Site fidelity and migration patterns of the Southern Streaked Flycatcher breeding in urban and rural areas of Brazil
- 1Spatial Ecology and Conservation Lab (LEEC), Department of Biodiversity, Institute of Biosciences, São Paulo State University - UNESP, Rio Claro, São Paulo, Brazil
- 2Natural Resources Institute, Federal University of Itajubá, Itajubá, Minas Gerais, Brazil
- 3Environmental Resilience Institute, Indiana University, Bloomington, IN, United States
The Southern Streaked Flycatcher (Myiodynastes maculatus solitarius) is a common Neotropical austral migrant that breeds in both rural and urban environments of southeastern Brazil, providing a model to understand how songbirds are responding to an increasingly urban landscape in this region of the continent. We evaluated whether breeding site fidelity is related to sex and habitat type (urban versus rural). Because the annual cycle of migratory birds provides context to breeding season processes, we also described their migration timing and routes, and location of the non-breeding sites. Between 2016 and 2021, we captured, banded, and deployed GPS archival tags on Southern Streaked Flycatchers, and conducted systematic monitoring of 61 individuals in rural and urban areas in southeastern Brazil. Our results revealed that this population migrates from the Atlantic Forest to Amazonia, spending 15-40 days on fall migration. Moreover, we found i) that overall return rates to breeding sites are high (>52%), ii) that return rates did not vary with sex, and iii) that return rates varied with body condition and habitat, with birds in better condition having higher return rate in rural but not urban areas. More individual-level research on migratory birds in South American urban areas promises a novel perspective on how Neotropical austral migrants are responding to a rapidly changing landscape, and to inform future conservation initiatives in the increasingly expanding urban areas of southern Brazil.
1 Introduction
Urbanization converts natural habitats into a landscape dominated by artificial structures, resulting in declining bird richness and filtering species, depending on a variety of traits (Barbosa et al., 2020; Neate-Clegg et al., 2023). This may affect both resident and migratory species, with migratory birds tending to use a greater variety of habitats to meet their demands, changing the timing of migration or even changing their migration routes (La Sorte et al., 2014; Vitorio et al., 2019; Barbosa et al., 2021). In South America, several species may be under multiple pressures derived from the increasing urbanization that characterizes the region. However, research in this region has not explicitly evaluated how urbanization may influence the behavioral ecology of migratory birds throughout the year, such as habitat use and breeding-site fidelity.
About 220 species are part of the Neotropical austral migration system in South America, breeding at south temperate latitudes, such as Patagonia, and then migrating north towards the tropics, such as Amazonia, to spend the austral winter (Jahn et al., 2020). While New World flycatchers (Tyrannidae) are responsible for about one-third of Neotropical austral migrant species (Chesser and Levey, 1998), site fidelity in this family has only been poorly understood (McNeil, 1982; Rumboll et al., 2005; Brown et al., 2007; Jahn et al., 2009). In Brazil, the largest country in the continent, only a few studies mention site fidelity in these migrants (Jahn et al., 2009; Rozas Sia et al., 2020) and describe their migratory routes (Jahn et al., 2016; Bravo et al., 2017; Jahn et al., 2019).
The Streaked Flycatcher (Myiodynastes maculatus) is known to perform poorly understood migration movements in the Neotropical Austral system in part of its range (Fitzpatrick et al., 2004). It is widespread in evergreen and open second growth forest from southeastern Mexico to Argentina, and is one of only a few flycatchers that breed in tree cavities (Fitzpatrick et al., 2004). Among its seven subspecies, only the southernmost population, the Southern Streaked Flycatcher (Myiodynastes maculatus solitarius) is known to undergo migration (Fitzpatrick et al., 2004). This subspecies breeds in urban and rural areas of southern South America and overwinters in the northern portions of the continent (Kirwan et al., 2022), and in the breeding season it can be found in forests and in urban green habitats (Barbosa et al., 2021). Research on the ecology of this subspecies is increasing, especially in urbanized areas (Vitorio et al., 2019; Barbosa et al., 2021), but the extent of its non-breeding areas, migration routes and level of breeding-site fidelity between urban and rural are still unknown.
Breeding-site fidelity, in which individuals return to the same breeding location between years (Newton, 2008), is a widespread but complex behavior among migratory birds. It plays a central role in population demography, social behavior and mating success (Sedgwick, 2004; Brown et al., 2007), and often differs between sex and age, usually being more frequent in males and older individuals (Greenwood, 1980). The differences between sexes seem to be related to the differential roles in incubation, nest defense and parental care, with familiarity of the surroundings giving males an advantage in defending territories (Greenwood, 1980, Patton and Edwards, 1996). In contrast, females are more flexible in moving to new territories (Sedgwick, 2004). On the other hand, a major factor influencing site fidelity in females is previous breeding performance, with which it is positively correlated (Sedgwick, 2004). Younger birds may be less competitive for territories and more prone to dispersal, and not to return, in comparison to older individuals (Greenwood and Harvey, 1982; Serrano et al., 2001; Sedgwick, 2004). In general, the main benefit of breeding site fidelity appears to be an increased familiarity with a site and local resource availability (Brown et al., 2007). Therefore, if a breeding territory presents high-quality resources, it is presumably more advantageous for a bird to reclaim a familiar territory than having to find and defend a new territory (Greenwood, 1980; Bollinger and Gavin, 1989). Nevertheless, anthropogenically modified habitats can negatively influence site fidelity (Gibson et al., 2018), and migratory birds that breed in urban areas are often less site faithful as compared to those breeding in rural areas (Rodewald and Shustack, 2008). Moreover, return rates are positively associated with body condition (i.e., size-corrected mass, an indication of fuel reserves), since the latter can contribute to increased survival and successfully defending a territory (Warkentin and Hernández, 1996).
Our objective was to understand how migratory birds in southeastern Brazil are responding to an increasingly urban landscape. To do so, we compared breeding-site fidelity of the Southern Streaked Flycatcher in rural and urban areas of southern Brazil, and whether it is related to individual characteristics, such as sex and body condition. We expected a higher percentage of site fidelity in males than females, especially in rural areas comparing with urban areas, and for individuals in better body condition. We also provide a first description of its migration routes and location of non-breeding sites.
2 Methods
2.1 Study sites and species
We studied Southern Streaked Flycatchers (hereafter, “flycatchers”) in southeastern Brazil, which is characterized by the Atlantic Forest biome. Due to intensive habitat clearing for agriculture, pasture and urban expansion, the landscape is highly fragmented, with low natural vegetation coverage, where the forest remnants are small in size, isolated and subject to edge effects (Ribeiro et al., 2009). We worked across 6 rural and 10 urban sites within eight municipalities: São Paulo, Guarulhos, Guararema, Jundiaí, Cotia, Rio Claro, and Marília in the state of São Paulo, and Itajubá in southern Minas Gerais state (for more details about the study sites, see the spreadsheet with supporting information).
