Editorial: Skin-interfaced platforms for quantitative assessment in public health
- 1Department of Electronics and Information Convergence Engineering, Kyunghee University, Yongin, Republic of Korea
- 2Department of Electrical and Computer Engineering, University of California, Davis, Davis, CA, United States
- 3Department of Biomedical Engineering, Kyunghee University, Yongin, Republic of Korea
Editorial on the Research Topic
Skin-interfaced platforms for quantitative assessment in public health
The development of wearable sensors for health monitoring applications has been greatly facilitated by advancements in soft electronics. Soft skin-interfaced electronics refer to electronic components and circuits that are designed to be flexible, stretchable, and gently interfaced with the human body. This design scheme enables comfortable and non-invasive integration with wearable devices, revolutionizing the field of public healthcare by providing numerous benefits in terms of comfort, wearability, and accuracy of quantitative health monitoring.
One of the main advantages of skin-interfaced electronics as wearable sensors is their flexibility and stretchability. Existing rigid electronics pose obstacles for skin-interfaced sensing applications as they are intrinsically uncomfortable to wear and may restrict movement. Skin-interfaced electronics, on the other hand, are fully integrated into flexible and thin substrates, enabling comfortable and unrestricted movement along with an improved signal-to-noise ratio. This flexibility ensures a better fit for the body, improving the accuracy and reliability of health monitoring data. In addition, such electronics are adaptable to biocompatible materials, so they are suitable for long-term wear on the skin without causing irritation or discomfort. This is particularly important for continuous health monitoring applications, where users may need to wear the device for a long period of assessment time. Soft materials, such as silicone elastomers and hydrogels, provide a comfortable interface between the sensor and the skin, ensuring user comfort and device adhesion.
Besides comfort and wearability, skin-interfaced electronics can offer high sensitivity and precision through signal processing, particularly in the quantitative assessment of public health applications. Soft sensors can be designed to detect a wide range of biometrics, including electrical, mechanical, optical, and even acoustic, such as cardiac activity, respiratory activity, body motion, body temperature, blood pressure, and biochemical markers in sweat or interstitial fluid. These sensor platforms can provide real-time monitoring of vital signs, allowing for early detection of health issues and timely intervention. Furthermore, soft electronics enable seamless integration of multiple sensors into a single device, offering comprehensive health monitoring capabilities. For example, a single device could integrate sensing elements for heart rate, respiratory rate, temperature, and activity level assessments, which can provide a holistic view of an individual’s health status. This engineered integration, combined with automated operation and machine learning-powered signal processing, significantly enhances usability, making daily health monitoring for individuals more natural and unobtrusive. As the field of wearable platform technology continues to advance, soft skin-interfaced electronics are expected to play an essential role in implementing new applications, especially in personalized remote healthcare monitoring.
Research in the field of skin-interfaced healthcare platforms has made significant advancements, widening the range of target sensing signals, quantizing collected data, and optimizing platforms. For instance, Tan et al., 2023 present research on measuring arterial and venous pulse at the wrist simultaneously, highlighting the importance of venous blood oxygen saturation monitoring. This establishes a foundation for noninvasive arterial and venous blood oxygen saturation measurement, and such signal processing techniques enhance the effectiveness of skin-interfaced electronics by allowing for enhanced accuracy and diverse biometric acquisitions (Tan et al., 2023). Jang et al. introduce a soft electronic system equipped with a 3D helical network structure for health monitoring that bypasses engineering constraints and performance limitations set by traditional 2D designs. The 3D interconnect buckling provides unique mechanical properties and enables physiological health monitoring wirelessly and without the need for batteries. This emphasizes the potential for advancements in medical applications as a methodological approach in the research of soft electronics substrates (Jang et al., 2017). Furthermore, Kwon et al., 2023 discuss the integration of flexible materials with conventional FPCB circuits, implementing soft packaging and miniaturization. This study explores methods of classifying signals obtained from portable sensors utilizing machine learning techniques, highlighting that these approaches can assist in assessments by portable monitoring, moving beyond traditional tethered clinical monitoring (Kwon et al., 2023).
Conclusion
Recent developments have primarily focused on miniaturization and packaging. As mentioned earlier, implementing soft packaging with multiple sensors enhances flexibility, comfort, and accuracy. Furthermore, advancements in AI models enable classification or discrimination of health signals obtained from personal skin-interfaced devices to extract more meaningful data. In this context, advanced AI models may yield valuable signals using reduced hardware resources. For example, blood pressure estimation using electrocardiogram (ECG) and seismocardiogram (SCG) can be explored. In particular, the study described in Figure 1 trained an AI model using a bidirectional Long short-term memory (LSTM) neural network to estimate blood pressure from ECG and SCG inputs. This approach allows for the prediction of ECG based on the collected SCG input. By inputting SCG into the trained model, the predicted ECG can be obtained. Subsequently, using the predicted ECG and measured SCG, it becomes possible to estimate blood pressure. Thus, using only the SCG sensor, it is possible to estimate ECG through an AI model, followed by blood pressure estimation based on the ECG and SCG signals relationship. This exemplified approach suggests a direction for development that leverages the aforementioned advantages of skin-interfaced devices and machine learning algorithms to enable more powerful quantitative assessments for public health applications.
