A c-di-GMP-Modulating Protein Regulates Swimming Motility of Burkholderia cenocepacia in Response to Arginine and Glutamate
- 1Department of Microbiology, Faculty of Science, University of Manitoba, Winnipeg, MB, Canada
- 2Department of Chemistry, Faculty of Science, University of Manitoba, Winnipeg, MB, Canada
- 3Department of Medical Microbiology & Infectious Diseases, Max Rady College of Medicine, University of Manitoba, Winnipeg, MB, Canada
Burkholderia cenocepacia is an opportunistic bacterium that can thrive in different environments, including the amino acid-rich mucus of the cystic fibrosis (CF) lung. B. cenocepacia responds to the nutritional conditions that mimic the CF sputum by increasing flagellin expression and swimming motility. Individual amino acids also induce swimming but not flagellin expression. Here, we show that modulation of the second messenger cyclic dimeric guanosine monophosphate (c-di-GMP) levels by the PAS-containing c-di-GMP phosphodiesterase, BCAL1069 (CdpA), regulates the swimming motility of B. cenocepacia K56-2 in response to CF sputum nutritional conditions. Heterologous expression of WspR, a diguanylate cyclase, in B. cenocepacia K56-2 caused an increase in c-di-GMP levels and reduced swimming motility but did not affect flagellin expression or flagellar biosynthesis. After insertional mutagenesis of 12 putative genes encoding c-di-GMP metabolizing enzymes, one mutant of the locus BCAL1069 (cdpA), exhibited decreased swimming motility independent of flagellin expression in CF sputum nutritional conditions and an increase in intracellular c-di-GMP levels. The reduced swimming motility phenotype of the BCAL1069 mutant was observed in the presence of arginine and glutamate, but not of histidine, phenylalanine, or proline. The B. cenocepacia CdpA was also found to be involved in regulation of protease activity but not in biofilm formation. Altogether, these results highlight a role of B. cenocepacia BCAL1069 (CdpA) in sensing the nutritional conditions of the CF sputum and eliciting a pathogenic response that includes swimming motility toward amino acids and an increase in protease activity.
Introduction
Cyclic-dimeric-guanosine monophosphate or c-di-GMP is an intracellular second messenger present in a wide range of bacterial species (Camilli and Bassler, 2006). In response to a change in the bacterial milieu, temporal, and spatial c-di-GMP levels are controlled through sensing and regulatory mechanisms, which alter bacterial physiology and phenotypes (Shanahan and Strobel, 2012). Initially associated with exopolysaccharide synthesis and biofilm formation, the role of c-di-GMP is now established in the transitioning of bacterial lifestyle from planktonic to sessile, cellular development, host cell adherence, virulence, and motility (Ross et al., 1987; Paul et al., 2004; Simm et al., 2004; Lee et al., 2010; Römling et al., 2013). C-di-GMP is synthesized and degraded by proteins containing GGDEF and EAL domains, respectively, named after the conserved signature motifs Gly-Gly-Asp-Glu-Phe (GGDEF) and Glu-Ala-Leu (EAL) (Galperin et al., 2001; Galperin, 2005). The GGDEF and EAL domains are typically linked to non-enzymatic domains that are involved in signal transduction systems (Galperin et al., 2001; Henry and Crosson, 2011). For instance, the GGDEF and EAL domains can be present in conjunction with signaling sensory domains such as GAF, PAS, Cache, HAMP, and a receiver domain, REC (Galperin, 2005; Römling et al., 2013). The sensory domains recognize small molecules and subsequently activate the receiver domain, resulting in functional changes in bacterial physiology and virulence factors, including motility (Galperin et al., 2001; Römling et al., 2013). A systematic study showed that three signaling domain-containing c-di-GMP proteins regulate swimming motility and pathogenicity in the rice pathogen, Xanthomonas oryzae (Wei et al., 2016). In Salmonella Typhimurium, the amino acid arginine was demonstrated to modulate c-di-GMP levels through a Cache-GGDEF domain-containing protein, leading to cellulose secretion and biofilm formation (Mills et al., 2015). These findings suggest that pathogenic bacteria can respond to environmental/nutritional cues and regulate virulence factors by modulating c-di-GMP levels through signal transduction pathways.
Burkholderia cenocepacia is a species of the Burkholderia cepacia complex (Bcc), a group of successful opportunistic bacteria with extremely versatile metabolism (De Smet et al., 2015; Eberl and Vandamme, 2016). Bcc species have been isolated from various ecological niches, such as soil, water bodies, plant rhizosphere, pharmaceutical products, and the lungs of people with the genetic disease cystic fibrosis (CF) (Mahenthiralingam et al., 2005). The diversity of Bcc ecological niches suggests that Bcc can sense different environmental settings and successfully elicit a response. For example, B. cenocepacia must possess mechanisms to sense the change from a relatively poor nutrient environment to the amino acid rich mucus present in the lungs of CF and trigger a response that results in successful colonization of the lung (Palmer et al., 2007a). Transcriptomic analysis of B. cenocepacia in nutritional conditions of CF sputum showed upregulation of flagellar biosynthesis genes, suggesting the importance of sensing the nutrients of the CF lung and the role of flagella in colonizing new environmental niches (Drevinek et al., 2008; Yoder-Himes et al., 2009, 2010).
We previously showed that synthetic CF sputum medium (SCFM) (Palmer et al., 2007a), induces motility of the clinical isolate B. cenocepacia K56-2 by upregulating flagellin synthesis and the number of flagella (Kumar and Cardona, 2016). SCFM mimics the nutrients of the CF sputum and is rich in amino acids. Interestingly, motility assays performed in a medium with individual amino acids also showed induced motility but the levels of flagellin were not affected (Kumar and Cardona, 2016). Considering the role of c-di-GMP in signal transduction pathways related to motility, we examined whether c-di-GMP metabolic genes regulate the motility of B. cenocepacia in response to amino acids. To inspect the role of c-di-GMP genes, we performed a systematic analysis of 12 sensory domain-coding c-di-GMP genes and assessed their role in swimming motility of B. cenocepacia K56-2. We found that a putative phosphodiesterase homologous to the B. pseudomallei CdpA (Lee et al., 2010), modulates intracellular c-di-GMP levels in response to amino acids and activates swimming motility. These findings deepen our understanding of the role of c-di-GMP signaling mechanisms in the adaptation of opportunistic pathogens to the host environment.
