Vaccination with DNA Encoding Truncated Enterohemorrhagic Escherichia coli (EHEC) Factor for Adherence-1 Gene (efa-1′) Confers Protective Immunity to Mice Infected with E. coli O157:H7
- 1Laboratory of Molecular Immunology, Department of Microbiology, Faculty of Biological Sciences, Universidad de Concepción, Concepción, Chile
- 2Microbiology and Mycology Program, Faculty of Medicine, Institute of Biomedical Sciences, University of Chile, Santiago, Chile
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 is the predominant causative agent of hemorrhagic colitis in humans and is the cause of haemolytic uraemic syndrome and other illnesses. Cattle have been implicated as the main reservoir of this organism. Here, we evaluated the immunogenicity and protective efficacy of a DNA vaccine encoding conserved sequences of truncated EHEC factor for adherence-1 (efa-1′) in a mouse model. Intranasal administration of plasmid DNA carrying the efa-1′ gene (pVAXefa-1′) into C57BL/6 mice elicited both humoral and cellular immune responses. In animals immunized with pVAXefa-1′, EHEC-secreted protein-specific IgM and IgG antibodies were detected in sera at day 45. Anti-EHEC-secreted protein sIgA was also detected in nasal and bronchoalveolar lavages. In addition, antigen-specific T-cell-proliferation, IL-10, and IFN-γ were observed upon re-stimulation with either heat-killed bacteria or EHEC-secreted proteins. Vaccinated animals were also protected against challenge with E. coli O157:H7 strain EDL933. These results suggest that DNA vaccine encoding efa-1′ have therapeutic potential in interventions against EHEC infections. This approach could lead to a new strategy in the production of vaccines that prevent infections in cattle.
Introduction
Most strains of Escherichia coli form part of the normal microbiota of the gastrointestinal tract of mammals and birds. However, several highly adapted E. coli strains possess special virulence properties, allowing them to adapt to alternative niches and to cause a broad spectrum of diseases including diarrhea. These strains, named diarrheagenic E. coli (DEC), have been classified based on their known virulence properties (Kaper et al., 2004). Enterohemorrhagic E. coli (EHEC) are important intestinal zoonotic pathogens, causing sporadic, and epidemic E. coli infection outbreaks worldwide (Bosilevac and Koohmaraie, 2011). EHEC is a type of Shiga toxin-producing E. coli (STEC) that colonizes the human intestine and causes diarrheal illness that can progress to hemorrhagic colitis and, in several cases, life-threatening haemolytic uremic syndrome (HUS) (Kaper et al., 2004; Farfan and Torres, 2012). Ruminants, especially cattle, are the primary source of STEC O157:H7, the most commonly detected strain, and harbor non-O157 STEC strain (Pennington, 2010; Bosilevac and Koohmaraie, 2011), and beef is considered to be an important source of STEC O157 and non-O157 human infection (Caprioli et al., 2005).
EHEC and enteropathogenic E. coli (EPEC) are intestinal pathogens that have the ability to form attaching and effacing (A/E) lesions in host intestinal epithelium (Schmidt, 2010). A/E lesions are characterized by bacterial attachment with the formation of an actin pedestal-like structure and by destruction of epithelial microvilli (Goosney et al., 2000). This pathology is genetically determined by the locus of enterocyte effacement (LEE) (Crawford et al., 2002; Kaper et al., 2004), which is highly conserved in EHEC and EPEC. The LEE contains a significant number of genes associated with virulence, mainly encoding a type III secretion system (T3SS), and the eae gene encoding the outer membrane adhesin intimin that, along with the translocated intimin receptor (Tir), allows intimate bacterial binding to intestinal epithelium (Crawford et al., 2002). However, some genes coding for effector proteins and associated factors implicated in EPEC and EHEC pathogenesis are located outside the LEE island, forming part of a large pathogenicity island responsible for increased virulence (Klapproth and Meyer, 2009). In various EHEC and EPEC strains, these include lifA/efa-1 (lymphocyte inhibitory factor A/EHEC factor for adherence-1), that encodes a toxin of approximately 360 kDa, one of the largest proteins produced by E. coli. It contains glycosyltransferase and protease domain, both present also in Clostridial cytotixins (Klapproth, 2010). LifA/Efa1 protein has been detected at the surface of the EPEC JPN15 strain (Badea et al., 2003) and affects intestinal colonization and adhesion by modulating local mucosal immunity in the gut (Malstrom and James, 1998; Klapproth et al., 2000).
The lifA/efa-1 gene is present in all tested non-O157:H7 EHEC serotype and in related enteropathogens, such as Citrobacter rodentium and rabbit EPEC (REPEC) (Klapproth et al., 2000; Nicholls et al., 2000). Although this gene is not physically located in the LEE it has only been observed in eae-positive (Nicholls et al., 2000; Vidal et al., 2008) and LEE-positive strains isolated from humans and cattle (Galli et al., 2010). However, the intact efa-1 gene is absent from the E. coli O157:H7 strains that have been sequenced (Perna et al., 2001; Janka et al., 2002). E. coli O157:H7 possesses a truncated lifA/efa-1 pseudogene (efa-1′) which is located within the pathogenic island O122 and is predicted to encode proteins identical to amino acids 1–433 and 435–710 of open reading frame (ORF) z4332 and the contiguous ORF z4333 respectively (Perna et al., 2001). It has been reported that an E. coli O157:H7 mutant carrying a transposon insertion upstream of efa-1′ showed reduced adherence to human colon cells (Stevens et al., 2002), indicating that the truncated Efa-1 protein may have some of the properties of full-length Efa-1, whose last gene (efa-1′) could contribute to its virulence (Karmali et al., 2003).
A variety of prevention strategies have been proposed in order to reduce the prevalence of EHEC in animals (Potter et al., 2004; Sargeant et al., 2007; Babiuk et al., 2008; Rozema et al., 2009), and thereby also reduce the incidence of human infections. The use of adjuvants has greatly improved the antigenicity of immunogens. An example is the new generation of immune-stimulation complex (ISCOM) adjuvants such as AbISCO; this is a potent inducer of humoral and cellular immune response against antigens administered via the mucose (Picard et al., 2012). Although mice do not develop the symptoms associated with diarrheal disease observed in human (Roxas et al., 2010), they mice have proved to be useful for EHEC infection and disease (García-Angulo et al., 2014). This includes the C57BL/6 mice (Rhee et al., 2011). In this study, we sought to develop an anti-EHEC DNA-based vaccine utilizing the efa-1′ gene, contained in an expression vector, as an innovative strategy to prevent EHEC infections. Similar strategies have been widely used for anti-viral therapy as well as for protection against bacteria and parasites (Dhama et al., 2008). The present study describes the immune response induced by a DNA plasmid encoding sequences of the efa-1′ gene in an intranasal C57BL/6 mouse model.
