Primary Prevention Implantable Cardiac Defibrillators: A Townsville District Perspective
- 1College of Medicine & Dentistry, Heart, Trauma and Sepsis Research Laboratory, James Cook University, Townsville, QLD, Australia
- 2Cardiac Investigations, The Townsville University Hospital, Douglas, QLD, Australia
- 3Cardiology Clinic, Cairns Hospital, Cairns, QLD, Australia
Background: Despite major advances in treating patients with severe heart failure, deciding who should receive an implantable cardiac defibrillator (ICD) remains challenging.
Objective: To study the risk factors and mortality in patients after receiving an ICD (January 2008–December 2015) in a regional hospital in Australia.
Methods: Eighty-two primary prevention patients received an ICD for ischemic cardiomyopathy (ICM, n = 41) and non-ischemic cardiomyopathy (NICM, n = 40) with 4.8-yrs follow-up. One patient had mixed ICM/NICM indications. Ventricular arrhythmias were assessed using intracardiac electrograms. Statistical analysis compared the total population and ICM and NICM groups using Kaplan-Meier for survival, Cox regression for mortality predictors, and binary logistic regression for predictors of ventricular arrhythmias (p < 0.05).
Results: Major risk factors were hypercholesterolemia (70.7%), hypertension (47.6%), and obesity (41.5%). Severe obstructive sleep apnea (OSA) was found exclusively in NICM patients (23.7%, p = 0.001). Mortality was 30.5% after 4.8-yrs. The majority of patients (n=67) had no sustained ventricular arrhythmias yet 28% received therapy (n = 23), 18.51% were appropriate (n = 15), and 13.9% inappropriate (n = 11). Patients receiving ≥2 incidences of inappropriate shocks were 18-times more likely to die (p = 0.013). Three sudden cardiac deaths (SCD) (3.7%) were prevented by the ICD.
Conclusion: Patients implanted with an ICD in Townsville had 30.5% all-cause mortality after 4.8-yrs. Only 28% of patients received ICD therapy and 13.9% were inappropriate. OSA may have contributed to the fourfold increase in inappropriate therapy in NICM patients. Our study raises important efficacy, ethical and healthcare cost questions about who should receive an ICD, and possible regional and urban center disparities.
Introduction
Heart failure (HF) represents a global healthcare pandemic that affects over 26 million people worldwide including 5.8 million in the United States, 10 million in Europe, up to 9 million in South East Asia, and around 300,000 in Australia (1–4). Despite major advances in patient management and drug therapies, treating patients with severe HF remains challenging. The problem is formidable given that 30–50% of this group will experience a cardiac arrest (5), which has an out-of-hospital survival rate of <10% (3, 5). To address this problem, 40 years ago Mirowski and colleagues were among the first to introduce implantable cardiac defibrillators (ICDs) into cardiac arrest patients as a preventive measure to reduce further attacks (6), which has now become a global standard practice in developed countries (7, 8).
Today, ICDs are also recommended for primary prevention patients with ischemic cardiomyopathy (ICM), usually from a previous myocardial infarction, and patients with non-ischemic cardiomyopathy (NICM) of a dilated or non-dilated origin with a left ventricular ejection fraction (LVEF) ≤35% (3, 5, 9, 10). ICM is the most common type of dilated cardiomyopathy where the left ventricle has been enlarged, dilated, and weakened usually from ischemia associated with coronary artery disease and myocardial infarction. NICM is defined as disease of the myocardium associated with mechanical or electrical dysfunction from ventricular hypertrophy or dilatation with a strong genetic component (3, 10, 11).
Notwithstanding the small clinical risks of device insertion (12), the most common problems of the ICD include lead failure, premature battery life, failure of algorithms to discriminate ventricular from supraventricular arrhythmias, device activation when shocks are not required, and patient anxiety issues (e.g., anticipatory “phantom shocks”) (8, 13). Despite the potential of ICDs to save lives, there is increasing controversy about who should receive a device, and their clinical efficacy (8, 14). Differences in patient outcomes appear to reflect the complexity of multiple phenotypes of heart failure with different comorbidities, the selection criteria based on ejection fraction classification, and lack of consensus guidelines for patient selection (4, 8, 13, 15). The aim of this study was to define risk factors, arrhythmia incidence, and mortality in primary prevention heart failure patients in the Townsville district in northern Australia. Secondary objectives were to investigate differences between ICM and NICM patients, and to compare findings with published data from larger metropolitan centers. This is, to the best of our knowledge, the first such analysis of ICD patients in regional Australia.
Materials and Methods
Ethical Considerations
This study was conducted in full conformance with the principles of the National Statement on Ethical Conduct in Human Research (Updated 2018). The study was approved by the Townsville Hospital and Health Service (THHS) Human Research Ethics Committee (HREC) (HREC/18/QTHS/57) and James Cook University HREC (H7438).
Study Population
The study population consisted of 82 patients within the THHS District who had a primary prevention ICD implanted at Townsville University Hospital (TUH) between January 1st, 2008 and December 31st, 2015. The study population included new implants and generator changes, which in a few cases required a device history prior to 2008. Primary prevention ICD generator changes were included as this reflects normal clinical practice.
Primary prevention patients who had an ICM or NICM indication were included for analysis, while all secondary prevention ICD patients were excluded. Angiography was used to exclude coronary artery disease as a cause of cardiomyopathy in NICM patients. All patients had coronary angiography with the exception of five patients who had a genetic or familial indication for ICD, e.g., long QT syndrome, non-compaction, sarcoidosis. In order to reduce the heterogeneity of non-ischemic group we identified and separated channelopathy patients with preserved ejection fraction. Exclusion criteria included incomplete chart information or ICD follow-up data. Patients were identified as indicated by implant report or clinic letter by the implanting Cardiac Electrophysiologist. Primary prevention indications were those with a history of coronary artery disease (CAD) or myocardial infarction, or the absence of CAD with an LVEF ≤35% on maximal medical therapy and New York Heart Association (NYHA) functional class II-III, or NYHA class 1 and an LVEF ≤30%. There was no prior history of sustained ventricular arrhythmias subsequent to ICD implantation in this patient cohort.
Data Collection and Analyses
Data were retrospectively collected from TUH's medical records and Cardiobase (version 8.1) from which clinic letters and ICD follow-up information was obtained (Public Health Act Approval: RD007511). Demographic information, comorbidities, ejection fraction (EF), and medications prior to ICD implantation were extracted and recorded. Obesity status was indicated by clinic letter or with a body mass index (BMI) ≥30. New implant device follow-up information, including complications and all-cause mortality, was collected from time of implant up until December 31st, 2018. For patients who received an ICD generator change, follow-up details were included from the previous devices to obtain a complete ICD history.
