Metal-based nano-delivery platform for treating bone disease and regeneration
- State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, China
Owing to their excellent characteristics, such as large specific surface area, favorable biosafety, and versatile application, nanomaterials have attracted significant attention in biomedical applications. Among them, metal-based nanomaterials containing various metal elements exhibit significant bone tissue regeneration potential, unique antibacterial properties, and advanced drug delivery functions, thus becoming crucial development platforms for bone tissue engineering and drug therapy for orthopedic diseases. Herein, metal-based drug-loaded nanomaterial platforms are classified and introduced, and the achievable drug-loading methods are comprehensively generalized. Furthermore, their applications in bone tissue engineering, osteoarthritis, orthopedic implant infection, bone tumor, and joint lubrication are reviewed in detail. Finally, the merits and demerits of the current metal-based drug-loaded nanomaterial platforms are critically discussed, and the challenges faced to realize their future applications are summarized.
1 Introduction
To achieve superior bone repair effects, the development of strategies to treat different bone diseases, such as bone defects, bone infections, fractures, osteoarthritis, osteoporosis, and bone tumors, has become a major public health issue (Chindamo et al., 2020). Bone is the hardest connective tissue in body, and its diseases can lead to restricted movements or even death (Hou et al., 2020). However, there are still many challenges in the diagnosis and treatments of bone diseases. Some bone diseases such as early-stage bone tumors are difficult to be detected by conventional diagnostic methods. It usually requires large doses to reach bone tissue for drugs given by mouth or bolus (Carbone et al., 2017). In addition, the healing cycle of bone tissue is long. Therefore, strict requirements about the release kinetics of therapeutic molecules are necessary for treating bone infection, inflammation or defect. A few emerging technologies, including tissue engineering material transplantation (Liu J. Y. et al., 2021), stem cell technology (Shang et al., 2021), and nanomedicine (Qiao K. et al., 2022), are potential means to promote the treatment of complex bone diseases. In particular, nanomedicine, based on various nanobiomaterials has significantly accelerated the diagnosis research, treatment, and regeneration of bone diseases, and has obtained numerous achievements (Chen et al., 2020; Hagaman et al., 2021). There are a wide variety of nanobiomaterials, including carbon-based, metal-based, virus-based, lipid-based, polymer-based, liposomes, cubes, micelles, exosomes, and cell membrane coatings (Sharma et al., 2021). Nanobiomaterials exhibit numerous excellent properties: nanoscale, large specific surface area, adjustable volume, favorable biocompatibility, and abundant modifiable surfaces (Eivazzadeh-Keihan et al., 2020b; Anastasio et al., 2021). Therefore, they have various applications such as nano-delivery systems (Vangijzegem et al., 2019; Hu S. et al., 2021), biomaterial modification (Liu et al., 2020d; Zhong et al., 2020; Xue Y. et al., 2021), biosensors (Fiorani et al., 2019; Singh et al., 2022), in vivo tracking, and imaging agents (Liu Y. et al., 2021; Kalva et al., 2022), gas storage (Wang X. et al., 2021; Zhu et al., 2022), and chemical catalysis (Fang et al., 2021; Khalil et al., 2021). Owing to these excellent properties, nanobiomaterials, particularly nanodelivery platforms, are attractive for the diagnosis and treatment of bone diseases as well as tissue repair. -The characteristic diagnosis and treatment of bone diseases by nanomaterials is particularly attractive. Nanomaterials have large comparative area and surface activity, so can achieve flexible bone-targeting binding, in vivo imaging and other functions through physical or chemical modification. Combing nanomaterials with either computed tomography, magnetic resonance imaging (MRI), and/or photoluminescence imaging for specific localization in vivo is helpful for the early diagnosis of bone disease (Wang S. et al., 2020). Nanomaterials have excellent loading capacity for bioactive factors, antibacterial agents, antitumor drugs, antibiotic drugs, gene molecules, etc. Required for the treatment of bone diseases (Kumar and Madhumathi, 2016; Li et al., 2019a; Zhao et al., 2020; Halim et al., 2021). More importantly, nanomaterials play a prominent role in reducing drug toxicity, increasing bioavailability, and improving pharmacokinetics and biodistribution, thus providing great potential for new breakthroughs in bone disease treatment (Liu G. et al., 2021; Li et al., 2022).
Compared with organic nanomaterials such as micelles, lipid-based and polymer-based, the metal-based nano-delivery platforms (MNPs) have unique features and advantages due to the inclusion of various metal elements. Considering the important role of metal ions in the metabolism and operation of the human body, particularly the physiological activities of bone tissues, the MNPs have exhibited considerable application potential in bone tissue engineering repair as well as the field of bone disease diagnosis and treatment (Fardjahromi et al., 2022). Metal elements exhibit excellent antibacterial and anti-oxidative stress effects (Makvandi et al., 2020), thereby facilitating synergistic anti-bone infection and osteoarthritis effects to the delivered components (Luo et al., 2021); however, a few other metal elements can also endow nano-delivery systems with special targeted delivery (Niculescu and Grumezescu, 2022), photodynamic therapy (PDT) (He et al., 2022), thermodynamic therapy (Boroushaki et al., 2022), bioimaging (Zhong et al., 2021), and stimuli-responsive (Hu et al., 2022) functions, which are more conducive to the efficient diagnosis and treatment of bone diseases or promote bone regeneration, and decrease the simple systemic side effects of drug use. A few of researchers reported that metal nanoparticles (NPs) can be used as nano-antibiotics because of their favorable antibacterial activities and significant potential to combat antibiotic resistance (Cheng et al., 2022). In addition, studies have demonstrated that metal-based nanomaterials possess excellent mechanical properties as well as the intrinsic ability to significantly promote osseointegration, osteoconductivity, and osteoinduction, which has become crucial factors for bone regeneration (Sobolev et al., 2019; Eivazzadeh-Keihan et al., 2020a). Moreover, MNPs can help achieve the controlled release of drugs or active molecules through various stimuli responses, including pH-triggered release systems (Duan et al., 2021), redox reactions (Duan et al., 2018), external thermal effects (Khodaei et al., 2022), magnetic field control (Dehghani et al., 2020), and ultrasonic dynamic stimulation (Li S. et al., 2020), or by the external activation of optical/mechanical stimuli (Feng et al., 2019).
In recent years, increasingly advanced methods have been attempted to use MNPs for the maintenance of bone tissue health. Komal et al. (Rao et al., 2018) placed Hesperidin into gum acacia-stabilized green silver (Ag)NPs for combating rheumatoid arthritis and achieved favorable results. Additional to loading an active ingredient, the co-encapsulation and simultaneous or sequential release of two or more active ingredients can be achieved using MNPs. For example, Yan (Jiang et al., 2021) used the Zn-based zeolitic imidazole framework (ZIF-8) as a carrier to deliver bone morphogenetic protein 2 (BMP-2) and cisplatin, thus defining different spatial distributions and environment-adaptive release patterns of osteogenic growth factors and anti-cancer drugs. In addition, metal-based nanomaterials can serve as bridges for integrating diagnostic and therapeutic components into a single platform. In a study (Wang et al., 2016b), the authors developed a novel core–shell PB@MIL-100(Fe) metal-organic frameworks (d-MOFs) NPs, which could serve as a contrast agent for MRI. Then the (d-MOFs) NPs was reported acting as an imaging agent for fluorescence optics as well as for achieving targeted tumor therapy in pH-responsive manner, and finally synergistically play the role of photothermal and chemotherapy (CT) for ablating tumors in mice. However, although the MNPs have been demonstrated to have significant potential in bone tissue engineering and related disease diagnosis and treatment, numerous questions regarding MNP have been posed. Titanium dioxide NPs have been reported to induce potential cytotoxicity (oxidative stress), genotoxicity, and immunotoxicity (Di Giampaolo et al., 2021). A few scholars have also proposed that the drug loading capacity of MNPs is predominantly unsatisfactory, and the high production costs limit the clinical translation process (Liu et al., 2020c). For oral administration, penetrating the mucus gel layer barrier to the absorption membrane is a significant test for MNPs to exert their efficacy, and MNPs within the 10–150 nm size range are considered to be the most favorable choice for enhancing permeability and exerting their effects (Murugan et al., 2015). Currently, only a few nanomedicines, such as Abraxane® (albumin-bound paclitaxel NPs) and Doxil (a PEGylated liposomal doxorubicin (DOX) formulation), have been approved for clinical use by the US Food and Drug Administration (FDA) (Barenholz, 2012).
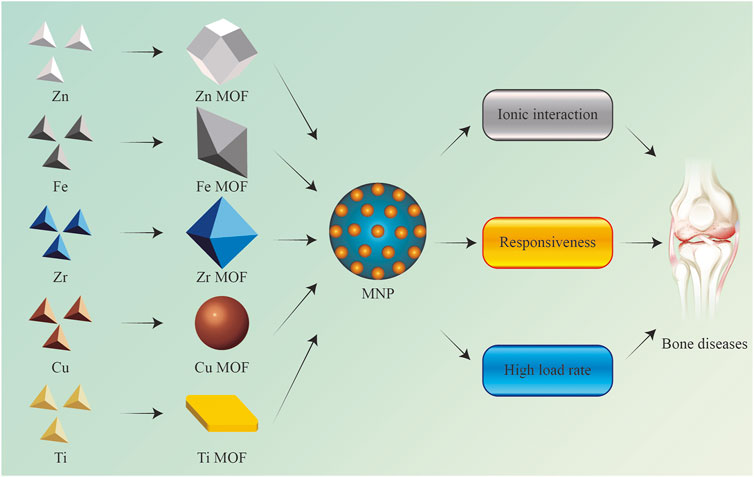
Herein, to comprehensively evaluate the status of MNP in the bone health maintenance field and interpret the advantages and disadvantages of such biomaterials for the diagnosis and treatment of bone diseases as well as tissue repair, it is urgently required to summarize the applications status of MNPs targeting bone tissues (Figure 1). This review summarizes the following four aspects: a complete classification and description of the MNPs; then, the method of the MNPs loading active factors is summarized; the application of MNPs to bone-related diseases is further introduced. Specific scenarios are discussed in the third section; the limitations of MNPs at this stage are critically discussed, and it is believed that this review can provide a reference for the application of MNPs in bone tissue-related research in the near future.
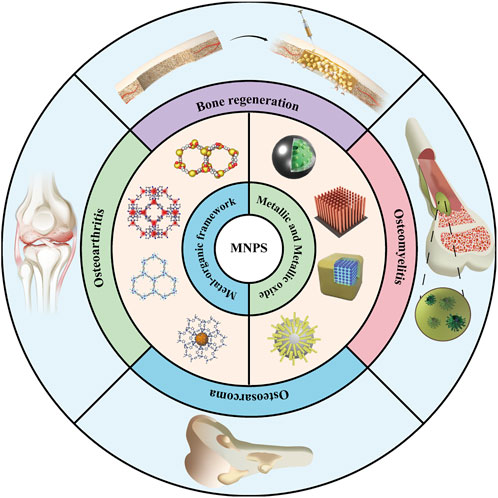
FIGURE 1. Application of metal-based nano-delivery platform for treating bone disease and regeneration.
2 Classification of MNPs
2.1 MOFs nano-delivery platform for bone disease and regeneration
MOF nanomaterials are a class of crystalline microporous materials that include endless lattices constituting metal ions or clusters and organic ligands connected via strong coordinate bonds (Fang et al., 2018). Compared with other non-metallic nanocarriers such as dendrimers, and mesoporous silica nanoparticles, MOFs have distinct and unique characteristics. First, different from the rigid structure of mesoporous silica nanoparticles, MOFs can flexibly adjust their structures by changing inorganic clusters and organic ligands (length, volume, bond angle, and chirality). Meanwhile, the pores of dendrimers and mesoporous silica nanoparticles are hydrophobic, while the pores of MOFs are amphiphilic (Sharma et al., 2019; Fernandes et al., 2021). All these features can ensure that MOFs can be more efficiently carried for different therapeutic molecules or drugs. Although the metal-free polymeric or liposomal nanocarriers have better stability and biocompatibility (Gupta et al., 2021; Liang et al., 2022). Inorganic metals such as Zn, Ti, Fe, Mg, Cu, Zr, and Co. contained by MOFs are not only essential elements for body, but also participating in important life activities such as cell proliferation, differentiation, and metabolism. More importantly, these inorganic metal elements can also endow MOFs with many other attractive functions, such as antibacterial properties, ionic interaction, magnetism, photothermal response, pH response, chemical catalysis, and enzymatic reactivity. The prevalent MOFs in the treatment of bone disease are classified by metal elements and discussed in the following (Figure 2).
2.1.1 Zn MOFs nano-delivery platform
Nano Zn MOFs comprises Zn (II) covalently linked with 1,4-bis(1H-pyrazol-4yL)-2-X-benzene (H2BDP_X; X = H, NO2, NH2, OH) (He et al., 2021) and possess various pores sizes in the range of 3.8–28.8 Ǻ. A major subfamily of Zn nano MOFs is the zeolitic imidazolate framework (ZIF), in which Zn (II) ions are connected via imidazolate or imidazole derivatives as organic ligands (Fardjahromi et al., 2022). Since Jiang et al. (Jiang et al., 2009) used an MOF as the Au nanoparticle carrier for the first time, Zn-based MOFs have been widely explored as a nano-delivery platform, and ZIF-8 application is the most representative. The ZIF-8 nano-platform for delivery of biologically active factors or drugs has been used in bone-related diseases or bone tissue engineering regeneration. ZIF-8 is a space-filling packing with the topology of truncated octahedrons and a pore size of 11.6 Ǻ (He et al., 2021). It has been reported that ZIF-8 can effectively deliver one or more small-molecule drugs such as alendronate (Ald), DOX (Xue X. H. et al., 2021), curcumin (Wang Y. T. et al., 2020), risedronate (Cheng et al., 2020), dexamethasone (Ran et al., 2018), 5-fluorouracil, and indocyanine green (ICG) (Ting et al., 2022), and simvastatin (Qiao M. et al., 2022). ZIF-8 has also been demonstrated to be an effective non-viral vector for delivering small noncoding RNAs such as microRNAs (miRNAs) in addition to viral vectors (Feng et al., 2022). Nano ZIF-8 can also be used to simultaneously transport drugs and proteins together; for example, Jiang et al. (Jiang et al., 2021) successfully loaded two substances, BMP-2 and cisplatin, within ZIF-8 to build MNPs for bone regeneration. Furthermore, drugs and genes could be co-delivered by Zn-based MOFs, and Rabiee et al. (Rabiee et al., 2021) had used MOF-5 to simultaneously carry DOX and pCRISPR. Therefore, nano-Zn MOFs have broad prospects for delivering active ingredients, such as a variety of drugs, genes as well as proteins, and can be used as a powerful reserve to accelerate the treatment of bone tissue diseases and bone regeneration.
2.1.2 Fe MOFs nano-delivery platform
Fe-based MOFs is an important constituent member of the Materials of Institute Lavoisier (MIL)-n family (Zhong et al., 2021). Common Fe-based MOFs are MIL -53(Fe), MIL-88A (Fe), MIL-88B(Fe), MIL-100(Fe), and MIL-101(Fe), which are composed of Fe centers and a terephthalate-based linker (1,4-dicarboxylic acid) connected by six oxygen atoms (Al Haydar et al., 2017). Fe-based MIL has been widely studied in biosensing, bioimaging, antibacterial, and drug delivery owing to its excellent properties such as low toxicity, biodegradability, biocompatibility, large pore volume, and surface area with high drug loading capability (Zhong et al., 2021). Fe-based MIL has significant potential as a nano-delivery platform. In particular, except delivering small molecule drugs, such as methotrexate (Ahmadijokani et al., 2021), protocatecholic acid (Xiong et al., 2020), curcumin (Faaizatunnisa et al., 2022), Ald (Golmohamadpour et al., 2018), flurbiprofen (Al Haydar et al., 2017), artemisinin (Wang et al., 2016b), acetaminophen (Pattappan et al., 2022), progesterone, and stavudine (Gordon et al., 2015), MIL family can serve as carrier for the sustained release of small molecule active metabolites, such as WR-1065 (active metabolite of amifostine), and peptides, such as glutathione (Cao et al., 2020). Therefore, Fe MOFs are also functionally rich nano-delivery platforms which can be used to overcome the challenges of bone regeneration and repair as well as bone disease diagnosis and treatment.
2.1.3 Zr MOFs nano-delivery platform
Zr MOFs are considered as promising MOFs for bone tissue engineering and bone disease-related applications owing to their low toxicity as well as high mechanical, thermal, acidic, and aqueous stability (Lazaro et al., 2017). In 2008, Cavka discovered the first Zr MOF, namely Universitetet i Oslo (Uio-66) (Cavka et al., 2008), which comprises inorganic Zr metal and organic ligand 1,4-benzene dicarboxylic acid. Additional to UiO-66, MOF-525, MOF-545 (also known as PCN-222), PCN-221, PCN-223, PCN-224, PCN-225, and NU-902 are commonly used Zr-based MOFs (Yu et al., 2021). The size, porosity, and release behavior of Zr MOFs can be regulated via the synthesis of different functional groups such as -NH2 and NO2 (Li Z. et al., 2019). Zr MOFs can be used to deliver different drugs or active ingredients when treating different bone diseases. For example, small molecule drugs can be delivered using UiO-66-NH2 NPs such as DOX, cisplatin, temozolomide, and curcumin (Ma et al., 2020; Fytory et al., 2021; Wan et al., 2021; Hu et al., 2022). In addition, a few single metal atoms (Pt, Au, Cu, Ru) can be delivered using PCN-222 (Yu et al., 2022). Moreover, it has been reported that a few immunostimulatory oligonucleotides, such as cytosine–phosphate–guanosine, can be successfully delivered using UiO-66 (Pang et al., 2020). Therefore, owing to their excellent delivery ability, the Zr MOFs nanoplatform is a new candidate as tissue engineering materials for diagnosing and treating bone tissue diseases.
