Paraguay’s approach to biotechnology governance: a comprehensive guide
- 1Departamento de Biotecnología, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Asunción, San Lorenzo, Paraguay
- 2Departamento de Relaciones Humanas, Ministerio de Salud Pública y Bienestar Social, Asunción, Paraguay
- 3Departamento de Biotecnología, Facultad de Ciencias Químicas, Universidad Nacional de Asunción, San Lorenzo, Paraguay
- 4Departamento de Biología Molecular y Biotecnología, Instituto de Investigaciones en Ciencias de la Salud, Universidad Nacional de Asunción, San Lorenzo, Paraguay
- 5Mycology Investigation and Safety Team, Centro Multidisciplinario de Investigaciones Tecnológicas, Universidad Nacional de Asunción, San Lorenzo, Paraguay
This study analyzes Paraguay’s biotechnology regulatory framework and its alignment with international standards amid biotechnological advancements. It also identifies areas of improvement for enhancing framework effectiveness. Through this work, we aim to provide a resource for policymakers, stakeholders, and researchers navigating Paraguay’s biotechnology regulation.
1 Introduction
The regulation of biotechnology products is a complex process involving various institutions with different protection goals to ensure their safety and efficacy (Wolt and Wolf, 2018). Therefore, it is essential to have a sound regulatory framework to monitor and evaluate biotechnology developments and their applications (Xue and Shang, 2022).
This study presents a compilation of Paraguay’s current regulatory framework for biotechnology focusing on recombinant DNA-derived products, vaccines, and biopharmaceuticals. It examines the country’s efforts to create a regulatory environment that aligns with international standards while accommodating rapid biotechnological advancements. Even though any attempt to summarize and analyze regulations runs the risk of being incomplete and outdated, we are confident that it can serve as a resource for policymakers, researchers, and other stakeholders working to understand and navigate this complex regulatory landscape. We aim to provide an analysis of this regulatory framework, focusing on the roles and responsibilities of the various government bodies involved (Table 1). Finally, we aim to highlight potential areas for improvement and collaboration among stakeholders.
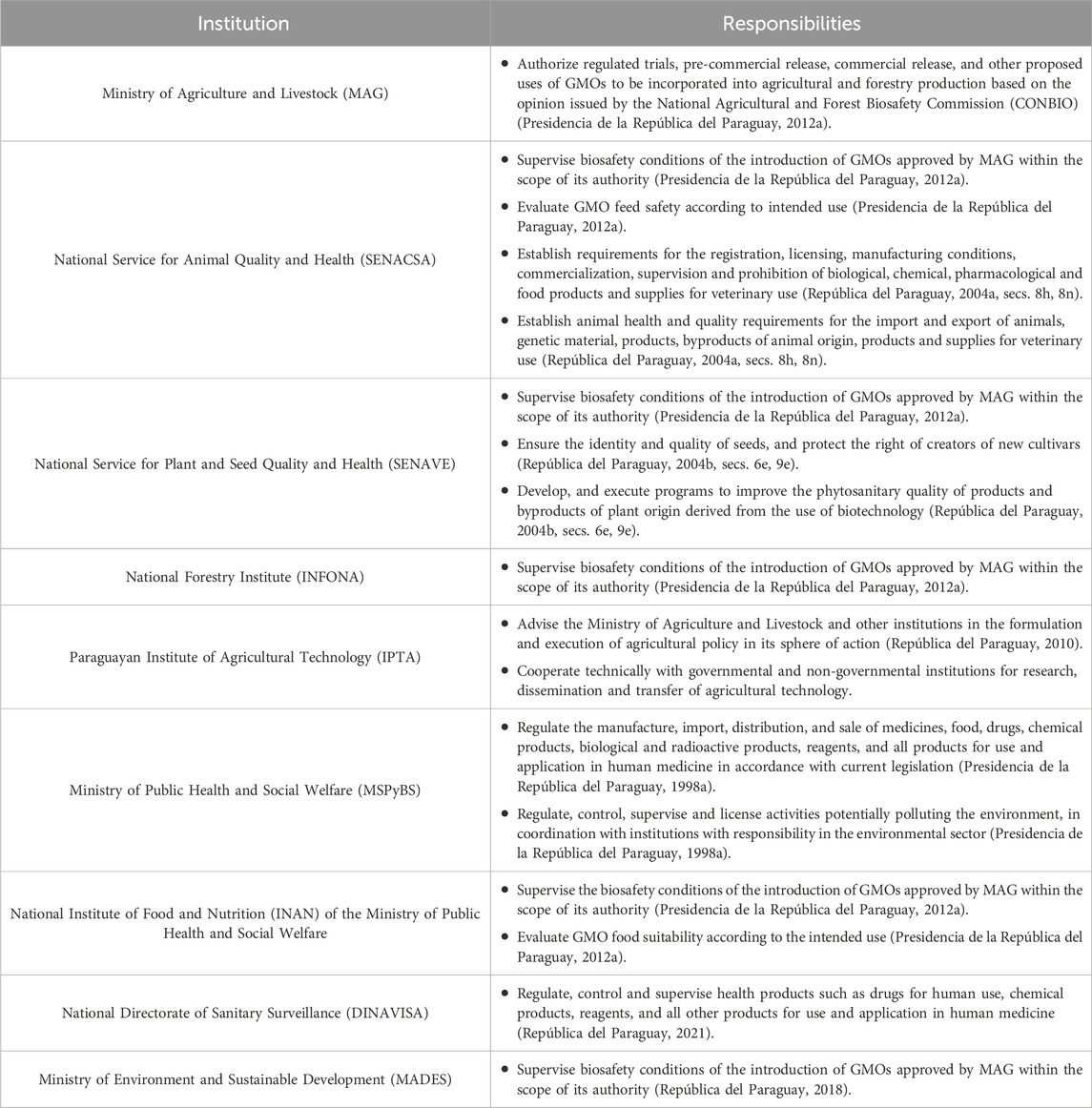
Table 1. Paraguayan government bodies involved in the regulation of biotechnology and their responsibilities relevant to the topic.
2 International treaties
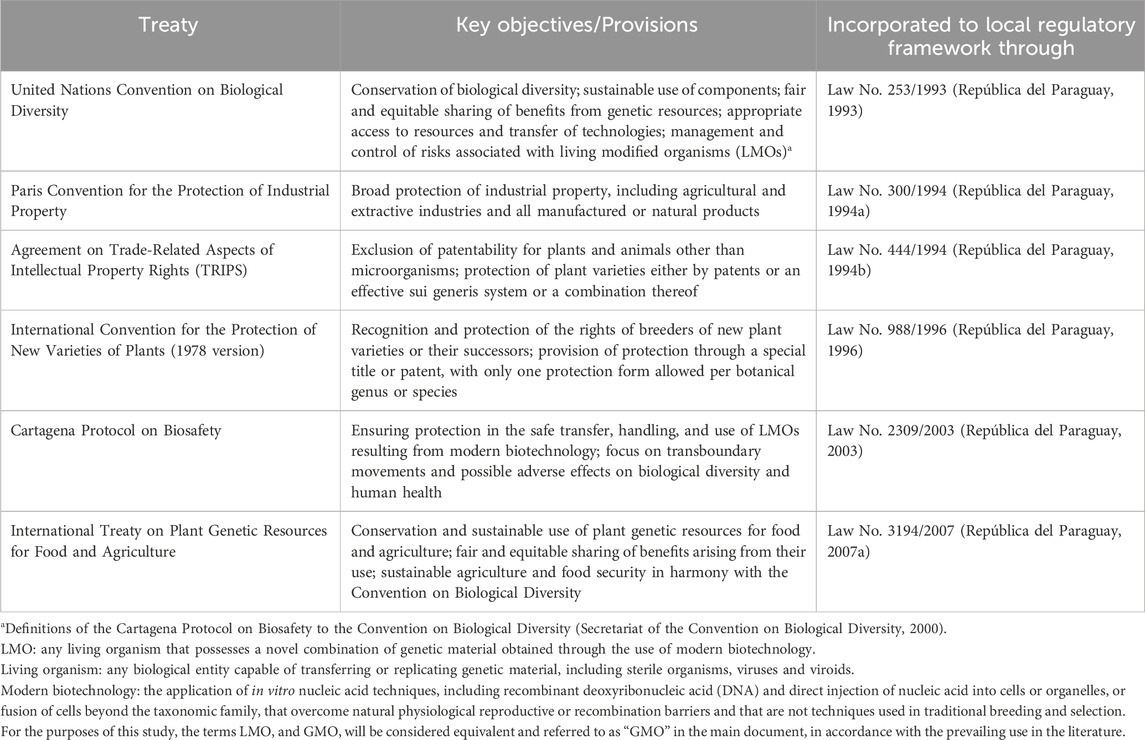
Paraguay has signed several international treaties related to modern biotechnology which emphasize the conservation of biological diversity, the sustainable use of resources, the equitable sharing of the benefits of genetic resources and the safe handling of genetically modified organisms (GMOs). They also address intellectual property rights and plant variety protection (Table 2).