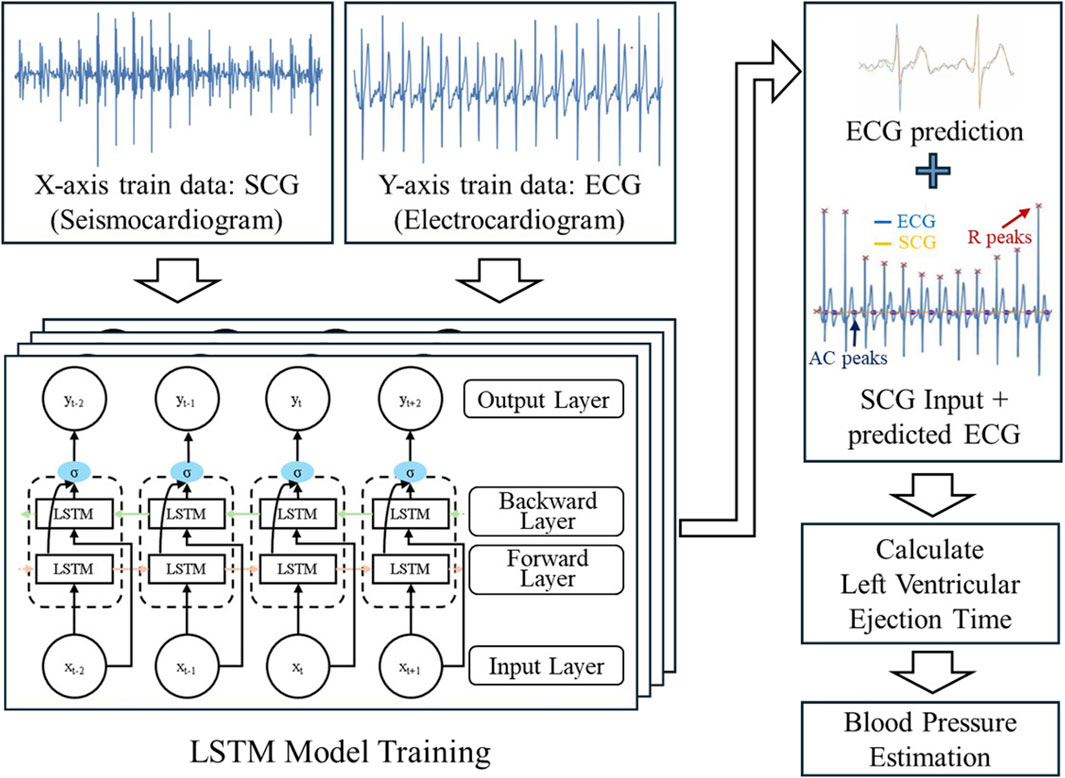
Figure 1. Illustration of the process for training a model utilizing a bidirectional Long short-term memory (LSTM) neural network to estimate blood pressure from electrocardiogram (ECG) and seismocardiogram (SCG) signals. The diagram at the top visualizes the network structure, including the input, bidirectional LSTM layers, and output layer. The results of the blood pressure estimation are displayed in the table on the bottom right.
Author contributions
SH: Conceptualization, Visualization, Writing–review and editing. CK: Visualization, Writing–review and editing. TK: Investigation, Writing–review and editing. HJ: Conceptualization, Writing–original draft, Writing–review and editing. SL: Conceptualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP; Ministry of Science, ICT and Future Planning, No. NRF-2021R1F1A1049758) and partially funded by the BK21 FOUR program of the National Research Foundation of Korea. HJ was supported by the National Institute on Aging of the National Institutes of Health under Award Number P30AG072972. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Jang, K. I., Li, K., Chung, H. U., Xu, S., Jung, H. N., Yang, Y., et al. (2017). Self-assembled three dimensional network designs for soft electronics. Nat. Commun. 8 (1), 15894–15910. doi:10.1038/ncomms15894
Kwon, S., Kim, H. S., Kwon, K., Kim, H., Kim, Y. S., Lee, S. H., et al. (2023). At-home wireless sleep monitoring patches for the clinical assessment of sleep quality and sleep apnea. Sci. Adv. 9 (21), eadg9671. doi:10.1126/sciadv.adg9671
Tan, P., Wang, E., Tamma, S., Bhattacharya, S., and Lu, N. (2023). “Towards simultaneous noninvasive arterial and venous oxygenation monitoring with wearable E-tattoo,” in 2023 45th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Sydney, Australia, 24-27 July 2023. doi:10.1109/EMBC40787.2023.10340010
Keywords: skin-interfaced platforms, health monitoring, Bi-LSTM, blood pressure estimation, personalized healthcare
Citation: Han S, Kim C, Kim T, Jeong H and Lee S (2024) Editorial: Skin-interfaced platforms for quantitative assessment in public health. Front. Bioeng. Biotechnol. 12:1406483. doi: 10.3389/fbioe.2024.1406483
Received: 25 March 2024; Accepted: 27 March 2024;
Published: 09 April 2024.
Edited and reviewed by:
Guozhen Liu, The Chinese University of Hong Kong, ChinaCopyright © 2024 Han, Kim, Kim, Jeong and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sangmin Lee, sangmlee@khu.ac.kr; Hyoyoung Jeong, ecejeong@ucdavis.edu
 Seungju Han1
Seungju Han1  Hyoyoung Jeong
Hyoyoung Jeong Sangmin Lee
Sangmin Lee