Antimicrobial activity of dietary supplements based on bacterial lysate of Lactobacillus rhamnosus DV
- 1Educational and Scientific Centre, Institute of Biology and Medicine, Taras Shevchenko National University of Kyiv, Kyiv, Ukraine
- 2Department of Normal Physiology Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
- 3Endocrinology Department, Bogomolets National Medical University, Kyiv, Ukraine
- 4Scientific Department, Medical Laboratory CSD, Kyiv, Ukraine
- 5Department of Biotechnology and Microbiology, National University of Food Technologies, Kyiv, Ukraine
- 6Scientific Department, MirImmunoPharm LLC, Kyiv, Ukraine
Introduction: According to WHO, antibiotic resistance is increasing to hazardous levels worldwide. Candidiasis often occurs after taking antibiotics. Therefore, antibiotic resistance is a global problem and searching for antibacterial agents is necessary.
Aim: To determine the antimicrobial activity of bacterial lysate of Lactobacillus (L.) rhamnosus DV separately and with plant extracts against bacterial and yeast test cultures.
Material and methods: Antimicrobial activity of Del-Immune V® (cell wall and DNA fragments from a L. rhamnosus DV) separately and with cinnamon, beetroot, and blackcurrant extracts was determined by the minimum inhibitory concentration (MIC). Twofold serial dilutions determined the MIC in previously prepared meat-peptone broth (MPB) for bacteria and liquid wort for yeast. In the study, gram-negative (Escherichia coli IEM-1, Proteus vulgaris PА-12, Pseudomonas sp. MI-2, L. rhamnosus 13/2) and gram-positive (Bacillus (B.) subtilis BТ-2, Staphylococcus aureus BМС-1) bacteria, as well as yeast (Candida (C.) albicans D-6, C. tropicalis PE-2, C. utilis BVS-65) were used as test cultures.
Results: The MIC for the studied bacterial test cultures after application of L. rhamnosus DV bacterial lysates was from 1.0 ± 0.05 mg/mL to 12.5 ± 0.63 mg/mL, which was significantly less than that of the thermally inactivated control (MIC from 125.0 ± 6.25 mg/mL to 250.0 ± 12.5 mg/mL). B. subtilis BT-2 culture was the least sensitive to the action of the bacterial lysate (MIC—12.5 ± 0.63 mg/mL). It showed the best antibacterial and antifungal effect bacterial lysate with the phytonutrient blackcurrant.
Conclusions: It was demonstrated that bacterial lysate of lactic acid bacteria L. rhamnosus DV exhibits antibacterial and antifungal properties during direct contact with pathogenic agents.
Introduction
The scientific literature points to the beneficial properties of probiotics in the process of regulating metabolism (Falalyeyeva et al., 2022; Kobyliak et al., 2022; Falalyeyeva et al., 2023; Lazarenko et al., 2023), yet at the same time, some scientific papers question the effectiveness and safety of probiotics (Merenstein et al., 2023). In turn, postbiotics and metabiotics are preparations of inanimate microorganisms and/or their components, which are directly identified with the safety of their use and the health benefits of the host (Bourebaba et al., 2022). Due to the chemical structure of postbiotics and metabiotics, it is found that they have many health benefits; in particular, they have a local effect on certain tissues of the intestinal epithelium and influence on many other organs and tissues. Postbiotics metabolites and metabiotics structural cell fragments create the appearance of a therapeutic effect of probiotics, which, in turn, limits the risk of introducing living microorganisms into a weakened immune defense. It should also be pointed out that postbiotics and metabiotics are more stable and have a longer shelf life. Inactivation of bacterial cells can be achieved by physical means (mechanical destruction, high hydrostatic pressure, γ or UV irradiation, heat treatment, freeze drying, ultrasonication) or chemical methods (acid deactivation) (De Almada et al., 2016).
In the current scientific discussion, the term “postbiotic” also comprises cell-free supernatants with bacterial metabolites. These include soluble factors (products or by-products of metabolism) released by living bacteria or released after bacterial lysis. The term “metabiotics” is often used for determining probiotic cell wall fragments and DNA motifs after probiotic cell enzymatic destruction (Oberg et al., 2011; Cicenia et al., 2014). Such soluble factors have been isolated from a number of strains of bacteria. They are short-chain fatty acids (SCFA), peptides, enzymes, teichoic acids, muropeptides derived from peptidoglycans, endo- and exopolysaccharides, cell surface proteins, plasmalogens, vitamins, and organic acids (Chen and Hoover, 2003; Cavallari et al., 2017). Nisin, a widely used preservative in several foods (dairy products, infant formula, canned soups), can be produced by Lactococcus lactis subsp. lactis (Tomasik and Tomasik, 2020).