In South America, the Southern Common Market (Mercosur) has been actively developing and implementing regulations for biotechnology products (Figure 1). Mercosur countries recognize the importance of cooperation and harmonization in biotechnology regulation which aim to establish consistent regulations across member states and facilitate trade, while maintaining safety and effectiveness standards (Dellepiane and Pagliusi, 2019). For each of the following sections, we have included Mercosur regulations that address these efforts.
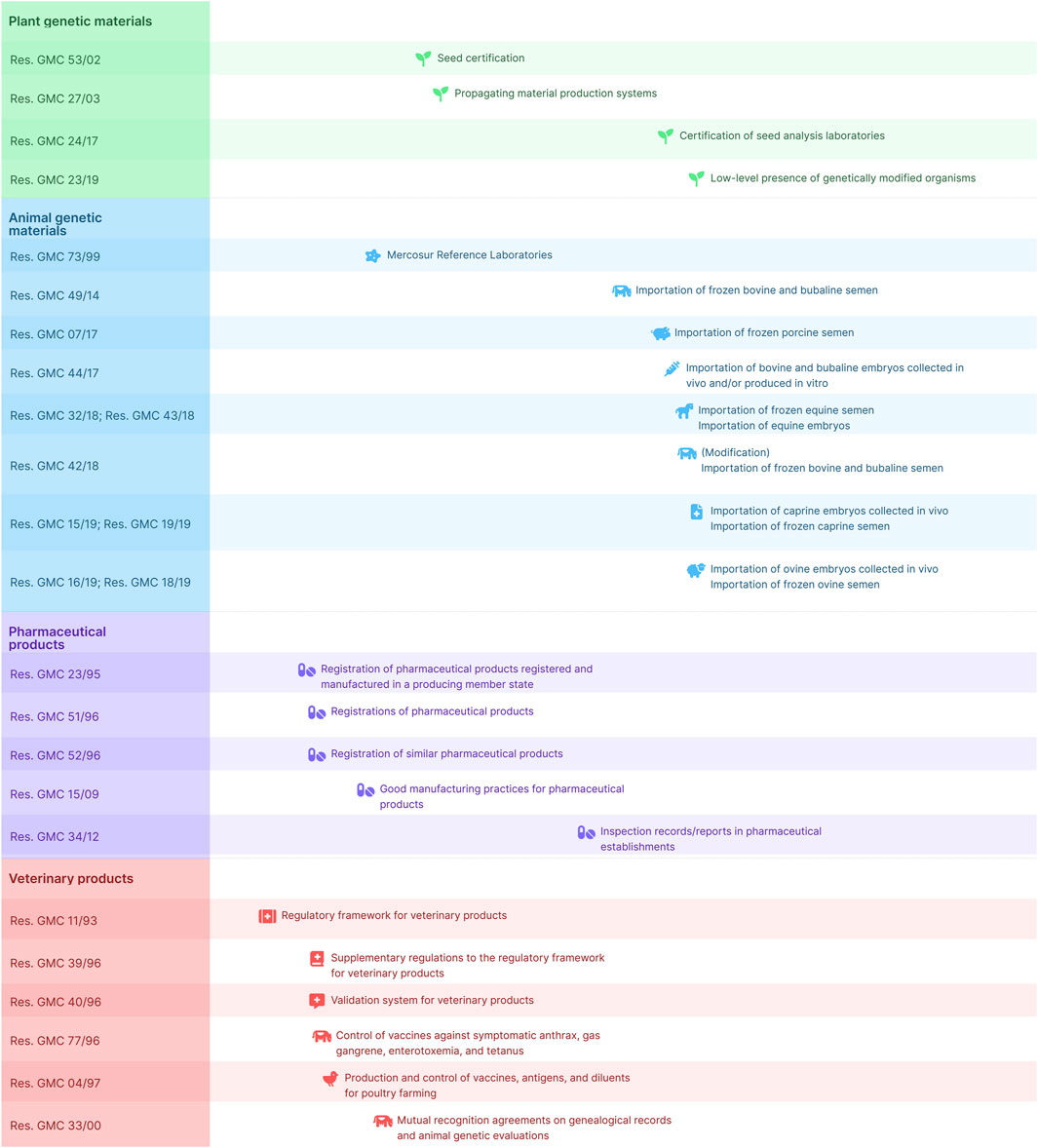
Figure 1. Chronological synthesis of current Mercosur Resolutions. The figure depicts the timeframe and extent of resolutions adopted by the Mercosur Common Market Group concerning biotechnology, offering an overview of key regulatory achievements in the region.
Mercosur Common Market Group (GMC) Resolutions have the goal of setting standards for the harmonization of regulations within the group, namely, once a GMC Resolution is incorporated into a country’s regulatory framework, said country must adjust its internal regulation to fit the standards set by that Resolution, and thus facilitate mutual recognition. To that end, GMC has also passed Resolutions on the procedures to elaborate, revise and revoke technical regulations (Mercosur GMC, 2017), and for mutual recognition of control systems (Mercosur GMC, 1998).
Mercosur’s GMC has long been incorporating Codex Alimentarius guidelines for the harmonization of food safety standards (FAO, 1995; De F Toledo, 2014). However, as of the date of submission of this paper, no Codex guidelines referring specifically to products of biotechnology have been incorporated into the Mercosur framework, and thus the use of said guidelines is left up to each member state.
3 Crop biotechnology
MAG plays a central role in authorizing regulated trials, pre-commercial releases, commercial releases, and other proposed uses of genetically modified (GM) crops based on the opinion issued by CONBIO (Figure 2).
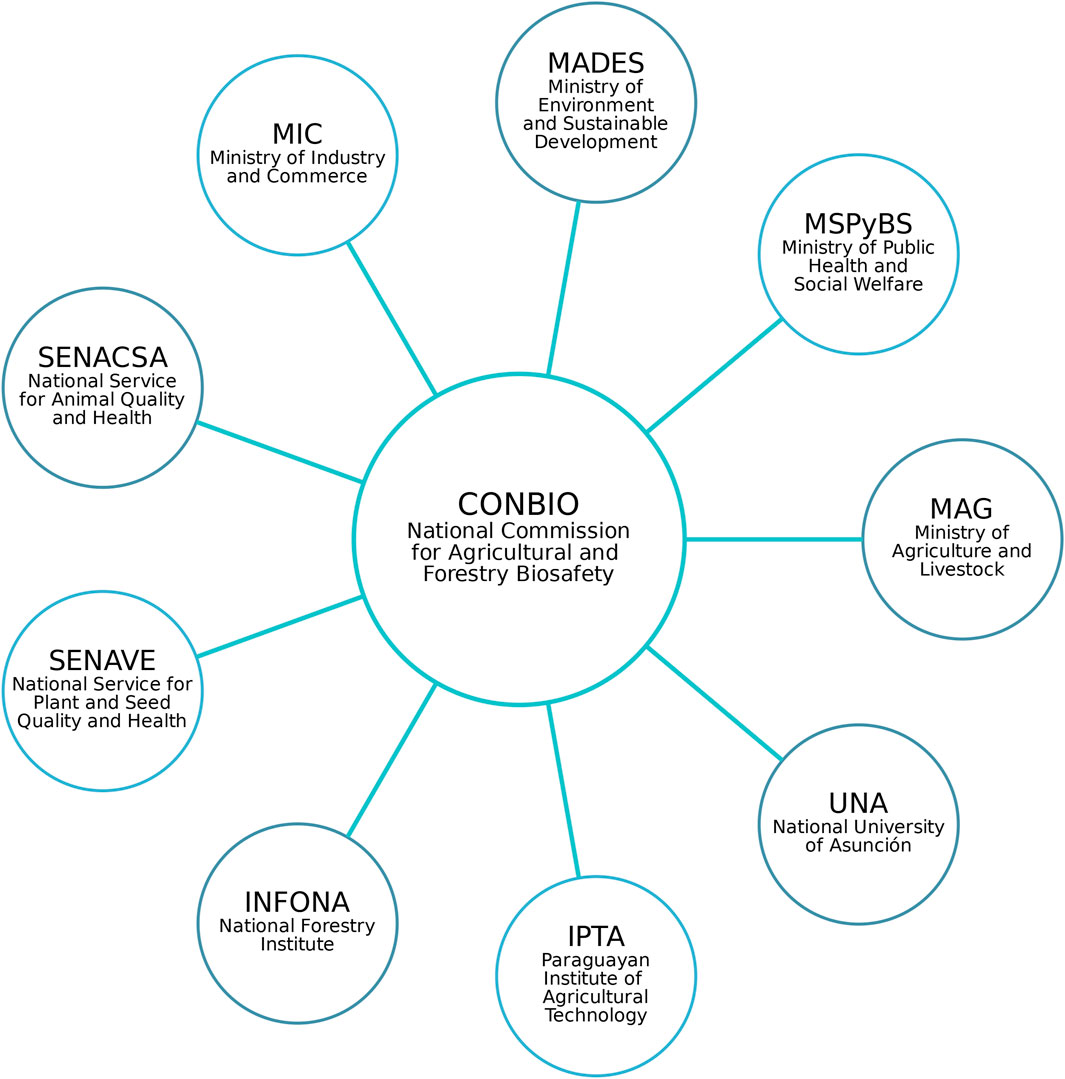
Figure 2. Composition of Paraguay’s National Commission for Agricultural and Forestry Biosafety (Presidencia de la República del Paraguay, 2012a).