2.1.4 Cu MOFs nano-delivery platform
In recent years, nano Cu MOFs have been frequently explored in the field of tissue engineering and nano-drug loading (Telgerd et al., 2019; Wang T.-L. et al., 2021; Binaeian et al., 2022). Benzene-1,3,5tricarboxylate linkers and Cu ions are connected to form Hong Kong University of Science and Technology-1 (HKUST-1), which is a widely used nano-Cu MOF (Lin et al., 2012). Owing to their numerous advantages, Cu MOFs are suitable candidates for the development of nano-delivery platforms: I) they provide a unique broad-spectrum antibacterial effect (Lee et al., 2021; Wang Z. et al., 2022); II) they possess the ability to stimulate endothelial cell proliferation and differentiation, thereby promoting angiogenesis by simulating hypoxia (Dang et al., 2020); III) they possess an open skeleton framework and excellent chemical stability (Liu et al., 2019); IV) they possess a unique near-infrared (NIR) absorption ability, which can be used to develop photo-thermal therapy (PTT) nano-drug system (Weng et al., 2020; Wang L. et al., 2021; Geng et al., 2022); V) they have a favorable loading capacity and are a good choice for developing chemodynamic therapy (CDT) nanocarriers (Hao et al., 2021b). Recently, small-molecule drugs, such as chlorhexidine (Soltani and Akhbari, 2022), DOX (Gharehdaghi et al., 2021), 5-fluorouracil (Liu W. et al., 2020), methotrexate (Nezhad-Mokhtari et al., 2019), diclofenac sodium, chlorpromazine hydrochloride, amodiaquin dihydrochloride (Liu et al., 2019), and ibuprofen (Javanbakht et al., 2019), have been successfully loaded into nano-drug delivery systems through Cu MOFs. Enzymatically active molecules, such as horseradish peroxidase and glucose oxidase, can also be effectively loaded by Cu MOFs and exhibit superior stabilities than those in the free state (Hao et al., 2021a; Lin et al., 2021). Therefore, nano-Cu MOFs are the widely explored nano-drug delivery platform in recent years.
2.1.5 Ti MOFs nano-delivery platform
Owing to the superior biocompatibility and photocatalytic performance of Ti compared with other metals, Ti MOFs exhibit significant potential for bone tissue engineering and nano-drug loading. In 2009, Dan-Hardi et al. (Dan-Hardi et al., 2009) first reported MIL-125(Ti), which comprises Ti octahedra and terephthalate dianions with accessible pore diameters of 6.13 and 12.55 Å. MIL-125(Ti) has attracted considerable attention in nano-drug loading, and it has been demonstrated to solely load small molecule drugs, such as DOX (Rengaraj et al., 2017), aspirin (Rojas et al., 2019), and ibuprofen (Xie et al., 2018), Ag NPs (Arenas-Vivo et al., 2019) as well as carbon monoxide gas (Jin et al., 2018). Thus, Ti MOFs are also a favorable choice for nano-delivery platform materials.
2.1.6 Other metal MOFs nano-delivery platforms
In addition to the several MOFs mentioned above, other MOF nano-delivery platforms, such as Mg-, Co-, and Ca-based MOFs have potential applications in the bone tissue engineering field. Although further research is required to expand the contents of these MOFs on nano-drug delivery, these MOFs have been attracting increasing research attention in recent years. Mg MOFs have been demonstrated to effectively load small molecule drugs such as icariin (Wang W. et al., 2022), ibuprofen, curcumin (Lawson et al., 2022), alpha-cyano-4-hydroxycinnamate (Hu J. Q. et al., 2021), and IL4 cytokines (Zheng et al., 2020). Additionally, Co. MOFs were used to load drugs, enzymes, and bioactive molecules, such as dimethyloxalylglycine (DMOG) (Li J. K. et al., 2020), olsalazine (Levine et al., 2016), glucose oxidase (Fang et al., 2020), and 4-chloro-N-cyclohexyl-N-(phenylmethyl)-benzamide (Sun et al., 2022). Among the metal elements predominantly present in bone, Ca is also observed to build MOFs serving as nanocarriers for loading drugs such as 5-fluorouracil (Li D. et al., 2020), ibuprofen, and guaiacol (Wei et al., 2017). Overall, further analysis and studies of Mg, Co., and Ca MOFs are required because of their significant potential applications in nano-delivery platforms to overcome bone-related problems.
2.2 Metallic and metallic oxide nano-delivery platform
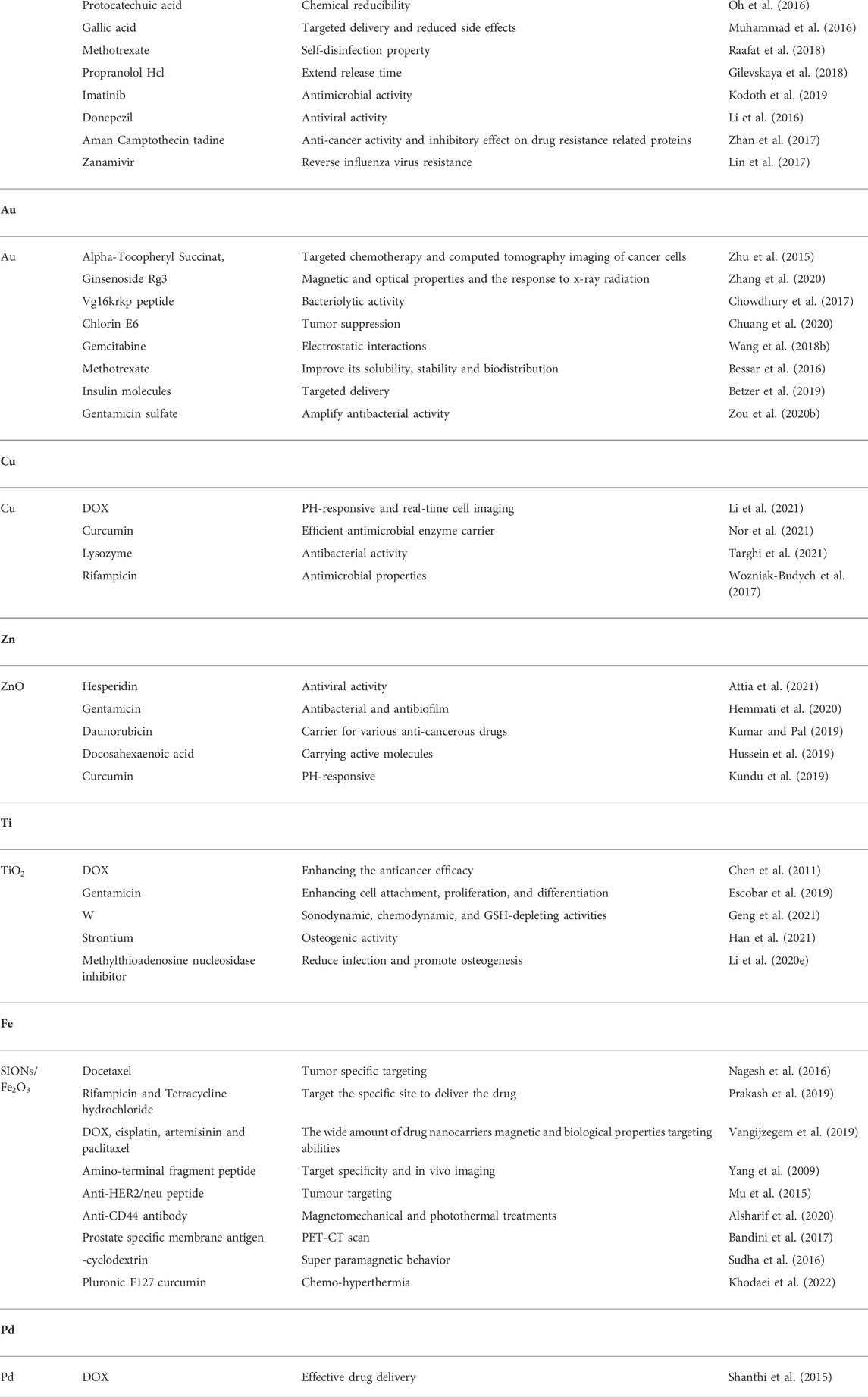
In recent years, metallic and metallic oxide nanoparticles have attracted significant attention. Although non-metallic NPs such as ceramic nanoparticles and polymeric nanoparticles can load with many therapeutic drugs, growth factors or genetic materials, metallic and metallic oxide nanoparticles are still irreplaceable for bone disease treatment. As we all known, metallic and metallic oxide NPs have unique advantages in the prevention or treatment of some infectious bone diseases because not only their good drug delivery ability, but also the excellent antibacterial properties. Not exactly the same as the antibacterial mechanism of metal ions from metal inorganic salts, in fact, the antibacterial properties of metallic and metallic oxide NPs are not only owing to the metal ions they release. Nanoscale size, morphology, and mediated generation of reactive oxide species (ROS) are all important factors for the antibacterial mechanism of this class of MNPs (Godoy-Gallardo et al., 2021). Besides, metallic and metallic oxide NPs have numerous advantages unlike traditional nanomaterials: excellent physical and chemical properties, osseointegration ability, cell labeling and imaging, photothermal response as well as magnetic actuation. Currently, the metallic and metallic oxides being used in nano-delivery platforms are also classified by metal elements and discussed in the following sections (Table 1).
2.2.1 Ag and Ag oxide nano-delivery platforms
Owing to the powerful bactericidal function, anti-inflammatory effect, mechanical strength, osteoinductive properties, and enhancement of cell proliferation rate (Shuai et al., 2018), Ag and Ag oxide (Ag2O) NPs are promising candidate materials for bone tissue engineering material coatings (Khan et al., 2020; Nardo et al., 2021), scaffold fillers (Xu et al., 2016), bactericides (Cao et al., 2018), and nano-delivery platforms (Raafat et al., 2018). The particle sizes, morphologies, surface chemistries, aggregation levels, and doses of Ag and Ag2O NPs affect the cellular response when used as carriers. Ag and Ag2O NPs can be used as delivery platforms for various drugs. For example, Zeng et al. used nanographene oxide (NGO)-coated Ag NPs (Ag@NGO) as a nanocarrier to deliver DOX (Zeng et al., 2018). Protocatechuic acid, gallic acid (Oh et al., 2016), methotrexate (Muhammad et al., 2016), propranolol HCl (Raafat et al., 2018), imatinib (Gilevskaya et al., 2018), donepezil (Kodoth et al., 2019), amantadine (Li et al., 2016), camptothecin (Zhan et al., 2017), zanamivir (Lin et al., 2017), and other drugs have been successfully delivered using Ag NPs.
2.2.2 Au nano-delivery platform
Au NPs with excellent biocompatibility, antibacterial ability, physicochemical properties, osseointegration ability, and abundant modifiable surfaces have wide ranging applications such as biocoatings, antimicrobials against drug resistance, and targeted drug carriers (Lee et al., 2018). Au NPs are known to be excellent nano-delivery vehicles for various drug molecules, peptides, proteins, plasmid deoxynucleic acid (pDNA), small interfering ribonucleic acid (siRNA), and chemotherapeutic agents (Li et al., 2015; Mallakpour et al., 2021). Au NPs have attracted considerable attention because of their excellent properties. Numerous teams have successfully achieved the delivery of antioxidant alpha-tocopheryl succinate (α-TOS) (Zhu et al., 2015), ginsenoside Rg3 (Zhang et al., 2020), VG16KRKP antimicrobial peptide (Chowdhury et al., 2017), chlorin e6 (Chuang et al., 2020), gemcitabine (Wang Z. et al., 2018), methotrexate (Bessar et al., 2016), insulin molecules (Betzer et al., 2019), gentamicin sulfate (Zou Y. et al., 2020), as well as various active molecules or drugs, such as gentamicin and ampicillin, demonstrating that Au NPs can be used as a reliable nano-delivery platform.
2.2.3 Cu nano-delivery platform
Compared to Au and Ag, Cu is an inexpensive and readily available metal and among the essential trace elements present in most living organisms. Simultaneously, Cu has gradually become a research hotspot owing to its excellent physical, chemical, electrical and optical properties as well as favorable antibacterial properties. The stable interactions between Cu NPs and various drugs (DOX, curcumin, lysozyme, rifampicin) can form nano-delivery systems with superior performance (Wozniak-Budych et al., 2017; Li et al., 2021; Nor et al., 2021; Targhi et al., 2021).
2.2.4 Zn oxide nano-delivery platform
Zn oxide (ZnO) exists in various forms, such as ZnO nanospheres, nanosheets, nanorods, and nanowires (Carofiglio et al., 2020; Wojnarowicz et al., 2020). Zn ions have been confirmed to effectively promote the mineralization of the extracellular matrix of bone marrow mesenchymal stem cells (BMSCs) (Wang Q. et al., 2018), implant osseointegration (Chen et al., 2017), migration and tube formation of vascular endothelial cells (Nakamura et al., 2020), and antibacterial effects (Yang et al., 2020). ZnO is a high-profile candidate material for bone tissue engineering material modification, bone regeneration, angiogenesis, as well as antibacterial and anti-infection effects. In addition, ZnO has broad prospects as nanocarriers. The loading of ZnO for various drugs can be realized using simple and quick methods. Currently, researchers have successfully used ZnO to load hesperidin (Attia et al., 2021), gentamicin (Hemmati et al., 2020), daunorubicin (Kumar and Pal, 2019), docosahexaenoic acid (Hussein et al., 2019), curcumin (Kundu et al., 2019), and other drugs.
2.2.5 Ti dioxide nano-delivery platform
It is well known that the Ti metal has excellent biocompatibility and osseointegration ability. Ti or Ti alloys have been widely used as implant materials in implants, bone substitute materials, fracture fixation screws, meshes, etc. Based on the excellent properties of Ti, Ti dioxide (TiO2) nanomaterials have attracted the attention of researchers. Numerous nanostructures of TiO2, such as TiO2 nanotubes, NPs, matrices, rods, and whiskers, have emerging applications as delivery platforms for different drugs or active factors. For example, TiO2 nanomaterials can deliver multiple small-molecule drugs, such as dexamethasone, temozolomide, curcumin, cisplatin, gambogic acid, valproic acid, DOX, and daunorubicin, as well as effectively deliver methylthioadenosine nucleosidase inhibitor, biologically active protein human recombinant BMP-2 and metal tungsten, strontium, etc. (Chen et al., 2011; Escobar et al., 2019; Li et al., 2020c; Geng et al., 2021; Han et al., 2021). Evidently, TiO2 is a powerful nano-delivery platform and an extremely promising material for bone health maintenance.
2.2.6 Iron oxide nano-delivery platform
Superparamagnetic iron oxide NPs (SIONs) are multifunctional nanomaterials of significance in bone disease diagnosis, targeted delivery, and bone replacement material modification because of their unique magnetic and biological properties. Iron oxide NPs have been approved for clinical applications. Magnetic iron oxide NPs have excellent advantages as nanocarriers, because they can efficiently load various drugs and also be imaged or reach target sites in vivo via an external magnetic force, enhancing the bioavailability of therapeutic compounds. Studies have demonstrated that SIONs can effectively deliver cisplatin, curcumin, lauric acid, mitoxantrone, artemisinin, docetaxel, paclitaxel, dopamine, 10-hydroxycamptothecin, docetaxel, DOX, rifampicin, tetracycline hydrochloride, and other drugs (Nagesh et al., 2016; Prakash et al., 2019; Vangijzegem et al., 2019). In addition, a few peptides, antibodies, or organic small molecules, such as amino-terminal fragment peptide (Yang et al., 2009), anti-HER2/neu peptide (Mu et al., 2015), anti-CD44 antibody (Alsharif et al., 2020), prostate specific membrane antigen (Bandini et al., 2017), β-cyclodextrin (Sudha et al., 2016), and pluronic F127 polymer (Khodaei et al., 2022)were conjugated on the SION surfaces, consequently enhancing the targeting function of the SIONs delivery system, increasing the specificity and therapeutic effect.
2.2.7 Palladium nano-delivery platform
Palladium is a precious metal material with high mechanical strength, high porosity, as well as anti-microbial, anti-oxidation, and anti-cancer properties. It is a worthy target for various applications including medical biomaterials, nano-delivery carriers, and bone disease treatment. Palladium is an effective nano-delivery platform, which has been demonstrated in several studies, and drugs, such as DOX, has been successfully loaded using palladium NPs (Shanthi et al., 2015).
