Bricks, trusses and superstructures: Strategies for skeletal reinforcement in batoid fishes (rays and skates)
- 1Image and Analysis Centre, Core Research Labs, London, United Kingdom
- 2Department of Biomaterials, Max Planck Institute of Colloids and Interfaces, Potsdam, Germany
- 3Natural History Museum, London, United Kingdom
- 4Department of Earth and Planetary Sciences, Birkbeck, University of London, London, United Kingdom
- 5Centre for Craniofacial and Regenerative Biology, Dental Institute, King’s College, London, United Kingdom
- 6Department of Infectious Diseases and Public Health, City University of Hong Kong, Kowloon Tong, Hong Kong SAR, China
Crushing and eating hard prey (durophagy) is mechanically demanding. The cartilage jaws of durophagous stingrays are known to be reinforced relative to non-durophagous relatives, with a thickened external cortex of mineralized blocks (tesserae), reinforcing struts inside the jaw (trabeculae), and pavement-like dentition. These strategies for skeletal strengthening against durophagy, however, are largely understood only from myliobatiform stingrays, although a hard prey diet has evolved multiple times in batoid fishes (rays, skates, guitarfishes). We perform a quantitative analysis of micro-CT data, describing jaw strengthening mechanisms in Rhina ancylostoma (Bowmouth Guitarfish) and Rhynchobatus australiae (White-spotted Wedgefish), durophagous members of the Rhinopristiformes, the sister taxon to Myliobatiformes. Both species possess trabeculae, more numerous and densely packed in Rhina, albeit simpler structurally than those in stingrays like Aetobatus and Rhinoptera. Rhina and Rhynchobatus exhibit impressively thickened jaw cortices, often involving >10 tesseral layers, most pronounced in regions where dentition is thickest, particularly in Rhynchobatus. Age series of both species illustrate that tesserae increase in size during growth, with enlarged and irregular tesserae associated with the jaws’ oral surface in larger (older) individuals of both species, perhaps a feature of ageing. Unlike the flattened teeth of durophagous myliobatiform stingrays, both rhinopristiform species have oddly undulating dentitions, comprised of pebble-like teeth interlocked to form compound “meta-teeth” (large spheroidal structures involving multiple teeth). This is particularly striking in Rhina, where the upper/lower occlusal surfaces are mirrored undulations, fitting together like rounded woodworking finger-joints. Trabeculae were previously thought to have arisen twice independently in Batoidea; our results show they are more widespread among batoid groups than previously appreciated, albeit apparently absent in the phylogenetically basal Rajiformes. Comparisons with several other durophagous and non-durophagous species illustrate that batoid skeletal reinforcement architectures are modular: trabeculae can be variously oriented and are dominant in some species (e.g. Rhina, Aetobatus), whereas cortical thickening is more significant in others (e.g. Rhynchobatus), or both reinforcing features can be lacking (e.g. Raja, Urobatis). We discuss interactions and implications of character states, framing a classification scheme for exploring cartilage structure evolution in the cartilaginous fishes.
Introduction
Among cartilaginous fishes (Chondrichthyes), the consumption of hard prey (durophagy) is most common in the clade of skates and rays (Batoidea; Elasmobranchii), particularly in the subfamilies Rhinopterinae and Myliobatinae (both Myliobatiformes: Myliobatidae), which contain only durophagous taxa (Figure 1). Durophagy in batoid fishes takes a variety of forms: diets can involve comparatively thin-shelled crustaceans, thick-shelled molluscs and/or prey with softer, tougher exoskeletons (e.g. shrimp or even insects) (Wilga and Motta, 2000; Kolmann et al., 2015b, 2016). Hard prey processing has not been extensively surveyed in batoid fishes, but at least two strategies exist (Figure 1): what we will call “chemical durophagy,” where predators rely on low stomach pH or chinitase to break down prey exoskeletons (Fänge et al., 1979; Holmgren and Nilsson, 1999; Cortés et al., 2008; Anderson et al., 2010) and “mechanical durophagy,” where predators crush prey before ingestion (Summers, 2000; Summers et al., 2004; Kolmann et al., 2015b; Rutledge et al., 2019; Ajemian et al., 2021). The limited current knowledge of the phylogenetic distribution of these two strategies suggests they could be mutually exclusive in batoid fishes (Figure 1), perhaps also indicating that these prey processing modes demand a level of anatomical and physiological specialization, in gut physiology for chemical durophagy and in skeletal reinforcement for mechanical durophagy.
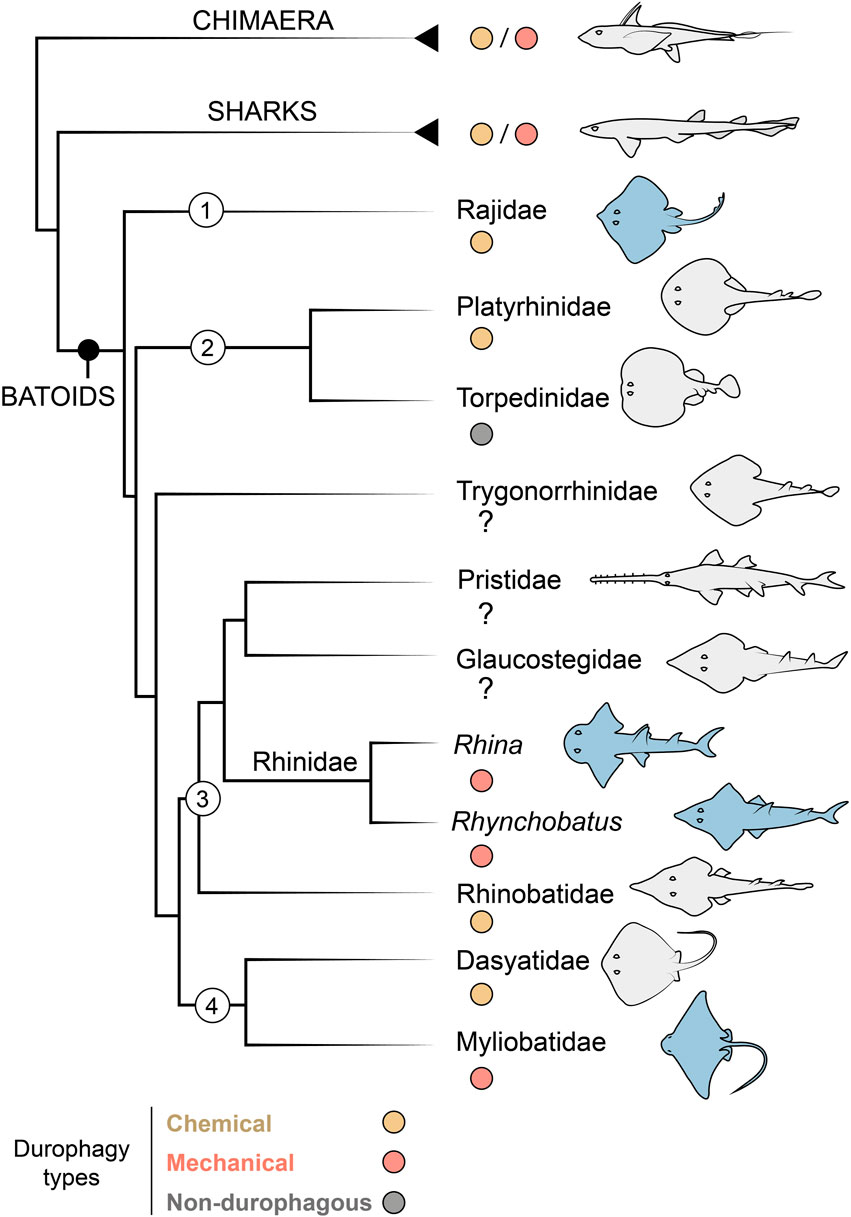
FIGURE 1. Durophagy in Chondrichthyes, with a focus on batoid relationships. Taxa of interest are indicated with numbers as follows: 1) Rajiformes, 2) Torpediniformes, 3) Rhinopristiformes and 4) Myliobatiformes. The Rhinidae is shown to genus level to indicate the two genera investigated in this study (Rhina and Rhynchobatus). Species examined in this study are highlighted in blue. In groups with some durophagous members, the most common types are highlighted, either chemical (where shelled prey is digested) or mechanical (where shelled prey is crushed). Groups where it is unclear if durophagy exists are marked with a question mark. Phylogenetic relationships based on (Franklin et al., 2014; Underwood et al., 2015).
From an anatomical perspective, mechanical durophagy is a particularly impressive dietary mode for elasmobranch fishes, as shark and ray skeletons are composed predominantly of unmineralized cartilage covered by a mineralized crust of blocks called tesserae, typically arranged in a monolayer merely hundreds of microns thick (Maisey, 2013; Atake and Eames, 2021; Berio et al., 2021; Maisey et al., 2021). A variety of morphological features have been found to be associated with mechanical durophagy (e.g. Summers, 2000; Summers et al., 2004; Dean et al., 2007, 2015; Herbert and Motta, 2018; Rutledge et al., 2019; Seidel et al., 2021; Huie et al., 2022), particularly within durophagous myliobatiform stingrays (Myliobatiformes): flat pavement-like teeth; large adductor muscles; relatively shortened jaws with high leverage, fused at the midline symphysis; and structural reinforcements of the jaw tissues in the form of thickening of the mineralized cortex (the tesseral layer) and/or mineralized struts (trabeculae) coursing through the unmineralized cartilage. Some of these structural features, however, may support functions not associated with durophagy. For example, the cownose ray (Rhinoptera bonasus; Myliobatiformes) was previously considered to be an obligate durophage, its jaws bearing all the anatomical indicators of durophagy, yet this species has also been shown to suction feed opportunistically on soft-bodied prey (Collins et al., 2007). Conversely, the jaws of the non-durophagous electric ray, Narcine brasilinensis (Torpediniformes) have a thickened cortex and trabeculae, but these features likely support this species’ predation on buried polychaetes, preventing the highly protrusible jaws from buckling when they are used in benthic excavation of prey (Dean et al., 2006). The disparate feeding modes and phylogenetic positions of Myliobatiformes and Torpediniformes (Dean et al., 2006, 2007; Aschliman et al., 2012) suggest that reinforcing features such as trabeculae and cortical thickening may be more widespread in Batoidea than currently appreciated (Figure 1).
In the last ∼20 years, much of the research into both elasmobranch skeletal biology and the functional morphology of durophagy has centered on myliobatiform stingrays (e.g. Summers, 2000; Summers et al., 2004; Dean et al., 2009; Kolmann et al., 2015a, 2015b; Seidel et al., 2016, 2017, 2021; Rutledge et al., 2019). Yet, batoid taxa offer a valuable diversity of species for exploring links between skeletal anatomy and ecology and clarifying how a cartilage skeleton can be modified through evolution to meet diverse functional demands. In this study, we use X-ray microcomputed tomography (micro-CT) to investigate whether jaw and dentition characters associated with stingray durophagy are also present in two durophagous members of the Rhinopristiformes, sister taxon to the Myliobatiformes (Dean et al., 2007; Aschliman et al., 2012; Aschliman, 2014) (Figure 1). We investigate two rhinopristiform species, Rhina ancylostoma (Bowmouth guitarfish; Rhinidae) and Rhynchobatus australiae (White-spotted wedgefish; Rhynchobatidae), large-bodied species with small, rounded, and ornamented teeth. In both species, these teeth form an unusual and striking dental battery, where multiple teeth are arrayed into spheroidal “meta-teeth”—bulbous structures constructed from multiple teeth, and particularly massive in Rhina—fitting into concave regions in the opposing jaw (Figures 2, 3A, 4A). These undulating dentitions are conspicuously different from the familiar, flat pavements of myliobatiform stingray teeth (e.g. Underwood et al., 2015), suggesting that the Rhinopristiformes may employ alternative anatomical strategies for durophagy.

FIGURE 2. Anatomical terminology used in this study. 3D reconstruction (top) and cross-sections of the lower jaw (bottom) of Rhina indicate the different anatomical positions and orientation terminology used in the text.