Materials and Methods
Animals
Eight-week-old female C57BL/6 mice (purchased from Instituto de Salud Pública, Santiago, Chile) were acclimated and randomly assigned to experimental groups. Mice were handled and disposed of according to the guidelines of the Universidad de Concepción Institutional Ethics Committee. According to the experimental procedure, mice were housed in individually cages with free access to food and water in temperature-controlled under 12 h light/12 h dark cycle, an environment free of specific pathogens. The Bioethics and security committee of the Faculty of Biological Sciences in the Universidad de Concepción approved this study. All efforts were made to minimize animal suffering.
Bacterial Strains and Culture Conditions
The E. coli EDL933 (Mohawk and O'Brien, 2011), the prototypical strain E. coli O157:H7, was used for experimental infection, for oral inoculation studies, this bacterial strain were amplified in brain heart infusion broth for 18 h at 37°C with shaking. E. coli strain DH5α (Life Technology, Gaithersburg, MD) was used to propagate plasmids. E. coli DH5α cultures were routinely grown at 37°C in Luria-Bertani broth or agar supplemented, when required, with Kanamycin 100 μg/ml.
Construction of the DNA Vaccine
DNA vaccine constructs expressing efa-1′ from the O-island 122 of E. coli O157:H7 were prepared as described below. The coding region for this antigen was PCR amplified from E. coli EDL933 chromosomal DNA. Primer sequences are listed in Table S1. PCR products were ligated into the pVAX-cloning vector (Invitrogen). The resulting plasmids were designated pVAXefa-1′. pVAX is a plasmid vector designed for use in the development of DNA vaccine and it is characterized by high-level transient expression of the protein of interest in most mammalian cells (Invitrogen, USA). Large amount of endotoxin-free plasmid DNA were prepared and purified using the Endo-free plasmid Giga Kit (Qiagen, Valencia, CA), following the manufacturer's instructions. The analysis of Efa-1′ protein expression was carried out by western blot from Cos7 cell transfected with the plasmid pVAXefa-1′. Immunodetection of proteins was carried out by the use of a mouse Flag specific monoclonal antibody (Sigma-Aldrich, Inc.) as the primary antibody (data not shown).
Purification of EHEC-Secreted Proteins
Secreted proteins by the Type III Secretion System (TTSS) were prepared from supernatants obtained from E. coli EDL933 cultures, as previously described (Niebuhr and Ebel, 2003). Briefly, E. coli strain O157:H7 EDL933 was cultivated in Luria-Bertani medium (LB) at 37°C overnight. This culture was then diluted in M-9 minimal medium supplemented with 44 mM NaHCO, 8 mM MgSO4, glucose and 0.1% Casamino Acids (Difco Laboratories); these culture conditions optimize the production of Type III Secretion System proteins. The culture was incubated at 37°C in an atmosphere with 5% CO2 until the optical density reached 0.7–0.8 at 600 nm. Bacteria were pelleted by centrifugation at 3500 g for 15 min; the supernatant was concentrated by precipitating with trichloroacetic acid (TCA), then 10% (v/v) 100% TCA was added and left overnight at 4°C. We then centrifuged at 4000 g for 20 min, discarded the supernatant, and the pellet was re-suspended in 200 μL Tris-HCl 1.5M. Proteins were stored at −20°C for later use as antigens in ELISA and lymphocyte proliferation assays.
Immunization
Ten mice per group were anesthetized using a solution of 10 mg/ml ketamine and 250 μg/ml acepromazine and immunized intranasally with a solution containing 50 μg of the recombinant plasmid pVAXefa-1′, 12 μg of the adjuvant AbISCO-100® (ISCONOVA AB, Uppsala, Sweden), plus PBS to complete a total volume of 50 μl for the appropriate preparation of the recombinant vector. As negative controls, groups of mice were immunized with empty pVAX vector vaccine, as internal control of plasmid or PBS plus adjuvant respectively. Three doses of vaccine were administered in 14-day intervals (Li et al., 2000). Assays were performed in duplicate; the results are a representative date set.
Evaluation of Antibody Response
Mouse serum samples were obtained every 2 weeks prior to each immunization and 2 weeks after the last administration of the DNA vaccine. The presence of serum immunoglobulin (Ig) G, IgM and IgA isotypes with specificity for Efa-1′ was determined by an enzyme-linked immunosorbent assay (ELISA) (Li et al., 2000). For this purpose, 2.5 μg/ml of EHEC-secreted protein diluted in carbonate buffer (pH 9.6) was used to coat the wells of a polystyrene plate at 4°C overnight. The plates were washed three times in PBS, 0.05% Tween 20 (PBST) and the non-specific sites were blocked with 3% gelatin in PBST. After 1 h incubation at 37°C and repeated washing, the plate was incubated with serial dilutions of sera from immunized mice (from 1:500 to 1:20,000) in PBST. They were then added to the ELISA plates and incubated at 37°C for 2 h. Then the plate were repeated washed and anti-mouse isotype HRP-conjugates (1:1000 in PBST, ICN Biomedical, Inc., Ohio, USA) was added, incubated a 37°C for 30 min and washed. After 30 min of incubation, 100 μl of substrate OPD was added (Sigma, USA) to each well. The reaction was stopped with 100 μl of 2 M H2SO4 and the OD450 was read on a microplate reader (Victor X3. PerkinElmer, USA). The result was expressed as endpoint titer of the last dilution, which gave an optical density at 450 nm of two times above the value of the negative control ± SEM.
In addition, the amount of total specific IgA present in nasal and bronchoalveolar lavages (NAL and BAL respectively) with specificity to EHEC secreted proteins were determined by ELISA (Oñate et al., 2003). Antibody titers were estimated as the reciprocals of the last sample dilution giving an absorbance (A450) value above the cut-off. To compensate for potential variations in the efficiency of recovery of secretory antibodies between animals, the results were normalized according to the total IgA content of the sample. The cut-off value for the assay was calculated as the mean specific OD450 plus standard error of the means (SEM) for 10 NAL or 10 BAL from non-immunized mice assayed at a dilution of 1:10 respectively. Thus, results were expressed as ELISA units (EU), namely the endpoint titer of antigen-specific IgA divided by the total concentration in μg of the IgA present in the sample.
