Conjunctival Microbiome-Host Responses Are Associated With Impaired Epithelial Cell Health in Both Early and Late Stages of Trachoma
- 1Clinical Research Department, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 2Disease Control and Elimination Theme, MRC Unit the Gambia at LSHTM, Banjul, Gambia
Background: Trachoma, a neglected tropical disease, is the leading infectious cause of blindness and visual impairment worldwide. Host responses to ocular chlamydial infection resulting in chronic inflammation and expansion of non-chlamydial bacteria are hypothesized risk factors for development of active trachoma and conjunctival scarring.
Methods: Ocular swabs from trachoma endemic populations in The Gambia were selected from archived samples for 16S sequencing and host conjunctival gene expression. We recruited children with active trachoma and adults with conjunctival scarring, alongside corresponding matched controls.
Findings: In children, active trachoma was not associated with significant changes in the ocular microbiome. Haemophilus enrichment was associated with antimicrobial responses but not linked to active trachoma. Adults with scarring trachoma had a reduced ocular bacterial diversity compared to controls, with increased relative abundance of Corynebacterium. Increased abundance of Corynebacterium in scarring disease was associated with innate immune responses to the microbiota, dominated by altered mucin expression and increased matrix adhesion.
Interpretation: In the absence of current Chlamydia trachomatis infection, changes in the ocular microbiome associate with differential expression of antimicrobial and inflammatory genes that impair epithelial cell health. In scarring trachoma, expansion of non-pathogenic bacteria such as Corynebacterium and innate responses are coincident, warranting further investigation of this relationship. Comparisons between active and scarring trachoma supported the relative absence of type-2 interferon responses in scarring, whilst highlighting a common suppression of re-epithelialization with altered epithelial and bacterial adhesion, likely contributing to development of scarring pathology.
Introduction
Ocular Chlamydia trachomatis (Ct) infection causes trachoma, the leading infectious cause of blindness worldwide. The pathophysiology of trachoma is complex and multifactorial (Hu et al., 2013; Ramadhani et al., 2016a). The factors involved in the inflammatory responses to repeated Ct infection that lead to conjunctival scarring, trichiasis, corneal opacity and blindness remain poorly understood. In addition to Ct infection, other factors, including the type and quality of the conjunctival host immune responses (Holland et al., 2010; Natividad et al., 2010; Burton et al., 2011b; Ramadhani et al., 2017), host genetic background (Roberts et al., 2014b), infections with other ocular pathogens and changes in overall bacterial community composition (Burton et al., 2011a; Hu et al., 2011a, 2018; Zhou et al., 2014) have each been linked to the different stages of trachomatous disease. Thus far there have been a limited number of studies that have investigated the interaction between the non-chlamydial ocular microbiota and conjunctival immune response in trachoma (Burton et al., 2010; Hu et al., 2012).
Culture-dependent methods have been used extensively to study the ocular surface microbiome, the first descriptions dating back to 1930 (Keilty, 1930). Initial reports generally considered between 20 and 80% of normal healthy eyes to be sterile. The ocular surface is still generally considered to harbor a paucibacillary community, although bacteria have been isolated from higher proportions of healthy conjunctivae by using more intensive modern culture techniques (Perkins et al., 1975). Efforts to define the normal ocular flora in African populations were initially conducted in rural Sierra Leone. The authors found several microbial species, including Staphylococcus spp., Pseudomonas spp., other Gram-negative populations and fungal species (Capriotti et al., 2009). Subsequent studies utilizing approaches to sequence prokaryotic 16S ribosomal RNA genes (16S rRNA) for identification of ocular bacterial communities confirmed the presence of Staphylococcus and Pseudomonas spp., amongst others, and further developed our understanding of the ocular microbiome beyond those species detectable by in vitro culture. Specifically, studies of the healthy human conjunctival microbiome have consistently identified Pseudomonas spp., Propionibacterium spp., Acinetobacter spp., Corynebacterium spp., Staphylococci, Micrococcus spp., and Streptococci (Dong et al., 2011a; Willcox, 2013; Huang et al., 2016; Shin et al., 2016; Ozkan et al., 2017).
Congruent with observations in other anatomical sites, several studies proposed a link between the ocular microbiota, ocular health and susceptibility to infections. Using culture-dependent techniques, bacteria were more frequently identified and at higher abundance in samples from bacterial conjunctivitis patients compared to healthy controls. However, cultured species predominantly overlapped with those identified by others as present in healthy conjunctival samples (Aoki et al., 2013). Two recent studies in murine models of ocular infection have demonstrated an important role for the ocular microbiota in bolstering local immune responses and increasing resistance to infectious challenge. Firstly, Pseudomonas aeruginosa–induced keratitis resistant mice became susceptible in the absence of ocular microbiota. The protection afforded was mediated by a microbiota-induced IL-1β-dependent mechanism (Kugadas et al., 2016). Secondly, a constituent of the ocular commensal flora, Corynebacterium mastitidis, was shown to mediate protection against ocular fungal (Candida albicans) and bacterial (P. aeruginosa) challenge infection in mice. In this case, C. mastitidis was found to elicit a local IL-17 response that was central to neutrophil recruitment and release of antimicrobials into the tears, leading to increased resistance (St. Leger et al., 2017). In addition to stimulating local immune responses, Corynebacterium spp., which are consistently found as a major constituent of the ocular microbiome, may also protect their ecological niche against specific pathogens such as Streptococcus pneumoniae (Sp) via release of antibacterial free fatty acids that inhibit their growth (Bomar et al., 2016).
In typical cases of active trachoma with proven Ct infection, such as reported in historical studies in The Gambia, the conjunctival response to Ct infection is characterized by epithelial cell reorganization, immune cell infiltration and secretion of anti-microbial peptides (Natividad et al., 2010). Similarly, trachomatous inflammation follicular (TF) and trachomatous scarring (TS) were also associated with expression of innate pro-inflammatory markers in Ethiopian and Tanzanian populations (Burton et al., 2011a,b; Hu et al., 2012; Ramadhani et al., 2017). However, clinical signs of trachoma are often prevalent in the relative absence of Ct infection (Burton et al., 2011a; Burr et al., 2013; Ramadhani et al., 2016b; Butcher et al., 2017). Additionally, longitudinal cohort studies in Tanzania and Ethiopia in adults with progressive conjunctival scarring found that concurrent Ct infection was virtually absent (Hu et al., 2018). In these studies, non-chlamydial bacteria were often prevalent and associated with trachomatous disease. These non-chlamydial bacteria include pathogens, such as Sp and Haemophilus influenzae (Hi), and commensals, such as Corynebacterium spp. (Burton et al., 2011a; Hu et al., 2011a, 2018; Burr et al., 2013). Non-chlamydial infections have also been associated with immunofibrogenic immune responses thought to drive scarring trachoma (Hu et al., 2012). Overall, studies from different trachoma endemic populations suggest that non-chlamydial bacterial species are significant factors in trachoma pathogenesis (Burton et al., 2011a; Hu et al., 2012, 2018; Burr et al., 2013).
Using culture independent methods, we have previously shown differences in conjunctival microbiome diversity between individuals with trachomatous scarring and controls, with elevated abundance of Corynebacterium in adults with scarring and trichiasis (Zhou et al., 2014). Here, we investigate the relationship between the conjunctival microbiome and host conjunctival-associated lymphoid tissue responses, additionally testing the influence of host genotype on the ocular microbiome in different clinical stages of trachoma in Gambians. Our data demonstrate significant associations between ocular microbiota and the host immune-response linked to specific bacteria and trachomatous disease.
Materials and Methods
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki. Permission for collection of samples and genotyping was granted by the relevant local and national ethics committees of the London School of Hygiene and Tropical Medicine, The Gambian Government/Medical Research Council Unit and The Gambia Joint Ethics Committee. Written, informed consent prior to a participant's enrolment was obtained from all adult participants and from a parent or a guardian for participants under 18 years of age.
Study Populations
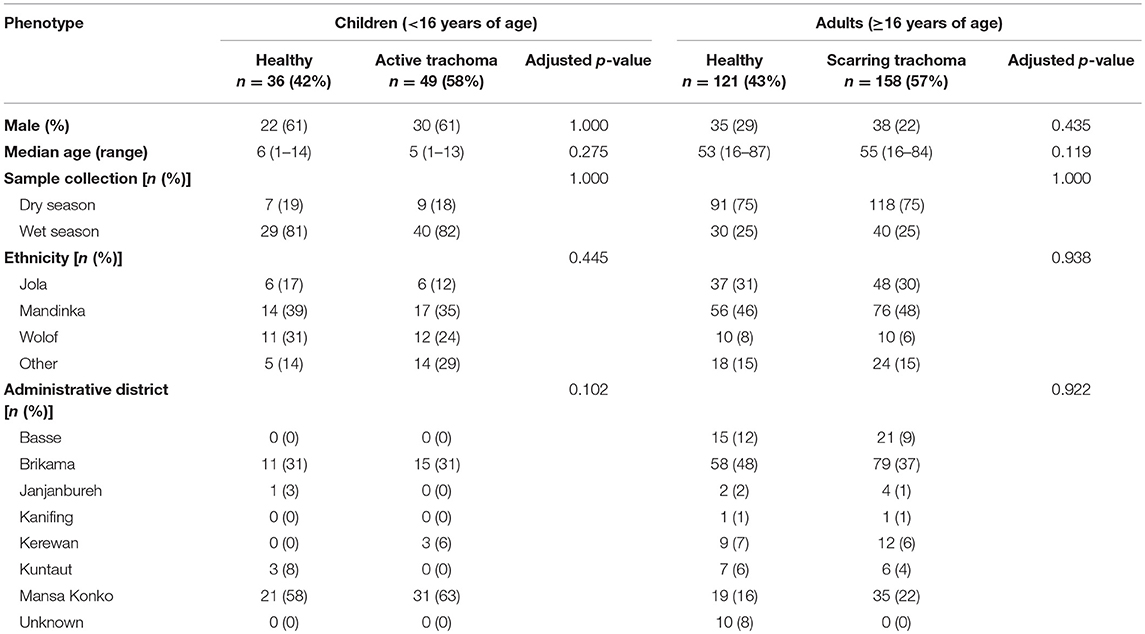
Samples (n = 361) were selected based on case-control status (trachoma clinical signs) from a larger archive of ocular swabs collected from individuals in communities across The Gambia between 2009 and 2011. This study included children with a normal, healthy conjunctiva (F0|P0) or active trachoma (F>0 ± P>0), and adults with a normal, healthy conjunctiva (F0|P0|C0) or scarring trachoma (C>0). Conjunctival microbiome data for a subset of samples (n = 220) has been published previously (Zhou et al., 2014). Cases of active or scarring trachoma were identified from screening records, community ophthalmic nurse referral, and opportunistic rapid screening. Control individuals with normal conjunctivae were selected by matching for age, sex, ethnicity and location. Samples were classified as collected during the Gambian dry season (December–April) or wet season (July–October). No samples were collected in May, June or November of any year. Subject demographics are shown in Table 1.
