Survey of Rickettsia species in hematophagous arthropods from endemic areas for Japanese spotted fever in China
- 1Affiliation of Disinfection and Vector Control, Wuhan Center for Disease Control and Prevention, Wuhan, Hubei, China
- 2Clinical Laboratory, Jiangxia Center for Disease Control and Prevention, Wuhan, Hubei, China
- 3National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
Japanese spotted fever (JSF) is caused by Rickettsia japonica, mainly vectored by hard ticks. However, whether R. japonica can be transmitted by other arthropods remains unknown. Moreover, it is of interest to investigate whether other Rickettsia species cause spotted fever in endemic areas. In this study, a survey of Rickettsia species was performed in hematophagous arthropods (mosquitoes, tabanids, and ticks) from endemic areas for JSF in Hubei Province, central China. The results showed that the diversity and prevalence of Rickettsia species in mosquitoes are low, suggesting that mosquitoes may not be the vector of zoonotic Rickettsia species. A novel Rickettsia species showed a high prevalence (16.31%, 23/141) in tabanids and was named “Candidatus Rickettsia tabanidii.” It is closely related to Rickettsia from fleas and mosquitoes; however, its pathogenicity in humans needs further investigation. Five Rickettsia species were identified in ticks. Rickettsia japonica, the agent of JSF, was detected only in Haemaphysalis longicornis and Haemaphysalis hystricis, suggesting that they may be the major vectors of R. japonica. Notably, two novel species were identified in H. hystricis ticks, one belonging to the spotted fever group and the other potentially belonging to the ancestral group. The latter one named “Candidatus Rickettsia hubeiensis” may provide valuable insight into the evolutionary history of Rickettsia.
Introduction
Spotted fever is caused by the spotted fever group Rickettsia species, which are mainly vectored and transmitted by ticks. In China, Japanese spotted fever (JSF) caused by Rickettsia japonica is a common disease in eastern and central areas such as Zhejiang, Hubei, and Henan provinces (Lu et al., 2018; Li et al., 2019; Li and Liu, 2021; Gao et al., 2022; Zhou et al., 2023). The main manifestations of JSF are fever, erythema, and eschar (Noguchi et al., 2018). Occasionally, JSF leads to inflammation of the central nervous system or even death (Gao et al., 2022; Zhou et al., 2023). Rickettsia japonica is transmitted by hard ticks; it has been detected in multiple tick species such as Haemaphysalis longicornis, Haemaphysalis hystricis, Haemaphysalis flava, and Rhipicephalus microplus (Zhao et al., 2021). However, some studies indicate that several patients infected with JSF report no tick bite history (Li et al., 2019). Therefore, it is important to investigate whether JSF is transmitted by other hematophagous arthropods. It should be also noted that the high R. japonica seroprevalence in rural populations in some endemic areas (Li et al., 2018) may also result from immune cross-reaction with other spotted fever group Rickettsia circulating in these areas. However, the prevalence of Rickettsia species in many arthropods from these areas remains largely unknown. To date, only several studies investigated the Rickettsia species in mosquitoes in China (Guo et al., 2016; Zhang et al., 2016, 2019; Li et al., 2022; Lu et al., 2023), and some of those reported Rickettsia bellii and Rickettsia monacensis in endemic areas of JSF. Meanwhile, no Rickettsia species have been reported in tabanids in China. The goal of this study was to investigate the presence of Rickettsia species in hematophagous arthropods (mosquitoes, tabanids, and ticks) collected from endemic areas for JSF in Hubei Province.
Methods
Sample collection and DNA extraction
In the summer and autumn of 2023, mosquitoes, blood-sucking flies, and ticks were collected from four endemic areas for JSF in Hubei Province: 1) Zigui County (110.98°E, 30.83°N) in Yichang City, 2) Xingshan County (110.75°E, 31.35°N) in Yichang City, 3) Macheng County (115.01°E, 31.17°N) in Huanggang City, and 4) Xinzhou District (114.80°E, 30.84°N) in Wuhan City (Figure 1). Mosquitoes were collected in hog pens, sheepfolds, and residential areas using a handheld mosquito trap (Li et al., 2022). Tabanids were collected in cowsheds using a handheld sweep net. We did not find suitable sampling areas for collecting insects in Xingshan, and there were no mosquito traps set in Macheng. Ticks were also collected from the bodies of domestic and roadkill animals opportunistically. All samples were frozen immediately in dry ice and then transported to the Wuhan Center for Disease Control and Prevention Laboratory, where morphological identification was performed (Teng and Jiang, 1991; Jiang et al., 2011; Changbunjong et al., 2021) and DNA was extracted. The entire specimen of mosquitoes and ticks was used for DNA extraction, while only the abdomen of tabanids was used. All samples were individually washed twice using phosphate-buffered saline and then homogenized in a mixer mill (Retsch MM400). DNA extraction was individually performed using the Omega Mollusc DNA kit.
