Unifying Virulence Evaluation in Toxoplasma gondii: A Timely Task
- 1SALUVET, Animal Health Department, Faculty of Veterinary Sciences, Complutense University of Madrid, Madrid, Spain
- 2Disease Control Department, Moredun Research Institute, Edinburgh, United Kingdom
- 3Department of Microbiology, University of Tennessee, Knoxville, TN, United States
Toxoplasma gondii, a major zoonotic pathogen, possess a significant genetic and phenotypic diversity that have been proposed to be responsible for the variation in clinical outcomes, mainly related to reproductive failure and ocular and neurological signs. Different T. gondii haplogroups showed strong phenotypic differences in laboratory mouse infections, which provide a suitable model for mimicking acute and chronic infections. In addition, it has been observed that degrees of virulence might be related to the physiological status of the host and its genetic background. Currently, mortality rate (lethality) in outbred laboratory mice is the most significant phenotypic marker, which has been well defined for the three archetypal clonal types (I, II and III) of T. gondii; nevertheless, such a trait seems to be insufficient to discriminate between different degrees of virulence of field isolates. Many other non-lethal parameters, observed both in in vivo and in vitro experimental models, have been suggested as highly informative, yielding promising discriminatory power. Although intra-genotype variations have been observed in phenotypic characteristics, there is no clear picture of the phenotypes circulating worldwide; therefore, a global overview of T. gondii strain mortality in mice is presented here. Molecular characterization has been normalized to some extent, but this is not the case for the phenotypic characterization and definition of virulence. The present paper proposes a baseline (minimum required information) for the phenotypic characterization of T. gondii virulence and intends to highlight the needs for consistent methods when a panel of T. gondii isolates is evaluated for virulence.
Introduction
Toxoplasma gondii is an apicomplexan parasite, globally distributed, with a heteroxenous life cycle that virtually comprises all homoeothermic animals, including humans, as intermediate hosts and felids as definitive hosts. The zoonotic, abortifacient and foodborne nature of the parasite makes toxoplasmosis a relevant public and animal health concern worldwide. Although the disease course is normally asymptomatic, immunocompromised and pregnant hosts are important risk groups. Clinical toxoplasmosis is mostly due to tachyzoite invasion and proliferation in different cells of the host, with subsequent destruction and necrotization of the infected tissues.
Toxoplasma gondii possesses a significant genetic and phenotypic diversity that have been proposed to be partly responsible for the variation in clinical presentations. Similar to genetic markers, parasite strain specific differences could be defined by “phenotypic markers” (Dardé et al., 2020). In the context of T. gondii, phenotype is related to virulence and lethality in laboratory mice, which is well defined for the three archetypal clonal types of the organism. Toxoplasma gondii type I (belonging to haplogroup 1) strains have been traditionally classified as highly virulent (100% cumulative mortality, LD100 = 1), type II (belonging to haplogroup 2) strains are considered of intermediate virulence (99-30%, LD50 ≥ 103), and type III (belonging to haplogroup 3) strains are defined as non-virulent (< 30%, LD50 > 105) (Sibley and Boothroyd et al., 1992; Su et al., 2002; Dardé et al., 2020). Likewise, most of the South American divergent strains (haplogroups 4 - 10) have been characterized as highly virulent using virulence in mice as a phenotypic marker (Grigg and Suzuki, 2003; Khan et al., 2007).
Quantitative trait locus (QTL) mapping analyses of virulence in mice of a F1 progeny, derived from sexual recombination experiments of representative strains of the three T. gondii archetypal genotypes (I×II, I×III and II×III crosses), resulted in the identification of some members of a family of serine/threonine protein kinases, found in rhoptries, as key determinants of acute virulence in mice (Saeij et al., 2006; Taylor et al., 2006; Behnke et al., 2011; Reese et al., 2011; Behnke et al., 2015). The ROP18, ROP5, and ROP16 genes encode three polymorphic rhoptry protein kinases that in a different but synergic manner contribute to the evasion of host immune response controlling the accumulation of interferon-γ induced immunity-related GTPases (IRGs) on parasitophorous vacuole (PV) membranes and subsequent parasite destruction (Behnke et al., 2012; Niedelman et al., 2012). The proven role played by these effectors motivated the interest to develop molecular typing markers based on their sequences to quickly infer the degree of virulence of T. gondii strains. Subsequent studies concluded that the allelic combination of ROP18/ROP5 is highly predictive of virulence in mice across globally distributed T. gondii strains (Dubey et al., 2014; Shwab et al., 2016). Nonetheless, there is growing evidence that this correlation is inconsistent for some genotypes (Bernstein et al., 2021; Fernández-Escobar et al., 2021).
In practice, parasite virulence assessment follows a simplistic and host-centred criterion, based mainly on the pathogenicity in mice. In most cases this is reduced to the calculation of mortality rate. A much more comprehensive definition of the virulence of T. gondii strains should combine the study of infection effects on the hosts (e.g., mortality, morbidity, immune responses dynamics) and other parameters of the parasite’s own fitness, such as success in transmission (e.g., cystogenesis, oocyst production rate) or the rate of asexual multiplication (e.g., invasion and proliferation rate) (Poulin and Combes, 1999).
Toxoplasma gondii genetic characterization methodologies have been largely standardized in the past two decades. However, phenotypic characterization procedures have not been subject to the same criticism and standardization due to its complexity. When it comes to phenotypic characterization of strains, many aspects, including environmental factors, host species, genetics of a given host species, and parasite stage can influence the outcome of the infection (Mukhopadhyay et al., 2020). There is wide evidence that long-term laboratory conditions (i.e., regular passages in cell culture or mice) can determine the biological behaviour of the parasite (Khan et al., 2009; Saraf et al., 2017; Sánchez-Sánchez et al., 2019). In addition, different hosts can have completely different infection outcomes even if challenged with the same isolate, and different infection routes can also affect the infection consequences (Oliveira et al., 2016; Yang et al., 2017; Taniguchi et al., 2018; Hassan et al., 2019; Sánchez-Sánchez et al., 2019). There was an attempt to standardize the calculation of cumulative mortality rate by Saraf et al. (2017), accepted as a consensus for virulence in mice assessment, but the fact is that this protocol has been applied in very few publications (Costa Viegas de Lima et al., 2019; Fernández-Escobar et al., 2020; Uzelac et al., 2020; Fernández-Escobar et al., 2021). Current animal welfare policies, which strictly frame scientific research, aim to minimize the use of laboratory animals and to refine the experiments. In the published scientific literature, there is a lack of concordance in the biological parameters measured as well as in the experimental conditions such as doses, infection routes or duration of the study. Consequently, it is difficult to derive general conclusions and to make comparisons of virulence of isolates presented in different studies, and it becomes evident that an in-depth review of methodologies is needed.
In Vivo Models for Virulence Assessment: State and Limitations
Animal models have been widely used to characterize T. gondii virulence and host responses to the infection by this parasite. Small rodents are natural hosts of T. gondii and likely play a major role as intermediate host for transmission of T. gondii infection. Most reported studies used laboratory mice as animal models due to their relative ease for handling and management. Domestic animals such as sheep, pig and chickens were also used mainly to assess host responses to parasite infection.
Current Normalized In Vivo Mouse Method Based on Mortality Ratio
Until now, mortality in mice has been considered the main parameter for the virulence evaluation of T. gondii strains, and it was established as the ratio between casualties and the number of infected animals challenged with the strain under study. Different subspecies of mouse have been used for T. gondii infection in vivo modelling, such as Swiss Webster, CD-1, C57BL/6, BALB/c or Kunming strains, among many others (Wang et al., 2013a; Taniguchi et al., 2018; Fukumoto et al., 2020; Uzelac et al., 2020). Toxoplasma gondii infection has been widely studied using cell-culture derived tachyzoites (or zoites grown in the mouse peritoneal cavity) that are intraperitoneally (IP) or subcutaneously (SC) inoculated into naïve laboratory mice (Howe et al., 1996). Despite not constituting a natural infection route, this model has the advantages of reproducibility, ease of inoculation, and accurate administration of challenge dose (Sibley et al., 1999). However, variants of this procedure using other parasite stages have been also implemented, including IP injection of bradyzoites contained in tissue cysts (Taniguchi et al., 2018; Gatkowska et al., 2019), or per os (PO) inoculation of tissue cysts (McLeod et al., 1984; Khan et al., 2007; Taniguchi et al., 2018; Arcon et al., 2021) and oocysts (Yang et al., 2017; Chiebao et al., 2021). These variants are inherently less reproducible due to the variable bradyzoite content of a given tissue cyst, or the difficulties in guaranteeing the oral dosage, as well as less feasible due to the complexity of oocysts production; but on the other hand, oocyst- or bradyzoite-induced infections are much more representative of what occurs in nature (Sibley et al., 1999). Consistent with the T. gondii life cycle, oocyst-mediated infections are known to be more pathogenic than bradyzoite- and finally, tachyzoite-induced infections (Dubey et al., 1977; Dubey et al., 1981; Dubey, 2006; Saraf et al., 2017). Logically, the doses tested (number of parasites inoculated) vary greatly between experimental designs depending on the parasite life cycle stage used, but they also do so between experiments that use the same parasite stage. All these mentioned factors (the route of inoculation, the parasite stage, the dose, and the host species or even subspecies) along with the number of passages in mice or cell culture, have been demonstrated to drastically affect the degree of parasite virulence (Saraf et al., 2017). Currently, there is a greater awareness that it is important to keep passages of evaluated isolates low before they become lab adapted, but the use of strains maintained for a long time under laboratory conditions (“laboratory strains”) or for which the passage number is not known still remains widespread in literature (Khan et al., 2014). Therefore, despite the numerous studies published on virulence evaluation (Supplementary Table S1), the large diversity of conditions and methodologies implemented, with heterogeneous interpretations and fragmented data, makes it difficult to draw conclusions about the real T. gondii population structure in terms of virulence.
After initial attempts for normalization (Su et al., 2002; Taylor et al., 2006), the only available standard operating procedure for the evaluation of cumulative mortality rates was recently published (Saraf et al., 2017). According to the authors, cumulative mortality rate calculation implies the use of outbred mice (e.g., Swiss Webster [SW] or CD-1 mouse strains), at least three consecutive doses of IP inoculated tachyzoites, and the recording of casualties among those successfully infected animals by day 28 post-inoculation (dpi). It should be pointed out that in some contexts, animal welfare regulations prevent getting ethical approval when mouse assays include several inoculation groups. In most publications, mortality rate in mice is the only parameter evaluated, which implies an overly simplistic and narrow view of virulence. Until Saraf’s publication, there was no consensus in the literature about how the mortality rate in mice should be calculated, so it is frequent to find lethality estimations/assumptions based on animal casualties during isolation procedures (Pena et al., 2006; Clementino Andrade et al., 2013; Shwab et al., 2016; Vilares et al., 2017), and even valuable attempts have been made to quantify the parasites in the inoculum (Mercier et al., 2010). Despite being a substantial attempt to standardize the procedure, some limitations should be pointed out. A parameter originally valued in the strain’s virulence assessment was the median lethal dose (LD50; Probit tests: https://probitanalysis.wordpress.com/2016/07/07/first-blog-post/; Finney, 1971; Shittu et al., 2020), especially since the discovery of absolute lethal doses (LD100) of a single parasite in the case of the strains related to haplogroup 1 (Sibley and Boothroyd, 1992; Su et al., 2002; Salman et al., 2021). However, this calculation has fallen out of favour, similarly to duration of survival post infection analyses (Wang et al., 2013a; Costa Viegas de Lima et al., 2019). Accurately determined, these parameters could offer new insights into dose-dependency and infection dynamics.