Ocular Swab Collection
Swab samples were taken from the everted left and right tarsal conjunctiva of each study participant using standard methodology (Keenan et al., 2010; Last et al., 2014). A separate swab was used for each eye and each swab was horizontally passed across the tarsal conjunctiva three times, rotating the swab by 1/3 with each pass. Individual swabs were immediately placed into sterile tubes filled with 250 μl RNAlater® (Ambion) and stored in a cool-box filled with ice packs in the field, then transferred to −80°C storage in the laboratory.
Photographs and Clinical Scoring
Subjects were examined for clinical signs of trachoma in the field. High resolution digital photographs were taken of each conjunctival surface at the time of sample collection and an FPC score [1981 WHO Trachoma Grading System; FPC—follicles, papillae, cicatricae (Dawson et al., 1981)] assigned to each sample by two experienced trachoma graders, as described previously (Zhou et al., 2014). For analyses in this study, the presence of follicles was defined as an F score > 0. Presence of papillae (indicative of inflammation) was graded as a P score > 0. Conjunctival scarring was defined as a C score > 0. Participants with normal, healthy conjunctivae, as defined by a score of F0|P0|C0, served as controls.
Genomic DNA and RNA Extraction From Ocular Swabs
Ocular swabs were removed from RNALater® and placed into 400 μl Norgen lysis buffer (Norgen Biotek), vortexed for 1 min and spun down for 30 s at 13,000 rpm. Lysates were transferred into a new RNAse/DNAse free tubes. Extraction of nucleic acid material was performed using the Norgen total RNA/DNA purification kit according to the manufacturer's instructions. β-mercaptoethanol was added to lysis buffers to inhibit RNAses. RNA and gDNA were stored at −80° C until further processing.
cDNA Synthesis
Generation of cDNA from RNA was performed using the SuperScript® VILO cDNA Synthesis Kit and Master Mix (Invitrogen) according to the manufacturer's instructions. Total RNA-derived cDNA (which includes cDNA derived from miRNA) was generated using the miScript II RT Kit (Qiagen) using the hiFlex buffer according to the manufacturer's instructions. cDNA was used immediately or stored at −20°C until further processing.
Taqman® Low Density Array (TLDA)
Analysis of ocular swab cDNA was performed using TLDA Microfluidic Cards (Applied Biosystems) according to the manufacturer's instructions. Custom array cards were used to interrogate expression profiles of immune transcripts (Supplementary Table 1). Arrays were run on an ABI PRISM® 7900HT thermal cycler (Applied Biosystems) using SDS software. Cycling conditions were as follows: 2 min at 50°C | 10 min at 94.5°C | 40 cycles (30 s at 97°C | 1 min at 59.7°C). Data were collected at 97 and 59.7°C. Cycle threshold (CT) was set to a standard mid-exponential phase amplification point. Delta CT values relative to housekeeping genes (GAPDH and HPRT1) were calculated using R (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org). Genes and individuals with ≥10% missing data were excluded. Fold-change was calculated using the delta-delta CT method. Modules of co-expressed genes were identified using the Weighted Correlation Network Analysis (WGCNA) package and putative functions were assigned manually based on known functions of genes in each module (Langfelder and Horvath, 2008, 2012). All genes in each module were included in a principal component analysis (PCA), the first component of which was used as an expression score per individual for each module.
miRNA qPCR
qPCR was carried out using miScript Primer Assays and the miScript SYBR Green PCR kit according to the manufacturer's instructions (Qiagen) and data were acquired on an ABI PRISM® 7900HT thermal cycler using SDS software. Primer assays used were: Hs_RNU6-2_11 (MS00033740), Hs_miR-1285_2 (MS00031367), Hs_miR-147b_1 (MS00008729), Hs_miR-155_2 (MS00031486), and Hs_miR-184_1 (MS00003640). Cycling conditions were as follows: 15 min at 95°C | 40 cycles (15 s at 94°C | 30 s at 55°C | 30 s at 70°C). Data were collected at 94 and 70°C. CT was determined automatically, or adjusted manually to cross amplification curves in the mid-exponential phase where not placed correctly by the software. Delta CT values relative to housekeeping genes (Hs_RNU6-2_11) were calculated using R.
Quantification of Bacterial and Human DNA by Droplet DigitalTM PCR
Detection and quantitation of Chlamydia trachomatis (Ct) plasmid and human DNA in ocular swab samples was performed using the Bio-rad QX100TM droplet digital PCR (ddPCR) platform. Primers and protocols for the specific amplification of Homo sapiens ribonuclease P/MRP 30 kDa subunit (RPP30), Ct plasmid, Haemophilus influenzae and Streptococcus pneumoniae were all described previously (Carvalho Mda et al., 2007; Meyler et al., 2012; Roberts et al., 2013; Butcher et al., 2017).
Prokaryotic 16S Ribosomal RNA Gene (16S rRNA) Sequencing
Ocular bacterial community composition was determined previously by 454 pyrosequencing (Zhou et al., 2014). Additional samples were analyzed by 16S rRNA sequencing using the MiSeq Next Generation Sequencing Platform (Illumina). Amplicon PCR was performed using the Phusion High-Fidelity PCR Master Mix (New England Biolabs) and previously described barcoded primers (Dong et al., 2011b) to amplify the V1-V3 region of bacterial 16S rDNA. Cycling conditions were as follows: 30 s at 98°C | 31 cycles (10 s at 98°C | 30 s at 62°C | 15 s at 72°C) | 7 min at 72°C | hold at 4°C. PCR products (~600 bp) were confirmed in a subset of samples using agarose gel electrophoresis. Purification of PCR products was performed using the Agencourt AMPure XP system (Beckman Coulter) and DNA quantified using the Qubit® 2.0 Fluorometer according to the manufacturer's instructions (ThermoFisher Scientific). Samples were pooled into a DNA library, which was denatured and run on the MiSeq sequencer at a final concentration of 5 pM alongside a 5 pM PhiX control (Illumina). Raw reads generated by MiSeq were error-corrected and filtered using DADA2 through QIIME2 (https://qiime2.org) (Caporaso et al., 2010). Filtered reads were clustered de novo into Operational Taxonomic Units (OTUs) at 97% sequence similarity. OTUs were then assigned taxonomy using a Naive Bayes classifier trained on the SILVA 16S database. Both processes were performed with QIIME2. Manual filtering of classified OTUs was performed using R as described below. OTUs were retained if they had been classified as bacteria, had a genus-level classification and constituted >0.005% of the total number of reads (Bokulich et al., 2013). Samples with <1,000 reads were excluded. Final read counts were rarefied to 1,000 reads per sample using the R package vegan. 16S rRNA sequencing data (genus counts) generated by Roche-454 (n = 220) published previously (Zhou et al., 2014) were included in the analysis. Read counts in the combined datasets were converted to relative abundance of each phyla or genera per individual for all analyses. Univariate analyses included genera with an abundance >1%.
KLRC2 Genotyping
KLRC2 genotypes, which code for the NK cell triggering receptor NKG2C, were determined by touchdown PCR using the Phusion High Fidelity PCR kit (New England Biolabs) using previously described methods (Miyashita et al., 2004; Goodier et al., 2014) and primers (Goncalves et al., 2016). Touchdown PCR was carried out as previously described (Goodier et al., 2014; Goncalves et al., 2016). Cycling conditions were as follows: 3 min at 95°C | 10 cycles (30 s at 94°C | 30 s from 65 to 55°C reducing by 1°C per cycle) | 30 s at 72°C | 26 cycles (30 s at 94°C | 30 s at 55°C | 30 s at 72°C). PCR products were separated and identified using agarose gel electrophoresis.
KIR Copy Number Variation (CNV) Assay
Determination of KIR2DL2 and KIR2DL3 alleles was performed by ddPCR (BioRad) on buccal brush extracted DNA (Roberts et al., 2014a). Each allele was tested for separately in parallel with the human target RPP30. Primers and probes were mixed to a final concentration of 3 μM each of primer and 1 μM of probe except in the case of KIR2DL2 (0.5 μM). Primer sequences were as previously described (Roberts et al., 2014b). Samples were digested with 1 unit of BamHI-HF enzyme (NEB) prior to running the PCR. PCR reactions contained 4 μl of digested sample, 2 μl of primer probe master mix, 4 μl molecular grade water and 10 μl of 2x ddPCR master mix (BioRad). Cycling conditions were as follows: 10 min 95°C | 43 cycles (15 s 95°C | 60 s 60°C) | 12 min 98°C.
HLA-C Typing
Extracted DNA from ocular swabs was used for HLA-C1/C2 epityping by allelic discrimination on an ABI 7900HT. Primer and probes used in the reaction were as follows; Forward primer HLA-C-JS_C1C2F 5′-TATTGGGACCGGGAGACACA-3′, Reverse primer HLA-C-3C26-R 5′-GGAGGGGTCGTGACCTGCGC-3′, C1 probe BARI-C1 6FAM-CCGAGTGAGCCTGC-MGBNFQ, C2 probe BARI-C2 VIC-CCGAGTGAACCTGC-MGBNFQ (Bari et al., 2011). Each PCR reaction contained 3 μl Taqman genotyping mastermix (Applied Biosystems), 1.7 μl molecular grade water, 0.3 μl primer and probe mix (JS C1C2F, HLA-3C26, Bari-C probes, all at 1 μM) and 1 μl template. Cycling conditions were as follows: 10 min at 95°C | 50 cycles (15 s 95°C | 60 s 60°C). Allele calls were made using SDS software v2.4.