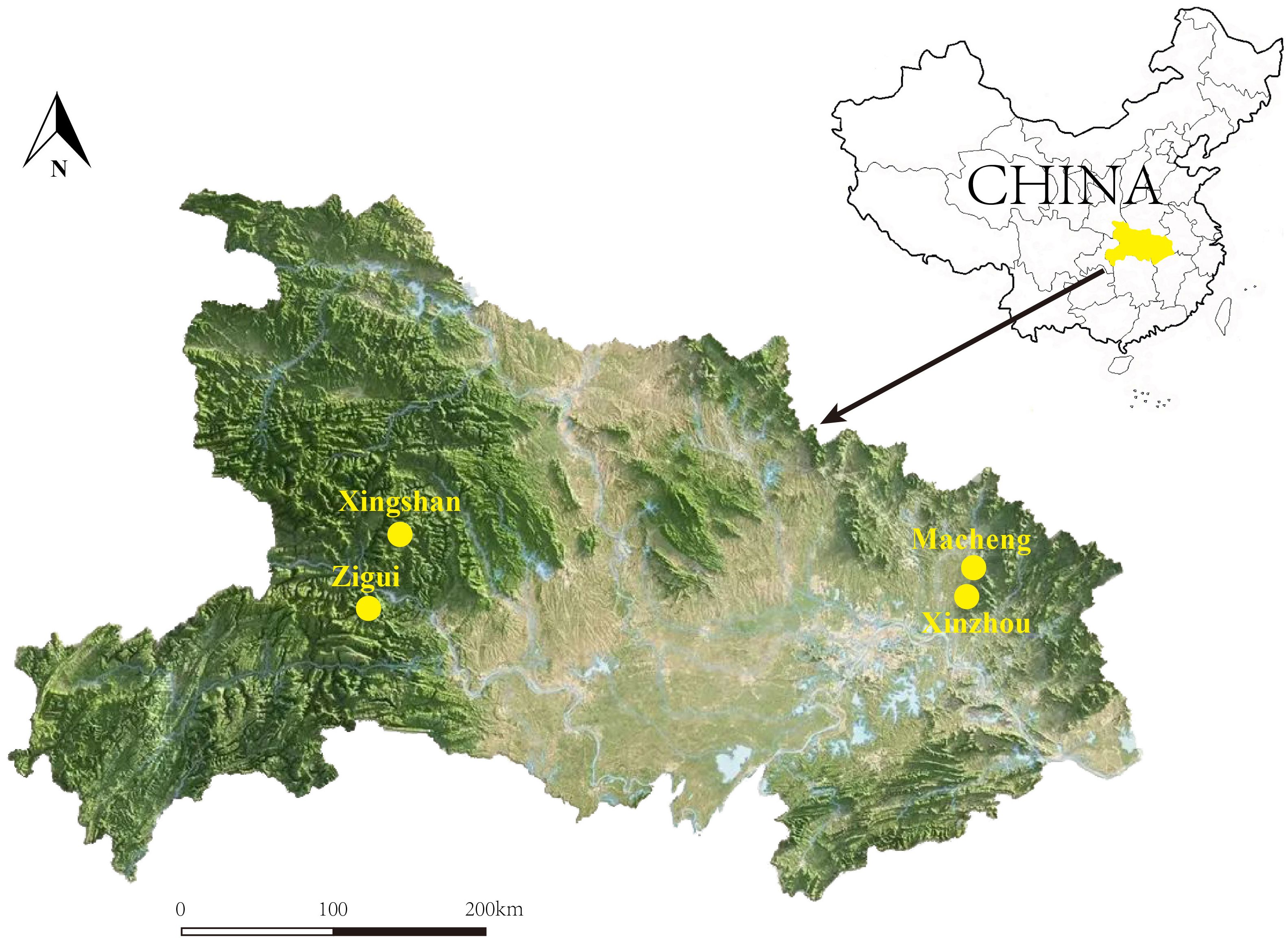
Figure 1 A map showing the locations of Zigui (110.98°E, 30.83°N), Macheng (115.01°E, 31.17°N), Xingshan (110.75°E, 31.35°N), and Xinzhou (114.80°E, 30.84°N) in Hubei Province, where the samples were collected.
Detection of Rickettsia and amplification of key genes
All DNA samples were screened for Rickettsia species by hemi-nested PCR amplifying an approximately 900-bp sequence of the 16S rRNA gene. PCR products with the expected size were subjected to sequencing. The sequences were aligned with those in the GenBank Database using BLASTn (https://blast.ncbi.nlm.nih.gov) to identify the potential Rickettsia species. Subsequently, the gltA (citrate synthase), groEL (60 kDa chaperonin), and ompB (outer member protein B) partial gene sequences were amplified from Rickettsia-positive samples for further analysis. Additionally, the ompA gene was amplified from spotted fever group Rickettsia strains. All primers used are shown in Table 1.
