Targeting Bruton’s Tyrosine Kinase in Inflammatory and Autoimmune Pathologies
- Department of Pulmonary Medicine, Erasmus MC, University Medical Center, Rotterdam, Netherlands
Bruton’s tyrosine kinase (BTK) was discovered due to its importance in B cell development, and it has a critical role in signal transduction downstream of the B cell receptor (BCR). Targeting of BTK with small molecule inhibitors has proven to be efficacious in several B cell malignancies. Interestingly, recent studies reveal increased BTK protein expression in circulating resting B cells of patients with systemic autoimmune disease (AID) compared with healthy controls. Moreover, BTK phosphorylation following BCR stimulation in vitro was enhanced. In addition to its role in BCR signaling, BTK is involved in many other pathways, including pattern recognition, Fc, and chemokine receptor signaling in B cells and myeloid cells. This broad involvement in several immunological pathways provides a rationale for the targeting of BTK in the context of inflammatory and systemic AID. Accordingly, numerous in vitro and in vivo preclinical studies support the potential of BTK targeting in these conditions. Efficacy of BTK inhibitors in various inflammatory and AID has been demonstrated or is currently evaluated in clinical trials. In addition, very recent reports suggest that BTK inhibition may be effective as immunosuppressive therapy to diminish pulmonary hyperinflammation in coronavirus disease 2019 (COVID-19). Here, we review BTK’s function in key signaling pathways in B cells and myeloid cells. Further, we discuss recent advances in targeting BTK in inflammatory and autoimmune pathologies.
Introduction
Loss of immunological tolerance associated with the activation of autoreactive B cells and their differentiation into autoantibody-producing cells are important pathogenic features in human systemic autoimmune disease (AID). B cell receptor (BCR) signaling is crucial for B cell activation, survival and differentiation and, therefore, reflects a potential therapeutic target for AID. Bruton’s tyrosine kinase (BTK) is a cytoplasmic protein belonging to the family of TEC (tyrosine kinase expressed in hepatocellular carcinoma) kinases. BTK is renowned for its critical role in BCR signaling and was originally identified as the gene defective in X-linked agammaglobulinemia (XLA) patients (Tsukada et al., 1993; Vetrie et al., 1993). Based on the therapeutic benefit of the anti-CD20 antibody rituximab to deplete mature B cells in AID, strategies were developed to discover selective BTK inhibitors (BTKi) for the treatment of rheumatoid arthritis (RA; Pan et al., 2007). These BTKi were designed to covalently and irreversibly bind BTK at the cysteine 481 residue in the catalytic domain. Interestingly, the high potential of these inhibitors, such as ibrutinib and acalabrutinib, to modulate BCR signaling led to their rapid implementation in the treatment of several B cell malignancies (Hendriks et al., 2014).
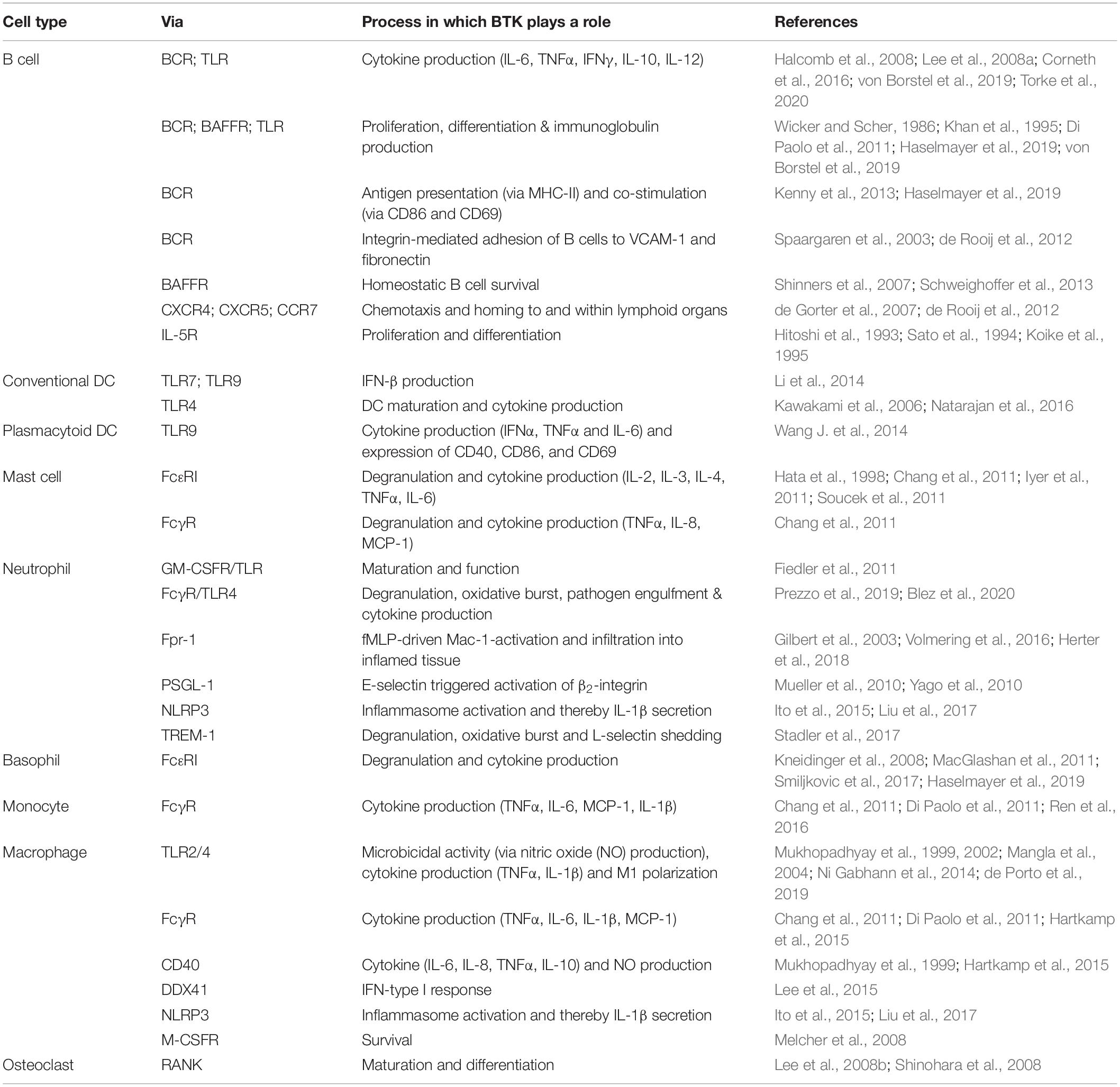
Soon after the discovery of its crucial function in BCR signaling, the involvement of BTK in several other signaling routes in B cells and myeloid cells was demonstrated (Table 1). This fueled research into the effects of BTKi in the context of inflammatory and AID by solely targeting BCR signaling or by targeting multiple pathways in several cell types simultaneously. The potential use of BTKi for the treatment of AID is currently being explored in vitro, and in vivo in animal models and clinical trials. In this review, we summarize BTK’s function in key signaling pathways in B cells and myeloid cells, and we discuss recent advances in targeting BTK in inflammatory and autoimmune pathologies.
BTK Signaling Pathways
Prosurvival Signaling in B Cells
B cell development takes place in the bone marrow. It is characterized by the ordered rearrangement of immunoglobulin heavy and light chain gene segments, leading to expression of a unique BCR. The random nature of this V(D)J recombination process inevitably generates BCRs that recognize self-antigen. However, multiple checkpoints ensure counterselection of these autoreactive B cells during development based on BCR specificity (Wardemann et al., 2003). These checkpoints are critical because autoreactive B cells, when activated, can have multiple pathogenic functions. These include the production of autoantibodies and pro-inflammatory cytokines, stimulation of tertiary lymphoid organ formation and antigen presentation to autoreactive T cells.
The survival of circulating B cells requires signals from both BCR and B cell activating factor (BAFF) receptor (BAFFR; Lam et al., 1997; Sasaki et al., 2004). This BCR prosurvival signaling is antigen-independent and is referred to as “tonic” signaling. It differs from stronger signals induced by cognate antigen binding, leading to activation and proliferation of B cells. BAFF-transgenic mice and mice with B cell-specific BTK overexpression (CD19-hBtk) develop autoimmune pathology resembling human systemic lupus erythematosus (SLE) and primary Sjögren’s syndrome (pSS; Thien et al., 2004; Kil et al., 2012).
B cell receptor engagement initiates intracellular signaling that leads to the phosphorylation of spleen tyrosine kinase (SYK; Figure 1). Subsequent recruitment of BTK to the cell membrane enables SYK to activate BTK through phosphorylation at Y551, followed by BTK autophosphorylation at Y223 (Rawlings et al., 1996). Active BTK can then phosphorylate phospholipase Cγ2 (PLCγ2). The formation of this BCR signalosome generates a Ca2+ influx and leads to activation of multiple downstream signaling pathways and transcription factors, including nuclear factor of activated T cells (NFAT), extracellular signal-regulated kinase (ERK), and nuclear factor (NF)-κB. These are crucial for B cell survival, proliferation, and differentiation. BTK has a central role in the BCR signaling pathway given the phenotype of XLA patients and the finding that BTK inhibition leads to a block in downstream signaling (Honigberg et al., 2010).
