Thy-1 in Integrin Mediated Mechanotransduction
- Department of Biomedical Engineering, University of Virginia, Charlottesville, VA, United States
The glycosylphosphatidylinositol (GPI) anchored glycoprotein Thy-1 has been prevalently expressed on the surface of various cell types. The biological function of Thy-1 ranges from T cell activation, cell adhesion, neurite growth, differentiation, metastasis and fibrogenesis and has been extensively reviewed elsewhere. However, current discoveries implicate Thy-1 also functions as a key mechanotransduction mediator. In this review, we will be focusing on the role of Thy-1 in translating extracellular mechanic cues into intracellular biological cascades. The mechanotransduction capability of Thy-1 relies on trans and cis interaction between Thy-1 and RGD-binding integrins; and will be discussed in depth in the review.
As the smallest member of the immunoglobulin superfamily, Thy-1 (CD90) is a 25–37 kDa glycosyl phosphatidylinositol (GPI) anchored cell membrane protein that bears critical biological functions. The glycoprotein is expressed across many different cell types including fibroblasts, endothelial cells, neuron and hematopoietic cells (Craig et al., 1993; Rege and Hagood, 2006b). Since its discovery decades ago, extensive scrutiny on the glycoprotein has established Thy-1 as an important player in almost every aspect in cellular biology including adhesion, migration, apoptosis, wound healing, tumorigenesis and fibrogenesis (Barker et al., 2004; Sanders et al., 2007, 2008; Barker and Hagood, 2009; Lee et al., 2013). More recently, studies have connected Thy-1 with mechanotransduction, specifically through its interaction with integrins. This mini review will focus on the role of Thy-1 in integrin mediated mechanotransduction, with a broader scope on Thy-1 driven physiological responses via mediating conversion of extracellular biophysical cues into intracellular biochemical signals.
Thy-1-Integrin Interaction, cis and trans
Integrins are a group of adhesion receptors connecting the extracellular matrix (ECM) with the cell cytoskeleton through their bulky, dimeric head domain, a type I transmembrane domain and a relatively small cytoplasmic domain (Luo et al., 2007). Integrins have long been regarded as critical mechanotransducers since the direct engagement between integrins and their ECM ligands is the prerequisite of formation of focal adhesions and cellular contractility. The evidence of Thy-1-integrin interaction began to emerge in the past decade. Potential interactions between Thy-1 and integrin αvβ5 has been proposed as the mechanism of Thy-1 mediated signaling that blocks activation of TGF-β (Herrera-Molina et al., 2013). Similarly, Thy-1 positive lung fibroblasts are resistant to TGF-β activation induced lung fibrosis, implicating possible role of Thy-1 in suppressing αvβ6 mediated TGF-β activation (Zhou et al., 2004). Direct interactions have been shown between Thy-1 and integrin αvβ3 on astrocytes (Leyton et al., 2001). The interaction is mediated through the RLD motif on the recombinant Thy-1-FC molecule and the engagement between Thy-1 and αvβ3 can promote focal adhesion formation as well as FAK phosphorylation. Another study later discovered that this trans interaction between Thy-1 and integrin αvβ3 induces Thy-1 microclustering and colocalization with Csk-binding protein (CBP) while displacing Src kinase from these clusters at the same time (Maldonado et al., 2017). Melanoma cells have also been seen to exploit Thy-1 expressed by vascular endothelial cells for adhesion and subsequent tumor metastasis, presumably through Thy-1- αvβ3 interaction (Schubert et al., 2013). αvβ3 is not the only integrin that has shown capability to interact with Thy-1. Thy-1-α5β1 and syndecan4 can form triplex and behave as a catch bond (Fiore et al., 2014).
While trans interactions between Thy-1 and integrin apparently mediates mechanotransduction, little is known regarding the impact of cis interaction until lately. In a study published by Fiore and his colleagues, Thy-1 is found to interact with integrin αvβ3 in cis on the surface of lung fibroblasts (Fiore et al., 2015). The interaction helps to keep the integrin in a low affinity, bent conformation. Moreover, the interaction facilitates Fyn, a member of SFK critical in mechanosignaling, recruitment to focal adhesions while also keeps c-Src activity under check through recruitment of CBP.
