An Overview of Immunotherapeutic Approaches Against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy
- 1Laboratory of Biology of Cell Interactions, Department of Morphology, Institute of Biological Sciences, Federal University of Minas Gerais, Belo Horizonte, Brazil
- 2Laboratory of Immuno-Proteome and Parasite Biology, Department of Parasitology, Institute of Biological Sciences, Federal University of Minas Gerais, Belo Horizonte, Brazil
- 3Laboratory of Biotechnology of Microorganisms, Federal University of São João Del-Rei, Divinópolis, Brazil
- 4Laboratory of Diagnostic and Monitoring Biomarkers, René Rachou Institute, FIOCRUZ-Minas, Belo Horizonte, Brazil
Visceral leishmaniasis (VL), caused by digenetic protozoa of the genus Leishmania, is the most severe form of leishmaniasis. Leishmania infantum is one of the species responsible for VL and the disease caused is considered a zoonosis whose main reservoir is the dog. Canine visceral leishmaniasis (CVL) can lead to the death of the animal if left untreated. Furthermore, the available pharmocologial treatment for CVL presents numerous disadvantages, such as relapses, toxicity, drug resistance, and the fact treated animals continue to be reservoirs when treatment fails to achieve parasitological cure. Moreover, the available VL control methods have not been adequate when it comes to controlling parasite transmission. Advances in immune response knowledge in recent years have led to a better understanding of VL pathogenesis, allowing new treatments to be developed based on immune system activation, often referred to as immunotherapy. In fact, well-defined protocols have been described, ranging from the use of immunomodulators to the use of vaccines. This treatment, which can also be associated with chemotherapy, has been shown to be effective in restoring or inducing an adequate immune response to reduce parasitic burden, leading to clinical improvement. This review focuses on immunotherapy directed at dogs infected by L. infantum, including a literature review of what has already been done in dogs. We also introduce a promising strategy to improve the efficacy of immunotherapy.
Introduction
Leishmaniasis is a group of infectious parasitic diseases caused by protozoa of the Leishmania genus (Rossi and Fasel, 2017). Visceral leishmaniasis (VL) is the most severe form, which can result in a high mortality rate in humans if untreated (Alemayehu and Alemayehu, 2017). It is known that three species are responsible for causing VL; Leishmania (Leishmania) donovani (Laveran and Mesnil, 1903) and Leishmania (Leishmania) infantum (Nicolle, 1908) are found in the Old World, while Leishmania (Leishmania) chagasi (Cunha and Chagas, 1937) is found in the New World. Although they have different names and different geographical origins, molecular data suggest that L. infantum and L. chagasi are the same species (Maurício et al., 2000).
In recent years, cases of human VL have been reported in 76 countries (Organização Pan-Americana da Saúde, 2018) and, in 2017, 95% of the new cases occurred in seven countries: Brazil, Ethiopia, India, Kenya, Somalia, South Sudan, and Sudan (World Health Organization, 2018). Brazil accounts for 96% of the number of human VL cases in Latin America (Organização Pan-Americana da Saúde, 2018).
The VL, caused by L. infantum, is a zoonosis in which the dog (Canis familiaris) serves as the main domestic reservoir (World Health Organization, 2010; Roatt et al., 2014; Duarte et al., 2016). The disease in dogs may be manifested by inducing apparent clinical signs that, when present, may range from mild to severe, causing death (Maia-Elkhoury et al., 2008; Reis et al., 2009). During VL urbanization (Da Silva et al., 2017), dogs became responsible for spreading the disease throughout the Brazilian countryside, resulting in a rising number of human VL cases (Reis et al., 2010). Notably, cases of canine visceral leishmaniasis (CVL) precede human cases (Leite et al., 2018).
The applied VL control measures are not adequate when it comes to interrupting the spread of the disease. Moreover, Leishmania antigens are not able to induce a high immunogenicity regarding protection against infection in dogs (Giunchetti et al., 2019). Although, CVL treatment cannot induce parasite clearance, this measure has been largely employed, thus demonstrating the dogs' close relationship in our society. In this sense, immunotherapeutic treatments have shown to be promising against CVL, with the main objective of reestablishing dog immunity and, therefore, parasite control (Roatt et al., 2017). This approach can be performed alone or in combination with chemotherapy (Singh and Sundar, 2014). The focus of this review is on the immunotherapy methods already described for the CVL treatment, whether or not associated with chemotherapy. Taking into account the complexity of CVL transmission, we discuss some current aspects regarding immunology, resistance and susceptibility biomarkers, as well as available control measures and disease treatment.
General Aspects of the Immunological Profile and Biomarkers Regarding Susceptibility and Resistance in Canine Visceral Leishmaniasis
The immune response in CVL is of great importance for understanding the pathogenesis of the disease (Alvar et al., 2004; Ribeiro et al., 2018; Giunchetti et al., 2019). The immune response profile can trigger a resistance or susceptibility pattern during the parasite infection, resulting in different clinical forms of the disease (Moreno and Alvar, 2002; Leal et al., 2014; Giunchetti et al., 2019).
With regard to vector contact with the canine host, in addition to local lesion formation induced by vector feeding (Solano-Gallego et al., 2001; Giunchetti et al., 2006; Jacintho et al., 2018), the deposition of infective L. infantum promastigotes takes place in the dermis along with salivary content vector. This process recruits phagocytic cells to the site, such as neutrophils, macrophages, and dendritic cells, creating a pro-inflammatory environment (Soulat and Bogdan, 2017).
An in vitro study demonstrated that neutrophils are effector cells with the ability to control the initial infection, resulting in reduced parasite viability (Pereira et al., 2017). Furthermore, it has been observed that neutrophils have an ability to produce high levels of IFN-γ when stimulated with soluble antigen of L. infantum (Leal et al., 2014). Moreover, other molecules of the innate immunity have been correlated with ongoing CVL, such as TLRs (Toll-like receptors) (Hosein et al., 2015; Pereira-Fonseca et al., 2017) and chemokines (Menezes-Souza et al., 2012; Solcà et al., 2016).
It is known that the main immune response against the parasite is induced by the adaptive response, especially the type 1 immune response, characterized by IFN-γ, TNF-α, and IL-2 production related to the resistance profile. This type of immune response is related to the upregulation of the anti-leishmanial activity in macrophages (Koutinas and Koutinas, 2014), this being the main effector mechanism of the intracellular death of Leishmania amastigotes (Baneth et al., 2008). In this sense, the type 1 immune response induces cytokines, such as IFN-γ and TNF-α, predominant in asymptomatic dogs, demonstrating their protective potential against the disease (Costa-Pereira et al., 2015). Solano-Gallego et al. (2016) demonstrated that infected dogs presenting high levels of IFN-γ had lower parasite loads when compared to infected dogs that did not produce this cytokine. Dogs lacking this cytokine have more severe clinical symptoms, with higher parasitemia (Martínez-Orellana et al., 2017). Similarly, Th17 cells induce L. infantum control growth (Nascimento et al., 2015; Rodriguez-Cortes et al., 2017).
In contrast, the type 2 immune response, characterized IL-4, IL-5, IL-10, and TGF-β cytokines, is related to susceptibility in CVL (Sanches et al., 2014; Rodríguez-Cortés et al., 2016; Rodriguez-Cortes et al., 2017; Rossi et al., 2016; Solano-Gallego et al., 2016; Solcà et al., 2016; Tonin et al., 2016; De Martini et al., 2018). These susceptible dogs manifest a common pattern in the progression of clinical signs, with severity and variety of signs increasing with disease progression, in which most clinicopathological changes become evident after 12 months of infection (Foglia Manzillo et al., 2013). The type 2 immune response provides an anti-inflammatory cytokine microenviroment deactivating the cellular immune response against L. infantum infection (Rodriguez-Cortes et al., 2017). Moreover, a pronounced anti-Leishmania humoral response leads to the production of high levels of non-immunoprotective antibodies (Barbiéri, 2006; Gradoni, 2015), highlighting the polyclonal B cell response characteristic of susceptibility in CVL (Koutinas and Koutinas, 2014). There is still no consensus as to which IgG subclass is related to resistance or susceptibility in CVL (Lima et al., 2017; Chaabouni et al., 2018). Furthermore, excessive activation of humoral immunity may lead to the production of autoantibodies (Koutinas and Koutinas, 2014), such as antiactin and antitubulin (Pateraki et al., 1983), antinuclear (Smith et al., 2004; Ginel et al., 2008), and antitransferrin (Chaabouni et al., 2018).
