The Safety and Efficacy of Inspiratory Muscle Training for Patients With Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention: Study Protocol for a Randomized Controlled Trial
- 1Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2Department of Biostatistics, School of Public Health, Southern Medical University, Guangzhou, China
Background: Uncommonly high rates of pneumonia in patients with acute myocardial infarction (AMI) undergoing primary percutaneous coronary intervention (PCI) have been observed during recent years. Inspiratory muscle training (IMT) could reduce pneumonia in patients undergoing coronary artery bypass grafting and other cardiac surgeries. The relationship between IMT and AMI is unknown. Here, we describe the feasibility and potential benefit of IMT in patients at high risk for pneumonia with AMI who have undergone primary PCI.
Methods: Our study is a prospective, randomized, controlled, single-center clinical trial. A total of 60 participants will be randomized into an IMT group and control group with 30 participants in each group. Participants in the IMT group will undergo training for 15 min per session, twice a day, from 12 to 24 h after primary PCI, until 30 days post-randomization; usual care will be provided for the control group. The primary endpoint is the change in inspiratory muscle strength, the secondary endpoint included feasibility, pneumonia, major adverse cardiovascular events, length of stay, pulmonary function tests measure, and quality of life.
Discussion: Our study is designed to evaluate the feasibility of IMT and its effectiveness in improving inspiratory muscle strength in participants with AMI who have undergone primary PCI.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT04491760.
Introduction
Uncommonly high rates (2–7%) of pneumonia have been reported in patients with acute myocardial infarction (AMI), thus increasing the mortality of such patients (1–4). However, other than antibiotic therapy, there are only a few treatment options against infection risk. Therefore, there is an urgent need for developing novel preventive strategies to decrease the risk of pneumonia in these patients. Maximal inspiratory pressure (MIP) is a simple, non-invasive way to evaluate respiratory muscle strength, and it is widely used clinically (5). Inspiratory muscle training (IMT) could significantly increase MIP and has been considered a feasible method to reduce pneumonia in patients undergoing cardiac surgery including coronary artery bypass grafting (6, 7). However, the effect of IMT on patients with AMI is poorly understood. Matos-Garcia and colleagues showed that respiratory muscle strength was impaired in patients with AMI compared to healthy subjects (8), and that such impairment might also later lead to pneumonia. To the best of our knowledge, no research has been performed to evaluate the value of IMT on patients with AMI. Therefore, we aim to conduct 30 days of IMT in patients with AMI who are at high risk of pneumonia and have undergone primary percutaneous coronary intervention (PCI), to evaluate the effectiveness and feasibility of IMT in this population.
Materials and Methods
Study Design
This proposed protocol involves a single-center, prospective, randomized study. The study is expected for enrollment from December 1st 2020 to April 30th 2021. All of the participants would be followed up until 30 days post-randomization. Our study protocol and patient information documents have been approved by the ethics committee of Guangdong Provincial People's Hospital [No. GDREC2019521H(R1)] and was registered in clinical trials (www.clinicaltrials.gov, NCT04491760). This study will be performed in compliance with the Code of Ethics of the 1964 Declaration of Helsinki and its later amendments. The study would comply with the Consolidated Standards of Reporting Trials Statements (CONSORT) (9), and provided CONSORT checklist when reported the study results.
Study Oversight
A specific data safety committee has been guaranteed for the current research, consisting of two cardiologists and one statistician. The cardiologists will be responsible for the review of the in-hospital course and follow-up adverse events of the participants. Then, a safety analysis will be conducted by the statistician at the time the 20th participant is randomized, and the data will be divided into IMT-associated events or non-IMT-associated events.
Study Population
The inclusion criteria are as follows: (1) age ≥ 18 years; patients with AMI undergoing primary PCI, admitted to the cardiac intensive care unit and at a high risk for pneumonia; and (2) be able to understand and agree with informed consent.
The exclusion criteria are: (1) a history of cerebrovascular accident; (2) have been treated with immunosuppressive medication for 30 days before the study; (3) have a neuromuscular disorder; (4) have cardiovascular instability (such as aortic dissection or unstable hemodynamics); (5) a history of coronary artery bypass grafting (CABG); (6) expected survival is < 6 months due to non-cardiogenic disease; (7) participated in other drugs or devices studies within 30 days; (8) confirmed ventricular aneurysm; and (9) other conditions not suitable for the study after the discussion among the researchers (e.g., poor compliance and unable to cooperate for the training).
After reviewing the results of previous studies and the clinical risk factors for pneumonia (1, 2, 7, 10), the following parameters will be used for pneumonia risk calculation: (1) age > 55 years; (2) history of diabetes mellitus, (3) present smoker; (4) chronic kidney disease [estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2)]; and (5) forced expiratory volume in the first second of expiration (FEV1) <80% predicted and FEV1/forced vital capacity (FVC) <70% predicted. Having two or more of these parameters is regarded as being high risk for pneumonia.
