Enzootic Trypanosoma cruzi infection by Rhodnius prolixus shows transmission to humans and dogs in Vichada, Colombia
- 1Grupo Biología y Control de Enfermedades Infecciosas - BCEI, Universidad de Antioquia (UdeA), Medellín, Colombia
- 2Programa de Promoción, Prevención y Control de Enfermedades Transmitidas por Vectores, Secretaría de Salud del Vichada, Puerto Carreño, Colombia
- 3Laboratory of Trypanosomatid Biology, Oswaldo Cruz Institute, Fundaçao Oswaldo Cruz (FIOCRUZ), Rio de Janeiro, Brazil
Background: Rhodnius prolixus is considered the most relevant Trypanosoma cruzi vector in Colombia and Venezuela due it is responsible for domestic transmission in both countries. However, a wild population of this species is distributed in the eastern plains of the Orinoco region and Amazonia jungle, where its epidemiological importance has not been sufficiently elucidated. This study aimed to assess epidemiological parameters of T. cruzi transmission in the Department of Vichada, Colombia.
Methods: We determined the characteristics of T. cruzi transmission using entomological studies in domestic and sylvatic ecotopes. We analyzed the T. cruzi infection in triatomine insects, identified blood meal sources, and conducted a serological determination of T. cruzi infection in scholar-aged children, domestic dogs, and wild hosts.
Results: Fifty-four triatomine bugs, 40 T. maculata and 14 R. prolixus were collected in peridomestic and sylvatic ecotopes. Infected R. prolixus was observed in La Primavera, Santa Rosalia, and Cumaribo municipalities. All the T. maculata bugs were not infected. Serological analysis indicated that two of 3,425 children were T. cruzi positive. The seroprevalence in domestic dogs was 10,5% (49/465). Moreover, 22 synanthropic mammals were sampled, being Didelphis marsupialis the most common. TcI genotype was detected in seropositive dogs, R. prolixus, and D. marsupialis.
Conclusion: The present work describes extra domestic R. prolixus and D. marsupialis in a sylvatic T. cruzi transmission cycle with transmission to humans and domestic dogs in Colombia’s Vichada Department.
1 Introduction
Chagas disease is a zoonosis caused by the protozoan hemoflagellate Trypanosoma cruzi, which is transmitted to humans mainly by insects of the triatominae subfamily (Hemiptera: Reduviidae) (Chagas, 1909). About 8 million people are currently infected with the parasite, and at least 10,000 deaths related to this disease occur each year (WHO, 2015). T. cruzi presents enormous genetic diversity and has been divided into six Discrete Typing Units (DTUs) called TcI to TcVI (Zingales et al., 2012). More recently, another genotype found in bats called TcBat was also reported (Ramirez et al., 2014). These genotypes are frequently associated with different clinical manifestations, geographical distribution, and transmission cycles (domestic, peridomestic, and sylvatic). TcI is the most widely distributed DTU in Colombia (Cura et al., 2010; Guhl and Ramirez, 2013).
In Colombia, 27 species of triatomines have been reported in 423 municipalities within 31 departments, where infected species participate in the domestic, peridomestic, and enzootic transmission cycles (Guhl, 2007a; Guhl et al., 2007; Ramírez et al., 2012; Cantillo-Barraza et al., 2014; Rendon et al., 2015). Rhodnius prolixus is the T. cruzi primary vector species in this country due to its domiciliation (Guhl et al., 2007; Guhl, 2007b). A national program for the interruption of the transmission by intradomestic populations of this species has been designed and implemented (Ministerio de la protección social, 2012). However, in the eastern plains of the Orinoco and Amazon regions, R. prolixus sylvatic populations in Attalea butyracea and oil palm plantations (Elaeis guineensis) have been described as part of enzootic transmission (Guhl et al., 2009; Rendon et al., 2015).
Approximately 2% of the Colombian population is infected with this parasite Llau et al., 2019; Olivera et al., 2019). However, there are still large geographical areas where the proportion of infected people has not been explored (Llau et al., 2019). Domestic and wild mammals, which play a fundamental role in local transmission in unstudied areas, are also poorly defined (Guhl et al., 2007; Rodríguez-Monguí et al., 2019). In this country, Canis lupus familiaris and Didelphis marsupialis are mammalian species with the highest epidemiological importance in Chagas disease (Guhl and Ramirez, 2013; Rodríguez-Monguí et al., 2019). Domestic dogs actively participate in the domestic cycle and play essential roles as synanthropic mammals (Ramírez et al., 2012; Rodríguez-Monguí et al., 2019; Cantillo-Barraza et al., 2020a). However, D. marsupialis is the species with the highest prevalence, and T. cruzi infection with an active role in the sylvatic transmission cycle was previously reported (Rodríguez-Monguí et al., 2019; Cantillo-Barraza et al., 2020a).
The Vichada department, located in the Orinoco region on the border with Venezuela, has ecological and geographical features appropriated to T. cruzi transmission. The north and west zones contain oil palm plantations, and the south and southeast are comprised of the amazon jungle (Tropical humid biome of the Amazon). The Vichada department is surrounded in its northern section by Chagas endemic departments of the eastern Colombian plains such as Casanare, Arauca, and Meta, and the Venezuelan state of Apure to the west. All these regions have been reported with domiciliation of R. prolixus (Feliciangeli et al., 2007; Guhl et al., 2007; Ceccarelli et al., 2018). Also, the jungle subregion of Vichada is a neighbor of the Guainia department in Colombia and the Amazonas state in Venezuela, where non-domiciled vectors such as Panstrongylus geniculatus, P. lignarius, and extra domestic R. prolixus have been observed (Solis-Medina et al., 2021). Although in the Vichada department, R. prolixus and other non-domiciled vectors, such as T. maculata, P. geniculatus, Erathyrus mucrunatus, and P. lignarius, have been reported, few studies related to its relevance in the parasite transmission have been carried out. Therefore, the present study aimed to a) estimate the T. cruzi seroprevalence in children, dogs, and synanthropic mammals; b) identify vector triatomine and calculate the natural infection rate, c) describe the blood-meal source in triatomines bugs; and d) determine the T. cruzi genotypes present in the region.