Although the cellular and humoral immunity parameters help to understand the progression of CVL, as well as the mechanisms related to resistance or susceptibility, integrated studies of several biomarkers are needed for a better understanding of the disease (Solcà et al., 2016). In asymptomatic dogs, hematological and biochemical parameters usually remain unchanged, while in symptomatic dogs changes may occur (Maia and Campino, 2018). Symptomatic dogs showed a significant decrease in red cells, lymphocytes, eosinophils, and platelets (Lopes et al., 2018). The biochemical parameters can be used to assess the general health status in CVL. Ongoing CVL is characterized by hyperproteinemia, hypoalbuminemia, and changes in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, urea, and creatinine concentrations (Heidarpour et al., 2012; Ribeiro et al., 2018). These parameters are interesting markers for therapeutic monitoring, especially those related to the kidney, since damage to this organ associated with the disease is almost unavoidable (Ribeiro et al., 2018). All of the biomarkers included in this section and regarding resistence or susceptibility in CVL are summarized in Figure 1.
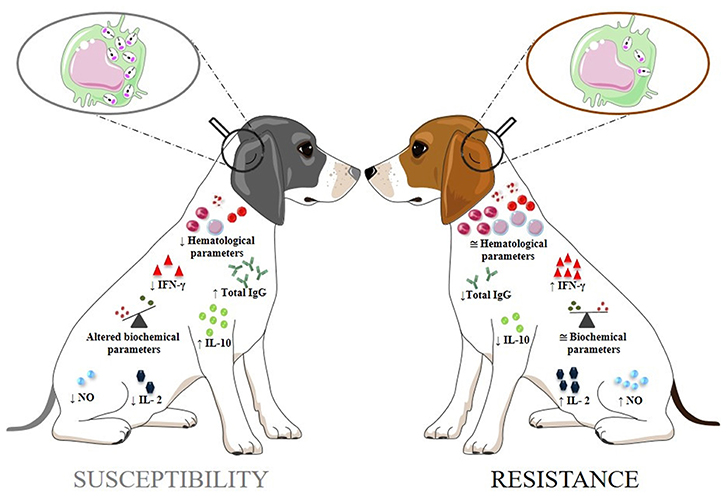
Figure 1. The biomarkers of canine visceral leishmaniasis related to susceptibility or resistance. The arrows (↑ and ↓) indicate the increase and decrease in biomarker levels, respectively; ≅: approximate normal levels; ↓ Hematological parameters: decreased in red blood cells, lymphocytes, eosinophils, and platelets; Altered Biochemical parameters: hyperproteinemia, hypoalbuminemia, increased in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, urea, and creatinine levels.
Current Control Methods Based on Sandfly Interference to Block Canine Visceral Leishmaniasis Transmission
The approach to visceral leishmaniasis control needs to consider all elements in the transmission network, such as (i) sandfly vector, (ii) parasite reservoirs, and (iii) human health. In this sense, health control and surveillance measures, based on the Brazilian National Visceral Leishmaniasis Program of the Ministry of Health, determine: (i) the use of chemical insecticides and (ii) environmental management for vector population control and vector-human contact reduction, (iii) canine serological surveys, (iv) euthanasia of positive cases and timely diagnosis, and (v) adequate treatment of human cases to prevent severe forms of disease and death (Ministério da Saúde, 2014). However, it has been reported that an urgent revision in this control program is required, as its effectiveness ranges from low to moderate (Werneck et al., 2014).
In an attempt to reduce the adaptation of the vector population to the peridomic environment, the environmental management associated with chemical spraying can be used as a preventive action (Lara-Silva et al., 2017). However, this strategy is unsustainable in the long term due to the size of the area to be treated (Otranto and Dantas-Torres, 2013). The use of insecticides/repellents (mainly pyrethroids), impregnated in dog collars or used for individual human protection on the skin and/or clothing (Alexander and Maroli, 2003) aims to prevent contact with the vector. Deltamethrin, lead representative, impregnated in dog collars induced a reduction from 53 to 59% in the CVL incidence rate of infected sandflies (Kazimoto et al., 2018). In addition, uncollared dogs showed a higher frequency of clinical signs with faster progression when compared to collared dogs, demonstrating the anti-feeding effect (Foglia Manzillo et al., 2006), presenting an interesting combination of disease control and cost-effectiveness (Shimozako et al., 2017). Another type II pyrethroid, Flumethrin, applied pour-on in dogs resulted in a significant reduction on total mortality rate and in the blood-feeding index of sandflies (Jalilnavaz et al., 2016). Furthermore, the systemic insecticide Fluralaner (Bravecto®, MSD animal health) (Gomez and Picado, 2017; Miglianico et al., 2018) used in dogs has demonstrated induction of 40–60% mortality of phlebotomines using a membrane feeding assay (Gomez et al., 2018a) and 90% mortality when the vector was direct feeding (Gomez et al., 2018b). Moreover, sandfly feeding in vaccinated dogs with CaniLeish® resulted in a marked reduction in Phlebotomus perniciosus infection (Bongiorno et al., 2013).
Recently, a newly patented vaccine using non-salivary antigens from sandflies has shown promise as a vector control strategy because it impairs its life cycle in addition to blocking Leishmania infection in sandflies. This approach has been considered as the next vaccine frontier for controlling vector-borne diseases (Graciano et al., 2019).
Despite all existing control measures, preventing the spread of VL has been ineffective in Brazil (Romero and Boelaert, 2010). In this context, researchers advocate alternative control measures, such as mass vaccination and treatment of dogs, since these approaches are able to induce reduction in the parasite load and block L. infantum transmission in sandflies, thus providing evidence for reducing new canine and human VL cases (Pessoa-e-Silva et al., 2019).
Conventional Canine Visceral Leishmaniaisis Treatment
Treatment of CVL is characterized by high rates of relapse, regardless of the antileishmanial drugs used, either as a single drug or in combined drug therapy (Ribeiro et al., 2018). Moreover, clinical and parasitological cure is rarely achieved, not to mention the possibility of drug resistance (Travi, 2014; Marcondes and Day, 2019).
Drug therapy using miltefosine was originally developed as an anticancer agent in the 1990s and was first recorded for VL treatment in 2002 in India (Dorlo et al., 2012). In 2016, the Brazilian Ministry of Health and the Ministry of Agriculture Livestock and Supply approved the registration of Milteforan® (Virbac, Brazil) (Brasil, 2016). Although there was a notable improvement in the clinical symptoms when using this drug, it was not accompanied by parasitological clearance, suggesting that treatment with miltefosine should not be recommended (Andrade et al., 2011). Recently, miltefosine treatment against CVL revealed clinical improvement with a reduction in infectivity from L. infantum-infected dogs (Dos Santos Nogueira et al., 2019).
Allopurinol has a parasitostatic activity and its long-term administration maintains low parasite loads, thus contributing to the prevention of canine relapse (Koutinas et al., 2001). The association of this drug with miltefosine showed to be a promising combination for CVL treatment (Foglia Manzillo et al., 2009). However, induced resistance is also a problem associated with the use of allopurinol (Yasur-Landau et al., 2017).
In most parts of the world, meglumine antimoniate is the most commonly used treatment for human and canine leishmaniasis. Meglumine antimonate, combined with allopurinol, is considered the most effective therapy for CVL (Solano-Gallego et al., 2009); however, CVL treatment with the same human-used drugs is not recommended since it may induce parasite resistance (Travi, 2014).
The great challenge of CVL treatment is to identity a drug that (i) is not used in VL human treatment, (ii) does not induce kidney damage or any other adverse effect, (iii) provides a parasite load control, (iv) interferes in the sandflies' life cycle, and (v) blocks parasite transmission. In this sense, other treatment options should be studied, such as immunotherapy, in an attempt to improve CVL treatment efficacy.
