- 1School of Chemistry and Environmental Engineering, Sichuan University of Science and Engineering, Zigong, China
- 2Guangxi Key Laboratory of Chemistry and Engineering of Forest Products, Guangxi University for Nationalities, College of Chemistry and Chemical Engineering, Nanning, China
- 3Dongguan Key Laboratory of Drug Design and Formulation Technology, Key Laboratory of Research and Development of New Medical Materials of Guangdong Medical University, School of Pharmacy, Guangdong Medical University, Dongguan, China
- 4Department of Chemistry, University of Delhi, New Delhi, India
- 5Department of Chemistry, Faculty of Science, University of Lucknow, Lucknow, India
The precise unification of functional groups and photoluminescence properties can give rise to MOFs that can offer diverse applications like selective detection of nitroaromatic compounds (NACs) which are considered to be an important ingredient of explosive as well as cation and anion sensing. Hence, a new 3D metal-organic framework (MOF) [Cd2(btc)(bib)(HCOO)(H2O)·H2O]n (1) has been synthesized using mixed ligand strategy by solvothermal reaction of cadmium acetate with two ligands viz. 1,3,5-benzenetricarboxylic acid (H3btc) and 1,4-bis(2-methyl-imidazol-1-yl)butane (bib). The MOF 1 possesses highly 10-connected network which is based on {Cd4(btc)2(bib)4} molecular building block. The studies showed that 1 could be taken as the fluorescent sensor for sensitive recognition of NACs, in particular 2,4,6-trinitrophenol (TNP) with notable quenching (Ksv = 5.42 × 104 M−1) and LOD of 1.77 ppm. Additionally, 1 also displayed selective sensing for Fe3+ ions with Ksv = 6.05 × 103 M− 1 and LOD = 1.56 ppm. Also, this dual sensor displayed excellent reusability toward the detection of TNP and Fe3+ ion. Theoretical calculations have been performed to propose the probable mechanism for the sensing luminescence intensity. Calculations indicated that because of the charge transfer and weak interaction that is operating between NACs and MOF, the weakening in the photoluminescence intensity resulted.
Introduction
Currently, tremendous amount of efforts have been devoted in developing new metal-organic frameworks (MOFs) which display photoluminescent properties and hence this class of luminescence materials can be in demand for solving the pollution problems and can offer potential application as luminescent sensors (Hua et al., 2015; Li et al., 2015; Shi et al., 2015; Wang et al., 2015, 2017; Liu et al., 2016a,b, 2017; Chen et al., 2017; Lu et al., 2017; Ma et al., 2017). To prepare useful and excellent luminescent MOF based sensors, the ligand-based strategy had been proposed in which the precise incorporation of functional groups in the π-conjugated organic ligands and their coordination with d10-metal ions deliver MOFs which can be used as luminescent materials (Ma et al., 2013; Zheng et al., 2014; Wang et al., 2016; Guo et al., 2017; Chen et al., 2018a,b; Shen et al., 2018). Up to now, numerous luminescent MOFs have been documented which had been utilized to detect metal ions and small organic compounds (Cui et al., 2012). Sun et al. had synthesized two Cd(II)-based MOFs which demonstrated selectivity to detect acetone (Yi et al., 2012). Chen et al. proposed that the choice of metal centers play a crucial role in molecular recognition by binding interactions of metal sites of the host MOF with guest molecules (Chen et al., 2007). Bu et al. reported a highly sensitive luminescent Cd(II)-based MOF that quenched at 100 ppm of TNP and display a high quenching efficiency of 92.5% (Tian et al., 2014). Thus, luminescence quenching based detection of compounds offers an alternative that is simple, sensitive, and convenient in nature. The possible mechanism associated with the sensing properties of these materials depend on monitoring of the transmission signals generated during interactions that are taking place between sensors (guest) and substrates (host) (Gole et al., 2011; Pramanik et al., 2011; Chen et al., 2013; Balamurugan et al., 2014).
The recent investigations have proved that MOFs, constructed from mixed organic ligands comprising of dicarboxylate and N-donor linkers display interesting dynamic properties (Zhan et al., 2014). Bearing these aspects in mind and in continuation to our efforts in the area of metal-organic frameworks (MOFs) (Li et al., 2016; Liu et al., 2016c; Jin et al., 2017), which were aimed toward the syntheses of multifunctional MOFs we have chosen a flexible 1,4-bis(2-methyl-imidazol-1-yl)butane (bib) ligand (Hou et al., 2014a,b; Shi et al., 2014), and a rigid 1,3,5-benzenetricarboxylic acid (H3btc) ligand as well as d10 transition-metal center (Cd2+) to develop new structures with potential applications as luminescence sensor. The choice of using this strategy has been based on the following considerations (Marin et al., 2006; Kent et al., 2010; Saini and Das, 2012; Son et al., 2013; Banerjee et al., 2014; Li et al., 2014; Park and Lee, 2015; Wen et al., 2015; Park et al., 2016; Das and Mandal, 2018): (1) the binding capacity between bib ligand and metal centers not only induces flexible and diverse structures but also facilitates the surface functionalities of the materials; (2) rigid ligand btc with aromatic rings could effectively favor intra-ligand interactions and induce luminescent character. Herein, we are presenting a new Cd(II) MOF having formula[Cd2(btc)(bib)(HCOO)(H2O)·H2O]n (1) which have been utilized as dual photoluminescent sensor for the selective detection of nitroaromatics (NACs) especially TNP and ferric ions.
Materials and Methods
The instrumental, X-ray crystallographic, and computational details are presented in the Supplementary Information.
Synthesis of [Cd2(btc)(bib)(HCOO)(H2O)·H2O]n (1)
A mixture of H3btc (0.10 mmol, 0.021 g), bib (0.10 mmol, 0.022 g), Cd(NO3)2·4H2O (0.15 mmol, 0.027 g) in 20 mL DMF/H2O mixture (v/v = 3:1) was stirred for 30 min. This mixture was then transferred in a 25-mL Teflon-lined reactor, sealed and heated up to 120°C and this temperature was maintained for 72 h. The mixture was cooled down to room temperature with a cooling rate of 5°C/h. Yellow block type crystals of 1 were obtained in 74% yield on the basis of cadmium. Elemental analysis (% calc/found): C: 35.17/34.92, H:3.37/3.28, N:7.81/7.55.