The state of São Paulo is the most industrialized and populated region of Brazil (IBGE, 2018). It is located in the transition between the Cerrado and Atlantic Forest domains, with roughly 16% of the original vegetation cover remaining, mostly restricted to fragments within a larger matrix of pastures, sugar cane, eucalyptus, orange and coffee plantations. São Paulo city is one of the most populous cities in the world, where more than 12 million people occupy an area of 1,521 km² (IBGE, 2018) and covers a vast area, merging with nearby cities (e.g., Guarulhos and Cotia). Even though urban structures dominate São Paulo’s land cover, the city contains numerous small parks and is bordered by two large blocks of forest, the Serra da Cantareira to the northwest and the Serra do Mar to the southwest. Moreover, land cover in São Paulo city is comprised of approximately 32% tree cover, 51% urban structures (e.g., buildings and impervious surface), with many migratory and resident birds that use the small green spaces (Barbosa et al., 2020). Guararema, Marília and Rio Claro, in São Paulo state and Itajubá in Minas Gerais state, are smaller urban areas with under 400,000 inhabitants. Their regions are characterized by a landscape dominated mostly by pastures, sugar cane and coffee plantations, with the remaining original vegetation restricted to small semideciduous Atlantic Forest fragments.
2.2 Bird captures and monitoring
We captured flycatchers during the breeding season (August to January) from 2016 to 2021 using nylon mist-nets (12 m and 18 m x 2.6 m, 36 mm and 38 mm mesh size). To increase the chances of capture, we placed the mist-net 8 m high and placed a decoy model of the species next to the net, along with a portable speaker emitting the species’ calls. Captured flycatchers were marked with numbered metal bands provided by CEMAVE (the Brazilian federal banding agency) and color bands for individual identification. Measurements of culmen, tarsus length, and body mass were taken to measure the body condition. Before release, we tagged a subset of 12 individuals with 1.2 g GPS archival tags (model PinPoint-10, Lotek Wireless, Inc.). The devices weighed less than 3% of the mass of the flycatchers on which they were deployed, and were deployed using a leg-loop harness made of Stretch Magic thread using methods described in Jahn et al. (2019) in Rio Claro, Marília and Itajubá. Individual flycatchers were sexed using molecular assays of blood samples (Unigen Labs; collection permit: SISBIO-53860), since Southern Streaked Flycatcher is not sexually dimorphic.
To quantify site fidelity, we searched during each season for previously marked individuals through playback experiments up to 500 m from the original point of capture, from sunrise (~6 am) to 10:00 am. We visited each study site at least twice per season, searching with the aid of binoculars and portable speakers. The perimeter for searching for birds were delimited based on authors previous experience with the species, that it was found just in the same place where it was banded. Moreover, we also used the citizen scientists to help to find the banded birds, announcing the research on local popular journal and in social medias, as in the region there is the highest number of birdwatchers in Brazil, especially in parks where birds were banded (Barbosa et al., 2020).
2.3 Analyses
We calculated the scaled mass index (SMI; hereafter, “body condition”) following Peig and Green (2009), in order to quantify body mass relative to body size, using linear regression of log-mass on log-length. For this, we first tested which measures (tarsus or culmen) had a higher correlation with body mass for each sex. We found that culmen for females and tarsus for males were the best fit. We used R language version 3.4.1 (R Core Team, 2017) to run χ2 tests to evaluate differences in return rates by sex and habitat, and a logistic regression with a binomial distribution to evaluate all variable combinations (habitat*SMI+sex*habitat+sex*SMI) and interactions between return rates with the single variables. We also calculated the confint of the models that shows the 95% of the confidence interval.
2.4 Tracking data
In order to describe migration routes and location of non-breeding sites, flycatchers with GPS tags were recaptured, the tags were removed, and the data were downloaded and processed. All data was processed in PinPoint Host program (Lotek Wireless, Inc.), and was saved in kml format.
3 Results
We banded 61 Southern Streaked Flycatchers (17 females, 26 males, and 18 of unknown sex) in six cities in São Paulo state and four (two males and two females) in one city in Minas Gerais state. Those of unknown sex were due to insufficient blood sample volume to test it. We deployed tags on twelve birds from 2018 to 2020 and ultimately obtained data from three tags (Figure 1).
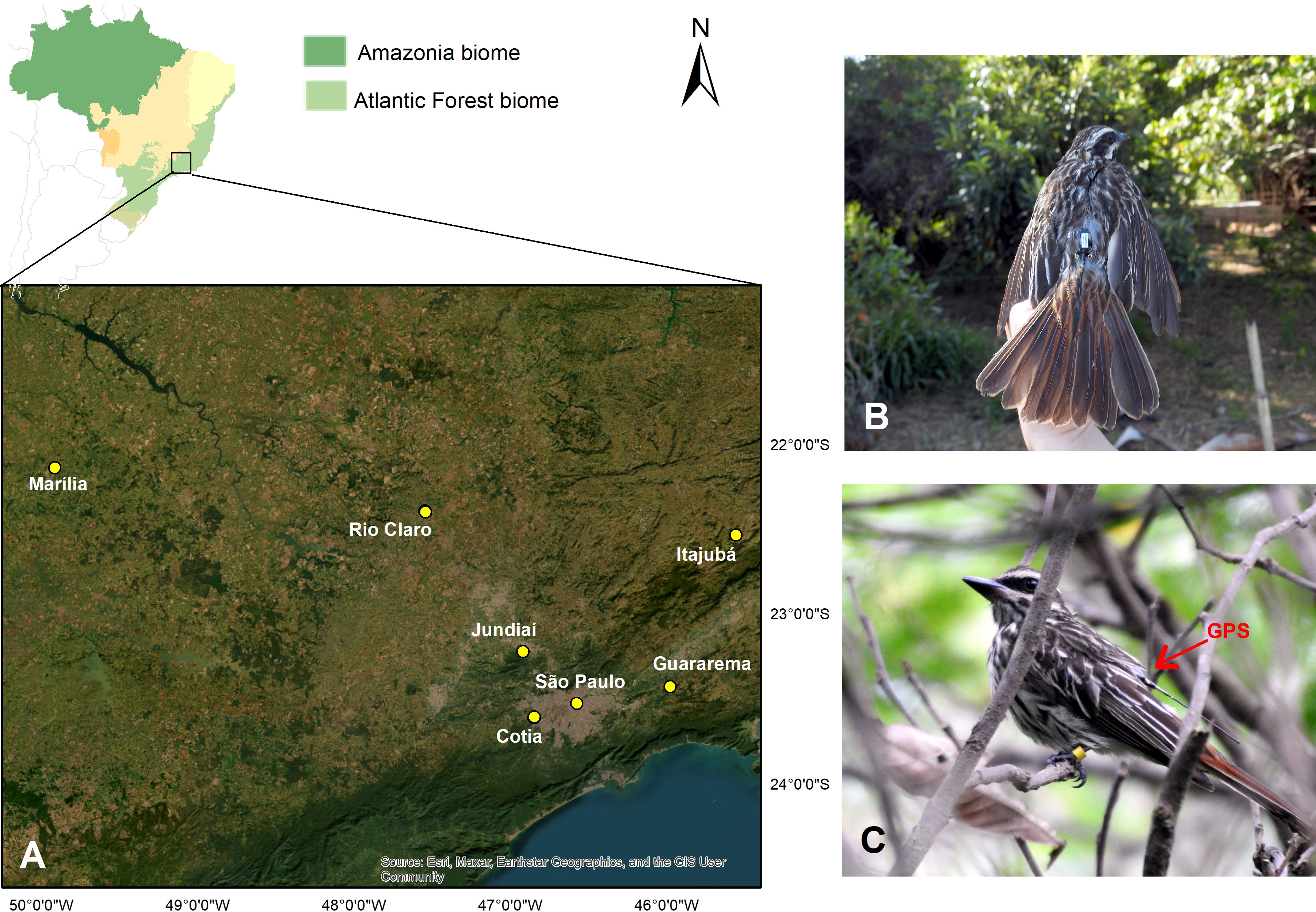
Figure 1 Banded Southern Streaked Flycatchers (A) Cities in the states of São Paulo (Jundiaí, Cotia, Guararema, Rio Claro, Marília and São Paulo) and Minas Gerais (Itajubá), Brazil, where 61 individuals were banded; (B) Southern Streaked Flycatcher with GPS; (C) The species with colored bands and GPS.
