Hidden in Plain Sight: Detecting Invasive Species When They Are Morphologically Similar to Native Species
- 1Department of Biology, La Sierra University, Riverside, CA, United States
- 2U.S. Geological Survey, Western Ecological Research Center, San Diego, CA, United States
- 3Department of Herpetology and Urban Nature Research Center, Natural History Museum of Los Angeles County, Los Angeles, CA, United States
Early detection and rapid response (EDRR) can help mitigate and control invasive species outbreaks early on but its success is dependent on accurate identification of invasive species. We evaluated a novel outbreak in San Diego County, California of the Sonoran Spotted Whiptail (Aspidoscelis sonorae) in order to confirm their spread as well as quantify how to better detect and potentially manage this invasive species in California. We found that A. sonorae went undetected for over two years due to its morphological similarity to native whiptails and that it has spread rapidly since they were first observed. There are two species of native California whiptails with which A. sonorae can be confused locally, the Orange-throated Whiptail (Aspidoscelis hyperythrus), and to a lesser extent the Tiger Whiptail (Aspidoscelis tigris). We review key diagnostic features to distinguish A. sonorae from native California whiptails. We also discuss how to efficiently use widely available community science tools to rapidly assess a novel invasive species outbreak and outline suggestions to help manage cryptic invasive species.
Introduction
Early detection and rapid response (EDRR) can be critical to controlling, understanding, and stopping the spread of invasive species (Reaser et al., 2020). While there are many methods to detect incipient invasions, community science has seen recent widespread use in helping reduce detection times and mapping the spread of invasive species (Delaney et al., 2008; Gallo and Waitt, 2011; Larson et al., 2020). However, community science approaches can be less effective at certain tasks, such as detecting non-native species when there are similar looking native species (Crall et al., 2011; Aceves-Bueno et al., 2017; Pauly and Gavit, 2019; Pauly et al., 2020) or when the non-natives are difficult to sample or too small to be easily documented through photography (Caley et al., 2020). This can slow detection allowing time for the spread of incipient invasive species. Fortunately, many of these issues can be resolved by increasing awareness amongst community scientists about these difficult-to-identify invasive species. Researchers and community science practitioners can inform potential observers about the diagnostic characters that are useful in the field as well as characters to include in photo vouchers so others can confirm identifications (Crall et al., 2011; Caley et al., 2020). Here, we report on the introduction and spread of the Sonoran Spotted Whiptail (Aspidoscelis sonorae Lowe and Wright, 1964), in San Diego County, California as a case study examining the challenges of rapidly detecting invasive species that look similar to native species. Further, we identify key characters for field identification and photo verification and use this ongoing invasion as an example to highlight strategies that may improve initial detection times for other non-native species.
Much of Southern California resides within the California Floristic Province (CFP), which is one of the Earth's 36 recognized biodiversity hotspots (Mittermeier et al., 2011; Noss et al., 2015). Globally, biodiversity hotspots are at an increased risk for the establishment of invasive reptiles (Li et al., 2016), and this makes detection efforts in these regions a critical conservation priority (Reaser et al., 2020). The CFP is a hotspot because of its remarkable biodiversity and the impacts on it from habitat loss or modification resulting from ranching, agriculture, and/or urban development (Mittermeier et al., 2011). Rapid urbanization within the CFP has given rise to large urban areas (e.g., 18.7 million people in the Greater Los Angeles Area and 3.3 million people in the San Diego-Chula Vista-Carlsbad metropolitan area). Due to the high numbers of people and high volume of goods moving into and throughout urban areas, there are increased chances for the introduction and establishment of non-native species (Spear et al., 2017; Santana Marques et al., 2020). Thus, efforts to improve the early detection of non-natives are especially relevant in and around the major metropolitan areas of the CFP.
Currently, the CFP is home to at least 11 established species of non-native lizards and three species of non-native snakes, most of which are found in urbanized areas (Palmer and Fisher, 2010; Pauly and Borthwick, 2015; Pauly et al., 2015; Reed et al., 2016; Fisher et al., 2020, 2021; Putman et al., 2020). In contrast there are ~45 species of native lizards and snakes in the CFP, with only a few species occurring in urban areas (Fisher, 2016a,b). Common pathways for the introduction and spread of non-native reptiles include the pet trade, the nursery plant trade, and cargo shipments (Kraus, 2009). In California, plant nurseries can harbor multiple species of invasive reptiles and serve as epicenters of spread (Fisher et al., 2020; Pauly and Fisher, unpubl. data). Generally, invasive reptile species that have successfully invaded and expanded their ranges in Southern California are from similar climates (Mediterranean/desert; e.g., Aspidoscelis, Hemidactylus, Tarentola) or have a short time to maturity (e.g., Anolis, Aspidoscelis, Hemidactylus, Indotyphlops) as predicted by Van Wilgen and Richardson (2012). Two of these non-native lizards that are spreading in California are parthenogenetic species. These include the Indo-Pacific Gecko (Hemidactylus garnotii Duméril and Bibron, 1836; Pauly et al., 2015) and the Sonoran Spotted Whiptail (Aspidoscelis sonorae; Winkleman and Backlin, 2016). Parthenogenetic reptiles tend to be quite successful at establishing non-native populations because all that is needed is for a single female individual to be introduced and find conditions suitable for growth and asexual reproduction (Kraus, 2009).
Aspidoscelis sonorae has been present in Orange County in Southern California since at least 2010 (Winkleman and Backlin, 2016; Erickson and Burt, 2019). Within whiptails, A. sonorae represents the fifth known species to become introduced (Witmer et al., 2007; Weaver et al., 2011) and is one of the few known diurnal parthenogenetic lizards to become invasive (Kraus, 2009). Additionally, A. sonorae is the first non-native lizard in Southern California with native congeneric species present in the state. Field surveys (Fisher and Fisher, unpubl. data) and observations on the iNaturalist community science platform show that A. sonorae has been rapidly spreading across Orange County (Figure 1A; www.inaturalist.org). This species is native to desert habitats of Arizona, New Mexico, and adjacent Mexico, and seems to do well in the Mediterranean climates of Southern California, especially in urbanized landscapes. However, the current distribution of this species in Southern California is poorly understood due to the difficulty of large scale urban surveys. There are two native whiptail species in Southern California, and both are sexual species—the Tiger Whiptail (Aspidoscelis tigris Baird and Girard, 1852) and the Orange-throated Whiptail (Aspidoscelis hyperythrus Cope, 1863). Both native species have been declining in Southern California in areas with increasing urbanization (Case and Fisher, 2001; Thomson et al., 2016; Amburgey et al., 2021), and A. sonorae might compete with and possibly also consume the native whiptail species leading to further declines. Aspidoscelis sonorae is morphologically very similar to the native A. hyperythrus, and this makes tracking the spread of the invasive even more challenging. Naturalists and professional biologists assuming a whiptail lizard is an A. hyperythrus may not document a non-native species. Additionally, even if a photo voucher is uploaded to a community science platform or deposited in a museum photo collection, the lizard may be incorrectly identified as the more familiar native species.