Immunotherapy and Immunochemotherapy as Strategies for Improving Canine Visceral Leishmaniasis Treatment Efficacy
Immunotherapy involves the use of biological substances or molecules to modulate immune responses for the purpose of achieving prophylactic and/or therapeutic success (Okwor and Uzonna, 2009; Musa et al., 2010; Khadem and Uzonna, 2014; Roatt et al., 2014; Singh and Sundar, 2014). For instance, immunotherapeutic agents exert their effect by directly or indirectly augmenting the host's natural defenses, restoring the impaired effector functions or reducing the host's excessive response (Oldham and Smalley, 1983; Okwor and Uzonna, 2009).
Since Leishmania is able to persist in host cells by evading or exploiting their immune mechanisms, the ability to develop a specific immune response could induce parasite replication control (Gupta et al., 2013). Thus, triggering the immune system with antigens or immunomodulators could be an alternative approach to combatting distinct infections such as leishmaniasis (Scott and Novais, 2016). In fact, cutaneous leishmaniasis (CL) immunotherapy treatment was evaluated by Avila et al. (1982) using glucan immunotherapy, but without satisfactory results. In Brazil, the first study was carried out by Badaro et al. (1990), which demonstrated the immunotherapeutic ability of IFN-γ when concomitantly administered with pentavalent antimony in human visceral leishmaniasis. Notably, Mayrink et al. (1992) proposed immunotherapy using a mixture of five Leishmania strains and observed a 76% cure rate in human CL.
Distinct therapeutic approaches in CVL discussed in this section are summarized in Table 1. Since immunotherapeutic treatment against Leishmania infection has been successfully proved, the first study in dogs was performed by Neogy et al. (1994) using LiF2 antigen alone or combined with N-methylglucamine antimonate. These authors described that the immunochemotherapy protocol was more efficient for CVL treatment, demonstrating a 100% clinical cure rate, in which they did not observe any parasite in direct microscopic examination of bone-marrow aspirates. Another study demonstrated that the association of N-methyl D-glucamine antimoniate and L. infantum antigens (soluble antigen) showed an increase in the proportion of T lymphocytes; however, lymphnode aspirates remained positive (Guarga et al., 2002). Treatment using L. braziliensis promastigotes, alone or in association with Glucantime®, showed that chemotherapy alone was more effective, since the dogs had the lowest parasite load (Melo et al., 2002). Similarly, the L. major promastigote antigens and heat-killed Mycobacterium vaccae (SRL172) were compared to Glucantime® chemotherapy and revealed that both treatments were able to control parasitism, albeit slower in immunotherapy than in chemotherapy treatment (Jamshidi et al., 2011).
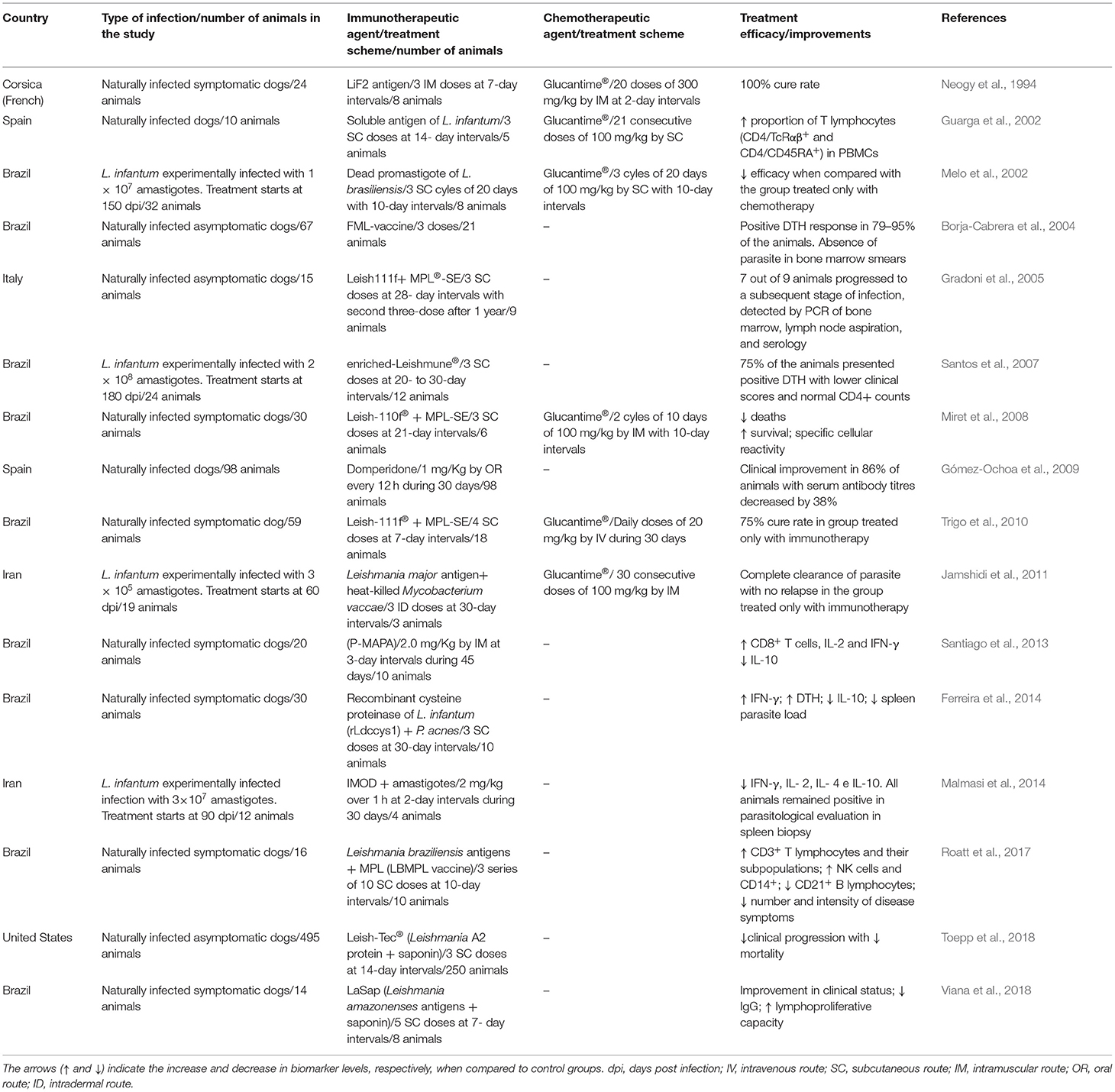
Table 1. Major immunotherapy and immunochemotherapy treatments evaluated in dogs against L. infantum infection.
Immunomodulators have been described as triggering the immune system against Leishmania infection resulting in parasite control (Taslimi et al., 2016). Domperidone, for example, was able to induce clinical improvement in CVL in 86% of the animals with multiple clinical signs, with serum antibody titres decreased by 38% (Gómez-Ochoa et al., 2009). Moreover, the protein aggregate of magnesium–ammonium phospholinoleate–palmitoleate anhydride (P-MAPA) was used as a immunomodulator approach against CVL, inducing partial immunocompetence in symptomatic dogs (Santiago et al., 2013). Contrarily, the IMOD (Novel Herbal Immunomodulator Drug) used as immunotherapeutic treatment in experimental CVL did not trigger a proinflammatory immune response or induce parasite control, resulting in low therapeutic efficacy (Malmasi et al., 2014).
Vaccine therapy terminology has been employed in immunotherapy treatment, since the authors described the vaccinal antigens used for inducing cell-mediated immune response against CVL. Borja-Cabrera et al. (2004) evaluated the immunotherapeutic efficacy of FML-vaccine in asymptomatic dogs, which induced a positive DTH response in 79–95% of the animals and parasite control in bone marrow. Contrarily, vaccination with Leish111f (MML polyprotein) plus MPL®-SE failed to deter disease progression (Gradoni et al., 2005). Santos et al. (2007) administered enriched-Leishmune® vaccine (FML-Saponin) in symptomatic dogs, resulting in a reduction in clinical signs and parasitic burden on the liver, spleen, bone marrow, and blood. Immunotherapy using Leish-110f® with the adjuvant MPL-SE (Monophosphoril Lipid A), alone or in combination with Glucantime® (immunochemotherapy) in symptomatic dogs, was able to reduce the number of deaths, increase survival probability, and trigger specific cellular reactivity for parasite antigens (Miret et al., 2008). Beyond that, the recombinant polyprotein using Leish-111f® antigen with MPL-SE® provided a 75% cure rate, which was higher as compared to dogs treated with chemotherapy (64%) or immunochemotherapy (50%) (Trigo et al., 2010).