Immune function and alleviation of digestive problems is also the relevant area of use of postbiotics today. According to some studies, postbiotic supplements can help with irritable bowel syndrome (IBS) and the symptoms of inflammatory bowel disease (IBD), as well as prevent respiratory tract infections (Thorakkattu et al., 2022). Although the exact processes are not yet well understood or unknown, in vitro studies have shown that postbiotics exhibit antibacterial, anti-inflammatory, immunomodulatory, antiproliferative, and antioxidant effects (Cuevas-González et al., 2020; Thorakkattu et al., 2022). The use of postbiotics as functional food has several advantages in the industrial processing and commercialization of food products. This contributes to the growth of the functional food market. From a practical standpoint, postbiotics are more stable and safer for food and pharmaceutical purposes than the living bacteria from which they are produced, because their viability is not actually required for large-scale production or consumption (Barros et al., 2020).
It is worth noting that changing the processing conditions of microbial suspensions or scaling can lead to significant structural modifications and variations in the physiological functions of postbiotics, and this will complicate the production of postbiotics on a large scale. Previously obtained results demonstrated no side effects, therapeutic potency, or probiotic lysates’ bifidogenic activity, indicating a more intense activation of endogenous immune protective factors following postbiotics. The probiotic lysates classified as postbiotics demonstrate indisputable advantages for their use as a safe and potent bio-therapeutics source with high immunotherapeutic potency involving cellular and humoral immunity (Sichel et al., 2013). Given the above, using food products as a “vehicle” for postbiotics is promising and relevant today, although it has quite a few unexplored problems.
According to the World Health Organization, antibiotic resistance is rising to dangerously high levels in all parts of the world (World Health Organization, 2020). New resistance mechanisms are emerging and spreading globally, threatening our ability to treat common infectious diseases. Candidiasis often occurs after taking antibiotics. Therefore, searching for new antimicrobial agents of natural origin is an extraordinary global problem. The work aimed to determine the antimicrobial activity of bacterial lysate of L. rhamnosus DV separately and with plant extracts against bacterial and yeast test cultures.
Material and methods
Research object
In research, the cell lysates of lactic acid bacterial strain L. rhamnosus DV were investigated, which were included in the Del-Immune V® dietary supplement produced by MirImmunoPharm LLC (Ukraine) in cooperation with Stellar Biotics, LLC (USA).
Content: L. rhamnosus DV lysate lyophilized powder: protein 100 mg/g; DNA 118 mg/g; muramyl peptides 15.0–18.0 mg/g; pH of 1% suspension 6.5–7.5; moisture (%), no more 5.0. The dietary supplement was represented by cell lysates of lactic acid bacterial strain L. rhamnosus DV in capsules containing lyophilisate of cells.
Also, we checked bacterial lysate (lyophilized enzymatic lysate of cells of the lactic acid bacterial strain Lactobacillus rhamnosus DV—50.0 mg (mg)) with cinnamon (cinnamon extract (5:1)—200 mg (mg)), beetroot (beet root extract (10:1)—500 mg (mg)), and blackcurrant (blackcurrant extract (10:1)—200 mg (mg)) extracts. Samples of thermally treated substances devoid of biological activity were used as a control.
Preparation of test samples
In studies, samples containing cell lysates of the lactic acid bacterial strain L. rhamnosus DV of various concentrations were used as postbiotics. For this, a sample of the postbiotic in 25 mg was dissolved in a sterile phosphate buffer (0.1 M, pH 7.0) to an initial volume of 25 mL. Dilution was done with the same buffer to the required concentration.
Test culture
Bacterial strains (Escherichia (E.) coli IEM-1, Staphylococcus aureus BMS-1, Bacillus (B.) subtilis BT-2, Proteus (P.) vulgaris PA-12, Pseudomonas sp. MI-2, L. rhamnosus 13/2) and yeast (Candida (C.) albicans D-6, C. tropicalis PE-2, C. utilis BVS-65) from the collection of live cultures of the Department of Biotechnology and Microbiology of the National University of Food Technologies (Ukraine) were used as test cultures in determining the antimicrobial and antifungal activity of the postbiotic.