Device Settings
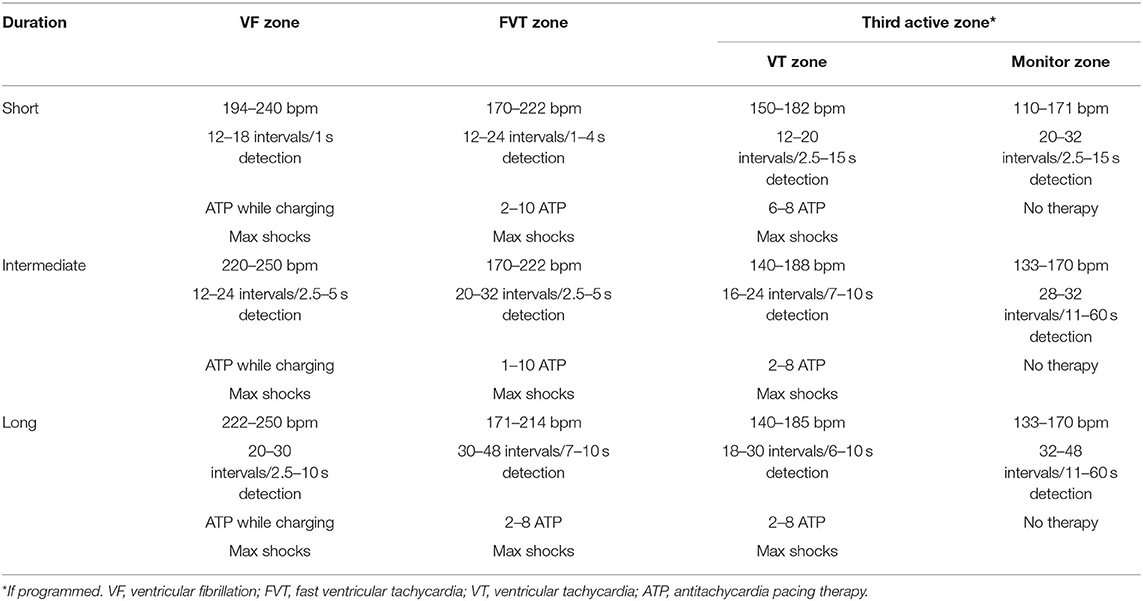
All devices were programmed at the discretion of the implanting cardiac electrophysiologist and had atrial arrhythmia algorithms turned on. Subcutaneous ICDs had a shock zone from 220–240 bpm and a conditional shock zone from 180–200 bpm with full output shocks programmed. Devices from Boston Scientific, Abbott, and Biotronik predominantly had a two-zone active therapy configuration with a monitor zone, whereas Medtronic devices mostly had three active therapy zones and a monitor zone programmed. Examples of short, intermediate, and long duration detection endocardial device settings can be found in Table 1.
Interpretation of ICD Therapy
Therapy was deemed “appropriate” for ventricular arrhythmias or “inappropriate” for supraventricular arrhythmias or device and/or lead malfunction. The terms “therapy” in this study refer to anti-tachycardia pacing (ATP) and shocks, and “shocks” refer to defibrillation from the ICD. Interpretation of therapy occurred in one of two ways: (1) From electrocardiograms in the clinical letters, which were interpreted by a certified cardiac device specialist (CCDS) and checked by a consultant medical specialist (KN), or (2) from individual device intracardiac electrograms of patients that were not found in the clinical letters, and interpreted by the same investigators (CCDS and KN). Single chamber devices were interpreted using onset and stability as well as electrogram morphology match score. When this was not optioned on the device the high voltage electrogram (far field) was analyzed for changes during onset and offset to identify changes to distinguish supraventricular tachycardia from ventricular tachycardia. For dual chamber devices ventricular tachycardia was identified by ventricular electrogram rate greater than atrial electrogram rate (V>A). When tachycardia was 1:1, the chamber of tachycardia onset was used together with onset (gradual or sudden) and stability (regular or irregular). Electrogram morphology was also used as a discriminator. As for single chamber devices, if unavailable the high voltage electrogram was assessed to differentiate supraventricular from ventricular tachycardia.
Sudden Cardiac Death Criteria
Sudden cardiac death was defined as spontaneously occurring ventricular tachycardia or ventricular fibrillation >240 beats per minute based on published work (16). If ventricular tachycardia was caused by anti-tachycardia pacing leading to an acceleration of heart rate >240 it was excluded from our criteria of sudden cardiac death. ICD intracardiac electrograms were assessed to identify patients who met this criterion in our study. This criterion relates to arrhythmic death only and does not account for all cases of sudden death that may also have occurred from non-arrhythmogenic pump failure because of the severely reduced LVEF found in this patient population (16).
Statistical Analysis
Statistical analysis was conducted using IBM SPSS version 24. Descriptive analysis is reported as frequencies, mean (standard deviation) or median (interquartile range). Variables were compared within the total population, and between ICM and NICM patients with Chi-squared test for categorical data and analysis of variance (ANOVA) with Levene's test of homogeneity of variance for continuous variables. One patient had a mixed indication for ICD and was not included in ICM vs NICM analysis. Kaplan–Meier test was used for survival analysis, with log-rank test for comparison between ICM and NICM patients. Cox proportional hazard analysis was performed to determine predictors of mortality, and binary logistic regression was used to assess associations between patient variables and appropriate ICD therapy. Multivariate regression analysis was conducted for parameters showing statistical significance in univariate tests. A p < 0.05 was considered statistically significant.