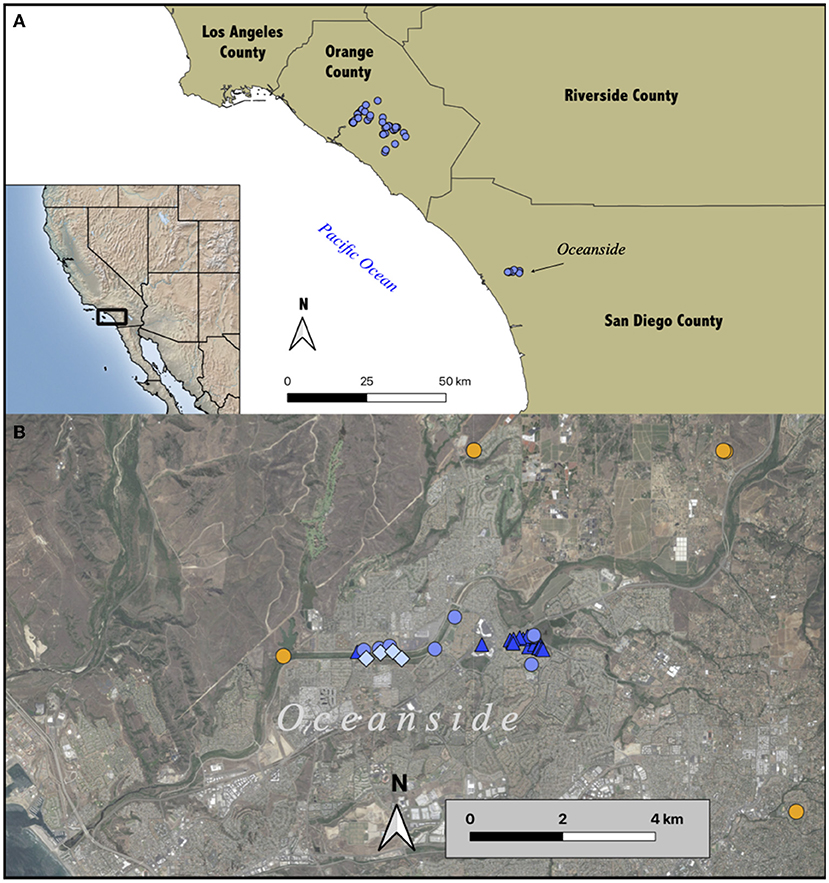
Figure 1. Distribution of Aspidoscelis sonorae in Southern California. (A) Confirmed A. sonorae in Orange and San Diego counties based on research-grade iNaturalist observations. (B) The distribution of the invasive A. sonorae (medium blue circles for iNaturalist observations; dark blue triangles for field observations by authors), probable A. sonorae based upon iNaturalist observations that cannot be confidently identified to species (pale blue diamonds), and native Orange-throated Whiptails (A. hyperythrus; orange circles).
In Southern California, recent detections of non-native species have resulted from observations by community scientists, especially via the iNaturalist platform (e.g., Pauly and Borthwick, 2015; Pauly et al., 2015; Pauly and Gavit, 2019; Fisher et al., 2020, 2021). Efforts by the Natural History Museum of Los Angeles County, the San Diego Natural History Museum, the U. S. Geological Survey, numerous nature centers, and other museums and universities across the region have encouraged high levels of participation in community science platforms such as Herpetological Education and Research Project (H.E.R.P.), HerpMapper, and iNaturalist (Fisher, 2016a). The Reptiles and Amphibians of Southern California (RASCals) project on iNaturalist (for which GBP is the lead scientist) has further accelerated the documentation of reptiles and amphibians (Spear et al., 2017) as has the San Diego Invasive Species Watch on iNaturalist (Richmond et al., in prep.). Thus, there is a large community of passionate naturalists and community scientists that are already helping to reduce detection times for potential invasive species. Despite these efforts, documenting the introduction and spread of A. sonorae in Southern California has proven especially challenging relative to the other non-native lizards introduced to California. This is mainly because they are difficult to differentiate from the native A. hyperythrus. Further compounding this issue, lizards in the genus Aspidoscelis are fast moving, active foragers which makes getting high quality photographs to post on community science platforms especially problematic. Even with high quality pictures, it can be difficult to accurately distinguish between these two species. This lack of diagnosability leads to an inaccurate tracking of the spread of A. sonorae at the edges of known localities, prevents new localities from being easily found, and can confound the true range of the native species because the invasive species can occupy suboptimal native habitat.
Here we use an incipient invasion of A. sonorae as an example to show how misidentifications can be reduced when dealing with morphologically similar species using community science. This study is motivated by the photo-documentation of A. sonorae more than 50 km south of the nearest known A. sonorae in Orange County by iNaturalist user J. Fishinger in August, 2020 (iNaturalist 55755922; originally identified only as Aspidoscelis and then identified within a few days as A. sonorae by GBP and others). This observation triggered examination of other iNaturalist observations, which revealed that the species had been photographed 3.3 km west of iNaturalist 55755922 26 months earlier but misidentified as the native A. hyperythrus (iNaturalist 13276378). We review key morphological characteristics necessary to reduce misidentifications, provide suggestions for characters to include in photographs, and document this species expansion into San Diego County for the first time. We conclude with suggestions for other researchers, invasive species' biologists, and community science practitioners who hope to use community science to reduce detection times of non-native species.