The procedure of study design
The antimicrobial activity of the postbiotics samples was analyzed according to the minimum inhibitory concentration (MIC) (Pirog et al., 2005; GoneliMali et al., 2018). The MIC was determined by twofold serial dilutions in meat-peptone broth (MPB) for bacteria and in liquid wort for yeast. Under sterile conditions, 1 mL of the medium was introduced into 10 test tubes, 1 mL of the sample solution of the maximum concentration was added to the first one, after which it was stirred, and 1 mL was taken and transferred to the next test tube. Dilution was similarly carried out for the following 12 test tubes. From the last test tube, 1 mL of solution was taken. Thus, the final volume of each tube was 1 mL (MPB or wort and postbiotic sample solution), and the concentration of the postbiotic in each subsequent tube was reduced by half. As a control, 1 mL of the postbiotic sample was used after sterilization in an autoclave (Р = 0.05, MPa = 30 min). Next, 0.1 mL of test culture suspension (105–106 CFU/mL) was added to each of the test tubes and stirred. The tubes were incubated for 24 h at 28°C–30°C for bacteria and 24°C–26°C for yeast. The results were evaluated by the optical density in percentage. The MIC was considered the lowest concentration inhibiting the respective microorganisms’ growth. All assays were performed in triplicate (GoneliMali et al., 2018).
The antimicrobial activity of L. rhamnosus 13/2 was investigated by the methods “paper disks,” “agar blocks,” and “well” methods (Hong-Thao et al., 2016). The dosage of the live culture is in CFU/mL. The results were registered visually according to the presence/absence of growth retardation zones of the test cultures on dense nutrient media.
Statistical analysis
Statistical processing of the experimental data was carried out as previously described (Chebbi et al., 2017). The statistical analysis was performed using STATISTICA software (Version 13.3). Data were presented in simple measures of frequency, percentage, mean, standard deviation, and range (minimum–maximum values). The significance of the difference was tested using Student’s t-test for the difference between two independent means. A p-value equal to or less than 0.05 was considered statistically significant.
Results
The data presented in Table 1 indicate that the MIC for the studied bacterial test cultures after application of L. rhamnosus DV bacterial lysates was from 1.0 ± 0.05 to 12.5 ± 0.63 mg/mL, which was significantly less than that of the thermally inactivated control (MIC from 125.0 ± 6.25 to 250.0 ± 12.5 mg/mL). It should be noted that the cell lysate of the lactic acid bacterial strain L. rhamnosus DV exhibited a high antimicrobial effect not only against gram-positive (B. subtilis BТ-2, S. aureus BMS-1) but also against gram-negative (E. coli IEM-1, P. vulgaris PА-12, Pseudomonas sp. МІ-2) bacteria. The culture of B. subtilis BT-2 appeared to be the least sensitive to the postbiotic (MIC—12.5 ± 0.63 mg/mL). The MIC ranged from 1.0 ± 0.05 to 4.07 ± 0.2 mg/mL for other cultures.