Results
Clinical Characteristics
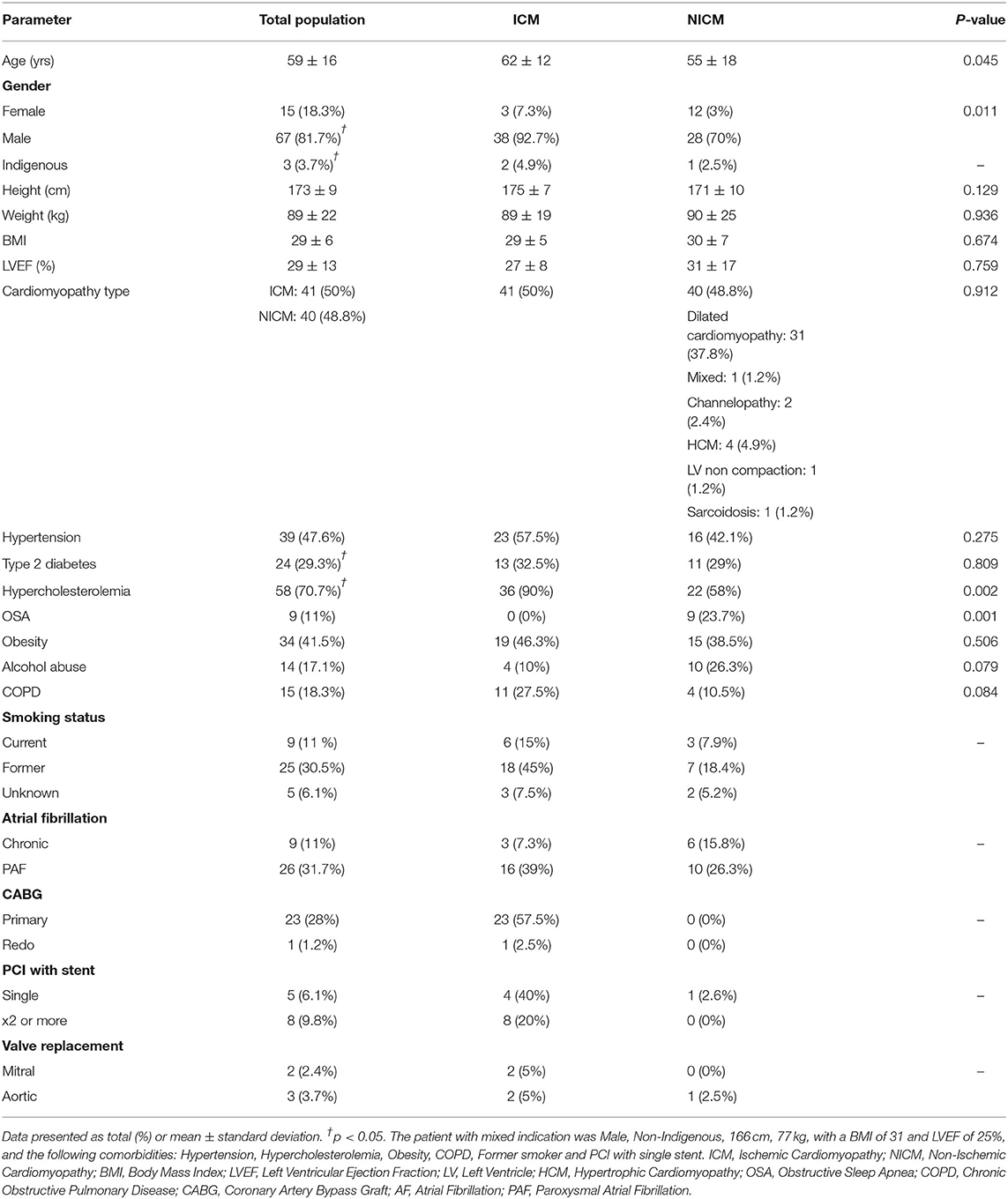
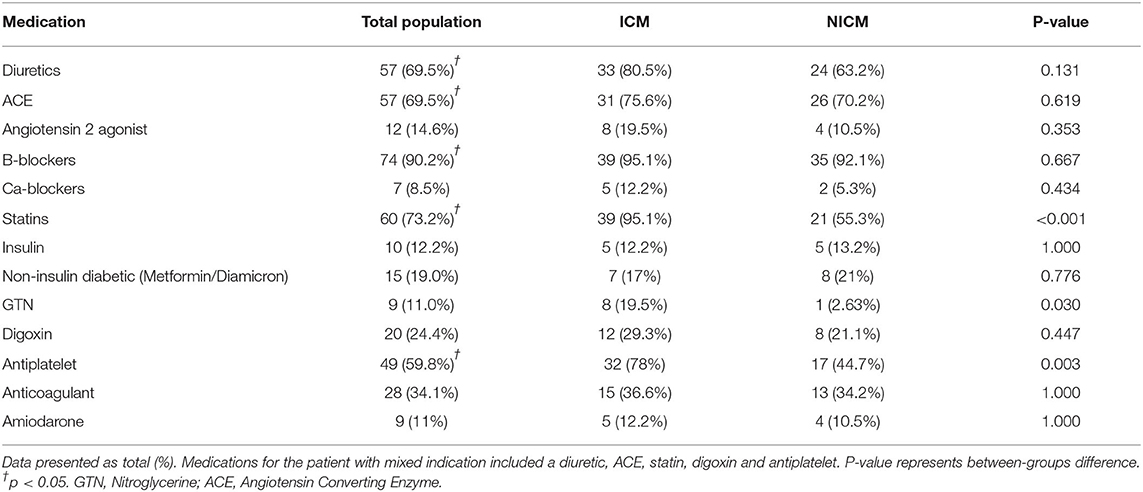
The mean age of patients was 59 ± 16 years although the NICM patient cohort was significantly younger (55 ± 18 years, p=0.045). A significantly higher proportion of males received ICDs in this district (81.7%; p=0.011). There was an even distribution of ICM and NICM patients (50 vs. 48.8%, p = 0.912; Table 2). Only three patients were identified as Indigenous. Risk factors in our cohort included hypercholesterolemia (70.7%, p < 0.05), hypertension (47.6%, p < 0.05), obesity (41.5%), and Type 2 diabetes (29.3%) (Table 2). ICM patients had higher hypercholesterolemia (90%; p = 0.002), higher smoking history, coronary artery bypass grafting, valve surgery, and percutaneous coronary intervention (Table 2). Severe obstructive sleep apnea was found exclusively in the NICM group (23.7%, p = 0.001; Table 2). Most patients received beta blockade medical therapy (90.2%) with more ICM patients receiving statins (95.1%, p < 0.001) and antiplatelet (78%, p = 0.003) medications (Table 3). Diuretics and ACE inhibitors were commonly prescribed, with 34.1% of all patients receiving an anticoagulant. Nitroglycerine was more commonly prescribed in patients with coronary artery disease (19.5%, p = 0.030) compared to those with a NICM (Table 3).