Phenotypic Diversity of the Global Toxoplasma gondii Population
The assessment of virulence in mice for a large number of T. gondii strains worldwide is contained in literature. The present section aims to critically examine the available phenotypic data, defined as mortality in mice, from T. gondii isolates worldwide, trying to provide an overview of the virulence profiles of T. gondii populations in the different continents. The PubMed database (https://pubmed.ncbi.nlm.nih.gov/) was searched combining the terms “Toxoplasma gondii”, “virulence characterization” and “pathogenicity”; 2644 investigations published until December 2021 were found. Only T. gondii isolate virulence studies involving assays measuring tachyzoite stage induced mortality in mice were considered, while studies focusing on in vitro assays or other parasite stages (oocysts or bradyzoites) were excluded. Infections based on these other stages account for only a minority of investigations, which is further limited by methodological variations (see previous section). Other rodent in vivo models (e.g., rats) were not included. Data from laboratory strains (e.g., RH, GT1, CTG, ME49, PRU, VEG) were not covered (except when obtained shortly after strain isolation), in order to better describe the real phenotypic diversity of the T. gondii populations globally. In total 62 studies were selected, involving 311 isolates (see Supplementary Table S1). Isolate IDs, host of origin, available genetic (e.g., ToxoDB#, ROP18/ROP5 alleles) and geographical (e.g., country, continent) features, experimental conditions for mouse mortality assessment, and other evaluated parameters, were extracted for each reference. Due to the heterogeneity of data presentation, mortality rate was re-calculated in each reference (when possible) based on Saraf et al. (2017) criteria (analysis of three sequential inoculation dosages, with the lowest dose resulting in only partial infection of the animals), and isolates were classified into “Highly virulent” (100% mortality rate), “Intermediate virulent” (99–30%) and “Non-virulent” (<30%) categories according to Su et al. (2002). Calculations considering 4 or more doses when available were also included. In the Figure 1, only studies in which mortality in outbred mice was assessed implementing at least 3 doses of IP or SC inoculated tachyzoites (serial 10-fold dilutions from 1 to 106 parasites/mouse), and 28-dpi animal monitoring were filtered (Saraf et al., 2017) (33 studies; 204 isolates), in order to map the T. gondii virulence diversity more accurately by increasing methodological homogeneity. Data is geographically biased; it is worth noting the sizable proportion of South American (including Caribbean) isolates (n=130) compared to the scarcity of those from Europe (n=17), North America (n=22) or Asia (n=34) and the extremely poor representation of the African continent (n=1). Generally, the T. gondii population in South America is mostly considered highly virulent, in association with a notably diverse, endemic genetic structure (Shwab et al., 2014; Shwad et al., 2018). However, present data show a proportion of South American highly virulent isolates that are not more common than those found in North America (37% vs. 45%) (Figure 1A). In both regions, almost one in four strains proved to be non-virulent (22-23%). In contrast, European isolates are broadly considered non-virulent, in relation with the prevalent genetic clonality found in the continent (involving mainly genotypes II and III) (Shwab et al., 2014; Shwab et al., 2018). Nonetheless, present data showed a not insignificant percentage of highly virulent strains in Europe (18%) (Figure 1A). Interestingly, the global/worldwide T. gondii virulence in mice distribution displays a certain balance between highly-, intermediate- and non-virulent strains (33%, 35% and 32%, respectively) (Figure 1A). Collected virulence data distribution within the different hosts of origin is also strongly biased; the vast majority of isolates assessed were obtained from domestic animals (n=129/204), while only 18 were isolated from wild animals (Figure 1B and Figure S1). Ignoring this fact, phenotypic data extracted showed an almost equal proportion of the different virulence degrees in strains infecting animals. In detail, isolates from domestic animals seem to be slightly less virulent than isolates from wildlife, which could correspond with the apparent more virulent character of “wild” strains vs. “domestic” strains described in North America (Jiang et al., 2018). Data fromhuman isolates (n=57/204) is partially biased as most of them came from clinical cases, which could explain that only 5% of the strains evaluated showed a non-virulent profile, while more than a half presented a highly virulent character (Figure 1B). Nevertheless, although a certain phenotype is probably being selected in isolates of human origin, it should be pointed out that there is no clear link between virulence in mice and in humans. On the other hand, researchers deal with an extra issue when it comes to human isolates due to the uncertainty of the geographical origin of some infections, with importation or migration related cases, only solvable by comprehensive epidemiological surveys.
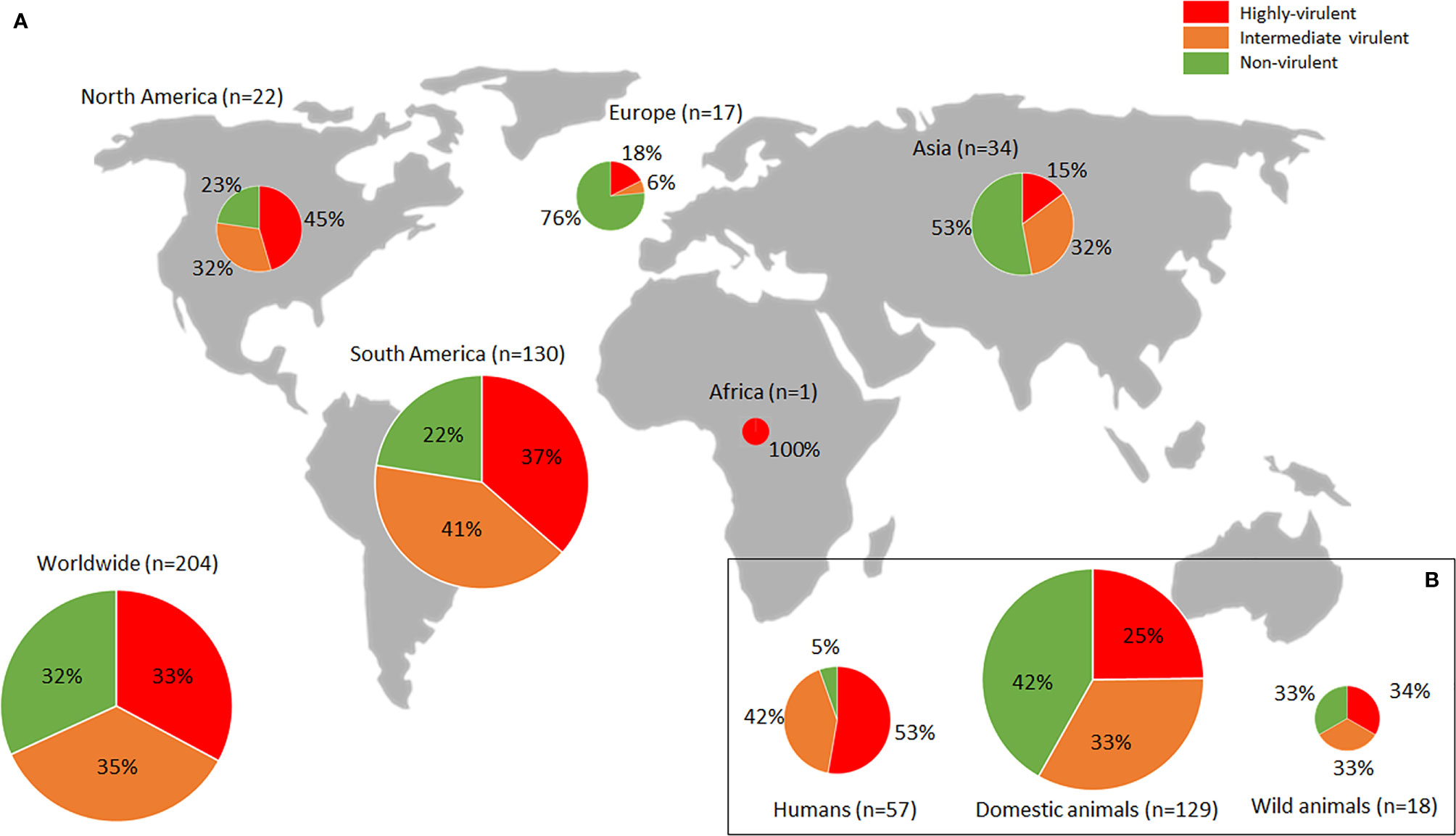
Figure 1 Worldwide Toxoplasma gondii phenotypic diversity distribution. (A) Proportion of highly-, intermediate- and non-virulent isolates (based on mortality rates in mice) found in each continent. (B) Figures observed pertaining to infected hosts (humans, domestic or wild animals). Sizes of pie charts correlate with total number of isolates (n). Only studies in which mortality in outbred mice was assessed, implementing at least 3 doses of IP or SC inoculated tachyzoites (serial 10-fold dilutions from 1 to 106 parasites/mouse), and 28-dpi animal monitoring (Saraf et al., 2017) were considered. Data used are compiled in Supplementary Table S1.
Present data collection probably provides a view of T. gondii worldwide population phenotypic diversity more realistic than previously described (Shwab et al., 2016; Shwab et al., 2018) based mainly on mortality rates calculated during the isolation process. The bioassay of digested tissue samples in mice cannot be considered as a method for virulence evaluation due to the lack of parasite quantification or control of inoculum composition, the assessment of a single “dose” and the widespread use of a very low numbers of mice (typically 3). Nonetheless, it should be noted that since standardized models are relatively expensive assays as they involve the use of a higher number of experimental animals (quite restrictive and regularized), it is common for the strains subjected to these experiments to have been pre-selected due to their more disparate behavior, which may result in an extra bias specially in underrepresented continents such as Europe. Data extracted from standardized mouse models (Supplementary Table S1) identified 40.1% of highly virulent strains in the southern hemisphere, while this accounted for 25.3% in the northern hemisphere. This strongly contrasts with figures described in the Americas by Shwab et al. (2018), where 61% of 427 isolates from South/Central America were categorized as highly virulent in mice, while only 7% of 193 isolates from North America were found to be virulent.
Other In Vivo Experimental Models
Little is known about how parasite virulence in mice extrapolates to other relevant hosts. Since the first characterization studies of T. gondii strains, there has always been an interest in associating virulence in mice with an outcome in human clinical infections (Sibley and Boothroyd, 1992). However, there are only a few comparative studies available, which indicate that drastically different infection outcomes occur in different hosts challenged with the same isolate (Taniguchi et al., 2018; Hassan et al., 2019; Sánchez-Sánchez et al., 2019; Xia et al., 2020).
Rodents
Efforts have been made to investigate if other rodents can be used as animal models to characterize virulence in T. gondii. In contrast to laboratory mice, which are highly susceptible to toxoplasmosis (Zenner et al., 1998), rats have been repeatedly demonstrated to be resistant to acute infection, remaining asymptomatic or even resistant to chronic infection by preventing tissue cysts formation in the case of some rat breeds (Sergent et al., 2005; Cavaillès et al., 2006). Therefore, it is considered that the laboratory rat infection model represents a better system for studying the immune resistance of humans to T. gondii infection than the mouse model (Loeuillet et al., 2019). In an experiment carried out in the USA, chronic toxoplasmosis was induced in Sprague Dawley female rats after oral inoculation with oocysts of 11 T. gondii strains of seven different genotypes. After 60 days, distribution, location and size of tissue cysts and pathological lesions in their brains were assessed by immunohistochemistry to investigate whether the parasite genotype could affect these virulence parameters (Dubey et al., 2016). Interesting differences between strains were found and some aspects of the parasite infection dynamics in rats were clarified. In a French study, Lewis (LEW) and Fischer (F344) rats were inoculated intraperitoneally with 107 tachyzoites of the GUY008-ABE (haplotype 5, ToxoDB # unknown) or the Prugniaud (PRU; haplotype 2, ToxoDB #3) strains; weight loss, survival time, parasite dissemination and histological lesions were assessed. Resistant LEW and susceptible congenic LEW.BN.c10-F rat infections were also carried out to study the number of brain cysts developed by each strain 2 months after inoculation. Complementarily, parasite proliferation was evaluated in vitro in primary rat peritoneal macrophages. Final findings in this model demonstrated the hypervirulent phenotype of the South American (French Guiana) GUY008-ABE strain in contrast to the avirulent profile of PRU laboratory strain (Loeuillet et al., 2019). Recently, guinea pigs were also proposed as a suitable model for human congenital toxoplasmosis in experimental infections of pregnant guinea pigs being administered 10, 100 or 500 oocysts of T. gondii strain ME49 at different time points during gestation (Grochow et al., 2021). The impact of the dose, the duration of infection and the gestational stage at infection on the seroconversion, survival rate of dams, fate of the offspring, parasite loads in various offspring tissues and organs and the integrity of the brains of the offspring were assessed. This model, together with those developed in pregnant mice (Liu et al., 2013; Müller et al., 2017; Sánchez-Sánchez et al., 2019), are examples of how pregnant rodent models are considered good options to study human congenital toxoplasmosis due to the haemochorial placentation that primates (including humans) and rodents have in common.
Sheep
Among domestic animals, sheep are sensitive to toxoplasmosis. Thanks to the development of standardized experimental sheep infection models, the knowledge of the pathogenesis of ovine toxoplasmosis has increased considerably in recent years (Benavides et al., 2011; Castaño et al., 2014; Castaño et al., 2016; Benavides et al., 2017). A recent study (Sánchez-Sánchez et al., 2019) compared the in vivo phenotype of a recently obtained type II isolate (TgShSp1; ToxoDB #3) with the type II reference ME49 (ToxoDB #1) in pregnant and non-pregnant mice, as well as in pregnant sheep. Although the in vivo non-pregnant mouse infections and complementary in vitro assays indicated that the laboratory isolate ME49 was clearly more virulent than TgShSp1, there were no differences between these two isolates for fetal/lamb mortality, lesions, or number of T. gondii-positive lambs when pregnant ewes were challenged with oocysts. Reviewing the literature revealed that virulence assessments in sheep models are scarce.
Pig
Pigs are also considered sensitive to toxoplasmosis. Although several experimental attempts to reproduce congenital toxoplasmosis in pigs have been reported, these have not been consistently successful (Moller et al., 1970; Dubey et al., 1990; Jungersen et al., 2001; Basso et al., 2015; Basso et al., 2017). The pathogenicity of different T. gondii strains of diverse host origin was compared after intravenous (IV) inoculation of 104 tachyzoites in 7-week-old pigs (Jungersen et al., 1999), assessing parameters such as rectal temperature, weight loss, histopathological lesions in several organs, IgG and IgM antibody levels, haptoglobin and TNF-α serum levels, among others. Later, an experimental infection in pregnant minipigs inoculated intravenously with 3 × 104 tachyzoites of different strains (Jungersen et al., 2001) showed marked differences in acute illness, associated abortions, and evidence of the parasite in the gilts or their foetuses. BR-1 mini pigs infection was proposed as a suitable model for human toxoplasmosis (Miranda et al., 2015), with groups of animals intramuscularly inoculated with 107 RH tachyzoites or orally infected with 660 ME49 tissue cysts, where clinical signs, parasitaemia, parasite burden in diverse organs, histopathological lesions, haematology or serum biochemistry, among other parameters, were evaluated. In another study (Taniguchi et al., 2018) micro minipigs were dosed orally with 900 tissue cysts of the Japanese isolate TgCatJpGi1/TaJ, previously classified as type III (ToxoDB# not provided), resulting in no clinical signs of infection. When tachyzoites of the same isolate were IP inoculated into laboratory mice no clinical signs of infection were observed either but 100-80% lethality was found in mice orally inoculated with low doses (100, 50 and 10) of tissue cysts of this isolate. Furthermore, a recently obtained Japanese isolate (TgCatJpOk4) showed notable mortality (60%) and morbidity (80%) rates when micro minipigs were IP inoculated with 107 tachyzoites (Taniguchi et al., 2019), which is in concordance with its previously demonstrated 100% mortality in mice (Fukumoto et al., 2020). In an interesting comparative study (Xia et al., 2020), the virulence of a type PRU (ToxoDB #3) Chinese isolate (TgPIG-WH1) obtained from an aborted piglet was assessed in mice and pigs. TgPIG-WH1 was less virulent than the RH and ME49 reference strains in mice (35%, 100% and 80% mortality rate, respectively), but showed a strong pathogenicity in pigs with higher mortality, more severe pathological lesions, and higher IgG levels in serum in comparison to infections with the ME49 strain. Overall, T. gondii virulence assessments in pig models are not standardized, and comparison of results from different studies is difficult.