3.1 Breeding-site fidelity
Among 61 banded flycatchers, 32 (52%) returned in subsequent years to the breeding sites where they were first captured, of which eight were females (47%), 17 males (65%), and seven of unknown sex (39%), with no significant difference in return rate between the sexes (X2 = 173 3.27, df = 2, P = 0.19 - Figure 2). Among these, at least eight (including two females and one male) returned in two subsequent years after banding. One banded female was observed two years after banding at a distance of 1 km from the capture site; it is the only individual found to have dispersed. Her social mate returned to the original capture site during two years after capture and was seen accompanying other females. Citizen scientists located and reported to us ringed birds in Guararema and São Paulo.
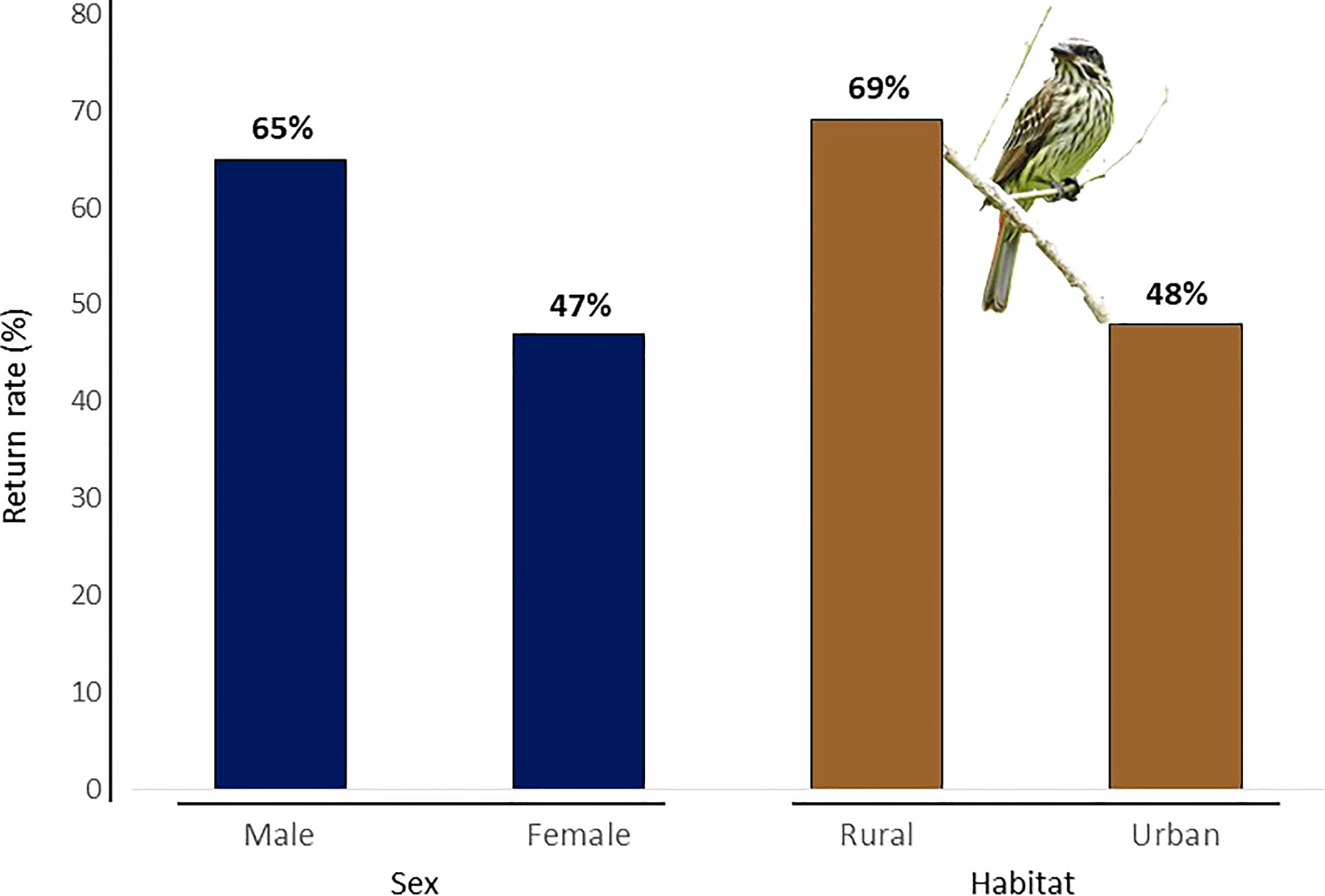
Figure 2 Differences in return rates (%) of Southern Streaked Flycatchers according to sex [males: 26 ringed, 17 returned (65%); females: 17 ringed, 8 returned (47%)] and habitat [rural: 13 ringed, 9 returned (69%); urban: 48 ringed, 23 returned (48%)].
We banded 48 individuals in 10 urban green spaces and 13 individuals in six rural areas, of which 23 returned to urban green spaces (48% return rate), and nine to rural areas (69% - Figure 2), which was not a significant difference in return rates between urban vs. rural areas (X2 = 1.11, df = 1, p-value = 0.29). The probability of returning to the breeding site was significantly related to body condition (t = 63.74, df = 53, P = << 0.0001), but not significantly different between the sexes (X2 = 104, df = 102, P = 0.42 – Figure 3).