Submissions for approval of both regulated field trials and commercial release of plant GM events are received by MAG, and derived for analysis to CONBIO. MAG Resolution No. 27/2015 (MAG, 2015) contains both Form 1, for submission of regulated field trial applications; and Form 2, for submission of commercial release applications for GM crops (Fernandez Rios et al., 2018). Different treatments are established for events submitted for approval, based on their characteristics (Benitez Candia et al., 2020; Fernandez Rios et al., 2024).
In the case of the differentiated procedure established for events previously approved by sound and experienced regulatory systems (MAG, 2019a; MAG, 2019b), it is worth noting that Paraguay has not established a formal definition of an “experienced” regulatory system. However, the country recognizes that such a system employs scientifically sound methodologies for risk assessments to evaluate the safety of GMOs as food and feed and to assess their potential environmental impact. These systems are typically accompanied by several key features (McHughen, 2016), such as regulations that are commensurate with the level of risk and are based on a comparative risk analysis; the development of a rational risk hypothesis; the requirement of scientifically valid data of sufficient quality and quantity to inform on relative safety; and a clear distinction between scientific, political, ethical, and economic considerations.
The agri-food regulatory system is structured around a problem formulation approach, where protection goals are identified and plausible risk hypotheses are formulated. These hypotheses are tested with robust testing methodology. Such tests are conducted either in the laboratory or in confined field trials and follow a tiered-testing approach. For example, for non-target organisms (NTOs) the process starts with highly conservative early-tier tests in the laboratory and progresses to more complex, higher-tier experiments when necessary. Risk assessors must select data that can evaluate potential effects by translating policies and protection goals into risk assessment operational goals (Garcia-Alonso and Raybould, 2014; Wach et al., 2016). Experience has taught local regulators that most countries where GM crops have already been approved have protection goals that apply to a common set of valued ecological functions. One of the key elements that enabled Paraguay to develop a differentiated procedure for GM crops already approved in third countries was the utility of early tier tests using surrogate species for predicting field effects (Wach et al., 2016). The choice of appropriate surrogates for the assessment of NTOs is influenced by scientific factors, such as understanding the mode of action and spectrum of activity, and protection goals based on ecosystem services, including biological control, pollination, and decomposition of organic matter. Furthermore, the Paraguay agri-food regulatory system considers that the results from the use of surrogate species in tiered testing are transportable, as such testing can be replicated in other laboratories (Wach et al., 2016).
When Paraguay updated the regulatory framework for GM crops in 2015, it incorporated the concept of data transportability, which became a useful tool for avoiding redundant confined field trials (CFT). Through collaboration with the regulatory agency from Argentina, risk assessors learned that CFTs’ site selection with a focus on the diversity of environments tested within the production zone of the crop of interest is a key element. At the same time, the appropriate methodology and agronomic management of the studies and the measured endpoints are relevant (Vesprini et al., 2020; Vesprini et al., 2022). If these conditions are met, and if through the application of problem formulation there were no risk hypotheses related to Paraguay’s agro-climatic conditions, not only the data (as informative studies), but also the conclusions of the CFT are transportable, and applicable to risk assessments for Paraguay, without the need to generate additional CFT data.
If a relevant risk hypothesis related to Paraguay’s agro-climatic conditions is formulated, risk assessors scientifically assess the need (or not) for a new specific confined field trial that provides necessary unavailable data and answers that specific risk hypothesis. If a new confined field trial is required, risk assessors need to identify putative agro-climatic zones (Melnick et al., 2023) where this specific confined field trial could be conducted (e.g., where the production of that crop is common, and understanding the importance of any particular agro-climate for the production of a specific crop). This contributes to providing only relevant data that answer a specific risk hypothesis that persists even when all available data are analyzed.
Since 2014, SENAVE has maintained a registry of companies operating with regulated GMOs in the agricultural sector. To apply for registration, companies must fill Forms DBA-01 and DBA-02 (SENAVE, 2014b), whereby they declare relevant data about their legal representatives, technical advisors, and location where the companies operate. SENAVE has also approved a procedure for the risk management of activities with regulated GMOs in the agricultural sector, through Resolution SENAVE No. 283/2014 (SENAVE, 2014a) (Supplementary Table 1). Meanwhile, a Mercosur GMC Resolution has been incorporated into Paraguay’s regulatory framework which approves a mechanism to reduce the low-level presence of unapproved GMOs (Mercosur GMC, 2019; Presidencia de la República del Paraguay, 2021).
4 Genetically modified microorganisms
The regulation of genetically modified microorganisms (GMMs) in the agri-food system is governed by the same norm as crop biotechnology and subject to analysis by CONBIO, which includes conducting case-by-case risk assessments of activities involving GMMs and identifying potential environmental risks or safety concerns to human and/or animal health resulting from the use of GMMs and their byproducts (Presidencia de la República del Paraguay, 2012a). However, the differentiated mechanism for events previously assessed in third countries is not currently applicable. Paraguay has approved the use of several GM yeasts for ethanol production (OECD, 2023). Because yeast-derived products and distiller’s dried grains with solubles (DDGS) can be used as animal feed, a CONBIO safety assessment is required.
5 Animal products of biotechnology
GM animals have a wide range of applications, from laboratory research to agriculture and public health. The regulation varies depending on these applications. In the context of the agri-food system, an assessment by CONBIO is necessary, and genetically modified animals are regulated according to the same standards as crop biotechnology.
Paraguay has recently granted the first commercial release of a GM insect, Spodoptera frugiperda, containing a self-limiting gene which allows for the production of male-only insects (MAG, 2024). Once released into the environment, these modified males will seek out and mate with wild females. The self-limiting gene will be transmitted to offspring, preventing female offspring from reaching maturity and reproducing. By continuously releasing GM males in a specific area, there will be a decrease in the number of wild females and consequently a reduction in the overall population of these insects (Reavey et al., 2022).
For applications related to public health, an evaluation by the MSPyBS may be required in some cases. However, there are currently no established guidelines outlining the assessment process in the public health context.
One particular case is that of synthetic beef (and plant-based meat substitutes). The use of the word “meat” (carne) is regulated by Law No. 6916/2022 (República del Paraguay, 2022), and is reserved for the edible muscular part of animals slaughtered and declared fit for human consumption by the official veterinary inspection, consisting of the soft tissues surrounding the skeleton, including their fat covering, tendons, vessels, nerves, aponeurosis, the skin of swine and poultry (except that of the order Struthioniformes) and all those tissues not separated during the slaughter operation. The diaphragm is also considered meat. At the time of submission of this paper, a draft bill is being studied in Congress to ban synthetic meat altogether (Franco Alfaro, 2023). These laws were promoted by the agribusiness sector as a way to combat market competition for conventional meat, claiming that by calling alternatives ‘meat’, competitors are fooling consumers (La Nacion, 2022). France has a similar policy against using meat-related terms for plant-based products (Carreno, 2022). However, research suggests that labeling these products as “meat” does not inherently cause confusion (Gleckel, 2020; Profeta et al., 2021; Tosun et al., 2021).
Several Mercosur GMC Resolutions have been integrated into the Paraguayan regulatory framework, focusing on animal health requirements for importing various types of animal genetic materials. This includes frozen semen and embryos from bovines, bubalines, porcines, equines, caprines, and ovines (Supplementary Table 2).
6 Biologics for human use
The registry of biologics for human use1 is made by DINAVISA, following public health policies from MSPyBS and is regulated by the Presidential Decree No. 6611/2016 (Presidencia de la República del Paraguay, 2016b; Presidencia de la República del Paraguay, 2016a). DINAVISA’s webpage makes available all forms required for the registry of biologics (DINAVISA, 2023).
Decree No. 6611/2016 categorizes requirements into two segments: general requirements (Table 3); and additional requirements (Table 4) for the registry of vaccines, hemoderivatives, innovative biologics, biosimilars, biologics for orphan diseases, and biologics obtained through recombinant DNA technology. Paraguay does not have specific regulation for biotechnological pharmaceutical products; their regulation is established within that of biologics.

Table 3. Paraguay’s general requirements for the sanitary registry of biologics for human use according to Decree No. 6611/2016 (Presidencia de la República del Paraguay, 2016a).