Materials and Methods
Species Background
In Southern California there are two native species of whiptail lizards, the Orange-throated Whiptail (Aspidoscelis hyperythrus) and the Tiger Whiptail (Aspidoscelis tigris), which have recently been evaluated as to their conservation status (Thomson et al., 2016). Additionally, the Sonoran Spotted Whiptail (Aspidoscelis sonorae) has been introduced into this region and is known from Orange County, California (Winkleman and Backlin, 2016; Erickson and Burt, 2019). Within California, the invasive spotted whiptails were classified as part of the Aspidoscelis flagellicauda/sonorae complex because available morphological variation and mtDNA sequence data were insufficient to identify the exact species. However, since Taylor et al. (2018) synonymized A. flagellicauda with A. sonorae, the invasive species in California is now referred as A. sonorae. This species is native to Arizona, New Mexico, and northern Mexico occurring in higher elevation montane desert habitat (Taylor et al., 2018).
Within California, the native Aspidoscelis hyperythrus has a small distribution from southwestern San Bernardino County and Orange County southward through western Riverside County and San Diego County (Ver Hoef et al., 2001; Stebbins, 2003). Its range extends southward through most of the Baja California Peninsula. Previously A. hyperythrus was listed as a Species of Special Concern within California (Thomson et al., 2016) but is now on the California Department of Fish and Wildlife watch list (California Natural Diversity Database, 2022). Of the two native whiptail species, A. hyperythrus is more likely to be confused with the invasive A. sonorae. Aspidoscelis tigris, the other native whiptail, is a wide ranging species that is composed of multiple subspecies; three subspecies reside in California (Stebbins, 2003). The species occurs widely across coastal habitats but is also found inland, through the mountains, and into and across the desert reaches of California (Stebbins, 2003). Aspidoscelis tigris occurs widely across the western United States and northeastern Mexico. It is larger than A. hyperythrus and has been declining in parts of its range due to urbanization (Thomson et al., 2016). This species is less likely to be confused with the invasive A. sonorae.
Database Surveys
To look for new records of Aspidoscelis sonorae in San Diego, we examined various community science platforms including the Herpetological Education and Research Project (H.E.R.P.; www.naherp.com), HerpMapper—Global Herp Atlas (www.herpmapper.org), and iNaturalist (www.inaturalist.org). The first two platforms are typically used by skilled identifiers who list the identification as they enter the record; there is little option for the community to confirm species identifications on these platforms, though others can comment on identifications, and as needed, they can be updated. The iNaturalist platform is used by these higher skilled identifiers as well as those with less taxonomic expertise. The original observer may or may not identify an organism to species, but the iNaturalist community can easily contribute identifications leading to a community-supported identification. Because of these differences, we searched these platforms differently. For H.E.R.P. and HerpMapper, we simply checked for San Diego County observations of A. sonorae. For iNaturalist, to find observations of A. sonorae within San Diego County, we looked at all whiptail (Aspidoscelis) occurrences. Occurrences were searched until the date of June 1, 2021 for H.E.R.P. and HerpMapper and through October 31, 2021 for iNaturalist. Aspidoscelis sonorae has mainly been misidentified as A. hyperythrus so we especially scrutinized records of A. hyperythrus in order to find pictures with potentially misidentified A. sonorae. Some individuals could not be positively identified to species level due to the quality of photographs, and were identified only to the genus Aspidoscelis.
Field Surveys
Field surveys targeted the two locations in Oceanside, San Diego County, where we identified A. sonorae from iNaturalist observations (iNaturalist 55755922 and 13276378). These two locations are on opposite sides of the San Luis Rey River and could represent population expansion following a single introduction or separate introduction events. For sampling, we treated these records as separate sites, and conducted surveys on foot between 10 am and 5 pm. We conducted surveys on 15 August 2020, 27 August 2020, 12 October 2020, and 2 June 2021. We surveyed in the immediate area of the two original iNaturalist locations to determine range boundaries and then surveyed points of interest between these two initial sites to determine whether this was one connected population. Sampling of intermediate sites was also done because lizards of the genus Aspidoscelis have large home range sizes >500 m (Eifler and Eifler, 1998), which can make detecting individuals more difficult. We conducted surveys primarily from sidewalks as most habitat is private property (house lots) and inaccessible to us.
Morphological Characters
We followed Taylor et al. (2018) for guidance on diagnosing invasive Aspidoscelis sonorae within Southern California. We used museum specimens of A. hyperythrus and A. tigris to look for diagnosable differences between the species that could be seen on photos. We also referred to Burt (1931; also Wright and Lowe, 1967) to identify characters useful for distinguishing between species groups within Aspidoscelis. We looked at snout vent length (SVL) for the three species to ascertain any size differences that might supplement other characters in a useful diagnostic key. For the two native species, we used previously collected measurements from the USGS Pit-fall trap studies across Southern California (Case and Fisher, 2001; Fisher et al., 2008; Fisher, 2016b; Amburgey et al., 2021). Using these data, we defined SVL for adults as >80 mm for A. tigris (Goldberg, 1976) and >50 mm for A. hyperythrus (Bostic, 1966). For the invasive A. sonorae, we used measurements for some of the pattern classes presented in Taylor et al. (2018); these authors only measured the largest individuals available (all above 77 mm SVL), allowing us to compare the maximum sizes across groups but not to compare adult size distributions. We used R package software to show body size versus species/pattern class.
Results
Database Surveys
We found no records for A. sonorae in either H.E.R.P. or HerpMapper for San Diego County. Within the iNaturalist platform, research-grade means that the observation has a photo, locality and date data, and a community supported identification. For San Diego County we found 10 observations of A. sonorae and four observations of whiptails that could not be confidently identified to species due to photo quality and/or key characters not being visible (Table 1). Of the 10 A. sonorae observations, three had been misidentified as Aspidoscelis hyperythrus, including iNaturalist 13276378 which was observed 7/9/2018 and achieved research-grade status as A. hyperythrus before being correctly identified 26 months later as A. sonorae. This represents the earliest known record of this invasive species in San Diego County. Thus, in this case, this was a misidentification. Similarly, iNaturalist 81581452 achieved research grade status as A. hyperythrus before being identified by us as A. sonorae, and iNaturalist 78404592 was also initially misidentified as A. hyperythrus (Table 1). With increasing awareness of the presence of A. sonorae in the Oceanside area of San Diego County, other observations were correctly identified as A. sonorae (iNaturalist 58354052, 75232275, and 84300160) or were identified by the initial observer only to genus and then by us as A. sonorae (iNaturalist 87635967, 90301782).

Table 1. iNaturalist observations that are confirmed or possible invasive Aspidoscelis sonorae in northwestern San Diego County.