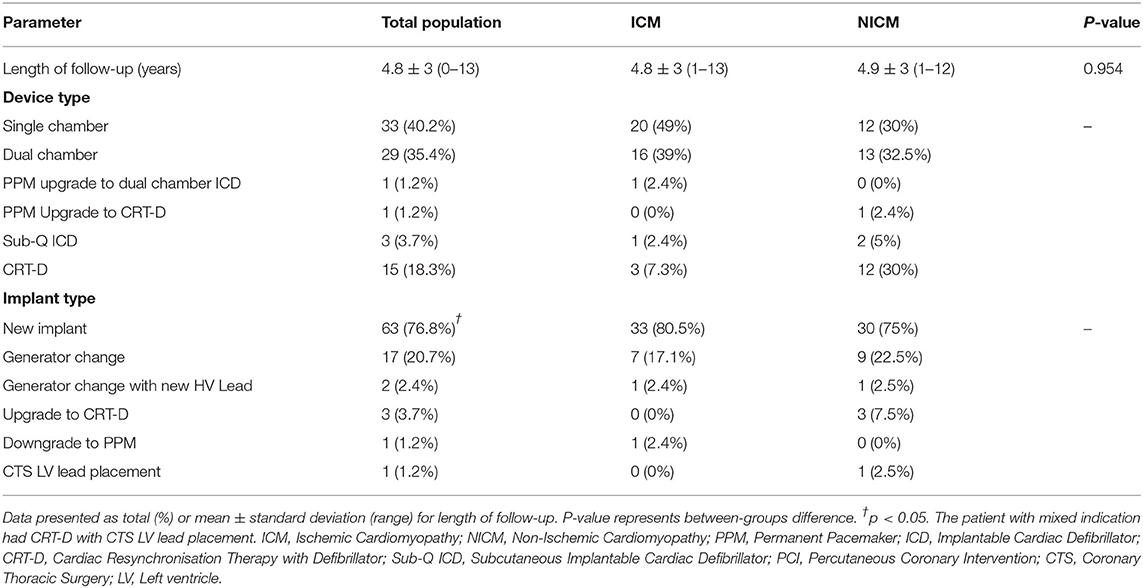
The mean follow-up time following ICD implantation was 4.8 ± 3 years (range: 0–13 years). New implants were received by 76.8% of patients and 20.7% had a generator change (Table 4). ICDs included single chamber devices (40.2%) and dual (35.4%). Cardiac resynchronisation therapy with defibrillation (CRT-D) was fourfold higher in the NICM than ICM patients (12 vs. 3) (Table 4).
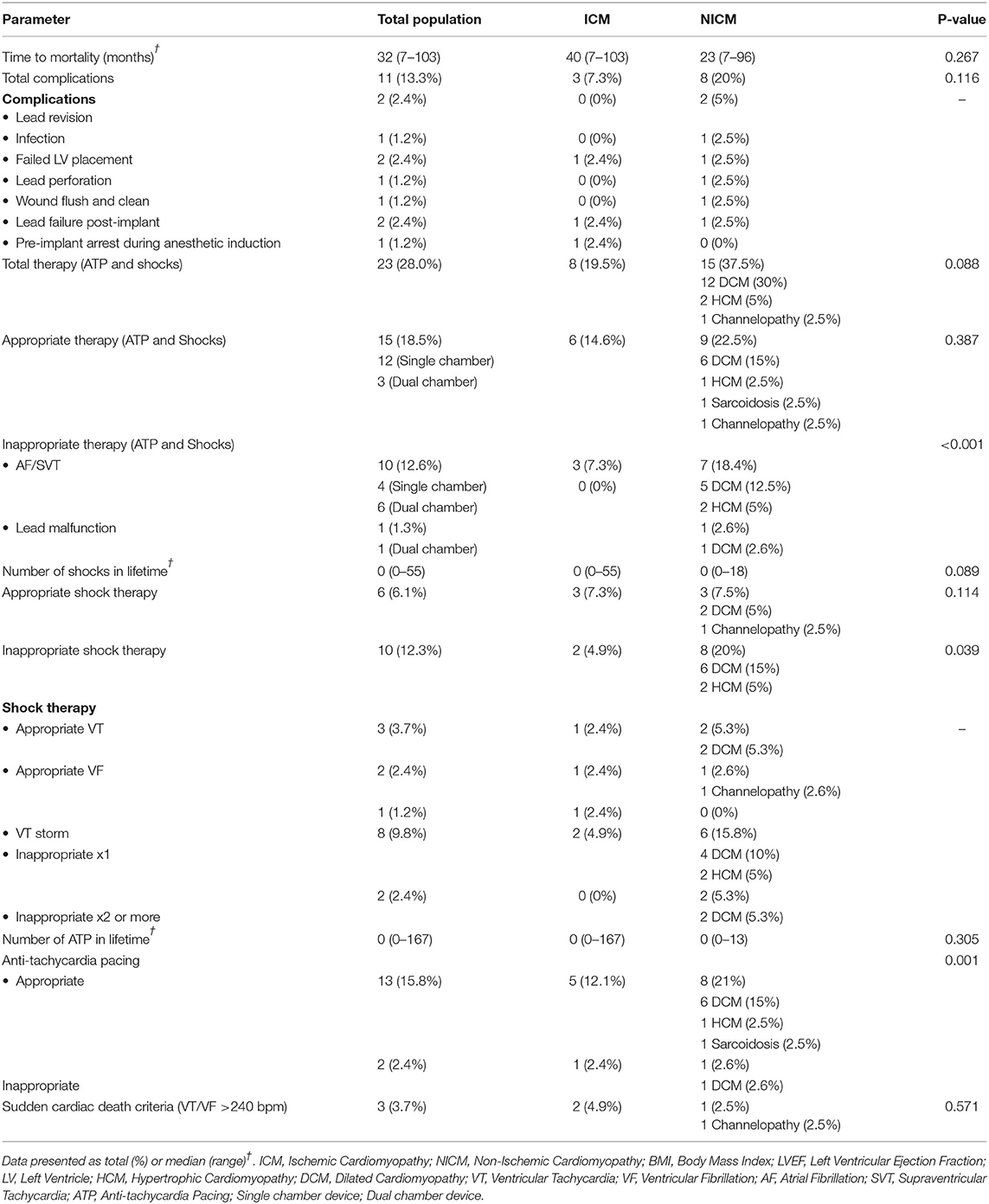
Complications occurred in 13% of total patients (8 were ICM and 3 NICM patients, p = 0.116; Table 5). Two patients had lead revision, failed LV placement, and lead failure post-implant, and one patient had an infection, lead perforation, wound flush-and-clean and pre-implant arrest during the anesthetic induction. Twenty-five patients (30.5%) died during the follow-up period, with a mean survival time of 36.7 months (range: 26.4–47.1 months) (Figure 1). The ICM group had a 10-month longer survival time compared to the NICM group (41.2 vs. 31.0 months) but this was not significant [Log Rank (Mantel Cox) χ2 = 1.393, df = 1, p = 0.238; Figure 2].