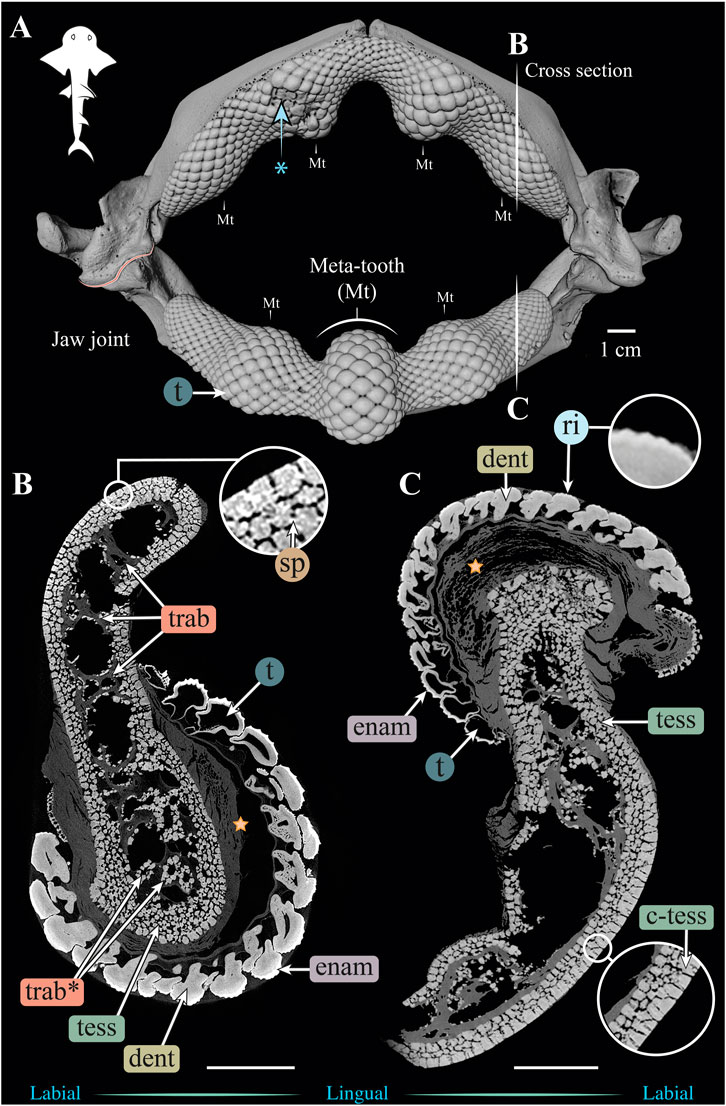
FIGURE 3. 3D reconstruction and cross sections of a Rhina ancylostoma jaw. (A) 3D reconstruction of upper and lower jaws, showing the distinctive undulating dentition and several bulbous “meta-teeth” (Mt) on the upper and lower jaw. Asterisk indicates an area of the jaw with broken teeth. Vertical white lines indicate the position in the jaw of cross sections shown in (B,C). Slices of the upper (B) and lower (C) jaws show trabeculae running parallel (trab) and perpendicular (trab*) to the section plane. Both globular (tess) and columnar (c-tess) tesserae form the jaw cortex, with hypermineralized “spokes” (sp) visible as regions of higher grayscale intensity, reinforcing the joints between tesserae. Tooth developmental stages can be distinguished in both sections, where newly formed teeth are hollow and progressively filled with mineralized dentin. Teeth typically exhibit surface ornamentation in the form of eight ridges, sculpted from the enameloid of each tooth crown. A bulk of unmineralized connective tissue (including jaw perichondrium and dental ligament) is visible between the teeth and tesserae (star). All scale bars 1 cm. Abbreviations: dent, dentin; enam, enameloid; ri, ridges; sp, spokes; tess, tesserae; c-tess, columnar tesserae; t, tooth; trab, trabeculae. Images from sample BMNH 2015.1.25.5 (24 cm).
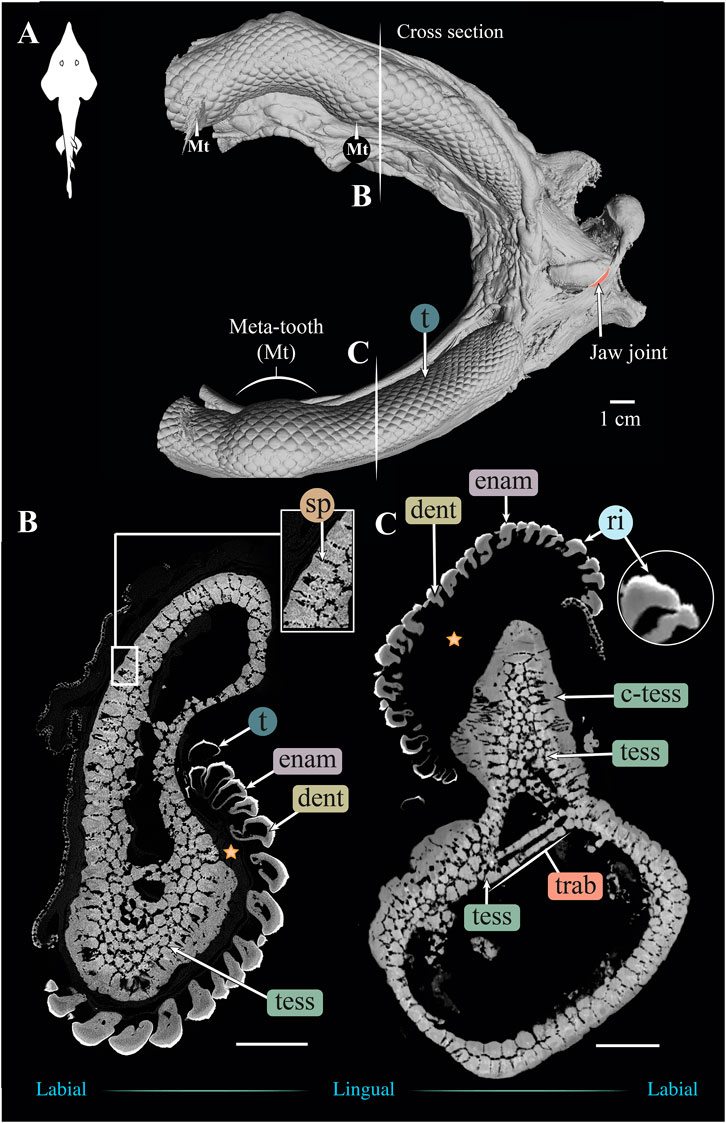
FIGURE 4. 3D reconstruction and cross section of a Rhynchobatus australiae jaw. (A) 3D reconstruction of upper and lower jaws, showing the distinctive undulating dentition and several bulbous “meta-teeth” (Mt) on the upper and lower jaw. Vertical white lines indicate the position in the jaw of cross sections shown in (B,C). Slices of the upper (B) and lower (C) jaws show trabeculae (trab) running largely parallel to the section plane (compare with the Rhina jaw in Figures 3B,C). Both globular (tess) and columnar (c-tess) tesserae form the jaw cortex, with hypermineralized “spokes” (sp) visible as regions of higher grayscale intensity, reinforcing the joints between tesserae (compare with the cortex of the Rhina jaw in Figure 3B, constructed from more numerous and smaller tesserae). Tooth developmental stages can be distinguished in both sections, where newly formed teeth are hollow and progressively filled with mineralized dentin. Teeth typically exhibit surface ornamentation in the form of low ridges, sculpted from the enameloid of each tooth crown. A bulk of unmineralized connective tissue (including jaw perichondrium and dental ligament) is visible between the teeth and tesserae (star). All scale bars 1 cm. Abbreviations: dent, dentin; enam, enameloid; ri, ridges; sp, spokes; tess, tesserae; c-tess, columnar tesserae; t, tooth; trab, trabeculae. Images from sample BMNH 2017.7.11.1 (28 cm).
Given that much previous research into cartilaginous fish durophagy has focused on myliobatiform stingrays, the Rhinopristiformes offer great potential for understanding the degree to which known anatomical modifications for a hard prey diet are group-specific or more general tissue-level modifications. Biomaterials and functional morphology studies (e.g. Summers, 2000; Dean et al., 2006; Liu et al., 2014; Seidel et al., 2019, 2020) have shown that tessellated cartilage (at least in some stingrays) has a distinct multi-scale mechanical anisotropy, with tesserae oriented parallel to the direction of loading (e.g. the biting direction) having a higher stiffness than those oriented perpendicular to it. Similarly, excised blocks of jaw cartilage are stiffer when they contain trabecular struts, and when the struts (and the tesserae forming them) are oriented in-line with the direction of applied load (Summers et al., 1998). The effect of tesserae orientation on skeletal stiffness presents a structural conundrum: the jaws of myliobatiform stingrays must have an appreciably broad, flat area to accommodate their pavement-like dentitions, yet this necessitates a wide skeletal surface where tesserae are oriented perpendicular to the biting load (i.e. in their less stiff orientation). This may explain the incredibly high density of trabeculae supporting the jaws in myliobatiform rays (i.e. buttressing occlusal areas with more tesserae oriented in-line with biting loads), while also suggesting that such supporting mechanisms may be less relevant for those durophagous batoids that lack flattened platform regions on their jaws (e.g. Rhinopristiformes) and/or that structural reinforcement may be accomplished by other means. It is possible, for example, that the shape of tesserae beneath the dentition may be altered, perhaps taking on the dome-like “voussoir” tesserae morphology known to be associated with arched skeletal surfaces (Maisey et al., 2021). Additionally, the jaw’s cortex could be reinforced by accessory tesseral layers (>10 have been described in some large species; Dean et al., 2015, 2017). We dissect these options in the current study, providing the first three-dimensional characterization of jaw-strengthening anatomies in batoids, comparing features among the range of durophagous and non-durophagous species examined in this study and previous works, to synopsize the diversity of strategies by which cartilage has been modified throughout elasmobranch evolution to meet varied performance demands.
Materials and methods
Sample selection and X-ray tomography acquisition
The dried jaw specimens examined in this study are from the Life Sciences Collections, Natural History Museum, London (BMNH) (Supplementary Table S1). Two specimens were used for the bulk of detailed analysis—Rhina ancylostoma (BMNH 2015.1.25.5) and Rhynchobatus australiae (BMNH 2017.7.11.1)—with additional higher-resolution scans focused on the regions of interest at the proximal ends of the lower and upper jaws. Other specimens of different sizes—Rhina (BMNH 2014.11.11.1) and Rhynchobatus (NHMUK PV P4048 and two unregistered specimens) — were scanned to investigate how tesserae and jaw trabeculae vary with age. The original body sizes of the animals from which specimens came were unknown and so jaw size (i.e. the outer jaw width at the jaw joints) was used as a proxy for age (i.e. larger jaws were assumed to come from larger and therefore older animals). A previous study (Dean et al., 2017) estimated Rhynchobatus jaw width to be ∼7–11% of total length and our measurements from two intact Rhina specimens (95.5 and 147 cm TL) and dried jaws from six specimens from animals of known total length suggest a similar ratio (∼11–15% of TL). Based on available size at maturity information for both species (Last et al., 2016; Purushottama et al., 2020), the jaw specimens used in our study (Supplementary Table S1) are likely all from mature individuals, an assertion supported by the high degree of mineralization of the skeleton (Seidel et al., 2016). It should be noted that two of the Rhynchobatus jaws could not be confidently identified to species; although available information indicates that all Rhynchobatus species include some amount of hard-shelled prey in their diet (e.g. Darracott, 1977; Moazzam and Osmany, 2020; Purushottama et al., 2020). Additionally, for comparative purposes, the upper and lower jaws of the durophagous stingray Aetobatus ex. gr. narinari (BMNH 2015.1.25.4; Myliobatiformes) and of the non-durophagous skate Raja clavata (BMNH 2015.1.25.2; Rajiformes) were scanned and examined.
Micro-CT imaging was performed at the Imaging and Analysis Centre, Natural History Museum, London, using a Nikon Metrology HMX ST 225 with a reflection target. The eight specimens listed above were scanned, as well as several selected regions imaged at smaller voxel sizes in both Rhina and Rhynchobatus, producing 12 separate data sets. X-ray source conditions ranged from 100 to 190 kV adjusted for field of view differences and sample density. A range of voxel sizes were achieved (26–121 µm), with smaller image pixel sizes utilized for quantifying tesserae dimensions. For a detailed list of micro-CT scan parameters and specimen information, see Supplementary Information, Supplementary Table S1.
Image processing
All scans were processed, rendered, and analysed using Avizo/Amira (version 9.4 and above or Amira ZIB Edition). Each acquisition was enhanced using a “low level” non-local means filter to reduce imaging noise from the data. Individual jaw elements were separated (segmented) for comparative analysis, using volume editing tools to isolate the lower jaw (Meckel’s cartilage) from the upper jaw (palatoquadrate), and to separate the left and right jaw moieties at the symphyses.
