The Role of the Interleukin-17 Axis and Neutrophils in the Pathogenesis of Endemic and Systemic Mycoses
- 1Medical and Experimental Mycology Group, Corporación para Investigaciones Biológicas (CIB), Universidad de Antioquia, Medellín, Colombia
- 2School of Health Sciences, Universidad Pontificia Bolivariana, Medellín, Colombia
- 3Max Planck Tandem Group in Nanobioengineering, Universidad de Antioquia, Medellin, Colombia
- 4Basic and Applied Microbiology Research Group (MICROBA), School of Microbiology, Universidad de Antioquia, Medellin, Colombia
Systemic and endemic mycoses are considered life-threatening respiratory diseases which are caused by a group of dimorphic fungal pathogens belonging to the genera Histoplasma, Coccidioides, Blastomyces, Paracoccidioides, Talaromyces, and the newly described pathogen Emergomyces. T-cell mediated immunity, mainly T helper (Th)1 and Th17 responses, are essential for protection against these dimorphic fungi; thus, IL-17 production is associated with neutrophil and macrophage recruitment at the site of infection accompanied by chemokines and proinflammatory cytokines production, a mechanism that is mediated by some pattern recognition receptors (PRRs), including Dectin-1, Dectine-2, TLRs, Mannose receptor (MR), Galectin-3 and NLPR3, and the adaptor molecules caspase adaptor recruitment domain family member 9 (Card9), and myeloid differentiation factor 88 (MyD88). However, these PRRs play distinctly different roles for each pathogen. Furthermore, neutrophils have been confirmed as a source of IL-17, and different neutrophil subsets and neutrophil extracellular traps (NETs) have also been described as participating in the inflammatory process in these fungal infections. However, both the Th17/IL-17 axis and neutrophils appear to play different roles, being beneficial mediating fungal controls or detrimental promoting disease pathologies depending on the fungal agent. This review will focus on highlighting the role of the IL-17 axis and neutrophils in the main endemic and systemic mycoses: histoplasmosis, coccidioidomycosis, blastomycosis, and paracoccidioidomycosis.
Introduction
Systemic fungal infections are characterized by their ability to produce a potentially life-threatening respiratory disease. These systemic mycoses are caused by a group of thermally dimorphic fungal pathogens belonging to different genera of several species including Histoplasma capsulatum, Coccidioides spp., Blastomyces spp., Paracoccidioides spp., Talaromyces marneffei and the newly described pathogen Emergomyces spp. (Sepúlveda et al., 2017; Turissini et al., 2017; Kirkland and Fierer, 2018; Cao et al., 2019; Schwartz et al., 2019; Schwartz and Kauffman, 2020). Additionally, these mycoses are usually geographically restricted; thus, histoplasmosis is found worldwide, coccidioidomycosis is endemic in some regions of the United States and some countries of Latin America, blastomycosis is endemic in North America and Africa, paracoccidioidomycosis is restricted to Latin America, talaromycosis is endemic in Asian countries, while emergomycosis has been reported in Africa, Europe, Asia, and North America (Sepúlveda et al., 2017; Turissini et al., 2017; Kirkland and Fierer, 2018; Cao et al., 2019; Schwartz et al., 2019; Schwartz and Kauffman, 2020).
In general, these systemic mycoses are acquired by inhalation of the conidia or spores that are produced in the mold phase; in the lungs, a temperature-dependent transformation occurs to the yeast phase, except for Coccidioides spp., which undergoes isotropic growth to form spherules initials (Hung et al., 2007). These fungal morphotypes are phagocytized by macrophages and can spread hematogenously to various organs, causing disseminated infection; nonetheless, the clinical presentation could vary from self-limited, or mild, to severe infection, which, in turn, depends on several factors including the immune response and the inoculum size, among others.
Several studies have confirmed the T-cell mediated immune response to some of these dimorphic fungal pathogens, especially those associated with T helper (Th)1 and Th17 responses that are essential for protection (Wüthrich et al., 2011; Nanjappa et al., 2012; Wu et al., 2013; Ketelut-Carneiro et al., 2019). Of note, Th17 and IL-17 protective responses, which also participate during the primary infections in the nonimmune host, are associated with recruiting and activating neutrophils and macrophages to the site of infection as well as with chemokine and proinflammatory cytokines production, a mechanism mediated by the fungal recognition of pattern recognition receptors (PRRs) present on the surface of the host cells, which lead to the activation of adaptor molecules and the subsequent downstream signaling (Wüthrich et al., 2011; Nanjappa et al., 2012; Wu et al., 2013; Ketelut-Carneiro et al., 2019). Nonetheless, both the Th17/IL-17 axis and neutrophils appear to play a dual role, being beneficial mediating fungal controls and detrimentally promoting disease pathology depending on the fungal agent (Loures et al., 2009; Wüthrich et al., 2011; Pino-Tamayo et al., 2016; Puerta-Arias et al., 2016; Ketelut-Carneiro et al., 2019).
In this review, we will discuss the current findings regarding the role of the IL-17 axis and neutrophils in the immune response against dimorphic fungal pathogens with special emphasis on the most studied endemic and systemic mycoses: histoplasmosis, coccidioidomycosis, blastomycosis, and paracoccidioidomycosis. Of note, the role of IL-17 and neutrophils on talaromycosis and emergomycosis have not been investigated so far or are incipient, reasons why these mycoses were not included in this review.
IL-17: Sources and Function
The IL-17 family is a group of pleiotropic cytokines secreted mainly by a subset of CD4+T helper cells (Th) known as Th17 cells (Harrington et al., 2006). The differentiation and stimulation of Th17 from naïve CD4+ T-cells occurs in secondary lymphoid organs with the participation of IL-1β, IL-6, transforming growth factor β (TGFβ), and IL-23. The stimuli with cytokines trigger the downstream STAT3, promoting the activation of RORγt, the master transcriptional factor, which modulates the production of the hallmark cytokines IL-17A, IL-17F, and other cytokines such as IL-21, IL-22, and granulocyte-macrophage colony-stimulation factor (GM-CSF) (Hartupee et al., 2009; Isailovic et al., 2015; Monin and Gaffen, 2018). Moreover, polarized Th17 cells express CC chemokine receptor 6 (CCR6), which allows their migration into mucosal barrier sites (Abusleme and Moutsopoulos, 2017).
Moreover, other cell populations were also reported as important sources of IL-17A and IL-17F, such as the CD8+ T-cells and the innate immune cells including γδ T-cells (Takatori et al., 2008), innate lymphoid cells subset 3 (ILC3) (Geremia et al., 2011; Villanova et al., 2014), invariant natural killer cells (iNKT) (Michel et al., 2007), IL-17 innate lymphoid cells (ILC17) (Buonocore et al., 2010), and natural killer T (NKT) cells (Cella et al., 2009). Additionally, macrophages and dendritic cells are also important sources of IL-23 and IL-17, which are produced in response to the microorganism’s invasion and inflammatory cytokines stimulation. Neutrophils and mast cells also contribute to IL-17 production (Hoshino et al., 2008; Lin et al., 2011; Monin and Gaffen, 2018; Schön and Erpenbeck, 2018).
The IL-17 family includes six related proteins, namely IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25), and IL-17F (Monin and Gaffen, 2018), IL-17A (commonly known as IL-17) being the most studied member of the IL-17 family. The functions of IL-17 are crucial to maintaining mucosal immunity against extracellular and intracellular pathogens through the induction of antimicrobial proteins and the recruitment of neutrophils to the site of infections. Furthermore, IL-17 increases mucosal barrier repair and maintenance by the production of tight junction proteins and the stimulation of epithelial cell proliferation (Valeri and Raffatellu, 2016).
IL-17 is recognized by the family of the IL-17 receptors (IL-17R), which is a multimeric receptor constituted by two subunits with five members: IL-17A-IL17E. The IL-17R is composed of a common IL-17RA chain and a second chain that determines the ligand and downstream signal. (Yao et al., 1995; Toy et al., 2006; Rickel et al., 2008; Ramirez-Carrozzi et al., 2011; Zhu et al., 2011). The IL-17RA is expressed on the surface of leukocytes, keratinocytes, fibroblasts, epithelial, mesothelial, and vascular endothelial cells. The expression of IL-17RA induces granulopoiesis, neutrophil recruitment, and inflammatory response (Tristão et al., 2017). The IL-17A and IL-17F share a high degree of similarity, with both playing a central role in the adaptive immune response, especially against bacteria and fungi. IL-17A and IL-17F induce an inflammatory response by stimulating the expression of proinflammatory cytokines and chemokines and matrix metalloproteinase (MMP) production, thus promoting a potent immune response with the recruitment of immune cells to the site of infection, mainly neutrophil accumulation (Tristão et al., 2017). IL-17A induces the production of the chemokines CXCL1, CXCL2, and CXCL8 (IL-8), which in turn attract neutrophils. In addition, IL17A appears to have a protecting role against microorganisms through the induction of antimicrobial peptides, including β-defensins, S100A8, and lipocalin 2 (Onishi and Gaffen, 2010; Chen and Kolls, 2017).
Fungal infections have been particularly associated with the regulation of the Th17 immune response by the activation of CD4+ T - antigen-presenting cells via recognition of the components of the fungal cell wall by pattern recognition receptors (PRRs). The cell walls of fungal pathogens contain three major polysaccharides types: β-glucan, chitin, and mannan (Netea et al., 2008); meanwhile, PRRs include Dectin-1, Dectin-2, Dectin-3, Mincle, mannose receptor (MR), and Toll-like receptors (TLRs), among others; thus, Dectin-1 recognizes fungi via β-1,3-glucan. Dectin-2 and Mincle recognize mannose-like structures, while TLR2 recognizes mainly β-glucan and zymosan, and TLR4 recognizes mannan components (McGreal et al., 2006; Netea et al., 2006; Reid et al., 2009; Yamasaki et al., 2009; Saijo et al., 2010; Ishikawa et al., 2013). Once PRRs recognize fungal cells, these interactions trigger a cascade of signaling events, with the participation of cytosolic adaptors [mainly caspase adaptor recruitment domain family member 9 (Card9) and myeloid differentiation factor 88 (MyD88)] that transduce signals from these PRRs, that in turn activate the secretion of proinflammatory cytokines and the induction of T-cell differentiation (Drummond et al., 2011; Loures et al., 2011; Wüthrich et al., 2012).
On the whole, the activation of the Th17 immune response against fungal infection depends upon which receptors are involved and the degree of interaction (Netea et al., 2008; van de Veerdonk et al., 2009; Wang et al., 2016).
The Role of IL-17 Axis in The Dimorphic Fungal Infections
It is known that the development of Th1 cells is crucial for protective immunity against dimorphic fungal pathogens including H. capsulatum, Coccidioides spp., Blastomyces spp., and Paracoccidioides spp.; however, the roles of the Th17 cell and IL-17 are controversial. In models of infection with the above fungal pathogens, some studies have shown that Th17/IL-17 axis mediate resistance, while others have shown that they promote disease pathology (Deepe and Gibbons, 2009; Loures et al., 2009; Loures et al., 2009; Wüthrich et al., 2011; Nanjappa et al., 2012; Wu et al., 2013; Wang et al., 2014; Pino-Tamayo et al., 2016; Puerta-Arias et al., 2016; Ketelut-Carneiro et al., 2019). In the case of histoplasmosis, coccidioidomycosis, and blastomycosis, it has been reported that mice vaccinated against these three fungal pathogens showed that Th1 immunity was dispensable, whereas the fungal-specific Th17 cells were sufficient for inducing protection against these systemic mycoses (Wüthrich et al., 2011). Subsequently, these results were confirmed using hosts lacking CD4+ cells, where CD8+ T-cell derived IL-17 was indispensable to develop immunity protection against these three endemic and systemic mycoses (Nanjappa et al., 2012).
In the dimorphic fungal infections, the induction of Th17 cells and the subsequent IL-17 production depends on the interactions of the PRRs present on the host cells’ surfaces. The fungal pathogen-associated molecular patterns (PAMPs), TLRs and C-Type Lectin [including Dectin-1, Dectin-2, MR and Mincle] receptors have been the most studied PPRs so far. These interactions induce the secretion of proinflammatory cytokines and T cell differentiation. Figure 1 shows the interactions of the main dimorphic fungal pathogens and PRRs with the subsequent signaling activation (with adaptor molecules participation), Th17 differentiation, and IL-17 production.