3 Loading method of MNPs
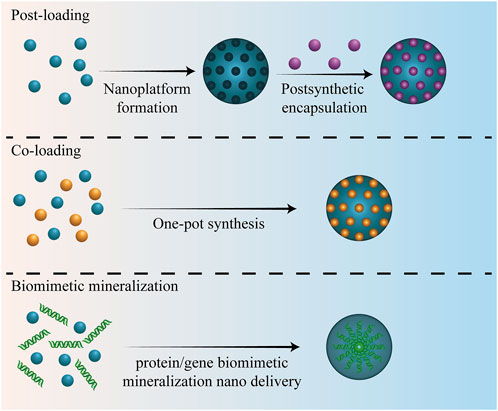
The MNP loading method affects the loading efficiency and release rule of therapeutic molecules, further affecting the diagnosis and treatment of bone-related diseases or bone regeneration. There are three commonly used methods for MNP loading with therapeutic molecules: post-loading, Co-loading, and biomimetic mineralization (Liu et al., 2020c; Lawson et al., 2021). Different loading methods are suitable for different kinds of MNPs. The three loading methods of MNPs are elaborated in the following subsections (Figure 3).
3.1 Post-loading
Post-loading can also be referred to as “postsynthetic encapsulation/loading,” which is a strategy of first fabricating nanocarriers and then loading drugs to achieve high drug loading nano drug-loading systems. Using this method, non-porous nanomaterials can be combined with therapeutic molecules via electrostatic attraction, non-covalent hydrophobic interactions, π–π stacking, and hydrogen bonding. Benefiting from tunable pore, high specific surface areas, and active surfaces, some porous NPs can also be loaded using drugs via this method (Chen et al., 2021). Therefore, postsynthetic encapsulation/loading is a suitable method for most MNPs; however, the difference is that for MNPs with pores, small drug molecules can be encapsulated inside the MNPs through the pore size; for MNPs without sufficient pore sizes or if the pores are already occupied, drug molecules can only be loaded onto the nanomaterial surface or functionalized modified surfaces. This post-loading method is particularly suitable for loading additional therapeutic molecules into MOFs that have already encapsulated the drug. The MNP pore size, drug-to-pore size ratio, and strength of interactions, such as coordination bonds, hydrophobic interactions, electrostatic attraction, and π–π stacking, between the drug and MNPs can all affect the loading efficiency of post-loading (Cao et al., 2020; Lawson et al., 2021). Farhad et al. (Ahmadijokani et al., 2021) post-loaded methotrexate with three MOFs, MIL-53, NH2-MIL-53 and NH2-MIL-101, and observed polar amine groups, larger surface area and pore volume, high positive zeta potential, and NH2-MIL-101 exhibited the highest loading capacity, 457.69 mg/g, of the drug. They proposed that electrostatic interactions, π–π stacking interactions, and H-bonding are the primary mechanisms by which methotrexate is successfully post-loaded. Hu et al. (Hu J. Q. et al., 2021) loaded CHC on Mg-MOF-74 using the “post-loading” method and achieved a loading of 625 mg g−1. Post-loading metal NPs can also be used to achieve high drug loading. A few researchers used Polyethylene glycol–poly (ethylene imine) -functionalized Au NPs to load chlorin e6, and the loading content reached 46.4 ± 0.4% (Chuang et al., 2020).
3.2 Co-loading
Co-loading, also known as “one-pot synthesis”, refers to the strategy of loading or encapsulating drugs during MNP formation. This loading method is primarily applicable to MOFs, and the therapeutic molecules can be used as building blocks to contribute to MOF formation along with metal ions. The size of the therapeutic molecule affects physical properties, such as the size and charge of the final MOF drug-carrying system, to an extent. One-pot synthesis is simple, convenient, and can facilitate the uniform distribution of drug molecules in the whole MOFs as well as effectively prevent the rapid release of drug molecules when the pore size of MOFs is smaller than the particle size of the drug. Electrostatic adsorption is of great significance to the nano-delivery system formed using one-pot synthesis, which is often used in the synthesis of nanomedicines for bone regeneration or bone disease treatment. Risedronate was successfully loaded into ZIF-8 using one-pot synthesis, and an encapsulation rate of 64.21 ± 2.48% was achieved (Cheng et al., 2020). Sun et al. (Sun et al., 2022) also loaded 4-chloro-N-cyclohexyl-N-(phenylmethyl)-benzamide (FPS-ZM1) in a Co-based MOF (ZIF-67) using one-pot synthesis. The results demonstrated that post loading of FPS-ZM1 with ZIF-67, the particle size changed from 386.0 to 466.3 nm, the zeta potential changed from 3.63 to 3.10 mV, and the ZIF-67 delivery platform achieved a favorable sustained release effect of FPS-ZM1.
3.3 Biomimetic mineralization
Biomimetic mineralization is a loading method similar to Co-loading, in which active molecules and MNPs are mixed. However, biomimetic mineralization is predominantly used to load biomolecules, such as nucleic acids and proteins, and relies on biomolecules as nucleation sites for MNPs crystallization (Wang J. et al., 2020). The encapsulation mechanism is forming bonds/interactions between the MNPs building blocks and loading biomolecules to facilitate nucleation. In this encapsulated state, biomolecules can be protected from harsh chemical environments, heat, and degrading enzymes, while the simultaneous delayed activity or slow release of biomolecules mediated by MNPs disintegration may occur (Zou D. et al., 2020; Ha et al., 2021). The tobacco mosaic virus was loaded onto ZIF-8 via biomimetic mineralization, and its thermal and chemical stability was significantly enhanced after encapsulation (Li et al., 2018). Li et al. (Li et al., 2019b) also successfully encapsulated pDNA into ZIF-8 via biomimetic mineralization, where pDNA was uniformly distributed within the ZIF-8 nanostructure, consequently protecting it against enzymatic degradation. In a study on biomimetic mineralization of Fe3O4 NPs (Liu L. et al., 2019), 14-mer bi-functional copolypeptide was used as a template and a ginger extract was applied as an antioxidant and a size-conditioning agent. Under the cooperative effect of the peptide and ginger extract, the size and dispersibility of Fe3O4 crystals were effectively controlled. Tong et al. (Tong et al., 2015) loaded TiO2 NPs onto C3N4 nanosheets via the arginine-enabled biomimetic mineralization, and made Ag NPs nucleate and grow on the surface of TiO2 NPs.
4 Application scenarios of MNPs in bone diseases
4.1 Bone regeneration
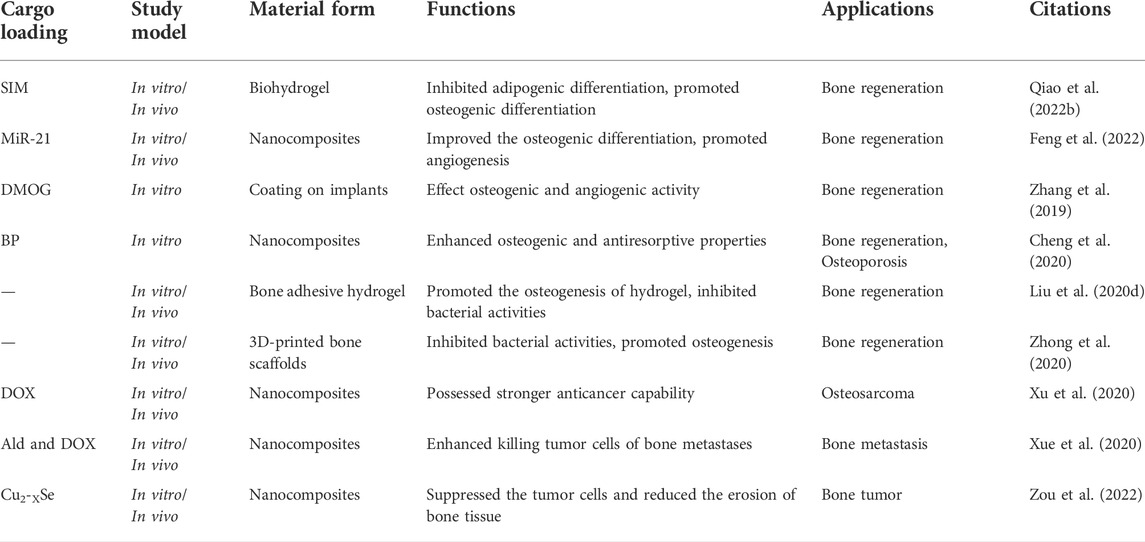
The regenerative treatment of large-area bone defects imposes strict requirements on tissue engineering materials, particularly in the conditions accompanying basic diseases, such as osteoporosis, hyperlipidemia, diabetes, infection, and vascular necrosis, and the performance of the tissue engineering materials is crucial. In these challenging bone-repair scenarios, MNPs, particularly the Zn-based NPs, exhibit satisfactory application potential and are widely used for enhancing or modifying bone tissue engineering materials. For example, MNPs comprising ZIF-8 with Zn as the coordination core have assisted numerous novel bone tissue engineering materials in the completion of complex bone repair tasks (Figure 4). In the face of bone metabolism disorders and hyperlipidemia, Qiao et al. (Qiao M. et al., 2022) used ZIF-8 to encapsulate the small-molecule drug, simvastatin, to reduce serum cholesterol levels to form SIM@ZIF-8 nanocarrier particles, and SIM@ZIF-8 for enhancing the performance of poly (ethylene glycol) diacrylate (PEGDA) and sodium alginate (SA) to form SIM@ZIF-8/PEGDA/SA (nSZPS) composite biogels. The corresponding in vivo and in vitro experiments confirmed that using SIM@ZIF-8, nSZPS can inhibit adipogenic differentiation and promote osteogenic differentiation of BMSCs in vitro, as well as promote blood lipid lowering and osseointegration of bone in hyperlipidemia rats (Figure 3). For treating bone defects under ischemic conditions, miR-21 or DMOG, which are active components for activating blood vessels, and can also be loaded using ZIF-8, which protect and intactly deliver drugs or nucleic acids into cells, thereby completing the activation of the blood supply to the damaged bone tissue area (Zhang et al., 2019; Feng et al., 2022). In addition, for treating osteoporosis and other bone immune behavior disorders, ZIF-8 can deliver bisphosphonates (BP) to combat excessive osteoclast behavior (Cheng et al., 2020). In addition, metals, such as Ti, which are consistent with bone implants are commonly used for designing MNPs, for the design of bone tissue engineering materials (Chen et al., 2017). Saha et al. (Saha et al., 2021) used curcumin-loaded TiO2 nanotubes to modify Ti6Al4V implant surfaces and obtained a novel bone implant with both drug release and support properties. This Ti-based MNP-optimized implant can significantly inhibit Escherichia coli and Staphylococcus aureus, as well as effectively promote the cell adhesion, proliferation, and osteogenic differentiation of mesenchymal stem cells, which is beneficial for solving infection-related problems of Ti bone implant and its surrounding. Recently, Xue et al. (Xue et al., 2022) endowed 3D-printed polycaprolactone (PCL) scaffolds with excellent photothermal properties by loading dexamethasone sodium phosphate on CuS nanoparticle. This design can controllably release drugs under exposure to 1,064 nm NIR light irradiation and effectively promote the osteogenic differentiation of BMSCs.
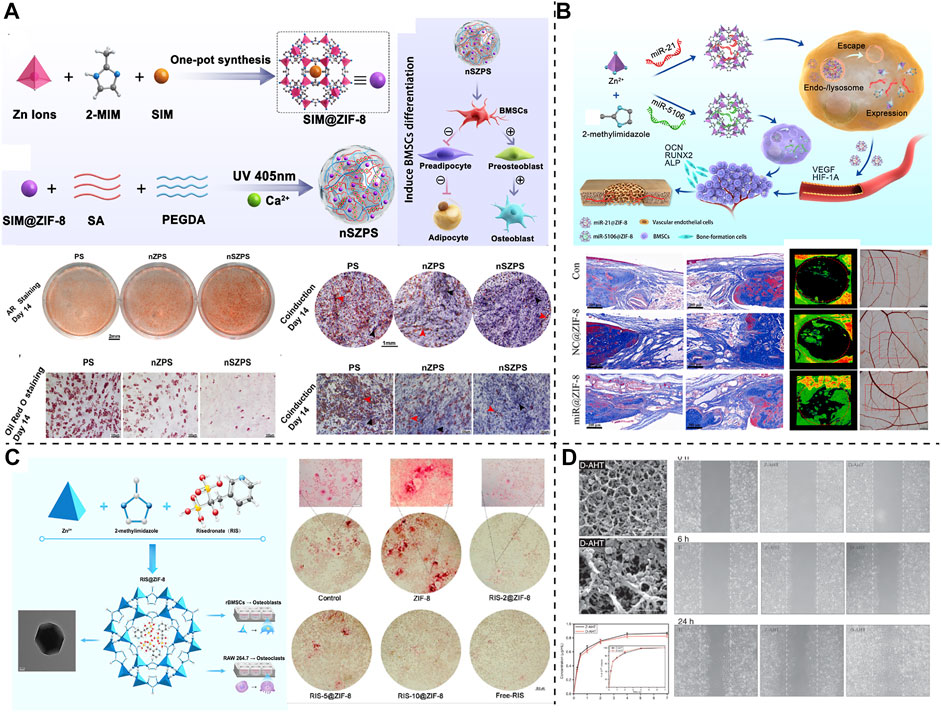
FIGURE 4. ZIF-8-based MNPs were demonstrated for building bone tissue engineering materials. (A) SIM@ZIF-8 carried hydrogel designed for regulating the balance of osteogenic and adipogenic differentiation, copyright 2022 Elsevier. (B) A ZIF-8 loading miRNA promoting vascularized bone formation, copyright 2021 Elsevier. (C) A ZIF-8-based MNP aiming at preventing osteoclasts, copyright 2019 ACS Publications. (D) Bone implants modified with DMOG@ZIF-8, copyright 2019 SAGE Publications.
Notably, the high loading capacity and stable delivery ability are not the only reasons why MNPs are preferred as bone tissue engineering modification materials. The drug release or controlled release capability of MNPs facilitates the possibility for multi-level, multi-stage, and multi-purpose bone regeneration and repair. Moreover, the metal elements in MNPs influence the physiological activities of bones. First, few metal elements (such as Zn) directly accelerate osteogenic differentiation (Table 2). For example, Liu et al. (2020c) used nano-ZIF-8 directly as a catechol–chitosan enhancer to prepare a multifunctional bone adhesive hydrogel, and the sustained-release Zn ions up-regulated the production and secretion of alkaline phosphatase, collagen1 and osteocalcin promoted the osteogenic differentiation of BMSCs and accelerated bone remodeling. Similar applications have also been observed in a few 3D-printed bone scaffolds or implants (Zhong et al., 2020). Similarly, the nano-ZnO particles also have a direct role in promoting bone repair. Studies have demonstrated that the osteogenic effect of these Zn-based nanomaterials may be achieved via upregulating the Wnt/β-catenin pathway (Gao et al., 2021). In addition to direct osteogenesis, accelerate vascularized bone repair has been achieved using NPs derived from Fe, Cu, and Mg (Zhu et al., 2020), thereby providing advantages for their constructed MNPs for bone repair. In addition, the potential hindrance of the osteoclast process is also of interest to researchers. Bai et al. (Bai et al., 2020) studied the effect of Au NPs without any drug loading on osteoclasts and observed that Au NPs can interact with the V0 domain of vacuolar-type H + -ATPase, consequently preventing its recruitment of the V1 domain, impairing osteoclast acid secretion, and inhibiting the proteolytic enzyme secretion by osteoclasts to degrade bone matrix. The inhibitory effect of Au NPs on bone resorption was demonstrated in a lipopolysaccharide-induced bone erosion mouse model. Therefore, the active regulation of osteogenesis-related processes using metal elements significantly facilitates the application of MNPs in the field of bone repair.
4.2 Osteomyelitis
Osteomyelitis is an inflammatory/infectious bone disease and considerably affects the daily life of patients, and it is commonly occurring in people that are older, diabetic, or with poor general health. Osteomyelitis is often caused by pyogenic bacteria, such as Staphylococcus aureus and Staphylococcus epidermidis, and fungal infections, such as Haemophilus influenzae and Brucella suis fungi. It may also be a complication of orthopedic surgery, tooth extraction, or facial plastic surgery. The predominant treatment approach currently employed is the administration of systemic antibiotics, which is less effective at the localized infection site. The topical use of MNPs encapsulated with antibiotics or MNPs with targeted functions is favorable choice to enhance the therapy of osteomyelitis, delay drug action time, reduce the systemic side effects, and reduce osteonecrosis. Abdulrehman et al. (Abdulrehman et al., 2020) synthesized Ag–Cu–boron (ACB) alloy NPs and obtained ACB–OBAb nano-delivery system via the Cadherin-11 antibody (OBAb) coupling. It has been demonstrated that ACB–OBAb can target osteoblasts and effectively inhibit Staphylococcus aureus inside and outside the infected osteoblasts. This study demonstrates the significance of MNPs in reducing systemic toxicity and targeting bone infection and inflammation. Escobar et al. (Escobar et al., 2019) used Ti dioxide film (MTF) loaded with gentamicin and conjugated human recombinant BMP-2 on its surface. This delivery platform can effectively inhibit the colonization of Staphylococcus aureus as well as significantly enhance the MC3T3-E1 preosteoblastic cell attachment, proliferation, and differentiation. Liu et al. (Liu et al., 2020b) used the Au NPs synthesized using Acetobacter and Gluconobacter to load ginsenoside compound K (CK) and surface-conjugated CopA3 to obtain the GNP–CK–CopA3 nano-delivery system. GNP–CK–CopA3 improved the LPS-induced production of nitric oxide (NO) and ROS and inhibited the mRNA and protein expressions of pro-inflammatory cytokines in macrophages, combined with significant anti-inflammatory effects.