Terminology
Anatomical terminology used is presented visually in Figure 2. “Proximal” indicates a position or direction toward the jaw joint and “distal” toward the jaw symphysis. “Symphyseal” refers to the (distal) midline joint between the two jaw halves, with “parasymphyseal” regions flanking the jaw symphysis laterally (e.g. Underwood et al., 2015). “Oral” is towards the biting surface of the jaw or teeth, with “aboral” indicating the opposing surface. “Labial” refers to the outer surface of the jaw, and “lingual” to the inner (pharyngeal) surface of the jaw. “Cortical” refers to a position or direction toward the jaw’s cortex, the outer mineralized rind comprising single or multiple layers of tesserae. “Perichondral” is used similarly, to indicate tesserae or portions of tesserae associated with the unmineralized, collagenous perichondrium that wraps the outer surface of tessellated cartilage skeletons (Seidel et al., 2020, 2021).
Measurements
Cortical and tooth thickness
The thickness of the jaw cortex and the dentition was measured from the full jaws of Rhina (Figure 3) and Rhynchobatus (Figure 4), on a mesh generated from the segmented volumes of the whole jaw specimen scans. Meshes were generated for the upper and lower jaws, and the upper and lower dentitions for each species; the upper and lower jaw cartilages were analysed independently from the dentition. The use of meshes simplified the process of bulk linear measurements and allowed thickness to be color-coded over the entire jaw surface: meshed surfaces were minimally smoothed and simplified to reduce computational resources needed, then thickness was determined by measuring the distance between two opposing vertices in the mesh that were both nearest and parallel (or very close to parallel) to each other. To visualize thickness variation, measurements were then represented by a surface scalar field for each vertex and a physics color map with a range of 0 to >5 mm, with thickness above 5 mm represented as red (Figures 5, 6). This process allowed quantification and visualization of the bulk thickness of the jaw cortex (regardless of the number of tesseral layers) and the dentition (i.e. tooth height, including the contributions of enameloid and dentin). For comparative purposes, additional meshes were generated and similarly quantified for the upper and lower jaw cartilages and dentitions of Aetobatus ex. gr. narinari (Figure 7) and Raja clavata (Figure 8).
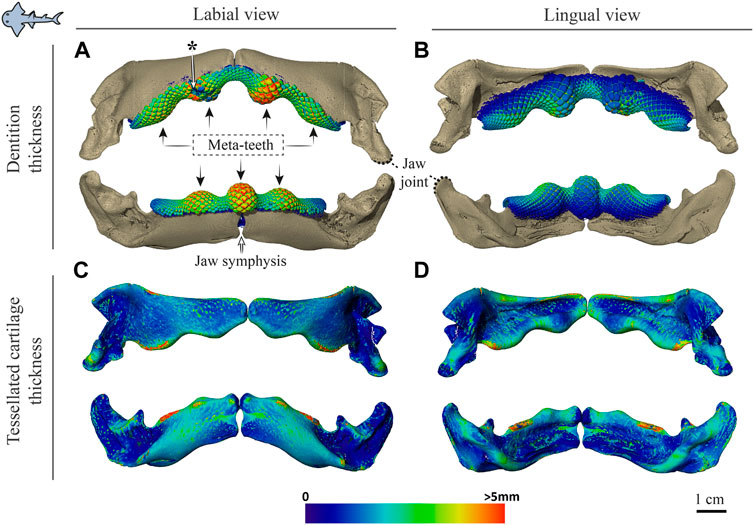
FIGURE 5. Dentition and tessellated cartilage thickness in the jaws of Rhina ancylostoma. (A,B) Color-coded dentition thickness, and (C,D) jaw cortex (tessellated cartilage) thickness. Both upper and lower jaws are shown in labial (A,C) and lingual (B,D) views. Black arrows in (A) indicate the positions of the meta-teeth in the lower and upper jaw. Asterisk in (A) indicates a region of tooth breakage (see also Figure 3A). Note also the gap between left and right jaw moieties, illustrating the lack of symphyseal fusion. Thickness is represented by a physics color map, with regions in red being thicker than 5 mm. Images from sample BMNH 2015.1.25 (24 cm).
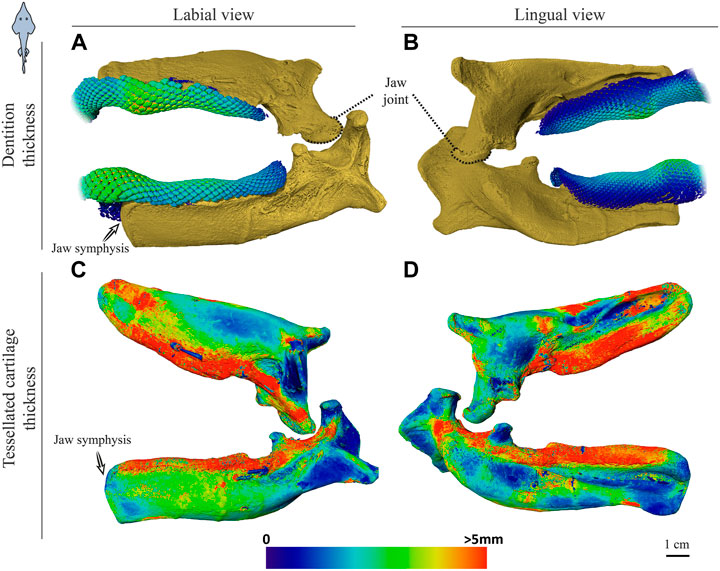
FIGURE 6. Dentition and tessellated cartilage thickness of the left jaw moieties of Rhynchobatus australiae. (A,B) Color-coded dentition thickness, and (C,D) jaw cortex (tessellated cartilage) thickness. Both upper and lower jaws are shown in labial (A,C) and lingual (B,D) views. As in the Rhina jaws (Figures 5C,D), the symphysis is unfused (note the anatomical edge of the jaw halves). Thickness is represented by a physics color map, with regions in red being thicker than 5 mm. Images from sample BMNH 2017.7.11.1 (28 cm).
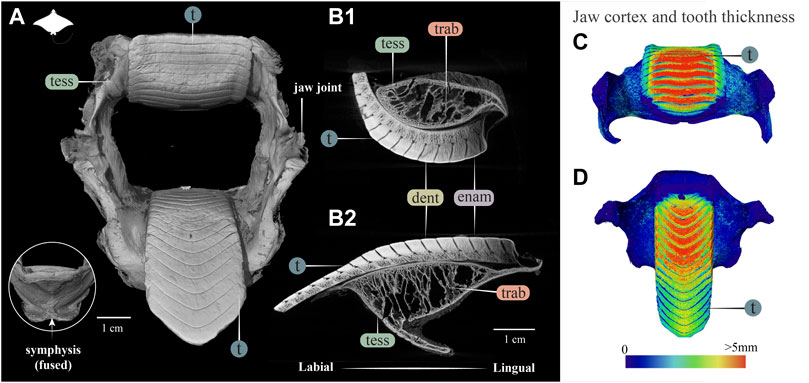
FIGURE 7. 3D reconstruction, cross section and thickness analysis of the jaw of an eagle ray jaw (Aetobatus ex. gr. narinari). (A) 3D reconstruction of upper and lower jaw and teeth in labial view. The inset shows the fused symphysis beneath the dentition. (B) Slices through the opposing upper (B1) and lower (B2) jaws, showing a cross section of the jaw skeleton, comprising a cortex of tesserae and internal trabeculae, and surmounted by interlocked teeth. Note that the cortex is comprised of fewer layers of tesserae and trabeculae exhibit more of a hierarchical branching pattern than in the jaws of Rhina (Figure 3) and Rhynchobatus (Figure 4). A visible progression of teeth development can be seen (moving from right to left in B1 and B2), characterized by a more porous dentin in newly formed teeth, infilled with mineralized dentin as teeth become functional. (C,D) Jaw cortex and tooth thickness are represented by a physics color map with regions in red being thicker than 5 mm. Abbreviations: enam, enameloid; dent, dentin; tess, tesserae; t, tooth; trab, trabeculae. Images from sample BMNH 2015.25.4 (7.5 cm).

FIGURE 8. 3D reconstruction, cross section and thickness analysis of a skate jaw (Raja clavata). 3D reconstruction of upper and lower jaw with labial (A) and lingual (B) views of the jaws and teeth. Vertical white lines in A indicate the position in the jaw of cross sections shown in C1 and C2. (C) Slices show cross sections of the upper and lower jaws at two positions (C1,C2). Tooth developmental stages can be distinguished in both sections, where newly formed teeth are hollow and progressively filled with mineralized dentin. Note that the cortex comprises only a single layer of tesserae, with a single potential trabecula (trab?) passing through the jaw. (D) Jaw cortex and tooth thickness are represented by a physics color map with regions in red being thicker than 5 mm (D1 labial view, D2 lingual view). All scale bars 1 cm. Abbreviations: enam, enameloid; dent, dentin; tess, tesserae; t, tooth; trab?, potential trabecula. Images from sample BMNH 2015.25.2 (9 cm).
Tesserae
Qualitative evaluation of the arrangements and morphologies of tesserae in the jaw cortex were performed on scan slice data and volume-renderings. To quantify aspects of tesseral morphology, the smallest possible voxel size was necessary to resolve tesserae boundaries successfully, but this came at the expense of scan volume: whereas entire jaws could be scanned from smaller specimens (i.e. the two smallest Rhynchobatus jaws, 11 and 18 cm [unregistered specimens]), ROI-scans were necessary to quantify tesserae morphometrics in larger specimens (lower right jaws of Rhynchobatus, NHMUK PV P4048 and BMNH 2015.1.25.5; lower right jaw of Rhina, BMNH 2014.11.11.1 and 2015.1.25.5; Supplementary Table S1). The dimensions of individual tesserae were investigated in the entire jaws of the small specimens and across four ROIs at the proximal ends of both the upper and lower jaws of the larger Rhina and Rhynchobatus (Figures 9, 10 and Supplementary Table S2); these regions were chosen for their comparatively simple cross-sections and the fact that they could be consistently compared across individuals and species (avoiding the undulating morphologies of the symphyseal regions, which become more pronounced with age). The mineralized tissue was segmented and individual tesserae isolated using the Separate Objects module with a marker extent of “2” (relating to the size of the seeds marking objects for separation). Segmentation of individual tesserae was possible due to the narrow gaps between tesserae (i.e. intertesseral joints) and with a high level of accuracy, particularly where high magnification was achieved. The Separate Objects module applies the Chamfer method which splits volumetric bodies that touch only partially with neighbouring bodies (e.g. at the “intertesseral contact zones,” where tesserae abut; Seidel et al., 2016; Jayasankar et al., 2020). Using this method, structures with strong overlap are not separated. In our data, once tesserae were segmented from one another, we recorded their locations (X, Y and Z coordinates of their centroids) and also approximated their sizes by measuring the major, intermediate and minor axis lengths of a bounding box enclosing all of the voxels belonging to each tessera (Supplementary Figure S1). Although this high-throughput size estimation calculates the length, width and thickness of every tessera’s bounding box, it does not determine how the bounding box’s orientation is linked to the tessera’s anatomical orientation. In other words, the method can calculate, for example, the longest dimension of a tessera’s bounding box, but not whether this is the tessera’s “width” or its “thickness” (i.e. the dimension parallel to the surface of the jaw vs perpendicular to it; see Supplementary Figure S1); addressing this challenge requires a method for identifying which tesseral face is associated with the skeletal surface, a feature unavailable in the segmentation and analysis software. As a result, in the current study, we only report the bounding box’s smallest linear dimension as an indication of “tesseral size.” We found this to be a reliable and usefully conservative general measurement for illustrating local variations in tesseral size, particularly since the longest bounding box dimension is heavily biased by tesseral fusions (see Results) and since length is a more anatomically intuitive metric than volume for tesserae. Measured values for tesserae size (Supplementary Table S2) were in reasonable agreement with 2D measurements from previous works (e.g. Dean et al., 2009; Seidel et al., 2016, 2021; Maisey et al., 2021), supporting the validity of our method. Comparisons of the various possible tesseral measurements and the development of methods for anatomical orientation of bounding boxes will be addressed in a future work (B. Yang et al., in preparation). Tesserae size distributions were plotted for all specimens of both species across eight size bins, distributed equally between each species’ minimum and maximum tesseral sizes (Figure 11). Tesseral size was also represented in jaw volume renderings with color maps grading from dark red to pale yellow (Figures 9, 10 and Supplementary Figures S1, S2, Supplementary Table S2).