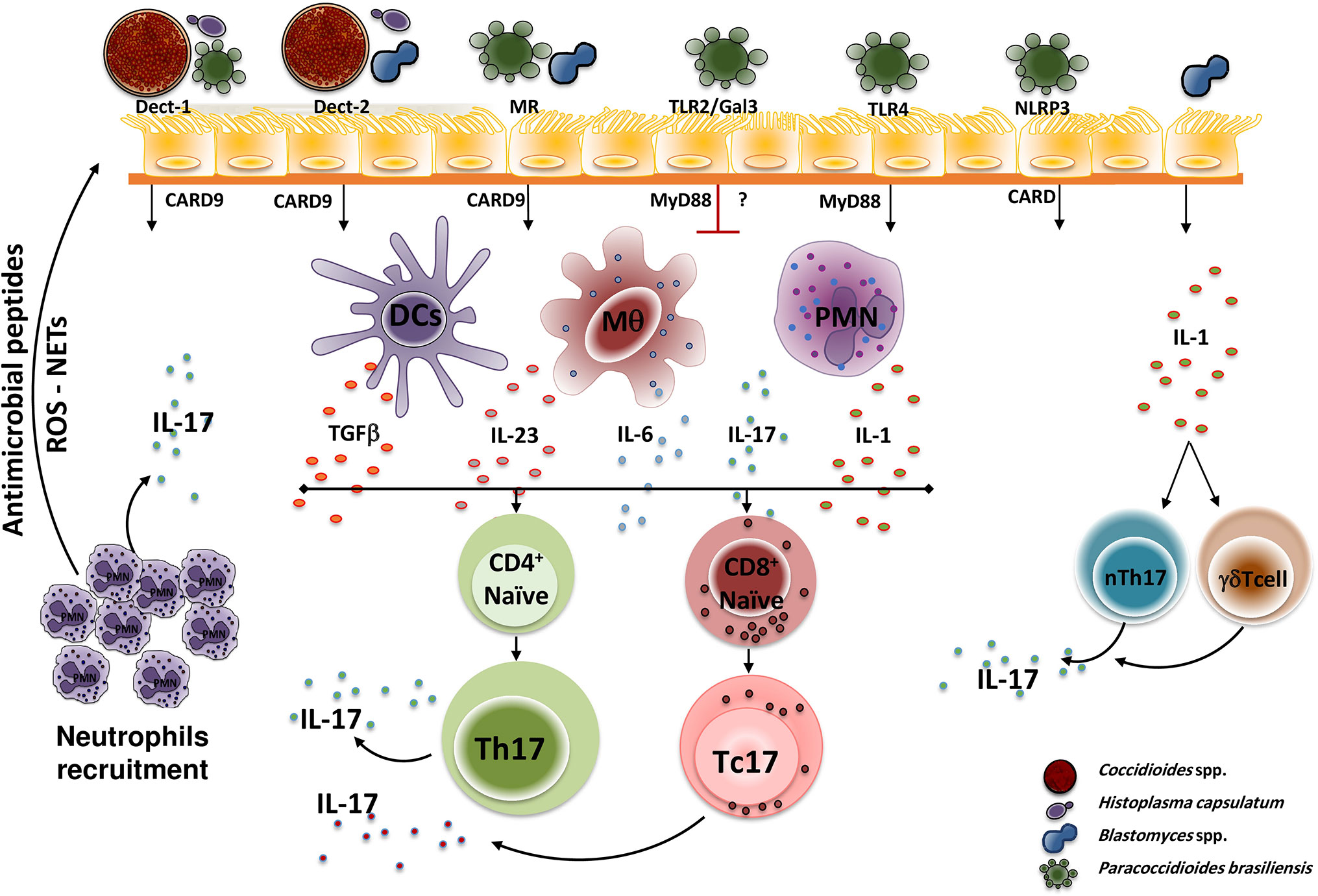
Figure 1 Dimorphic fungal pathogen recognition by PPRs. During initial infection, fungal PAPMs are recognized by different host PPRs present in antigen-presenting cells (mainly Dendritic cells, macrophages, and neutrophils), interactions that trigger a downstream signal that induces the T cell naïve (CD4+ or CD8+) differentiation to Th17 cells (in the presence of TGFβ, IL-1, IL-6, and IL-23) and the subsequent production of IL-17 which in turn amplified the inflammatory response by recruiting neutrophils at the site of infection. However, each PRR plays a distinctly different role for each dimorphic fungus. Thus, H. capsulatum and C. posadasii are recognized by Dectin-1 and Dectin-2, P. brasiliensis is recognized by Dectin-1, TLR4, MR, and NLRP3, whereas B. dermatitidis is only recognized by Dectin-2. After recognition, the PRRs activate downstream adaptor molecules, including MyD88 and CARD9, that finally activate transcription factors that induce the specific genes, especially this coding for IL-17. Once the IL-17 is produced, it induces the recruitment and activation of neutrophils, which in turn exhibit microbicidal mechanisms mediated by antimicrobial peptides release, reactive oxygen species (ROS) production, and NETs formation. Of note, TLR2 and Gal3 negatively regulate IL-17 production after P. brasiliensis recognition. Moreover, the interaction of fungal cells (i.e. Blastomyces) with lung epithelial cells, induces the release of IL-1 which in turn activates innate cells that then induce activation and IL-17 production by innate cells, mainly nTh17 and γδTcells. PAMP, Pathogen-associated molecular pattern; PRR, pattern recognition receptor; Th, T helper; Tc, T cytotoxic; Dect, Dectin; MR, mannose receptor; TLR, Toll-Like receptor; Gal3, Galectin-3; NLRP3, NOD-like receptor P3; CARD9, adaptor recruitment domain family member 9; MyD88, myeloid differentiation factor 88; DCs, Dendritic cells; Mq, macrophages; PMN, polymorphonuclear neutrophils; IL, interleukin; TGFβ, transforming growth factor β; ROS, reactive oxygen species; NETs, extracellular traps; nTh17, natural T helper cells (innate cells).
Histoplasmosis
Histoplasmosis is the most common endemic mycosis reported worldwide. The infection is acquired by the inhalation of aerosolized microconidia and mycelial fragments, but the severity of illness and the presence of clinical manifestations depend on the intensity of fungal burden exposure and the host´s immune status (Wheat et al., 2016). It is known that Th17 and its signature cytokine IL-17 play an important role and mediate a response in H. capsulatum infection; however, despite IL-17 not being necessary for survival, its neutralization alters inflammatory cell recruitment and elevates fungal burden in a murine model of histoplasmosis; therefore, it was demonstrated that this cytokine participates in the control of this fungal infection, particularly in the absence of IFN-γ (Deepe and Gibbons, 2009).
Among the different PRRs that participate in the recognition of Histoplasma, it has been reported that Dectin-1 and Dectin-2, but not Mincle, recognize and induce a protective Th17 immune response against H. capsulatum (Wüthrich et al., 2011; Viriyakosol et al., 2013; Wang et al., 2014); moreover, these interactions are mediated by Card9 and MyD88 signaling, which are indispensable for the development of this Th17 protective immune response (Wüthrich et al., 2011; Wang et al., 2014). Additionally, it has been demonstrated that the Galectin-3 (gal3) receptor, a member of the galectin family, negatively regulates IL-17A response through the inhibition of IL-23/IL-17 axis cytokine production by dendritic cells (DC) when infected with H. capsulatum (Wu et al., 2013). Similarly, it has been reported that the induction of IL-23 producing DCs depended on the activation of Dectin-1, which is mediated by β-glucan exposed in the cell walls of the fungal pathogens. Interestingly, the yeast form of Histoplasma, which lacks cell wall exposure of β-glucan, failed to induce IL-23 producing DCs, a fact that was confirmed using a mutant of Histoplasma in which β-glucan present in its cell wall was unmasked; thus, the interaction of DC with this mutant not only abrogated the pathogenicity of this fungus but also triggered the induction of IL-23 producing DCs (Chamilos et al., 2010); this study indicated that β-glucan exposure in the fungal cell wall is essential for the generation of IL-23 producing DCs and Th17 immune responses and may represent an evasion mechanism exerted by Histoplasma.
It is known that the differentiation of Th17 cells and their IL-17 production are mediated by IL-6 and TGF-β signaling, a process that is amplified and sustained by IL-21 and IL-23, respectively (Korn et al., 2009; Zhu et al., 2010). Along these lines, in Histoplasma infection, it has been described that the Th17 response is associated with cytokines that include IL-6, IL-23, and IL-17 and that CD4+ and CD8+ T cells expressing CD25 are the predominant sources of IL-17 (Deepe and Gibbons, 2009; Kroetz and Deepe, 2012). Moreover, the infection model for histoplasmosis showed that the development of the IL-17 protective response required IL-6 but not the participation of IL-1 receptor signaling (Nanjappa et al., 2012). More recently, it was reported that IL-22 deficiency was associated with a reduction of IFN-γ or IL-17-producing CD4+ cells in the lungs of mice infected with H. capsulatum, suggesting an inflammatory loop between IL-22 and a Th1/Th17 response (Prado et al., 2020).
In addition, some chemokines and their receptors also participate in the development of a Th17 response in Histoplasma infection; thus, the absence of CCR5 or CCL4 neutralization was associated with the impaired infiltration of the lungs of Histoplasma-infected mice by inflammatory cells. Those mice resolved the infection. The absence of CCR5 or CCL4 neutralization was also associated with the beneficial role of IL-17 accelerating the pathogen resolution (Kroetz and Deepe, 2010); moreover, mice lacking the CCR5 and treated with a monoclonal antibody against IL-17 showed an increase in the fungal burden and Treg cells, suggesting that this CCR5/CCL4 axis regulates the balance between Treg and Th17 cells in this fungal infection (Kroetz and Deepe, 2010). The important role of IL-17 in the Histoplasma infection has been clearly demonstrated, especially in immunization studies where this cytokine has been associated with the development of a protective immune response (Wüthrich et al., 2011; Deepe et al., 2018).
Coccidioidomycosis
Coccidioidomycosis, commonly known as San Joaquin Valley fever, is a systemic fungal disease caused by the inhalation of the airborne spores of Coccidioides immitis or C. posadasii (Galgiani et al., 2005). The development of a protective immune response in Coccidioides infection is similar to that observed in histoplasmosis. Thus, Th1 and Th17 immune patterns are pivotal in mounting an effective control of coccidioidal infection (Hung et al., 2011; Wüthrich et al., 2011). Several studies employing different coccidioidal antigens and adjuvants for immunization protocols have been reported; these antigens include: a genetically-engineered mutant strain (Δcts2/ard1/cts3) used as a live attenuated vaccine (ΔT) (Xue et al., 2009); a ΔT conjugated with the adjuvant EP67; a peptide agonist of the biologically active C-terminal region of human complement component C5a (Hung et al., 2012); a multivalent recombinant Coccidioides polypeptide antigen (rCpa1) that consists of three previously identified antigens (Ag2/Pra, Cs-Ag, and Pmp1) and five pathogen-derived peptides with high affinity for human major histocompatibility complex class II (MHC-II) molecules (Hurtgen et al., 2012; Hung et al., 2018; Campuzano et al., 2020); and a rCpa1 encapsulated into glucan-chitin particles (GCP) or β-glucan particles (GP) (Campuzano et al., 2020). Additionally, transgenic mice expressing a human major histocompatibility complex class II (MHC II) receptor have also been employed to study the immune response to coccidioidal vaccines (Hurtgen et al., 2012; Hurtgen et al., 2016; Hung et al., 2018). All the above studies confirmed the protective effect addressed by Th1 and Th17 expansion with higher production of the signature cytokines, IFN-γ, and IL-17, respectively. Nonetheless, a higher expression of RORc, the hallmark transcription factor of the Th17 pathway combined with higher amounts of IL-23 and IL-6 and accompanied by a down-regulated expression of Foxp3, which promotes the differentiation of Treg cells, suggests the development of a biased Th17 protective immune response to coccidioidal infection (Hung et al., 2011; Wüthrich et al., 2011; Hung et al., 2012). Moreover, an additional study showed that IFN-γ−/− knock-out mice immunized with the ΔT vaccine could still be protected (100% survival), while only 40% of ΔT-vaccinated IL17 receptor A-deficient mice survived (Hung et al., 2011); again, this study shows that Th17/IL-17 axis contributes strongly to protection against Coccidioides infection.
