Deciphering the epidemiological dynamics: Toxoplasma gondii seroprevalence in mainland China’s food animals, 2010-2023
- 1Key Laboratory of Zoonosis Prevention and Control of Guangdong Province, College of Veterinary Medicine, South China Agricultural University, Guangzhou, Guangdong, China
- 2Kerry Rehabilitation Medicine Research Institute, Shenzhen, China
- 3College of Agriculture, South China Agricultural University, Guangzhou, Guangdong, China
- 4Key Laboratory of Zoonosis of Ministry of Agriculture and Rural Affairs, South China Agricultural University, Guangzhou, Guangdong, China
Background: Toxoplasma gondii (T. gondii) is a significant protozoan pathogen among food animals. Despite the threat to public health by T. gondii infections, there’s limited understanding of its seroprevalence and trends in food animals across mainland China. This study aimed to estimate the seroprevalence of T. gondii infections among swine, sheep, goats, chickens, and cattle in mainland China from 2010 to 2023.
Methods: We searched cross-sectional studies published between 2010 and 2023 that reported the prevalence of T. gondii in food animals from databases including PubMed, Embase, Web of Science, China Biology Medicine Disc (CBM), China National Knowledge Infrastructure (CNKI), Wanfang data, and the China Science and Technology Journal Database (CQVIP). We performed subgroup analyses to explore the impact of different factors on the seroprevalence of T. gondii. Pooled estimates of T. gondii seroprevalence were calculated with a random-effects model.
Results: An analysis of 184 studies involving 211985 animals revealed a T. gondii overall seroprevalence of 15.3% (95% CI: 13.1-17.8). Although the seroprevalence of food animals across mainland China was relatively stable from 2010 to 2023, notable variations were observed across different animal types and regions (P < 0.01), along with changes in geographical distribution. Sample type, detection method, animal age, and history of abortion were identified as key risk factors for T. gondii seroprevalence.
Conclusion: The study conducted a meta-analysis on the seroprevalence of T. gondii in mainland China’s Food Animals from 2010 to 2023, and identified key risk factors. These findings advance our understanding of T. gondii infection dynamics, offering critical insights for developing control strategies and guiding public health policies.
1 Introduction
Toxoplasma gondii, a coccidian parasite, has been considered as an important zoonotic protozoan parasite in food animals, which can cause large economic losses (Dubey, 2010; Schlüter et al., 2014). It is estimated that one-third of the world population is infected with T. gondii (Montoya and Liesenfeld, 2004). All warm-blooded species, including people, livestock, and wildlife, serve as intermediate hosts for T. gondii, with cats and other felids as its only known definitive host (Dubey, 2009; Gardner et al., 2010). T. gondii can invade the nucleated cells of these species, where it survives and replicates (Jones and Dubey, 2012). Pregnant women and immunocompromised individuals are considered the main risk groups for T. gondii infection, which can cause severe health problems (Weiss and Dubey, 2009; Dubey, 2010).
Humans typically contract T. gondii infection by ingesting food or water contaminated with sporulated oocysts shed by the primarily infected felines or consuming undercooked or raw meat containing tissue cysts (Dubey et al., 2005). In farming environments, common food animals such as cows, sheep, and swine may contract T. gondii by consuming feed and water contaminated with sporulated oocysts. This infection results in the formation of cysts in their tissues, which can then be transmitted to humans if the meat is not thoroughly cooked. In addition to meat, other food items, such as milk, marine products, and vegetables can also serve as potential sources of T. gondii infection (Marín-García et al., 2022). Furthermore, T. gondii can cross the placenta, which may result in vertical transmission and congenital toxoplasmosis, especially in sheep, goats, and humans.
Over the past ten years, T. gondii infections in food animals have raised serious concerns among Chinese consumers (Wang et al., 2013). After the acute T. gondii infection, these animals typically progress to a chronic phase and carrying tissue cysts that can be transmitted to humans. Cultural factors significantly impact the differences in the prevalence of T. gondii between different countries, especially regarding dietary habits (Tenter et al., 2000; Robert-Gangneux and Dardé, 2012). A significant association exists between human T. gondii infections and consuming raw or undercooked meat (Kapperud et al., 1996; Belluco et al., 2018). In traditional Chinese cuisine, the staple foods typically consist of slowly simmered meats, rice, noodles, and various cooked vegetables, with meats having become the major and favored food in the past decades. Although most Chinese people don’t often eat undercooked meats or animal-by products, regional foods like hotpot, BBQ, and raw milk products are popular, especially with raised living standards (Li et al., 2009; Gao and Tang, 2018).
According to the National Bureau of Statistics of China (2023) (Stat. Com. of China, 2023), mainland China’s total pork, beef, mutton, and poultry output increased by 3.8% compared to the previous year, reaching 92.27 million tons in 2022, indicating an expanding preference for various animal-based foods. The consumption of different food animals is especially indicative of this rising need; chickens and ducks are the most common species among poultry, while the most commonly consumed mammals are cattle, goats, sheep, and swine. Unfortunately, conventional meat inspection methods cannot detect T. gondii infections, and little attention is paid to implement preventative measures within the food supply chain (Dorny et al., 2009). The absence of specific inspection strategies and standards for handling infected meat poses a potential public health concern.
There is a substantial study gap in studies on the prevalence and dynamics of T. gondii in food animals, especially in the last ten years, even though there has been a great deal of epidemiological research on the T. gondii in humans, livestock, and pets in mainland China. Significantly, existing studies primarily concentrated on evaluating the prevalence of animal T. gondii in a broad sense, without specifically focusing on its prevalence in food animals raised for human consumption. These studies might include diseased or already succumbed animals in their analyses, raising concern since the findings may not accurately represent the true prevalence of T. gondii in meat and meat products intended for human consumption.
Our research carefully excluded studies that examined sick or dead animals and instead concentrated on T. gondii infections in food animals produced for human consumption in mainland China between 2010 and 2023. This period has not only witnessed a substantial increase in the consumption of animal products but also brought to light the imperative need for a more profound understanding of food safety and zoonotic diseases, especially in the context of global and localized disease outbreaks. Additionally, further influenced by climate change and resultant extreme weather events in China (Li and Gu, 2020; Zhu and Fan, 2021; Cai et al., 2022; Zheng et al., 2023), these factors collectively signal the potential shifts in infectious disease dynamics. In order to address the changing health concerns in this important field, our research used meta-analysis to determine the effects of these environmental and public health changes on T. gondii seroprevalence.
2 Materials and methods
This study followed PRISMA guidelines, and the registration application for PROSPERO has been approved (ID: CRD42023437036).
2.1 Search strategy
To capture the majority of relevant articles, we systematically searched databases including PubMed, Embase, Web of Science, China Biology Medicine Disc (CBM), China National Knowledge Infrastructure (CNKI), Wanfang data, and the China Science and Technology journal database (CQVIP) for studies published from January 1, 2010 to October 13, 2023, with no language restrictions. The specific search steps for all databases are detailed in Supplementary Table S1-4. Additionally, we reviewed the reference lists of the analyzed studies and recent reviews.
2.2 Study selection and data extraction
In this study, the following inclusion criteria were considered: (1) The research subjects were common edible animals in mainland China (swine, cattle, goats, sheep, chickens). (2) Studies detected the prevalence of Toxoplasma gondii with the sample size and the number of positive cases. (3) Research published between 2010-2023 with a sample size of 50 or more. (4) Only serology detection methods, such as IHA, MAT, and ELISA, are acceptable. (5) Only cross-sectional study types are included.
The standard exclusion criteria included (1) Articles with reviews, abstracts, conference abstracts, and studies without raw data. (2) Studies that test multiple or mixed samples on a single animal individual. (3) Studies involving wildlife, sick animals, or dead animals. (4) Only the most comprehensive one will be included in duplicate publications or multiple articles using the same data.
Two investigators (ZP Yang and Hao Yuan) carried out the search strategy and assessed all article titles and abstracts by the eligibility standards. The information to be extracted included first author, year of publication, region, animal species, sample type, detection method, sample size, number of positive cases, sample selection, animal age, season, and location of sampling. From a food safety perspective, yaks, cattle, and dairy cows were grouped as ‘cattle’ to simplify raw data processing and enable easier comparisons among food animal species.
After selecting full articles, data was extracted individually by the two investigators using a standard form. The third investigator (ZG Yuan) resolved any assessment discrepancies with an agreement.
2.3 Quality assessment
The quality assessment follows the quality evaluation criteria from a similar study (Wang et al., 2021), which was based on the GRADE criteria (Guyatt et al., 2008). Four categories are scored: detection method, sample size, sample collection method, and the presence of four or more risk factors. Each category can score up to 1 point, totaling 4 points. A score of 0-1 indicates low quality, 2 is moderate quality, and 3-4 indicates high quality.
