkamp F., Kemp B., Molenaar R., et al. (2021). Effects of pen enrichment on leg health of fast and slower-growing broiler chickens. PLoS One 16, e0254462. doi: 10.1371/journal.pone.0254462
Hartcher K. M., Lum H. K. (2020). Genetic selection of broilers and welfare consequences: a review. World’s Poult. Sci. J. 76, 154–167. doi: 10.1080/00439339.2019.1680025
Haskell M. J., Li B., Prentice P. M. P., Dwyer C. M. (2023) Determining potential impacts of precision breeding on animal welfare, Final Report. Available online at: https://sciencesearch.defra.gov.uk/ProjectDetails?ProjectId=21137 (Accessed 9.1.24).
Heinola K., Latvala T., Niemi J. K. (2023). Consumer trust and willingness to pay for establishing a market-based animal welfare assurance scheme for broiler chickens. Poult. Sci. 102, e102765. doi: 10.1016/j.psj.2023.102765
Hendricksen C., Morton D., Cussler K. (2010). Use of Humane Endpoints to Minimise Suffering. The COST Manual of Laboratory Animal Care and Use (Oxfordshire: CRC Press).
Horsted K., Henning J., Hermansen J. E. (2005). Growth and sensory characteristics of organically reared broilers differing in strain, sex and age at slaughter. Acta Agriculturae Scandinavica Section A-Animal Sci. 55, 149–157. doi: 10.1080/09064700500520779
Julian R. J. (2005). Production and growth related disorders and other metabolic diseases of poultry – a review. Vet. J. 169, 350–369. doi: 10.1016/j.tvjl.2004.04.015
Kang S. W., Christensen K. D., Kidd M. Y., Orlowski S. K. (2023). Effects of a variable light intensity program on the welfare and performance of commercial broiler chickens. Front. Physiol. 14. doi: 10.3389/fphys.2023.1059055
Kappell D. N. R. G., Hill W. G., Neeteson A.-M., McAdam J., Avendano S. (2012). Twenty five years of selection for improved leg health in purebred broiler lines and underlying genetic parameters. Poult. Sci. 91, 3032–3043. doi: 10.3382/ps.2012-02578
Kestin S. C., Knowles T. G., Tinch A. E., Gregory N. E. (1992). The prevalence of leg weakness in broiler chickens assessed by gait scoring and its relationship to genotype. Veterinary Rec. 131, 190–194. doi: 10.1136/vr.131.9.190
Kestin S. C., Su G., Sorensen P. (1999). Different commercial broiler crosses have different susceptibilities to leg weakness. Poult. Sci. 78, 1085–1090. doi: 10.1093/ps/78.8.1085
Kjaer J. B., Su G., Nielsen B. L., Sorensen P. (2006). Growth and sensory characteristics of organically reared broilers differing in strain, sex and age at slaughter. Poult. Sci. 98, 2326–2337. doi: 10.3382/ps/pez023
Knowles T. G., Kestin S. C., Haslam S. M., Brown S. N., Green L. E., Butterworth A., et al. (2008). Leg disorders in broiler chickens: prevalence, risk factors and prevention. PLoS One 3, e1545. doi: 10.1371/journal.pone.0001545
Lichnikova M., Hampel D., Nedmova S., Kupcikova L., Anderle V. (2017). The effect of genotype on the behaviour of free range chickens. J. Cent. Eur. Agric. 18, 632–645. doi: 10.5513/JCEA01/18.3.1937
Lindholm C., Karlsson L., Johansson A., Altimiras J. (2017). Higher fear of predators does not decrease outdoor range use in free-range Rowan Ranger broiler chickens. Acta Agriculturae Scandinavica Section A-Animal Sci. 66, 231–238. doi: 10.1080/09064702.2017.1337214
Littin K., Acevedo A., Browne W., Edgar J. L., Mendl M., Owen D., et al. (2008). Towards humane endpoints: behavioural changes precede clinical signs of disease in a Huntington’s Disease model. Proc. R. Soc. Section B 275, 1865–1874. doi: 10.1098/rspb.2008.0388
Louton H., Keppler C., Erhard M., van Tuijl O., Bachmeier J., Damme K., et al. (2019). Animal-based welfare indicators of 4 slow-growing broiler genotypes for the approval in an animal welfare label program. Poult. Sci. 98, 2326–2337. doi: 10.3382/ps/pez023
Malchow J., Dudde A., Berk J., Krause E. T., Sanders O., Puppe B., et al. (2019a). Is the rotarod test an objective alternative to the gait score for evaluating walking ability in chickens. Anim. Welfare 28, 261–269. doi: 10.7120/109627286.28.3.261
Malchow J., Puppe B., Berk J., Schrader L. (2019b). Effects of elevated grids on growing male chickens differing in growth performance. Front. Vet. Sci. 6, e203. doi: 10.3389/fvets.2019.00203