Materials and Methods
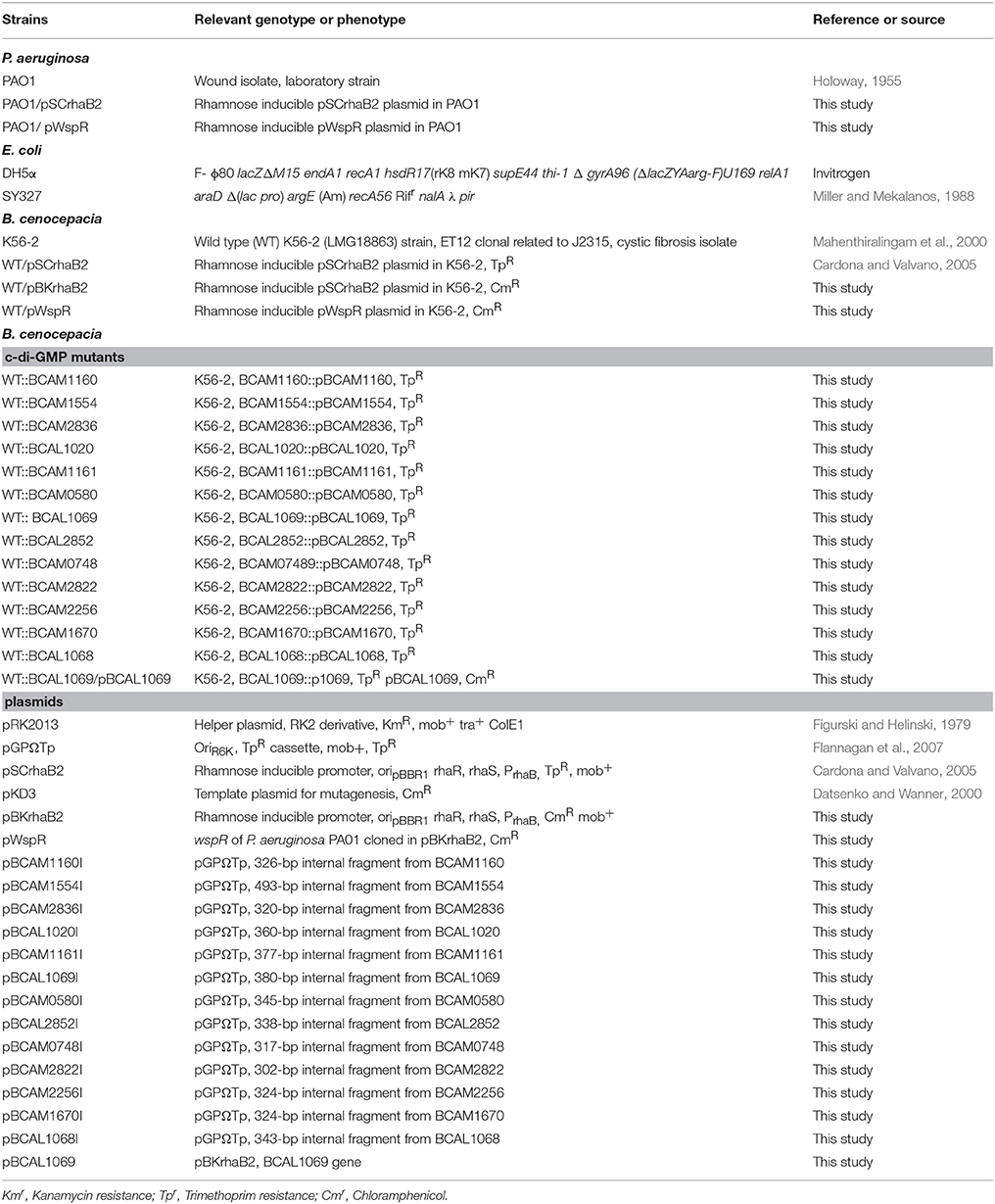
Bacterial Strains, Plasmids, and Growth Conditions
The bacterial strains used in this study are summarized in Table 1. Primers are listed in Supplementary Table 1. The B. cenocepacia K56-2 wild type (WT) strain is a clinical isolate obtained from a CF patient and belongs to Rapid Amplification of DNA polymorphism (RAPD) and electrophoretic type (ET) 12. MOPS-glucose 20 mM and SCFM were prepared as described previously (Palmer et al., 2007a). The bacterial strains were grown in MOPS-glucose 20 mM or SCFM overnight, as indicated. Cells were washed in PBS and inoculated in SCFM, or MOPS-glucose 5 mM (glucose-minimal medium) with or without amino acids according to the experimental design. When appropriate, media was supplemented with trimethoprim (100 μg/ml for B. cenocepacia or 50 μg/ml for Escherichia coli), chloramphenicol (200 μg/ml for B. cenocepacia or 20 μg/ml for E. coli), kanamycin (25 μg/ml for E. coli), or rhamnose at 0.2% final concentration. To study the effect of amino acids, MOPS-glucose 5 mM was supplemented with individual amino acids at the same concentration present in SCFM.
For genetic manipulations in B. cenocepacia K56-2 via triparental mating, the helper strain E. coli pRK2013 was used. E. coli SY327 Z-competent cells (Zymo Research, USA) were used to maintain the pBK1 plasmid. Polymerase chain reactions (PCR) were carried out with either Taq DNA polymerase (Qiagen) or HotStar HiFidelity Taq polymerase (Qiagen) with optimized conditions for each pair of primers. The DNA ligase and restriction enzymes (New England Biolabs) were used as recommended by the manufacturer. QIAquick purification kit (Qiagen) and QIAprep Miniprep kit (Qiagen) were used to purify PCR products and plasmids, respectively.
Growth Assays
Growth experiments were performed in 96-well format in a total volume of 200 μl. Bacterial cells were inoculated at a starting optical density at 600 nm (OD600) of 0.04 in triplicate. The 96-well microtiter plates were incubated at 37°C with continuous shaking for 24 h in a BioTek Synergy 2 plate reader. Readings were taken hourly at OD600 and values were converted to 1-cm-path-length OD600 by prior calibration with a GeneQuant™ III 4283, version 4283V1.6.
Cloning of c-di-GMP Genes in B. cenocepacia
To construct c-di-GMP modulating B. cenocepacia K56-2 (WT) strains, the wspR gene was PCR-amplified using Pseudomonas aeruginosa PAO1 genome as a template (primers described in Supplementary Table 1). The PCR amplicon and plasmid were digested using NdeI and XbaI, and the digested genes and plasmid were ligated using T4 ligase enzyme. This resulted in cloning of wspR gene into pSCrhaB2 under the rhamnose-inducible promoter. The resulting pWspR plasmid was introduced into the B. cenocepacia K56-2 WT strain, resulting in WT/pWspR.
Insertional Mutagenesis of c-di-GMP Metabolizing Genes
Insertional mutagenesis with the plasmid pGPΩTp was utilized (Flannagan et al., 2007). First, an internal fragment (~300–400 bp) of the gene of interest was PCR-amplified using forward and reverse primers (Supplementary Table 1). The PCR-amplified internal fragment and pGPΩTp were digested with the restriction enzymes, XbaI and EcoRI. The digested internal fragments and plasmid were ligated with T4 ligase enzyme, and the ligation products transformed into E. coli SY327. The resulting plasmids (Table 1) were introduced into B. cenocepacia K56-2 WT strain, generating exconjugants that were selected for trimethoprim resistance. Colony PCR was performed to confirm the insertion of the suicide plasmid in the target gene by using specific primers upstream to 5′ of the internal fragment and a primer that anneals with the plasmid (p53 reverse primer). The c-di-GMP mutants were named after the interrupted gene, for instance, WT::BCAL1069 mutant indicated insertional mutagenesis in the BCAL1069 gene.
Complementation of the WT::BCAL1069 Mutant
Plasmid pBKrhaB2 was created using pSCrhaB2 as the backbone. The dihydrofolate reductase gene of pSCrhaB2 was replaced with the chloramphenicol acetyltransferase (CAT) gene, which was amplified from pKD3 using primers, 876 and 877. The amplified CAT gene and pBKrhaB2 were digested with EcoRV and NsiI. The digested CAT gene was ligated into digested pBKrhaB2 using T4 ligase enzyme. The resulting plasmid pBKrhaB2 was utilized for complementation experiments. To complement the WT::BCAL1069 mutant, the BCAL1069 gene was PCR-amplified using primers 851 and 852. The PCR-amplified BCAL1069 and pBKrhaB2 were digested with NdeI and XbaI, and the digested gene and plasmids were ligated using T4 ligase. The pBCAL1069 plasmid was introduced into the BCAL1069 mutant strain, creating the WT::BCAL1069/pBCAL1069 strain.
Western Blot
The WT and c-di-GMP mutant strains were grown overnight in SCFM. To ensure equal amounts of proteins were loaded, cell cultures were adjusted to an OD600 of 1.0 before taking equal volumes. To prepare the whole cell lysates, the cells were thawed, pelleted and resuspended in 2X SDS loading dye. After boiling, the whole cell lysates were separated by 12% SDS-PAGE to separate protein and transferred onto PVDF membrane. Then, the FliC protein production level was detected with the FliC specific primary polyclonal anti-flagellin antibody by incubating the membrane for 45 min at 4°C on the shaker. The polyclonal anti-flagellin antibody was raised against B. pseudomallei in rabbit (kindly gifted by Dr. David Speert). The membrane was then incubated with the secondary specific rabbit antibody tagged with alkaline phosphatase (Sigma-Aldrich, USA) for 45 min on a shaker. The ratio of primary and secondary antibodies dissolved in blocking buffer were 1:20,000 and 1:30,000, respectively. Finally, the FliC-primary antibody complex and secondary antibody interaction were detected with an NBT/BCIP detection kit (Roche, USA).
Swimming Motility Assay
Overnight grown cell cultures were washed twice in PBS and the cell OD600 was adjusted to 1.0. To examine swimming motility of the WT and c-di-GMP mutants, 5 μl of bacterial inoculum was stabbed on 0.3% agar semi-solid plates. The plates, which were prepared on the same day, were then incubated statically at 37°C for 24 h. The motility halos were recorded quantitatively by measuring the circular zone of turbidity, which corresponds to the bacteria swimming away from the point of the inoculation. Motility halos of WT and mutants were compared and statistically analyzed. To examine mutants swimming motility, antibiotics were not added in the motility plates; however, rhamnose was added as inducer for gene expression.
Electron Microscopy
For electron microscopy sample preparation, a drop of the diluted overnight bacterial culture grown in SCFM was spotted on carbon-coated grids and stained with 2% uranyl acetate for 30 s. After drying the grids for 30 min, they were observed under a Hitachi H-7000 Transmission Electron Microscope at an operating voltage of 75 kV.
Protease Activity Assay
Extracellular protease activity of WT and mutants were measured qualitatively using 1.5% agar containing 2% skim milk. The plates were inoculated with 5 μl of bacterial cell culture adjusted to an OD600 of 3.0 and incubated for 48 h at 37°C. Protease activity was indicated by a clear (lysis) zone around the bacterial colony. Protease activity of WT and mutants were compared and statistically analyzed.