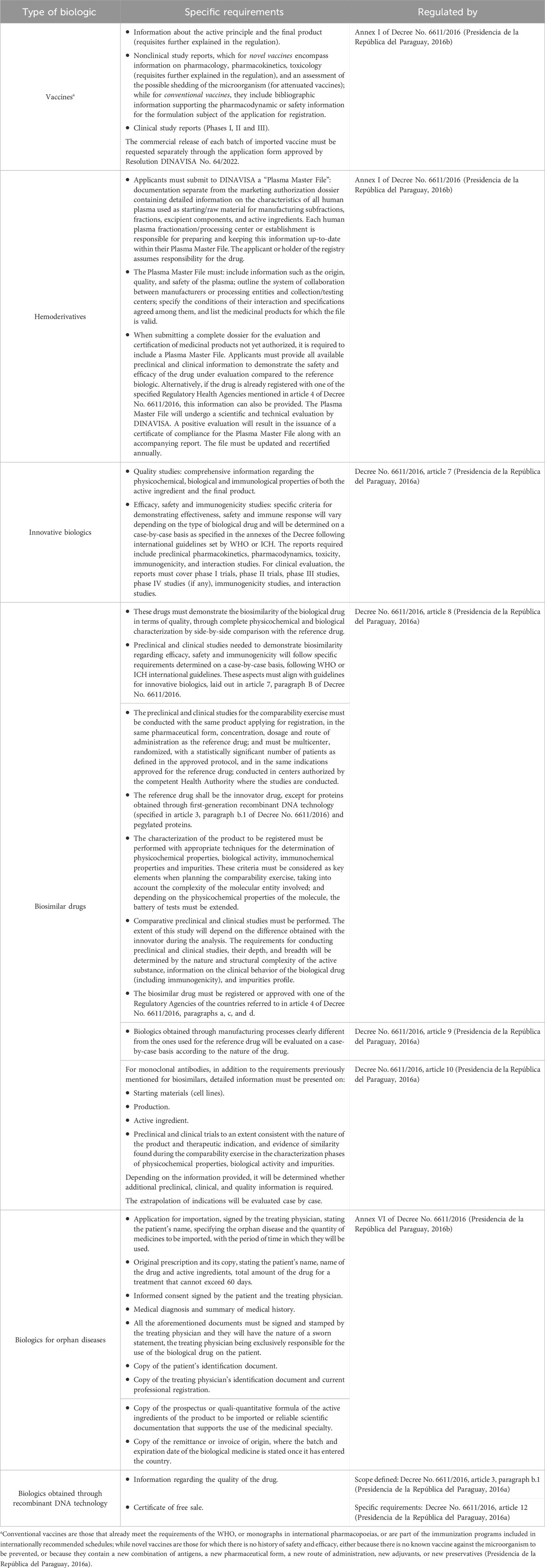
Table 4. Paraguay’s specific additional requirements for the registry of vaccines, hemoderivatives, innovative biologics, biosimilars, biologics for orphan diseases, and biologics obtained through recombinant DNA technology.
The registration of all products requires preclinical and clinical trial information, and in the case of biosimilars, said studies must be done in a comparative manner. Paraguay does not require the performance of local clinical trials. The biosimilarity study seeks to rationally predict the same safety and clinical efficiency between the innovator product and the biosimilar. The process must define the quality and safety attributes necessary for comparison and must include preclinical and clinical studies. The scope of this study shall be defined based on the characteristics of the product and the differences in the production and purification mechanisms, and the results obtained may determine the need for additional studies for biosimilarity testing. The World Health Organization (WHO) has recently issued a new guideline on biosimilars (Kurki et al., 2022; WHO, 2022), thus we can expect local regulation instruments to be updated soon.
Vaccines present different situations. In the case of novel vaccines, preclinical and clinical studies are required; while for conventional and combined vaccines developed by new manufacturers, the demonstration of non-inferiority with marketed vaccines of proven efficacy and safety is required. When new vaccination schedules or new indications are proposed, they must be accompanied by the corresponding clinical trials.
Good Manufacturing and Control Practices are required in all cases. The guidelines for the certification of compliance of Good Manufacturing and Control Practices were approved through MSPyBS Resolution No. 020/2015 (MSPyBS, 2015a; MSPyBS, 2015b).
Sanitary registries are valid for 5 years, and can be renewed for similar periods. The renewal of sanitary registries for biologics necessitates compliance with the requirements specified in article 6 of the Decree, ensuring that there have been no changes in the production process from the active ingredient to the final product, modifications of the therapeutic indications, change of manufacturer, and other changes that DINAVISA considers essential to maintain the quality, safety, efficacy and immunogenicity of the biologic2. If there have been changes in the production process from the active ingredient to the final product, modifications of the therapeutic indications, change of manufacturer and other changes that DINAVISA considers essential to maintain the quality, safety, efficacy and immunogenicity of the biologic, a new application for sanitary registry must be submitted.
Regarding pharmaceutical products in Mercosur, several important resolutions have been incorporated into the Paraguayan regulatory framework. These include standards for registering pharmaceutical products across member states (Mercosur GMC, 1995; Presidencia de la República del Paraguay, 1997), criteria for company registration (Mercosur GMC, 1996c; Presidencia de la República del Paraguay, 1997), and detailed requirements for documentation and information necessary for registration (Mercosur GMC, 1996d; Presidencia de la República del Paraguay, 1997). In addition, standards for good manufacturing practices have been established to ensure product quality and safety (Mercosur GMC, 2009; Presidencia de la República del Paraguay, 2012b).
7 Biologics for veterinary use
Paraguay’s regulation of veterinary products, supervised by SENACSA, is characterized by a multilayered approach aimed at ensuring the safety and efficacy of veterinary biologics. The foundational legal framework, established by Law No. 667/1995 (República del Paraguay, 1995) and amended by Law No. 2426/2004 (República del Paraguay, 2004a), mandates the registration of all veterinary products and entities dealing with such products.
Meanwhile, Decree No. 6991/2017 (Presidencia de la República del Paraguay, 2017) entrusts SENACSA with exclusive jurisdiction over the authorization, operation, and supervision of entities dealing with veterinary products. The registration of all activities related to veterinary products is managed through SENACSA’s SIGOR (Regional Office Management Information System) online platform (SENACSA, 2021b; SENACSA, 2021c).
Resolution SENACSA No. 2803/2011 (SENACSA, 2011) outlines the criteria for the registration and licensing of importers. In addition, Resolution SENACSA No. 199/2012 (SENACSA, 2012a; SENACSA, 2018a) sets operational standards for vaccine-vending houses and distribution centers, while Resolution SENACSA No. 785/2012 (SENACSA, 2012b) sets comprehensive guidelines covering a wide array of operational aspects of laboratories including personnel management, facility layout, and quality control processes.
Biosafety information requirements for the registration of veterinary biologics include biological and chemical composition, specifications and methods of control for components of the formula and for culture media, substrates and other biological materials used, methodology of product manufacturing, method of control of the finished product, and evidence of safety and efficacy (literature review and clinical trials, when applicable) (SENACSA, 2020; SENACSA, 2021b).
The registration process for subunit immunogens produced via biotechnological processes mandates comprehensive control of the components and final product, ensuring their safety, quality, and efficacy. This encompasses chemical and biological characterization, manufacturing processes, and quality control protocols. The registration process focuses specifically on managing biological risks, emphasizing the prevention of public, animal, and environmental health hazards during production (SENACSA, 2021b; SENACSA, 2021a).
Disease control is another critical aspect addressed. Resolutions SENACSA No. 687/2017, No. 1641/2017, and No. 124/2018 focus on foot-and-mouth disease vaccine production standards, while Resolution SENACSA No. 690/2017 outlines standards for Brucella abortus vaccine production. These standards include infrastructure requirements for the manufacturers, dosage and specific strains to be used in production, and quality control (SENACSA, 2017b; SENACSA, 2017c; SENACSA, 2017a; SENACSA, 2018b).
Mercosur GMC Resolutions scaffold the harmonization of regional regulatory frameworks for veterinary products. In particular, vaccines against diseases such as symptomatic anthrax and gas gangrene are addressed, along with vaccine production in poultry. The adoption of Mercosur resolutions into Paraguay’s regulatory framework indicates an effort to align veterinary health standards and promote a unified strategy for animal healthcare (Table 5).

Table 5. Resolutions dealing with veterinary products approved by the Mercosur Common Market Group and incorporated into the Paraguayan regulatory framework.
8 Intellectual property of products of biotechnology
Patents are regulated in Paraguay through Law No. 1630/2000 (República del Paraguay, 2000), which establishes the requirements for the obtention of a patent, types of patent, matters excluded from patent protection, duration of the patent, and other relevant regulations. In particular, article 5 states that plants and animals (except microorganisms), and processes that are essentially biological for the production of plants or animals, are excluded from patent protection.
In addition, article 16 determines that during the application for a patent, when the invention refers to a product or procedure related to some biological material that is not available to the public and cannot be described in such a way that the invention can be implemented by a person skilled in the matter, the description shall be complemented by the deposit of said material in a deposit institution recognized by the General Directorate of Industrial Property. Such deposit shall not be required if it has already been made in any state member of the World Trade Organization or if the examination of novelty has already been carried out by the authority of any such country. The executing body of intellectual property policy is the National Directorate of Intellectual Property (República del Paraguay, 2012).
In compliance with article 27 section 3.b of Law No. 444/1994 (incorporation of TRIPS agreement) (República del Paraguay, 1994b), Law No. 385/1994 (República del Paraguay, 1994c) establishes several instruments for the protection of plant varieties, which apply to GM crops. Its implementing authority is SENAVE since the passing of Law No. 2459/2004 (República del Paraguay, 2004b). Further specifications on the use of these instruments can be found in Decree No. 7797/2000 (Presidencia de la República del Paraguay, 2000).