More recently, a primary dendritic cell (DC)-vaccine [DC-vaccine (Ag2-DC) that was prepared by non-virally transfecting the primary bone marrow-derived DCs with a plasmid DNA encoding Ag2/PRA (protective epitope of Coccidioides)] was evaluated, and healthy mice treated with the DC-vaccine showed IL-17, IFN-γ and IL-4 cytokine-secreting cells in the lungs and lymph nodes after immunization (Awasthi et al., 2019).
Regarding PRRs that participate in the recognition of Coccidioides and the subsequent stimulation and development of a protective Th17 immunity response, it has been demonstrated that this mechanism is mediated by the activation of both MyD88 and Card9-associated Dectin-1 and Dectin-2 signal pathways (Wüthrich et al., 2011; Viriyakosol et al., 2013; Wang et al., 2014; Campuzano et al., 2020). Moreover, it was also demonstrated that the IL-1 receptor, but not TLR2, is essential to developing a Th17 immunity response against Coccidioides infection, a mechanism mediated by MyD88 (Hung et al., 2016).
Blastomycosis
Blastomycosis refers to a disease caused by the dimorphic fungi Blastomyces dermatitidis; this systemic disease, like endemic mycosis, has a T-cell and macrophage-mediated immune response (Chang et al., 2000). The clinical spectrum of this illness is broad, from asymptomatic patients to acute, chronic, or disseminated disease (Chapman et al., 2008). As described previously, in histoplasmosis and coccidioidomycosis, a Th1 and Th17 immune responses exert an important role in the control of infection by Blastomyces (Wüthrich et al., 2011; Wang et al., 2014). Thus, after the host cells recognize Blastomyces´ fungal cell wall or its antigens by PRRs, activation and differentiation, mainly of Th17 cells, take place. Among the different PRRs that participate in Blastomyces recognition, Dectin-2 and Dectin-3 (also known as MCL) appear to be indispensable to developing a Th17 protective immune response and resistance to this fungal pathogen (Wüthrich et al., 2011; Wang et al., 2014; Wang et al., 2015; Wang et al., 2017). Of note, MCL regulates the development, expansion and, differentiation of Th17 through a mechanism dependent on the adaptor FcRγ (Wang et al., 2015). Furthermore, once MR recognizes a mannan-like structure on the B. dermatitidis cell wall, it also activates and differentiates naïve T cells into Th17 effector cells, which are pivotal to the protection of an immunized host against Blastomyces (Wang et al., 2016). Additional adaptor molecules, including Card9 and MyD88, are also indispensable for the development of a Th17 protective immune response against Blastomyces (Wüthrich et al., 2011; Wang et al., 2014; Nanjappa et al., 2015; Wang et al., 2017).
On the other hand, IL-1 and IL-6 appear also to play an important role in the development of a Th17 immune response against blastomycosis (Nanjappa et al., 2012; Wüthrich et al., 2013; Merkhofer et al., 2019). More recently, studies in animals and human beings have revealed that IL-6 had a pivotal role in the development of adaptive immunity and resistance to B. dermatitidis infection, through induction of a Th17 pattern. Thus, genetic analysis of an Asian population (The Hmong) that show an elevated incidence of blastomycosis in comparison with those of European ancestry (168 vs 13 per 100,000 inhabitants, respectively), demonstrated that in addition to mice that had lost IL-6 signaling, the presence of polymorphisms on the IL-6 gene increased susceptibility to developing blastomycosis, a fact that was associated with lower levels of IL-6, IL-17, and RAR-related orphan receptor gamma t [(RORγt), the hallmark transcription factor of IL-17-producing T cells] in humans and low recruited Th17 producing cells in lungs of IL-6−/− mice infected with B. dermatitidis (Merkhofer et al., 2019). By the same token, it has been reported that the use of exogenous IL-1 enhanced the protection of weak vaccines against lethal B. dermatitidis infection, promoting the development of fungus-specific Th17 cells (Wüthrich et al., 2013).
Recently, it has been described that lung epithelial cells are essential for immunity against Blastomyces; thus, the interaction of these epithelial cells with the fungus triggers a NF-κB signaling with a subsequent increase in the number of IL-17-producing innate lymphocytes, mainly CCR6+ natural Th17 cells (nTh17) and γδT-cells, a mechanism dependent on CCL20 chemokine production, which in turn is induced by IL-1α/IL1R signaling (Hernández-Santos et al., 2018).
Finally, vaccination against Blastomyces induces the development of memory Tc17 cells with different requirements for long-term persistence than Tc1 cells; thus, these anti-fungal Tc17 cells retained the expression of RORγt and showed higher proliferative renewal and lower levels of anti-apoptotic molecule Bcl-2, but required hypoxia-inducible factor 1α (HIF-1α) for their homeostasis (Nanjappa et al., 2017).
Paracoccidioidomycosis
Paracoccidioidomycosis (PCM) is a fungal infection caused by the dimorphic fungus from the genus Paracoccidioides and is one of the most prevalent systemic mycoses in Latin America (Restrepo et al., 2019). In PCM, cellular immunity exhibits a protective role; thus, CD4+ T cells exert a protective effect through the regulation of antibody production and delayed-type hypersensitivity (DTH) reactivity, while CD8+ T cells control fungal burden (Chiarella et al., 2007). Furthermore, using a PCM model, it has been demonstrated that infected P. brasiliensis-infected mice showed increased levels of IL-17A accompanied by the Th17 associated cytokines, IL-6 and IL-23; moreover, deficiency of these Th17-associated cytokines or IL-17RA conferred susceptibility during infection associated with reduced concentrations of TNF-α, IFN-γ, and inducible nitric oxide synthase (iNOS) expression (Tristão et al., 2017).
Regarding participation of PRRs on Th17 development in PCM, it was demonstrated that TLR2 acts as a negative regulator of Th17 cells, thus a deficiency of TLR2 was associated with a lower fungal burden, a prevalent Th17 immunity (higher levels of IL-17, IL-6, IL-23, and TGF-b), and an increased number of neutrophils; nonetheless, a exacerbated pulmonary inflammation was also observed, which was associated with a diminished expansion of regulatory T cells (Loures et al., 2009). Similarly, TLR3 also acts as a negative regulator of Tc17 cells (IL-17-CD8+ producing cells); thus, in TLR3 deficient mice infected with P. brasiliensis, an increased number of Tc17 and Tc1 cells associated with higher levels of IL-17, IL-1β, IL-6, and IFN-γ were observed (Jannuzzi et al., 2019).
Conversely, in vitro studies showed that neutrophils primed with the 43kDa glycoprotein (gp43) from P. brasiliensis express higher levels of TLR2 and IL-17 (Gardizani et al., 2019). It is noteworthy that MyD88 (the adaptor molecule used by TLRs) is required to mount an efficient innate and adaptive immune response; thus, MyD88 deficient mice showed an association between disease severity and reduced Th17 response and IL-1β production (Loures et al., 2011).
Regarding CTL receptors, it has been reported that Dectin-1 has a critical influence in the differentiation and migration of Tc17 cells; thus, Dectin-1 deficient mice infected with P. brasiliensis showed a more severe infection, enhanced tissue pathology, and mortality rates, accompanied with reduced differentiation of T cells to Tc17 phenotype, increased expansion of Treg cells, impaired production of Th1, Th2, and Th17 cytokines, and migration of Tc17 and neutrophils to the site of infection (Loures et al., 2014). Moreover, monocytes from healthy individuals produced IL-17A after incubation with P. brasiliensis yeasts via activation of the Dectin-1 receptor (Romagnolo et al., 2018). Of note, dendritic cells stimulated with P. brasiliensis induced the differentiation of Th17/Tc17 by a mechanism mediated by TLR4, Dectin-1 and MR in a synergistic fashion (Loures et al., 2015).
Furthermore, activation of the NOD-like receptor P3 (NLRP3), which is related to the inflammasome, was associated with the development of protective immunity against P. brasiliensis by a mechanism mediated by Th1 and Th17 (Feriotti et al., 2017).
In another study using the experimental model of pulmonary paracoccidioidomycosis, it has been reported that IL-1α deficiency was associated with a reduction of Th17 cells and a diminished number of neutrophils in a mechanism mediated by caspase 11 (Ketelut-Carneiro et al., 2019). Moreover, the important role of IL-6 for the development of a protective Th17 immune response has been demonstrated; thus, the adoptive transfer of IL-6 competent macrophages restored the resistance in P. brasiliensis-infected IL-6- and IL-17RA-deficient mice (Tristão et al., 2017).
Interestingly, the results of other studies have been controversial; thus, we reported that during the early stages of infection, the depletion of neutrophils using a specific monoclonal antibody was associated with an exacerbation of the inflammatory response and high fungal burden. This neutrophil depletion was also accompanied by a decreased level of IL-17; moreover, it was confirmed that neutrophils were an essential source of IL-17 during the early stages of P. brasiliensis infection (Pino-Tamayo et al., 2016). Conversely, neutrophil depletion during the chronic course of paracoccidioidomycosis promotes the resolution of pulmonary inflammation and fibrosis accompanied by a reduced fungal burden. These results were associated with a decrease of proinflammatory cytokines, including IL-17, TNF-α, and TGF-β (Puerta-Arias et al., 2016). Additionally, the most severe form of PCM has been characterized by a predominant Th17/Th22 response, along with substantial participation of Th1 cells (de Castro et al., 2013). On these lines, it has been reported that P. brasiliensis-infected mice treated with a recombinant 60-kDa heat shock protein of this fungal pathogen increased the concentrations of proinflammatory cytokines, including IL-17, TNF-α, and IFN-γ, which lead to severe inflammation, host tissue damage, impaired granuloma formation, and fungal dissemination (Fernandes et al., 2016). The above results indicate that IL-17 plays a dual role in PCM.
Finally, in addition to CD4+Th cells, it has been demonstrated that other cell populations are important sources of IL-17, induced by dimorphic fungal pathogens; these cells include Tc17, nTh17, γδT-cells, and neutrophils (Pino-Tamayo et al., 2016; Nanjappa et al., 2017; Tristão et al., 2017; Hernández-Santos et al., 2018).
Altogether, the above findings suggest that the antifungal effect exerted by the Th17/IL-17 axis is mediated by the recruitment and activation of neutrophils and macrophages to the site of infection (Wüthrich et al., 2011; Nanjappa et al., 2012; Hernández-Santos et al., 2018).
The Role of Neutrophils in The Dimorphic Fungal Infections
Neutrophils are the most abundant type of immune cells and constitute the first line of defense against infections by different pathogens. As multifunctional cells of the immune response, neutrophils actively participate in the development of innate and adaptative immunity (Kolaczkowska and Kubes, 2013), and exert a variety of effector functions including phagocytosis, intra and extra-cellular pathogen killing via oxidative and non-oxidative cytotoxic mechanisms, extracellular release of microbicidal molecules stored in their intracellular granules, production of immune mediators including pro-inflammatory cytokines and chemokines, and formation of neutrophil extracellular traps (NETs) (Mócsai, 2013; Wang and Arase, 2014; Tecchio and Casatella, 2016; Yang et al., 2017). The latter is one of the most important microbicidal mechanisms, mainly against certain pathogens that are difficult to phagocytose due to their large size (Brinkmann et al., 2004).
The microbicidal effect of NETs has been reported in different animal and human models with infections by fungal pathogens, parasites, bacteria, and viruses. This mechanism can comprise two phases: i) the trapping and immobilization of the pathogen to prevent the spread to tissues, organs, and systems; and ii) the elimination of the pathogen by microbicidal action of the proteins present in the NETs (Brinkmann et al., 2004). Such proteins exert their effect by degrading virulence factors, damaging the cell wall, or forming a complex with metal ions important in the life cycle of some dimorphic fungi (Brinkmann et al., 2004; Urban et al., 2006; Bianchi et al., 2009; Byrd et al., 2013; Hoeksema et al., 2016). However, although NETs can prevent the pathogens’ spread or directly eliminate the trapped microorganism, some studies have reported that certain peptides, as well as histones linked to the traps, trigger a high cytotoxic effect in the tissue, especially when the clearance mechanisms of the host are ineffective, thus causing a continuous inflammation as observed in several disorders or infectious diseases (Leffler et al., 2012; Cheng and Palaniyar, 2013).