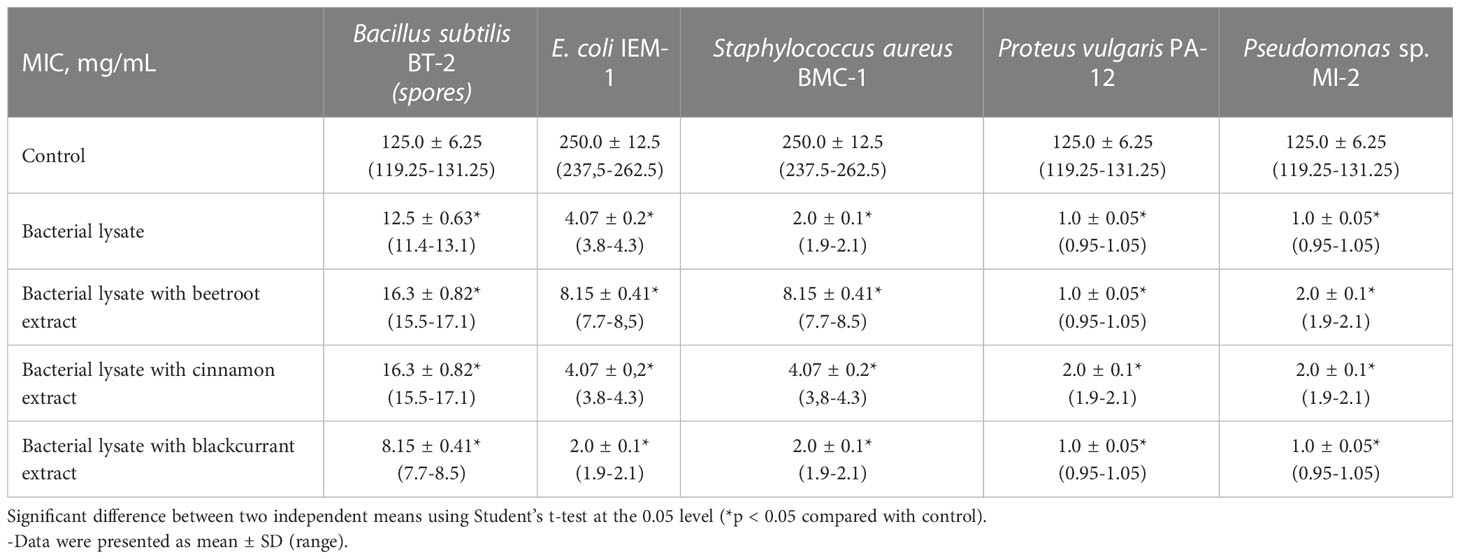
Table 1 Antibacterial activity of bacterial lysates of lactic acid bacterial strain L. rhamnosus DV separately and with the addition of phytonutrient from beetroot, cinnamon, and blackcurrant.
It has also been shown that supplements that add plant extracts showed different antibacterial activities. The L. rhamnosus DV bacterial lysates with the phytonutrients beetroot and cinnamon had less antibacterial activity than a supplement without additives. It may be due to the extracts’ protective substances for bacterial test strains. Comparing these compositions showed the best antimicrobial effect of the bacterial lysates L. rhamnosus DV with the phytonutrient blackcurrant.
The study of the antifungal action showed that the bacterial lysates L. rhamnosus DV are characterized by a pronounced fungicidal activity against Candida yeast. Data on the study of the action of antifungal substances of bacterial lysates of various postbiotics are presented in Table 2.

Table 2 Antifungal activity of bacterial lysates of lactic acid bacterial strain L. rhamnosus DV separately and with the addition of phytonutrient from beetroot, cinnamon, and blackcurrant.
These findings indicate that the bacterial cell lysates L. rhamnosus DV (with and without plant extracts) show less antifungal activity against test microorganisms than bacterial test microorganisms. Thus, the MIC was in the range of 65.2 ± 3.25 to 125.0 ± 6.25 mg/mL, which was 4 (p < 0.05) to 8 (p < 0.05) times less than the control. Despite this, we may consider these concentrations therapeutic, given the low toxicity level of the postbiotic. However, it should be noted that the supplements with extracts were more active against yeast test cultures. The bacterial cell lysates L. rhamnosus DV with the phytonutrient blackcurrant had more profound antifungal activity (Table 2).
Experiments were conducted in vitro, so we can talk about the direct effect of the postbiotic on microorganisms. It was necessary to compare the influence of probiotic lysate with the living cells L. rhamnosus. Since it is impossible to check the activity of living cells using the method specified in the article, it was decided to use the following methods: “paper disks,” “agar blocks,” and “well” methods. Dosage of the drug was carried out in mg/mL, and that in the case of live culture was in CFU/mL.
It was shown that when testing the activity by the “well method,” a live culture of L. rhamnosus prevents the growth of S. aureus BMS-1 at a dilution of 105 CFU/mL. The application dose was 200 μL (2*104 CFU in 200 μL). All other cultures showed resistance to the first dilution, which was at the level of 107 CFU/mL. The “paper disk” and “agar block” methods showed no growth retardation of the test cultures due to the insignificant amounts of living cells in the agar block and paper disks. Because the units of measurement cannot be directly compared, we can only conclude the qualitative and not the quantitative component of antimicrobial activity in relation to the test cultures of microorganisms.