In addition to the treatment of osteomyelitis via high-efficiency drug loading, anti-inflammatory or antibacterial activities are attributes of most MNPs that can release metal ions, making them effective candidates for the topical treatment of osteomyelitis. The powerful antibacterial, antifungal, and osteoinductive properties of Ag-based nano-delivery platforms have been extensively validated and are exceedingly the most commonly used commercial NPs (Hauser and Nowack, 2021). In addition, there is significant evidence that Cu-based, Ti-based, Zn-based, Palladium-based, iron-based, Co-based and other nanomaterials exhibit favorable bactericidal properties (Rojas-Andrade et al., 2017). Additionally, compared with traditional antibiotics, MNPs avoids the limitations caused by drug resistance. Marsich et al. (Marsich et al., 2013) prepared alginate/hydroxyapatite composite scaffolds containing Ag NPs and demonstrated that the presence of Ag NPs endowed the scaffolds with favorable sterilization against both Gram+ and Gram− bacterial strains and did not affect the ability of the scaffold to promote the proliferation of osteoblasts. Arenas-Vivo et al. (Arenas-Vivo et al., 2019) formed nanocomposites with MIL-125(Ti)NH2 composite Ag NPs. This nanocomposite exhibits a favorable effect against Staphylococcus aureus biofilm under the triple action of the intrinsic bactericidal activity of MIL-125(Ti), bactericidal properties of Ag NPs, and photoactivity of UVA irradiation. This study provides a substantial basis for MNPs to inhibit bone implant biofilm formation and treat osteomyelitis caused by bacterial infection. According to reports (Krumdieck et al., 2019), TiO2 can be used as an effective antibacterial coating material owing to its unique photocatalytic properties, which can effectively reduce E. coli. In a study, magnetic Co. ferrite NPs were demonstrated to exhibit inhibitory effects on Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus, Candida parapsilosis, and Candida albicans. Moreover, the antibacterial effect was improved with increasing the Co2+ content (Zalneravicius et al., 2018). The cooperation of different metal NPs sometimes brings new ideas for the treatment of deep osteomyelitis. Fe3O4 NPs and Au NPs were combined to act as the nuclear component of an engineered macrophage with a nuclear–membrane structure. The nuclear component can produce abundant ROS and heat under microwave irradiation thus suppressing inflammatory responses, killing bacteria in situ, simultaneously promoting osteoblast differentiation for osteomyelitis (Fu et al., 2021).
4.3 Bone tumor
There are several commonly occurring types of bone tumors, such as osteosarcoma and bone metastases originating from breast or prostate cancer. The common symptoms of bone tumors are severe pain, pathological fractures, bone marrow aplasia, and hypercalcemia, which induce considerable pain in patients. CT, radiotherapy, and ablation are the commonly used non-surgical methods for treating bone tumors. DOX, cisplatin, ifosfamide, BP, tetracycline, denosumab, cabozantinib, mesna, methotrexate, etc. Are commonly used as CT drugs for bone tumors. To reach bone tissues via systemic administration, drugs require to penetrate the blood–bone marrow barrier including the clefts of bone marrow sinusoidal capillaries with diameters of 80–100 nm (Sarin, 2010). Therefore, MNPs are favored by bone tumor specialists because of their small sizes and high loading capacities. Fang et al. (Fang et al., 2020) synthesized a nanoscale Co–ferrocene MOF (Co–Fc NMOF) and loaded glucose oxidase with this nanoplatform to construct Co–Fc@GOx, which plays the role of a cascade enzymatic/Fenton catalytic platform to cure cancer via CDT. In addition, a few scholars (Raghubir et al., 2020) delivered riluzole through two different shapes of iron oxide NPs (nanocages or nanospheres, 15 ± 2.5 nm), and significantly induced tumor tissue apoptosis in the osteosarcoma model mice. Mokhtari et al. (Nezhad-Mokhtari et al., 2019) loaded methotrexate into the empty face-centered cubic lattice and two-dimensional tunnels of Cu-MOF to form nano-delivery ions (Cu-MOF/MTX), and further inserted Cu-MOF/MTX into a series of synthesis experiments of novel microspheres (Cu-MOF/MTX@GM) from pH-sensitive gelatin microsphere biopolymers, demonstrating that the microspheres are suitable for targeted anticancer drug delivery. In a report (Sha et al., 2021), metal manganese (Mn) was in situ doped into Au core mesoporous silica NPs to construct a multifunctional MNPs (Au@MMSN). Au@MMSN was further loaded with Ald and DOX (DOX@Au@MMSN-Ald) for osteosarcoma treatment. It was confirmed that the Au and Mn ions released from the nanocomposite could be used for computer tomography and MR dual-modality imaging for in vivo localization. At the same time, DOX@Au@MMSN-Ald can release antitumor drugs responsively to realize the combined treatment of CT and CDT for osteosarcoma.
MNPs can load drugs for bone tumor CTP and show considerable potential in PTT, PDT, sonodynamic therapy, and other new noninvasive solid tumor therapy or multimode combined therapy. Recently, Geng et al. (Geng et al., 2021) reported ultrafine W-doped TiO2 (W- TiO2) nanorods for sonodynamic−chemodynamic combination tumor therapy. They observed that W doping narrows the band gap from 3.2 to 2.3 eV, enhancing the TiO2 acoustodynamic properties; W5+ doping endows W- TiO2 nanorods with Fenton-like reactivity; W6+ doping promotes the transfer of endogenous transformation of glutathione to W5+ ions, thereby enhancing the chemical kinetic activity of W- TiO2 and changing the tumor microenvironment. In vivo experiments demonstrated that W- TiO2 enhances tumor eradication in an osteosarcoma model under single ultrasound irradiation. Cheng et al. (Cheng et al., 2021) used a mixed-metal Cu/Zn-MOF for integrating Mn2+ and MnO2 and loaded the photosensitizer ICG to form nanocomposites. The contained ICG can realize photothermal imaging and PTT under laser irradiation. The nanocomposite exhibits fluorescence imaging and PDT capacity by releasing ICG upon reaching the tumor site and can produce cytotoxic OH by releasing Cu+/Mn2+ and scavenging glutathione to exert CDT effect. The study demonstrates that MNPs are excellent candidate materials for PTT/PDT/CDT of bone tumors. Recently, Zou et al. (Zou et al., 2022) developed a composite nanoplatform using ZIF-8–capped Cu2-XSe and used it for CDT and PTT of bone tumors. Cu2-XSe was released with the cleavage of ZIF-8 under acidic microenvironment in tumor and subsequently degrade into Cu+ and Cu2+ to initiate a Fenton-like reaction inducing CDT. At the same time, Cu2-XSe can also induce PTT effect and inhibit tumor cells and osteoclasts. This ZIF8-capped nanomedicine effectively demonstrates the potential value of MNPs for advanced therapy in bone tumors.
4.4 Osteoarthritis
Osteoarthritis is a chronic disease characterized by the progressive degeneration of articular cartilage, abnormal reduction of joint lubrication, and synovial inflammation. Joint pain as well as joint damage and dysfunction are the primary clinical symptoms of osteoarthritis (Litwic et al., 2013). Several common drugs, including anti-rheumatic drugs, non-steroidal anti-inflammatory drugs, glucocorticoids, and newly discovered biological agents, are used to treat osteoarthritis (Mackenzie and MacDonald, 2010). Although intra-articular drug injection is a characteristic administration mode for treating osteoarthritis, the free drug is rapidly cleared from the joint cavity, leading to increased complications and decreased drug bioavailability (Kompel et al., 2019). Based on this, MNPs have been extensively explored as a drug-loading vehicle for osteoarthritis (Table 3). Xiong et al. (Xiong et al., 2020) developed a pH-responsive Fe-based MOF system MOF@HA@PCA loaded with an anti-inflammatory protocatechuic acid (PCA) and modified with hyaluronic acid (HA), which can respond to the acidic microenvironment and gradually released for treating osteoarthritis PCA, which downregulates the expression of osteoarthritis inflammatory markers and promotes the expression of cartilage-specific markers, significantly reducing IL-1β-induced synovial inflammation in both joints and chondrocytes. Li et al. (Li et al., 2020b) generated five poly (amidoamine) dendrimer-entrapped Au NPs (Au DENPs) that simultaneously delivered antioxidant α-TOS and anti-inflammatory antiTNF-α siRNA (Au DENPs/TNF-α siRNA complex thing). The complex can significantly enhance the antioxidant capacities of macrophages and down-regulate inflammatory cytokines in arthritis mouse models, thereby achieving a combined antioxidant and anti-inflammatory therapy for joint inflammation. MNPs also can contribute uniquely to stem cell therapy for osteoarthritis. The CuS@MnO2 NPs loaded with metformincan could be targeted for uptake by MSCs which further were used as stem cell therapy for osteoarthritis. MSCs modified with this MNPs were validated to exhibit an increased capability of anti-inflammation and chondrogenesis, and effectively relieve osteoarthritis symptoms (Lu et al., 2022).
Similarly, MNPs provide new avenues for osteoarthritis treatment owing to their excellent light responsiveness, strong plasticity, and self-anti-inflammatory properties. Huang et al. (Huang et al., 2021) reported a metal/semiconductor composite (Au NR@CuS) comprising octahedral Cu sulfide shell and Au nanorod core, loaded with vasoactive intestinal peptide and HA to form VIP–HA–Au NR@CuS NPs. It was experimentally demonstrated that the nanocomposite generated a photothermal effect under laser irradiation and introduced additional OH for PDT under the integration of a Fenton-like reaction and Au NR and CuS semiconductor photocatalysts; targeting synovial cells under the action of active intestinal peptides and HA, thereby exerting the combined effect of PTT, PDT, and CT, significantly removing the hyperplastic synovium, reducing joint inflammation symptoms. In addition, it has been reported (Carneiro et al., 2020) that Au-coated superparamagnetic iron oxide NPs could more significantly reduce the number of immunostaining positive cells of TNF-α and IL-1β in synovium compared with the drug methotrexate alone, thereby inhibiting joint edema and inflammation.
5 Challenges of MNPs
Although the applications of MNPs in bone tissue engineering and the management of various bone-related diseases have been well reported, only a few MNPs have entered the market evaluation stage and received final approvals. Numerous challenges remain to be overcome in the translation of MNPs from basic experiments to clinical applications in orthopedics, which may originate from multiple aspects and fields. We analyzed the reasons and summarized them into the following points:
5.1 Agglomeration
Several reports have mentioned the agglomeration of MNPs, which limits MNPs functionality in the nano-scale, as well as their dispersibility and stability. A few scholars believe that electrostatic interactions, which is size-dependent, cause this agglomeration (Chandrakala et al., 2022). Agglomeration in vivo hinders the dispersibility of MNPs, which challenges the targeting efficacy and biosecurity of MNPs. Apparently, agglomeration may also affect the in vivo behavior of MNPs, such as the cellular uptake, inter-tissue transport, penetration, diffusion, and biocompatibility (Sengul and Asmatulu, 2020), which hinder the application of MNPs as active molecules or therapeutic agent delivery vehicles. Surface functional modification of MNPs is currently a commonly used method to overcome the limitations of agglomeration instability and improve biological applications. The surface functionalization of MNPs can be achieved via techniques such as coordination binding of unsaturated metal sites, ligand exchange, and covalent binding with pre-functionalized linkers (Kainz and Reiser, 2014; Nolte et al., 2017). The thermal and chemical stability of MOFs can be significantly improved using amino-modified MOFs in 2-aminoterephthalic acid ligands (Chen et al., 2019). Surface PEGylation is an FDA-approved technique (Veronese and Pasut, 2005). In addition, complexation with other nanomaterials (polymer NPs, liposomes, cubes, etc.) can reduce MNP agglomeration (Phuong et al., 2019). These methods impose strict requirements on process flow, cost, and technical parameters. Moreover, all MNPs cannot overcome the agglomeration limitation via the aforementioned methods. The surface chemical properties of MNPs are significantly vary, and the chemical groups or compounds that can be matched are complex. Methods to overcome MNP agglomeration remains a topic worthy of future research.
5.2 Toxicity
The toxicity of MNPs has always been a hot research topic. In addition to the agglomeration phenomenon mentioned above, there are several reasons for MNP toxicity. The recognized high specific surface area of MNPs leads to high surface reactivity, which is a double-edged sword that may bring unexpected toxicity mechanisms to MNPs (Mahana et al., 2021). The small sizes of MNPs promote the cellular uptake rate, which subsequently increases bioavailability as well as enhances toxicity (Yang et al., 2013). In addition, MNPs release numerous metal ions with a bactericidal effect, but excessive metal ions can impair the electrolyte balance of the body fluid environment and the normal ion exchange of cell membranes, consequently resulting in cytotoxicity (Wang et al., 2016a). It has been reported that heavy metal ions accumulated in osteoblasts and chondrocytes can lead to cellular dysfunction by replacing essential elements in enzymes and disrupting the conformation of the active site, thereby increasing the risk of osteoporosis and osteoarthritis (Krizkova et al., 2016). Organic linkers, metal ions, solvents, and chemical residues formed during MNP synthesis also cause toxicity, which still requires attention and should be resolved. Although a few scholars (Huang et al., 2020; Ting et al., 2022) have functionally modified MNP surfaces by targeting ligands, attempting to reduce systemic toxicity through the targeting of MNPs, MNP toxicity remains a major problem that limits its clinical application.
5.3 Complexity
The application of MNPs in orthopedics, particularly in tissue engineering materials, often requires the cooperation of materials such as scaffolds, gels, and biofilms. The type of disease, drug resistance, implantation site, mechanical properties, and several other requirements should be comprehensively considered. This requires the precise control of MNPs by nanomedicine experts, as well as a high degree of coordination among orthopedic surgeons, biomedical scientists, and biomechanical engineers (Schuemann et al., 2020). In addition, the repair/treatment requirements of bone defects/diseases in different zones of the epiphysis, diaphysis, metaphysis, and joints are different. The most important requirement for diaphyseal healing is the establishment of mechanical strength; for the metaphysis, joints, both mechanical strength and flexibility require to be considered. Therefore, the mechanical, physiological, pathological, immune biochemical, and surgical conditions for the design and implementation of MNPs into scaffolds should be comprehensively considered. That is, for different orthopedic problems, appropriate MNPs should be specially designed for the corresponding needs. Particularly, the selection of optimal drugs or active ingredients, metal ions, organic ligands, supporting biomaterials, machines, preparation techniques is a complex interdisciplinary task.
6 Conclusion
The rapid development of MNPs provides new avenues for innovating the diagnosis and treatment of bone diseases and regeneration. The advantages of MNPs are significant: high loading rate, excellent antibacterial properties, functionally modified surface, tunable pore volume and size, acceptable biocompatibility, and strong mechanical properties as well as a few unique features such as magnetic properties, light responsiveness, and pH responsiveness. Various types of MNPs, such as MOFs, metal nanoplatforms, and metal oxide nanoplatforms, exist. Ag NPs and Ag2O NPs are exceedingly the most commonly used commercial nanoplatforms. The current mainstream research is focused on the development and application of MOFs. In addition to small-molecule drug MNPs, various therapeutic agents, such as proteins, viruses, peptides, and RNA, can be effectively delivered. Moreover, several delivery methods can be used, such as post-loading, Co-loading, and biomimetic mineralization. MNPs can be used in various orthopedic scenarios, most commonly in bone tissue engineering regeneration and can also play a favorable role in bone regeneration in a few osteoporotic diseases. In addition, MNPs have significant potential in treating diseases, such as osteomyelitis caused by various infections, bone metastases from different sources, osteosarcoma, and osteoarthritis. However, MNPs still have limitations; for instance, the problems of aggregation and toxicity require to be further overcome. In the future, multidisciplinary efforts are required to promote the clinical application of MNPs in orthopedics.
Author contributions
YL wrote the main paper. YL, ZX, and MQ drawn pictures and tables. HC and ZZ analyzed the data and revised the paper. HC and ZZ conceived and designed the paper. All authors critically reviewed and approved the paper.