Clearly, it is well known that the neutrophils are crucial in the immunity against invasive infection caused by fungal pathogens of the genus Candida and Aspergillus (Desai and Lionakis, 2018). However, in other fungal infections caused by a heterogeneous group of dimorphic fungi, including Histoplasma capsulatum, Coccidioides spp., Paracoccidioides spp., and Blastomyces dermatitidis (Hsu et al., 2010), this immune-cell apparently does not play an important role (Lionakis et al., 2017; Lionakis and Levitz, 2018). For this group of fungal infections, it has been suggested that these phagocytic cells may play a paradoxical function which depends on both the infection phase (acute or chronic) or certain conditions of the host; thus, neutrophils could exert a beneficial effect by controlling the infection, or, on the contrary, they could induce a detrimental effect with a poor prognosis of the infection/disease and worse outcomes. In Table 1, we summarized the role of neutrophils in the main dimorphic fungal infections.
Histoplasmosis
Although the T-cell activated macrophages play a central role in the pathogenesis of histoplasmosis, it has also been suggested that H. capsulatum yeasts are recognized by different phagocytic cells, including neutrophils (Deepe et al., 2008). Earlier studies in murine models of histoplasmosis have described the presence of neutrophils during the first 36 h post-infection (Procknow et al., 1960; Baughman et al., 1986). Thus, recognition of Histoplasma yeast cells by neutrophils is mediated by PRRs present on their surface; these PRRs include Dectin-2, NLRP3, and macrophage integrin or integrin αMβ2 (Mac-1 or CD11b/CD18) (Coxon et al., 1990; Patin et al., 2019). Notably, phagocytosis of H. capsulatum yeast did not induce a respiratory burst response in neutrophils; however, the production of superoxide anion was observed only when fungal cells were opsonized, indicating that the activation of this microbicidal mechanism needs the participation of either complement or Fc receptors (Schnur and Newman, 1990). Later, Newman et al. (1993) demonstrated that recognition and phagocytosis of opsonized yeast of H. capsulatum by neutrophils was via complement receptor (CR) type 1 (CR1), CR3, and FcRIII (CD16).
Additionally, Medeiros et al. (2015) demonstrated that during the acute pulmonary histoplasmosis, inflammatory mediators such as leukotriene B4 (LTB4), LTC4, platelet-activating factor (PAF), KC (murine IL-8 homolog), and TNF-α induce a strong recruitment of neutrophils into the lungs and others remote localized inflammatory sites, where these cells exhibit fungistatic activity against H. capsulatum. Likewise, other reports have demonstrated the limited fungicidal effect of neutrophils against H. capsulatum (Brummer et al., 1991; Kurita et al., 1991). Newman et al. (2000) reported that the azurophilic granules present inside neutrophils contain defensins and serprocidins, molecules that exert a fungistatic effect against H. capsulatum yeast. It has also been demonstrated that H. capsulatum inhibits apoptosis in neutrophils from both human and mouse hosts, which correlates with decreased cell-surface Mac-1 expression (Medeiros et al., 2002). Overall, it could be suggested that H. capsulatum can evade microbicidal mechanisms or is able to survive inside the phagocytic cells during the early phase of infection.
Moreover, a recent report has shown that NETs response promotes the loss of yeast viability and exerts a fungicidal activity, as a dependent mechanism of ROS, SyK/Src Kinase pathway, and CD18 (Thompson-Souza et al., 2020).
Although it has been described that neutrophils participate in the innate and adaptive immune response, the role of these phagocytic cells in the cell-mediated immune response against H. capsulatum at early times of infection is still unclear.
Coccidioidomycosis
The immunity against coccidioidomycosis is mediated mainly by macrophages and T cells, and relatively little is known about the role of neutrophils in the inflammatory response. Although the capacity of mononuclear cells to locate around parasitic-phase structures of the fungi has been shown, some studies have suggested that neutrophils may participate during the early course of the disease; thus histopathological examinations of the infected lungs of mice at the first two weeks postchallenge have shown that neutrophils are the predominant inflammatory cells located adjacent to mature spherules that have ruptured and released their endospores (Galgiani et al., 2005; Shubitz et al., 2008; Xue et al., 2009). It has been suggested that these phagocytic cells respond to the contents of spherules in a chemotaxis-like fashion, and the intense inflammatory response observed at infection sites may contribute to lung tissue damage, which could exacerbate the course of the disease (Hung et al., 2005). Moreover, it has been hypothesized that neutrophils could recognize the spherules and endospores of Coccidioides spp. via TLR2 or C-type lectin receptors, including Dectin-1, and inhibit their growth through NETs or granuloma formation. In the latter structure, the neutrophils are organized to form a necrotic center accompanied by eosinophilic debris and macrophages (Shubitz et al., 2008; Gonzalez et al., 2011; Lee et al., 2015). However, it is important to mention that in a mouse model of coccidioidomycosis, it was observed that lung-infiltrated neutrophils produce high amounts of IL-10, a fact that was associated with impairment of resistance to coccidioidal infection due to a suppression of Th1, Th2, and Th17 immunity mediated by this anti-inflammatory cytokine (Hung et al., 2013).
Paradoxically, Hung et al. (2014) observed that neutrophil-depleted mice infected with spores of the virulent isolate of C. posadasii did not show a difference in the fungal burden or the survival rate in comparison with control mice, indicating that neutrophils are dispensable for defense against this mycosis. Nonetheless, when the mice were immunized with a live-attenuated vaccine against coccidioidomycosis, the vaccine-induced protection promoted early recruitment and elevated numbers of neutrophils to the infection site, suggesting that the role of these phagocytic cells depends on prior exposure of the host to Coccidioides spp. (Hung et al., 2014).
In additional studies using mice deficient of NADPH oxidase 2 (NOX2), it was reported that NOX2 deficient mice infected with Coccidioides showed a reduced survival accompanied by a high and sustained number of lung-infiltrated neutrophils on days 7 and 11 postchallenge compared to infected WT mice. This evidence suggests that NOX2 production plays a role in limiting neutrophil recruitment and the subsequent pathogenic inflammation in this murine model of coccidioidomycosis (Gonzalez et al., 2011).
Blastomycosis
In contrast with the other dimorphic fungal infections, immunosuppression like HIV/aids does not appear to be a risk factor in developing blastomycosis (Pappas et al., 1993; Schwartz and Kauffman, 2020); thus, neutrophils and other innate immune cells might be enough to control the infection.
Early in vitro studies with murine neutrophils have been controversial. Although the ability of neutrophils to kill B. dermatitidis has been demonstrated (Brummer and Stevens, 1984; Brummer et al., 1986; Morrison et al., 1989), some reports have shown that neutrophils enhance and allow the replication of this fungal pathogen, a fact that was associated with an exacerbation of the infection by accumulation and death of neutrophils in the tissue lesions (Brummer and Stevens, 1983). In this sense, the presence of a fungal chemotactic factor in serum-free culture filtrated of B. dermatitidis has been demonstrated (Sixbey et al., 1979; Thurmond and Mitchell, 1984). Lorenzini et al. (2017) also demonstrated that Blastomyces produces a peptidase (DppIVA) that cleaves chemokines, specifically those belonging to the CXC family, including the CXCL-2, which is the most potent chemoattractant molecule for neutrophils, thus improving neutrophil migration and promoting a pyogranulomatous response, a typical reaction during blastomycosis infection.
The function of a subset of neutrophils has recently been described in a murine model of pulmonary blastomycosis, which shows the capability of transdifferentiation in a neutrophil-dendritic cell hybrid that was associated with a better fungicidal function, NETs formation, and a higher expression of PRRs and the production of reactive oxygen species than canonical neutrophils (Fites et al., 2018). Although the role of these cells in other fungal infections is still unknown, these cells could be expected to contribute significantly due to their ability to improve both the innate and the adaptative immunity.
Paracoccidioidomycosis
In contrast to other endemic mycoses, several studies have suggested the dual role played by neutrophils during PCM infection. The functionality of these phagocytic cells appears to depend on some factors, including the genetic pattern of the host or the stage of infection. Pina et al. (2006) observed a significant difference, in the role of neutrophils, between resistant and susceptible mice; thus, in susceptible mice, these phagocytic cells have low fungicidal activity, but in contrast, neutrophils from resistant mice are more abundant in the lesion areas and efficient to control infection. Similar results were obtained by Sperandio et al. (2015), who using resistant and susceptible mice to PCM showed that in susceptible mice, the infection was able to disseminate to their bone marrow, impairing the production and maturation of neutrophils, which is different from what was observed in resistant mice.
Similarly, it has been suggested that neutrophils are essential during the acute inflammatory phase since they represent more than 85% of inflammatory cells and could positively modulate the innate immune response through the production of pro-inflammatory cytokines and lipidic mediators in the infected lung tissue (Gonzalez and Cano, 2001; Gonzalez et al., 2003; Balderramas et al., 2014). Along these lines, using an experimental model of pulmonary PCM in mice with intermediate susceptibility to infection and treated with a monoclonal antibody specific to neutrophils, we reported that compared to control mice, infected and neutrophil-depleted mice showed decreased survival rates during the early stage of infection, accompanied by an increase in both the fungal burden and the inflammatory response with an exaggerated production of several chemokines and proinflammatory cytokines, suggesting the pivotal role of these phagocytic cells in this fungal infection during the early course of infection (Pino-Tamayo et al., 2016).
Conversely, in studies of chronic pulmonary PCM in those mice with intermediate susceptibility, it was reported that treatment with the antifungal itraconazole or the immunomodulator pentoxifylline, alone or in combination, was associated with a decreased number of neutrophils as well as with an improved outcome of the disease, suggesting that these phagocytic cells appear to play a deleterious effect during the chronic stages of PCM (Naranjo et al., 2010., Naranjo et al., 2011; Lopera et al., 2015). Subsequently, we reported that those mice treated with the monoclonal antibodies specific to neutrophils during the chronic stages of infection showed better control of infection correlated with a reduction not only on the fungal burden but also on the inflammatory response and pulmonary fibrosis, suggesting that these phagocytic cells appear to play a detrimental effect during the chronic course of pulmonary PCM (Puerta-Arias et al., 2016).
Additional studies confirmed the presence of two different subsets of murine neutrophils, the type I neutrophils associated with a pro-inflammatory response, and the type II neutrophils associated with an anti-inflammatory response. Thus, a greater number of type II neutrophils were observed, which could be related to the incapacity of the host to control P. brasiliensis infection without the appropriate treatment (Puerta-Arias et al., 2018).
Moreover, other reports have described the capacity of P. brasiliensis to survive inside neutrophils and extend the lifetime of these cells via IL-8 production triggered by the fungus, suggesting that P. brasiliensis could evade the antifungal mechanisms allowing its replication (Brummer et al., 1989; Acorci et al., 2008). Along the same lines, it has been reported that paracoccin, a lectin expressed by P. brasiliensis, induces IL-8, IL-1β, and ROS production. It also induces the release of DNA and inhibits apoptosis (Ricci-Azevedo et al., 2018). In contrast, it has been reported that neutrophils exhibit antifungal activity against P. brasiliensis yeast only when these phagocytic cells are priming with IFN-γ, GM-CSF, IL-1β, and TNF-α (Kurita et al., 2000; Rodrigues et al., 2007). Similar results were obtained when human neutrophils were activated with IL-15, which increases P. brasiliensis killing by a mechanism dependent on H2O2 and superoxide anion (Tavian et al., 2007).