2.4 Statistical analysis
Pooled seroprevalence of T. gondii and its 95% confidence intervals (CI) were calculated by a “metaprop” command provided in Stata software (version 14.0). We utilized Cochran’s Q test and I² test for heterogeneity assessment (Higgins et al., 2003). When the results indicated no significant heterogeneity among studies (P ≥ 0.05, I² ≤ 50%), we employed the fixed effects model. Otherwise, we used the random effects model (P < 0.05, I² > 50%). Subgroup analyses were performed to explore the impact of different factors on T. gondii seroprevalence. The differences between the pooled seroprevalence of T. gondii across subgroups were analyzed using the Z-test. The funnel plots and Egger tests were used to assess publishing bias (Egger et al., 1997). The “ggplot2” R package was used to draw curves of the pooled prevalence of T. gondii across different years and their 95% confidence intervals. The “ComplexHeatmap” R package was used to visualize the pooled prevalence of T. gondii in food animals. From a food safety perspective, we include ‘cattle’ yaks, cattle, and dairy cows, facilitating consolidated comparisons among food animal species.
3 Results
3.1 Search results and eligible studies
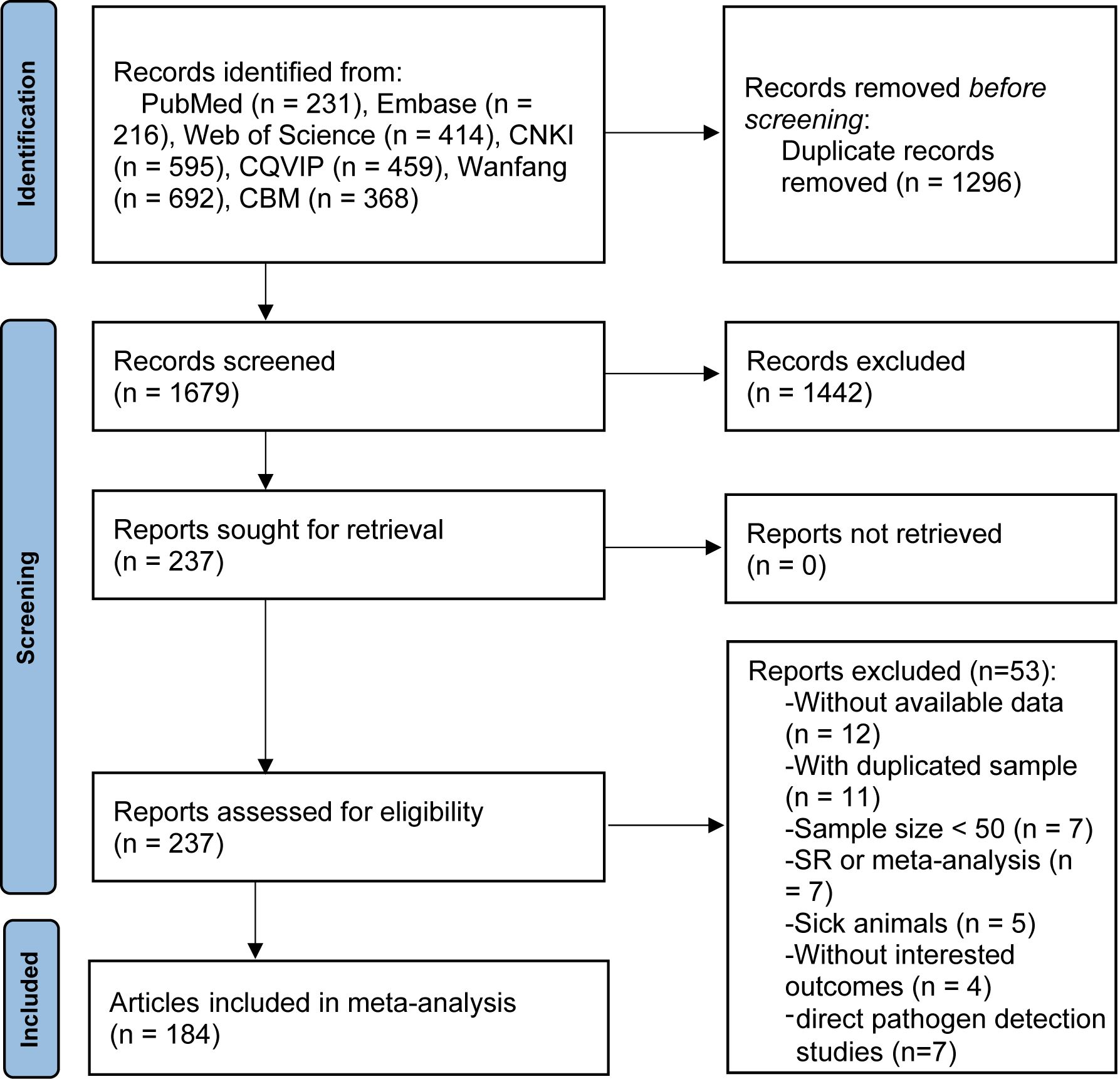
Figure 1 presents a flow-process diagram outlining the selection of relevant studies. Initially, we retrieved 2975 articles from electronic databases according to the literature search strategies. After eliminating 1296 duplicate articles, 1679 articles remained. After reviewing titles and abstracts, 1442 articles were excluded for failing to meet the inclusion criteria. Consequently, 184 articles published from 2010 to 2023, comprising 67 in English and 117 in Chinese, were deemed qualified and included in the meta-analysis. The basic characteristics of the included studies are summarized in Supplementary Table S5.
3.2 Research characteristics and quality assessment
The meta-analysis included 184 articles, which encompassed 221 studies, with total number of positive animals ranging from 50 to 30024 cases and 211,985 total tested animals. The quality assessment result of the included studies (Supplementary Tables S5, S6) revealed 154 high-quality reports, 25 medium-quality reports, and 5 low-quality reports.
3.3 Seroprevalence of Toxoplasma gondii in food animals in mainland China
As shown in Figure 2, the pooled seroprevalence of T. gondii infection among the swine, sheep, goats, chickens, and cattle in mainland China between 2010-2023 was 15.3% (95% CI: 13.1-17.8; 40810/211985). Subsequently, a random-effects model was used in this study (I2 = 99.553%, P < 0.001).
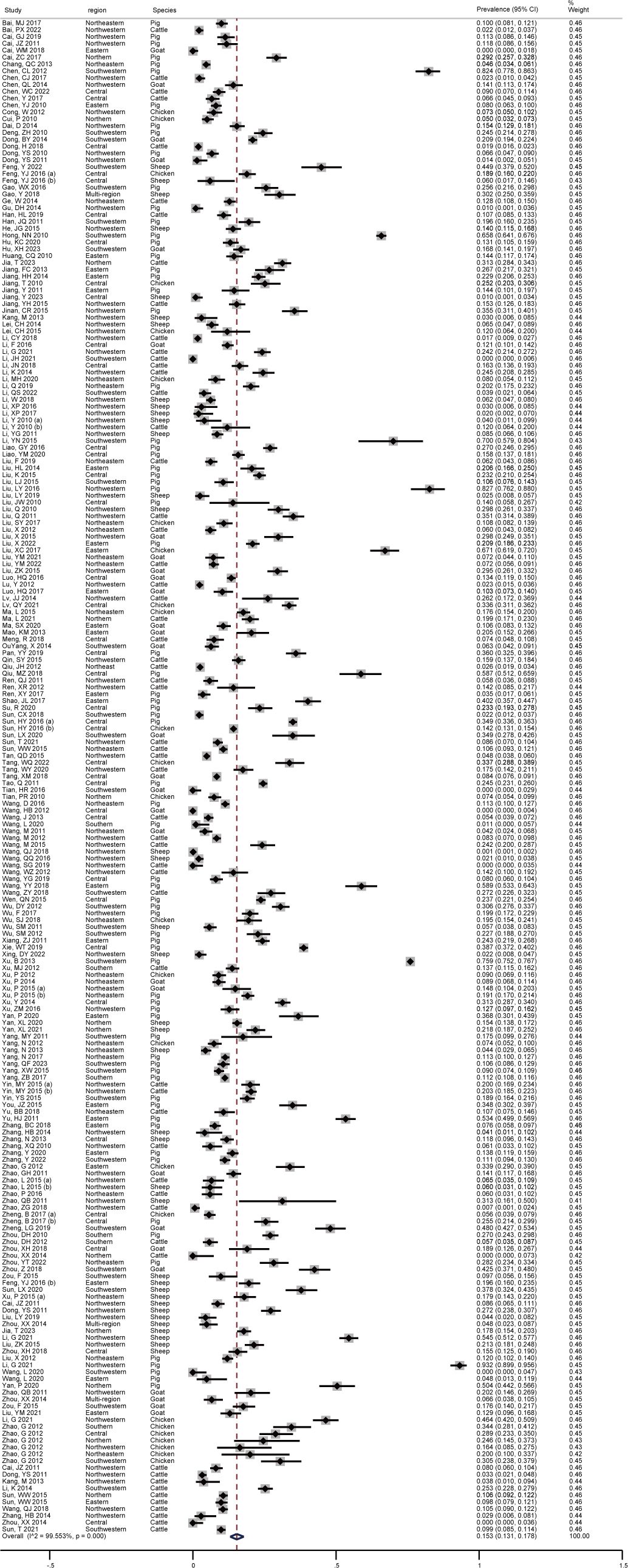
Figure 2 Forest plot of T. gondii seroprevalence of food animals in mainland China between 2010-2023.