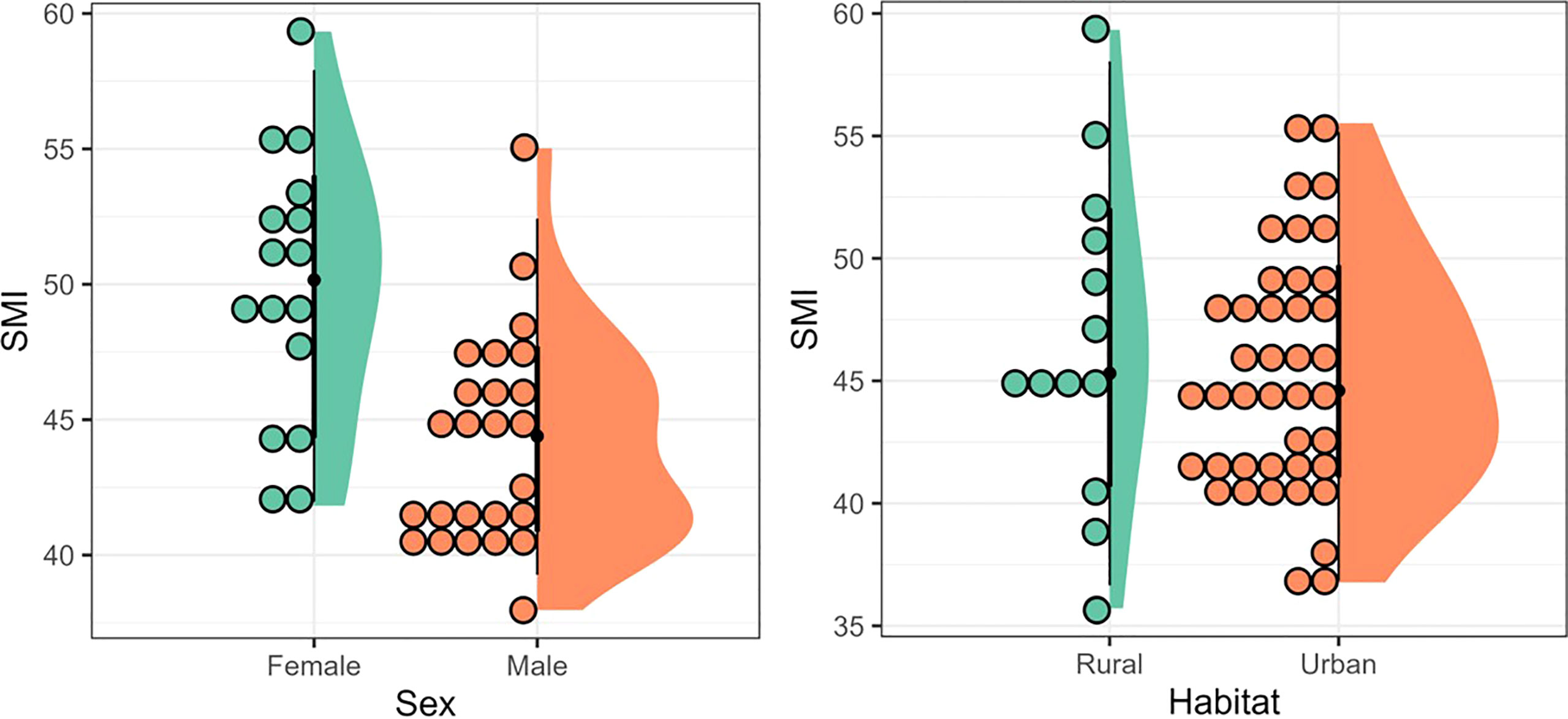
Figure 3 A boxplot showing the relation between body condition (SMI) and habitat (rural and urban) or sex, in return rates of 61 Southern Streaked Flycatchersringed in Brazil.
The Generalized Linear Model analyses with binomial distribution (returned~habitat*SMI+sex*habitat+sex*SMI) showed no effect of sex on return probability (sex*habitat F = 43.417, df= 35, P = 0.99 and sex*SMI F=40.866, df=35, P=0.204). On the other hand, the interaction between habitat and body condition (SMI) had a significant effect in return rates (F=12.974, P=0.0009) (CI 95% = -395.52, -28.41). In rural habitat, there is a positive effect in return rates and in urban habitat the effect was negative (Figure 4). To evaluate the combined effects of habitat and body condition on return rates, we also used a quasibinomial GLM (for more details, see the supporting information 2), which showed that flycatchers in better body condition have a higher return probability in the rural but not the urban habitat (rural 0.75, urban: 0.54).
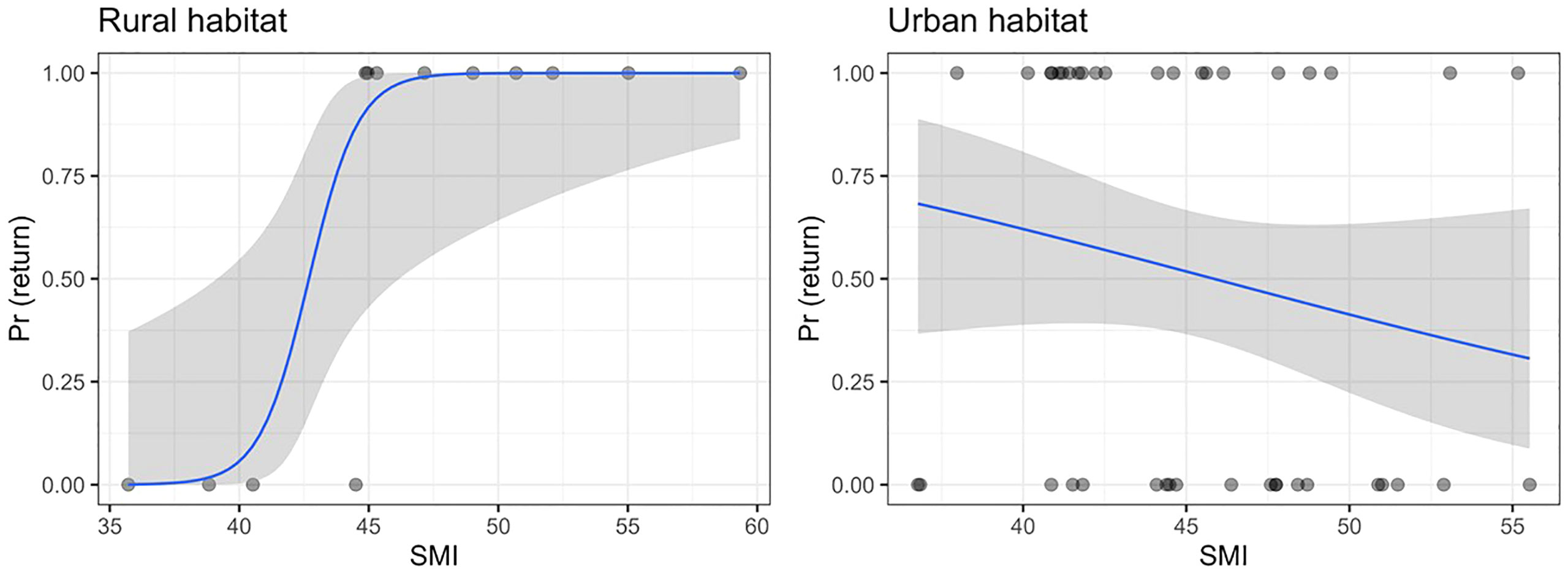
Figure 4 Logistic regression of the body condition (SMI) in relation to habitat (Rural and Urban) in the probability to return - Pr (return) - of 61 ringed Southern Streaked Flycatchers in Brazil.
3.2 Movement tracking data
We deployed GPS tags on 12 individuals (four in 2018, seven in 2019 and on one in 2020). From those tagged in 2018, we recaptured two in 2019, but did not obtain data from the tags (one in Rio Claro did not return with the tag and one in São Paulo city did not record data). In October of 2020, we recaptured three flycatchers that had been tagged in 2019, one of which did not return with the tag. We recovered data from the other two: one captured on the campus of UNESP University (Rio Claro) and the other in Carmo State Park (São Paulo city). In 2021, we recaptured four tagged flycatchers (one of which had been tagged in 2019), but one (from Rio Claro) did not return with the tag and another (from Itajubá) had technical problems and data was not recovered (Table 1).