Trans Interaction Between Thy-1 and Integrin αvβ3 Mediates Mechanotransduction
Thy-1 has been shown to support cell adhesion through trans interaction with integrin. Immobilized Thy-1 is capable to function as ligand for integrin αvβ3 and support cell adhesion in a Mn2+ dependent manner. On the cell membrane, interactions between αvβ3 on DITNC1 astrocytes and Thy-1 on neuron cells support cell adhesion but inhibit neuron cell differentiation and neurite extension (Herrera-Molina et al., 2012). Immobilized recombinant αvβ3-FC functions similarly and induces clustering of Thy-1 on neuron cell surface. It has been proposed that such a trans interaction triggered redistribution/clustering of Thy-1 leads to inactivation of Src through Thy-1 mediated CBP recruitment. Thy-1 mediated cell-cell interaction has also been found to be critical for melanoma cell adhesion and metastasis. Thy-1 deficient mice showed significantly reduced metastasis sites due to ablation of Thy-1 mediated melanoma cell adhesion on Endothelial cells (Schubert et al., 2013). When mediating cell-cell adhesion, Thy-1 not only needs to interact with integrin αvβ3, but also need to bring in Syndecan4, a lipid raft protein that binds to a heparin-binding domain on Thy-1. The interaction between Thy-1 and Syndecan4 itself is not sufficient to induce Rac-1 RhoGTPase activation; however, the binding is required for the Thy-1- αvβ3 interaction to support cell adhesion and migration (Kong et al., 2013). It’s worth noting that the Thy-1- αvβ3 interaction alone indeed triggers phosphorylation of Akt, indicating that cell-cell trans interaction through Thy-1 and integrin could promote cell viability/survival but is not sufficient to generate mechano-signal transduction. Interestingly, while surface Thy-1 clustering induced by integrin αvβ3 generates inhibitory signal in neuron cells, Thy-1 crosslinking by mAb induces Ca2+ influx and proliferation in T lymphocytes (Kroczek et al., 1986; Conrad et al., 2009). The seemingly paradoxical evidence implicates highly context dependent nature of Thy-1 function.
Thy-1-FC conjugated beads are sufficient to induce enhanced formation of focal adhesions and elevated tyrosine phosphorylation of p130cas and FAK (Leyton et al., 2001). A Thy-1-CBP-RhoA-ROCK axis has been proposed to induce astrocyte retraction and RhoA dependent actin stress fiber formation (Avalos et al., 2004; Maldonado et al., 2017). The phenomenon is induced via Thy-1-FC conjugated protein A beads, implicating that clustered Thy-1 is likely required to mediate the trans-interaction based mechanotransduction through integrin αvβ3. Unlike other traditional integrin ligands, Thy-1 is a monovalent molecule and its RLD motif likely binds with integrin at lower affinity. Therefore, clustering of Thy-1 and presence of potential binding partners in addition to integrin (e.g., syndecan4) could be essential for Thy-1 mediated cell-cell interaction and mechanotransduction. This is particularly plausible considering that immobilized Thy-1-FC can't support cell adhesion without the presence of Mn2+ whereas conjugated (and thus “clustered”) Thy-1-FC beads successfully induced focal adhesion assembly in a RhoA-ROCK dependent pathway (Leyton et al., 2001; Avalos et al., 2004). Interaction with ECM ligands induces integrin clustering which is the key event in cell adhesion and migration. It is known that integrin clustering is dependent on PI(4,5,)P2 and Talin (Cluzel et al., 2005; Saltel et al., 2009) while syndecan4 helps retention of PI(4,5,)P2 in cell membrane (Kwon et al., 2009). Therefore, Thy-1, integrin αvβ3 and syndecan4 work synergistically to mediate mechanotransduction through cell-cell interaction. Further downstream, this trimolecular complex also regulates RhoA GTPase mainly through modulating p190GAP phosphorylation and distribution. Syndecan4 and integrin α5β1 have been shown to regulate p190GAP membrane distribution and Src-dependent tyrosine phosphorylation, respectively (Bass et al., 2008). The coordinated interaction subsequently leads to suppressed RhoA activity and cell migration. However, introduction of Thy-1 causes a reduction of Src activity and downregulation of p190GAP, which leads to higher RhoA activity, stable adhesion and enhanced stress fiber formation (Barker et al., 2004). The phenomenon can be attributed, in part, to the recruitment of CBP by Thy-1 to integrin membrane proximity, which leads to inhibitory phosphorylation of Src kinase by CBP interacting Csk. More interestingly, the Thy-1- α5β1-Syndecan4 trimolecular complex not only delivers mechano-related biochemical signaling coordinately but also physically interprets force directly (Fiore et al., 2014). When Thy-1 binds to either α5β1 integrin or Syndecan4 alone, both interactions behave as classic slip bond, meaning that the lifetime of the interactions decreases with force application. However, the trimolecular bond expresses a unique catch bond feature–described as “dynamic catch” by the authors. The mechanism behind the phenomenal has been proposed as a sudden bond stiffening from an acquired contribution of the syndecan4-Thy-1 interaction, once the force load reaches a ~15 pN threshold. Before reaching the threshold, α5β1-Thy-1 interaction bears the majority of the force whereas after the threshold, due to force-induced extension of the GAG motif on Syndecan4, both α5β1 and Syndecan4 start to resist force at full load. Taken together, the Thy-1-α5β1-Syndecan4 complex mediates mechanotransduction both at the single molecule biophysical level and at the cell biochemical level.