2 Material and methods
2.1 Study area
The Vichada is the second largest Department in Colombia, with an area of 102,242 km2 and four municipalities with altitudes between 53 to 180 m.a.s.l (meters above sea level). This study was conducted between 2016 to 2017 on Colombian’s eastern plains of the Orinoco Region and Amazon jungle (2°43’37” and 06°21’18’’N) (67°24’24” and 71°05’28’’W). Three-quarters of the Department are savannahs, and the rest is a tropical jungle (Gallego Gallego, 2018). The weather is tropical, with defined dry (from January to March) and rainy (from April to December) seasons. An average annual temperature of 27°C and rainfall of 2,688 mm (Edgar et al., 2007). This Department is populated by around 73,702 inhabitants (30,660 in urban and 43,042 in rural areas, respectively). Indigenous people constitute the majority of the population, with five indigenous ethnicities present in this Department: Sikuany, Curripacos, Piaroas, Puinaves, and Piapocos (Ministerio del interior (2022).
The survey was carried out in four municipalities in the Department: Puerto Carreño (PC), La Primavera (LP), Santa Rosalia (SR), and Cumaribo (C). The first three are savannas and the last with savannas and jungle (Figure 1).
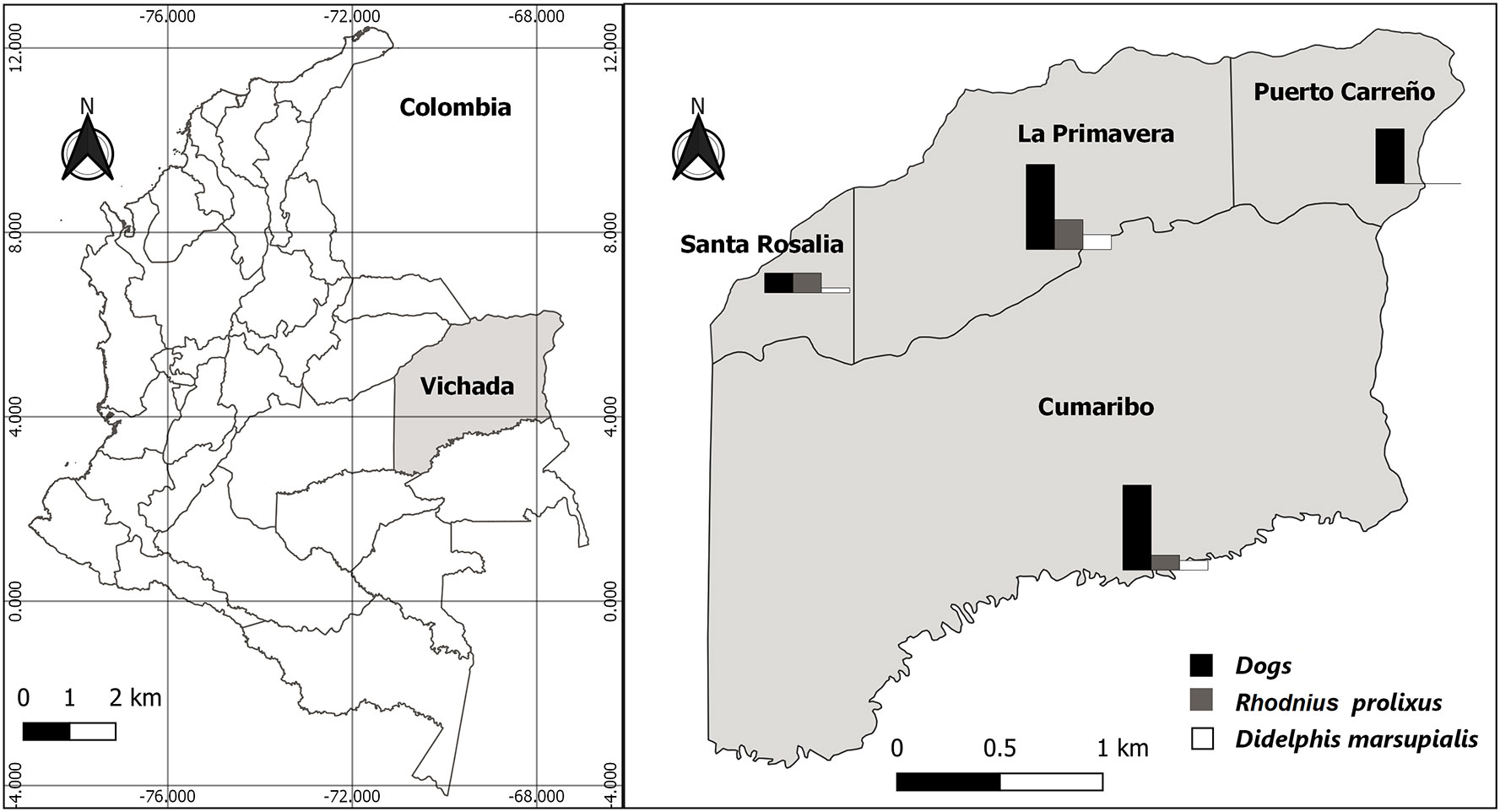
Figure 1 Study area and seroprevalence to Trypanosoma cruzi in dogs, frequency of infection of triatomine bugs, and Didelphis marsupialis distributed by each municipality in Vichada Department, Colombia.
2.2 Entomological samples
2.2.1 Intradomestic and peridomestic survey
Five entomological surveys, each around twenty days, were performed during the study period in the four municipalities. All procedures were carried out by technicians of the University of Antioquia, following the National Protocols of Entomological Surveillance. In brief, outdoor and indoor triatomine niches were searched for 30 minutes; flashlights were used to help see cracks and crevices throughout the fabric of buildings, behind pictures on the walls, furniture, in closets, and, especially, under bedding material. All households in neighborhoods with previous reports of triatomine bugs were visited in the urban area. In rural areas, all dwellings were inspected (Table 1).