Malchow J., Puppe B., Berk J., Schrader L. (2019c). Perches or grids? What do rearing chickens differing in growth performance prefer for roosting? Poult. Sci. 98, 29–38. doi: 10.3382/ps/pey320
Malchow J., Schrader L. (2021). Effects of an elevated platform on welfare aspects in male conventional broilers and dual-purpose chickens. Front. Vet. Sci. 8, e660602. doi: 10.3389/fvets.2021.660602
Mancinelli A. C., Mattioli S., Dal Bosco A., Aliberti A., Amata M. G., Castellini C. (2020). Performance, behavior, and welfare status of six different organically reared poultry genotypes. Animals 10, e550. doi: 10.3390/ani10040550
Mandel R., Nicol C. J., Whay H. R., Klement E. (2017). Detection and monitoring of metritis in dairy cows using an automated grooming device. J. Dairy Sci. 100, 5724–5728. doi: 10.3168/jds.2016-12201
Mason G., Mendl M. (1993). Why is there no simple way of measuring animal welfare. Anim. Welfare 2, 301–319. doi: 10.1017/S0962728600016092
Mellor D. J. (2017). Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 7, 60. doi: 10.3390/ani7080060
Montoro-Dasi L., Villagra A., Sevilla-Navarro S., Perez-Gracia M. T., Vega S., Marin C. (2020). The dynamic of antibiotic resistance in commensal Escherichia coli throughout the growing period in broiler chickens: fast-growing vs. slow-growing breeds. Poult. Sci. 99, 1591–1597. doi: 10.1016/j.psj.2019.10.080
Mulder M., Zomer S. (2017). Dutch consumers’ willingness to pay for broiler welfare. J. Appl. Anim. Welfare Sci. 2, 137–154. doi: 10.1080/10888705.2017.1281134
Neeteson A.-M., Avendano S., Koerhuis A., Duggan B., Souza E., Mason J., et al. (2023). Evolutions in commercial meat poultry breeding. Animals 13, e3150. doi: 10.3390/ani13193150
Nicol C. J., Caplen G., Edgar J., Richards G., Browne W. J. (2011). Relationships between multiple welfare indicators measured in individual chickens across different time periods and environments. Anim. Welfare 20, 133–143. doi: 10.1017/S0962728600002621
Nielsen B. L. (2012). Effects of ambient temperature and early open-field response on the behaviour, feed intake and growth of fast- and slow-growing broiler strains. Animal 6, 1460–1468. doi: 10.1017/S1751731112000353
Nielsen B. L., Juul-Madsen H. R., Steenfeldt S., Kjaer J. B., Sorensen P. (2010). Feeding activity in groups of newly hatched broiler chicks: Effects of strain and hatching time. Poult. Sci. 89, 1336–1344. doi: 10.3382/ps.2009-00544
Nielsen B. L., Kjaer J. B., Friggens N. C. (2004). Temporal changes in activity measured by passive infrared detection (PID) of broiler strains growing at different rates. Archiv Fur Geflugelkunde 68, 106–110.
Norring M., Kaukonen E., Valros A. (2016). The use of perches and platforms by broiler chickens. Appl. Anim. Behav. Sci. 184, 91–96. doi: 10.1016/j.applanim.2016.07.012
OECD-FAO (2021)Meat. In: Agricultural Outlook 2021-2030. Available online at: https://www/fao.org/3/cb5332en/Meat.pdf (Accessed 9.1.24).
Rauch E., Keppler C., Damme K., Hausleitner M., Bachmeier J., Hartmann J., et al. (2017). Test of different premium broiler genotypes under Animal Welfare Label conditions. Part II: Anim. Health Eur. Poult. Sci. 81. doi: 10.1399/eps.2017.169
Rayner A. C., Newberry R. C., Vas J., Mullan S. (2020). Slow-growing broilers are healthier and express more behavioural indicators of positive welfare. Sci. Rep. 10, e15151. doi: 10.1038/s41598-020-72198-x
Rezaei M., Yngvesson J., Gunnarsson S., Jonsson L., Wallenbeck A. (2018). Feed efficiency, growth performance, and carcass characteristics of a fast- and a slower-growing broiler hybrid fed low- or high-protein organic diets. Organic Agric. 8, 121–128. doi: 10.1007/s13165-017-0178-6
Riber A. B., Casey-Trott T. M., Herskin M. S. (2018). The influence of keel bone damage on welfare of laying hens. Front. Vet. Sci. 5. doi: 10.3389/fvets.2018.00006
Rocchi L., Mancinelli A. C., Paolotti L., Mattioli S., Boggia A., Papi F., et al. (2021). Sustainability of rearing system using multicriteria analysis: application in commercial poultry production. Animals 11, e3483. doi: 10.3390/ani11123483
RSPCA (2017). Broiler Welfare Assessment Protocol. Available online at: https://science.rspca.org.uk/sciencegroup/farmanimals/standards/chickens (Accessed 9.1.24).