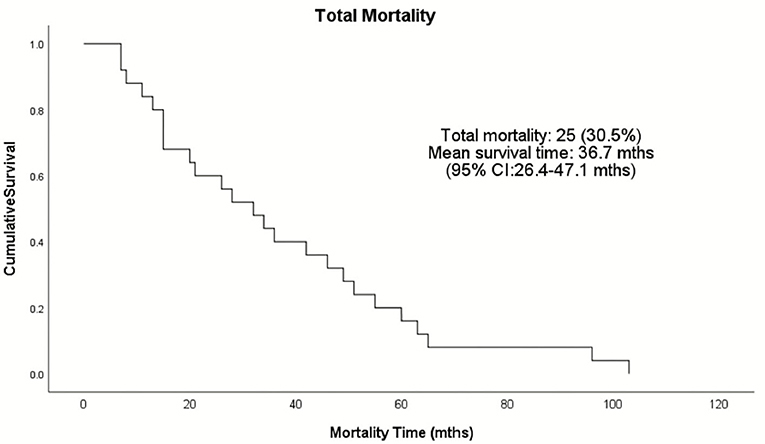
Figure 1. Kaplan–Meier survival analysis of the total patient population receiving ICDs in the Townsville District from January 2008–December 2015. Twenty-five patients died during the follow-up period as represented by the events on the Kaplan-Meier cumulative survival curve.
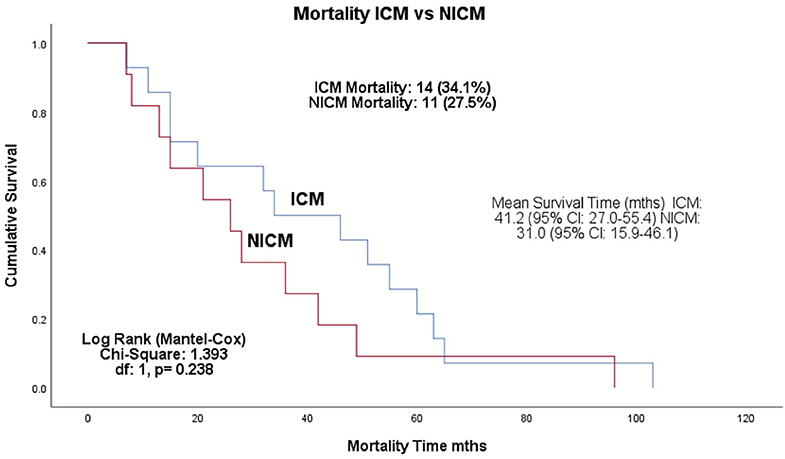
Figure 2. Kaplan–Meier survival analysis of the ICM and NICM patient cohorts in the Townsville District over 4.8-years follow-up from January 2008–December 2015. Log rank test shows no significant difference in survival between ICM and NICM patients (p = 0.238). ICM, ischemic cardiomyopathy; NICM, non-ischemic cardiomyopathy.
Risk Factors for Mortality and ICD Events
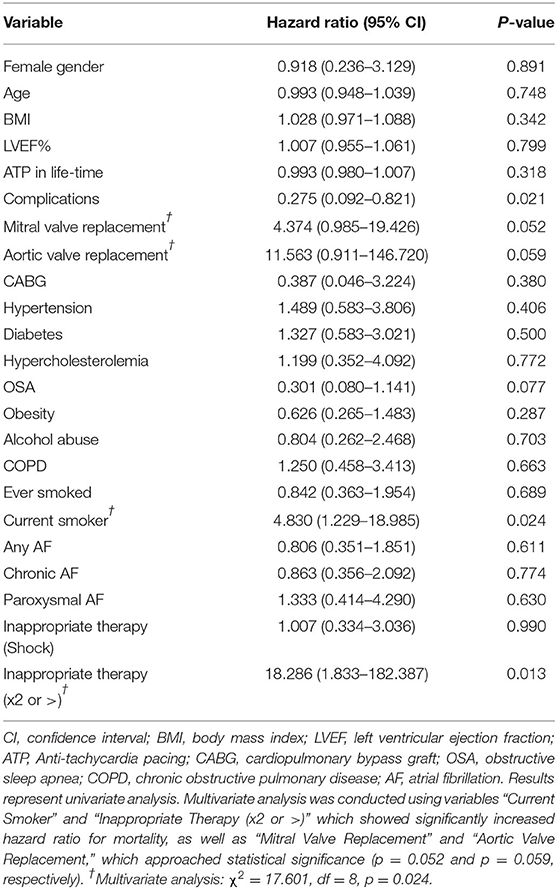
Age, gender, BMI, and ejection fraction were not significant predictors of mortality in this patient population (Table 6). Smokers were at a significantly higher risk of dying than non-smokers (HR: 4.830, 95% CI: 1.229–18.985, p = 0.024; Table 6). Aortic valve replacement carried a ~12-fold increased risk of death (p = 0.052) and mitral valve replacement a 4-fold risk (p = 0.059; Table 6). Patients receiving two or more incidences of inappropriate shocks were 18-fold more likely to die (HR: 18.286, 95% CI: 1.833–182.387, p = 0.013; Table 6). Interestingly, patients who experienced complications had a 73% reduced risk of mortality (HR: 0.275, 95% CI: 0.092–0.821, p = 0.021). Patients with obstructive sleep apnea had a 70% reduced risk (Table 6). Multivariate analysis of smoking history, inappropriate shocks, and valve replacement surgery showed a significant increase in mortality risk (χ2 = 17.601, df = 8, p = 0.024; Table 6).
Twenty-eight percent (n = 23) of all patients received therapy, either pacing or defibrillation (Table 5). Almost 40% of NICM patients received therapy compared to ~20% of ICM patients (p = 0.088). Of all patients who received therapy only 18.51% were appropriate for sustained ventricular arrhythmias. While 22.5% of NICM patients received appropriate therapy, 21.05% also received inappropriate therapy, which was significantly higher than in ICM patients (p = <0.001). Sudden cardiac death would have occurred in three patients (3.7%), two ICM and one NICM patient, if ICD defibrillation had not occurred (Table 5).