3.4 Subgroup analysis
3.4.1 Seroprevalence of Toxoplasma gondii in different species of food animals
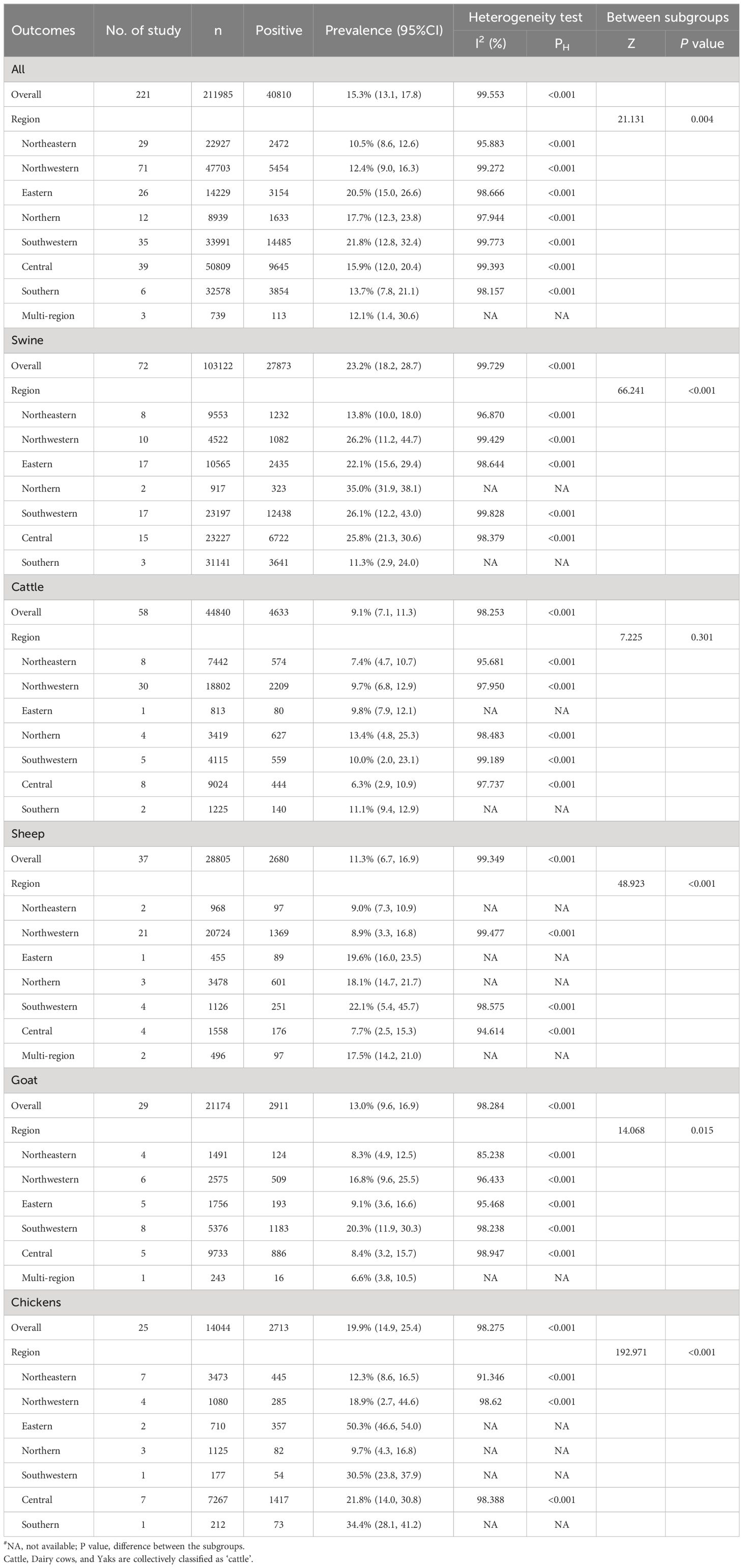
Figures 3A–E showed the pooled seroprevalence of T. gondii for swine, cattle, sheep, goats, and chickens, respectively. Specifically, there were 72 studies for swine, 58 for cattle, 37 for sheep, 29 for goats, and 25 for chickens. Among the five food animals in mainland China from 2010 to 2023, swine exhibited the highest seroprevalence of T. gondii at 23.2% (95% CI: 18.2, 28.7). Chickens followed this with a seroprevalence of 19.9% (95% CI: 14.9, 25.4), goats at 13.0% (95% CI: 9.6, 0.16.9), and sheep at 11.3% (95% CI: 6.7, 16.9). The lowest seroprevalence was observed in cattle, at 9.1% (95% CI: 7.1, 11.3).
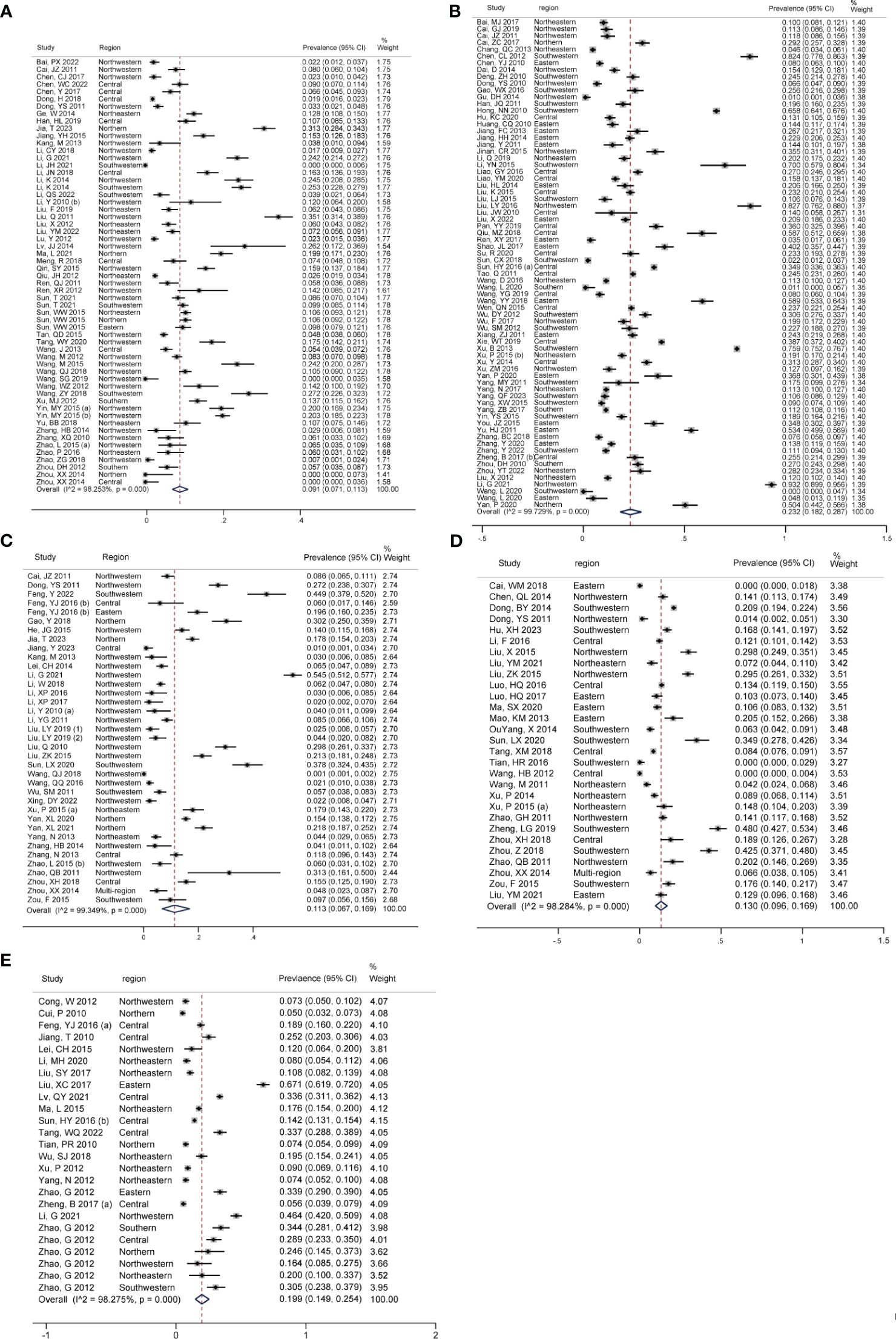
Figure 3 Forest plot of T. gondii seroprevalence in different food animals. The overall T. gondii seroprevalence in food animals, including (A) cattle, (B) swine, (C) sheep, (D) goats, and (E) chickens.