Mechanotransduction in cis
In contrast to only the induction of focal adhesions and promotion of FAK activation seen in trans, cis interaction between Thy-1 and integrin is more complicated, providing both a tonic inhibition, but also facilitating efficient mechanosignaling in the focal adhesion (Fiore et al., 2015). The Thy-1- αvβ3 interaction shifts the dynamic equilibrium of integrin conformation toward a bent-closed state. This effectively reduces integrin avidity for its extracellular ligand; Thy-1 is a weak inhibitor of integrin in cis. Remarkably, the Thy-1-αvβ3 interaction physically couples unbound integrin to lipid raft microdomains containing critical signaling molecules. Thus, Thy-1 facilitates co-clustering of lipid raft proteins with focal adhesions enabling proper mechanosensing in fibroblasts. Integrin mediated mechanotransduction relies on ECM ligand engagement and subsequent integrin clustering, which leads to self-activation of FAK and Src, resulting in downstream RhoA activation and cellular contractility (Hu and Luo, 2013). More specifically, by keeping integrin in the bend-low affinity conformation, Thy-1 not only constraints the ligand accessibility for integrin but also limits the likelihood of ECM ligand binding independent self-clustering and thus reduces the overall integrin avidity. Thy-1 keeps c-Src activity in check through recruitment of the lipid raft protein CBP, which leads to recruitment of Src inhibitor Csk; concurrently the lipid raft-associated Src-family member Fyn is brought to the focal adhesion enabling a prompt mechanosignaling response after ligand engagement (Fiore et al., 2015). It is worth noting that this Thy-1-mediated mechanosensing requires proper lipid raft location, as replacing the GPI anchor with a CD8 transmembrane domain greatly reduced the ability of cells to appropriately respond to environmental rigidity. Lipid rafts have been widely regarded as a critical participant in mechanotransduction (Head et al., 2014). Colocalization of Fyn, CBP and another Thy-1 interacting protein Reggie1/2 on non-caveolar lipid raft has been reported previously (Stuermer et al., 2001; Deininger et al., 2003). Moreover, Fyn has been shown to be able to both interact with FAK in early integrin mediated adhesion and phosphorylate CBP, resulting in subsequent recruitment/activation of Csk (Yasuda et al., 2002; Maksumova et al., 2005; Baillat et al., 2008). Adding the evidence together, Thy-1 likely functions as a lipid raft coupler, recruiting Fyn and CBP to the focal adhesion to regulate basal Src activity through Csk. In addition to promote RhoA activity through downregulating c-Src dependent p190GAP activity, Fyn has also shown to directly phosphorylate and activate Rho guanine nucleotide exchange factor (GEF) in response to integrin mediated force transduction, resulting in a more direct activation of RhoA (Guilluy et al., 2011). Importantly, the activity of Rho GTPase is required for ECM stiffness induced nucleus translocation of Yap/Taz, which drives mechano-activation of fibroblast and fibrosis (Dupont et al., 2011; Liu et al., 2015). Taking together, the direct and indirect regulatory role of Fyn over RhoA activity makes it a core modulator of force-induced cellular response.
The third way Thy-1 appears to impact mechanotransduction is through regulating the TGF-β pathway. TGF-β-SMAD2/3/4 is well established as the main signaling route to induce mechano-related cellular responses including proliferation, cellular contraction and ECM deposition. The signaling axis is also the main driving force in fibrosis. It has been reported that Thy-1 null c57BL/6 mice were more prone to develop severe lung fibrosis after bleomycin treatment (Hagood et al., 2005). Thy-1 negative fibroblasts are more responsive toward inflammatory cytokines like TGF-β whereas Thy-1 positive cells are resistant to similar treatments. The difference does not appear to be due to downstream signal transduction of TGF-β but instead to higher latent TGF-β activation in Thy-1 negative cells (Zhou et al., 2004), potentially through Thy-1 stabilization of integrin's bent conformation as described above. Likewise, induction of MMP9 by TGF-β has been observed in Thy-1 negative fibroblasts but not in Thy-1 positive fibroblasts, implicating Thy-1 as an important suppressor in MMP9 induced latent TGF-β activation– the positive feedback loop that efficiently enhances TGF-β signaling (Ramirez et al., 2011). The interaction between Thy-1 and integrin αvβ5 has been proposed as a mechanism to constrain latent TGF-β activation by the integrin (Zhou et al., 2010). The study, however, failed to reveal if the inhibition is caused by cis interaction between the two molecules or trans. It is conceivable that by keeping TGF-β activating integrins (αvβ5 and αvβ6) in a low affinity conformation, Thy-1 can reduce activation of endogenous TGF-β, enabling a cellular “brake” to TGF-β.