Saeed M., Abbas G., Alagawa M., Kamboh A. A., Abd El-Hack M. E., Khafaga A. F., et al. (2019). Heat stress management in poultry farms: a comprehensive review. J. Thermal Biol. 84, 414–425. doi: 10.1016/j.jtherbio.2019.07.025
Santos M. N., Widowski T. M., Kiarie E. G., Guerin M. T., Edwards A. M., Torrey S. (2022a). In pursuit of a better broiler: tibial morphology, breaking strength, and ash content in conventional and slower-growing strains of broiler chickens. Poult. Sci. 101, e101755. doi: 10.1016/j.psj.2022.101755
Santos M. N., Widowski T. M., Kiarie E. G., Guerin M. T., Edwards A. M., Torrey S. (2022b). In pursuit of a better broiler: walking ability and incidence of contact dermatitis in conventional and slower growing strains of broiler chickens. Poult. Sci. 101, e101768. doi: 10.1016/j.psj.2022.101768
Sarica M., Yamak U. S., Boz M. A. (2014). Effect of production systems on foot pad dermatitis (FPD) levels among slow-, medium- and fast-growing broilers. Eur. Poult. Sci. 78. doi: 10.1399/eps.2014.52
Sarica M., Yamak U. S., Boz M. A., Erensoy K., Cilavdaroglu E., Noubandiguim M. (2019). Performance of fast, medium and slow growing broilers in indoor and free-range production systems. South African. J. Anim. Sci. 49, 1127–1138. doi: 10.4314/sajas.v49i6.16
Sarmiento-Garcia A., Revilla I., Abecia J. A., Palacios C. (2021). Performance evaluation of two slow-medium growing chicken strains maintained under organic production system during different seasons. Animals 11, e1090. doi: 10.3390/ani11041090
Shepherd E. M., Fairchild B. D. (2010). Footpad dermatitis in poultry. Poult. Sci. 89, 2043–2051. doi: 10.3382/ps.2010-00770
Shynkaruk T., Long K., LeBlanc C., Schwean-Lardner K. (2023). Impact of stocking density on the welfare and productivity of broiler chickens reared to 34d of age. J. Appl. Poult. Res. 32, 1–12. doi: 10.1016/j.japr.2023.100344
Singh M., Lim A. J., Muir W. I., Groves P. J. (2021). Comparison of performance and carcass composition of a novel slow-growing crossbred broiler with fast-growing broiler for chicken meat in Australia. Poult. Sci. 100, e100966. doi: 10.1016/j.psj.2020.12.063
Snyder A. M., Riley S. P., Robison C. I., Karcher D. M., Wickware C. L., Johnson T. A., et al. (2022). Behavior and immune response of conventional and slow-growing broilers to Salmonella typhimurium. Front. Physiol. 13, e890848. doi: 10.3389/fphys.2022.890848
Souza A. P. O., Tuyttens F. A. M., Taconeli C. A., Biscarra J. C., Molento C. F. M. (2022). Ordinal or visual analogue scales for assessing aspects of broiler chicken welfare? J. Appl. Anim. Welfare Sci. doi: 10.1080/10888705.2022.2105648
Steenfeldt S., Sorensen P., Nielsen B. L. (2019). Effects of choice feeding and lower ambient temperature on feed intake, growth, foot health, and panting of fast- and slow-growing broiler strains. Poult. Sci. 98, 503–513. doi: 10.3382/ps/pey323
Sutherland D.-A. A. T., Honaker C. F., Dorshorst B., Andersson L., Brisbin I. L., Siegel P. B. (2018). Growth patterns for three generations of an intercross between red junglefowl and chickens selected for a low bodyweight. J. Anim. Breed. Genet. 135, 300–310. doi: 10.111/jbg.12336
Tahamtani F. M., Herskin M. S., Foldager L., Murrell J., Sandercock D. A., Riber A. B. (2021). Assessment of mobility and pain in broiler chickens with identifiable gait defects. Appl. Anim. Behav. Sci. 234, e105183. doi: 10.1016/j.applanim.2020.105183
Torrey S., Mohammadigheisar M., dos Santos M. N., Rothschild D., Dawson L. C., Liu Z. Z., et al. (2021). In pursuit of a better broiler: growth, efficiency, and mortality of 16 strains of broiler chickens. Poult. Sci. 100, e100955. doi: 10.1016/j.psj.2020.12.052