Results and Discussion
Crystal Structure Description
The single-crystal X-ray diffraction results indicate that 1 crystallizes in triclinic space group P-1. 1 is a 3D architecture having binuclear Cd(II) clusters as secondary building units(SBUs) which in turn is constructed by mixed ligands viz. btc and bib. 1 shows two types of Cd(II) centers with different coordination fashions. The SBUs of 1 are bonded by two bib, one μ2-η2:η2 formate anion and two bidentate chelating carboxylic anions and one bridging bidentate carboxylic anion (Figure 1A and Scheme S1). The formate anion was generated from DMF under acid condition. This conversion had already been reported previously many times (Lu et al., 2017). The Cd1 ion is hexa-coordinated where the CdO5N unit possess distorted octahedral geometry, while the Cd2 ion is seven-coordinated in which CdO6N moiety display distorted pentagonal bipyramidal geometry. The Cd1 coordinates to one carboxylate group from one btc, one O-atom of one bridged carboxylate from another btc, one μ2-O of one formate, one N from imidazole and is capped by the O of one H2O.The Cd2 center of 1 is connected to one carboxylate from one btc, one O of one bridged carboxylate from the other btc, three μ2-O of two formate anions and one N from imidazole. The dimeric units in SBU are formed by completely deprotonated btc anions to generate a 2D layered arrangement (Figure 1B and Figure S1). Further these 2D layers further linked by bib ligand to generate 3D framework (Figure 1C). Thus, the four Cd(II)centers are divulged by two bridged formates and two carboxylates of btc. Further it spreads out with six 3-connected btc and four linear imidazole-based bib ligands and in this way, this tetra-cadmium SBU can be simplified into a 10-connected node. The full motif of 1 can be taken as a 10-connected 3D network with (36.44.510. 512.68.65) topology (Figure 1D). The evacuated 1 shows theoretical porosity of 15.8% according to PLATON calculations with a probe radius of 1.65 Å (Spek, 2003). Thermogravimetric result indicates that 1 remains stable till 350°C (Figure S2). Also, PXRD experiment had been performed to assess whether the MOF is having phase purity in the solid state. The PXRD patterns confirm the phase purity of the bulk sample (Figure S3).
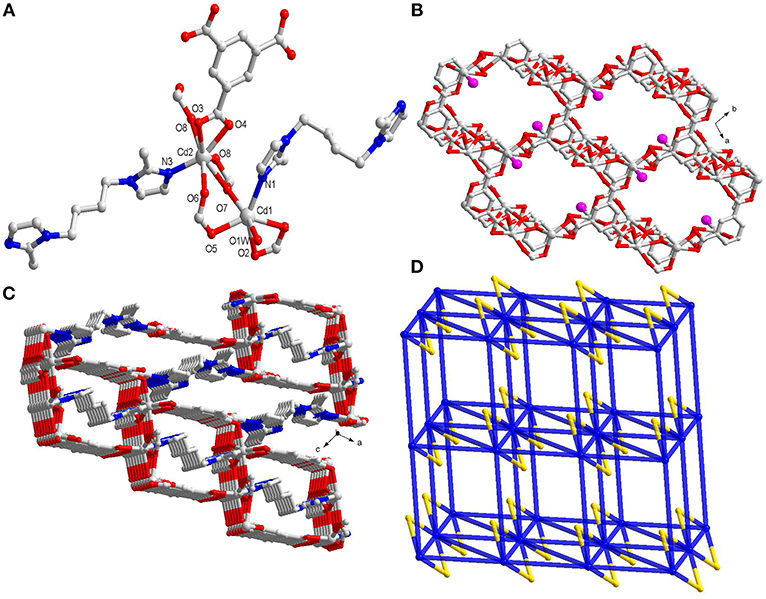
Figure 1. (A) The coordination environment around Cd(II) centers in 1; (B) Perspective view of 2D layer constructed by Cd(II) centers and btc linkers(the pink ball represents the uncoordinated carboxylate group); (C) The 3D framework connected by btc and bib ligands; (D) Schematic representation of the 3D 10-connected network(the yellow ball represents the formate linker).
Luminescence Sensing
In general, the MOFs based on d10-based metal centers and conjugated linkers are excellent candidates for the photoluminescent properties. It has been reported that the btc and bib exhibit emissions at 375 nm (λex = 300 nm) and 445 nm (λex = 370 nm), respectively (Li et al., 2015). 1 displayed emission with the maxima at 395 nm (λex = 300 nm).The red-shift in emission maximum in the case of 1 as compared to btc with concomitant enhancement in the intensity indicates that the electron transfer may be taking place between the ligands and metalcenters (Allendorf et al., 2009; Rocha et al., 2011; Heine and Buschbaum, 2013; Hu et al., 2015). The spectral feature associated with 1 provided us impetus to use this MOF as the luminescent sensor for the detection of organic compounds (Figure S4). Thus, the photoluminescent property of the emulsion of 1 in different solvent was investigated. It has been found that luminescent spectrum of 1 is largely dependent on nature of small solvents, especially nitrobenzene (NB) (Figure S5) where 1 exhibits the most significant quenching effect in intensity (Figure 2a). The possible mechanism of the quenching in photoluminescent intensity in presence of NB has been proposed to originate from the electron-withdrawing effect from its nitro groups (Chen et al., 2009; Zheng M. et al., 2013; Song et al., 2014). Such solvent reliant luminescence properties can be interesting for sensing of different derivatives of NB. Hence, the luminescence intensity of emulsion of 1 in presence of different nitroaromatic compounds (NACs), viz.2,6-dinitrotoluene (2,6-DNT), o-nitrophenol (ONP),2,4-dinitrophenol (2,4-DNP), 2,4,6-trinitrophenol (TNP), p-nitrophenol (PNP),2-nitrotoluene (2-NT), 4-nitrotoluene (4-NT), 1,3-dinitrobenzene (1,3-DNB),1,2,4-trimethylbenzene 1,3,5-trimethylbenzene (1,3,5-TMB),2,4-dinitrotoluene (2,4-DNT), were recorded. The experiments showed that the addition of same concentration of NACs to the emulsion of 1 will results in the decline in its luminescent intensity to different levels (Figure 2b and Figures S6–S26). However, aromatic compounds not having such electron withdrawing nitro group, viz.1,3,5-TMB and 1,2,4-TMB have displayed intensity enhancement in 1.
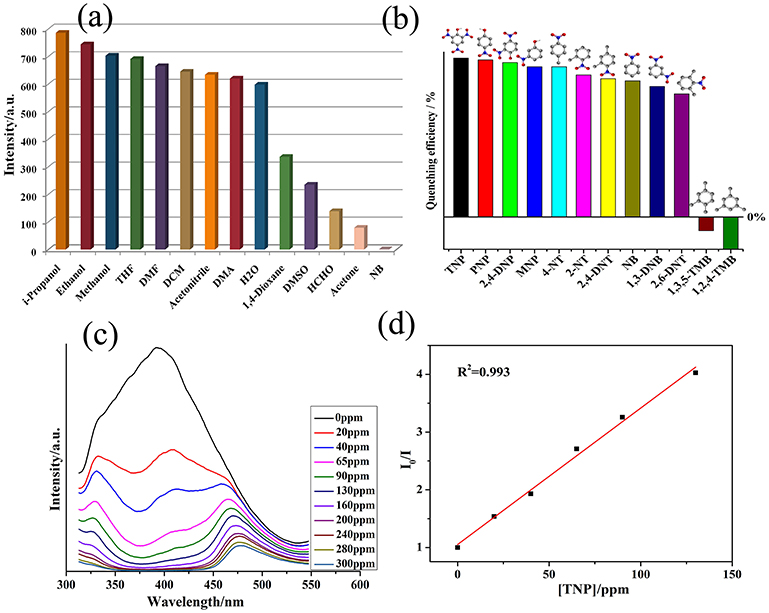
Figure 2. (a) The plots of varied luminescent intensity of 1 when dispersed in different small organic molecules (λex = 320 nm); (b) The quenching efficiency of different nitro compounds (Emission intensities at 375 nm were selected); (c) The luminescence intensity of 1 recorded after addition of 1 mM TNP (λex = 320 nm); (d) SV plot in the presence of 1 at different TNP concentrations.