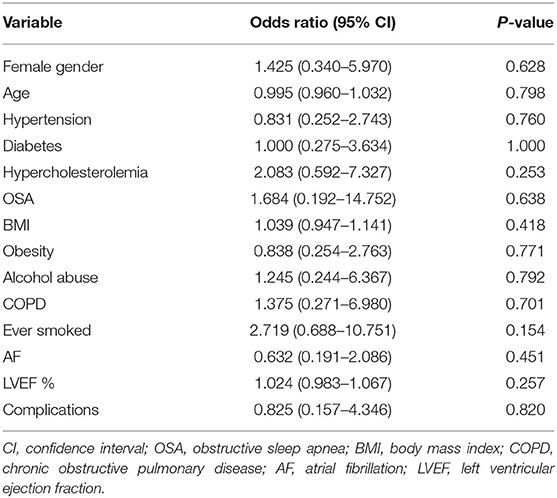
There were no significant associations between patient demographics or risk factors and ventricular arrhythmias (Table 7). However, if patients had a previous smoking history, they were 2.7 times more likely to receive an appropriate shock compared with patients who have never smoked (OR: 2.719, 95% CI: 0.688–10.75, p = 0.154). Patients with hypercholesterolemia also showed a two-fold increased likelihood of receiving therapy for ventricular arrhythmias when compared to patients with normal cholesterol levels (OR: 2.083, 95% CI: 0.592–7.327, p = 0.252; Table 7).
Discussion
The management of ventricular arrhythmias in heart failure patients remains challenging despite significant advances in ICD innovation, clinical research and cardiovascular-modifying drug therapies (7). We report that ICD patients serviced by the Townsville University Hospital monitored over a 4.8-year period had 30.5% all-cause mortality. We also report that 82% (67 patients) experienced no sustained ventricular arrhythmias, yet 28% (23 patients) received therapy. Eighteen per cent of device therapy were appropriate and 14% were inappropriate. No associations were found between ventricular arrhythmias and ejection fraction, hypercholesterolemia, hypertension or obesity. In NICM patients, sleep apnea was associated with a fourfold increase in inappropriate shocks compared to ICM patients. Interestingly, the overall risk of mortality was 73% lower in patients who had complications, which may be due to increased follow-up and medical care in this subset. Three patients would have almost certainly died without an ICD. These data raise a number of important questions about the utility and effectiveness of ICDs and the current guidelines and selection criteria on who should receive a device.
Mortality Was Higher in Regional North Queensland
All-cause mortality of 30.5% in the Townsville district was 1.2 to 2 times higher than mortality reported in other cohort studies in Australia, New Zealand, North America and Europe (15, 17–20). Although we found no significant difference in mortality between the ICM and NICM patients, the total mortality in ICM patients was 1.4 to 2.4 times those in the MADIT, MADIT II, and MUST trials (21, 22), and 1.3 to 2.8 times higher in the NICM patients compared to the CAT, AMIOVIRT, DEFINITE, and recent DANISH trials (23–26).
Currently, we do not know the reasons for the higher mortality but it may relate to differences in patient inclusion and exclusion criteria. For example, in contrast to the CAT, DEFINITE and DANISH trials, we did not exclude patients with valvular disease, those on anti-arrhythmics, or permanent atrial fibrillation patients, respectively (23, 25, 26). Increased mortality in our patient cohort may also be related to heart failure progression in our cohort or the type and severity of comorbidities (27). Cardiovascular disease, for example, is responsible for 30% more hospitalizations in outer regional and rural centers than urban centers. Age did not appear to be a factor (Table 2), which was different from many other published studies (15, 17, 18, 20). The possible role of regional, rural and urban health inequities to higher mortality requires further investigation.
Only 19% of Patients Received Appropriate ICD Therapy
Of the 82 patients implanted with an ICD only 15 received appropriate pacing/shock therapy. On face value 18% appears to be a low success rate. However, in our study 67 patients did not experience ventricular arrhythmias over the duration of 4.8 years, which needs to be taken into consideration when determining a therapy's success rate. An appropriate therapy rate of 18% sits at the lower end of the published range of 11.6 to 24% over 2.1–3.64 years follow-up (11, 15, 19, 20, 28–31). In a large randomized control trial of 2,521 patients Bardy showed a 21% appropriate shock rate in the ICD arm (32, 33). However, many of these studies don't report the percentage of patients who did not have sustained ventricular arrhythmias. A unique and distinguishing feature of our study was its analysis of intracardiac electrograms allowing such determinations.
Inappropriate ICD Therapy Was Associated With Poor Outcomes
We also found in our study that eleven patients (14%) received inappropriate therapy. This subset included 10 patients who had not experienced ventricular arrhythmias and one patient who had an arrhythmia. From our electrocardiogram analysis, inappropriate device therapy was also delivered in response to supraventricular tachycardias (Table 5). The inability of the device to distinguish between ventricular and atrial arrhythmias appears to be related to ICD programming and algorithms of rate parameters and detection times (34). This is an important area of future ICD development for primary prevention of sudden cardiac death because, in our study, those patients who received two or more inappropriate shocks were 18 times more likely to die than those who received only one event or no inappropriate therapy (Table 6). In a larger study, Van Rees similarly showed that inappropriate shock therapy was related to a 1.6-fold increase risk of mortality (35).
NICM Patients had Significantly Higher Inappropriate Shocks
Another standout finding from the above analysis was the higher rate of inappropriate shock therapy delivered to NICM patients (20%) compared to ICM patients (4.9%; p = 0.039). This suggests that our NICM patients were predisposed to higher incidences of supraventricular or atrial arrhythmias. This group also had significantly higher incidence of obstructive sleep apnea (OSA) (23.7%) than ICM patients (0%) (p = 0.001). This is interesting because sleep apnea is strongly associated with atrial fibrillation (36), and was a predictor of inappropriate shocks in the study of Fernandez-Cisnal (30). Kreuz et al. also reported that ICD patients with sleep disorders had two times the inappropriate therapy of those without sleep apnea (37). Unfortunately, we did not examine the association between atrial fibrillation and sleep apnea throughout the 4.8-year follow-up. Why primary prevention NICM patients are more vulnerable to inappropriate shocks than ICM patients requires further investigation (26, 38). In addition, there is an urgent need to clarify heart failure guidelines for this vulnerability in NICM patients (4, 39).