The immunotherapeutic protocol using L. infantum recombinant cysteine proteinase (rLdccys1) in combination with adjuvant Propionibacterium acnes induced high IFN-γ with low IL-10 cytokine production along with a reduction in the spleen parasite load (Ferreira et al., 2014). Notably, the vaccine composed of L. braziliensis antigens associated with MPL adjuvant (LBMPL vaccine) in symptomatic dogs was able to trigger increased CD3+ T lymphocytes and their subpopulations, a reduction in CD21+ B lymphocytes, and an increase in NK cells and CD14+ monocytes. Moreover, the dogs exhibited an important decline in the number and intensity of disease symptoms, increased body weight, reduced splenomegaly, and a drop in the parasite burden (Roatt et al., 2017). Similarly, Viana et al. (2018) demonstrated that L. amazonensis antigens, alone or in association with saponin (LaSap therapeutic vaccine), used in symptomatic dogs improved their clinical status, reduced IgG serum levels, and triggered a lymphoproliferative profile using L. infantum antigens, resulting in an outstanding reduction in parasite load. Furthermore, the vaccine Leish-Tec® (Leishmania A2 protein plus saponin adjuvant—Ceva Saúde Animal Ltda) used as immunotherapy in asymptomatic dogs induced a curtailment in clinical progression and in mortality (Toepp et al., 2018).
The different protocols used for immunotherapy or immunochemotherapy generally lead to an improvement in clinical signs with a possibility to further reduce the parasite burden by being activated in the immune system against Leishmania infection. Taken together, these results showed that immunotherapy is a promising strategy for the treatment of CVL. However, parasite clearance in CVL has not yet been achieved, irrespective of treatment, and this is the strongest negative aspect in these studies. The search for new immunotherapeutic agents to improve the results in this type of treatment is of great interest, given its aim to improve parasite control and develop approaches to blocking CVL transmission. All immunotherapy-related immunological aspects described above are summarized in Figure 2.
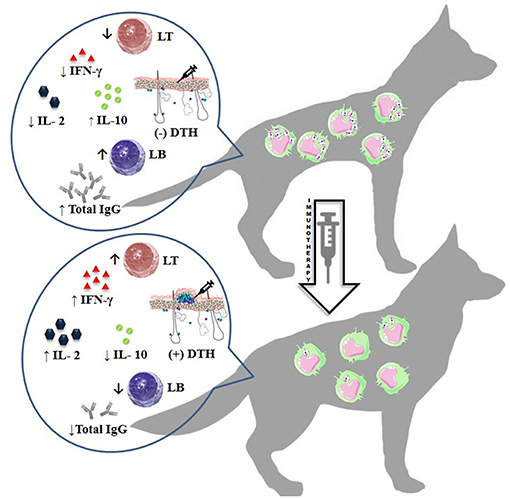
Figure 2. Immunotherapy-related immunological aspects. ↑, decreased; ↑, increased; IFN-γ, Interferon gamma; IL-2, Interleukin 2; IL-10, Interleukin 10; (+), positive; (–), negative; DTH, delayed-type hypersensitivity; IgG, Immunoglobulin G; LT, T lymphocyte; LB, B lymphocyte.
Discussion and Perspectives: Immunotherapeutic Strategies to Treat and Block Canine Visceral Leishmaniasis Transmission
Although the immunotherapeutic protocols described were able to induce clinical improvement, there is still a major impasse when it comes to obtaining parasitological cure, as the L. infantum-infected dogs continue to be parasite reservoirs for sandfly vectors. Therefore, new protocols are needed to achieve a better efficacy in CVL treatment. Furthermore, innovative strategies can be incorporated into immunotherapy to interfere with the dynamics of disease transmission.
Considering that the sandfly's blood meal and the parasite's interaction with the invertebrate host are determining factors for Leishmania transmission, our research group has been developing studies focused on these factors so as to interfere with the parasite transmission dynamic (Graciano et al., 2019). The incorporation of vector antigens into new immunobiologicals is a promising strategy designed to disrupt the sandflies' life cycle, as well as block L. infantum transmission (Graciano et al., 2019). In fact, our research group has already identified different formulations with these capabilities that are currently being analyzed in addition to Leishmania antigens. The combination of parasite antigens with sandfly antigens in a single formulation as an immunotherapeutic protocol would provide more appropriate treatment. However, this new immunotherapeutic approach has not yet been tested in dogs. Finally, this type of immunotherapy could promote clinical improvement and efficient control of the parasite load, in addition to significantly reducing the risk of VL transmission and, thereby, lessening the number of canine and human cases.
Author Contributions
AAG, JL, LR, and RM wrote the manuscript. AAG, PS, OM-J, HR, DO, DS, and TS reviewed the manuscript. AAG, AM, AG, OM-F, WD, DS-L, and RG drafted and critically evaluated the manuscript.
Funding
This research was financially supported through grants from CAPES (Coordination for the Improvement of High Higher Education Personnel, Brazil), CNPq (National Council for Scientific and Technological Development), Ministry of Health of Brazil (Decit/SCTIE/MS), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), and Quatree (Granvita).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would also like to thank the CNPq for a fellowship to AM, AG, OM-F, WD, DS-L, and RG.
References
Alemayehu, B., and Alemayehu, M. (2017). Leishmaniasis: a review on parasite, vector and reservoir host. Healt. Sci. J. 11, 1–6. doi: 10.21767/1791-809X.1000519
Alexander, B., and Maroli, M. (2003). Control of phlebotomine sandflies. Med Vet. Entomol. 17, 1–18. doi: 10.1046/j.1365-2915.2003.00420.x
Alvar, J., Cañavate, C., Molina, R., Moreno, J., and Nieto, J. (2004). Canine leishmaniasis. Adv. Parasitol. 57, 1–88. doi: 10.1016/S0065-308X(04)57001-X
Andrade, H. M., Toledo, V. P. C. P., Pinheiro, M. B., Guimarães, T. M. P. D., Oliveira, N. C., Castro, J. A., et al. (2011). Evaluation of miltefosine for the treatment of dogs naturally infected with L. infantum (= L. chagasi) in Brazil. Vet. Parasit. 181, 83–90. doi: 10.1016/j.vetpar.2011.05.009
Avila, J. L., Biondo, F., Monzón, H., and Convit, J. (1982). Cutaneous leishmaniasis in mice: resistance to glucan immunotherapy, either alone or combined with chemotherapy. Am J Trop Med Hyg. 31, 53–59. doi: 10.4269/ajtmh.1982.31.53
Badaro, R., Falcoff, E., Badaro, F. S., Carvalho, E. M., Pedral-Sampaio, D., Barral, A., et al. (1990). Treatment of visceral leishmaniasis with pentavalent antimony and interferon gamma. N. Engl. J. Med. 322, 16–21. doi: 10.1056/NEJM199001043220104
Baneth, G., Koutinas, A. F., Solano-Gallego, L., Bourdeau, P., and Ferrer, L. (2008). Canine leishmaniosis - new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 24, 324–330. doi: 10.1016/j.pt.2008.04.001
Barbiéri, C. L. (2006). Immunology of canine leishmaniasis. Parasite Immunol. 28, 329–337. doi: 10.1111/j.1365-3024.2006.00840.x
Bongiorno, G., Paparcone, R., Manzillo, V. F., Oliva, G., Cuisinier, A. M., and Gradoni, L. (2013). Vaccination with LiESP/QA-21 (CaniLeish®) reduces the intensity of infection in Phlebotomus perniciosus fed on Leishmania infantum infected dogs-A preliminary xenodiagnosis study. Vet. Parasitol. 197, 691–695. doi: 10.1016/j.vetpar.2013.05.008
Borja-Cabrera, G. P., Cruz, A., Paraguai, E., Souza, D., Hashimoto, L. Y., Antonio, F., et al. (2004). Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine 22, 2234–2243. doi: 10.1016/j.vaccine.2003.11.039
Brasil (2016). Ministério da Agricultura Pecuária e Abastecimento. Nota Técnica Conjunta n°001/2016. Available online at: http://www.sbmt.org.br/portal/wp-content/uploads/2016/09/nota-tecnica.pdf (accesed March 04, 2019).