On the other hand, other studies have reported that neutrophils recognize P. brasiliensis yeasts via Dectin-1, MR, TLR2, and TLR4 (Acorci-Valéro et al., 2010; Balderramas et al., 2014; Gardizani et al., 2019) and produce cytokines including IL-12, IL-10, PGE2, and LTB4 (Balderramas et al., 2014). Additionally, these interactions were able to induce NETs formation by either dependent or independent reactive oxygen species production, correlating with the fungal morphotype used for stimulation (conidia or yeast, respectively); however, the killing of the fungus by NETs was dependent on the fungal strain and previous activation by cytokines, including TNF-α, IFN-γ, and GM-CSF. In addition, NETs appear to prevent fungal dissemination (Mejía et al., 2015; Bachiega et al., 2016). Likewise, histopathological samples of cutaneous lesions from human PCM patients have revealed the production of NETs (Della-Coletta et al., 2015).
Taken all together, the above studies clearly demonstrated the important effector and immunomodulatory roles played by neutrophils during the early stages of infection, contributing to P. brasiliensis host resistance. Paradoxically, these phagocytic cells could also play a detrimental role during the chronic or advanced stages of infection.
Neutrophils and IL-17 Participation in The Granulomatous Inflammation and Pulmonary Fibrosis Development of Endemic Mycoses
The lungs are the primary organs affected by the dimorphic fungal pathogens; thus, once the conidia reach the alveoli, they transform into pathogenic morphotypes (yeast cells or spherules). Initially, these fungal propagules interact with lung epithelial cells or alveolar macrophages. Such interactions trigger the activation of these host cells, which, in turn, secret soluble mediator molecules, mainly chemokines and pro-inflammatory cytokines that induce the recruitment of pro-inflammatory cells, including neutrophils and other innate cells, into the lungs (Martin and Frever, 2005). During pulmonary inflammation, neutrophils can also interact with epithelial cells, lymphocytes, macrophages, and other granulocytes inducing the activation of the adaptive immune response (Siew et al., 2017). However, neutrophils may be self-defeating; thus, they could play a protective role but could also contribute to injury and tissue damage, especially in those cases of chronic inflammation due to a continuous activation mediated by the IL-17 (Parkos, 2016; Rosales et al., 2016). Neutrophils contain a great number of proteases, inflammatory mediators, and oxidants stored in their granules that lead to the progression of pulmonary complications such as asthma, chronic obstructive pulmonary disease (COPD), granulomatous lesions, and finally fibrosis (Liu et al., 2017).
In the case of systemic and endemic mycoses, there are scant studies that link the IL-17 and/or neutrophils with the development of a chronic inflammatory process or with fibrosis development. Some studies have demonstrated the presence of neutrophils in pulmonary infiltrating cells during infections by P. brasiliensis (Gonzalez and Cano, 2001; Gonzalez et al., 2003), H. capsulatum (Baughman et al., 1986), C. posadasii (Hung et al., 2005), and B. dermatitidis (Lorenzini et al., 2017). However, as mentioned above, the role of neutrophils during these dimorphic fungal infections is not clear; moreover, their participation in the immune response appears to depend on the phase of infection (acute or chronic) (Pino-Tamayo et al., 2016; Puerta-Arias et al., 2016). Only in an experimental model of paracoccidioidomycosis, the role of neutrophils in the pulmonary fibrosis development has been studied, where it was demonstrated that these phagocytic cells are detrimental and promote granulomatous inflammation and pulmonary fibrosis during the chronic course of the mycosis, a process that was also associated with the presence of IL-17 (Pino-Tamayo et al., 2016; Puerta-Arias et al., 2016). Moreover, it was demonstrated that the Th17-associated cytokines, IL-17, IL-6, and IL-23, are crucial for granuloma formation during experimental paracoccidioidomycosis; thus, deficiency of IL-6, IL-23, or IL-17RA impaired the compact granuloma formation and conferred susceptibility to infection (Tristão et al., 2017). In an additional study conducted by Heninger et al. (2006), it was reported that the granuloma lesions induced by Histoplasma infection are composed mainly of CD4+, CD8+, Dendritic cells, and macrophages, which are the principal sources of IFN-γ and IL-17; notably, neutrophils were not evidenced. In the other fungal endemic and systemic mycosis (coccidioidomycosis and blastomycosis), the role played by neutrophils and IL-17 in the development of the fibrosis process remains to be explored.
On the other hand, pulmonary fibrosis (PF) is the final result of a chronic inflammation caused by microbial pathogens and chemical or physical agents; thus, PF is a consequence of a repetitive process of injury and reparation of the alveolar epithelium, which leads to an exacerbated wound healing process, accompanied by an excess deposition of extracellular matrix (ECM) components and a scarring process of the lung (Rydell-Tormanen et al., 2012; Chioma and Drake, 2017). Additionally, during this PF process, an increased number of myofibroblasts and fibroblasts that are known to synthesize connective tissue proteins have been observed, mainly Type III Collagen, and matrix metalloproteinases (MMP) (Baum and Duffy, 2011; Wynn and Ramalingam, 2012). In paracoccidioidomycosis, the presence of neutrophil infiltration within the granuloma is observed during the chronic form, and especially surrounding the granulomas (González et al., 2008). Additional studies have shown that treatment with a combination of Itraconazole plus an immunomodulatory agent (Pentoxifylline) during the chronic pulmonary paracoccidioidomycosis, reduced the granulomatous inflammation and neutrophils (Naranjo et al., 2011). Along the same lines, in this fungal model and using a monoclonal antibody specific to neutrophils it was demonstrated that the depletion of these phagocytic cells was associated with an attenuation of the inflammation and the fibrotic process through a down-regulation of pro-fibrotic mediators including IL-17, TGF-β1, TNF-α, MMP-8 and the tissue inhibitor of metalloproteinases (TIMP)-2 (Puerta-Arias et al., 2016). The above studies clearly suggest that both neutrophils and IL-17 are responsible, in part, for the fibrosis development, evidence that supports the idea that neutrophils and/or IL-17 can be targets of a therapeutic intervention for the treatment of fibrosis as well as mycosis.
Future Questions and Conclusions
Over the last years, it has been learned that IL17 plays an important role and appears to be indispensable in developing a protective immunity against fungal infections including systemic and endemic mycoses, a mechanism that is mediated by the interactions between fungal PAMPs and PRRs with the subsequent activation and recruitment of neutrophils and macrophages at the site of infection. Furthermore, it has also been demonstrated that each PRR plays a distinctly different role for each dimorphic fungal pathogen. Nonetheless, both the IL17 and neutrophils play a detrimental role in inducing pathological disease, possibly due to an unrestrained inflammatory response. Future studies will need to focus on which specific fungal antigens or PAMPs are recognized by the different PRRs and confer immunity through this Th17 immune pattern or, on the contrary, which kind of interactions induce pathological disease. Additionally, it would be interesting to know if IL17 induce NETs formation as a microbicidal mechanism against these fungal dimorphic pathogens. Thus, understanding the specific interactions between fungal PAMPs and PRRs and their contributions to disease outcomes will provide potential insights for designing and developing immunotherapies to control these systemic and endemic mycoses.
Author Contributions
All authors contributed to the article and approved the submitted version.
Funding
Supported in part by Universidad de Antioquia; Basic and Applied Microbiology Research Group (MICROBA), School of Microbiology, Universidad de Antioquia, Medellin, Colombia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abusleme L., Moutsopoulos N. M. (2017). IL-17: overview and role in oral immunity and microbiome. Oral. Dis. 23, 854–865. doi: 10.1111/odi.12598
Acorci M. J., Dias-Melicio L. A., Golim M. A., Bordon-Graciani A. P., Peraçoli M. T. S., Soares Â.M.V. (2008). Inhibition of human neutrophil apoptosis by Paracoccidioides brasiliensis: role of Interleukin-8. Basic Immunol. 69, 73–79. doi: 10.1111/j.1365-3083.2008.02199.x
Acorci-Valério M. J., Bordon-Graciani A. P., Dias-Melicio L. A., de Assis Golim M., Nakaira-Takahagi E., de Campos Soares A. M. (2010). Role of TLR2 and TLR4 in human neutrophil functions against Paracoccidioides brasiliensis. Scand. J. Immunol. 71, 99–108. doi: 10.1111/j.1365-3083.2009.02351.x
Awasthi S., Vilekar P., Conkleton A., Rahman N. (2019). Dendritic cell-based immunization induces Coccidioides Ag2/PRA-specific immune response. Vaccine 37, 1685–1691. doi: 10.1016/j.vaccine.2019.01.034
Bachiega T. F., Dias-Melicio L. A., Fernandes R. K., de Almeida Balderramas H., Rodrigues D. R., Ximenes V. F., et al. (2016). Participation of dectin-1 receptor on NETs release against Paracoccidioides brasiliensis: Role on extracellular killing. Immunobiology 221, 228–235. doi: 10.1016/j.imbio.2015.09.003
Balderramas H. A., Penitenti M., Rodrigues D., Bachiega D., Fernandes R., Ikoma M., et al. (2014). Human neutrophils produce IL-12, IL-10, PGE2, and LTB4 in response to Paracoccidioides brasiliensis. Involvement of TLR2, mannose receptor and dectin-1. Cytokine 67, 36–43. doi: 10.1016/j.cyto.2014.02.004
Baughman R., Kim C., Vinegar A., Hendricks D., Schmidt J., Bullock E. (1986). The pathogenesis of experimental pulmonary histoplasmosis. Correlative studies of histopathology, bronchoalveolar lavage, and respiratory function. Am. Rev. Respir. Dis. 134, 771–776. doi: 10.1164/arrd.1986.134.4.771
Baum J., Duffy H. S. (2011). Fibroblasts and Myofibroblasts: what are we talking about? J. Cardiovasc. Pharmacol. 57, 376–379. doi: 10.1097/FJC.0b013e3182116e39
Bianchi M., Hakkim A., Brinkmann V., Siler U., Seger R. A., Zychlinsky A., et al. (2009). Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114, 2619–2622. doi: 10.1182/blood-2009-05-221606
Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., et al. (2004). Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. doi: 10.1126/science.1092385
Brummer E., Stevens D. A. (1983). Enhancing effect of murine polymorphonuclear neutrophils (PMN) on the multiplication of Blastomyces dermatitidis in vitro and in vivo. Clin. Exp. Immunol. 54, 587–594.
Brummer E., Stevens D. A. (1984). Activation of murine polymorphonuclear neutrophils for fungicidal activity with supernatants from antigen-stimulated immune spleen cell cultures. Infect. Immun. 45, 447–452. doi: 10.1128/IAI.45.2.447-452.1984
Brummer E., McEwen J. G., Stevens D. A. (1986). Fungicidal activity of murine inflammatory polymorphonuclear neutrophils: comparison with murine peripheral blood PMN. Clin. Exp. Immunol. 66, 681–690.
Brummer E., Hanson L. H., Restrepo A., Stevens D. A. (1989). Intracellular multiplication of Paracoccidioides brasiliensis in macrophages: killing and restriction of multiplication by activated macrophages. Infect. Immun. 57, 2289–2294. doi: 10.1128/IAI.57.8.2289-2294.1989
Brummer E., Kurita N., Yoshida S., Nishimura K., Miyaji M. (1991). Fungistatic activity of human neutrophils against Histoplasma capsulatum: correlation with phagocytosis. J. Infect. Dis. 164, 158–162. doi: 10.1093/infdis/164.1.158
Buonocore S., Ahern P. P., Uhlig H. H., Ivanov I. II, Littman D. R., Maloy K. J., et al. (2010). Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375. doi: 10.1038/nature08949
Byrd A. S., O’Brien X. M., Johnson C. M., Lavigne L. M., Reichner J. S. (2013). An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J. Immunol. 190, 4136–4148. doi: 10.4049/jimmunol.1202671
Campuzano A., Zhang H., Ostroff G. R., Dos Santos Dias L., Wüthrich M., Klein B. S., et al. (2020). CARD9-associated Dectin-1 and Dectin-2 are required for protective immunity of a multivalent vaccine against Coccidioides posadasii infection. J. Immunol. 204, 3296–3306. doi: 10.4049/jimmunol.1900793
Cao C., Xi L., Chaturvedi V. (2019). Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: Insights into the clinical trends of a major fungal disease 60 years after the discovery of the pathogen. Mycopathologia 184, 709–720. doi: 10.1007/s11046-019-00410-2
Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J. K. M., et al. (2009). A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725. doi: 10.1038/nature0753
Chamilos G., Ganguly D., Lande R., Gregorio J., Meller S., William E., et al. (2010). Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of th-17 responses. PloS One 5, e12955. doi: 10.1371/journal.pone.0012955
Chang W., Audet R., Aizenstein B., Hogna L., DeMars R., Klein B. (2000). T-Cell epitopes and human leukocyte antigen restriction elements of an immunodominant antigen of Blastomyces dermatitidis. Infect. Immun. 68, 502–510. doi: 10.1128/iai.68.2.502-510.2000
Chapman S., Dismukes W., Proia L., Bradsher R., Pappas P., Threlkeld M., et al. (2008). Clinical practice guidelines for the management of blastomycosis: 2008 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 46, 1801–1812. doi: 10.1086/588300
Chen K., Kolls J. K. (2017). Interluekin-17A (IL17A). Gene 614, 8–14. doi: 10.1016/j.gene.2017.01.016
Cheng O. Z., Palaniyar N. (2013). NET balancing: a problem in inflammatory lung diseases. Front. Immunol. 4, 1. doi: 10.3389/fimmu.2013.00001
Chiarella A. P., Arruda C., Pina A., Costa T. A., Ferreira R. C., Calich V. L. (2007). The relative importance of CD4+ and CD8+T cells in immunity to pulmonary paracoccidioidomycosis. Microbes Infect. 9, 1078–1088. doi: 10.1016/j.micinf.2007.04.016
Chioma O. S., Drake W. P. (2017). Role of microbial agents in pulmonary fibrosis. Yale J. Biol. Med. 90, 219–227.