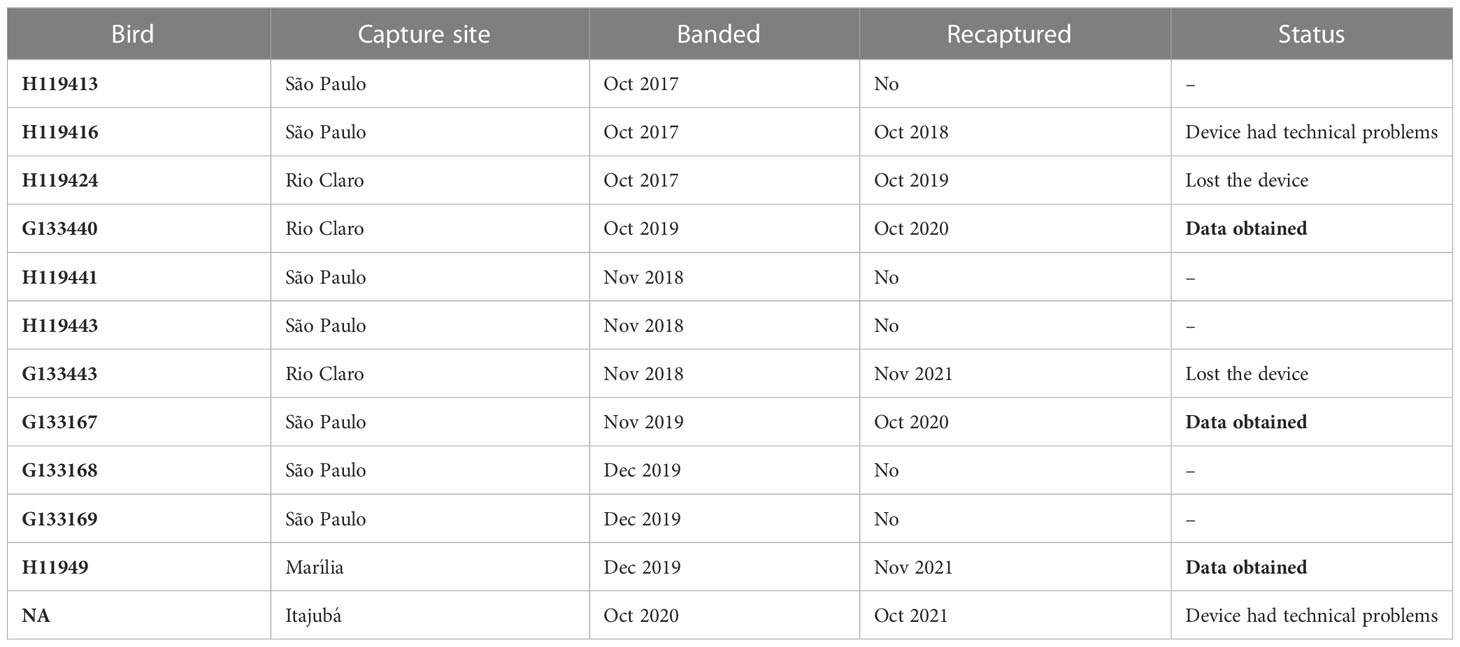
Table 1 Individual Southern Streaked Flycatchers (Myiodynastes maculatus solitarius) ringed and tracked with GPS tags in Brazil.
3.3 Migration routes and habitat size in breeding and non-breeding sites
We obtained data from three individuals of Southern Streaked Flycatcher, in three different cities: São Paulo, Rio Claro e Marília (Figure 5). Eight GPS points recorded by the tag of individual G133440 indicate that it was at the breeding site from January 15th to February 19th, and that its breeding area in the town of Rio Claro was 270 hectares in size (on the campus of university - UNESP - and adjacent protected area in Rio Claro). It initiated fall migration by February 19th and apparently used small green spaces to stopover at during fall migration. It arrived at Mulata National Forest, in the state of Pará, eastern Amazonia, by March 20th (a fall migration duration of ~20 days), after migrating ~2,600 km. It used a wintering area 6.3 hectares in size from March 20th to at least May 19th, when the tag stopped collecting data.
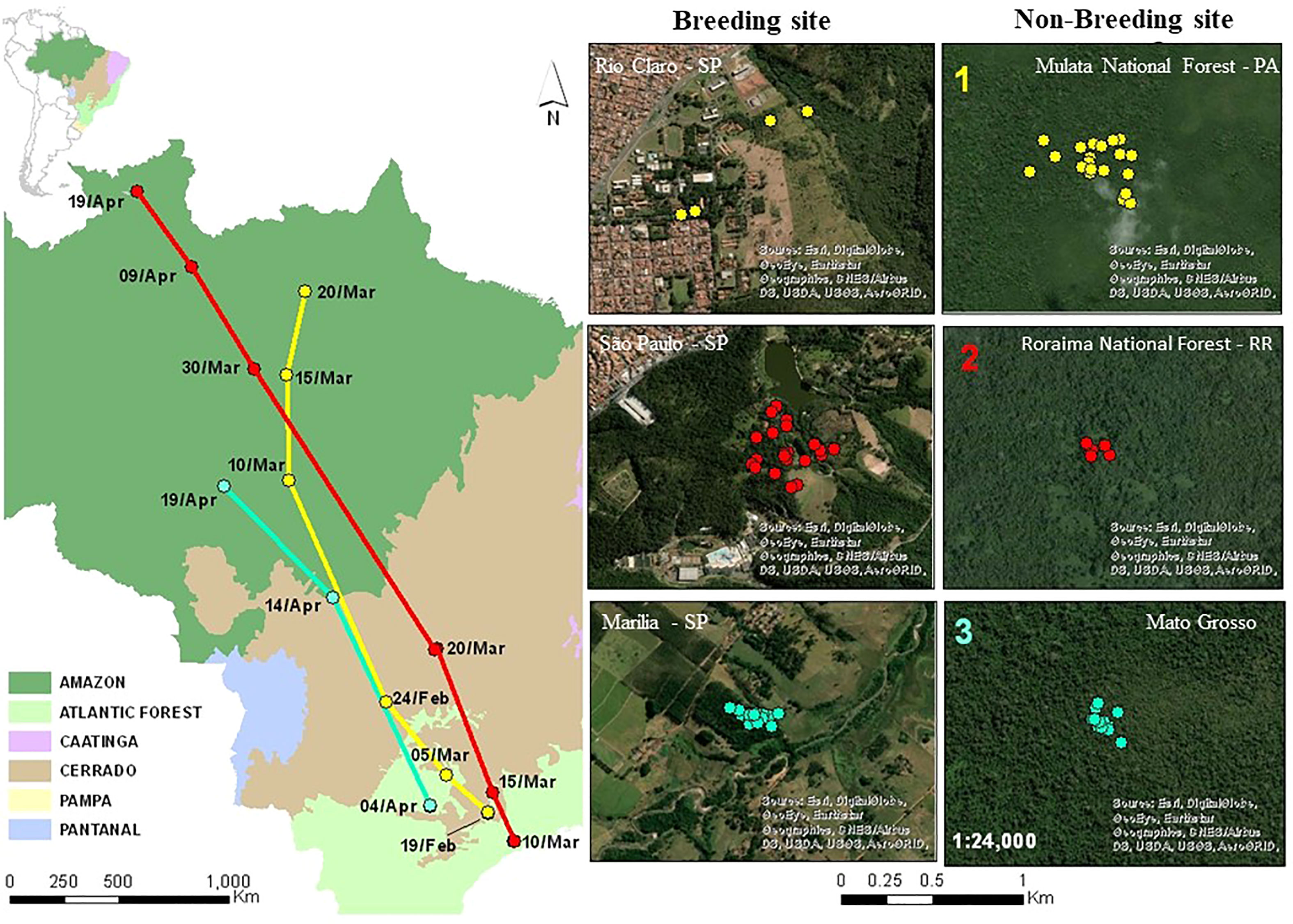
Figure 5 Migration routes of Southern Streaked Flycatcher (Myiodynastes maculatus solitarius) and breeding and non-breeding sites. Id of individuals: Route 1 - G133440, Route 2 - G133167 and Route 3 - H11949.