Chaabouni, A., Elandoulsi, R. B., Mhadhbi, M., Gharbi, M., and Sassi, A. (2018). Comparative analysis of the Leishmania infantum-specific antibody repertoires and the autoantibody repertoires between asymptomatic and symptomatic dogs. Vet. Parasitol. 261, 9–17. doi: 10.1016/j.vetpar.2018.07.011
Costa-Pereira, C., Moreira, M. L., Soares, R. P., Marteleto, B. H., Ribeiro, V. M., França-Dias, M. H., et al. (2015). One-year timeline kinetics of cytokine-mediated cellular immunity in dogs vaccinated against visceral leishmaniasis. BMC Vet. Res. 11, 1–10. doi: 10.1186/s12917-015-0397-6
Cunha, A. M., and Chagas, E. (1937). Estudos sobre o parasito. In: Leishmaniose visceral americana, nova entidade mórbida do homem na América do Sul. Mem. Inst. Oswaldo Cruz. 32, 329–337.
Da Silva, T. A. M., Coura-Vital, W., Barbosa, D. S., Oiko, C. S. F., Morais, M. H. F., Tourinho, B. D., et al. (2017). Spatial and temporal trends of visceral leishmaniasis by mesoregion in a southeastern state of Brazil, 2002-2013. PLoS Negl. Trop. Dis. 11:e0005950. doi: 10.1371/journal.pntd.0005950
De Martini, C. C., de Andrade, J. T., de Almeida, S. K. M., Silva, K. L. O., Eugenio, F. R., Santos, P. S. P., et al. (2018). Cellular apoptosis and nitric oxide production in PBMC and spleen from dogs with visceral leishmaniasis. Comp. Immunol. Microb. Infec. Dis. 57, 1–7. doi: 10.1016/j.cimid.2018.01.003
Dorlo, T. P. C., Balasegaram, M., Beijnen, J. H., and Vries, P. (2012). Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniosis. J. Antimicrob. Chemother. 67, 2576–2597. doi: 10.1093/jac/dks275
Dos Santos Nogueira, F., Avino, V. C., Galvis-Ovallos, F., Pereira-Chioccola, V. L., Moreira, M. A. B., Romariz, A. P. P. L., et al. (2019). Use of miltefosine to treat canine visceral leishmaniasis caused by Leishmania infantum in Brazil. Parasites Vect. 12:79. doi: 10.1186/s13071-019-3323-0
Duarte, M. C., Lage, D. P., Martins, V. T., Chávez-Fumagalli, M. A., Roatt, B. M., Menezes-Souza, D., et al. (2016). Recent updates and perspectives on approaches for the development of vaccines against visceral leishmaniasis. Rev. Soc. Bras. Med.Trop. 49, 398–407. doi: 10.1590/0037-8682-0120-2016
Ferreira, J. H. L., Silva, L. S., Longo-Maugéri, I. M., Katz, S., and Barbiéri, C. L. (2014). Use of a recombinant cysteine proteinase from Leishmania (Leishmania) infantum chagasi for the immunotherapy of canine visceral leishmaniasis. PLoS Negl. Trop. Dis. 8, 1–8. doi: 10.1371/journal.pntd.0002729
Foglia Manzillo, F., Paparcone, R., Cappiello, S., De Santo, R., Bianciardi, P., and Oliva, G. (2009). Resolution of tongue lesions caused by Leishmania infantum in a dog treated with the association miltefosine-allopurinol. Parasites Vect. 2, 4–7. doi: 10.1186/1756-3305-2-S1-S6
Foglia Manzillo, V., Di Muccio, T., Cappiello, S., Scalone, A., Paparcone, R., Fiorentino, E., et al. (2013). Prospective study on the incidence and progression of clinical signs in naïve dogs naturally infected by Leishmania infantum. PLoS Negl. Trop. 7:e2225. doi: 10.1371/journal.pntd.0002225
Foglia Manzillo, V., Oliva, G., Pagano, A., Manna, L., Maroli, M., and Gradoni, L. (2006). Deltamethrin-impregnated collars for the control of canine leishmaniasis: evaluation of the protective effect and influence on the clinical outcome of Leishmania infection in kennelled stray dogs. Vet. Parasitol. 142, 142–145. doi: 10.1016/j.vetpar.2006.06.029
Ginel, P. J., Camacho, S., and Lucena, R. (2008). Anti-histone antibodies in dogs with leishmaniasis and glomerulonephritis. Res. Vet. Sci. 85, 510–514. doi: 10.1016/j.rvsc.2008.01.007
Giunchetti, R. C., Mayrink, W., Genaro, O., Carneiro, C. M., Corrêa-Oliveira, R., Martins-Filho, O. A., et al. (2006). Relationship between canine visceral leishmaniosis and the Leishmania (Leishmania) chagasi burden in dermal inflammatory foci. J. Comp. Pathol. 135, 100–137. doi: 10.1016/j.jcpa.2006.06.005
Giunchetti, R. C., Silveira, P., Resende, L. A., Leite, J. C., de Oliveira Melo-Júnior, O. A., Rodrigues Alves, M. L., et al. (2019). Canine visceral leishmaniasis biomarkers and their employment in vaccines. Vet. Parasitol. 271, 87–97. doi: 10.1016/j.vetpar.2019.05.006
Gomez, S. A., Curdi, J. L., Hernandez, J. A. C., Peris, P. P., Gil, A. E., Velasquez, R. V. O., et al. (2018a). Phlebotomine mortality effect of systemic insecticides administered to dogs. Parasit. Vect. 11:230. doi: 10.1186/s13071-018-2820-x
Gomez, S. A., Lucientes, J., Castillo, J. A., Peris, M. P., Delacour, S., Ortega, P., et al. (2018b). A randomized, blinded, controlled trial to assess sand fly mortality of fluralaner administered orally in dogs. Parasit Vect. 11:627. doi: 10.1186/s13071-018-3231-8
Gomez, S. A., and Picado, A. (2017). Systemic insecticides used in dogs: potential candidates for phlebotomine vector control? Trop. Med. Int. Health 22, 755–764. doi: 10.1111/tmi.12870
Gómez-Ochoa, P., Castillo, J. A., Gascón, M., Zarate, J. J., Alvarez, F., and Couto, C. G. (2009). Use of domperidone in the treatment of canine visceral leishmaniasis: a clinical trial. Vet. J. 179, 259–263. doi: 10.1016/j.tvjl.2007.09.014
Graciano, R. C. D., Ribeiro, J. A. T., Macedo, A. K. S., Lavareda, J. P. S., Oliveira, P. R., Netto, J. B., et al. (2019). Recent patents applications in red biotechnology: a mini-review. Recent Pat Biotechnol. 13, 170–186. doi: 10.2174/1872208313666190114150511
Gradoni, L. (2015). Canine Leishmania vaccines: still a long way to go. Vet. Parasitol. 208, 94–100. doi: 10.1016/j.vetpar.2015.01.003
Gradoni, L., Manzillo, V. F., Pagano, A., Piantedosi, D., De Luna, R., Gramiccia, M., et al. (2005). Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine 23, 5245–5251. doi: 10.1016/j.vaccine.2005.07.001
Guarga, J. L., Moreno, J., Lucientes, J., Gracia, M. J., Peribanez, M. A., and Castillo, J. A. (2002). Evaluation of a specific immunochemotherapy for the treatment of canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 88, 13–20. doi: 10.1016/S0165-2427(02)00128-9
Gupta, G., Oghumu, S., and Satoskar, A. R. (2013). Mechanisms of immune evasion in leishmaniasis. Adv. Appl. Microb. 82, 155–184. doi: 10.1016/B978-0-12-407679-2.00005-3
Heidarpour, M., Soltani, S., Mohri, M., and Khoshnegah, J. (2012). Canine visceral leishmaniasis: relationships between oxidative stress, liver and kidney variables, trace elements, and clinical status. Parasitol. Res. 111, 1491–1496. doi: 10.1007/s00436-012-2985-8
Hosein, S., Rodríguez-Cortés, A., Blake, D. P., Allenspach, K., Alberola, J., and Solano-Gallego, L. (2015). Transcription of toll-like receptors 2, 3, 4 and 9, FoxP3 and Th17 cytokines in a susceptible experimental model of canine Leishmania infantum infection. PLoS ONE 10:e0140325. doi: 10.1371/journal.pone.0140325
Jacintho, A. P. P., Melo, G. D., Machado, G. F., Bertolo, P. H. L., Moreira, P. R. R., Momo, C., et al. (2018). Expression of matrix metalloproteinase-2 and metalloproteinase-9 in the skin of dogs with visceral leishmaniasis. Parasitol. Res. 117, 1819–1827. doi: 10.1007/s00436-018-5868-9
Jalilnavaz, M. R., Abai, M., Vatandoost, H., Mohebali, M., Akhavan, A., Zarei, Z., et al. (2016). Original article application of flumethrin pour-on on reservoir dogs and its efficacy against sand flies in endemic focus of visceral leishmaniasis. J Arthropod Borne Dis. 10, 78–86.