Biofilm Formation Assay
Bacterial attachment or biofilm formation were studied by measuring adherence to the wells of a 96-well polystyrene plastic plate using the method of Merritt et al., 2005. Overnight cultures were adjusted to initial inoculum of OD600 0.04 in SCFM in the wells of the 96-well plate. The plate was then incubated statically at 37°C for 48 h. After the incubation period, planktonic bacteria were removed, and adherent bacteria washed three times in PBS, and then stained with 0.1% crystal violet for 30 min. The stain was removed carefully; the attached cells were again washed with PBS, and the stained bacteria eluted with 20:80 (v/v) acetone: ethanol mixture. Eluted CV stained bacteria readings were measured at OD600 using the BioTek plate reader.
Extraction and Quantification of c-di-GMP
The extraction of c-di-GMP in B. cenocepacia K56-2 was performed as previously described (Roy et al., 2013). Briefly, bacterial cell cultures were grown for 24 h in SCFM with or without supplementation with 0.2% rhamnose. A 20 milliliter volume of cell culture was standardized to OD600 0.9, followed by washing the cells twice in ice cold H20 at 16,000 × g for 3 min. Cells were resuspended in 1,000 μl of H20 and boiled at 100°C for 10 min. To the same tube, 1,000 μl of ice cold 65% ethanol was added, and the suspension was vortexed for 15 s and centrifuged (16,000 × g, 3 min). The supernatant containing extracted c-di-GMP was transferred to a new microfuge tube, and the extraction procedure was repeated. The supernatant from the two extractions was dried using a vacuum centrifuge (Thermo, SpeedVac). The visible white pellet, nucleotide extract, was resuspended in 200 μl H20, followed by filtering (0.2 um filter, GE). The nucleotide extract was stored at −80°C until used. Using reverse-phase high-performance liquid chromatography (RP-HPLC), c-di-GMP was detected and quantified by injecting 40 μl of nucleotide extract into the column.
Detection and quantification of intracellular c-di-GMP was performed using a Waters HPLC Separation Module 2695 equipped with an autosampler, degasser and UV/Vis detector set to 256 nm (PDA Detector Model 2996). Separation of molecules in the extract was achieved by μBondapak™ Waters C18 (3.9 × 300 mm) column particle diameter of 15–20 μm, with 125 Å pores at a flow rate of 1 ml min−1. Solvents containing methanol 100% (solvent A) and trifluoroacetic acid 0.075% (solvent B) were used. To elute c-di-GMP, the following gradient was used: 0.01–10 min, 5% solvent A (= 5% solvent A and 95% solvent B); 10–20 min, 5–20% solvent A; 20–25 min, 20% solvent A, 25–30 min, 20–5% solvent A. The retention time of standard c-di-GMP was observed ~7.6 min and the UV spectra 200–600 nm was used to confirm the identity of c-di-GMP. The area under the curve or peak was used to quantify the c-di-GMP concentration, and change in the c-di-GMP levels in strains are represented in percentage.
Bioinformatics Analysis
The amino acid sequence of c-di-GMP related proteins were retrieved from the Burkholderia.com and UniProt databases (Wu et al., 2006; Winsor et al., 2008). Domains in putative c-di-GMP metabolizing proteins were predicted using the SMART (Simple Modular Architecture Research Tool) program (Schultz et al., 2000; Letunic et al., 2015). To perform alignment of amino acid sequence, a multiple sequence alignment program, MAFFT, was used (Katoh and Standley, 2013). Further, the alignment viewer tool AliView was utilized to observe GGDEF and EAL motifs in the multiple aligned sequence (Larsson, 2014).
Statistical Analysis
An unpaired Student's t-test was used to analyze data from two groups, and one way ANOVA followed by the Dunnett's multiple comparisons test was used to analyze data for more than two groups. P-values were calculated using GraphPad Prism version 6 for Windows 7, GraphPad Software (La Jolla California USA). Differences were considered significant when the P-value was less than 0.01.
Results
Increased c-di-GMP Levels Regulate Swimming Motility of B. cenocepacia K56-2
Modulation of c-di-GMP levels can change flagellar gene expression (Hickman and Harwood, 2008) or affect flagellar rotation, resulting in altered motility (Boehm et al., 2010; Baraquet and Harwood, 2013). In a previous study, we showed that the CF sputum nutritional conditions increased the swimming motility of B. cenocepacia K56-2 through upregulated flagellin expression and a change in the flagellation pattern (Kumar and Cardona, 2016). However, in the presence of individual amino acids, the motility of the K56-2 strain was upregulated independent of flagellin expression, possibly through the mechanism of regulation of flagellar function. To address whether c-di-GMP regulates the swimming motility of B. cenocepacia, we cloned the wspR gene from P. aeruginosa PAO1 and overexpressed it in B. cenocepacia K56-2 (WT). The wspR gene encodes a well-characterized diguanylate cyclase enzyme that modulate intracellular c-di-GMP levels by synthesizing c-di-GMP (Hickman et al., 2005). Next, we evaluated the swimming phenotype of B. cenocepacia overexpressing WspR in SCFM, which mimics CF sputum nutritional conditions (Palmer et al., 2007b). Figure 1A shows that the swimming motility of the WT/pWspR strain is reduced under the WspR protein overexpression condition. Similarly, when WspR was overexpressed in P. aeruginosa PAO1, overexpression of WspR decreased swimming motility (Supplementary Figure 1).
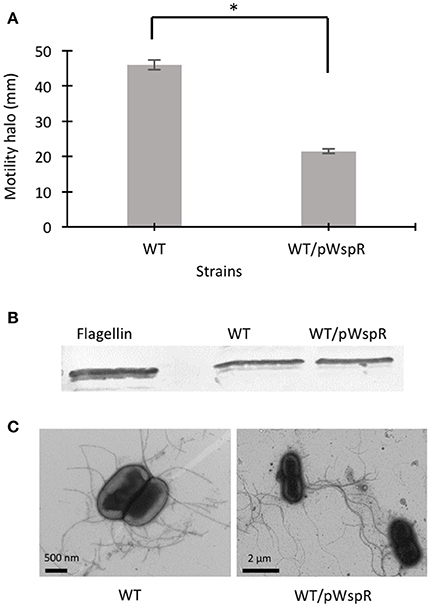
Figure 1. The effect of c-di-GMP modulating conditions on swimming motility, flagellin expression, and flagella biosynthesis of B. cenocepacia K56-2 WT. (A) Motility of the strains was examined in semi-solid SCFM 0.3% agar plates after 24 h at 37°C. Experiments were performed three-times independently in duplicate. *Denotes significant p-values (p < 0.01). (B) Whole cell lysates were used to separate proteins by 12% SDS-PAGE followed by detection of flagellin protein using anti-flagellin primary antibody and alkaline phosphatase crosslinked to secondary antibody. From two Western blots, one representative experiment is shown. (C) Electron micrographs of uranyl acetate stained WT strains displaying flagella under c-di-GMP varying conditions.
To confirm that overexpression of the WspR protein changed the c-di-GMP levels in the WT/pWspR strain, we measured intracellular c-di-GMP levels in the WT/pWspR strain by high-pressure liquid chromatography (HPLC). The chromatogram of the WT/pWspR nucleotide extract shows an increased peak at the retention time of ~7.6 min, which corresponds to the standard control elution time, in comparison to the WT nucleotide extract (Supplementary Figure 2). The UV trace of the c-di-GMP standard corresponds to the peak of WT/pWspR (Supplementary Figures 2B,C). To confirm that the observed peak corresponded to c-di-GMP, we spiked the nucleotide extracts of strains with c-di-GMP. The same peak with a higher intensity was observed, confirming that the elution time and UV trace corresponded to c-di-GMP.
To investigate if induced intracellular c-di-GMP levels affect flagellar function in the WT strain at the post-translational level, we detected flagellin protein expression by Western blot and observed the presence of flagella by electron microscopy. The flagellin levels in the WT/pWspR strain were similar to the WT strain (Figure 1B, Supplementary Figure 1). The slight difference between the migration of the native flagellin and the recombinant protein control expressed in E. coli is likely due to the flagellin glycosylation of B. cenocepacia (Hanuszkiewicz et al., 2014). In agreement with the lack of upregulation of flagellar biosynthesis, the flagellation patterns of the WT and c-di-GMP modulating strain WT/pWspR was similar, as observed by electron microscopy (Figure 1C). Taken together, these results indicate that the induced c-di-GMP levels reduce the motility of B. cenocepacia K56-2 (WT) strain through a mechanism that is independent of flagellin expression levels or changes in the flagellation pattern.