Splenocyte Cultures and Lymphocyte Proliferation
Two weeks after their last immunization, five mice per group were euthanized. Their spleens were removed and homogenized under aseptic conditions. Single-cell suspensions were prepared according to procedure described (Li et al., 2000). The splenocytes were cultured in RPMI 1640 medium (Sigma), 10% heat-inactivated fetal calf serum (GIBCO BRL), and penicillin-streptomycin (50 UI of penicillin; 50 μg/ml streptomycin) at 37°C with 5% CO2 in a 96-well flat-bottom plate at a concentration of 4 × 105 viable cells/well in the presence of no additive (unstimulated control) or one of the following stimulants: (1) 0.2 μg/well of heat-killed E. coli strain EDL933 or (2) 1 μg/well of EHEC-secreted proteins. The heat-killed E.coli EDL933 wet weight is measured by the cell pellet weight after the centrifugation of a culture obtained as follow; 100 ml of LB inoculated with 1% of ON bacterial culture, was incubated at 37°C with shaking until it reaches an OD600 of 0.8 (exponential phase). Then the culture was spun at 20,000 × g by 20 min, suspend to obtain 50 mg/ml (wet cell weight), then the bacteria were killed by heating at 65°C during 1 h, spun at 20,000 × g, and the wet cell pellet was weight and adjusted to the required final concentration.
The cells were cultured for 72 h and pulsed for 8 h with 0.4 μCi of thymidine (50 μCi/mmol; Amersham, UK) per well. The radioactivity incorporated into the DNA of proliferating cells was determined by scintillation counting. Lymphocyte proliferation data were expressed as mean counts per minute of triplicate cultures from a cell pool for each group (five mice per group). In addition, a stimulation index (SI) was calculated for each experimental group by dividing the counts per minute of cells with antigen by the counts per minute of cells without antigen.
Cytokine mRNA Profile
Splenocytes (4 × 105 cells per well) were stimulated with heat-killed E. coli strain EDL933 (0.2 μg/well) and EHEC-secreted proteins (1 μg/well) for 9 h (Fu et al., 2013). RNA was isolated from cells using TRIzol® (Invitrogen), as recommended by the manufacturer's instructions, and reverse transcribed to cDNA. Cytokine-specific real-time PCR for interleukin (IL)-4, IL-10 and INF-γ was carried out following standard procedures. The cytokine primer sequences are listed in Table S1. The gapdh gene was used as a control for constitutive gene expression. The amplification reaction was carried out for a total of 40 cycles as follows: 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with a pre-cycle of 95°C for 10 min and final extension at 72°C for 5 min. PCR products (5–10 μl) were analyzed by gel electrophoresis. Expression data were normalized against the housekeeping gapdh gene expression profile. Each value was analyzed for statistical difference according to the Bonferroni/Dunn method (Retamal-Díaz et al., 2014).
Challenge of Immunized Mice
The protection experiments were performed as described previously (Giulietti et al., 2001). Twenty-four hours before the challenge, mouse were treated with 1 mg/ml streptomycin, which was administered in water which they drank ad libitum. Five mice from each group were challenged through oral gavage 6 weeks after the last vaccine dose with 100 μl of a suspension containing 5 × 105 CFU/ml of E. coli strain EDL933. Two weeks later, the infected mice were euthanized and their distal and proximal colon were removed under aseptic conditions, homogenized and diluted into plates containing Sorbitol MacConkey agar (Oxoid, Basingstoke, UK), supplemented with 20 μg/ml nalidixic acid (Sigma, St. Louis, USA) and 2.5 μg/ml potassium tellurite (TN-SMAC) in order to determine the number of E. coli strain EDL933 CFU per ml. Some non-sorbitol-fermenting colonies were tested by PCR for O157 serogroup (primers are described in Table S1). Bacterial counts of challenged strain were determined in intestinal tissue homogenates and expressed as CFU ml-1 of homogenate. Log10 units of protection were obtained by subtracting the mean log10 CFU for the experimental group from the mean log10 CFU of the corresponding control group.
Statistical Analysis
Data for lymphocyte proliferation, evaluation of the level of antibody, and detection of cytokine were analyzed using ANOVA test with Bonferroni post-hoc test (P-value of 0.05 or less was considered statistically significant). The data derived from the protection experiment were analyzed using the Two-way ANOVA test and Sidak's multiple comparisons test with a confidence interval of 95% (α = 0.05).
Results
Immune Response of Mice Vaccinated with DNA Vaccines
We examined the immune response induced in mice intranasally immunized with pVAXefa-1′ (co-administered with adjuvant AbISCO). Systemic anti-Efa-1′ IgM, IgG and IgA were detected. As shown in Figure 1A, 15 days after priming the sera from mice immunized with pVAXefa-1′ contained a significant titer of anti-Efa-1′ IgM (P < 0.05 in comparison to the negative control groups PBS or pVAX). On days 30 and 45 after priming, the titers of anti-Efa-1′ IgM were again significantly higher as compared to the negative control groups PBS or pVAX (P < 0.01). Later, Efa-1′ IgG was determined in sera of vaccinated animals. The result showed that 15 and 30 days after immunization with pVAXefa-1′ the titer of anti-Efa-1′ IgG was higher, but not significantly, in comparison with the values obtained in mice from the negative control groups (Figure 1B; P > 0.05). At day 45, the level of IgG showed a higher increase compared with other days. This was statistically significant compared to the negative control groups (P < 0.01). The titer of anti-Efa-1′ IgA present in sera from mice immunized with the vector pVAXefa-1′ exhibited slight variation throughout the experiment, with no significant difference when compared with values from mice in the negative control groups (Figure 1C).
