Accessibility to Protected Areas Increases Primate Hunting Intensity in Bioko Island, Equatorial Guinea
- 1Department of Applied Sciences, University of the West of England, Bristol, United Kingdom
- 2Departamento de Tecnología Ambiental, Facultad de Medio Ambiente, Universidad Nacional de Guinea Equatorial, Malabo, Equatorial Guinea
- 3Bioko Biodiversity Protection Program, Academy of Natural Sciences of Drexel University, Philadelphia, PA, United States
- 4Facultad de Medio Ambiente, Universidad Nacional de Guinea Equatorial, Malabo, Equatorial Guinea
- 5Oficina de Investigación, Universidad Nacional de Guinea Equatorial, Malabo, Equatorial Guinea
- 6Department of Biology, Drexel University, Philadelphia, PA, United States
- 7Academy of Natural Sciences of Drexel University, Philadelphia, PA, United States
Bioko is one of the most important sites for African primate conservation; yet it has seen a severe decline in its primate populations due to illegal hunting to supply a thriving wildmeat trade. The completion in 2015 of a new road bisecting the Gran Caldera Scientific Reserve (GCSR), where rugged terrain and lack of infrastructure once served as a natural barrier, further threatened this last stronghold for Bioko's primates. Here we used passive acoustic monitoring to study factors affecting hunting patterns within GCSR through the automatic detection of shotgun sounds. Ten acoustic sensors were placed in locations that varied in terrain heterogeneity, distance to the new road, human settlements, research camps (i.e., Moraka and Moaba) and elevation. Sensors recorded continuously between January 2018 and January 2020, collecting 2,671 site-days of audio. In total 596 gunshots were detected, including in the most remote areas. There were significant differences in hunting rate between areas (Kruskal-Wallis, χ2 = 102.71, df = 9, p < 0.001). We also found there were significantly fewer gunshots during 2019 than during 2018 (V = 55, p < 0.001). Occupancy modeling showed that hunting increased with decreasing terrain heterogeneity and decreasing distance to roads and villages; and decreased with increasing proximity to Research Camps. These results demonstrated that increasing accessibility increased primate hunting in GCSR, which was exacerbated by the opening of the new road. We also demonstrated that research presence was effective at reducing primate hunting. Unless strict conservation interventions are implemented, including road checkpoints, increasing biomonitoring and hunting patrols, and an island-wide, enforced ban on firearms, GCSR will see a significant decrease in primate density over the next decade, including the potential extinction of Critically Endangered Pennant's red colobus, whose entire population is restricted to GCSR and is a primary target of hunters.
Introduction
The earth is in the midst of a biodiversity crisis. As the human population and the use of natural resources grows, so does the pressure on the Earth's ecosystem. In the case of primates, our closest relatives, 65% of species are currently threatened with extinction, and 87% have declining populations (Fernández et al., 2021; IUCN, 2021). One of the main drivers of primate population decline is hunting to supply a commercial trade in wildmeat, which affects 79% of species. Hunting is particularly severe in Africa, where it affects 91% of threatened species compared to 74% in the Americas, 67% in Madagascar, and 87% in Asia (IUCN, 2021).
Unsustainable levels of hunting have been described for each of the four regions where primates are found (Milner-Gulland and Bennett, 2003; Chapman and Peres, 2021). In Central Africa alone, it is estimated that between 1 and 4 million tons of wildmeat is extracted annually (Fa et al., 2002; Fa and Brown, 2009), which has decimated primates' and other mid- and large-bodied species' populations (Milner-Gulland and Bennett, 2003). The effect of hunting on primate numbers tends to be more pronounced outside of protected areas. For example, a study in Monte Alén National Park, continental Equatorial Guinea, did not detect any group of black colobus (Colobus satanas) around the village of Sendje, just ~10 km outside of the Park's boundaries, while they were the most abundant species inside the Park (Kümpel et al., 2010). Hunting, however, is also well documented inside protected areas, as many lack effective enforcement and are in essence paper parks (N'Goran et al., 2012). For instance, in Moukalaba Doudou National Park, Gabon, the abundance of western lowland gorilla (Gorilla gorilla gorilla) and central chimpanzee (Pan troglodytes troglodytes) decreased as hunting intensity increased (Kuehl et al., 2009). Similarly, in Korup National Park, Cameroon, drills (Mandrillus leucophaeus leucophaeus) disappeared in a region that had seen a threefold increase in primate offtake from 1990 to 2004 (Linder and Oates, 2011). Hunting is also the main driver behind the potential extinction of the Miss Waldron's red colobus (Piliocolobus waldronae) in Ghana (Oates et al., 2020).
Wildmeat hunting can have detrimental consequences on non-targeted species as well as the habitat more generally. For example, leopards (Panthera pardus) in Gabon showed lower population densities and a shift to smaller prey where wildmeat hunting is high, as both humans and leopards have high dietary niche overlap, resulting in strong exploitative competition between both species (Henschel et al., 2011). Similarly, as primates perform key ecological functions, primate hunting can have profound consequence on an ecosystem's structure and function (Bourlière, 1985; Poulsen et al., 2001; Chapman et al., 2013). For example, the loss of primate species has been linked to a reduction in angiosperm diversity, a general loss of plant diversity, and a takeover of pioneer species in tropical forests in the Central African Republic (Vanthomme et al., 2010). In Nigeria, the seedling-community of heavily hunted areas differed from that of protected sites, where primate-dispersed seedlings dominated (Effiom et al., 2013). Primate disappearance has also been linked to a reduction on a habitat's carbon storage potential. In the Amazon basin, the loss primates led to the decline of hardwood tree species, forest plant diversity, biomass and the overall forests' carbon storage capacity (Stevenson and Aldana, 2008; Peres et al., 2016). This same pattern has been seen in Central African tropical forests, where areas that were hunted commercially for wildmeat had significantly lower above-ground biomass than non-hunted forests (Poulsen et al., 2013). Additionally, the loss of primates can also affect the income and food security of human population living in these areas. For example, in Côte d'Ivoire and Uganda, 48 and 42% of plants dispersed by primates had economic and/or cultural value to local communities, respectively (Koné et al., 2008). Moreover, at current wildmeat extraction rates in the Congo Basin, it is predicted that—with the exception of Gabon—by 2050 there will not be enough wildmeat available to meet the daily protein requirements of its growing human population (Fa et al., 2003).
Given the dire threat presented by commercial wildmeat hunting for biodiversity conservation and environmental and human health, novel, cost-effective methods of understanding hunting patterns are required to help inform impactful conservation interventions. One such method is passive acoustic monitoring (PAM), the process of using acoustic data to inform on a species, ecosystem, or anthropogenic activity (Sugai et al., 2019). This is done by placing acoustic sensors (sound recorders) in an environment. These acoustic recordings can be then analyzed to assess a range of topics, including human activity (Sugai et al., 2019), making PAM a uniquely suited tool for assessing and understanding gun hunting in tropical forests. Astaras et al. (2017, 2020), for instance, used a 12-sensor grid to quantify gunshots in Korup National Park between June 2013 and 2016. They found that hunting was more prevalent during the dry season (December to February), peaking during the weeks leading up to Christmas Day and New Year's Day (Astaras et al., 2017). They also found that 68.6% of gun hunting took place at night, although it was negatively affective by moon illumination, and that hunting intensity increased over time (Astaras et al., 2017, 2020). Overall, the authors found that PAM was a more effective and less costly method to monitor hunting than foot patrols and camera traps (Astaras et al., 2017).
Bioko Island, Equatorial Guinea (Figure 1), in the Gulf of Guinea, has been identified as a hotspot for biodiversity conservation, particularly primates (Oates, 1996; Myers et al., 2000). Although relatively small (2,017 km2), Bioko harbors seven species of diurnal primates, including the endemic Pennant's red colobus (Piliocolobus pennatii), classified as Critically Endangered by the IUCN (Cronin, 2019). Bioko still contains large swaths of primary forest, and ~40% of its territory falls within one of the two existing protected areas: Pico Basilé National Park (330 km2), in the North; and Gran Caldera de Luba Scientific Reserve (GCSR, 510 km2), which covers most of the southern half of the island (Figure 1). Although Bioko's forest remains relatively intact, a long-stablished commercial wildmeat hunting trade has decimated Bioko's primate population, particularly during the last two decades (Albrechtsen et al., 2005; Reid et al., 2005; Cronin et al., 2015a,b, 2016). Carcass surveys conducted between 1997 and 2010 at the Malabo market, the largest wildmeat market in the island, recorded 197,000 animals for sale. Primates alone made up 18% (35,000 animals) of those of carcases, and were almost exclusively hunted with shotguns (Reid et al., 2005; Cronin et al., 2015b). Currently, GCRS is the only place in Bioko where all the seven species of diurnal primates remain, as an almost complete lack of human presence (the only settlement is the coastal village of Ureca, a small community of less than 80 occupants), combined with rugged terrain and heavy rain, has historically served as a natural barrier to hunters (Cronin et al., 2015a; Figure 1). This changed in 2015, when a new paved road connecting the village of Belebu to Ureca was completed (Figure 1), effectively bisecting GCRS into two forest regions and opening the area to hunters (Cronin et al., 2017).