9 Difficulties with data collection
One of the primary difficulties in Paraguay’s regulatory framework for biotechnology is the absence of a centralized database of regulations. Currently, these regulations are scattered across various platforms, which often leads to accessibility issues, and some are entirely unavailable online, meaning interested parties must make a written request of a physical copy at the respective government office, resulting in extended waiting periods.
This decentralization impedes the ability to monitor the development of regulations, thereby complicating the process of determining whether a norm is currently in effect, or has been repealed, replaced, or modified. Paraguay has a unified online portal of public information (Portal Paraguay - Acceso a la Información Pública, 2023) where such requests can also be made, but they are not always answered in a timely manner. Additionally, the absence of official signatures or letterheads on digitally available documents necessitates additional verification steps, which further diminishes the efficiency of the system. We gathered the regulations analyzed for this work and deposited them in a repository to ensure their availability to our readers (in Spanish) (Benitez Candia et al., 2024).
Mercosur GMC Resolutions present a specific challenge. We were unable to find a local incorporation instrument for Resolution GMC No. 33/00 (Table 5), which results in uncertainty about its domestic status.
10 Considerations on the situation of the regulatory framework for biotechnology in Paraguay
The biotechnology framework in Paraguay is closely aligned with Mercosur, yet the regulatory agencies of the member states exhibit distinct characteristics that may result in variations in their approaches (Magnuson et al., 2013; Mukherjee et al., 2022). Cooperation within the region is primarily sustained by the exchange of information and a certain level of harmonization of legal and regulatory requirements. However, effective harmonization necessitates the acceptance of common values and objectives, shared interests and challenges, mutual economic and other advantages, avoidance of disputes, collaboration on other concerns, and streamlining of procedures (McLean et al., 2002). Unfortunately, achieving this level of harmonization is a daunting task.
Moreover, regulatory systems have often been implemented on a “piece-by-piece” basis (McLean et al., 2002) in response to the urgent needs of the moment, and are more reactive than preventive systems. An inventory and evaluation of priorities, policies, existing regulatory regimes, and scientific and technical means is ideally a prerequisite to the development and implementation of policies and regulations (McLean et al., 2002; Schoemaker et al., 2020). However, building such a system and making it operational is complicated by the fact that there is no single best approach nor standard that reflects cultural, political, financial, and scientific heterogeneity. When establishing a regulatory framework, considerable attention must be paid to factors such as regulatory triggers, transparency, public involvement in policy-making and regulatory decision-making processes, and proportionate methods for assessing and managing risk.
While Paraguay has made efforts to improve its regulatory framework, the triggers for regulatory review are not adequately defined in several current norms. The country has recently implemented science-based approaches to assessing and managing risks in relation to GMOs. However, there is a deficiency in terms of public consultations and participation. Current processes for public engagement are lacking, resulting in a disconnect between regulatory bodies and the broader community. Establishing an effective public consultation process would not only enhance regulatory decision-making, but also promote a more transparent approach to biotechnology governance. It is essential to involve stakeholders and the public in discussions about biotechnology to ensure informed policymaking and foster trust.
Several regulations have proven particularly challenging in their interpretation. An example is article 10 of Law No. 3283/2007 (República del Paraguay, 2007b), which addresses the validation of evaluations for sanitary registration from specific countries. The wording of the law presents a challenge to understanding whether the procedure involves automatic acceptance from third-country assessments, and the extent of this provision. At the very least, interpretations are manifold. In addition, articles 4 and 5 of Decree No. 6611/2016 determine that for biologics with a sanitary registry from the regulatory agencies specified in the aforementioned law, therapeutic indications “can be recognized and expanded”, which makes interpretation of both regulations together even more challenging.
These difficulties have significant ramifications for a range of stakeholders, including researchers, industry experts, and policymakers; and can impede the development, authorization, and commercialization of biotechnology products, consequently affecting scientific progress. At minimum, the process and criteria for risk assessment and risk management must be widely published to instill trust in the system as credible and predictable among developers, stakeholders, and the public (Crow et al., 2016; Wolt and Wolf, 2018).
We suggest the evaluation of current scientific and technical capacity in Paraguay in order to facilitate the development of a more fit-for-purpose system. A sound regulatory system necessitates continuous updates on the latest scientific advancements; without such updates, the regulator’s knowledge base will have a limited lifespan. We hope that this initial assessment of current legislation will be the starting point for determining and implementing appropriate, scientifically sound regulations.
Author contributions
NB: Writing–review and editing, Writing–original draft, Visualization, Validation, Resources, Methodology, Investigation, Formal Analysis, Data curation. MU: Writing–original draft, Resources, Investigation. PS: Writing–review and editing, Validation, Resources, Investigation, Formal Analysis. EN: Writing–review and editing, Validation, Resources, Investigation, Formal Analysis. AA: Writing–review and editing, Writing–original draft, Validation, Supervision, Resources, Project administration, Formal Analysis, Conceptualization. DF: Writing–review and editing, Writing–original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by projects PIC-01-2024 and PIC-45-2023, both granted by Facultad de Ciencias Exactas y Naturales - Universidad Nacional de Asunción. The projects covered operational, material, and personnel expenses.
Acknowledgments
The authors express their sincere appreciation to María Florencia Goberna and Facundo Vesprini for his valuable feedback on this manuscript. They also extend their thanks to María Paz Corrales and Samuel Gabaglio for their kind contributions of country-specific legislation, and scientific references.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2024.1373473/full#supplementary-material
Footnotes
1Defined in Decree No. 6611/2016 as: “substances composed of or derived from proteins, nucleic acids, sugars or a complex combination of the above or living entities such as cells or tissues, obtained from living organisms or their tissues. They include viruses, therapeutic serums, toxins, antitoxins, vaccines, blood, blood components or derivatives, allergenic products, hormones, colony-stimulating factors, cytokines, antibodies, heparins, among others. The sources and methods of production include, but are not limited to cell culture, microorganisms, extraction from tissues or biological fluids, recombinant DNA techniques, transgenesis, hybridoma techniques, propagation of microorganisms in embryos or animals, etc. They are products used for the prevention, treatment, or in vivo diagnosis of certain diseases.”
2Except for products specified in article 3, paragraph b.1 of the Decree, which must follow requirements established in article 12, specified in the section for biologics obtained through recombinant DNA technology (Table 4).
References
Benitez Candia, N., Fernandez Rios, D., and Vicien, C. (2020). Paraguay’s path toward the simplification of procedures in the approval of GE crops. Front. Bioeng. Biotechnol. 8, 1023. doi:10.3389/fbioe.2020.01023
Benitez Candia, N., Ulke Mayans, M. G., Sotelo Torres, P. H., Nara Pereira, E. M., Arrua Alvarenga, A. A., and Fernandez Rios, D. (2024). Paraguay’s regulatory framework for products obtained through modern biotechnology as of February 2024. https://zenodo.org/records/10688936.
Carreno, I. (2022). France bans “meaty” terms for plant-based products: will the European union follow? Eur. J. risk Regul. 13, 665–669. doi:10.1017/err.2022.22
Crow, D. A., Albright, E. A., and Koebele, E. (2016). Public information and regulatory processes: what the public knows and regulators decide. Rev. Policy Res. 33, 90–109. doi:10.1111/ropr.12154
De F Toledo, M. C. (2014). A need for harmonized legislation: perspectives in South America: a need for harmonized legislation in South America. J. Sci. Food Agric. 94, 1958–1961. doi:10.1002/jsfa.6163
Dellepiane, N., and Pagliusi, S. (2019). Opportunities for improving access to vaccines in emerging countries through efficient and aligned registration procedures: an industry perspective. Vaccine 37, 2982–2989. doi:10.1016/j.vaccine.2019.03.025
DINAVISA (2023). Medicamentos biologicos. Direccion Nac. Vigil. Sanit. Parag., Available at: https://dinavisa.gov.py/medicamentos-biologicos/(Accessed December 13, 2023).
FAO (1995). “La normalización de alimentos y el Codex Alimentarius en el marco del MERCOSUR,” in Alimentacion, Nutricion y Agricultura. Inocuidad y comercio de los alimentos Alimentacion, Nutricion y Agricultura. Roma: organizacion de las Naciones Unidas para la Agricultura y la Alimentacion. FAO, Rome, Italy, Available at: https://www.fao.org/3/v9723t/v9723t0b.htm#la%20normalizaci%C3%B3n%20de%20alimentos%20y%20el%20codex%20alimentarius%20en%20el%20marco%20del%20mer (Accessed February 20, 2024).
Fernandez Rios, D., Benitez Candia, N., Soerensen, M., Goberna, M., and Arra, A. (2024). Regulatory landscape for new breeding techniques NBTs: insights from Paraguay. Front. Bioeng. Biotechnol. 12, 1332851. doi:10.3389/fbioe.2024.1332851
Fernandez Rios, D., Rubinstein, C., and Vicien, C. (2018). Capacities for the risk assessment of GMOs: challenges to build sustainable systems. Front. Bioeng. Biotechnol. 6, 40. doi:10.3389/fbioe.2018.00040
Franco Alfaro, L. F. (2023). Que prohibe la produccion, importacion y comercializacion de la carne cultivada en laboratorio, para consumo humano y en toda la cadena alimenticia, presentado por el Diputado Luis Federico Franco Alfaro. Available at: http://silpy.congreso.gov.py/web/expediente/129071 (Accessed December 18, 2023).