Jamshidi, S. H., Avizeh, R., Mohebali, M., and Bokaie, S. (2011). Immunotherapy using autoclaved L. major antigens and M. vaccae with meglumine antimoniate, for the treatment of experimental canine visceral leishmaniasis. Iranian J. Parasit. 6, 26–34.
Kazimoto, T. A., Amora, S. S. A., Figueiredo, F. B., Magalhaes, J. M. E., Freitas, Y. B. M., Sousa, M. L. R., et al. (2018). Impact of 4% deltamethrin-impregnated dog collars on the prevalence and incidence of canine visceral leishmaniasis. Vec. Borne Zoonotic Dis. 18, 356–363. doi: 10.1089/vbz.2017.2166
Khadem, F., and Uzonna, J. E. (2014). Immunity to visceral leishmaniasis: implications for immunotherapy. Future Microbiol. 9, 901–915. doi: 10.2217/fmb.14.43
Koutinas, A. F., and Koutinas, C. K. (2014). Pathologic mechanisms underlying the clinical findings in canine leishmaniosis due to Leishmania infantum/chagasi. Vet. Pathol. 51, 527–538. doi: 10.1177/0300985814521248
Koutinas, A. F., Saridomichelakis, M. N., Mylonakis, M. E., Leontides, L., Polizopoulou, Z., Billinis, C., et al. (2001). A randomized, blinded, placebo-controlled clinical trial with allopurinol in canine leishmaniosis. Vet. Parasitol. 98, 247–261. doi: 10.1016/S0304-4017(01)00399-5
Lara-Silva, F. O., Michalsky, E. M., Fotes-Dias, C. L., Fiuza, V. O. P., and Dias, E. S. (2017). Evaluation of chemical spraying and environmental management efficacy in areas with minor previous application of integrated control actions for visceral leishmaniasis in Brazil. Acta Trop. 176, 109–113. doi: 10.1016/j.actatropica.2017.07.029
Laveran, A., and Mesnil, F. (1903). Sur un protozaire nouveau (Piroplasma donovani Lav. & Mesn.). Parasite d' une fièvrew de I'Inde. Comp. R. Hébd. Séanc. Acad. Sci. 137, 957–961.
Leal, G. G. D. A., Roatt, B. M., Aguiar-Soares, R. D. O., Carneiro, C. M., Giunchetti, R. C., Teixeira-Carvalho, A., et al. (2014). Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. Vet. Parasitol. 205, 472–482. doi: 10.1016/j.vetpar.2014.08.022
Leite, B. M. C., Solcà, M. D. S., Santos, L. C. S., Coelho, L. B., Amorim, L. D. A. F., Donato, L. E., et al. (2018). The mass use of deltamethrin collars to control and prevent canine visceral leishmaniasis: a field effectiveness study in a highly endemic área. PLoS Negl. Trop. Dis. 12:e0006496. doi: 10.1371/journal.pntd.0006496
Lima, L. V. D. R., Carneiro, L. A., Campos, M. B., Santos, T. V., Ramos, P. K., Laurenti, M. D., et al. (2017). Further evidence associating IgG1, but not IgG2, with susceptibility to canine visceral leishmaniasis caused by Leishmania (L.) infantum chagasi-infection. Parasite 24:37. doi: 10.1051/parasite/2017039
Lopes, V. V., Belo, V. S., Pereira, D. A., Coelho, M. B., Pena, H. P., Alves, N. R., et al. (2018). IgG avidity index and complete blood count as biomarkers of clinical disease in naturally infected dogs with Leishmania infantum. Vet. Parasitol. 15, 96–103. doi: 10.1016/j.vetpar.2018.08.016
Maia, C., and Campino, L. (2018). Biomarkers associated with Leishmania infantum exposure, infection, and disease in dogs. Front. Cell. Infect. Microbiol. 8:302. doi: 10.3389/fcimb.2018.00302
Maia-Elkhoury, A. N., Alves, W. A., Sousa-Gomes, M. L., Sena, J. M., and Luna, E. A. (2008). Visceral leishmaniasis in Brazil: trends and challenges. Cad Saude Publica 24, 2941–2947. doi: 10.1590/S0102-311X2008001200024
Malmasi, A., Ardestani, B. Z., Bayanolhagh, S., Mohebali, M., Khorshid, H. K., Sadrpour, P., et al. (2014). Assessment of the effects of a novel herbal immunomodulator drug (IMOD) on cytokine profiles in experimental canine visceral leishmaniasis: a preliminary survey. Iran. J. Parasitol. 9, 292–301.
Marcondes, M., and Day, M. J. (2019). Current status and management of canine leishmaniasis in Latin America. Res. Vet. Sci. 123, 261–272. doi: 10.1016/j.rvsc.2019.01.022
Martínez-Orellana, P., Marí-Martorell, D., Montserrat-Sangrà, S., Ordeix, L., Baneth, G., and Solano-Gallego, L. (2017). Leishmania infantum-specific IFN-γ production in stimulated blood from dogs with clinical leishmaniosis at diagnosis and during treatment. Vet. Parasitol. 248, 39–47. doi: 10.1016/j.vetpar.2017.10.018
Maurício, I. L., Stothard, J. R., and Miles, M. A. (2000). The strange case of Leishmania chagasi. Parasitol. Today 16, 188–189. doi: 10.1016/S0169-4758(00)01637-9
Mayrink, W., Magalhaes, P. A., Michalick, M. S., da Costa, C. A., Lima Ade, O., Melo, M. N., et al. (1992). Immunotherapy as a treatment of American cutaneous leishmaniasis: preliminary studies in Brazil. Parassitologia. 34, 159–165.
Melo, M. A., França-Silva, J. C., Azevedo, E. O., Tabosa, I. M., Da Costa, R. T., Da Costa, C. A., et al. (2002). Clinical trial on the efficacy of the N-methyl glucamine associated to immunotherapy in dogs, experimentally infected with Leishmania (Leishmania) chagasi. Rev. Med. Vet. 153, 75–84.
Menezes-Souza, D., Guerra-Sa, R., Carneiro, C. M., Vitoriano-Souza, J., Giunchetti, R. C., Teixeira-Carvalho, A., et al. (2012). Higher expression of CCL2, CCL4, CCL5, CCL21, and CXCL8 chemokines in the skin associated with parasite density in canine visceral leishmaniasis. PLoS Negl. Trop. Dis. 6:e1566. doi: 10.1371/journal.pntd.0001566
Miglianico, M., Eldering, M., Slater, H., Ferguson, N., Ambrose, P., Lees, R. S., et al. (2018). Repurposing isoxazoline veterinary drugs for control of vector-borne human diseases. Proc. Natl. Acad. Sci. U.S.A. 115, E6920–6. doi: 10.1073/pnas.1801338115
Ministério da Saúde Secretaria de Vigilância em Saúde, and Departamento de Vigilância Epidemiológica. (2014). Manual de Vigilância e Controle da Leishmaniose Visceral. Série A. Normas e Manuais Técnicos (Brasília), 120.