Conclusion
Thy-1 bears a vast range of functionality, affecting T cell activation, proliferation, differentiation, neuron regeneration, adhesion and fibrosis (Rege and Hagood, 2006a,b). Interestingly, many of these functions are overlapping with integrin functionalities such as immunological synapse formation (αLβ2; Springer and Dustin, 2012), proliferation, adhesion, etc. The dual integrin interacting pattern (trans and cis) makes Thy-1 a key mechanoregulator through its integrin interaction capacity. The subsequent biological impacts of Thy-1-integrin interactions can be further categorized as either on the plasmamembrane or in the cytosol.
On the plasma membrane, Thy-1 exerts profound impact in a direct manner. On the surface of neurons, Thy-1 directly binds to astrocyte integrin αvβ3 in trans. The interaction triggers Thy-1 clustering and suppresses neuron outgrowth (Leyton et al., 2001). Thy-1 dependent cell adhesion and migration is also mediated through the trans interaction between Thy-1 and integrin, namely αvβ3, αXβ2, and αMβ2 (Rege and Hagood, 2006a). Therefore, the trans interaction mediates mechanotransduction in the context of cell-cell interaction, which result in either clustering of Thy-1 and subsequent suppression of c-Src (Figure 1 ①) or directly force transduction (Figure 1②). The cis interaction, on the other hand, plays a more inhibitive/regulatory role in integrin mediated mechanotransduction. Through direct binding to integrin through its RLD motif, Thy-1 can restrict integrin by promoting its bent conformation, due to the proximity of the RLD motif against the plasma membrane. Furthermore, through interaction with integrin, Thy-1 can also effectively reduce overall integrin avidity toward ECM ligands (Figure 1③a) and at the same time, inhibit integrin-mediated latent TGF-β activation (Figure 1④).
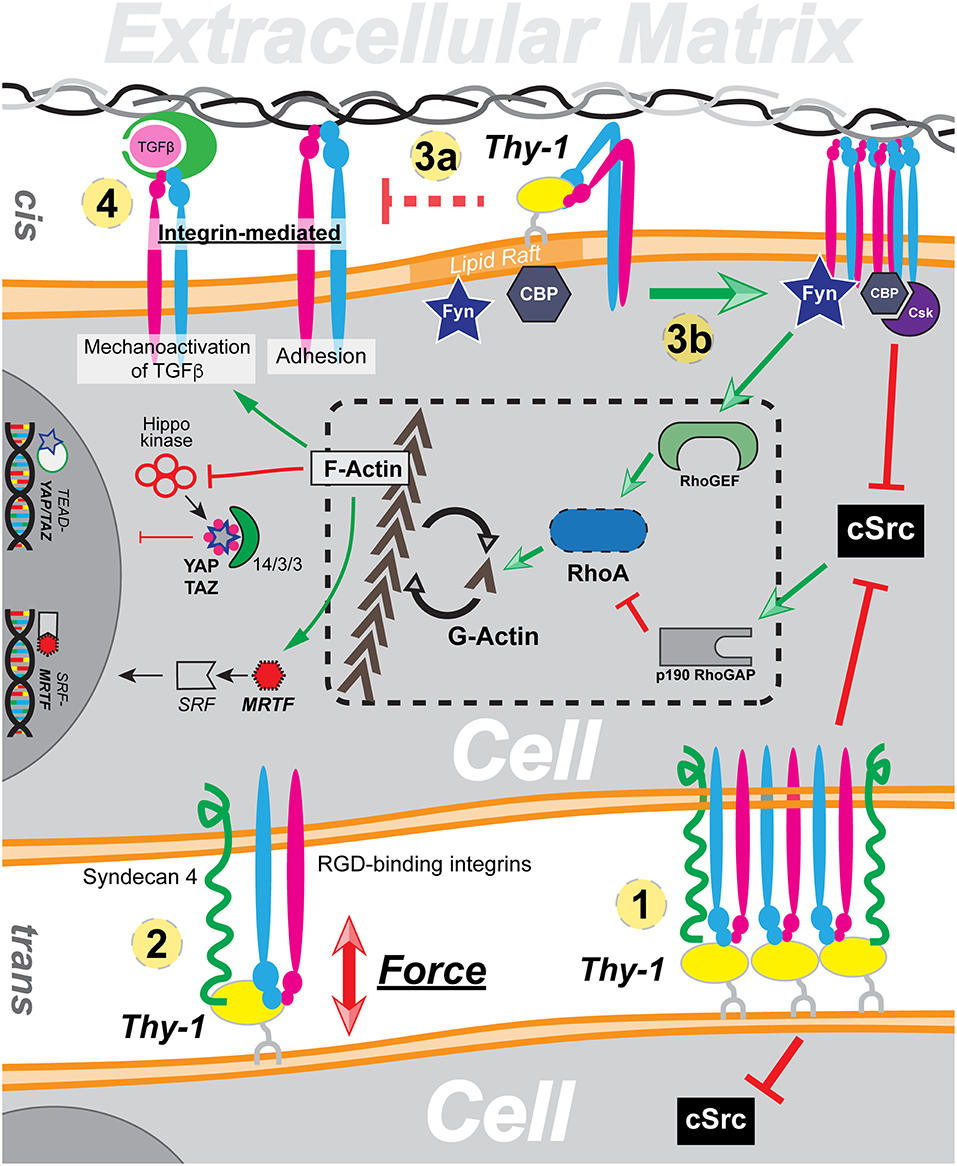
Figure 1. A global overview of Thy-1's functionality in mechanotransduction through cis and trans interaction with integrin. ① Trans interaction with integrin induces Thy-1 clustering and Src inhibition. ② Thy-1-integrin-Syndecan4 triplex directly responds to force by forming a dynamic catch bond. ③ Direct cis interaction between Thy-1 and integrin results in reduction of integrin affinity (a) and downstream regulation of integrin mediated mechanotransduction (b). ④ Thy-1-integrin cis interaction also suppresses integrin-dependent TGF-β activation.