Significant statistical heterogeneity was observed in the studies included for each animal: I2 > 50%, P < 0.001. Among all the food animals, there was a significant difference in T. gondii seroprevalence (Z = 37.505, P < 0.001).
3.4.2 The impact of geographic factors on Toxoplasma gondii seroprevalence
We conducted regional subgroup analyses for each animal type (Table 1), and the geographical distribution map of T. gondii seroprevalence in food animals is shown in Figure 4. The distribution of T. gondii serologic positivity rate varies across different regions in Mainland China. Notably, Southwestern China displayed the highest seroprevalence, with a pooled prevalence of 21.8% (95% CI: 12.8, 32.4). The pooled seroprevalences in other regions significantly also shape the national prevalence landscape, with Northeastern China reporting the lowest at 10.5% (95% CI: 8.6, 12.6) and the other regions showing intermediate values. Overall, there were significant differences in the T. gondii seroprevalence among food animals from different regions (Z = 21.131, P < 0.001).
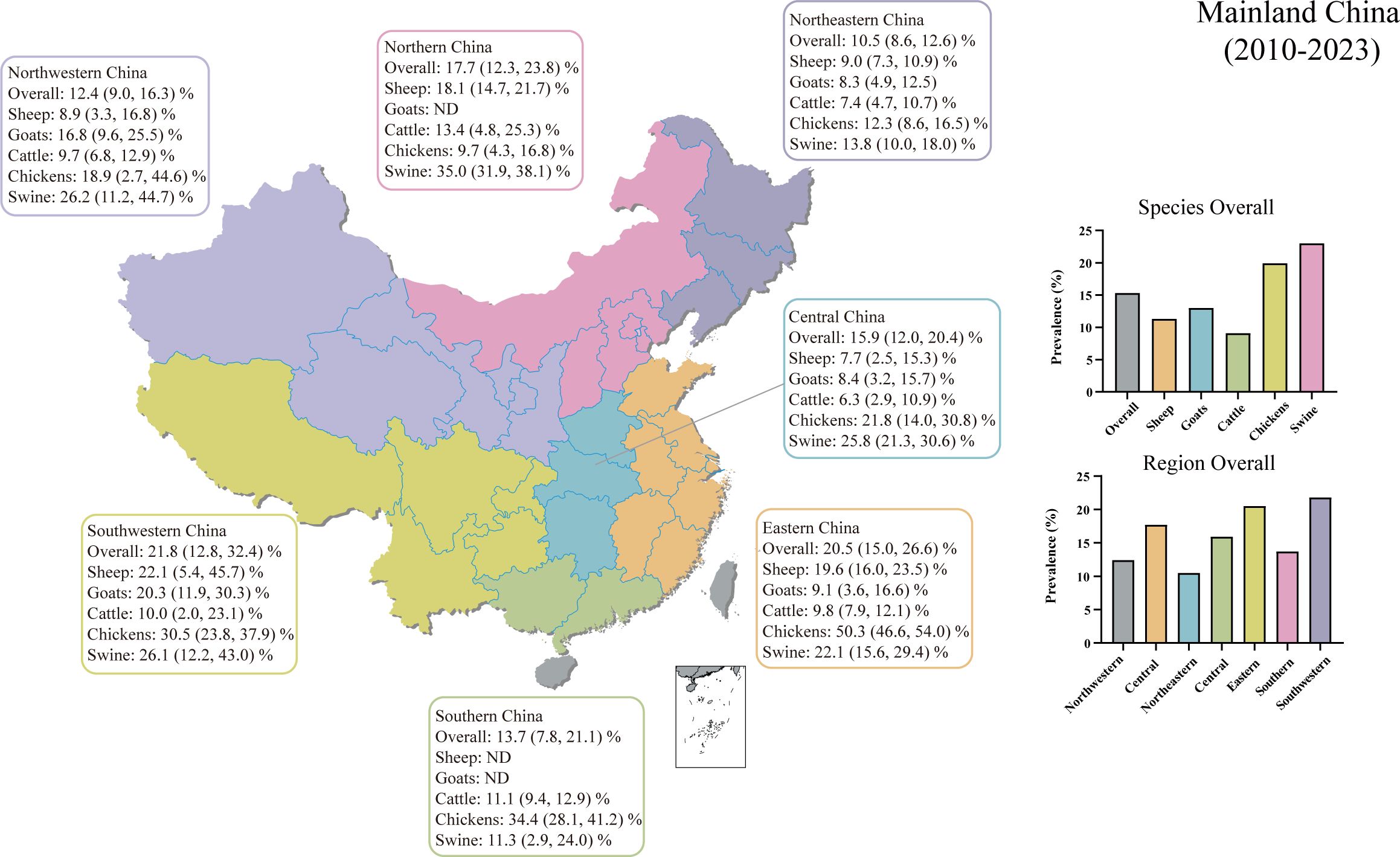
Figure 4 Geographical distribution map of T. gondii seroprevalence in sheep, goats, swine, chickens, and cattle from 2010–2023 in Mainland China. ND, not determined.
3.4.3 Trends in the seroprevalence of Toxoplasma gondii in food animals in mainland China from 2010 to 2023
To reflect the seroprevalence trends of T. gondii in food animals in mainland China, as reported in studies from 2010 to 2023, we compiled and visualized the serologic positivity rates of T. gondii infection (Figures 5A, B). Overall, between 2010 and 2023, the seroprevalence of T. gondii remained relatively stable, with no statistically significant differences in the pooled prevalence across the years (P > 0.05).
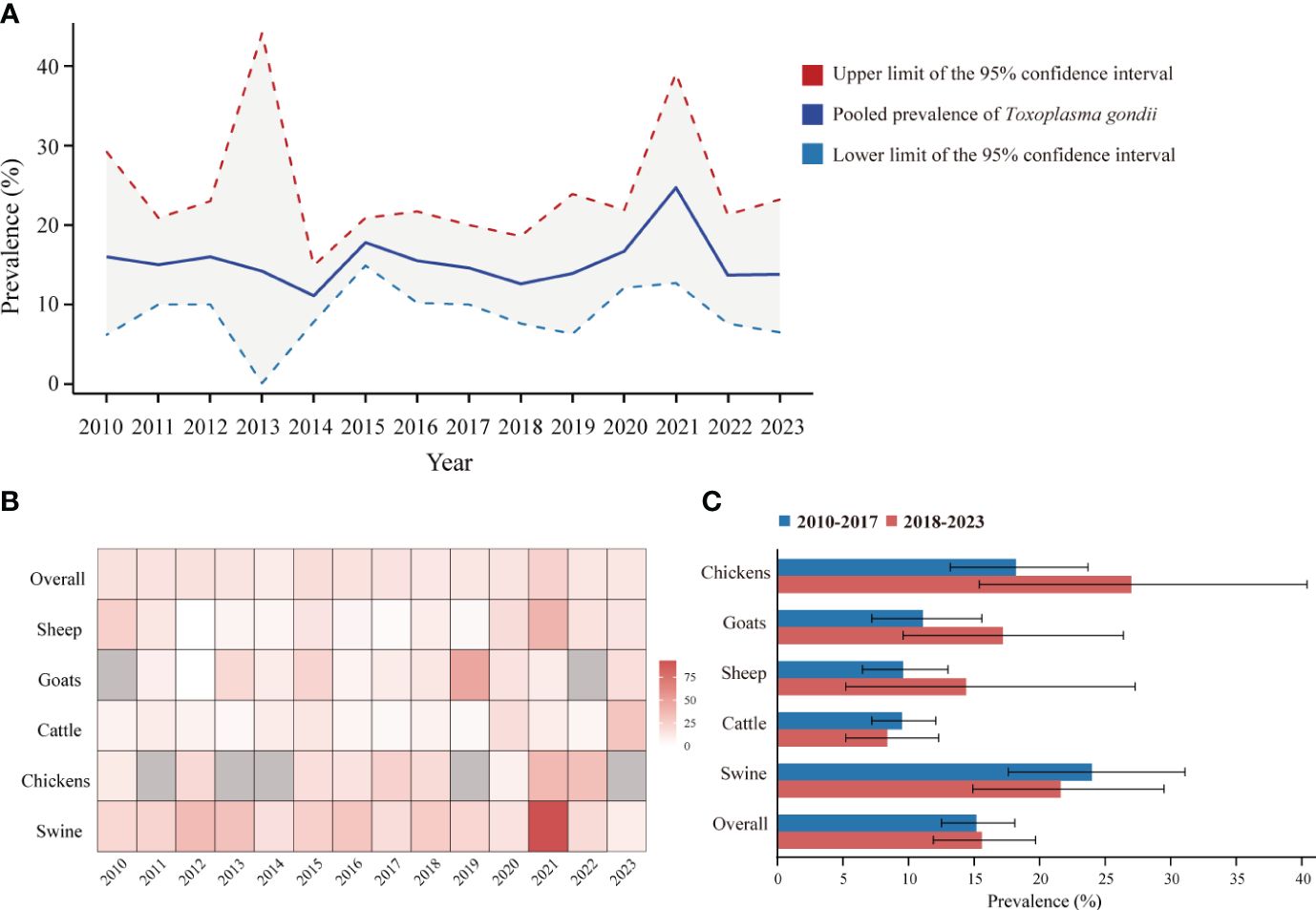
Figure 5 Dynamic Changes of T. gondii seroprevalence in sheep, goats, swine, chickens, and cattle from 2010 to 2023 in Mainland China. (A) Pooled seroprevalence of T. gondii from 2010 to 2023. (B) Heatmap of T. gondii seroprevalence changes in sheep, goats, swine, chickens, and cattle from 2010 to 2023. (C) T. gondii seroprevalence among different species of food animals between 2010 to 2017 and 2018 to 2023.