Garcia-Alonso, M., and Raybould, A. (2014). Protection goals in environmental risk assessment: a practical approach. Transgenic Res. 23, 945–956. doi:10.1007/s11248-013-9760-1
Gleckel, J. A. (2020). Are consumers really confused by plant-based food labels? An empirical study. Available at: https://papers.ssrn.com/abstract=3727710 (Accessed November 27, 2023).
Kurki, P., Kang, H.-N., Ekman, N., Knezevic, I., Weise, M., and Wolff-Holz, E. (2022). Regulatory evaluation of biosimilars: refinement of principles based on the scientific evidence and clinical experience. BioDrugs 36, 359–371. doi:10.1007/s40259-022-00533-x
La Nacion (2022). Paraguay, segundo pais en la region con Ley de defensa al consumidor de carnicos y sus derivados. Negocios. Available at: https://www.lanacion.com.py/negocios/2022/05/12/paraguay-segundo-pais-en-la-region-con-ley-de-defensa-al-consumidor-de-carnicos-y-sus-derivados/(Accessed October 9, 2023).
MAG (2015). Resolucion No 27/2015 Por la cual se aprueban los documentos Formulario 1: ensayos regulados, 2: liberacion comercial y de la guia para Formulario 2: liberacion comercial. Available at: https://conbio.mag.gov.py/media/ckfinder/files/Resolucion%20MAG%20N%2027_15%20Formularios_1.PDF (Accessed November 3, 2023).
MAG (2019a). Resolucion No 1030/2019 Por la cual se amplia el inc. (C) del Articulo 31 de la Resolucion MAG No 1348 Por la cual se reglamenta el Decreto No 9699 del 19 de Septiembre de 2012 ‘Que crea la Comision Nacional de Bioseguridad Agropecuaria y Forestal (CONBIO)’, de fecha 20 de Diciembre de 2012. Available at: https://conbio.mag.gov.py/media/ckfinder/files/RES.N1030%20DE%2023%20DE%20AGOSTO%20DE%202019Liberacion%20Comercial.pdf Accessed November 3, 2023.
MAG (2019b). Resolución No 1071/2019 Por la cual se reglamenta la Resolución MAG 1030/2019 "Por la cual se amplía el inc. C del Artículo 31 de la Resolución MAG No 1348 “Por la cual se reglamenta el Decreto No 9699 del 19 de Septiembre de 2012 ‘Que crea la Comisión Nacional de Bioseguridad Agropecuaria y Forestal CONBIO’, de fecha 20 de Diciembre de 2012”, de fecha 23 de Agosto de 2019. Available at: https://conbio.mag.gov.py/media/ckfinder/files/RES.N1071%203%20DE%20SETIEMBRE%20Aprobaciones%20ComercialDE%202019.pdf (Accessed November 3, 2023).
MAG (2024). Resolución No 92/2024 Por la cual se autoriza la liberacion comercial de organismo genéticamente modificado denominado OX5382G “Spodoptera frugiperda”, a favor de la empresa. UK: OXITEC LTD.
Magnuson, B., Munro, I., Abbot, P., Baldwin, N., Lopez-Garcia, R., Ly, K., et al. (2013). Review of the regulation and safety assessment of food substances in various countries and jurisdictions. Food Addit. Contam. Part A 30, 1147–1220. doi:10.1080/19440049.2013.795293
McHughen, A. (2016). A critical assessment of regulatory triggers for products of biotechnology: product vs. process, GM Crops Food 7, 125–158. doi:10.1080/21645698.2016.1228516
McLean, M. A., Frederick, R. J., Traynor, P. L., Cohen, J. I., and Komen, J. (2002). A conceptual framework for implementing biosafety: linking policy, capacity, and regulation. ISNAR Brief. Pap., 1–12.
Melnick, R. L., Jarvis, L., Hendley, P., Garcia-Alonso, M., Metzger, M. J., Ramankutty, N., et al. (2023). GEnZ explorer: a tool for visualizing agroclimate to inform research and regulatory risk assessment. Transgenic Res. 32, 321–337. doi:10.1007/s11248-023-00354-w
Mercosur GMC (1993). Resolución 11/93 Marco regulatorio para productos veterinarios. Available at: https://normas.mercosur.int/public/normativas/2204 (Accessed November 3, 2023).
Mercosur GMC (1995). Resolución 23/95 Requisitos para el registro de productos farmaceuticos registrados y elaborados en un estado parte productor, similares a productos registrados en el estado parte receptor. Available at: https://normas.mercosur.int/public/normativas/1791 (Accessed November 3, 2023).
Mercosur GMC (1996a). Resolución 39/96 Reglamentacion complementaria del marco regulatorio de productos veterinarios. Available at: https://normas.mercosur.int/public/normativas/1948 (Accessed November 3, 2023).
Mercosur GMC (1996b). Resolución 40/96 Reglamentacion del sistema de convalidacion para productos veterinarios. Available at: https://normas.mercosur.int/public/normativas/1949 (Accessed November 3, 2023).
Mercosur GMC (1996c). Resolución 51/96 Empresas y titularidad de registros: requisitos que deben reunir las empresas para ser autorizadas como titulares, en el estado parte receptor, de registros de productos farmacéuticos elaborados en otro estado parte del Mercosur. Available at: https://normas.mercosur.int/public/normativas/1792 (Accessed November 3, 2023).
Mercosur GMC (1996d). Resolución 52/96 Listado de informaciones y documentación requerida para el registro de productos farmacéuticos similares - resolución GMC no 23/95. Available at: https://normas.mercosur.int/public/normativas/1793 (Accessed November 3, 2023).
Mercosur GMC (1996e). Resolución 77/96 Reglamento técnico para el control de las vacunas contra el carbúnculo sintomático, gangrena gaseosa, enterotoxemia y tétano inactivadas y conservadas bajo refrigeración. Available at: https://normas.mercosur.int/public/normativas/2002 (Accessed November 3, 2023).
Mercosur GMC (1997). Resolución 4/97 Reglamento tecnico para la produccion y el control de vacunas, antigenos y diluyentes para avicultura. Available at: https://normas.mercosur.int/public/normativas/1620 (Accessed November 3, 2023).
Mercosur GMC (1998). Resolucion No 77/1998 Reconocimiento mutuo de equivalencia de sistemas de control. Available at: https://normas.mercosur.int/public/normativas/1612 (Accessed November 3, 2023).
Mercosur GMC (2000). Resolucion 33/00 Acuerdos de reconocimiento mutuo en materia de registros genealogicos y evaluaciones genéticas animales. Available at: https://normas.mercosur.int/public/normativas/1236 (Accessed November 3, 2023).
Mercosur GMC (2009). Resolución 15/09 Buenas prácticas de fabricación de productos farmacéuticos y mecanismo de implementación en el ámbito del Mercosur Derogación de las Res. GMC no 14/96 y 61/00. Available at: https://normas.mercosur.int/public/normativas/641 (Accessed November 3, 2023).
Mercosur GMC (2017). Resolución No 45/2017 Procedimientos para la elaboración, revisión y derogación de reglamentos técnicos mercosur y procedimientos mercosur de evaluación de la conformidad Derogación de la Res. GMC No 56/02. Available at: https://normas.mercosur.int/public/normativas/3509 (Accessed November 3, 2023).
Mercosur GMC (2019). Resolucion 23/19 Mecanismo para disminuir la ocurrencia de presencia en bajos niveles (PBN) de organismos geneticamente modificados OGM entre los estados partes. Available at: https://normas.mercosur.int/public/normativas/3736 (Accessed November 3, 2023).
MSPyBS (2015a). Guia de verificacion del cumplimiento de las Buenas Practicas de Fabricacion y Control de las industrias farmaceuticas. Available at: https://www.mspbs.gov.py/dependencias/dnvs/adjunto/f16a5d-RESOLUCINS.GN0202015GUADEINSPECCINDEBPFYCDEESPECIALIDADESFARMACUTICAS.pdf (Accessed November 3, 2023).
MSPyBS (2015b). Resolución No 020/2015 Por la cual se aprueba la Guía de Inspección para la Obtención del Certificado de Cumplimiento de Buenas Prácticas de Fabricación y Control para los laboratorios, importadoras, fraccionadoras y distribuidoras de la industria farmacéutica; y se deroga la Resolución S.G. No 17, del 20 de enero de 2011. Available at: https://www.mspbs.gov.py/dependencias/dnvs/adjunto/0f582a-RESOLUCINS.GN0202015.pdf (Accessed November 3, 2023).