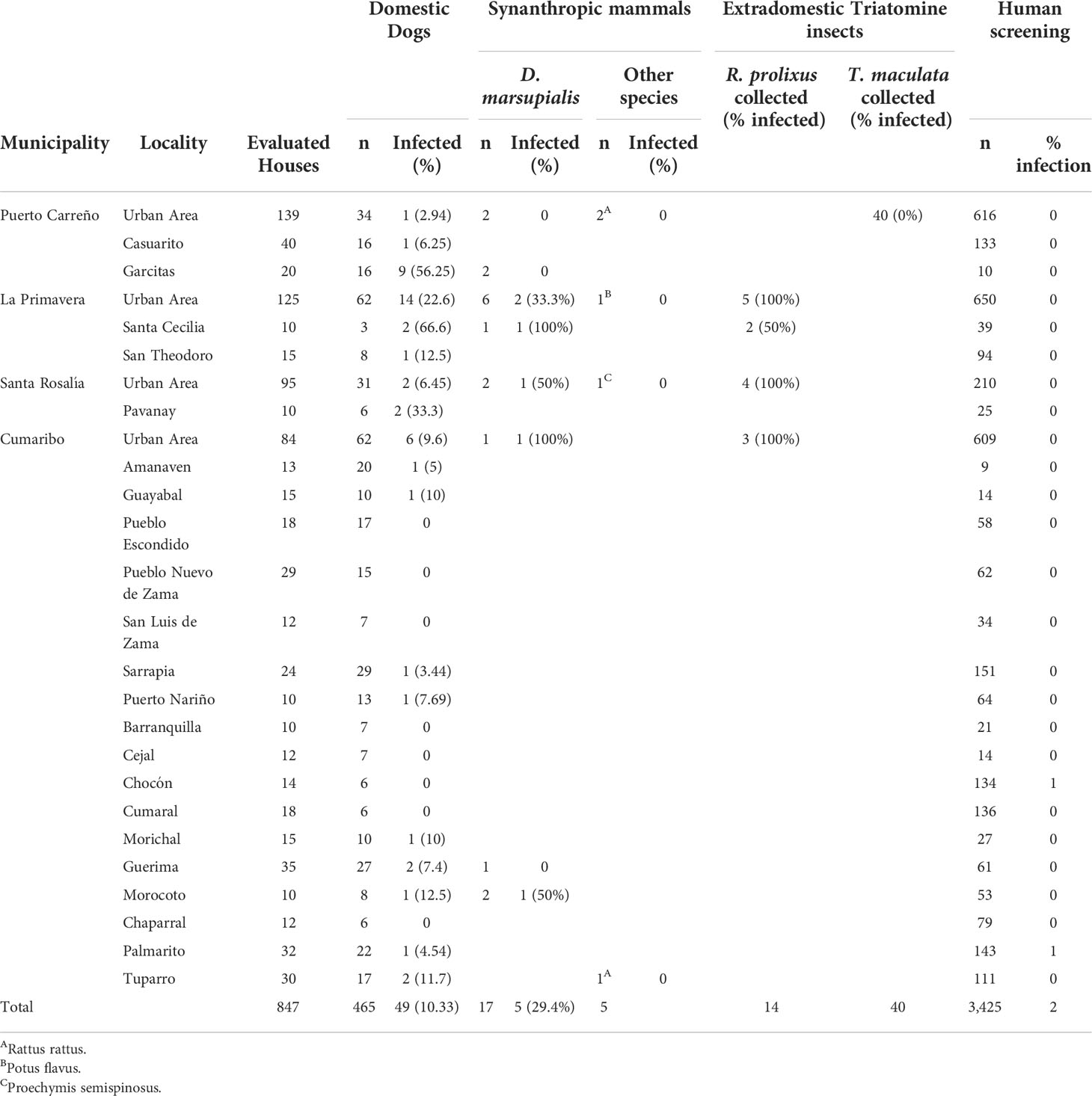
Table 1 Summary of capture and T. cruzi infection in triatomine bugs, seroprevalence in dogs, synanthropic mammals, and human T. cruzi infection in the study area: Puerto Carreño, La Primavera, Santa Rosalia, and Cumaribo.
2.2.2 Extradomestic survey
Palm trees (n=60), A. butyracea (Zuleta-Duenas et al., 2017), E. guineensis (Feliciangeli et al., 2007), and Maurita flexuosa (Guhl et al., 2007) located inside the studied town or found less than 500 meters from humans’ livings in peripheral areas were tested for triatomines with five live bait traps per palm/night. Additionally, dry, green leaves, organic debris, interfoliaceous meshes, and bracts were examined for the presence of triatomines with the help of a ladder. Insects were transported to the laboratory, registered, and identified using taxonomic keys (Lent and Wigodzinsky, 1979).
2.3 Blood-meal source determination
Genomic DNA was extracted from 200 µL of triatomine feces using the Genomic DNA purification kit (DNeasy Blood & Tissue kit Qiagen, Germantown, USA) following the manufacturer’s instructions. The DNA was subjected to a conventional PCR targeting the cytochrome b (cytb) gene vertebrates to identify blood-meal sources. The PCR was performed in a final volume of 25 µL containing 40-50 ng of genomic DNA, buffer 1X, 0,2mM of dNTP, 3mM MgCl2, 0,4 µM of each primer (cytbF and cytbR), and 0,025 U Taq polymerase (Invitrogen, California, USA). The amplifications were performed using a thermal cycler of initial denaturation at 95°C for 4 min, followed by 36 cycles at 95°C for 30 s, 60°C for 50 s, and 72°C for 40 s; and a final extension at 72°C for 5 min (Peña et al., 2012). Positive PCR products were purified and sequenced in both strands using the Sanger method at Macrogen, Seoul, South Korea. The sequences obtained were compared with sequences deposited in GenBank using the BLASTN search to identify the host species associated with triatomines.
2.4 Mammal host samples
2.4.1 Dog samples
Sampling was carried out in 26 places located in the four municipalities. The sample size was calculated using Epi info 7.0 (www.cdc.gov), considering a population of ~7,000 dogs in the Vichada department, a 22,5% probability of being infected according to (Jaimes-Dueñez et al., 2017), a confidence interval of 95%, and a margin of error of 4%. The estimated sample size was 435, but it was increased by 10% to compensate for sampling error. A non-probabilistic sample was conducted for taking blood samples of canines through a house-to-house strategy. Inclusion criteria for selected dogs were as follows: (i) born and grown in the study area, (ii) dogs with a recognizable owner, (iii) available information about the animal’s history (e.i. age, site of repose, often feeding and health).
Blood samples were taken from dorsal-tibial or radial veins under minimal stress with the owner’s help. The samples were centrifuged, serum was stored at -20°C for serological assays, and the remaining was used for DNA extraction and molecular diagnosis (see below).