Mutational Analysis of GGDEF/EAL-Signaling-Domain Coding Genes Reveals that BCAL1069 Plays a Role in the Swimming Motility of B. cenocepacia K56-2
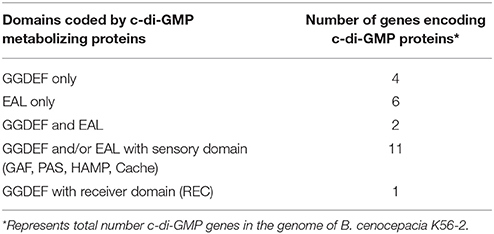
C-di-GMP metabolizing protein encoding genes are numerous, ranging from ~30 to 50 in pathogenic bacteria such as E. coli, P. aeruginosa, and Vibrio cholerae (Waters et al., 2008; Ha and O'Toole, 2015; Povolotsky and Hengge, 2015). Despite a plethora of c-di-GMP data on other pathogenic bacteria, our knowledge regarding the roles of putative c-di-GMP metabolic genes in adaptation to the host environment is limited in B. cenocepacia. We used the annotated genome of B. cenocepacia J2315 to identify putative c-di-GMP genes, which are assigned based on the presence of c-di-GMP metabolizing domains. The Burkholderia database (Winsor et al., 2008) was used to retrieve c-di-GMP related homologous genes from the genome of B. cenocepacia K56-2. In total, we identified 24 putative c-di-GMP-related genes in the genome of the K56-2 strain (Table 2). These genes encode GGDEF and/or EAL domain(s), but not HD-GYP domain, which is another c-di-GMP degrading domain. We then used the SMART bioinformatics software (Schultz et al., 2000; Letunic et al., 2015) to predict signaling (sensory or receiver) domains present in conjunction with the c-di-GMP metabolizing domains (Table 2). Twelve of the c-di-GMP genes encode sensory or receiver domains on their N-terminus in addition to the GGDEF and/or EAL domains (Figure 2A). Because we were interested in determining the role of signaling domain-containing c-di-GMP proteins in sensing nutritional cues present in CF sputum conditions and modulation of swimming motility, these 12 putative c-di-GMP metabolic proteins were chosen for further investigation. Among the sensory domains contained in the c-di-GMP proteins, the PAS and Cache domains are the most common (Table 2). Also, more than half of these proteins possess a transmembrane region, suggesting a periplasmic localization of some of these domains (Figure 2A). Among those, the only gene with an experimentally confirmed function in B. cenocepacia is rpfR (BCAM0580). RpfR is a PAS-GGDEF-EAL domain-containing protein that positively regulates swarming motility, protease activity, biofilm formation, and virulence (Deng et al., 2012). To find out whether the 12 sensory domain-containing c-di-GMP proteins mediate a change in intracellular c-di-GMP levels in response to CF sputum nutritional conditions, we created 12 c-di-GMP mutants using insertional mutagenesis and examined their swimming motility in SCFM. Except for BCAL1069, all other putative c-di-GMP synthesizing/degrading genes are not present in an operon. Therefore, a polar effect on downstream genes was not expected. All the c-di-GMP mutants showed growth kinetics similar to the WT strain in CF sputum nutritional conditions (Supplementary Figure 3). Among 12 c-di-GMP mutants, the WT::BCAM1161, WT::BCAM0580 (rpfR) and WT::BCAL1069 (cdpA) mutants exhibited a reduction in swimming motility in SCFM (Figure 2B). However, the WT::BCAL1069 (cdpA) mutant showed the most significant defect in swimming motility. The BCAL1069 protein is a homolog (86% identical) of CdpA that has been previously characterized in B. pseudomallei KHW (Lee et al., 2010). To rule out that phenotypes observed in WT::BCAL1069 (cdpA) could be caused by inactivation of the downstream gene BCAL1068, we created the insertional mutant WT::BCAL1068. The decrease in motility was not due to polar effects on BCAL1068 as the swimming motility of the WT::BCAL1068 mutant was similar to the WT strain (Supplementary Figure 4). To confirm that the reduced motility phenotype of WT::BCAL1069 (cdpA) mutant was due to the disruption of the BCAL1069 gene, we complemented the mutant with BCAL1069 (cdpA) in trans. The complemented strain showed partial restoration of the swimming motility, demonstrating that the defect in WT::BCAL1069 (cdpA) motility is due to the inactivation of the BCAL1069 (cdpA) gene (Figure 3A). In our previous study, we showed that SCFM conditions induced swimming motility through an increased flagellin protein expression (Kumar and Cardona, 2016). Then, we considered whether the mutation in BCAL1069 (cdpA) in the WT::BCAL1069 (cdpA) mutant affects flagellin expression and flagellation. Western blot and electron microscopy analysis demonstrated no difference in flagellin expression and flagella biosynthesis (Figures 3B,C, Supplementary Figure 6). Together, our results suggest that BCAL1069 (cdpA) positively regulates swimming motility of B. cenocepacia K56-2 in CF sputum nutritional conditions, without affecting flagellin expression.
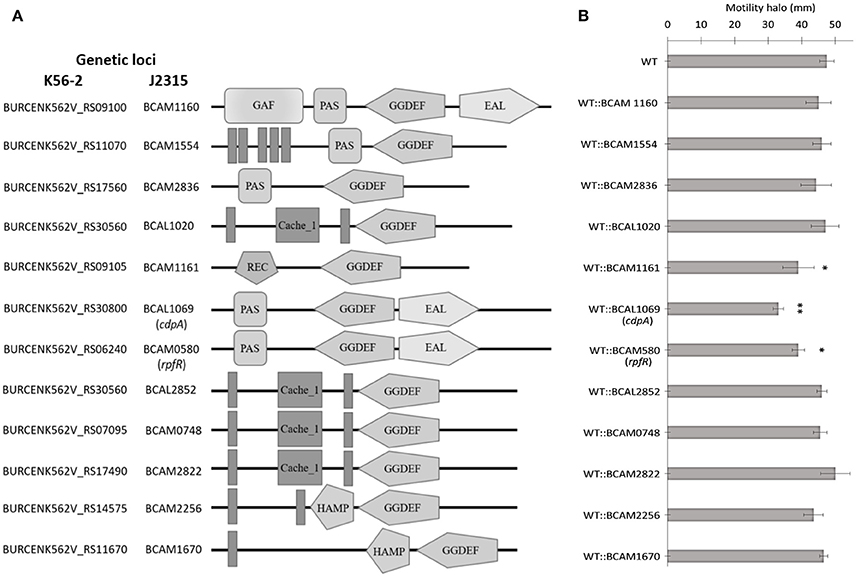
Figure 2. Predicted domains coded by c-di-GMP metabolic proteins and their role in swimming motility of B. cenocepacia K56-2 WT. (A) Putative proteins with c-di-GMP modulating and signal transduction domains as predicted by SMART. Amino acid sequences were obtained from the genome of B. cenocepacia J2315. EAL, putative diguanylate phosphodiesterase; GG(D/E)EF, putative diguanylate cyclase; PAS, Per/ARNT/Sim; GAF, cGMP-specific phosphodiesterase/Adenyly cyclase/FhlA; REC, cheY-homologous receiver domain; Cache, CAlcium channels and CHEmotaxis receptors; HAMP, Histidine kinases/Adenylate cyclases/Methyl accepting proteins/Phosphatases; Vertical bars, transmembrane domain. Domains are not drawn to scale. (B) The swimming motility of the mutants was examined in semi-solid SCFM 0.3% agar plates after 24 h at 37°C. Three independent experiments were performed in duplicates (*p < 0.01; **p < 0.001).