Field Surveys
During our August 2020 and June 2021 surveys, over 150 A. sonorae of all size classes were seen across the sites, suggesting a reproductive and expanding population. Only a single A. sonorae, a juvenile, was seen during the October 2020 survey. One juvenile was collected on 8/13/20 (LACM RNF), three additional specimens (one adult, one juvenile, one hatchling) were collected on 8/26/20 (LACM 194168–194170), and three additional adults were collected on 6/2/2021. There is a total distance of 4.02 km between the two farthest individuals detected. In our surveys, the lizards were not continuous across the 4-km survey area, but were concentrated on each end in the vicinity of the two original iNaturalist observations, and at an urban shopping center in the middle (Figure 1). However, community scientists have made several additional observations along the San Luis Rey River in this intervening area (Table 1; iNaturalist 80545340, 84300160). During the surveys, native Sceloporus occidentalis (Baird and Girard, 1852) and Uta stansburiana (Baird and Girard, 1852) were observed sympatrically with A. sonorae. No other species of Aspidoscelis were detected during the surveys (Figure 1).
Morphological Differences
We show the size differences (SVL) between the native species of whiptails in coastal Southern California compared with the native range of A. sonorae (Figure 2). The largest adult A. sonorae average between 83 and 89 mm depending on clone type with a maximum size of 93 mm from a total of 72 specimens (Taylor et al., 2018). This is larger than A. hyperythrus which has a maximum size of 75 mm measured from a total of 11,476 specimens. The A. tigris we documented had a maximum size of 117 mm measured from 1,910 specimens from coastal Southern California. Thus, adult A. sonorae and A. tigris overlap in body size, but A. tigris reaches a maximum length almost 25 mm longer than A. sonorae.
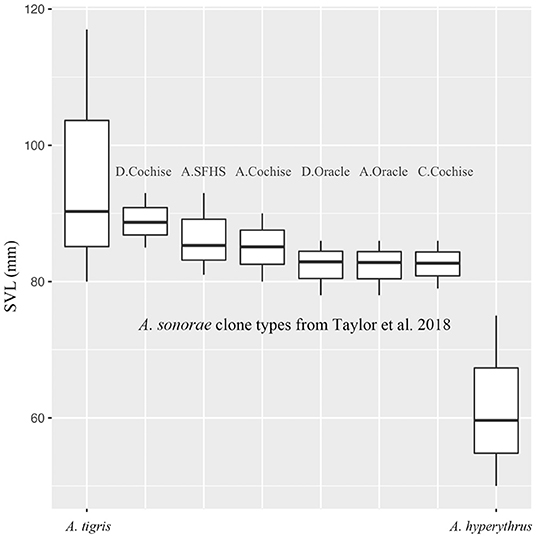
Figure 2. Body size (snout to vent length [SVL] in mm) for native species of Aspidoscelis from Southern California and pattern classes for Aspidoscelis sonorae (Taylor et al., 2018). Note that Taylor et al. (2018) only examined the largest adults in their populations (those over 77 mm SVL); thus, comparisons should only be made across maximum sizes of each group. Aspidoscelis sonorae from Taylor et al. (2018) are listed as the pattern class (A, C, or D) and then the location (Cochise, SFHS, or Oracle).
In addition to body size, a number of other morphological characteristics are useful in differentiating invasive A. sonorae from the two native whiptails (Table 2). There are four fairly easily differentiable characteristics between A. sonorae and A. hyperythrus: (1) young individuals of A. hyperythrus have a blue tail whereas A. sonorae have a slight orange or red coloration to the tail or the tail is slightly lighter but similar to the body coloration; (2) A. hyperythrus develops orange coloration under their throat and body as they mature; (3) A. hyperythrus (at least in Southern California) have paravertebral stripes which merge at the base of the tail or more anteriorly in the pelvic region becoming a single vertebral stripe that continues onto the tail, whereas A. sonorae have paravertebral stripes that remain parallel through the pelvic region and then fade on the upper tail; and (4) A. hyperythrus have an undivided frontoparietal scale; they sometimes have only three supraocular scales; and they sometimes have circumorbital scales that extend forward of the frontal-frontoparietal plate divide [Figure 3; note that Stebbins (2003) refers to these circumorbital scales as the supraorbital semicircle]. Aspidoscelis sonorae has a divided frontoparietal scale and three or four supraocular scales, and circumorbital scales which end at or posterior to the frontal and frontoparietal plate divide. Aspidoscelis tigris does not generally have much striping, as its pattern tends to be mottled; therefore, it is much easier to tell apart from both A. hyperythrus and A. sonorae. The head scales of A. tigris are similar to those of A. sonorae in that they have a divided frontoparietal plate, circumorbital scales which end at or posterior to the frontal and frontoparietal plate divide, and four supraocular scales.
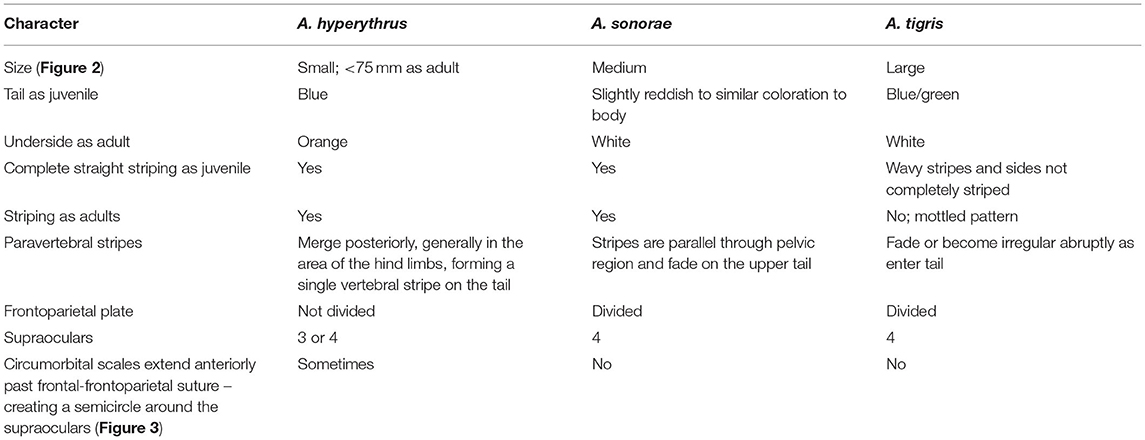
Table 2. Diagnostic characteristics to distinguish between the two native species of whiptail and the invasive Sonoran Spotted Whiptail (bold) in Southern California.