To further explore the sensing property of 1 toward TNP, the emission spectra of the suspension of 1 were recorded by gradual addition of batches of TNP solutions. The experiment indicated that the luminescence intensity of 1 declined with augmenting concentration of TNP (Figure 2c). The quenching efficiency was evaluated to be 44.5% for 20 ppm of TNP and 85% for 240 ppm of TNP. The Stern–Volmer (SV) equation which is used quantitatively to calculate the quenching efficiency was employed (Sanchez and Trogler, 2008; Sanchez et al., 2008; Zhang et al., 2015). The SV plot of TNP was almost linear at related low concentrations (Figure 2d), and its quenching constant (Ksv) of 1 is 5.42 × 104 M−1 and its LOD is 1.77 ppm, which was similar to some previously reported examples (Table S3). Additionally the Ksv value of 1 for TNP is 3.64 × 103 M−1 and LOD parameter is 1.35 ppm. These observations clearly indicates that the 1 under study may be a highly selective sensor for TNP in comparison to other NACs (Qin et al., 2014; Singh and Nagaraja, 2014). The PXRD pattern of the recovered sample after performing 5 cycles of sensing studies indicate high stability of 1 and hence it can be concluded that the integrity of 1 remained almost unchanged after operational sensing works (Figure S3). On the basis of the structural feature of 1 which indicated only 15.8% porosity (vide supra), the encapsulation of TNP into the channels of MOF cannot take place. Thus, the interaction of analytes with 1 are more inclined toward the surface interaction operating between the analytes and 1.
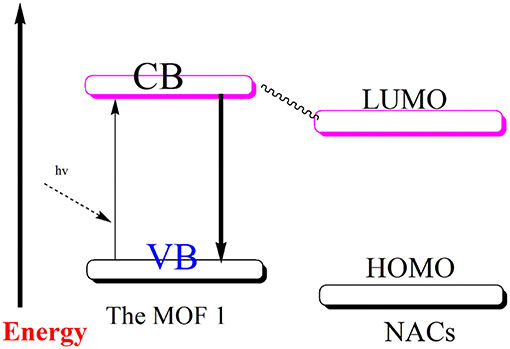
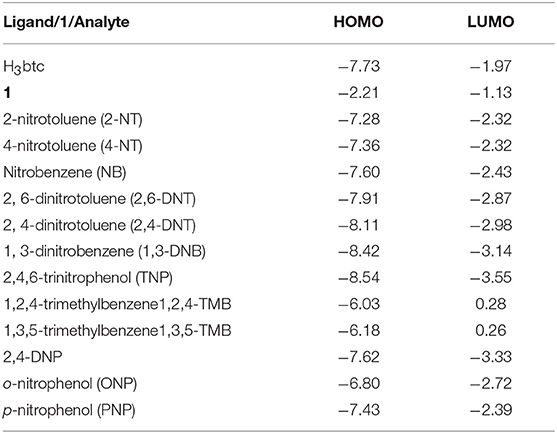
To offer the possible explanation for the decline in the photoluminescent intensity of MOF to varying magnitudes in the presence of different aromatic compounds, the computational investigation was done at the B3LYP level of theory (Table 1 and Figure S27). The weakening in photoluminescence intensity of MOFs when aromatic compounds comprising of –NO2 functions are added to them may be attributed to charge transfer that is taking place from conduction band of MOF to LUMO of these compounds (Scheme 1). To enable such transition the conduction band of MOFs should lie at higher energy scale than the conduction band of aromatic nitro-analytes (Lan et al., 2009; Zhang et al., 2010, 2011, 2016; Kreno et al., 2012; Li et al., 2013; Lin et al., 2016; Yang et al., 2016; Zhao et al., 2016, 2017). The Table 1 elucidatesthe conduction band energy levels of the full nitro-aromatic compounds, which are low than that of 1.
So, these nitro-aromatic analytes are in the apt position to accept the charge from 1 which is photo-excited. Hence, this phenomenon results in deterioration in photoluminescence intensity of the MOF to varying degree different nitro-aromatic compounds are added to it. Also, the Table 1 indicates that 1,3,5-TMB and 1,2,4-TMBare possessing higher LUMO energy level position than 1. Therefore, these aromatic compounds deprived of –NO2 functions display small effect on photoluminescence intensity of 1. However, the experimental order of decrement in photoluminescence intensity of 1 when different nitroaromatic analytes are added to it is not in accordance with their corresponding LUMO energy values. Therefore, it assessed that charge transfer may not be the solitary phenomenon responsible for decline in the photoluminescence. Additionally, along with the charge transfer phenomenon, weak interactions between the MOF and nitroaromatic analytes may also contribute toward the decline in the photoluminescence (Lan et al., 2009; Zhang et al., 2010, 2011; Kreno et al., 2012; Lin et al., 2016). Probably because of this reason the aromatic compounds 1,3,5-TMB and 1,2,4-TMB even though having relatively high LUMO energy values than 1 are slightly enhancing the photoluminescence intensity of 1 (Toal and Trogler, 2006; Kim et al., 2013; Wang et al., 2013; Zheng Q. et al., 2013; Hu et al., 2016).
Furthermore, to authenticate the selective sensing behavior of 1 toward TNP, the competitive experiments were executed by the adding different nitroaromatic compounds in the suspension of 1 followed by TNP (Figure S28). Results indicated that effective photoluminescent quenching was observed only when the TNP solution was added (Figure S28). These experiments validate the remarkable selectivity of 1 toward TNP. Further, to have better understanding into the selective TNP-sensing ability of 1, the electronic properties of MOF reported herein as well as the nitroaromatic compounds were analyzed. The mechanism for photoluminescent quenching through electronic migration from conduction band of probes MOFs to LUMO of NAC shaving electron deficient nature is well-established (Toal and Trogler, 2006; Lan et al., 2009; Zhang et al., 2010, 2011; Kreno et al., 2012; Kim et al., 2013; Wang et al., 2013; Zheng M. et al., 2013; Hu et al., 2016; Lin et al., 2016). In general, the conduction band of rich electric probes MOFs is having higher energy in comparison to the energies of LUMO corresponding to nitro-aromatic compounds. With downfall in the LUMO energy values of NACs, the tendency to accept electron by these analytes and photoluminescent quenching becomes higher. The selective sensing for TNP is in agreement with its lower LUMO energy in comparison to other NACs (Table 1). The disagreements between quenching of photoluminescent intensity for others NACs except for TNP and their LUMO energy parametersare conducted, in which may contain the electronic migration and/or resonance energy transfer (RET).The correlative factors responsible for photoluminescent intensity quenching process (Hu et al., 2016). Also, the efficiency of energy transfer depend on the extent of overlap between the emission spectrum of MOF and the electronic absorption spectrum of the analyte (Marin et al., 2006; Kent et al., 2010; Saini and Das, 2012; Son et al., 2013; Banerjee et al., 2014; Li et al., 2014; Park and Lee, 2015; Wen et al., 2015; Park et al., 2016; Das and Mandal, 2018). In sharp contrast to other NACs the absorption spectrum for TNP displays good full coverage the emission spectrum of 1 (Figure S29). This clearly suggests the electronic migration and energy conversion mechanisms are associated with the luminescence quenching in 1 by TNP, but electron transfer mechanism solely exists for other nitro-aromatic compounds. The existence of the dominating energy transfer between 1 and TNP has also been substantiated by the preferential quenching of the 375 nm band over 475 nm during the titration experiments (Figure 2c). The emission band at ~375 nm display spectral overlap with the electronic absorption spectrum of TNP which lead to efficient quenching of this band by an energy transfer mechanism (Figure S29). Antagonistically, the emission band at ~475 nm display poor spectral overlap with the electronic absorption spectrum of TNP because of which the quenching of this emission band occurs by a “less efficient” photo-induced electron transfer (PET) mechanism (Banerjee et al., 2014). In addition, there might be electrostatic interactions between TNP and the nitrogen centers of the ligands in 1 (Marin et al., 2006; Kent et al., 2010; Saini and Das, 2012; Son et al., 2013; Banerjee et al., 2014; Li et al., 2014; Park and Lee, 2015; Wen et al., 2015; Park et al., 2016; Das and Mandal, 2018) Hence due to combination of electron-transfer, energy-transfer and electrostatic interaction between TNP 1, the quenching efficiency for TNP gets significantly enhanced (Marin et al., 2006; Kent et al., 2010; Son et al., 2013; Li et al., 2014; Das and Mandal, 2018).