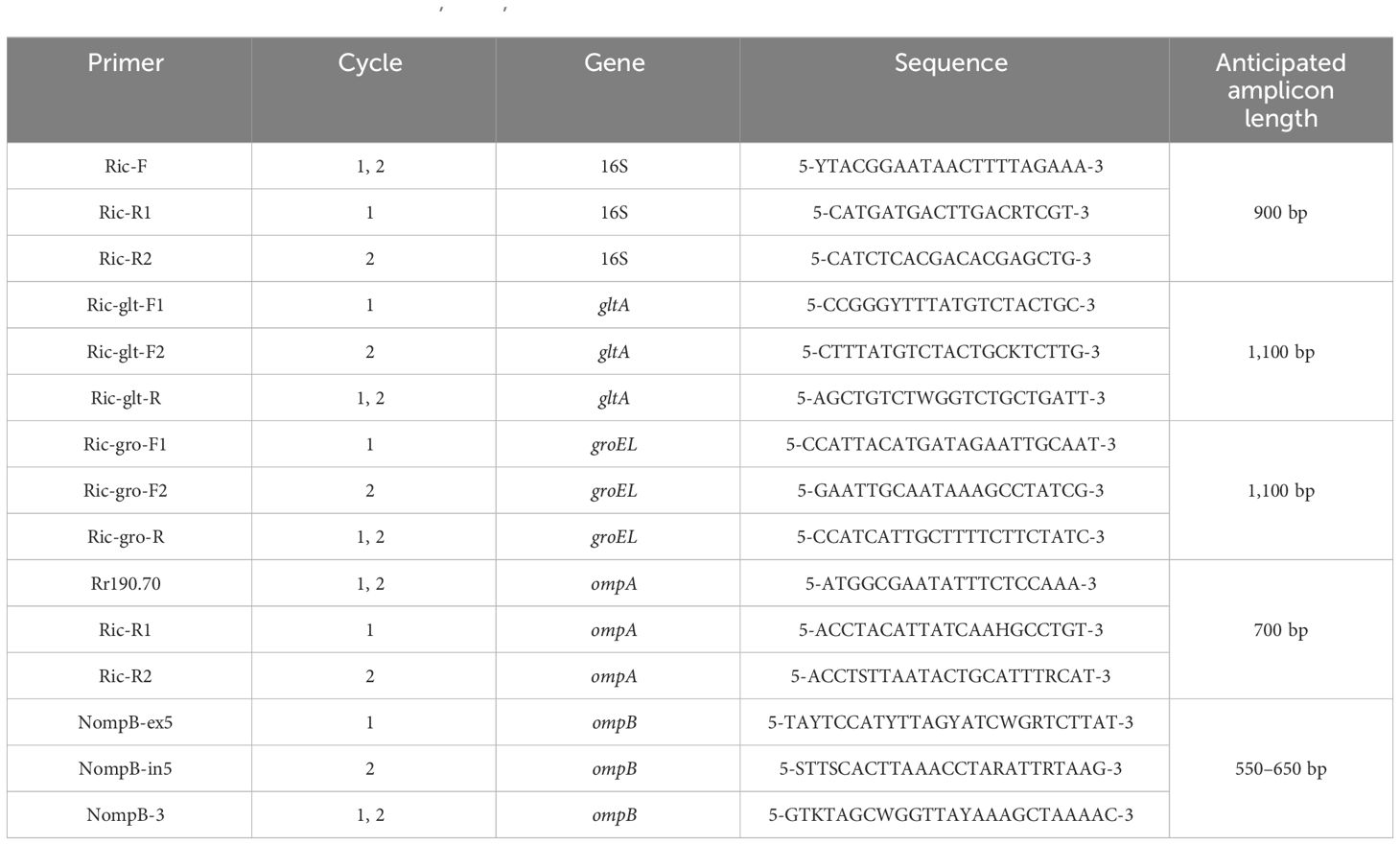
Table 1 The primers used to amplify 16S, gltA, groEL, ompA, and ompB genes from Rickettsia strains by hemi-nested PCR.
Sequence analysis
All the recovered sequences were sequenced in both directions. In addition, chimeras have been screened and removed. All sequences were compared with GenBank sequences using BLAST to determine nucleotide similarity. Sequences obtained in this study and reference sequences downloaded from the GenBank database were aligned using the MegAlign program in Lasergene (Burland, 2000). Maximum-likelihood (ML) trees were reconstructed using PhyML v3.0 (Guindon et al., 2009) under the best-fit substitution model determined by MEGA 7.0 (Kumar et al., 2016).
Results
Sample collections
Between July and October 2023, 527 adult mosquitoes, 141 adult tabanids, and 276 ticks (275 adult and 1 larva) were collected from four locations in Hubei Province. In Zigui County, 202 mosquitoes (48 Culex tritaeniorhynchus, 50 Armigeres subalbatus, 48 Aedes albopictus, 54 Culex quinquefasciatus, and 2 Anopheles sinensis), 67 tabanids (67 Tabanus spp.), and 196 ticks (88 H. longicornis, 58 H. flava, 49 R. microplus, and 1 H. hystricis) were collected (Table 2). In Macheng County, 325 mosquitoes (79 C. tritaeniorhynchus, 69 A. albopictus, 51 A. sinensis, 1 Coquillettidia crassipes, 3 Mansonia uniformis, 98 A. subalbatus, and 24 C. quinquefasciatus) were collected (Table 2). Additionally, 80 H. hystricis ticks were collected in Xingshan County, and 74 Tabanus spp. specimens were collected in Xinzhou District (Table 2). All species were morphologically identified by a trained taxonomist; however, tabanids were not identified at the species level. We tried to molecularly confirm the species of the tabanids, but the results were not decisive.