Mukherjee, A., Gómez-Sala, B., O’Connor, E. M., Kenny, J. G., and Cotter, P. D. (2022). Global regulatory frameworks for fermented foods: a review. Front. Nutr. 9, 902642. doi:10.3389/fnut.2022.902642
OECD (2023). “Paraguay,” in development in delegations on biosafety issues, june 2022 – april 2023 series on the harmonisation of regulatory oversight in biotechnology. Paris: OECD environment directorate, chemicals and biotechnology committee. Available at: https://one.oecd.org/document/ENV/CBC/MONO(2023)28/en/pdf.
Portal Paraguay - Acceso a la Información Pública (2023). Portal Unificado de Transparencia Activa, Available at: https://informacionpublica.paraguay.gov.py/portal/#!/buscar_informacion#busqueda (Accessed December 18, 2023).
Presidencia de la República del Paraguay (1997). Decreto No 17057/1997 Por el cual se dispone la vigencia en la República del Paraguay de las Resoluciones adoptadas por el Grupo Mercado Común del Mercosur, referentes a reglamentos técnicos. Available at: https://www.mspbs.gov.py/dependencias/dnvs/adjunto/5e1c98-DECRETON170571997INTERNALIZACIONDERESOLUCIONESDELGMC.pdf (Accessed November 3, 2023).
Presidencia de la República del Paraguay (1998a). Decreto No 21376/1998 Por el cual se establece la nueva organización funcional del Ministerio de Salud Pública y Bienestar Social. Available at: https://www.mspbs.gov.py/dependencias/portal/adjunto/94401d-2DECRETO21376ORGANIZACIONFUNCIONALDELMSPBS.pdf (Accessed November 3, 2023).
Presidencia de la República del Paraguay (1998b). Decreto No 891/1998 Por el cual se dispone la vigencia en la República del Paraguay de las Resoluciones adoptadas por el Grupo Mercado Común del Mercosur, referentes a armonizaciones de productos veterinarios y normas zoosanitarias. doi:10.5281/zenodo.10688936
Presidencia de la República del Paraguay (2000). Decreto No 7797/2000 Por el cual se reglamenta la Ley No 385/94 de semillas y protección de cultivares. Available at: http://web.senave.gov.py:8081/docs/web/decretos/Dto7797-00.pdf (Accessed November 3, 2023).
Presidencia de la República del Paraguay (2012a). Decreto Na 9699/2012 Por el cual se crea la Comisión Nacional de Bioseguridad Agropecuaria y Forestal CONBIO. Available at: https://conbio.mag.gov.py/media/ckfinder/files/Decreto%209699.pdf (Accessed November 3, 2023).
Presidencia de la República del Paraguay (2012b). Decreto No 10403/2012 Por el cual se incorporan al ordenamiento jurídico Nacional las Resoluciones No: 69/06, 15/09, 16/09, 38/09, 18/10, 19/10, 20/10, 21/10, 48/10, 07/11, 08/11, 09/11, 18/11, 19/11, 20/11, 21/11, 22/11, 23/11, 24/11 y 31/11 del Grupo Mercado Común, vinculadas al subgrupo de trabajo No 11 “salud” del Mercosur. Available at: https://www.mspbs.gov.py/dependencias/dnvs/adjunto/b07568-DECRETON104032012INTERNALIZACIONDERESOLUCIONESDELGMC.pdf (Accessed November 3, 2023).
Presidencia de la República del Paraguay (2016a). Decreto No 6611/2016 Por el cual se reglamenta el artículo 24 de la Ley No 1119/1997, “De productos para la salud y otros”, y se establecen los requisitos para el registro de medicamentos biológicos. Available at: https://www.mspbs.gov.py/dependencias/dnvs/adjunto/1a7b18-DecretoN661116.pdf (Accessed November 3, 2023).
Presidencia de la República del Paraguay (2016b). Anexo I del Decreto No 6611/2016. Available at: https://www.mspbs.gov.py/dependencias/dnvs/adjunto/ec7399-DecretoN6611anexo.pdf (Accessed November 3, 2023).
Presidencia de la República del Paraguay (2017). Decreto N° 6991/2017 Por el cual se deroga el Decreto N° 4.642 del 11 de Agosto de 1999 y su modificatoria el Decreto N° 4.320 del 6 de Mayo de 2010; y se encarga al Servicio Nacional De Calidad y Salud Animal SENACSA, la reglamentación de las normas para la habilitación, funcionamiento y fiscalización de empresas y/o laboratorios que elaboren, procesen, importen/exporten, distribuyan, comercialicen o almacenen productos e insumos biológicos, químicos o farmacológicos de uso veterinario como así mismo para la admisión, utilización y registro de dichos productos. doi:10.5281/zenodo.10688936
Presidencia de la República del Paraguay (2021). Decreto No 6532/2021 Por el cual se incorpora al ordenamiento jurídico nacional la Resolución del Grupo Mercado Común del Mercosur No 23/19 Mecanismo para disminuir la ocurrencia de presencia en bajos niveles (PBN) de organismos genéticamente modificados (OGM) entre los estados partes doi:10.5281/zenodo.10688936
Profeta, A., Baune, M.-C., Smetana, S., Bornkessel, S., Broucke, K., Van Royen, G., et al. (2021). Preferences of German consumers for meat products blended with plant-based proteins. Sustainability 13, 650. doi:10.3390/su13020650
Reavey, C. E., Walker, A. S., Joyce, S. P., Broom, L., Willse, A., Ercit, K., et al. (2022). Self-limiting fall armyworm: a new approach in development for sustainable crop protection and resistance management. BMC Biotechnol. 22, 5. doi:10.1186/s12896-022-00735-9
República del Paraguay (1993). Ley No 253/1993 Que aprueba el Convenio sobre Diversidad Biológica, adoptado durante la Conferencia de las Naciones Unidas sobre el Medio Ambiente y Desarrollo -La Cumbre para la Tierra-, celebrado en la Ciudad de Río de Janeiro, Brasil. Available at: https://faolex.fao.org/docs/pdf/par201179.pdf (Accessed November 3, 2023).
República del Paraguay (1994a). Ley No 300/1994 Que aprueba el Convenio de París para la protección industrial y sus revisiones y enmiendas. Available at: https://www.wipo.int/wipolex/es/text/225780 (Accessed November 3, 2023).
República del Paraguay (1994b). Ley No 444/1994 Que ratifica el Acta Final de la Ronda del Uruguay del Gatt. Available at: http://digesto.senado.gov.py/ups/leyes/4219%20.pdf (Accessed November 3, 2023).
República del Paraguay (1994c). Ley N° 385/1994 de semillas y protección de Cultivares. Available at: https://bacn.gov.py/archivos/2377/20140411124503.pdf (Accessed November 3, 2023).
República del Paraguay (1995). Ley No 667/1995 Que establece el régimen y fiscalización integral de los productos de uso veterinario y fija condiciones para desarrollar actividades de elaboración, fraccionamiento, distribución, importación, tenencia, expendio y uso de dichos productos. Available at: http://digesto.senado.gov.py/ups/leyes/5385%20.pdf (Accessed November 3, 2023).
República del Paraguay (1996). Ley N° 988/1996 que aprueba el Convenio Internacional para la Protección de las Obtenciones de Vegetales. Available at: https://www.wipo.int/wipolex/es/text/129455 (Accessed November 3, 2023).
República del Paraguay (2000). Ley N° 1630/2000 de patentes de Invenciones. Available at: https://www.wipo.int/wipolex/es/text/225598 (Accessed November 3, 2023).
República del Paraguay (2003). Ley No 2309/2003 Que aprueba el Protocolo de Cartagena sobre Seguridad de la Biotecnología del Convenio sobre la Diversidad Biológica. Available at: http://digesto.senado.gov.py/detalles&id=4147 (Accessed November 3, 2023).
República del Paraguay (2004a). Ley No 2426/2004 que crea el Servicio nacional de Calidad y salud animal (SENACSA). Available at: http://documentos.senacsa.gov.py/share/s/kR-O17yVRqOYWTw4xYSgGg (Accessed November 3, 2023).
República del Paraguay (2004b). Ley No 2459/2004 que crea el Servicio nacional de Calidad y sanidad vegetal y de Semillas SENAVE. Available at: https://www.wipo.int/wipolex/es/text/547430 (Accessed November 3, 2023).
República del Paraguay (2007a). Ley No 3194/2007 Que aprueba el Tratado Internacional sobre los Recursos Fitogenéticos para la Alimentación y la Agricultura. Available at: http://digesto.senado.gov.py/ups/leyes/4136%20.pdf (Accessed November 3, 2023).
República del Paraguay (2007b). Ley No 3283/2007 De protección de la información no divulgada y datos de prueba para los registros farmacéuticos. Available at: http://digesto.senado.gov.py/ups/leyes/10629%20.pdf (Accessed November 3, 2023).
República del Paraguay (2010). Ley No 3788/2010 que crea el Instituto paraguayo de Tecnología agraria. Available at: http://digesto.senado.gov.py/ups/leyes/7411.pdf (Accessed November 3, 2023).
República del Paraguay (2012). Ley N° 4798/2012 que crea la Dirección nacional de Propiedad intelectual (DINAPI). Available at: https://www.wipo.int/wipolex/es/text/316069 (Accessed November 3, 2023).