Chicken
Apart from experimental infections of sheep or pigs, other in vivo models could be found in the literature. Although chickens are considered resistant to clinical toxoplasmosis, and only a few reports of clinical toxoplasmosis are available worldwide, the fact that chickens are one of the most important meat resources for humans and the high seropositivity rates found in some areas, justifies an interest in developing infection models for this species (Dubey, 2021). Experimental infections of 7 to 28-day-old Broiler chickens by IP injection with 108 tachyzoites of the mouse-virulent RH and JS strains were conducted (Wang et al., 2014). Clinical signs, survival time, parasite detection in pooled tissues and histopathological lesions were evaluated. The mortality rate in 7-day-old chickens infected with the JS strain (100%) was higher than with the RH strain (70%), but the infections did not produce relevant clinical manifestations in the rest of the challenged animals, and similar results were found for the other parameters evaluated for both strains.
Complementary Approaches on the Determination of Virulence in Toxoplasma gondii Field Strains
Non-Lethal Parameters
Current animal welfare policies aim to minimize the use of laboratory animals, replace them when possible, and to refine the experiments to reduce animal suffering. In this regard, important improvements can be introduced for the evaluation of virulence using the mouse model by assessing additional non-lethal parameters involved in virulence (Table 1). A much more comprehensive view of the virulence of T. gondii strains should combine the study of infection effects on the host (e.g., lethality and tissue lesions) with other aspects inherent to the parasite’s own fitness. The oocyst production rate is a direct indicator of the parasite’s transmission success, and it has been studied in several publications in the past (Dubey et al., 2002; Dubey et al., 2003). The demonstrated loss of the capacity to produce oocysts, as a consequence of successive passages in cell culture or mice, is considered as a loss of virulence (Frenkel et al., 1976; Lindsay et al., 1991; Dubey et al., 1999). In particular, a study of oocyst shedding in domestic cats of several genetically diverse T. gondii strains from French Guiana showed difference of fecundity among them, which may potentially affect their transmission (Khan et al., 2014). However, the use of cat models has long been controversial and currently is not accepted by the wide scientific community, in ethical terms. Another useful way to measure parasite transmissibility is to evaluate parasite cystogenesis, the capacity and capability of T. gondii to form tissue cysts. The central nervous system (CNS) is the tissue par excellence to study the development of cysts during the chronic phase of the infection but the T. gondii tropism towards immune privileged organs also involves ocular or muscle tissues (Jiang et al., 2020; Yang et al., 2020; Dubey, 2021). Cysts can be quantified and even measured (diameter) by microscopic observation of fresh/unstained (Dubey et al., 2012) and immunostained fixed brain sections or brain homogenates (Masatani et al., 2020; Wang and Sibley, 2020). An indirect way to quantify the presence of cysts in the mouse brain is to measure the parasite burden in the CNS from 3 weeks pi by quantitative PCR (Fernández-Escobar et al., 2020; Fernández-Escobar et al., 2021; Salman et al., 2021). A literature review (Watson and Davis, 2019) compiled and described T. gondii experimental latent infections in murine models for the quantification of brain cysts, in order to find key factors on data variance and to propose optimized protocols; however, the conclusions were not as informative as expected because of the fragmentation of data gathered. Some non-archetypical strains showed decreased potential to develop into bradyzoites in vitro, bradyzoites with decreased resistance to pepsin treatment, and formation of a lower number and smaller tissue cysts, associated with a limited oral transmission (Fux et al., 2007).
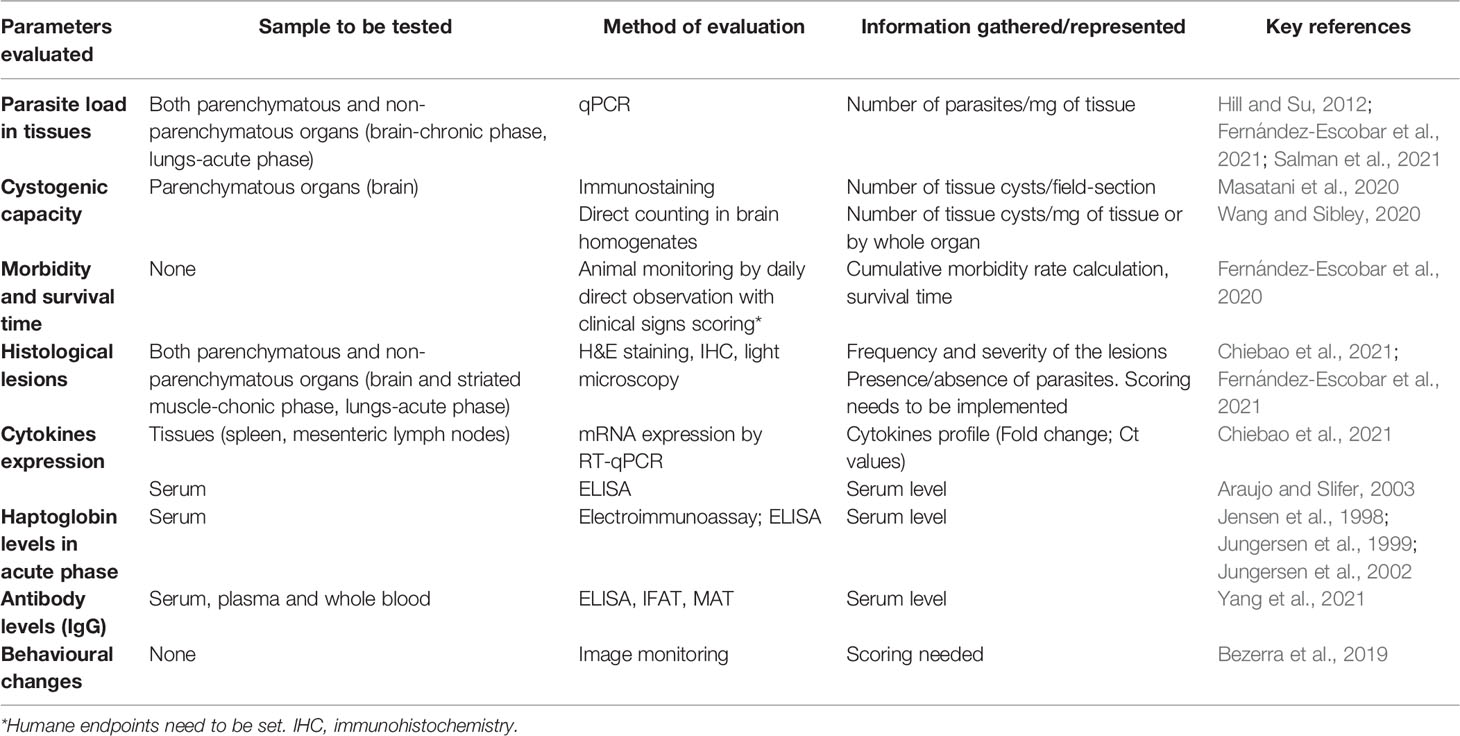
Table 1 Summary of non-lethal parameters for the assessment of in vivo virulence of Toxoplasma gondii strains (ordered by reliability and informativeness).
As a reflection of virulence in vivo, parasite burdens in different organs have been determined after short times post-inoculation in many studies. Apart from CNS, parasite burdens in lung, spleen, kidney, liver, ocular tissues, mesenteric lymph nodes, diaphragm or even blood has been also studied in literature (Zenner et al., 1998; Djurković-Djaković et al., 2012; Hill and Su, 2012; Hill et al., 2012; Wang et al., 2013b; Hamilton et al., 2019; Fernández-Escobar et al., 2020; Fernández-Escobar et al., 2021). Real-time PCR was used to monitor the distribution of T. gondii in different murine tissues during the infection with 102 or 106 tachyzoites (IP) of the RH strain, or 10 cysts (PO) of the ME49 strain (Djurković-Djaković et al., 2012); this study concluded that the level of parasite burden in the lungs seemed to be critical for mice survival/parasite virulence. This result agrees perfectly with what was observed previously (Loeuillet et al., 2019), where pneumomegaly, and the greater parasitic load and tissue destruction in the lungs were strongly associated with the hypervirulence of the non-canonical GUY008-ABE isolate (haplogroup 5, ToxoDB # unknown). On the other hand, some studies pointed out ocular tropism as a virulence related trait, with a more frequent parasite DNA detection in ocular tissues from mice infected with more virulent strains (Hamilton et al., 2019; Chiebao et al., 2021; Fernández-Escobar et al., 2021). These findings are in agreement with data from human ocular toxoplasmosis in Brazil, where lesions in the retina are the most common clinical manifestation (Dubey et al., 2012). From a practical point, all these parasite distribution assays could be carried out in parallel to mortality rate evaluation, by selecting a dose of interest for tropism evaluation (Fernández-Escobar et al., 2021). Histological and immunohistochemical examinations for describing pathological lesions in tissues after T. gondii infection have been carried out in several studies, and even though they are considered essential to demonstrate the definitive cause-effect in clinical diagnostic reports, for virulence assessment this technique has less discriminatory power (Yang et al., 2017; Hamilton et al., 2019; Fernández-Escobar et al., 2020; Chiebao et al., 2021).
Another interesting approach to assess differences in virulence of T. gondii strains is to study differential expression of cytokines and other markers of the immune response during infection. The levels of mRNA expression in spleen and mesenteric lymph nodes of IFNγ, IL-12, T-cells surface markers CD8, CD4 and CD25, as well as the receptor adapter MyD88 and the chemokine receptor CXCR3, were evaluated and compared during mice infection with the archetypal non-virulent M4 strain (type II variant, ToxoDB #3), a non-archetypal virulent strain (genotype BrI, #6) and a non-archetypal intermediate virulent isolate (genotype BrIII, #8) (Chiebao et al., 2021). In accordance with previous literature, the authors associate a strong and acute Th1 immune response (IFNγ, IL-12 higher levels) with highly lethal strains, whereas a longer and modulatory Th2 immune response was triggered by moderately virulent isolates (TLR-MyD88, CXCR3 expression). Similarly, serum levels of IFNγ, TNF-α, IL-12 p40, IL-10, IL-6, IL-4, and IL-2 were evaluated as virulence markers for the severity of toxoplasmic encephalitis (Araujo and Slifer, 2003).
The cumulative morbidity rate or the severity of clinical signs are additional non-lethal virulence parameters considered in literature. It requires trained personnel, cohesion in the evaluation criteria and, at best, a normalized clinical scoring criterion (Sánchez-Sánchez et al., 2019; Fernández-Escobar et al., 2020; Chiebao et al., 2021; Fernández-Escobar et al., 2021; Pena et al., 2021; Salman et al., 2021). The main clinical signs associated with T. gondii infections in mice are (in increasing order of severity) piloerection (ruffled coat), ascites, loss of body weight/condition, prostration, dyspnoea, and neurological signs such as motor incoordination, head tilting, or circling motion. The cumulative morbidity could be calculated based on the number of mice that present any clinical sign after inoculation divided by the number of infected mice (Fernández-Escobar et al., 2020). Another strategy to quantify such a subjective aspect is the representation of the daily scoring mean value variation and standard deviation according to the clinical findings; both aspects were successfully evaluated for mice experimentally infected with type II (#3), BrI (#9) or BrIII (#8) genotypes elsewhere (Chiebao et al., 2021).
In some Danish studies, levels of the acute-phase reactant haptoglobin in serum were considered for evaluation as an additional virulence parameter along mice infection with T. gondii (Jensen et al., 1998; Jungersen et al., 1999; Jungersen et al., 2002). Apparently, strains that caused more severe body weight loss also induced the highest serum haptoglobin and specific anti-T. gondii antibodies concentrations during the acute phase of the infection. Weight loss and anti-T. gondii IgG response have been used in other studies although, similarly to haptoglobin levels, not extensively, probably due to the excessive handling of animals required and a weak correlation with mortality rates (Kannan et al., 2010; Bezerra et al., 2019; Masatani et al., 2020; Salman et al., 2021; Yang et al., 2021). Interestingly, some studies also approached the analysis of behavioral changes in mice infected by different T. gondii reference strains, through the evaluation of learning and memory, locomotor activity, spatial working and aversion to feline odour, among other parameters (Kannan et al., 2010; Bezerra et al., 2019); however, such complex assessments require special devices to monitor mice activity. The study of behavioral changes as phenotypic markers associated with infection could be of special interest since the relationship between toxoplasmosis and human neurological disorders and psychiatric illnesses such as schizophrenia and bipolar disorder has been repeatedly demonstrated (Xiao et al., 2013; Chaudhury and Ramana, 2019; Wang et al., 2019).
Despite attempts to describe and incorporate new non-lethal virulence parameters, the main problem we face is the lack of homogeneity in the selection and in the experimental conditions between studies. Time points of infection, procedures and analytical methods vary completely between different investigations (Supplementary Table S1). However, despite this lack of consensus protocols, it is undeniable that there is a growing interest, as shown within the literature, in going beyond the mere mortality rate calculation in the assessment of virulence for different T. gondii strains using the mouse model.