The impacts of Thy-1 on cytoplasmic mechanotransduction pathway, on the other hand, are indirect due to the lack of a Thy-1 cytoplasmic domain. In addition to regulating integrin affinity/avidity through direct cis-interaction, Thy-1-integrin binding also facilitates phosphorylation and recruitment of CBP through Fyn, a lipid raft Src family kinase (SFK) recruited to the focal adhesion by Thy-1. Subsequently, Csk is recruited by CBP, leading to phosphorylate the c-terminus of and inactivate c-Src. The c-Src inhibition subsequently leads to reduced p190GAP activity and elevated RhoA-dependent actin stress fiber assembly. It has been widely described that RhoA/ROCK controlled cellular G-actin pool dynamics directly regulates nuclear translocation and activation of MRTF (Miralles et al., 2003; Fan et al., 2007; Vartiainen et al., 2007). Therefore, Thy-1 mediated downregulation of Src activity can result in nuclear accumulation of active MRTF in response to extracellular tension by reducing availability of MRTF-inhibitory G-actin (Figure 1③b). Similarly, both Hippo dependent and independent Yap/Taz signal transduction are also tightly regulated by RhoA mediated F-actin stress fiber assembly (Dupont et al., 2011; Sansores-Garcia et al., 2011; Wada et al., 2011). Besides a relatively “slower” pathway to regulate RhoA activity through Src, Fyn has also been shown to directly activate Rho GEF LARG and thus enables swift early cellular response toward extracellular mechanic cues through RhoA. In sum, through indirectly manipulating RhoA activity and subsequent equilibrium between G-actin and F-actin, Thy-1 functions as a key regulator of cellular mechanotransduction.
The impact of Thy-1 on mechanotransduction is likely the fundamental mechanism behind its broad functionality. This mechano-based regulatory mechanism not only affects cellular behavior but also profoundly influence tissue development and cell differentiation. Thy-1 negative fibroblasts are more sensitive to inflammatory cytokines and more likely to differentiate into myofibroblasts (Sanders et al., 2007). Thy-1 deficiency also leads to poor osteogenesis in mouse due to altered Wnt pathway (Picke et al., 2018). Without Thy-1, mouse mesenchymal stem cells (MSC) are more likely to differentiate into adipocytes instead of osteoblasts (Picke et al., 2018). These discoveries strongly suggest that Thy-1, through integrin mediated mechanotransduction, significantly influences differentiation and cell fate determination. Recently it has been reported that integrin αvβ3 signaling potentiates fibrotic activation of lung fibroblast (Fiore et al., 2018). In the study, Thy-1 KD induced fibroblast stiffening and promoted MRTF nucleus translocation with enhanced cellular contractility. Elevated αvβ3 staining was observed in both Thy-1 KD cells and in Thy-1 null mice treated with bleomycin to induce lung fibrosis, implicating strong correlation between lung fibrogenesis, Thy-1 loss and dysregulated integrin αvβ3 signaling.