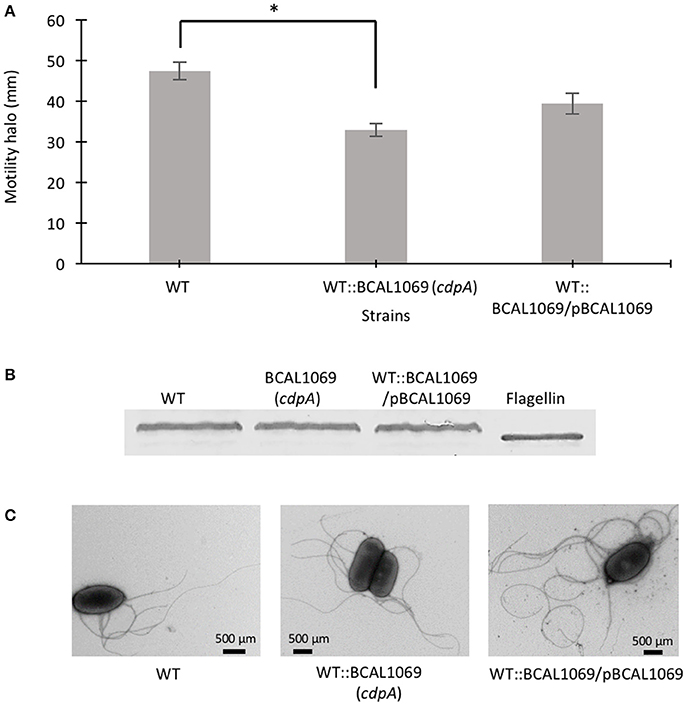
Figure 3. Role of BCAL1069 (cdpA) motility in swimming motility. (A) The swimming motility of the strains was examined in semi-solid SCFM 0.3% agar plates at 37°C after 24 h. The motility assay was performed three-times independently in duplicates (*p < 0.01). (B) To detect flagellin protein, proteins from whole cell lysates of strains were separated on 12% SDS-PAGE followed by flagellin detection using anti-flagellin primary antibody and alkaline phosphatase cross linked to secondary antibody. Western blots were performed twice. One representative experiment is shown. (C) The presence of flagella in the WT and mutant strains were observed using transmission electron microscope.
BCAL1069 Negatively Regulates Intracellular c-di-GMP Levels in B. cenocepacia K56-2
Further analysis of the BCAL1069 (cdpA) protein sequence shows that the protein contains a PAS (Per/Arnt/Sim) domain at the N-terminus, which is fused to the GGDEF and EAL domains. The protein has no predicted transmembrane-spanning regions, indicating a cytoplasmic location (Figure 2A). The PAS domain, which senses small molecule ligands, is widely present in sensory and signal transduction proteins in bacteria (Ulrich et al., 2005). A multiple sequence alignment of signature motifs (Katoh and Standley, 2013; Larsson, 2014) of the BCAL1069 protein (CdpA) and other GGDEF domain-containing proteins indicates that the GGDEF domain of BCAL1069 is an enzymatically inactive variant, since a degenerate GGDEF motif was observed (Figure 4A). In Caulobacter crescentus, a similar catalytically inactive variant of the GGDEF motif was detected in a c-di-GMP metabolizing protein, CC3396 (Christen et al., 2005). The presence of a PAS domain and an intact EAL motif in BCAL1069 suggests that this protein is likely a PDE in which the c-di-GMP degradation catalytic activity responds to an extracellular signal.
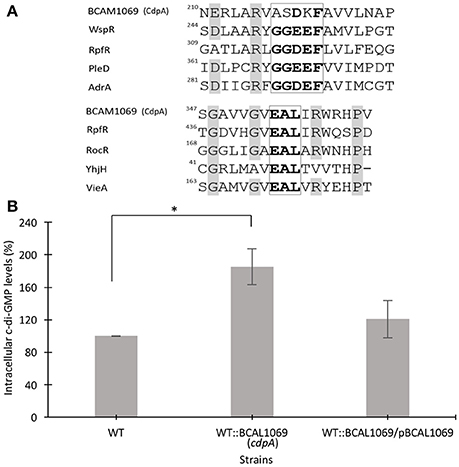
Figure 4. Multiple sequence alignment of residues of signature motifs of BCAL1069 (CdpA) and determination of intracellular levels of c-di-GMP in the BCAL1069 mutant. (A) Partial alignment of BCAL1069 amino acid sequence with GGDEF and EAL domain-containing proteins, retrieved from the UniProt database. The amino acids in the GGDEF and EAL motifs are highlighted in bold. Other conserved amino acids are shaded in gray. The numbers show the position of the amino acid in the protein. BCAL1069, UniProt Id B4ED05; WspR, UniProt Id Q9HXT9; RpfR, UniProt Id B4EKM4; PleD, UniProt Id Q9A515; AdrA, UniProt Id Q9L401; RocR, UniProt Id Q9HX69; YhjH, UniProt Id P37646; VieA, UniProt Id O68318. (B) Relative intracellular c-di-GMP levels in the WT:BCAL1069 mutant. Percentage represents area under the c-di-GMP curve of the strain relative to the WT strain. Three independent experiments were performed. *Denotes significant p-values (p < 0.01).
The presence of an inactive GGDEF and an active EAL domain in the BCAL1069 protein motivated us to investigate the intracellular c-di-GMP levels in the WT::BCAL11069 (cdpA) mutant. We extracted the nucleotide pools from the WT, WT::BCAL1069 (cdpA) and complemented WT::BCAL1069/pBCAL1069 strains grown in SCFM. The HPLC data showed that the insertional mutagenesis in BCAL1069 resulted in approximately two-fold increase in the intracellular c-di-GMP levels (Figure 4B). Moreover, in trans expression of the BCAL1069 gene in the mutant restored intracellular c-di-GMP levels similar to the WT strain, suggesting that the increased levels of c-di-GMP are caused by interruption of the BCAL1069 gene (Figure 4B). Taken together, the increased intracellular c-di-GMP levels of the WT::BCAL1069 mutant suggest that the BCAL1069 protein possesses PDE activity.
BCAL1069 Protein Regulates Swimming Motility in Response to Arginine and Glutamate Amino Acids
To identify if the BCAL1069 protein (CdpA) protein senses particular nutritional cues in CF sputum nutritional conditions, the swimming motility of the WT::BCAL1069 mutant was examined in glucose-minimal medium supplemented with individual amino acids at the same concentrations as found in SCFM. The amino acids, arginine, glutamate, histidine, phenylalanine, and proline, were chosen because the swimming motility of B. cenocepacia K56-2 was increased in the presence of those amino acids, compared with that of cells in the presence of glucose alone (Kumar and Cardona, 2016). The swimming motility of WT and WT::BCAL1069 was similar in the presence of glucose with or without histidine, phenylalanine, or proline (Figures 5A,D–F). However, the swimming motility of the BCAL1069 mutant was not influenced by arginine or glutamate as it was for the wild type (Figures 5A–C,G). Moreover, complementation with multicopy BCAL1069 restored the effect of arginine or glutamate to the mutant strain (Figure 5G). The lack of swimming motility changes of the WT::BCAL1069 mutant in response to arginine and glutamate was not due to impaired growth, since similar growth kinetics of the WT, WT::BCAL1069, and complemented mutant strains were observed in the medium (Figure 5H). Taken together, these results suggest that BCAL1069 is either directly or indirectly involved in sensing the presence of arginine and glutamate and induce swimming motility in B. cenocepacia K56-2.
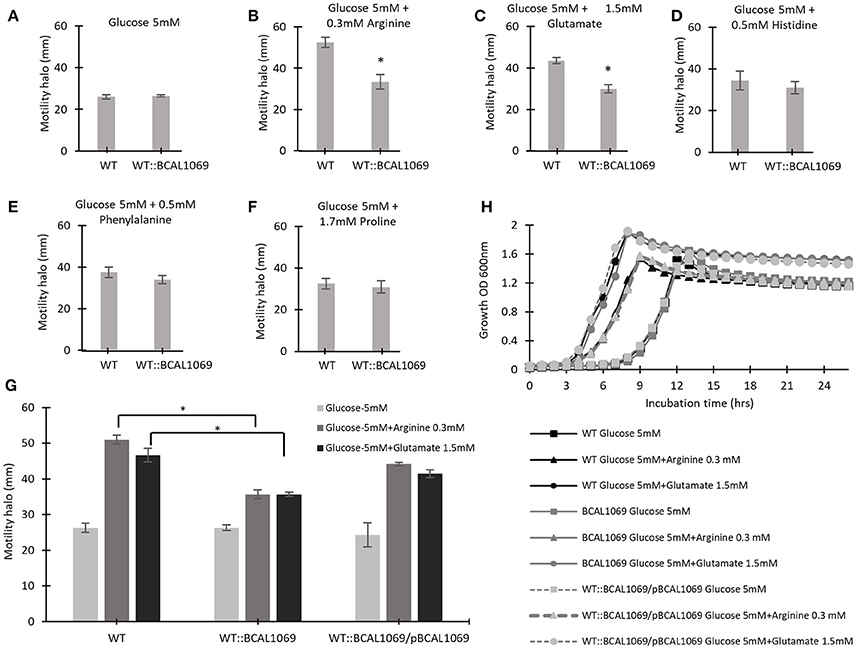
Figure 5. The effect of individual amino acids on the swimming motility of WT and WT::BCAL1069 mutant. (A–G) The motility halos of the indicated strains were examined in MOPS-glucose 5 mM with individual amino acids at the same concentration as present in SCFM, after incubation for 24 h at 37°C. Each experiment was performed three times independently in duplicate (*p < 0.01). (H) Growth curves of WT, WT::BCAL1069 and WT::BCAL1069/pBCAL1069 in MOPS-glucose 5 mM with individual amino acid at the same concentration as present in SCFM. The figure is representative of one from two independent experiments.