Corynebacterium rpoB Amplification and Sequencing
DNA samples that were positive for Corynebacterium from 16S sequencing were amplified using degenerate primers C2700 and C3130 (Khamis et al., 2004). Briefly, the PCR mix consisted of 1 x Ultra-red mix (PCR Biosystems), 400 nM of each primer and 2 μl of template DNA. Cycling conditions were as follows: 1 min at 95°C | 6 cycles (15 s at 95°C | 15 s at 68–62°C touchdown | 30 s at 72°C) | 35 cycles (15 s at 95°C | 15 s at 62°C | 30 s at 72°C) | 10 min at 72°C | hold at 4°C. PCR products were confirmed using agarose gel electrophoresis. Purification of PCR products was performed using the Agencourt AMPure XP system (Beckman Coulter) and DNA quantified using the Qubit® 2.0 Fluorometer according to the manufacturer's instructions (ThermoFisher Scientific). ABI Prism terminator reactions were then performed as per manufacturer's instructions, and consequently sequenced on the ABI 3700 capillary sequencer. Sequence data was analyzed within R by inputting the -ab files and trimming the end N bases before quality filtering to Q10. Sequences were classified using blastn sequence identity.
Bacterial Diversity Using Hill Numbers
Differences in alpha diversity of the ocular microbiome were examined using Hill numbers, which take into account both richness and evenness (Equation 1) (Chao et al., 2010). Where S is number of genera, pi is the proportion of genera per individual and q is the order of diversity. As the order of diversity (q) increases, greater weight is placed on the most abundant genera, reducing the Hill number. Within each value of q, higher values indicate increased diversity. Samples with few, dominant genera will have a lower Hill number, reflecting unevenness and reduced diversity. Samples with many, equally abundant genera will have a higher Hill number, reflecting increased diversity, and evenness.
Statistical Analyses
R was used for statistical analyses and graphical visualizations. Analyses were performed using the linear model function to compute p-values unless otherwise stated. Analyses of gene expression (GE) were conducted using a linear regression of GE as the dependent variable and host disease phenotype as the independent variable, adjusted for age and gender. Analyses of the ocular microbiome were conducted using a linear regression of microbial diversity or relative abundance as the dependent variable and host disease phenotype as the independent variable, adjusted for age, gender, and season. All analyses in adults were additionally adjusted for evidence of P-score > 0. P-values were adjusted using the false discovery rate (FDR) by the Benjamini-Hochberg procedure. Contingency analyses were performed by Chi-square test or Fisher's exact test. Scaled PCA was performed in R using the stats package. Hill numbers, diversity indices and non-metric multidimensional scaling were calculated using the vegan package. P-values were considered significant at <0.05 and are denominated in figures as follows: *p < 0.05; **p < 0.01; and ***p < 0.001.
Results
Participants
Thirty-six children (<16 years) with a normal, healthy conjunctiva (“N”; F0|P0) and 49 with active trachoma (“AT”; F > 0 ± P > 0) were included in this study. Evidence of scarring was not significantly different between N (4/36 [11%]) and AT (9/49 [18%]). Of the adults studied, 121 (≥16 years) had a normal, healthy conjunctiva (“N”; F0|P0|C0) and 158 had scarring trachoma (“ST”; C > 0). Additionally, 76/158 (48%) adults with ST had P-score > 0, which was adjusted for in all analyses. No demographic variables were significantly associated with AT in children or with ST in adults, and unless otherwise stated, downstream analyses were only adjusted for age and gender.
Gene Expression Patterns in Active and Scarring Trachoma
Gene expression (GE) was characterized using TLDA Microfluidic Cards for targeted immune transcripts and qPCR for miRNA transcripts previously identified as associated with trachoma (Natividad et al., 2010; Burton et al., 2011b; Derrick et al., 2013, 2016). GE data were available from 78/85 children (N = 32, AT = 46) and 147/279 adults (N = 69, ST = 78) (Supplementary Table 2).
The expression of seven genes was significantly upregulated in AT (Figure 1A; S100A7, DEFB4B, IL-17A, IL-23A, CD274, SRGN, and CD53). All seven genes were in the WCGNA defined GE module we termed “Active Trachoma Expression Module (ATEM) 2,” the members of which are putatively involved in the innate response to microbiota (Supplementary Table 3). Combined expression level of ATEM2 was also significantly upregulated in active trachoma (adj.p = 0.002, coef = 2.267 [se = 0.704]). Four genes were significantly downregulated in AT (Figure 1A; SPARCL1, TRAF6, MUC16, and TNFSF15). Similarly, all four genes were in the same GE module ATEM3, which is putatively involved in suppression of epithelial cell expansion and recovery (Supplementary Table 3).
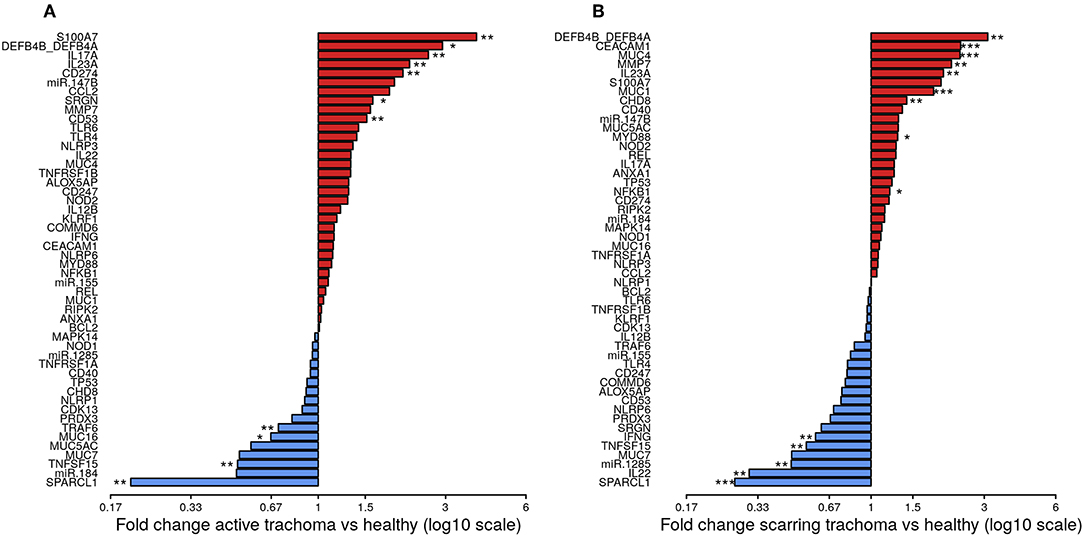
Figure 1. Fold-changes in conjunctival gene expression between cases and matched, healthy controls. Magnitude of fold-changes in conjunctival gene expression between children with active trachoma and healthy controls (A) and adults with scarring trachoma and healthy controls (B), shown by bars. Colors highlight increased (red) or decreased (blue) expression in cases. P-values were considered significant at <0.05 and are denominated as follows: *p < 0.05; **p < 0.01; and ***p < 0.001.
In ST, the expression of nine genes was significantly upregulated (Figure 1B; DEFB4B, CEACAM1, MUC4, MMP7, IL-23A, MUC1, CHD8, MYD88, and NFKB1). Six of nine genes were in GE module “Scarring Trachoma Expression Module (STEM) 4,” characteristically involved in responses to microbiota (Supplementary Table 4). Combined expression level of STEM4 was also significantly upregulated in ST (adj.p = 7.050 × 10−7, coef = 2.117 [se = 0.405]). Five genes were significantly downregulated in ST (Figure 1B; SPARCL1, IL-22, miR-1285, TNFSF15, and IFNy). Three of four genes were in the same GE module STEM1, involved in epithelial health (Supplementary Table 4).
To identify shared differential GE in active and scarring trachoma, fold-changes from significantly differentially expressed genes in the previously described comparisons (Figure 1) were contrasted. This highlighted three groups of genes that were (a) downregulated in AT and ST (Figure 2, blue hatched area), (b) upregulated in AT and ST (Figure 2, red hatched area), and (c) differentially regulated in AT and ST (Figure 2, green cross-hatched area). Co-downregulated genes (Figure 2; blue hatched area) are primarily involved in regulation of cellular expansion and migration. Of the differentially regulated genes (Figure 2, green cross-hatched area), those upregulated in AT and downregulated in ST are part of the host pro-inflammatory response. Co-upregulated genes (Figure 2; red hatched area) are mostly antimicrobial or pro-inflammatory. Within this latter set of co-upregulated genes, the magnitude of upregulation is higher in mucins and MMP7 in ST relative to AT. Conversely, the magnitude of upregulation is relatively higher in antimicrobials and pro-inflammatory IL-17A in AT.
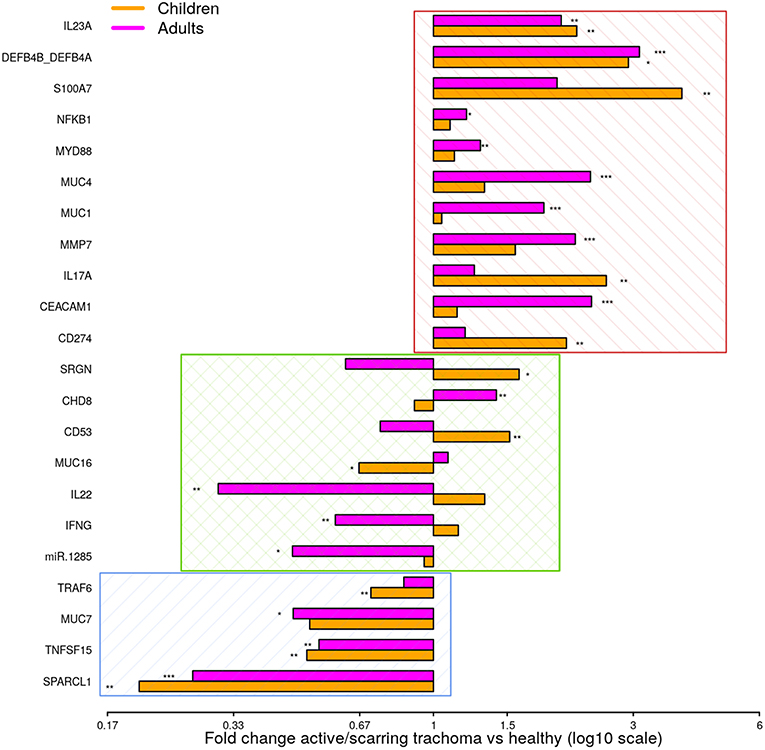
Figure 2. Comparison of fold-changes in conjunctival gene expression between active/scarring trachoma cases and healthy controls. Fold-changes in gene expression between children with active trachoma and healthy controls (orange bars) and adults with scarring trachoma and healthy controls (purple bars) are represented by bars with significance indicated as described below. Genes are sorted into three groups; downregulated in active and scarring trachoma (blue area), upregulated in active and scarring and trachoma (red area), and differentially regulated in active and scarring trachoma (green area). P-values were considered significant at <0.05 and are denominated as follows: *p < 0.05; **p < 0.01; and ***p < 0.001.