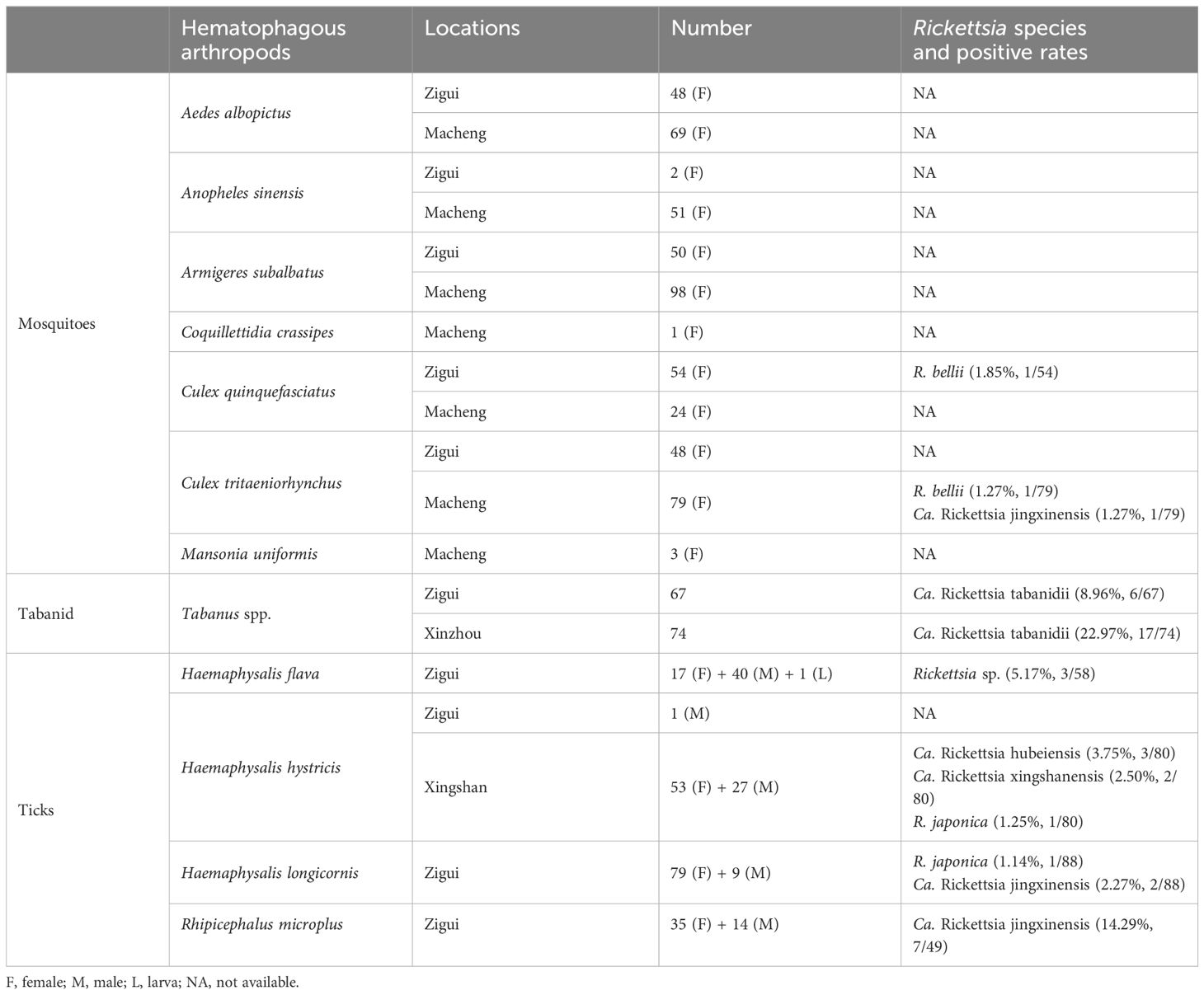
Table 2 Detection of Rickettsia spp. in hematophagous vector species from different sampling locations in Hubei Province, central China.
Rickettsia spp. in mosquitoes and tabanids
Only three mosquitoes (0.57%, 3/527) tested positive for Rickettsia species. Rickettsia bellii was detected in one C. tritaeniorhynchus (strain MCW67) from Macheng and one C. quinquefasciatus (strain ZGCQ1) from Zigui, respectively. The gltA and groEL sequences of the strain ZGCQ1 showed 98.73% and 96.04% nucleotide identity with those of other R. bellii strains. Moreover, Ca. Rickettsia jingxinensis was detected in one C. tritaeniorhynchus specimen.
Notably, tabanids from both locations were positive for a putative novel Rickettsia species (8.96% and 22.97% tabanids from Zigui and Macheng, respectively). Their 16S rRNA, gltA, groEL, and ompB sequences were identical between samples from the two locations despite the geographic distance. Genomic analysis showed that the 16S rRNA sequences had the highest identity (99.88%) with Rickettsia tamurae from ticks and Rickettsia endosymbiont of Curculio spp. The gltA sequences showed 99.14% similarity with Candidatus Rickettsia senegalensis identified in Ctenocephalides felis (fleas), whereas the groEL sequences were highly homologous (99.51%) to Candidatus Rickettsia yingkouensis previously identified in A. sinensis (mosquitoes) (Lu et al., 2023). The ompB sequences showed 98.83% similarity with Rickettsia asembonensis strains identified in C. canis (fleas). In the phylogenetic trees (Figure 2), these strains were clustered in an independent clade. According to the criteria established by Fournier et al. (2003), a novel Rickettsia species should have at most one of the following sequence similarity degrees when compared with validated Rickettsia species: 16S rDNA ≥99.8%, gltA genes ≥99.9%, and, when available, ≥98.8%, ≥99.2%, and ≥99.3% for the ompA, ompB, and sca4 genes, respectively. These results may propose that it represents a putative novel species. Herein, we named it “Candidatus Rickettsia tabanidii.”