Unlike other mechanotransducing molecules, Thy-1 is capable of mediating mechanotransduction through trans AND cis interactions with integrins, making the GPI anchored protein a unique mechano mediator. The Thy-1 mediated mechanotransduction is highly context dependent. The trans molecular coupling of Thy-1 with integrin and Syndecan4 is necessary to generate full strength of force as well as cellular contractile formation. Meanwhile, the lipid raft GPI anchor is an absolute requirement for Thy-1 cis mechanotransduction, emphasizing the importance of lipid environment for proper Thy-1 functionality. Considering that the function of Thy-1 is also highly cell type dependent, it is conceivable that differential membrane protein coupling and subtle change in lipid raft composition could serve as a fine-tuned regulatory mechanism of Thy-1 mediated mechanotransduction. Therefore, Thy-1 could potentially be coupling with other not-yet-identified lipid raft proteins directly or indirectly and thus regulating a wide range of mechano-related cellular response spanning from ECM remodeling to cell differentiation and determination. More studies are needed to fully understand the role of Thy-1 in the context of mechanotransduction.
Author Contributions
PH and TB conceived the idea and wrote the manuscript. TB conceptualized and created the figure.
Funding
We would like to acknowledge the United States National Institutes of Health, specifically the National Heart, Lung and Blood Institute for funding (R01 HL 127283 and R01 HL 132585).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Avalos, A. M., Arthur, W. T., Schneider, P., Quest, A. F., Burridge, K., and Leyton, L. (2004). Aggregation of integrins and RhoA activation are required for Thy-1-induced morphological changes in astrocytes. J. Biol. Chem. 279, 39139–39145. doi: 10.1074/jbc.M403439200
Baillat, G., Siret, C., Delamarre, E., and Luis, J. (2008). Early adhesion induces interaction of FAK and Fyn in lipid domains and activates raft-dependent Akt signaling in SW480 colon cancer cells. Biochim. Biophys. Acta 1783, 2323–2331. doi: 10.1016/j.bbamcr.2008.08.008
Barker, T. H., Grenett, H. E., MacEwen, M. W., Tilden, S. G., Fuller, G. M., Settleman, J., et al. (2004). Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp. Cell Res. 295, 488–496. doi: 10.1016/j.yexcr.2004.01.026
Barker, T. H., and Hagood, J. S. (2009). Getting a grip on Thy-1 signaling. Biochim. Biophys. Acta 1793, 921–923. doi: 10.1016/j.bbamcr.2008.10.004
Bass, M. D., Morgan, M. R., Roach, K. A., Settleman, J., Goryachev, A. B., and Humphries, M. J. (2008). p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J. Cell Biol. 181, 1013–1026. doi: 10.1083/jcb.200711129
Cluzel, C., Saltel, F., Lussi, J., Paulhe, F., Imhof, B. A., and Wehrle-Haller, B. (2005). The mechanisms and dynamics of αvβ3 integrin clustering in living cells. J. Cell Biol. 171, 383–392. doi: 10.1083/jcb.200503017
Conrad, D. M., Furlong, S. J., Doucette, C. D., Boudreau, R. T., and Hoskin, D. W. (2009). Role of mitogen-activated protein kinases in Thy-1-induced T-lymphocyte activation. Cell. Signal. 21, 1298–1307. doi: 10.1016/j.cellsig.2009.03.014
Craig, W., Kay, R., Cutler, R. L., and Lansdorp, P. M. (1993). Expression of Thy-1 on human hematopoietic progenitor cells. J. Exp. Med. 177, 1331–1342. doi: 10.1084/jem.177.5.1331
Deininger, S. O., Rajendran, L., Lottspeich, F., Przybylski, M., Illges, H., Stuermer, C. A., et al. (2003). Identification of teleost Thy-1 and association with the microdomain/lipid raft reggie proteins in regenerating CNS axons. Mol. Cell. Neurosci. 22, 544–554. doi: 10.1016/S1044-7431(03)00028-9
Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183. doi: 10.1038/nature10137
Fan, L., Sebe, A., Peterfi, Z., Masszi, A., Thirone, A. C., Rotstein, O. D., et al. (2007). Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol. Biol. Cell 18, 1083–1097. doi: 10.1091/mbc.e06-07-0602
