- School of Earth, Atmospheric and Life Sciences, University of Wollongong, Wollongong, NSW, Australia
The application of reproductive technologies to amphibian conservation breeding programs is gaining momentum and the field is poised to contribute significantly toward amphibian species recovery. We briefly discuss the opportunities for reproductive technologies to enhance conservation breeding outcomes, including their potential to enhance the genetic management, and in turn, the fitness of threatened species. Despite this potential, an important consideration that is not yet well understood is the degree to which specific reproductive technologies might influence (either enhance, or in some instances potentially decrease) individual fitness and lead to shifts in population viability. The development of a standardised approach to monitoring offspring throughout life-stages to detect changes to morphology, behaviour, physiology, survivorship, and developmental trajectories is essential. The primary focus of this review is to provide a ‘best-practise’ framework for quantifying key fitness determining traits expected to contribute to the fitness of individuals and long-term viability of populations, which will ultimately allow us to progress the field of amphibian reproductive technologies and assess the impact of protocol refinement.
1 Introduction
Under the current global scenario, where species continue to decline at an unprecedented rate, integrated, multidisciplinary approaches to support species recovery are needed more than ever (Silla and Kouba, 2022). It is through an inclusive, united effort towards conservation, involving the cooperative application of a suite of strategies within the conservationist’s toolbox, that the greatest promise of success for the recovery threatened species is held. The ultimate aim of threatened species recovery is to establish robust, self-sustaining populations with enough genetic diversity and adaptive potential to remain viable over time. Reproductive Technologies (RTs, also known as assisted reproductive technologies, ARTs) are a collection of techniques within the conservationist’s toolbox that have immense potential to improve reproductive outcomes, enhance genetic management, and safeguard valuable genetic resources for threatened species recovery (Silla and Byrne, 2019; Holt and Comizzoli, 2021; Bolton et al., 2022; Silla and Kouba, 2022). These technologies also afford valuable opportunities for interconnecting populations of threatened species and establishing an ex situ- in situ continuum (Bolton et al., 2022; Kouba and Julien, 2022). Despite the potential for reproductive technologies to assist threatened species recovery, in practice these technologies have been underutilized in amphibian conservation breeding programs (CBPs), primarily due to a need for species-specific optimisation and increased knowledge of the reproductive biology of many species required to successfully implement RTs. Over the past two-to-three decades, however, a surge in research focused on the development of amphibian reproductive technologies has progressed the field substantially and led to an increase in the application of reproductive technologies for threatened species recovery. This review begins by briefly detailing the opportunities and considerations of integrating reproductive technologies into conservation breeding programs. Next, we provide comprehensive detail on which fitness-determining traits to measure throughout amphibian life-stages in order to quantify offspring fitness and the viability of captive populations. Ultimately, the close monitoring of fitness, will allow us to progress the field of reproductive technologies and assess the impact of technological advances and protocol refinement.
2 Application of reproductive technologies to amphibian conservation breeding programs: opportunities and considerations
The application of reproductive technologies to amphibian threatened species has traditionally lagged behind that of human, agricultural and aquaculture species, however in recent years a surge of research in this field is closing the gap. Reproductive technologies encompass a complement of techniques, including those aimed at: i) monitoring the reproductive status and stress physiology of captive individuals, ii) hormone therapies to induce spawning in pairs/groups of amphibians, or induce sperm-release, or egg-release in isolated males and females, respectively, iii) cold storage for the short-term storage and transport of gametes, iv) cryopreservation and biobanking of sperm, tissue or cell lines, and v) assisted fertilisation (known as AF, or IVF). The reasons to adopt amphibian reproductive technologies and welfare considerations for their application have been recently reviewed (Silla et al., 2021), and together with numerous comprehensive technical reviews provides a guide for the accession of these technologies into conservation breeding programs (see; Kouba et al., 2013; Narayan, 2013; Vu and Trudeau, 2016; Browne et al., 2019; Silla and Byrne, 2019; Della Togna et al., 2020; Silla et al., 2021; Byrne and Silla, 2022; Clulow et al., 2022b; Graham and Kouba, 2022; Silla and Langhorne, 2022; Strand et al., 2022; Trudeau et al., 2022; Anastas et al., 2023; Upton et al., 2023). The potential for reproductive technologies to complement traditional methods of captive breeding and assist the conservation of threatened amphibians is increasingly being recognised and a steady increase in the application of RTs to conservation breeding programs is in motion.
While the application of reproductive technologies offers valuable opportunities to enhance propagation and the generation of higher numbers of individuals for assurance and reintroduction, the true value of RTs for wildlife conservation lies in their potential to enhance the genetic management of threatened species. Strategic and effectual genetic management is critical for the conservation of threatened species to prevent the loss of genetic diversity (through genetic drift and directional selection) and fitness (inbreeding depression) that typically compounds over generations and can heighten the risk of further decline (Frankham, 2008; Frankham et al., 2010). Reproductive technologies can be employed to assist genetic management by i) facilitating the generation of offspring from genetically valuable parental genotypes (through hormone therapies to induce spawning in male-female pairs, or through assisted fertilisation), ii) conducting assisted fertilisation to split clutches and ejaculates and increase the combination of parental genotypes to increase genetic diversity from a single mating event, iii) using assisted fertilisation to test for genetic compatibility and combining ability both within and between ex situ and in situ populations, iv) facilitating genetic exchange within and between ex situ and in situ populations (through gamete collection, transport and assisted fertilisation), and arguably the most powerful tool, v) employing biobanking and assisted fertilisation techniques to generate offspring from cryopreserved sperm to extend the reproductive lifespan of individuals and reinvigorate genetic diversity through the introgression of expired genotypes decades into the future (Silla and Byrne, 2019; Byrne and Silla, 2022; Anastas et al., 2023).
The enormous potential of reproductive technologies to enhance the genetic management, and in turn, the fitness of threatened species is evident. However, an important consideration that is not yet well understood is the degree to which specific reproductive technologies might influence (either enhance, or potentially decrease) individual fitness and lead to shifts in population viability. While improving and managing genetic diversity via the employment of reproductive technologies is predicted to lead to an overall increase in the fitness of populations, a standardised approach to monitoring offspring throughout life-stages to detect changes to morphology, behaviour, physiology, survivorship, and developmental trajectories are essential. An important consideration that has recently been highlighted is the potential for specific technical processes, including sperm cryopreservation, to impact fitness traits (Holt, 2023). Two primary mechanisms by which sperm cryopreservation could impact the fitness of offspring generated from AF with frozen-thawed sperm have been suggested. First, the cryopreservation process induces artificial selection on the ejaculate, as a proportion of sperm will not survive the freeze-thaw process. This selection pressure could positively or negatively impact the fitness of resultant offspring (Nusbaumer et al., 2019). Second, the process of sperm cryopreservation and/or the nature of incubation media have the capacity to influence the transcription of genetic and epigenetic information to the developing embryo (Nusbaumer et al., 2019; Holt, 2023). There is evidence in some species of fish and amphibians that cryopreservation leads to smaller offspring size or delayed growth (Nusbaumer et al., 2019; Poo and Hinkson, 2020; Poo et al., 2022), while larger size of cryo-derived offspring has been reported in other species (Bokor et al., 2015; Lampert et al., 2022).
At present, amphibian studies investigating and reporting on the fitness of offspring generated via reproductive technologies are limited. Important considerations for designing these experiments include selecting a model amphibian lacking parental care (to control for differential investment), and possessing a large clutch size and high sperm concentration. Importantly, these characteristics will allow the use of a split-clutch/split-ejaculate breeding design to control for maternal- and paternal- effects, and parental incompatibilities. Adopting this approach will permit comparison between protocol-induced effects, while controlling for parental effects. Following from the importance of a rigorous breeding design, is the value of a standardise framework for quantifying the fitness-determining traits of offspring throughout life-stages. Multi-institutional adoption of the proposed framework provided below, coupled with transparent research reporting, is expected to progress the field of amphibian reproductive technologies and allow practitioners to assess the impact of further protocol refinement.