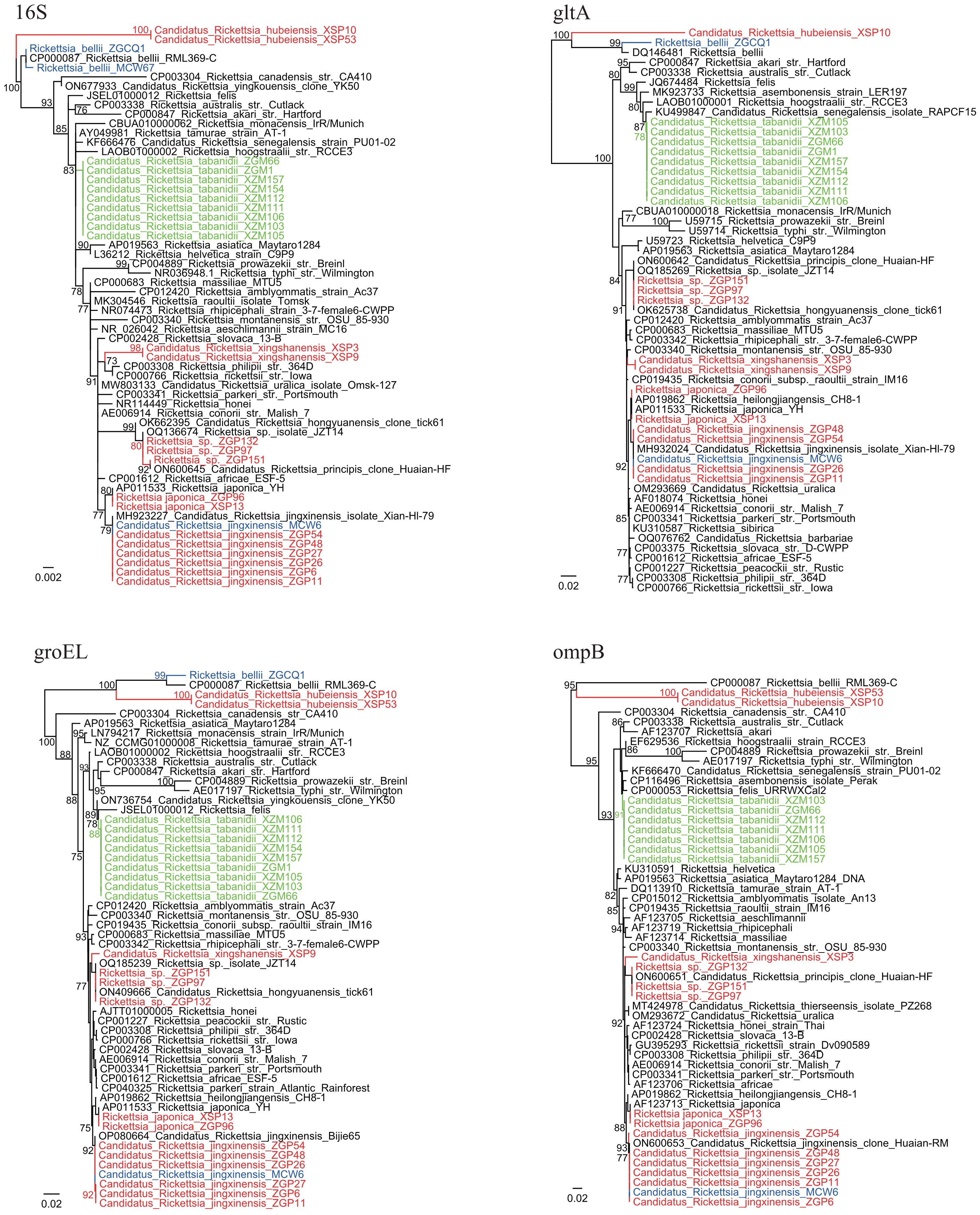
Figure 2 Phylogenetic trees based on the 16S (809 bp), gltA (792–947 bp), groEL (1,032–1,036 bp), and ompB (523–600 bp) sequences of the Rickettsia strains. Green: Rickettsia strains from tabanids. Blue: Rickettsia strains from mosquitoes. Red: Rickettsia strains from ticks.
Rickettsia spp. in ticks
Five Rickettsia species were identified in ticks. Only Ca. Rickettsia jingxinensis (14.29%, 7/49) was detected in R. microplus ticks. Similarly, only one Rickettsia species closely related to Ca. Rickettsia hongyuanensis and Ca. Rickettsia principis (5.17%, 3/58) was detected in H. flava ticks. Phylogenetic analysis of 16S rRNA and ompA sequences indicated certain genetic variations among these strains (Figure 2, Supplementary Figure S1). In H. longicornis ticks, R. japonica, the causative agent of JSF (1.14%, 1/88), as well as Ca. Rickettsia jingxinensis (2.27%, 2/88), was detected. Notably, three Rickettsia species were identified in H. hystricis ticks: R. japonica (1.25%, 1/80) and two novel species (Table 2). For one of these species (strains XSP3 and XSP9), the 16S rRNA sequences were 100% identical to uncultured Rickettsia sp. Hhy_2024 in H. hystricis ticks from Japan and 99.38% identical to Rickettsia peacockii str. Rustic. Moreover, the gltA sequences were 100% identical to Rickettsia sp. MT55-R identified in H. hystricis ticks from Japan and 98.73% identical to R. raoultii strains. The groEL and ompB sequences were also amplified and showed the highest nucleotide similarity to Ca. Rickettsia hongyuanensis tick61 (98.55%) and Rickettsia sp. strain RH-9699 (96.83%), respectively. According to the criteria established by Fournier et al. (2003), although its ompA gene was not amplifiable, our data suggested that it was a novel species belonging to the spotted fever group, and it was named “Candidatus Rickettsia xingshanensis.”