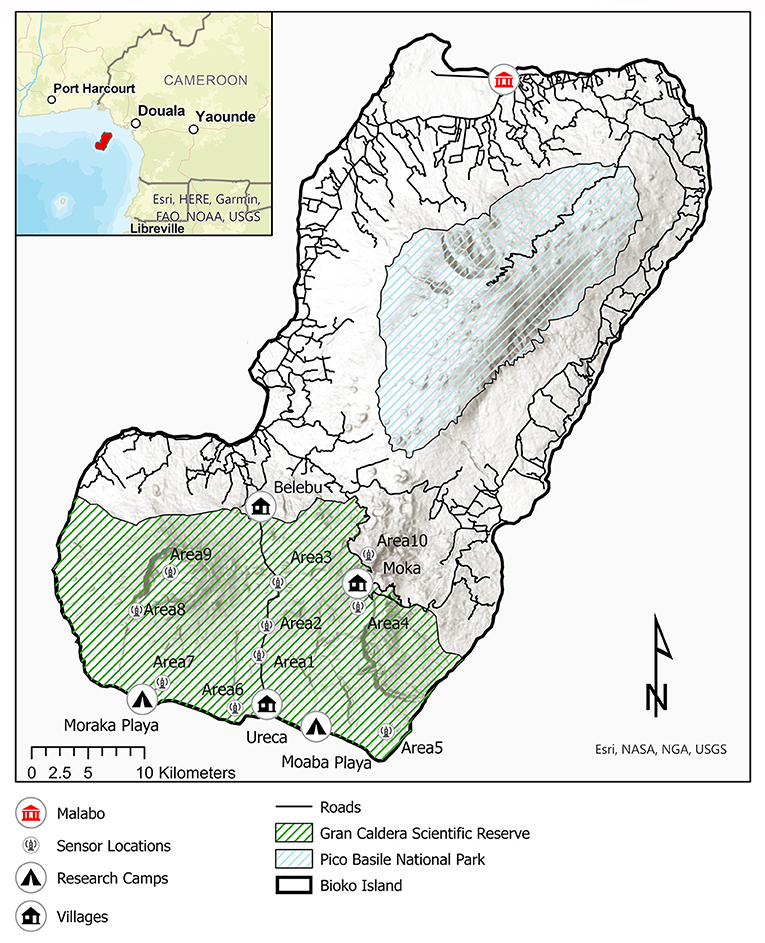
Figure 1. Distribution of protected areas, roads, villages, research camps and the 10 monitored areas inside the Gran Caldera de Luba Scientific Reserve (shaded green) in Bioko Island, Equatorial Guinea. Area 9 is located within the Gran Caldera Volcánica de Luba u Ose, one of the most isolated locations in Bioko. The city of Malabo, capital of Equatorial Guinea, harbors the largest wildmeat markets in Bioko.
Road construction and other infrastructure development has been linked to an increase in hunting in tropical forests, as these constructions open up previously inaccessible and difficult to reach areas to hunters (Wilkie et al., 1992; Abernethy et al., 2013). For instance, in Nigeria and Cameroon, increased road density has been linked a decreased in large-mammal density on the area (Fa et al., 2015). Similarly, in the Republic of Congo, the construction of logging infrastructure and industrial roads led to an increase in hunting, as the workforce migrated and settled along the new roads (Poulsen et al., 2009).
In this study we use PAM to measure hunting patterns across GCSR, including the effect of the new paved road. We predict that hunting intensity will increase with accessibility, being highest in areas closest to the new road and villages, and lowest in most remote areas, particularly inside the Gran Caldera de Luba (Figure 1: Area 9). We also predict hunting intensity would be low in areas close to the two existing research camps on the southern coast (Figure 1), as research presence has been shown to deter illegal activities (Junker et al., 2020).
Materials and Methods
Study Area
This study was conducted in GCSR, which comprises 25% of Bioko Island's area (Figure 1). GCSR is formed of two large steep sided volcanoes covering an elevation range from sea level to 2,261 m (Cronin et al., 2017). The climate is tropical/equatorial, with an average temperature of 26°C. GCSR receives rain throughout the year with annual rainfall exceeding 10,000 mm, however rainfall varies between the dry season (November–March) and the wet season (April–October), with a peak rainfall between July and September (Cronin et al., 2017). The elevational and rainfall ranges lead to varied habitat types in the GCSR, predominantly Guineo-Congolian rainforest at low elevation and Afromontane vegetation at higher elevations (Fa et al., 2000). The only human settlement within and around the GCSR are Ureca, on the south of the GCSR, and the villages of Belebu and Moka, on the north and northwest boundary of the GCSR, respectively (Figure 1). Additionally, as part of ongoing biomonitoring efforts by the Bioko Biodiversity Protection Program (BBPP, www.bioko.org), since 2013 there has been two research camps on the GCSR southern coast, one located at Moraka Playa, to the West, and one at Moaba Playa, to the East of the Reserve (Figure 1). These camps are occupied during the dry season by researchers, volunteers and occasionally by staff from the Equatoguinean Institute for Forestry Development (INDEFOR), a governmental body that oversees all activities within Protected Areas. From these camps, personnel conduct daily sea turtle and primate censuses (Cronin et al., 2017).
Due to different ecological and habitat requirements, as well as to historical hunting pressure (Fa et al., 2000; Reid et al., 2005; Cronin et al., 2017), primate diversity varies across the GCSR, with the highest diversity found inside Gran Caldera Volcánica de Luba u Ose, in the northeast of the park (Figure 1: Area 9), where all seven species of diurnal primates are found; and the lowest around the northern border of the protected area (Cronin et al., 2017). At lower altitudes and in less remote areas, primate diversity remains relatively high, however, except for the Preuss guenon (Allochrocebus preussi insularis), a high-altitude specialist; and the Pennant's red colobus, which is particularly sensitive to hunting and is only found at low densities (Cronin et al., 2016, 2017).
Data Collection
Data were collected using Ten SWIFT passive acoustic sensors (Bioacoustics Research Program at the Cornell Lab of Ornithology) from 19th January 2018 through 5th January 2020 (Figure 1). Sensors were deployed in areas 1–9 starting on 19th January 2018. After its initial deployment, the sensor in area 5 was thought lost and was not relocated until December 2018. In February 2019, an additional sensor was deployed in area 10 until the end of the study. Deployment locations were systematically chosen to ensure they varied in their accessibility. To avoid sampling overlap, all sensors were separated by at least 2.4 km, as previous studies suggest that SWIFT sensors have a shotgun sound detection rate of ~1.2 km (Astaras et al., 2017; Astaras, Pers. Comm.). The total monitored area was therefore 75 km2. Sensors were placed on tree trunks, approximate 200 cm off the ground, away from existing trails and water sources. Vegetation around the sensor was removed to prevent the vegetation from knocking against the sensors and disrupting the recording. Sensors were set up to record continuously on one channel, at a sampling frequency of 8 kHz and a gain of 32 dB. The audio collected was saved as uncompressed .wav files using SanDisk Extreme Pro SD card memory cards. Batteries and memory cards were changed every 2 months approximately, except sensors in areas 7, 8 and 9, which were not accessible during the rainy season. Overall, we collected > 2,671 site-days of audio.
Data Analysis
We used an automatic gunshot detection algorithm developed by the Elephant Listening Project at Cornell University (Wrege et al., 2017a,b) to identify putative gunshots in the audio files using Raven Pro Sound Analysis Software Version 1.6.1 (Yang, 2019). The detection algorithm was based on cross-correlation of sound spectrograms of nine shotgun shots sampled at 4 kHz in a forest habitat in Gabon and Cameroon (Wrege et al., 2017a,b; Wrege, Pers. Comm.). The algorithm assigned a score (ranging between 0 and 1) to each putative gunshot indicating the probability that the signal was an actual gunshot. Because gunshots are relatively rare events, we used the default score threshold of 0.53, as we prioritized true positive recall (the number of actual gunshots returned) over precision (Astaras et al., 2017; Wrege et al., 2017b, Astaras, Pers. Comm). Each putative gunshot with a score over the 0.53 threshold was verified acoustically and visually via the signal's spectrogram (Figure 2).
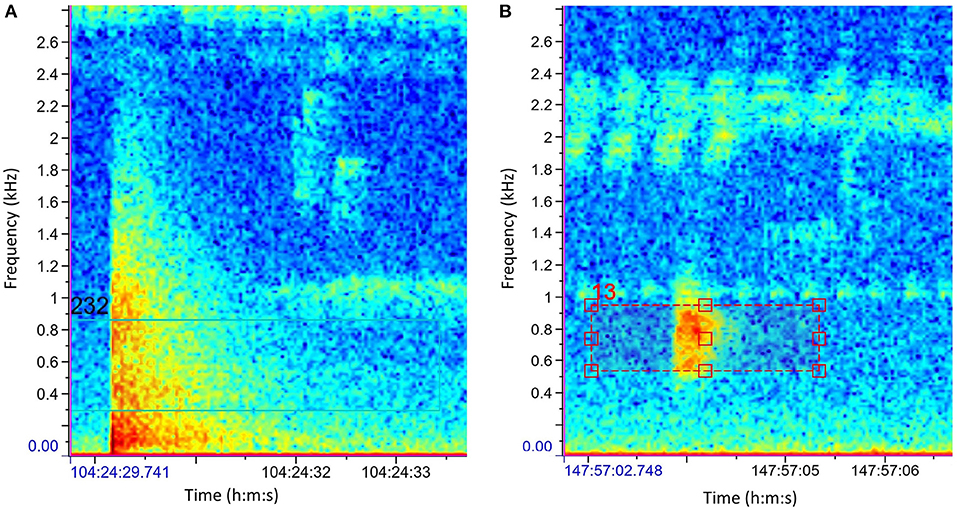
Figure 2. Output spectrograms of the Automatic Detection Algorithm and the score assigned by the algorithm to an actual gunshot (A) and a false positive, a bird call (B). Color indicates sound intensity, with blue being lowest and red highest. Gunshots are characterized by a broad, sudden increase in intensity followed by a gradual decreased.
Description of Hunting Patterns in GCRS
To describe spatio-temporal hunting patterns in GRCS and due to variation in sampling effort across areas, we defined hunting rate as the number of gunshots divided by the number of days or weeks recorded per area. We used gunshots/week to calculate variation in hunting frequency between years (2018 vs. 2019), season (dry vs. wet), month of the year and lunar intensity [i.e., the percentage of visible moon surface: 0–25% (New Moon), 25–75% (Waxing and Waning Moons) and 75–100% (Full Moon)]. We used gunshots/day to calculate hunting frequency during each day of the week and times of day (day vs. night).