3 How and where to rear offspring
Amphibians typically display high levels of phenotypic plasticity, whereby the environment experienced during development interacts with an individual’s genotype to alter phenotypic expression. Across a diversity of species profound changes in the timing of development and morphological features have been reported in response to heterogeneity in various environmental variables, including temperature, salinity, food availability, water level, larval density, disease, and predation risk (Newman, 1998; Parris and Cornelius, 2004; Tejedo et al., 2010; Hopkins and Brodie, 2015; Sinai et al., 2022). Consequently, it is critical that quantification of all fitness-determining traits takes place in facilities where both the rearing and test environments can be tightly controlled. We recommend that research takes place in biosecure disease-free facilities where researchers can control and monitor key environmental variables, such as temperature, humidity, water parameters, photoperiod and UV levels. As far as possible conditions should be standardised and ecologically appropriate, with decisions over what conditions to implement based on available knowledge of a study species’ natural history and life history (Kelleher et al., 2018). Beyond husbandry decisions regarding essential characteristics of the environment (i.e. ensuring appropriate temperature, lighting, humidity, water, and substrate), decisions over diet and nutrition are particularly important. In addition to emerging evidence that feeding regimes (amount of food and feeding frequency) can induce amphibian developmental plasticity (Courtney Jones et al., 2015), there is a growing understanding that an individual’s health, viability and performance can be significantly impacted by diet composition. In captivity, amphibians can be successfully reared to sexual maturity by feeding them a basic diet comprised of vegetable matter and fish food during the larval stage, and small cultured insects (e.g. crickets and mealworms) during the post-metamorphic life stages. However, diets lacking in key vitamins, nutrients, and micronutrients can lead to disease, developmental abnormalities and even mortality. For example, suboptimal development and performance has been linked to inadequate intake of dietary antioxidants such as Vitamin A (Ferrie et al., 2014; Rodríguez and Pessier, 2014) and carotenoids (Ogilvy et al., 2012; Silla et al., 2016), high cholesterol intake has been linked to ocular disease (Boss and Plummer, 2022), and diets deficient in calcium, phosphorous and vitamin D3 (coupled with inappropriate provision of UVB light and low temperatures) are known to cause metabolic bone disease and neurological and musculo-skeletal abnormalities (Ferrie et al., 2014). Although species will differ in their needs, and rearing protocols will need to be developed and optimised on a species- by- species basis, in recent years detailed guidelines for the husbandry and welfare of amphibians in captivity have emerged in the literature, providing an excellent basis for developing protocols (Ferrie et al., 2014; Tapley et al., 2015; Karlsdóttir et al., 2021; Linhoff et al., 2021; Van Zanten and Simpson, 2021).
Ideally individuals should be housed separately throughout life-stages to allow the tracking of individual fitness traits (Silla et al., 2016; Byrne and Silla, 2017; Keogh et al., 2018). While there may be welfare concerns over rearing individuals in isolation, there is currently very little research to indicate that such concerns are warranted. To the contrary, there is evidence that group housing may be an acute stressor for both tadpoles (Forsburg et al., 2019) and adult frogs (Cikanek et al., 2014). Provision of life-support systems to isolated individuals, such as oxygenation of water and environmental enrichment, can be achieved through the use of automated husbandry systems (e.g. irrigation systems and rain chambers to provide clean, oxygenated water), provision of plants and tunnels/tubes (for exploration and shelter), and use of transparent enclosures to allow visual social interactions among conspecifics. If it is not feasible to rear animals individually, housing offspring in sibling cohorts and randomly selecting a subset of individuals from each cohort for the quantification of fitness-determining traits is acceptable, but large sample sizes will be needed to ensure that sufficient variation is captured. It is also recommended that detailed studbooks are maintained to record mortality, reproduction and longevity (Brereton, 2024). Tracking individuals housed within groups may be possible, but the reliability and welfare impact of different methods needs to be considered. Microchipping, including passive integrated transponder (PIT) tags, are an option for larger amphibians (>40mm snout–vent length), but are not suitable for smaller species or tadpoles (Waudby et al., 2022). Toe-clipping is another option for juveniles and adult amphibians of some species (Waudby et al., 2022). Alternatively, tadpoles, juveniles and adults may be individually marked using visible implant alphanumeric (VIA) tags, or visible implant elastomer (VIE) markers injected subcutaneously (Fouilloux et al., 2020; Waudby et al., 2022). Each of these methods is considered invasive and animal welfare issues and potential impact on fitness requires careful consideration (Waudby et al., 2022). A less invasive approach is to draw on a species’ natural external markers for photo-based identification. For example, inter-individual variation in tail venation was recently shown to be effective at identifying green and golden bellfrog tadpoles (Gould et al., 2023) and ventral patterns have been successful in identifying individual brown toadlets (Byrne and Silla, 2023). Such biometric identification can be facilitated by the use of pattern recognition software (Ribeiro and Rebelo, 2011), with recent innovations in artificial intelligence and deep learning providing the opportunity to train software to identify individuals with extreme precision (Takaya et al., 2023).
4 What fitness-determining traits should be measured?
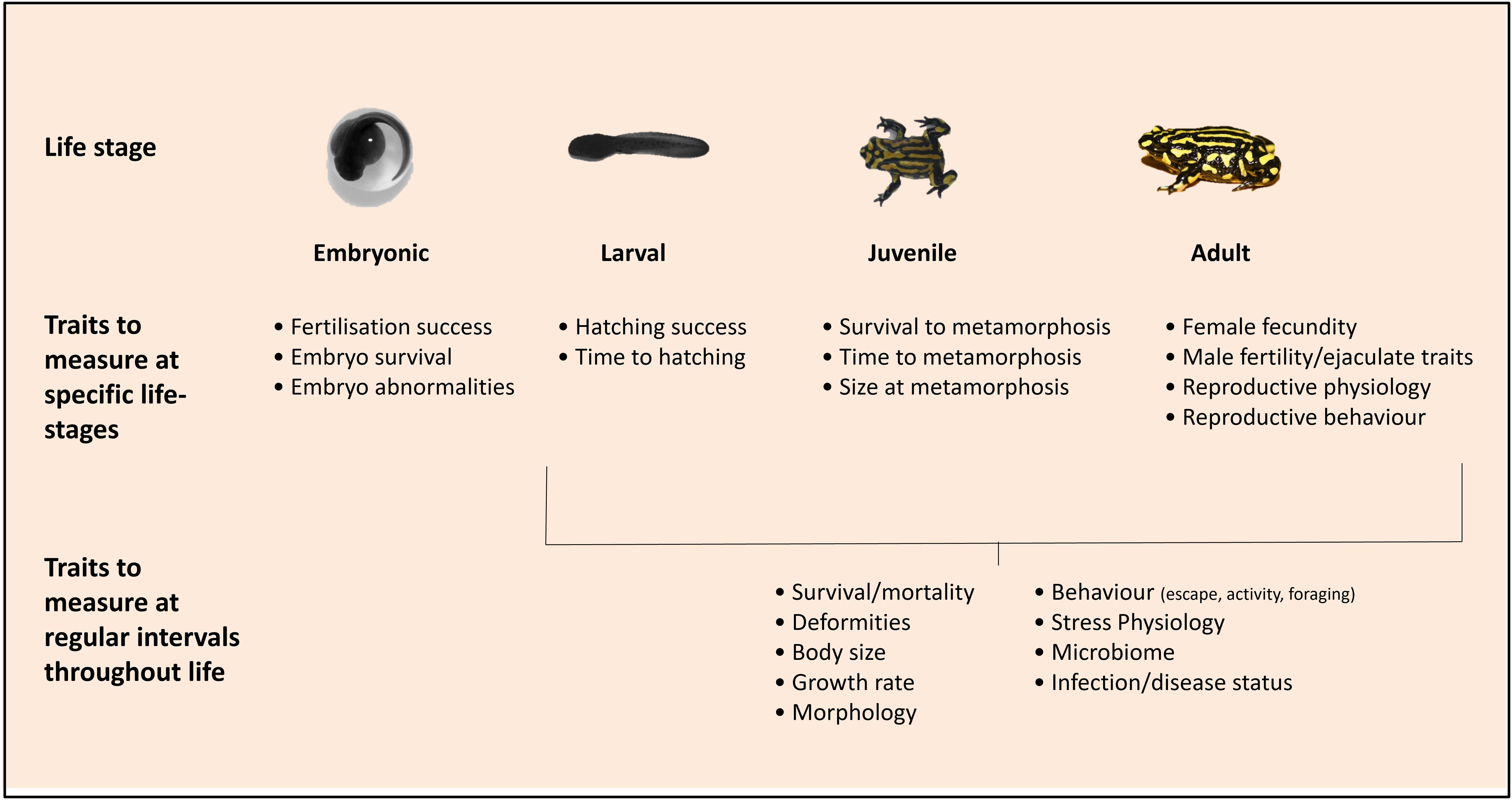
Multiple traits contribute to total fitness, that is the probability that an individual (population, or species) successfully reproduces and contributes genes to future generations. Therefore, practitioners should aim to measure a suite of traits that represent different components of fitness (e.g. survival or fecundity), as well as traits that are indirectly related to fitness (e.g. body size or growth rate) (see Figure 1). Below we discuss traits that are likely to be most critical to fitness in amphibians, and, in turn, those that are critically important in regulating population size, growth rate and persistence. We also detail the types of methods and assays required to ensure accurate and biologically relevant measurements are taken.