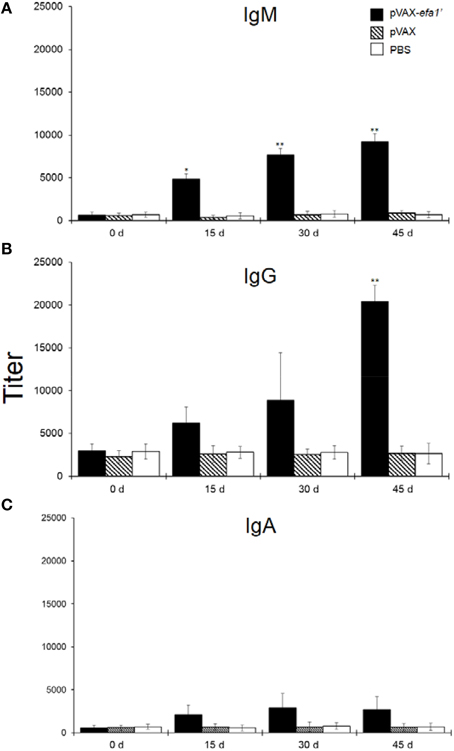
Figure 1. Efa-1′-specific serum antibodies. Five mice in each group were immunized by i.n. route with pVAXefa-1′, control pVAX and PBS. Serum samples were obtained on days 0, 15, 30, and 45 post-immunization, and Efa-1′-specific IgM (A), IgG (B), and IgA (C) levels in the samples were quantified. Endpoint titers are expressed as retrograde values of the last dilution that gave an OD450 of two times above the value of the negative control ± SEM. These results are representative of data from two independent experiments. Statistical significances are represented by asterisks (*P < 0.05, and **P < 0.01, as compared to the control PBS group).
In order to evaluate the induction of mucosal responses in vaccinated animals, EHEC-secreted specific sIgA titres were determined directly from nasal lavage (NAL) and bronchoalveolar lavages (BAL) (Figure 2). Results indicated that vaccination with pVAXefa-1′ induced significantly higher levels of antigen-specific mucosal IgA production in both NAL (Figure 2A) and BAL (Figure 2B) as compared to control mice receiving PBS or pVAX (P < 0.01).
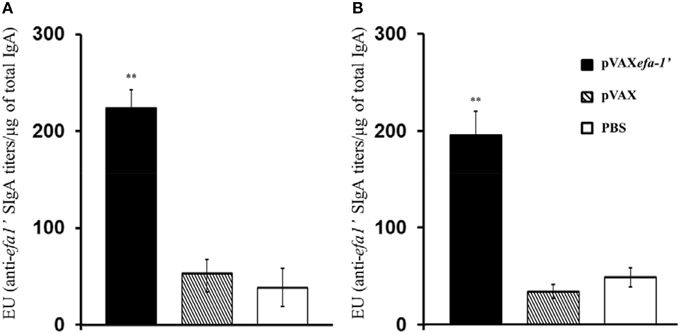
Figure 2. Efa-1′-specific mucosal sIgA antibodies in nasal lavages (A) and bronchoalveolar lavage (B) fluids. Efa-1′-specific antibody titers were estimated as the reciprocals of the last sample dilution giving an A450 value above the cut-off. Results were normalized according to the total IgA content of the sample. Results are expressed as ELISA units (EU), namely, the endpoint titer of SOD-specific IgA divided by the total concentration in μg of IgA present in the sample. Data are shown as mean ± SEM of two experiments. The statistical significances are represented by **P < 0.01.
In most cases the generation of an efficient and long lasting immune response requires the induction of a T-helper cell sub-set. Thus, we opted for examining the cellular mediated immunity (CMI) response to heat-killed E. coli and EHEC-secreted protein in the vaccinated mice. For this purpose, we measured the proliferative response of splenocyte following in vitro restimulation with the corresponding antigen (Figure 3). Splenocytes from mice immunized with pVAXefa-1′ exhibited a significant proliferative response to heat-killed E. coli EDL933 (P < 0.01 in comparison to the PBS or pVAX groups), with a stimulation index of 5.23. Equivalently, when the splenocytes from mice immunized with pVAXefa-1′ were stimulated in vitro with EHEC-secreted proteins, they showed a statistically significant increase compared to control groups (P < 0.05), with a stimulation index of 4.34. T cells from mice of all groups had similarly high levels of proliferative response to the mitogen Concanavalin A (data not shown).
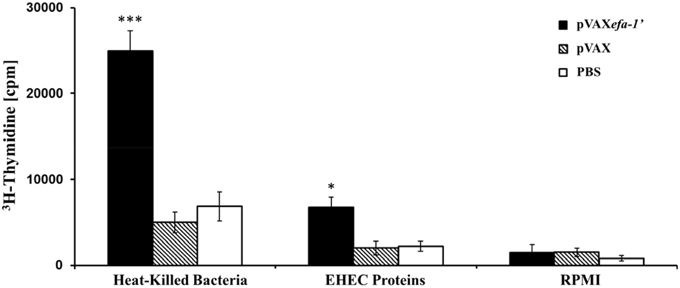
Figure 3. Lymphocyte proliferation assay. C57BL/6 mice were immunized with pVAXefa-1′, pVAX, or PBS. Efa-1′-T-cell proliferative responses were measured 2 weeks after the last immunization by [3H] thymidine incorporation. Splenocyte derived from animals in each group were pooled, and 4 × 105 cells for wells were restimulated in vitro with heat-killed E. coli (0.2 μg/well) or EHEC-secreted protein (1 μg/well). Each bar indicates the average number of counts per minute for triplicate cultures of cells ± standard deviation (error bar) obtained from five mice per group. Statistical significances are represented by asterisks (*P < 0.05, and ***P < 0.001).
To analyse the stimulated immune response in more detail, we also evaluated the cytokine production by spleen-derived lymphocytes after restimulation with the antigen. We used reverse transcription-polymerase chain reaction (RT-PCR) to quantify IL-10, IFN-γ and IL-4 mRNA levels in spleen-cell culture from pVAXefa-1′-inmunized animals. By RT-PCR we observed an 8-fold increase in IL-10 mRNA expression (Figure 4A) and a 15-fold increase in IFN-γ mRNA expression (Figure 4B), at 9 h post-stimulation with heat-killed E. coli, in splenocytes from pVAXefa-1′-vaccinated mice. This result was statistically significant compared to expression levels in unstimulated cells (P < 0.05). Upon stimulation with EHEC-secreted proteins, splenocytes from pVAXefa-1′-vaccinated mice expressed IL-10 and IFN-γ, but only IFN-γ levels were significant compared to unstimulated cells (P < 0.05) (Figure 4B). No significant IL-4 mRNA was detected in any of the splenocyte-stimulated groups (Figure 4C).