An additional approach to virulence degree prediction can be achieved based on molecular analyses. The CS3 locus has been described previously as a highly predictive marker of mortality in mice challenged with T. gondii isolates (Pena et al., 2008); high mortality rates associated with the type I or II alleles of the CS3 region, and low or null rates associated with the type III alleles have been reported (Pena et al., 2008; Wang et al., 2013b; Rocha et al., 2018). However, several subsequent findings contradicted such observations (Langoni et al., 2012; Rêgo et al., 2017; Fernández-Escobar et al., 2020). Currently, ROP18 and ROP5 are well-known virulence factors in Toxoplasma virulence in mice (Behnke et al., 2015; Xia et al., 2021), and their allelic combination was proposed as highly predictive of virulence in mice across globally distributed T. gondii isolates (Dubey et al., 2014; Shwab et al., 2016). As detailed in above studies, some allelic combination such as 2/2 or 4/4 has been found firmly associated with certain mortality degrees (0% or 100% lethality, respectively); however, other genetic combinations such as 3/1 or 4/3 are much less predictive, and ultimately, the combination 3/3 is the most unspecific profile due to its association with levels of mortality strongly varying from 100 to 0% (Shwab et al., 2016; Hamilton et al., 2019; Uzelac et al., 2020; Bernstein et al., 2021; Fernández-Escobar et al., 2021). In summary, although molecular tools seem useful to predict the virulence degree to some extent, additional genetic factors might be also involved. It should be taken into account that despite the large database used for these correlations at a global level (Shwab et al., 2016), with up to 240 records, it included mouse mortality data calculated at the time of strain isolation by bioassay in mice. In the light of gathered data (Supplementary Table S1), a re-assessment of genotype-phenotype correlation, considering only mice mortality data obtained from standardised mouse infection models, could yield more reliable results. A continuous collection of ROP18 and ROP5 allelic types and mouse virulence data will facilitate future studies to identify other virulence genes, and ultimately improve the prediction power of genotyping for virulence.
In Vitro Models
The current animal welfare policies not only highlight the necessity to refine the in vivo procedures but also to minimize and replace the use of laboratory animals. In this context, the use of in vitro models represents an excellent alternative for the study of intracellular organisms such as T. gondii. The in vitro models allow the study of the host cell infection process by the tachyzoite stage, namely the lytic cycle, which mimics the dissemination of the parasite during the acute phase of the infection (Black and Boothroyd, 2000). The lytic cycle of T. gondii is a tightly regulated process, which includes adhesion to the host cell, invasion, PV formation, multiplication, and egress steps (Sibley, 2010). It is important to note that in vitro experiments reflect the behavior of the parasite in the absence of selective pressures during an infection of a host, which explains that the results may easily not correspond to what occurs in an in vivo assay. In the case of T. gondii, given its enormous plasticity represented in a vast host range, in vitro experiments can provide a less biased, host-centered view, although it will always be necessary to contextualize the results (Poulin and Combes, 1999).
Proliferative stages of the parasite have been cultured in vitro employing a variety of cell culture lines (e.g., HeLa, Vero, HFF, BeWo) and primary cell cultures (Scheidegger et al., 2005; Müller and Hemphill, 2013), and among them, target cells or tissues (e.g., trophoblast and nervous cells, dendritic cells [DCs] or macrophages) should be highlighted (Guimarães et al., 2008; Dellacasa-Lindberg et al., 2011; Mammari et al., 2014; Witola et al., 2014; Barbosa et al., 2015; da Silva et al., 2017; Pacheco et al., 2020). Most of publications on T. gondii implementing in vitro assays are focused on safety and efficacy assessment of potential antiparasitic drugs (Basto et al., 2017; Murata et al., 2017; Radke et al., 2018) or on demonstrating the role of different host and parasite effectors in the T. gondii lytic cycle (Camejo et al., 2014; Bai et al., 2018; Guo et al., 2019; Wang et al., 2020). In vitro models are considered also suitable first approaches to phenotypically characterize apicomplexan parasite strains (Regidor-Cerrillo et al., 2011; Dellarupe et al., 2014; Frey et al., 2016; Jiménez-Pelayo et al., 2017; García-Sánchez et al., 2019). However, only a small proportion of the publications addresses the virulence characterization of non-laboratory T. gondii isolates in vitro (Loeuillet et al., 2019; Sánchez-Sánchez et al., 2019; Bernstein et al., 2020; Uzelac et al., 2020; Fernández-Escobar et al., 2021; Salman et al., 2021).
A systematic review of literature (Contreras-Ochoa et al., 2012) compared studies that had used mouse and human glial cell cultures to determine T. gondii invasion and replication rates in these cells. The wide experimental heterogeneity found hampers drawing definitive conclusions but type II strains (ME49 [#1] and PRU [#3]) seem to be less invasive of nervous system-derived cells than type I (RH [#10] and BK [genotype # unknown]) strains. Several publications have characterized some in vitro virulence parameters of the par excellence T. gondii strain RH in different cell types, and it has been extensively used as an experimental control. However, the RH strain has been maintained and passed through lab mice or cell culture for several decades and its biological behaviour has drastically changed (Khan et al., 2009). Comparative studies of other different laboratory strains have been also published (Appleford and Smith, 1997; Diana et al., 2004; Fux et al., 2007; Lambert et al., 2009; Cañedo-Solares et al., 2013; Mammari et al., 2014). Overall, it is generally claimed that type I strains present enhanced proliferation capacity and lower host immune system stimulation than type II isolates (Mammari et al., 2014). Regarding the in vitro virulence assessment of non-laboratory (field) isolates, the number of studies is relatively low; however, its use as a complement to the evaluation of virulence in mice has increased in recent years (Loeuillet et al., 2019; Sánchez-Sánchez et al., 2019; Bernstein et al., 2020; Fukumoto et al., 2020; Uzelac et al., 2020; Fernández-Escobar et al., 2021; Salman et al., 2021).
Reviewing the T. gondii literature revealed that in vitro phenotypic evaluation is mostly based on parameters such as parasite invasion rate, proliferation kinetics, tachyzoite yield (TY), or on the assessment of plaque formation, tachyzoite-bradyzoite conversion and spontaneous cyst-formation (Regidor-Cerrillo et al., 2011; Li et al., 2014; Loeuillet et al., 2019; Sánchez-Sánchez et al., 2019; Uzelac et al., 2020; Fernández-Escobar et al., 2021; Salman et al., 2021). Data on the use of the main virulence parameters in recent remarkable investigations are summarized in Table 2. Other interesting but less extended in vitro parameters can be found in the literature. For example, in a Chinese study (Zhang et al., 2013) activation and polarization of macrophages after infection with a highly virulent or a mildly virulent ToxoDB genotype #9 strains were studied in primary BMMφs and peritoneal Mφs from mice and Raw 264.7 cells (mouse macrophages), showing completely opposite phenotypes. In a complex study (Barragan and Sibley, 2002), in vitro migration of 20 T. gondii strains was measured using a transwell system based on polarized Madin-Darby canine kidney (MDCK) and human foreskin fibroblast (HFF) cell monolayers co-cultures, as well as in HFF cell monolayers covered with agarose. The transwell system consisted of the upper chamber with polarized MDCK and HFF cell monolayer on a filter and the lower chamber with a HFF cell monolayer. Freshly released parasites were added to the upper chamber of the transwell system and migration of parasites to the lower chamber HFF monolayer was quantified. Both approaches revealed a superior migratory capacity of Type I overtype II and type III strains. These in vitro assays need to be tested with more T. gondii strains to confirm their significance in predicting parasite virulence in the future.
One of the key issues is that there is no consensus in experimental conditions (e.g., multiplicity of infection [MOI], number of passages, time points for infection, cell culture lines, methods of analysis, among others), yielding non-comparable results (Table 2) (Contreras-Ochoa et al., 2012).
Ex Vivo Models
In vitro assays offer only a partial view of the processes triggered during infection, since the interaction between the different cell types that shape an organ or the immune system response, for example, are not reflected. Meanwhile, ex vivo models that preserve the cellular architecture and function of certain organs (e.g., placenta) during the in vitro study period might be an interesting alternative (Fry et al., 2019). Noteworthy studies on the proliferation of T. gondii in explants and organoid-derived monolayers have been carried out (Scheidegger et al., 2005; Robbins et al., 2012; Ander et al., 2018; Holthaus et al., 2021). Practical applications of placental explants in the study of host (human)-parasite (T. gondii) interactions ex vivo were reviewed recently (Pastor-Fernández et al., 2021), but until now no study has been focused on virulence evaluation of T. gondii strains using ex vivo approaches. An investigation that deserves attention was carried out by Robbins et al. (2012), in which the preferential infection of different structural components of the placenta and the proliferation capacity in a human placental explant model were evaluated for three reference strains of T. gondii of different genetic types (I, II, and III); nevertheless, no statistical significance was observed.
PROPOSED METHODOLOGY FOR VIRULENCE ASSESSMENT OF Toxoplasma gondii
Given the high heterogeneity within studies observed in the previous sections, it is clear that there is a need to harmonize criteria and methodologies when field strains are being subjected to virulence evaluation. In the present section, taking into consideration bioethical and practical aspects, we aimed to provide an easy-to-follow workflow (Figure 2) of reliable assays that may yield repeatable and comparable results.
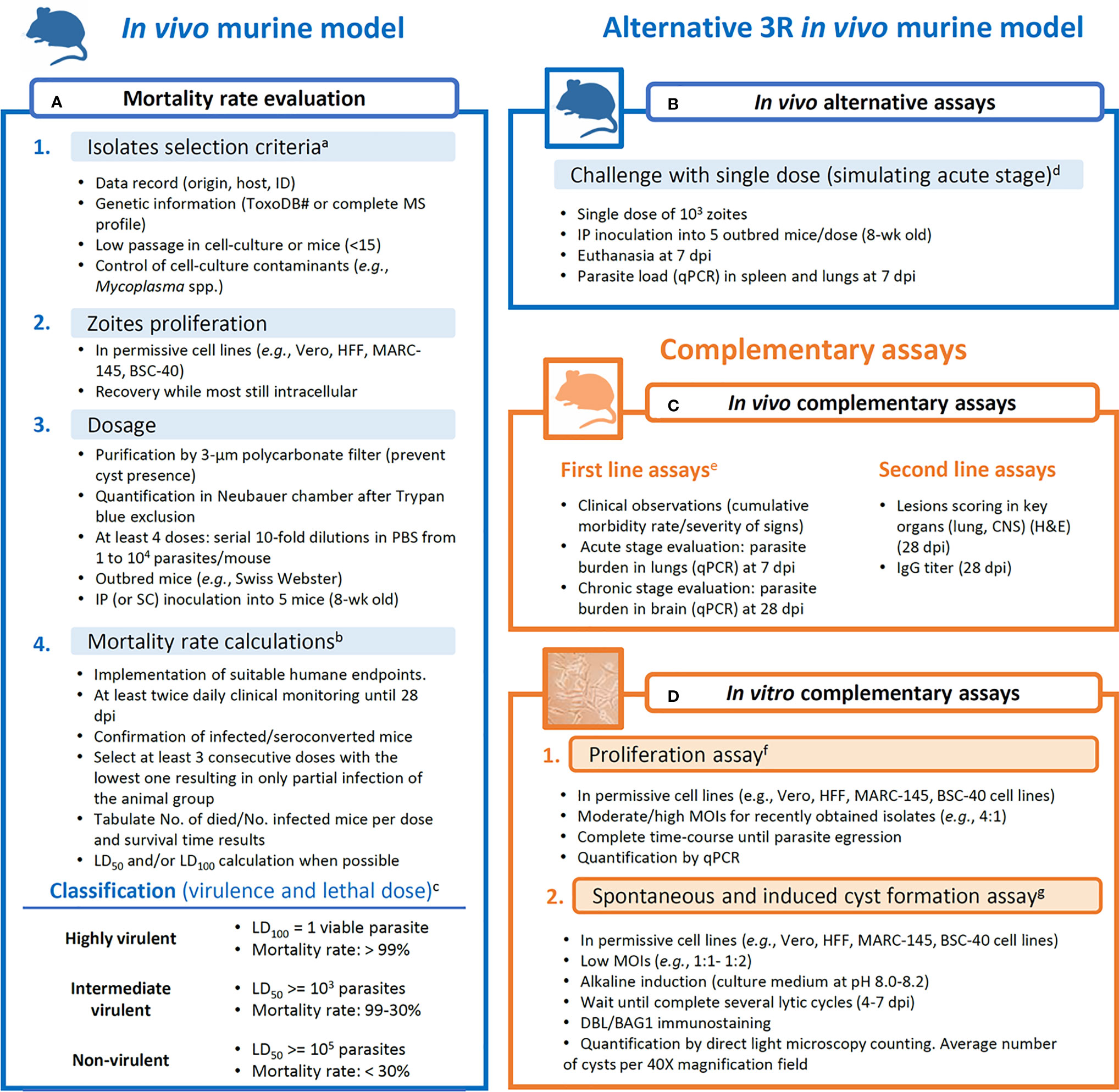
Figure 2 Proposed workflow for assays aiming at obtaining data for the evaluation of the virulence degree of Toxoplasma gondii strains. (A) Mortality rate calculation is based on Saraf et al. (2017), with complementary analysis like lethal dose calculations and survival time reports. (B) Alternative assays to the mortality rate evaluation. (C) Complementary in vivo assays are divided into first line (more informative) and second line (less informative) parameters/procedures. (D) In vitro assays are proposed as reliable complementary procedures that limit the inter-host variability. aROP18/5 allele combination and CS3 profile are considered to have predictive value for the virulence in mice (Pena et al., 2008; Shwab et al., 2016); isolates with low passage history (Khan et al., 2014). bMorbidity scoring and humane endpoint (Pena et al., 2021); tabulation of data (Jiang et al., 2020); LD50 calculations (Probit tests). cVirulence classification (Su et al., 2002; Dubey et al., 2014; Saraf et al., 2017). dParasite tropism and quantification (Hill and Su, 2012; Fernández-Escobar et al., 2020). eClinical scoring, weight loss, parasite load and histological lesions scoring in additional inoculation groups (103 tachyzoites/mouse) euthanized at 7 or 28 dpi (Fernández-Escobar et al., 2020; Fernández-Escobar et al., 2021). fProliferation evaluation (Fernández-Escobar et al., 2021). gDBL, Dolichos biflorus lectin; spontaneous conversion (Salman et al., 2021); induced conversion (Ribeiro-Andrade et al., 2019); quantification of cysts (Fux et al., 2007).