FIGURE 9. Tesserae size variation in the jaws of Rhina ancylostoma. (A,B) 3D rendering of Rhina ancylostoma jaws, in labial (A) and lingual (B) views, showing the regions of interest (yellow) used for tesserae size analysis. The scale for tesserae size color-coding is shown at the bottom of figure. (C–E) Tesserae color-coded according to their size in labial, cross-section and lingual views of the upper (C–E) and lower (F–H) jaw. The pores visible in the labial cortical surface are openings for trabeculae canals (C,H), and volume-rendered trabeculae can be seen running through the cross-section (D,G). (F–H) Columnar tesserae (c-tess) form the superficial portion of the cortex in both upper and lower jaws. Note that tessera size is not uniform, but rather varies across the cortical surface. All scale bars 1 cm. Abbreviations: c-tess, Columnar tesserae; tess, tesserae; trab, trabeculae. Full jaw images are from sample BMNH 2015.1.25.5 (24 cm) and regions of interest images are from sample BMNH 2014.11.11.1 (35 cm).
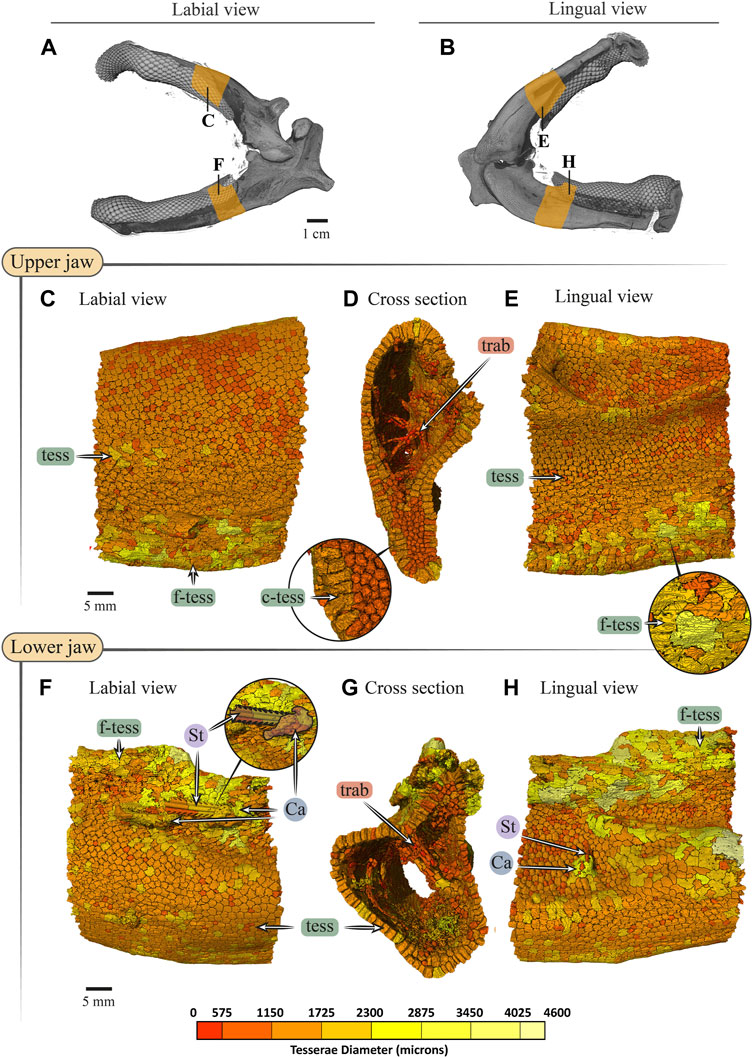
FIGURE 10. Tesserae size variation in the jaws of Rhynchobatus australiae. (A,B) 3D rendering of Rhynchobatus australiae jaws, in labial (A) and lingual (B) views, showing the regions of interest (yellow) used for tesserae size analysis. Tesserae color-coded according to their size in labial, cross section and lingual views of the upper (C–E) and lower (F–H) jaw. Volume-rendered trabeculae can be observed running across the cross sections of the upper (D) and lower jaws (G). Columnar tesserae (c-tess) form the superficial portion of the cortex (inset in D,G). In labial (F) and lingual (H) views, a barb/sting from a stingray can be observed embedded in the jaw, being surrounded with a mineralized callus (see Dean et al., 2017). Note that tessera size is not uniform, but rather varies across the cortical surface. Abbreviations: Ca, callus; St, sting; tess, tesserae; c-tess, columnar tesserae; f-tess, fused tesserae; trab, trabeculae. Full jaw images are from sample Unregistered specimen (11 cm) and region of interest Images are from sample BMNH 2017.7.11.1 (28 cm).
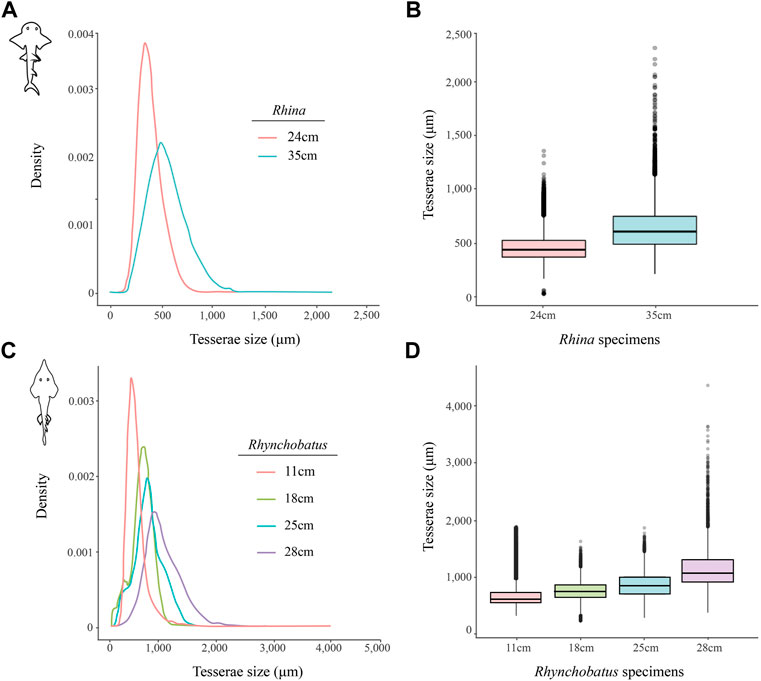
FIGURE 11. Variation of tesserae size across different jaw sizes (ages) in Rhina and Rhynchobatus. (A,B) Density graph (A) and boxplot (B) showing the distribution differences of tesserae size between two different Rhina specimens of different jaw sizes. (C,D) Density graph (C) and boxplot (D) showing the distribution differences of tesserae size among four Rhynchobatus specimens of different jaw sizes. All graphs illustrate an increase in tesseral size and a broadening of the size distributions with age. Note the size scale differences between the Rhina and Rhynchobatus graphs, with relatively larger tesserae in Rhynchobatus.
Trabeculae
Trabeculae position and orientation were examined from scan slice data and volume-renderings. In addition, trabeculae were manually segmented from the full jaw scans of both species—the right upper and lower jaw moieties in Rhina (BMNH 2015.1.25.5) and the left upper and lower jaw moieties in Rhynchobatus (BMNH 2017.7.11.1) — by combining region-growing and selective thresholding, using a brush tool to separate the trabeculae from the cortical mineralized cartilage. Trabeculae prevalence was then quantified as a percent volume fraction relative to the total volume of mineralized jaw cartilage (including trabeculae; Supplementary Table S3). To further visualize interspecies differences, trabeculae were also volume-rendered using a red to light yellow color map with the remaining (non-trabecular) mineralized tissue (i.e. the jaw cortex) visualised in transparent grayscale (Figure 12).
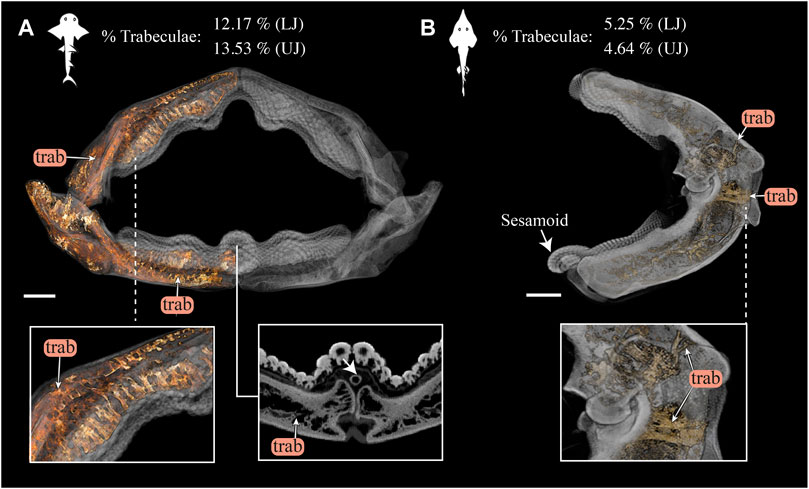
FIGURE 12. 3D rendering and volumetric measures of trabeculae in Rhina and Rhynchobatus jaws. (A) 3D rendering of the full jaw of Rhina ancylostoma, with trabeculae (trab) highlighted in orange. A higher magnification view (left inset) shows trabeculae of relatively consistent orientation in the parasymphyseal region and a cross-section of the meta-teeth (right inset) shows the sesamoid cartilage (white arrow). (B) 3D rendering of the left jaw moiety of Rhynchobatus australiae, showing that, although less dense than in Rhina, trabeculae are also present, especially in the region surrounding the jaw joint (bottom inset). The mandibular sesamoid of Rhynchobatus is visible in the volume rendering in (B) (white arrow). The interspecies difference in trabecular density is also quantified in terms of volume percentage of trabeculae at the top of the figure. All scale bars 1 cm. Abbreviations: trab, trabeculae. Images from samples BMNH 2015.1.25.5 (Rhina ancylostoma, 24 cm) and BMNH 2017.7.11.1 (Rhynchobatus australiae, 28 cm).
Results
Rhina and Rhynchobatus
Gross morphology, jaw cortex and cortical tesserae
Although similar-sized jaws were examined for Rhina and Rhynchobatus, jaw morphologies and tissue arrangements varied considerably (Figures 3, 4). Our scans revealed that the oral/occlusal edges of both species’ jaws echo the undulating morphology of their meta-tooth arrays. The symphyseal regions were exceptions to this, where the largest meta-teeth (on the lower jaw in both species) encircled the unfused symphyseal joint (labeled meta-tooth in Figures 2–4). The symphyseal mandibular meta-tooth was most striking in Rhina, forming a robust, denticulate torus around the symphysis (Figure 3). The meta-teeth themselves were not solid masses of mineralized tissue, but rather spheroidal constructions of slightly larger-than-average teeth (see section 3.1.3 below), forming a shell around an underlying mass of unmineralized dental ligament and perichondrium (star in Figures 3B,C, 4B,C). In both species, a single ovoid tessellated sesamoid cartilage was seen embedded in the connective tissue of the mandibular meta-tooth, between the tooth layer and symphysis (Figure 12 and Supplementary Figure S1).
Significant differences were also apparent between the species in the thickness of the jaw cortex, how this thickness was constructed, and in the morphologies of the tesserae themselves. The general gross tissue arrangements, however, were similar between the species: beneath the teeth, the jaws were roughly sigmoid or pear-shaped in cross-section, with a pronounced lingual depression housing the youngest portion of the tooth array (hollow, incipient teeth; see section 3.1.3 below) and with connective tissue linkages between the tooth array and jaw cortex as described above (dark areas marked with stars in Figures 3B,C, 4B,C). The thickness of the mineralized cortex (composed of tesserae) varied across regions of the jaws in both taxa, in general thickest along the oral jaw surfaces, and thinnest near the jaw joint and symphysis (Figures 3B,C, 4B,C, 5C,D, 6C,D). In Rhina, the cortex was generally between 0.5 and 1.5 mm thick, however with the cortex visibly thicker along the biting (oral) and contralateral aboral margins (∼2–3 mm and up to nearly 5 mm in some areas) (Figures 5C,D). The labial side of the lower jaw was also noticeably thicker than the contralateral lingual face (∼2–3 mm; Figures 5C,D), this thickened cortex continuing from the symphysis to the area of the proximal termination of the dentition, approaching the jaw joint. In Rhynchobatus, the cortex of the upper and lower jaws was overall notably thicker than in Rhina (in most areas >3 mm), with the labial cortex often more than 1 mm thicker than the lingual, and the oral jaw margins strikingly reinforced (>5 mm thick; Figures 6C,D).