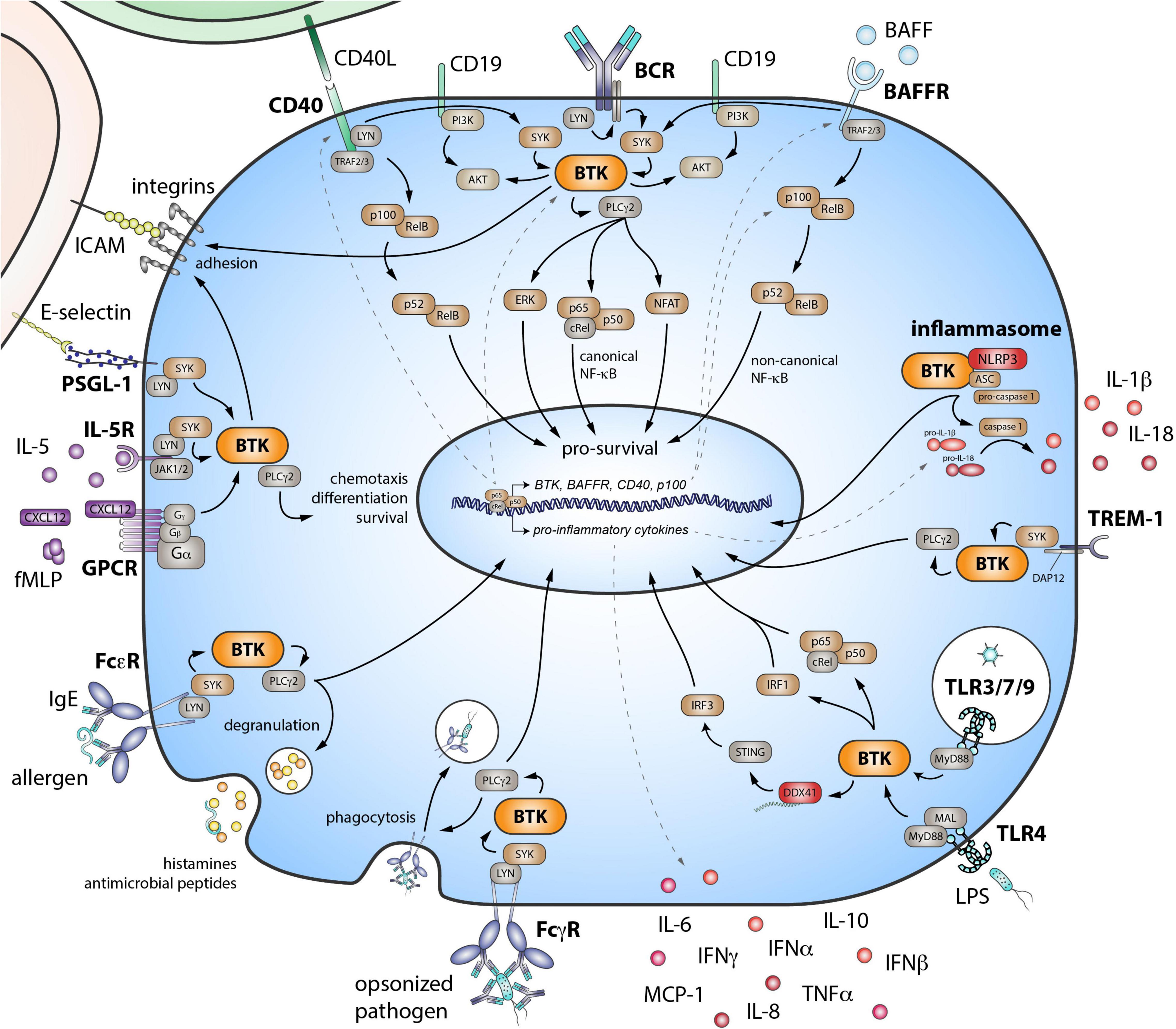
Figure 1. Role of Bruton’s tyrosine kinase (BTK) in various signaling pathways in B cells and myeloid cells. BTK is critical downstream of the B cell receptor (BCR). The BAFF receptor (BAFFR) transduces signals by coopting the BCR. The co-stimulatory receptor CD40 also signals via both the noncanonical NF-κB pathway and BTK. Eventually, these signaling pathways lead to the activation of downstream transcription factors—important for survival, differentiation, proliferation, and cytokine production of B cells. BTK also functions in inflammasome activation and in signaling downstream pattern recognition receptors, including triggering receptor expressed on myeloid cells 1 (TREM-1) and the Toll-like receptor (TLR) family. Activating FcγRs via BTK signaling, can stimulate cells to initiate cytokine production, phagocytosis, and microbicidal activity of engulfed pathogens. FcεRs can bind IgE and are mostly expressed on mast cells and basophils. When cross-linked, these receptors also signal via BTK, resulting in the quick release of histamines and antimicrobial peptides via degranulation. BTK is also involved in downstream signaling of G-protein coupled receptors (GPCR), such as chemokine and cytokine receptors. E-selectin–driven engagement of PSGL-1 induces downstream signaling via BTK to activate integrins. SYK, spleen tyrosine kinase; PLCγ2, phospholipase Cγ2; PI3K, phosphoinositide 3-kinase; ERK, extracellular signal-regulated kinase; NF-κB, nuclear factor-κB; TRAF, tumor necrosis factor receptor-associated factor; BAFF, B cell activating factor of the tumor necrosis factor superfamily; NLRP3, NLR family pyrin domain containing 3; ASC, apoptosis-associated speck like protein containing a caspase recruitment domain; DAP12, DNAX activation protein of 12 kDa; MyD88, myeloid differentiation primary response 88; MAL, MyD88 adaptor-like; IRF, interferon regulatory factor; DDX41, DEAD-box helicase 41; STING, stimulator of interferon genes; IL, interleukin; IFN, interferon; MCP-1, monocyte chemoattractant protein-1; TNFα, tumor necrosis factor α; FcγR, Fcγ receptor; FcεR, Fcε receptor; fMLP, N-Formylmethionyl-leucyl-phenylalanine; CXCL12, C-X-C-motif chemokine ligand 12; JAK, Janus kinase; PSGL-1, P-selectin glycoprotein ligand-1; ICAM, intercellular adhesion molecule.
B cell activating factor is a ligand for three receptors: transmembrane activator and CAML interactor (TACI), B cell maturation antigen (BCMA), and BAFFR. The latter is most important for survival of mature naïve B cells by activating the noncanonical NF-κB pathway. Studies show that the BAFFR also transduces its signals by crosstalk with the BCR, involving SYK and BTK, leading to canonical NF-κB signaling (Figure 1; Shinners et al., 2007; Schweighoffer et al., 2013).
An important co-stimulatory receptor for T cell dependent B cell responses is CD40. Its ligand CD154 (CD40L) is expressed by activated T cells and, next to homeostatic proliferation of the naïve B cell pool, supports B cell differentiation and maturation (Schwartz et al., 2014). Signaling downstream of CD40 activates the noncanonical NF-κB pathway (Figure 1). However, BTK is also activated (Brunner et al., 2002), and the expression of BCR signaling proteins—including BTK—is enhanced (Kil et al., 2012). Interestingly, BAFF stimulates CD40 expression and CD40L co-stimulation from T cells in a BAFFR-dependent way (Zhang et al., 2016).
Therefore, BCR/BAFFR/CD40 signaling acts in a self-amplifying loop, both directly as prosurvival signals and indirectly by enhancing transcription of these prosurvival receptors and their downstream signaling molecules (Smith and Cancro, 2003; Stadanlick et al., 2008; Yu et al., 2008; Castro et al., 2009; Smulski and Eibel, 2018). It is hypothesized that, in AID, a disbalance in survival signals causes escape of autoreactive B cells from negative checkpoints. Therefore, modulating these B cell signaling pathways simultaneously via BTKi offers potential in targeting pathogenic B cells and constraining overt B cell activation.
BTK Function Beyond B Cell Signaling
Pattern Recognition Receptors
Bruton’s tyrosine kinase is also implicated in signaling of various pattern recognition receptors (PRRs), a group of germline-encoded sensors expressed by innate and adaptive immune cells. PRRs have a pivotal role in sensing pathogen- and damage-associated molecular patterns and are expressed on the cell surface, intracellularly within endosomes or in the cytoplasm. BTK is involved in toll-like receptor (TLR) signaling, in which it interacts with their intracellular signaling domain and with the downstream adaptor molecules MyD88 (myeloid differentiation primary response 88) and MAL (MyD88-adaptor-like) (Hendriks et al., 2014). Similar to the BAFFR, TLR4 is thought to transduce signals through the BCR (Schweighoffer et al., 2017). BTK interacts directly with the cytoplasmic sensor NLR family pyrin domain containing 3 (NLRP3) and its adaptor ASC (apoptosis-associated speck like protein containing a caspase recruitment domain) and is, thus, involved in inflammasome and caspase-1 activation and subsequent interleukin (IL)-1β and IL-18 production (Ito et al., 2015). Engagement of triggering receptor expressed on myeloid cells-1 (TREM-1) induces BTK activation and leads to inflammatory responses (Ormsby et al., 2011). Last, BTK phosphorylates DEAD-box helicase 41 (DDX41) and stimulates its binding to dsDNA and activation of the STING (stimulator of interferon genes) pathway (Lee et al., 2015). All these pathways lead to downstream signaling and activation of transcription factors, such as activator protein 1 (AP-1), NF-κB, and/or interferon regulatory factors (IRFs), promoting differentiation, survival, and pro-inflammatory cytokine production (Figure 1; Weber et al., 2017).
Because BTK functions in both TLR and BCR signaling, B cell activation with TLR ligands can lead to synergistic effects when combined with BCR stimulation (Kenny et al., 2013). TLR signaling via NF-κB enhances expression of BTK and BAFFR (Kil et al., 2012; Abu-Rish et al., 2013). Hence, TLR triggering adds to this self-amplifying loop of multiple stimuli (BCR/CD40/BAFF) that support B cell survival. A disbalance in these signals can result in a disturbed survival of pathogenic B cells. Therefore, BTKi may show potential in the treatment of inflammatory and AID by inhibitory effects on PRR signaling in both B and myeloid cells.
Fc, Cytokine, and Chemokine Receptors and Integrin Activation
Antibodies exert their function—pathogen neutralization and opsonization—by complement activation and via Fc receptor (FcR) signaling involving BTK (Chang et al., 2011; Fiorcari et al., 2016; Figure 1). Activation of FcRs on myeloid cells induces release of antimicrobial factors via degranulation, and it stimulates de novo cytokine production, phagocytosis, and antigen presentation. Although these tools are crucial in the clearing of pathogens, these can be pathogenic in AID. Last, BTK also functions in downstream signaling of chemokine receptors (de Gorter et al., 2007), cytokine receptors (Sato et al., 1994; Matsuda et al., 1995) and in integrin activation (Spaargaren et al., 2003; Yago et al., 2010; Volmering et al., 2016; Herter et al., 2018). Thus, for both B and myeloid cells, BTK is important in processes controlling cell localization, survival, and adhesion and migration into site of inflammation.
BTK and BTKi in B Cell–Mediated Autoimmune Disease
Following the production of first-line BTKi, such as ibrutinib, second-generation (mostly covalent) inhibitors with higher specificity, lower side effects, and their own unique properties were developed (Estupiñán et al., 2021; von Hundelshausen and Siess, 2021). Next to these small-molecule inhibitors, other types of inhibitors are being investigated, such as small-interfering RNAs targeting BTK production (Zhao et al., 2019). In this next section, we discuss the most important and most recent advances in the targeting of BTK in the context of inflammatory and AID.