República del Paraguay (2018). Ley No 6123/2018 Que eleva al rango de Ministerio a la Secretaría del Ambiente y pasa a denominarse Ministerio del Ambiente y Desarrollo Sostenible. Available at: http://digesto.senado.gov.py/ups/leyes/10215.pdf (Accessed November 3, 2023).
República del Paraguay (2021). Ley No 6788/2021 Que establece la competencia, atribuciones y estructura orgánica de la Dirección Nacional de Vigilancia Sanitaria. Available at: http://digesto.senado.gov.py/ups/leyes/11869.pdf (Accessed November 3, 2023).
República del Paraguay (2022). Ley No 6916/2022 De protección al consumidor de productos cárnicos y sus derivados. Available at: http://digesto.senado.gov.py/ups/leyes/12063.pdf (Accessed November 3, 2023).
Schoemaker, C. G., Van Loon, J., Achterberg, P. W., Den Hertog, F. R. J., Hilderink, H., Melse, J., et al. (2020). Four normative perspectives on public health policy-making and their preferences for bodies of evidence. Health Res. Policy Sys 18, 94. doi:10.1186/s12961-020-00614-9
Secretariat of the Convention on Biological Diversity (2000). Cartagena protocol on biosafety to the convention on biological diversity: text and annexes. Montreal: secretariat of the convention on biological diversity. Available at: https://www.cbd.int/doc/legal/cartagena-protocol-en.pdf.
SENACSA (2011). Resolución N° 2803/2011 Por la cual se establecen requisitos para el registro y habilitación de personas físicas y jurídicas que importan productos de uso veterinario al territorio de la República del Paraguay. doi:10.5281/zenodo.10688936
SENACSA (2012a). Resolución No 199/2012 Por la cual se establecen los requisitos par la habilitación de casas expendedoras y centros de distribución de vacuna antiaftosa de la Comisión de Salud Animal (CSA); y se autoriza la implementación del módulo “Casas expendedoras de vacuna” del Sistema Informático SIGOR III. doi:10.5281/zenodo.10688936
SENACSA (2012b). Resolución N° 785/2012 Por la cual se establecen requisitos para la certificación en Buenas Prácticas de Manufactura BPM, de laboratorios productores de biológicos de uso veterinario. doi:10.5281/zenodo.10688936
SENACSA (2017a). Resolución N° 1641/2017 Por la cual se modifica parcialmente la Resolución SENACSA N° 687 del 2 de Mayo de 2017, “Por la cual se establece reglamentación para habilitación, funcionamiento y fiscalización de empresas y/o laboratorios que elaboren, importen/exporten y comercialicen productos biológicos utilizados en los programas de control y erradicación de la fiebre aftosa.” doi:10.5281/zenodo.10688936
SENACSA (2017b). Resolución N° 687/2017 Por la cual se establece reglamentación para habilitación, funcionamiento y fiscalización de empresas y/o laboratorios que elaboren, importen/exporten y comercialicen productos biológicos utilizados en los programas de control y erradicación de la fiebre aftosa. doi:10.5281/zenodo.10688936
SENACSA (2017c). Resolución N° 690/2017 Por la cual se establece reglamentación para la habilitación, funcionamiento y fiscalización de empresas y/o laboratorios que elaboren, importen/exporten y comercialicen productos biológicos a ser utilizados en el programa nacional de control y erradicación de la brucelosis, conforme al art. 2° del Decreto del Poder Ejecutivo N° 6.991 del 3 de Abril del 2017. doi:10.5281/zenodo.10688936
SENACSA (2018a). Resolución No 910/2018 Por la cual se deja sin efecto la Resolución del SENACSA No 537 del 06 de Abril de 2018 Por la cual se establecen requisitos para casas expendedoras de productos biológicos y centros de distribución de vacunas de las Comisiones de Salud Animal (CSA)/FUNDASSA.” doi:10.5281/zenodo.10688936
SENACSA (2018b). Resolución N° 124/2018 Por la cual se modifica parcialmente la Resolución SENACSA N° 687 del 2 de Mayo de 2017, “Por la cual se establece reglamentación para habilitación, funcionamiento y fiscalización de empresas y/o laboratorios que elaboren, importen/exporten y comercialicen productos biológicos utilizados en los programas de control y erradicación de la fiebre aftosa.” doi:10.5281/zenodo.10688936
SENACSA (2020). Formulario de inscripción para productos biológicos de uso veterinario. Available at: http://documentos.senacsa.gov.py/share/s/GKFu5QS-QBG3j6k4-Vrfug (Accessed November 3, 2023).
SENACSA (2021a). Formulario para el registro de inmunógenos de subunidades obtenidos por métodos biotecnológicos. doi:10.5281/zenodo.10688936
SENACSA (2021b). Resolución 900/2021 Por la cual se autoriza la implementación gradual del módulo de registro y receta electrónica del sistema SIGOR y la utilización obligatoria del mismo, para todos los establecimientos que desarrollan actividades de elaboración, fraccionamiento, distribución, tenencia, importación, exportación, expendio de productos de uso veterinario. Available at: http://documentos.senacsa.gov.py/share/s/UmLk63L2TiO48PrLtejDXA (Accessed November 3, 2023).
SENACSA (2021c). Sistema SIGOR. Servicio Nacional de Calidad y Salud Animal de Paraguay. Available at: https://www.sigor.gov.py/web/modulos.html (Accessed November 3, 2023).
SENAVE (2014a). Resolución No 283/2014 Por la cual se aprueba el procedimiento para la gestión del riesgo en actividades con organismos genéticamente modificados regulados en el ámbito agrícola. Available at: http://web.senave.gov.py:8081/docs/resoluciones/senave/Res283-14.pdf (Accessed November 3, 2023).
SENAVE (2014b). Resolución No 925/2014 Por la cual se implementa el registro de empresas que operan con organismos genéticamente modificados regulados en el ámbito agrícola. Available at: http://web.senave.gov.py:8081/docs/resoluciones/senave/Res925-14.pdf (Accessed November 3, 2023).
Tosun, P., Yanar, M., Sezgin, S., and Uray, N. (2021). Meat substitutes in sustainability context: a content analysis of consumer attitudes. J. Int. Food and Agribus. Mark. 33, 541–563. doi:10.1080/08974438.2020.1840475
Vesprini, F., Maggi, A. I., López Olaciregui, M., and Módena, N. A. (2020). Transportability of conclusions from confined field trials: a case study using the virus resistant transgenic bean developed in Brazil. Front. Bioeng. Biotechnol. 8, 815. doi:10.3389/fbioe.2020.00815
Vesprini, F., Whelan, A. I., Goberna, M. F., Murrone, M. L., Barros, G. E., Frankow, A., et al. (2022). Update of Argentina’s regulatory policies on the environmental risk assessment. Front. Bioeng. Biotechnol. 9, 834589. doi:10.3389/fbioe.2021.834589
Wach, M., Hellmich, R. L., Layton, R., Romeis, J., and Gadaleta, P. G. (2016). Dynamic role and importance of surrogate species for assessing potential adverse environmental impacts of genetically engineered insect-resistant plants on non-target organisms. Transgenic Res. 25, 499–505. doi:10.1007/s11248-016-9945-5
WHO (2022). Guidelines on evaluation of biosimilars. Replacement of annex 2 of WHO technical report series, No. 977. Available at: https://www.who.int/publications/m/item/guidelines-on-evaluation-of-biosimilars (Accessed December 28, 2023).
Wolt, J. D., and Wolf, C. (2018). Policy and governance perspectives for regulation of genome edited crops in the United States. Front. Plant Sci. 9, 1606. doi:10.3389/fpls.2018.01606
Keywords: Mercosur, genetically modified organisms, recombinant DNA, biologics, vaccines
Citation: Benítez Candia N, Ulke Mayans MG, Sotelo PH, Nara Pereira E, Arrúa Alvarenga AA and Fernández Ríos D (2024) Paraguay’s approach to biotechnology governance: a comprehensive guide. Front. Bioeng. Biotechnol. 12:1373473. doi: 10.3389/fbioe.2024.1373473
Received: 19 January 2024; Accepted: 05 March 2024;
Published: 27 March 2024.
Edited by:
Karen Hokanson, Agriculture and Food Systems Institute, United StatesReviewed by:
Monica Garcia-Alonso, Estel Consult Ltd, United KingdomClara Rubinstein, Institute for Scientific Cooperation in Environment and Health (ICCAS), Argentina
Copyright © 2024 Benítez Candia, Ulke Mayans, Sotelo, Nara Pereira, Arrúa Alvarenga and Fernández Ríos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danilo Fernández Ríos, dfernandez@facen.una.py; Andrea Alejandra Arrúa Alvarenga, andrea.arrua@cemit.una.py
 Nidia Benítez Candia
Nidia Benítez Candia María Gabriela Ulke Mayans
María Gabriela Ulke Mayans Pablo Hernán Sotelo
Pablo Hernán Sotelo Eva Nara Pereira
Eva Nara Pereira Andrea Alejandra Arrúa Alvarenga
Andrea Alejandra Arrúa Alvarenga Danilo Fernández Ríos
Danilo Fernández Ríos