In both Rhina (Figures 3B,C, Supplementary Figures S2B,C, Supplementary Movies S1, S2) and Rhynchobatus (Figures 4B,C, Supplementary Figures S1B,C, Supplementary Movie S3), the jaw cortex is formed from multiple monolayers of tesserae. In virtual sections, a high variation in tesseral shape and size is apparent, with tesserae noticeably larger and fewer in number in Rhynchobatus than in Rhina, even for jaws of similar sizes (Figures 3B,C versus Figures 4B,C). Unlike the thin plate-like tesserae described for other batoid species (e.g. see Fig. 10 in Seidel et al., 2016), in Rhina and Rhynchobatus, tesserae shapes tended toward columnar (tall and narrow; c-tess in Figures 3C, 4C, 9G, 10D) or globular forms (more common; Figures 3B,C, 4B,C), but varied locally across the cross-sections of both species’ jaws. In Rhina, tesserae in the labial jaw cortex tended to be larger and columnar in shape (Figures 3B,C), in comparison with tesserae of the lingual cortex, which were smaller and arranged in fewer layers, particularly in the lower jaw (Figure 3C). In Rhynchobatus, labial and lingual tesserae were more comparable in size (Figures 4B,C).
Within the multiple tesseral layers of the jaw cortex, the outermost/surface layer typically had a more regular morphology (in size and shape) relative to the inner layers, readily distinguishable by eye (see below; e.g. Figures 3B,C, 4B,C). Most jaw regions examined in both Rhina and Rhynchobatus exhibited from three to seven layers of tesserae, with the most numerous tesseral layers observed in association with the jaw’s oral surface (beneath the teeth; Figures 3B,C, 4B,C). Oral (sub-dental) multi-layers were observed even in the smallest specimen examined (Rhynchobatus, Supplementary Figures 1B,C), although in the largest Rhina, the number of oral tesseral layers appeared reduced, compared to the medium Rhina (compare Figures 3B,C to Supplementary Figures 2B,C). In sub-dental regions, the number of tesseral layers was challenging to count, as the smaller tesserae there formed a disorganized scree nearly filling the narrow, labiolingually-compressed interior of the jaw (Figures 3C, 4C, 9D,G, 10D,G). This “tesseral scree” was also occasionally visible in the narrow aboral regions of the jaw (e.g. Figure 4B). Although a multi-layered tesseral cortex was the norm in both taxa, monolayers were present in localized areas of the aboral-lingual margin of the lower jaw of Rhynchobatus (Figure 4C). Convex regions of the jaw cortex in all scans had abundant columnar tesserae, often twice as thick as tesserae in flatter areas of the skeleton (Figures 3C, 4C, 9D,G, 10D,G). These tesserae were often somewhat wedge-shaped, slightly wider at their perichondral side.
The tesserae of both species were typically hundreds of micrometers wide, with those of Rhynchobatus on average larger than those of Rhina (note the scale differences in the color-coding in Figures 9–11 and Supplementary Table S2). Although only region-of-interest scans were performed for larger specimens, all datasets examined included tens of thousands of tesserae (and more than 100,000 tesserae in one case), with the small age series examined exhibiting the same trend for both species: with increasing jaw (and, therefore, animal) size, the distribution of tesseral sizes broadened and shifted toward larger tesserae (Figure 11). Compared to the 24 cm Rhina jaw, for example, tesserae in the 35 cm Rhina jaw were nearly 40% larger (from 456.8 ± 120.3 µm to 632.3 ± 199.7 µm: mean ± standard deviation; Supplementary Table S2). Similarly, in Rhynchobatus, tesserae showed almost a twofold average size increase from the smallest animal to the largest, ranging from 606.6 ± 186.4 µm in the 11 cm jaw to 1086.4 ± 338.6 µm in the 28 cm jaw (Supplementary Table S2). The largest tesserae recorded in both species (2315.4 µm in Rhina, 4504.6 µm in Rhynchobatus) represent the tesserae we observed beneath the teeth, fused together into mineralized sheets (see below; Figures 9, 10).
In jaw cross sections, tesserae showed differences in orientation and connectivity within and among the different tesseral layers (Figures 3B,C, 4B,C and Supplementary Figures S1B,C, S2B,C). In the outermost layer, the contacts between adjacent columnar tesserae appeared tightest peripherally (nearest the perichondrium), especially compared to the looser packing of scree tesserae in deeper layers. Throughout the jaw cortex, minute projections were regularly seen bridging adjacent tesserae (see insets in Figures 3B, 4B); these were often very bright in tomographic slices, indicating they are the hypermineralized “spokes” known to reinforce intertesseral joints in other species (Seidel et al., 2016, 2019, 2020). In Rhina and Rhynchobatus, multiple spokes could often be seen spanning a single joint space, particularly in the perichondral layer (e.g. Figures 3B, 4B insets). Spokes appeared irregularly distributed in both taxa (i.e. not visible between every tessera in every slice) and were more numerous in Rhynchobatus. Spokes were not as apparent in the smallest individual sampled (Rhynchobatus, Supplementary Figures 1B,C), nor in the largest (Rhina, Supplementary Figures S2B,C). It is unclear from the current data whether this was a function of the spokes being less prevalent (as in younger animals; Seidel et al., 2016), or their presence being masked by the lower resolution resulting from the large field of view needed to scan the largest specimen (Supplementary Table S1).
The density of tesserae (based on gray values, calculated from the attenuation coefficient) appeared generally consistent within datasets, similar to tooth dentin and skin denticles, but less mineral-dense than tooth enameloid (e.g. Figures 3B,C, 4B,C). Whereas smaller specimens of Rhina and Rhynchobatus showed more consistent density contrast in all tesseral layers (Figures 3B,C and Supplementary Figures S1B,C), in the largest Rhina (Supplementary Figures S2B,C) and especially the largest Rhynchobatus (Figures 4B,C), tesserae had a visibly lower density in areas directly below the dentition, implying a lower degree of mineralization. In these regions in Rhynchobatus and in the very large Rhina, tesserae at the oral surface were large and often partially fused together into irregular mineralized masses (e.g. Supplementary Figure S2), in extreme cases resulting in the tesseral pattern being obliterated and replaced by a nearly homogeneous tissue of lower mineral density (Figures 4B,C).
Trabeculae and trabecular tesserae
Trabeculae (reinforcing struts passing through the core of the jaws) were present in both species (Figures 3, 4, 9D,G, 10D,G, 12 and Supplementary Figures 1B,C, 2B,C, Supplementary Movies S1–S3). Trabeculae in Rhynchobatus were tessellated tubes with walls one tesseral layer thick (Figures 4B,C, 10D,G). In contrast, in Rhina, trabeculae often appeared only partially mineralized (Figures 3B,C), as darker gray (less-mineralized) tubes studded with tesserae. It was impossible to determine, however, the degree to which these trabeculae might have collapsed, lost tesserae, or become degraded as these museum specimens were dried and the internal cartilage pulled away during dehydration.
In both species, trabeculae typically ran between the labial and lingual cortical surfaces (e.g. Figures 3C,D, 4C,D, 12), but with some exceptions (see below). The internal canals formed by trabeculae were often open at each end, communicating to the perichondrium through visible pores, particularly in Rhina (e.g. Figures 9C,H and Supplementary Figure 2G). In Rhina, trabeculae represented 13.6% and 12.2% of the volume of the mineralized tissue in the upper and lower jaws, respectively (Figure 12A and Supplementary Table S3). In contrast, trabeculae were far less dominant in Rhynchobatus, representing only 5.2% and 4.7% of the upper and lower jaw volumes, respectively (Figure 12B and Supplementary Table S3). The abundance of trabeculae in Rhina was particularly apparent in the distal jaw region associated with teeth, where trabeculae dominated the interior of the jaw, orientated in numerous directions (oral-aboral, labio-lingual; Figures 9D,G, 12A), and even appearing to course parallel to the cortex of the oral surface (i.e. in the proximo-distal direction) in some areas (e.g. circular trabecular sections in Figures 3B, 12A [trab*] and Supplementary Movies S1–S3). In contrast, trabeculae in Rhynchobatus, tended to be oriented labio-lingually, although some proximo-distal trabeculae were also observed (Figures 4B,C, 10D,G, 12B).
Teeth
Our tomographic slices provided clear views of the process of tooth development in both species (Figures 3B,C, 4B,C and Supplementary Figures S1B,C, 2B,C). New teeth arose at the lingual side of the jaw, appearing in our micro-CT data as hollow shells covered by dense enameloid caps and anchored to the dental lamina by thin dentin bases. As the teeth developed and progressed labially, the enameloid layers became thicker and the interior pulp cavities were gradually filled with mineralized dentin, before the teeth reached their functional positions. In some sections, the enameloid layer of post-functional teeth appeared slightly thinner, suggesting some wear. The enameloid layer was thicker on average in Rhina (Figures 3B,C versus Figures 4B,C). Both species showed surface ornamentation on their teeth: a single latero-medial ridge on each tooth crown of Rhynchobatus (occasionally with smaller secondary ridges visible in cross section; inset in Figure 4B), in contrast to a series of labio-lingual ridges in Rhina (typically eight per tooth; inset in Figure 3C). These ornamentations were sculpted predominantly from enameloid, with only a slight associated undulation of the enameloid-dentin junction. In cross-section, points of direct labio-lingual contact were visible between teeth in a tooth file (labio-lingual series; Figures 3B,C, 4B,C), the teeth touching at only a single point in Rhynchobatus, but effectively interlocking in Rhina. In both species, points of contact always appeared to be between enameloid-coated tooth regions, not where dentin was exposed.
Teeth in both taxa were largest and the dentitions thickest where associated with the meta-teeth: at the symphysis on the lower jaw and parasymphyseally on the upper jaw in both species, with additional less pronounced meta-teeth also present parasymphyseally on the lower jaw in Rhina (Figures 3A,B, 4A,B, 5A,B, 6A,B). Conversely, the concave regions of the undulating dentition (i.e. those that “received” the meta-teeth) were covered by comparatively small teeth. The largest teeth (those forming the meta-teeth) were also slightly more bulbous, whereas the teeth associated with concave regions of the jaws and the proximal end of the dentition were slightly flatter (Figures 3, 4). In jaws of similar size (e.g. Figures 5A,B, 6A,B), the dentition of Rhina was more robust (typically ≥1 mm thicker).
Myliobatiformes (Aetobatus), Rajiformes (Raja)
The dentition of the durophagous stingray Aetobatus (Myliobatiformes) consists of a single symphyseal file of elongate teeth in both the upper and lower jaws (Figure 7). Aetobatus teeth were much thicker than the jaw’s cortex (Figures 7C,D), which bore several tesseral layers in the upper jaw (four to five layers, in some areas), but fewer in the lower jaw (Figures 7B1,B2 and Supplementary Movie S4). As with Rhina and Rhynchobatus, the regions with the most cortical tesseral layers were directly beneath the functional teeth, although the number of cortical tesseral layers was generally far fewer than in the rhinopristiform taxa. Aetobatus tesserae ranged in size from ∼100 to 500 μm, with no irregularly-shaped tesserae observed (e.g. the perichondral columnar tesserae of Rhina and Rhynchobatus). The teeth were capped by a thin layer of enameloid (<300 µm thick), with the bulk of the tooth thickness provided by dentin. As with Rhina and Rhynchobatus, gray values of micro-CT data show that internal teeth tissues gradually mineralize during development, with the dentin of the newest forming teeth being poorly mineralized, compared to the more highly mineralized functional teeth. In Aetobatus, however, older teeth were considerably worn, being only ∼10% their starting height (apparently due to removal of both enameloid and dentin). Trabeculae were far more numerous than in either Rhina or Rhynchobatus, representing 37.1% and 33.2% of the mineralized tissue volume of the upper and lower jaws, respectively. Aetobatus trabeculae occurred throughout the jaw (Figures 7B1,B2 and Supplementary Movies S1–S6), running primarily from the oral to aboral jaw surfaces. Trabeculae in this species were also more irregularly-shaped than in either rhinopristiform species, appearing to branch and anastomose toward the oral jaw surface. Although trabeculae were tessellated near the oral surface of the jaw, aborally, trabeculae appeared to lack tesserae (as in Rhina, described above).