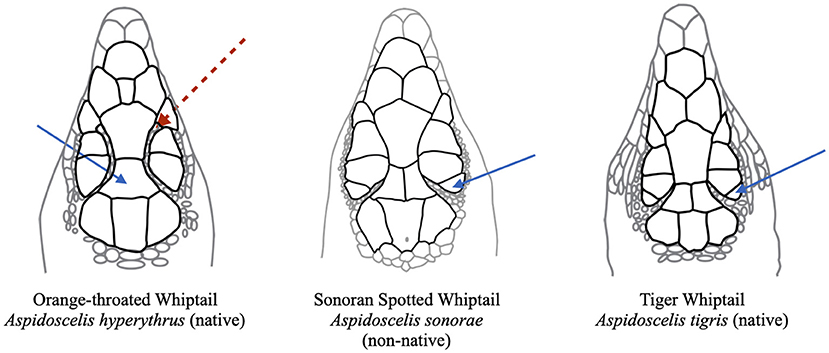
Figure 3. Left is a head drawing of Aspidoscelis hyperythrus, middle is A. sonorae, and right is A. tigris. Blue arrow on left shows the non-divided frontoparietal that is diagnostic of A. hyperythrus. Blue arrows on middle and right drawings show additional supraocular scale that is sometimes absent from A. hyperythrus. The circumorbital scales are the small scales between the supraocular scales and the frontoparietal scale. Only in A. hyperythrus do the circumorbital scales sometimes extend forward of the frontal-frontoparietal suture (dashed red arrow in left panel).
In reviewing iNaturalist observations of whiptails from across Southern California, at least three (A, C, D) of the A. sonorae pattern classes recognized in Taylor et al. (2018) are present in Southern California. However, we only observed pattern classes A and C in San Diego County, per the definitions of Taylor et al. (2018).
Discussion
Sonoran Spotted Whiptails in Southern California
This study documents the first known record of Aspidoscelis sonorae in San Diego County, and shows that this species is continuing to spread in Southern California. We found evidence for a spreading and reproductive population center that remained undetected for at least 2 years. The nearest known population of invasive A. sonorae is ~50 km away, suggesting either that this species was moved 50 km by humans or that there are other pathways by which the species is repeatedly introduced to California. Care should be taken to understand these pathways and potentially close them, especially if this species proves to affect native species. Multiple individuals were found on both sides of the San Luis Rey River, which has been shown to be a biogeographical break for certain species (Vandergast et al., 2008). As such we are not able to assess if the A. sonorae are all from the same founding event. Urbanization can cause biogeographical barriers to weaken, so this could be one continuous population. Because A. sonorae is parthenogenetic, it is also possible they have only crossed the river once and are spreading as one invasion but in disjunct suitable habitats. While this river may have once inhibited some native species from crossing, it remains to be seen if invasive species will have that same issue. Alternatively, there could be two separate introduction events to Oceanside, each giving rise to the areas of denser observations on the west and east end of our survey area, with individuals slowly spreading into the intervening region where there are fewer observations at present. Molecular analyses are currently in progress with individuals from across the Oceanside area and other parts of California to assess the number of independent introduction events and to help identify the geographic region of the source population(s).
In Oceanside, the invasive A. sonorae co-occur with at least three native lizards, the Side-blotched Lizard (U. stansburiana), the Western Fence Lizard (S. occidentalis), and the Southern Alligator Lizard (Elgaria multicarinata Blainville, 1835). We observed the first two during our surveys but not the more secretive E. multicarinata, which has been documented by others in the area (iNaturalist 10220595, 15585294, and 50495145). These three species are also found in and around A. sonorae in Orange County. While A. sonorae is currently found in urban parts of Oceanside where there are no known native whiptail lizards, both native species of whiptail are not far from the expanding range of this population. In Orange County, the native and invasive whiptails are overlapping or in proximity to each other. Further work is needed to establish any impacts that A. sonorae may have on the native species of lizards and other potential competitors and prey species.
An additional threat presented by A. sonorae is potential hybridization with the native whiptails. Sonoran Spotted Whiptails are known to hybridize with A. tigris in their native range (Lowe et al., 1970). It is currently unknown whether A. sonorae can hybridize with the native A. hyperythrus. In areas where there are already reduced numbers of native whiptails (e.g., urban areas; Case and Fisher, 2001), the native sexual species could be negatively impacted if hybridization between these species frequently occurs. Additional molecular studies with increased sampling within and around the introduced populations will be especially helpful if hybridization events with native Aspidoscelis occur.
The establishment and rapid spread of A. sonorae in Southern California is somewhat unexpected given the level of relatedness and phenotypic similarity (i.e., same genus) to native whiptails (Van Wilgen and Richardson, 2012). Currently A. sonorae is only found in urbanized areas where the native species have been displaced (Case and Fisher, 2001). It is possible that A. sonorae will not be able to invade more natural habitats where it will be excluded by native sexual species as described in the weed hypothesis (Wright and Lowe, 1968), which suggests that parthenogenetic whiptail species tend to be found in marginal or disturbed habitats or in ecotones. Here again, iNaturalist observations have an important potential role as these recent species occurrence records can be used for ecological modeling studies to understand habitat use by the native and invasive whiptails.
Differentiating Native and Non-native Whiptails in California
Community science tools like iNaturalist are useful for identifying and detecting invasive species, although there are challenges in detecting non-native species that are extremely similar morphologically to co-occurring native species or that cannot be easily photographed. Documentation of Aspidoscelis sonorae is hampered for both of these reasons—it looks similar to native species and it is also fast and only seasonally-active (Routman and Hulse, 1984), making obtaining high quality photos a challenge during the warmer months when the species is most active. By creating a diagnostic key, we hope to inform community science users and land managers about how to distinguish between A. sonorae and the native whiptails (see also Supplemental Figure 1). In the Results and in Table 2, we list characters that are useful in differentiating the invasive A. sonorae from the native A. hyperythrus and A. tigris. The scale characters (final three characters in Table 2) are useful once an animal or preserved specimen is in hand, but these characters are unlikely to be useful when someone is only taking a photograph. For community scientists taking photographs that others will scrutinize to confirm species identification, it is especially helpful to photograph the tails of juveniles, the throat and ventral region of adults, and the dorsal striping especially near the hind limbs and base of the tail. Photographs showing these characters should allow correct identification of adults and juveniles.