2.4.2 Dogs serological diagnostic
Detection of anti - T. cruzi antibodies was conducted using the Indirect Immunofluorescence Antibody Test (IFAT) and the Enzyme-Linked Immunosorbent Assay (ELISA, Bio-Manguinhos, FIOCRUZ, Rio de Janeiro, RJ, Brazil). The cut-off criteria for a reactive test were a titer of 1/40 for IFAT and optical absorbance ≥ 0,200 (mean +/- 3 SD) for the ELISA test. Animals were defined as seropositive when samples were reactive for both IFAT and ELISA tests. To evaluate cross-reactions and mixed infection by T. cruzi and Leishmania spp., dog sera were also assayed for antigens derived from a mixture of L. infantum and L. panamensis using IFAT and the Rapid Test for Diagnosis of Canine Visceral Leishmaniasis (CVL) (TR DPP®, Bio-Manguinhos, FIOCRUZ, Rio de Janeiro, RJ, Brazil) (DPP).
The IFAT cut-off value adopted for T. cruzi infection was 1/40 when the IFAT result for L. infantum was lower than 1/40, and the DPP result was negative. On the other hand, for L. infantum positive dogs, positive T. cruzi infection was considered only when the IFAT titer was 1/80 or higher. For L. infantum infection, the adopted IFAT cut-off value was 1/40 when the infection was confirmed by DPP and 1/80 when the DPP assay was negative.
2.4.3 Synanthropic mammal capture and diagnostic
Synanthropic mammals were captured using traps (Tomahawk) baited with a mixture of peanut, banana oat, and fish. At each locality, the traps were set for three nights in the forests where palms were sampled and were distributed in linear transects, with capture points established every 20 mts. To detect T. cruzi, trapped animals were anesthetized (ketamine, 100mg/kg), and their blood was collected by cardiac puncture. Two tubes containing NNN medium, covered with a LIT overlay, were inoculated with 0,2 mL of blood from each specimen. They were examined for epimastigote forms presence weekly for three months. The remaining samples were stored for DNA extraction and molecular diagnosis (See below).
2.5 Trypanosoma cruzi infection in humans
With the previous written informed consent of one or both parents and following the University of Antioquia Ethics committee (08–012–185), blood sampling was obtained on 3,425 students between 5 to 20 years old. All schools in the study area were chosen to sign the written informed consent and blood sampling. Approximately 5 ml of whole blood was collected by venipuncture centrifuged, and the obtained serum was stored under refrigeration until further processing.
2.5.1 Serologic analysis
All participants were evaluated by two Enzyme-Linked Immunosorbent Assay (ELISA) tests with different principles, according to the recommendations of the National Institute of Health, Colombia. Anti - T. cruzi IgG was detected by two serological tests: (i) for all samples, one initial screening by ELISA test (enzyme-linked immunosorbent assay) based on crude parasite extract using two T. cruzi isolates (I.RHO/CO/00/CAS-15.CAS; I.TRI/CO/03/MG-8.MAG) were used. The optical density (OD) values of previously confirmed positive and negative controls were used to define the limits for seropositivity and seronegativity in this assay. OD values higher than 2 SD of the OD average for negatives control were considered ELISA-positives. (ii) ELISA test with recombinant antigens (Dia Pro Diagnostic Bioprobes T. cruzi-AB), following the manufacturer’s instructions. The incongruent samples were analyzed by one additional serological test: Indirect Immunofluorescence Assay (IFAT). The incongruent samples, reactive to at least one of two complementary tests, were considered positive.
2.6 Trypanosoma cruzi detection in triatomines, domestics dogs, and synanthropic mammals
Using parasitological and molecular methods, all triatomines collected were evaluated for T. cruzi infection. Feces were obtained by abdominal compression, diluted in 300 μL of sterile PBS pH 7.2, and used for DNA extraction. A 10 µL aliquot was examined under an optical microscope at 400X for flagellated forms. The remaining samples were used for genomic DNA extraction with a DNA purification kit (DNeasy Blood & Tissue kit Qiagen, Germantown, USA) and T. cruzi genetic typing (see below).
Total DNA extracted from feces of captured triatomines and blood from domestic dogs and synanthropic mammals was used for T. cruzi molecular diagnosis. All DNA preparations were screened to test for T. cruzi using a conventional PCR targeting satellite DNA (Moser et al., 1989). The PCR was performed in a final volume of 25µL containing 40-50 ng of genomic DNA, 1X of a buffer, 0,04 mM of dNTP, 1,5 mM MgCl2, 0,4 µM of each primer (TCZ1 and TCZ2), and 0,05 U of Taq polymerase (Invitrogen, California, USA). The thermal cycling conditions were as follows: pre-heating at 95°C for 15 min, 40 cycles at 95°C for 10 s, 55°C for 15 s, and 72°C for 10 s in a thermal cycler. Positive T. cruzi samples were analyzed for molecular discrimination of T. cruzi DTUs based on the amplification spliced leader intergenic region (SL-IR) gene using the primers TCC, TC1, and TC2, previously reported (Souto et al., 1996). The PCR was performed in a final volume of 25µL containing 40-50 ng of genomic DNA, 1X of a buffer, 0.25 mM of dNTP, 2 mM MgCl2, 0.4µM of each primer, 0.05 U of Taq polymerase (Invitrogen, California, USA). The thermal cycling conditions were as follows: pre-heating at 94°C for 5 min, 35 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 45 s in a thermal cycler, and a final extension at 72°C for 5 min. Amplification products were run on a 1.5% agarose gel stained by ethidium bromide and visualized under UV light. PCR products were purified and sequenced using the Sanger methodology at Macrogen sequencing service, Seoul, South Korea, for direct sequencing of the SL-IR region.
3 Results
3.1 The natural infection rate in triatomines
Fifty-four triatomine bugs, 40 T. maculata and 14 R. prolixus were collected. T. maculata was captured in Puerto Carreño in chicken coops associated with the peridomestic area. However, no T. cruzi infection was registered in this species. R. prolixus was collected in La Primavera, Santa Rosalia, and Cumaribo in A. butyracea and Maurita flexuosa palms (Table 1). T. cruzi infection in this species was found in all municipalities. On the other hand, 847 households were visited, none of which triatomine bugs were found inside the home (Table 1).