In Raja, the teeth were small and pointed with a thin enameloid cover (Figure 8; a male specimen, in females the teeth are also small, but flatter and rounded; Underwood et al., 2015), and of similar thickness to the jaw cortex (Figure 10D). Tesserae in Raja range between 400 and 900 μm in size, having the regular prismatic shape previously described for batoid fishes (Seidel et al., 2020). In cross section, the cortical tesserae appeared thicker than those of Aetobatus, although only a single layer was present in Raja, except beneath the dentition, where additional layers of smaller tesserae occurred (Figure 8C). Trabeculae were absent, although what appeared to be an unmineralized or poorly mineralized strut was visible in one section (Figure 8C2).
Discussion
The rhinopristiform species investigated here demonstrate multiple strategies for jaw reinforcement against a hard prey diet, indicating species- and order-level differences, while also illustrating that the accepted anatomical correlates of durophagy in elasmobranchs (e.g. Summers, 2000; Dean et al., 2006; Seidel et al., 2021) are more varied and modular than previously appreciated.
Tesserae
Although closely related, Rhina and Rhynchobatus exhibited clear differences in overall thickness of the jaw cortex; in degrees of mineralization in tesserae associated with the jaw cartilage; in size, shape and arrangement of individual tesserae; in number, location, orientation and degree of mineralization of trabeculae; and in thickness of enameloid and the functional dentition (Figures 1, 3, 4). Both species, however, shared an obvious reinforcement of dental and skeletal features in association with the occlusal regions of the jaw. In both taxa, larger jaws exhibit greater cortical thickness distally along the jaw at the oral surface (Figures 5, 6). In Rhynchobatus, the cortical thickness below the dentition (in the form of multiple layers of tesserae) can be more than double that of Rhina (Figures 3–6, 9, 10). This thickening, however, is not the result of Rhynchobatus possessing more layers of tesserae, but rather having larger tesserae overall (Figures 9,10 and Supplementary Table S2). In contrast, in Rhina, local cortical thickening is accomplished through assemblies of smaller tesserae arranged into a thickened, disorganized scree (Figure 3).
In the rhinopristiform species we examined, dentition-associated cortical thickening apparently becomes more pronounced with age, suggestive of an adaptive response. Tessellated cartilage is believed to have limited to no remodeling ability (Clement, 1992; Dean et al., 2015; Marconi et al., 2020) and therefore the mineralized layer can only grow through addition of new material (Dean et al., 2009, 2017; Seidel et al., 2016). Across the Rhina and Rhynchobatus specimens examined, the distribution of tesseral sizes broadens and shifts in the direction of increasingly larger tesserae as animals increase in size (Figure 11 and Supplementary Table S2). These data therefore provide the first broadscale support of a tessellated cartilage growth hypothesis based previously only on 2D slices from limited numbers of tesserae, arguing that skeletal growth is accomplished (at least in part) by tesserae increasing in size with age (Dean et al., 2009; Seidel et al., 2016, 2019). Growth of the cortex could also be a function of the addition of new (small) tesserae interstitially in the tesseral layer; however, the rightward shifting of our tesseral size distributions away from smaller tesserae argues this mechanism is either not occurring or is comparatively uncommon.
Our data also suggest that tesserae may not be growing at uniform rates. In Rhynchobatus, tesseral size appears to become more heterogeneous with age: in comparison with the smaller individuals, tesserae size in the larger Rhynchobatus specimen is more variable (Figures 11C, D and Supplementary FigureS1). In both Rhynchobatus and Rhina, the perichondral (outermost) tesseral layers involve massive tesserae with striking columnar morphologies (Figures 3, 4, 9, 10). Such high-aspect-ratio tesserae have been likened by Maisey et al. (2021) to the “voussoir” stones used by stonemasons to build the curved portions of archways; similarly, most images of voussoir tesserae suggesting they have a quite local distribution, associated with skeletal ridges and the margins of foramina in tessellated cartilage (Seidel et al., 2016; Maisey et al., 2021). Our data, however, show columnar tesserae to be the primary tesseral morphology of the perichondral tesseral layer in the jaws of durophagous Rhynchobatus and Rhina, not only limited to curved regions. This argues that these large tesserae may be important for resisting high mechanical loads, as well as for constructing strongly curved surfaces (i.e. regions with small radii of curvature). Also, accepting that tesserae increase in size with age, the larger size of voussoir tesserae could indicate that the perichondral tesseral layer is the oldest in the jaw skeleton. This would suggest that the inner (chondral) layers of smaller scree tesserae developed after perichondral tesserae, within the unmineralized cartilage (Maisey et al., 2021), their disorganization perhaps suggesting a more rapid development in response to increased feeding stresses as the animals grew. Alternatively, columnar tesserae might be larger due to more rapid growth (e.g. in response to high mechanical loads) and scree layering could simply be a constructional constraint of building highly curved cross-sections from individual brick-like elements (tesserae). The hypothesis that larger tesserae and/or a thicker jaw cortex develop in response to load (or at least are involved in resisting higher loads) is also supported by the occurrence of thicker tesseral layers below the dentition (in Rhina and Rhynchobatus, but also in Aetobatus and Raja) and associated with the jaw joint (Rhina, Rhynchobatus, Raja) (Figures 5C,D, 6C,D, 7C,D, 8D). The presence of tessellated sesamoid cartilages beneath the symphyseal meta-teeth in both rhinopristiform species is further suggestive of high local mechanical stresses (Sarin et al., 1999; Fontenelle et al., 2018).
The largest tesserae we measured were irregular and less-mineralized, observed in the oral cortex of larger Rhina and especially Rhynchobatus specimens (Figures 9, 10 and Supplementary Figure S2, Supplementary Table S2). We believe these to be the product of fusions of individual tesserae. It is possible that these structures instead represent groups of tesserae with particularly narrow gaps between them (i.e. beyond the resolutions of our scans), but we find this unlikely, given that individual tesserae were successfully resolved in all other scan regions. The irregular shape of these tesserae is a significant departure from the polygonal (or at least symmetrical) tesserae of other batoid taxa (e.g. Urobatis, Aetobatus, Raja; Seidel et al., 2016, 2020, 2021; Atake and Eames, 2021) and of the smaller Rhina and Rhynchobatus sampled here (Figures 9, 10 and Supplementary Figure S2). Similar irregular tesserae, fused together at their perichondral surfaces, have been observed in the jaw cortices of other species, either in healthy tissues (Maisey, 2013; Maisey et al., 2021) or associated with a callus-building damage response (Dean et al., 2017). Maisey (2013) hypothesized this morphology represented a breakdown of the inhibition of mineralization in the joints between tesserae, noting that this morphology did not always occur in regions of high stress. The similar irregular mineralization we observed in larger Rhina and Rhynchobatus could therefore be associated with age, however, the lower level of mineralization in these tesserae is more difficult to explain. Perhaps these morphologies indicate tesserae with an especially high organic content (e.g. particularly large Sharpey’s fibers; Seidel et al., 2017), as might be needed where the fibrous dental ligament is anchored into the skeleton.
Trabeculae
In addition to surface reinforcements of the skeleton, trabeculae were present in the jaws of all durophagous species examined (Rhynchobatus, Rhina and Aetobatus), but absent in Raja and also Urobatis (see summary diagram, Figure 13). In both Rhynchobatus and Rhina, trabeculae were simple, relatively linear tubes (Figure 12). Whereas trabeculae were typically covered by a single layer of stout tesserae in Rhynchobatus, they apparently bore only a patchy tessellated covering in some regions in Rhina, although trabeculae in this species were roughly three times as densely packed as in Rhynchobatus (Figures 3, 4 and Supplementary Movies S1–S3). The patchy tessellation of Rhina trabeculae is similar to the trabeculae of the Aetobatus jaw we examined (Figure 7 and Figures 9, 10 and Supplementary Movie S4), where trabeculae appeared to lack tesserae at their aboral ends (but see caveat above about the dried nature of the specimens). In contrast to the rhinopristiform species, the trabeculae of Aetobatus were hierarchically branched and far more densely populated.
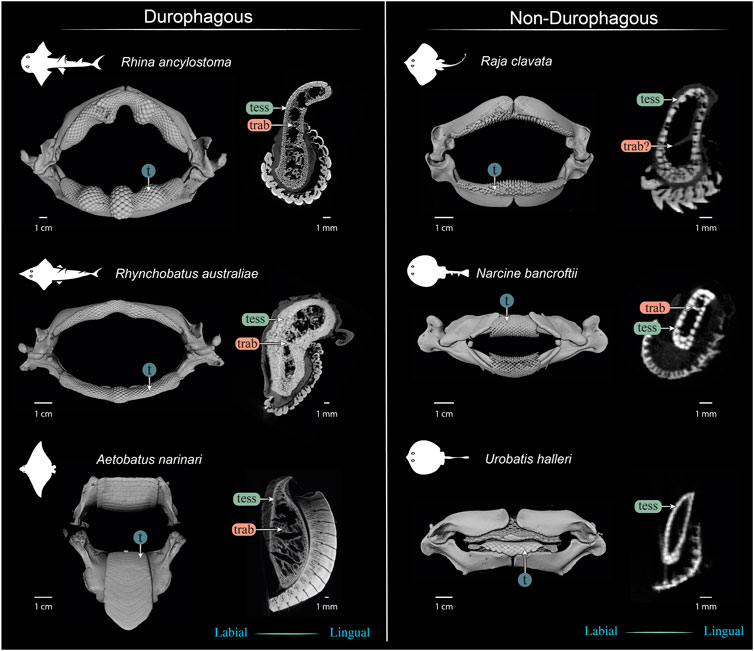
FIGURE 13. Comparison of jaws and upper jaw cross sections of durophagous (left) and non-durophagous batoid species (right). The durophagous batoid jaws shown here are highly “structured,” involving large tesserae and/or numerous tesseral layers reinforcing the cortex, and well-defined tessellated trabeculae (trab), but the preponderance of these characters varies among the species shown here. The non-durophagous jaws are simpler in their cross-sectional shape and sheathed mostly in a single layer of tesserae, but some regions bear features previously associated only with durophagous taxa. For example, multiple tesseral layers are visible in the jaw cortex at the oral ends of all jaw cross sections (beneath the teeth) and sparse load-leading trabeculae are visible in the jaws of Narcine bancroftii and perhaps in Raja clavata (trab?), that of the latter albeit not surrounded by tesserae. In contrast, the jaws of Urobatis halleri were entirely devoid of trabeculae and bore a comparatively thin cortex. The Narcine and Urobatis scans are from unregistered specimens, scanned for other studies.
The orientations of trabeculae in all three durophagous species suggest differences in loading patterns associated with feeding. “Load leading” trabeculae (oriented in-line with the bite force; Dean et al., 2006) were situated beneath the dentition in both the upper and lower jaw cartilages of Rhina and Aetobatus; the durophagous diets of these species can explain the need for such support, preventing collapse of the jaw cortex when exerting the force necessary to fracture invertebrate exoskeletons. The irregular and branching nature of Aetobatus trabeculae may compensate for their comparatively thin walls, but may also indicate this species employs more diverse (e.g. multi-axial) prey-processing behaviors. Conversely, the apparent lack of load-leading trabeculae in Rhynchobatus may be accounted for by the species’ particularly robust jaw cortex. Both Rhina and Rhynchobatus also exhibited trabeculae oriented perpendicular to the direction of bite force, “truss trabeculae” bridging the labial and lingual jaw cortices (Dean et al., 2006), particularly in narrow regions of the jaw cross-section (Figures 3B,C, 4B,C, 9D,G, 10D,G). As with horizontal tie-bars used to brace and strengthen brickwork walls (e.g. Spina et al., 2004; Zielińska, 2017), truss trabeculae likely help maintain the jaw shape during biting by preventing structural buckling (Dean et al., 2006). Whereas truss trabeculae were largely found in the mid-shaft (parasymphyseal regions) of the jaw in both rhinopristiform species, load-leading trabeculae were absent in Rhynchobatus and concentrated closer to the symphysis in Rhina, suggesting some local division of labor in the jaws, perhaps associated with the undulating dentitions of these species. A third class of trabeculae was observed in both rhinopristiform species, perpendicular to both load-leading and truss trabeculae, in line with the long (proximo-distal) axis of the jaws (trab* in Figure 3B and Figures 9, 10 and Supplementary Movies S1–S6). To our knowledge, such trabeculae have not been previously described. Although their role is unclear, their orientation could argue they provide either additional structural support (e.g. like longitudinal bars in steel-reinforced concrete beams) or even a non-mechanical function (e.g. nutrient transport). Mapping the full trabecular network in hydrated samples would help to clarify the true diversity of their functional roles.