Coxon A., Rieu P., Barkalow F. J., Askari S., Sharpe A. H., von Andrian U. H., et al. (1990). A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 5, 653–666. doi: 10.1016/s1074-7613(00)80278-2
de Castro L. F., Ferreira M. C., da Silva R. M., Blotta M. H., Longhi L. N., Mamoni R. L. (2013). Characterization of the immune response in human paracoccidioidomycosis. J. Infect. 67, 470–485. doi: 10.1016/j.jinf.2013.07.019
Deepe G. S. Jr., Gibbons R. S. (2009). Interleukins 17 and 23 influence the host response to Histoplasma capsulatum. J. Infect. Dis. 200, 142–151. doi: 10.1086/599333
Deepe G., Gibbons R., Smulian G. (2008). Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs. J. Leukoc. Biol. 84, 669–678. doi: 10.1189/jlb.0308154
Deepe G. S. Jr, Buesing W. R., Ostroff G. R., Abraham A., Specht C. A., Huang H., et al. (2018). Vaccination with an alkaline extract of Histoplasma capsulatum packaged in glucan particles confers protective immunity in mice. Vaccine 36, 3359–3367. doi: 10.1016/j.vaccine.2018.04.047
Della-Coletta A. M., Bachiega T. F., de Quaglia e Silva J. C., Soares Â.M.V., de C., De Faveri J., et al. (2015). Neutrophil extracellular traps identification in tegumentary lesions of patients with paracoccidioidomycosis and different patterns of NETs generation in vitro. PloS Negl. Trop. Dis. 9, e0004037. doi: 10.1371/journal.pntd.0004037
Desai J., Lionakis M. (2018). The role of neutrophils in host defense against invasive fungal infections. Curr. Clin. Microbiol. Rep. 5, 181–189. doi: 10.1007/s40588-018-0098-6
Drummond R. A., Saijo S., Iwakura Y., Brown G. D. (2011). The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur. J. Immunol. 41, 276–281. doi: 10.1002/eji.201041252
Feriotti C., de Araújo E. F., Loures F. V., da Costa T. A., Galdino N. A., Zamboni D. S., et al. (2017). NOD-Like receptor P3 inflammasome controls protective Th1/Th17 immunity against pulmonary paracoccidioidomycosis. Front. Immunol. 8, 786. doi: 10.3389/fimmu.2017.00786
Fernandes F. F., Oliveira L. L., Landgraf T. N., Peron G., Costa M. V., Coelho-Castelo A. A., et al. (2016). Detrimental effect of fungal 60-kda heat shock protein on experimental Paracoccidioides brasiliensis infection. PloS One 11, e0162486. doi: 10.1371/journal.pone.0162486
Fites J., Gui M., Kernien J., Negoro P., Dagher Z., Sykes D., et al. (2018). An unappreciated role for neutrophil-DC hybrids in immunity to invasive fungal infections. PloS Pathog. 14, e1007073. doi: 10.1371/journal.ppat.1007073
Galgiani J., Ampel N., Blair J., Catanzaro A., Johnson R., Stevens D., et al. (2005). Coccidioidomycosis. Clin. Infect. Dis. 41, 1217–1223. doi: 10.1086/496991
Gardizani T. P., Della Coletta A. M., Romagnoli G. G., Puccia R., Serezani A. P. M., de Campos Soares Â.M.V., et al. (2019). 43 kDa Glycoprotein (gp43) from Paracoccidioides brasiliensis induced IL-17A and PGE2 production by human polymorphonuclear neutrophils: Involvement of TLR2 and TLR4. J. Immunol. Res. 2019, 1790908. doi: 10.1155/2019/1790908
Geremia A., Arancibia-Cárcamo C. V., Fleming M. P. P., Rust N., Singh B., Mortensen N. J., et al. (2011). IL-23–responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 208, 1127–1133. doi: 10.1084/jem.20101712
Gonzalez A., Cano L. (2001). Participation of the polymorphonuclear neutrophils in the immune response against Paracoccidioides brasiliensis. [Participación del polimorfonuclear neutrófilo en la respuesta inmune contra Paracoccidioides brasiliensis]. Biomedica 21, 264–274. doi: 10.7705/biomedica.v21i3.1117
Gonzalez A., Sahaza J., Ortiz B., Restrepo A., Cano L. (2003). Production of pro-inflammatory cytokines during the early stages of experimental Paracoccidioides brasiliensis infection. Med. Mycol. 41, 391–399. doi: 10.1080/13693780310001610038
González A., Lenzi H. L., Motta E. M., Caputo L., Restrepo A., Cano L. E. (2008). Expression and arrangement of extracellular matrix proteins in the lungs of mice infected with Paracoccidioides brasiliensis conidia. Int. J. Exp. Pathol. 89, 106–116. doi: 10.1111/j.1365-2613.2008.00573.x
Gonzalez A., Hung C.-Y., Cole G. (2011). Absence of phagocyte NADPH oxidase 2 leads to severe inflammatory response in lungs of mice infected with Coccidioides. Microb. Pathog. 51, 432–441. doi: 10.1016/j.micpath.2011.08.003
Harrington L. E., Mangan P. R., Weaver C. T. (2006). Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 18, 349–356. doi: 10.1016/j.coi.2006.03.017
Hartupee J., Liu C., Novotny M., Sun D., Li X., Hamilton T. A. (2009). IL-17 signaling for mRNA stabilization does not require TNF receptor-associated factor 6. J. Immunol. 182, 1660–1666. doi: 10.4049/jimmunol.182.3.1660
Heninger E., Hogan L. H., Karman J., Macvilay S., Hill B., Woods J. P., et al. (2006). Characterization of the Histoplasma capsulatum-induced granuloma. J. Immunol. 177, 3303–3313. doi: 10.4049/jimmunol.177.5.3303
Hernández-Santos N., Wiesner D. L., Fites J. S., McDermott A. J., Warner T., Wüthrich M., et al. (2018). Lung epithelial cells coordinate innate lymphocytes and immunity against pulmonary fungal infection. Cell Host Microbe 23, 511–522.e5. doi: 10.1016/j.chom.2018.02.011
Hoeksema M., van Eijk M., Haagsman H. P., Hartshorn K. L. (2016). Histones as mediators of host defense, inflammation and thrombosis. Future Microbiol. 11, 441–453. doi: 10.2217/fmb.15.151
Hoshino A., Nagao T., Nagi-Miura N., Ohno N., Yasuhara M., Yamamoto K., et al. (2008). MPO-ANCA induces IL-17 production by activated neutrophils in vitro via its Fc region- and complement-dependent manner. J. Autoimmun. 31, 79–89. doi: 10.1016/j.jaut.2008.03.006
Hsu L., Ng E., Koh L. (2010). Common and emerging fungal pulmonary infections. Infect. Dis. Clin. North Am. 24, 557–577. doi: 10.1016/j.idc.2010.04.003
Hung C. Y., Seshan K. R., Yu J. J., Schaller R., Xue J., Basrur V., et al. (2005). A metalloproteinase of Coccidioides posadasii contributes to evasion of host detection. Infect. Immun. 73, 6689–6703. doi: 10.1128/IAI.73.10.6689-6703.2005
Hung C. Y., Xue J., Cole G. T. (2007). Virulence mechanisms of Coccidioides. Ann. N. Y. Acad. Sci. 1111, 225–235. doi: 10.1196/annals.1406.020
Hung C. Y., Gonzalez A., Wüthrich M., Klein B. S., Cole G. T. (2011). Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect. Immun. 79, 4511–4522. doi: 10.1128/IAI.05726-11
Hung C. Y., Hurtgen B. J., Bellecourt M., Sanderson S. D., Morgan E. L., Cole G. T. (2012). An agonist of human complement fragment C5a enhances vaccine immunity against Coccidioides infection. Vaccine 30, 4681–4690. doi: 10.1016/j.vaccine.2012.04.084
Hung C. Y., Castro-Lopez N., Cole G. T. (2013). Vaccinated C57BL/6 mice develop protective and memory t cell responses to Coccidioides posadasii infection in the absence of interleukin-10. Infect. Immun. 82, 903–913. doi: 10.1128/IAI.01148-13
Hung Y., Jiménez-Alzate M., del P., Gonzalez A., Wuthrich M., Klein S., et al. (2014). Interleukin-1 receptor but not Toll-like receptor 2 is essential for MyD88-dependent Th17 immunity to Coccidioides infection. Infect. Immun. 82, 2106–2114. doi: 10.1128/IAI.01579-13
Hung C. Y., Castro-Lopez N., Cole G. T. (2016). Card9- and MyD88-mediated gamma interferon and nitric oxide production is essential for resistance to subcutaneous Coccidioides posadasii infection. Infect. Immun. 84, 1166 –11175. doi: 10.1128/IAI.01066-15
Hung C. Y., Zhang H., Castro-Lopez N., Ostroff G. R., Khoshlenar P., Abraham A., et al. (2018). Glucan-chitin particles enhance Th17 response and improve protective efficacy of a multivalent antigen (rCpa1) against pulmonary Coccidioides posadasii infection. Infect. Immun. 86, e00070–e00018. doi: 10.1128/IAI.00070-18
Hurtgen B. J., Hung C. Y., Ostroff G. R., Levitz S. M., Cole G. T. (2012). Construction and evaluation of a novel recombinant T cell epitope-based vaccine against Coccidioidomycosis. Infect. Immun. 80, 3960–3974. doi: 10.1128/IAI.00566-12
Hurtgen B. J., Castro-Lopez N., Jiménez-Alzate M. D. P., Cole G. T., Hung C. Y. (2016). Preclinical identification of vaccine induced protective correlates in human leukocyte antigen expressing transgenic mice infected with Coccidioides posadasii. Vaccine 34, 5336–5343. doi: 10.1016/j.vaccine.2016.08.078
Isailovic N., Daigo K., Mantovani A., Selmi C. (2015). Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 60, 1–11. doi: 10.1016/j.jaut.2015.04.006
Ishikawa T., Itoh F., Yoshida S., Saijo S., Matsuzawa T., Gonoi T., et al. (2013). Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe 13, 477–488. doi: 10.1016/j.chom.2013.03.008
Jannuzzi G. P., de Almeida J. R. F., Amarante-Mendes G. P., Romera L. M. D., Kaihami G. H., Vasconcelos J. R., et al. (2019). TLR3 is a negative regulator of immune responses against Paracoccidioides brasiliensis. Front. Cell Infect. Microbiol. 8, 426. doi: 10.3389/fcimb.2018.00426
Ketelut-Carneiro N., Souza C. O. S., Benevides L., Gardinassi L. G., Silva M. C., Tavares L. A., et al. (2019). Caspase-11-dependent IL-1α release boosts Th17 immunity against Paracoccidioides brasiliensis. PloS Pathog. 15, e1007990. doi: 10.1371/journal.ppat.1007990
Kirkland T. N., Fierer J. (2018). Coccidioides immitis and posadasii; A review of their biology, genomics, pathogenesis, and host immunity. Virulence 9, 1426–1435. doi: 10.1080/21505594.2018.1509667
Kolaczkowska E., Kubes P. (2013). Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. doi: 10.1038/nri3399
Korn T., Bettelli E., Oukka M., Kuchroo V. K. (2009). IL-17 and Th17 Cells. Annu. Rev. Immunol. 27, 485–517. doi: 10.1146/annurev.immunol.021908.132710
Kroetz D. N., Deepe G. S. Jr (2010). CCR5 dictates the equilibrium of proinflammatory IL-17+ and regulatory Foxp3+ T cells in fungal infection. J. Immunol. 184, 5224–5231. doi: 10.4049/jimmunol.1000032
Kroetz D. N., Deepe G. S. (2012). The role of cytokines and chemokines in Histoplasma capsulatum infection. Cytokine 58, 112–117. doi: 10.1016/j.cyto.2011.07.430