Fiore, V. F., Ju, L., Chen, Y., Zhu, C., and Barker, T. H. (2014). Dynamic catch of a Thy-1-α5bβ1+syndecan-4 trimolecular complex. Nat. Commun. 5, 4886. doi: 10.1038/ncomms5886
Fiore, V. F., Strane, P. W., Bryksin, A. V., White, E. S., Hagood, J. S., and Barker, T. H. (2015). Conformational coupling of integrin and Thy-1 regulates Fyn priming and fibroblast mechanotransduction. J. Cell Biol. 211, 173–190. doi: 10.1083/jcb.201505007
Fiore, V. F., Wong, S. S., Tran, C., Tan, C., Xu, W., Sulchek, T., et al. (2018). αvβ3 Integrin drives fibroblast contraction and strain stiffening of soft provisional matrix during progressive fibrosis. JCI Insight 3:97597. doi: 10.1172/jci.insight.97597
Guilluy, C., Swaminathan, V., Garcia-Mata, R., O'Brien, E. T., Superfine, R., and Burridge, K. (2011). The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13, 722–727. doi: 10.1038/ncb2254
Hagood, J. S., Prabhakaran, P., Kumbla, P., Salazar, L., MacEwen, M. W., Barker, T. H., et al. (2005). Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am. J. Pathol. 167, 365–379. doi: 10.1016/S0002-9440(10)62982-3
Head, B. P., Patel, H. H., and Insel, P. A. (2014). Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 1838, 532–545. doi: 10.1016/j.bbamem.2013.07.018
Herrera-Molina, R., Frischknecht, R., Maldonado, H., Seidenbecher, C. I., Gundelfinger, E. D., Hetz, C., et al. (2012). Astrocytic αVβ3 integrin inhibits neurite outgrowth and promotes retraction of neuronal processes by clustering Thy-1. PLoS ONE 7:e34295. doi: 10.1371/journal.pone.0034295
Herrera-Molina, R., Valdivia, A., Kong, M., Alvarez, A., Cardenas, A., Quest, A. F., et al. (2013). Thy-1-interacting molecules and cellular signaling in cis and trans. Int. Rev. Cell Mol. Biol. 305, 163–216. doi: 10.1016/B978-0-12-407695-2.00004-4
Hu, P., and Luo, B. H. (2013). Integrin bi-directional signaling across the plasma membrane. J. Cell. Physiol. 228, 306–312. doi: 10.1002/jcp.24154
Kong, M., Munoz, N., Valdivia, A., Alvarez, A., Herrera-Molina, R., Cardenas, A., et al. (2013). Thy-1-mediated cell-cell contact induces astrocyte migration through the engagement of αVβ3 integrin and syndecan-4. Biochim. Biophys. Acta 1833, 1409–1420. doi: 10.1016/j.bbamcr.2013.02.013
Kroczek, R. A., Gunter, K. C., Germain, R. N., and Shevach, E. M. (1986). Thy-1 functions as a signal transduction molecule in T lymphocytes and transfected B lymphocytes. Nature 322, 181–184. doi: 10.1038/322181a0
Kwon, S., Son, H., Choi, Y., Lee, J. H., Choi, S., Lim, Y., et al. (2009). Syndecan-4 promotes the retention of phosphatidylinositol 4,5-bisphosphate in the plasma membrane. FEBS Lett. 583, 2395–2400. doi: 10.1016/j.febslet.2009.06.039
Lee, M. J., Shin, J. O., and Jung, H. S. (2013). Thy-1 knockdown retards wound repair in mouse skin. J. Dermatol. Sci. 69, 95–104. doi: 10.1016/j.jdermsci.2012.11.009
Leyton, L., Schneider, P., Labra, C. V., Ruegg, C., Hetz, C. A., Quest, A. F., et al. (2001). Thy-1 binds to integrin β3 on astrocytes and triggers formation of focal contact sites. Curr. Biol. 11, 1028–1038. doi: 10.1016/S0960-9822(01)00262-7
Liu, F., Lagares, D., Choi, K. M., Stopfer, L., Marinkovic, A., Vrbanac, V., et al. (2015). Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L344–357. doi: 10.1152/ajplung.00300.2014
Luo, B. H., Carman, C. V., and Springer, T. A. (2007). Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647. doi: 10.1146/annurev.immunol.25.022106.141618
Maksumova, L., Le, H. T., Muratkhodjaev, F., Davidson, D., Veillette, A., and Pallen, C. J. (2005). Protein tyrosine phosphatase α regulates Fyn activity and Cbp/PAG phosphorylation in thymocyte lipid rafts. J. Immunol. 175, 7947–7956. doi: 10.4049/jimmunol.175.12.7947
Maldonado, H., Calderon, C., Burgos-Bravo, F., Kobler, O., Zuschratter, W., Ramirez, O., et al. (2017). Astrocyte-to-neuron communication through integrin-engaged Thy-1/CBP/Csk/Src complex triggers neurite retraction via the RhoA/ROCK pathway. Biochim Biophys Acta Mol Cell Res 1864, 243–254. doi: 10.1016/j.bbamcr.2016.11.006