BCAL1069 Protein Regulates Protease Activity in B. cenocepacia K56-2
Protease activity and biofilm formation have been associated with c-di-GMP regulatory networks (Deng et al., 2012; Ryan et al., 2015). To study the functional relationship between the BCAL1069 (CdpA) protein and virulence factors, we investigated whether BCAL1069 regulates protease activity and biofilm formation in B. cenocepacia K56-2. To examine protease activity, a clear zone around inoculated bacterial spots in the skim milk plates was measured at two different time points. After 24 h, low level of protease activity was observed for WT and complemented strains. To quantify the protease activity of the mutants, clear zones were observed for the strains after 48 h of incubation (Figure 6A). The WT::BCAL1069 (cdpA) mutant showed a significant reduction in protease activity in comparison to the WT strain (Figure 6B). In the complemented WT::BCAL1069/pBCAL1069 strain, the defect in protease activity was restored to WT levels, suggesting the reduced protease activity is caused by insertional mutagenesis of BCAL1069 (Figure 6B). When the biofilm formation ability of the WT::BCAL1069 (cdpA) mutant was analyzed in comparison to the WT strain, there was no significant difference observed (Supplementary Figure 5). Taken together, the BCAL1069 regulates protease activity, but not biofilm formation in B. cenocepacia K56-2.
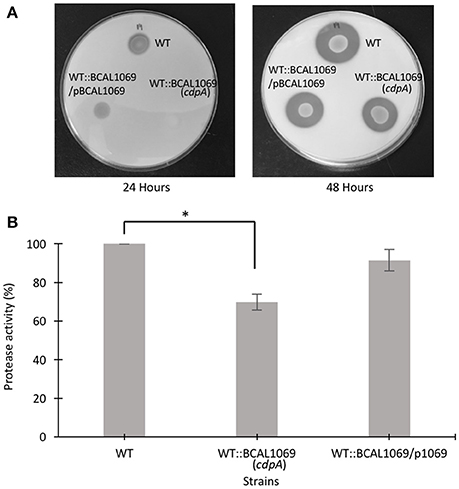
Figure 6. Protease activity of B. cenocepacia K56-2 WT, WT::BCAL1069 c-di-GMP mutant and complemented mutant. (A) Protease activity of strains on 2% skim milk agar plates after 24 and 48 h at 37°C. One representative of four independent experiments is shown. (B) Quantitative analysis of proteolytic activity after 48 h on 2% skim milk agar plates. Protease activity is represented in percentage relative to the WT strain. The protease assay was performed four times independently with three technical replicates per experiment (*p < 0.01).
Discussion
C-di-GMP, a second messenger intracellular signaling molecule, is involved in the regulation of a wide range of bacterial processes and its metabolizing proteins are part of signal transduction pathways (Römling et al., 2013). The intracellular levels of this molecule are modulated by GGDEF or EAL domain-containing c-di-GMP proteins. The c-di-GMP metabolizing domains, GGDEF, and/or EAL, have been identified in conjunction with signaling (sensory and receiver) domains. Sensory and receiver domains are an integral part of signal transduction pathways and mechanisms (Galperin et al., 2001). For instance, in P. aeruginosa, WspR and RocR c-di-GMP proteins are comprised of a receiver (REC) in addition to the GGDEF or EAL domain (Hickman et al., 2005; Rao et al., 2008). On the contrary, the STM1987 protein of S. Typhimurium possesses sensory Cache1 and GGDEF domains and is involved in cellulose synthesis associated with biofilm formation (Mills et al., 2015). Other sensory domains are also linked to the c-di-GMP metabolizing domains, such as HK, PAS, GAF, HAMP, and REC (Galperin et al., 2001; Galperin, 2006).
Studies have shown that c-di-GMP plays a role in swimming and swarming motility, protease activity, biofilm formation, and virulence in several bacteria (Deng et al., 2012; Wei et al., 2016; Plumley et al., 2017). For instance, P. aeruginosa induces c-di-GMP levels through two c-di-GMP genes, sadC and siaD, to upregulate biofilm formation and counter stress when exposed to tellurite (Chua et al., 2015). A systematic analysis of 11 GGDEF, EAL, and signaling domain-containing proteins identified two c-di-GMP mutants (XOC_2393 and XOC_4190), which positively modulated swimming motility and virulence in the rice pathogen, X. oryzae (Wei et al., 2016). In B. pseudomallei, two PDE, I2284::T24 (cdpA) and I2285::T24, encoding genes positively modulate swimming motility irrespective of temperature (30°C and 37°C), whereas, a diguanylate cyclase mutant, II2523::T24, regulates biofilm formation in a temperature dependent manner (Plumley et al., 2017). Moreover, CdpA regulated flagellum biosynthesis, motility, biofilm formation, aggregation, and cell invasion (Lee et al., 2010). Despite the established role of c-di-GMP genes in motility and the presence of 24 c-di-GMP genes in Burkholderia genomes, only one c-di-GMP gene, rpfR (BCAM0580), has been characterized in B. cenocepacia previously to this study. This gene encodes PAS-GGDEF-EAL domains. The interaction of Burkholderia diffusible signal factor with the PAS domain of RpfR resulted in decreased intracellular c-di-GMP levels, which consequently regulated swarming motility, biofilm formation, and protease activity (Deng et al., 2012). However, the genome of B. cenocepacia K56-2 encodes 12 c-di-GMP proteins that also code for sensory domain, including the RpfR (BCAM0580) protein (Table 2 and Figure 2A). The identification of sensory domains in c-di-GMP-related proteins in B. cenocepacia indicates the presence of signal transduction pathways that may sense external signals to control swimming motility through a c-di-GMP turnover.
In the present study, we created 12 c-di-GMP insertional mutants and examined their swimming motility in CF sputum nutritional conditions. Three c-di-GMP mutants, WT::BCAM0580 (rpfR), WT::BCAM1161, and WT::BCAL1069, demonstrated a reduction in the swimming motility phenotype (Figure 2B). We chose the WT::BCAL1069 mutant, a homolog of the cdpA gene in B. pseudomallei KHW, for further studies, since the WT::BCAL1069 mutant displayed the most significant swimming motility defect (Figure 2B). Like rpfR, the BCAL069 (cdpA) gene is involved in the regulation of protease activity; however, BCAL1069 is not associated to biofilm formation in B. cenocepacia under the conditions tested (Figure 6 and Supplementary Figure 5). Unlike the BCAL1069 protein (CdpA) in B. cenocepacia K56-2, the B. pseudomallei KHW CdpA positively modulated biofilm formation (Lee et al., 2010). Moreover, the BCAL1069 mutant showed an increase in intracellular c-di-GMP levels, which could be explained by the presence of an active EAL domain and a degenerate GGDEF domain (Figure 4). The presence of a sensory PAS domain in the BCAL1069 protein (CdpA; Figure 2A), suggested that signal molecules may act to induce conformational changes to its linked enzymatic domain (Ulrich et al., 2005; Deng et al., 2012). We then hypothesized that the BCAL1069 protein responds to specific nutritional cues in CF sputum conditions, namely amino acids. To investigate if the PAS domain-containing BCAL1069 protein senses any specific amino acids in CF sputum conditions, swimming motility of the WT::BCAL1069 mutant was examined in glucose-minimal medium supplemented with individual amino acids. Our results suggested that swimming motility of the WT::BCAL1069 mutant was not influenced in the presence of arginine and glutamate (Figure 5). These results indicate that BCAL1069 (CdpA) has a role in mediating B. cenocepacia swimming motility by sensing the amino acids arginine and glutamate. It would be useful to further demonstrate if the PAS domain of BCAL1069 (CdpA) protein directly senses amino acids, arginine and glutamate. Other c-di-GMP proteins have been shown to directly or indirectly sense amino acids to modulate intracellular c-di-GMP levels, transducing the signal into an increase in cellulose synthesis (Mills et al., 2015) and biofilm dispersion (Basu Roy and Sauer, 2014).