Funding
This review was supported by the National Natural Science Foundation of China (Nos. 82101076), the Postdoctoral Research Foundation of China (No. 2020M683334), the Research Funding from West China School/Hospital of Stomatology Sichuan University (No. RCDWJS 2021-7), the Research and Develop Program from West China School/Hospital of Stomatology Sichuan University (No. RD-02-202109, No. RD-02-202111).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdulrehman, T., Qadri, S., Skariah, S., Sultan, A., Mansour, S., Azzi, J., et al. (2020). Boron doped silver-copper alloy nanoparticle targeting intracellular S. Aureus in bone cells. PLoS ONE 15 (4), e0231276. doi:10.1371/journal.pone.0231276
Ahmadijokani, F., Tajahmadi, S., Rezakazemi, M., Sehat, A. A., Molavi, H., Aminabhavi, T. M., et al. (2021). Aluminum-based metal-organic frameworks for adsorptive removal of anti-cancer (methotrexate) drug from aqueous solutions. J. Environ. Manage. 277, 111448. doi:10.1016/j.jenvman.2020.111448
Al Haydar, M., Abid, H. R., Sunderland, B., and Wang, S. (2017). Metal organic frameworks as a drug delivery system for flurbiprofen. Drug Des. devel. Ther. 11, 2685–2695. doi:10.2147/dddt.S145716
Alsharif, N. A., Aleisa, F. A., Liu, G., Ooi, B. S., Patel, N., Ravasi, T., et al. (2020). Functionalization of magnetic nanowires for active targeting and enhanced cell-killing efficacy. ACS Appl. Bio Mat. 3 (8), 4789–4797. doi:10.1021/acsabm.0c00312
Anastasio, A. T., Paniagua, A., Diamond, C., Ferlauto, H. R., and Fernandez-Moure, J. S. (2021). Nanomaterial nitric oxide delivery in traumatic orthopedic regenerative medicine. Front. Bioeng. Biotechnol. 8, 592008. doi:10.3389/fbioe.2020.592008
Arenas-Vivo, A., Amariei, G., Aguado, S., Rosal, R., and Horcajada, P. (2019). An Ag-loaded photoactive nano-metal organic framework as a promising biofilm treatment. Acta Biomater. 97, 490–500. doi:10.1016/j.actbio.2019.08.011
Attia, G. H., Moemen, Y. S., Youns, M., Ibrahim, A. M., Abdou, R., El Raey, M. A., et al. (2021). Antiviral zinc oxide nanoparticles mediated by hesperidin and in silico comparison study between antiviral phenolics as anti-sars-cov-2. Colloids Surfaces B Biointerfaces 203, 111724. doi:10.1016/j.colsurfb.2021.111724
Bai, X., Gao, Y., Zhang, M. Y., Chang, Y. N., Chen, K., Li, J., et al. (2020). Carboxylated gold nanoparticles inhibit bone erosion by disturbing the acidification of an osteoclast absorption microenvironment. Nanoscale 12 (6), 3871–3878. doi:10.1039/c9nr09698a
Bandini, M., Gandaglia, G., Fossati, N., Montorsi, F., and Briganti, A. (2017). An explanatory case on the limitations of lymph node staging in recurrent prostate cancer. Urol. Case Rep. 12, 34–36. doi:10.1016/j.eucr.2017.02.011
Barenholz, Y. (2012). Doxil ®-- the first FDA-approved nano-drug: Lessons learned. J. Control. Release 160 (2), 117–134. doi:10.1016/j.jconrel.2012.03.020
Bessar, H., Venditti, I., Benassi, L., Vaschieri, C., Azzoni, P., Pellacani, G., et al. (2016). Functionalized gold nanoparticles for topical delivery of methotrexate for the possible treatment of psoriasis. Colloids Surfaces B Biointerfaces 141, 141–147. doi:10.1016/j.colsurfb.2016.01.021
Betzer, O., Shilo, M., Motiei, M., and Popovtzer, R. (2019). “Insulin-coated gold nanoparticles as an effective approach for bypassing the blood-brain barrier,” Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XVI. San Francisco, CA, 3–4 February 2019.
Binaeian, E., Motaghedi, N., Maleki, S., and Arjmandi, M. (2022). Ibuprofen uptake through dimethyl ethylenediamine modified mof: Optimization of the adsorption process by response surface methodology technique. J. Dispers. Sci. Technol. 43 (1), 1–14. doi:10.1080/01932691.2020.1810696
Boroushaki, T., Dekamin, M. G., Hashemianzadeh, S. M., Naimi-Jamal, M. R., and Koli, M. G. (2022). A molecular dynamic simulation study of anticancer agents and uio-66 as a carrier in drug delivery systems. J. Mol. Graph. Model. 113, 108147. doi:10.1016/j.jmgm.2022.108147
Cao, D., Xu, Z., Chen, Y., Ke, Q., Zhang, C., Guo, Y., et al. (2018). Ag-loaded mgsrfe-layered double hydroxide/chitosan composite scaffold with enhanced osteogenic and antibacterial property for bone engineering tissue. J. Biomed. Mat. Res. 106 (2), 863–873. doi:10.1002/jbm.b.33900
Cao, J., Li, X. J., Wang, X. X., Li, K., Liu, Y. H., Tian, H. Q., et al. (2020). Surface pegylation of mil-101(Fe) nanoparticles for Co-delivery of radioprotective agents. Chem. Eng. J. 384, 123363. doi:10.1016/j.cej.2019.123363
Carbone, E. J., Rajpura, K., Allen, B. N., Cheng, E., Ulery, B. D., Lo, K. W. H., et al. (2017). Osteotropic nanoscale drug delivery systems based on small molecule bone-targeting moieties. Nanomedicine Nanotechnol. Biol. Med. 13 (1), 37–47. doi:10.1016/j.nano.2016.08.015
Carneiro, M. F. H., Machado, A. R. T., Antunes, L. M. G., Souza, T. E., Freitas, V. A., Oliveira, L. C. A., et al. (2020). Gold-coated superparamagnetic iron oxide nanoparticles attenuate collagen-induced arthritis after magnetic targeting. Biol. Trace Elem. Res. 194 (2), 502–513. doi:10.1007/s12011-019-01799-z
Carofiglio, M., Barui, S., Cauda, V., and Laurenti, M. (2020). Doped zinc oxide nanoparticles: Synthesis, characterization and potential use in nanomedicine. Appl. Sci. (Basel). 10 (15), 5194. doi:10.3390/app10155194
Cavka, J. H., Jakobsen, S., Olsbye, U., Guillou, N., Lamberti, C., Bordiga, S., et al. (2008). A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130 (42), 13850–13851. doi:10.1021/ja8057953
Chandrakala, V., Aruna, V., and Angajala, G. (2022). Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. emergent Mat. 4, 1–23. doi:10.1007/s42247-021-00335-x
Chen, H. F., Fu, Y., Feng, K., Zhou, Y. F., Wang, X., Huang, H. H., et al. (2021). Polydopamine-coated uio-66 nanoparticles loaded with perfluorotributylamine/tirapazamine for hypoxia-activated osteosarcoma therapy. J. Nanobiotechnol. 19 (1), 298. doi:10.1186/s12951-021-01013-0
Chen, J., Zhang, X., Huang, C., Cai, H., Hu, S., Wan, Q., et al. (2017). Osteogenic activity and antibacterial effect of porous titanium modified with metal-organic framework films. J. Biomed. Mat. Res. A 105 (3), 834–846. doi:10.1002/jbm.a.35960
Chen, Y., Wan, Y., Wang, Y., Zhang, H., and Jiao, Z. (2011). Anticancer efficacy enhancement and attenuation of side effects of doxorubicin with titanium dioxide nanoparticles. Int. J. Nanomedicine 6, 2321–2326. doi:10.2147/ijn.S25460
Chen, Z., Yu, C., Xi, J., Tang, S., Bao, T., Zhang, J., et al. (2019). A hybrid material prepared by controlled growth of a covalent organic framework on amino-modified mil-68 for pipette tip solid-phase extraction of sulfonamides prior to their determination by hplc. Microchim. Acta 186 (6), 393. doi:10.1007/s00604-019-3513-7
Chen, Z., Yu, H., Lu, W., Shen, J., Wang, Y., Wang, Y., et al. (2020). Bone-seeking albumin-nanomedicine for in vivo imaging and therapeutic monitoring. ACS Biomater. Sci. Eng. 6 (1), 647–653. doi:10.1021/acsbiomaterials.9b01195
Cheng, X., Pei, X., Xie, W., Chen, J., Li, Y., Wang, J., et al. (2022). pH-triggered size-tunable silver nanoparticles: Targeted aggregation for effective bacterial infection therapy. Small 18 (22), e2200915. doi:10.1002/smll.202200915
Cheng, X. T., Zhu, Z., Liu, Y. H., Xue, Y. Y., Gao, X. M., Wang, J., et al. (2020). Zeolitic imidazolate framework-8 encapsulating risedronate synergistically enhances osteogenic and antiresorptive properties for bone regeneration. ACS Biomater. Sci. Eng. 6 (4), 2186–2197. doi:10.1021/acsbiomaterials.0c00195
Cheng, Y., Wen, C., Sun, Y. Q., Yu, H., and Yin, X. B. (2021). Mixed-metal mof-derived hollow porous nanocomposite for trimodality imaging guided reactive oxygen species-augmented synergistic therapy. Adv. Funct. Mat. 31 (37), 2104378. doi:10.1002/adfm.202104378
Chindamo, G., Sapino, S., Peira, E., Chirio, D., Gonzalez, M. C., Gallarate, M., et al. (2020). Bone diseases: Current approach and future perspectives in drug delivery systems for bone targeted therapeutics. Nanomaterials 10 (5), 875. doi:10.3390/nano10050875
Chowdhury, R., Ilyas, H., Ghosh, A., Ali, H., Ghorai, A., Midya, A., et al. (2017). Multivalent gold nanoparticle-peptide conjugates for targeting intracellular bacterial infections. Nanoscale 9 (37), 14074–14093. doi:10.1039/c7nr04062h
Chuang, C.-C., Chen, Y.-N., Wang, Y.-Y., Huang, Y.-C., Lin, S.-Y., Huang, R.-Y., et al. (2020). Stem cell-based delivery of gold/chlorin E6 nanocomplexes for combined photothermal and photodynamic therapy. ACS Appl. Mat. Interfaces 12 (27), 30021–30030. doi:10.1021/acsami.0c03446
Dan-Hardi, M., Serre, C., Frot, T., Rozes, L., Maurin, G., Sanchez, C., et al. (2009). A new photoactive crystalline highly porous titanium(iv) dicarboxylate. J. Am. Chem. Soc. 131 (31), 10857–10859. doi:10.1021/ja903726m
Dang, W., Ma, B., Li, B., Huan, Z., Ma, N., Zhu, H., et al. (2020). 3d printing of metal-organic framework nanosheets-structured scaffolds with tumor therapy and bone construction. Biofabrication 12 (2), 025005. doi:10.1088/1758-5090/ab5ae3
Dehghani, S., Hosseini, M., Haghgoo, S., Changizi, V., Javar, H. A., Khoobi, M., et al. (2020). Multifunctional mil-Cur@Fc as a theranostic agent for magnetic resonance imaging and targeting drug delivery: In vitro and in vivo study. J. Drug Target. 28 (6), 668–680. doi:10.1080/1061186x.2019.1710839
Di Giampaolo, L., Zaccariello, G., Benedetti, A., Vecchiotti, G., Caposano, F., Sabbioni, E., et al. (2021). Genotoxicity and immunotoxicity of titanium dioxide-embedded mesoporous silica nanoparticles (TiO2@Msn) in primary peripheral human blood mononuclear cells (pbmc). Nanomaterials 11 (2), 270. doi:10.3390/nano11020270
Duan, F., Wang, J., Li, Z., Zhang, T., Li, Z., Zhou, X., et al. (2021). pH-responsive metal-organic framework-coated mesoporous silica nanoparticles for immunotherapy. ACS Appl. Nano Mat. 4 (12), 13398–13404. doi:10.1021/acsanm.1c02908
Duan, Y., Ye, F., Huang, Y., Qin, Y., He, C., Zhao, S., et al. (2018). One-pot synthesis of a metal-organic framework-based drug carrier for intelligent glucose-responsive insulin delivery. Chem. Commun. 54 (42), 5377–5380. doi:10.1039/c8cc02708k
Eivazzadeh-Keihan, R., Bahojb Noruzi, E., Khanmohammadi Chenab, K., Jafari, A., Radinekiyan, F., Hashemi, S. M., et al. (2020a). Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 14 (12), 1687–1714. doi:10.1002/term.3131
Eivazzadeh-Keihan, R., Noruzi, E. B., Chenab, K. K., Jafari, A., Radinekiyan, F., Hashemi, S. M., et al. (2020b). Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 14 (12), 1687–1714. doi:10.1002/term.3131
Escobar, A., Muzzio, N., Coy, E., Liu, H., Bindini, E., Andreozzi, P., et al. (2019). Antibacterial mesoporous titania films with embedded gentamicin and surface modified with bone morphogenetic protein 2 to promote osseointegration in bone implants. Adv. Mat. Interfaces 6 (9), 1801648. doi:10.1002/admi.201801648
Faaizatunnisa, N., Lestari, W. W., Saputra, O. A., Saraswati, T. E., Larasati, L., Wibowo, F. R., et al. (2022). Slow-release of curcumin induced by core-shell mesoporous silica nanoparticles (msns) modified mil-100(Fe) composite. J. Inorg. Organomet. Polym. Mat. 32 (5), 1744–1754. doi:10.1007/s10904-022-02230-2
Fang, C., Deng, Z., Cao, G. D., Chu, Q., Wu, Y. L., Li, X., et al. (2020). Co-ferrocene mof/glucose oxidase as cascade nanozyme for effective tumor therapy. Adv. Funct. Mat. 30 (16), 1910085. doi:10.1002/adfm.201910085
Fang, C., Liu, L., Weng, J., Zhang, S., Zhang, X., Ren, Z., et al. (2021). Modifiers versus channels: Creating shape-selective catalysis of metal nanoparticles/porous nanomaterials. Angew. Chem. Int. Ed. 60 (2), 976–982. doi:10.1002/anie.202011866
Fang, X., Zong, B., and Mao, S. (2018). Metal-organic framework-based sensors for environmental contaminant sensing. Nanomicro. Lett. 10 (4), 64. doi:10.1007/s40820-018-0218-0
Fardjahromi, M. A., Nazari, H., Tafti, S. M. A., Razmjou, A., Mukhopadhyay, S., Warkiani, M. E., et al. (2022). Metal-organic framework-based nanomaterials for bone tissue engineering and wound healing. Mat. Today Chem. 23, 100670. doi:10.1016/j.mtchem.2021.100670
Feng, H., Li, Z., Xie, W., Wan, Q., Guo, Y., Chen, J., et al. (2022). Delivery of therapeutic mirnas using nanoscale zeolitic imidazolate framework for accelerating vascularized bone regeneration. Chem. Eng. J. 430, 132867. doi:10.1016/j.cej.2021.132867
Feng, J., Xu, Z., Dong, P., Yu, W. Q., Liu, F., Jiang, Q. Y., et al. (2019). Stimuli-responsive multifunctional metal-organic framework nanoparticles for enhanced chemo-photothermal therapy. J. Mat. Chem. B 7 (6), 994–1004. doi:10.1039/c8tb02815j
Fernandes, C. S., Nordin, N., Bilad, M. R., Matsuura, T., Putra, Z. A., Wirzal, M. D. H., et al. (2021). Explication of hydrophobic silica as effective pore former for membrane fabrication. Appl. Surf. Sci. Adv. 3, 100051. doi:10.1016/j.apsadv.2020.100051
Fiorani, A., Merino, J. P., Zanut, A., Criado, A., Valenti, G., Prato, M., et al. (2019). Advanced carbon nanomaterials for electrochemiluminescent biosensor applications. Curr. Opin. Electrochem. 16, 66–74. doi:10.1016/j.coelec.2019.04.018
Fu, J. N., Li, Y., Zhang, Y., Liang, Y. Q., Zheng, Y. F., Li, Z. Y., et al. (2021). An engineered pseudo-macrophage for rapid treatment of bacteria-infected osteomyelitis via microwave-excited anti-infection and immunoregulation. Adv. Mat. 33 (41), e2102926. doi:10.1002/adma.202102926
Fytory, M., Arafa, K. K., El Rouby, W. M. A., Farghali, A. A., Abdel-Hafiez, M., El-Sherbiny, I. M., et al. (2021). Dual-ligated metal organic framework as novel multifunctional nanovehicle for targeted drug delivery for hepatic cancer treatment. Sci. Rep. 11 (1), 19808. doi:10.1038/s41598-021-99407-5
Gao, X., Xue, Y., Zhu, Z., Chen, J., Liu, Y., Cheng, X., et al. (2021). Nanoscale zeolitic imidazolate framework-8 activator of canonical mapk signaling for bone repair. ACS Appl. Mat. Interfaces 13 (1), 97–111. doi:10.1021/acsami.0c15945