3.2 Blood-meal sources identification
Eight R. prolixus and five T. maculata were analyzed for blood meal identification by PCR and sequencing of the cyt B gene. One of the three amplified sequences of R. prolixus showed an identity of 99.9%, with sequences derived from chicken (Gallus gallus). The remaining two showed the highest identity, with sequences derived from the Ceracris kiangsu reptile (Table 2). No amplification was found in T. maculata.

Table 2 Stage, capture site, blood meals, GenBank accession number, infection state, and identity (%) with the reference genotype.
3.3 Trypanosoma cruzi infection in dogs
Most of the dogs sampled for this study live in houses not/enclosed by fences made of solid material, which allows animals to roam freely outside. All 465 dogs evaluated were creole breeds. The mean age of dogs was 3,65 ± 2,2 years (ranging from 6 months to 10 years old). Forty-nine dogs (10.53%, 95% CI=7.04-12.47%) were positive for T. cruzi by the ELISA and IFAT tests. The highest proportion of T. cruzi infection was in La Primavera, 23.28% (17/73), while the lowest infection was in Cumaribo, 8.4% (14/289) (Table 1).
3.4 Trypanosoma cruzi infection in synanthropic mammals and Trypanosoma cruzi genotyping
Twenty-two mammals were captured in and around the studied towns. D. marsupialis was the most abundant (Rodríguez-Monguí et al., 2019), followed by Rattus rattus (WHO, 2015), Proechymis semispinosus (Chagas, 1909), and Potus flavus (Chagas, 1909). Of the 17 tested D. marsupialis, eight were positive for T. cruzi by haemocultures and molecular tools (Table 1). No infection was detected in the other mammals.
Discrimination between DTUs by SL-IR analysis revealed only the presence of DTUI in the samples of R. prolixus, D. marsupialis, and infected dogs.
3.5 Seropositivity in scholar students
A total of 3,425 kindergartens, primary and secondary students, were assessed. Two patients, twelve and twenty years old, residents of Cumaribo were positive, leading to an overall T. cruzi infection ratio of 0.06% (95% CI: 0.007-0.211) (Table 1).
4 Discussion
Rhodnius prolixus has been considered the primary vector species of T. cruzi in Colombia, especially in the Andean and Eastern Plains regions, where domestic populations have been linked to the Chagas disease domestic transmission cycle (Guhl and Ramirez, 2013). After extensive control efforts across this country, about half of municipalities considered at high risk were certified as free of T. cruzi transmission mediated by domestic R. prolixus (PAHO - Pan American Health Organization, 2019). However, in recent years, some authors have provided evidence of the participation of wild populations of this species, with less epidemiological relevance and involved in occurrences of an oral outbreak (Rendon et al., 2015; Zuleta-Duenas et al., 2017). These events have illustrated the species’ ability to participate in non-domiciliary transmission, suggesting the need to implement entomological surveillance instead of traditional programs of spraying domestic populations (Guhl et al., 2009; Cantillo-Barraza et al., 2021).
In this study, we highlighted some relevant facts in the Vichada Department: (i) the presence of R. prolixus exclusively in A. butyracea and M. flexuosa palms, (ii) the infection of this vector with TcI sylvatic, (iii) and reptiles as a food source, which support the presence of the sylvatic T. cruzi transmission cycle in this region of Colombia. This condition of non-domiciliation of this vector and the sylvatic cycle’s existence has been related to slow transmission to humans and moderate transmission to domestic dogs (Rendon et al., 2015). In concordance with this idea, this work showed low T. cruzi transmission to humans and domestic dogs in the Vichada department and extended area of Eastern Plains regions of Colombia without domiciliation and active participation of D. marsupialis.
The presence of R. prolixus in plantations of African oil palm (E. guineensis) in the Plains regions of Colombia and Venezuela has given rise to a novel epidemiological scenario for T. cruzi transmission (Cantillo-Barraza et al., 2021). In the present work, we did not evaluate the infestation of R. prolixus in African Oil Palms because these were located far from the assessed towns. However, this issue must be studied in the future because the municipalities of Santa Rosalia and La Primavera contain approximately 9,000 hectares of these crops (Fedepalma, 2020).
Triatoma maculata is one of the most widely distributed Triatoma species in northern South America, reported in Brazil (Luitgards-Moura et al., 2005), Colombia, Venezuela, Guyana, Suriname, French Guiana, and some Caribbean islands (Monsalve et al., 2016). This species is the most widely distributed secondary vector in Colombia after Panstrongylus geniculatus (Guhl et al., 2007). T. maculata is a species with heterogeneous epidemiological relevance in Colombia and Venezuela. It has been found with high infection levels and an active role in T. cruzi transmission in the Caribbean region (Garcia-Alzate et al., 2014; Cantillo-Barraza et al., 2015; Hernandez et al., 2016). However, it has been reported without infection in other areas and is associated with birds (Guhl et al., 2007). In the study area, T. maculata was found without infection and associated with chicken coops. Therefore, it could be considered low epidemiological relevance for this area (Luitgards-Moura et al., 2005).
Furthermore, we reported a domestic dog infection frequency of 10.33% in the Vichada department. This result showed a significant intensity of T. cruzi transmission to this species. However, this value is lower than those reported in other Colombian regions, such as the Caribbean (70.1%), Andean (34%), and Eastern Plains regions (25.6%), where triatomines are present in the domestic and peridomestic areas (Cantillo-Barraza et al., 2015; Cantillo-Barraza et al., 2020a; Jaimes-Dueñez et al., 2020). The low intensity of T. cruzi infection reported here is congruent with reports in countries such as Panama, Brazil, and Venezuela, with enzootic transmission by triatomines species in palms (Calzada et al., 2006; Morocoima et al., 2010; Malavazi et al., 2020). Moreover, a similar situation was described by Rendon et al. in palm forests with sylvatic R. prolixus in the Casanare department, Colombia, with a regular sylvatic enzootic cycle.
In Colombia, different studies have demonstrated that domestic dogs play a role as synanthropic reservoirs that link domestic and sylvatic environments (Ramirez et al., 2013; Cantillo-Barraza et al., 2020b; Jaimes-Dueñez et al., 2020). TcI sylvatic in dogs supports this role in an area with sylvatic R. prolixus. In the Vichada department, the T. cruzi transmission has the extra domestic palms as microfoci; therefore, the infections could occur when domestic dogs enter the forest to hunt or accompany their owners. This absence of infestation may be related to the low intensity of transmission.