Ocular Microbial Changes in Active and Scarring Trachoma
The ocular microbial community was characterized by sequencing of the V1-V3 region of the 16S gene, combining previously published data generated by 454 method (Zhou et al., 2014) and new data generated by MiSeq sequencing. Non-metric multidimensional scaling of the compiled datasets showed no significant difference between samples sequenced on the two platforms (p = 0.436). 16S data was available from 72/85 children (N = 31, AT = 41) and 235/279 adults (N = 105, ST = 130) (Supplementary Table 5).
We identified 382 genera from 29 phyla. As found previously, the ocular microbiome community diversity decreased with age (p = 0.0007, coef = −0.007 [se = 0.002]). Phyla with an abundance >1% were identical between children and adults. Actinobacteria and Firmicutes accounted for the majority of the observed microbes (Figure 3). Eleven genera had relative abundance >1% in both children and adults, 10 genera were >1% in children only and one genus was >1% in adults only (Figure 4).
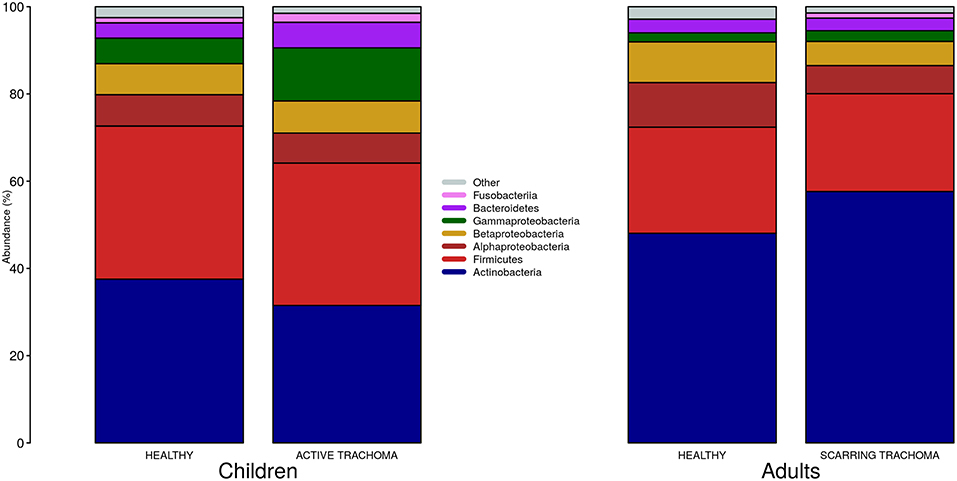
Figure 3. Relative abundance of major phyla in children and adults by case-control status. Phyla with relative abundance >1% in either children or adults are shown. Phyla with relative abundance ≤1% are grouped into “Other.”
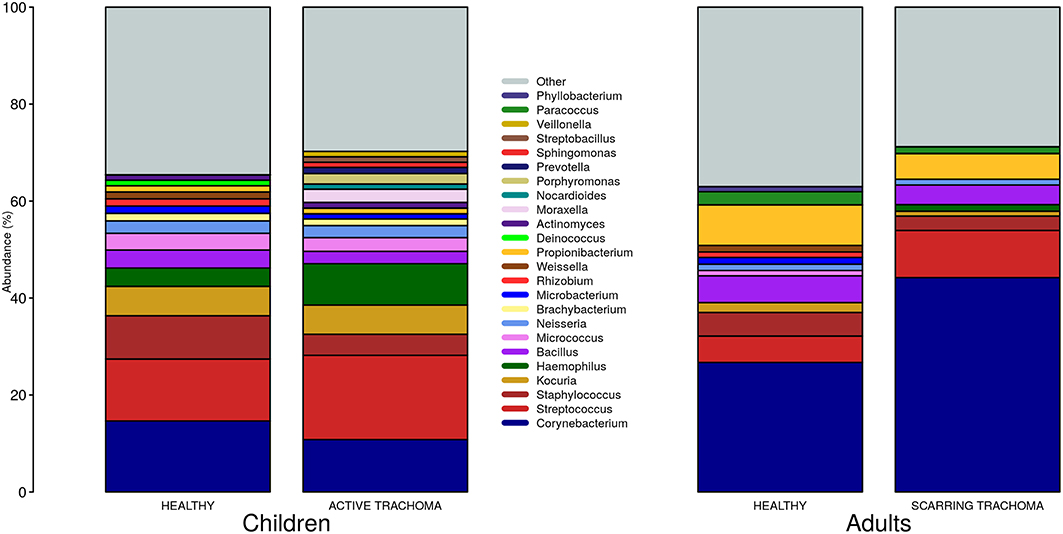
Figure 4. Relative abundance of major genera in children and adults by case-control status. Genera with relative abundance >1% in either children or adults are shown. Genera with relative abundance ≤1% are grouped into “Other.”
Global differences in the ocular microbiome were examined using Hill numbers, details are provided in the Methods. At order of diversity = 0 children with AT tended to have reduced diversity compared to healthy controls; Hill number did not significantly differ between AT cases and normal children when order of diversity was 0.5 or higher (Figure 5A). At order of diversity = 0, adults with ST were indistinguishable from normal, healthy adults (Figure 5B). From order of diversity = 0.5 upwards, adults with ST had significantly reduced diversity. The level of significance continued to increase with increasing order of diversity, suggesting dominance of a small number of genera.
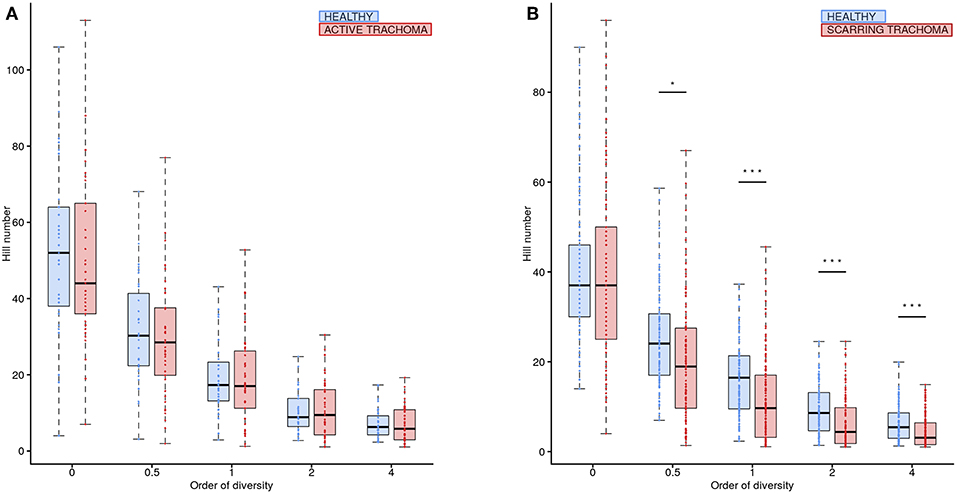
Figure 5. Ocular microbiome diversity in children and adults by case-control status. Hill number at corresponding order of diversity are shown for cases (red) and healthy controls (blue) in children (A) and adults (B). Boxes represent the interquartile range, with median indicated (blue line). Outer bars represent the range. P-values were considered significant at <0.05 and are denominated as follows: *p < 0.05; **p < 0.01; and ***p < 0.001.
In univariate analyses there were no differentially abundant genera between children with AT and normal, healthy children. In adults with scarring trachoma, Corynebacterium abundance was significantly greater (adj.p = 0.0002, coef = 0.156 [se = 0.036]) and Staphylococcus abundance was significantly lower than in healthy controls (adj.p = 0.0011, coef =−0.022 [se = 0.006]) (Supplementary Figure 1). For both genera, prevalence (read count > 0) was equivalent between N and ST (Corynebacterium; N = 105/105 [100%] and ST = 129/130 [99%]. Staphylococcus; N = 90/105 [86%] and ST = 109/103 [84%]).
Individual direct tests for Ct, Haemophilus influenzae (Hi), and S. pneumoniae (Sp) were performed using ddPCR. Hi and Sp are non-chlamydial conjunctivitis-associated bacterial pathogens. In children, we identified seven cases of current Ct infection (7/82 [8.5%]). However, Ct infection was not associated with AT (p = 0.200 [N = 5/32, AT = 2/46]). No adults had detectable current Ct infection. The overall prevalence of Hi was 43/85 (51%) in children and 25/279 (9%) in adults. Sp prevalence was 28/85 (33%) in children and 12/279 (4%) in adults. In children, there were no differences in the proportion of AT cases and controls who were positive for Hi (p = 0.203 [N = 14/31, AT = 26/41]) or Sp (p = 0.333 [N = 8/31, AT = 18/41]). Using ddPCR as the reference, 16S sequencing had reasonable sensitivity but poor specificity (Table 2) for these bacteria. The high proportion of ddPCR-negative, 16S-positive samples suggests that additional members of the Haemophilus and Streptococcus genera were present on a significant number of conjunctivae. Correlation between ddPCR concentration and 16S relative abundance was also poor for both species (Hi p = 0.174, Sp p = 0.207). Abundance was higher however in PCR-positive samples, significantly so for Sp (Hi p = 0.081, Sp p = 0.002).
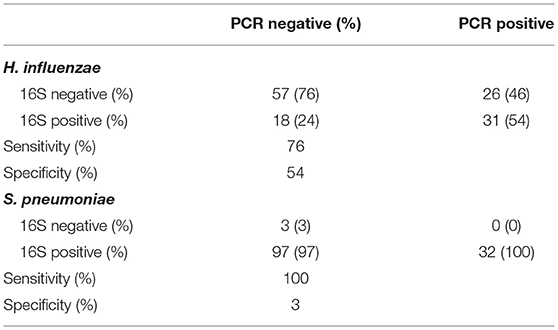
Table 2. Comparison of species-specific, ddPCR based test, and 16S genus-level operational taxonomic unit (OTU) classification.