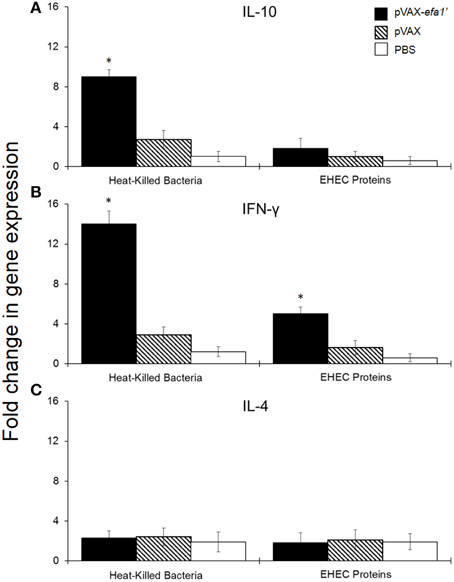
Figure 4. Relative mRNA expression of IL-10 (A) and IFN-γ (B) and IL-4 (C) produced by mouse splenocytes. Fold variation of encoded cytokines is shown 9 h after stimulation with 0.2 μg/well of heat-killed E. coli and 1 μg/well of EHEC secreted proteins. *Significant differences with respect to the group inoculated with PBS (P < 0.05).
Efficacy of pVAXefa-1′ Immunization in Generating Protective Immunity against E. coli EDL933
Immune protection experiments were carried out by challenging vaccinated and control mice by oral gavage with virulent E. coli strain EDL933. The level of infection was evaluated by bacterial counts in intestinal tissue homogenates and expressed as CFU/ml-1 of homogenate. The results showed that immunization with pVAXefa-1′ induced a high degree of protection, 3.51 log10 less bacterial in this group, compared with the PBS negative control groups, (P < 0.001) (Table 1).
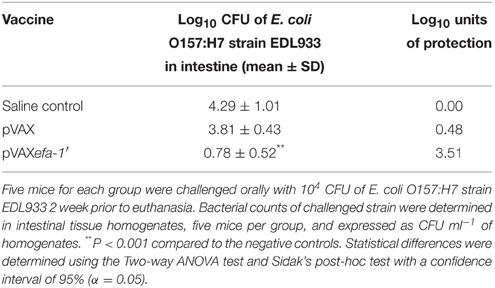
Table 1. Protection of mice against challenge with E. coli O157:H7 after immunization with DNA vaccine.
Discussion
EHEC were first recognized as a cause of human disease in 1982 (Asahara et al., 2004) and are associated with diarrhea, hemorrhagic colitis, and life-threatening haemolytic uremic syndrome (Riley et al., 1983). The incidence of EHEC varies by age group, with the highest incidence occurring in children under 15 years of age (0.7 cases per 100,000 in USA). Potential complications associated with EHEC include HUS, the principal cause of acute kidney failure in children. Most cases of HUS are caused by E. coli O157:H7 (Tarr et al., 2005). In the case of EHEC infection, antibiotic therapy is not usually recommended because antibiotics may induce toxin release from the pathogen as a result of an increased systemic exposure to the adverse effects of the potent nephrotoxin (Wong et al., 2000; Zhang et al., 2000) and there is a need for new strategies to control EHEC colonization, including from animal reservoirs. Particular focus should be placed on protecting cattle, the largest reservoir worldwide, as well as swine, another significant reservoir in many countries (Fremaux et al., 2006; Kagambèga et al., 2012).
The proteins encoded within the LEE have been used as subunit vaccines to generate an increase in serum antibody production and lymphocyte proliferation in murine (Momtaz et al., 2013) and cattle models (Cataldi et al., 2008). Not all strains of EHEC and EPEC harbor the same virulence factors. For example, STEC strains lacking intimin (eae), isolated from patients with hemorrhagic colitis or HUS, may carry iha, efa-1, lpfA, and/or saa genes (Vidal et al., 2008). Therefore, it is necessary to find other potential immunogens capable of inducing or improving a wide-range of protective immunity to EHEC. Based on our studies Efa-1′ meet the required criteria, because it is conserved among various EHEC serogrups and elicit a strong immune response. Intramuscular immunization of cattle with the truncated recombinant protein (Efa-1′) has been shown to induce a humoral response to the specific antigen, but not protective immunity (McNeilly et al., 2010). Nevertheless, it cannot be excluded that under different conditions, such as route of administration or dose of challenge, different results could be obtained.
The use of immunostimulating complexes (ISCOMs) has been shown to induce robust cellular and humoral immune responses against antigens administered intranasally (van Diemen et al., 2007). Prominent IL-12 production by innate immune cells is a characteristic reaction induced by ISCOMs, promoting the development of a strong Th1 response. After intranasal or intestinal mucosal administration, the ISCOM induces a strong specific mucosal IgA response on local and remote mucosal surfaces, with stimulation of the secretion of proinflammatory cytokines such as IL-1 and TNF-α, or anti-inflammatory cytokines such as IL-10 (Hu et al., 2001).
DNA vaccination has already proved affective against E. coli O157:H7 (Morein et al., 2004). We examined the effectiveness of the DNA vaccine transport efa-1′ administered together with AbISCO adjuvant, in generating immunity and protection against experimental infection with the E. coli strain EDL933 in mice. With EHEC-secreted proteins as antigen, the plasmid pVAXefa-1′ induced the highest titers of IgM and IgG production compared with negative control groups pVAX and PBS. A strong antibody response against LEE-encoded proteins has been reported after experimental infection with EHEC O157:H7 (Morein et al., 2004). Nevertheless, it is known that in natural EHEC infections in cattle, the serum antibody response is not essential to generate an efficient protective response (Shariati Mehr et al., 2012). At the mucosal level, intranasal immunization with our vaccine resulted in the production of higher antigen-specific IgA titers (sIgA) in BAL than in NAL. Several studies have demonstrated that such locally produced antibodies, mainly sIgA, which prevents the binding of bacteria and toxin action on epithelial cells, most likely provide protective immunity (Kaper et al., 2004). These results are correlated with the high rate of lymphocyte stimulation observed in the splenocytes of animals immunized with the efa-1′ gene, with the production of INF-γ, IL-10 and significant protection. Contrarily, it has been reported that various strains of EHEC are capable of inhibiting the INF-γ pathway through inhibition of Stat-1 phosphorylation in epithelial cells, contributing to immune evasion by these microorganisms (Bretschneider et al., 2007). The high expression of INF-γ in vaccinated mice may offset the inhibitory effects caused by EHEC infection. Moreover, the expression of the anti-inflammatory cytokine IL-10 may contribute to the significant mucosal immune response observed by immunization with pVAXefa-1′. B cells are the major source of IgA precursor cells. They undergo a class switch recombination to IgA secreting cells, which are heavily dependent on cytokines secreted by activated T cells, such as IL-10, which in lamina propria promotes conversion of sIgA B cells to mature sIgA secreting plasma cells (Ho et al., 2012), potentiating a Th2-biased immunological response. Moreover, high level of IL-10 expression has been reported in mice vaccinated with Shiga toxins (Stxs) fusion proteins from EHEC (Fagarasan et al., 2010), demonstrating that production of IL-10 is a natural response against E. coli pathogenic infection. Thus, immunization with pVAXefa-1′ induces significant production of IFN-γ associated with immune protection against microbial pathogens, with the production of IL-10 cytokine that is crucial in preventing inflammation, and thus protecting tissues from damage. This is essential for the maintenance of gut homeostasis and the recovery of the intestinal epithelial barrier. Additionally, this protects gut mucosal tissues from colitis (Cai et al., 2011). Therefore, the joint action of sIgA in mucosa together with the high production of INF-γ and IL-10 observed in pVAXefa-1′ immunized mice, may explain the high protective response observed in this group. Further, the production of IL-10 in conjunction with IFN-γ could be interpreted as a regulatory immune mechanism that prevents uncontrolled TH type 1 immune responses that could be potentially harmful (Li et al., 2014) o beneficial agianst to EHEC (Ghasemi et al., 2014).