Miralles, F., Posern, G., Zaromytidou, A. I., and Treisman, R. (2003). Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329–342. doi: 10.1016/S0092-8674(03)00278-2
Picke, A. K., Campbell, G. M., Bluher, M., Krugel, U., Schmidt, F. N., Tsourdi, E., et al. (2018). Thy-1 (CD90) promotes bone formation and protects against obesity. Sci. Transl. Med. 10:eaao6806. doi: 10.1126/scitranslmed.aao6806
Ramirez, G., Hagood, J. S., Sanders, Y., Ramirez, R., Becerril, C., Segura, L., et al. (2011). Absence of Thy-1 results in TGF-β induced MMP-9 expression and confers a profibrotic phenotype to human lung fibroblasts. Lab. Invest. 91, 1206–1218. doi: 10.1038/labinvest.2011.80
Rege, T. A., and Hagood, J. S. (2006a). Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 20, 1045–1054. doi: 10.1096/fj.05-5460rev
Rege, T. A., and Hagood, J. S. (2006b). Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim. Biophys. Acta 1763, 991–999. doi: 10.1016/j.bbamcr.2006.08.008
Saltel, F., Mortier, E., Hytonen, V. P., Jacquier, M. C., Zimmermann, P., Vogel, V., et al. (2009). New PI(4,5)P2- and membrane proximal integrin-binding motifs in the talin head control β3-integrin clustering. J. Cell Biol. 187, 715–731. doi: 10.1083/jcb.200908134
Sanders, Y. Y., Kumbla, P., and Hagood, J. S. (2007). Enhanced myofibroblastic differentiation and survival in Thy-1(-) lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 36, 226–235. doi: 10.1165/rcmb.2006-0178OC
Sanders, Y. Y., Pardo, A., Selman, M., Nuovo, G. J., Tollefsbol, T. O., Siegal, G. P., et al. (2008). Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 39, 610–618. doi: 10.1165/rcmb.2007-0322OC
Sansores-Garcia, L., Bossuyt, W., Wada, K., Yonemura, S., Tao, C., Sasaki, H., et al. (2011). Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335. doi: 10.1038/emboj.2011.157
Schubert, K., Gutknecht, D., Koberle, M., Anderegg, U., and Saalbach, A. (2013). Melanoma cells use Thy-1 (CD90) on endothelial cells for metastasis formation. Am. J. Pathol. 182, 266–276. doi: 10.1016/j.ajpath.2012.10.003
Springer, T. A., and Dustin, M. L. (2012). Integrin inside-out signaling and the immunological synapse. Curr. Opin. Cell Biol. 24, 107–115. doi: 10.1016/j.ceb.2011.10.004
Stuermer, C. A., Lang, D. M., Kirsch, F., Wiechers, M., Deininger, S. O., and Plattner, H. (2001). Glycosylphosphatidyl inositol-anchored proteins and fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and−2. Mol. Biol. Cell 12, 3031–3045. doi: 10.1091/mbc.12.10.3031
Vartiainen, M. K., Guettler, S., Larijani, B., and Treisman, R. (2007). Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752. doi: 10.1126/science.1141084
Wada, K., Itoga, K., Okano, T., Yonemura, S., and Sasaki, H. (2011). Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914. doi: 10.1242/dev.070987
Yasuda, K., Nagafuku, M., Shima, T., Okada, M., Yagi, T., Yamada, T., et al. (2002). Cutting edge: fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J. Immunol. 169, 2813–2817. doi: 10.4049/jimmunol.169.6.2813
Zhou, Y., Hagood, J. S., Lu, B., Merryman, W. D., and Murphy-Ullrich, J. E. (2010). Thy-1-integrin αvβ5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-β1 activation and myofibroblast differentiation. J. Biol. Chem. 285, 22382–22393. doi: 10.1074/jbc.M110.126227
Keywords: Thy-1, integrin, trans interaction, cis interaction, mechanotransduction
Citation: Hu P and Barker TH (2019) Thy-1 in Integrin Mediated Mechanotransduction. Front. Cell Dev. Biol. 7:22. doi: 10.3389/fcell.2019.00022
Received: 29 November 2018; Accepted: 05 February 2019;
Published: 25 February 2019.
Edited by:
Emanuela Felley-Bosco, University of Zurich, SwitzerlandReviewed by:
Luca Azzolin, Università di Padova, ItalyVladimir Sytnyk, University of New South Wales, Australia
Copyright © 2019 Hu and Barker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas H. Barker, thomas.barker@virginia.edu
 Ping Hu
Ping Hu Thomas H. Barker
Thomas H. Barker