Reads corresponding to the Corynebacterium OTU were detected in 332/364 (91.2%) samples. There was sufficient residual DNA for Corynebacterium specific PCR amplification and sequencing in 112/332 samples. Within the 112 samples that could be tested, there were 71 ST cases and 41 age matched controls, of which 18 samples (16 cases and two controls) passed Q10 filtering and returned species level identification of Corynebacterium. Alignment and phylogenetic analysis identified four species, C. accolens (10/18), C. mastitidis (1/18), C. tuberculostericum (5/18), and C. simulans (2/18). The small sample size and skewed case-control status limited any meaningful tests for association.
Relationship Between Ocular Microbial Community and Host Genotype
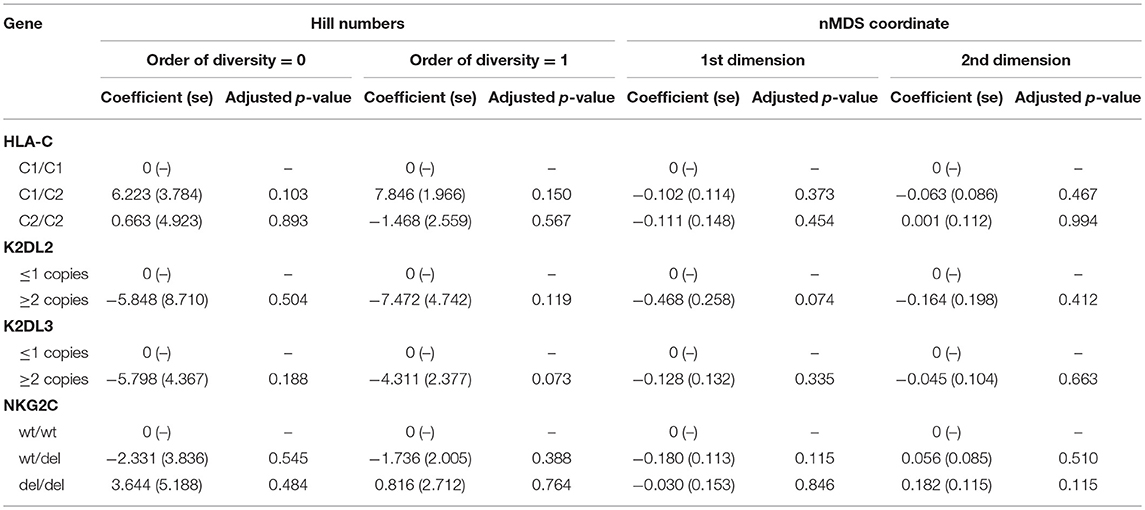
We have previously found an association between HLA-C2 copy number, KIR2DL2/KIR2DL3 heterozygosity, and conjunctival scarring (Roberts et al., 2014b). KLRC2 (NKG2C) was also investigated as it is an activating receptor of NK cells and T cells that could potentially impact bacterial community structure or host responses. Polymorphisms in expression of this receptor have previously been shown not to be associated with trachoma however (Goncalves et al., 2016). Relationships between these host genetic factors and ocular microbiome in adults were determined by Hill numbers and non-metric-multidimensional scaling (nMDS) of complete microbial communities. No significant associations were identified (Table 3). Details of host genotype by trachomatous disease stage are available in the supplement (Supplementary Table 6).
Relationship Between Gene Expression, Microbial Community, and Evidence of Trachomatous Disease
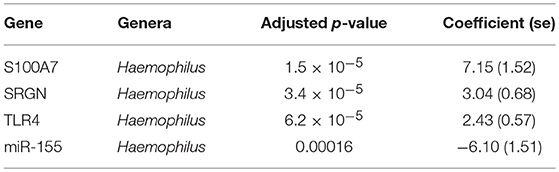
Previous studies have implicated interplay between host gene expression and microbiome in disease pathogenesis (Carvalho Mda et al., 2007; Langfelder and Horvath, 2012; Meyler et al., 2012; Roberts et al., 2013). We now evaluate the interplay of host ocular microbiome and conjunctival gene expression using a linear regression of GE on relative abundance, with previously detailed adjustments. In children, univariate analyses identified a number of genes associated with abundance of Haemophilus (Table 4). S100A7, SRGN, and TLR4 were upregulated in children with increased abundance of Haemophilus. These genes were all within module ATEM2, the expression of which was also significantly upregulated in children with increased abundance of Haemophilus (adj.p = 0.0001, coef = 7.777 [se = 1.898]). Visualization of the relationship between gene expression and microbiome suggested the association between Haemophilus abundance and expression levels of ATEM2 was not linked to active trachoma (Figure 6). No further associations between microbiome and GE were identified.
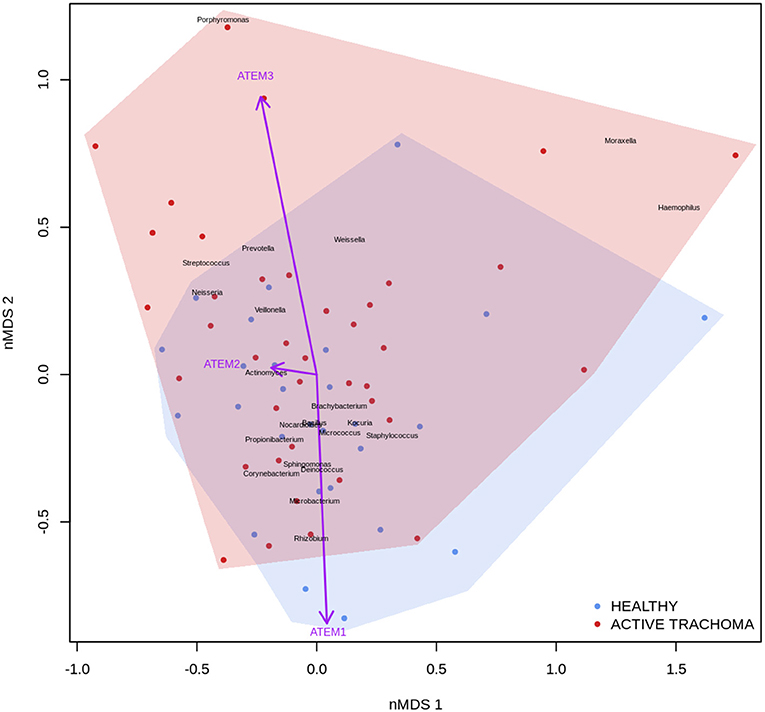
Figure 6. Plot of the ocular microbiome, modular conjunctival gene expression, and trachomatous disease in children. Non-metric multidimensional scaling of the complete ocular microbial community was used to position samples (points), enrichment of genera with relative abundance >1% are shown (black text). Arrows represent conjunctival gene expression modules (purple text), arrow coordinates indicate increased expression in surrounding samples. Shaded areas highlight the distribution of active trachoma (red) and healthy control (blue) samples.
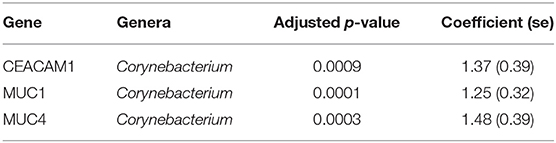
In adults, univariate analyses identified 3 genes upregulated with increasing abundance of Corynebacterium (Table 5). These genes were all within module STEM4, expression of which was also significantly upregulated with increased abundance of Corynebacterium (adj.p = 0.0003, coef = 2.472 [se = 0.672]). Visualization of the relationship between gene expression and microbiome showed greater separation of scarring trachoma and healthy individuals than observed in children with active trachoma and healthy individuals (Figures 6, 7). Adults with ST clustered in the negative space of the first dimension of nMDS. Expression of STEM4 and abundance of Corynebacterium were both increased in this space populated exclusively by individuals with ST, suggesting the association between them is linked to ST. No further associations between microbiome and GE were identified.
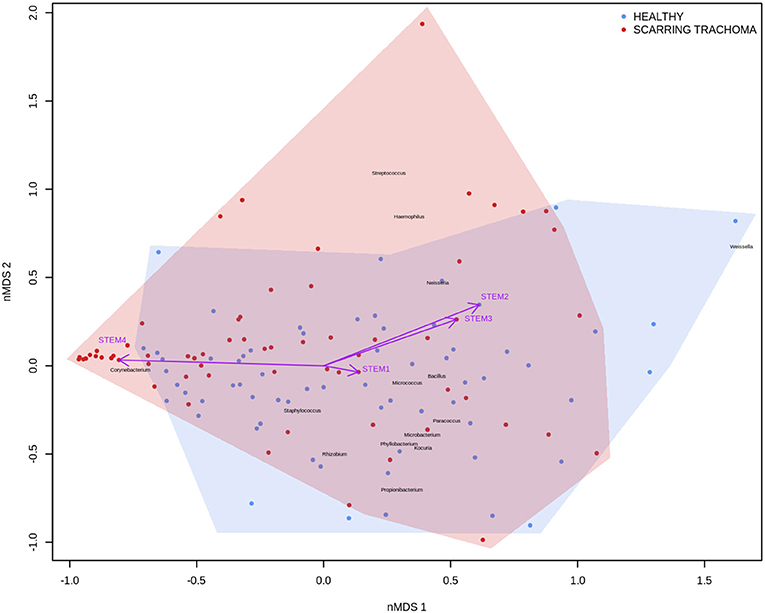
Figure 7. Plot of the ocular microbiome, modular conjunctival gene expression, and trachomatous disease in adults. Non-metric multidimensional scaling of the complete ocular microbial community was used to position samples (points), enrichment of genera with relative abundance >1% are shown (black text). Arrows represent conjunctival gene expression modules (purple text), arrow coordinates indicate increased expression in surrounding samples. Shaded areas highlight the distribution of scarring trachoma (red) and healthy control (blue) samples.