The overall T. gondii seroprevalence for food animals was 15.2% (95% CI: 12.5, 18.1) for 2010-2017 and 15.6% (95% CI: 11.9, 19.7) for 2018-2023, with no significant difference (P > 0.05). Despite we observed fluctuation in T. gondii seroprevalence within each animal species between the periods 2010-2017 and 2018-2023, pairwise comparisons were systematically conducted between all possible subgroup pairs, revealing no significant differences (Figure 5C) (P > 0.05).
Comparing the 2010-2017 and 2018-2023 periods, shifting was observed in the regional distribution of T. gondii seroprevalence across mainland China (Figure 6). During 2010-2017, the highest seroprevalence was recorded in Southwestern China at 25.5% (95% CI: 13.8, 39.3), with Eastern China closely following at 23.4% (95% CI: 16.3, 31.3). However, the period of 2018-2023 showed Northern China rise to the highest seroprevalence at 25.2% (95% CI: 18.0, 33.1), while Southwestern China’s rate declined to the second highest at 16.7% (95% CI: 9.3, 25.6). Additionally, the region with the lowest seroprevalence changed from the Northeastern (at 9.8%, 95% CI: 7.7, 12.1) in 2010-2017 to the Northwestern (at 12.1%, 95% CI: 4.1, 23.4) in 2018-2023, highlighting the dynamic nature of T. gondii seroprevalence distribution over time. Details on the pooled seroprevalence across different regions for periods 2010-2017 and 2018-2023 are available in Supplementary Table S7.
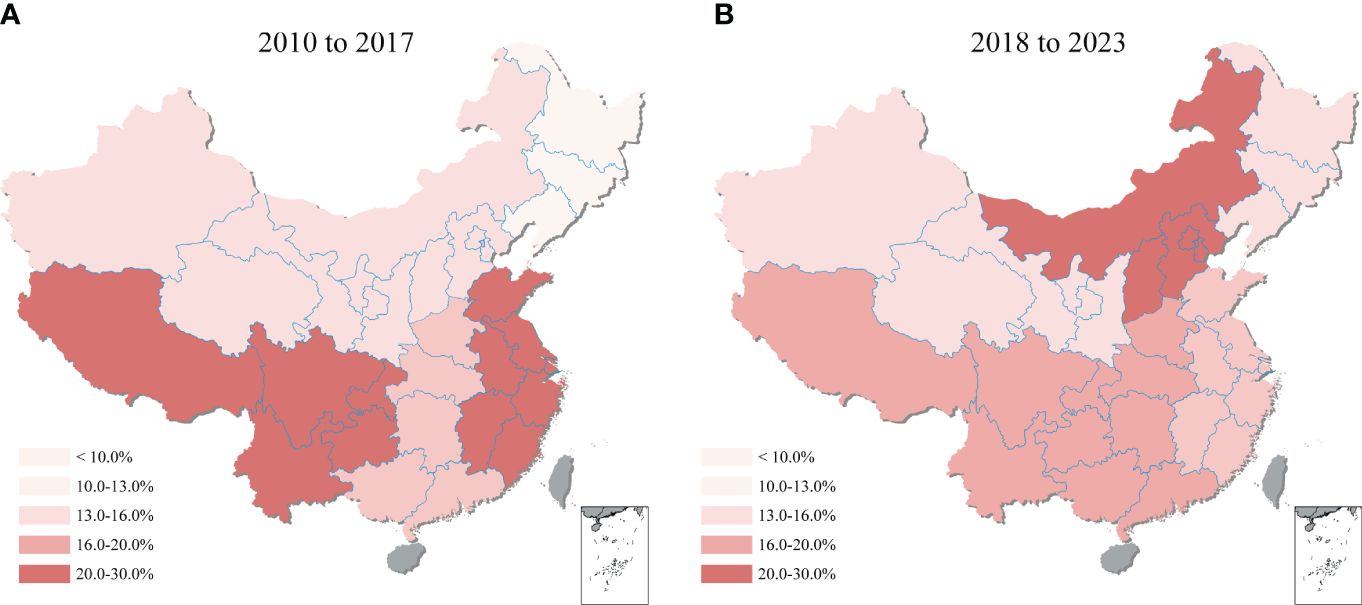
Figure 6 Geographical heatmap of T. gondii seroprevalence. The heatmap showed the seroprevalence of sheep, goats, swine, chickens, and cattle in mainland China from (A) 2010 to 2017 and (B) 2018 to 2023.
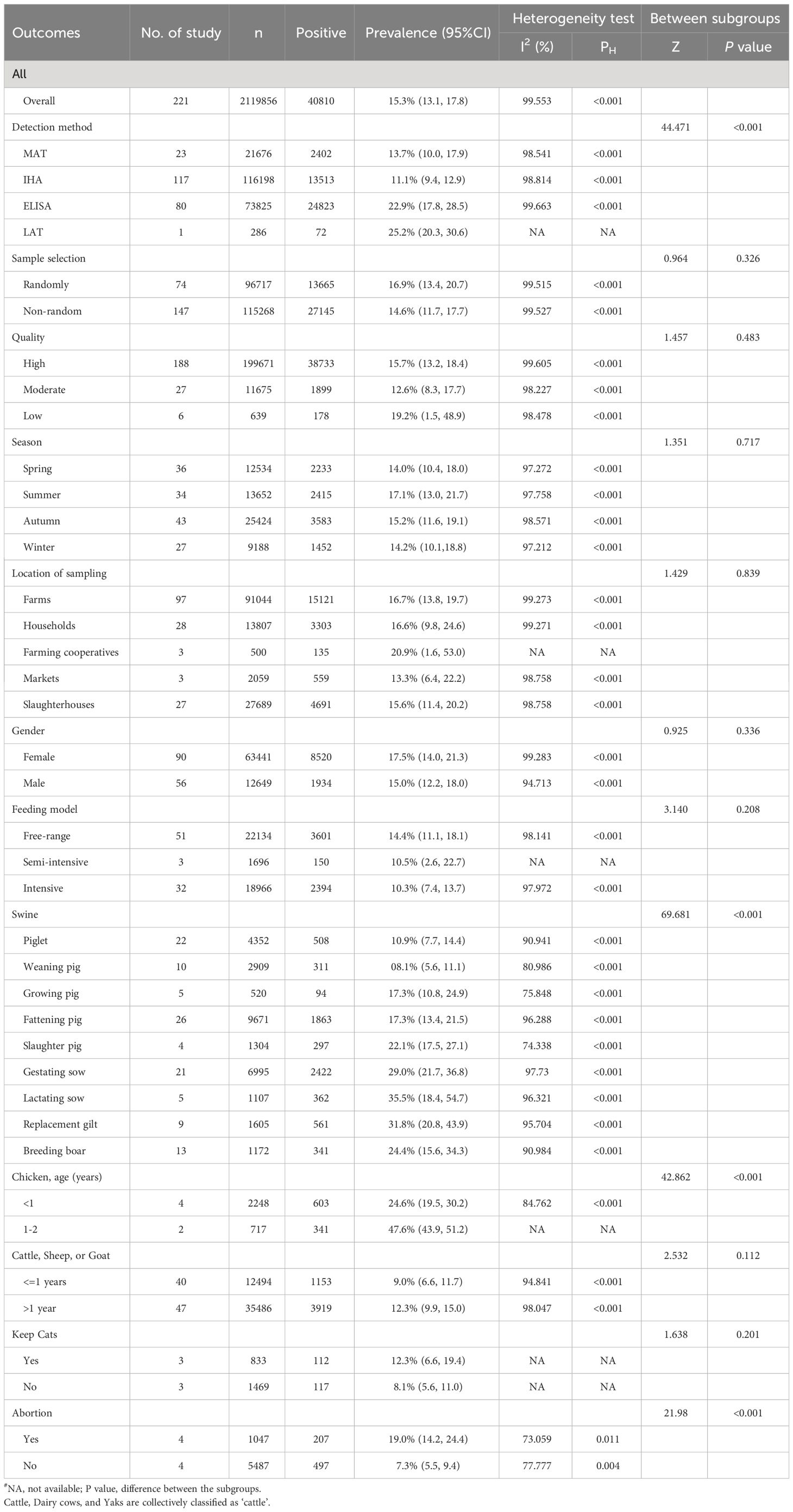
We have completed subgroup analyses for the following factors: sample type, detection method, sample selection, quality, season, location of sampling, gender, feeding model, age, cat ownership, and history of abortion (Table 2). Sample type, detection method, age groups, and history of abortion were significantly associated with the seroprevalence of T. gondii (P < 0.01).