Hunting frequency was highly zero inflated and not normally distributed. To examine differences in hunting rates within an area, the Mann-Whitney U test was used for comparisons between years (2018 vs. 2019), seasons (dry vs. rainy), and times of day (day vs. night); while the Kruskal-Wallis test was used for comparisons among months, days of the week, and lunar intensities. Differences across areas, on the other hand, were tested using the Wilcoxon-matched pair test to compare variation between years, seasons and times of day; and the Kruskal-Wallis test to examine differences between months, days of the week and lunar intensity.
Occupancy Modeling
To understand what factors affected the occurrence and abundance of gunshots in the GCSR we used single-season, single-species occupancy modeling (MacKenzie, 2017; Ferreguetti et al., 2018; Dias et al., 2020). Occupancy models are formed from two parts, covariates that affect detectability and covariates that affect occupancy (MacKenzie, 2017), thus it is possible to create an estimate of where hunting is most likely to happen (occupancy, Ψ) and where hunting is likely to be most frequent (detectability, p) (Ferreguetti et al., 2018). In total, we selected four spatial covariates, all shown to affect the frequency of illegal wildmeat hunting (Fa et al., 2015; Ferreguetti et al., 2018; McNamara et al., 2019; Dias et al., 2020): the Euclidean distance to the nearest main road (Road), the Euclidean distance to the nearest village (Village), the Euclidean distance to the nearest research camp in the GCSR (Camps), and the average Terrain Heterogeneity (i.e., terrain ruggedness, Hetro) using a 1.2 km buffer radius from each sensor, as that distance is the estimated sensors' gunshot detection rate (Astaras et al., 2017, Astaras Pers. Comm.). In addition to the spatial covariates, we also included as a covariate lunar intensity averaged over a 7-day period (Moon).
All covariate data were collected using ArcGIS Pro. Euclidean distances were calculated using the Euclidian Distance function. The elevation data were sourced from World Elevation Terrain service by Esri. Terrain heterogeneity was created using a high-resolution elevation raster with 0.25 m cells, each cell holding an elevation value. We first calculated differences in elevation values between neighboring cells using the Calculate Topographic Heterogeneity function in the SDMtoolbox (Brown et al., 2017). These differences were then averaged within a 1.2 km radius buffer around each sensor to account for the sensors' gunshot detection rate. The resulting averaged valued was used as the terrain heterogeneity for each sensor. Correlations between all covariates were tested with Pearson's Correlation Coefficient and all were found to be ≤ 0.6, indicating was no autocorrelation between covariates.
Because areas 7, 8 and 9 were not accessible to hunters during the rainy season, we used only data from the 5-month dry season between November 2018 and March 2019, for this analysis. Data during this period were divided into 22 discrete sampling periods of 7 days to create a consistent detection history of whether hunting took place during a given week. Selection of the best-fitting model followed a two-step process, whereby we first determined the best-fitting model for detectability (p) while keeping occupancy (Ψ) constant. Next, we determined the best-fitting model for occupancy (Ψ) while modeling detectability (p) as indicated by the best-fit model identified in step 1 (Ferreguetti et al., 2018). In total we built 1,054 models using the UNMARKED package in R (Fiske and Chandler, 2011). Top ranked models were selected using Akaike's Information Criterion corrected for small sample size (AICc) and the model weight (AICcw). All models with a difference in AICc (ΔAICc) of less than 2 were considered equivalent and averaged. The goodness of fit of the selected model was tested using a MacKenzie and Bailey Goodness-of-Fit Test for single season occupancy models (MacKenzie and Bailey, 2004). Occupancy models were created in R (R Core Team, 2018).
The averaged occupancy model [Ψ(Hetro + Roads + Village + Camps); p(Hetro + Moon + Camps + Road + Village)] was used to calculate the occupancy and intensity of hunting for a 100 m2 cell map across GCSR using the LATTICE package in R (Sarkar, 2008). To factor the inaccessibility of the rugged terrain (e.g., ravines, walls of the caldera) we created around each cell buffer circles with a radius of 1.2 km and averaged the terrain heterogeneity of each cell within the buffer. All other covariates were predicted using a 100 m2 cell size.
Results
A total of 2,671 site-days were recorded during the study, with 26,102 detections with a score higher than 0.53 initially detected by the detection algorithm across all 10 Areas. All these detections were manually verified, which resulted in 595 being actual gunshots. While manually reviewing the automatic detections 1 true gunshot was found that had not been detected, this gunshot was added to the count, totalling 596 shots across the study period (Table 1; Supplementary Table 1).
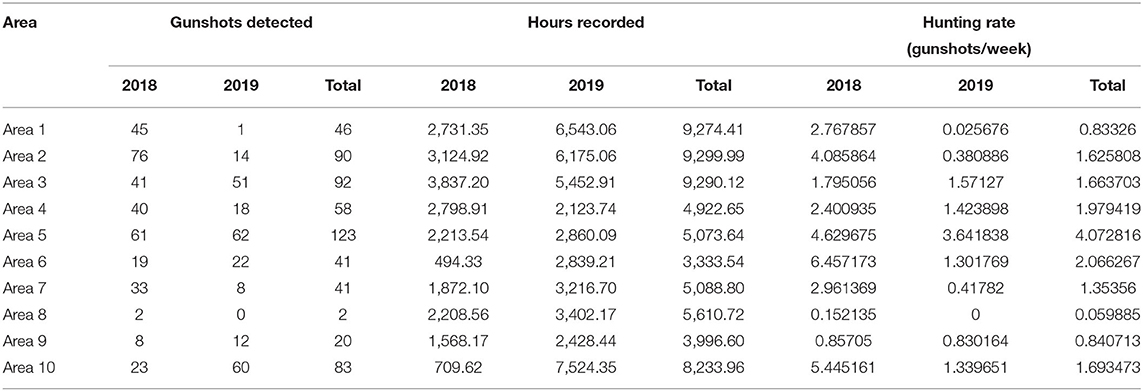
Table 1. Total number of shots identified, total number of recorded hours and hunting rate (shots/week) for year of the study and overall.
Hunting Patterns in GCRS
Shots were detected across all 10 Areas (Table 1). Overall, when controlling for the number of hours sampled, there was an average of 1.62 ± 1.00SD shots/week. There were, however, significant differences in hunting rate across sites (Kruskal-Wallis, χ2 = 102.71, df = 9, p < 0.001), with hunting rates ranging from of 4.07 shots/week (Area 5) to 0.06 shots/week (Area 8).
The effects of temporal factors on hunting rate varied among sites (Table 2; Supplementary Table 1). In Area 1, for instance, there was significantly more hunting in 2018 compared to 2019 (Mann-Whitney U test, W = 19,119, p < 0.001), during the wet compared to during the dry season (Mann-Whitney U test, W = 10,157, p < 0.001), and during the month of May (Kruskal-Wallis, χ2 = 29.111, df = 10, p < 0.001), but not significant differences among days of the week, time of day, or phase of the moon (Table 2). In Area 10, on the other hand, there was also a significant decrease in hunting from 2018 to 2019 (Mann-Whitney U test, W = 5,989.5, p < 0.001), but hunting rate was significantly higher in December rather than in May (Kruskal-Wallis, χ2 = 48.551, df = 11, p < 0.001), and there was more hunting at night time than at daytime (Mann-Whitney U test, W = 35.5, p < 0.05; Table 2; Supplementary Table 1).
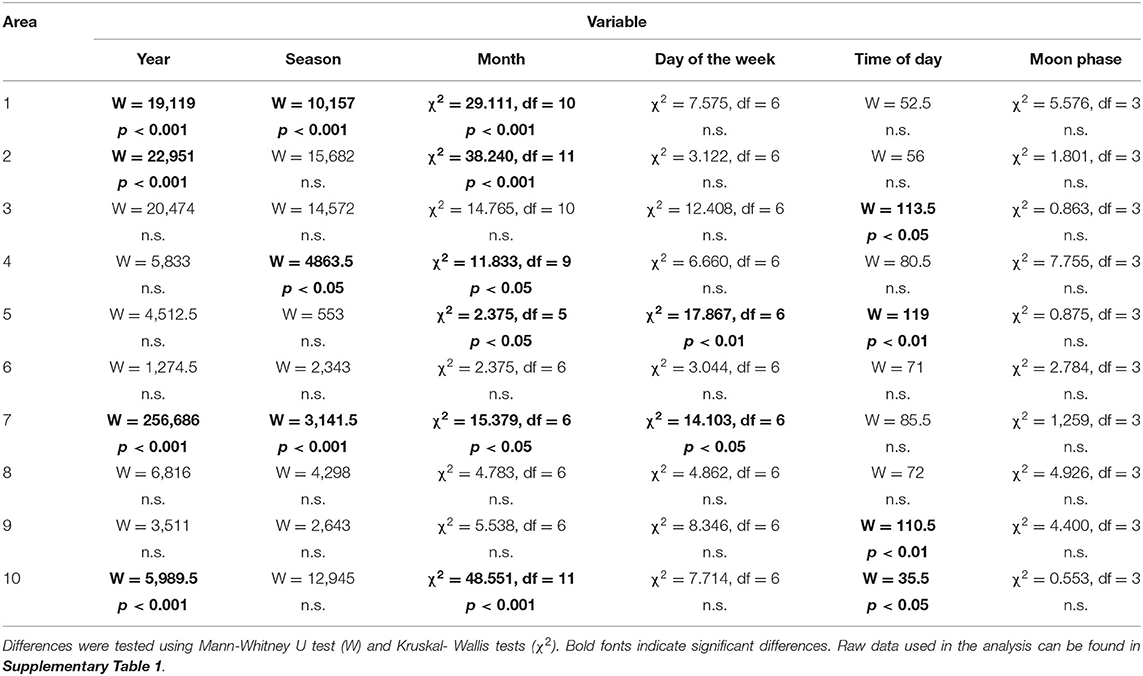
Table 2. Effect of temporal factors, including year (2018 vs. 2019), season (dry vs. wet), month, day of the week, time of day (night vs. day), and moon phase (full vs. waxing vs. new vs. waning) on hunting rate across study areas.