Rheumatoid Arthritis
Bruton’s tyrosine kinase-deficiency in mice is protective in several experimental autoimmune arthritis models (Jansson and Holmdahl, 1993; Nyhoff et al., 2016). Protection appeared to be largely attributable to its role in B cells although BTK may also contribute to disease through macrophages (Horwood et al., 2006; Ni Gabhann et al., 2014) and osteoclasts/osteoblasts (Hayer et al., 2008; Shinohara et al., 2008). This is also evident from animal studies in which BTKi ameliorated B cell–dependent but also B cell–independent myeloid-mediated arthritis (Chang et al., 2011; Di Paolo et al., 2011; Caldwell et al., 2019; Haselmayer et al., 2019; Angst et al., 2020; Liu et al., 2021).
In human RA, dysregulated BCR signaling may lead to aberrant B cell activation and loss of tolerance. BTK protein and phosphorylation (pBTK) were increased in peripheral blood B cells of anti-citrullinated protein antibody positive RA patients (Corneth et al., 2017). These protein levels correlated with pathogenic T cell subsets and pBTK expression correlated with rheumatoid factor levels in circulation (Wang et al., 2015). Furthermore, RA synovial tissue cultured with BTKi showed decreased pro-inflammatory cytokine production (Hartkamp et al., 2015). Together, these data suggest BTKi may be a beneficial therapeutic option in RA. Indeed, fenebrutinib showed efficacy at higher doses, comparable to tumor necrosis factor (TNF)α-inhibitor adalimumab, and reduced pro-inflammatory cytokine and autoantibody levels (Cohen et al., 2020). However, several other studies with BTKi showed only mild effects on disease severity, including spebrutinib (Schafer et al., 2020), evobrutinib (NCT02784106, NCT03233230), and tirabrutinib (NCT02626026).
Primary Sjögren’s Syndrome
In an IL-14α-driven mouse model of pSS, BTK-deficiency did not protect against disease development (Shen et al., 2016). However, CD19-hBtk mice develop a spontaneous pSS/SLE-like autoimmune phenotype, including lymphocytic infiltrates in the salivary glands (Kil et al., 2012). This phenotype is dependent on B–T cell interaction (Corneth et al., 2016). Similarly, a subset of patients with active pSS had increased BTK protein and pBTK levels in circulating B cell subsets, which correlated with numbers of infiltrating T cells in the parotid gland and normalized following abatacept treatment (Corneth et al., 2017). Interestingly, enhanced BTK expression was already present in transitional and naïve B cells, which had a more activated phenotype and showed loss of tolerance in pSS (Corneth et al., 2017; Glauzy et al., 2017). Integrated BCR, TLR, and TACI signaling can induce autoantibody production by transitional B cells (Du et al., 2018). B cell–depleting studies in pSS have yielded contradicting results, possibly due to persistence of pathogenic B cells in the salivary glands, linked to high BAFF levels (Hamza et al., 2012; Cornec et al., 2016; Dorner et al., 2019). Importantly, BTK overexpression may be associated with increased risk of lymphoma development in pSS (Duret et al., 2019). BTKi may, therefore, be an interesting therapeutic strategy in pSS. Currently, a phase II clinical trial with remibrutinib in pSS is recruiting (NCT04035668).
Systemic Lupus Erythematosus
Bruton’s tyrosine kinase-deficient mice and BTKi-treated mice are protected in a wide range of experimental models of systemic SLE (Rip et al., 2018). Efficacy is attributed not only to inhibition of BCR signaling—thereby reducing autoantibody levels—but also to TLR and FcR signaling in monocytes and macrophages, important drivers of renal damage in SLE. BAFF transgenic and CD19-hBTK mice develop a spontaneous pSS/SLE-like phenotype, featuring antinuclear antibodies and immunoglobulin deposition in the kidneys (Mackay et al., 1999; Kil et al., 2012). In SLE patients, increased BTK expression in peripheral B cells was linked to lupus nephritis and correlated with disease severity (Kong et al., 2018). In a mouse model of lupus nephritis, BTKi lead to remission (Chalmers et al., 2017). BTKi in SLE are currently being tested in several clinical trials (NCT02537028, NCT04305197, NCT03878303, and NCT02829541). A phase II clinical trial with the noncovalent BTKi fenebrutinib did not meet its primary end point (Isenberg et al., 2019) although strong immunomodulatory effects were shown. More studies are required to ascertain efficacy of BTKi in SLE.
Systemic Sclerosis
Systemic sclerosis (SSc) is a very heterogeneous disease of unknown etiology. However, as >90% of patients harbor autoantibodies, B cells are thought to play a major role in SSc (Sakkas and Bogdanos, 2016). Genetic susceptibility studies implicate BCR signaling in disease pathogenesis (Dieude et al., 2009; Gourh et al., 2010; Rueda et al., 2010). Circulating BAFF levels were increased in SSc patients (Matsushita et al., 2007) and BAFF blockade modulated scleroderma phenotype in a bleomycin-mediated mouse model (Matsushita et al., 2018). In vitro treatment of SSc B cells with ibrutinib reduced IL-6, TNFα, and SSc-specific autoantibody production following TLR stimulation (Einhaus et al., 2020). Though further research is needed, these results indicate BTKi may be a therapeutic option in SSc.
Multiple Sclerosis
Multiple sclerosis (MS) is a demyelinating AID of the central nervous system (CNS). B cells are thought to play an important role in MS pathogenesis as shown by the clinical success of rituximab treatment (Kinzel and Weber, 2016). In experimental autoimmune encephalitis, a mouse model for MS, BTKi ameliorated disease (Torke et al., 2020). Compared with other AID and healthy controls, MS B cells did not show increased BTK protein expression or pBTK levels upon BCR stimulation (Torke et al., 2020). A phase II clinical trial with evobrutinib showed promising clinical results at the highest dose (Montalban et al., 2019). Trials with tolebrutinib in relapsing and progressive forms of MS are currently running (NCT04410978, NCT04410991, and NCT04458051). As BTKi are small-molecule inhibitors, they may be better suited in entering the CNS and reaching pathogenic B cells than therapeutic antibodies such as rituximab (Dolgin, 2021).
Type I Diabetes
In non-obese diabetic (NOD) mice, BTK-deficiency ameliorated disease by increasing BCR editing, thereby reducing the number of autoreactive BCRs, and so a loss in pathogenic autoantibodies. However, autoreactive B cells were still able to escape selection, and the phenotype could be restored by provision of an insulin-specific BCR (Kendall et al., 2009; Bonami et al., 2014). In another study, treatment of NOD mice with a SYK inhibitor delayed the onset and progression of the anti-insulin response (Colonna et al., 2010). These data suggest that targeting BCR signaling, and BTK in particular, could be beneficial in diabetes patients.
Granulomatosis With Polyangiitis
In granulomatosis with polyangiitis (GPA) patients, BTK levels were increased in peripheral B cells of patients with active disease but not patients in remission, indicating its association with disease activity (von Borstel et al., 2019). Newly emerging transitional and naïve B cells were more responsive to BCR stimulation as pBTK and pPLCγ2 stimulation ratios were increased compared with healthy controls. In vitro incubation of patients’ B cells with acalabrutinib reduced cytokine production and plasma cell differentiation, although this reduction was smaller than in B cells from healthy controls (von Borstel et al., 2019). Nevertheless, targeting BCR signaling through BTKi could be a new treatment option in GPA.
Pemphigus
Pemphigus and pemphigoid are AID characterized by blistering and erosions of the skin or mucosal membranes and associated with IgG autoantibodies targeting structural proteins in epithelia. Therapy involves high-dose corticosteroids and rituximab, which achieves remissions in ∼80% of patients (Bieber et al., 2021). Because of the prominent role of autoantibodies, BTKi were evaluated in canine pemphigus foliaceus and facilitated good responses (Goodale et al., 2020a, b). Efficacy of BTKi is currently evaluated in phase II (NCT02704429) and III (NCT03762265) clinical trials.
Immune Thrombocytopenic Purpura
Immune thrombocytopenic purpura (ITP) is an AID characterized by autoantibodies targeting thrombocytes. BTKi showed effectivity in a mouse model (Langrish et al., 2017) and a phase I/II clinical trial is currently ongoing (NCT03395210) with first results indicating clinical activity (Kuter et al., 2020).
Idiopathic Pulmonary Fibrosis
Increased BTK expression was found in circulating B cells in a fraction of patients with idiopathic pulmonary fibrosis (IPF; Heukels et al., 2019). However, BTKi showed divergent effects in bleomycin mouse models for pulmonary fibrosis, likely due to off-target effects and multi-kinase inhibition (Gu et al., 2018; Sun et al., 2020).
BTK and BTKi Beyond the B Cell Compartment
Psoriasis
Psoriasis is an autoinflammatory disease of the skin characterized by epidermal hyperplasia and parakeratosis. Hereby, TLR-activated myeloid cells produce cytokines critical for differentiation of IL-17 and IL-22-producing T cells. The finding that BTKi attenuated TLR7-driven psoriasis-like inflammation in mice, most likely by acting on innate immune cells (Al-Harbi et al., 2020; Nadeem et al., 2020), points to BTKi as a promising therapeutic option.
Chronic Graft-Versus-Host Disease
Chronic graft-versus-host disease (GvHD) is a serious and life-threatening complication of allogeneic hematopoietic stem cell transplantation. Although primarily mediated by donor T cells, an important role for B cells in the disease is supported by clinical benefit of B cell depletion by rituximab. BTKi by ibrutinib could could reverse established GvHD in various T cell-driven and alloantibody-driven mouse models (Dubovsky et al., 2014; Schutt et al., 2015). A phase Ib/II clinical trial with ibrutinib in active chronic GvHD patients shows substantial clinical responses with effects on both B and T cells (Miklos et al., 2017). Based on the observed efficacy and acceptable safety, ibrutinib has been FDA-approved for treatment of GvHD patients in which prior therapy failed.