3.4.4 Risk of bias assessment
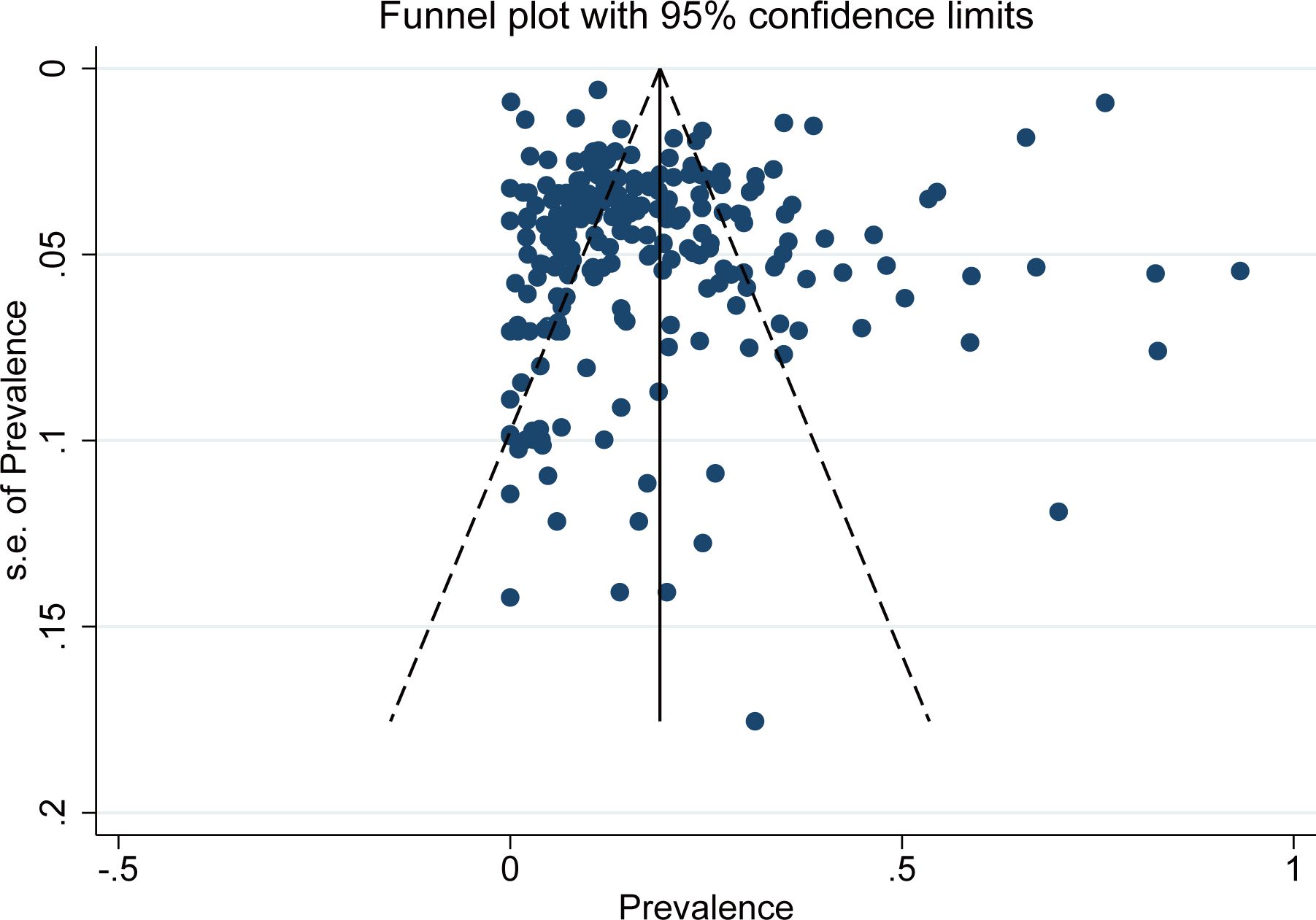
The bias funnel diagram is shown in Figure 7. The Egger test result indicates no significant publication bias in the included studies (P = 0.202).
4 Discussion
The zoonotic illness toxoplasmosis, which affects both humans and animals, is especially dangerous for animal husbandry and public health (Montoya and Liesenfeld, 2004). The lifecycle of T. gondii emphasizes the role of food animals in human transmission, directly linking to the critical risk posed by consuming undercooked meat harboring T. gondii cysts (Dubey, 2010). Strong food safety regulations and all-encompassing approaches, including enhanced monitoring, public awareness campaigns, and more stringent food processing guidelines, are required to combat the threat and reduce the risk of T. gondii infection.
From 2010 to 2023, mainland China faced significant public health challenges, including COVID-19, African swine fever, and avian influenza. These challenges impacted human and animal behavior patterns (Barrett et al., 2015; Watts et al., 2017; Jalloh et al., 2020) and influenced livestock practices (Taylor et al., 2020; Brown et al., 2021; Liu et al., 2021; Petrovan et al., 2021), thereby highlighting the importance of researching T. gondii dynamics and understanding zoonotic diseases more broadly (Stoffel et al., 2020). Against this backdrop, gaining a deeper understanding of the epidemiology of T. gondii is imperative, especially in a country like China with a vast and diverse food-animal sector.
To our knowledge, this is the first meta-analysis to evaluate the seroprevalence of T. gondii in China’s most common food animals (cattle, sheep, goats, swine, and chickens) from 2010 to 2023. The study’s findings offer valuable insights into T. gondii seroprevalence, crucial for public health experts and policymakers for designing and implementing effective control strategies, particularly in food safety and animal disease management. Our research lays the groundwork for further exploration into T. gondii’s transmission mechanisms and preventive measures. Additionally, it may serve as a reference point for other researchers delving deeper into this field, enhancing the understanding of the global epidemiology of toxoplasmosis.
The seroprevalence of T. gondii among food animals in mainland China from 2010 to 2023 was 15.3%, which is lower than the reported rates for worldwide livestock and poultry between 2000-2019 (28.3%) (Hajimohammadi et al., 2022), and the seroprevalence observed in China’s food animals between 2000-2017 (23.7%) (Dong et al., 2018), and China’s pigs from 1990-2017 (24%) (Foroutan et al., 2019). The difference may be attributed to the differences in the number of studies included, methodological differences in studies, sample selection biases, and notably, the influence of evolving environmental and societal factors over time. Despite the absence of specific food safety measures targeting T. gondii in China, it’s crucial to consider that environmental and societal factors influence T. gondii transmission by altering critical conditions for its survival, such as humidity and temperature (Robert-Gangneux and Dardé, 2012). Additionally, a series of epidemic control measures may weaken the transmission of T. gondii. For instance, wildlife protection measures reduce contact between natural hosts of T. gondii and livestock, while closed management and all-in-all-out policies could decrease the spread of pathogens within livestock populations.
To better understand the changes in T. gondii seroprevalence, we divided our data into two periods: 2010 to 2017 and 2018 to 2023. Although the seroprevalence remained relatively stable between 2010-2023, significant regional differences in distribution between different periods were observed. The China Meteorological Administration’s 2023 Blue Book (Press, 2023) reveals considerable climate changes across various regions of China since 2010, including an increase in average annual precipitation and a continuous rise in temperatures from 2015 to 2022, ranking among the hottest eight years since 1961. Additionally, it highlights the rise in extreme weather events, which has raised the climate risk index considerably. The regional variations in these environmental factors may have influenced the distribution of T. gondii. Since 2018, compared to the previous period, outbreaks of COVID-19 and African Swine Fever have profoundly affected public health security in mainland China, likely leading to substantial changes in animal management and transportation strategies, such as enhanced wildlife protection measures, stricter animal transport restrictions, and quarantine measures. In addition, the impact of the disease outbreak has shifted consumer preferences towards consuming locally or regionally-produced meat. These comprehensive factors could influence the T. gondii seroprevalence and its distribution in food animals across specific regions.