Additionally, D. marsupialis is the main reservoir in Colombia’s T. cruzi sylvatic transmission cycle (Rodríguez-Monguí et al., 2019). Recently, it has been suggested that this species play a relevant role as a synanthropic reservoir due to its behavior in some Colombian and Ecuador areas (Souto et al., 1996; Ocana-Mayorga et al., 2010; Betancourt-Echeverri et al., 2021). Our study showed that D. marsupialis was the most common sylvatic mammal with an infection level of 29.4% (5/17), reaffirming its importance in maintaining T. cruzi transmission in the study area. Moreover, the results support the species’ significance as a vehicle connecting the microfoci of T. cruzi transmission in forest palms with the villages where indigenes and colonist people of the Vichada department (Cantillo-Barraza et al., 2015; Rendon et al., 2015).
The serological evaluation results of the school-aged population in this study showed a low frequency of infection (0,06%, 95%CI: 0.007-0.211), suggesting a low level of contact among residents with T. cruzi transmission cycles. A similar situation was described by the serological results of school children in the Casanare department, where the infection was 1,25%, revealing an infrequent contact with the sylvatic cycle (Rendon et al., 2015; Olivera et al., 2019). The present study represents the most considerable collection effort and serological evaluation recorded in Colombia under field conditions after the National Study of Chagas between 1998 to 2002 (WHO. Grupo de trabajo científico, 2007). A total of 3,425 people throughout the Vichada department were included in this study, which allowed the inclusion of the entire indigenous population that voluntarily declared to participate. Our results contrast with the high T. cruzi prevalence reported in neighboring departments such as Casanare and Arauca (Llau et al., 2019; Olivera et al., 2019). However, epidemiological differences exist between these departments because, in the Vichada, the R. prolixus domiciliation was not registered in this study. It is important to highlight that this study was carried out during 2016-2017, data that can serve as an epidemiological baseline. Still, continuous surveillance must be established in this study area to keep the distribution and appearance of new cases updated.
In conclusion, the entomological, serological, and molecular evaluation of humans, domestic and synanthropic mammals showed that the Vichada department presents a non-domiciled and complete sylvatic cycle of T. cruzi transmission mediated by R. prolixus infesting palms of A. butyracea and M. flexuosa. These results show that R. prolixus wild populations have less epidemiological relevance than domiciled populations but require new approaches to control.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
With previous informed consent signed by both parents or legal tutors in the boarding school and following the requirement of the University of Antioquia (License 08-012-185), blood samples were collected from school-aged children, and assent forms were signed by the participants. Individuals over 18 years signed their consent forms/provided. Also, we had written permits from authorities of five indigenous ethnicities. The design and development of the study were carried out following the guidelines of the Declaration of Helsinki 2002 and the International Ethical Standards for Research in Humans and health research. All procedures were designed to reduce animal suffering. All owners were informed about the risks of Chagas disease, both for the human and canine populations. All animals were handled in strict accordance with the Colombian code of practice for the care and use of animals for scientific purposes, established by law 84 of 1989. Ethical approval (Act No 2223) for analyzing animal species was obtained from the animal ethics committee of Antioquia University.
Author contributions
Conceptualization: OC-B and OT-C. Data curation: OC-B, MO, AM-J and SX. Formal analysis: OC-B, CS, AZ, RH, AM-J & OT-C. Funding acquisition: OC-B, CS, AZ and OT-C. Investigation: OC-B, CS, RH, MO, EG, AM-J and OT-C. Methodology: OC-B, CS, RH, MO, EG, SX, AM-J and OT-C. Project administration: OC-B and OT-C. Resources: OC-B, AZ and OT-C. Supervision: OC-B and OT-C. Validation: OC-B and OT-C. Writing – review & editing: OC-B, CS & OT-C. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Universidad de Antioquia (UdeA), and SGR (Sistema General de Regalías), convenio interadministrativo 391 Gobernación del Vichada- Universidad de Antioquia.
Acknowledgments
We are very grateful to the staff of the Health Secretary from the Vichada Department for the help collecting samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Betancourt-Echeverri, A., Pereira-Patiño, A., Quintero-Garcia, W., López-Rueda, P., Uribe-Delgado, N. (2021). Identification de helmintos en didelphis marsupialis (Didelphidae) y rattus rattus (Muridae) en el área metropolitana de bucaramanga - Colombia. Actual Biol. 43, 1–13. doi: 10.17533/udea.acbi.v43n114a03
Calzada, J. E., Pineda, V., Montalvo, E., Alvarez, D., Santamaría, A. M., Samudio, F., et al. (2006). Human trypanosome infection and the presence of intradomicile Rhodnius pallescens in the western border of the Panama canal, Panama. Am. J. Trop. Med. Hyg 74, 762–765. doi: 10.4269/ajtmh.2006.74.762
Cantillo-Barraza, O., Bedoya, S. C., Xavier, S. C. C., Zuluaga, S., Salazar, B., Vélez-Mira, A., et al. (2020a). Trypanosoma cruzi infection in domestic and synanthropic mammals such as potential risk of sylvatic transmission in a rural area from north of antioquia, Colombia. Parasite Epidemiol. Control 1, 11. doi: 10.1016/j.parepi.2020.e00171
Cantillo-Barraza, O., Chaverra, D., Marcet, P. L., Arboleda-Sánchez, S., Triana-Chávez, O. (2014). Trypanosoma cruzi transmission in a Colombian Caribbean region suggests that secondary vectors play an important epidemiological role. Parasites Vectors 7, 381. doi: 10.1186/1756-3305-7-381
Cantillo-Barraza, O., Garcés, E., Gómez-Palacio, A., Cortés, L. A., Pereira, A., Marcet, P. L., et al. (2015). Eco-epidemiological study of an endemic chagas disease region in northern Colombia reveals the importance of Triatoma maculata (Hemiptera: Reduviidae), dogs and Didelphis marsupialis in Trypanosoma cruzi maintenance. Parasites Vectors 8, :482. doi: 10.1186/s13071-015-1100-2
Cantillo-Barraza, O., Medina, M., Zuluaga, S., Valverde, C., Motta, C., Ladino, A., et al. (2020b). Eco-epidemiological study reveals the importance of Triatoma dimidiata in the Trypanosoma cruzi transmission, in a municipality certified without transmission by Rhodnius prolixus in Colombia. Acta Trop. 209, 105550. doi: 10.1016/j.actatropica.2020.105550
Cantillo-Barraza, O., Torres, J., Hernández, C., Romero, Y., Zuluaga, S., Correa-Cárdenas, C. A., et al. (2021). The potential risk of enzootic trypanosoma cruzi transmission inside four training and re-training military battalions (BITER) in Colombia. Parasites Vectors 14, 519. doi: 10.1186/s13071-021-05018-4
Ceccarelli, S., Balsalobre, A., Medone, P., Cano, M. E., Gonçalves, R. G., Feliciangeli, D., et al. (2018). DataTri, a database of American triatomine species occurrence. Sci. Data 5, 180071. doi: 10.1038/sdata.2018.71
Chagas, C. (1909). Nova Tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz 1, 159–218. doi: 10.1590/S0074-02761909000200008
Cura, C. I., Mejia-Jaramillo, A. M., Duffy, T., Burgos, J. M., Rodriguero, M., Cardinal, M. V., et al. (2010). Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. Int. J. Parasitol. 40, 1599–1607. doi: 10.1016/j.ijpara.2010.06.006
Fedepalma (2020) No title Fedepalma,2020. Available at: https://web.fedepalma.org/.