Conclusions
This study shows that genetic vaccines containing efa-1′ from E. coli O157:H7 result in the induction of mucosal and systemic immune responses. This approach is able to confer efficient protection against challenge with the enterohemorrhagic E. coli EDL933 in the mouse model studied. Overall, these results indicate that mucosal inoculation with DNA vaccines is a valid vaccination approach for the induction of immuno-mediated protection against EHEC infections.
Author Contributions
RR, writing and discussion of the results, perform cytokines qPCR assays. AR, writing the paper, performs immunization trials, antibodies evaluation by ELISA and lymphocyte proliferation assays. DS, he was performing the recombinant protein, cloning and immunization. PF, conducted evaluation tests of immunity at mucosal level and statistical analysis of the results. FD, cloning, discussion, and writing of the manuscript. JS, discuss and determine the growth culture conditions for optimal protein purification from E. coli EDL933. GO, he analyzed the efa-1′ gene sequence in enterohemorrhagic E. coli isolates from patients, animal and foods. RV, define the truncated region of enterohemorrhagic E. coli (EHEC) factor for adherence-1 gene (efa-1′) for protective immunogenic analysis, programming and monitoring the experiments, collecting the results, performing the analysis, and discussion and writing the manuscript. AO, programming and monitoring the experiments, collecting the results, performing the analysis and discussion and writing the manuscript. RV and AO are principal investigators at the FONDECYT grant that funded this work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grant 1110260 and 1130093 from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), Santiago, Chile.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fcimb.2015.00104
References
Asahara, T., Shimizu, K., Nomoto, K., Hamabata, T., Ozawa, A., and Takeda, Y. (2004). Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 72, 2240–2247. doi: 10.1128/IAI.72.4.2240-2247.2004
Babiuk, S., Asper, D. J., Rogan, D., Mutwiri, G. K., and Potter, A. A. (2008). Subcutaneous and intranasal immunization with type III secreted proteins can prevent colonization and shedding of Escherichia coli O157:H7 in mice. Microb. Pathog. 45, 7–11. doi: 10.1016/j.micpath.2008.01.005
Badea, L., Doughty, S., Nicholls, L., Sloan, J., Robins-Browne, R. M., and Hartland, E. L. (2003). Contribution of Efa1/LifA to the adherence of enteropathogenic Escherichia coli to epithelial cells. Microb. Pathog. 34, 205–215. doi: 10.1016/S0882-4010(03)00026-3
Bosilevac, J. M., and Koohmaraie, M. (2011). Prevalence and characterization of non-O157 shiga toxin- producing Escherichia coli isolated from commercial ground beef in the United States. Appl. Environ. Microbiol. 77, 2103–2112. doi: 10.1128/AEM.02833-10
Bretschneider, G., Berberov, E. M., and Moxley, R. A. (2007). Isotype-specific antibody responses against Escherichia coli O157:H7 locus of enterocyte effacement proteins in adult beef cattle following experimental infection. Vet. Immunol. Immunopathol. 118, 229–238. doi: 10.1016/j.vetimm.2007.06.005
Cai, K., Gao, X., Li, T., Wang, Q., Hou, X., Tu, W., et al. (2011). Enhanced immunogenicity of a novel Stx2Am-Stx1B fusion protein in a mice model of enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine 29, 946–952. doi: 10.1016/j.vaccine.2010.11.035
Caprioli, A., Morabito, S., Brugère, H., and Oswald, E. (2005). Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet. Res. 36, 289–311. doi: 10.1051/vetres:2005002
Cataldi, A., Yevsa, T., Vilte, D. A., Schulze, K., Castro-Parodi, M., Larzábal, M., et al. (2008). Efficient immune responses against Intimin and EspB of enterohaemorragic Escherichia coli after intranasal vaccination using the TLR2/6 agonist MALP-2 as adjuvant. Vaccine 26, 5662–5667. doi: 10.1016/j.vaccine.2008.07.027
Crawford, J. A., Blank, E. T., and Kaper, J. B. (2002). “The LEE-encoded type III secretion system in EPEC and EHEC: assembly, function and regulation,” in Escherichia coli: Virulence Mechanisms of a Versatile Pathogen, ed M. S. Donnenberg (London: Academic Press), 337–359.
Desmarchelier, P. M., Bilge, S. S., Fegan, N., Mills, L., Vary, J. C. Jr., and Tarr, P. I. (1998). A PCR specific for Escherichia coli O157 based on the rfb locus encoding O157 lipopolysaccharide. J. Clin. Microbiol. 36, 1801–1804.