Notably, another novel Rickettsia species (strains XSP10, XSP53, and XSP76) located at the base of the phylogenetic trees was characterized and named “Candidatus Rickettsia hubeiensis” (Figure 2). The 16S rRNA sequences showed the highest identity (98.49%) with Rickettsia honei and 97.89% identity with R. bellii. The gltA, groEL, and ompB sequences were all successfully recovered, showing a nucleotide identity of 92.81% with Rickettsia endosymbiont of Amblyomma patinoi, 89.45% with R. bellii, and 79.09% with R. asembonensis (93% coverage), respectively. Notably, its ompB gene appeared to be truncated. In the phylogenetic trees, this species formed a sister clade with the ancestral Rickettsia species R. bellii.
Discussion
In this study, we investigated whether Rickettsia species were harbored by hematophagous arthropods (mosquitoes, tabanids, and ticks) collected from endemic areas for JSF in China. A total of seven Rickettsia species were identified, including R. japonica—the causative agent of JSF—and three putative novel Rickettsia species. Of the studied arthropods, ticks, especially H. hystricis ticks, were demonstrated to harbor the most diverse Rickettsia species.
Previous studies have suggested that mosquitoes harbor several Rickettsia species belonging to various groups, and the role of mosquitoes in the transmission of Rickettsia has been speculated (Guo et al., 2016; Zhang et al., 2019; Pollio et al., 2022). However, the present study identified only two Rickettsia species in mosquitoes. It is surprising that Ca. Rickettsia jingxinensis, a spotted fever group Rickettsia frequently detected in ticks (Guo et al., 2019; Wang et al., 2021; Lu et al., 2022), was identified in one C. tritaeniorhynchus sample. Natural infection of mosquitoes with Ca. Rickettsia jingxinensis is not likely. In addition, the Ca. Rickettsia jingxinensis-positive mosquito sample was engorged. Therefore, we speculate that the finding is attributable to a blood meal that the mosquitoes derived from a natural animal host of Ca. Rickettsia jingxinensis. In previous studies, the positive rate of R. belli in mosquitoes may vary from 0.52% to 24.49% (Guo et al., 2016; Li et al., 2022). Although some serological investigations showed that animals (dogs, horses, etc.) may be exposed to R. bellii (de Oliveira et al., 2019; Neves et al., 2020), no infection case has been reported in animals or humans. In other words, there is no concrete evidence for the pathogenicity of these two Rickettsia species. Additionally, the positive rates of both Ca. Rickettsia jingxinensis and R. belli were extremely low in mosquitoes. Therefore, we speculate that mosquitoes do not act as the vector of zoonotic Rickettsia species in this area.
It is out of our expectation that tabanids harbor a novel Rickettsia species. Compared with the positive rate reported by Keita et al. (5.1%), the prevalence of Ca. Rickettsia tabanidii in tabanids is quite high (16.31%, 23/141). Although it is well known that tabanids bite humans, tabanids have long been overlooked as a potential vector of zoonotic pathogens. In 2020, Keita et al. detected Rickettsia africae, Rickettsia slovaca, and Rickettsia montanensis in the tabanid species Atylotus fuscipes from Senegal, West Africa (Keita et al., 2020). To our knowledge, this is the only report to date of tabanids harboring Rickettsia species. In the present study, the high prevalence of Ca. Rickettsia tabanidii in samples from two locations suggests that it might have a broad distribution and Rickettsia species could be more frequently carried by tabanids than is reported. More attention should be paid to its human pathogenicity and the associated threat to public health.
Five Rickettsia species were identified in ticks. Rickettsia japonica, the agent of JSF, was detected only in H. longicornis and H. hystricis ticks, suggesting that these two tick species may be the main vectors of R. japonica. This finding is consistent with previous reports (Zhao et al., 2021). Moreover, Candidatus Rickettsia jingxinensis, also named Candidatus Rickettsia longicornii (Guo et al., 2019), was detected in H. longicornis and R. microplus ticks from Zigui County. This finding was not unexpected because it is a widely distributed spotted fever group Rickettsia species and has previously been detected in R. microplus and H. longicornis ticks from multiple provinces in China (Guo et al., 2019; Wang et al., 2021; Lu et al., 2022). Additionally, two spotted fever group Rickettsia species—Ca. Rickettsia xingshanensis and an unidentified Rickettsia closely related to both Ca. Rickettsia hongyuanensis and Ca. Rickettsia principis—were identified in H. hystricis and H. flava, respectively. Although there is still no evidence that they infect humans, their close relationship with pathogenic species suggests a potential risk. Both H. hystricis and H. flava have been reported to bite humans (Grassman et al., 2004; Kim et al., 2022), which potentially increases the risk. Therefore, it is necessary to investigate whether these Rickettsia species are the causative agents of spotted fever in patients in this area.