Discussion
This is the first study that has examined conjunctival gene expression in the context of a culture-free characterization of the ocular microbiome in active and scarring trachoma in comparison with healthy controls. We found pro-inflammatory, antimicrobial, and tissue-remodeling gene expression was associated with AT and ST, as previously described (Burton et al., 2011b; Hu et al., 2012; Ramadhani et al., 2017). Novel comparisons between AT and ST highlighted the importance of pro-inflammatory responses and tissue-remodeling in these pathogeneses. ST was associated with reduced microbial diversity and dominance of Corynebacterium whilst AT was not associated with detectable changes in the microbiota, both of which have been shown previously (Hu et al., 2011a, 2018; Zhou et al., 2014). Disease-associated changes in gene expression were strongly associated with imbalances in the microbiota, notably with increased abundance of Haemophilus in children and Corynebacterium in adults. In children, this relationship was independent of AT. Conversely, in adults, the relationship between changes in gene expression and Corynebacterium abundance were strongly associated with ST. Host genotype of targeted innate immune genes were not associated with ocular microbiome diversity or composition.
We found patterns of differential gene expression typical of AT and ST, as described previously in The Gambia and independently in other populations including Ethiopia and Tanzania (Burton et al., 2011b; Hu et al., 2012; Ramadhani et al., 2017). In AT, we found anti-microbial peptides (S100A7 and DEFB) had the largest significant up-regulation. SPARCL1, an inhibitor of extracellular matrix remodeling, was the most reduced transcript in both AT and ST, possibly indicating cellular remodeling of the conjunctiva taking place in both stages of disease. Upregulation of IL17A/IL23A and suppression of MUC16 was also clearly evident in AT. This expression pattern, indicative of a type 17 response, is generally considered to be triggered by extracellular bacterial pathogens, fungi and some commensal bacterial species that bind to epithelial cells. These type 17-associated cytokines can be both beneficial (promoting mucosal barrier function) or detrimental (being associated with chronic inflammatory disorders) (Guglani and Khader, 2010; Onishi and Gaffen, 2010; Valeri and Raffatellu, 2016). The gene expression pattern reflected in ST is also dominated by up-regulation of DEFB expression, accompanied by MUC1/MUC4 and smaller differences in IL23A and S100A7, with little change in IL17A expression. Perhaps the most interesting observation is the reduction in IL22 expression and the lack of IL17A differential expression in adults, expression of which are required for maintaining epithelial cell barrier health and recruitment of neutrophils (Schreiber et al., 2015), respectively. In support of the protective properties of IL-22, a study of corneal abrasion in mice found IL-22 production promoted epithelial wound healing through enhanced recruitment of neutrophils and platelets (Li et al., 2011). Similarly, increased levels of IL-22 in lacrimal fluid of individuals with dry eye disease (DED) correlates with reduction in disease (Ji et al., 2017). It is plausible that reduced expression of IL-22 impairs healing of conjunctival scarring and may exacerbate disease through increased infiltration of Th17 cells, as found in IL-22 knock-out mice (Ji et al., 2017). In ST, the epithelial cell layer is typically thinned and denuded in parts (Hu et al., 2011b) which is thought to be permissive or conducive to further bacterial colonization and changes to the bacterial microbiome (dysbiosis), downregulation of IL-22 may be a factor in this epithelial damage.
Profiling both children with AT and adults with ST permits us to characterize the primary features of gene expression between the early and later stages of clinical disease. The largest differences between disease stages were the relatively reduced levels of expression of IL22, IFNG, and miR-1285 in adults with ST compared to children with AT. This is consistent with a damaged epithelial cell layer, cellular proliferation and tissue re-organization in the conjunctivae of adults. The relative absence of IFNG is consistent with previous transcriptome and targeted gene expression results in Ethiopian and Tanzanian adult trachoma cases with scarring or trichiasis (Burton et al., 2011b; Hu et al., 2012; Ramadhani et al., 2017). This lack of immune interferon suggests a lack of a dominant Th1 response in ST, unlike in AT, in which Th1, and type-2 interferon response profiles have been described as critical for clearance of Ct infection (Faal et al., 2006; Natividad et al., 2010).
We found no evidence of significant changes in the ocular bacterial communities of children with AT compared to matched, healthy controls. Overall the prevalence of ocular Ct infection was low in AT cases, which is consistent with the observed decline in AT and infection prevalence seen in The Gambia in these districts prior to the mass distribution of Zithromax (Harding-Esch et al., 2009). The V1-V3 16S rRNA sequencing method used in this and our previous studies is not capable of resolving Chlamydiae taxa, which is a limitation of this study, however in AT cases there was no evidence of an overall dysbiosis.
The most substantial changes in relative abundance across each disease phenotype are in Corynebacterium. In children with AT there was a non-significant decrease in relative abundance whilst in adults with ST there was a significant increase. In adults with ST, there is almost a 1/3 increase in Corynebacterium relative abundance compared to controls. This is consistent with culture results in trachoma where adults with ST are consistently found to have an increased proportion of Corynebacterium isolated (Hu et al., 2011a). A longitudinal study of progressive scarring disease suggested that presence of commensal bacteria by microbiological culture, including Corynebacterium spp., is marginally associated with further development of disease, although the effect was enhanced when in the presence of an ocular pathogen (Hu et al., 2018).
There are >120 species within the Corynebacterium genus. This genus is almost always isolated in ocular culture and identified in sequence studies of both healthy and diseased conjunctiva. Corynebacterium is largely disregarded as either a commensal, skin contaminant, or a secondary infection without a significant role in disease. Speciation of Corynebacterium in healthy eyes is not frequently investigated; one of the few reported studies found 23 of 92 healthy individuals positive for Corynebacterium, consisting of 5 lipophilic species (C. macginleyi, C. afermentans subsp. lipophilum, C. accolens, unspeciated lipophilic corynebacteria, and C. jeikeium) (von Graevenitz et al., 2001). Our samples were dominated by C. accolens and C. tuberculostericum. The identification of Corynebacterium species not commonly resident on skin suggests that these are not contaminants from surrounding facial skin, but species which find a niche on the conjunctiva.
We found both individual genes and modules (combinations of genes) that were differentially expressed and associated with the relative abundance of a number of genera. In children, three transcripts (S100A7, SRGN, and TLR4) and miR-155 expression were associated with presence of Haemophilus. These genes were members of ATEM2, an expression module with 24 genes; the expression of which is characteristic of innate responses to the microbiota. In vitro studies have shown that Haemophilus influenzae can stimulate release of S100A7 from human conjunctival epithelial cells (Garreis et al., 2011) but also may have increased resistance to most classes of antimicrobial peptides (Mason et al., 2006). It is therefore possible that Haemophilus may induce an inflammatory response while resisting clearance. However, combined analysis of gene expression, ocular microbiome, and disease status suggested that the association between Haemophilus abundance and ATEM2 expression was not related to AT. This supports the lack of independent association between Haemophilus abundance and AT. Increased ATEM2 expression in AT may be driven by a recently cleared bacterial infection or an unknown non-bacterial pathogen. It is also possible that previous infections have caused epigenetic changes which predispose individuals to inflammatory responses in the absence of classical stimuli. A larger sample size is required to investigate these hypotheses.
In adults, increased relative abundance of Corynebacterium was associated with increased expression of mucins (MUC1/MUC4) and the adhesion molecule CEACAM1. CEACAM1 in particular is exploited in mucosal colonization by both pathogenic and non-pathogenic bacteria, increased expression may support ocular expansion of otherwise transient bacteria (Tchoupa et al., 2014). MUC1 is thought to prevent damage and infection of the ocular surface epithelium through interactions with galectin-3 (Baudouin et al., 2018), however animal models and in vivo work have found inconsistent expression and functions of MUC1 in ocular infection and disease. MUC1 knock-out mice in two different strains, respectively showed no effect on infectivity after ocular challenge with Pseudomonas aeruginosa and increased infectivity after ocular challenge with Corynebacteria and coagulase-negative Staphylococci (Mantelli and Argueso, 2008). In humans, MUC1 expression is increased in allergic keratoconjunctivitis (Baudouin et al., 2018) and Sjögren's syndrome but decreased in DED (Hodges and Dartt, 2013). These inconsistencies may be in part due to splice variants in MUC1 and others (Imbert-Fernandez et al., 2011; Xie et al., 2014). The frequency of two MUC1 variants have been implicated in DED through modulation of the local inflammatory response (Imbert-Fernandez et al., 2011), it is possible changes in the frequency of variants may increase susceptibility to ST. These 3 genes are members of a larger module, STEM4, containing 12 genes whose expression is driven by the ocular microbiota. In this case, the majority of increased STEM4 expression was associated with increased relative abundance of Corynebacterium, which was further enhanced in ST. A proposed hypothesis from work in mice is that C. mastiditis is required to stimulate innate resistance (St. Leger et al., 2017), whilst in humans C. accolens is able to competitively generate metabolites that inhibit the growth of ocular pathogens such as S. pneumonia (Bomar et al., 2016). In contrast, our data suggest Corynebacterium spp. found in the ocular niche may contribute to altered mucin expression, along with bacterial and epithelial cell adhesion which are important factors in ST. To determine if increased Corynebacterium abundance is a promoter of or a result of ST requires longitudinal investigation.
The primary limitation of this study is that individuals were sampled cross-sectionally. Longitudinal sampling would help identify causal relationships between conjunctival gene expression, microbiome, and trachomatous disease which is difficult cross-sectionally. It is also possible that evaluating gene expression more comprehensively, for example by RNA-Seq, may have provided additional insights into the relationship between expression profiles and the ocular microbiome. However, the target transcripts were chosen based on associations with trachomatous disease based on previous micro-array studies (Natividad et al., 2010; Burton et al., 2011b), therefore likely provide a reliable representation of differentially regulated pathways. Ocular bacteria were comprehensively profiled in this study, the role of non-bacterial microorganisms was not investigated. There may be a fungal and/or viral component to non-chlamydial trachoma that could be elucidated through pan-fungal and metagenome sequencing.
The combined examination of the ocular microbiome and conjunctival host response suggests contrasting profiles in AT and ST. In children, in the absence of current Ct infection, ocular pathogens such as Haemophilus are prevalent and associate with damaging inflammatory responses that impair epithelial cell health. However, these pathogens are not independently associated with AT, suggesting there may be undiscovered factors promoting inflammation. In adults, expansion of a non-pathogenic or commensal bacterium such as Corynebacterium, at the cost of bacterial community diversity, is associated with innate responses thought to drive ST. This response in adults was associated with increased expression of mucins and genes involved in matrix adhesion of epithelial cells. Enhanced cell matrix adhesion could contribute to fibrosis, whilst increased mucin expression may modulate inflammatory responses. Longitudinal studies are critical to the further understanding of progression of AT to ST. In AT, longitudinal studies are needed to understand how long inflammatory responses are sustained after clearance of an infection. In ST, longitudinal studies are required to investigate innate responses and Corynebacterium abundance and whether they are drivers or outcomes of scarring.