Geng, B. J., Yang, X., Li, P., Shi, W. Y., Pan, D. Y., Shen, L. X., et al. (2021). W-doped TiO2 nanorods for multimode tumor eradication in osteosarcoma models under single ultrasound irradiation. ACS Appl. Mat. Interfaces 13 (38), 45325–45334. doi:10.1021/acsami.1c14701
Geng, P., Yu, N., Macharia, D. K., Meng, R., Qiu, P., Tao, C., et al. (2022). Mof-derived cus@Cu-mof nanocomposites for synergistic photothermal-chemodynamic-chemo therapy. Chem. Eng. J. 441, 135964. doi:10.1016/j.cej.2022.135964
Gharehdaghi, Z., Rahimi, R., Naghib, S. M., and Molaabasi, F. (2021). Cu (Ii)-Porphyrin metal-organic framework/graphene oxide: Synthesis, characterization, and application as a pH-responsive drug carrier for breast cancer treatment. J. Biol. Inorg. Chem. 26 (6), 689–704. doi:10.1007/s00775-021-01887-3
Gilevskaya, K. S., Ignatovich, Z. V., Golubeva, M. B., Koroleva, E. V., and Agabekov, V. E. (2018). Preparation and properties of protamine/pectin-Ag biopolymer microcapsules containing a 2-arylaminopyrimidine derivative. Pharm. Chem. J. 51 (10), 922–927. doi:10.1007/s11094-018-1717-5
Godoy-Gallardo, M., Eckhard, U., Delgado, L. M., Puente, Y., Hoyos-Nogues, M., Gil, F. J., et al. (2021). Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mat. 6 (12), 4470–4490. doi:10.1016/j.bioactmat.2021.04.033
Golmohamadpour, A., Bahramian, B., Shafiee, A., and Ma'mani, L. (2018). Slow released delivery of alendronate using beta-cyclodextrine modified Fe-mof encapsulated porous hydroxyapatite. J. Inorg. Organomet. Polym. Mat. 28 (5), 1991–2000. doi:10.1007/s10904-018-0871-2
Gordon, J., Kazemian, H., and Rohani, S. (2015). Mil-53 (Fe), mil-101, and sba-15 porous materials: Potential platforms for drug delivery. Mater. Sci. Eng. C 47, 172–179. doi:10.1016/j.msec.2014.11.046
Gupta, P. K., Gahtori, R., Govarthanan, K., Sharma, V., Pappuru, S., Pandit, S., et al. (2021). Recent trends in biodegradable polyester nanomaterials for cancer therapy. Mater. Sci. Eng. C 127, 112198. doi:10.1016/j.msec.2021.112198
Ha, L., Ryu, U., Kang, D.-C., Kim, J.-K., Sun, D., Kwon, Y.-E., et al. (2021). Rapid single-step growth of mof exoskeleton on mammalian cells for enhanced cytoprotection. ACS Biomater. Sci. Eng. 7 (7), 3075–3081. doi:10.1021/acsbiomaterials.1c00539
Hagaman, D. E., Damasco, J. A., Perez, J. V. D., Rojo, R. D., and Melancon, M. P. (2021). Recent advances in nanomedicine for the diagnosis and treatment of prostate cancer bone metastasis. Molecules 26 (2), 384. doi:10.3390/molecules26020384
Halim, N. a. A., Hussein, M. Z., and Kandar, M. K. (2021). Nanomaterials-upconverted hydroxyapatite for bone tissue engineering and a platform for drug delivery. Int. J. Nanomedicine 16, 6477–6496. doi:10.2147/ijn.S298936
Han, T. X., Xue, P., Ju, S. Y., Zhang, Z. T., Zeng, J. Y., Zhang, Y. M., et al. (2021). Rna-seq reveals correlations between cytoskeleton-related genes and the osteogenic activity of mesenchymal stem cells on strontium loaded titania nanotube Arrays. Mater. Sci. Eng. C 122, 111939. doi:10.1016/j.msec.2021.111939
Hao, Y.-N., Qu, C.-C., Shu, Y., Wang, J.-H., and Chen, W. (2021a). Construction of novel nanocomposites (Cu-Mof/God@Ha) for chemodynamic therapy. Nanomaterials 11 (7), 1843. doi:10.3390/nano11071843
Hao, Y.-N., Zhang, W.-X., Gao, Y.-R., Wei, Y.-N., Shu, Y., Wang, J.-H., et al. (2021b). State-of-the-Art advances of copper-based nanostructures in the enhancement of chemodynamic therapy. J. Mat. Chem. B 9 (2), 250–266. doi:10.1039/d0tb02360d
Hauser, M., and Nowack, B. (2021). Probabilistic modelling of nanobiomaterial release from medical applications into the environment. Environ. Int. 146, 106184. doi:10.1016/j.envint.2020.106184
He, S., Wu, L., Li, X., Sun, H., Xiong, T., Liu, J., et al. (2021). Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 11 (8), 2362–2395. doi:10.1016/j.apsb.2021.03.019
He, X., Dai, L., Ye, L., Sun, X., Enoch, O., Hu, R., et al. (2022). A vehicle-free antimicrobial polymer hybrid gold nanoparticle as synergistically therapeutic platforms for Staphylococcus aureus infected wound healing. Adv. Sci. (Weinh). 9 (14), e2105223. doi:10.1002/advs.202105223
Hemmati, F., Salehi, R., Ghotaslou, R., Kafil, H. S., Hasani, A., Gholizadeh, P., et al. (2020). The assessment of antibiofilm activity of chitosan-zinc oxide-gentamicin nanocomposite on Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Biol. Macromol. 163, 2248–2258. doi:10.1016/j.ijbiomac.2020.09.037
Hou, J., He, C., He, W., Yang, M., Luo, X., Li, C., et al. (2020). Obesity and bone health: A complex link. Front. Cell Dev. Biol. 8, 600181. doi:10.3389/fcell.2020.600181
Hu, J. Q., Chen, Y., Zhang, H., and Chen, Z. X. (2021a). Controlled syntheses of Mg-Mof-74 nanorods for drug delivery. J. Solid State Chem. 294, 121853. doi:10.1016/j.jssc.2020.121853
Hu, L. F., Xiong, C. X., Wei, G. H., Yu, Y. H., Li, S. H., Xiong, X. X., et al. (2022). Stimuli-responsive charge-reversal Mof@Polymer hybrid nanocomposites for enhanced Co-delivery of chemotherapeutics towards combination therapy of multidrug-resistant cancer. J. Colloid Interface Sci. 608, 1882–1893. doi:10.1016/j.jcis.2021.10.070
Hu, S., Pei, X., Duan, L., Zhu, Z., Liu, Y., Chen, J., et al. (2021b). A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat. Commun. 12 (1), 1689. doi:10.1038/s41467-021-21989-5
Huang, R. Q., Zhang, C. Y., Bu, Y. Y., Li, Z., Zheng, X., Qiu, S., et al. (2021). A multifunctional nano-therapeutic platform based on octahedral yolk-shell Au Nr@Cus: Photothermal/photodynamic and targeted drug delivery tri-combined therapy for rheumatoid arthritis. Biomaterials 277, 121088. doi:10.1016/j.biomaterials.2021.121088
Huang, Y. J., Xiao, Z. H., Guan, Z. L., Zeng, Z. S., Shen, Y. F., Xu, X. Y., et al. (2020). Bone-seeking nanoplatform Co-delivering cisplatin and zoledronate for synergistic therapy of breast cancer bone metastasis and bone resorption. Acta Pharm. Sin. B 10 (12), 2384–2403. doi:10.1016/j.apsb.2020.06.006
Hussein, J., Attia, M. F., El Bana, M., El-Daly, S. M., Mohamed, N., El-Khayat, Z., et al. (2019). Solid state synthesis of docosahexaenoic acid-loaded zinc oxide nanoparticles as a potential antidiabetic agent in rats. Int. J. Biol. Macromol. 140, 1305–1314. doi:10.1016/j.ijbiomac.2019.08.201
Javanbakht, S., Nezhad-Mokhtari, P., Shaabani, A., Arsalani, N., and Ghorbani, M. (2019). Incorporating Cu-based metal-organic framework/drug nanohybrids into gelatin microsphere for ibuprofen oral delivery. Mater. Sci. Eng. C 96, 302–309. doi:10.1016/j.msec.2018.11.028
Jiang, H. L., Liu, B., Akita, T., Haruta, M., Sakurai, H., Xu, Q., et al. (2009). Au@Zif-8: Co oxidation over gold nanoparticles deposited to metal-organic framework. J. Am. Chem. Soc. 131 (32), 11302–11303. doi:10.1021/ja9047653
Jiang, Y., Pan, X., Yao, M., Han, L., Zhang, X., Jia, Z., et al. (2021). Bioinspired adhesive and tumor microenvironment responsive nanomofs assembled 3d-printed scaffold for anti-tumor therapy and bone regeneration. Nano Today 39, 101182. doi:10.1016/j.nantod.2021.101182
Jin, Z. K., Zhao, P. H., Zhang, J. H., Yang, T., Zhou, G. X., Zhang, D. H., et al. (2018). Intelligent metal carbonyl metal-organic framework nanocomplex for fluorescent traceable H2o2-triggered Co delivery. Chem. Eur. J. 24 (45), 11667–11674. doi:10.1002/chem.201801407
Kainz, Q. M., and Reiser, O. (2014). Polymer- and dendrimer-coated magnetic nanoparticles as versatile supports for catalysts, scavengers, and reagents. Acc. Chem. Res. 47 (2), 667–677. doi:10.1021/ar400236y
Kalva, S. K., Sanchez-Iglesias, A., Dean-Ben, X. L., Liz-Marzan, L. M., and Razansky, D. (2022). Rapid volumetric optoacoustic tracking of nanoparticle kinetics across murine organs. ACS Appl. Mat. Interfaces 14 (1), 172–178. doi:10.1021/acsami.1c17661
Khalil, M., Kadja, G. T. M., and Ilmi, M. M. (2021). Advanced nanomaterials for catalysis: Current progress in fine chemical synthesis, hydrocarbon processing, and renewable energy. J. Ind. Eng. Chem. 93, 78–100. doi:10.1016/j.jiec.2020.09.028
Khan, M. U. A., Al-Thebaiti, M. A., Hashmi, M. U., Aftab, S., Abd Razak, S. I., Abu Hassan, S., et al. (2020). Synthesis of silver-coated bioactive nanocomposite scaffolds based on grafted beta-glucan/hydroxyapatite via freeze-drying method: Anti-microbial and biocompatibility evaluation for bone tissue engineering. Materials 13 (4), 971. doi:10.3390/ma13040971
Khodaei, A., Jahanmard, F., Hosseini, H. R. M., Bagheri, R., Dabbagh, A., Weinans, H., et al. (2022). Controlled temperature-mediated curcumin release from magneto-thermal nanocarriers to kill bone tumors. Bioact. Mat. 11, 107–117. doi:10.1016/j.bioactmat.2021.09.028
Kodoth, A. K., Ghate, V. M., Lewis, S. A., Prakash, B., and Badalamoole, V. (2019). Pectin-based silver nanocomposite film for transdermal delivery of donepezil. Int. J. Biol. Macromol. 134, 269–279. doi:10.1016/j.ijbiomac.2019.04.191
Kompel, A. J., Roemer, F. W., Murakami, A. M., Diaz, L. E., Crema, M. D., Guermazi, A., et al. (2019). Intra-articular coricosteroid injections in the nip and knee: Perhaps not as safe as we thought? Radiology 293 (3), 656–663. doi:10.1148/radiol.2019190341
Krizkova, S., Kepinska, M., Emri, G., Rodrigo, M. a. M., Tmejova, K., Nerudova, D., et al. (2016). Microarray analysis of metallothioneins in human diseases-a review. J. Pharm. Biomed. Anal. 117, 464–473. doi:10.1016/j.jpba.2015.09.031
Krumdieck, S. P., Boichot, R., Gorthy, R., Land, J. G., Lay, S., Gardecka, A. J., et al. (2019). Nanostructured TiO2 anatase-rutile-carbon solid coating with visible light antimicrobial activity. Sci. Rep. 9, 1883. doi:10.1038/s41598-018-38291-y
Kumar, D., and Pal, S. (2019). Optical band gap and crystallite size investigations of anticancer drug loaded zno nanoparticles. AIP Conf. Proc. 2142, 180004. doi:10.1063/1.5122627
Kumar, T. S., and Madhumathi, K. (2016). Antibiotic delivery by nanobioceramics. Ther. Deliv. 7 (8), 573–588. doi:10.4155/tde-2016-0025
Kundu, M., Sadhukhan, P., Ghosh, N., Chatterjee, S., Manna, P., Das, J., et al. (2019). pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized zno nanoparticles for breast cancer therapy. J. Adv. Res. 18, 161–172. doi:10.1016/j.jare.2019.02.036
Lawson, H. D., Walton, S. P., and Chan, C. (2021). Metal-organic frameworks for drug delivery: A design perspective. ACS Appl. Mat. Interfaces 13 (6), 7004–7020. doi:10.1021/acsami.1c01089
Lawson, S., Rownaghi, A. A., and Rezaei, F. (2022). Combined ibuprofen and curcumin delivery using Mg-Mof-74 as a single nanocarrier. ACS Appl. Bio Mat. 5 (1), 265–271. doi:10.1021/acsabm.1c01067
Lazaro, I. A., Haddad, S., Sacca, S., Orellana-Tavra, C., Fairen-Jimenez, D., Forgan, R. S., et al. (2017). Selective surface pegylation of uio-66 nanoparticles for enhanced stability, cell uptake, and pH-responsive drug delivery. Chem 2 (4), 561–578. doi:10.1016/j.chempr.2017.02.005
Lee, D., Heo, D. N., Nah, H. R., Lee, S. J., Ko, W.-K., Lee, J. S., et al. (2018). Injectable hydrogel composite containing modified gold nanoparticles: Implication in bone tissue regeneration. Int. J. Nanomedicine 13, 7019–7031. doi:10.2147/ijn.S185715
Lee, D. N., Gwon, K., Kim, Y., Cho, H., and Lee, S. (2021). Immobilization of antibacterial copper metal-organic framework containing glutarate and 1, 2-bis(4-Pyridyl)Ethylene ligands on polydimethylsiloxane and its low cytotoxicity. J. Ind. Eng. Chem. 102, 135–145. doi:10.1016/j.jiec.2021.07.002
Levine, D. J., Runcevski, T., Kapelewski, M. T., Keitz, B. K., Oktawiec, J., Reed, D. A., et al. (2016). Olsalazine-based metal–organic frameworks as biocompatible platforms for H2 adsorption and drug delivery. J. Am. Chem. Soc. 138 (32), 10143–10150. doi:10.1021/jacs.6b03523
Li, D., Li, L. F., Zhang, Z. F., Xue, D., Pan, L., Liu, Y., et al. (2020a). Ultrasound-assisted synthesis of a new nanostructured Ca(Ii)-Mof as 5-fu delivery system to inhibit human lung cancer cell proliferation, migration, invasion and induce cell apoptosis. J. Coord. Chem. 73 (2), 266–281. doi:10.1080/00958972.2020.1727895
Li, J., Chen, L., Xu, X. Y., Fan, Y., Xue, X., Shen, M. W., et al. (2020b). Targeted combination of antioxidative and anti-inflammatory therapy of rheumatoid arthritis using multifunctional dendrimer-entrapped gold nanoparticles as a platform. Small 16 (49), 2005661. doi:10.1002/smll.202005661
Li, J., Du, N., Tan, Y., Hsu, H.-Y., Tan, C., Jiang, Y., et al. (2021). Conjugated polymer nanoparticles based on copper coordination for real-time monitoring of pH-responsive drug delivery. ACS Appl. Bio Mat. 4 (3), 2583–2590. doi:10.1021/acsabm.0c01564
Li, J. K., Lv, F., Li, J. X., Li, Y. X., Gao, J. D., Luo, J., et al. (2020d). Cobalt-based metal-organic framework as a dual cooperative controllable release system for accelerating diabetic wound healing. Nano Res. 13 (8), 2268–2279. doi:10.1007/s12274-020-2846-1
Li, J., Mutreja, I., Hooper, G. J., Clinch, K., Lim, K., Evans, G., et al. (2020c). Combined infection control and enhanced osteogenic differentiation capacity on additive manufactured Ti-6al-4v are mediated via titania nanotube delivery of novel biofilm inhibitors. Adv. Mat. Interfaces 7 (7), 1901963. doi:10.1002/admi.201901963
Li, L., Qi, Z., Han, S., Li, X., Liu, B., Liu, Y., et al. (2022). Advances and applications of metal-organic framework nanomaterials as oral delivery carriers: A review. Mini Rev. Med. Chem. 22. doi:10.2174/1389557522666220330152145
Li, M., Li, Y., Huang, X., and Lu, X. (2015). Captopril-polyethyleneimine conjugate modified gold nanoparticles for Co-delivery of drug and gene in anti-angiogenesis breast cancer therapy. J. Biomaterials Sci. Polym. Ed. 26 (13), 813–827. doi:10.1080/09205063.2015.1057991
Li, S., Dharmarwardana, M., Welch, R. P., Benjamin, C. E., Shamir, A. M., Nielsen, S. O., et al. (2018). Investigation of controlled growth of metal-organic frameworks on anisotropic virus particles. ACS Appl. Mat. Interfaces 10 (21), 18161–18169. doi:10.1021/acsami.8b01369
Li, S., Tan, L., and Meng, X. (2020e). Nanoscale metal-organic frameworks: Synthesis, biocompatibility, imaging applications, and thermal and dynamic therapy of tumors. Adv. Funct. Mat. 30 (13), 1908924. doi:10.1002/adfm.201908924