C-di-GMP regulatory networks are comprised of signal transduction systems, where c-di-GMP regulate a biological process by binding to diverse c-di-GMP receptors at multiple steps (Orr et al., 2016). Once c-di-GMP is bound to its receptors, the resulting complex controls and affects bacterial phenotype(s) or cellular processes at the level of transcription, translation or post translation (Shanahan and Strobel, 2012). Swimming motility is one of the bacterial phenotypes regulated by c-di-GMP at the transcriptional and post-translational level under elevated c-di-GMP levels. For instance, in P. aeruginosa, the FleQ master regulator downregulates expression of flagellar genes (Hickman and Harwood, 2008; Baraquet and Harwood, 2013). Whereas, in E. coli, the interaction of c-di-GMP with flagella motors and YcgR proteins control functioning of flagella (Fang and Gomelsky, 2010; Paul et al., 2010). We demonstrated that the high intracellular c-di-GMP levels in the WT/pWspR strain reduce swimming motility without affecting the flagellin expression and flagellar biosynthesis, suggesting a post translational regulation of swimming motility in B. cenocepacia K56-2 (Figure 1 and Supplementary Figure 2). Further, our results showed that the swimming motility of WT::BCAL1069 (cdpA) mutant was reduced without changing the flagellin expression and flagellation, while the mutant displayed increased intracellular levels of c-di-GMP (Figures 3, 4). This indicates that c-di-GMP regulates swimming motility by induced intracellular c-di-GMP levels independent of flagellin expression. Noticeably, in B. pseudomallei KHW, the cdpA mutant exhibited aflagellate phenotype (Lee et al., 2010), which is in contrast to the WT:: BCAL1069 (cdpA) mutant flagellate phenotype observed in this study (Figure 3). This difference in swimming motility at the level of flagella biosynthesis in the two cdpA mutants suggests that c-di-GMP signaling transduction pathways can regulate motility through gene expression, assembly of flagellar proteins or flagellar functioning despite belonging to two species of the same genus. Bacteria residing in diverse ecological niches develop more complex signaling systems than those that dwell in stable environmental settings (Galperin, 2005). Burkholderia species have been isolated from diverse ecological niches, such as soil, water bodies, rhizosphere, and lungs of CF patients (Mahenthiralingam et al., 2005). When B. cenocepacia encounters the host environment, there must be mechanisms in place that allow the cells to sense the lung nutritional conditions, which is rich in amino acids. Once B. cenocepacia establishes an infection in the lungs of CF patients, bacteria may undergo distinct adaptive strategies, including mutations in motility related regulators (Lee et al., 2017). These late genetic mutations indicate that B. cenocepacia can adapt to the CF lungs and loss motility phenotype during chronic infection to evade host immune response. The host Toll-like receptor 5 recognizes flagellin as a ligand to induce the inflammatory defense response to eradicate pathogens (Hayashi et al., 2001). Previously, we demonstrated that the CF sputum conditions induce swimming motility, flagellin expression and multiple flagellation through the flhF gene in B. cenocepacia K56 (Kumar and Cardona, 2016). In this study, we unravel another link between B. cenocepacia swimming motility and nutritional cues in CF conditions, by establishing the integration of the second signal messenger c-di-GMP into the swimming motility response to amino acids. We propose that sensing of nutritional cues in the CF lung, followed by an increase in c-di-GMP-mediated swimming motility, plays an important role in the establishment of the initial stage of B. cenocepacia infection. It remains to be determined if the other c-di-GMP genes, not investigated in this study, also have a role in virulence and swimming motility.
Author Contributions
BK: Designed and performed the experiments, interpreted the data, and wrote the manuscript; JS: Overviewed the HPLC experiments and edited the final version of the manuscript; SC: Conceived the research approach, contributed to writing, and edited the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Tasia Lightly and Andrew Hogan for critically reading this manuscript. We also thank to Dr. David Speert for sharing the flagellin antibody. This study was supported by a discovery grant from the Natural Sciences and Engineering Research Council (NSERC) to SC. BK was supported by funding provided by Faculty of Science, University of Manitoba, Graduate Enhancement of Tri-Council Stipends, and TransCanada Pipelines Graduate Fellowship.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2018.00056/full#supplementary-material
References
Baraquet, C., and Harwood, C. S. (2013). Cyclic diguanosine monophosphate represses bacterial flagella synthesis by interacting with the Walker A motif of the enhancer-binding protein FleQ. Proc. Natl. Acad. Sci. U.S.A. 110, 18478–18483. doi: 10.1073/pnas.1318972110
Basu Roy, A., and Sauer, K. (2014). Diguanylate cyclase NicD-based signalling mechanism of nutrient-induced dispersion by Pseudomonas aeruginosa. Mol. Microbiol. 94, 771–793. doi: 10.1111/mmi.12802
Boehm, A., Kaiser, M., Li, H., Spangler, C., Kasper, C. A., Ackermann, M., et al. (2010). Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141, 107–116. doi: 10.1016/j.cell.2010.01.018
Camilli, A., and Bassler, B. L. (2006). Bacterial small-molecule signaling pathways. Science 311, 1113–1116. doi: 10.1126/science.1121357
Cardona, S. T., and Valvano, M. A. (2005). An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid 54, 219–228. doi: 10.1016/j.plasmid.2005.03.004
Christen, M., Christen, B., Folcher, M., Schauerte, A., and Jenal, U. (2005). Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 280, 30829–30837. doi: 10.1074/jbc.M504429200
Chua, S. L., Sivakumar, K., Rybtke, M., Yuan, M., Andersen, J. B., Nielsen, T. E., et al. (2015). C-di-GMP regulates Pseudomonas aeruginosa stress response to tellurite during both planktonic and biofilm modes of growth. Sci. Rep. 5:10052. doi: 10.1038/srep10052
Datsenko, K. A., and Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645. doi: 10.1073/pnas.120163297
Deng, Y., Schmid, N., Wang, C., Wang, J., Pessi, G., Wu, D., et al. (2012). Cis-2-dodecenoic acid receptor RpfR links quorum-sensing signal perception with regulation of virulence through cyclic dimeric guanosine monophosphate turnover. Proc. Natl. Acad. Sci. U.S.A. 109, 15479–15484. doi: 10.1073/pnas.1205037109
De Smet, B., Mayo, M., Peeters, C., Zlosnik, J. E., Spilker, T., Hird, T. J., et al. (2015). Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov., two novel Burkholderia cepacia complex species from environmental and human sources. Int. J. Syst. Evol. Microbiol. 65, 2265–2271. doi: 10.1099/ijs.0.000251
Drevinek, P., Holden, M. T., Ge, Z., Jones, A. M., Ketchell, I., Gill, R. T., et al. (2008). Gene expression changes linked to antimicrobial resistance, oxidative stress, iron depletion and retained motility are observed when Burkholderia cenocepacia grows in cystic fibrosis sputum. BMC Infect. Dis. 8:121. doi: 10.1186/1471-2334-8-121
Eberl, L., and Vandamme, P. (2016). Members of the genus Burkholderia: good and bad guys. F1000Res 5:F1000 Faculty Rev-1007. doi: 10.12688/f1000research.8221.1
Fang, X., and Gomelsky, M. (2010). A post-translational, c-di-GMP-dependent mechanism regulating flagellar motility. Mol. Microbiol. 76, 1295–1305. doi: 10.1111/j.1365-2958.2010.07179.x
Figurski, D. H., and Helinski, D. R. (1979). Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U.S.A. 76, 1648–1652. doi: 10.1073/pnas.76.4.1648
Flannagan, R. S., Aubert, D., Kooi, C., Sokol, P. A., and Valvano, M. A. (2007). Burkholderia cenocepacia requires a periplasmic HtrA protease for growth under thermal and osmotic stress and for survival in vivo. Infect. Immun. 75, 1679–1689. doi: 10.1128/IAI.01581-06
Galperin, M. Y. (2005). A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 5:35. doi: 10.1186/1471-2180-5-35
Galperin, M. Y. (2006). Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J. Bacteriol. 188, 4169–4182. doi: 10.1128/JB.01887-05
Galperin, M. Y., Nikolskaya, A. N., and Koonin, E. V. (2001). Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol. Lett. 203, 11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x
Ha, D. G., and O'Toole, G. A. (2015). c-di-GMP and its effects on biofilm formation and dispersion: a Pseudomonas aeruginosa review. Microbiol. Spectr. 3:MB-0003–2014. doi: 10.1128/microbiolspec.MB-0003-2014
Hanuszkiewicz, A., Pittock, P., Humphries, F., Moll, H., Rosales, A. R., Molinaro, A., et al. (2014). Identification of the flagellin glycosylation system in Burkholderia cenocepacia and the contribution of glycosylated flagellin to evasion of human innate immune responses. J. Biol. Chem. 289, 19231–19244. doi: 10.1074/jbc.M114.562603
Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., et al. (2001). The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103. doi: 10.1038/35074106
Henry, J. T., and Crosson, S. (2011). Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu. Rev. Microbiol. 65, 261–286. doi: 10.1146/annurev-micro-121809-151631
Hickman, J. W., and Harwood, C. S. (2008). Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 69, 376–389. doi: 10.1111/j.1365-2958.2008.06281.x
Hickman, J. W., Tifrea, D. F., and Harwood, C. S. (2005). A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc. Natl. Acad. Sci. U.S.A. 102, 14422–14427. doi: 10.1073/pnas.0507170102
Holoway, B. W. (1955). Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13, 572–581. doi: 10.1099/00221287-13-3-572
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kumar, B., and Cardona, S. T. (2016). Synthetic cystic fibrosis sputum medium regulates flagellar biosynthesis through the flhF gene in Burkholderia cenocepacia. Front. Cell. Infect. Microbiol. 6:65. doi: 10.3389/fcimb.2016.00065
Larsson, A. (2014). AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30, 3276–3278. doi: 10.1093/bioinformatics/btu531
Lee, A. H., Flibotte, S., Sinha, S., Paiero, A., Ehrlich, R. L., Balashov, S., et al. (2017). Phenotypic diversity and genotypic flexibility of Burkholderia cenocepacia during long-term chronic infection of cystic fibrosis lungs. Genome Res. 27, 650–662. doi: 10.1101/gr.213363.116
Lee, H. S., Gu, F., Ching, S. M., Lam, Y., and Chua, K. L. (2010). CdpA is a Burkholderia pseudomallei cyclic di-GMP phosphodiesterase involved in autoaggregation, flagellum synthesis, motility, biofilm formation, cell invasion, and cytotoxicity. Infect. Immun. 78, 1832–1840. doi: 10.1128/IAI.00446-09
Letunic, I., Doerks, T., and Bork, P. (2015). SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 43, D257–D260. doi: 10.1093/nar/gku949
Mahenthiralingam, E., Coenye, T., Chung, J. W., Speert, D. P., Govan, J. R., Taylor, P., et al. (2000). Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J. Clin. Microbiol. 38, 910–913.