When we compared temporal hunting patterns across sites, we found a significant decrease in hunting rate between 2018 and 2019 (Wilcoxon matched-pairs test, V = 55, p < 0.001). All other temporal variables tested, including night vs. day (Wilcoxon matched-pairs test, V = 27, n.s.), day of the week (Kruskal-Wallis, χ2 = 5.4623, df = 6, n.s.), month of the year (Kruskal-Wallis, χ2 = 9.3618, df = 11, n.s.), dry vs. wet season (Wilcoxon matched-pairs test, V = 37, n.s.), and phase of the moon (Kruskal-Wallis, χ2 = 1.7833, df = 3, n.s.), were not significant.
Occupancy Modeling
The best covariates for predicting detection probability of illegal gun hunting in the GCSR were the Euclidian distance to roads, distance to research camps and terrain heterogeneity, which had a positive effect on hunting detection probability; and the Euclidian distance to the nearest village and lunar intensity, which had a negative effect (Figure 3; Table 3). To predict the occupancy probability the best covariates were the Euclidian distance research camps, which had a positive effect, and the Euclidean distance to the main road, distance to the nearest villages and terrain heterogeneity, which had a negative effect (Figure 4; Table 3). The goodness-of-fit test did not indicate overdispersion (c = 0.51; p = 0.809).
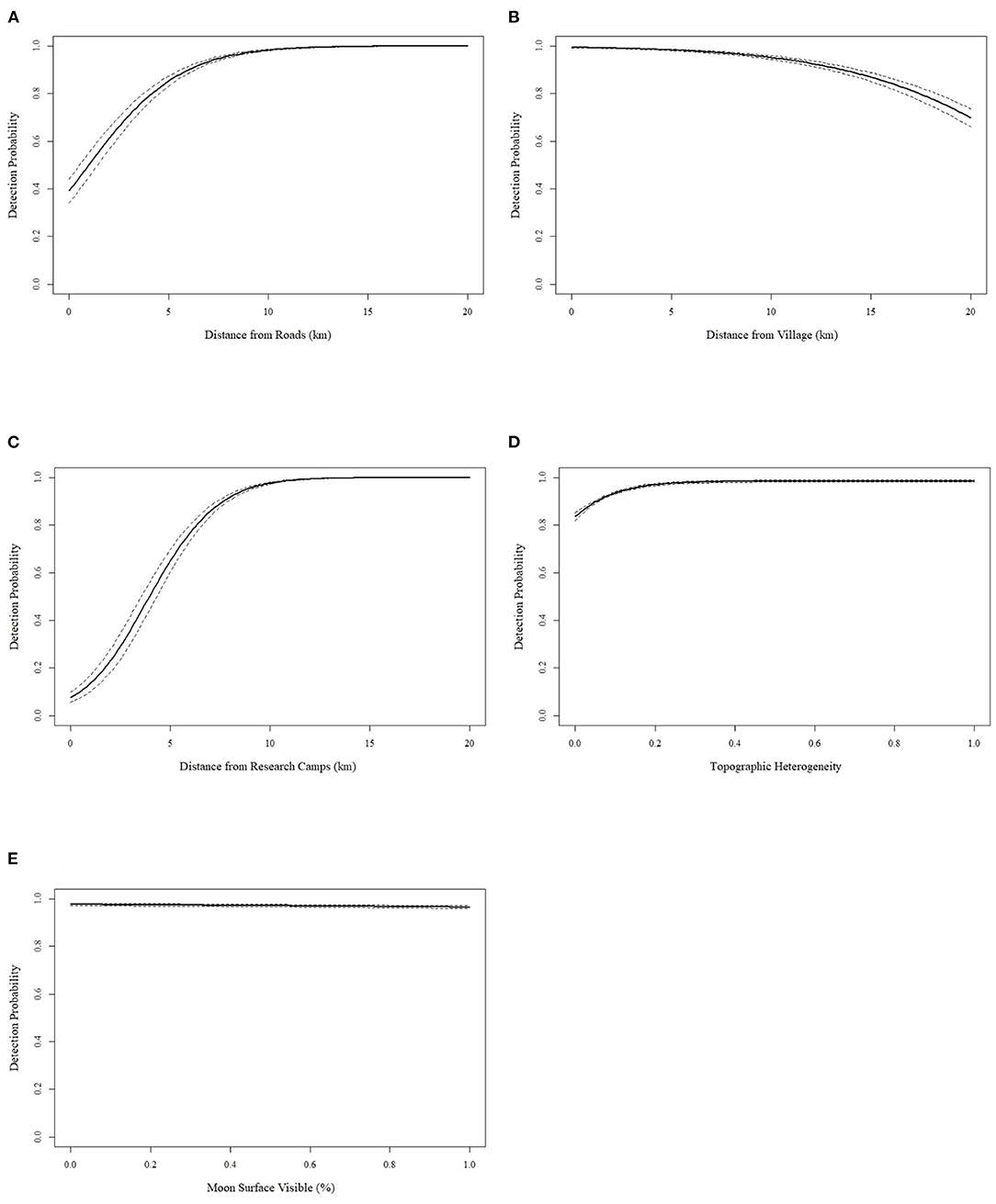
Figure 3. Probability (±95%) of hunting detection as a function of distance to road (A), distance to village (B), distance to nearest research camp (C), topographic heterogeneity (D) and moon intensity (E).
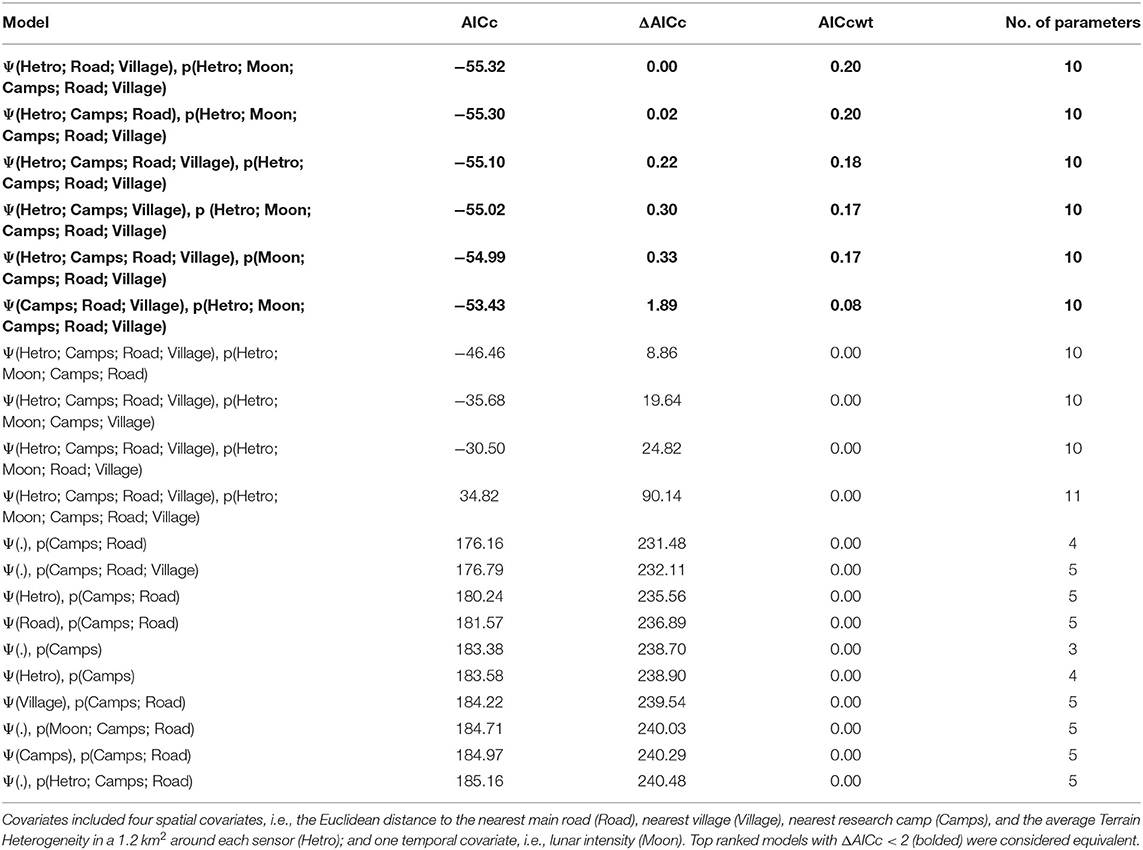
Table 3. Top 20 best fitting models used to detect hunting detectability (p) and occupancy (Ψ) across the Gran Caldera de Luba Scientific Reserve, Bioko Island, Equatorial Guinea.