Asthma and Chronic Obstructive Pulmonary Disease
In line with the critical role of BTK and IL-2-inducible T cell kinase (ITK) in mast cell degranulation and of ITK in T cell activation (Liao and Littman, 1995; Forssell et al., 2005), ibrutinib suppressed allergic airway inflammation in mice (Phillips et al., 2016; Nadeem et al., 2019) and blocked allergen-induced contraction of human bronchi (Dispenza et al., 2020). Furthermore, BTKi suppressed the alveolar changes related to chronic obstructive pulmonary disease (COPD) progression in mice following cigarette smoke exposure (Florence et al., 2018b), possibly by affecting airway neutrophils. Therefore, BTK/ITK inhibition in models of airway inflammation may affect both B, T, and myeloid cell activation.
Atherosclerosis
Evidence was provided that BTKi targeting glycoprotein GPIb and GPVI signal transduction in platelets blocked atherosclerotic plaque-selective platelet aggregation but spared physiologic hemostasis (Busygina et al., 2018), implying that BTKi holds therapeutic promise in atherosclerosis.
Coronavirus Disease 2019
Bruton’s tyrosine kinase has emerged as a potential therapeutic target to dampen the hyperinflammatory response in coronavirus disease 2019 (COVID-19). A dysregulated response by macrophages recognizing the single-stranded RNA of SARS-coronavirus-2 via TLRs is thought to be damaging to the host in severe COVID-19 disease (Merad and Martin, 2020). This is likely to involve BTK-dependent pathways including NF-κB and NLRP3 inflammasome activation, resulting in pro-inflammatory cytokine secretion.
Several lines of evidence suggest that BTKi may reduce COVID-19 symptoms. First, BTKi protected against fatal lung injury in bacterial or influenza-induced acute respiratory distress syndrome mouse models (Krupa et al., 2014; Florence et al., 2018). Second, an unexpectedly mild course of COVID-19 was seen in XLA patients (Miloševiæ et al., 2020; Quinti et al., 2020; Soresina et al., 2020) and in BTKi-treated COVID-19 patients with a B cell malignancy (Thibaud et al., 2020; Treon et al., 2020). Third, in a prospective study of hospitalized COVID-19 patients, acalabrutinib was administered off-label, and oxygenation improved, lymphopenia recovered, and inflammatory parameters normalized (Roschewski et al., 2020).
Nevertheless, very recent studies involving larger cohorts of COVID-19 patients with chronic lymphocytic leukemia indicated BTKi exerted only a modest protective effect and did not impact survival (Mato et al., 2020; Scarfò et al., 2020). A randomized phase II clinical trial of acalabrutinib in hospitalized patients was initiated (NCT04346199) but failed to meet the primary end point of increasing the proportion of patients remaining alive and free of respiratory failure. Other BTKi, including abivertinib and ibrutinib, are currently investigated in various clinical trials (NCT04528667, NCT04440007, and NCT04439006). These studies are expected to reveal whether—and at what stage of the disease—BTKi may show efficacy. Evidently, these should include the analysis of the effects of BTKi on the virus-specific antibody response and B cell memory formation.
Conclusion and Future Perspectives
Bruton’s tyrosine kinase was discovered for its crucial role in B cell development. Ever since then, its function in very different signaling pathways and cell types has been studied in health and disease. Concordantly, multiple BTKi were developed in the context of B cell–mediated disease, especially for B cell malignancies. This spotlight is now extended toward AID, in which BTK plays an important role in pro-inflammatory activation pathways in both B and myeloid cells. This fuels further research into BTK’s exact pathogenic role in AID. These studies remain challenging because of developmental problems in BTK-deficient mice or off-target effects of BTK-inhibiting compounds, such as ibrutinib, may obscure an accurate picture of the effects of specific BTKi. Nonetheless, preliminary studies in human AID and animal models show potential clinical effectiveness of BTKi. Additionally, they not only show potential in the field of AID, but also in other diseases showing hyperinflammation, such as COVID-19, or in which autoimmunity may not be that prominent, including IPF.
Future research should aim at gaining more knowledge on the pathogenic role of BTK signaling and the effects of its inhibition in inflammatory and AID. Importantly, these disorders often involve aberrant activation of other cell types of the immune system, T cells in particular. BTK is normally not expressed in T cells,1 but some of the currently available BTKi have considerable off-target effects on signaling molecules expressed in T cells, including TEC, ITK, Janus kinase 3 (JAK3), or lymphocyte-specific protein tyrosine kinase (LCK; Estupiñán et al., 2021). It is attractive to explore the benefits of BTKi that show additional specificity to these related kinases as was observed in the treatment of human chronic lymphocytic leukemia or GvHD in mice (Schutt et al., 2015; Long et al., 2017). Likewise, BTK inhibition was shown to dampen inflammatory arthritis by blocking B cell activation and proliferation as well as by abolishing FcγR-induced production of pro-inflammatory cytokines in macrophages (Di Paolo et al., 2011). However, inhibitors with lower specificity also have greater off-target effects. Ibrutinib-related adverse events, including atrial fibrillation and hemorrhage, were not observed during treatment with acalabrutinib, which has improved specificity (Byrd et al., 2016). To prevent adverse events, BTKi with higher specificity are currently being developed and tested in the clinic (Estupiñán et al., 2021; von Hundelshausen and Siess, 2021). Effectiveness of strategies using combinational therapies should also be explored. In treatment of B cell malignancies, such combinational or sequential therapeutic strategies are often focused on combinations with other inhibitors acting on B cells [e.g., idelalisib targeting phosphoinositide 3-kinase (PI3K; de Rooij et al., 2015; Pal Singh et al., 2018)]. In the field of AID, however, a combination with inhibitors of T cell activation or general immunomodulatory therapies may yield high efficacy (Gillooly et al., 2017). Further studies are warranted to learn which AID patient benefits most from which therapeutic strategy.
Author Contributions
SN, RH, and OC wrote the manuscript. All authors agreed to be accountable for the content of the work.
Funding
This project was funded by grants from the Target2B consortium, Erasmus MC MRace, and the Dutch Arthritis Association.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Abu-Rish, E. Y., Amrani, Y., and Browning, M. J. (2013). Toll-like receptor 9 activation induces expression of membrane-bound B-cell activating factor (BAFF) on human B cells and leads to increased proliferation in response to both soluble and membrane-bound BAFF. Rheumatology (Oxford). 52, 1190–1201. doi: 10.1093/rheumatology/ket006
Al-Harbi, N. O., Nadeem, A., Ahmad, S. F., Bakheet, S. A., El-Sherbeeny, A. M., Ibrahim, K. E., et al. (2020). Therapeutic treatment with Ibrutinib attenuates imiquimod-induced psoriasis-like inflammation in mice through downregulation of oxidative and inflammatory mediators in neutrophils and dendritic cells. Eur. J. Pharmacol. 877:173088. doi: 10.1016/j.ejphar.2020.173088
Angst, D., Gessier, F., Janser, P., Vulpetti, A., Wälchli, R., Beerli, C., et al. (2020). Discovery of LOU064 (Remibrutinib), a potent and highly selective covalent inhibitor of bruton’s tyrosine kinase. J. Med. Chem. 63, 5102–5118. doi: 10.1021/acs.jmedchem.9b01916
Bieber, K., Kridin, K., Emtenani, S., Boch, K., Schmidt, E., and Ludwig, R. J. (2021). Milestones in personalized medicine in pemphigus and pemphigoid. Front. Immunol. 11:3294. doi: 10.3389/fimmu.2020.591971
Bonami, R. H., Sullivan, A. M., Case, J. B., Steinberg, H. E., Hoek, K. L., Khan, W. N., et al. (2014). Bruton’s tyrosine kinase promotes persistence of mature anti-insulin B cells. J. Immunol. 192, 14591470. doi: 10.4049/jimmunol.1300125
Brunner, C., Avots, A., Kreth, H. W., Serfling, E., and Schuster, V. (2002). Bruton’s tyrosine kinase is activated upon CD40 stimulation in human B lymphocytes. Immunobiology 206, 432–440. doi: 10.1078/0171-2985-00192
Busygina, K., Jamasbi, J., Seiler, T., Deckmyn, H., Weber, C., Brandl, R., et al. (2018). Oral Bruton tyrosine kinase inhibitors selectively block atherosclerotic plaque–triggered thrombus formation in humans. Blood 131, 2605–2616. doi: 10.1182/blood-2017-09-808808
Byrd, J. C., Harrington, B., O’Brien, S., Jones, J. A., Schuh, A., Devereux, S., et al. (2016). Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 323–332. doi: 10.1056/NEJMoa1509981
Caldwell, R. D., Qiu, H., Askew, B. C., Bender, A. T., Brugger, N., Camps, M., et al. (2019). Discovery of evobrutinib: an oral, potent, and highly selective, covalent bruton’s tyrosine kinase (BTK) inhibitor for the treatment of immunological diseases. J. Med. Chem. 62, 7643–7655. doi: 10.1021/acs.jmedchem.9b00794
Castro, I., Wright, J. A., Damdinsuren, B., Hoek, K. L., Carlesso, G., Shinners, N. P., et al. (2009). B cell receptor-mediated sustained c-Rel activation facilitates late transitional B cell survival through control of B cell activating factor receptor and NF-kappaB2. J. Immunol. 182, 7729–7737. doi: 10.4049/jimmunol.0803281
Chalmers, S. A., Doerner, J. L., Bosanac, T., Khalil, S., Smith, D., Harcken, C., et al. (2017). Remission induction of established nephritis by therapeutic administration of a novel BTK inhibitor in an inducible model of lupus nephritis. J. Immunol. 198, 224.16.