Miret, J., Nascimento, E., Sampaio, W., França, J. C., Fujiwara, R. T., Vale, A., et al. (2008). Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime®) and the recombinant Leish-110f®+MPL-SE® vaccine to treat canine visceral leishmaniasis. Vaccine 26, 1585–1594. doi: 10.1016/j.vaccine.2008.01.026
Moreno, J., and Alvar, J. (2002). Canine leishmaniasis: epidemiological risk andthe experimental model. Trends Parasitol. 18, 399–405. doi: 10.1016/S1471-4922(02)02347-4
Musa, A. M., Noazin, S., Khalil, E. A. G., and Modabber, F. (2010). Immunological stimulation for the treatment of leishmaniasis: a modality worthy of serious consideration. Trans. R. Soc. Trop. Med. Hyg. 104, 1–2. doi: 10.1016/j.trstmh.2009.07.026
Nascimento, M. S. L., Albuquerque, T. D., Nascimento, A. F., Caldas, I. S., Do-Valle-Matta, M. A., Souto, J. T., et al. (2015). Impairment of interleukin-17A expression in canine visceral leishmaniosis is correlated with reduced interferon-γ and inducible nitric oxide synthase expression. J. Comp. Pathol. 153, 197–2015. doi: 10.1016/j.jcpa.2015.10.174
Neogy, A. B., Vouldoukis, I., da Costa, J. M., and Monjour, L. (1994). Exploitation of parasite-derived antigen in therapeutic success against canine visceral leishmaniosis. Vet. Parasitol. 54, 367–373. doi: 10.1016/0304-4017(94)90003-5
Nicolle, C. H. (1908). Culture des corps de Leishman isoles de la rate dans trois cãs d'anemicsptenique infantile. Bull. Soc. Pathol. 1:121.
Okwor, I., and Uzonna, J. E. (2009). Immunotherapy as a strategy for treatment of leishmaniasis: a review of the literature. Immunotherapy 1, 765–776. doi: 10.2217/imt.09.40
Oldham, R. K., and Smalley, R. V. (1983). Immunotherapy: the old and the new. J. Biol. Response Mod. 2, 1–37.
Organização Pan-Americana da Saúde (2018). Leishmaniasis. Epidemiological Report of the Americas. Available online at: https://www.paho.org/hq/index.php?option=com_topics&view=article&id=29&Itemid=40754&lang=pt (accesed January 17, 2019).
Otranto, D., and Dantas-Torres, F. (2013). The prevention of canine leishmaniasis and its impact on public health. Trends Parasitol. 29, 339–345. doi: 10.1016/j.pt.2013.05.003
Pateraki, E., Portocala, I. R., and Labrousse, H. (1983). Antiactin and antitubulin antibodies in canine visceral leishmaniasis. Infect. Immunol. 42, 496–500.
Pereira, M., Valério-Bolas, A., Santos-Mateus, D., Alexandre-Pires, G., Santos, M., Rodrigues, A., et al. (2017). Canine neutrophils activate effector mechanisms in response to Leishmania infantum. Vet. Parasitol. 248, 10–20. doi: 10.1016/j.vetpar.2017.10.008
Pereira-Fonseca, D. C. M., Oliveira-Rovai, F. M., Rodas, L. A. C., Beloti, C. A. C., Torrecilha, R. B. P., Ito, P. K. R. K., et al. (2017). Dog skin parasite load, TLR-2, IL-10 and TNF-α expression and infectiousness. Parasite Immunol. 39, 1–7. doi: 10.1111/pim.12493
Pessoa-e-Silva, R., Souza, V. V. A., Andrade, T. A. S., Silva, A. C. O., Oliveira, G. A., Trajano-Silva, L. A. M., et al. (2019). The diagnosis of canine visceral leishmaniasis in Brazil: confronting old problems. Exp. parasitol. 199, 9–16. doi: 10.1016/j.exppara.2019.02.012
Reis, A. B., Giunchetti, R. C., Carrillo, E., Martins-Filho, O. A., and Moreno, J. (2010). Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasit. 26, 341–349. doi: 10.1016/j.pt.2010.04.005
Reis, A. B., Martins-Filho, O. A., Teixeira-Carvalho, A., Giunchetti, R. C., Carneiro, C. M., Mayrink, W., et al. (2009). Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 128, 87–95. doi: 10.1016/j.vetimm.2008.10.307
Ribeiro, R. R., Michalick, M. S. M., da Silva, M. E., Dos Santos, C. C. P., Frézard, F. J. G., and da Silva, S. M. (2018). Canine leishmaniasis: an overview of the current status and strategies for control. BioMed Res. Int. 2018:3296893. doi: 10.1155/2018/3296893
Roatt, B. M., Aguiar-Soares, R. D., Coura-Vital, W., Ker, H. G., Moreira, N., Vitoriano-Souza, J., et al. (2014). Immunotherapy and immunochemotherapy in visceral leishmaniasis: promising treatments for this neglected disease. Front. Immunol. 5:272. doi: 10.3389/fimmu.2014.00272
Roatt, B. M., Aguiar-Soares, R. D., Reis, L. E., Cardoso, J. M., Mathias, F. A., de Brito, R. C., et al. (2017). A vaccine therapy for canine visceral leishmaniasis promoted significant improvement of clinical and immune status with reduction in parasite burden. Front. Immunol. 8:217. doi: 10.3389/fimmu.2017.00217
Rodríguez-Cortés, A., Carrillo, E., Martorell, S., Todolí, F., Ojeda, A., Martínez-Flórez, A., et al. (2016). Compartmentalized immune response in leishmaniasis: changing patterns throughout the disease. PLoS ONE 11:e0155224. doi: 10.1371/journal.pone.0155224
Rodriguez-Cortes, A., Martori, C., Martinez-Florez, A., Clop, A., Amills, M., Kubejko, J., et al. (2017). Canine leishmaniasis progression is associated with Vitamin D deficiency. Sci. Rep. 7, 1–10. doi: 10.1038/s41598-017-03662-4
Romero, G. A., and Boelaert, M. (2010). Control of visceral leishmaniasis in Latin America - a systematic review. PLoS Negl. Trop. Dis. 4:e584. doi: 10.1371/journal.pntd.0000584
Rossi, C. N., Tomokane, T. Y., Batista, L. F., Marcondes, M., Larsson, C. E., and Laurenti, M. D. (2016). In situ cutaneous cellular immune response in dogs naturally affected by visceral leishmaniasis. Rev. Inst. Med. Trop. São Paulo 58, 3–10. doi: 10.1590/S1678-9946201658048
Rossi, M., and Fasel, N. (2017). How to master the host immune system? Leishmania parasites have the solutions! Int. Immunol. 30, 103–111. doi: 10.1093/intimm/dxx075
Sanches, F. P., Tomokane, T. Y., Da Matta, V. L., Marcondes, M., Corbett, C. E., and Laurenti, M. D. (2014). Expression of inducible nitric oxide synthase in macrophages inversely correlates with parasitism of lymphoid tissues in dogs with visceral leishmaniasis. Acta Vet. Scand. 7, 56–57. doi: 10.1186/s13028-014-0057-z
Santiago, M. E. B., Neto, L. S., Alexandre, E. C., Munari, D. P., Andrade, M. M. C., Somenzari, M. A., et al. (2013). Improvement in clinical signs and cellular immunity of dogs with visceral leishmaniasis using the immunomodulator P-MAPA. Acta Trop. 127, 174–180. doi: 10.1016/j.actatropica.2013.04.005
Santos, F. N., Borja-Cabrera, G. P., Miyashiro, L., Grechi, J., Reis, A. B., Moreira, M. A. B., et al. (2007). Immunotherapy against experimental canine visceral leishmaniasis with the saponin enriched-Leishmune® vaccine. Vaccine 25, 6176–6190. doi: 10.1016/j.vaccine.2007.06.005