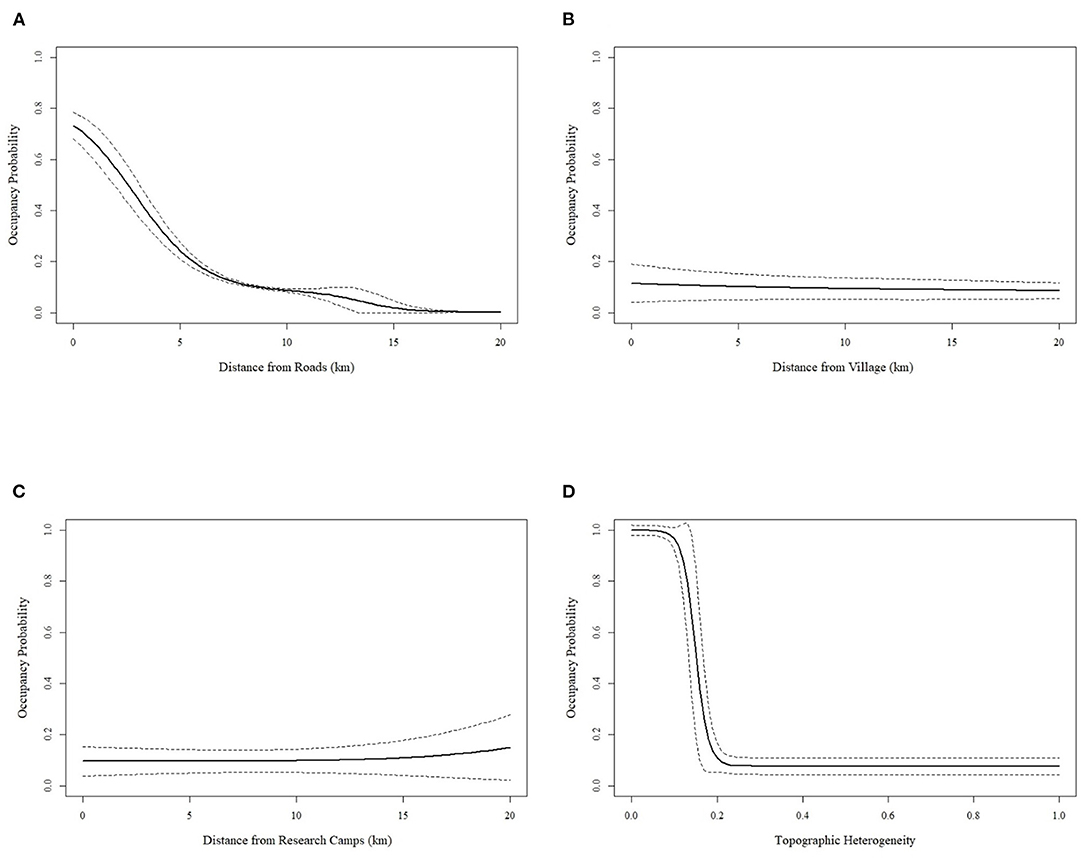
Figure 4. Probability (±95%) of hunting occupancy as a function of distance to road (A), distance to village (B), distance to nearest research camp (C) and topographic heterogeneity (D).
The maps produced showed that hunting was most likely to occur (i.e., occupancy, Ψ) in flatter, less heterogenous areas, with likelihood being highest on the northeast of GCSR, along the new paved road, the southern coast and the eastern boundary of the Reserve; and inside the Caldera Volcánica de Luba (Figure 5). Hunting intensity (detectability, p) was predicted to be highest on the north half of the Reserve, including the first ca. 5 miles of the new paved road, and along the southeast corner of the Reserve, including the southeaster coast; and lowest around both research camps (Figure 5).
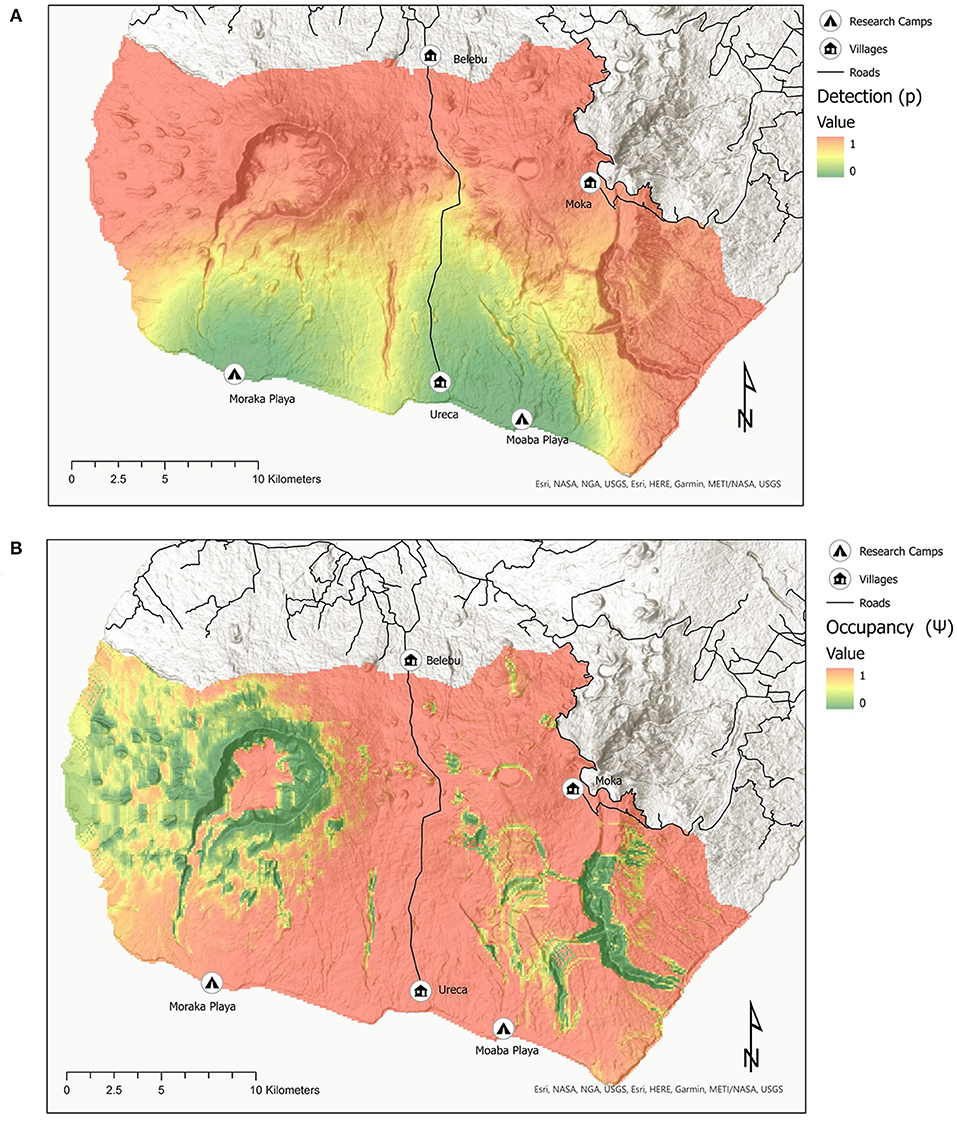
Figure 5. Maps showing the best fitted occupancy model [Ψ(Hetro + Roads + Village + Camps); p(Hetro + Moon + Camps + Road + Village] predicting detectability (A) and occupancy (B) of hunting across the Gran Caldera de Luba Scientific Reserve.
Discussion
In this study we used passive acoustic monitoring to record environmental sounds within GCSR. We then used an automatic detection algorithm to identify gunshots within the audio recordings to describe hunting and to model what factors affected the distribution and intensity of this illegal activity. We detected hunting activity in all sites, including the most remote locations, such as inside the Caldera de Luba (Figure 1: Area 9; Table 1). This is in stark contrasts to previous work that examined hunting intensity in GCSR, which found that after the road was completed hunting increased along the road but not inside the Caldera. For example, during surveys conducted in 2011 and 2012, before the road was complete, Cronin et al. (2016) found 5.8 times more signs of shotgun hunting in and around the village of Ureca (~Areas 3 and 6 in this study), than in the more remote Playa Moraka (~Areas 7, 8 and 9 in this study). That study also did not find any evidence of hunting inside the Caldera. Similarly, repeated censuses conducted by BBPP before (2011–2013) and after (2016–2017) the construction of the new road on transects perpendicular to the road and inside the Caldera, found hunting signs near the road increased from 0.15 ± 0.20 cartridges/km (Years: 2011–12, 30.91 km censused) to 0.52 ± 0.58 cartridges/km (Years: 2016–2017, 21.8 km censused), while inside the Caldera censuses did not find any hunting sings before (Years: 2011–2013, 105.1 km censused) or after (Year: 2017, 5.9 km censused; BBPP, unpublished; Fernández et al., 2018). While hunting signs inside the Caldera were occasionally detected before the road was built (BBPP, unpublished; Fernández, Pers. Obs.), the fact that >105 km of systematic censuses carried out before the road was completed failed to find any hunting signs suggest that hunting inside the Caldera was rare historically. Our results indicate, however, that the construction of the new road has led to a steep increase in hunting throughout GCSR, and to regular hunting inside the Caldera. As shotgun hunting has been shown to affect primate abundance and community composition in Bioko (Cronin et al., 2016), we expect primate numbers to have declined in remote areas since 2015. This decline would have been particularly pronounced for those species that are more sensitive to hunting, namely the Bioko drill, M. l. poensis, the black colobus (C. s. satanas) and the Pennant's red colobus. This is particularly worrying for the latter, a Critically Endangered species endemic to the Island and now believed to be restricted to GCRS following local extinction in Pico Basile National Park due to hunting (Cronin et al., 2017; Linder et al., 2021).
Overall, we did not find any significant effect in any of the temporal factors we examined across sites, except for a decrease in hunting from the first to the second year of the study. Data from carcass sales in Malabo, however, indicate that hunting increased over time (Venditti et al., in prep.; BBPP, unpublished). The observed decrease in hunting intensity from 2018 to 2019 in our study, therefore, may reflect hunters' response to a local depletion of primate populations, as they move from monitored areas—where primates decreased in numbers from the first to the second year of the study—to areas where primates are more abundant in order to keep up with wildmeat demand in Malabo. A similar response has been documented in the Republic of Congo, where hunters increased travel distance to meet a growing demand from wildmeat as the human population in local forest logging villages increased (Poulsen et al., 2009). Whether our observation that hunting decreased from 2018 to 2019 was due to hunters moving away from areas with decreasing primate density or to an actual decrease in overall hunting in GCSR, however, requires further research.