Chang, B. Y., Huang, M. M., Francesco, M., Chen, J., Sokolove, J., Magadala, P., et al. (2011). The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res. Ther. 13:R115. doi: 10.1186/ar3400
Cohen, S., Tuckwell, K., Katsumoto, T. R., Zhao, R., Galanter, J., Lee, C., et al. (2020). Fenebrutinib versus Placebo or Adalimumab in rheumatoid arthritis: a randomized, double-blind, phase II Trial (ANDES Study). Arthritis Rheumatol. 72, 1435–1446. doi: 10.1002/art.41275
Colonna, L., Catalano, G., Chew, C., D’Agati, V., Thomas, J. W., Wong, F. S., et al. (2010). Therapeutic targeting of Syk in autoimmune diabetes. J. Immunol. 185, 1532–1543. doi: 10.4049/jimmunol.1000983
Cornec, D., Costa, S., Devauchelle-Pensec, V., Jousse-Joulin, S., Marcorelles, P., Berthelot, J. M., et al. (2016). Blood and salivary-gland BAFF-driven B-cell hyperactivity is associated to rituximab inefficacy in primary Sjogren’s syndrome. J. Autoimmun. 67, 102–110. doi: 10.1016/j.jaut.2015.11.002
Corneth, O. B. J., de Bruijn, M. J. W., Rip, J., Asmawidjaja, P. S., Kil, L. P., and Hendriks, R. W. (2016). Enhanced expression of bruton’s tyrosine kinase in B cells drives systemic autoimmunity by disrupting T Cell Homeostasis. J. Immunol. 197, 58–67. doi: 10.4049/jimmunol.1600208
Corneth, O. B. J., Verstappen, G. M. P., Paulissen, S. M. J., de Bruijn, M. J. W., Rip, J., Lukkes, M., et al. (2017). Enhanced Bruton’s Tyrosine Kinase activity in peripheral blood B lymphocytes from patients with autoimmune disease. Arthritis Rheumatol. (Hoboken, N.J.) 69, 1313–1324. doi: 10.1002/art.40059
Blez, D., Blaize, M., Soussain, C., Boissonnas, A., Meghraoui-Kheddar, A., Menezes, N., et al. (2020). Ibrutinib induces multiple functional defects in the neutrophil response against Aspergillus fumigatus. Haematologica 105, 478–489. doi: 10.3324/haematol.2019.219220
de Gorter, D. J. J., Beuling, E. A., Kersseboom, R., Middendorp, S., van Gils, J. M., Hendriks, R. W., et al. (2007). Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity 26, 93–104. doi: 10.1016/j.immuni.2006.11.012
de Porto, A. P., Liu, Z., de Beer, R., Florquin, S., de Boer, O. J., Hendriks, R. W., et al. (2019). Btk inhibitor ibrutinib reduces inflammatory myeloid cell responses in the lung during murine pneumococcal pneumonia. Mol. Med. 25:3. doi: 10.1186/s10020-018-0069-7
de Rooij, M. F. M., Kuil, A., Geest, C. R., Eldering, E., Chang, B. Y., Buggy, J. J., et al. (2012). The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 119, 2590–2594. doi: 10.1182/blood-2011-11-390989
de Rooij, M. F. M., Kuil, A., Kater, A. P., Kersten, M. J., Pals, S. T., and Spaargaren, M. (2015). Ibrutinib and idelalisib synergistically target BCR-controlled adhesion in MCL and CLL: a rationale for combination therapy. Blood 125, 2306–2309. doi: 10.1182/blood-2014-12-619163
Di Paolo, J. A., Huang, T., Balazs, M., Barbosa, J., Barck, K. H., Bravo, B. J., et al. (2011). Specific Btk inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat. Chem. Biol. 7, 41–50. doi: 10.1038/nchembio.481
Dieude, P., Wipff, J., Guedj, M., Ruiz, B., Melchers, I., Hachulla, E., et al. (2009). BANK1 is a genetic risk factor for diffuse cutaneous systemic sclerosis and has additive effects with IRF5 and STAT4. Arthritis Rheum. 60, 3447–3454. doi: 10.1002/art.24885
Dispenza, M. C., Krier-Burris, R. A., Chhiba, K. D., Undem, B. J., Robida, P. A., and Bochner, B. S. (2020). Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J. Clin. Invest. 130, 4759–4770. doi: 10.1172/JCI138448
Dolgin, E. (2021). BTK blockers make headway in multiple sclerosis. Nat. Biotechnol. 39, 3–5. doi: 10.1038/s41587-020-00790-710.1038/s41587-020-00790-7
Dorner, T., Posch, M. G., Li, Y., Petricoul, O., Cabanski, M., Milojevic, J. M., et al. (2019). Treatment of primary Sjogren’s syndrome with ianalumab (VAY736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann. Rheum. Dis. 78, 641–647. doi: 10.1136/annrheumdis-2018-214720
Du, S. W., Jacobs, H. M., Arkatkar, T., Rawlings, D. J., and Jackson, S. W. (2018). Integrated B Cell, Toll-like, and BAFF receptor signals promote autoantibody production by transitional B Cells. J. Immunol. 201, 3258–3268. doi: 10.4049/jimmunol.1800393
Dubovsky, J. A., Flynn, R., Du, J., Harrington, B. K., Zhong, Y., Kaffenberger, B., et al. (2014). Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J. Clin. Invest. 124, 4867–4876. doi: 10.1172/JCI75328
Duret, P., Ye, T., Ng, W., Saraux, A., Devauchelle Pensec, V., Seror, R., et al. (2019). BTK overexpression is associated with the risk of lymphoma in primary sjögren’s syndrome: data from whole blood transcriptome of 346 patients followed-up prospectively for 10 years [abstract]. Arthritis Rheumatol. 71.
Einhaus, J., Pecher, A.-C., Asteriti, E., Schmid, H., Secker, K.-A., Duerr-Stoerzer, S., et al. (2020). Inhibition of effector B cells by ibrutinib in systemic sclerosis. Arthritis Res. Ther. 22:66. doi: 10.1186/s13075-020-02153-8
Estupiñán, H. Y., Berglöf, A., Zain, R., and Smith, C. I. E. (2021). Comparative Analysis of BTK Inhibitors and Mechanisms Underlying Adverse Effects. Front. Cell Dev. Biol. 9:630942. doi: 10.3389/fcell.2021.630942
Fiedler, K., Sindrilaru, A., Terszowski, G., Kokai, E., Feyerabend, T. B., Bullinger, L., et al. (2011). Neutrophil development and function critically depend on Bruton tyrosine kinase in a mouse model of X-linked agammaglobulinemia. Blood 117, 1329–1339. doi: 10.1182/blood-2010-04-281170
Fiorcari, S., Maffei, R., Audrito, V., Martinelli, S., Ten Hacken, E., Zucchini, P., et al. (2016). Ibrutinib modifies the function of monocyte/macrophage population in chronic lymphocytic leukemia. Oncotarget 7, 65968–65981. doi: 10.18632/oncotarget.11782
Florence, J. M., Krupa, A., Booshehri, L. M., Davis, S. A., Matthay, M. A., and Kurdowska, A. K. (2018). Inhibiting Bruton’s tyrosine kinase rescues mice from lethal influenza-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 315, L52–L58. doi: 10.1152/ajplung.00047.2018
Florence, J. M., Krupa, A., Booshehri, L. M., Gajewski, A. L., and Kurdowska, A. K. (2018b). Disrupting the Btk pathway suppresses COPD-like lung alterations in atherosclerosis prone ApoE(-/-) mice following regular exposure to cigarette smoke. Int. J. Mol. Sci. 19:343. doi: 10.3390/ijms19020343
Forssell, J., Sideras, P., Eriksson, C., Malm-Erjefält, M., Rydell-Törmänen, K., Ericsson, P.-O., et al. (2005). Interleukin-2-inducible T cell kinase regulates mast cell degranulation and acute allergic responses. Am. J. Respir. Cell Mol. Biol. 32, 511–520. doi: 10.1165/rcmb.2004-0348OC
Gilbert, C., Levasseur, S., Desaulniers, P., Dusseault, A.-A., Thibault, N., Bourgoin, S. G., et al. (2003). Chemotactic factor-induced recruitment and activation of Tec family kinases in human neutrophils. II. Effects of LFM-A13, a specific Btk inhibitor. J. Immunol. 170, 5235–5243. doi: 10.4049/jimmunol.170.10.5235
Gillooly, K. M., Pulicicchio, C., Pattoli, M. A., Cheng, L., Skala, S., Heimrich, E. M., et al. (2017). Bruton’s tyrosine kinase inhibitor BMS-986142 in experimental models of rheumatoid arthritis enhances efficacy of agents representing clinical standard-of-care. PLoS One 12:e0181782. doi: 10.1371/journal.pone.0181782
Glauzy, S., Sng, J., Bannock, J. M., Gottenberg, J. E., Korganow, A. S., Cacoub, P., et al. (2017). Defective Early B cell tolerance checkpoints in sjogren’s syndrome patients. Arthritis Rheumatol. 69, 2203–2208. doi: 10.1002/art.40215
Goodale, E. C., Varjonen, K. E., Outerbridge, C. A., Bizikova, P., Borjesson, D., Murrell, D. F., et al. (2020a). Efficacy of a bruton’s tyrosine kinase inhibitor (PRN-473) in the treatment of canine pemphigus foliaceus. Vet. Dermatol. 31, 291–e71. doi: 10.1111/vde.12841
Goodale, E. C., White, S. D., Bizikova, P., Borjesson, D., Murrell, D. F., Bisconte, A., et al. (2020b). Open trial of Bruton’s tyrosine kinase inhibitor (PRN1008) in the treatment of canine pemphigus foliaceus. Vet. Dermatol. 31:410–e110. doi: 10.1111/vde.12878
Gourh, P., Agarwal, S. K., Martin, E., Divecha, D., Rueda, B., Bunting, H., et al. (2010). Association of the C8orf13-BLK region with systemic sclerosis in North-American and European populations. J. Autoimmun. 34, 155–162. doi: 10.1016/j.jaut.2009.08.014
Gu, Y., Huang, B., Yang, Y., Qi, M., Lu, G., Xia, D., et al. (2018). Ibrutinib exacerbates bleomycin-induced pulmonary fibrosis via promoting inflammation. Inflammation 41, 904–913. doi: 10.1007/s10753-018-0745-3
Halcomb, K. E., Musuka, S., Gutierrez, T., Wright, H. L., and Satterthwaite, A. B. (2008). Btk regulates localization, in vivo activation, and class switching of anti-DNA B cells. Mol. Immunol. 46, 233–241. doi: 10.1016/j.molimm.2008.08.278
Hamza, N., Bootsma, H., Yuvaraj, S., Spijkervet, F. K., Haacke, E. A., Pollard, R. P., et al. (2012). Persistence of immunoglobulin-producing cells in parotid salivary glands of patients with primary Sjogren’s syndrome after B cell depletion therapy. Ann. Rheum. Dis. 71, 1881–1887. doi: 10.1136/annrheumdis-2011-201189
Hartkamp, L. M., Fine, J. S., van Es, I. E., Tang, M. W., Smith, M., Woods, J., et al. (2015). Btk inhibition suppresses agonist-induced human macrophage activation and inflammatory gene expression in RA synovial tissue explants. Ann. Rheum. Dis. 74, 1603–1611. doi: 10.1136/annrheumdis-2013-204143