Data Availability
Anonymized 16S sequencing data are available from the Sequence Read Archive (SRA) at the National Center for Biotechnology Information (NCBI) under accession numbers PRJNA248889 and PRNA515408.
Author Contributions
DM, RB, MB, CR, SB, and MH contributed to the study design. PM, HJ, TD, SB, and MH contributed to sample collection. HP, CP, JH, TD, and AG contributed to data collection. HP, CP, JH, TD, AG, CR, SB, and MH contributed to data analysis. HP, CP, JH, PM, HJ, TD, AG, DM, RB, MB, CR, SB, and MH contributed to the manuscript preparation.
Funding
This study was funded by grants from the Wellcome Trust (079246/Z/06/Z and 097330/Z/11/Z). CR was funded by the Wellcome Trust Institutional Strategic Support Fund (105609/Z/14/Z).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our thanks to the study participants, field team, and support services in The Gambia. We also extend our thanks to Dr. David Nelson and Dr. Evelyn Toh of Indiana University for providing 16S rRNA primer sequences and valuable advice.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00297/full#supplementary-material
References
Aoki, R., Fukuda, K., Ogawa, M., Ikeno, T., Kondo, H., Tawara, A., et al. (2013). Identification of causative pathogens in eyes with bacterial conjunctivitis by bacterial cell count and microbiota analysis. Ophthalmology 120, 668–676. doi: 10.1016/j.ophtha.2012.10.001
Bari, R., Leung, M., Turner, V. E., Embrey, C., Rooney, B., Holladay, M., et al. (2011). Molecular determinant-based typing of KIR alleles and KIR ligands. Clin. Immunol. 138, 274–281. doi: 10.1016/j.clim.2010.12.002
Baudouin, C., Rolando, M., Benitez Del Castillo, J. M., Messmer, E. M., Figueiredo, F. C., Irkec, M., et al. (2018). Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog. Retin. Eye Res. 71, 68–87. doi: 10.1016/j.preteyeres.2018.11.007
Bokulich, N. A., Subramanian, S., Faith, J. J., Gevers, D., Gordon, J. I., Knight, R., et al. (2013). Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 10, 57–59. doi: 10.1038/nmeth.2276
Bomar, L., Brugger, S. D., Yost, B. H., Davies, S. S., and Lemon, K. P. (2016). Corynebacterium accolens releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. MBio 7:e01725-15. doi: 10.1128/mBio.01725-15
Burr, S. E., Hart, J. D., Edwards, T., Baldeh, I., Bojang, E., Harding-Esch, E. M., et al. (2013). Association between ocular bacterial carriage and follicular trachoma following mass azithromycin distribution in the Gambia. PLoS Negl. Trop. Dis. 7:e2347. doi: 10.1371/journal.pntd.0002347
Burton, M. J., Bailey, R. L., Jeffries, D., Rajak, S. N., Adegbola, R. A., Sillah, A., et al. (2010). Conjunctival expression of matrix metalloproteinase and proinflammatory cytokine genes after trichiasis surgery. Invest. Ophthalmol. Vis. Sci. 51, 3583–3590. doi: 10.1167/iovs.09-4550
Burton, M. J., Hu, V. H., Massae, P., Burr, S. E., Chevallier, C., Afwamba, I. A., et al. (2011a). What is causing active trachoma? The role of nonchlamydial bacterial pathogens in a low prevalence setting. Invest. Ophthalmol. Vis. Sci. 52, 6012–6017. doi: 10.1167/iovs.11-7326
Burton, M. J., Rajak, S. N., Bauer, J., Weiss, H. A., Tolbert, S. B., Shoo, A., et al. (2011b). Conjunctival transcriptome in scarring trachoma. Infect. Immun. 79, 499–511. doi: 10.1128/IAI.00888-10
Butcher, R. M. R., Sokana, O., Jack, K., Kalae, E., Sui, L., Russell, C., et al. (2017). Active trachoma cases in the solomon islands have varied polymicrobial community structures but do not associate with individual non-chlamydial pathogens of the eye. Front. Med. 4:251. doi: 10.3389/fmed.2017.00251
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Capriotti, J. A., Pelletier, J. S., Shah, M., Caivano, D. M., and Ritterband, D. C. (2009). Normal ocular flora in healthy eyes from a rural population in Sierra Leone. Int. Ophthalmol. 29, 81–84. doi: 10.1007/s10792-008-9196-4
Carvalho Mda, G., Tondella, M. L., McCaustland, K., Weidlich, L., McGee, L., Mayer, L. W., et al. (2007). Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J. Clin. Microbiol. 45, 2460–2466. doi: 10.1128/JCM.02498-06
Chao, A., Chiu, C. H., and Jost, L. (2010). Phylogenetic diversity measures based on Hill numbers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 3599–3609. doi: 10.1098/rstb.2010.0272
Dawson, C. R., Jones, B. R., and Tarizzo, M. L. (1981). Guide to Trachoma Control in Programmes for the Prevention of Blindness. World Health Organisation.
Derrick, T., Last, A. R., Burr, S. E., Roberts, C. H., Nabicassa, M., Cassama, E., et al. (2016). Inverse relationship between microRNA-155 and−184 expression with increasing conjunctival inflammation during ocular Chlamydia trachomatis infection. BMC Infect. Dis. 16:60. doi: 10.1186/s12879-016-1367-8
Derrick, T., Roberts, C., Rajasekhar, M., Burr, S. E., Joof, H., Makalo, P., et al. (2013). Conjunctival MicroRNA expression in inflammatory trachomatous scarring. PLoS Negl. Trop. Dis. 7:e2117. doi: 10.1371/journal.pntd.0002117
Dong, Q., Brulc, J. M., Iovieno, A., Bates, B., Garoutte, A., Miller, D., et al. (2011a). Diversity of bacteria at healthy human conjunctiva. Invest. Ophthalmol. Vis. Sci. 52, 5408–5413. doi: 10.1167/iovs.10-6939
Dong, Q., Nelson, D. E., Toh, E., Diao, L., Gao, X., Fortenberry, J. D., et al. (2011b). The microbial communities in male first catch urine are highly similar to those in paired urethral swab specimens. PLoS ONE 6:e19709. doi: 10.1371/journal.pone.0019709
Faal, N., Bailey, R. L., Jeffries, D., Joof, H., Sarr, I., Laye, M., et al. (2006). Conjunctival FOXP3 expression in trachoma: do regulatory T cells have a role in human ocular Chlamydia trachomatis infection? PLoS Med. 3:e266. doi: 10.1371/journal.pmed.0030266
Garreis, F., Gottschalt, M., Schlorf, T., Glaser, R., Harder, J., Worlitzsch, D., et al. (2011). Expression and regulation of antimicrobial peptide psoriasin (S100A7) at the ocular surface and in the lacrimal apparatus. Invest. Ophthalmol. Vis. Sci. 52, 4914–4922. doi: 10.1167/iovs.10-6598
Goncalves, A., Makalo, P., Joof, H., Burr, S., Ramadhani, A., Massae, P., et al. (2016). Differential frequency of NKG2C/KLRC2 deletion in distinct African populations and susceptibility to Trachoma: a new method for imputation of KLRC2 genotypes from SNP genotyping data. Hum. Genet. 135, 939–951. doi: 10.1007/s00439-016-1694-2
Goodier, M. R., White, M. J., Darboe, A., Nielsen, C. M., Goncalves, A., Bottomley, C., et al. (2014). Rapid NK cell differentiation in a population with near-universal human cytomegalovirus infection is attenuated by NKG2C deletions. Blood 124, 2213–2222. doi: 10.1182/blood-2014-05-576124
Guglani, L., and Khader, S. A. (2010). Th17 cytokines in mucosal immunity and inflammation. Curr. Opin. HIV AIDS 5, 120–127. doi: 10.1097/COH.0b013e328335c2f6
Harding-Esch, E. M., Edwards, T., Sillah, A., Sarr, I., Roberts, C. H., Snell, P., et al. (2009). (). Active trachoma and ocular Chlamydia trachomatis infection in two Gambian regions: on course for elimination by 2020? PLoS Negl. Trop. Dis. 3:e573. doi: 10.1371/journal.pntd.0000573
Hodges, R. R., and Dartt, D. A. (2013). Tear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp. Eye Res. 117:62–78. doi: 10.1016/j.exer.2013.07.027
Holland, M. J., Jeffries, D., Pattison, M., Korr, G., Gall, A., Joof, H., et al. (2010). Pathway-focused arrays reveal increased matrix metalloproteinase-7 (matrilysin) transcription in trachomatous trichiasis. Invest. Ophthalmol. Vis. Sci. 51, 3893–3902. doi: 10.1167/iovs.09-5054
Hu, V. H., Holland, M. J., and Burton, M. J. (2013). Trachoma: protective and pathogenic ocular immune responses to Chlamydia trachomatis. PLoS Negl. Trop. Dis. 7:e2020. doi: 10.1371/journal.pntd.0002020
Hu, V. H., Macleod, D., Massae, P., Afwamba, I., Weiss, H. A., Mabey, D. C. W., et al. (2018). Non-chlamydial bacterial infection and progression of conjunctival scarring in trachoma. Invest. Ophthalmol. Vis. Sci. 59, 2339–2344. doi: 10.1167/iovs.17-23381
Hu, V. H., Massae, P., Weiss, H. A., Chevallier, C., Onyango, J. J., Afwamba, I. A., et al. (2011a). Bacterial infection in scarring trachoma. Invest. Ophthalmol. Vis. Sci. 52, 2181–2186. doi: 10.1167/iovs.10-5829
Hu, V. H., Massae, P., Weiss, H. A., Cree, I. A., Courtright, P., Mabey, D. C., et al. (2011b). In vivo confocal microscopy of trachoma in relation to normal tarsal conjunctiva. Ophthalmology 118, 747–754. doi: 10.1016/j.ophtha.2010.08.029
Hu, V. H., Weiss, H. A., Ramadhani, A. M., Tolbert, S. B., Massae, P., Mabey, D. C., et al. (2012). Innate immune responses and modified extracellular matrix regulation characterize bacterial infection and cellular/connective tissue changes in scarring trachoma. Infect. Immun. 80, 121–130. doi: 10.1128/IAI.05965-11