Feliciangeli, M. D., Sanchez-Martin, M., Marrero, R., Davies, C., Dujardin, J. P. (2007). Morphometric evidence for a possible role of Rhodnius prolixus from palm trees in house re-infestation in the state of barinas (Venezuela). Acta Trop. 101, 169–177. doi: 10.1016/j.actatropica.2006.12.010
Gallego Gallego, E. J. (2018). Nivelación geodésica del instituto geográfico agustin codazzi (IGAC). Bogota: Universidad Distrital Francisco Jose de Caldas.
Garcia-Alzate, R., Lozano-Arias, D., Reyes-Lugo, R. M., Morocoima, A., Herrera, L., Mendoza-Leon, A. (2014). Triatoma maculata, the vector of Trypanosoma cruzi, in venezuela. phenotypic and genotypic variability as potential indicator of vector displacement into the domestic habitat. Front. Public Healh 2, 170. doi: 10.3389/fpubh.2014.00170
Guhl, F. (2007a). Chagas disease in Andean countries. Mem Inst Oswaldo Cruz 102, 29–38. doi: 10.1590/S0074-02762007005000099
Guhl, F. (2007b). Chagas disease in Andean countries. Mem Inst Oswaldo Cruz 102, 29–38. doi: 10.1590/S0074-02762007005000099
Guhl, F., Aguilera, G., Pinto, N., Vergara, D. (2007). Updated geographical distribution and ecoepidemiology of the triatomine fauna (Reduviidae: Triatominae) in Colombia. Biomedica 27 Suppl 1, 143–162. doi: 10.7705/biomedica.v27i1.258
Guhl, F., Pinto, N., Aguilera, G. (2009). Sylvatic triatominae: a new challenge in vector control transmission. Mem Inst Oswaldo Cruz 104, 71–75. doi: 10.1590/S0074-02762009000900012
Guhl, F., Ramirez, J. D. (2013). Retrospective molecular integrated epidemiology of chagas disease in Colombia. Infect. Genet. Evol. 20c, 148–154. doi: 10.1016/j.meegid.2013.08.028
Hernandez, C., Salazar, C., Brochero, H., Teheran, A., Buitrago, L. S., Vera, M., et al. (2016). Untangling the transmission dynamics of primary and secondary vectors of Trypanosoma cruzi in Colombia: parasite infection, feeding sources and discrete typing units. Parasit Vectors 9, 620. doi: 10.1186/s13071-016-1907-5
Jaimes-Dueñez, J., Cantillo-Barraza, O., Triana-Chávez, O., Mejia-Jaramillo, A. M. (2020). Molecular surveillance reveals bats from eastern Colombia infected with Trypanosoma theileri and Trypanosoma wauwau like parasites. Prev. Vet. Med., 184, 105159. doi: 10.1016/j.prevetmed.2020.105159
Jaimes-Dueñez, J., Triana-Chávez, O., Cantillo-Barraza, O., Hernández, C., Ramírez, J. D., Góngora-Orjuela, A. (2017). Molecular and serological detection of Trypanosoma cruzi in dogs (Canis lupus familiaris) suggests potential transmission risk in areas of recent acute chagas disease outbreaks in Colombia. Prev. Vet. Med., 141, 141. doi: 10.1016/j.prevetmed.2017.03.009
Lent, H., Wigodzinsky, P. (1979). Revision of the triatominae (Hemiptera, reduviidae) and their significance as vector of chagas disease. Bull. Am. Nat. Hist 163, 123–520.
Llau, A. F., Tejada, C. E., Ahmed, N. U. (2019). Chagas disease prevalence in Colombia: A meta-analysis and systematic review. Vector Borne Zoonotic Dis. 19, 81–89. doi: 10.1089/vbz.2018.2308
Luitgards-Moura, J. F., Vargas, A. B., Almeida, C. E., Magno-Esperança, G., Agapito-Souza, R., Folly-Ramos, E., et al. (2005). A Triatoma maculata (Hemiptera, reduviidae, triatominae) population from roraima, Amazon region, Brazil, has some bionomic characteristics of a potential chagas disease vector. Rev. Inst Med. Trop. Sao Paulo 47, 131–137. doi: 10.1590/S0036-46652005000300003
Malavazi, P. F. N. S., Daudt, C., Melchior, L. A. K., Meneguetti, D. U. O., Xavier, S. C. C., Jansen, A. M., et al. (2020). Trypanosomes of vectors and domestic dogs in Trypanosoma cruzi transmission areas from Brazilian southwestern Amazon: new mammalian host for trypanosoma janseni. Acta Trop. 210, 105504. doi: 10.1016/j.actatropica.2020.105504
Ministerio de la protección social (2012). Guía para la atención clínica integral del paciente con enfermedad de chagas. Medicina Laboratorio 18, 1–2.
Ministerio del interior (2022). Dirección de asuntos indigenas, Rom y Minorías. Available at: https://www.mininterior.gov.co/direccion-de-asuntos-indigenas-rom-y-minorias/vichada/.