Flycatcher G133167 departed its breeding site in an urban park in São Paulo city by March 10th and arrived at Roraima National Forest (state of Roraima) in the northern Amazon Basin by April 19th (~40 days on fall migration). The traveled distance between breeding and wintering sites was ~3,400 km. According to 20 GPS points recorded from 6 December to 10 March, it used a breeding area 10.3 hectares in size (in the urban park in Carmo) and a wintering area 0.72 hectares in size, which it occupied from April 19th to at least May 19th, when the tag stopped collecting data.
Flycatcher H11949 departed on fall migration by April 4th from a rural area in Marília and arrived in the northern part of Mato Grosso State, in the southern Amazon Basin, by April 19th (~15 days of fall migration). The traveled distance between breeding and wintering sites was ~1,760 km. According to 31 GPS points recorded from January 15th to April 4th, it used an area 1.3 hectares in size (in a green rural area), and a wintering area 1.9 hectares in size. It used the latter from April 24th to at least June 23th, when the tag stopped collecting data.
4 Discussion
Southern Streaked Flycatcher represents a valuable model to understand the effects of urbanization on migratory behavior in the Neotropical austral migratory system, being common in both urban and rural areas, nesting in tree-cavities and frequently recorded by birdwatchers (Barbosa et al., 2021). Our results revealed that flycatchers in better condition have higher breeding site fidelity in rural but not urban areas, and are the first to describe the migration timing, routes and wintering areas of individual Streaked flycatchers in urban and natural areas.
Both wintering or breeding-site fidelity have been documented for only 10 Neotropical austral migrants (McNeil, 1982; Rumboll et al., 2005; Brown et al., 2007; Jahn et al., 2009), even though there are more than 220 Neotropical austral migrant species in South America (Chesser and Levey, 1998; Somenzari et al., 2018). Approximately 50% of the marked flycatchers in our study returned to the same breeding site between years, which is the first evidence of site fidelity among breeding Neotropical austral migrants in Brazil. These results also show higher return rates than those reported for migratory birds in other parts of the continent. In a study conducted in Argentina, the return rates for seven migrant species were <10% (Jahn et al., 2009). Other studies have also shown lower return rates, with 11.4% breeding-site fidelity for White-crested Elaenia (Elaenia albiceps, Brown et al., 2007), and 9.3% wintering site fidelity for Small-billed Elaenia (E. parvirostris, McNeil, 1982). The relatively high return rates we found may be due to the fact that few previous studies used color bands, whereas we intensively searched for color-banded individuals in subsequent seasons.
In north-temperate regions, breeding-site return rates of flycatcher species were higher, and in some cases, similar to those of our study. In North America, 52.0% of Willow Flycatcher (Empidonax traillii) males and 51.3% of females returned (Sedgwick, 2004), whereas in Spain, the return rates of European Pied Flycatchers (Ficedula hypoleuca) were 53% for males and 42% for females (Kern et al., 2014). Return rates of other families of Nearctic-Neotropical migrants vary between 30 and 40% (Wunderle and Latta, 2000).
For most migratory passerines, return rates are positively related to age (Gauthreaux, 1982), possibly due to the high mortality of juveniles, competition with adults, or more likely, high rates of juvenile dispersal (Greenwood, 1980). The fact that none of the three juvenile Southern Streaked Flycatchers we banded returned to their hatching site suggests a role for juvenile dispersal and/or mortality, which calls for further research on that within this system.
4.1 Male vs. female site fidelity
We found no significant difference in breeding-site fidelity between males and females, even though the return rates of males were numerically higher, a similar pattern as to what is found in other passerines (Bollinger and Gavin, 1989; Hoover, 2003; Sedgwick, 2004). The causes of sex-dependent differences in return rates are generally unknown, but are likely associated with the different roles of males and females during breeding. Males establish and defend territories, while females are often more flexible in their selection of breeding-sites (Greenwood, 1980), eventually leading to differential return rates. Nonetheless, females may return to the same nest site, usually following a previously successful breeding attempt, even when their previous social mate is currently paired with another female (Sedgwick, 2004). None of the five pairs of flycatchers we banded remained together in subsequent seasons.
4.2 Influence of habitat quality and body condition on return rate
Breeding-site fidelity is often related to habitat quality (Bollinger and Gavin, 1989; Warkentin and Hernández, 1996; Ortega et al., 2006). In urban landscapes, green areas can provide habitat for numerous resident and migratory bird species (Barbosa et al., 2020), and the Southern Streaked Flycatcher has been reported using small urban green areas (10 hectares), even in the megacity of São Paulo (Barbosa et al., 2021). Breeding-site fidelity is often related to habitat quality (Bollinger and Gavin, 1989; Warkentin and Hernández, 1996; Ortega et al., 2006). Urban green spaces are often embedded within an anthropogenically altered landscape that is less permeable as compared to more rural habitat (Shimazaki et al., 2016); nevertheless, our results show that return rates, albeit being numerically higher in the rural areas, are not affected by habitat type alone. Although we found a significative interaction between habitat type and body condition, with a lower return probability for individuals in lower body condition in rural areas; in urban sites, return rates were not as decisively affected by body condition. Thus, although urban areas are typically a low-quality habitat (Bollinger and Gavin, 1989), potentially with limited resources for foraging as compared to a rural sites, urban sites appear to provide sufficient resources for this species to successfully breed. Urban sites are also expected to fill up more quickly than rural sites, especially considering that Southern Streaked Flycatchers require cavities in which to nest and require 5.4 hectares of breeding area (Vitorio et al., 2019; Barbosa et al., 2021). Although the Southern Streaked Flycatcher can nest in a wide variety of cavities made by other species or people (Fitzpatrick et al., 2004), small urban parks usually have only a few old or dead trees that can provide nesting cavities, which could potentially decrease their breeding success (Cockle et al., 2017). These urban birds may therefore be more limited than rural birds in their ability to acquire potential breeding sites with natural cavities, negatively influencing return rates, especially if all potential territories are already occupied. Overall, migratory bird species that breed in urban areas have different constraints than those in rural areas. For example, nest predation levels are sometimes higher in urban sites (Rodewald and Shustack, 2008). If so, protected rural areas may provide better breeding conditions as compared to small urban parks, where domestic cats and other invasive predators are prevalent, which can influence breeding-site fidelity (Rodewald and Shustack, 2008).