In Figure 2A, an improved and thorough version of the virulence in mice evaluation procedure described by Saraf et al. (2017) is presented. Details on isolates selection criteria or data presentation, among others, are described. As commented earlier, due to national regulations, some animal welfare policies prevent getting ethical approval for the development of mouse assays that include several inoculation groups, and alternatives need to be searched. Although the cumulative mortality approach suggested can provide a base to compare mouse-virulence results from different studies, an alternative to reduce the number of animals in the assays can be solved by selecting an alternative in vivo assay (Figure 2B). A compromise could be using a single intermediate dose inoculum (103 tachyzoites/animal) and determining parasite load in target tissues such as spleen and lung at 7 dpi. Previous studies showed that T. gondii mouse virulence is highly associated with parasite tissue burden, providing an alternative to the cumulative mortality method (Fernández-Escobar et al., 2020, Hill and Su, 2012). From this point, complementary in vivo and/or in vitro assays are desirable (Figures 2C, D). Clinical scoring, as well as parasite load and histological lesions scoring determined in key organs have demonstrated to be useful for phenotypic diversity description even between closely related isolates (Figure 2C) (Fernández-Escobar et al., 2020; 2021). On the other hand, in vitro assays are valuable tools to determine parasite proliferative capacities (proliferation rate and tachyzoite-bradyzoite conversion ability) negating the inter-host variability (Figure 2D) (Regidor-Cerrillo et al., 2011; Fernández-Escobar et al., 2021).
Concluding Remarks
In the present article, a view of the reliable data on the virulence degree of T. gondii has been conducted resulting in two main conclusions: first, virulence evaluation is a complex task that should be addressed from multiple approaches, and second, harmonized evaluation criteria and procedures are urgently needed. In addition, an apparent broken linkage between genotype and phenotype have shaken the traditional conceptualization of the virulence degree classification. Here we proposed the baseline for a comprehensive evaluation of T. gondii strains virulence by methodologies that should be accessible to most T. gondii research laboratories that, if adopted, should result in comparable results between different studies.
Many gaps remain to be solved, and some of these should be taken into consideration when planning further experiments: a) a wider (complete) definition of virulence is necessary and it should combine the study of infection effects on the host and parameters of the parasite’s own fitness; b) in combination with the conventional in vivo mouse model, complementary in vitro models are valuable strategies to describe host- independent parasite proliferative features; c) and further deep molecular analyses (e.g., whole-genome sequencing and epigenetic studies) are needed to identify new virulence factors involved in the different phenotypes (virulence degrees) observed.
Currently, all approaches need to be planned under the 3Rs (replacement, reduction and refinement) ethical principles and are compulsorily implemented along with strict animal welfare policies (e.g., Regulation [EU] 2019/1010 of the European Parliament and of the Council of 5 June 2019). Furthermore, active searches for non-lethal parameters are of major importance for in vivo research to reduce animal suffering. Steps should be taken for increasing the number of isolates evaluated by integrated models since isolates from many geographical areas remain unexplored; a considerable lack of information regarding isolates of human origin is particularly relevant, especially as T. gondii is a major zoonotic agent and an excellent example of the One Health concept.
To sum up, through the implementation of integrated methods, a thorough panel of parameters will be available to compare isolates worldwide, and this information will contribute to a risk evaluation assessment for circulating Toxoplasma strains in a given area. It is undeniable that interesting future challenges remain for researchers in the field.
Author Contributions
RC-B, MF-E, and LO-M conceived and designed the manuscript. RC-B, and MF-E extracted data. RC-B, MF-E, FK, CS, and LO-M drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
MF-E is funded by UCM-POP 2021 post-doctoral grants. RC-B, MF-E, and LO-M are part of the TOXOSOURCES consortium supported by the funding from the European Union’s Horizon 2020 Research and Innovation Programme under the grant agreement No. 773830: One Health European Joint Programme. FK is supported by funding from the Scottish Government’s Rural and Environment Science and Analytical Services Division (RESAS) and the Moredun Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor JS declared a past co-authorship with the authors CS, MFE, and LOM.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.868727/full#supplementary-material
Supplementary Table 1 | Summary of the available literature reporting virulence evaluation of Toxoplasma gondii strains using dosage of tachyzoites in a mouse model.
Supplementary Figure 1 | Proportion of Toxoplasma gondii isolates subjected to virulence evaluation in a normalized mouse model according to One Health compartment of origin. Note that no isolates obtained from environmental matrices were available. Data used are compiled in Supplementary Table S1.
References
Ander, S. E., Rudzki, E. N., Arora, N., Sadovsky, Y., Coyne, C. B., Boyle, J. P. (2018). Human Placental Syncytiotrophoblasts Restrict Toxoplasma gondii Attachment and Replication and Respond to Infection by Producing Immunomodulatory Chemokines. mBio 9 (1), e01678–e01617. doi: 10.1128/mBio.01678-17
Appleford, P. J., Smith, J. E. (1997). Toxoplasma gondii: The Growth Characteristics of Three Virulent Strains. Acta Trop. 65 (2), 97–104. doi: 10.1016/s0001-706x(97)00656-6
Araujo, F. G., Slifer, T. (2003). Different Strains of Toxoplasma gondii Induce Different Cytokine Responses in CBA/Ca Mice. Infect. Immun. 71 (7), 4171–4174. doi: 10.1128/IAI.71.7.4171-4174.2003
Arcon, N., Picchio, M. S., Fenoy, I. M., Moretta, R. E., Soto, A. S., Perrone Sibilia, M. D., et al. (2021). Synergistic Effect of GRA7 and Profilin Proteins in Vaccination Against Chronic Toxoplasma gondii Infection. Vaccine 39 (6), 933–942. doi: 10.1016/j.vaccine.2020.12.072
Bai, M. J., Wang, J. L., Elsheikha, H. M., Liang, Q. L., Chen, K., Nie, L. B., et al. (2018). Functional Characterization of Dense Granule Proteins in Toxoplasma gondii RH Strain Using CRISPR-Cas9 System. Front. Cell. Infect. Microbiol. 8, 300. doi: 10.3389/fcimb.2018.00300
Barbosa, B. F., Lopes-Maria, J. B., Gomes, A. O., Angeloni, M. B., Castro, A. S., Franco, P. S., et al. (2015). IL10, TGF Beta1, And IFN Gamma Modulate Intracellular Signaling Pathways and Cytokine Production to Control Toxoplasma gondii Infection in Bewo Trophoblast Cells. Biol. Reprod. 92 (3), 82. doi: 10.1095/biolreprod.114.124115
Barragan, A., Sibley, L. D. (2002). Transepithelial Migration of Toxoplasma gondii Is Linked to Parasite Motility and Virulence. J. Exp. Med. 195, 1625–1633. doi: 10.1084/jem.20020258
Basso, W., Grimm, F., Ruetten, M., Djokic, V., Blaga, R., Sidler, X., et al. (2017). Experimental Toxoplasma gondii Infections in Pigs: Humoral Immune Response, Estimation of Specific IgG Avidity and the Challenges of Reproducing Vertical Transmission in Sows. Vet. Parasitol. 236, 76–85. doi: 10.1016/j.vetpar.2017.01.026
Basso, W., Handke, M., Sydler, T., Borel, N., Grimm, F., Sidler, X., et al. (2015). Involvement Of Toxoplasma gondii in Reproductive Disorders in Swiss Pig Farms. Parasitol. Int. 64 (2), 157–160. doi: 10.1016/j.parint.2014.11.017
Basto, A. P., Müller, J., Rubbiani, R., Stibal, D., Giannini, F., Süss-Fink, G., et al. (2017). Characterization of the Activities of Dinuclear Thiolato-Bridged Arene Ruthenium Complexes Against Toxoplasma gondii. Antimicrob. Agents Chemother. 61 (9), e01031–e01017. doi: 10.1128/AAC.01031-17
Behnke, M. S., Fentress, S. J., Mashayekhi, M., Li, L. X., Taylor, G. A., Sibley, L. D. (2012). The Polymorphic Pseudokinase ROP5 Controls Virulence in Toxoplasma gondii by Regulating the Active Kinase Rop18. PLoS Pathog. 8 (11), e1002992. doi: 10.1371/journal.ppat.1002992
Behnke, M. S., Khan, A., Lauron, E. J., Jimah, J. R., Wang, Q., Tolia, N. H., et al. (2015). Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of Toxoplasma gondii. PLoS Genet. 11 (8), e1005434. doi: 10.1371/journal.pgen.1005434
Behnke, M. S., Khan, A., Wootton, J. C., Dubey, J. P., Tang, K., Sibley, L. D. (2011). Virulence Differences in Toxoplasma Mediated by Amplification of a Family of Polymorphic Pseudokinases. Proc. Natl. Acad. Sci. U. S. A. 108 (23), 9631–9636. doi: 10.1073/pnas.1015338108
Benavides, J., Fernández, M., Castaño, P., Ferreras, M. C., Ortega-Mora, L., Pérez, V. (2017). Ovine Toxoplasmosis: A New Look at Its Pathogenesis. J. Comp. Pathol. 157 (1), 34–38. doi: 10.1016/j.jcpa.2017.04.003
Benavides, J., Maley, S., Pang, Y., Palarea, J., Eaton, S., Katzer, F., et al. (2011). Development of Lesions and Tissue Distribution of Parasite in Lambs Orally Infected With Sporulated Oocysts of Toxoplasma gondii. Vet. Parasitol. 179 (1-3), 209–215. doi: 10.1016/j.vetpar.2011.03.001
Bernstein, M., Pardini, L., Bello Pede Castro, B., Unzaga, J. M., Venturini, M. C., Moré, G. (2021). ROP18 and ROP5 Alleles Combinations Are Related With Virulence of T. Gondii Isolates From Argentina. Parasitol. Int. 83, 102328. doi: 10.1016/j.parint.2021.102328
Bernstein, M., Pardini, L., Campero, L. M., Helman, E., Unzaga, J. M., Venturini, M. C., et al. (2020). Evaluation of Biological Behavior of Toxoplasma gondii Atypical Isolates 14 And 163. Exp. Parasitol. 211, 107860. doi: 10.1016/j.exppara.2020.107860
Bezerra, E. C. M., Dos Santos, S. V., Dos Santos, T. C. C., de Andrade, H. F., Jr., Meireles, L. R. (2019). Behavioral Evaluation Of BALB/C (Mus Musculus) Mice Infected With Genetically Distinct Strains of Toxoplasma gondii. Microb. Pathog. 126, 279–286. doi: 10.1016/j.micpath.2018.11.021
Black, M. W., Boothroyd, J. C. (2000). Lytic Cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64 (3), 607–623. doi: 10.1128/MMBR.64.3.607-623.2000
Brenier-Pinchart, M. P., Bertini, R. L., Maubon, D., Pelloux, H. (2010). In Vitro Differential Phenotypic Characteristics Among Type-II Toxoplasma gondii Strains From Congenital Toxoplasmosis in Humans. J. Parasitol. 96 (4), 798–799. doi: 10.1645/GE-2405.1
Camejo, A., Gold, D. A., Lu, D., McFetridge, K., Julien, L., Yang, N., et al. (2014). Identification of Three Novel Toxoplasma gondii Rhoptry Proteins. Int. J. Parasitol. 44 (2), 147–160. doi: 10.1016/j.ijpara.2013.08.002