Risk/Benefit and Cost Considerations of ICD Implantation
Considering that 82% of total patients did not have sustained ventricular arrhythmias over the 4.8-year period, our study, although small, raises ethical questions about the pros and cons of having a device implanted, and validity of current ICD selection criteria. Notwithstanding, the life-saving potential of having a device, the clinical criteria for deciding who needs a primary prevention ICD is imprecise (8, 14, 40). Notwithstanding these important issues, three patients would have likely died without their ICD (Table 5). Healthcare costs are another consideration. The average procedural cost for ICD implantation in Australia is ~$22,000 (without complications), and $47,000 (with complications) (3, 41). In the Townsville district, the cost to healthcare providers for the 67 patients who did not need a device (no sustained ventricular arrhythmias) is estimated to be at least $1.5M, and if our data is representative of other centers in Australia, the national savings could be 82% of $155M per year (2011–2014) or $127M per annum (41).
Limitations of the Study
A limitation of the present study was that it was a retrospective, observational analysis on a small population at a single center, regional tertiary hospital, that included both new implants and generator changes. A larger population of primary prevention ICD patients including more hospitals and service areas in Northern Australia, and a comparator from an urban center, would provide more information on the different rates of outcomes and to better evaluate this regional area compared to larger hospitals in metropolitan centers. Another limitation was that there was no analysis of ECGs performed in the patients who received appropriate shocks prior to implant which may have contributed to a more detailed assessment of a patient's risk. This is a topic of future research. Lastly, a detailed cost-effective analysis would provide important data to inform providers to the benefit and health-related quality of life of ICD recipients.
Conclusions
Primary prevention ICD patients implanted at the Townsville University Hospital had high rates of mortality and low rates of sustained ventricular arrhythmias. The incidence of inappropriate ICD therapy was comparable to appropriate therapy, and was associated with increased mortality.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Townsville Hospital and Health Service Human Research Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
NE, GD, KN, and HL contributed equally to literature search, study design, data interpretation, and writing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would particularly like to thank the College of Medicine and Dentistry at James Cook University, Townsville, and the Townsville University Hospital for their support of the study. NE would like to thank the Townsville Hospital and Health Service Human Research Ethics Committee and James Cook University Ethics Committee for providing access to the data that formed the basis for this retrospective study.
References
1. Savarese G, Lund LH. Global public health burden of heart failure. Cardiiac Failure Review. (2017) 3:7–11. doi: 10.15420/cfr.2016:25:2
2. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American college of cardiology/american heart association task force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm. (2018) 15:e190–252. doi: 10.1161/CIR.0000000000000548
3. Atherton JJ, Sindone A, De Pasquale CG, Al E. National heart foundation of Australia and cardiac society of Australia and New Zealand: Australian clinical guidelines for the management of heart failure 2018. Med J Aust. (2018) 209:363–9. doi: 10.5694/mja18.00647
4. Lau DH, Kalman JM, Sanders P. Primary prevention implantable cardioverter defibrillators in non-ischaemic cardiomyopathy: challenging the Australian heart failure guidelines. Med J Aust. (2019) 211:154–5.e1. doi: 10.5694/mja2.50248
5. Packer M. Major reduction in the risk of sudden cardiac death in patients with chronic heart failure with the use of drug and device combinations that favourably affect left ventricular structure. Eur J Heart Fail. (2019) 21:823–6. doi: 10.1002/ejhf.1501
6. Deyell MW, Tung S, Ignaszewski A. The implantable cardioverter-defibrillator: from Mirowski to its current use. BC Med J. (2010) 52:248–53. Available online at: https://bcmj.org/articles/implantable-cardioverter-defibrillator-mirowski-its-current-use>
7. Santangeli P, Rame JE, Birati EY, Marchlinski FE. Management of ventricular arrhythmias in patients with advanced heart failure. J Am Coll Cardiol. (2017) 69:1842–60. doi: 10.1016/j.jacc.2017.01.047
8. Disertori M, Mase M, Rigoni M, Nollo G, Ravelli F. Declining clinical benefit of ICD in heart failure patients: Temporal trend of mortality outcomes from randomized controlled trials. J Cardiol. (2020) 75:148–54. doi: 10.1016/j.jjcc.2019.06.001
9. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european society of cardiology (ESC). endorsed by: association for european paediatric and congenital cardiology (AEPC). Eur Heart J. (2015) 36:2793–867. doi: 10.1093/eurheartj/ehv316
10. Chieng D, Paul V, Denman R. Current device therapies for sudden cardiac death prevention – the ICD, subcutaneous ICD and wearable ICD. Heart Lung Circ. (2019) 28:65–75. doi: 10.1016/j.hlc.2018.09.011
11. Foo FS, Lee M, Looi KL, Larsen P, Clare GC, Heaven D, et al. Implantable cardioverter defibrillator and cardiac resynchronization therapy use in New Zealand. (ANZACS-QI 33). J Arrhythm. (2020) 36:153–63. doi: 10.1002/joa3.12244
12. Wong JA, Devereaux PJ. Cardiac device implantation complications: a gap in the quality of care? Ann Intern Med. (2019) 171:368–9. doi: 10.7326/M19-1895
13. Kleemann T, Strauss M, Kouraki K, Lampropoulou E, Fendt A, Werner N, et al. Contemporary benefit-harm profile over two decades in primary prophylactic ICD-therapy. Clin Cardiol. (2019) 42:866–72. doi: 10.1002/clc.23234
14. Spartalis M, Iliopoulos DC, Spartalis E, Athanasiou A, Paschou SA, Voudris V, et al. Is the clinical benefit of implantable cardioverter-defibrillators in heart failure patients declining? J Cardiol. (2020) 75:583–4. doi: 10.1016/j.jjcc.2020.02.002