Haselmayer, P., Camps, M., Liu-Bujalski, L., Nguyen, N., Morandi, F., Head, J., et al. (2019). Efficacy and pharmacodynamic modeling of the BTK inhibitor evobrutinib in autoimmune disease models. J. Immunol. 202, 28882906. doi: 10.4049/jimmunol.1800583
Hata, D., Kawakami, Y., Inagaki, N., Lantz, C. S., Kitamura, T., Khan, W. N., et al. (1998). Involvement of Bruton’s tyrosine kinase in FcepsilonRI-dependent mast cell degranulation and cytokine production. J. Exp. Med. 187, 1235–1247. doi: 10.1084/jem.187.8.1235
Hayer, S., Polzer, K., Brandl, A., Zwerina, J., Kireva, T., Smolen, J. S., et al. (2008). B-cell infiltrates induce endosteal bone formation in inflammatory arthritis. J. Bone Min. Res. 23, 1650–1660. doi: 10.1359/jbmr.080508
Hendriks, R. W., Yuvaraj, S., and Kil, L. P. (2014). Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat. Rev. Cancer 14, 219–232. doi: 10.1038/nrc3702
Herter, J. M., Margraf, A., Volmering, S., Correia, B. E., Bradshaw, J. M., Bisconte, A., et al. (2018). PRN473, an inhibitor of Bruton’s tyrosine kinase, inhibits neutrophil recruitment via inhibition of macrophage antigen-1 signalling. Br. J. Pharmacol. 175, 429–439. doi: 10.1111/bph.14090
Heukels, P., van Hulst, J. A. C., van Nimwegen, M., Boorsma, C. E., Melgert, B. N., and von (2019). Enhanced Bruton’s tyrosine kinase in B-cells and autoreactive IgA in patients with idiopathic pulmonary fibrosis. Respir. Res. 20:232. doi: 10.1186/s12931-019-1195-7
Hitoshi, Y., Sonoda, E., Kikuchi, Y., Yonehara, S., Nakauchi, H., and Takatsu, K. (1993). IL-5 receptor positive B cells, but not eosinophils, are functionally and numerically influenced in mice carrying the X-linked immune defect. Int. Immunol. 5, 1183–1190. doi: 10.1093/intimm/5.9.1183
Honigberg, L. A., Smith, A. M., Sirisawad, M., Verner, E., Loury, D., Chang, B., et al. (2010). The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U.S.A. 107, 13075–13080. doi: 10.1073/pnas.1004594107
Horwood, N. J., Page, T. H., McDaid, J. P., Palmer, C. D., Campbell, J., Mahon, T., et al. (2006). Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J. Immunol. 176, 3635–3641. doi: 10.4049/jimmunol.176.6.3635
Isenberg, D., Furie, R., Jones, N., Guibord, P., Galanter, J., Lee, C., et al. (2019). Efficacy, safety, and pharmacodynamic effects of the bruton’s tyrosine kinase inhibitor, fenebrutinib (GDC-0853), in moderate to severe systemic lupus erythematosus: results of a phase 2 randomized controlled trial [abstract]. Arthritis Rheumatol. 71.
Ito, M., Shichita, T., Okada, M., Komine, R., Noguchi, Y., Yoshimura, A., et al. (2015). Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 6:7360. doi: 10.1038/ncomms8360
Iyer, A. S., Morales, J. L., Huang, W., Ojo, F., Ning, G., Wills, E., et al. (2011). Absence of Tec family kinases interleukin-2 inducible T cell kinase (Itk) and Bruton’s tyrosine kinase (Btk) severely impairs Fc epsilonRI-dependent mast cell responses. J. Biol. Chem. 286, 9503–9513. doi: 10.1074/jbc.M110.165613
Jansson, L., and Holmdahl, R. (1993). Genes on the X chromosome affect development of collagen-induced arthritis in mice. Clin. Exp. Immunol. 94, 459–465. doi: 10.1111/j.1365-2249.1993.tb08218.x
Kawakami, Y., Inagaki, N., Salek-Ardakani, S., Kitaura, J., Tanaka, H., Nagao, K., et al. (2006). Regulation of dendritic cell maturation and function by Bruton’s tyrosine kinase via IL-10 and Stat3. Proc. Natl. Acad. Sci. U.S.A. 103, 153–158. doi: 10.1073/pnas.0509784103
Kendall, P. L., Moore, D. J., Hulbert, C., Hoek, K. L., Khan, W. N., and Thomas, J. W. (2009). Reduced diabetes in btk-deficient nonobese diabetic mice and restoration of diabetes with provision of an anti-insulin IgH chain transgene. J. Immunol. 183, 6403–6412. doi: 10.4049/jimmunol.0900367
Kenny, E. F., Quinn, S. R., Doyle, S. L., Vink, P. M., van Eenennaam, H., and O’Neill, L. A. J. (2013). Bruton’s tyrosine kinase mediates the synergistic signalling between TLR9 and the B cell receptor by regulating calcium and calmodulin. PLoS One 8:e74103. doi: 10.1371/journal.pone.0074103
Khan, W. N., Alt, F. W., Gerstein, R. M., Malynn, B. A., Larsson, I., Rathbun, G., et al. (1995). Defective B cell development and function in Btk-deficient mice. Immunity 3, 283–299. doi: 10.1016/1074-7613(95)90114-0
Kil, L. P., de Bruijn, M. J. W., van Nimwegen, M., Corneth, O. B. J., van Hamburg, J. P., Dingjan, G. M., et al. (2012). Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 119, 3744–3756. doi: 10.1182/blood-2011-12-397919
Kinzel, S., and Weber, M. S. (2016). B cell-directed therapeutics in multiple sclerosis: rationale and clinical evidence. CNS Drugs 30, 1137–1148. doi: 10.1007/s40263-016-0396-6
Kneidinger, M., Schmidt, U., Rix, U., Gleixner, K. V., Vales, A., Baumgartner, C., et al. (2008). The effects of dasatinib on IgE receptor-dependent activation and histamine release in human basophils. Blood 111, 3097–3107. doi: 10.1182/blood-2007-08-104372
Koike, M., Kikuchi, Y., Tominaga, A., Takaki, S., Akagi, K., Miyazaki, J., et al. (1995). Defective IL-5-receptor-mediated signaling in B cells of X-linked immunodeficient mice. Int. Immunol. 7, 21–30. doi: 10.1093/intimm/7.1.21
Kong, W., Deng, W., Sun, Y., Huang, S., Zhang, Z., Shi, B., et al. (2018). Increased expression of Bruton’s tyrosine kinase in peripheral blood is associated with lupus nephritis. Clin. Rheumatol. 37, 43–49. doi: 10.1007/s10067-017-3717-3
Krupa, A., Fol, M., Rahman, M., Stokes, K. Y., Florence, J. M., Leskov, I. L., et al. (2014). Silencing Bruton’s tyrosine kinase in alveolar neutrophils protects mice from LPS/immune complex-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L435–L448. doi: 10.1152/ajplung.00234.2013
Kuter, D. J., Efraim, M., Mayer, J., Trneny, M., McDonald, V., Bird, R., et al. (2020). Phase I/II, open-label, ongoing study of PRN1008 (Rilzabrutinib), an oral bruton tyrosine kinase inhibitor, in patients with heavily pretreated immune thrombocytopenia (ITP) [abstract]. Res Pr. Thromb. Haemost 4.
Lam, K.-P., Kühn, R., and Rajewsky, K. (1997). In Vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90, 1073–1083. doi: 10.1016/S0092-8674(00)80373-6
Langrish, C. L., Bradshaw, J. M., Owens, T. D., Campbell, R. L., Francesco, M. R., Karr, D. E., et al. (2017). PRN1008, a reversible covalent BTK inhibitor in clinical development for immune thrombocytopenic purpura. Blood 130:1052. doi: 10.1182/blood.V130.Suppl_1.1052.1052
Lee, K.-G., Kim, S. S.-Y., Kui, L., Voon, D. C.-C., Mauduit, M., Bist, P., et al. (2015). Bruton’s tyrosine kinase phosphorylates DDX41 and activates its binding of dsDNA and STING to initiate type 1 interferon response. Cell Rep. 10, 1055–1065. doi: 10.1016/j.celrep.2015.01.039
Lee, K.-G., Xu, S., Wong, E.-T., Tergaonkar, V., and Lam, K.-P. (2008a). Bruton’s tyrosine kinase separately regulates NFkappaB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B Cells. J. Biol. Chem. 283, 11189–11198. doi: 10.1074/jbc.M708516200
Lee, S. H., Kim, T., Jeong, D., Kim, N., and Choi, Y. (2008b). The tec family tyrosine kinase Btk Regulates RANKL-induced osteoclast maturation. J. Biol. Chem. 283, 11526–11534. doi: 10.1074/jbc.M708935200
Li, Y.-F., Lee, K.-G., Ou, X., and Lam, K.-P. (2014). Bruton’s tyrosine kinase and protein kinase C μ are required for TLR7/9-induced IKKα and IRF-1 activation and interferon-β production in conventional dendritic cells. PLoS One 9:e105420. doi: 10.1371/journal.pone.0105420
Liao, X. C., and Littman, D. R. (1995). Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity 3, 757–769. doi: 10.1016/1074-7613(95)90065-9
Liu, X., Pichulik, T., Wolz, O.-O., Dang, T.-M., Stutz, A., Dillen, C., et al. (2017). Human NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome activity is regulated by and potentially targetable through Bruton tyrosine kinase. J. Allergy Clin. Immunol. 140, 1054–1067.e10. doi: 10.1016/j.jaci.2017.01.017
Liu, Y. T., Ding, H. H., Lin, Z. M., Wang, Q., Chen, L., Liu, S. S., et al. (2021). A novel tricyclic BTK inhibitor suppresses B cell responses and osteoclastic bone erosion in rheumatoid arthritis. Acta Pharmacol. Sin. doi: 10.1038/s41401-020-00578-0
Long, M., Beckwith, K., Do, P., Mundy, B. L., Gordon, A., Lehman, A. M., et al. (2017). Ibrutinib treatment improves T cell number and function in CLL patients. J. Clin. Invest. 127, 3052–3064. doi: 10.1172/JCI89756
MacGlashan, D. Jr., Honigberg, L. A., Smith, A., Buggy, J., and Schroeder, J. T. (2011). Inhibition of IgE-mediated secretion from human basophils with a highly selective Bruton’s tyrosine kinase, Btk, inhibitor. Int. Immunopharmacol. 11, 475–479. doi: 10.1016/j.intimp.2010.12.018
Mackay, F., Woodcock, S. A., Lawton, P., Ambrose, C., Baetscher, M., Schneider, P., et al. (1999). Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190, 1697–1710. doi: 10.1084/jem.190.11.1697
Mangla, A., Khare, A., Vineeth, V., Panday, N. N., Mukhopadhyay, A., Ravindran, B., et al. (2004). Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood 104, 1191–1197. doi: 10.1182/blood-2004-01-0207
Mato, A. R., Roeker, L. E., Lamanna, N., Allan, J. N., Leslie, L., Pagel, J. M., et al. (2020). Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood 136, 1134–1143. doi: 10.1182/blood.2020006965
Matsuda, T., Takahashi-Tezuka, M., Fukada, T., Okuyama, Y., Fujitani, Y., Tsukada, S., et al. (1995). Association and activation of Btk and Tec tyrosine kinases by gp130, a signal transducer of the interleukin-6 family of cytokines. Blood 85, 627–633.