4.1 Fertilisation success
Among sexually reproducing animals, including amphibians, fertilisation is controlled by the interaction of gamete recognition proteins located on the surface of sperm and eggs (Tian et al., 1997). Incompatibilities between these proteins (reflecting genetic incompatibilities) can cause significant drops in fertilisation success, with direct implications for reproductive success and fitness in both sexes. Fertilisation success can be estimated within hours of AF by scoring the proportion of eggs that have rotated. Eggs are characterised by two hemispheres; the animal pole (typically darker in colour) and the vegetal pole (typically lighter in colour). When a sperm penetrates an egg, the axis between the poles will rotate to align with the gravitational field, resulting in the animal pole facing upwards. The only downside of this approach is that rotating eggs can sometimes cause unfertilised neighbouring eggs to rotate, inflating estimates of fertilisation success. To avoid this problem, fertilisation success should be confirmed using optical microscopy. To ensure a standardised approach, eggs should always be staged using generalised staging systems (Gosner, 1960). In anurans fertilisation is normally scored between the 2-cell stage (Gonser stage 3) and the neural-plate stage (Gosner stage 13). Not all studies score fertilisation success, but this is a mistake because it can lead to inaccurate estimates of early embryo mortality (Sagvik et al., 2005). Where possible, eggs should be removed from terrariums/aquariums as soon as possible following oviposition and fertilisation success scored (a dissection microscope can aid in looking for cell division). Amphibian eggs are generally quite robust due to their protective jelly coat, so using sterile plastic spoons and tweezers to carefully manipulate the eggs to view and score fertilisation is possible.
4.2 Embryo survival and embryo abnormalities
Among amphibians it is well established that mortality risk is highest during embryonic and larvae life stages (Matthews et al., 2013), and that changes to this risk can influence population persistence (Biek et al., 2002). Genetic incompatibilities that reduce offspring viability are expected to manifest, and compound, during early development. Therefore, it is important to continuously monitor developing embryos and score exactly when mortality occurs (using generalised staging systems to record the stage of death). Importantly, in certain species, development can be suspended prior to hatching (embryonic diapause). Therefore, embryos should only be discarded once they show clear signs of death. Embryos should also undergo routine pathology to monitor fungal and viral pathogens (Wright and Whitaker, 2001). For example, aquatic oomycetes (known as “water molds”) can cause the disease saprolegniosis, which is known to infect wild and captive amphibian embryos. Infertile and dead eggs are readily infected with saprolegniosis postmortem (Costa and Lopes, 2022). Developing embryos are also known to be susceptible to infection if they have been exposed to trauma (physical or chemical) to the protective egg jelly capsule, or through contact infection between adjacent eggs (Costa and Lopes, 2022). Reducing the risk of infection during embryonic development is another reason that rearing offspring individually from the point of fertilisation is encouraged. Where clutches are kept together, they should be monitored regularly for embryo mortality and infection and embryos removed that exhibit symptoms. Surviving embryos should also be monitored for abnormalities that provide an indication of genetic incompatibility. Common embryonic disorders and malformations include oedema, severely bent trunk axis, axial flexures and shrunken, enlarged, or missing eyes and tails.
4.3 Hatching success and time to hatching
Scoring the proportion of eggs hatching is a reliable way to estimate offspring viability. Hatching failure is common in amphibians, and generally indicates that embryos have died just prior to hatching, or have fatigued and died during the strenuous process of exiting the egg capsule. Hatching in amphibians is normally triggered by a specific environmental cue (e.g. vibration or hypoxia), and is also typically asynchronous. As such, before eggs are scored as failed, they should be checked for signs of life (e.g. reflex movement or a heartbeat) and decomposition. Hatching success per family should be scored by calculating the number of eggs successfully hatched as a proportion of the number of eggs fertilised. The time between stimulation of hatching and successful hatching can also be used to score time to hatching. Time to hatching can have important fitness consequences as individuals that hatch faster are expected to access resources required for larval development more rapidly. The only caveat here is that there may be a tradeoff between time to hatching and body size at hatching (Stearns, 1992). If individuals that hatch quickly are smaller, this could have flow on affects that negatively impact other developmental milestones that influence fitness (time to metamorphosis and size at metamorphosis). The potential for such life history tradeoffs, and flow on effects, should be considered on a species-by-species basis as this may inform decisions regarding optimal rearing conditions.
4.4 Larval survival, deformity, growth rate, body size and shape
Mortality in amphibians can be extreme during the larvae phase as many individuals fail to thrive. Therefore, this is a critical time to measure survivorship. Of note, mortality at this life-stage may be due to numerous reasons that are often undetermined. Histopathology and/or necropsy to attempt to determine cause of death of tadpoles is encouraged and full procedural details are provided elsewhere (Wright and Whitaker, 2001). Larval survival can be measured at specific time points (e.g. daily or weekly intervals) or specific developmental points (e.g. emergence of the first hind limb or forearm). Larval viability can also be assessed by recording the appearance of deformities, which are expected to compromise survival and fitness (see Pakkasmaa et al., 2003). Abnormalities normally present as lateral, concave or convex curvature of the spine (scoliosis, lordosis, kyphosis), abnormal accumulation of fluid in tissue (oedema), malformed heads or tails, missing tails, or missing, shrunken or misplaced eyes. Photos can be taken of tadpoles (next to a scale) at regular intervals, and images analysed using image-analysis software. Sophisticated landmark based geometric morphometrics offer a powerful way to quantify variation in morphology in amphibians (see Vidal-García et al., 2018), but even very basic measures of head and tail length and width and total length will allow estimates of variation in body size and shape (see Pakkasmaa et al., 2003; Rudin-Bitterli et al., 2018, 2020). These measures can be informative because tadpole size often correlates with size at metamorphosis (Pakkasmaa et al., 2003). Moreover, tadpole shape is known to influence ability to escape predators (Relyea, 2001), which is one of the most important sources of mortality during the larval stage in the wild (Wells, 2010). Taking photos at defined time points also provides the opportunity to measure growth rate, which is often inter-related with time to metamorphosis and body size at metamorphosis. From a practical perspective, it is best to photograph tadpoles in situ to avoid handling stress. This can often be achieved by taking the photograph from above, after dropping the water to avoid size distortions as tadpoles move through the water column, and placing grid paper under the container to provide a scale for measurement (Keogh et al., 2018; Mcinerney et al., 2019). If dropping the water level prior to capturing photographs, it is important that tadpoles remain fully submerged, and movement is unrestricted (a minimum depth of 2–5cm depending on tadpole size) to avoid adverse impacts on amphibian welfare. Water levels in each aquarium should be returned to normal immediately after photographs have been taken (Keogh et al., 2018). Where tank design doesn’t allow photographs from above, removal of tadpoles may be necessary. This can be achieved using a fine mesh fish net and placing tadpoles in an individual water dish under a camera set up, noting that tadpoles are very delicate in the first week or two following hatching.