The MOF1 was suspended in distilled water having 1 × 102 M M(NO3)n to form the Mn+@1 to perform sensing experiments for the detection of metal cations. The intensities were enhanced upon adding Na+, K+, and Ca2+ ions in solutions when compared to the blank experiment (Figure 3A), while other metal ions exhibited different levels of quenching effects. Notably in the presence of Fe3+ ions the significant quenching in the luminescence intensity of MOF was observed. Thus, the relationship between the concentrations of Fe3+ ions upon the intensity of 1 was explored by varying the concentration of Fe3+ (Figure 3B). When the concentration of ferric ion in the emulsion of 1 was 500 ppm, then the luminescent intensity was completely disappeared. The mechanism pertaining to the quenching effect caused by Fe3+could be explicated on the basis of electronic migration operating from the organic ligands of the MOF which behave as donor toward the metal ions which act as acceptor (Wu et al., 2015). The crystal structure investigation indicates that in1 the uncoordinated oxygens of the carboxylate groups of btc ligands can behave as potential electrons donors.
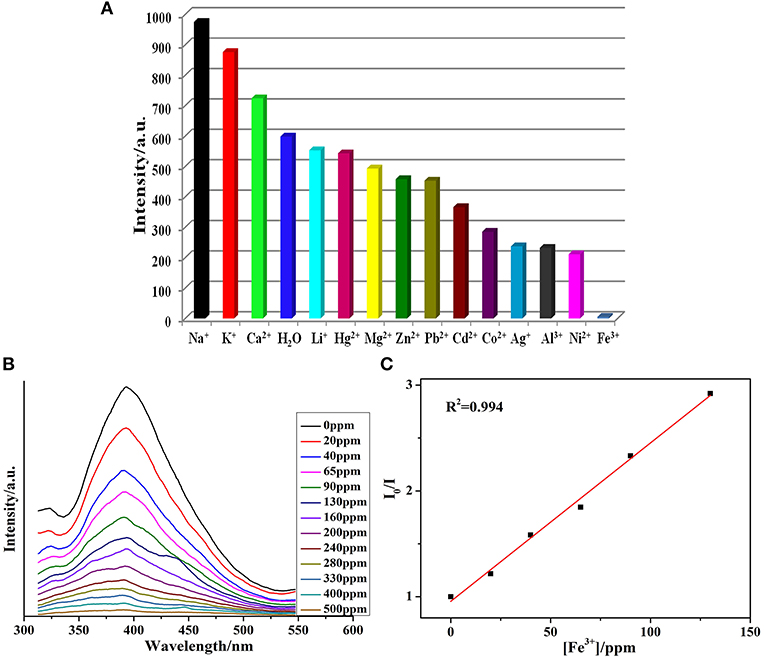
Figure 3. (A) Photoluminescence intensity of 1 when it was dispersed in different metal ions solutions in H2O (λex = 320 nm); (B) Quenching in photoluminescence intensity after adding 1 mM Fe3+ solution; (C) SV plot after adding different Fe3+ concentrations.
Based on the above results, 1 shows a highly selective to Fe3+. The photoluminescent intensity of 1 was nearly nullified at a Fe3+ concentration of 6.05 × 103 M−1. Further, the LOD was calculated ca. 1.56 ppm, which is comparable to the reported examples (Table S4) (Xiang et al., 2010; Jayaramulu et al., 2012; Liu et al., 2014; Cao et al., 2015; Xu et al., 2015). Moreover, the PXRD patterns of 1 after accomplishing sensing experiments indicated that the MOF retain its structural integrity and hence can be reused as a probe to detect ferric ions (Figure S3). The highly selective sensing for Fe3+ ions stimulated us to further inspect the effect of the related metal ions on Fe3+ sensing. This had been checked by the addition of metal ions other than Fe3+ to 1 followed by Fe3+. The experiments revealed that the luminescence intensity of 1 will be completely quenched while the Fe3+ ion (10−2 M) is added to 1. The Ksv of Fe3+ in the above systems is little greater that the value obtained for the pure 1 (Figure S30).
The possible sensing mechanism associated with the luminescence quenching of in the presence of Fe3+ has been further examined. Till date, some pertinent reasons for quenching in the luminescence intensity are: (Xu et al., 2011; Dang et al., 2012; Tang et al., 2013; Yang et al., 2013; Hu et al., 2014; Zhou et al., 2014; Xu and Yan, 2015) (I) the breakdown of framework; (II) ion exchange operating between sensing ions and the metal centers of probes; (III) resonance energy transfer (RET); and (IV) the weak interactions that are operating between metal cations and the functional groups from the organic ligands. The PXRD patterns (Figure S3) indicates that the MOF 1 even when immersed in metal ion solutions maintain its structural integrity. To substantiate the probable mechanism, the ICP has also been performed which showed that negligible Cd content is present in the filtrate obtained after suspending 1 in the Fe3+ solution for 1 day. This rules out that the exchange of Fe3+ with the Cd2+ of the MOF during sensing experiment (Table S5). Additionally, UV-Vis absorption data have been also conducted (Figure S31). The absorption spectrum of Fe3+ solution covers most of the emission spectrum of 1, while in the absorption spectra of other metal ions no such spectral overlap have been observed. So, the competitive absorption of excitation wavelength between aqueous solution of Fe3+ and 1 may be plausible reason for the quenching in photoluminescnece (Tang et al., 2013; Yang et al., 2013; Hu et al., 2014). Furthermore, the fluorescence lifetime of 3.6 ms in 1 was reduced to 1.9 ms using 1.0 mM Fe3+ solution (Figure S32). Hence, energy transfer is the prime phenomenon because of which loss in photoluminescent intensity is observed. To further explicate the possible mechanism for luminescence quenching in 1 by Fe3+ ion, the X-ray photoelectron spectroscopy (XPS) on Fe3+@1 showed that the energy of Fe 2p1 shifts to 712.6 eV, which may demonstrate the weak interaction between them (Figure S33) (Buragohain et al., 2016; Santra et al., 2016; Wu et al., 2017; Wang et al., 2018). In the time-dependent luminescence intensity measurement experiment (Figure S34), the final results indicated that it follows the first-order exponential decay curve. The character showed that the collision between Fe3+ and 1 may be faster than the static process. Hence, the static interaction might be incomplete instantly and acts as a continuous process which leads to a gradual decrease in luminescence intensity.