In H. hystricis ticks, Ca. Rickettsia hubeiensis, a putative novel Rickettsia species located in the ancestral position in the phylogenetic trees, was identified. On the basis of phylogenomic analysis, the genus Rickettsia can be separated into five groups: two spotted fever groups, a typhus group, a canadensis group, and an ancestral bellii group (El Karkouri et al., 2022). The bellii group contains R. bellii, and it is the earliest diverging group among the genus Rickettsia. In phylogenetic trees based on sequences obtained in this study, the Ca. Rickettsia hubeiensis strains formed a sister clade with R. bellii and formed the outgroup for all other Rickettsia members. Although its genome sequences remain unavailable, we propose that it may be a novel member of the bellii group. The study of its genome sequence may elucidate the evolutionary history of Rickettsia.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
JT: Writing – review & editing, Resources, Investigation, Funding acquisition. JingL: Writing – review & editing, Investigation. JinL: Writing – review & editing. ML: Writing – review & editing, Methodology. XC: Writing – review & editing, Investigation. KL: Writing – review & editing, Writing – original draft, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Natural Science Foundation of Hubei Province of China (2023AFB1011), the National Key Research and Development Program of China (2021YFC2301202), and the Medical Youth Top Talent Project of Hubei (EWT2019048).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1384284/full#supplementary-material
Supplementary Figure S1 | Phylogenetic tree based on the ompA (677-696 bp) sequences of the spotted fever group Rickettsia strains. Blue: Rickettsia strains from mosquitoes. Red: Rickettsia strains from ticks.
References
Burland, T. G. (2000). DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 132, 71–91. doi: 10.1385/1-59259-192-2:71
Changbunjong, T., Prakaikowit, N., Maneephan, P., Kaewwiset, T., Weluwanarak, T., Chaiphongpachara, T., et al. (2021). Landmark data to distinguish and identify morphologically close Tabanus spp. (Diptera: Tabanidae). Insects 12, 974. doi: 10.3390/insects12110974
de Oliveira, P. B., Harvey, T. V., Fehlberg, H. F., Rocha, J. M., Martins, T. F., da Acosta, I. C. L., et al. (2019). Serologic and molecular survey of Rickettsia spp. in dogs, horses and ticks from the Atlantic rainforest of the state of Bahia, Brazil. Exp. Appl. Acarol. 78, 431–442. doi: 10.1007/s10493-019-00397-x
El Karkouri, K., Ghigo, E., Raoult, D., Fournier, P. E. (2022). Genomic evolution and adaptation of arthropod-associated Rickettsia. Sci. Rep. 12, 3807. doi: 10.1038/s41598-022-07725-z
Fournier, P. E., Dumler, J. S., Greub, G., Zhang, J., Wu, Y., Raoult, D. (2003). Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 41, 5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003
Gao, S., Li, L., Zhou, X., Dai, X., Lu, L., Chen, Y., et al. (2022). Fatal rickettsia japonica infection complicating disseminated intravascular coagulation in yichang, China. Infect. Drug Resist. 15, 6613–6623. doi: 10.2147/IDR.S383917
Grassman, L. I., Jr, Sarataphan, N., Tewes, M. E., Silvy, N. J., Nakanakrat, T. (2004). Ticks (Acari: Ixodidae) parasitizing wild carnivores in Phu Khieo Wildlife Sanctuary, Thailand. J. Parasitol. 90, 657–659. doi: 10.1645/GE-3327RN
Guindon, S., Delsuc, F., Dufayard, J. F., Gascuel, O. (2009). Estimating maximum likelihood phylogenies with PhyML. Methods Mol. Biol. 537, 113–137. doi: 10.1007/978-1-59745-251-9_6
Guo, W. P., Tian, J. H., Lin, X. D., Ni, X. B., Chen, X. P., Liao, Y., et al. (2016). Extensive genetic diversity of Rickettsiales bacteria in multiple mosquito species. Sci. Rep. 6, 38770. doi: 10.1038/srep38770
Guo, W. P., Wang, Y. H., Lu, Q., Xu, G., Luo, Y., Ni, X., et al. (2019). Molecular detection of spotted fever group rickettsiae in hard ticks, northern China. Transbound Emerg. Dis. 66, 1587–1596. doi: 10.1111/tbed.13184
Jiang, Z. K., Zheng, Z. M., Wang, Z. C. (2011). Management of Hygienic Pest (Beijing: People’s Medical Publishing House Co., Ltd).