Suggestions for Detecting Cryptic Invasive Species via Community Science
Community science efforts, and especially iNaturalist, can be useful in decreasing detection times for novel invasive species and for tracking the spread of ongoing invasions. However, for species that are morphologically similar to co-occurring native species, are temporally or climatically limited in their activity periods, or are otherwise difficult to photograph, invasive species biologists and community science practitioners can take additional steps to increase the likelihood that new records are documented and correctly identified. Based on our experiences working with community scientists in detecting A. sonorae and other non-native species in California, we provide the following suggestions:
1. Identify diagnostic characters that can be used to differentiate native and non-native species when the specimen is in hand, and especially when it is at a slight distance and is only being photographed.
2. Increase awareness among biologists, landscape managers, and especially community scientists about the potential non-native species, similar-looking native species, key characters for differentiating native and non-native species, and key characters to include in voucher photographs to assist in subsequent identifications. Share this information broadly, such as through social media, traditional media, communications to colleagues, journal entries on iNaturalist projects, and directly with iNaturalist users through personal messages and comments on relevant observations.
3. Biologists and community science practitioners concerned about potential invasive species could actively monitor relevant community science platforms for observations correctly identified as a non-native species and, perhaps more importantly, for observations of non-native species that are misidentified as a native species. Active community scientists can also be encouraged to do the same.
4. For species that are especially difficult to document, invasive species biologists likely cannot rely solely on photo-vouchering through community science to reduce detection times. Instead, they can use additional trapping and monitoring efforts including potentially partnering with community members to set up monitoring stations on private property otherwise inaccessible to the biologists.
In our study of A. sonorae, we have used many of the approaches above. Following the 2020 observation of this species in Oceanside, we scrutinized hundreds of whiptail observations in San Diego and Orange counties, routinely commenting on observations and sending personal messages to observers. Subsequent observations posted to iNaturalist were typically uploaded with identifications only to genus (i.e., Aspidoscelis) when the observer could not confidently differentiate between the native and non-native species or as A. sonorae when the observer was able to correctly identify the invasive species.
Longer-term, another way to improve the detection of cryptic invasive species in community-science generated photographs is to use machine-learning algorithms trained to detect the species of interest (Wäldchen and Mäder, 2018; Weinstein, 2018). At present, iNaturalist uses machine-learning algorithms for automated species/taxon identification. The algorithm is trained on an existing set of research-grade observations. Modifications to this algorithm or separate algorithms dedicated to particular taxa or identification challenges could automate the review of new observations, “flagging” records of suspected non-natives that can then be scrutinized by experts. Not only is accurate recognition and identification of species important for tracking the spread of A. sonorae within California, it is also important for researchers who may be using inaccurate occurrence data, e.g., data with multiple species under the umbrella of only one species.
Conclusion
We found that Aspidoscelis sonorae is present and reproducing within San Diego County. We suggest that photographing the throat and ventral region of adults, the dorsal region of the head, dorsal striping especially near the hind limbs and base of the tail, and the tails of juveniles can assist with identifications from photos. Focusing on these key characters to increase accuracy of identifications could also improve tracking the spread of this invasive species. To this end, we have created a diagnostic key to help land managers and community scientists to properly identify and photograph these lizards (Table 2; Figure 3 and Supplemental Figure 1). Currently within Southern California, there may be more unknown populations of A. sonorae, hidden in plain sight.
Data Availability Statement
Data are available via iNaturalist and from the corresponding authors and/or collections staff, Department of Herpetology, Natural History Museum of Los Angeles County.
Ethics Statement
The animal study was reviewed and approved by UC Davis ACUC.
Author Contributions
SF, RF, and GP contributed to writing, design, and implementation of the study. All authors contributed to the article and approved the submitted version.
Funding
The LACM supported GP's fieldwork and writing. SanDAG and Ecosystems Mission Area of the United States Geological Survey provided funding support for RF.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are especially thankful to Jonny Fishinger and Deborah Rank Sozzani who were the first to contribute photographs of Sonoran Spotted Whiptails in Oceanside to iNaturalist. We also thank the many community scientists using iNaturalist whether that is to photograph or to make identifications of whiptail lizards in Southern California. We are grateful to Cynthia Hitchcock for making the line drawings, Lelani del Pinto for help with figures, Carlton Rochester for data QA/QC and to Susan Wynn for help with field work. We thank members of the Urban Nature Research Center (UNRC) at the Natural History Museum of Los Angeles County (LACM) for providing feedback on an earlier version of this manuscript. The LACM, SanDAG, and Ecosystems Mission Area of the U. S. Geological Survey provided funding. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2022.846431/full#supplementary-material
References
Aceves-Bueno, E., Adeleye, A. S., Feraud, M., Huang, Y., Tao, M., Yang, Y., et al. (2017). The accuracy of citizen science data: a quantitative review. Bull. Ecol. Soc. Am. 98, 278–290. doi: 10.1002/bes2.1336
Amburgey, S. M., Miller, D. A. W., Rochester, C. J., Delaney, K. S., Riley, S. P. D., Brehme, C. S., et al. (2021). The influence of species life history and distribution characteristics on species responses to habitat fragmentation in an urban landscape. J. Anim. Eco. 90, 685–697. doi: 10.1111/1365-2656.13403
Baird, S. F., and Girard, C. (1852). Descriptions of new species of reptiles, collected by the US Exploring Expedition under the command of Capt. Charles Wilkes, USN: First part: Including the species from the Western Coast of America. Proc. Acad. Nat. Sci. Philadelphia. 6, 68–70.
Blainville, H. M. D. (1835). Description de quelques espèces de reptiles de la Californie précédée de l'analyse d'un système général d'erpétologie et d'amphibiologie. Nouv. Ann. Mus. Hist. Nat. Paris 4, 233–296.
Bostic, D. L. (1966). A preliminary report of reproduction in the teiid lizard, Cnemidophorus hyperythrus beldingi. Herpetologica 22, 81–90.