Discussion
Impaired wound healing is a growing medical problem, and very few approved drugs with documented clinical efficacy are available. Lactic acid bacteria exhibit antibacterial activity due to the production of lactic acid, acetic acid, hydrogen peroxide, diacetyl, CO2, and bacteriocins. However, reports on using lactic acid bacteria in bioconservation are limited due to their antifungal effects (Maier et al., 2010). It has been suggested that topical lactic acid bacteria can improve skin health or combat disease. It has been shown that specific lactobacilli strains have a beneficial role in the wound healing process, in defense against the inflammatory processes that affect the skin, and in resistance to infections by interfering with pathogens (Baquerizo Nole et al., 2014; Lukic et al., 2017; Knackstedt et al., 2020). CXCL12-expressing lactic acid bacteria, Limosilactobacillus reuteri (ILP100-Topical), has been demonstrated to accelerate wound healing in controlled preclinical models (Öhnstedt et al., 2022). In the clinical study, ILP100-Topical was safe and well-tolerated in all individuals and doses with no systemic exposure. A combined cohort analysis showed a significantly more significant proportion of healed wounds by multi-dosing of ILP100-Topical when compared with placebo. The time to first registered healing was shortened by 6 days on average and by 10 days at the highest dose. ILP100-Topical increased the density of CXCL12+ cells in the wounds and local wound blood perfusion (Öhnstedt et al., 2023).
The International Scientific Association for Probiotics and Prebiotics raised questions regarding the safety of probiotics, especially among vulnerable populations (such as newborns, pregnant women, patients with short bowel syndrome, and immunocompromised). The authors noted that the most significant danger is the possibility of antibiotic resistance gene transfer via transformation, the potential impact of probiotic−induced changes in microbiomes, and drug interactions (Merenstein et al., 2023). Recent scientific studies on the stated issues point to the benefits of some postbiotics in treating metabolic disorders (Bourebaba et al., 2022). The practical use of probiotics and the study of the mechanism of their action were made lately to find that a certain level of biological activity is preserved by dead probiotic cells and even their lysates, which are the natural mixes of metabiotic and postbiotic substances—a biological activity which is strongly oriented toward gut health and immune system regulation. Because probiotic lysates demonstrated biological activity without any of the potential adverse side effects associated with live bacterial cells, one of the future goals is research of the novel postbiotics and metabiotics substances as well as their individual structures and biological characteristics for understanding their way of communications with host cells and microbiota representatives. That is why studies conducted to study the antibacterial activity of postbiotics are extremely important.
Data about using probiotic culture lysates in medical practice to control infectious diseases are not widely presented. It has also been shown that L. rhamnosus LR lysate improves skin barrier function in a reconstructed human epidermis model (Jung et al., 2019). In our study, supplements containing bacterial lysates of lactic acid bacterial strain L. rhamnosus DV exhibit antibacterial and antifungal activity. The spectrum of antimicrobial activity of the studied medications concerned gram-positive and gram-negative bacteria, along with yeast-like fungi of the genus Candida. Antimicrobial activity was demonstrated by bacterial lysate samples that were thermally inactivated, indicating that the active substances are thermolabile.
It is widely accepted that bioactive compounds extracted from plants significantly impact human health. As a result, it is crucial to comprehend the antimicrobial effectiveness and mode of operation of these plant-derived bioactive compounds (Kha and Le, 2021). Plants with medicinal properties contain various phytochemicals like flavonoids, alkaloids, tannins, and terpenoids. These natural compounds have antimicrobial and antioxidant properties (GoneliMali et al., 2018). Many plant species have been extensively studied for their antimicrobial properties. Cinnamon, beetroot, blackcurrant, garlic, basil, curry, ginger, sage, mustard, and other herbs have crude extracts that exhibit antimicrobial effects against a wide range of Gram-positive and Gram-negative bacteria (Alzoreky and Nakahara, 2003; Castro et al., 2008; Ikuta et al., 2012; Suriyaprom et al., 2022). In this study, the bacterial lysate added with plant extracts (beetroot and cinnamon) exhibited less antimicrobial activity against bacterial test cultures, which may be associated with the protective properties of the extract components. However, in general, minimal inhibitory doses can also be considered therapeutic. It showed the best antimicrobial effect bacterial lysates of lactic acid bacterial strain L. rhamnosus DV with the phytonutrient blackcurrant. Probiotic lysate showed high activity against gram-positive and gram-negative bacteria and Candida yeasts. Instead, the live culture L. rhamnosus showed an inhibitory effect only of a gram-positive bacterium—S. aureus BMS-1.