We used occupancy modeling to understand factors affected hunting in GCSR. We found that occupancy i.e., the chance of an area being hunted; increased with accessibility, as it decreased with increasing distances to the new road, villages and with increasing heterogeneity (Figure 4). We also found that both, occupancy and detectability (i.e., the intensity of hunting), were lower near research camps (Figures 3, 4). In addition, detectability increased away from roads, near villages, and during periods of low lunar intensity (Figure 3). Increasing accessibility associated with infrastructure development, such as roads, is the main factor behind the steep, continuous increase in commercial hunting that tropical forests have undergone over the last several decades (Wilkie et al., 1992; Laurance et al., 2006, 2009, 2017; Fa and Brown, 2009; Estrada et al., 2017). In Gabon, for example, large mammal density increased with increasing distance to roads (Laurance et al., 2006). Similarly, in Boqueirão da Onça, Brazil, a neotropical dry forest, distance to roads was negatively correlated with hunting intensity, and positively associated with hunting hotspots (Dias et al., 2020). In the case of GCSR, the construction of the new road effectively removed the costs imposed by the rugged terrain, which has been argued to serve as a natural barrier to hunters and to contribute to the relatively high primate density in GCSR compared to other parts of the island (Cronin et al., 2016, 2017). The new road, therefore, made hunting in GCSR much more cost-effective. For example, before the road to Ureca was built, hunters would have to access GCSR by foot using a ~22 km forest trail, where they would remain for an average of 2.9 days/week. Then, hunters would have to hike back out and send the meat to Malabo market's boyanselars (“buy-and-sellers” in pidgin, the women who sell bushmeat at the market) using local transport (Reid et al., 2005). In contrast, now hunters based in Malabo, in the north coast of Bioko, can reach the core of GCSR within 1.5 h using a regular vehicle or a taxi—which are regularly seen parked by the side of the road (Fernández, Pers. Obs.)—hunt through the day, and return the same evening to sell fresh meat directly to boyanselars, restaurants or private consumers (Figure 1). Therefore, unless access to this new road is regulated, over the next few years we will see a steep decline in primate density across GCSR.
It should be noted, however, that while distance to road negatively affected occupancy, hunting detectability (i.e., intensity) was higher at increasing distance from the road. This disparity is probably a reflection of lower primate density near the road caused by intense hunting pressure, so that while hunters were more common near roads, most hunting happened away from them, where primate density was higher. A similar “hunting halo” effect has been described for rural villages, where persistent hunting radiating from human settlements depletes large-bodied mammals of nearby forests (Koerner et al., 2017). In Gabon, for example, this hunting halo was concentrated ca. 10 km around villages (Koerner et al., 2017), and the animal community within this area was dominated by non-hunted, small-bodied species (Koerner et al., 2017; Beirne et al., 2019), as a result, hunters had to travel farther to find wildmeat (Beirne et al., 2019).
Although our occupancy model showed that distance to the road had a negative effect on the distribution of hunting, it is worrying to see relatively high hunting rates also occurring in the most remote areas of GCSR, particularly inside the Caldera (Figure 1: Area 9). This remote and isolated area not only harbors the highest primate densities in Bioko, but is also the only location in the entire island were all seven diurnal primates remains (Cronin et al., 2016, 2017). The Caldera also contains one of the most pristine forests in Africa, where animals have had so little interaction with humans that primates and duikers do not flee upon encountering people (Fernández, Pers. Obs.). As part of BBPP's ongoing monitoring activities, a local census team conducts primate censuses in this area for ~2 weeks/month during the dry season, the only time of the year when it is accessible. Hunters, however, seem to specifically target this area, which requires overnight stay and advance planning to avoid entering the Caldera while the primate census team are working. To ensure this important biodiversity area is effectively protected, more frequent and random censuses should be organized to disincentive hunters from accessing this unique ecosystem.
Distance to either research camp, in Moraka Playa or Moaba Playa (Figure 1) had a negative effect of hunting. Research presence has been shown to be an effective primate conservation tool (Junker et al., 2020). For example, a study in Conkouati Reserve, Republic of Congo, found that 3.5 years after the reintroduction of 20 wild-born orphaned central chimpanzees, none of the individuals had been hunted, which authors linked to their continuous monitoring by researchers and assistants (Goossens et al., 2015). Similarly, in the Dja Region, Cameroon, the continuous presence for research activities has been associated to lower rates of human activity and hunting, and with higher western lowland gorillas and central chimpanzees abundance (Tagg et al., 2015). Both Moaba and Moraka research camps, which have been in operation for over a decade, are active during the dry season (October–March). Staffed by a team of 7–12 people, including UNGE graduates, expatriate researchers and volunteers and occasionally INDEFOR technicians, these camps carry out daily primate and sea turtle censuses. They do not patrol to monitor illegal activity, although they do report to local authorities whenever they witness direct hunting evidence of either primates or sea turtles. Thus, our findings provide further evidence that research and monitoring presence in itself is enough to effectively deter hunting (Junker et al., 2020). This deterrence effect of research camps at GCSR could be increased by adding several, cost-effective measures, including (1) the establishment of randomized forest patrols to monitor illegal activity, (2) permanent staffing of research camps by personnel with authority to make arrests, and (3) better communication with local authorities to ensure a rapid and timely response from law enforcement.
Hunting within protected areas is illegal in Equatorial Guinea, and hunting, trade and consumption of primates across the national territory has been banned since 2007 (Republic of Equatorial Guinea, 2003, 2007). However, wildmeat trade is not only well-established in Bioko, where the market in Malabo have been in operation for over 20 years, but wildmeat demand seems to be increasing (Venditti et al., in prep.). For example, since 2017 several new secondary markets have opened in Malabo and Luba, and the number of direct sales from hunters to restaurants have also increased (BBPP, unpublished). The drop in global oil prices in 2014 severely affected Equatorial Guinea's economy, which is heavily reliant on crude exports (Reid et al., 2005), and by 2016 the country's GDP had dropped 51.7%, from $21.77 billion to $11.24 billion (World Bank, 2021). This economic downturn caused the government to shut down most infrastructure projects, leaving thousands of young Equatoguineans unemployed. Many of these young men (primarily) were forced to return to their villages and undertake hunting and trapping as their only source of income (McLaughlin et al., 2020). The Covid-19 pandemic has only exacerbated this situation. In 2020, Equatorial Guinea's GDP had further decreased to $10.02 billion, the lowest it has been since 2006 (World Bank, 2021); and there are reports that since March 2020 there has been an increase in hunting in Monte Alen National Park, Equatorial Guinea's largest protected area (Bindang, pers. comm.). Therefore, unless the demand for wildmeat is reduced, we will expect a continuous escalation of this activity in the future.
It is important to note that our study has most likely underestimated the level of hunting. For instance, sensors covered a total area of ~75 km2, or 14.7% of the total GCSR area, leaving large swaths of forest unmonitored. Similarly, the northwest of GCSR, which is relatively flat, accessible from villages just outside the border of GCSR and close to paved roads, was predicted to have high levels of hunting. We did not monitor this area, however, but given the high hunting intensity in Area 5, which has similar accessibility in terms of terrain heterogeneity but with not paved road nearby, it is very likely that hunting levels in Northwest of GCRS are significantly higher than those predicted by our model.
We would also like to recognize that while PAM represents a significant improvement over traditional methods to monitor shotgun hunting, which relies on gathering indirect evidence (e.g., gunshots shells) through forest transects, wildmeat market monitoring, and/or hunter surveys; it is still constrains by financial and logistical barriers. On the one hand, PAM allows for continuous monitoring of large areas, provides an objective quantification of this activity and allows for the detection of changes of hunting intensity over time and space. In contrast, PAM first requires a significant initial investment to acquire the necessary equipment (sensors, memory cards, hard drives, locks, etc). The largest financial investment, however, is the running costs of maintaining the sensors and replacing memory cards and batteries. In this study we used alkaline batteries, which powered the sensors for ~6 weeks. Using lithium batteries would have prolonged running time significantly, but lithium batteries costs ~15 times more than alkaline batteries (www.batterystation.co.uk), are not available locally and are difficult to ship due to strict air safety regulations. Additionally, analysis of PAM data is still relatively slow, even when using an automatic detection algorithm as we did. In our study, the computer we used to run the algorithm took ~30 days to go through the data (~3.4 TB). Manual verification of the algorithm output took an additional 30 days. A potential solution to improve the effectiveness of PAM as a tool to monitor gunshot hunting would be to combine thresholding recording to save battery life and memory card used (Prince et al., 2019), with sustainable power sources, such as solar panels (Rainforest Connection, https://www.rfcx.org/), and with wireless data transmission (Aide et al., 2013). Additionally, using methods that improve automatic signal classification, such as machine learning, would significantly reduce analysis time. These advances would allow a much faster and targeted anti-hunting response, significantly improving field conservation efforts (Gibb et al., 2019).
Most of the monitored area in this study has been described as a “critical zone” for primate conservation, where a no-take policy should apply (Cronin et al., 2017) due to this area containing all seven diurnal primates, which are also at higher densities here than anywhere else on Bioko. For example, this area has the highest densities of the Bioko drill and black colobus, and the entire population of the Penntant's red colobus (Cronin et al., 2016, 2017). Unless effective and strict interventions to deter hunting are implemented, the survival of these three species is at serious risk. These interventions should include (1) establishing government-run, anti-poaching road checkpoints at the entry points and key access routes GCSR to stem the flow of wildmeat to markets; (2) increasing biomonitoring activities across GCSR; (3) establishing random anti-poaching patrols across GCSR, particularly around access points and hunting hotspots, such as the Luba-Ureca road, the southwestern beaches, and the northeast and northwest of GCSR; (4) and a strict country-wide ban on shotgun ownership. These interventions should be accompanied by activities that would provide opportunities for professional development and foster economic independence for Equatoguineans that rely directly or indirectly on income from hunting, with particular focus on residents of villages within and in proximity to GCSR and other protected areas. Only once these interventions are in effect will the future of primates and other species affected by wildmeat hunting be certain.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by UWE Animal Welfare and Ethics Committee (AWEC) (Record number: R50).