Burt, C. E. (1931). A study of the teiid lizards of the genus Cnemidophorus with special reference to their phylogenetic relationships. Bull. Am. Mus. Nat. 155, 1–286. doi: 10.5479/si.03629236.154.1
Caley, P., Welvaert, M., and Barry, S. C. (2020). Crowd surveillance: estimating citizen science reporting probabilities for insects of biosecurity concern. J. Pest Sci. 93, 543–550. doi: 10.1007/s10340-019-01115-7
California Natural Diversity Database (2022). Special Animals List. (CNDDB). Available online at: https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=109406&inline (accessed April 1, 2022). doi: 10.1063/PT.6.3.20220407a
Case, T. J., and Fisher, R. N. (2001). “Measuring and predicting species presence: coastal sage scrub case study,” in Spatial Uncertainty in Ecology: Implications for Remote Sensing and GIS Applications, eds C. T. Hunsaker, M. F. Goodchild, M. A. Friedl, and T. J. Case (New York, NY: Springer-Verlag), 47–71. doi: 10.1007/978-1-4613-0209-4_3
Cope, E. D. (1863). Descriptions of new American squamata in the museum of the Smithsonian Institution. Proc. Acad. Nat. Sci. Philadelphia 15, 100–106.
Crall, A. W., Newman, G. J., Stohlgren, T. J., Holfelder, K. A., Graham, J., and Waller, D. M. (2011). Assessing citizen science data quality: an invasive species case study. Conserv. Lett. 4, 433–442. doi: 10.1111/j.1755-263X.2011.00196.x
Delaney, D. G., Sperling, C. D., Adams, C. S., and Leung, B. (2008). Marine invasive species: validation of citizen science and implications for national monitoring networks. Biol. Invasions. 10, 117–128. doi: 10.1007/s10530-007-9114-0
Duméril, A. M. C., and Bibron, C. (1836). Erpetologie Générale ou Histoire Naturelle Complete des Reptiles. Vol. 3. Libr. Paris: Encyclopédique Roret. p. 528.
Eifler, D. A., and Eifler, M. A. (1998). Foraging behavior and spacing patterns of the lizard Cnemidophurus uniparens. J. Herpetol. 32, 24–33. doi: 10.2307/1565475
Erickson, R. A., and Burt, W. G. (2019). Additional information on a nonnative whiptail population (Aspidoscelis flagellicauda/sonorae complex) in suburban Orange County, California. Bull. South. Calif. Acad. Sci. 118, 76–78. doi: 10.3160/0038-3872-118.1.76
Fisher, R. N. (2016a). “Chapter 2. Planning and setting objectives in field studies,” in Reptile Ecology and Conservation a Handbook of Techniques, ed C. K. Dodd (Oxford: Oxford University Press), 16–31. doi: 10.1093/acprof:oso/9780198726135.003.0002
Fisher, R. N. (2016b). “Urban and suburban areas,” in Habitat Management Guidelines for Amphibians and Reptiles of the Southwestern United States, eds L. L. C. Jones, K. J. Halama, and R. E. Lovich (Birmingham, AL: Partners in Amphibian and Reptile Conservation, Technical Publication HMG-5), 154–157.
Fisher, R. N., Stokes, D., Rochester, C., Brehme, C., Hathaway, S., and Case, T. (2008). Herpetological monitoring using a pitfall trapping design in Southern California. U.S. Geol. Survey Techniq. Methods 2-A5:44. doi: 10.3133/tm2A5
Fisher, S. R., Del Pinto, L. A., and Fisher, R. N. (2020). Establishment of brown anoles (Anolis sagrei) across a Southern California county and potential interactions with a native lizard species. PeerJ. 8:e8937. doi: 10.7717/peerj.8937
Fisher, S. R., Martin, C. E., and Fisher, R. N. (2021). Geographic distribution: USA, California, Orange County: Tarentola annularis. Herpetol. Rev. 52:85.
Gallo, T., and Waitt, D. (2011). Creating a successful citizen science model to detect and report invasive species. BioScience 61, 459–465. doi: 10.1525/bio.2011.61.6.8
Goldberg, S. R. (1976). Reproduction in a mountain population of the coastal whiptail lizard, Cnemidophorus tigris multiscutatus. Copeia 1976, 260–266. doi: 10.2307/1443945
Kraus, F. (2009). Alien Reptiles and Amphibians: A Scientific Compendium and Analysis, Vol. 4. Berlin: Springer Science and Business Media. doi: 10.1007/978-1-4020-8946-6
Larson, E. R., Graham, B. M., Achury, R., Coon, J. J., Daniels, M. K., Gambrell, D. K., et al. (2020). From eDNA to citizen science: emerging tools for the early detection of invasive species. Front. Ecol. Environ. 18, 194–202. doi: 10.1002/fee.2162
Li, X., Liu, X., Kraus, F., Tingley, R., and Li, Y. (2016). Risk of biological invasions is concentrated in biodiversity hotspots. Front. Ecol. Environ. 14, 411–417. doi: 10.1002/fee.1321
Lowe, C. H., and Wright, J. W. (1964). Species of the Cnemidophorus exsanguis subgroup of whiptail lizards. J. Ariz. Acad. Sci. 3, 78–80.
Lowe, C. J., Wright, J. W., Cole, C. J., and Bezy, R. L. (1970). Natural hybridization between the teiid lizards Cnemidophorus sonorae (parthenogenetic) and Cnemidophorus tigris (bisexual). Syst. Zool. 19, 114–127. doi: 10.2307/2412449
Mittermeier, R. A., Turner, W. R., Larsen, F. W., Brooks, T. M., and Gascon, C. (2011). “Global biodiversity conservation: the critical role of hotspots,” in Biodiversity Hotspots, eds F. E. Zachosand, and J. C. Habel (Berlin, Heidelberg: Springer), 3–22. doi: 10.1007/978-3-642-20992-5_1
Noss, R. F., Platt, W. J., Sorrie, B. A., Weakley, A. S., Means, D. B., Costanza, J., et al. (2015). How global biodiversity hotspots may go unrecognized: lessons from the North American Coastal Plain. Divers. Distrib. 21, 236–244. doi: 10.1111/ddi.12278
Palmer, D. D., and Fisher, R. N. (2010). Geographic distribution: Ramphotyphlops braminus. Herpetol. Rev. 41:518.
Pauly, G. B., and Borthwick, D. B. (2015). Geographic distribution: USA, California, Los Angeles County: Anolis carolinensis. Herpetol. Rev. 46:567.
Pauly, G. B., and Gavit, P. D. (2019). Geographic distribution: USA, California, Los Angeles County: Trachylepis quinquetaeniata. Herpetol. Rev. 50, 103–104.