Huang, Y., Yang, B., and Li, W. (2016). Defining the normal core microbiome of conjunctival microbial communities. Clin. Microbiol. Infect. 22, 643.e7–643.e12. doi: 10.1016/j.cmi.2016.04.008
Imbert-Fernandez, Y., Radde, B. N., Teng, Y., Young, W. W. Jr., Hu, C., and Klinge, C. M. (2011). MUC1/A and MUC1/B splice variants differentially regulate inflammatory cytokine expression. Exp. Eye Res. 93, 649–657. doi: 10.1016/j.exer.2011.08.004
Ji, Y. W., Mittal, S. K., Hwang, H. S., Chang, E. J., Lee, J. H., Seo, Y., et al. (2017). Lacrimal gland-derived IL-22 regulates IL-17-mediated ocular mucosal inflammation. Mucosal Immunol. 10, 1202–1210. doi: 10.1038/mi.2016.119
Keenan, J. D., Lakew, T., Alemayehu, W., Melese, M., Porco, T. C., Yi, E., et al. (2010). Clinical activity and polymerase chain reaction evidence of chlamydial infection after repeated mass antibiotic treatments for trachoma. Am. J. Trop. Med. Hyg. 82, 482–487. doi: 10.4269/ajtmh.2010.09-0315
Keilty, R. A. (1930). The bacterial flora of the normal conjunctiva with comparative nasal culture study. Am. J. Ophthalmol. 13, 876–879. doi: 10.1016/S0002-9394(30)92437-3
Khamis, A., Raoult, D., and La Scola, B. (2004). rpoB gene sequencing for identification of Corynebacterium species. J. Clin. Microbiol. 42, 3925–3931. doi: 10.1128/JCM.42.9.3925-3931.2004
Kugadas, A., Christiansen, S. H., Sankaranarayanan, S., Surana, N. K., Gauguet, S., Kunz, R., et al. (2016). Impact of microbiota on resistance to ocular pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 12:e1005855. doi: 10.1371/journal.ppat.1005855
Langfelder, P., and Horvath, S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. doi: 10.1186/1471-2105-9-559
Langfelder, P., and Horvath, S. (2012). Fast R functions for robust correlations and hierarchical clustering. J. Stat. Softw. 46:i11. doi: 10.18637/jss.v046.i11
Last, A. R., Burr, S. E., Weiss, H. A., Harding-Esch, E. M., Cassama, E., Nabicassa, M., et al. (2014). Risk factors for active trachoma and ocular Chlamydia trachomatis infection in treatment-naive trachoma-hyperendemic communities of the Bijagos Archipelago, Guinea Bissau. PLoS Negl. Trop. Dis. 8:e2900. doi: 10.1371/journal.pntd.0002900
Li, Z., Burns, A. R., Miller, S. B., and Smith, C. W. (2011). CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB J. 25, 2659–2668. doi: 10.1096/fj.11-184804
Mantelli, F., and Argueso, P. (2008). Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 8, 477–483. doi: 10.1097/ACI.0b013e32830e6b04
Mason, K. M., Bruggeman, M. E., Munson, R. S., and Bakaletz, L. O. (2006). The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol. Microbiol. 62, 1357–1372. doi: 10.1111/j.1365-2958.2006.05460.x
Meyler, K. L., Meehan, M., Bennett, D., Cunney, R., and Cafferkey, M. (2012). Development of a diagnostic real-time polymerase chain reaction assay for the detection of invasive Haemophilus influenzae in clinical samples. Diagn. Microbiol. Infect. Dis. 74, 356–362. doi: 10.1016/j.diagmicrobio.2012.08.018
Miyashita, R., Tsuchiya, N., Hikami, K., Kuroki, K., Fukazawa, T., Bijl, M., et al. (2004). Molecular genetic analyses of human NKG2C (KLRC2) gene deletion. Int. Immunol. 16, 163–168. doi: 10.1093/intimm/dxh013
Natividad, A., Freeman, T. C., Jeffries, D., Burton, M. J., Mabey, D. C., Bailey, R. L., et al. (2010). Human conjunctival transcriptome analysis reveals the prominence of innate defense in Chlamydia trachomatis infection. Infect. Immun. 78, 4895–4911. doi: 10.1128/IAI.00844-10
Onishi, R. M., and Gaffen, S. L. (2010). Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129, 311–321. doi: 10.1111/j.1365-2567.2009.03240.x
Ozkan, J., Nielsen, S., Diez-Vives, C., Coroneo, M., Thomas, T., and Willcox, M. (2017). Temporal stability and composition of the ocular surface microbiome. Sci. Rep. 7:9880. doi: 10.1038/s41598-017-10494-9
Perkins, R. E., Kundsin, R. B., Pratt, M. V., Abrahamsen, I., and Leibowitz, H. M. (1975). Bacteriology of normal and infected conjunctiva. J. Clin. Microbiol. 1, 147–149.
Ramadhani, A. M., Derrick, T., Holland, M. J., and Burton, M. J. (2016a). Blinding trachoma: systematic review of rates and risk factors for progressive disease. PLoS Negl. Trop. Dis. 10:e0004859. doi: 10.1371/journal.pntd.0004859
Ramadhani, A. M., Derrick, T., Macleod, D., Holland, M. J., and Burton, M. J. (2016b). The relationship between active trachoma and ocular Chlamydia trachomatis infection before and after mass antibiotic treatment. PLoS Negl. Trop. Dis. 10:e0005080. doi: 10.1371/journal.pntd.0005080
Ramadhani, A. M., Derrick, T., Macleod, D., Massae, P., Mtuy, T., Jeffries, D., et al. (2017). Immunofibrogenic gene expression patterns in tanzanian children with ocular Chlamydia trachomatis infection, active trachoma and scarring: baseline results of a 4-year Longitudinal study. Front. Cell. Infect. Microbiol. 7:406. doi: 10.3389/fcimb.2017.00406
Roberts, C. H., Jiang, W., Jayaraman, J., Trowsdale, J., Holland, M. J., and Traherne, J. A. (2014a). Killer-cell immunoglobulin-like receptor gene linkage and copy number variation analysis by droplet digital PCR. Genome Med. 6:20. doi: 10.1186/gm537
Roberts, C. H., Last, A., Molina-Gonzalez, S., Cassama, E., Butcher, R., Nabicassa, M., et al. (2013). Development and evaluation of a next-generation digital PCR diagnostic assay for ocular Chlamydia trachomatis infections. J. Clin. Microbiol. 51, 2195–2203. doi: 10.1128/JCM.00622-13
Roberts, C. H., Molina, S., Makalo, P., Joof, H., Harding-Esch, E. M., Burr, S. E., et al. (2014b). Conjunctival scarring in trachoma is associated with the HLA-C ligand of KIR and is exacerbated by heterozygosity at KIR2DL2/KIR2DL3. PLoS Negl. Trop. Dis. 8:e2744. doi: 10.1371/journal.pntd.0002744
Schreiber, F., Arasteh, J. M., and Lawley, T. D. (2015). Pathogen resistance mediated by IL-22 signaling at the epithelial-microbiota interface. J. Mol. Biol. 427, 3676–3682. doi: 10.1016/j.jmb.2015.10.013
Shin, H., Price, K., Albert, L., Dodick, J., Park, L., and Dominguez-Bello, M. G. (2016). Changes in the eye microbiota associated with contact lens wearing. MBio 7:e00198. doi: 10.1128/mBio.00198-16
St. Leger, A. J., Desai, J. V., Drummond, R. A., Kugadas, A., Almaghrabi, F., Silver, P., et al. (2017). An ocular commensal protects against corneal infection by driving an interleukin-17 response from mucosal gammadelta T cells. Immunity 47, 148.e5–158.e5. doi: 10.1016/j.immuni.2017.06.014
Tchoupa, A. K., Schuhmacher, T., and Hauck, C. R. (2014). Signaling by epithelial members of the CEACAM family - mucosal docking sites for pathogenic bacteria. Cell Commun. Signal. 12:27. doi: 10.1186/1478-811X-12-27
Valeri, M., and Raffatellu, M. (2016). Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 74:ftw111. doi: 10.1093/femspd/ftw111
von Graevenitz, A., Schumacher, U., and Bernauer, W. (2001). The corynebacterial flora of the normal human conjunctiva is lipophilic. Curr. Microbiol. 42, 372–374. doi: 10.1007/s002840010232
Willcox, M. D. (2013). Characterization of the normal microbiota of the ocular surface. Exp. Eye Res. 117:99–105. doi: 10.1016/j.exer.2013.06.003
Xie, K., Zhi, X., Tang, J., Zhu, Y., Zhang, J., Li, Z., et al. (2014). Upregulation of the splice variant MUC4/Y in the pancreatic cancer cell line MIA PaCa-2 potentiates proliferation and suppresses apoptosis: new insight into the presence of the transcript variant of MUC4. Oncol. Rep. 31, 2187–2194. doi: 10.3892/or.2014.3113
Keywords: trachoma, immune response, microbiome, innate immunity, conjunctival diseases
Citation: Pickering H, Palmer CD, Houghton J, Makalo P, Joof H, Derrick T, Goncalves A, Mabey DCW, Bailey RL, Burton MJ, Roberts CH, Burr SE and Holland MJ (2019) Conjunctival Microbiome-Host Responses Are Associated With Impaired Epithelial Cell Health in Both Early and Late Stages of Trachoma. Front. Cell. Infect. Microbiol. 9:297. doi: 10.3389/fcimb.2019.00297
Received: 02 April 2019; Accepted: 31 July 2019;
Published: 21 August 2019.
Edited by:
Matam Vijay-Kumar, University of Toledo, United StatesReviewed by:
Daniel Champlin Propheter, UT Southwestern Medical Center, United StatesFernando L. Leite, Boehringer Ingelheim Animal Health Business Unit, United States
Copyright © 2019 Pickering, Palmer, Houghton, Makalo, Joof, Derrick, Goncalves, Mabey, Bailey, Burton, Roberts, Burr and Holland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harry Pickering, harry.pickering@lshtm.ac.uk
†These authors have contributed equally to this work
 Harry Pickering
Harry Pickering Christine D. Palmer1†
Christine D. Palmer1†  Tamsyn Derrick
Tamsyn Derrick Matthew J. Burton
Matthew J. Burton Chrissy H. Roberts
Chrissy H. Roberts Martin J. Holland
Martin J. Holland