Kurita N., Terao K., Brummer E., Ito E., Nishimura K., Miyaji M. (1991). Resistance of Histoplasma capsulatum to killing by human neutrophils. Mycopathologia 115, 207–213. doi: 10.1007/BF00462229
Kurita N., Oarada M., Miyaji M., Ito E. (2000). Effect of cytokines on antifungal activity of human polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med. Mycol. 38, 177–182. doi: 10.1080/mmy.38.2.177.182
Lee C. Y., Thompson III G., Hastey C., Hodge G., Lunetta J., Pappagianis D., et al. (2015). Coccidioides endospores and spherules draw strong chemotactic, adhesive, and phagocytic responses by individual human neutrophils. PloS One 10, e0129522. doi: 10.1371/journal.pone.0129522
Leffler J., Martin M., Gullstrand B., Tydén H., Lood C., Truedsson L., et al. (2012). Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 188, 3522–3531. doi: 10.4049/jimmunol.1102404
Lin A. M., Rubin C. J., Khandpur R., Wang J. Y., Riblett M., Yalavarthi S., et al. (2011). Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 187, 490–500. doi: 10.4049/jimmunol.110012
Lionakis M., Levitz S. (2018). Host control of fungal infections: Lessons from basic studies and human cohorts. Annu. Rev. Immunol. 36, 157–191. doi: 10.1146/annurev-immunol-042617-053318
Lionakis M., Iliev I., Hohl T. (2017). Immunity against fungi. JCI Insights 2, e93156. doi: 10.1172/jci.insight.93156
Liu J., Pang Z., Wang G., Guan X., Fang K., Wang Z., et al. (2017). Advanced role of neutrophils in common respiratory diseases. J. Immunol. Res. 2017:6710278. doi: 10.1155/2017/6710278
Lopera D. E., Naranjo T. W., Hidalgo J. M., Echeverri L., Patiño J. H., Moreno Á.R., et al. (2015). Pentoxifylline immunomodulation in the treatment of experimental chronic pulmonary paracoccidioidomycosis. Fibrogenesis Tissue Repair 8:10. doi: 10.1186/s13069-015-0027-8
Lorenzini J., Fites J. S., Nett J., Klein B. (2017). Blastomyces dermatitidis serine protease(DppIVA) cleaves ELR+CXC chemokines altering their effects on neutrophils. Cell Microbiol. 19:10. doi: 10.1111/cmi.12741
Loures F. V., Pina A., Felonato M., Calich V. L. (2009). TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J. Immunol. 183, 1279–1290. doi: 10.4049/jimmunol.0801599
Loures F. V., Pina A., Felonato M., Feriotti C., de Araujo E. F., Calich V. L. (2011). MyD88 signaling is required for efficient innate and adaptive immune responses to Paracoccidioides brasiliensis infection. Infect. Immun. 79, 2470–2480. doi: 10.4049/jimmunol.0801599
Loures F. V., Araújo E. F., Feriotti C., Bazan S. B., Costa T. A., Brown G. D., et al. (2014). Dectin-1 induces M1 macrophages and prominent expansion of CD8+IL-17+ cells in pulmonary paracoccidioidomycosis. J. Infect. Dis. 210, 762–773. doi: 10.1093/infdis/jiu136
Loures F. V., Araújo E. F., Feriotti C., Bazan S. B., Calich V. L. G. (2015). TLR-4 cooperates with Dectin-1 and mannose receptor to expand Th17 and Tc17 cells induced by Paracoccidioides brasiliensis stimulated dendritic cells. Front. Microbiol. 6, 261. doi: 10.3389/fmicb.2015.00261
Martin T., Frever C. (2005). Innate immunity in the lung. Proc. Am. Thorac. Soc 2, 403–411. doi: 10.1513/pats.200508-090JS
McGreal E. P., Rosas M., Brown G. D., Zamze S., Wong S. Y., Gordon S., et al. (2006). The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16, 422–430. doi: 10.1093/glycob/cwj077
Medeiros A. II, Bonato V. L., Malheiro A., Dias A. R., Silva C. L., Faccioli L. H. (2002). Histoplasma capsulatum inhibits apoptosis and Mac-1 expression in leucocytes. Scand. J. Immunol. 56, 392–398. doi: 10.1046/j.1365-3083.2002.01142.x
Medeiros A. II, Secatto A., Bélanger C., Sorgi P. B., Marleau S., Faccioli L. (2015). Impairment of neutrophil migration to remote inflammatory site during lung histoplasmosis. BioMed. Res. Int. 2015, 409309. doi: 10.1155/2015/409309
Mejía S. P., Cano L. E., López J. A., Hernandez O., González Á. (2015). Human neutrophils produce extracellular traps against Paracoccidioides brasiliensis. Microbiology 161, 1008–1017. doi: 10.1099/mic.0.000059
Merkhofer R. M. Jr, O’Neill M. B., Xiong D., Hernandez-Santos N., Dobson H., Fites J. S., et al. (2019). Investigation of genetic susceptibility to blastomycosis reveals interleukin-6 as a potential susceptibility locus. mBio. 10, e01224–e01219. doi: 10.1128/mBio.01224-19
Michel M.-L., Keller A. C., Paget C., Fujio M., Trottein F., Savage P. B., et al. (2007). Identification of an IL-17–producing NK1.1neg iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204, 995–1001. doi: 10.1084/jem.20061551
Mócsai A. (2013). Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 210, 1283–1299. doi: 10.1084/jem.20122220
Monin L., Gaffen S. L. (2018). Interleukin 17 family cytokines: Signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb. Perspect. Biol. 10:a028522. doi: 10.1101/cshperspecta028522
Morrison C. J., Brummer E., Stevens D. (1989). In vivo activation of peripheral blood polymorphonuclear neutrophils by gamma interferon results in enhanced fungal killing. Infect. Immun. 57, 2953–1958. doi: 10.1128/IAI.57.10.2953-2958.1989
Nanjappa S. G., Heninger E., Wüthrich M., Gasper D. J., Klein B. S. (2012). Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PloS Pathog. 8, e1002771. doi: 10.1371/journal.ppat.1002771
Nanjappa S. G., Hernández-Santos N., Galles K., Wüthrich M., Suresh M., Klein B. S. (2015). Intrinsic MyD88-Akt1-mTOR signaling coordinates disparate Tc17 and Tc1 responses during vaccine immunity against fungal pneumonia. PloS Pathog. 11, e1005161. doi: 10.1371/journal.ppat.1005161
Nanjappa S. G., McDermott A. J., Fites J. S., Galles K., Wüthrich M., Deepe G. S. Jr, et al. (2017). Antifungal Tc17 cells are durable and stable, persisting as long-lasting vaccine memory without plasticity towards IFNγ cells. PloS Pathog. 13, e1006356. doi: 10.1371/journal.ppat.1006356
Naranjo T. W., Lopera D. E., Diaz-Granados L. R., Duque J. J., Restrepo A., Cano L. E. (2010). Histopathologic and immunologic effects of the itraconazole treatment in a murine model of chronic pulmonary paracoccidioidomycosis. Microbes Infect. 12, 1153–1162. doi: 10.1016/j.micinf.2010.07.013
Naranjo T. W., Lopera D. E., Diaz-Granados L. R., Duque J. J., Restrepo A. M., Cano L. E. (2011). Combined itraconazole-pentoxifylline treatment promptly reduces lung fibrosis induced by chronic pulmonary paracoccidioidomycosis in mice. Pulm. Pharmacol. Ther. 24, 81–91. doi: 10.1016/j.pupt.2010.09.005
Netea M. G., Van der Meer J. W., Kullberg B. J. (2006). Role of the dual interaction of fungal pathogens with pattern recognition receptors in the activation and modulation of host defence. Clin. Microbiol. Infect. 12, 404–409. doi: 10.1111/j.1469-0691.2006.01388.x
Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. R. (2008). An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6, 67–78. doi: 10.1038/nrmicro1815
Newman S. L., Gootee L., Gabay J. E. (1993). Human neutrophil-mediated fungistasis against Histoplasma capsulatum. Localization of fungistatic activity to the azurophil granules. J. Clin. Invest. 92, 624–631. doi: 10.1172/JCI116630
Newman S., Gootee L., Gabay J., Selsted M. (2000). Identification of constituents of human neutrophil azurophil granules that mediate fungistasis against Histoplasma capsulatum. Infect. Immun. 68, 5668–5672. doi: 10.1128/iai.68.10.5668-5672.2000
Onishi R. M., Gaffen S. L. (2010). Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 129, 311–321. doi: 10.1111/j.1365-2567.2009.03240.x
Pappas P., Threlkeld M., Bedsole G., Cleveland K. O., Gelfand M. S., Dismukes W. E. (1993). Blastomycosis in immunocompromised patients. Med. (Baltimore) 72, 311–325. doi: 10.1097/00005792-199309000-00003
Parkos C. A. (2016). Neutrophil-epithelial interactions: A double-edged sword. Am. J. Pathol. 186, 1404–1416. doi: 10.1016/j.ajpath.2016.02.001
Patin E. M., Thompson A., Selinda J. O. (2019). Pattern recognition receptors in fungal immunity. Semin. Cell Dev. Biol. 89, 24–33. doi: 10.1016/j.semcdb.2018.03.003
Pina A., Saldiva P., Restrepo L. E., Calich V. L. (2006). Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts. J. Leukoc. Biol. 79, 1202–1213. doi: 10.1189/jlb.0106052
Pino-Tamayo P., Puerta-Arias J., Lopera D., Urán-Jiménez M., Gonzalez A. (2016). Depletion of neutrophils exacerbates the earlyi Immune response in lungs of mice infected with Paracoccidioides brasiliensis. Mediators Inflamm. 2016:3183285. doi: 10.1155/2016/3183285
Prado M. K. B., Fontanari C., Souza C. O. S., Gardinassi L. G., Zoccal K. F., de Paula-Silva F. W. G., et al. (2020). IL-22 promotes IFN-γ-mediated immunity against Histoplasma capsulatum Infection. Biomolecules 10, 865. doi: 10.3390/biom10060865
Procknow J. J., Page M. II, Loossli C. G. (1960). Early pathogenesis of experimental histoplasmosis. Arch. Pathol. 69, 420–428.
Puerta-Arias J., Pino-Tamayo P., Arango J., Gonzalez A. (2016). Depletion of neutrophils promotes the resolution of pulmonary inflammation and fibrosis in mice infected with Paracoccidioides brasiliensis. PloS One 11, e0163985. doi: 10.1371/journal.pone.0163985
Puerta-Arias J. D., Pino-Tamayo P. A., Arango J. C., Salazar-Peláez L. M., González A. (2018). Itraconazole in combination with neutrophil depletion reduces the expression of genes related to pulmonary fibrosis in an experimental model of paracoccidioidomycosis. Med. Mycol. 56, 579–590. doi: 10.1093/mmy/myx087
Ramirez-Carrozzi V., Sambandam A., Luis E., Lin Z., Jeet S., Lesch J., et al. (2011). IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 12, 1159–1166. doi: 10.1038/ni.2156
Reid D. M., Gow N. A., Brown G. D. (2009). Pattern recognition: recent insights from Dectin-1. Curr. Opin. Immunol. 21, 30–37. doi: 10.1016/j.coi.2009.01.003
Restrepo A., Tobón A. M., González A. (2019). “Paracoccidioidomycosis,” in Principles and Practice of Infectious Diseases, 9. Eds. Mandell G. L., Douglas, Bennett’s J. E. (Philadelphia, PA, USA: Elsevier), 3211–3221.