Jung et al. (2019) noted that it would be interesting to examine whether the application of live L. rhamnosus can manifest skin barrier-protective effects. However, cosmetics must not be contaminated with microorganisms including probiotic species. The FDA regulates that acceptable limits for total (not pathogenic) microorganisms in cosmetics are 500 colony forming units (CFU) per gram in eye-area products and 1,000 CFU/g for other area products (Herrera, [[NoYear]]). Therefore, using live probiotic strains in cosmetic products must overcome this hurdle whereas different biotherapeutic approaches beyond cosmetics are ongoing with live probiotics (Jung et al., 2019). Indeed, in our work, we have shown that a live culture of L. rhamnosus 13/2 prevents the growth of S. aureus BMS-1 at a huge concentration—105 CFU/mL. All other cultures showed resistance to the first dilution, which was at the level of 107 CFU/mL. Therefore, the obtained data indicate the ineffectiveness of a live culture of L. rhamnosus 13/2 in inhibiting the growth of test cultures of microorganisms.
Given the above, bacterial lysate, which refers to postbiotics, can present the possibility of developing new and more effective therapeutic strategies with a more advanced safety profile while circumventing the risks related to the use of living microorganisms.
Conclusion
Therefore, it was demonstrated that bacterial lysate of lactic acid bacteria L. rhamnosus DV exhibits antibacterial and antifungal properties during direct contact with pathogenic agents. The bacterial lysate of lactic acid bacteria L. rhamnosus DV showed the best antibacterial and antifungal effects of bacterial lysate with the phytonutrient blackcurrant. Obtained results opened a new area for bacterial lysates of lactic acid bacterial strain L. rhamnosus DV topical application, combined with modulation of innate skin immunity with direct impact on infectious agents.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YP, MS, TF, and NK contributed to the conceptualization and the original idea of this manuscript. IK and DO contributed to methodology and reviewed the literature. SO, OT, and OK were involved in validation and revised and validated the literature findings. TF and YP performed formal analysis. YP and IK contributed to investigation. TF and NK were involved in data curation. NK, YP, MS, and TF did the writing—original draft preparation. TF, YP, and MS did the writing—review and editing. NK did visualization. TF and NK were involved in supervision. TF, NK, FG, and YP contributed to project administration. All authors contributed to the article and approved the submitted version.
Conflict of interest
Author FG is employed by MirImmunoPharm LLC, Kyiv, Ukraine
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alzoreky, N., Nakahara, K. (2003). Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 80, 223–230. doi: 10.1016/S0168-1605(02)00169-1
Baquerizo Nole, K. L., Yim, E., Keri, J. E. (2014). Probiotics and prebiotics in dermatology. J. Am. Acad. Dermatol. 71, 814–821. doi: 10.1016/j.jaad.2014.04.050
Barros, C. P., Guimarães, J. T., Esmerino, E. A., Duarte, M. C. K., Silva, M. C., Silva, R., et al. (2020). Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr. Opin. Food Sci. 32, 1–8. doi: 10.1016/j.cofs.2019.12.003
Bourebaba, Y., Marycz, K., Mularczyk, M., Bourebaba, L. (2022). Postbiotics as potential new therapeutic agents for metabolic disorders management. BioMed. Pharmacother. 153, 113138. doi: 10.1016/j.biopha.2022.113138
Castro, S. B. R., Leal, C. A. G., Freire, F. R., Carvalho, D. A., Oliveira, D. F., Figueiredo, H. C. P. (2008). Antibacterial activity of plant extracts from Brazil against fish pathogenic bacteria. Braz. J. Microbiol. 39, 756–760. doi: 10.1590/S1517-838220080004000030