4.5 Survival to metamorphosis and metamorph deformities
Metamorphosis is a physiologically demanding transformation and is typically accompanied by a spike in mortality, so survival to metamorphosis is a good indicator of viability. Survival can be measured at various points, but the most common are; 1) the point of complete forelimb emergence (Gosner stage 42), 2) the point when individuals leave the water (though this can be hard to monitor), and 3) the point of complete tail resorption (Gosner stage 46) (Rudin-Bitterli et al., 2018; Székely et al., 2020; Weber et al., 2024). Of note, metamophs can easily drown, so care should be taken to drop water levels and provide a suitable substrate to allow metamorphs to exit the water. Failing to do so can mask genetic causes of mortality. As metamorphs grow, they should be checked for possible congenital abnormalities, such as lateral deviation of the spine (scoliosis), polymelia (presence of extra limbs), ectromelia (missing limbs), ectroddactly (missing digits), ectopic appendages, missing or misplaced eyes, microcephaly (shrunken head), bicephaly (presence of two heads), and shortened lower jaw (brachygnathia). Definitions of the diversity of deformities observed in amphibians, and practical guide to their identification and causes, have been detailed elsewhere (Meteyer, 2000; Lunde and Johnson, 2012).
4.6 Time to metamorphosis and body size at metamorphosis
It is a central tenant of life history theory that individuals entering adulthood faster, and at a larger body size, will have higher lifetime fitness, and be selectively favoured (Stearns, 1992). In line with this notion, there is evidence that TTM and SAM are effective predictors of post-metamophic survival, fecundity, and performance in various amphibians (Pakkasmaa et al., 2003; Earl and Whiteman, 2015). Broadly speaking, however, meta-analysis has indicated that SAM is a much better fitness predictor than TTM, and that the value of these measures as measures of fitness appears to be highly species and system specific (Earl and Whiteman, 2015). For instance, there is evidence that SAM predicts fitness more accurately in species in which there is a shorter amount of time between metamorphosis and reproductive maturity (Earl and Whiteman, 2015). Nevertheless, we recommend that TTM and SAM are routinely recorded because they can be measured quickly and accurately and have the potential to be highly informative (especially if the life history and ecology of the target species is well known). TTM can be measured at various points (see above) and body size can be estimated by weighing individuals immediately after tail resorption to estimate body mass, and using callipers or photos to measure snout-vent length (SVL). Mass-length relationships can also be used to calculate a body condition index (Maccracken and Stebbings, 2012), which can be an important fitness determinant in amphibians. Saying this, if time and funds permit, more direct measures of body composition (e.g. lipids, proteins, fatty acids, amino acids, micronutrients) may provide a better estimate of the resources available to support metabolic activity, or other fitness related characters, such as muscles mass (Wilder et al., 2016).
4.7 Adult survival
As animals age there will be progressive mortality, and we should expect that rates of mortality will be higher for individuals of poor genetic quality (Dziminski et al., 2008). As such, there is value in keeping careful records of when individuals die. Histopathology and/or necropsy to determine cause of death is encouraged and full procedural details are provided elsewhere (Wright and Whitaker, 2001). We also recommend the postmortem extraction and biobanking of gonadal tissue, including gonadal cell lines, testes macerates, or oocytes (Kaurova et al., 2021; Clulow et al., 2022a; Strand et al., 2022).
4.8 Adult body size
Across amphibians, there is a growing body of evidence that larger individuals have a greater chance of survival, which has been linked to impacts on risk of desiccation, infection, predation, starvation, and competition for resources (Wells, 2010; Cabrera-Guzmán et al., 2013). There is also evidence for strong positive relationships between body size and reproductive success, largely due to relationships between body size and fecundity and fertility, as well as between body size and attractiveness in mate choice, and success in male-male competition (see Amézquita and Lüddecke, 1999; Kupfer, 2007; Wells, 2010). As such, recording body size (ideally as weight and SVL) for all experimental animals at regular time intervals during adult life (in addition to larval and juvenile life-stages) is recommended. Keeping a photographic record of body size throughout life-stages is also encouraged as this provides the opportunity for morphometric analysis of body traits linked to fitness (see Vidal-García et al., 2018). It is also important to note that sexual size dimorphism (SSD) is strong in amphibians, with female-biased SSD reported in 91% of anurans, 79% of salamanders, and 81% of caecilians (Pincheira-Donoso et al., 2021). As such, it is recommended that body size and growth rate be quantified for each sex independently (see Linhoff et al., 2022 for a review of methods for identifying sex in amphibians).
4.9 Female fecundity and male fertility
Fecundity is known to be a significant contributor to lifetime reproductive success in amphibians. For some species fecundity will correlate tightly with body size (allowing body size to be used as proxy), but this won’t always be the case. As such, if animals are reared to sexual maturity, we advise keeping long term records of clutch number and clutch size produced for each female. For males, the non-invasive collection of spermic urine, milt, or spermatophores is encouraged (Silla and Langhorne, 2022), though if animals die unexpectedly or need to be euthanized for welfare reasons, testes should be extracted post-mortem to generate testes macerates (Silla et al., 2017; Kaurova et al., 2021). Following sperm collection, we advise measuring a range of characteristics known to influence fertilisation capacity and male reproductive success in amphibians, including sperm concentration, viability (proportion live/dead, DNA integrity), motility parameters (velocity, percentage motility, forward progression, longevity), and morphology (head length, tail length, morphological deformities). Detailed methods for the measurement of these sperm traits are provided in Silla and Langhorne (2022).