Scott, P., and Novais, F. O. (2016). Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nature Rev. Immunol. 16, 581–592. doi: 10.1038/nri.2016.72
Shimozako, H. J., Wu, J., and Massad, E. (2017). The preventive control of zoonotic visceral leishmaniasis: efficacy and economic evaluation. Comput. Math. Methods Med. 2017:e4797051. doi: 10.1155/2017/4797051
Singh, O. P., and Sundar, S. (2014). Immunotherapy and targeted therapies in treatment of visceral leishmaniasis: current status and future prospects. Front. Immunol. 5:296. doi: 10.3389/fimmu.2014.00296
Smith, B. E., Tompkins, M. B., and Breitschwerdt, E. B. (2004). Antinuclear antibodies can be detected in dog sera reactive to Bartonella vinsonii subsp. berkhoffii, Ehrlichia canis, or Leishmania infantum Antigens. J. Vet. Intern. Med. 18, 47–51. doi: 10.1111/j.1939-1676.2004.tb00134.x
Solano-Gallego, L., Koutinas, A., Miró, G., Cardoso, L., Pennisi, M. G., Ferrer, L., et al. (2009). Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 165, 1–18. doi: 10.1016/j.vetpar.2009.05.022
Solano-Gallego, L., Montserrrat-Sangrà, S., Ordeix, L., and Martínez-Orellana, P. (2016). Leishmania infantum-specific production of IFN-γ and IL-10 in stimulated blood from dogs with clinical leishmaniosis. Parasit. Vect. 9, 1–10. doi: 10.1186/s13071-016-1598-y
Solano-Gallego, L., Riera, C., Roura, X., Iniesta, L., Gallego, M., Valladares, J. E., et al. (2001). Leishmania infantum- specific IgG, IgG1 and IgG2 antibody responses in healthy and ill dogs from endemic areas. Evolution in the course of infection and after treatment. Vet. Parasitol. 96, 265–276. doi: 10.1016/S0304-4017(00)00446-5
Solcà, M. S., Andrade, B. B., Abbehusen, M. M., Teixeira, C. R., Khouri, R., Valenzuela, J. G., et al. (2016). Circulating biomarkers of immune activation, oxidative stress and inflammation characterize severe canine visceral leishmaniasis. Sci. Rep. 6:32619. doi: 10.1038/srep32619.
Soulat, D., and Bogdan, C. (2017). Function of macrophage and parasite phosphatases in leishmaniasis. Front. Immunol. 8:1838. doi: 10.3389/fimmu.2017.01838
Taslimi, Y., Zahedifard, F., and Rafati, S. (2016). Leishmaniasis and various immunotherapeutic approaches. Parasitology 145, 497–507. doi: 10.1017/S003118201600216X
Toepp, A., Larson, M., Wilson, G., Grinnage-Pulley, T., Bennett, C., Leal-Lima, A., et al. (2018). Randomized, controlled, double-blinded field trial to assess Leishmania vaccine effectiveness as immunotherapy for canine leishmaniosis. Vaccine 36, 6433–6441. doi: 10.1016/j.vaccine.2018.08.087
Tonin, A. A., Calado, A. M., Bottari, N. B., Dalenogare, D., Thomé, G. R., Duarte, T., et al. (2016). Novel markers of inflammatory response and hepatic dysfunction in canine leishmaniasis. Comp. Immunol. Microb. Infec. Dis. 44, 61–64. doi: 10.1016/j.cimid.2015.09.004
Travi, B. L. (2014). Ethical and epidemiological dilemmas in the treatment of dogs for visceral leishmaniosis in latin America. Biomedica 34, 7–12. doi: 10.7705/biomedica.v34i1.2153
Trigo, J., Abbehusen, M., Netto, E. M., Nakatani, M., Pedral-Sampaio, G., Jesus, R. S., et al. (2010). Treatment of canine visceral leishmaniasis by the vaccine Leish-111f+MPL-SE. Vaccine 28, 3333–3340. doi: 10.1016/j.vaccine.2010.02.089
Viana, K. F., Lacerda, G., Teixeira, N. S., Rodrigues Cangussu, A. S., Sousa Aguiar, R. W., and Giunchetti, R. C. (2018). Therapeutic vaccine of killed Leishmania amazonensis plus saponin reduced parasite burden in dogs naturally infected with Leishmania infantum. Vet. Parasitol. 254, 98–104. doi: 10.1016/j.vetpar.2018.03.010
Werneck, G. L., Costa, C. H. N., de Carvalho, F. A. A., Pires e Cruz, M., do, S., Maguire, J. H., and Castro, M. C. (2014). Effectiveness of insecticide spraying and culling of dogs on the incidence of Leishmania infantum infection in humans: a cluster randomized trial in Teresina, Brazil. PLoS Negl. Trop. Dis. 8:e3172. doi: 10.1371/journal.pntd.0003172
World Health Organization (2010). Control of the Leishmaniases. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases (Geneva). Available online at: http://whqlibdoc.who.int/trs/WHO_TRS_949_eng.pdf?ua=1 (accesed January 23, 2019).
World Health Organization (2018). Epidemiological Situation, 2018. Available online at: https://www.who.int/leishmaniasis/burden/en/ (accessed January 17, 2019).
Keywords: canine visceral leishmaniasis, Leishmania infantum, biomarkers, treatment, immunotherapy
Citation: Gonçalves AAM, Leite JC, Resende LA, Mariano RMdS, Silveira P, Melo-Júnior OAdO, Ribeiro HS, de Oliveira DS, Soares DF, Santos TAP, Marques AF, Galdino AS, Martins-Filho OA, Dutra WO, da Silveira-Lemos D and Giunchetti RC (2019) An Overview of Immunotherapeutic Approaches Against Canine Visceral Leishmaniasis: What Has Been Tested on Dogs and a New Perspective on Improving Treatment Efficacy. Front. Cell. Infect. Microbiol. 9:427. doi: 10.3389/fcimb.2019.00427
Received: 02 September 2019; Accepted: 29 November 2019;
Published: 18 December 2019.
Edited by:
Herbert Leonel de Matos Guedes, Federal University of Rio de Janeiro, BrazilReviewed by:
Valentina Foglia Manzillo, University of Naples Federico II, ItalyEva Spada, University of Milan, Italy
Yasuyuki Goto, The University of Tokyo, Japan
Angamuthu Selvapandiyan, Jamia Hamdard University, India
Copyright © 2019 Gonçalves, Leite, Resende, Mariano, Silveira, Melo-Júnior, Ribeiro, de Oliveira, Soares, Santos, Marques, Galdino, Martins-Filho, Dutra, da Silveira-Lemos and Giunchetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodolfo Cordeiro Giunchetti, giunchetti@icb.ufmg.br; giunchetti@gmail.com
 Ana Alice Maia Gonçalves
Ana Alice Maia Gonçalves Jaqueline Costa Leite
Jaqueline Costa Leite Lucilene Aparecida Resende
Lucilene Aparecida Resende Reysla Maria da Silveira Mariano
Reysla Maria da Silveira Mariano Patricia Silveira
Patricia Silveira Otoni Alves de Oliveira Melo-Júnior
Otoni Alves de Oliveira Melo-Júnior Helen Silva Ribeiro
Helen Silva Ribeiro Diana Souza de Oliveira
Diana Souza de Oliveira Diogo Fonseca Soares
Diogo Fonseca Soares Thaiza Aline Pereira Santos
Thaiza Aline Pereira Santos Alexandre Ferreira Marques
Alexandre Ferreira Marques Alexsandro Sobreira Galdino
Alexsandro Sobreira Galdino Olindo Assis Martins-Filho
Olindo Assis Martins-Filho Walderez Ornelas Dutra
Walderez Ornelas Dutra Denise da Silveira-Lemos
Denise da Silveira-Lemos Rodolfo Cordeiro Giunchetti
Rodolfo Cordeiro Giunchetti