Although the seroprevalence of T. gondii within each species of food animals shows no statistically significant differences across the two time periods, the observed fluctuations should not be overlooked. These variations, while not currently significant, have the potential to manifest more clearly in studies with larger sample sizes or longer durations, highlighting the necessity for ongoing surveillance. Effective and targeted prevention and control techniques for various locations and species must be implemented in future public health policies and animal disease management plans.
We conducted extensive subgroup analyses to dissect the risk factors of T. gondii infection. Animal species, geographical location, year-to-year variations, sample type, detection method, age groups, and history of abortion are identified as key risk factors influencing the T. gondii seroprevalence. China is one of the countries with the highest biodiversity in the world, boasting various landscapes and animal populations. The variability in T. gondii prevalence among different species can be attributed to differences in susceptibility, which is largely influenced by the host immune system and how it is modulated by parasitic factors (Innes, 1997; Mukhopadhyay et al., 2020). Among the food animal species we monitored, swine and cattle had the highest and lowest rates of T. gondii serological positivity, respectively, while goats and sheep had intermediate rates, also supported by Pan et al (Pan et al., 2017). Significantly, our findings reveal a rising trend of T. gondii prevalence in chickens, particularly in the southern regions, which marks an emerging concern when compared to past reviews (Pan et al., 2017; Wang et al., 2021). However, it should be noted that the scarcity of available data for certain species might lead to discrepancies between our results and the actual seroprevalence, particularly when examining specific regions. Accurately determining T. gondii prevalence for various species, especially in regions where studies are limited, necessitates increased involvement from researchers across diverse regions in future research efforts. While data from 2010 to 2023 indicate a stability of T. gondii seroprevalence, year-specific fluctuations in pooled prevalence highlight the need for ongoing monitoring, which is particularly evident between certain years. Additionally, the 95% confidence interval variability for the pooled prevalence, whether within a single year or across different years, underscores the influence of various factors on disease prevalence at different times and locations. This variability further supports the necessity of ongoing monitoring of these local dynamics.
We observed that swine and chickens of greater age, specifically adults, tend to have higher positivity of T. gondii, suggesting the possibility of widespread chronic infections due to breeding conditions and environmental factors in these species. The subgroup analyses suggest that replacement gilts, lactating sows, and gestating sows exhibit the highest prevalence of T. gondii among different types of pigs. Vertical transmission of T. gondii mainly occurs during the initial acquisition of the infection by immunologically naive pregnant individuals (Tenter et al., 2000). Considering our findings of lowest seroprevalence in piglets and weaning pigs, we infer that most gestating sows are likely infected with T. gondii before pregnancy, providing a certain level of protection to the piglets. To control the spread of T. gondii, breeding institutions should focus on closely monitoring and screening these high-risk groups and strictly manage the environment of pig farms and pens. While a similar trend is observed in chickens of different ages, research on this aspect is extremely scarce. Therefore, future research should focus more on this issue, especially on whether chickens of different ages exhibit significantly different prevalences of T. gondii.
T. gondii infection can lead to abortions or fetal developmental disorders in small ruminants and pregnant women (Dubey, 2010). Despite the subgroup analysis on the history of abortion involving only a limited number of studies, evidence indicates a strong correlation between T. gondii infection and the incidence of miscarriages. In China, the seroprevalence of T. gondii among pregnant entities is significantly higher in animals than in women, with pigs at 24% and chickens and ruminants at 20%, in contrast to 5.0% or less in pregnant women (Deng et al., 2018). Additionally, variations in T. gondii seroprevalence were observed based on different detection methods. Although there are recognized methods for detecting T. gondii, standardization of detection methods in the food industry is still needed (Koutsoumanis et al., 2018; Marín-García et al., 2022). Given the current limitations in detecting infected animals before slaughter, including the economic and practical infeasibility of employing the most sensitive methods like PCR on a large scale, it’s crucial to explore and adopt a multifaceted approach. We advocate for integrating feasible testing strategies within a broader inspection and quarantine framework, aiming to enhance food safety by mitigating risks associated with meat and related products from edible animals.
To reduce the risks posed by T. gondii to public health and food safety, governments and researchers need to monitor its prevalence trends and craft region-specific prevention strategies. Establishing standardized detection methods for this parasite, supported nationally, is critical for accurate toxoplasmosis diagnosis. We encourage quarantine departments to periodically employ standardized methods for monitoring T. gondii in food animals to track its prevalence. Furthermore, incorporating T. gondii detection into the pre-slaughter quarantine of livestock following the monitoring of localized outbreaks is a measure to ensure public health.
In summary, this study comprehensively analyzes T. gondii seroprevalence in China’s food animals based on extensive research and a substantial, high-quality dataset, ensuring reliable results. It paves the way for further investigations into T. gondii’s transmission and prevention, offering valuable insights for researchers and advancing global understanding of toxoplasmosis epidemiology.
However, our study has limitations. Firstly, our results demonstrate a high degree of heterogeneity, the sources we could not identify through sensitivity or subgroup analysis. Secondly, due to data limitations, our study could not account for the variability in sensitivity and specificity among detection methods used in the original research, leading to uncertainty or variance in seroprevalence rates. Such variability introduces additional uncertainty to the outcomes of this study. Therefore, future research should establish universal detection methods and standards, especially for tailored measures in different regions. Thirdly, some articles lack critical information such as age, gender, breeding methods, cat ownership, and history of abortion, which limits the ability to conduct more detailed analyses on specific subgroups. Lastly, caution is required when generalizing these findings globally since all included studies are conducted in China. This geographical limitation suggests a need for broader, international research to validate and extend our findings.
5 Conclusion
In mainland China, the seroprevalence of T. gondii in food animals was 15.3% (95% CI: 13.1-17.8) between 2010 and 2023, with observed species and regional variations. Although the overall trend of T. gondii seroprevalence remained stable, a notable shift was observed across different areas. Thus, it is advised to implement and uphold stringent preventative and control measures, such as increased monitoring and better food safety procedures.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZY: Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Methodology, Software, Supervision, Visualization. HY: Investigation, Methodology, Writing – review & editing. LN: Formal analysis, Methodology, Writing – review & editing. QW: Writing – review & editing, Visualization. HL: Formal analysis, Methodology, Writing – review & editing. LY: Formal analysis, Methodology, Writing – review & editing. YS: Data curation, Methodology, Writing – review & editing. XL: Visualization, Writing – review & editing. XZ: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. Z-GY: Conceptualization, Supervision, Writing – review & editing, Resources, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Natural Science Foundation of Guangdong Province (2023A1515011795), the National Natural Science Foundation of China (31972707).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1381537/full#supplementary-material
References
Barrett, B., Charles, J. W., Temte, J. L. (2015). Climate change, human health, and epidemiological transition. Prev. Med. 70, 69–75. doi: 10.1016/j.ypmed.2014.11.013
Belluco, S., Simonato, G., Mancin, M., Pietrobelli, M., Ricci, A. (2018). Toxoplasma gondii infection and food consumption: A systematic review and meta-analysis of case-controlled studies. Crit. Rev. Food Sci. Nutr. 58, 3085–3096. doi: 10.1080/10408398.2017.1352563
Brown, V. R., Miller, R. S., McKee, S. C., Ernst, K. H., Didero, N. M., Maison, R. M., et al. (2021). Risks of introduction and economic consequences associated with African swine fever, classical swine fever and foot-and-mouth disease: A review of the literature. Transbound Emerg. Dis. 68, 1910–1965. doi: 10.1111/tbed.13919
Cai, W., Zhang, C., Zhang, S., Bai, Y., Callaghan, M., Chang, N., et al. (2022). The 2022 China report of the Lancet Countdown on health and climate change: leveraging climate actions for healthy ageing. Lancet Public Health 7, e1073–e1090. doi: 10.1016/s2468-2667(22)00224-9
China, N.B.o.S.o (2023) STATISTICAL COMMUNIQUÉ OF THE PEOPLE'S REPUBLIC OF CHINA ON THE 2022 NATIONAL ECONOMIC AND SOCIAL DEVELOPMENT. Available online at: https://www.stats.gov.cn/english/PressRelease/202302/t20230227_1918979.html (Accessed February 28, 2023).