Keita, M. L., Medkour, H., Sambou, M., Dahmana, H., Mediannikov, O. (2020). Tabanids as possible pathogen vectors in Senegal (West Africa). Parasit Vectors. 13, 500. doi: 10.1186/s13071-020-04375-w
Kim, Y. J., Seo, J. Y., Kim, S. Y., Lee, H. I. (2022). Molecular detection of Anaplasma phagocytophilum and Ehrlichia species in ticks removed from humans in the Republic of Korea. Microorganisms 10, 1224. doi: 10.3390/microorganisms10061224
Kumar, S., Stecher, G., Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Li, J., Hu, W., Wu, T., Li, H. B., Hu, W., Sun, Y., et al. (2018). Japanese spotted fever in Eastern Chin. Emerg. Infect. Dis. 24, 2107–2109. doi: 10.3201/eid2411.170264
Li, W., Liu, S. N. (2021). Rickettsia japonica infections in Huanggang, China, in 2021. IDCases 26, e01309. doi: 10.1016/j.idcr.2021.e01309
Li, F., Tian, J., Wang, L., Yang, Z., Lu, M., Qin, X., et al. (2022). High prevalence of Rickettsia bellii in mosquitoes from Eastern China. J. Med. Entomol. 59, 390–393. doi: 10.1093/jme/tjab177
Li, H., Zhang, P. H., Du, J., Yang, Z. D., Cui, N., Xing, B., et al. (2019). Rickettsia japonica infections in humans, Xinyang, China 2014-2017. Emerg. Infect. Dis. 25, 1719–1722. doi: 10.3201/eid2509.171421
Lu, M., Chen, S., Meng, C., Wang, W., Li, H., Sun, Y., et al. (2023). A novel Rickettsia species closely related to Rickettsia felis in Anopheles mosquitoes from Yingkou City, Northeast China. Zoonoses Public Health 70, 568–571. doi: 10.1111/zph.13043
Lu, M., Tian, J., Wang, W., Zhao, H., Jiang, H., Han, J., et al. (2022). High diversity of Rickettsia spp., Anaplasma spp., and Ehrlichia spp. in ticks from Yunnan Province, Southwest China. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1008110
Lu, Q., Yu, J., Yu, L., Zhang, Y., Chen, Y., Lin, M., et al. (2018). Rickettsia japonica infections in humans, Zhejiang Province, Chin. Emerg. Infect. Dis. 24, 2077–2079. doi: 10.3201/eid2411.170044
Neves, L. C., Barreto, A. L. G., Souza, M. X., Martins, D. B., Barbieri, A. R. M., Serpa, M. C. A., et al. (2020). Serosurvey on rickettsiae of the spotted fever group and Rickettsia bellii among dogs in the state of Goiás, Brazil. Rev. Bras. Parasitol. Vet. 29, e021419. doi: 10.1590/s1984-29612020018
Noguchi, M., Oshita, S., Yamazoe, N., Miyazaki, M., Takemura, Y. C. (2018). Important clinical features of Japanese spotted fever. Am. J. Trop. Med. Hyg. 99, 466–469. doi: 10.4269/ajtmh.17-0576
Pollio, A. R., Jiang, J., Lee, S. S., Gandhi, J. S., Knott, B. D., Chunashvili, T., et al. (2022). Discovery of Rickettsia spp. in mosquitoes collected in Georgia by metagenomics analysis and molecular characterization. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.961090
Teng, K. F., Jiang, Z. J. (1991). Economic insect fauna of China, Fasc 39 Acari: Ixodidae (in Chinese) (China: Scienece Press Beijing).
Wang, Q., Guo, W. B., Pan, Y. S., Jiang, B. G., Du, C. H., Que, T. C., et al. (2021). Detection of novel spotted fever group Rickettsiae (Rickettsiales: Rickettsiaceae) in ticks (Acari: Ixodidae) in Southwestern China. J. Med. Entomol. 58, 1363–1369. doi: 10.1093/jme/tjaa294
Zhang, J., John Kelly, P., Lu, G., Cruz-Martinez, L., Wang, C. (2016). Rickettsia in mosquitoes, yangzhou, China. Emerg. Microbes Infect. 5, e108. doi: 10.1038/emi.2016.107
Zhang, J., Lu, G., Li, J., Kelly, P., Li, M., Wang, J., et al. (2019). Molecular Detection of Rickettsia felis and Rickettsia bellii in Mosquitoes. Vector Borne Zoonotic Dis. 19, 802–809. doi: 10.1089/vbz.2019.2456
Zhao, G. P., Wang, Y. X., Fan, Z. W., Ji, Y., Liu, M. J., Zhang, W. H., et al. (2021). Mapping ticks and tick-borne pathogens in China. Nat. Commun. 12, 1075. doi: 10.1038/s41467-021-21375-1
Keywords: mosquitoes, tabanids, ticks, Japanese spotted fever, Candidatus Rickettsia tabanidii, Candidatus Rickettsia xingshanensis, Candidatus Rickettsia hubeiensis
Citation: Tian J, Liu J, Liu J, Lu M, Chen X and Li K (2024) Survey of Rickettsia species in hematophagous arthropods from endemic areas for Japanese spotted fever in China. Front. Cell. Infect. Microbiol. 14:1384284. doi: 10.3389/fcimb.2024.1384284
Received: 09 February 2024; Accepted: 08 April 2024;
Published: 25 April 2024.
Edited by:
Adela Oliva Chavez, Texas A and M University, United StatesReviewed by:
Benjamin Cull, University of Minnesota Twin Cities, United StatesJulia González, Texas A and M University, United States
Copyright © 2024 Tian, Liu, Liu, Lu, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun Li, likun@icdc.cn
†These authors have contributed equally to this work
 Junhua Tian1†
Junhua Tian1†  Miao Lu
Miao Lu Kun Li
Kun Li