Dhama, K., Mahendran, M., Gupta, P. K., and Rai, A. (2008). DNA vaccines and their applications in veterinary practice: current perspectives. Vet. Res. Commun. 32, 341–356. doi: 10.1007/s11259-008-9040-3
Fagarasan, S., Kawamoto, S., Kanagawa, O., and Suzuki, K. (2010). Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 28, 243–273. doi: 10.1146/annurev-immunol-030409-101314
Farfan, M. J., and Torres, A. G. (2012). Molecular mechanisms that mediate colonization of Shiga toxin-producing Escherichia coli strains. Infect. Immun. 80, 903–913. doi: 10.1128/IAI.05907-11
Fremaux, B., Raynaud, S., Beutin, L., and Rozand, C. V. (2006). Dissemination and persistence of Shiga toxin-producing Escherichia coli (STEC) strains on French dairy farms. Vet. Microbiol. 117, 180–191. doi: 10.1016/j.vetmic.2006.04.030
Fu, Y., Zhou, E., Liu, Z., Li, F., Liang, D., Liu, B., et al. (2013). Staphylococcus aureus and Escherichia coli elicit different innate immune responses from bovine mammary epithelial cells. Vet. Immunol. Immunopathol. 155, 245–252. doi: 10.1016/j.vetimm.2013.08.003
Galli, L., Miliwebsky, E., Irino, K., Leotta, G., and Rivas, M. (2010). Virulence profile comparison between LEE-negative Shiga toxin-producing Escherichia coli (STEC) strains isolated from cattle and humans. Vet. Microbiol. 143, 307–313. doi: 10.1016/j.vetmic.2009.11.028
García-Angulo, V. A., Kalita, A., Kalita, M., Lozano, L., and Torres, A. G. (2014). Comparative genomics and immunoinformatics approach for the identification of vaccine candidates for enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 82, 2016–2026. doi: 10.1128/IAI.01437-13
Ghasemi, A., Zarnani, A. H., Ghoodjani, A., Rezania, S., Salari, M. H., and Jeddi-Tehrani, M. (2014). Identification of a new immunogenic candidate conferring protection against Brucella melitensis infection in mice. Mol. Immunol. 62, 142–149. doi: 10.1016/j.molimm.2014.06.017
Giulietti, A., Overbergh, L., Valckx, D., Decallonne, B., Bouillon, R., and Mathieu, C. (2001). An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25, 386–401. doi: 10.1006/meth.2001.1261
Goosney, D. L., Gruenheid, S., and Finlay, B. B. (2000). Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu. Rev. Cell Dev. Biol. 16, 173–189. doi: 10.1146/annurev.cellbio.16.1.173
Ho, N. K., Ossa, J. C., Silphaduang, U., Johnson, R., Johnson-Henry, K. C., and Sherman, P. M. (2012). Enterohemorrhagic Escherichia coli O157:H7 Shiga toxins inhibit gamma interferon-mediated cellular activation. Infect. Immun. 80, 2307–2315. doi: 10.1128/IAI.00255-12
Hu, K., Lövgren-Bengtsson, K., and Morein, B. (2001). Immunostimulating complexes (ISCOMs) for nasal vaccination. Adv. Drug Deliv. Rev. 51, 149–159. doi: 10.1016/S0169-409X(01)00165-X
Janka, A., Bielaszewska, M., Dobrindt, U., and Karch, H. (2002). Identification and distribution of the enterohemorrhagic Escherichia coli factor for adherence (efa1) gene in sorbitol-fermenting Escherichia coli O157: H-. Int. J. Med. Microbiol. 292, 207–214. doi: 10.1078/1438-4221-00206
Kagambèga, A., Martikainen, O., Siitonen, A., Traoré, A. S., Barro, N., and Haukka, K. (2012). Prevalence of diarrheagenic Escherichia coli virulence genes in the feces of slaughtered cattle, chickens, and pigs in Burkina Faso. Microbiologyopen 1, 276–284. doi: 10.1002/mbo3.30
Kaper, J. B., Nataro, J. P., and Mobley, H. L. (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140. doi: 10.1038/nrmicro818
Karmali, M. A., Mascarenhas, M., Shen, S., Ziebell, K., Johnson, S., Reid-Smith, R., et al. (2003). Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 41, 4930–4940. doi: 10.1128/JCM.41.11.4930-4940.2003
Klapproth, J. M. (2010). The role of lymphostatin/EHEC factor for adherence-1 in the pathogenesis of gram negative infection. Toxins (Basel) 2, 954–962. doi: 10.3390/toxins2050954
Klapproth, J. M., and Meyer, F. (2009). Multitalented lymphostatin. Dtsch. Med. Wochenschr. 134, 417–420. doi: 10.1055/s-0029-1208066
Klapproth, J. M., Scaletsky, I. C., McNamara, B. P., Lai, L. C., Malstrom, C., James, S. P., et al. (2000). A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect. Immun. 68, 2148–2155. doi: 10.1128/IAI.68.4.2148-2155.2000
Li, B., Alli, R., Vogel, P., and Geiger, T. L. (2014). IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 7, 869–878. doi: 10.1038/mi.2013.103
Li, Y., Frey, E., Mackenzie, A. M., and Finlay, B. B. (2000). Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect. Immun. 68, 5090–5095. doi: 10.1128/IAI.68.9.5090-5095.2000
Malstrom, C., and James, S. (1998). Inhibition of murine splenic and mucosal lymphocyte function by enteric bacterial products. Infect. Immun. 66, 3120–3127.
McNeilly, T. N., Mitchell, M. C., Rosser, T., McAteer, S., Low, J. C., Smith, D. G., et al. (2010). Immunization of cattle with a combination of purified intimin-531, EspA and Tir significantly reduces shedding of Escherichia coli O157:H7 following oral challenge. Vaccine 28, 1422–1428. doi: 10.1016/j.vaccine.2009.10.076
Mohawk, K. L., and O'Brien, A. D. (2011). Mouse models of Escherichia coli O157:H7 infection and shiga toxin injection. J. Biomed. Biotech. 2011, 1–17. doi: 10.1155/2011/258185
Momtaz, H., Safarpoor Dehkordi, F., Rahimi, E., Ezadi, H., and Arab, R. (2013). Incidence of Shiga toxin-producing Escherichia coli serogroups in ruminant's meat. Meat Sci. 95, 381–388. doi: 10.1016/j.meatsci.2013.04.051
Morein, B., Hu, K. F., and Abusugra, I. (2004). Current status and potential application of ISCOMs in veterinary medicine. Adv. Drug Deliv. Rev. 56, 1367–1382. doi: 10.1016/j.addr.2004.02.004
Nicholls, L., Grant, T. H., and Robins-Browne, R. M. (2000). Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35, 275–288. doi: 10.1046/j.1365-2958.2000.01690.x
Niebuhr, K., and Ebel, F. (2003). Generation of monoclonal antibodies against secreted proteins of STEC. Methods Mol. Med. 73, 125–135. doi: 10.1385/1-59259-316-X:125