Matsushita, T., Hasegawa, M., Matsushita, Y., Echigo, T., Wayaku, T., Horikawa, M., et al. (2007). Elevated serum BAFF levels in patients with localized scleroderma in contrast to other organ-specific autoimmune diseases. Exp. Dermatol. 16, 87–93. doi: 10.1111/j.1600-0625.2006.00485.x
Matsushita, T., Kobayashi, T., Mizumaki, K., Kano, M., Sawada, T., Tennichi, M., et al. (2018). BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci. Adv. 4:eaas9944. doi: 10.1126/sciadv.aas9944
Melcher, M., Unger, B., Schmidt, U., Rajantie, I. A., Alitalo, K., and Ellmeier, W. (2008). Essential roles for the Tec family kinases Tec and Btk in M-CSF receptor signaling pathways that regulate macrophage survival. J. Immunol. 180, 8048–8056. doi: 10.4049/jimmunol.180.12.8048
Merad, M., and Martin, J. C. (2020). Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 20, 355–362. doi: 10.1038/s41577-020-0331-4
Miklos, D., Cutler, C. S., Arora, M., Waller, E. K., Jagasia, M., Pusic, I., et al. (2017). Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 130, 2243–2250. doi: 10.1182/blood-2017-07-793786
Miloševiæ, I., Jovanoviæ, J., and Stevanovic, O. (2020). Atypical course of COVID-19 in patient with Bruton agammaglobulinemia. J. Infect. Dev. Ctries. 14, 1248–1251. doi: 10.3855/jidc.13840
Montalban, X., Arnold, D. L., Weber, M. S., Staikov, I., Piasecka-Stryczynska, K., Willmer, J., et al. (2019). Placebo-Controlled trial of an oral BTK inhibitor in multiple sclerosis. N. Engl. J. Med. 380, 2406–2417. doi: 10.1056/NEJMoa1901981
Mueller, H., Stadtmann, A., Van Aken, H., Hirsch, E., Wang, D., Ley, K., et al. (2010). Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood 115, 3118–3127. doi: 10.1182/blood-2009-11-254185
Mukhopadhyay, S., George, A., Bal, V., Ravindran, B., and Rath, S. (1999). Bruton’s tyrosine kinase deficiency in macrophages inhibits nitric oxide generation leading to enhancement of IL-12 induction. J. Immunol. 163, 1786–1792.
Mukhopadhyay, S., Mohanty, M., Mangla, A., George, A., Bal, V., Rath, S., et al. (2002). Macrophage effector functions controlled by bruton’s tyrosine kinase are more crucial than the cytokine balance of t cell responses for microfilarial clearance. J. Immunol. 168, 2914–2921. doi: 10.4049/jimmunol.168.6.2914
Nadeem, A., Ahmad, S. F., Al-Harbi, N. O., El-Sherbeeny, A. M., Alasmari, A. F., Alanazi, W. A., et al. (2020). Bruton’s tyrosine kinase inhibitor suppresses imiquimod-induced psoriasis-like inflammation in mice through regulation of IL-23/IL-17A in innate immune cells. Int. Immunopharmacol. 80:106215. doi: 10.1016/j.intimp.2020.106215
Nadeem, A., Ahmad, S. F., Al-Harbi, N. O., Ibrahim, K. E., Siddiqui, N., Al-Harbi, M. M., et al. (2019). Inhibition of Bruton’s tyrosine kinase and IL-2 inducible T-cell kinase suppresses both neutrophilic and eosinophilic airway inflammation in a cockroach allergen extract-induced mixed granulocytic mouse model of asthma using preventative and therapeutic. Pharmacol. Res. 148:104441. doi: 10.1016/j.phrs.2019.104441
Natarajan, G., Terrazas, C., Oghumu, S., Varikuti, S., Dubovsky, J. A., Byrd, J. C., et al. (2016). Ibrutinib enhances IL-17 response by modulating the function of bone marrow derived dendritic cells. Oncoimmunology 5:e1057385. doi: 10.1080/2162402X.2015.1057385
Ni Gabhann, J., Hams, E., Smith, S., Wynne, C., Byrne, J. C., Brennan, K., et al. (2014). Btk regulates macrophage polarization in response to lipopolysaccharide. PLoS One 9:e85834. doi: 10.1371/journal.pone.0085834
Nyhoff, L. E., Barron, B. L., Johnson, E. M., Bonami, R. H., Maseda, D., Fensterheim, B. A., et al. (2016). Bruton’s Tyrosine Kinase Deficiency inhibits autoimmune arthritis in mice but fails to block immune complex-mediated inflammatory arthritis. Arthritis Rheumatol. 68, 1856–1868. doi: 10.1002/art.39657
Ormsby, T., Schlecker, E., Ferdin, J., Tessarz, A. S., Angelisová, P., Köprülü, A. D., et al. (2011). Btk is a positive regulator in the TREM-1/DAP12 signaling pathway. Blood 118, 936–945. doi: 10.1182/blood-2010-11-317016
Pal Singh, S., Dammeijer, F., and Hendriks, R. W. (2018). Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer 17:57. doi: 10.1186/s12943-018-0779-z
Pan, Z., Scheerens, H., Li, S.-J., Schultz, B. E., Sprengeler, P. A., Burrill, L. C., et al. (2007). Discovery of selective irreversible inhibitors for bruton’s tyrosine kinase. ChemMedChem 2, 58–61. doi: 10.1002/cmdc.200600221
Phillips, J. E., Renteria, L., Burns, L., Harris, P., Peng, R., Bauer, C. M. T., et al. (2016). Btk Inhibitor RN983 delivered by dry powder nose-only aerosol inhalation inhibits bronchoconstriction and pulmonary inflammation in the ovalbumin allergic mouse model of asthma. J. Aerosol Med. Pulm. Drug Deliv. 29, 233–241. doi: 10.1089/jamp.2015.1210
Prezzo, A., Cavaliere, F. M., Bilotta, C., Pentimalli, T. M., Iacobini, M., Cesini, L., et al. (2019). Ibrutinib-based therapy impaired neutrophils microbicidal activity in patients with chronic lymphocytic leukemia during the early phases of treatment. Leuk. Res 87:106233. doi: 10.1016/j.leukres.2019.106233
Quinti, I., Lougaris, V., Milito, C., Cinetto, F., Pecoraro, A., Mezzaroma, I., et al. (2020). A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J. Allergy Clin. Immunol. 146, 211–213.e4. doi: 10.1016/j.jaci.2020.04.013
Rawlings, D. J., Scharenberg, A. M., Park, H., Wahl, M. I., Lin, S., Kato, R. M., et al. (1996). Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science 271, 822–825. doi: 10.1126/science.271.5250.822
Ren, L., Campbell, A., Fang, H., Gautam, S., Elavazhagan, S., Fatehchand, K., et al. (2016). Analysis of the Effects of the Bruton’s tyrosine kinase (Btk) inhibitor ibrutinib on monocyte Fcγ Receptor (FcR) function. J. Biol. Chem. 291, 3043–3052. doi: 10.1074/jbc.M115.687251
Rip, J., Van Der Ploeg, E. K., Hendriks, R. W., and Corneth, O. B. J. (2018). The role of bruton’s tyrosine kinase in immune cell signaling and systemic autoimmunity. Crit. Rev. Immunol. 38, 17–62. doi: 10.1615/CritRevImmunol.2018025184
Roschewski, M., Lionakis, M. S., Sharman, J. P., Roswarski, J., Goy, A., Monticelli, M. A., et al. (2020). Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 5:eabd0110. doi: 10.1126/sciimmunol.abd0110
Rueda, B., Gourh, P., Broen, J., Agarwal, S. K., Simeon, C., Ortego-Centeno, N., et al. (2010). BANK1 functional variants are associated with susceptibility to diffuse systemic sclerosis in Caucasians. Ann. Rheum. Dis. 69, 700–705. doi: 10.1136/ard.2009.118174
Sakkas, L. I., and Bogdanos, D. P. (2016). Systemic sclerosis: new evidence re-enforces the role of B cells. Autoimmun. Rev. 15, 155–161. doi: 10.1016/j.autrev.2015.10.005
Sasaki, Y., Casola, S., Kutok, J. L., Rajewsky, K., and Schmidt-Supprian, M. (2004). TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J. Immunol. 173, 2245–2252. doi: 10.4049/jimmunol.173.4.2245
Sato, S., Katagiri, T., Takaki, S., Kikuchi, Y., Hitoshi, Y., Yonehara, S., et al. (1994). IL-5 receptor-mediated tyrosine phosphorylation of SH2/SH3-containing proteins and activation of Bruton’s tyrosine and Janus 2 kinases. J. Exp. Med. 180, 2101–2111. doi: 10.1084/jem.180.6.2101
Scarfò, L., Chatzikonstantinou, T., Rigolin, G. M., Quaresmini, G., Motta, M., Vitale, C., et al. (2020). COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia 34, 2354–2363. doi: 10.1038/s41375-020-0959-x
Schafer, P. H., Kivitz, A. J., Ma, J., Korish, S., Sutherland, D., Li, L., et al. (2020). Spebrutinib (CC-292) affects markers of B cell activation, chemotaxis, and osteoclasts in patients with rheumatoid arthritis: results from a mechanistic study. Rheumatol. Ther. 7, 101–119. doi: 10.1007/s40744-019-00182-7
Schutt, S. D., Fu, J., Nguyen, H., Bastian, D., Heinrichs, J., Wu, Y., et al. (2015). Inhibition of BTK and ITK with ibrutinib is effective in the prevention of chronic graft-versus-host disease in mice. PLoS One 10:e0137641. doi: 10.1371/journal.pone.0137641