4.10 Behavioural and physiological performance
Animal behaviour, the measurable response of an individual to external or internal stimuli, plays a crucial role in determining fitness (an individual’s ability to survive, reproduce and pass genes to the next generation). Assays of escape performance, exercise/locomotory performance, foraging performance, general-movement behaviour and sexual display, can all provide estimates of individual viability. The behavioural ecology literature is replete with examples of these types of assays, and they range from being very simple and easy to run, to highly complex (Kelleher et al., 2018). Some relatively simple tests include observing or video recording test subjects and measuring sensorimotor performance, such as quantifying foraging efficiency (Mcinerney et al., 2016), locomotory performance (Székely et al., 2020), activity levels in familiar home environments (Videlier et al., 2014; Urszán et al., 2015), and exploration and boldness behaviour in novel environments (Brodin et al., 2013; Carlson and Langkilde, 2013; Kelleher et al., 2019). For many species, anti-predatory escape responses (quantified as escape burst speed and distance travelled) can also be measured following a simulated predator attack. This is commonly achieved by imposing a light physical stimulus to larvae or adults to induce a flight response (Touchon and Wojdak, 2014), or by imposing either; 1) a chemical threat stimulus, whereby a test subject is presented with chemical stimuli associated with a predator (Sih et al., 2003; Wilson and Krause, 2012; Carlson and Langkilde, 2014) or 2) a physical threat stimulus, whereby a test subject is threatened by a live or model predator (Koprivnikar et al., 2012; Urszán et al., 2015; Kelleher et al., 2017; Mcinerney et al., 2017). More complex assays include the use of robotics (Klein et al., 2012), sophisticated 3D animations (Bian et al., 2018), behavioural tracking software and remote videography (Hughey et al., 2018) to standardise stimuli and capture subtle or long term inter-individual behavioural differences in foraging behaviour, movement behaviour, anti-predatory behaviour, and mating behaviour (reviewed in Kelleher et al., 2018). In regard to physiological performance, the capacity to withstand fatigue during sustained activity (endurance) provides a good proxy for dispersal ability and is often easily measured by running larvae or adults in linear or circular race tracks and recording the time and distance moved (Herrel et al., 2014; Araspin et al., 2023). When testing adults, researchers will also often measure fatigue using a ‘righting ability test’, whereby individuals are flipped on their back and the time to right is recorded (Herrel et al., 2014; Silla et al., 2016). Beyond such tests, increasingly sophisticated physiological measures of performance can also be highly informative. One widely used approach is to measure basal metabolic rate, defined as the overall energetic costs required to fulfil physiological needs (Padilla et al., 2023). Basal metabolic rate is accurately measured using a respiratory chamber and flow throw respirometry and is strongly linked to fitness due to direct impacts on growth, development, reproduction, and general functioning (Santos and Cannatella, 2011; Padilla et al., 2023). Another increasingly used physiological test involves measuring the maximal jump force of frogs (linked to performance in dispersal, foraging, predator escape and competition), quantified using a jump plate and charge amplifier (Herrel et al., 2014; Araspin et al., 2023). It is also important to recognise that there can be sex-specific differences in behavioural performance (Herrel et al., 2014), making it essential to quantify experimental variables for both sexes independently.
There is also a growing interest in non-invasive methods for measuring stress physiology (Narayan et al., 2019; Narayan, 2022). In captive contexts, sources of stress can include excessive handling, overcrowding, competitive interactions/aggression, overstimulation (e.g. from external stimuli such as vibration, light and sound), and lack of refugia (Ferrie et al., 2014). With the advent of non-invasive methods for monitoring of acute stress in captive amphibians, such as quantifying urinary, faecal, water-borne, and dermal secretions (Cikanek et al., 2014; Santymire et al., 2018; Forsburg et al., 2019; Narayan et al., 2019; Narayan, 2022), it is now possible to track the stress responses of individuals in various contexts (including in response to the behavioural assays described above). Of note, knowledge of causes of stress can also be used to optimise husbandry protocols and rearing conditions (see section 3. above).
5 Conclusion
Reproductive technologies encompass a complement of techniques aimed at understanding and enhancing reproductive outcomes and genetic management, and the application of these technologies to amphibian conservation breeding programs is gaining momentum. In order to assess the degree to which RTs influence individual fitness, and to allow practitioners to assess the impact of protocol refinement, a standardised approach to monitoring the fitness-determining traits of offspring throughout life-stages is critical. Acquiring this information will help conservation practitioners run cost-benefit analyses to inform decisions surrounding the integration of reproductive technologies into the recovery plans for declining species.
Author contributions
AS: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. PB: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The support of the following funding grants is also acknowledged, of which AS and PB were in receipt during the preparation of this manuscript, Australian Research Council; Discovery Early Career Researcher Award (DE210100812), and Zoo and Aquarium Association’s Wildlife Conservation Fund, with ancillary funding support from the Environmental Futures Research Centre at the University of Wollongong.
Acknowledgments
The traditional custodians and cultural knowledge holders of the land on which the research summarised in this chapter was conducted are acknowledged and we pay respect to Elders both past, present, and emerging.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amézquita A., Lüddecke H. (1999). Correlates of intrapopulational variation in size at metamorphosis of the high-andean frog hyla labialis. Herpetologica 55, 295–303.
Anastas Z. M., Byrne P. G., O’brien J. K., Hobbs R. J., Upton R., Silla A. J. (2023). The increasing role of short-term sperm storage and cryopreservation in conserving threatened amphibian species. Animals 13, 2094. doi: 10.3390/ani13132094
Araspin L., Measey J., Herrel A. (2023). Does aquatic performance predict terrestrial performance: A case study with an aquatic frog, xenopus laevis. J. Of Exp. Biol. 226, Jeb246545. doi: 10.1242/jeb.246545
Bian X., Chandler T., Laird W., Pinilla A., Peters R. (2018). Integrating evolutionary biology with digital arts to quantify ecological constraints on vision-based behaviour. Methods In Ecol. And Evol. 9, 544–559. doi: 10.1111/2041-210X.12912
Biek R., Funk W. C., Maxell B. A., Mills L. S. (2002). What is missing in amphibian decline research: insights from ecological sensitivity analysis. Conserv. Biol. 16, 728–734. doi: 10.1046/j.1523-1739.2002.00433.x
Bokor Z., Ittzés I., Mosonyi G., Kotrik L., Müller T., Urbányi B., et al. (2015). Survival and growth rates of wels catfish (S iluris glanis L innaeus 1758) larvae originating from fertilization with cryopreserved or fresh sperm. J. Of Appl. Ichthyol. 31, 164–168. doi: 10.1111/jai.12730
Bolton R. L., Mooney A., Pettit M. T., Bolton A. E., Morgan L., Drake G. J., et al. (2022). Resurrecting biodiversity: advanced assisted reproductive technologies and biobanking. Reprod. And Fert. 3, R121–R146. doi: 10.1530/RAF-22-0005
Boss C., Plummer C. E. (2022). “Ophthalmology of amphibia: caecilians, salamanders, frogs, toads, and relatives,” in Wild and exotic animal ophthalmology: volume 1: invertebrates, fishes, amphibians, reptiles, and birds (Cham, Switzerland: Springer). doi: 10.1007/978-3-030-71302-7
Brereton J. E. (2024). What is the role of the studbook in zoo and aquarium research? Zoo Biol. 43, 22–31. doi: 10.1002/zoo.21797
Brodin T., Lind M. I., Wiberg M. K., Johansson F. (2013). Personality trait differences between mainland and island populations in the common frog (Rana temporaria). Behav. Ecol. And Sociobiol. 67, 135–143. doi: 10.1007/s00265-012-1433-1
Browne R. K., Silla A. J., Upton R., Della-Togna G., Marcec-Greaves R., Shishova N. V., et al. (2019). Sperm collection and storage for the sustainable management of amphibian biodiversity. Theriogenology 133, 187–200. doi: 10.1016/j.theriogenology.2019.03.035
Byrne P. G., Silla A. (2023). Lifetime breeding-site and nest-site fidelity in a declining terrestrial toadlet: evidence for a win-stay/lose-shift strategy. Front. In Conserv. Sci. 4, 1226658. doi: 10.3389/fcosc.2023.1226658
Byrne P. G., Silla A. J. (2017). Testing the effect of dietary carotenoids on larval survival, growth and development in the critically endangered southern corroboree frog. Zoo Biol. 36, 161–169. doi: 10.1002/zoo.21352
Byrne P. G., Silla A. J. (2022). “Genetic management of threatened amphibians: using artificial fertilisation technologies to facilitate genetic rescue and assisted gene flow,” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A. J., Kouba A., Heatwole H. (Melbourne, Australia: CSIRO Publishing).