Ricci-Azevedo R., Gonçales R. A., Roque-Barreira M. C., Girard D. (2018). Human neutrophils are targets to paracoccin, a lectin expressed by Paracoccidioides brasiliensis. Inflammation Res. 67, 31–41. doi: 10.1007/s00011-017-1093-8
Rickel E. A., Siegel L. A., Yoon B.-R. P., Rottman J. B., Kugler D. G., Swart D. A., et al. (2008). Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J. Immunol. 181, 4299–4310. doi: 10.4049/jimmunol.181.6.4299
Rodrigues D. R., Dias-Melicio L. A., Calvi S. A., Peraçoli M. T., Soares A. M. (2007). Paracoccidioides brasiliensis killing by IFN-gamma, TNF-alpha and GM-CSF activated human neutrophils: role for oxygen metabolites. Med. Mycol. 45, 27–33. doi: 10.1080/13693780600981676
Romagnolo A. G., de Quaglia E., Silva J. C., Della Coletta A. M., Gardizani T. P., Martins A. T. L., et al. (2018). Role of Dectin-1 receptor on cytokine production by human monocytes challenged with Paracoccidioides brasiliensis. Mycoses 6, 222–230. doi: 10.1111/myc.12725
Rosales C., Demaurex N., Lowell C. A., Uribe-Querol E. (2016). Neutrophils: Their role in innate and adaptive immunity. J. Immunol. Res. 2016:1469780. doi: 10.1155/2016/1469780
Rydell-Tormanen K., Andréasson K., Hesselstrand R., Risteli J., Heinegård T., Saxne T., et al. (2012). Extracellular matrix alterations and acute inflammation; developing in parallel during early induction of pulmonary fibrosis. Lab. Invest. 92, 917–925. doi: 10.1038/labinvest.2012.57
Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., et al. (2010). Dectin-2 recognition of alphamannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691. doi: 10.1016/j.immuni.2010.05.001
Schnur R. A., Newman S. L. (1990). The respiratory burst response to Histoplasma capsulatum by human neutrophils. Evidence for intracellular trapping of superoxide anion. J. Immunol. 144, 765–772.
Schön M. P., Erpenbeck L. (2018). The Interleukin-23/Interleukin-17 Axis Links Adaptive and Innate Immunity in Psoriasis. Front. Immunol. 9, 1323. doi: 10.3389/fimmu.2018.01323
Schwartz I. S., Kauffman C. A. (2020). Blastomycosis. Semin. Respir. Crit. Care Med. 41, 31–41. doi: 10.1055/s-0039-3400281
Schwartz I. S., Govender N. P., Sigler L., Jiang Y., Maphanga T. G., Toplis B., et al. (2019). Emergomyces: The global rise of new dimorphic fungal pathogens. PloS Pathog. 15, e1007977. doi: 10.1371/journal.ppat.1007977
Sepúlveda V. E., Márquez R., Turissini D. A., Goldman W. E., Matute D. R. (2017). Genome sequences reveal cryptic speciation in the human pathogen Histoplasma capsulatum. mBio 8, e01339–e01317. doi: 10.1128/mBio.01339-17
Shubitz L. F., Dial S., Perrill R., Casement R., Galgiani J. (2008). Vaccine-induced cellular immune responses differ from innate responses in susceptible and resistant strains of mice infected with Coccidioides posadasii. Infect. Immun. 76, 5553–5564. doi: 10.1128/IAI.00885-08
Siew L. Q., Wu S. Y., Ying S., Corrigan C. J. (2017). Cigarette smoking increases bronchial mucosal IL-17A expression in asthmatics, which acts in concert with environmental aeroallergens to engender neutrophilic inflammation. Clin. Exp. Allergy 47, 740–750. doi: 10.1111/cea.12907
Sixbey J. W., Fields B. T., Sun C. N., Clark R. A., Nolan C. M. (1979). Interactions between human granulocytes and Blastomyces dermatitidis. Infect. Immun. 23, 41–44. doi: 10.1128/IAI.23.1.41-44.1979
Sperandio F. F., Fernandes G. P., Mendes A. C. S. C., Bani G., de A. C., Calich V. L. G., et al. (2015). Resistance to P. brasiliensis experimental infection of inbred mice is associated with an efficient neutrophil mobilization and activation by mediators of inflammation. Mediators Inflamm. 2015, 430425. doi: 10.1155/2015/430525
Takatori H., Kanno Y., Watford W. T., Tato C. M., Weiss G., Ivanov I. I., et al. (2008). Lymphoid tissue inducer–like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206, 35–41. doi: 10.1084/jem.20072713
Tavian E. G., Dias-Melicio L. A., Acorci M. J., Bordon-Graciani A. P., Peraçoli M. T. S., Soares A. M. V. (2007). Interleukin-15 increases Paracoccidioides brasiliensis killing by human neutrophils. Cytokine 41, 48–53. doi: 10.1016/j.cyto.2007.10.011
Tecchio C., Casatella M. (2016). Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 28, 119–128. doi: 10.1016/j.smim.2016.04.003
Thompson-Souza G., Santos G., Silva J., Muniz V., Braga Y., Figueiredo R., et al. (2020). Histoplasma capsulatum-induced extracellular DNA trap release in human neutrophils. Cell. Microbiol. 22, e13195. doi: 10.1111/cmi.13195
Thurmond L. M., Mitchell T. (1984). Blastomyces dermatitidis chemotactic factor: kinetics of production and biological characterization evaluated by a modified neutrophil chemotaxis assay. Infect. Immunity 46, 87–93. doi: 10.1128/IAI.46.1.87-93.1984
Toy D., Kugler D., Wolfson M., Vanden Bos T., Gurgel J., Derry J., et al. (2006). Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 177, 36–39. doi: 10.4049/jimmunol.177.1.36
Tristão F. S. M., Rocha F. A., Carlos D., Ketelut-Carneiro N., Souza C. O. S., Milanezi C. M., et al. (2017). Th17-inducing cytokines IL-6 and IL-23 are crucial for granuloma formation during experimental paracoccidioidomycosis. Front. Immunol. 8, 949. doi: 10.3389/fimmu.2017.00949
Turissini D. A., Gomez O. M., Teixeira M. M., McEwen J. G., Matute D. R. (2017). Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 106, 9–25. doi: 10.1016/j.fgb.2017.05.007
Urban C. F., Reichard U., Brinkmann V., Zychlinsky A. (2006). Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 8, 668–676. doi: 10.1111/j.1462-5822.2005.00659.x
Valeri M., Raffatellu M. (2016). Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 74, ftw111. doi: 10.1093/femspd/ftw111
van de Veerdonk F. L., Gresnigt M. S., Kullberg B. J., van der Meer J. W., Joosten L. A., Netea M. G. (2009). Th17 responses and host defense against microorganisms: an overview. BMB Rep. 42, 776–787. doi: 10.5483/bmbrep.2009.42.12.776
Villanova F., Flutter B., Tosi I., Grys K., Sreeneebus H., Perera G. K., et al. (2014). Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Invest. Dermatol. 134, 984–991. doi: 10.1038/jid.2013.477
Viriyakosol S., Jimenez Mdel P., Gurney M. A., Ashbaugh M. E., Fierer J. (2013). Dectin-1 is required for resistance to coccidioidomycosis in mice. MBio 4, e00597–e12. doi: 10.1128/mBio.00597-12
Wang J., Arase H. (2014). Regulation of immune responses by neutrophils. Ann. N. Y. Acad. Sci. 1319, 66–81. doi: 10.1111/nyas.12445
Wang H., LeBert V., Hung C. Y., Galles K., Saijo S., Lin X., et al. (2014). C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America. J. Immunol. 192, 1107–1119. doi: 10.4049/jimmunol.1302314
Wang H., LeBert V., Li M., Lerksuthirat T., Galles K., Klein B., et al. (2016). Mannose receptor is required for optimal induction of vaccine-induced T-helper Type 17 cells and resistance to Blastomyces dermatitidis Infection. J. Infect. Dis. 213, 1762–1766. doi: 10.1093/infdis/jiw010
Wang H., Li M., Lerksuthirat T., Klein B., Wüthrich M. (2015). The C-type lectin receptor MCL mediates vaccine-induced immunity against infection with Blastomyces dermatitidis. Infect. Immun. 84, 635–642. doi: 10.1128/IAI.01263-15
Wang H., Lee T. J., Fites S. J., Merkhofer R., Zarnowski R., Brandhorst T., et al. (2017). Ligation of Dectin-2 with a novel microbial ligand promotes adjuvant activity for vaccination. PloS Pathog. 13, e1006568. doi: 10.1371/journal.ppat.1006568
Wheat L. J., Azar M. M., Bahr N. C., Spec A., Relich R. F., Hage C. (2016). Histoplasmosis. Infect. Dis. Clin. North. Am. 30, 207–227. doi: 10.1016/j.idc.2015.10.009
Wu S. Y., Yu J. S., Liu F. T., Miaw S. C., Wu-Hsieh B. A. (2013). Galectin-3 negatively regulates dendritic cell production of IL-23/IL-17-axis cytokines in infection by Histoplasma capsulatum. J. Immunol. 190, 3427–3437. doi: 10.4049/jimmunol.1202122
Wüthrich M., Gern B., Hung C. Y., Ersland K., Rocco N., Pick-Jacobs J., et al. (2011). Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest. 121, 554–568. doi: 10.1172/JCI43984
Wüthrich M., Deepe G., Klein B. (2012). Adaptive immunity to fungi. Annu. Rev. Immunol. 30, 115–148. doi: 10.1146/annurev-immunol-020711-074958
Wüthrich M., LeBert V., Galles K., Hu-Li J., Ben-Sasson S. Z., Paul W. E., et al. (2013). Interleukin 1 enhances vaccine-induced antifungal T-helper 17 cells and resistance against Blastomyces dermatitidis infection. J. Infect. Dis. 208, 1175–1182. doi: 10.1093/infdis/jit283
Wynn T. A., Ramalingam T. R. (2012). Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040. doi: 10.1038/nm.2807
Xue J., Chen X., Selby D., Hung C. Y., Yu J. J., Cole G. T. (2009). A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect. Immun. 77, 3196–3208. doi: 10.1128/IAI.00459-09
Yamasaki S., Matsumoto M., Takeuchi O., Matsuzawa T., Ishikawa E., Sakuma M., et al. (2009). C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Nat. Acad. Sci. U. S. A. 106, 1897–1902. doi: 10.1073/pnas.0805177106
Yang F., Feng C., Zhang X., Lu J., Zhao Y. (2017). The diverse biological functions of neutrophils, beyond the defense against infections. Inflammation 40, 311.23. doi: 10.1007/s10753-016-0458-4
Yao Z., Painter S. L., Fanslow W. C., Ulrich D., Macduff B. M., Spriggs M. K., et al. (1995). Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155, 5483–5486.
Zhu J., Yamane H., Paul W. E. (2010). Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489. doi: 10.1146/annurev-immunol-030409-101212
Keywords: IL-17, Neutrophils, Paracoccidioides spp., Coccidioides spp., Histoplasma capsulatum, Blastomyces spp
Citation: Puerta-Arias JD, Mejía SP and González Á (2020) The Role of the Interleukin-17 Axis and Neutrophils in the Pathogenesis of Endemic and Systemic Mycoses. Front. Cell. Infect. Microbiol. 10:595301. doi: 10.3389/fcimb.2020.595301
Received: 15 August 2020; Accepted: 13 November 2020;
Published: 14 December 2020.
Edited by:
Yong-Sun Bahn, Yonsei University, South KoreaReviewed by:
Chiung-Yu Hung, University of Texas at San Antonio, United StatesBruce Klein, University of Wisconsin-Madison, United States
Copyright © 2020 Puerta-Arias, Mejía and González. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ángel González, angel.gonzalez@udea.edu.co
 Juan David Puerta-Arias1,2
Juan David Puerta-Arias1,2  Ángel González
Ángel González