15. Boveda S, Narayanan K, Jacob S, Providencia RUI. Temporal trends over a decade of defibrillator therapy for primary prevention in community practice. J cardiovasc Electrophys. (2017) 28:666–73. doi: 10.1111/jce.13198
16. Böcker D, Block M, Isbruch F, Wietholt D, Hammel D, Borggrefe M, et al. Do patients with an implantable defibrillator live longer? J Am Coll Cardiol. (1993) 21:1638–44. doi: 10.1016/0735-1097(93)90380-J
17. Bradshaw PJ, Stobie P, Briffa T, Hobbs MST. Use and long-term outcomes of implantable cardioverter-defibrillators, 1990 to 2009. Am Heart J. (2013) 165:816–22. doi: 10.1016/j.ahj.2013.02.007
18. Fauchier L, Marijon E, Defaye P, Piot O, Sadoul N, Perier M-C, et al. Effect of age on survival and causes of death after primary prevention implantable cardioverter-defibrillator implantation. Am J Cardiol. (2015) 115:1415–22. doi: 10.1016/j.amjcard.2015.02.031
19. Friedman DJ, Fudim M, Overton R, Shaw LK, Patel D, Pokorney SD, et al. The relationship between baseline and follow-up left ventricular ejection fraction with adverse events among primary prevention ICD patients. Am Heart J. (2018) 201:17–24. doi: 10.1016/j.ahj.2018.03.017
20. Looi K-L, Sidhu K, Cooper L, Dawson L, Slipper D, Gavin A, et al. Long-term outcomes of heart failure patients who received primary prevention implantable cardioverter-defibrillator: an observational study. J Arrhythm. (2018) 34:46–54. doi: 10.1002/joa3.12027
21. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. (2002) 346:877–83. doi: 10.1056/NEJMoa013474
22. Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, et al. Clinical research: limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease. lessons from the MUSTT study. J Am College Cardiol. (2007) 50:1150–7. doi: 10.1016/j.jacc.2007.04.095
23. Bänsch D, Antz M, Boczor S, Volkmer M, Tebbenjohanns J, Seidl K, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy. Circulation. (2002) 105:1453. doi: 10.1161/01.CIR.0000012350.99718.AD
24. Strickberger SA, Strickberger SA, Hummel JD, Bartlett TG, Frumin HI. Amiodarone versus implantable cardioverter-defibrillator: randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia - AMIOVIRT. J Am Coll Cardiol. (2003) 41:1707–12. doi: 10.1016/S0735-1097(03)00297-3
25. Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. (2004) 350:2151–8. doi: 10.1056/NEJMoa033088
26. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. (2016) 375:1221–30. doi: 10.1056/NEJMoa1608029
27. Butler J, Yang M, Manzi MA, Hess GP, Patel MJ, Rhodes T, et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol. (2019) 73:935–44. doi: 10.1016/j.jacc.2018.11.049
28. Smith T. Long-term follow-up of prophylactic implantable cardioverter-defibrillator-only therapy: comparison of ischemic and nonischemic heart disease. Clin Cardiol. (2011) 34:761–7. doi: 10.1002/clc.20970
29. Verma A. Predictors of appropriate implantable cardioverter defibrillator. (ICD) therapy in primary prevention patients with ischemic and nonischemic cardiomyopathy. Pacing Clin Electrophysiol. (2011) 33:320–9. doi: 10.1111/j.1540-8159.2009.02566.x
30. Fernández-Cisnal A, Arce-León Á, Arana-Rueda E, Rodríguez-Mañero M, González-Cambeiro C, Moreno-Arribas J, et al. Analyses of inappropriate shocks in a Spanish ICD primary prevention population: predictors and prognoses. Int J Cardiol. (2015) 195:188–94. doi: 10.1016/j.ijcard.2015.05.146
31. Fontenla A, Martinez-Ferrer JB, Alzueta J, Vinolas X, Garcia-Alberola A, Brugada J, et al. Incidence of arrhythmias in a large cohort of patients with current implantable cardioverter-defibrillators in Spain: results from the UMBRELLA registry. Europace. (2016) 18:1726–34. doi: 10.1093/europace/euv393
32. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med. (2005) 352:225–37. doi: 10.1056/NEJMoa043399
33. Bardy G. Long-term follow-up in the sudden cardiac death heart failure trial (SCD-HEFT). Heart Rhythm. (2012) 9:1579–1579. doi: 10.1016/j.hrthm.2012.06.018
34. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. (2016) 13:e50–86. doi: 10.1016/j.hrthm.2015.11.018
35. Van Rees JB, Borleffs CJW, De Bie MK, Stijnen T, Van Erven L, Bax JJ, et al. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. (2011) 57:556–62. doi: 10.1016/j.jacc.2010.06.059
36. D'onofrio A, La Rovere MT, Emdin M, Capucci A, Sinagra G, Bianchi V, et al. Implantable cardioverter-defibrillator-computed respiratory disturbance index accurately identifies severe sleep apnea: the DASAP-HF study. Heart Rhythm. (2018) 15:211–7. doi: 10.1016/j.hrthm.2017.09.038
37. Kreuz J, Skowasch D, Horlbeck F, Atzinger C, Schrickel JW, Lorenzen H, et al. Usefulness of sleep-disordered breathing to predict occurrence of appropriate and inappropriate implantable-cardioverter defibrillator therapy in patients with implantable cardioverter-defibrillator for primary prevention of sudden cardiac death. Am J Cardiol. (2013) 111:1319–23. doi: 10.1016/j.amjcard.2013.01.277
38. Pathak RK, Sanders P, Deo R. Primary prevention implantable cardioverter-defibrillator and opportunities for sudden cardiac death risk assessment in non-ischaemic cardiomyopathy. Eur Heart J. (2018) 39:2859–66. doi: 10.1093/eurheartj/ehy344
39. Munawar DA, Mahajan R, Linz D, Wong GR, Khokhar KB, Thiyagarajah A, et al. Predicted longevity of contemporary cardiac implantable electronic devices: a call for industry-wide “standardized” reporting. Heart Rhythm. (2018) 15:1756–63. doi: 10.1016/j.hrthm.2018.07.029
40. Goldberger JJ, Subacius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. (2014) 63:1879–89. doi: 10.1016/j.jacc.2013.12.021
Keywords: heart failure, implantable cardiac defibrillator, primary prevention, arrhythmia, sudden cardiac death, shocks
Citation: Engstrom N, Dobson GP, Ng K and Letson HL (2020) Primary Prevention Implantable Cardiac Defibrillators: A Townsville District Perspective. Front. Cardiovasc. Med. 7:577248. doi: 10.3389/fcvm.2020.577248
Received: 29 June 2020; Accepted: 14 September 2020;
Published: 27 October 2020.
Edited by:
Shimon Rosenheck, Meir Medical Center, IsraelReviewed by:
Antonio Sorgente, EpiCURA, BelgiumOsmar Antonio Centurion, National University of Asunción, Paraguay
Copyright © 2020 Engstrom, Dobson, Ng and Letson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hayley L. Letson, hayley.letson@jcu.edu.au
 Nathan Engstrom
Nathan Engstrom Geoffrey P. Dobson
Geoffrey P. Dobson Kevin Ng
Kevin Ng Hayley L. Letson
Hayley L. Letson