Body condition is a key factor determining return rates of many migratory birds (Warkentin and Hernández, 1996), but often also depends on sex and habitat type. Individuals Southern Streaked Flycatchers in rural areas had higher body condition than those in urban areas, and there was no significant difference in body condition between sexes. In a North American study, females were in marginally better condition than males, but their condition was not related significantly to urbanization or sex (Rodewald and Shustack, 2008). In Costa Rica, return rates were positively related with mass and body condition in overwintering Northern Waterthrushes (Parkesia noveboracensis), a Nearctic Neotropical migratory songbird (Warkentin and Hernández, 1996), suggesting that individuals that are in better condition have higher interannual survival rates and a higher chance to return to maintain their winter territories. Nevertheless, species with high interannual site fidelity may be less able to adapt to habitat degradation and loss, and as the distance between patches of suitable habitat increases, numbers of such species tend to decline (Warkentin and Hernández, 1996). Better body condition in rural areas may be related to adequate food resources, and previous familiarity with a place may save time and energy (Switzer, 1993). Given that breeding-site fidelity of flycatchers in rural vs urban areas is not a straightforward process, further research into these processes is needed to gain insights into the ecology and population dynamics of migratory birds in areas undergoing rapid urbanization, such as southern Brazil.
4.3 Fall migration route
Although our sample size is small, the GPS tracking data we obtained showed for the first time that some Streaked Flycatchers overwinter >3,000 km distant from their breeding site in the Atlantic Forest biome, using humid forests of the Amazon Basin. Remarkably, these wintering habitats are drastically distinct, in terms of anthropogenic habitat disturbance, compared to their breeding sites in either rural or urban areas in southeastern Brazil. Fall migration can take up to 40 days, occurring mainly through central Brazil. The duration of fall migration is similar that of Fork-tailed Flycatchers (Tyrannus s. savana), another Neotropical austral migrant species that also breed in southeastern Brazil (Jahn et al., 2016).
The recovery rate of archival tags in Southern Streaked Flycatchers (58%) was higher than that of Fork-tailed Flycatcher. Although some tags had technical problems or the bird lost the GPS, the capture method we employed is promising for future research on Streaked Flycatchers.
5 Conclusions
Southern Streaked Flycatchers that breed in southeastern Brazil migrate as far as the Amazon Basin to overwinter, using habitats drastically different than those they breed in (i.e., small urban parks and secondary Atlantic Forest fragments versus primary, continuous Amazon Forest). They also exhibited relatively higher breeding-site fidelity than that of other previously studied Neotropical austral migrants, with return rates affected by the interaction between habitat type and body condition. We still know little about the basic movement patterns of most intra-tropical migratory birds, nor how bird migration within the Neotropical region is related to other life history strategies (Jahn et al., 2020). In urban landscapes, the anthropogenic effects on migratory birds are generally poorly understood, but appear to result in decreasing species richness, suggesting that the dynamic nature of urban areas poses a challenging scenario for those species. Further research on the movement ecology of migratory birds in South America is needed to understand how the conversion of natural landscapes to urban areas may affect the dynamics of these species in the long term, including breeding and migration timing. Studies focusing on these aspects in the Neotropics offer a broader perspective on how bird migration evolved generally, and the challenges that migratory birds face on a rapidly changing planet.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Comitê de Ética em Pesquisa - UNESP.
Author contributions
KB and AJ conceived and planned the experiments. KB and TC carried out the experiments. KB and MR planned and conducted the data analyses. KB, AJ and TC contributed to the interpretation of the results. KB took the lead in writing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
KB received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nı́vel Superior - Brasil (CAPES) - Finance Code 001. AJ received a fellowship from the Prepared for Environmental Change Grand Challenge Initiative at Indiana University. MR received financial support from FAPESP (processes #2013/50421-2; #2020/01779-5; #2021/08534-0; 2021/10195-0) and National Council for Scientific and Technological Development - CNPq (processes #442147/2020-1; #402765/2021-4; 313016/2021-6).
Acknowledgments
We are grateful to all the volunteers who helped with this project, especially those who helped capture and recapture birds, and to Diego Tuero and Maurício Vancine for assistance with analyses. We are also indebted to two anonymous reviewers for their helpful comments that greatly improved the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbirs.2023.1214432/full#supplementary-material
References
Barbosa K. V. C., Develey P. F., Ribeiro M. C., Jahn A. E. (2021). The contribution of citizen science to research on migratory and urban birds in Brazil. Ornithol Res. 29, 1–11. doi: 10.1007/s43388-020-00031-0
Barbosa K. V. C., Rodewald A. D., Ribeiro M. C., Jahn A. E. (2020). Noise level and water distance drive resident and migratory bird species richness within a Neotropical megacity. Landscape Urban Plann. 197, 103769. doi: 10.1016/j.landurbplan.2020.103769
Bollinger E. K., Gavin T. A. (1989). The effects of site quality on breeding-site fidelity in bobolinks. Auk 106, 584–594.
Bravo S. P., Cueto V. R., Gorosito C. A. (2017). Migratory timing, rate, routes and wintering areas of White-crested Elaenia (Elaenia albiceps chilensis), a key seed disperser for Patagonian Forest regeneration. PloS One 12 (2), e0170188. doi: 10.1371/journal.pone.0170188
Brown C. E., Anderson C. B., Ippi S., Sherriffs M. F., Charlin R., McGehee S., et al. (2007). The autecology of the Fio-Fio (Elaenia albiceps Lafresnaye & D´Orbigny) in subantarctic forests of the Cape Horn Biosphere Reserve, Chile. Anales Instituto Patagonia (Chile) 35 (2), 29–40.
Chesser R. T., Levey D. J. (1998). Austral migrants and the evolution of migration in new world birds: diet, habitat, and migration revisited. Am. Nat. 152 (2), 311–319. doi: 10.1086/286171
Cockle K. L., Martin K., Bodrati A. (2017). Persistence and loss of tree cavities used by birds in the subtropical Atlantic Forest. For. Ecol. Manage. 384, 200–207. doi: 10.1016/j.foreco.2016.10.052
Fitzpatrick J. W., Bates J. M., Bostwick K. S., Caballero I. C., Clock B. M., Farnsworth A., et al. (2004). Family tyrannidae (tyrant-flycatchers). Handb. birds World 9, 170–462.
Gauthreaux S. A. Jr. (1982). “The ecology and evolution of avian migration systems,” in Avian Biology, vol. 6 . Eds. Farner D. S., King J. R. (New York and London: Academic Press), 93–167.
Gibson D., Chaplin M. K., Hunt K. L., Friedrich M. J., Weithman C. E., Addison L. M. (2018). Impacts of anthropogenic disturbance on body condition, survival, and site fidelity of nonbreeding Piping Plovers. The Condor 120 (3), 566–580.
Greenwood P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28 (4), 1140–1162. doi: 10.1016/S0003-3472(80)80103-5
Greenwood P. J., Harvey P. H. (1982). The natal and breeding dispersal of birds. Annu. Rev. Ecol. Systematics 13, 1–21. doi: 10.1146/annurev.es.13.110182.000245
Hoover J. P. (2003). Decision rules for site fidelity in a migratory bird, the prothonotary wabler. Ecology 84 (2), 416–430. doi: 10.1890/0012-9658(2003)084[0416:DRFSFI]2.0.CO;2
IBGE – Instituto Brasileiro de Geografia e Estatística (2018) Brazilian government geographic and statistics – data from 2018. Available at: https://cidades.ibge.gov.br/brasil/sp/sao-paulo/.