Conclusion
A new 10-connected Cd(II)-based MOF with (36.44.510.512.68.65) topology have been synthesized. Moreover, 1 reveals the selective and sensitive photoluminescent quenching by TNP. A combined experimental and mechanistic investigation substantiates that the quenching of the photoluminescent intensity of MOF in presence of analytes can be credited to the simultaneous charge transfer and weak interactions that are operating between 1 and analytes. Additional investigation of 1 to detect metal-ions indicated extremely sensitive luminescent quenching of 1 framework by Fe3+ ion over other metal ions. The good quenching constant (6.05 × 103 M−1) and very low limit of detection (LOD) (1.56 ppm), validates that 1 can be the potential detector for Fe3+. The excellent reusability/recyclability of MOF with respect to both TNP and Fe3+ sensing is certainly remarkable and this makes 1 as anew dual channel sensor for detection of TNP and Fe3+ ions.
Author Contributions
JuW wrote the manuscript. JiW, LL, HX, and MT designed and synthesised the materials. AK, JL, and MZ checked the full materials.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge financial assistance from Sichuan University of Science and Engineering (nos. 2015RC23, 2017RCL02), the Education Committee of Sichuan Province (nos. 17ZA0265, 18ZB0422 and 18ZB0425), the Department of Science and Technology of Sichuan Province (nos. 2017JY0129), the Project of Zigong Science & Technology (No. 2016HG04, 2018YYJC01), the Opening Project of Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education (no: LZJ1705) and the Student's Platform for Innovation and Entrepreneurship Training Program (201810622053). AK is grateful to CSIR, New Delhi for the project no. 01(2899)/17/EMR-II.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00244/full#supplementary-material
References
Allendorf, M. D., Bauer, A., Bhakta, R. K., and Houk, R. J. T. (2009). Luminescent metal-organic frameworks. Chem. Soc. Rev. 38, 1330–1352. doi: 10.1039/B802352M
Balamurugan, A., Kumar, V., and Jayakannan, M. (2014). Triple action polymer probe: carboxylic distilbene fluorescent polymer chemosensor for temperature, metal-ions and biomolecules. Chem.Commun. 50, 842–845. doi: 10.1039/C3CC45274C
Banerjee, D., Hu, Z. C., and Li, J. (2014). Luminescent metal-organic frameworks as explosive sensors. Dalton Trans. 43, 10668–10685. doi: 10.1039/C4DT01196A
Buragohain, A., Yousufuddin, M., Sarma, M., and Biswas, S. (2016). 3D luminescent amide-functionalized cadmium tetrazolate framework for selective detection of 2,4,6-trinitrophenol. Cryst. Growth Des. 16, 842–851. doi: 10.1021/acs.cgd.5b01427
Cao, K. L., Xia, Y., Wang, G. X., and Feng, Y. L. (2015). A robust luminescent Ba (II) metal-organic framework based on pyridine carboxylate ligand for sensing of small molecules. Inorg. Chem. Commun. 53, 42–45. doi: 10.1016/j.inoche.2015.01.021
Chen, B., Yang, Y., Zapata, F., Lin, G., Qian, G., and Lobkovsky, E. B. (2007). Luminescent open metal sites within a metal-organic framework for sensing small molecules. Adv. Mater. 19, 1693–1696. doi: 10.1002/adma.200601838
Chen, B. L., Wang, L. B., Xiao, Y. Q., Fronczek, F. R., Xue, M., Cui, Y. J., et al. (2009). A luminescent metal-organic framework with lewis basic pyridyl sites for the sensing of metal ions. Angew. Chem. Int. Ed. 48, 508–511. doi: 10.1002/ange.200805101
Chen, W. M., Meng, X. L., Zhuang, G. L., Wang, Z., Kurmoo, M., Zhao, Q. Q., et al. (2017). A superior fluorescent sensor for Al3+and UO based on a Co(II) metal–organic framework with exposed pyrimidyl Lewis base sites. J. Mater. Chem. A 5, 13079–13085. doi: 10.1039/C7TA01546A
Chen, Z., Mi, X., Lu, J., Wang, S., Li, Y., Dou, J., et al. (2018a). From 2D → 3D interpenetration to packing: N coligand-driven structural assembly and tuning of luminescent sensing activities towards Fe3+ and Cr2 O ions. Dalton Trans. 47, 6240–6249. doi: 10.1039/C8DT00909K
Chen, Z., Mi, X., Wang, S., Lu, J., Li, Y., Li, D., et al. (2018b). Two novel penetrating coordination polymers based on flexible S-containing dicarboxylate acid with sensing properties towards Fe3+ and Cr2O ions. J. Solid State Chem. 261, 75–85. doi: 10.1016/j.jssc.2018.02.008
Chen, Z., Sun, Y. W., Zhang, L. L., Sun, D., Liu, F. L., Meng, Q. G., et al. (2013). A tubular europium-organic framework exhibiting selective sensing of Fe3+ and Al3+ over mixed metal ions. Chem. Commun. 49, 11557–11559. doi: 10.1039/C3CC46613B
Cui, Y. J., Yue, Y. F., Qian, G. D., and Chen, B. L. (2012). Luminescent functional metal–organic frameworks. Chem. Rev. 112, 1126–1162. doi: 10.1021/cr200101d
Dang, S., Ma, E., Sun, Z. M., and Zhang, H. J. (2012). A layer-structured Eu-MOF as a highly selective fluorescent probe for Fe3+ detection through a cation-exchange approach. J. Mater. Chem. 22, 16920–16926. doi: 10.1039/C2JM32661B
Das, P., and Mandal, S. K. (2018). Strategic design and functionalization of an amine-decorated luminescent metal organic framework for selective gas/vapor sorption and nanomolar sensing of 2,4,6-trinitrophenol in water. ACS Appl. Mater. Interfaces 10, 25360–25371. doi: 10.1021/acsami.8b06339
Gole, B., Bar, A. K., and Mukherjee, S. (2011). Fluorescent metal–organic framework for selective sensing of nitroaromatic explosives. Chem. Commun. 47, 1137–12139. doi: 10.1039/c1cc15594f
Guo, L. Y., Su, H. F., Kurmoo, M., Wang, X. P., Zhao, Q. Q., Lin, S. C., et al. (2017). Multifunctional triple-decker inverse 12-metallacrown-4 sandwiching halides. ACS Appl. Mater. Interfaces 9, 19980–19987. doi: 10.1021/acsami.7b05191
Heine, J., and Buschbaum, K. M. (2013). Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 42, 9232–9242. doi: 10.1039/c3cs60232j
Hou, Y. F., Liu, B., Yue, K. F., Zhou, C. S., Wang, Y. M., Yan, N., et al. (2014a). Five solvent-induced cadmium coordination polymers (CPs) based on the same mixed ligands. CrystEngComm 16, 9560–9567. doi: 10.1039/C4CE01359J