Author Contributions
DF conceived and designed the study, co-led data collection, wrote the paper, and acquired funding. DB analyzed the data, made the figures, and wrote the paper. SM contributed to study design and co-led data collection. LM, MS, FM, AM, PO, and JN collected the data and assisted with logistics. BF, DM, JE, and MF supported to logistics and permit acquisition. MG acquired funding, contributed to study design and data collection, and facilitated permit acquisition. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by the Mohammed Bin Zayed Species Conservation Fund (172516206) and the U.S. Fish and Wildlife Service (F17AP00425). Additional financial support provided by the University of the West of England's Department of Applied Sciences. BBPP is supported by continued support from the ExxonMobil Foundation, Mobil Equatorial Guinea.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the Government of Equatorial Guinea, the National University of Equatorial Guinea and the Equatoguinean National Institute of Forestry Development (INDEFOR) for permission to conduct this research. We thank Peter Wrege for his support and advise with SWIFT sensors and the Automatic Selection Algorithm. Robert Adee Koch provided SWIFT troubleshooting. We also thank Orume Robinson for delivering a Passive Acoustics Monitoring Training Workshop for the research team and Christos Astaras for advise on SWIFT deployment, data collection and analysis. We are grateful to Angeliki Savvantoglou for guidance using ArcGIS and mapping, to Gráinne McCabe for proofreading the manuscript, to Mark Steer and Stephanie Sargeant for giving feedback on an earlier version of this manuscript, and to two reviewers. Additional support was provided by Mackenzie Grapes, Steve Miller, Miguel Ángel Bikuy Ondo Mangue and Dana Venditti.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.780162/full#supplementary-material
References
Abernethy, K. A., Coad, L., Taylor, G., Lee, M. E., and Maisels, F. (2013). Extent and ecological consequences of hunting in Central African rainforests in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 368. doi: 10.1098/rstb.2012.0303
Aide, T. M., Corrada-Bravo, C., Campos-Cerqueira, M., Milan, C., Vega, G., and Alvarez, R. (2013). Real-time bioacoustics monitoring and automated species identification. Peerj 1, e103. doi: 10.7717/peerj.103
Albrechtsen, L., Fa, J. E., Barry, B., and Macdonald, D. W. (2005). Contrasts in availability and consumption of animal protein in Bioko Island, West Africa: the role of bushmeat. Environ. Conserv. 32, 340–348. doi: 10.1017/S0376892906002694
Astaras, C., Linder, J. M., Wrege, P., Orume, R., Johnson, P. J., and MacDonald, D. W. (2020). Boots on the ground: the role of passive acoustic monitoring in evaluating anti-poaching patrols. Environ. Conserv. 47, 213–216. doi: 10.1017/S0376892920000193
Astaras, C., Linder, J. M., Wrege, P., Orume, R. D., and Macdonald, D. W. (2017). Passive acoustic monitoring as a law enforcement tool for Afrotropical rainforests. Front. Ecol. Environ. 15, 233–234. doi: 10.1002/fee.1495
Beirne, C., Meier, A. C., Mbele, A. E., Menie, G. M., Froese, G., Okouyi, J., et al. (2019). Participatory monitoring reveals village-centered gradients of mammalian defaunation in central Africa. Biol. Conserv. 233, 228–238. doi: 10.1016/j.biocon.2019.02.035
Bourlière, F. Ç. (1985). Primate communities: their structure and role in tropical ecosystems. Int. J. Primatol. 6, 1–26. doi: 10.1007/BF02693694
Brown, J. L., Bennett, J. R., and French, C. M. (2017). SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 5, e4095. doi: 10.7717/peerj.4095
Chapman, C. A., Bonnell, T. R., Gogarten, J. F., Lambert, J. E., Omeja, P. A., Twinomugisha, D., et al. (2013). Are primates ecosystem engineers? Int. J. Primatol. 34, 1–14. doi: 10.1007/s10764-012-9645-9
Chapman, C. A., and Peres, C. A. (2021). Primate conservation: Lessons learned in the last 20 years can guide future efforts. Evol. Anthropol. Issues News Rev. 30, 345–361. doi: 10.1002/evan.21920
Cronin, D. T. (2019). Piliocolobus pennantii. The IUCN red list of threatened species 2019: e.T41025A92653653. doi: 10.2305/IUCN.UK.2019-3.RLTS.T41025A92653653.en
Cronin, D. T., Bocuma Meñe, D., Perella, C., Fernández, D., Hearn, G. W., and Gonder, M. K. (2015a). The Future of the Biodiversity of the Gran Caldera Scientific Reserve: Translating Science into Policy to Develop an Effective Management Plan. Available online at: https://uwe-repository.worktribe.com/output/832673/the-future-of-the-biodiversity-of-the-gran-caldera-scientific-reserve-translating-science-into-policy-to-develop-an-effective-management-plan (accessed September 17, 2021).
Cronin, D. T., Riaco, C., Linder, J. M., Bergl, R. A., Gonder, M. K., O'Connor, M. P., et al. (2016). Impact of gun-hunting on monkey species and implications for primate conservation on Bioko Island, Equatorial Guinea. Biol. Conserv. 197, 180–189. doi: 10.1016/j.biocon.2016.03.001
Cronin, D. T., Sesink Clee, P. R., Mitchell, M. W., Bocuma Meñe, D., Fernández, D., Riaco, C., et al. (2017). Conservation strategies for understanding and combating the primate bushmeat trade on Bioko Island, Equatorial Guinea. Am. J. Primatol. 79, 22663. doi: 10.1002/ajp.22663
Cronin, D. T., Woloszynek, S., Morra, W. A., Honarvar, S., Linder, J. M., Gonder, M. K., et al. (2015b). Long-term urban market dynamics reveal increased bushmeat carcass volume despite economic growth and proactive environmental legislation on Bioko Island, Equatorial Guinea. PLoS ONE 10, e0134464. doi: 10.1371/journal.pone.0134464
Dias, D. de M., Ferreguetti, Á. C., and Rodrigues, F. H. G. (2020). Using an occupancy approach to identify poaching hotspots in protected areas in a seasonally dry tropical forest. Biol. Conserv. 251, 108796. doi: 10.1016/J.BIOCON.2020.108796
Effiom, E. O., Nuñez-Iturri, G., Smith, H. G., Ottosson, U., and Olsson, O. (2013). Bushmeat hunting changes regeneration of African rainforests. Proc. R. Soc. B Biol. Sci. 280. doi: 10.1098/rspb.2013.0246
Estrada, A., Garber, P. A., Rylands, A. B., Roos, C., Fernandez-Duque, E., Fiore, A. D., et al. (2017). Impending extinction crisis of the world's primates: Why primates matter. Sci. Adv. 3, e1600946. doi: 10.1126/sciadv.1600946
Fa, J. E., and Brown, D. (2009). Impacts of hunting on mammals in African tropical moist forests: a review and synthesis. Mamm. Rev. 39, 231–264. doi: 10.1111/J.1365-2907.2009.00149.X
Fa, J. E., Currie, D., and Meeuwig, J. (2003). Bushmeat and food security in the Congo Basin: linkages between wildlife and people's future. Environ. Conserv. 30, 71–78. doi: 10.1017/S0376892903000067
Fa, J. E., Olivero, J., Farfán, M. A., Márquez, A. L., Duarte, J., Nackoney, J., et al. (2015). Correlates of bushmeat in markets and depletion of wildlife. Conserv. Biol. 29, 805–815. doi: 10.1111/cobi.12441
Fa, J. E., Peres, C. A., and Meeuwig, J. (2002). Bushmeat exploitation in tropical forests: An intercontinental comparison. Conserv. Biol. 16, 232–237. doi: 10.1046/j.1523-1739.2002.00275.x
Fa, J. E., Yuste, J. E. G., and Castelo, R. (2000). Bushmeat markets on bioko island as a measure of hunting pressure. Conserv. Biol. 14, 1602–1613. doi: 10.1111/J.1523-1739.2000.99067.X
Fernández, D., Cronin, D., Miller, S. C., Montgomery, D., Etingüe, A. M., Venditti, D., et al. (2018). A new silent forest? Primate hunting in newly accessible areas decreases overall biodiversity in Bioko Island, Equatorial Guinea. International Primatological Society's Meeting, Nairobi, Kenya.
Fernández, D., Kerhoas, D., Dempsey, A., Billany, J., McCabe, G., and Argirova, E. (2021). The current status of the world's primates: Mapping threats to understand priorities for primate conservation. Int. J. Primatol. 1–25. doi: 10.1007/s10764-021-00242-2. [Epub ahead of Print].
Ferreguetti, Á. C., Pereira-Ribeiro, J., Prevedello, J. A., Tomás, W. M., Rocha, C. F. D., and Bergallo, H. G. (2018). One step ahead to predict potential poaching hotspots: Modeling occupancy and detectability of poachers in a neotropical rainforest. Biol. Conserv. 227, 133–140. doi: 10.1016/j.biocon.2018.09.009
Fiske, I. J., and Chandler, R. B. (2011). Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 43, 1–23. doi: 10.18637/jss.v043.i10
Gibb, R., Browning, E., Glover-Kapfer, P., and Jones, K. E. (2019). Emerging opportunities and challenges for passive acoustics in ecological assessment and monitoring. Methods Ecol. Evol. 10, 169–185. doi: 10.1111/2041-210x.13101
Goossens, B., Ancrenaz, M., Vidal, C., Latour, S., Paredes, J., Vacher-Vallas, M., et al. (2015). The release of wild-born orphaned chimpanzees Pan troglodytes into the Conkouati Reserve, Republic of Congo. African Primates 5, 42–46. Available online at: http://dspace.stir.ac.uk/handle/1893/22176 (accessed September 17, 2021).
Henschel, P., Hunter, L. T. B., Coad, L., Abernethy, K. A., and Mühlenberg, M. (2011). Leopard prey choice in the Congo Basin rainforest suggests exploitative competition with human bushmeat hunters. J. Zool. 285, 11–20. doi: 10.1111/j.1469-7998.2011.00826.x
IUCN (2021). Red List of Threatened Species. Available online at: https://www.iucnredlist.org/ (accessed September 17, 2021).