Cabrera-Guzmán E., Crossland M. R., Brown G. P., Shine R. (2013). Larger body size at metamorphosis enhances survival, growth and performance of young cane toads (Rhinella marina). PloS One 8, E70121. doi: 10.1371/journal.pone.0070121
Carlson B. E., Langkilde T. (2013). Personality traits are expressed in bullfrog tadpoles during open-field trials. J. Of Herpetol. 47, 378–383. doi: 10.1670/12-061
Carlson B. E., Langkilde T. (2014). No evidence of selection by predators on tadpole boldness. Behaviour 151, 23–45. doi: 10.1163/1568539X-00003121
Cikanek S. J., Nockold S., Brown J. L., Carpenter J. W., Estrada A., Guerrel J., et al. (2014). Evaluating group housing strategies for the ex-situ conservation of harlequin frogs (Atelopus spp.) using behavioral and physiological indicators. PloS One 9, E90218. doi: 10.1371/journal.pone.0090218
Clulow S., Clulow J., Marcec-Greaves R., Della Togna G., Calatayud N. E., Yuan Y. (2022b). Common goals, different stages: the state of the arts for reptile and amphibian conservation. Reproduct. Fert. And Dev. 34, I–Ix. doi: 10.1071/RDv34n5_FO
Clulow J., Upton R., Clulow S. (2022a). “Cryopreservation of amphibian genomes: targeting the holy grail, cryopreservation of maternal-haploid and embryonic-diploid genomes,” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A. J., Kouba A., Heatwole H. (Melbourne, Australia: CSIRO Publishing).
Costa S., Lopes I. (2022). Saprolegniosis in amphibians: an integrated overview of A fluffy killer disease. J. Of Fungi 8, 537. doi: 10.3390/jof8050537
Courtney Jones S. K., Munn A. J., Penman T. D., Byrne P. G. (2015). Long-term changes in food availability mediate the effects of temperature on growth, development and survival in striped marsh frog larvae: implications for captive breeding programmes. Conserv. Physiol. 3, Cov029. doi: 10.1093/conphys/cov029
Della Togna G., Howell L. G., Clulow J., Langhorne C. J., Marcec-Greaves R., Calatayud N. E. (2020). Evaluating amphibian biobanking and reproduction for captive breeding programs according to the amphibian conservation action plan objectives. Theriogenology 150, 412–431. doi: 10.1016/j.theriogenology.2020.02.024
Dziminski M. A., Roberts J. D., Simmons L. W. (2008). Fitness consequences of parental compatibility in the frog crinia Georgiana. Evolution 62, 879–886. doi: 10.1111/evo.2008.62.issue-4
Earl J. E., Whiteman H. H. (2015). Are commonly used fitness predictors accurate? A meta-analysis of amphibian size and age at metamorphosis. Copeia 103, 297–309. doi: 10.1643/CH-14-128
Ferrie G. M., Alford V. C., Atkinson J., Baitchman E., Barber D., Blaner W. S., et al. (2014). Nutrition and health in amphibian husbandry. Zoo Biol. 33, 485–501. doi: 10.1002/zoo.21180
Forsburg Z. R., Goff C. B., Perkins H. R., Robicheaux J. A., Almond G. F., Gabor C. R. (2019). Validation of water-borne cortisol and corticosterone in tadpoles: recovery rate from an acute stressor, repeatability, and evaluating rearing methods. Gen. And Comp. Endocrinol. 281, 145–152. doi: 10.1016/j.ygcen.2019.06.007
Fouilloux C. A., Garcia-Costoya G., Rojas B. (2020). Visible implant elastomer (Vie) success in early larval stages of A tropical amphibian species. Peerj 8, E9630. doi: 10.7717/peerj.9630
Frankham R. (2008). Genetic adaptation to captivity in species conservation programs. Mol. Ecol. 17, 325–333. doi: 10.1111/j.1365-294X.2007.03399.x
Frankham R., Ballou J. D., Briscoe D. A. (2010). Introduction to conservation genetics (Cambridge University Press: Cambridge). doi: 10.1017/CBO9780511809002
Gosner K. L. (1960). A simplified table for staging anuran embryos and larvae with notes on identification. Herpetol. League 16, 183–190.
Gould J., Taylor J., Davies B., Donelly R., Schmahl K., Bugir C. K., et al. (2023). Tadpole fingerprinting: using tail venation patterns to photo-identify tadpole individuals of A threatened frog. Austral Ecol. 48, 585–599. doi: 10.1111/aec.13286
Graham K. M., Kouba C. K. (2022). “Ultrasonography to assess female reproductive status and inform hormonally induced ovulation,” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A. J., Kouba A. J., Heatwole H. (Melbourne, Australia: CSIRO Publishing).
Herrel A., Vasilopoulou-Kampitsi M., Bonneaud C. (2014). Jumping performance in the highly aquatic frog, xenopus tropicalis: sex-specific relationships between morphology and performance. Peerj 2, E661. doi: 10.7717/peerj.661
Holt W. V. (2023). Biobanks, offspring fitness and the influence of developmental plasticity in conservation biology. Anim. Reprod. 20, E20230026. doi: 10.1590/1984-3143-ar2023-0026
Holt W. V., Comizzoli P. (2021). Perspective: genome resource banking for wildlife conservation: promises and caveats. Cryoletters 42, 309–320.
Hopkins G. R., Brodie E. D. Jr. (2015). Occurrence of amphibians in saline habitats: A review and evolutionary perspective. Herpetol. Monogr. 29, 1–27. doi: 10.1655/HERPMONOGRAPHS-D-14-00006
Hughey L. F., Hein A. M., Strandburg-Peshkin A., Jensen F. H. (2018). Challenges and solutions for studying collective animal behaviour in the wild. Philos. Trans. Of R. Soc. B: Biol. Sci. 373, 20170005. doi: 10.1098/rstb.2017.0005
Karlsdóttir B., Knight A. T., Johnson K., Dawson J. (2021). Lessons from practitioners for designing and implementing effective amphibian captive breeding programmes. Oryx 55, 382–392. doi: 10.1017/S0030605320000332
Kaurova S. A., Uteshev V. K., Gapeyev A. B., Shishova N. V., Gakhova E. N., Browne R. K., et al. (2021). Cryopreservation of spermatozoa obtained postmortem from the european common frog rana temporaria. Reproduct. Fert. And Dev. 33, 588–595. doi: 10.1071/RD20336
Kelleher S. R., Silla A. J., Byrne P. G. (2018). Animal personality and behavioral syndromes in amphibians: A review of the evidence, experimental approaches, and implications for conservation. Behav. Ecol. And Sociobiol. 72, 1–26. doi: 10.1007/s00265-018-2493-7
Kelleher S. R., Silla A. J., Dingemanse N. J., Byrne P. G. (2017). Body size predicts between-individual differences in exploration behaviour in the southern corroboree frog. Anim. Behav. 129, 161–170. doi: 10.1016/j.anbehav.2017.05.013
Kelleher S. R., Silla A. J., Niemelä P. T., Dingemanse N. J., Byrne P. G. (2019). Dietary carotenoids affect the development of individual differences and behavioral plasticity. Behav. Ecol. 30, 1273–1282. doi: 10.1093/beheco/arz074
Keogh L. M., Silla A. J., Mcfadden M. S., Byrne P. G. (2018). Dose and life stage-dependent effects of dietary beta-carotene supplementation on the growth and development of the booroolong frog. Conserv. Physiol. 6, Coy052. doi: 10.1093/conphys/coy052
Klein B. A., Stein J., Taylor R. C. (2012). Robots in the service of animal behavior. Communicat. Integr. Biol. 5, 466–472. doi: 10.4161/cib.21304
Koprivnikar J., Gibson C. H., Redfern J. C. (2012). Infectious personalities: behavioural syndromes and disease risk in larval amphibians. Proc. R. Soc. B: Biol. Sci. 279, 1544–1550. doi: 10.1098/rspb.2011.2156
Kouba C. K., Julien A. R. (2022). “Linking in situ and ex-situ populations of threatened amphibian species using genome resource banks,” in Reproductive technologies and biobanking for the conservation of amphibians. Silla J., Kouba A., Heatwole H. Eds. (Melbourne, Australia: CSIRO Publishing).