Teeth
The teeth of durophagous species are in a battle of contact and fracture mechanics with their prey, working to cause damage to prey shells and exoskeletons, but without tooth materials being damaged in return (Lucas et al., 2008; Amini et al., 2020). The nature of contact between tooth and prey is a deciding factor in which surface is damaged (e.g. tooth or shell) with the radius of curvature of the contacting tooth being hugely important to the type of damage caused (Lucas et al., 2008; Crofts and Summers, 2014). For a given prey item, as teeth become flatter (i.e. with larger radius of curvature), they will tend to cause more far-field damage (at a distance from the contact point) and through-thickness shell failure (Lucas et al., 2008). These are more destructive damage modes than produced by near-contact stresses (Lucas et al., 2008), although smaller indenters, like pointed tooth cusps, can be quite effective in initiating cracks (Crofts and Summers, 2014). Furthermore, for a given tooth diameter and prey item, flat and domed teeth require less force than concave ones to initiate crack propagation in prey exoskeletons, although this is conversely also dependent on the size of the prey item, relative to the concavity (Crofts and Summers, 2014).
The distinctive curved dentitions of Rhina and Rhynchobatus take advantage of the geometric factors facilitating prey fracture. Although containing smaller teeth with far smaller radii of curvature than myliobatiform stingray dentitions (this study; Kolmann et al., 2015a), the bulbous meta-teeth of the rhinopristiform species, particularly pronounced in Rhina, massively increase the radius of curvature of contact with prey items, creating larger “effective cusps” more suited for pulverizing shells. Additionally, although the cross-sectional shape of myliobatiform jaws has been shown to have little impact on shell crushing performance (Kolmann et al., 2015a), the undulating oral jaw surfaces of Rhina and Rhynchobatus provide a sculptured loading platform for prey items, the biological equivalent of an engineering three-point bending rig, allowing the meta-teeth to act as large, local stress concentrators.
Compared to most other examined batoid fishes, Rhina and Rhynchobatus have thicker enameloid, comprising a compact outer layer of randomly-orientated crystallites and an inner parallel-organized layer, with crystallites orientated perpendicular to the tooth surface (Enault et al., 2013; Manzanares et al., 2016). This thickened microstructure is believed to impart compression resistance to the enameloid (Gillis and Donoghue, 2006; Enault et al., 2013; Amini et al., 2020). Coupled with teeth being interlocked, and tooth ridges and meta-teeth surely enhancing the grip on prey (particularly in Rhina), these dental features create a stabilized platform for crushing behaviors. The comparatively large teeth that comprise meta-teeth (Figures 3–6) and the broken cusps observed in two of our specimens’ meta-teeth (asterisk in Figures 3A, 5A) are perhaps indications of the exceptional local stresses generated in these areas. The robust and self-supporting nature of Rhina and Rhynchobatus dentitions is also demonstrated by their spanning of the jaw symphyses, which we show are unfused and flanked by the distal jaw tips, which have surprisingly thin-walled cortices (Figures 3, 4). This is in stark contrast to the jaws of Aetobatus, where the jaw halves are fused at the midline into stout, single elements (Figure 7). In Rhina and Rhynchobatus, the dentitions (and perhaps, sesamoid cartilages) must therefore also act as structural girders to support the jaws at the midline, a function not typically attributed to teeth.
Toward a synthesis of elasmobranch durophagy
It is clear that “durophagy” is too general a descriptor for the diverse diets and morphologies of elasmobranch fishes typically placed in this category. For example, whereas the diet of Aetobatus is dominated by hard-shelled molluscs (Schluessel et al., 2010), Rhina is known to feed on fish, prawns, and cephalopods, in addition to crabs and bivalves (Compagno and Last, 1999; Raje, 2006). The stomach contents of Rhynchobatus, by comparison, indicate a predominantly shrimp-based diet, with fish and crabs eaten only by larger individuals (Nasir, 2000; Abdurahiman et al., 2010), while a recent study, based on spines embedded in the jaws (see also Figure 10F), suggested that Rhynchobatus may also prey on smaller stingrays (Dean et al., 2017). Anyone who has eaten seafood knows that the strategies for crushing mollusc shells differ considerably from those for processing crustaceans like shrimp. Yet to date, no experimental study has looked broadly enough at durophagous feeding anatomy and performance in elasmobranchs to resolve more subtle ecomorphological connections.
Our data, combined with anatomical data from previous works (e.g. Summers, 2000; Kolmann et al., 2015a; Rutledge et al., 2019) begin to frame a more holistic view of elasmobranch durophagy, mapping out a suite of modular morphological characters, which can be diversely combined to reinforce against extreme feeding loads. A comparison of several of the batoid fishes most-studied in anatomical research (Figure 13) illustrates the potential interrelationships of morphological characters, underlining differences in diet, tesseral shape and layering, trabecular presence and orientation, and dentition (including enameloid thickness and surface ornamentation). Cortical tesserae, for example, can vary in their size and shape, and in how orderly and numerous their layers are. Thicker cortices are certainly associated with regions of high load, even in non-durophagous species, but this can be variously achieved, by employing massive tesserae (e.g. perichondral columnar tesserae), numerous tesseral layers, or both. Such reinforcements seem to come with departures from the “typical” polygonal tesseral forms, in perichondral and chondral tesseral layers. Symphyseal fusion, more massive and flatter teeth, and teeth interacting to form superstructures (e.g. meta-teeth) also occur in species experiencing high feeding stresses. Trabeculae, also present in their “truss” (labio-lingual) orientation in non-durophagous species, occur in far higher densities and with telltale load-leading (oral-aboral) alignment in species with molluscs in their diets.
These characters can all be involved in jaw reinforcement for durophagy, yet they appear to trade-off in their preponderance: Rhynchobatus possesses a thicker jaw cortex (comprised of fewer, but more massive tesserae, fused into mineralized concretions beneath the teeth), but no load-leading trabeculae, an unfused symphysis, and shorter teeth with a low degree of interlocking and tooth superstructuring (i.e. assembly into meta-teeth). Rhina’s unfused symphysis and thinner jaw cortex (from smaller, but more numerous tesserae), is compensated for by thick-walled trabeculae (albeit in some regions only partially tessellated), oriented in line with loading and a more robust dentition, with thicker teeth, thicker enameloid and massive meta-teeth. The jaws of Aetobatus have a comparatively thin cortex (comprised of several layers of thin, platelike tesserae), but are filled with a dense stand of load-leading trabeculae (thin-walled, but branching), the jaws fused at the symphysis, and the extremely thick and interlocking teeth creating a monolithic dental plate.
Previous comparisons of durophagous shark and ray species have suggested that elasmobranch lineages invest to different degrees in shape-vs. structure-vs. material-solutions for jaw reinforcement (Summers et al., 2004; Huie et al., 2022). Including our data, these observations of varied reinforcement strategies argue that durophagy is an interestingly multivariate problem in elasmobranchs with diverse solutions, yet the pressures driving the evolution of the different character combinations are unclear. The key to clarifying this is multi-disciplinary: by examining and integrating feeding behavior and mechanics, gut content, anatomical, and biological materials data, we can better resolve the factors that have shaped extreme feeding modes and determine their links to phylogeny, prey co-evolution and biogeography.
Data availability statement
Renderings of the primary microCT datasets are provided in annotated videos in the Supplementary Material. Additional microCT data is available from the authors by request.
Ethics statement
Ethical review and approval was not required for the animal study because all samples examined were fixed or dried specimens from research collections (Natural History Museum, London).
Author contributions
BC, ZJ, MMS, and MD devised the project and experiments. BC, ZJ, and CU procured and scanned specimens and rendered scan data. BC, JC, and MD analyzed data. BC, JC, and ZJ generated the figures and tables. BC, MD, ZJ, and JC wrote the manuscript. All authors edited the manuscript.
Funding
Several of the study’s micro-CT scans were performed via a SYNTHESYS project collections-based research grant (GB-TAF-6706; http://www.synthesys.info) to MD, financed by the European Community Research Infrastructure Action under the FP7 Integrating Activities Programme.
Acknowledgments
We thank Yannis Papastamatiou and Carol Bucking for insights into elasmobranch digestive enzymes and Matt Kolmann for always-inspiring discussions about elasmobranch durophagy and all things batoid. Frederik Mollen (Elasmobranch Research Belgium) kindly provided Rhina specimens to help in estimating maturity from jaw width. Daniel Werner, Binru Yang and Dominique Adriaens helped arrange the Urobatis and Narcine scans for Figure 13. We also thank James MacLaine, Oliver Crimmen and Emma Bernard (Natural History Museum, London) for access to collections under their care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2022.932341/full#supplementary-material
References
Abdurahiman, K. P., Nayak, T. H., Zacharia, P. U., and Mohamed, K. S. (2010). Trophic organisation and predator–prey interactions among commercially exploited demersal finfishes in the coastal waters of the southeastern Arabian Sea. Estuar. Coast. Shelf Sci. 87, 601–610. doi:10.1016/j.ecss.2010.03.002
Ajemian, M. J., Lamboy, C., Ibrahim, A., DeGroot, B. C., Bassos-Hull, K., Mann, D. A., et al. (2021). Capturing shell-crushing by large mobile predators using passive acoustics technology. J. Exp. Mar. Biol. Ecol. 535, 151497. doi:10.1016/j.jembe.2020.151497
Amini, S., Razi, H., Seidel, R., White, W., Weaver, J. C., Dean, M. N., et al. (2020). Staying sharp despite erosion: Lessons from the micro-architectural heterogeneities in Port Jackson shark tooth enameloid. Nat. Commun. 11, 5971. doi:10.1038/s41467-020-19739-0
Anderson, W. G., Dasiewicz, P. J., Liban, S., Ryan, C., Taylor, J. R., Grosell, M., et al. (2010). Gastro-intestinal handling of water and solutes in three species of elasmobranch fish, the white-spotted bamboo shark, Chiloscyllium plagiosum, little skate, Leucoraja erinacea and the clear nose skate Raja eglanteria. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 155, 493–502. doi:10.1016/j.cbpa.2009.09.020
Aschliman, N. C., Claeson, K. M., and McEachran, J. D. (2012). “Phylogeny of Batoidea,” in Biology of sharks and their relatives. Editors J. C. Carrier, J. A. Musick, and M. R. Heithaus (CRC Press), 57.