Hou, Y. F., Yan, N., Yue, K. F., Shi, J. T., He, T., and Li, X. Y. (2014b). Syntheses and characterization of Co(II) and Cu(II) coordination polymers (CPs) based on mixed flexible and rigid ligands. Inorg. Chem.Commun. 48, 44–47. doi: 10.1016/j.inoche.2014.08.011
Hu, X. L., Liu, F. H., Qin, C., Shao, K. Z., and Su, Z. M. (2015). A 2D bilayered metal–organic framework as a fluorescent sensor for highly selective sensing of nitro explosives. Dalton. Trans. 44, 7822–7827. doi: 10.1039/C5DT00515A
Hu, Y., Ding, M., Liu, X., Sun, L., and Jiang, H. (2016). Rational synthesis of an exceptionally stable Zn(II) metal-organic framework for the highly selective and sensitive detection of picric acid. Chem. Commun. 52, 5734–5737. doi: 10.1039/C6CC01597B
Hu, Z. C., Deibert, B. J., and Li, J. (2014). Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 43, 5815–5840. doi: 10.1039/C4CS00010B
Hua, J. A., Zhao, Y., Kang, Y. S., Lu, Y., and Sun, W. Y. (2015). Solvent-dependent zinc(II) coordination polymers with mixed ligands: selective sorption and fluorescence sensing. Dalton Trans. 44, 11524–11532. doi: 10.1039/C5DT01386K
Jayaramulu, K., Narayanan, R. P., George, S. J., and Maji, T. K. (2012). Luminescent microporous metal-organic framework with functional lewis basic sites on the pore surface: specific sensing and removal of metal ions. Inorg. Chem. 51, 10089–10091. doi: 10.1021/ic3017547
Jin, J. C., Wu, X. R., Luo, Z. D., Deng, F. Y., Liu, J. Q., Singh, A., et al. (2017). Luminescent sensing and photocatalytic degradation properties of an uncommon (4,5,5)-connected 3D MOF based on 3,5-di(3′,5′-dicarboxylphenyl)benzoic acid. CrystEngComm 19, 4368–4377. doi: 10.1039/C7CE01012E
Kent, C. A., Mehl, B. P., Ma, L., Papanikolas, J. M., Meyer, T. J., and Lin, W. (2010). Energy transfer dynamics in metal-organic frameworks. J. Am. Chem. Soc. 132, 12767–12769. doi: 10.1021/ja102804s
Kim, T. K., Lee, J. H., Moon, D., and Moon, H. R. (2013). Luminescent Li-based metal–organic framework tailored for the selective detection of explosive nitroaromatic compounds: direct observation of interaction sites. Inorg. Chem. 52, 589–595. doi: 10.1021/ic3011458
Kreno, L. E., Leong, K., Farha, O. K., Allendorf, M., Van Duyne, R. P., and Hupp, J. T. (2012). Metal-organic framework materials as chemical sensors. Chem. Rev. 112, 1105–1125. doi: 10.1021/cr200324t
Lan, A., Li, K., Wu, H., Olson, D. H., Emge, T. J., Ki Hong, W., et al. (2009). A Luminescent Microporous Metal-Organic Framework for the Fast and Reversible Detection of High Explosives. Angew. Chem. Int. Ed. 48, 2370–2374. doi: 10.1002/ange.200804853
Li, B. H., Wu, J., Liu, J. Q., Gu, C. Y., Xu, J. W., Luo, M. M., et al. (2016). A luminescent zinc(II) metal-organic framework for selective detection of nitroaromatics, Fe3+ and CrO: a versatile threefold fluorescent sensor. ChemPlusChem 81, 885–892. doi: 10.1002/cplu.201600304
Li, J., Yang, G. P., Hou, L., Cui, L., Li, Y. P., Wang, Y. Y., et al. (2013). Three new solvent-directed 3D lead(II)-MOFs displaying the unique properties of luminescence and selective CO2 sorption. Dalton Trans. 42, 13590–13598. doi: 10.1039/c3dt51203g
Li, X. Y., Liu, M., Yue, K. F., Wu, Y. P., He, T., Yan, N., et al. (2015). A series of reaction-controlled coordination polymers constructed from bis(imidazole) and tetrafluoroterephthalic acid ligands: syntheses, structural diversities, properties. CrystEngComm 17, 8273–8281. doi: 10.1039/C5CE01423A
Li, Y., Song, H., Chen, Q., Liu, K., Zhao, F. Y., Ruan, W. J., et al. (2014). Two coordination polymers with enhanced ligand-centered luminescence and assembly imparted sensing ability for acetone. J. Mater. Chem. A 2, 9469–9473. doi: 10.1039/c4ta00944d
Lin, R. B., Liu, S. Y., Ye, J. W., Li, X. Y., and Zhang, J. P. (2016). Photoluminescent metal-organic frameworks for gas sensing. Adv. Sci. 3:1500434. doi: 10.1002/advs.201500434
Liu, B., Wu, W. P., Hou, L., and Wang, Y. Y. (2014). Four uncommon nanocage-based Ln-MOFs: highly selective luminescent sensing for Cu2+ ions and selective CO2 capture. Chem. Commun. 50, 8731–8734. doi: 10.1039/c4cc03049d
Liu, J. Q., Li, G. P., Liu, W. C., Li, Q. L., Li, B. H., Gable, R. W., et al. (2016b). Two unusual nanocage-based Ln-MOFs with triazole sites: highly fluorescent sensing for Fe3+ and Cr2O, and selective CO2 capture. ChemPlusChem 81, 1299–1304. doi: 10.1002/cplu.201600289
Liu, J. Q., Liu, G. L., Gu, C. Y., Liu, W. C., Xu, J. W., Li, B. H., et al. (2016a). Rational synthesis of a novel 3,3,5-c polyhedral metal-organic framework with high thermal stability and hydrogen storage capability. J. Mater. Chem. A 4, 11630–11634. doi: 10.1039/C6TA03675A
Liu, J. Q., Wang, W. J., Luo, Z. D., Li, B. H., and Yuan, D. Q. (2017). Microporous metal-organic framework based on ligand-truncation strategy with high performance for gas adsorption and separation. Inorg. Chem. 56, 10215–10219. doi: 10.1021/acs.inorgchem.7b00851
Liu, J. Q., Wu, J., Li, F. M., Liu, W. C., Li, B. H., Wang, J., et al. (2016c). Luminescent sensing from a new Zn(II) metal–organic framework. RSC. Adv. 6, 31161–31166. doi: 10.1039/C6RA01709F
Lu, L., Wu, J., Wang, J., Liu, J. Q., Li, B. H., Singh, A., et al. (2017). An uncommon 3D 3,3,4,8-c Cd(II) metal-organic framework for highly efficient luminescent sensing and organic dye adsorption: experimental and theoretical insight. CrystEngComm 19, 7057–7067. doi: 10.1039/C7CE01638G
Ma, D. X., Li, B. Y., Zhou, X. J., Zhou, Q., Liu, K., Zeng, G., et al. (2013). A dual functional MOF as a luminescent sensor for quantitatively detecting the concentration of nitrobenzene and temperature. Chem. Commun. 49, 8964–8966. doi: 10.1039/c3cc44546a
Ma, R., Chen, Z., Wang, S., Yao, Q., Li, Y., Lu, J., et al. (2017). Solvent-induced assembly of two helical Eu(III) metal-organic frameworks and fluorescence sensing activities towards nitrobenzene and Cu2+ ions. J. Solid State Chem. 252, 142–151. doi: 10.1016/j.jssc.2017.05.018