Kouba A. J., Lloyd R. E., Houck M. L., Silla A. J., Calatayud N., Trudeau V. L., et al. (2013). Emerging trends for biobanking amphibian genetic resources: the hope, reality and challenges for the next decade. Biol. Conserv. 164, 10–21. doi: 10.1016/j.biocon.2013.03.010
Kupfer A. (2007). Sexual size dimorphism in amphibians: an overview. Sex Size And Gender Roles: Evolutionary Stud. Of Sexual Size Dimorphism 5, 50–60.
Lampert S., Burger I., Chen D., Kouba A., Kouba C. (2022). Comparison of the effect of frozen-thawed versus fresh sperm on offspring growth in tiger salamanders (Ambystoma tigrinum). Cryobiology 109, 50. doi: 10.1016/j.cryobiol.2022.11.160
Linhoff L., Germano J., Molinia F. (2022) “Methods of identifying the sex of amphibians and of conditioning captive brood stock for assisted reproduction” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A. J., Kouba A., Heatwole H. Melbourne, Australia: CSIRO Publishing.
Linhoff L., Soorae P., Harding G., Donnelly M., Germano J., Hunter D., et al (2021). Iucn guidelines for amphibian reintroductions and other conservation translocations. first edition. Iucn: Gland Switzerland, 1–81.
Lunde K. B., Johnson P. T. J. (2012). A practical guide for the study of malformed amphibians and their causes. J. Of Herpetol. 46, 429–441, 13. doi: 10.1670/10-319
Maccracken J. G., Stebbings J. L. (2012). Test of A body condition index with amphibians. J. Of Herpetol. 46, 346–350. doi: 10.1670/10-292
Matthews J. H., Funk W. C., Ghalambor C. K. (2013). Demographic approaches to assessing climate change impact: an application to pond-breeding frogs and shifting hydropatterns. In Brodie J. F., Post E. S., Doak D. F.. Wildlife Conservation in a Changing Climate. Chicago, USA: University of Chicago Press, 58–85.
Mcinerney E. P., Byrne P. G., Silla A. J. (2017). The effect of dietary antioxidants and exercise training on the escape performance of southern corroboree frogs. Behav. Processes 144, 46–50. doi: 10.1016/j.beproc.2017.08.012
Mcinerney E. P., Silla A. J., Byrne P. G. (2016). The influence of carotenoid supplementation at different life-stages on the foraging performance of the southern corroboree frog (Pseudophryne corroboree): A test of the silver spoon and environmental matching hypotheses. Behav. Processes 125, 26–33. doi: 10.1016/j.beproc.2016.01.008
Mcinerney E. P., Silla A. J., Byrne P. G. (2019). Effect of carotenoid class and dose on the larval growth and development of the critically endangered southern corroboree frog. Conserv. Physiol. 7, Coz009. doi: 10.1093/conphys/coz009
Meteyer C. U. (2000). Field guide to malformations of frogs and toads: with radiographic interpretations (U.S. Geological Survey BSR 2000-0005), 20 p. Available at: https://pubs.er.usgs.gov/publication/53882.
Narayan E. J. (2013). Non-invasive reproductive and stress endocrinology in amphibian conservation physiology. Conserv. Physiol. 1, Cot011. doi: 10.1093/conphys/cot011
Narayan E. J (2022) “Non-invasive monitoring of stress physiology during management and breeding of amphibians in captivity,” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A. J., Kouba A., Heatwole H.. Melbourne, Australia: CSIRO Publishing.
Narayan E., Gabor C. R., Forsburg Z. R., Davis D. (2019). Non-invasive methods for measuring and monitoring stress physiology in imperiled amphibians. Front. In Ecol. And Evol. 7, 431. doi: 10.3389/fevo.2019.00431
Newman R. A. (1998). Ecological constraints on amphibian metamorphosis: interactions of temperature and larval density with responses to changing food level. Oecologia 115, 9–16. doi: 10.1007/s004420050485
Nusbaumer D., Marques Da Cunha L., Wedekind C. (2019). Sperm cryopreservation reduces offspring growth. Proc. Of R. Soc. B 286, 20191644. doi: 10.1098/rspb.2019.1644
Ogilvy V., Preziosi R., Fidgett A. (2012). A brighter future for frogs? The influence of carotenoids on the health, development and reproductive success of the red-eye tree frog. Anim. Conserv. 15, 480–488. doi: 10.1111/j.1469-1795.2012.00536.x
Padilla P., Herrel A., Denoël M. (2023). What makes A great invader? Anatomical traits as predictors of locomotor performance and metabolic rate in an invasive frog. J. Exp. Biol. 226 (24), jeb246717. doi: 10.1242/jeb.246717
Pakkasmaa S., Merilä J., O'hara R. (2003). Genetic and maternal effect influences on viability of common frog tadpoles under different environmental conditions. Heredity 91, 117–124. doi: 10.1038/sj.hdy.6800289
Parris M. J., Cornelius T. O. (2004). Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology 85, 3385–3395. doi: 10.1890/04-0383
Pincheira-Donoso D., Harvey L. P., Grattarola F., Jara M., Cotter S. C., Tregenza T., et al. (2021). The multiple origins of sexual size dimorphism in global amphibians. Global Ecol. And Biogeograp. 30, 443–458. doi: 10.1111/geb.13230
Poo S., Bogisich A., Mack M., Lynn B. K., Devan-Song A. (2022). Post-release comparisons of amphibian growth reveal challenges with sperm cryopreservation as A conservation tool. Conserv. Sci. And Pract. 4, E572. doi: 10.1111/csp2.572
Poo S., Hinkson K. M. (2020). Amphibian conservation using assisted reproductive technologies: cryopreserved sperm affects offspring morphology, but not behavior, in A toad. Global Ecol. And Conserv. 21, E00809. doi: 10.1016/j.gecco.2019.e00809
Relyea R. A. (2001). Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82, 523–540. doi: 10.1890/0012-9658(2001)082[0523:MABPOL]2.0.CO;2
Ribeiro J., Rebelo R. (2011). Survival of alytes cisternasii tadpoles in stream pools: A capture-recapture study using photo-identification. Amphibia-Reptilia 32, 365–374. doi: 10.1163/017353711X584186
Rodríguez C. E., Pessier A. P. (2014). Pathologic changes associated with suspected hypovitaminosis A in amphibians under managed care. Zoo Biol. 33, 508–515. doi: 10.1002/zoo.21161
Rudin-Bitterli T. S., Evans J. P., Mitchell N. J. (2020). Geographic variation in adult and embryonic desiccation tolerance in A terrestrial-breeding frog. Evolution 74, 1186–1199. doi: 10.1111/evo.13973
Rudin-Bitterli T. S., Mitchell N. J., Evans J. P. (2018). Environmental stress increases the magnitude of nonadditive genetic variation in offspring fitness in the frog crinia Georgiana. Am. Nat. 192, 461–478. doi: 10.1086/699231
Sagvik J., Uller T., Olsson M. (2005). Outbreeding depression in the common frog, rana temporaria. Conserv. Genet. 6, 205–211. doi: 10.1007/s10592-004-7829-3
Santos J. C., Cannatella D. C. (2011). Phenotypic integration emerges from aposematism and scale in poison frogs. Proc. Of Natl. Acad. Of Sci. 108, 6175–6180. doi: 10.1073/pnas.1010952108
Santymire R. M., Manjerovic M. B., Sacerdote-Velat A. (2018). A novel method for the measurement of glucocorticoids in dermal secretions of amphibians. Conserv. Physiol. 6 (1), coy008. doi: 10.1093/conphys/coy008
Sih A., Kats L. B., Maurer E. F. (2003). Behavioural correlations across situations and the evolution of antipredator behaviour in A sunfish–salamander system. Anim. Behav. 65, 29–44. doi: 10.1006/anbe.2002.2025
Silla A. J., Byrne P. G. (2019). The role of reproductive technologies in amphibian conservation breeding programs. Annu. Rev. Of Anim. Biosci. 7, 499–519. doi: 10.1146/annurev-animal-020518-115056
Silla A. J., Calatayud N. E., Trudeau V. L. (2021). Amphibian reproductive technologies: approaches and welfare considerations. Conserv. Physiol. 9, Coab011. doi: 10.1093/conphys/coab011
Silla A. J., Keogh L. M., Byrne P. G. (2017). Sperm motility activation in the critically endangered booroolong frog: the effect of medium osmolality and phosphodiesterase inhibitors. Reproduct. Fert. And Dev. 29, 2277–2283. doi: 10.1071/RD17012
Silla A., Kouba A. J. (2022). “Integrating reproductive technologies into the conservation toolbox for the recovery of amphibian species,” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A., Kouba A., Heatwole H. (Melbourne, Australia: CSIRO Publishing).