Tuyttens F., Heyndrickx M., De Boeck M., Moreels A., Van Nuffel A., Van Poucke E., et al. (2008). Broiler chicken health, welfare and fluctuating asymmetry in organic versus conventional production systems. Livest. Sci. 113, 123–132. doi: 10.1016/j.livsci.2007.02.019
van der Eijk J. A. J., Gunnink H., Melis S., van Riel J. W., de Jong I. C. (2022). Reducing stocking density benefits behaviour of fast- and slower-growing broilers. Appl. Anim. Behav. Sci. 257, e105754. doi: 10.1016/j.applanim.2022.105754
van der Eijk J. A. J., van Harn J., Gunnink H., Melis S., van Riel J. W., de Jong I. C. (2023). Fast- and slower-growing broilers respond similarly to a reduction in stocking density with regard to gait, hock burn, skin lesions, cleanliness, and performance. Poult. Sci. 102, e102603. doi: 10.1016/j.psj.2023.102603
Wallenbeck A., Wilhelmsson S., Jonsson L., Gunnarsson S., Yngvesson J. (2016). Behaviour in one fast-growing and one slower-growing broiler (Gallus gallus domesticus) hybrid fed a high-or low-protein diet during a 10-week rearing period. Acta Agriculturae Scandinavica Section A-Animal Sci. 66, 168–176. doi: 10.1080/09064702.2017.1303081
Webster A. J. F. (2016). Animal welfare: Freedoms, dominions and a life worth living. Animals 6, 35. doi: 10.3390/ani6060035
Weeks C. A., Danbury T. D., Davies H. C., Hunt P., Kestin S. C. (2000). The behaviour of broiler chickens and its modification by lameness. Appl. Anim. Behav. Sci. 67, 111–125. doi: 10.1016/S0168-1591(99)00102-1
Weeks C. A., Knowles T. G., Gordon R. G., Kerr A. E., Peyton S. T., Tilbrook N. T. (2002). New method for objectively assessing lameness in broiler chickens. Vet. Rec. 151, 762–764.
Weimer S. L., Mauromoustakos A., Karcher D. M., Erasmus M. A. (2020). Differences in performance, body conformation, and welfare of conventional and slow-growing broiler chickens raised at 2 stocking densities. Poult. Sci. 99, 4398–4407. doi: 10.1016/j.psj.2020.06.009
Wilhelmsson S., Yngvesson J., Jonsson L., Gunnarsson S., Wallenbeck A. (2019). Welfare quality (R) assessment of a fast-growing and a slower-growing broiler hybrid, reared until 10 weeks and fed a low-protein, high-protein or mussel-meal diet. Livest. Sci. 219, 71–79. doi: 10.1016/j.livsci.2018.11.010
Williams L. K., Sait L. C., Trantham E. K., Cogan T. A., Humphrey T. J. (2013). Campylobacter infection has different outcomes in fast- and slowgrowing broiler chickens. Avian Dis. 57, 238–241. doi: 10.1637/10442-110212-Reg.1
Yang Y.-C., Hong C.-Y. (2019). Taiwanese consumers’ willingness to pay for broiler welfare improvement. Animals 9, e231. doi: 10.3390/ani9050231
Yngvesson J., Wedin M., Gunnarsson S., Jonsson L., Blokhuis H., Wallenbeck A. (2018). Let me sleep! Welfare of broilers (Gallus gallus domesticus) with disrupted resting behaviour. Acta Agriculturae Scandinavica Section A-Animal Sci. 67, 123–133. doi: 10.1080/09064702.2018.1485729
Keywords: Broiler, Animal Welfare, Leg health, Slower-growing chickens, Mortality, Contact Dermatitis, Lameness
Citation: Nicol CJ, Abeyesinghe SM and Chang Y-M (2024) An analysis of the welfare of fast-growing and slower-growing strains of broiler chicken. Front. Anim. Sci. 5:1374609. doi: 10.3389/fanim.2024.1374609
Received: 22 January 2024; Accepted: 07 March 2024;
Published: 22 March 2024.
Edited by:
Maria José Hötzel, Federal University of Santa Catarina, BrazilReviewed by:
Laura Dixon, Scotland’s Rural College, United KingdomOluwaseun Serah Iyasere, Federal University of Agriculture, Abeokuta, Nigeria
Copyright © 2024 Nicol, Abeyesinghe and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine Janet Nicol, Y25pY29sQHJ2Yy5hYy51aw==
 Christine Janet Nicol
Christine Janet Nicol Siobhan Maya Abeyesinghe
Siobhan Maya Abeyesinghe Yu-Mei Chang
Yu-Mei Chang