Cañedo-Solares, I., Calzada-Ruiz, M., Ortiz-Alegría, L. B., Ortiz-Muñiz, A. R., Correa, D. (2013). Endothelial Cell Invasion by Toxoplasma gondii: Differences Between Cell Types and Parasite Strains. Parasitol. Res. 112 (8), 3029–3033. doi: 10.1007/s00436-013-3476-2
Castaño, P., Fuertes, M., Ferre, I., Fernández, M., Ferreras, M. C., Moreno-Gonzalo, J., et al. (2014). Placental Thrombosis in Acute Phase Abortions During Experimental Toxoplasma gondii Infection in Sheep. Vet. Res. 45 (1), 9. doi: 10.1186/1297-9716-45-9
Castaño, P., Fuertes, M., Regidor-Cerrillo, J., Ferre, I., Fernández, M., Ferreras, M. C., et al. (2016). Experimental Ovine Toxoplasmosis: Influence Of The Gestational Stage on the Clinical Course, Lesion Development and Parasite Distribution. Vet. Res. 47, 43. doi: 10.1186/s13567-016-0327-z
Cavaillès, P., Sergent, V., Bisanz, C., Papapietro, O., Colacios, C., Mas, M., et al. (2006). The Rat Toxo1 Locus Directs Toxoplasmosis Outcome and Controls Parasite Proliferation and Spreading by Macrophage-Dependent Mechanisms. Proc. Natl. Acad. Sci. U. S. A. 103 (3), 744–749. doi: 10.1073/pnas.0506643103
Chai, J. Y., Lin, A., Shin, E. H., Oh, M. D., Han, E. T., Nan, H. W., et al. (2003). Laboratory Passage and Characterization of an Isolate of Toxoplasma gondii From an Ocular Patient in Korea. Korean J. Parasitol. 41 (3), 147–154. doi: 10.3347/kjp.2003.41.3.147
Chaudhury, A., Ramana, B. V. (2019). Schizophrenia and Bipolar Disorders: The Toxoplasma Connection. Trop. Parasitol. 9 (2), 71–76. doi: 10.4103/tp.TP_28_19
Chiebao, D. P., Bartley, P. M., Chianini, F., Black, L. E., Burrells, A., Pena, H. F. J., et al. (2021). Early Immune Responses And Parasite Tissue Distribution In Mice Experimentally Infected With Oocysts of Either Archetypal or Non-Archetypal Genotypes of Toxoplasma gondii. Parasitology 148 (4), 464–476. doi: 10.1017/S0031182020002346
Clementino Andrade, M. M., Pinheiro, B. V., Cunha, M. M., Carneiro, A. C., Andrade Neto, V. F., Vitor, R. W. (2013). New Genotypes of Toxoplasma gondii Obtained From Farm Animals in Northeast Brazil. Res. Vet. Sci. 94 (3), 587–589. doi: 10.1016/j.rvsc.2013.01.006
Contreras-Ochoa, C. O., Lagunas-Martínez, A., Belkind-Gerson, J., Correa, D. (2012). Toxoplasma gondii Invasion and Replication in Astrocyte Primary Cultures and Astrocytoma Cell Lines: Systematic Review of the Literature. Parasitol. Res. 110 (6), 2089–2094. doi: 10.1007/s00436-012-2836-7
Costa Viegas de Lima, D., de Melo, R. P. B., Campos de Almeida, J., Rodrigues Magalhães, F. J., Ribeiro Andrade, M., de Morais Pedrosa, C., et al. (2019). Toxoplasma gondii in Invasive Animals on the Island of Fernando De Noronha in Brazil: Molecular Characterization and Mouse Virulence Studies of New Genotypes. Comp. Immunol. Microbiol. Infect. Dis. 67, 101347. doi: 10.1016/j.cimid.2019.101347
Dardé, M. L., Mercier, A., Su, C., Khan, A., Grigg, M. E. (2020). “Chapter 3: Molecular Epidemiology and Population Structure of Toxoplasma gondii,” in Toxoplasma gondii: The Model Apicomplexan - Perspectives and Methods, Third Edition. Eds. Weiss, L. M., Kim, K. (New York, NY: Academic Press). doi: 10.1016/C2011-0-07157-0
da Silva, R. J., Gomes, A. O., Franco, P. S., Pereira, A. S., Milian, I. C. B., Ribeiro, M., et al. (2017). Enrofloxacin and Toltrazuril Are Able to Reduce Toxoplasma gondii Growth in Human BeWo Trophoblastic Cells and Villous Explants From Human Third Trimester Pregnancy. Front. Cell. Infect. Microbiol. 7, 340. doi: 10.3389/fcimb.2017.00340
Dellacasa-Lindberg, I., Fuks, J. M., Arrighi, R. B., Lambert, H., Wallin, R. P., Chambers, B. J., et al. (2011). Migratory Activation of Primary Cortical Microglia Upon Infection With Toxoplasma gondii. Infect. Immun. 79 (8), 3046–3052. doi: 10.1128/IAI.01042-10
Dellarupe, A., Regidor-Cerrillo, J., Jiménez-Ruiz, E., Schares, G., Unzaga, J. M., Venturini, M. C., et al. (2014). Comparison of Host Cell Invasion and Proliferation Among Neospora Caninum Isolates Obtained From Oocysts and From Clinical Cases of Naturally Infected Dogs. Exp. Parasitol. 145, 22–28. doi: 10.1016/j.exppara.2014.07.003
Diana, J., Persat, F., Staquet, M. J., Assossou, O., Ferrandiz, J., Gariazzo, M. J., et al. (2004). Migration and Maturation of Human Dendritic Cells Infected With Toxoplasma gondii Depend on Parasite Strain Type. FEMS Immunol. Med. Microbiol. 42 (3), 321–331. doi: 10.1016/j.femsim.2004.06.021
Djurković-Djaković, O., Djokić, V., Vujanić, M., Zivković, T., Bobić, B., Nikolić, A., et al. (2012). Kinetics of Parasite Burdens in Blood and Tissues During Murine Toxoplasmosis. Exp. Parasitol. 131 (3), 372–376. doi: 10.1016/j.exppara.2012.05.006
Dubey, J. P. (2006). Comparative Infectivity of Oocysts and Bradyzoites of Toxoplasma gondii for Intermediate (Mice) and Definitive (Cats) Hosts. Vet. Parasitol. 140 (1-2), 69–75. doi: 10.1016/j.vetpar.2006.03.018
Dubey, J. P. (2021). Toxoplasmosis Of Animals And Humans. 3rd ed. (Boca Raton, FL: CRC Press). doi: 10.1201/9781003199373
Dubey, J. P., Christie, E., Pappas, P. W. (1977). Characterization of Toxoplasma gondii From the Feces of Naturally Infected Cats. J. Infect. Dis. 136 (3), 432–435. doi: 10.1093/infdis/136.3.432
Dubey, J. P., Ferreira, L. R., Alsaad, M., Verma, S. K., Alves, D. A., Holland, G. N., et al. (2016). Experimental Toxoplasmosis in Rats Induced Orally With Eleven Strains of Toxoplasma gondii of Seven Genotypes: Tissue Tropism, Tissue Cyst Size, Neural Lesions, Tissue Cyst Rupture Without Reactivation, and Ocular Lesions. PloS One 11 (5), e0156255. doi: 10.1371/journal.pone.0156255
Dubey, J. P., Graham, D. H., Blackston, C. R., Lehmann, T., Gennari, S. M., Ragozo, A. M., et al. (2002). Biological and Genetic Characterisation of Toxoplasma gondii Isolates From Chickens (Gallus Domesticus) From São Paulo, Brazil: Unexpected Findings. Int. J. Parasitol. 32 (1), 99–105. doi: 10.1016/s0020-7519(01)00364-2
Dubey, J. P., Graham, D. H., da Silva, D. S., Lehmann, T., Bahia-Oliveira, L. M. (2003). Toxoplasma gondii Isolates of Free-Ranging Chickens From Rio De Janeiro, Brazil: Mouse Mortality, Genotype, and Oocyst Shedding by Cats. J. Parasitol. 89 (4), 851–853. doi: 10.1645/GE-60R
Dubey, J. P., Lago, E. G., Gennari, S. M., Su, C., Jones, J. L. (2012). Toxoplasmosis in Humans and Animals in Brazil: High Prevalence, High Burden Of Disease, and Epidemiology. Parasitology 139 (11), 1375–1424. doi: 10.1017/S0031182012000765
Dubey, J. P., Schlafer, D. H., Urban, J. F., Jr., Lindsay, D. S. (1990). Lesions in Fetal Pigs With Transplacentally-Induced Toxoplasmosis. Vet. Pathol. 27 (6), 411–418. doi: 10.1177/030098589902700605
Dubey, J. P., Sharma, S. P., Juranek, D. D., Sulzer, A. J., Teutsch, S. M. (1981). Characterization of Toxoplasma gondii Isolates From an Outbreak of Toxoplasmosis in Atlanta, Georgia. Am. J. Vet. Res. 42 (6), 1007–1010.
Dubey, J. P., Shen, S. K., Kwok, O. C., Frenkel, J. K. (1999). Infection and Immunity With the RH Strain of Toxoplasma gondii in Rats and Mice. J. Parasitol. 85 (4), 657–662. doi: 10.2307/3285739
Dubey, J. P., Van Why, K., Verma, S. K., Choudhary, S., Kwok, O. C., Khan, A., et al. (2014). Genotyping Toxoplasma gondii From Wildlife in Pennsylvania and Identification of Natural Recombinants Virulent to Mice. Vet. Parasitol. 200 (1-2), 74–84. doi: 10.1016/j.vetpar.2013.11.001
Fernández-Escobar, M., Calero-Bernal, R., Regidor-Cerrillo, J., Vallejo, R., Benavides, J., Collantes-Fernández, E., et al. (2020). Isolation, Genotyping, and Mouse Virulence Characterization of Toxoplasma gondii From Free Ranging Iberian Pigs. Front. Vet. Sci. 7, 604782. doi: 10.3389/fvets.2020.604782
Fernández-Escobar, M., Calero-Bernal, R., Regidor-Cerrillo, J., Vallejo, R., Benavides, J., Collantes-Fernández, E., et al. (2021). In Vivo and In Vitro Models Show Unexpected Degrees of Virulence Among Toxoplasma gondii Type II and III Isolates From Sheep. Vet. Res. 52 (1), 82. doi: 10.1186/s13567-021-00953-7
Frenkel, J. K., Dubey, J. P., Hoff, R. L. (1976). Loss of Stages After Continuous Passage of Toxoplasma gondii and Besnoitia Jellisoni. J. Protozool. 23 (3), 421–424. doi: 10.1111/j.1550-7408.1976.tb03799.x
Frey, C. F., Regidor-Cerrillo, J., Marreros, N., García-Lunar, P., Gutiérrez-Expósito, D., Schares, G., et al. (2016). Besnoitia Besnoiti Lytic Cycle in Vitroi and Differences in Invasion and Intracellular Proliferation Among Isolates. Parasitol. Vectors 9, 115. doi: 10.1186/s13071-016-1405-9
Fry, R. C., Bangma, J., Szilagyi, J., Rager, J. E. (2019). Developing Novel In Vitro Methods for the Risk Assessment of Developmental and Placental Toxicants in the Environment. Toxicol. Appl. Pharmacol. 378, 114635. doi: 10.1016/j.taap.2019.114635
Fukumoto, J., Yamano, A., Matsuzaki, M., Kyan, H., Masatani, T., Matsuo, T., et al. (2020). Molecular and Biological Analysis Revealed Genetic Diversity and High Virulence Strain of Toxoplasma gondii in Japan. PloS One 15 (2), e0227749. doi: 10.1371/journal.pone.0227749
Fux, B., Nawas, J., Khan, A., Gill, D. B., Su, C., Sibley, L. D. (2007). Toxoplasma gondii Strains Defective in Oral Transmission Are Also Defective in Developmental Stage Differentiation. Infect. Immun. 75 (5), 2580–2590. doi: 10.1128/IAI.00085-07
García-Sánchez, M., Jiménez-Pelayo, L., Horcajo, P., Regidor-Cerrillo, J., Ólafsson, E. B., Bhandage, A. K., et al. (2019). Differential Responses of Bovine Monocyte-Derived Macrophages to Infection by Neospora Caninum Isolates of High and Low Virulence. Front. Immunol. 10, 915. doi: 10.3389/fimmu.2019.00915
Gatkowska, J., Dzitko, K., Ferra, B. T., Holec-Gąsior, L., Kawka, M., Dziadek, B. (2019). The Impact of the Antigenic Composition of Chimeric Proteins on Their Immunoprotective Activity Against Chronic Toxoplasmosis in Mice. Vaccines (Basel) 7 (4), 154. doi: 10.3390/vaccines7040154
Grigg, M. E., Suzuki, Y. (2003). Sexual Recombination and Clonal Evolution of Virulence in Toxoplasma. Microbes Infect. 5 (7), 685–690. doi: 10.1016/s1286-4579(03)00088-1
Grochow, T., Beck, B., Rentería-Solís, Z., Schares, G., Maksimov, P., Strube, C., et al. (2021). Establishment and Validation of a Guinea Pig Model for Human Congenital Toxoplasmosis. Parasitol. Vectors 14 (1), 389. doi: 10.1186/s13071-021-04890-4
Guimarães, E. V., de Carvalho, L., Barbosa, H. S. (2008). Primary Culture Of Skeletal Muscle Cells as a Model for Studies of Toxoplasma gondii Cystogenesis. J. Parasitol. 94 (1), 72–83. doi: 10.1645/GE-1273.1
Guo, H., Gao, Y., Jia, H., Moumouni, P. F. A., Masatani, T., Liu, M., et al. (2019). Characterization of Strain-Specific Phenotypes Associated With Knockout of Dense Granule Protein 9 in Toxoplasma gondii. Mol. Biochem. Parasitol. 229, 53–61. doi: 10.1016/j.molbiopara.2019.01.003
Hamilton, C. M., Black, L., Oliveira, S., Burrells, A., Bartley, P. M., Melo, R. P. B., et al. (2019). Comparative Virulence of Caribbean, Brazilian and European Isolates of Toxoplasma gondii. Parasitol. Vectors 12 (1), 104. doi: 10.1186/s13071-019-3372-4