Silla A. J., Langhorne C. J. (2022). “Protocols for hormonally induced spermiation, and the cold storage, activation, and assessment of amphibian sperm,” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A. J., Kouba A. J., Heatwole H. (Melbourne, Australia: CSIRO Publishing).
Silla A. J., Mcinerney E. P., Byrne P. G. (2016). Dietary carotenoid supplementation improves the escape performance of the southern corroboree frog. Anim. Behav. 112, 213–220. doi: 10.1016/j.anbehav.2015.12.012
Sinai N., Glos J., Mohan A. V., Lyra M. L., Riepe M., Thöle E., et al. (2022). Developmental plasticity in amphibian larvae across the world: investigating the roles of temperature and latitude. J. Of Thermal Biol. 106, 103233. doi: 10.1016/j.jtherbio.2022.103233
Strand J., Fraser B., Houck M. L., Clulow S. (2022). “Culturing and biobanking of amphibian cell lines for conservation applications,” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A. J., Kouba A. J., Heatwole H. (Melbourne, Australia: CSIRO Publishing).
Székely D., Cogălniceanu D., Székely P., Armijos-Ojeda D., Espinosa-Mogrovejo V., Denoël M. (2020). How to recover from A bad start: size at metamorphosis affects growth and survival in A tropical amphibian. BMC Ecol. 20, 24. doi: 10.1186/s12898-020-00291-w
Takaya K., Taguchi Y., Ise T. (2023). Individual identification of endangered amphibians using deep learning and smartphone images: case study of the Japanese giant salamander (Andrias japonicus). Sci. Rep. 13, 16212. doi: 10.1038/s41598-023-40814-1
Tapley B., Rendle M., Baines F. M., Goetz M., Bradfield K. S., Rood D., et al. (2015). Meeting ultraviolet B radiation requirements of amphibians in captivity: A case study with mountain chicken frogs (Leptodactylus fallax) and general recommendations for pre-release health screening. Zoo Biol. 34, 46–52. doi: 10.1002/zoo.21170
Tejedo M., Marangoni F., Pertoldi C., Richter-Boix A., Laurila A., Orizaola G., et al. (2010). Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Climate Res. 43, 31–39. doi: 10.3354/cr00878
Tian J., Gong H., Thomsen G. H., Lennarz W. J. (1997). Xenopus laevissperm–egg adhesion is regulated by modifications in the sperm receptor and the egg vitelline envelope. Dev. Biol. 187, 143–153. doi: 10.1006/dbio.1997.8607
Touchon J. C., Wojdak J. M. (2014). Plastic hatching timing by red-eyed treefrog embryos interacts with larval predator identity and sublethal predation to affect prey morphology but not performance. PloS One 9, E100623. doi: 10.1371/journal.pone.0100623
Trudeau V. L., Raven B. H., Pahuja H. K., Narayan E. J. (2022). “Hormonal control of amphibian reproduction,” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A. J., Kouba A. J., Heatwole H. (Melbourne, Australia: CSIRO Publishing).
Upton R., Clulow S., Colyvas K., Mahony M., Clulow J. (2023). Paradigm shift in frog sperm cryopreservation: reduced role for non-penetrating cryoprotectants. Reproduction 165, 583–592. doi: 10.1530/REP-22-0486
Urszán T. J., Török J., Hettyey A., Garamszegi L. Z., Herczeg G. (2015). Behavioural consistency and life history of rana dalmatina tadpoles. Oecologia 178, 129–140. doi: 10.1007/s00442-014-3207-0
Van Zanten T. C., Simpson S. C. (2021). Managing the health of captive groups of reptiles and amphibians. Veterinary Clinics: Exotic Anim. Pract. 24, 609–645. doi: 10.1016/j.cvex.2021.05.005
Vidal-García M., Bandara L., Keogh J. S. (2018). Shaperotator: an R tool for standardized rigid rotations of articulated three-dimensional structures with application for geometric morphometrics. Ecol. Evol. 8, 4669–4675. doi: 10.1002/ece3.4018
Videlier M., Bonneaud C., Cornette R., Herrel A. (2014). Exploration syndromes in the frog X enopus (S ilurana) tropicalis: correlations with morphology and performance? J. Zool. 294, 206–213. doi: 10.1111/jzo.12170
Vu M., Trudeau V. L. (2016). Neuroendocrine control of spawning in amphibians and its practical applications. Gen. And Comp. Endocrinol. 234, 28–39. doi: 10.1016/j.ygcen.2016.03.024
Waudby H. P., Burns P. A., Jensen M. A., Hampton J. O., Hunter D., Mcknight D. T., et al. (2022). “Wildlife marking methods,” in Wildlife research in Australia: practical and applied methods. Eds. Smith B. P., Waudby H. P., Alberthsen C., Hampton J. O. (Melbourne, Australia: CSIRO Publishing).
Weber T., Ozgul A., Schmidt B. R. (2024). Density-dependent performance of larval and juvenile toads: implications for amphibian conservation. Basic And Appl. Ecol. 75, 12–17. doi: 10.1016/j.baae.2024.02.001
Wells K. D. (2010). The ecology and behavior of amphibians (Chicago, USA: University Of Chicago Press). doi: 10.7208/9780226893334
Wilder S. M., Raubenheimer D., Simpson S. J. (2016). Moving beyond body condition indices as an estimate of fitness in ecological and evolutionary studies. Funct. Ecol. 30, 108–115. doi: 10.1111/1365-2435.12460
Wilson A. D., Krause J. (2012). Personality and metamorphosis: is behavioral variation consistent across ontogenetic niche shifts? Behav. Ecol. 23, 1316–1323. doi: 10.1093/beheco/ars123
Keywords: amphibian, captive breeding, conservation, threatened species, reproductive technologies, biobanking, IVF, fitness traits
Citation: Silla AJ and Byrne PG (2024) The importance of quantifying fitness-determining traits throughout life to assess the application of reproductive technologies for amphibian species recovery. Front. Conserv. Sci. 5:1378624. doi: 10.3389/fcosc.2024.1378624
Received: 30 January 2024; Accepted: 11 March 2024;
Published: 22 March 2024.
Edited by:
William Holt, The University of Sheffield, United KingdomReviewed by:
Lola Brookes, National Centre for the Replacement Refinement and Reduction of Animals in Research, United KingdomCecilia Langhorne, Royal Zoological Society of Scotland, United Kingdom
Copyright © 2024 Silla and Byrne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aimee J. Silla, YXNpbGxhQHVvdy5lZHUuYXU=
†These authors have contributed equally to this work
 Aimee J. Silla
Aimee J. Silla Phillip G. Byrne
Phillip G. Byrne