Li, Y., Bolinger, J., Yu, Y., Glass, Z., Shi, N., Yang, L., et al. (2019a). Intracellular delivery and biodistribution study of crispr/cas9 ribonucleoprotein loaded bioreducible lipidoid nanoparticles. Biomater. Sci. 7 (2), 596–606. doi:10.1039/c8bm00637g
Li, Y., Lin, Z., Zhao, M., Guo, M., Xu, T., Wang, C., et al. (2016). Reversal of H1n1 influenza virus-induced apoptosis by silver nanoparticles functionalized with amantadine. RSC Adv. 6 (92), 89679–89686. doi:10.1039/c6ra18493f
Li, Y., Zhang, K., Liu, P., Chen, M., Zhong, Y., Ye, Q., et al. (2019b). Encapsulation of plasmid DNA by nanoscale metal-organic frameworks for efficient gene transportation and expression. Adv. Mat. 31 (29), 1901570. doi:10.1002/adma.201901570
Li, Z., Guo, R., Gu, Z., Wang, X., Wang, Y., Xu, H., et al. (2019c). Identification of a promoter element mediating kisspeptin-induced increases in gnrh gene expression in sheep. Gene 699, 1–7. doi:10.1016/j.gene.2019.03.006
Liang, W., Dong, Y., Shao, R., Zhang, S., Wu, X., Huang, X., et al. (2022). Application of nanoparticles in drug delivery for the treatment of osteosarcoma: Focussing on the liposomes. J. Drug Target. 30 (5), 463–475. doi:10.1080/1061186x.2021.2023160
Lin, C., Du, Y., Wang, S., Wang, L., and Song, Y. (2021). Glucose oxidase@Cu-hemin metal-organic framework for colorimetric analysis of glucose. Mater. Sci. Eng. C 118, 111511. doi:10.1016/j.msec.2020.111511
Lin, K.-S., Adhikari, A. K., Ku, C.-N., Chiang, C.-L., and Kuo, H. (2012). Synthesis and characterization of porous hkust-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 37 (18), 13865–13871. doi:10.1016/j.ijhydene.2012.04.105
Lin, Z., Li, Y., Guo, M., Xu, T., Wang, C., Zhao, M., et al. (2017). The inhibition of H1n1 influenza virus-induced apoptosis by silver nanoparticles functionalized with zanamivir. RSC Adv. 7 (2), 742–750. doi:10.1039/c6ra25010f
Litwic, A., Edwards, M. H., Dennison, E. M., and Cooper, C. (2013). Epidemiology and burden of osteoarthritis. Br. Med. Bull. 105 (1), 185–199. doi:10.1093/bmb/lds038
Liu, G., Yang, L., Chen, G., Xu, F., Yang, F., Yu, H., et al. (2021a). A review on drug delivery system for tumor therapy. Front. Pharmacol. 12, 735446. doi:10.3389/fphar.2021.735446
Liu, J. Y., Rafiq, N. B. M., Wong, L. M., and Wang, S. J. (2021b). Surface treatment and bioinspired coating for 3d-printed implants. Front. Chem. 9, 768007. doi:10.3389/fchem.2021.768007
Liu, L., Pu, X. M., Yin, G. F., Chen, X. C., Yin, J., Wu, Y. H., et al. (2019a). Biomimetic mineralization of magnetic iron oxide nanoparticles mediated by Bi-functional copolypeptides. Molecules 24 (7), 1401. doi:10.3390/molecules24071401
Liu, W. C., Shen, X., Han, Y. Y., Liu, Z. H., Dai, W., Dutta, A., et al. (2019). Selective adsorption and removal of drug contaminants by using an extremely stable Cu(Ii)-Based 3d metal-organic framework. Chemosphere 215, 524–531. doi:10.1016/j.chemosphere.2018.10.075
Liu, W., Zhong, Y., Wang, X., Zhuang, C., Chen, J., Liu, D., et al. (2020a). A porous Cu(Ii)-Based metal-organic framework carrier for pH-controlled anticancer drug delivery. Inorg. Chem. Commun. 111, 107675. doi:10.1016/j.inoche.2019.107675
Liu, Y., Naumenko, E., Akhatova, F., Zou, Q., Fakhrullin, R., Yan, X., et al. (2021c). Self-assembled peptide nanoparticles for enhanced dark-field hyperspectral imaging at the cellular and invertebrate level. Chem. Eng. J. 424, 130348. doi:10.1016/j.cej.2021.130348
Liu, Y., Perumalsamy, H., Kang, C. H., Kim, S. H., Hwang, J. S., Koh, S. C., et al. (2020b). Intracellular synthesis of gold nanoparticles by gluconacetobacter liquefaciens for delivery of peptide CopA3 and ginsenoside and anti-inflammatory effect on lipopolysaccharide-activated macrophages. Artif. Cells Nanomed. Biotechnol. 48 (1), 777–788. doi:10.1080/21691401.2020.1748639
Liu, Y., Yang, G. Z., Jin, S., Xu, L. T., and Zhao, C. X. (2020c). Development of high-drug-loading nanoparticles. Chempluschem 85 (9), 2143–2157. doi:10.1002/cplu.202000496
Liu, Y., Zhu, Z., Pei, X., Zhang, X., Cheng, X., Hu, S., et al. (2020d). Zif-8-Modified multifunctional bone-adhesive hydrogels promoting angiogenesis and osteogenesis for bone regeneration. ACS Appl. Mat. Interfaces 12 (33), 36978–36995. doi:10.1021/acsami.0c12090
Luo, J. C., Zhang, Y., Zhu, S. B., Tong, Y., Ji, L. C., Zhang, W., et al. (2021). The application prospect of metal/metal oxide nanoparticles in the treatment of osteoarthritis. Schmiedeb. Arch. Pharmacol. 394 (10), 1991–2002. doi:10.1007/s00210-021-02131-0
Ma, P. H., Zhang, J. L., Liu, P., Wang, Q., Zhang, Y., Song, K. S., et al. (2020). Computer-assisted design for stable and porous metal-organic framework (mof) as a carrier for curcumin delivery. Lwt-Food. Sci. Technol. 120, 108949. doi:10.1016/j.lwt.2019.108949
Mackenzie, I. S., and Macdonald, T. M. (2010). Treatment of osteoarthritis in hypertensive patients. Expert Opin. Pharmacother. 11 (3), 393–403. doi:10.1517/14656560903496422
Mahana, A., Guliy, O. I., and Mehta, S. K. (2021). Accumulation and cellular toxicity of engineered metallic nanoparticle in freshwater microalgae: Current status and future challenges. Ecotoxicol. Environ. Saf. 208, 111662. doi:10.1016/j.ecoenv.2020.111662
Makvandi, P., Wang, C. Y., Zare, E. N., Borzacchiello, A., Niu, L. N., Tay, F. R., et al. (2020). Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mat. 30 (22), 1910021. doi:10.1002/adfm.201910021
Mallakpour, S., Azadi, E., and Hussain, C. M. (2021). Recent advancements in synthesis and drug delivery utilization of polysaccharides-based nanocomposites: The important role of nanoparticles and layered double hydroxides. Int. J. Biol. Macromol. 193, 183–204. doi:10.1016/j.ijbiomac.2021.10.123
Marsich, E., Bellomo, F., Turco, G., Travan, A., Donati, I., Paoletti, S., et al. (2013). Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: Preparation, characterization and biological properties. J. Mat. Sci. Mat. Med. 24 (7), 1799–1807. doi:10.1007/s10856-013-4923-4
Mu, Q., Kievit, F. M., Kant, R. J., Lin, G., Jeon, M., Zhang, M., et al. (2015). Anti-Her2/Neu peptide-conjugated iron oxide nanoparticles for targeted delivery of paclitaxel to breast cancer cells. Nanoscale 7 (43), 18010–18014. doi:10.1039/c5nr04867b
Muhammad, Z., Raza, A., Ghafoor, S., Naeem, A., Naz, S. S., Riaz, S., et al. (2016). Peg capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur. J. Pharm. Sci. 91, 251–255. doi:10.1016/j.ejps.2016.04.029
Murugan, K., Choonara, Y. E., Kumar, P., Bijukumar, D., Du Toit, L. C., Pillay, V., et al. (2015). Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures. Int. J. Nanomedicine 10, 2191–2206. doi:10.2147/ijn.S75615
Nagesh, P. K. B., Johnson, N. R., Boya, V. K. N., Chowdhury, P., Othman, S. F., Khalilzad-Sharghi, V., et al. (2016). Psma targeted docetaxel-loaded superparamagnetic iron oxide nanoparticles for prostate cancer. Colloids Surfaces B Biointerfaces 144, 8–20. doi:10.1016/j.colsurfb.2016.03.071
Nakamura, T., Yoshida, E., Hara, T., Fujie, T., Yamamoto, C., Fujiwara, Y., et al. (2020). Zn(Ii)2, 9-dimethyl-1, 10-phenanthroline stimulates cultured bovine aortic endothelial cell proliferation. RSC Adv. 10 (69), 42327–42337. doi:10.1039/d0ra06731h
Nardo, T., Chiono, V., Carmagnola, I., Fracchia, L., Ceresa, C., Tabrizian, M., et al. (2021). Mussel-inspired antimicrobial coating on ptfe barrier membranes for guided tissue regeneration. Biomed. Mat. 16 (3), 035035. doi:10.1088/1748-605X/abf27e
Nezhad-Mokhtari, P., Arsalani, N., Javanbakht, S., and Shaabani, A. (2019). Development of gelatin microsphere encapsulated Cu-based metal-organic framework nanohybrid for the methotrexate delivery. J. Drug Deliv. Sci. Technol. 50, 174–180. doi:10.1016/j.jddst.2019.01.020
Niculescu, A.-G., and Grumezescu, A. M. (2022). Novel tumor-targeting nanoparticles for cancer treatment-a review. Int. J. Mol. Sci. 23 (9), 5253. doi:10.3390/ijms23095253
Nolte, T. M., Hartmann, N. B., Kleijn, J. M., Garnaes, J., Van De Meent, D., Hendriks, A. J., et al. (2017). The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat. Toxicol. 183, 11–20. doi:10.1016/j.aquatox.2016.12.005
Nor, Y. A., Johari, F., Meka, A. K., Hao, S., Jimat, D. N., Jamal, N. A., et al. (2021). Preparation of porous core shell copper zinc oxide nanospheres for lysozyme delivery. Int. J. Nanoparticles 13 (3), 159. doi:10.1504/ijnp.2021.118107
Oh, S., Jang, M., Kim, J., Lee, J., Zhou, H., Lee, J., et al. (2016). Synthesis of silver nanoparticles using analogous reducibility of phytochemicals. Curr. Appl. Phys. 16 (7), 738–747. doi:10.1016/j.cap.2016.04.013
Pang, Y. C., Fu, Y., Li, C., Wu, Z. X., Cao, W. C., Hu, X., et al. (2020). Metal-organic framework nanoparticles for ameliorating breast cancer-associated osteolysis. Nano Lett. 20 (2), 829–840. doi:10.1021/acs.nanolett.9b02916
Pattappan, D., Kavya, K. V., Vargheese, S., Kumar, R. T. R., and Haldorai, Y. (2022). Graphitic carbon nitride/nh2-mil-101(Fe) composite for environmental remediation: Visible-Light-Assisted photocatalytic degradation of acetaminophen and reduction of hexavalent chromium. Chemosphere 286, 131875. doi:10.1016/j.chemosphere.2021.131875
Phuong, T., Pyo, Y.-C., Kim, D.-H., Lee, S.-E., Kim, J.-K., Park, J.-S., et al. (2019). Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics 11 (3), 132. doi:10.3390/pharmaceutics11030132
Prakash, N., Sadaf, M., Salomi, A., and Daniel, E. C. (2019). Cytotoxicity of functionalized iron oxide nanoparticles coated with rifampicin and tetracycline hydrochloride on Escherichia coli and Staphylococcus aureus. Appl. Nanosci. 9 (6), 1353–1366. doi:10.1007/s13204-019-00973-y
Qiao, K., Xu, L., Tang, J., Wang, Q., Lim, K. S., Hooper, G., et al. (2022a). The advances in nanomedicine for bone and cartilage repair. J. Nanobiotechnol. 20 (1), 141. doi:10.1186/s12951-022-01342-8
Qiao, M., Xu, Z., Pei, X., Liu, Y., Wang, J., Chen, J., et al. (2022b). Nano Sim@Zif-8 modified injectable high-intensity biohydrogel with bidirectional regulation of osteogenesis and anti-adipogenesis for bone repair. Chem. Eng. J. 434, 134583. doi:10.1016/j.cej.2022.134583
Raafat, A. I., Mahmoud, G. A., Ali, A. E.-H., Badawy, N. A., and Elshahawy, M. F. (2018). In vitro evaluation of mucoadhesive and self-disinfection efficiency of (acrylic acid/polyethylene glycol)-silver nanocomposites for buccal drug delivery. J. Bioact. Compat. Polym. 33 (1), 95–115. doi:10.1177/0883911517710665
Rabiee, N., Bagherzadeh, M., Jouyandeh, M., Zarrintaj, P., Saeb, M. R., Mozafari, M., et al. (2021). Natural polymers decorated mof-mxene nanocarriers for Co-delivery of doxorubicin/pcrispr. ACS Appl. Bio Mat. 4 (6), 5106–5121. doi:10.1021/acsabm.1c00332
Raghubir, M., Rahman, C. N., Fang, J., Matsui, H., and Mahajan, S. S. (2020). Osteosarcoma growth suppression by riluzole delivery via iron oxide nanocage in nude mice. Oncol. Rep. 43 (1), 169–176. doi:10.3892/or.2019.7420
Ran, J. B., Zeng, H., Cai, J., Jiang, P., Yan, P., Zheng, L. Y., et al. (2018). Rational design of a stable, effective, and sustained dexamethasone delivery platform on a titanium implant: An innovative application of metal organic frameworks in bone implants. Chem. Eng. J. 333, 20–33. doi:10.1016/j.cej.2017.09.145
Rao, K., Aziz, S., Roome, T., Razzak, A., Sikandar, B., Jamali, K. S., et al. (2018). Gum Acacia stabilized silver nanoparticles based nano-cargo for enhanced anti-arthritic potentials of hesperidin in adjuvant induced arthritic rats. Artif. Cells Nanomed. Biotechnol. 46 (Suppl. 1), 597–607. doi:10.1080/21691401.2018.1431653
Rengaraj, A., Puthiaraj, P., Heo, N. S., Lee, H., Hwang, S. K., Kwon, S., et al. (2017). Porous nh2-mil-125 as an efficient nano-platform for drug delivery, imaging, and ros therapy utilized low-intensity visible light exposure system. Colloids Surfaces B Biointerfaces 160, 1–10. doi:10.1016/j.colsurfb.2017.09.011
Rojas, S., Guiliou, N., and Horcajada, P. (2019). Ti-based nanomof as an efficient oral therapeutic agent. ACS Appl. Mat. Interfaces 11 (25), 22188–22193. doi:10.1021/acsami.9b06472
Rojas-Andrade, M. D., Chata, G., Rouholiman, D., Liu, J., Saltikov, C., Chen, S., et al. (2017). Antibacterial mechanisms of graphene-based composite nanomaterials. Nanoscale 9 (3), 994–1006. doi:10.1039/c6nr08733g
Saha, S., Pramanik, K., and Biswas, A. (2021). Antibacterial activity and biocompatibility of curcumin/TiO2 nanotube Array system on Ti6al4v bone implants.Mat. Technol. 36 (4), 221–232. doi:10.1080/10667857.2020.1742984
Sarin, H. (2010). Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res. 2, 14. doi:10.1186/2040-2384-2-14
Schuemann, J., Bagley, A. F., Berbeco, R., Bromma, K., Butterworth, K. T., Byrne, H. L., et al. (2020). Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 65 (21), 21RM02. doi:10.1088/1361-6560/ab9159
Sengul, A. B., and Asmatulu, E. (2020). Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 18 (5), 1659–1683. doi:10.1007/s10311-020-01033-6
Sha, Z., Yang, S., Fu, L., Geng, M., Gu, J., Liu, X., et al. (2021). Manganese-doped gold core mesoporous silica particles as a nanoplatform for dual-modality imaging and chemo-chemodynamic combination osteosarcoma therapy. Nanoscale 13 (9), 5077–5093. doi:10.1039/d0nr09220g
Shang, F., Yu, Y., Liu, S., Ming, L., Zhang, Y., Zhou, Z., et al. (2021). Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mat. 6 (3), 666–683. doi:10.1016/j.bioactmat.2020.08.014
Shanthi, K., Vimala, K., Gopi, D., and Kannan, S. (2015). Fabrication of a pH responsive dox conjugated pegylated palladium nanoparticle mediated drug delivery system: An in vitro and in vivo evaluation. RSC Adv. 5 (56), 44998–45014. doi:10.1039/c5ra05803a
Sharma, S., Mittal, D., Verma, A. K., and Roy, I. (2019). Copper-gallic acid nanoscale metal-organic framework for combined drug delivery and photodynamic therapy. ACS Appl. Bio Mat. 2 (5), 2092–2101. doi:10.1021/acsabm.9b00116
Sharma, S., Parveen, R., and Chatterji, B. P. (2021). Toxicology of nanoparticles in drug delivery. Curr. Pathobiol. Rep. 9 (4), 133–144. doi:10.1007/s40139-021-00227-z