Deng, H., Devleesschauwer, B., Liu, M., Li, J., Wu, Y., van der Giessen, J. W. B., et al. (2018). Seroprevalence of Toxoplasma gondii in pregnant women and livestock in the mainland of China: a systematic review and hierarchical meta-analysis. Sci. Rep. 8, 6218. doi: 10.1038/s41598-018-24361-8
Dong, H., Su, R., Lu, Y., Wang, M., Liu, J., Jian, F., et al. (2018). Prevalence, risk factors, and genotypes of toxoplasma gondii in food animals and humans, (2000-2017) from China. Front. Microbiol. 9, 2108. doi: 10.3389/fmicb.2018.02108
Dorny, P., Praet, N., Deckers, N., Gabriel, S. (2009). Emerging food-borne parasites. Vet. Parasitol. 163, 196–206. doi: 10.1016/j.vetpar.2009.05.026
Dubey, J. P. (2009). History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 39, 877–882. doi: 10.1016/j.ijpara.2009.01.005
Dubey, J. P., Hill, D. E., Jones, J. L., Hightower, A. W., Kirkland, E., Roberts, J. M., et al. (2005). Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J. Parasitol. 91, 1082–1093. doi: 10.1645/ge-683.1
Egger, M., Davey Smith, G., Schneider, M., Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Foroutan, M., Fakhri, Y., Riahi, S. M., Ebrahimpour, S., Namroodi, S., Taghipour, A., et al. (2019). The global seroprevalence of Toxoplasma gondii in pigs: A systematic review and meta-analysis. Vet. Parasitol. 269, 42–52. doi: 10.1016/j.vetpar.2019.04.012
Gao, J., Tang, Z. (2018). Changes in Chinese residents food consumption based on food equivalent. Food Nutr. China 24, 63–67.
Gardner, I. A., Greiner, M., Dubey, J. P. (2010). Statistical evaluation of test accuracy studies for Toxoplasma gondii in food animal intermediate hosts. Zoonoses Public Health 57, 82–94. doi: 10.1111/j.1863-2378.2009.01281.x
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336, 924–926. doi: 10.1136/bmj.39489.470347.AD
Hajimohammadi, B., Ahmadian, S., Firoozi, Z., Askari, M., Mohammadi, M., Eslami, G., et al. (2022). A meta-analysis of the prevalence of toxoplasmosis in livestock and poultry worldwide. Ecohealth 19, 55–74. doi: 10.1007/s10393-022-01575-x
Higgins, J. P., Thompson, S. G., Deeks, J. J., Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Innes, E. A. (1997). Toxoplasmosis: comparative species susceptibility and host immune response. Comp. Immunol. Microbiol. Infect. Dis. 20, 131–138. doi: 10.1016/s0147-9571(96)00038-0
Jalloh, M. F., Sengeh, P., Bunnell, R. E., Jalloh, M. B., Monasch, R., Li, W., et al. (2020). Evidence of behaviour change during an Ebola virus disease outbreak, Sierra Leone. Bull. World Health Organ 98, 330–340b. doi: 10.2471/blt.19.245803
Jones, J. L., Dubey, J. P. (2012). Foodborne toxoplasmosis. Clin. Infect. Dis. 55, 845–851. doi: 10.1093/cid/cis508
Kapperud, G., Jenum, P. A., Stray-Pedersen, B., Melby, K. K., Eskild, A., Eng, J. (1996). Risk factors for Toxoplasma gondii infection in pregnancy. Results of a prospective case-control study in Norway. Am. J. Epidemiol. 144, 405–412. doi: 10.1093/oxfordjournals.aje.a008942
Koutsoumanis, K., Allende, A., Alvarez-Ordóñez, A., Bolton, D., Bover-Cid, S., Chemaly, M., et al. (2018). Public health risks associated with food-borne parasites. Efsa J. 16, e05495. doi: 10.2903/j.efsa.2018.5495
Li, C., Gu, H. (2020). Climate change and mortality evolution in China. J. Environ. Manage 267, 110622. doi: 10.1016/j.jenvman.2020.110622
Li, X. M., Ouyang, Y., Yang, Y. C., Lin, R., Xu, H. B., Xie, Z. Y., et al. (2009). [Distribution of food-borne parasitic diseases and dietary habits in human population in Guangxi]. Chin. J. Parasitol. Parasitic Dis. 27, 151–155.
Liu, Y., Zhang, X., Qi, W., Yang, Y., Liu, Z., An, T., et al. (2021). Prevention and control strategies of african swine fever and progress on pig farm repopulation in China. Viruses 13. doi: 10.3390/v13122552
Marín-García, P. J., Planas, N., Llobat, L. (2022). Toxoplasma gondii in foods: prevalence, control, and safety. Foods 11. doi: 10.3390/foods11162542
Montoya, J. G., Liesenfeld, O. (2004). Toxoplasmosis. Lancet 363, 1965–1976. doi: 10.1016/s0140-6736(04)16412-x
Mukhopadhyay, D., Arranz-Solís, D., Saeij, J. P. J. (2020). Influence of the host and parasite strain on the immune response during toxoplasma infection. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.580425
Pan, M., Lyu, C., Zhao, J., Shen, B. (2017). Sixty years, (1957-2017) of research on toxoplasmosis in China-an overview. Front. Microbiol. 8, 1825. doi: 10.3389/fmicb.2017.01825
Petrovan, S. O., Aldridge, D. C., Bartlett, H., Bladon, A. J., Booth, H., Broad, S., et al. (2021). Post COVID-19: a solution scan of options for preventing future zoonotic epidemics. Biol. Rev. Camb Philos. Soc. 96, 2694–2715. doi: 10.1111/brv.12774
Press, C.M.N (2023) Blue Book on Climate Change of China unveiled. Available online at: https://www.cma.gov.cn/en2014/climate/ClimateUpdate/202307/t20230719_5657000.html (Accessed July 19, 2023).
Robert-Gangneux, F., Dardé, M. L. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296. doi: 10.1128/cmr.05013-11
Schlüter, D., Däubener, W., Schares, G., Groß, U., Pleyer, U., Lüder, C. (2014). Animals are key to human toxoplasmosis. Int. J. Med. Microbiol. 304, 917–929. doi: 10.1016/j.ijmm.2014.09.002
Stoffel, C., Schuppers, M., Buholzer, P., Muñoz, V., Lechner, I., Sperling, U., et al. (2020). The ongoing crises in China illustrate that the assessment of epidemics in isolation is no longer sufficient. Transbound Emerg. Dis. 67, 1043–1044. doi: 10.1111/tbed.13536
Taylor, C. A., Boulos, C., Almond, D. (2020). Livestock plants and COVID-19 transmission. Proc. Natl. Acad. Sci. U.S.A. 117, 31706–31715. doi: 10.1073/pnas.2010115117
Tenter, A. M., Heckeroth, A. R., Weiss, L. M. (2000). Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30, 1217–1258. doi: 10.1016/s0020-7519(00)00124-7
Wang, L., Cheng, H. W., Huang, K. Q., Xu, Y. H., Li, Y. N., Du, J., et al. (2013). Toxoplasma gondii prevalence in food animals and rodents in different regions of China: isolation, genotyping and mouse pathogenicity. Parasit Vectors 6, 273. doi: 10.1186/1756-3305-6-273
Wang, W., Gong, Q. L., Li, M. H., Wei, X. Y., Chen, Y., Jiang, J., et al. (2021). The prevalence of Toxoplasma gondii in sheep in China: A systematic review and meta-analysis. Res. Vet. Sci. 138, 19–29. doi: 10.1016/j.rvsc.2021.05.016
Watts, N., Adger, W. N., Ayeb-Karlsson, S., Bai, Y., Byass, P., Campbell-Lendrum, D., et al. (2017). The Lancet Countdown: tracking progress on health and climate change. Lancet 389, 1151–1164. doi: 10.1016/s0140-6736(16)32124-9
Weiss, L. M., Dubey, J. P. (2009). Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 39, 895–901. doi: 10.1016/j.ijpara.2009.02.004
Zheng, J., Lin, H., Ling, J., Huang, J., Li, D. (2023). The trends of disease burden due to high temperature in Mainland China from 1990 to 2019 and its prediction to 2030. Sci. Rep. 13, 22238. doi: 10.1038/s41598-023-49491-6
Keywords: Toxoplasma gondii, parasitology, epidemiology, public health, meta-analysis
Citation: Yang Z, Yuan H, Nie L, Wen Q, Li H, Yang L, Song Y, Luo X, Zhang X-X and Yuan Z-G (2024) Deciphering the epidemiological dynamics: Toxoplasma gondii seroprevalence in mainland China’s food animals, 2010-2023. Front. Cell. Infect. Microbiol. 14:1381537. doi: 10.3389/fcimb.2024.1381537
Received: 03 February 2024; Accepted: 20 March 2024;
Published: 03 April 2024.
Edited by:
Olgica Djurkovic-Djakovic, University of Belgrade, SerbiaReviewed by:
Ivana Klun, University of Belgrade, SerbiaCristina Cabanacan Salibay, De La Salle University – Dasmariñas, Philippines
Huifang Deng, Utrecht University, Netherlands
Copyright © 2024 Yang, Yuan, Nie, Wen, Li, Yang, Song, Luo, Zhang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xun Luo, larryluoxun@163.com; Xiu-Xiang Zhang, xiuxiangzh@scau.edu.cn; Zi-Guo Yuan, ziguoyuan@scau.edu.cn
†These authors have contributed equally to this work and share first authorship
 Zipeng Yang
Zipeng Yang Hao Yuan
Hao Yuan Linchong Nie1
Linchong Nie1  Yining Song
Yining Song Xun Luo
Xun Luo Zi-Guo Yuan
Zi-Guo Yuan