Mahenthiralingam, E., Urban, T. A., and Goldberg, J. B. (2005). The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3, 144–156. doi: 10.1038/nrmicro1085
Merritt, J. H., Kadouri, D. E., and O'Toole, G. A. (2005). Growing and analyzing static biofilms. Curr. Protoc. Microbiol. Chapter 1:Unit 1B.1. doi: 10.1002/9780471729259.mc01b01s00
Miller, V. L., and Mekalanos, J. J. (1988). A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170, 2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988
Mills, E., Petersen, E., Kulasekara, B. R., and Miller, S. I. (2015). A direct screen for c-di-GMP modulators reveals a Salmonella Typhimurium periplasmic L-arginine-sensing pathway. Sci. Signal. 8:ra57. doi: 10.1126/scisignal.aaa1796
Orr, M. W., Galperin, M. Y., and Lee, V. T. (2016). Sustained sensing as an emerging principle in second messenger signaling systems. Curr. Opin. Microbiol. 34, 119–126. doi: 10.1016/j.mib.2016.08.010
Palmer, K. L., Aye, L. M., and Whiteley, M. (2007a). Nutritional cues control Pseudomonas aeruginosa multi-cellular behavior in cystic fibrosis sputum. J. Bacteriol. 189, 8079–8087. doi: 10.1128/JB.01138-07
Palmer, K. L., Brown, S. A., and Whiteley, M. (2007b). Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J. Bacteriol. 189, 4449–4455. doi: 10.1128/JB.00162-07
Paul, K., Nieto, V., Carlquist, W. C., Blair, D. F., and Harshey, R. M. (2010). The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol. Cell 38, 128–139. doi: 10.1016/j.molcel.2010.03.001
Paul, R., Weiser, S., Amiot, N. C., Chan, C., Schirmer, T., Giese, B., et al. (2004). Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 18, 715–727. doi: 10.1101/gad.289504
Plumley, B. A., Martin, K. H., Borlee, G. I., Marlenee, N. L., Burtnick, M. N., Brett, P. J., et al. (2017). Thermoregulation of biofilm formation in Burkholderia pseudomallei is disrupted by mutation of a putative diguanylate cyclase. J. Bacteriol. 199:e00780–16. doi: 10.1128/JB.00780-16
Povolotsky, T. L., and Hengge, R. (2015). Genome-based comparison of cyclic Di-GMP signaling in pathogenic and commensal Escherichia coli strains. J. Bacteriol. 198, 111–126. doi: 10.1128/JB.00520-15
Rao, F., Yang, Y., Qi, Y., and Liang, Z. X. (2008). Catalytic mechanism of cyclic di-GMP-specific phosphodiesterase: a study of the EAL domain-containing RocR from Pseudomonas aeruginosa. J. Bacteriol. 190, 3622–3631. doi: 10.1128/JB.00165-08
Römling, U., Galperin, M. Y., and Gomelsky, M. (2013). Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 77, 1–52. doi: 10.1128/MMBR.00043-12
Ross, P., Weinhouse, H., Aloni, Y., Michaeli, D., Weinberger-Ohana, P., Mayer, R., et al. (1987). Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325, 279–281. doi: 10.1038/325279a0
Roy, A. B., Petrova, O. E., and Sauer, K. (2013). Extraction and quantification of cyclic Di-GMP from P. aeruginosa. Bio Protoc. 3:e828. doi: 10.21769/BioProtoc.828
Ryan, R. P., An, S. Q., Allan, J. H., McCarthy, Y., and Dow, J. M. (2015). The DSF family of Cell-cell signals: an expanding class of bacterial virulence regulators. PLoS Pathog. 11:e1004986. doi: 10.1371/journal.ppat.1004986
Schultz, J., Copley, R. R., Doerks, T., Ponting, C. P., and Bork, P. (2000). SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 28, 231–234. doi: 10.1093/nar/28.1.231
Shanahan, C. A., and Strobel, S. A. (2012). The bacterial second messenger c-di-GMP: probing interactions with protein and RNA binding partners using cyclic dinucleotide analogs. Org. Biomol. Chem. 10, 9113–9129. doi: 10.1039/c2ob26724a
Simm, R., Morr, M., Kader, A., Nimtz, M., and Römling, U. (2004). GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53, 1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x
Ulrich, L. E., Koonin, E. V., and Zhulin, I. B. (2005). One-component systems dominate signal transduction in prokaryotes. Trends Microbiol. 13, 52–56. doi: 10.1016/j.tim.2004.12.006
Waters, C. M., Lu, W., Rabinowitz, J. D., and Bassler, B. L. (2008). Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 190, 2527–2536. doi: 10.1128/JB.01756-07
Wei, C., Jiang, W., Zhao, M., Ling, J., Zeng, X., Deng, J., et al. (2016). A systematic analysis of the role of GGDEF-EAL domain proteins in virulence and motility in Xanthomonas oryzae pv. oryzicola. Sci. Rep. 6:23769. doi: 10.1038/srep23769
Winsor, G. L., Khaira, B., Van Rossum, T., Lo, R., Whiteside, M. D., and Brinkman, F. S. (2008). The Burkholderia genome database: facilitating flexible queries and comparative analyses. Bioinformatics 24, 2803–2804. doi: 10.1093/bioinformatics/btn524
Wu, C. H., Apweiler, R., Bairoch, A., Natale, D. A., Barker, W. C., Boeckmann, B., et al. (2006). The Universal Protein Resource (UniProt): an expanding universe of protein information. Nucleic Acids Res. 34, D187–D191. doi: 10.1093/nar/gkj161
Yoder-Himes, D. R., Chain, P. S., Zhu, Y., Wurtzel, O., Rubin, E. M., Tiedje, J. M., et al. (2009). Mapping the Burkholderia cenocepacia niche response via high-throughput sequencing. Proc. Natl. Acad. Sci. U.S.A. 106, 3976–3981. doi: 10.1073/pnas.0813403106
Keywords: cystic fibrosis, SCFM, Burkholderia cenocepacia, c-di-GMP, motility, BCAL1069, cdpA, Burkholderia cepacia complex
Citation: Kumar B, Sorensen JL and Cardona ST (2018) A c-di-GMP-Modulating Protein Regulates Swimming Motility of Burkholderia cenocepacia in Response to Arginine and Glutamate. Front. Cell. Infect. Microbiol. 8:56. doi: 10.3389/fcimb.2018.00056
Received: 12 November 2017; Accepted: 12 February 2018;
Published: 28 February 2018.
Edited by:
Amal O. Amer, The Ohio State University College of Medicine, United StatesReviewed by:
Wenli Chen, Huazhong Agricultural University, ChinaDinesh Sriramulu, Shres Consultancy (Life Sciences), India
Copyright © 2018 Kumar, Sorensen and Cardona. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia T. Cardona, silvia.cardona@umanitoba.ca
 Brijesh Kumar
Brijesh Kumar John L. Sorensen
John L. Sorensen Silvia T. Cardona
Silvia T. Cardona