Aschliman, N. C. (2014). Interrelationships of the durophagous stingrays (Batoidea: Myliobatidae). Environ. Biol. Fishes 97, 967–979. doi:10.1007/s10641-014-0261-8
Atake, O. J., and Eames, B. F. (2021). Mineralized cartilage and bone-like tissues in chondrichthyans offer potential insights into the evolution and development of mineralized tissues in the vertebrate endoskeleton. Front. Genet. 12, 762042. doi:10.3389/fgene.2021.762042
Berio, F., Broyon, M., Enault, S., Pirot, N., López-Romero, F. A., and Debiais-Thibaud, M. (2021). Diversity and evolution of mineralized skeletal tissues in chondrichthyans. Front. Ecol. Evol. 9, 660767. doi:10.3389/fevo.2021.660767
Clement, J. G. (1992). Re-Examination of the fine structure of endoskeletal mineralization in chondrichthyans: Implications for growth, ageing and calcium homeostasis. Mar. Freshw. Res. 43, 157–181. doi:10.1071/mf9920157
Collins, A., Heupel, M., Hueter, R., and Motta, P. J. (2007). Hard prey specialists or opportunistic generalists? An examination of the diet of the cownose ray. Mar. Freshw. Res. 58, 135–144. doi:10.1071/mf05227
Compagno, L. J. V., and Last, P. R. (1999). FAO species identification guide for fishery purposes. The living marine resources of the western central pacific. Norfolk, Virginia, USA: Food and Agriculture Organization of the United Nations–FAO, 1418
Cortés, E., Papastamatiou, Y. P., Carlson, J. K., Ferry-Graham, L., Wetherbee, B. M., Cyrino, J. E. P., et al. (2008). An overview of the feeding ecology and physiology of elasmobranch fishes. Feeding and Digestive Functions of Fishes. London: CRC Press, 393
Crofts, S. B., and Summers, A. P. (2014). How to best smash a snail: The effect of tooth shape on crushing load. J. R. Soc. Interface 11, 20131053. doi:10.1098/rsif.2013.1053
Darracott, A. (1977). Availability, morphometrics, feeding and breeding activity in a multi-species, demersal fish stock of the Western Indian Ocean. J. Fish. Biol. 10, 1–16. doi:10.1111/j.1095-8649.1977.tb04036.x
Dean, M. N., Bizzarro, J. J., Clark, B., Underwood, C. J., and Johanson, Z. (2017). Large batoid fishes frequently consume stingrays despite skeletal damage. R. Soc. Open Sci. 4, 170674–170611. doi:10.1098/rsos.170674
Dean, M. N., Bizzarro, J. J., and Summers, A. P. (2007). The evolution of cranial design, diet, and feeding mechanisms in batoid fishes. Integr. Comp. Biol. 47, 70–81. doi:10.1093/icb/icm034
Dean, M. N., Ekstrom, L., Monsonego-Ornan, E., Ballantyne, J., Witten, P. E., Riley, C., et al. (2015). Mineral homeostasis and regulation of mineralization processes in the skeletons of sharks, rays and relatives (Elasmobranchii). Semin. Cell Dev. Biol. 46, 51–67. doi:10.1016/j.semcdb.2015.10.022
Dean, M. N., Huber, D. R., and Nance, H. A. (2006). Functional morphology of jaw trabeculation in the lesser electric ray Narcine brasiliensis, with comments on the evolution of structural support in the Batoidea. J. Morphol. 267, 1137–1146. doi:10.1002/jmor.10302
Dean, M. N., Mull, C. G., Gorb, S. N., and Summers, A. P. (2009). Ontogeny of the tessellated skeleton: Insight from the skeletal growth of the round stingray Urobatis halleri. J. Anat. 215, 227–239. doi:10.1111/j.1469-7580.2009.01116.x
Enault, S., Cappetta, H., and Adnet, S. (2013). Simplification of the enameloid microstructure of large stingrays (Chondrichthyes: Myliobatiformes): A functional approach. Zool. J. Linn. Soc. 169, 144–155. doi:10.1111/zoj.12059
Fänge, R., Lundblad, G., Lind, J., and Slettengren, K. (1979). Chitinolytic enzymes in the digestive system of marine fishes. Mar. Biol. 53, 317–321. doi:10.1007/bf00391614
Fontenelle, J. P., Loboda, T. S., Kolmann, M., and De Carvalho, M. R. (2018). Angular cartilage structure and variation in Neotropical freshwater stingrays (Chondrichthyes: Myliobatiformes: Potamotrygonidae), with comments on their function and evolution. Zool. J. Linn. Soc. 183, 121–142. doi:10.1093/zoolinnean/zlx054
Franklin, O., Palmer, C., and Dyke, G. (2014). Pectoral fin morphology of batoid fishes (Chondrichthyes: Batoidea): Explaining phylogenetic variation with geometric morphometrics. J. Morphol. 275, 1173–1186. doi:10.1002/jmor.20294
Gillis, J. A., and Donoghue, P. C. J. (2006). The homology and phylogeny of chondrichthyan tooth enameloid. J. Morphol. 268, 33–49. doi:10.1002/jmor.10501
Herbert, A. M., and Motta, P. J. (2018). Biomechanics of the jaw of the durophagous bonnethead shark. Zoology 129, 54–58. doi:10.1016/j.zool.2018.07.001
Holmgren, S., and Nilsson, S. (1999). Sharks, skates and rays: The biology of elasmobranch fishes. Editor W. C. Hamlett (Baltimore, London: The Johns Hopkins University Press), 144
Huie, J. M., Summers, A. P., and Kawano, S. M. (2022). SegmentGeometry: A tool for measuring second moment of area in 3D slicer. Integr. Org. Biol. 4, obac009. doi:10.1093/iob/obac009
Jayasankar, A. K., Seidel, R., Hosny, A., Weaver, J. C., Fratzl, P., Chen, J., et al. (2020). Multi-scale modeling and mechanical performance characterization of stingray skeleton-inspired tessellations. J. Mech. Phys. Solids 138, 103906. doi:10.1016/j.jmps.2020.103906
Kolmann, M. A., Crofts, S. B., Dean, M. N., Summers, A. P., and Lovejoy, N. R. (2015a). Morphology does not predict performance: Jaw curvature and prey crushing in durophagous stingrays. J. Exp. Biol. 218, 3941–3949. doi:10.1242/jeb.127340
Kolmann, M. A., Huber, D. R., Motta, P. J., and Grubbs, R. D. (2015b). Feeding biomechanics of the cownose ray, Rhinoptera bonasus, over ontogeny. J. Anat. 227, 341–351. doi:10.1111/joa.12342
Kolmann, M. A., Welch, K. C., Summers, A. P., and Lovejoy, N. R. (2016). Always chew your food: Freshwater stingrays use mastication to process tough insect prey. Proc. Biol. Sci. 283, 20161392–20161399. doi:10.1098/rspb.2016.1392
Last, P., Naylor, G., Séret, B., White, W., de Carvalho, M., and Stehmann, M. (2016). Rays of the world. Australia, New Zealand: Csiro Publishing.
Liu, X., Dean, M. N., Youssefpour, H., Summers, A. P., and Earthman, J. C. (2014). Stress relaxation behavior of tessellated cartilage from the jaws of blue sharks. J. Mech. Behav. Biomed. Mat. 29, 68–80. doi:10.1016/j.jmbbm.2013.08.014
Lucas, P., Constantino, P., Wood, B., and Lawn, B. (2008). Dental enamel as a dietary indicator in mammals. Bioessays 30, 374–385. doi:10.1002/bies.20729
Maisey, J. G., Denton, J. S. S., Burrow, C., and Pradel, A. (2021). Architectural and ultrastructural features of tessellated calcified cartilage in modern and extinct chondrichthyan fishes. J. Fish. Biol. 98, 919–941. doi:10.1111/jfb.14376
Maisey, J. G. (2013). The diversity of tessellated calcification in modern and extinct chondrichthyans. Rev. Paléobiologie, Genève 32, 355.
Manzanares, E., Rasskin-Gutman, D., and Botella, H. (2016). New insights into the enameloid microstructure of batoid fishes (Chondrichthyes). Zool. J. Linn. Soc. 177, 621–632. doi:10.1111/zoj.12377
Marconi, A., Hancock-Ronemus, A., and Andrew Gillis, J. (2020). Adult chondrogenesis and spontaneous cartilage repair in the skate, Leucoraja erinacea. eLife 9, e53414. doi:10.7554/elife.53414
Moazzam, M., and Osmany, H. B. (2020). Species composition, commercial landings, distribution and some aspects of biology of guitarfish and wedgefish (Class Pisces: Order Rhinopristiformes) from Pakistan. J. Biol. Biotechnol. 17, 469
Nasir, N. A. (2000). The food and feeding relationships of the fish communities in the inshore waters of Khor Al-Zubair, northwest Arabian Gulf. Cybium Int. J. Ichthyology 24 (1), 89
Purushottama, G. B., Raje, S. G., -T., Akhilesh, K. V., Kizhakudan, S. J., and Zacharia, P. U. (2020). Reproductive biology and diet composition of Rhynchobatus laevis (bloch and schneider, 1801) (Rhinopristiformes: Rhinidae) from the northern Indian ocean. Indian J. Fish. 67. doi:10.21077/ijf.2020.67.4.95636-02
Raje, S. G. (2006). Skate fishery and some biological aspects of five species of skates off Mumbai. Indian J. Fish. 53, 431
Rutledge, K. M., Summers, A. P., and Kolmann, M. A. (2019). Killing them softly: Ontogeny of jaw mechanics and stiffness in mollusk-feeding freshwater stingrays. J. Morphol. 280, 796–808. doi:10.1002/jmor.20984
Sarin, V. K., Erickson, G. M., Giori, N. J., Bergman, A. G., and Carter, D. R. (1999). Coincident development of sesamoid bones and clues to their evolution. Anat. Rec. 257, 174–180. doi:10.1002/(SICI)1097-0185(19991015)257:5<174::AID-AR6>3.0.CO;2-O
Schluessel, V., Bennett, M. B., and Collin, S. P. (2010). Diet and reproduction in the white-spotted eagle ray Aetobatus narinari from queensland, Australia and the penghu islands, taiwan. Mar. Freshw. Res. 61, 1278–1289. doi:10.1071/mf09261
Seidel, R., Blumer, M., Chaumel, J., Amini, S., and Dean, M. N. (2020). Endoskeletal mineralization in chimaera and a comparative guide to tessellated cartilage in chondrichthyan fishes (sharks, rays and chimaera). J. R. Soc. Interface 17, 20200474. doi:10.1098/rsif.2020.0474
Seidel, R., Blumer, M., Pechriggl, E.-J., Lyons, K., Hall, B. K., Fratzl, P., et al. (2017). Calcified cartilage or bone? Collagens in the tessellated endoskeletons of cartilaginous fish (sharks and rays). J. Struct. Biol. 200, 54–71. doi:10.1016/j.jsb.2017.09.005
Seidel, R., Jayasankar, A. K., and Dean, M. N. (2021). The multiscale architecture of tessellated cartilage and its relation to function. J. Fish. Biol. 98, 942–955. doi:10.1111/jfb.14444
Seidel, R., Lyons, K., Blumer, M., Zaslansky, P., Fratzl, P., Weaver, J. C., et al. (2016). Ultrastructural and developmental features of the tessellated endoskeleton of elasmobranchs (sharks and rays). J. Anat. 229, 681–702. doi:10.1111/joa.12508
Seidel, R., Roschger, A., Li, L., Zhang, Q., Yin, J., Yang, T., et al. (2019). Mechanical properties of stingray tesserae: High-resolution correlative analysis of mineral density and indentation moduli in tessellated cartilage. Acta Biomater. 96, 421–435. doi:10.1016/j.actbio.2019.06.038
Spina, G., Ramundo, F., and Mandara, A. (2004). Masonry strengthening by metal tie-bars, a case study. Structural Analysis of Historical Constructions. Padova, Italy, 10. Available at: http://www.hms.civil.uminho.pt/sahc/2004/1207.pdf
Summers, A. P., Ketcham, R., and Rowe, T. (2004). Structure and function of the horn shark (Heterodontus francisci) cranium through ontogeny - the development of a hard prey specialist. J. Morphol. 260, 1–12. doi:10.1002/jmor.10141
Summers, A. P., Koob, T. J., and Brainerd, E. L. (1998). Stingray jaws strut their stuff. Nature 395, 450–451. doi:10.1038/26649
Summers, A. P. (2000). Stiffening the stingray skeleton—an investigation of durophagy in myliobatid stingrays (Chondrichthyes, Batoidea, Myliobatidae). J. Morphol. 243, 113–126. doi:10.1002/(SICI)1097-4687(200002)243:2<113::AID-JMOR1>3.0.CO;2-A
Underwood, C. J., Johanson, Z., Welten, M., Metscher, B., Rasch, L. J., Fraser, G. J., et al. (2015). Development and evolution of dentition pattern and tooth order in the skates and rays (Batoidea; Chondrichthyes). PLoS One 10, e0122553–19. doi:10.1371/journal.pone.0122553
Wilga, C. D., and Motta, P. J. (2000). Durophagy in sharks: Feeding mechanics of the hammerhead Sphyrna tiburo. J. Exp. Biol. 203, 2781–2796. doi:10.1242/jeb.203.18.2781
Keywords: Batoidea, durophagy, jaw, trabeculae, tesserae, tessellated cartilage, skeletal biomaterials
Citation: Clark B, Chaumel J, Johanson Z, Underwood C, Smith MM and Dean MN (2022) Bricks, trusses and superstructures: Strategies for skeletal reinforcement in batoid fishes (rays and skates). Front. Cell Dev. Biol. 10:932341. doi: 10.3389/fcell.2022.932341
Received: 29 April 2022; Accepted: 22 August 2022;
Published: 12 October 2022.
Edited by:
Sylvain Marcellini, University of Concepcion, ChileReviewed by:
Tatjana Haitina, Uppsala University, SwedenJake Leyhr, Uppsala University, Sweden, in collaboration with reviewer TH
Jonathan Huie, George Washington University, United States
Matthew Vickaryous, University of Guelph, Canada
Copyright © 2022 Clark, Chaumel, Johanson, Underwood, Smith and Dean. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mason N. Dean, mndean@cityu.edu.hk, mason.dean@mpikg.mpg.de
†These authors have contributed equally to this work and share first authorship
 Brett Clark1†
Brett Clark1†  Júlia Chaumel
Júlia Chaumel Zerina Johanson
Zerina Johanson Moya M. Smith
Moya M. Smith Mason N. Dean
Mason N. Dean