Marin, V., Holder, E., Hoogenboom, R., Tekin, E., and Schubert, U. S. (2006). Light-emitting iridium(III) and ruthenium(II) polypyridyl complexes containing quadruple hydrogen-bonding moieties. Dalton Trans. 13, 1636–1644. doi: 10.1039/b513957k
Park, I. H., Mulijanto, C. E., Lee, H. H., Kang, Y., Lee, E., Chanthapally, A., et al. (2016). Influence of interpenetration in diamondoid metal-organic frameworks on the photoreactivity and sensing properties. Cryst. Growth Des. 16, 2504–2508. doi: 10.1021/acs.cgd.6b00354
Park, S., and Lee, S. Y. (2015). Significant enhancement of curcumin photoluminescence by a photosensitizing organogel: an optical sensor for pyrrole detection. Sens. Actuators B 220, 318–325. doi: 10.1016/j.snb.2015.05.078
Pramanik, S., Zheng, C., Zhang, X., Emge, T. J., and Li, J. (2011). New microporous metal-organic framework demonstrating unique selectivity for detection of high explosives and aromatic compounds. J. Am. Chem. Soc. 133, 4153–4155. doi: 10.1021/ja106851d
Qin, J. S., Bao, S. J., Li, P., Xie, W., Du, D. Y., Zhao, L., et al. (2014). A stable porous anionic metal-organic framework for luminescence sensing of Ln3+ ions and detection of nitrobenzene. Chem. Asian J. 9, 749–753. doi: 10.1002/asia.201301531
Rocha, J., Carlos, L. D., Paz, F. A. A., and Ananias, D. (2011). Luminescent multifunctional lanthanides-based metal-organic frameworks. Chem. Soc. Rev. 40, 926–940. doi: 10.1039/C0CS00130A
Saini, R. K., and Das, K. J. (2012). Picosecond spectral relaxation of curcumin excited state in a binary solvent mixture of toluene and methanol. Phys. Chem. B 116, 10357–10363. doi: 10.1021/jp305447y
Sanchez, J. C., and Trogler, W. C. (2008). Efficient blue-emitting silafluorene-fluorine-conjugated copolymers: selective turn-off/turn-on detection of explosives. J. Mater. Chem. 18, 3143–3156. doi: 10.1039/B802623H
Sanchez, J. C., Urbas, S. A., Toal, S. J., DiPasquale, A. G., Rheingold, A. L., and Trogler, W. C. (2008). Catalytic hydrosilylation routes to divinylbenzene bridged silole and silafluorene polymers. Applications to surface imaging of explosive particulates. Macromolecules 41, 1237–1245. doi: 10.1021/ma702274c
Santra, D. C., Bera, M. K., Sukul, P. K., and Malik, S. (2016). Charge- transfer-induced fluorescence quenching of anthracene derivatives and selective detection of picric acid. Chem. Eur. J. 22, 2012–2019. doi: 10.1002/chem.201504126
Shen, J., Wang, Z., Sun, D., Xia, C., Yuan, S., Sun, P., et al. (2018). pH-responsive nanovesicles with enhanced emission co-assembled by Ag(I) nanoclusters and polyethyleneimine as a superior sensor for Al3+. ACS Appl. Mater. Interfaces 10, 3955–3963. doi: 10.1021/acsami.7b16316
Shi, B. B., Zhong, Y. H., Guo, L. L., and Li, G. (2015). Two dimethylphenyl imidazole dicarboxylate-based lanthanide metal-organic frameworks for luminescence sensing of benzaldehyde. Dalton Trans. 44, 4362–4369. doi: 10.1039/C4DT03326D
Shi, J. T., Yue, K. F., Liu, B., Zhou, C. S., Liu, Y. L., Fang, Z. G., et al. (2014). Two porous metal-organic frameworks (MOFs) based on mixed ligands: synthesis, structure and selective gas adsorption. CrystEngComm 16, 3097–3102. doi: 10.1039/c3ce41557k
Singh, D., and Nagaraja, C. M. (2014). A luminescent 3D interpenetrating metal–organic framework for highly selective sensing of nitrobenzene. Dalton Trans. 43, 17912–17915. doi: 10.1039/C4DT02841D
Son, H. J., Jin, S., Patwardhan, S., Wezenberg, S. J., Jeong, N. C., So, M. Hupp, J. T., et al. (2013). Light-harvesting and ultrafast energy migration in porphyrin-based metal–organic frameworks. J. Am. Chem. Soc. 135, 862–869. doi: 10.1021/ja310596a
Song, X. Z., Song, S. Y., Zhao, S. N., Hao, Z. M., Zhu, M., Meng, X., et al. (2014). Single-crystal-to-single-crystal transformation of a europium(III) metal-organic framework producing a multi-responsive luminescent sensor. Adv. Funct. Mater. 24, 4034–4041. doi: 10.1002/adfm.201303986
Spek, A. L. (2003). Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 36, 7–13. doi: 10.1107/S0021889802022112
Tang, Q., Liu, S. X., Liu, Y. W., Miao, J., Li, S. J., Zhang, L., et al. (2013). Cation sensing by a luminescent metal-organic framework with multiple lewis basic sites. Inorg. Chem. 52, 2799–2801. doi: 10.1021/ic400029p
Tian, D., Li, Y., Chen, R. Y., Chang, Z., Wang, G. Y., and Bu, X. H. (2014). A luminescent metal-organic framework demonstrating ideal detection ability for nitroaromatic explosives. J. Mater. Chem. A 2, 1465–1470. doi: 10.1039/C3TA13983B
Toal, S. J., and Trogler, W. C. (2006). Polymer sensors for nitroaromatic explosives detection. J. Mater. Chem. 116, 2871–2883. doi: 10.1039/b517953j
Wang, G. Y., Yang, L. L., Li, Y., Song, H., Ruan, W. J., Chang, Z., et al. (2013). A luminescent 2D coordination polymer for selective sensing of nitrobenzene. Dalton Trans. 42, 12865–12868. doi: 10.1039/c3dt51450a
Wang, J., Wu, X. R., Liu, J. Q., Li, B. H., Singh, A., Kumar, A., et al. (2017). An uncommon (5,5)-connected 3D metal organic material for selective and sensitive sensing of nitroaromatics and ferric ion: experimental studies and theoretical analysis. CrystEngComm 19, 3519–3525. doi: 10.1039/C7CE00912G
Wang, X. L., Luan, J., Lin, H. Y., Lu, Q. L., Le, M., Liu, G. C., et al. (2015). Metal(II)-Organic coordination polymers modulated by two isomeric semirigid Bis-Pyridyl-Bis-Amide ligands: structures, fluorescent sensing behavior, and selective photocatalysis. ChemPlusChem 79, 1691–1702. doi: 10.1002/cplu.201402193
Wang, X. P., Han, L. L., Wang, Z., Guo, L. Y., and Sun, D. (2016). Microporous Cd(II) metal-organic framework as fluorescent sensor for nitroaromatic explosives at the sub-ppm level. J. Mol. Struct. 1107, 1–6. doi: 10.1016/j.molstruc.2015.11.018
Wang, X. S., Li, L., Yuan, D., Huang, Q. Y. B., and Cao, R. (2018). Fast, highly selective and sensitive anionic metal-organic framework with nitrogen-rich sites fluorescent chemosensor for nitro explosives detection. J. Hazard Mater. 344, 283–290. doi: 10.1016/j.jhazmat.2017.10.027