Cavallari, J. F., Fullerton, M. D., Duggan, B. M., Foley, K. P., Denou, E., Smith, B. K., et al. (2017). Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4. Cell Metab. 25, 1063–1074.e3. doi: 10.1016/j.cmet.2017.03.021
Chebbi, A., Elshikh, M., Haque, F., Ahmed, S., Dobbin, S., Marchant, R., et al. (2017). Rhamnolipids from Pseudomonas aeruginosa strain W10; as antibiofilm/antibiofouling products for metal protection. J. Basic Microbiol. 57, 364–375. doi: 10.1002/jobm.201600658
Chen, H., Hoover, D. G. (2003). Bacteriocins and their food applications. Compr. Rev. Food Sci. Food Saf. 2, 82–100. doi: 10.1111/j.1541-4337.2003.tb00016.x
Cicenia, A., Scirocco, A., Carabotti, M., Pallotta, L., Marignani, M., Severi, C. (2014). Postbiotic activities of lactobacilli-derived factors. J. Clin. Gastroenterol. 48, S18–S22. doi: 10.1097/MCG.0000000000000231
Cuevas-González, P. F., Liceaga, A. M., Aguilar-Toalá, J. E. (2020). Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 136, 109502. doi: 10.1016/j.foodres.2020.109502
De Almada, C. N., Almada, C. N., Martinez, R. C. R., Sant’Ana, A. S. (2016). Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 58, 96–114. doi: 10.1016/j.tifs.2016.09.011
Falalyeyeva, T., Chornenka, N., Cherkasova, L., Tsyryuk, O., Molchek, N., Kovalchuk, O., et al. (2022). “Gut Microbiota Interactions With Obesity,” in Reference Module in Food Science. (Elsevier). doi: 10.1016/B978-0-12-819265-8.00030-9
Falalyeyeva, T., Kobyliak, N., Korotkyi, O., Meleshko, T., Sulaieva, O., Hryshchenko, I., et al. (2023). Microbiome and Obesity. (Elsevier, UK: Springer Science and Business Media LLC), 101–131. doi: 10.1007/978-3-031-19564-8_5
GoneliMali, F. D., Lin, J., Miao, W., Xuan, J., Charles, F., Chen, M., et al. (2018). Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.01639
Herrera, A. G. (2004). “Microbiological Analysis of Cosmetics” in Public Health Microbiology (New Jersey: Humana Press), 293–296. doi: 10.1385/1-59259-766-1:293
Hong-Thao, P. T., Mai-Linh, N. V., Hong-Lien, N. T., Van Hieu, N. (2016). Biological Characteristics and Antimicrobial Activity of Endophytic Streptomyces sp. TQR12-4 Isolated from Elite Citrus nobilis Cultivar Ham Yen of Vietnam. Int. J. Microbiol. 2016, 1–7. doi: 10.1155/2016/7207818
Ikuta, K., Hashimoto, K., Kaneko, H., Mori, S., Ohashi, K., Suzutani, T. (2012). Anti-viral and anti-bacterial activities of an extract of blackcurrants ( Ribes nigrum L. ). Microbiol. Immunol. 56, 805–809. doi: 10.1111/j.1348-0421.2012.00510.x
Jung, Y.-O., Jeong, H., Cho, Y., Lee, E.-O., Jang, H.-W., Kim, J., et al. (2019). Lysates of a probiotic, lactobacillus rhamnosus, can improve skin barrier function in a reconstructed human epidermis model. Int. J. Mol. Sci. 20, 4289. doi: 10.3390/ijms20174289
Kha, T. C., Le, L. T. P. (2021). Plant Extracts: Antimicrobial Properties, Mechanisms of Action and Applications. (Springer Science and Business Media LLC), 257–283. doi: 10.1007/978-981-15-7098-8_11
Knackstedt, R., Knackstedt, T., Gatherwright, J. (2020). The role of topical probiotics in skin conditions: A systematic review of animal and human studies and implications for future therapies. Exp. Dermatol. 29, 15–21. doi: 10.1111/exd.14032
Kobyliak, N., Falalyeyeva, T., Kyriachenko, Y., Tseyslyer, Y., Kovalchuk, O., Hadiliia, O., et al. (2022). Akkermansia muciniphila as a novel powerful bacterial player in the treatment of metabolic disorders. Minerva Endocrinol. 47 (2). doi: 10.23736/S2724-6507.22.03752-6
Lazarenko, L., Melnykova, O., Babenko, L., Bubnov, R., Beregova, T., Falalyeyeva, T., et al. (2023). Probiotic Concepts of Predictive, Preventive, and Personalized Medical Approach for Obesity: Lactic Acid Bacteria and Bifidobacteria Probiotic Strains Improve Glycemic and Inflammation Profiles. (Springer Science and Business Media LLC), 371–390. doi: 10.1007/978-3-031-19564-8_14