Junker, J., Kühl, H. S., Orth, L., Smith, R. K., Petrovan, S. O., and Sutherland, W. J. (2020). Global evidence for the effects of interventions. Prim. Conserv. 7, 431–482. doi: 10.11647/OBP.0191.07
Koerner, S. E., Poulsen, J. R., Blanchard, E. J., Okouyi, J., and Clark, C. J. (2017). Vertebrate community composition and diversity declines along a defaunation gradient radiating from rural villages in Gabon. J. Appl. Ecol. 54, 805–814. doi: 10.1111/1365-2664.12798
Koné, I., Lambert, J. E., Refisch, J., and Bakayoko, A. (2008). Primate seed dispersal and its potential role in maintaining useful tree species in the Taï Region, Côte-d'Ivoire: implications for the conservation of forest fragments. Trop. Conserv. Sci. 1, 293–305. doi: 10.1177/194008290800100309
Kuehl, H. S., Nzeingui, C., Le Duc Yeno, S., Huijbregts, B., Boesch, C., and Walsh, P. D. (2009). Discriminating between village and commercial hunting of apes. Biol. Conserv. 142, 1500–1506. doi: 10.1016/j.biocon.2009.02.032
Kümpel, N. F., Milner-Gulland, E. J., Cowlishaw, G., and Rowcliffe, J. M. (2010). Incentives for hunting: the role of bushmeat in the household economy in Rural Equatorial Guinea. Hum. Ecol. 38, 251–264. doi: 10.1007/s10745-010-9316-4
Laurance, W. F., Campbell, M. J., Alamgir, M., and Mahmoud, M. I. (2017). Road expansion and the fate of Africa's tropical forests. Front. Ecol. Evol. 0, 75. doi: 10.3389/FEVO.2017.00075
Laurance, W. F., Croes, B. M., Tchignoumba, L., Lahm, S. A., Alonso, A., Lee, M. E., et al. (2006). Impacts of roads and hunting on central African rainforest mammals. Conserv. Biol. 20, 1251–1261. doi: 10.1111/j.1523-1739.2006.00420.x
Laurance, W. F., Goosem, M., and Laurance, S. G. W. (2009). Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evolut. 24, 659–669. doi: 10.1016/j.tree.2009.06.009
Linder, J. M., Cronin, D. T., Ting, N., Abwe, E. E., Davenport, T. R. B., Detwiler, K., et al. (2021). Red colobus (Piliocolobus) conservation action plan 2021–2026. Gland: IUCN. doi: 10.2305/IUCN.CH.2021.08.en
Linder, J. M., and Oates, J. F. (2011). Differential impact of bushmeat hunting on monkey species and implications for primate conservation in Korup National Park, Cameroon. Biol. Conserv. 144, 738–745. doi: 10.1016/J.BIOCON.2010.10.023
MacKenzie, D. I. (2017). Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence. New York: Academic Press.
MacKenzie, D. I., and Bailey, L. L. (2004). Assessing the fit of site-occupancy models. J. Agric. Biol. Environ. Stat. 9, 300–318. doi: 10.1198/108571104X3361
McLaughlin, P., Fernández, D., and McCabe, G. (2020). A long-term monitoring programme for Great Apes in Monte Alén National Park. Available online at: https://uwe-repository.worktribe.com/output/6666811/a-long-term-monitoring-programme-for-great-apes-in-monte-alen-national-park
McNamara, J., Fa, J. E., and Ntiamoa-Baidu, Y. (2019). Understanding drivers of urban bushmeat demand in a Ghanaian market. Biol. Conserv. 239, 108291. doi: 10.1016/j.biocon.2019.108291
Milner-Gulland, E. J., and Bennett, E. L. (2003). Wild meat: the bigger picture. Trends Ecol. Evol. 18, 351–357. doi: 10.1016/s0169-5347(03)00123-x
Myers, N., Mittermeler, R. A., Mittermeler, C. G., Da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
N'Goran, P. K., Boesch, C., Mundry, R., N'Goran, E. K., Herbinger, I., Yapi, F. A., et al. (2012). Hunting, law enforcement, and African primate conservation. Conserv. Biol. 26, 565–571. doi: 10.1111/j.1523-1739.2012.01821.x
Oates, J. F., Koné, I., McGraw, S., and Osei, D. (2020). Piliocolobus waldroni (amended version of 2019 assessment). The IUCN Red list of threatened species 2020. e.T18248A166620835. doi: 10.2305/IUCN.UK.2020-1.RLTS.T18248A166620835.en
Peres, C. A., Emilio, T., Schietti, J., Desmoulière, S. J. M., and Levi, T. (2016). Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc. Natl. Acad. Sci. USA 113, 892–897. doi: 10.1073/pnas.1516525113
Poulsen, J. R., Clark, C. J., Mavah, G., and Elkan, P. W. (2009). Bushmeat supply and consumption in a tropical logging concession in northern Congo. Conserv. Biol. 23, 1597–1608. doi: 10.1111/j.1523-1739.2009.01251.x
Poulsen, J. R., Clark, C. J., and Palmer, T. M. (2013). Ecological erosion of an Afrotropical forest and potential consequences for tree recruitment and forest biomass. Biol. Conserv. 163, 122–130. doi: 10.1016/J.BIOCON.2013.03.021
Poulsen, J. R., Clark, C. J., and Smith, T. B. (2001). Seed dispersal by a diurnal primate community in the Dja Reserve, Cameroon. J. Trop. Ecol. 17, 787–808. doi: 10.2307/3068616
Prince, P., Hill, A., Covarrubias, E. P., Doncaster, P., Snaddon, J. L., and Rogers, A. (2019). Deploying acoustic detection algorithms on low-cost, open-source acoustic sensors for environmental monitoring. Sensors. 19, 553. doi: 10.3390/s19030553
R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/
Reid, J., Morra, W., Posa Bohome, C., and Fernández Sobrado, D. (2005). The economics of the primate trade in Bioko, Equatorial Guinea. Conserv. Strateg. Fund. Available online at: https://uwe-repository.worktribe.com/output/1045785/the-economics-of-the-primate-trade-in-bioko-equatorial-guinea (accessed September 17, 2021).
Republic of Equatorial Guinea (2003). Environmental Regulation Law in the Republic of Equatorial Guinea. Law Num. 7/2003.
Republic of Equatorial Guinea (2007). Hunting and Consumption of Monkeys and Other Primates in the Republic of Equatorial Guinea Is Prohibited. Decree Num. 72/2007.
Stevenson, P. R., and Aldana, A. M. (2008). Potential effects of ateline extinction and forest fragmentation on plant diversity and composition in the western Orinoco Basin, Colombia. Int. J. Primatol. 29, 365–377. doi: 10.1007/s10764-007-9177-x
Sugai, L. S. M., Silva, T. S. F., Ribeiro, J. W., and Llusia, D. (2019). Terrestrial passive acoustic monitoring: review and perspectives. Bioscience 69, 5–11. doi: 10.1093/biosci/biy147
Tagg, N., Willie, J., Duarte, J., Petre, C.-A., and Fa, J. E. (2015). Conservation research presence protects: a case study of great ape abundance in the Dja region, Cameroon. Anim. Conserv. 18, 489–498. doi: 10.1111/acv.12212
Vanthomme, H., Bellé, B., and Forget, P.-M. (2010). Bushmeat hunting alters recruitment of large-seeded plant species in Central Africa. Biotropica 42, 672–679. doi: 10.1111/j.1744-7429.2010.00630.x
Wilkie, D. S., Sidle, J. G., and Boundzanga, G. C. (1992). Mechanized logging, market hunting, and a bank loan in Congo. Conserv. Biol. 6, 570–580. doi: 10.1046/J.1523-1739.1992.06040570.X
World Bank (2021). World Development Indicators. DataBank (2021). Available online at: https://databank.worldbank.org/source/world-development-indicators (accessed September 17, 2021).
Wrege, P. H., Rowland, E. D., Keen, S., and Shiu, Y. (2017b). Acoustic monitoring for conservation in tropical forests: examples from forest elephants. Methods Ecol. Evol. 8, 1292–1301. doi: 10.1111/2041-210x.12730
Wrege, P. W., Rowland, E. D., and Keen, S. (2017a). guns8_templateDetector_installer.exeFigshare. doi: 10.6084/m9.figshare.4560895.v4
Keywords: bushmeat, conservation, passive acoustic monitoring, PAM, wildlife trade, road, Central Africa, infrastructure
Citation: Branch D, Moka Sharpe S, Maho LM, Silochi Pons MÁ, Mitogo Michá F, Motove Etingüe A, Nze Avomo JCO, Owono Nchama PO, Esara Echube JM, Fero Meñe M, Featherstone B, Montgomery D, Gonder MK and Fernández D (2022) Accessibility to Protected Areas Increases Primate Hunting Intensity in Bioko Island, Equatorial Guinea. Front. Conserv. Sci. 3:780162. doi: 10.3389/fcosc.2022.780162
Received: 20 September 2021; Accepted: 03 March 2022;
Published: 08 April 2022.
Edited by:
Jonah Henri Ratsimbazafy, Madagascar Primate Study and Research Group, MadagascarReviewed by:
Thomas H. White, United States Fish and Wildlife Service, Puerto RicoXiaoli Shen, Institute of Botany (CAS), China
Copyright © 2022 Branch, Moka Sharpe, Maho, Silochi Pons, Mitogo Michá, Motove Etingüe, Nze Avomo, Owono Nchama, Esara Echube, Fero Meñe, Featherstone, Montgomery, Gonder and Fernández. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Fernández, david.fernandez@uwe.ac.uk
†Present Address: Juan Cruz Ondo Nze Avomo, Institute of Conservation Science and Learning, Bristol Zoological Society, Bristol, United Kingdom
 Douglas Branch1
Douglas Branch1  Amancio Motove Etingüe
Amancio Motove Etingüe Maximiliano Fero Meñe
Maximiliano Fero Meñe Mary Katherine Gonder
Mary Katherine Gonder David Fernández
David Fernández