Wen, L. L., Xu, X. Y., Lv, K. L., Huang, Y. M., Zheng, X. F., Zhou, L., et al. (2015). Metal-organic frameworks constructed from d-camphor acid: bifunctional properties related to luminescence sensing and liquid-phase separation. ACS Appl. Mater. Interfaces 7, 4449–4455. doi: 10.1021/acsami.5b00160
Wu, Y. L., Yang, G. P., Zhou, X., Li, J., Ning, Y., and Wang, Y. Y. (2015). Three new luminescent Cd(II)-MOFs by regulating the tetracarboxylate and auxiliary co-ligands, displaying high sensitivity for Fe3+ in aqueous solution. Dalton Trans. 44, 10385–10391. doi: 10.1039/C5DT00492F
Wu, Y. P., Xu, G. W., Dong, W. W., Zhao, J., Li, D. S., Zhang, J., et al. (2017). Anionic lanthanide MOFs as a platform for iron-selective sensing, systematic color tuning, and efficient nanoparticle catalysis. Inorg. Chem. 56, 1402–1411. doi: 10.1021/acs.inorgchem.6b02476
Xiang, S., Zhou, W., Zhang, Z., Green, M. A., Liu, Y., and Chen, B. (2010). Open metal sites within isostructural metal–organic frameworks for differential recognition of acetylene and extraordinarily high acetylene storage capacity at room temperature. Angew. Chem. Int. Ed. 49, 4719–4722. doi: 10.1002/ange.201000094
Xu, H., Hu, H. C., Cao, C. S., and Zhao, B. (2015). Lanthanide organic framework as a regenerable luminescent probe for Fe3+. Inorg. Chem. 54, 4585–4587. doi: 10.1021/acs.inorgchem.5b00113
Xu, H., Liu, F., Cui, Y., Chen, B., and Qian, G. (2011). A luminescent nanoscale metal-organic framework for sensing of nitroaromatic explosives. Chem. Commun. 47, 3153–3155. doi: 10.1039/c0cc05166g
Xu, X. Y., and Yan, B. (2015). Eu(III)-functionalized MIL-124 as fluorescent probe for highly selectively sensing ions and organic small molecules especially for Fe(III) and Fe(II). ACS Appl. Mater. Interfaces 7, 721–729. doi: 10.1021/am5070409
Yang, C. X., Ren, H. B., and Yan, X. P. (2013). Fluorescent metal-organic framework MIL-53(Al) for highly selective and sensitive detection of Fe3+ in aqueous solution. Anal. Chem. 85, 7441–7446. doi: 10.1021/ac401387z
Yang, Y. J., Wang, M. J., and Zhang, K. L. (2016). A novel photoluminescent Cd(II)–organic framework exhibiting rapid and efficient multi-responsive fluorescence sensing for trace amounts of Fe3+ ions and some NACs, especially for 4-nitroaniline and 2-methyl-4 –nitroaniline. J. Mater. Chem. C 4, 11404–11418. doi: 10.1039/C6TC04195G
Yi, F. Y., Yang, W. T., and Sun, Z. M. (2012). Highly selective acetone fluorescent sensors based on microporous Cd(II) metal–organic frameworks. J. Mater. Chem. 22, 23201–23209. doi: 10.1039/C2JM35273G
Zhan, C., Ou, S., Zou, C., Zhao, M., and Wu, C. D. (2014). A luminescent mixed-lanthanide-organic framework sensor for decoding different volatile organic molecules. Anal. Chem. 86, 6648–6653. doi: 10.1021/ac5013442
Zhang, C., Che, Y., Zhang, Z., Yang, X., and Zang, L. (2011). Fluorescent nanoscale zinc(II)-carboxylate coordination polymers for explosive sensing. Chem. Commun. 47, 2336–2338. doi: 10.1039/C0CC04836D
Zhang, C., Sun, L., Yan, Y., Li, J., Song, X., Liu, Y., et al. (2015). A luminescent cadmium metal-organic framework for sensing of nitroaromatic explosives. Dalton Trans. 44, 230–236. doi: 10.1039/C4DT02227K
Zhang, L. L., Kang, Z. X., Xin, X. L., and Sun, D. F. (2016). Metal-organic frameworks based luminescent materials for nitroaromatics sensing. CrystEngComm 18, 193–206. doi: 10.1039/C5CE01917F
Zhang, Z., Xiang, S., Rao, X., Zheng, Q., Fronczek, F. R., Qian, G., et al. (2010). A rod packing microporous metal-organic framework with open metal sites for selective guest sorption and sensing of nitrobenzene. Chem. Commun. 46, 7205–7207. doi: 10.1039/c0cc01236j
Zhao, S., Ding, J. G., Zheng, T. R., Li, K., Li, B. L., and Wu, B. (2017). The 3D and 2D cadmium coordination polymers as luminescent sensors for detection of nitroaromatics. J. Lumin. 188, 356–364. doi: 10.1016/j.jlumin.2017.04.044
Zhao, S., Lv, X. X., Shi, L. L., Li, B. L., and Wu, B. (2016). An unusual (4,4)-connected 3D porous cadmium metal-organic framework as a luminescent sensor for detection of nitrobenzene. RSC. Adv. 6, 56035–56041. doi: 10.1039/C6RA10664A
Zheng, M., Tan, H. Q., Xie, Z. G., Zhang, L. G., Jing, X. B., and Sun, Z. C. (2013). Fast response and high sensitivity europium metal organic framework fluorescent probe with chelating terpyridine sites for Fe3+. ACS Appl. Mater. Interfaces 5, 1078–1083. doi: 10.1021/am302862k
Zheng, Q., Yang, F., Deng, M., Ling, Y., Liu, X., Chen, Z., et al. (2013). A porous metal–organic framework constructed from carboxylate–pyrazolate shared heptanuclear zinc clusters: synthesis, gas adsorption, and guest-dependent luminescent properties. Inorg. Chem. 52, 10368–10374. doi: 10.1021/ic401092j
Zheng, X. F., Zhou, L., Huang, Y. M., Wang, C. G., Duan, J. G., Wen, L. L., et al. (2014). A series of metal-organic frameworks based on 5-(4-pyridyl)-isophthalic acid: selective sorption and fluorescence sensing. J. Mater. Chem. A 2, 12413–12422. doi: 10.1039/C4TA01900H
Keywords: MOF, nitroaromatics, sensor, theoretical calculation, topology
Citation: Wang J, Wu J, Lu L, Xu H, Trivedi M, Kumar A, Liu J and Zheng M (2019) A New 3D 10-Connected Cd(II) Based MOF With Mixed Ligands: A Dual Photoluminescent Sensor for Nitroaroamatics and Ferric Ion. Front. Chem. 7:244. doi: 10.3389/fchem.2019.00244
Received: 18 February 2019; Accepted: 26 March 2019;
Published: 16 April 2019.
Edited by:
Carlos Lodeiro, Universidade NOVA de Lisboa, PortugalReviewed by:
Di Sun, Shandong University, ChinaSalah S. Massoud, University of Louisiana at Lafayette, United States
Copyright © 2019 Wang, Wu, Lu, Xu, Trivedi, Kumar, Liu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Wang, c2N3YW5nanVuMjAxMUAxMjYuY29t
Abhinav Kumar, YWJoaW5hdm1hcnNoYWxAZ21haWwuY29t
Jianqiang Liu, amlhbnFpYW5nbGl1MjAxMEAxMjYuY29t
Mingbin Zheng, bWluZ2JpbnpoZW5nQDEyNi5jb20=
 Jun Wang1*
Jun Wang1* Manoj Trivedi
Manoj Trivedi Abhinav Kumar
Abhinav Kumar Jianqiang Liu
Jianqiang Liu