Monsalve, Y., Panzera, F., Herrera, L., Triana-Chávez, O., Gómez-Palacio, A. (2016). Population differentiation of the chagas disease vector Triatoma maculata (Erichson, 1848) from Colombia and Venezuela. J. Vector Ecol. 41, 72–79. doi: 10.1111/jvec.12196
Morocoima, A., Chique, J., Zavala-Jaspe, R., Diaz-Bello, Z., Ferrer, E., Urdaneta-Morales, S., et al. (2010). Commercial coconut palm as an ecotope of chagas disease vectors in north-eastern Venezuela. J. Vector Borne Dis. 47, 76–84.
Moser, D. R., Kirchhoff, L. V., Donelson, J. E. (1989). Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J. Clin. Microbiol. 27, 1477–1482. doi: 10.1128/jcm.27.7.1477-1482.1989
Ocana-Mayorga, S., Llewellyn, M. S., Costales, J. A., Miles, M. A., Grijalva, M. J. (2010). Sex, subdivision, and domestic dispersal of Trypanosoma cruzi lineage I in southern Ecuador. PLos Negl. Trop. Dis. 4, 1–8. doi: 10.1371/journal.pntd.0000915
Olivera, M. J., Fory, J. A., Porras, J. F., Buitrago, G. (2019). Prevalence of chagas disease in Colombia: A systematic review and meta-analysis. PloS One 14, e0210156. doi: 10.1371/journal.pone.0210156
PAHO - Pan American Health Organization (2019) Evaluación internacional de la situación epidemiológica y de control de chagas en 34 municipios de los departamentos de arauca, boyacá, casanare, norte santander, santander y vichada, Colombia. Available at: https://www.minsalud.gov.co/sites/rid/Lists/Biblio-tecaDigital/RIDE/VS/PP/ET/informe-verificacion-interrupcion-transmision-vectorial-chagas-2019.pdf.
Peña, V., Fernández, G., Gómez-Palacio, A. M., Mejía-Jaramillo, A. M., Cantillo, O., Triana-Chávez, O. (2012). High-resolution melting (HRM) of the cytochrome b gene: a powerful approach to identify blood-meal sources in chagas disease vectors. Plos Negl. Trop. Dis. 6 (2), e1530. doi: 10.1371/journal.pntd.0001530
Ramírez, J. D., Guhl, F., Messenger, L. A., Lewis, M. D., Montilla, M., Cucunuba, Z., et al. (2012). Contemporary cryptic sexuality in trypanosoma cruzi. Mol. Ecol. 17, 4216–4226. doi: 10.1111/j.1365-294X.2012.05699.x
Ramirez, J. D., Hernandez, C., Montilla, M., Zambrano, P., Florez, A. C., Parra, E., et al. (2014). First report of human Trypanosoma cruzi infection attributed to TcBat genotype. Zoonoses Public Health 61 (7), 477–479. doi: 10.1111/zph.12094
Ramirez, J. D., Turriago, B., Tapia-Calle, G., Guhl, F. (2013). Understanding the role of dogs (Canis lupus familiaris) in the transmission dynamics of Trypanosoma cruzi genotypes in Colombia. Vet. Parasitol. 196, 216–219. doi: 10.1016/j.vetpar.2012.12.054
Rendon, L. M., Guhl, F., Cordovez, J. M., Erazo, D. (2015). New scenarios of Trypanosoma cruzi transmission in the Orinoco region of Colombia. Mem Inst Oswaldo Cruz 110, 283–288. doi: 10.1590/0074-02760140403
Rodríguez-Monguí, E., Cantillo-Barraza, O., Prieto-Alvarado, F. E., Cucunubá, Z. M. (2019). Heterogeneity of Trypanosoma cruzi infection rates in vectors and animal reservoirs in Colombia: A systematic review and meta-analysis. Parasites Vectors 12, 308. doi: 10.1186/s13071-019-3541-5
Solis-Medina, C., Zuluaga Aguirre, S., Triana-Chavez, O., Cantillo-Barraza, O. (2021). Infección natural por Trypanosoma cruzi (TRYPANOSOMATIDAE) en triatominos intradomesticos del departamento de guainía. Acta Biol. Colomb 26, 127–130. doi: 10.15446/abc.v26n1.84343
Souto, R. P., Fernandes, O., Macedo, A. M., Campbell, D. A., Zingales, B. (1996). DNA Markers define two major phylogenetic lineages of trypanosoma cruzi. Mol. Biochem. Parasitol. 83, 141–152. doi: 10.1016/S0166-6851(96)02755-7
WHO (2015). Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol. Rec 90 (6), 33–43.
WHO. Grupo de trabajo científico (2007). Reporte sobre la enfermedad de Chagas.17-20 de abril de 2005. Ed. Lazdins-Helds, G. F. Geneva: World Health Organization
Zingales, B., Miles, M. A., Campbell, D. A., Tibayrenc, M., Macedo, A. M., Teixeira, M. M., et al. (2012). The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2, 240–253. doi: 10.1016/j.meegid.2011.12.009
Keywords: Chagas disease, Colombia, Rhodnius prolixus, Triatoma maculata, Trypanosoma cruzi, Didelphis marsupialis
Citation: Cantillo-Barraza O, Solis C, Zamora A, Herazo R, Osorio MI, Garcés E, Xavier S, Mejía-Jaramillo AM and Triana-Chávez O (2022) Enzootic Trypanosoma cruzi infection by Rhodnius prolixus shows transmission to humans and dogs in Vichada, Colombia. Front. Cell. Infect. Microbiol. 12:999082. doi: 10.3389/fcimb.2022.999082
Received: 20 July 2022; Accepted: 28 September 2022;
Published: 18 October 2022.
Edited by:
Ana Gonçalves Domingos, New University of Lisbon, PortugalReviewed by:
Wilfredo Quiñones, Universidad de Los Andes, VenezuelaAlessandra Aparecida Guarneri, René Rachou Institute, Oswaldo Cruz Foundation (FIOCRUZ), Brazil
Copyright © 2022 Cantillo-Barraza, Solis, Zamora, Herazo, Osorio, Garcés, Xavier, Mejía-Jaramillo and Triana-Chávez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omar Triana-Chávez; omar.triana@udea.edu.co
 Omar Cantillo-Barraza
Omar Cantillo-Barraza Cesil Solis
Cesil Solis Alexander Zamora2
Alexander Zamora2  Samanta Xavier
Samanta Xavier Ana María Mejía-Jaramillo
Ana María Mejía-Jaramillo Omar Triana-Chávez
Omar Triana-Chávez