Pauly, G. B., Shaulsky, M. C., Barley, A. J., Kennedy-Gold, S., Stewart, S. C., Keeney, S., et al. (2020). Morphological change during rapid population expansion confounds leopard frog identifications in the southwestern United States. Copeia. 108, 299–308. doi: 10.1643/CH-19-222
Pauly, G. B., Yoshida, G. S., and Worrell, R. (2015). Geographic distribution: USA, California: Hemidactylus garnotii. Herpetol. Rev. 46:569.
Putman, B. J., Pauly, G. B., and Blumstein, D. T. (2020). Urban invaders are not bold risk-takers: a study of three invasive lizards in Southern California. Curr. Zool. 66, 657–665. doi: 10.1093/cz/zoaa015
Reaser, J. K., Brantley, K. A., Kirkey, J., Burgiel, S. W., Veatch, S. D., and Rodriguez-Burgos, J. (2020). The early detection of and rapid response (EDRR) to invasive species: a conceptual framework and federal capacities assessment. Biol. Invasions 22, 1–19. doi: 10.1007/s10530-019-02156-w
Reed, R. N., Todd, B. D., Miano, O. J., Canfield, M., Fisher, R. N., and McMartin, L. (2016). Ecology and control of an introduced population of Southern Watersnakes (Nerodia fasciata) in Southern California. Herpetologica 72, 130–136. doi: 10.1655/HERPETOLOGICA-D-14-00061
Routman, E., and Hulse, A. (1984). Ecology and reproduction of a parthenogenetic lizard, Cnemidophorus sonorae. J. Herpetol. 18, 381–386. doi: 10.2307/1564100
Santana Marques, P., Resende Manna, L., Clara Frauendorf, T., Zandon,à, E., Mazzoni, R., and El-Sabaawi, R. (2020). Urbanization can increase the invasive potential of alien species. J. Anim. Ecol. 89, 2345–2355. doi: 10.1111/1365-2656.13293
Spear, D. M., Pauly, G. B., and Kaiser, K. (2017). Citizen science as a tool for augmenting museum collection data from urban areas. Front. Ecol. Evol. 5:86. doi: 10.3389/fevo.2017.00086
Stebbins, R. C. (2003). A Field Guide to Western Reptiles and Amphibians. Boston, MA: Houghton Mifflin.
Taylor, H. L., Cole, C. J., and Townsend, C. R. (2018). Relegation of Aspidoscelis flagellicaudus to the synonymy of the parthenogenetic teiid lizard A. sonorae based on morphological evidence and a review of relevant genetic data. Herpetol. Rev. 49, 636–653.
Thomson, R. C., Wright, A. N., and Shaffer, H. B. (2016). California Amphibian and Reptile Species of Special Concern. Berkeley, CA: University of California Press.
Van Wilgen, N. J., and Richardson, D. M. (2012). The roles of climate, phylogenetic relatedness, introduction effort, and reproductive traits in the establishment of non-native reptiles and amphibians. Conserv. Biol. 26, 267–277. doi: 10.1111/j.1523-1739.2011.01804.x
Vandergast, A. G., Bohonak, A. J., Hathaway, S. A., Boys, J., and Fisher, R. N. (2008). Are hotspots of evolutionary potential adequately protected in Southern California? Biol. Conserv. 141, 1648–1664. doi: 10.1016/j.biocon.2008.04.009
Ver Hoef, J. M., Cressie, N., Fisher, R. N., and Case, T. J. (2001). “Uncertainty and spatial linear models for ecological data,” in Spatial Uncertainty in Ecology: Implications for Remote Sensing and GIS Applications, eds C. T. Hunsaker, M. F. Goodchild, M. A. Friedl, and T. J. Case (New York, NY: Springer-Verlag), 214–237. doi: 10.1007/978-1-4613-0209-4_10
Wäldchen, J., and Mäder, P. (2018). Machine learning for image based species identification. Methods Ecol. Evol. 9, 2216–2225. doi: 10.1111/2041-210X.13075
Weaver, R. E., O'Connor, A. P., Wallace, J. L., King, J. M., and Walker, J. M. (2011). Discovery of the parthenogenetic Colorado Checkered Whiptail, Aspidoscelis neotesselata (Squamata: Teiidae), in Washington State. Northwestern Nat. 92, 233–236. doi: 10.1898/1051-1733-92.3.233
Weinstein, B. G. (2018). A computer vision for animal ecology. J. Anim. Ecol. 87, 533–545. doi: 10.1111/1365-2656.12780
Winkleman, R. S., and Backlin, A. R. (2016). Geographic distribution: Asidoscelis flagellicauda/sonorae complex (Spotted Whiptail). Herpetol. Rev. 47, 256–257.
Witmer, G. W., Burke, P. W., Pitt, W. C., and Avery, M. L. (2007). “Management of invasive vertebrates in the United States: an overview,” in Managing Vertebrate Invasive Species: An International Symposium, eds G. Witmer, W. Pitt, K. Fagerstone (Fort Collins, CL: USDA/APHIS/WS National Research Center), 127–137.
Wright, J. W., and Lowe, C. H. (1967). Hybridization in nature between parthenogenetic and bisexual species of whiptail lizards (Genus Cnemidophorus). Am. Mus. Novit. 2286, 1–36.
Keywords: Sonoran Spotted Whiptail (Aspidoscelis sonorae), citizen science, community science, iNaturalist, parthenogenesis (asexual reproduction), species occurrence data, diagnostic key, San Diego County
Citation: Fisher S, Fisher RN and Pauly GB (2022) Hidden in Plain Sight: Detecting Invasive Species When They Are Morphologically Similar to Native Species. Front. Conserv. Sci. 3:846431. doi: 10.3389/fcosc.2022.846431
Received: 31 December 2021; Accepted: 07 April 2022;
Published: 16 May 2022.
Edited by:
Mohammad Farhadinia, University of Oxford, United KingdomReviewed by:
Félix Manuel Medina, Cabildo Insular de La Palma, SpainSharlene E. Sing, USDA Forest Service Rocky Mountain Research Station, United States
Gonzalo Collado, University of the Bío Bío, Chile
Yu Dan, Wuhan University, China
Copyright © 2022 Fisher, Fisher and Pauly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert N. Fisher, rfisher@usgs.gov; Gregory B. Pauly, gpauly@nhm.org
 Samuel Fisher
Samuel Fisher Robert N. Fisher
Robert N. Fisher Gregory B. Pauly
Gregory B. Pauly