Hassan, M. A., Olijnik, A. A., Frickel, E. M., Saeij, J. P. (2019). Clonal and Atypical Toxoplasma Strain Differences in Virulence Vary With Mouse Sub-Species. Int. J. Parasitol. 49 (1), 63–70. doi: 10.1016/j.ijpara.2018.08.007
Hill, R. D., Gouffon, J. S., Saxton, A. M., Su, C. (2012). Differential Gene Expression in Mice Infected With Distinct Toxoplasma Strains. Infect. Immun. 80 (3), 968–974. doi: 10.1128/IAI.05421-11
Hill, R. D., Su, C. (2012). High Tissue Burden of Toxoplasma gondii Is the Hallmark of Acute Virulence in Mice. Vet. Parasitol. 187 (1-2), 36–43. doi: 10.1016/j.vetpar.2012.01.001
Holthaus, D., Delgado-Betancourt, E., Aebischer, T., Seeber, F., Klotz, C. (2021). Harmonization of Protocols for Multi-Species Organoid Platforms to Study the Intestinal Biology of Toxoplasma gondii and Other Protozoan Infections. Front. Cell. Infect. Microbiol. 10, 610368. doi: 10.3389/fcimb.2020.610368
Howe, D. K., Summers, B. C., Sibley, L. D. (1996). Acute Virulence in Mice Is Associated With Markers on Chromosome VIII in Toxoplasma gondii. Infect. Immun. 64 (12), 5193–5198. doi: 10.1128/iai.64.12.5193-5198.1996
Jensen, L., Petersen, E., Henriksen, S. A., Dietz, H. H., Lind, P. (1998). Monoclonal Antibodies to Toxoplasma gondii Strain 119 Identify Recently Isolated Danish Strains as One Group. Int. J. Parasitol. 28 (8), 1305–1313. doi: 10.1016/s0020-7519(98)00073-3
Jiang, T., Shwab, E. K., Martin, R. M., Gerhold, R. W., Rosenthal, B. M., Dubey, J. P., et al. (2018). A Partition of Toxoplasma gondii Genotypes Across Spatial Gradients and Among Host Species, and Decreased Parasite Diversity Towards Areas of Human Settlement in North America. Int. J. Parasitol. 48 (8), 611–619. doi: 10.1016/j.ijpara.2018.01.008
Jiang, N., Xin, S., Li, J., Su, C., Zhang, L., Yang, Y. (2020). Isolation and Characterization of Toxoplasma gondii From Captive Caracals (Caracal caracal). Int. J. Parasitol. Parasites Wildl. 13, 196–201. doi: 10.1016/j.ijppaw.2020.10.006
Jiménez-Pelayo, L., García-Sánchez, M., Regidor-Cerrillo, J., Horcajo, P., Collantes-Fernández, E., Gómez-Bautista, M., et al. (2017). Differential Susceptibility of Bovine Caruncular and Trophoblast Cell Lines to Infection With High and Low Virulence Isolates of Neospora Caninum. Parasitol. Vectors 10 (1), 463. doi: 10.1186/s13071-017-2409-9
Jungersen, G., Bille-Hansen, V., Jensen, L., Lind, P. (2001). Transplacental Transmission of Toxoplasma gondii in Minipigs Infected With Strains of Different Virulence. J. Parasitol. 87 (1), 108–113. doi: 10.1645/0022-3395(2001)087[0108:TTOTGI]2.0.CO;2
Jungersen, G., Jensen, L., Rask, M. R., Lind, P. (2002). Non-Lethal Infection Parameters in Mice Separate Sheep Type II Toxoplasma gondii Isolates by Virulence. Comp. Immunol. Microbiol. Infect. Dis. 25 (3), 187–195. doi: 10.1016/s0147-9571(01)00039-x
Jungersen, G., Jensen, L., Riber, U., Heegaard, P. M., Petersen, E., Poulsen, J. S., et al. (1999). Pathogenicity of Selected Toxoplasma gondii Isolates in Young Pigs. Int. J. Parasitol. 29 (8), 1307–1319. doi: 10.1016/s0020-7519(99)00078-8
Kannan, G., Moldovan, K., Xiao, J. C., Yolken, R. H., Jones-Brando, L., Pletnikov, M. V. (2010). Toxoplasma gondii Strain-Dependent Effects on Mouse Behaviour. Folia Parasitol. (Praha) 57 (2), 151–155. doi: 10.14411/fp.2010.019
Khan, A., Ajzenberg, D., Mercier, A., Demar, M., Simon, S., Dardé, M. L., et al. (2014). Geographic Separation of Domestic and Wild Strains of Toxoplasma gondii in French Guiana Correlates With a Monomorphic Version of Chromosome1a. PloS Negl. Trop. Dis. 8 (9), e3182. doi: 10.1371/journal.pntd.0003182
Khan, A., Behnke, M. S., Dunay, I. R., White, M. W., Sibley, L. D. (2009). Phenotypic and Gene Expression Changes Among Clonal Type I Strains of Toxoplasma gondii. Eukaryot. Cell. 8 (12), 1828–1836. doi: 10.1128/EC.00150-09
Khan, A., Fux, B., Su, C., Dubey, J. P., Darde, M. L., Ajioka, J. W., et al. (2007). Recent Transcontinental Sweep of Toxoplasma gondii Driven by a Single Monomorphic Chromosome. Proc. Natl. Acad. Sci. U. S. A. 104 (37), 14872–14877. doi: 10.1073/pnas.0702356104
Lambert, H., Vutova, P. P., Adams, W. C., Loré, K., Barragan, A. (2009). The Toxoplasma gondii-Shuttling Function of Dendritic Cells Is Linked to the Parasite Genotype. Infect. Immun. 77 (4), 1679–1688. doi: 10.1128/IAI.01289-08
Langoni, H., Matteucci, G., Medici, B., Camossi, L. G., Richini-Pereira, V. B., Silva, R. C. (2012). Detection and Molecular Analysis of Toxoplasma gondii and Neospora Caninum From Dogs With Neurological Disorders. Rev. Soc Bras. Med. Trop. 45 (3), 365–368. doi: 10.1590/s0037-86822012000300016
Li, M., Mo, X. W., Wang, L., Chen, H., Luo, Q. L., Wen, H. Q., et al. (2014). Phylogeny and Virulence Divergency Analyses of Toxoplasma gondii Isolates From China. Parasitol. Vectors 7, 133. doi: 10.1186/1756-3305-7-133
Lindsay, D. S., Dubey, J. P., Blagburn, B. L., Toivio-Kinnucan, M. (1991). Examination of Tissue Cyst Formation by Toxoplasma gondii in Cell Cultures Using Bradyzoites, Tachyzoites, and Sporozoites. J. Parasitol. 77 (1), 126–132. doi: 10.2307/3282569
Liu, T., Zhang, Q., Liu, L., Xu, X., Chen, H., Wang, H., et al. (2013). Trophoblast Apoptosis Through Polarization of Macrophages Induced by Chinese Toxoplasma gondii Isolates With Different Virulence in Pregnant Mice. Parasitol. Res. 112 (8), 3019–3027. doi: 10.1007/s00436-013-3475-3
Loeuillet, C., Mondon, A., Kamche, S., Curri, V., Boutonnat, J., Cavaillès, P., et al. (2019). Toxoplasma Hypervirulence in the Rat Model Parallels Human Infection and Is Modulated by the Toxo1 Locus. Front. Cell. Infect. Microbiol. 9, 134. doi: 10.3389/fcimb.2019.00134
Mammari, N., Vignoles, P., Halabi, M. A., Darde, M. L., Courtioux, B. (2014). In Vitro Infection of Human Nervous Cells by Two Strains Of Toxoplasma gondii: A Kinetic Analysis of Immune Mediators and Parasite Multiplication. PloS One 9 (6), e98491F. doi: 10.1371/journal.pone.0098491
Masatani, T., Oyamada, S., Inoue, R., Tsujio, M., Hatai, H., Matsui, T., et al. (2020). In Vivo Characterization of a Toxoplasma gondii Strain TgCatJpTy1/k-3 Isolated From a Stray Cat in Japan. Parasitol. Int. 74, 101995. doi: 10.1016/j.parint.2019.101995
McLeod, R., Estes, R. G., Mack, D. G., Cohen, H. (1984). Immune Response of Mice to Ingested Toxoplasma gondii: A Model of Toxoplasma Infection Acquired by Ingestion. J. Infect. Dis. 149 (2), 234–244. doi: 10.1093/infdis/149.2.234
Mercier, A., Devillard, S., Ngoubangoye, B., Bonnabau, H., Bañuls, A. L., Durand, P., Ajzenberg, D., Dardé, P., Durand, M. L. (2010). Additional Haplogroups Of Toxoplasma Gondii Out Of Africa: Population Structure And Mouse-virulence Of Strains From Gabon. PLoS Negl. Trop. Dis 4. (11). doi: 10.1371/journal.pntd.0000876
Miranda, F. J., Souza, D. B., Frazão-Teixeira, E., Oliveira, F. C., Melo, J. C., Mariano, C. M., et al. (2015). Experimental Infection With the Toxoplasma gondii ME-49 Strain in the Brazilian BR-1 Mini Pig Is a Suitable Animal Model for Human Toxoplasmosis. Mem. Inst. Oswaldo Cruz 110 (1), 95–100. doi: 10.1590/0074-02760140318
Moller, T., Fennestad, K. L., Eriksen, L., Work, K., Siim, J. C. (1970). Experimental Toxoplasmosis in Pregnant Sows. Acta Pathol. Microbiol. Scand. A. 78 (3), 241–255. doi: 10.1111/j.1699-0463.1970.tb03299.x
Mukhopadhyay, D., Arranz-Solís, D., Saeij, J. P. J. (2020). Influence of the Host and Parasite Strain on the Immune Response During Toxoplasma Infection. Front. Cell. Infect. Microbiol. 10, 580425. doi: 10.3389/fcimb.2020.580425
Müller, J., Aguado-Martínez, A., Ortega-Mora, L. M., Moreno-Gonzalo, J., Ferre, I., Hulverson, M. A., et al. (2017). Development of a Murine Vertical Transmission Model for Toxoplasma gondii Oocyst Infection and Studies on the Efficacy of Bumped Kinase Inhibitor (BKI)-1294 and The Naphthoquinone Buparvaquone Against Congenital Toxoplasmosis. J. Antimicrob. Chemother. 72 (8), 2334–2341. doi: 10.1093/jac/dkx134
Müller, J., Hemphill, A. (2013). In Vitro Culture Systems for The Study of Apicomplexan Parasites in Farm Animals. Int. J. Parasitol. 43 (2), 115–124. doi: 10.1016/j.ijpara.2012.08.004
Murata, Y., Sugi, T., Weiss, L. M., Kato, K. (2017). Identification of Compounds That Suppress Toxoplasma gondii Tachyzoites and Bradyzoites. PloS One 12 (6), e0178203. doi: 10.1371/journal.pone.0178203
Niedelman, W., Gold, D. A., Rosowski, E. E., Sprokholt, J. K., Lim, D., Farid Arenas, A., et al. (2012). The Rhoptry Proteins ROP18 and ROP5 Mediate Toxoplasma gondii Evasion of the Murine, But Not the Human Interferon-Gamma Response. PloS Pathog. 8 (6), e1002784. doi: 10.1371/journal.ppat.1002784
Oliveira, C. B., Meurer, Y. S., Andrade, J. M., Costa, M. E., Andrade, M. M., Silva, L. A., et al. (2016). Pathogenicity and Phenotypic Sulfadiazine Resistance of Toxoplasma gondii Isolates Obtained From Livestock in Northeastern Brazil. Mem. Inst. Oswaldo Cruz 111 (6), 391–398. doi: 10.1590/0074-02760150459
Pacheco, A. O. L., Amaral, M. P., de Farias, I. S., Bottino, L. Z. M. F., Bortoluci, K. R. (2020). Concomitant Isolation of Primary Astrocytes and Microglia for Protozoa Parasite Infection. J. Vis. Exp. 157. doi: 10.3791/60680
Pastor-Fernández, I., Collantes-Fernández, E., Jiménez-Pelayo, L., Ortega-Mora, L. M., Horcajo, P. (2021). Modeling the Ruminant Placenta-Pathogen Interactions in Apicomplexan Parasites: Current and Future Perspectives. Front. Vet. Sci. 7, 634458. doi: 10.3389/fvets.2020.634458
Pena, H. F. J., Ferreira, M. N., Gennari, S. M., de Andrade, H. F., Jr., Meireles, L. R., Galisteo, A. J., Jr. (2021). Toxoplasma gondii Isolated From a Brazilian Patient With Rare Pulmonary Toxoplasmosis has a Novel Genotype and Is Closely Related to Amazonian Isolates. Parasitol. Res. 120 (3), 1109–1113. doi: 10.1007/s00436-020-07008-4
Pena, H. F., Gennari, S. M., Dubey, J. P., Su, C. (2008). Population Structure and Mouse-Virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol. 38 (5), 561–569. doi: 10.1016/j.ijpara.2007.09.004
Pena, H. F., Soares, R. M., Amaku, M., Dubey, J. P., Gennari, S. M. (2006). Toxoplasma gondii Infection in Cats From São Paulo State, Brazil: Seroprevalence, Oocyst Shedding, Isolation in Mice, and Biologic and Molecular Characterization. Res. Vet. Sci. 81 (1), 58–67. doi: 10.1016/j.rvsc.2005.09.007
Poulin, R., Combes, C. (1999). The Concept of Virulence: Interpretations and Implications. Parasitol. Today 15 (12), 474–475. doi: 10.1016/s0169-4758(99)01554-9
Radke, J. B., Burrows, J. N., Goldberg, D. E., Sibley, L. D. (2018). Evaluation of Current and Emerging Antimalarial Medicines for Inhibition of Toxoplasma gondii Growth in Vitro. ACS Infect. Dis. 4 (8), 1264–1274. doi: 10.1021/acsinfecdis.8b00113
Reese, M. L., Zeiner, G. M., Saeij, J. P., Boothroyd, J. C., Boyle, J. P. (2011). Polymorphic Family of Injected Pseudokinases Is Paramount in Toxoplasma Virulence. Proc. Natl. Acad. Sci. U. S. A. 108 (23), 9625–9630. doi: 10.1073/pnas.1015980108
Regidor-Cerrillo, J., Gómez-Bautista, M., Sodupe, I., Aduriz, G., Álvarez-García, G., Del Pozo, I., et al. (2011). In Vitro Invasion Efficiency and Intracellular Proliferation Rate Comprise Virulence-Related Phenotypic Traits of Neospora Caninum. Vet. Res. 42 (1), 41. doi: 10.1186/1297-9716-42-41