Schwartz, M. A., Kolhatkar, N. S., Thouvenel, C., Khim, S., and Rawlings, D. J. (2014). CD4 T Cells and CD40 participate in selection and homeostasis of peripheral B cells. J. Immunol. 193, 3492–3502. doi: 10.4049/jimmunol.1400798
Schweighoffer, E., Nys, J., Vanes, L., Smithers, N., and Tybulewicz, V. L. J. (2017). TLR4 signals in B lymphocytes are transduced via the B cell antigen receptor and SYK. J. Exp. Med. 214, 1269–1280. doi: 10.1084/jem.20161117
Schweighoffer, E., Vanes, L., Nys, J., Cantrell, D., McCleary, S., Smithers, N., et al. (2013). The BAFF receptor transduces survival signals by Co-opting the B cell receptor signaling pathway. Immunity 38, 475–488. doi: 10.1016/j.immuni.2012.11.015
Shen, L., Gao, C., Suresh, L., Xian, Z., Song, N., Chaves, L. D., et al. (2016). Central role for marginal zone B cells in an animal model of Sjogren’s syndrome. Clin. Immunol. 168, 30–36. doi: 10.1016/j.clim.2016.04.008
Shinners, N. P., Carlesso, G., Castro, I., Hoek, K. L., Corn, R. A., Woodland, R. T., et al. (2007). Bruton’s tyrosine kinase mediates NF-kappa B activation and B cell survival by B cell-activating factor receptor of the TNF-R family. J. Immunol. 179, 3872–3880. doi: 10.4049/jimmunol.179.6.3872
Shinohara, M., Koga, T., Okamoto, K., Sakaguchi, S., Arai, K., Yasuda, H., et al. (2008). Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell 132, 794–806. doi: 10.1016/j.cell.2007.12.037
Smiljkovic, D., Blatt, K., Stefanzl, G., Dorofeeva, Y., Skrabs, C., Focke-Tejkl, M., et al. (2017). BTK inhibition is a potent approach to block IgE-mediated histamine release in human basophils. Allergy 72, 1666–1676. doi: 10.1111/all.13166
Smith, S. H., and Cancro, M. P. (2003). Cutting edge: B cell receptor signals regulate BLyS receptor levels in mature B cells and their immediate progenitors. J. Immunol. 170, 5820–5823. doi: 10.4049/jimmunol.170.12.5820
Smulski, C. R., and Eibel, H. (2018). BAFF and BAFF-receptor in B cell selection and survival. Front. Immunol. 9:2285. doi: 10.3389/fimmu.2018.02285
Soresina, A., Moratto, D., Chiarini, M., Paolillo, C., Baresi, G., Focà, E., et al. (2020). Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 31, 565–569. doi: 10.1111/pai.13263
Soucek, L., Buggy, J. J., Kortlever, R., Adimoolam, S., Monclús, H. A., Allende, M. T. S., et al. (2011). Modeling pharmacological inhibition of mast cell degranulation as a therapy for insulinoma. Neoplasia 13, 1093–1100. doi: 10.1593/neo.11980
Spaargaren, M., Beuling, E. A., Rurup, M. L., Meijer, H. P., Klok, M. D., Middendorp, S., et al. (2003). The B cell antigen receptor controls integrin activity through Btk and PLCgamma2. J. Exp. Med. 198, 1539–1550. doi: 10.1084/jem.20011866
Stadanlick, J. E., Kaileh, M., Karnell, F. G., Scholz, J. L., Miller, J. P., and Quinn, W. J. III (2008). Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat. Immunol. 9, 1379–1387. doi: 10.1038/ni.1666
Stadler, N., Hasibeder, A., Lopez, P. A., Teschner, D., Desuki, A., Kriege, O., et al. (2017). The Bruton tyrosine kinase inhibitor ibrutinib abrogates triggering receptor on myeloid cells 1-mediated neutrophil activation. Haematologica 102, e191–e194. doi: 10.3324/haematol.2016.152017
Sun, B., Liu, X., Zheng, X., Wang, C., Meng, Q., Sun, H., et al. (2020). Novel pyrimidines as multitarget protein tyrosine kinase inhibitors for the treatment of idiopathic pulmonary fibrosis (IPF). ChemMedChem 15, 182–187. doi: 10.1002/cmdc.201900606
Thibaud, S., Tremblay, D., Bhalla, S., Zimmerman, B., Sigel, K., and Gabrilove, J. (2020). Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID-19. Br. J. Haematol. 190, e73–e76. doi: 10.1111/bjh.16863
Thien, M., Phan, T. G., Gardam, S., Amesbury, M., Basten, A., Mackay, F., et al. (2004). Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20, 785–798. doi: 10.1016/j.immuni.2004.05.010
Torke, S., Pretzsch, R., Häusler, D., Haselmayer, P., Grenningloh, R., Boschert, U., et al. (2020). Inhibition of Bruton’s tyrosine kinase interferes with pathogenic B-cell development in inflammatory CNS demyelinating disease. Acta Neuropathol. 140, 535–548. doi: 10.1007/s00401-020-02204-z
Treon, S. P., Castillo, J. J., Skarbnik, A. P., Soumerai, J. D., Ghobrial, I. M., Guerrera, M. L., et al. (2020). The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 135, 1912–1915. doi: 10.1182/blood.2020006288
Tsukada, S., Saffran, D. C., Rawlings, D. J., Parolini, O., Allen, R. C., Klisak, I., et al. (1993). Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72, 279–290. doi: 10.1016/0092-8674(93)90667-f
Vetrie, D., Voøechovský, I., Sideras, P., Holland, J., Davies, A., Flinter, F., et al. (1993). The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 361, 226–233. doi: 10.1038/361226a0
Volmering, S., Block, H., Boras, M., Lowell, C. A., and Zarbock, A. (2016). The Neutrophil Btk signalosome regulates integrin activation during sterile inflammation. Immunity 44, 73–87. doi: 10.1016/j.immuni.2015.11.011
von Borstel, A., Abdulahad, W. H., Sanders, J. S., Rip, J., Neys, S. F. H., Hendriks, R. W., et al. (2019). Evidence for enhanced Bruton’s tyrosine kinase activity in transitional and naïve B cells of patients with granulomatosis with polyangiitis. Rheumatology (Oxford). 58, 2230–2239. doi: 10.1093/rheumatology/kez205
von Hundelshausen, P., and Siess, W. (2021). Bleeding by bruton tyrosine kinase-inhibitors: dependency on drug type and disease. Cancers 13:1103. doi: 10.3390/cancers13051103
Wang, J., Lau, K.-Y., Jung, J., Ravindran, P., and Barrat, F. J. (2014). Bruton’s tyrosine kinase regulates TLR9 but not TLR7 signaling in human plasmacytoid dendritic cells. Eur. J. Immunol. 44, 1130–1136. doi: 10.1002/eji.201344030
Wang, S.-P., Iwata, S., Nakayamada, S., Niiro, H., Jabbarzadeh-Tabrizi, S., Kondo, M., et al. (2015). Amplification of IL-21 signalling pathway through Bruton’s tyrosine kinase in human B cell activation. Rheumatology (Oxford). 54, 1488–1497. doi: 10.1093/rheumatology/keu532
Wardemann, H., Yurasov, S., Schaefer, A., Young, J. W., Meffre, E., and Nussenzweig, M. C. (2003). Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377. doi: 10.1126/science.1086907
Weber, A. N. R., Bittner, Z., Liu, X., Dang, T.-M., Radsak, M. P., and Brunner, C. (2017). Bruton’s Tyrosine Kinase: An Emerging Key Player in Innate Immunity. Front. Immunol. 8:1454. doi: 10.3389/fimmu.2017.01454
Wicker, L. S., and Scher, I. (1986). X-linked immune deficiency (xid) of CBA/N mice. Curr. Top. Microbiol. Immunol. 124, 87–101. doi: 10.1007/978-3-642-70986-9_6
Yago, T., Shao, B., Miner, J. J., Yao, L., Klopocki, A. G., Maeda, K., et al. (2010). E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood 116, 485–494. doi: 10.1182/blood-2009-12-259556
Yu, L., Mohamed, A. J., Simonson, O. E., Vargas, L., Blomberg, K. E. M., Björkstrand, B., et al. (2008). Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-B. Blood 111, 4617–4626. doi: 10.1182/blood-2007-10-121137
Zhang, F., Song, S., Shu, J., Li, Y., Wu, Y., Wang, Q., et al. (2016). BAFF upregulates CD28/B7 and CD40/CD154 expression and promotes mouse T and B cell interaction in vitro via BAFF receptor. Acta Pharmacol. Sin. 37, 1101–1109. doi: 10.1038/aps.2016.15
Keywords: Bruton’s tyrosine kinase (BTK), B cells, myeloid cells, inflammation, autoimmunity, small-molecule inhibitor
Citation: Neys SFH, Hendriks RW and Corneth OBJ (2021) Targeting Bruton’s Tyrosine Kinase in Inflammatory and Autoimmune Pathologies. Front. Cell Dev. Biol. 9:668131. doi: 10.3389/fcell.2021.668131
Received: 15 February 2021; Accepted: 07 April 2021;
Published: 04 June 2021.
Edited by:
Cornelia Brunner, Ulm University Medical Center, GermanyReviewed by:
Kamalakannan Rajasekaran, Genentech, Inc., United StatesSonika Patial, Louisiana State University, United States
Copyright © 2021 Neys, Hendriks and Corneth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rudi W. Hendriks, r.hendriks@erasmusmc.nl; Odilia B. J. Corneth, o.corneth@erasmusmc.nl
†These authors have contributed equally to this work
 Stefan F. H. Neys
Stefan F. H. Neys Rudi W. Hendriks
Rudi W. Hendriks Odilia B. J. Corneth
Odilia B. J. Corneth