Jahn A. E., Cereghetti J., Cueto V. R., Hallworth M. T., Levey D. J., Marini M.Â., et al. (2019). Breeding latitude predicts timing but not rate of spring migration in a widespread migratory bird in South America. Ecol. Evol. 9 (10), 5752–5765. doi: 10.1002/ece3.5159
Jahn A. E., Cueto V. R., Fontana C. S., Guaraldo A. C., Levey D. J., Marra P. P., et al. (2020). Bird migration within the Neotropics. Auk 137 (4), ukaa033. doi: 10.1093/auk/ukaa033
Jahn A. E., Cueto V. R., Sagario M. C., Mamani A. M., Vidoz J. Q., Casenave J. L., et al. (2009). Breeding and winter site fidelity among eleven Neotropical austral migrant bird species. Ornitologia Neotropical 20, 275–283.
Jahn A. E., Seavy N. E., Bejarano V., Guzmán M. B., Provinciato I. C., Pizo M. A., et al. (2016). Intra-tropical migration and wintering areas of Fork-tailed Flycatchers (Tyrannus savana) breeding in São Paulo, Brazil. Rev. Bras. Ornitologia 24 (2).
Kern M., Slater F., Cowie R. (2014). Return rates and dispersal distances of Welsh Pied Flycatchers Ficedula hypoleuca and factors that influence them. Ringing Migration 29 (1), 1–9. doi: 10.1080/03078698.2014.932617
Kirwan G. M., Shah S. S., Barbosa K. V. C. (2022). “Streaked Flycatcher (Myiodynastes maculatus), version 2.0,” in Birds of the World. Eds. Schulenberg T. S., Keeney B. K. (Ithaca, NY, USA: Cornell Lab of Ornithology). doi: 10.2173/bow.strfly1.02
La Sorte F. A., Tingley M. W., Hurlbert A. H. (2014). The role of urban and agricultural areas during avian migration: an assessment of within-year temporal turnover. Glob. Ecol. Biogeogr. 23, 1225–1234. doi: 10.1111/geb.12199
McNeil R. (1982). Winter resident repeats and returns of austral and boreal migrant birds banded in Venezuela. J. Field Ornithol. 53 (2), 125–132.
Neate-Clegg M. H. C., Tonelli B. A., Youngflesh C., Wu J. X., Montgomery G. A., Sexekercioglu Ç.H., et al. (2023). Traits shaping urban tolerance in birds differ around the world. Curr. Biol. 33 (9), 1677–1688.e6. doi: 10.1016/j.cub.2023.03.024
Ortega Y. K., McKelvey K. S., Six D. L. (2006). Invasion of an exotic forb impacts reproductive success and site fidelity of a migratory songbird. Oecologia 149, 340–351. doi: 10.1007/s00442-006-0438-8
Patton P. W. C., Edwards T. C. Jr. (1996). Factors affecting interannual movements of Snowy Plovers. Auk 113, 534–543. doi: 10.2307/4088973
Peig J., Green A. (2009). New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883—1891. doi: 10.1111/j.1600-0706.2009.17643.x
R Core Team (2017). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Ribeiro M. C., Metzger J. P., Martensen A. C., Ponzoni F. J., Hirota M. M. (2009). The Brazilian Atlantic Forest: How much is left and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 142 (6), 1141–1153. doi: 10.1016/j.biocon.2009.02.021
Rodewald A. D., Shustack D. P. (2008). Consumer resource-matching in urbanizing landscapes: Are synanthropic species over-matching? Ecology 89, 515–521. doi: 10.1890/07-0358.1
Rozas Sia M. G., Gonzales E., Segura L. N. (2020). High levels of natal philopatry and no difference in reproductive success between philopatric and non-philopatric songbirds breeding in native forests of east-central Argentina. Stud. Neotropical Fauna Environ. 57, 165–171. doi: 10.1080/01650521.2020.1829888
Rumboll M., Capllonch P., Lobo R., Punta. G. (2005). Sobre el anillado en la Argentina: recuperaciones y recapturas. Nuestras Aves 50, 21–24.
Sedgwick J. A. (2004). Site fidelity, territory fidelity, and natal philopatry in Willow Flycatchers (Empidonax traillii). Auk 121 (4), 1103–1121. doi: 10.1642/0004-8038(2004)121[1103:SFTFAN]2.0.CO;2
Serrano D. L., Tella J. T., Forero M. G., Donázar J. A. (2001). Factors affecting breeding dispersal in the facultatively colonial Lesser Kestrel: Individual experience vs. conspecific cues. J. Anim. Ecol. 70, 568–578. doi: 10.1046/j.1365-2656.2001.00512.x
Shimazaki A., Yamaura Y., Senzaki M., Yabuhara Y., Akasaka T., Nakamura R. (2016). Urban permeability for birds: An approach combining mobbing-callexperiments and circuit theory. Urban Forestry Urban Greening 19, 167–175. doi: 10.1016/j.ufug.2016.06.024
Somenzari M., Amaral P. P., Cueto V. R., Guaraldo A. C., Jahn A. E., Lima D. M., et al. (2018). A review of Brazilian migratory birds. Pap. Avulsos Zool 58, 1–66. doi: 10.11606/1807-0205/2018.58.03
Switzer P. V. (1993). Site fidelity in predictable and unpredictable habitats. Evolutionary Ecol. 7 (6), 533–555. doi: 10.1007/BF01237820
Vitorio J. G., Frenedozo R. C., Barbosa K. V. C. (2019). Habitat use and home range of a migratory bird, Myiodynastes maculatus solitarius, in an urban park in the Atlantic Forest, Brazil. Braz. J. Ornithol 27 (2), 115–121. doi: 10.1007/BF03544455
Warkentin I. G., Hernández D. (1996). The conservation implications of site fidelity: a case study involving Nearctic-Neotropical migrant songbirds wintering in a Costa Rican mangrove. Biol. Conserv. 77, 143–150. doi: 10.1016/0006-3207(95)00146-8
Keywords: urbanization, Neotropical, migratory bird, flycatcher, GPS, body condition, Atlantic Forest, Amazonia
Citation: Barbosa KVC, Costa TVV, Ribeiro MC and Jahn AE (2023) Site fidelity and migration patterns of the Southern Streaked Flycatcher breeding in urban and rural areas of Brazil. Front. Bird Sci. 2:1214432. doi: 10.3389/fbirs.2023.1214432
Received: 29 April 2023; Accepted: 14 July 2023;
Published: 01 August 2023.
Edited by:
Benjamin Zuckerberg, University of Wisconsin-Madison, United StatesReviewed by:
Luciano Segura, National University of La Plata, ArgentinaDavid John Green, Simon Fraser University, Canada
Copyright © 2023 Barbosa, Costa, Ribeiro and Jahn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karlla Vanessa de Camargo Barbosa, barbosa.karlla@gmail.com; Alex Edward Jahn, alexjahn77@yahoo.com
 Karlla Vanessa Camargo Barbosa
Karlla Vanessa Camargo Barbosa Thiago Vernaschi Vieira Costa2
Thiago Vernaschi Vieira Costa2