Oñate, A. A., Céspedes, S., Cabrera, A., Rivers, R., González, A., Muñoz, C., et al. (2003). A DNA vaccine encoding Cu,Zn superoxide dismutase of Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 71, 4857–4861. doi: 10.1128/IAI.71.9.4857-4861.2003
Pennington, H. (2010). Escherichia coli O157. Lancet 376, 1428–1435. doi: 10.1016/S0140-6736(10)60963-4
Perna, N. T., Plunkett, G. III, Burland, V., Mau, B., Glasner, J. D., Rose, D. J., et al. (2001). Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409, 529–533. doi: 10.1038/35054089
Picard, M. D., Cohane, K. P., Gierahn, T. M., Higgins, D. E., and Flechtner, J. B. (2012). High-throughput proteomic screening identifies Chlamydia trachomatis antigens that are capable of eliciting T cell and antibody responses that provide protection against vaginal challenge. Vaccine 30, 4387–4393. doi: 10.1016/j.vaccine.2012.01.017
Potter, A. A., Klashinsky, S., Li, Y., Frey, E., Townsend, H., Rogan, D., et al. (2004). Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22, 362–369. doi: 10.1016/j.vaccine.2003.08.007
Retamal-Díaz, A., Riquelme-Neira, R., Sáez, D., Rivera, A., Fernández, P., Cabrera, A., et al. (2014). Use of S-[2,3-bispalmitoyiloxy-(2R)-propyl]-R-cysteinyl-amido-monomethoxy polyethyleneglycol as an adjuvant improved protective immunity associated with a DNA vaccine encoding Cu,Zn superoxide dismutase of Brucella abortus in mice. Clin. Vaccine Immunol. 21, 1474–1480. doi: 10.1128/CVI.00554-14
Rhee, K. J., Cheng, H., Harris, A., Morin, C., Kaper, J. B., and Hecht, G. (2011). Determination of spatial and temporal colonization of enteropathogenic E. coli and enterohemorrhagic E. coli in mice using bioluminescent in vivo imaging. Gut Microbes. 2, 34–41. doi: 10.4161/gmic.2.1.14882
Riley, L. W., Remis, R. S., Helgerson, S. D., McGee, H. B., Wells, J. G., Davis, B. R., et al. (1983). Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308, 681–685. doi: 10.1056/NEJM198303243081203
Roxas, J. L., Koutsouris, A., Bellmeyer, A., Tesfay, S., Royan, S., Falzari, K., et al. (2010). Enterohemorrhagic, E. coli alters murine intestinal epithelial tight junction protein expression and barrier function in a Shiga toxin independent manner. Lab Invest. 90, 1152–1168. doi: 10.1038/labinvest.2010.91
Rozema, E. A., Stephens, T. P., Bach, S. J., Okine, E. K., Johnson, R. P., Stanford, K., et al. (2009). Oral and rectal administration of bacteriophages for control of Escherichia coli O157:H7 in feedlot cattle. J. Food Prot. 72, 241–250.
Sargeant, J. M., Amezcua, M. R., Rajic, A., and Waddell, L. (2007). Pre-harvest interventions to reduce the shedding of E. coli O157 in the faeces of weaned domestic ruminants: a systematic review. Zoonoses Public Health. 54, 260–277. doi: 10.1111/j.1863-2378.2007.01059.x
Schmidt, M. A. (2010). LEE ways: tales of EPEC, ATEC and EHEC. Cell. Microbiol. 12, 1544–1552. doi: 10.1111/j.1462-5822.2010.01518.x
Shariati Mehr, K., Mousavi, S. L., Rasooli, I., Amani, J., and Rajabi, M. (2012). A DNA vaccine against Escherichia coli O157:H7. Iran Biomed. J. 16, 133–139. doi: 10.6091/ibj.1059.2012
Stevens, M. P., van Diemen, P. M., Frankel, G., Phillips, A. D., and Wallis, T. S. (2002). Efa1 influences colonization of the bovine intestine by shiga toxin-producing Escherichia coli serotypes O5 and O111. Infect. Immun. 70, 5158–5166. doi: 10.1128/IAI.70.9.5158-5166.2002
Tarr, P. I., Gordon, C. A., and Chandler, W. L. (2005). Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365, 1073–1086. doi: 10.1016/s0140-6736(05)71144-2
van Diemen, P. M., Dziva, F., Abu-Median, A., Wallis, T. S., van den Bosch, H., Dougan, G., et al. (2007). Subunit vaccines based on intimin and Efa-1 polypeptides induce humoral immunity in cattle but do not protect against intestinal colonisation by enterohaemorrhagic Escherichia coli O157:H7 or O26:H-. Vet. Immunol. Immunopathol. 116, 47–58. doi: 10.1016/j.vetimm.2006.12.009
Vidal, M., Prado, V., Whitlock, G. C., Solari, A., Torres, A. G., and Vidal, R. M. (2008). Subtractive hybridization and identification of putative adhesins in a Shiga toxin-producing eae-negative Escherichia coli. Microbiology 154, 3639–3648. doi: 10.1099/mic.0.2008/021212-0
Wong, C. S., Jelacic, S., Habeeb, R. L., Watkins, S. L., and Tarr, P. I. (2000). The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342, 1930–1936. doi: 10.1056/NEJM200006293422601
Keywords: DNA vaccine, truncated efa-1 gene, enterohemorragic E. coli, O157:H7 serotype, protective to mice
Citation: Riquelme-Neira R, Rivera A, Sáez D, Fernández P, Osorio G, del Canto F, Salazar JC, Vidal RM and Oñate A (2016) Vaccination with DNA Encoding Truncated Enterohemorrhagic Escherichia coli (EHEC) Factor for Adherence-1 Gene (efa-1′) Confers Protective Immunity to Mice Infected with E. coli O157:H7. Front. Cell. Infect. Microbiol. 5:104. doi: 10.3389/fcimb.2015.00104
Received: 01 October 2015; Accepted: 21 December 2015;
Published: 20 January 2016.
Edited by:
Nora Lía Padola, Universidad Nacional del Centro de la Provincia de Buenos Aires, ArgentinaReviewed by:
Analía Inés Etcheverría, Universidad Nacional del Centro de la Provincia de Buenos Aires, ArgentinaMarina Sandra Palermo, Instituto de Medicina Experimental- National Council of Scientific and Technical Research-CONICET, Argentina
Copyright © 2016 Riquelme-Neira, Rivera, Sáez, Fernández, Osorio, del Canto, Salazar, Vidal and Oñate. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto M. Vidal, rvidal@med.uchile.cl;
Angel Oñate, aonate@udec.cl
†These authors have contributed equally to this work.
 Roberto Riquelme-Neira
Roberto Riquelme-Neira Alejandra Rivera
Alejandra Rivera Darwin Sáez1
Darwin Sáez1  Pablo Fernández
Pablo Fernández Gonzalo Osorio
Gonzalo Osorio Felipe del Canto
Felipe del Canto Juan C. Salazar
Juan C. Salazar Roberto M. Vidal
Roberto M. Vidal Angel Oñate
Angel Oñate