Lukic, J., Chen, V., Strahinic, I., Begovic, J., Lev-Tov, H., Davis, S. C., et al. (2017). Probiotics or pro-healers: the role of beneficial bacteria in tissue repair. Wound Repair Regener. 25, 912–922. doi: 10.1111/wrr.12607
Maier, E., Kurz, K., Jenny, M., Schennach, H., Ueberall, F., Fuchs, D. (2010). Food preservatives sodium benzoate and propionic acid and colorant curcumin suppress Th1-type immune response in vitro. Food Chem. Toxicol. 48, 1950–1956. doi: 10.1016/j.fct.2010.04.042
Merenstein, D., Pot, B., Leyer, G., Ouwehand, A. C., Preidis, G. A., Elkins, C. A., et al. (2023). Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 15, 2185034. doi: 10.1080/19490976.2023.2185034
Oberg, T. S., Steele, J. L., Ingham, S. C., Smeianov, V. V., Briczinski, E. P., Abdalla, A., et al. (2011). Intrinsic and inducible resistance to hydrogen peroxide in Bifidobacterium species. J. Ind. Microbiol. Biotechnol. 38, 1947–1953. doi: 10.1007/s10295-011-0983-y
Öhnstedt, E., Lofton Tomenius, H., Frank, P., Roos, S., Vågesjö, E., Phillipson, M. (2022). Accelerated wound healing in minipigs by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Pharmaceutics 14, 229. doi: 10.3390/pharmaceutics14020229
Öhnstedt, E., Vågesjö, E., Fasth, A., Lofton Tomenius, H., Dahg, P., Jönsson, S., et al. (2023). Engineered bacteria to accelerate wound healing: an adaptive, randomised, double-blind, placebo-controlled, first-in-human phase 1 trial. eClinicalMedicine 60, 102014. doi: 10.1016/j.eclinm.2023.102014
Pirog, T., Shevchuk, T., Voloshina, I. G. N. (2005). Use of claydite-immobilized oil-oxidizing microbial cells for purification of water from oil. Appl. Biochem. Microbiol. 41, 51–55. doi: 10.1007/s10438-005-0010-z
Sichel, L., Timoshok, N., Pidgorsky V, S. N. (2013). Study of interferonogenous activity of the new probiotic formulation del-immune V®. J. Probiotics Heal 01, 1–6. doi: 10.4172/2329-8901.1000107
Suriyaprom, S., Mosoni, P., Leroy, S., Kaewkod, T., Desvaux, M., Tragoolpua, Y. (2022). Antioxidants of fruit extracts as antimicrobial agents against pathogenic bacteria. Antioxidants 11, 602. doi: 10.3390/antiox11030602
Thorakkattu, P., Khanashyam, A. C., Shah, K., Babu, K. S., Mundanat, A. S., Deliephan, A., et al. (2022). Postbiotics: current trends in food and pharmaceutical industry. Foods 11, 3094. doi: 10.3390/foods11193094
Tomasik, P., Tomasik, P. (2020). Probiotics, non-dairy prebiotics and postbiotics in nutrition. Appl. Sci. 10, 1470. doi: 10.3390/app10041470
World Health Organization (2020). Antibiotic resistance. Available at: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.
Keywords: bacterial lysate, postbiotics, metabiotics, lactic acid bacteria, antimicrobial activity, plant extracts
Citation: Penchuk Y, Savytska M, Kobyliak N, Ostapchenko D, Kolodiy I, Onysenko S, Tsyryuk O, Korotkyi O, Grygoriev F and Falalyeyeva T (2023) Antimicrobial activity of dietary supplements based on bacterial lysate of Lactobacillus rhamnosus DV. Front. Cell. Infect. Microbiol. 13:1211952. doi: 10.3389/fcimb.2023.1211952
Received: 25 April 2023; Accepted: 31 July 2023;
Published: 25 August 2023.
Edited by:
Zhendong Cai, Ningbo University, ChinaReviewed by:
Zhenshang Xu, Qilu University of Technology, ChinaBiswaranjan Pradhan, Indian Institute of Technology Bhubaneswar, India
Aleksandra Maria Kocot, University of Gdansk, Poland
Copyright © 2023 Penchuk, Savytska, Kobyliak, Ostapchenko, Kolodiy, Onysenko, Tsyryuk, Korotkyi, Grygoriev and Falalyeyeva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tetyana Falalyeyeva, tfalalyeyeva@gmail.com
 Yurii Penchuk1
Yurii Penchuk1  Nazarii Kobyliak
Nazarii Kobyliak Svitlana Onysenko
Svitlana Onysenko Tetyana Falalyeyeva
Tetyana Falalyeyeva