Shuai, C., Guo, W., Wu, P., Yang, W., Hu, S., Xia, Y., et al. (2018). A graphene oxide-Ag Co-dispersing nanosystem: Dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem. Eng. J. 347, 322–333. doi:10.1016/j.cej.2018.04.092
Singh, K. R. B., Rathee, S., Nagpure, G., Singh, J., and Singh, R. P. (2022). Smart and emerging nanomaterials-based biosensor for sars-cov-2 detection. Mat. Lett. 307, 131092. doi:10.1016/j.matlet.2021.131092
Sobolev, A., Valkov, A., Kossenko, A., Wolicki, I., Zinigrad, M., Borodianskiy, K., et al. (2019). Bioactive coating on Ti alloy with high osseointegration and antibacterial Ag nanoparticles. ACS Appl. Mat. Interfaces 11 (43), 39534–39544. doi:10.1021/acsami.9b13849
Soltani, S., and Akhbari, K. (2022). Cu-btc metal-organic framework as a biocompatible nanoporous carrier for chlorhexidine antibacterial agent. J. Biol. Inorg. Chem. 27 (1), 81–87. doi:10.1007/s00775-021-01912-5
Sudha, N., Yousuf, S., Israel, E. V. M. V., Paulraj, M. S., and Dhanaraj, P. (2016). On the accessibility of surface-bound drugs on magnetic nanoparticles. Encapsulation of drugs loaded on modified dextran-coated superparamagnetic iron oxide by beta-cyclodextrin. Colloids Surfaces B Biointerfaces 141, 423–428. doi:10.1016/j.colsurfb.2016.02.020
Sun, Y., Bao, B. B., Zhu, Y., Shen, J. J., Liu, X. Z., Gao, T., et al. (2022). An fps-zm1-encapsulated zeolitic imidazolate framework as a dual proangiogenic drug delivery system for diabetic wound healing. Nano Res. 15 (6), 5216–5229. doi:10.1007/s12274-022-4106-z
Targhi, A. A., Moammeri, A., Jamshidifar, E., Abbaspour, K., Sadeghi, S., Lamakani, L., et al. (2021). Synergistic effect of curcumin-Cu and curcumin-Ag nanoparticle loaded niosome: Enhanced antibacterial and anti-biofilm activities. Bioorg. Chem. 115, 105116. doi:10.1016/j.bioorg.2021.105116
Telgerd, M. D., Sadeghinia, M., Birhanu, G., Daryasari, M. P., Zandi-Karimi, A., Sadeghinia, A., et al. (2019). Enhanced osteogenic differentiation of mesenchymal stem cells on metal-organic framework based on copper, zinc, and imidazole coated poly-L-lactic acid nanofiber scaffolds. J. Biomed. Mat. Res. A 107 (8), 1841–1848. doi:10.1002/jbm.a.36707
Ting, G., Zhang, W. W., Fei, G., Zhu, L. B., Ping, S., Li, W. Z., et al. (2022). A bone-targeting drug delivery vehicle of a metal-organic framework conjugate with zoledronate combined with photothermal therapy for tumor inhibition in cancer bone metastasis. Biomater. Sci. 10 (7), 1831–1843. doi:10.1039/d1bm01717a
Tong, Z. W., Yang, D., Sun, Y. Y., and Jiang, Z. Y. (2015). Biomimetic synthesis of C3n4/tio2/Ag nanosheet composites with high visible-light photocatalytic performance. RSC Adv. 5 (70), 56913–56921. doi:10.1039/C5ra06980g
Vangijzegem, T., Stanicki, D., and Laurent, S. (2019). Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 16 (1), 69–78. doi:10.1080/17425247.2019.1554647
Veronese, F. M., and Pasut, G. (2005). Pegylation, successful approach to drug delivery. Drug Discov. Today 10 (21), 1451–1458. doi:10.1016/s1359-6446(05)03575-0
Wan, Z., Li, C., Gu, J., Qian, J., Zhu, J., Wang, J., et al. (2021). Accurately controlled delivery of temozolomide by biocompatible uio-66-nh2 through ultrasound to enhance the antitumor efficacy and attenuate the toxicity for treatment of malignant glioma. Int. J. Nanomedicine 16, 6905–6922. doi:10.2147/ijn.S330187
Wang, D., Lin, Z., Wang, T., Yao, Z., Qin, M., Zheng, S., et al. (2016a). Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mat. 308, 328–334. doi:10.1016/j.jhazmat.2016.01.066
Wang, D., Zhou, J., Chen, R., Shi, R., Zhao, G., Xia, G., et al. (2016b). Controllable synthesis of dual-mofs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 100, 27–40. doi:10.1016/j.biomaterials.2016.05.027
Wang, J., Bao, M., Wei, T., Wang, Z., and Dai, Z. (2020a). Bimetallic metal-organic framework for enzyme immobilization by biomimetic mineralization: Constructing a mimic enzyme and simultaneously immobilizing natural enzymes. Anal. Chim. Acta X. 1098, 148–154. doi:10.1016/j.aca.2019.11.039
Wang, L., Li, S.-R., Chen, Y.-Z., and Jiang, H.-L. (2021a). Encapsulating copper nanocrystals into metal-organic frameworks for cascade reactions by photothermal catalysis. Small 17 (22), e2004481. doi:10.1002/smll.202004481
Wang, Q., Tang, P., Ge, X., Li, P., Lv, C., Wang, M., et al. (2018a). Experimental and simulation studies of strontium/zinc-codoped hydroxyapatite porous scaffolds with excellent osteoinductivity and antibacterial activity. Appl. Surf. Sci. 462, 118–126. doi:10.1016/j.apsusc.2018.08.068
Wang, S., Lv, J., Meng, S., Tang, J., and Nie, L. (2020b). Recent advances in nanotheranostics for treat-to-target of rheumatoid arthritis. Adv. Healthc. Mat. 9 (6), e1901541. doi:10.1002/adhm.201901541
Wang, T.-L., Zhou, Z.-F., Liu, J.-F., Hou, X.-D., Zhou, Z., Dai, Y.-L., et al. (2021b). Donut-like mofs of copper/nicotinic acid and composite hydrogels with superior bioactivity for Rh-bfgf delivering and skin wound healing. J. Nanobiotechnol. 19 (1), 275. doi:10.1186/s12951-021-01014-z
Wang, W., Xiong, Y. Z., Zhao, R. L., Li, X., and Jia, W. T. (2022a). A novel hierarchical biofunctionalized 3d-printed porous Ti6al4v scaffold with enhanced osteoporotic osseointegration through osteoimmunomodulation. J. Nanobiotechnol. 20 (1), 68. doi:10.1186/s12951-022-01277-0
Wang, X., He, T., Hu, J., and Liu, M. (2021c). The progress of nanomaterials for carbon dioxide capture via the adsorption process. Environ. Sci. Nano 8 (4), 890–912. doi:10.1039/d0en01140a
Wang, Y. T., Ying, T., Li, J. X., Xu, Y. F., Wang, R. Q., Ke, Q. F., et al. (2020c). Hierarchical micro/nanofibrous scaffolds incorporated with curcumin and zinc ion eutectic metal organic frameworks for enhanced diabetic wound healing via anti-oxidant and anti-inflammatory activities. Chem. Eng. J. 402, 126273. doi:10.1016/j.cej.2020.126273
Wang, Z., Chen, L., Chu, Z., Huang, C., Huang, Y., Jia, N., et al. (2018b). Gemcitabine-loaded gold nanospheres mediated by albumin for enhanced anti-tumor activity combining with ct imaging. Mater. Sci. Eng. C 89, 106–118. doi:10.1016/j.msec.2018.03.025
Wang, Z., Guo, W., Zhang, K., Ye, Y., Wang, Y., Sui, D., et al. (2022b). Two-dimensional copper metal-organic frameworks as antibacterial agents for biofilm treatment. Sci. China Technol. Sci. 65 (5), 1052–1058. doi:10.1007/s11431-021-1963-3
Wei, L. Q., Lu, J. Y., Li, Q. Q., Zhou, Y. L., Tang, L. L., Li, F. Y., et al. (2017). A porous Ca-mof with nano-sized {Ca-11} as building unit: Structure, drug loading and release properties. Inorg. Chem. Commun. 78, 43–47. doi:10.1016/j.inoche.2017.02.010
Weng, Y., Guan, S., Wang, L., Lu, H., Meng, X., Waterhouse, G. I. N., et al. (2020). Defective porous carbon polyhedra decorated with copper nanoparticles for enhanced nir-driven photothermal cancer therapy. Small 16 (1), e1905184. doi:10.1002/smll.201905184
Wojnarowicz, J., Chudoba, T., and Lojkowski, W. (2020). A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 10 (6), 1086. doi:10.3390/nano10061086
Wozniak-Budych, M. J., Przysiecka, L., Langer, K., Peplinska, B., Jarek, M., Wiesner, M., et al. (2017). Green synthesis of rifampicin-loaded copper nanoparticles with enhanced antimicrobial activity. J. Mat. Sci. Mat. Med. 28 (3), 42. doi:10.1007/s10856-017-5857-z
Xie, Y., Liu, X. J., Ma, X. X., Duan, Y. F., Yao, Y. W., Cai, Q., et al. (2018). Small titanium-based mofs prepared with the introduction of tetraethyl orthosilicate and their potential for use in drug delivery. ACS Appl. Mat. Interfaces 10 (16), 13325–13332. doi:10.1021/acsami.8b01175
Xiong, F., Qin, Z. N., Chen, H. M., Lan, Q. M., Wang, Z. T., Lan, N. H., et al. (2020). pH-responsive and hyaluronic acid-functionalized metal-organic frameworks for therapy of osteoarthritis. J. Nanobiotechnol. 18 (1), 139. doi:10.1186/s12951-020-00694-3
Xu, Z.-L., Lei, Y., Yin, W.-J., Chen, Y.-X., Ke, Q.-F., Guo, Y.-P., et al. (2016). Enhanced antibacterial activity and osteoinductivity of Ag-loaded strontium hydroxyapatite/chitosan porous scaffolds for bone tissue engineering. J. Mat. Chem. B 4 (48), 7919–7928. doi:10.1039/c6tb01282e
Xue, X. H., Yu, J. C., Lu, F. F., Jiang, H. Y., and Wang, X. Y. (2021a). Enhancement of cancer chemotherapeutic efficacy via bone-targeted drug delivery carrier in bone metastases. Drug Des. devel. Ther. 15, 4455–4468. doi:10.2147/dddt.S333999
Xue, X., Zhang, H., Liu, H., Wang, S. C., Li, J. D., Zhou, Q. R., et al. (2022). Rational design of multifunctional cus nanoparticle-peg composite soft hydrogel-coated 3d hard polycaprolactone scaffolds for efficient bone regeneration. Adv. Funct. Mat. 2022, 2202470. doi:10.1002/adfm.202202470
Xue, Y., Zhu, Z., Zhang, X., Chen, J., Yang, X., Gao, X., et al. (2021b). Accelerated bone regeneration by mof modified multifunctional membranes through enhancement of osteogenic and angiogenic performance. Adv. Healthc. Mat. 10 (6), e2001369. doi:10.1002/adhm.202001369
Yang, L., Peng, X.-H., Wang, Y. A., Wang, X., Cao, Z., Ni, C., et al. (2009). Receptor-targeted nanoparticles for in vivo imaging of breast cancer. Clin. Cancer Res. 15 (14), 4722–4732. doi:10.1158/1078-0432.Ccr-08-3289
Yang, T., Wang, D., and Liu, X. (2020). Antibacterial activity of an nir-induced Zn ion release film. J. Mat. Chem. B 8 (3), 406–415. doi:10.1039/c9tb02258a
Yang, W. J., Lee, J. H., Hong, S. C., Lee, J., Lee, J., Han, D.-W., et al. (2013). Difference between toxicities of iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials 6 (10), 4689–4706. doi:10.3390/ma6104689
Yu, K., Won, D. I., Lee, W. I., and Ahn, W. S. (2021). Porphyrinic zirconium metal-organic frameworks: Synthesis and applications for adsorption/catalysis. Korean J. Chem. Eng. 38 (4), 653–673. doi:10.1007/s11814-020-0730-z
Yu, Y., Cheng, Y., Tan, L., Liu, X., Li, Z., Zheng, Y., et al. (2022). Theory-screened mof-based single-atom catalysts for facile and effective therapy of biofilm-induced periodontitis. Chem. Eng. J. 431, 133279. doi:10.1016/j.cej.2021.133279
Zalneravicius, R., Paskevicius, A., Mazeika, K., and Jagminas, A. (2018). Fe(Ii)-Substituted cobalt ferrite nanoparticles against multidrug resistant microorganisms. Appl. Surf. Sci. 435, 141–148. doi:10.1016/j.apsusc.2017.11.028
Zeng, F. Y., Xu, D. D., Zhan, C., Liang, C. Y., Zhao, W. W., Zhang, J. H., et al. (2018). Surfactant-free synthesis of graphene oxide coated silver nanoparticles for sers biosensing and intracellular drug delivery. ACS Appl. Nano Mat. 1 (6), 2748–2753. doi:10.1021/acsanm.8b00444
Zhan, H., Zhou, X., Cao, Y., Jagtiani, T., Chang, T.-L., Liang, J. F., et al. (2017). Anti-cancer activity of camptothecin nanocrystals decorated by silver nanoparticles. J. Mat. Chem. B 5 (14), 2692–2701. doi:10.1039/c7tb00134g
Zhang, W., Zhao, X., Yuan, Y., Miao, F., Li, W., Ji, S., et al. (2020). Microfluidic synthesis of multimode Au@Cofeb-Rg3 nanomedicines and their cytotoxicity and anti-tumor effects. Chem. Mat. 32 (12), 5044–5056. doi:10.1021/acs.chemmater.0c00797
Zhang, X., Wang, J., Wu, J. X., Jiang, X. G., Pei, X. B., Chen, J. Y., et al. (2019). Dimethyloxalylglycine improves angiogenesis of zif-8-coated implant. J. Biomater. Appl. 34 (3), 396–407. doi:10.1177/0885328219850976
Zhao, K., Rong, G., Teng, Q., Li, X., Lan, H., Yu, L., et al. (2020). Dendrigraft poly-L-lysines delivery of DNA vaccine effectively enhances the immunogenic responses against H9n2 avian influenza virus infection in chickens. Nanomedicine Nanotechnol. Biol. Med. 27, 102209. doi:10.1016/j.nano.2020.102209
Zheng, Z. W., Chen, Y. H., Guo, B., Wang, Y., Liu, W., Sun, J., et al. (2020). Magnesium-organic framework-based stimuli-responsive systems that optimize the bone microenvironment for enhanced bone regeneration. Chem. Eng. J. 396, 125241. doi:10.1016/j.cej.2020.125241
Zhong, L., Chen, J., Ma, Z., Feng, H., Chen, S., Cai, H., et al. (2020). 3d printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale 12 (48), 24437–24449. doi:10.1039/d0nr06297a
Zhong, Y., Liu, W., Rao, C., Li, B., Wang, X., Liu, D., et al. (2021). Recent advances in Fe-mof compositions for biomedical applications. Curr. Med. Chem. 28 (30), 6179–6198. doi:10.2174/0929867328666210511014129
Zhu, J., Fu, F., Xiong, Z., Shen, M., and Shi, X. (2015). Dendrimer-entrapped gold nanoparticles modified with rgd peptide and alpha-tocopheryl succinate enable targeted theranostics of cancer cells. Colloids Surfaces B Biointerfaces 133, 36–42. doi:10.1016/j.colsurfb.2015.05.040
Zhu, Y., Chen, C., Cheng, P., Ma, J., Yang, W., Yang, W., et al. (2022). Recent advances in hydrothermal synthesis of facet-controlled ceo2-based nanomaterials. Dalton Trans. 51 (17), 6506–6518. doi:10.1039/d2dt00269h
Zhu, Z., Jiang, S., Liu, Y., Gao, X., Hu, S., Zhang, X., et al. (2020). Micro or nano: Evaluation of biosafety and biopotency of magnesium metal organic framework-74 with different particle sizes. Nano Res. 13, 511–526. doi:10.1007/s12274-020-2642-y
Zou, B., Xiong, Z., He, L., and Chen, T. (2022). Reversing breast cancer bone metastasis by metal organic framework-capped nanotherapeutics via suppressing osteoclastogenesis. Biomaterials 285, 121549. doi:10.1016/j.biomaterials.2022.121549
Zou, D., Yu, L., Sun, Q., Hui, Y., Tengjisi, , Liu, Y., et al. (2020a). A general approach for biomimetic mineralization of mof particles using biomolecules. Colloids Surfaces B Biointerfaces 193, 111108. doi:10.1016/j.colsurfb.2020.111108
Keywords: metal-based nanocarriers, drug deivlery, bone disease, bone regeneration, tissue engineering
Citation: Liu Y, Xu Z, Qiao M, Cai H and Zhu Z (2022) Metal-based nano-delivery platform for treating bone disease and regeneration. Front. Chem. 10:955993. doi: 10.3389/fchem.2022.955993
Received: 29 May 2022; Accepted: 07 July 2022;
Published: 09 August 2022.
Edited by:
Junyu Chen, Sichuan University, ChinaReviewed by:
Yu Han, Shanghai Jiao Tong University, ChinaWeiqi Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2022 Liu, Xu, Qiao, Cai and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: He Cai, caihe@foxmail.com; Zhou Zhu, jujoy1202@163.com
 Yanhua Liu
Yanhua Liu  He Cai
He Cai Zhou Zhu
Zhou Zhu