Rêgo, W. M. F., Costa, J. G. L., Baraviera, R. C. A., Pinto, L. V., Bessa, G. L., Lopes, R. E. N., et al. (2017). Association of ROP18 and ROP5 Was Efficient as a Marker of Virulence in Atypical Isolates of Toxoplasma gondii Obtained From Pigs and Goats in Piauí, Brazil. Vet. Parasitol. 247, 19–25. doi: 10.1016/j.vetpar.2017.09.015
Ribeiro-Andrade, M., de Crasto Souza Carvalho, J., Amorim da Silva, R., da Conceição Carvalho, M., Nascimento Porto, W. J., Mota, R. A. (2019). Inter- and Intra-Genotype Differences in Induced Cystogenesis of Recombinant Strains of Toxoplasma gondii Isolated From Chicken and Pigs. Exp. Parasitol. 207, 107775. doi: 10.1016/j.exppara.2019.107775
Robbins, J. R., Zeldovich, V. B., Poukchanski, A., Boothroyd, J. C., Bakardjiev, A. I. (2012). Tissue Barriers of the Human Placenta to Infection With Toxoplasma gondii. Infect. Immun. 80 (1), 418–428. doi: 10.1128/IAI.05899-11
Rocha, D. S., Nilsson, M. G., Maciel, B. M., Pena, H. F. J., Alves, B. F., Silva, A. V., et al. (2018). Genetic Diversity of Toxoplasma gondii Isolates From Free-Range Chickens in Bahia, Brazil. J. Parasitol. 104 (4), 377–382. doi: 10.1645/18-9
Saeij, J. P., Boyle, J. P., Coller, S., Taylor, S., Sibley, L. D., Brooke-Powell, E. T., et al. (2006). Polymorphic Secreted Kinases Are Key Virulence Factors in Toxoplasmosis. Science 314 (5806), 1780–1783. doi: 10.1126/science.1133690
Salman, D., Mahmoud, M. E., Pumidonming, W., Mairamkul, T., Oohashi, E., Igarashi, M. (2021). Characterization of a Spontaneous Cyst-Forming Strain of Toxoplasma gondii Isolated From Tokachi Subprefecture in Japan. Parasitol. Int. 80, 102199. doi: 10.1016/j.parint.2020.102199
Sánchez-Sánchez, R., Ferre, I., Regidor-Cerrillo, J., Gutiérrez-Expósito, D., Ferrer, L. M., Arteche-Villasol, N., et al. (2019). Virulence in Mice of a Toxoplasma gondii Type II Isolate Does Not Correlate With the Outcome of Experimental Infection in Pregnant Sheep. Front. Cell. Infect. Microbiol. 8, 436. doi: 10.3389/fcimb.2018.00436
Saraf, P., Shwab, E. K., Dubey, J. P., Su, C. (2017). On the Determination of Toxoplasma gondii Virulence in Mice. Exp. Parasitol. 174, 25–30. doi: 10.1016/j.exppara.2017.01.009
Scheidegger, A., Vonlaufen, N., Naguleswaran, A., Gianinazzi, C., Müller, N., Leib, S. L., et al. (2005). Differential Effects of Interferon-Gamma and Tumor Necrosis Factor-Alpha on Toxoplasma gondii Proliferation in Organotypic Rat Brain Slice Cultures. J. Parasitol. 91 (2), 307–315. doi: 10.1645/GE-379R
Sergent, V., Cautain, B., Khalife, J., Deslée, D., Bastien, P., Dao, A., et al. (2005). Innate Refractoriness Of The Lewis Rat to Toxoplasmosis Is a Dominant Trait That Is Intrinsic to Bone Marrow-Derived Cells. Infect. Immun. 73 (10), 6990–6997. doi: 10.1128/IAI.73.10.6990-6997.2005
Shittu, O., Opeyemi, O. A., Salawu, M. K., Ashiru, A. A., Medaiyese, S. A., Asogwa, N., et al. (2020). Alterations in Histological, Biochemical and Hematological Parameters in Plasmodium Berghei NK-65 Infected Balb/C Mice Treated With Bridelia Ferruginea Stem Bark Extract. J. Complement. Integr. Med. 18 (1), 93–105. doi: 10.1515/jcim-2018-0219
Shwab, E. K., Jiang, T., Pena, H. F., Gennari, S. M., Dubey, J. P., Su, C. (2016). The ROP18 and ROP5 Gene Allele Types Are Highly Predictive of Virulence in Mice Across Globally Distributed Strains of Toxoplasma gondii. Int. J. Parasitol. 46 (2), 141–146. doi: 10.1016/j.ijpara.2015.10.005
Shwab, E. K., Saraf, P., Zhu, X. Q., Zhou, D. H., McFerrin, B. M., Ajzenberg, D., et al. (2018). Human Impact on the Diversity and Virulence of the Ubiquitous Zoonotic Parasite Toxoplasma gondii. Proc. Natl. Acad. Sci. U. S. A. 115 (29), E6956–E6963. doi: 10.1073/pnas.1722202115
Shwab, E. K., Zhu, X. Q., Majumdar, D., Pena, H. F., Gennari, S. M., Dubey, J. P., et al. (2014). Geographical Patterns of Toxoplasma gondii Genetic Diversity Revealed by Multilocus PCR-RFLP Genotyping. Parasitology 141 (4), 453–461. doi: 10.1017/S0031182013001844
Sibley, L. D. (2010). How Apicomplexan Parasites Move in and Out of Cells. Curr. Opin. Biotechnol. 21 (5), 592–598. doi: 10.1016/j.copbio.2010.05.009
Sibley, L. D., Boothroyd, J. C. (1992). Virulent Strains of Toxoplasma gondii Comprise a Single Clonal Lineage. Nature 359 (6390), 82–85. doi: 10.1038/359082a0
Sibley, L. D., Mordue, D., Howe, D. K. (1999). Experimental Approaches to Understanding Virulence in Toxoplasmosis. Immunobiology 201 (2), 210–224. doi: 10.1016/S0171-2985(99)80061-8
Su, C., Howe, D. K., Dubey, J. P., Ajioka, J. W., Sibley, L. D. (2002). Identification of Quantitative Trait Loci Controlling Acute Virulence in Toxoplasma gondii. Proc. Natl. Acad. Sci. U. S. A. 99 (16), 10753–10758. doi: 10.1073/pnas.172117099
Taniguchi, Y., Appiah-Kwarteng, C., Murakami, M., Fukumoto, J., Nagamune, K., Matsuo, T., et al. (2018). Atypical Virulence in a Type III Toxoplasma gondii Strain Isolated in Japan. Parasitol. Int. 67 (5), 587–592. doi: 10.1016/j.parint.2018.05.010
Taniguchi, Y., Yanagihara, I., Nakura, Y., Ichikawa, C., Saito, T., Appiah-Kwarteng, C., et al. (2019). A Toxoplasma gondii Strain Isolated in Okinawa, Japan Shows High Virulence in Microminipigs. Parasitol. Int. 72, 101935. doi: 10.1016/j.parint.2019.101935
Taylor, S., Barragan, A., Su, C., Fux, B., Fentress, S. J., Tang, K., et al. (2006). A Secreted Serine-Threonine Kinase Determines Virulence in the Eukaryotic Pathogen Toxoplasma gondii. Science 314 (5806), 1776–1780. doi: 10.1126/science.1133643
Uzelac, A., Klun, I., Ćirković, V., Djurković-Djaković, O. (2020). In Vivo and In Vitro Virulence Analysis of Four Genetically Distinct Toxoplasma gondii Lineage III Isolates. Microorganisms 8 (11), 1702. doi: 10.3390/microorganisms8111702
Vilares, A., Gargaté, M. J., Ferreira, I., Martins, S., Gomes, J. P. (2017). Molecular and Virulence Characterization of Toxoplasma gondii Strains Isolated From Humans in Portugal. Parasitol. Res. 116 (3), 979–985. doi: 10.1007/s00436-017-5374-5
Wang, J. L., Bai, M. J., Elsheikha, H. M., Liang, Q. L., Li, T. T., Cao, X. Z., et al. (2020). Novel Roles of Dense Granule Protein 12 (GRA12) in Toxoplasma gondii Infection. FASEB J. 34 (2), 3165–3178. doi: 10.1096/fj.201901416RR
Wang, L., Cheng, H. W., Huang, K. Q., Xu, Y. H., Li, Y. N., Du, J., et al. (2013b). Toxoplasma gondii Prevalence in Food Animals and Rodents in Different Regions of China: Isolation, Genotyping and Mouse Pathogenicity. Parasitol. Vectors 6, 273. doi: 10.1186/1756-3305-6-273
Wang, L., Chen, H., Liu, D., Huo, X., Gao, J., Song, X., et al. (2013a). Genotypes and Mouse Virulence of Toxoplasma gondii Isolates From Animals and Humans in China. PloS One 8 (1), e53483. doi: 10.1371/journal.pone.0053483
Wang, Q., Sibley, L. D. (2020). Assays for Monitoring Toxoplasma gondii Infectivity in the Laboratory Mouse. Methods Mol. Biol. 2071, 99–116. doi: 10.1007/978-1-4939-9857-9_5
Wang, T., Sun, X., Qin, W., Zhang, X., Wu, L., Li, Y., et al. (2019). From Inflammatory Reactions to Neurotransmitter Changes: Implications for Understanding the Neurobehavioral Changes in Mice Chronically Infected With Toxoplasma gondii. Behav. Brain Res. 359, 737–748. doi: 10.1016/j.bbr.2018.09.011
Wang, S., Zhao, G., Wang, W., Xie, Q., Zhang, M., Yuan, C., et al. (2014). Pathogenicity of Two Toxoplasma gondii Strains in Chickens of Different Ages Infected Via Intraperitoneal Injection. Avian Pathol. 43 (1), 91–95. doi: 10.1080/03079457.2013.874007
Watson, G. F., Davis, P. H. (2019). Systematic Review and Meta-Analysis of Variation in Toxoplasma gondii Cyst Burden in the Murine Model. Exp. Parasitol. 196, 55–62. doi: 10.1016/j.exppara.2018.12.003
Witola, W. H., Bauman, B., McHugh, M., Matthews, K. (2014). Silencing of GRA10 Protein Expression Inhibits Toxoplasma gondii Intracellular Growth and Development. Parasitol. Int. 63 (5), 651–658. doi: 10.1016/j.parint.2014.05.001
Xia, N. B., Lu, Y., Zhao, P. F., Wang, C. F., Li, Y. Y., Tan, L., et al. (2020). Genotyping and Characterization of Toxoplasma gondii Strain Isolated From Pigs in Hubei Province, Central China. Trop. Biomed. 37 (2), 489–498.
Xiao, J., Li, Y., Jones-Brando, L., Yolken, R. H. (2013). Abnormalities of Neurotransmitter and Neuropeptide Systems in Human Neuroepithelioma Cells Infected by Three Toxoplasma Strains. J. Neural Transm. (Vienna) 120 (12), 1631–1639. doi: 10.1007/s00702-013-1064-3
Xia, J., Venkat, A., Bainbridge, R. E., Reese, M. L., Le Roch, K. G., Ay, F., et al. (2021). Third-Generation Sequencing Revises the Molecular Karyotype for Toxoplasma gondii and Identifies Emerging Copy Number Variants in Sexual Recombinants. Genome Res. 31 (5), 834–851. doi: 10.1101/gr.262816.120
Yang, Y. R., Feng, Y. J., Lu, Y. Y., Dong, H., Li, T. Y., Jiang, Y. B., et al. (2017). Antibody Detection, Isolation, Genotyping, and Virulence of Toxoplasma gondii in Captive Felids From China. Front. Microbiol. 8, 1414. doi: 10.3389/fmicb.2017.01414
Yang, Y., Feng, Y., Yao, Q., Wang, Y., Lu, Y., Liang, H., et al. (2017). Seroprevalence, Isolation, Genotyping, and Pathogenicity of Toxoplasma gondii Strains From Sheep in China. Front. Microbiol. 8, 136. doi: 10.3389/fmicb.2017.00136
Yang, Y., Jiang, N., Xin, S., Zhang, L. (2020). Toxoplasma gondii Infection in White Spoonbills (Platalea Leucorodia) From Henan Province, China. Emerg. Microbes Infect. 9 (1), 2619–2621. doi: 10.1080/22221751.2020.1854057
Yang, Y., Ren, H., Xin, S., Jiang, N. (2021). Comparative Immunological Response and Pathobiology of Mice Inoculated With Toxoplasma gondii Isolated From Different Hosts. J. Parasitol. 107 (2), 179–181. doi: 10.1645/20-107
Zenner, L., Darcy, F., Capron, A., Cesbron-Delauw, M. F. (1998). Toxoplasma gondii: Kinetics of the Dissemination in the Host Tissues During the Acute Phase of Infection of Mice and Rats. Exp. Parasitol. 90 (1), 86–94. doi: 10.1006/expr.1998.4301
Keywords: Toxoplasma gondii, virulence, phenotype, harmonization, lethal parameters, non-lethal parameters
Citation: Calero-Bernal R, Fernández-Escobar M, Katzer F, Su C and Ortega-Mora LM (2022) Unifying Virulence Evaluation in Toxoplasma gondii: A Timely Task. Front. Cell. Infect. Microbiol. 12:868727. doi: 10.3389/fcimb.2022.868727
Received: 03 February 2022; Accepted: 28 March 2022;
Published: 28 April 2022.
Edited by:
Jeroen P. J. Saeij, University of California, Davis, United StatesReviewed by:
Marie-Laure Dardé, University of Limoges, FranceMelissa Lodoen, University of California, Irvine, United States
Jon P. Boyle, University of Pittsburgh, United States
Copyright © 2022 Calero-Bernal, Fernández-Escobar, Katzer, Su and Ortega-Mora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rafael Calero-Bernal, r.calero@ucm.es; Luis Miguel Ortega-Mora, luis.ortega@ucm.es
 Rafael Calero-Bernal
Rafael Calero-Bernal Mercedes Fernández-Escobar
Mercedes Fernández-Escobar Frank Katzer
Frank Katzer Chunlei Su
Chunlei Su Luis Miguel Ortega-Mora
Luis Miguel Ortega-Mora