Randomization
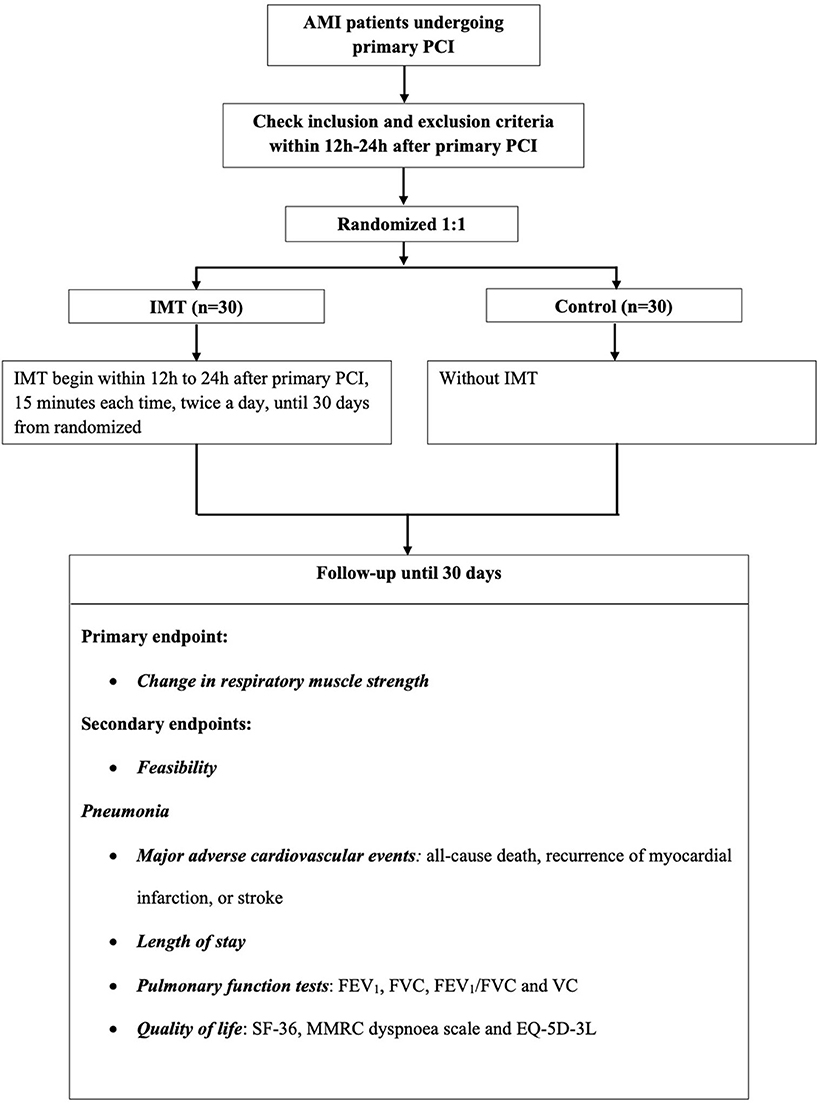
A specific computer-generated, randomized number will be placed in a sealed envelope. Researchers will be blinded to the information in envelopes and will select consecutive envelopes to distribute to consecutive participants, thus randomly assigning them to either the IMT group (n = 30) or the control group (n = 30). See study flow Figure 1.
Invention: IMT
For the IMT group, IMT will be performed for 15 min per session, twice a day, with an initial load of 30% of MIP using a threshold inspiratory muscle trainer (c-type, Shengchang medical equipment factory, Yuyao City, China). During the hospital period, the resistance will increase incrementally, based on the rate of perceived exertion scored on the Borg scale (11). If the rate of perceived exertion is <5, the resistance of the inspiratory threshold trainer will then be increase incrementally by 5%. To facilitate training for participants, the load for home-based IMT training will be set at the highest training load achieved while the patient was in hospital. All of the participants will be required to complete maneuvers in the seated position during training, wearing nose-clips. IMT will be performed every day from 12 to 24 h after primary PCI and the final session will be performed 30 days post-randomization. Participants in the control group will receive standard care after primary PCI according to the current clinical practice guidelines (12), which included risk stratification, continuous monitoring and specific nursing. And we also encourage an early ambulation, based on the complications, symptoms, and capacity.
Baseline Characteristics and Medicine
Electrocardiogram will be performed before primary PCI, and repeated testing will also be required. Transthoracic echocardiography will be performed within 24 h after primary PCI, mainly for early identification of cardiac rupture or ventricular aneurysm. X-ray and/or pulmonary computed tomography will be performed to diagnose pneumonia, and repetitive testing should be considered if necessary. A loading dose of antiplatelet agents will be given before coronary angiography and all of the catheter techniques will be performed according to guidelines (13). During the intervention procedure, intra-aortic balloon pump or other invasive equipment will be used if necessary. For the in-hospital stay, medications, such as antiplatelet agents, angiotensin-converting enzyme inhibitors, and β-blockers, will be prescribed by the cardiologists as needed.
Follow-Up
Participants will be followed by two experienced researchers every week through phone interviews after discharge. Details of IMT will be documented, quality assessment will be performed, and any reported adverse events will be followed up, with assistance provided if necessary. All of the participants will be asked to return to the clinic for follow-up visits at 30 days post-randomization.
Objectives
The aim is to assess the effectiveness of IMT by measures including the improvement of inspiratory muscle strength, pulmonary function, and quality of life, and by the avoidance of clinically adverse events. We will also assess the feasibility of IMT by examining the safety of training performance and through use of a satisfaction questionnaire.
Study Endpoints
Primary Endpoint
Change in Inspiratory Muscle Strength
Static MIP will be selected for the estimation of inspiratory muscle strength. A standard measurement according to the ERS Statement on Respiratory Muscle Testing at Rest and during Exercise published by the European Respiratory Society (ERS) will be taken (14) (Powerbreathe® K5). The MIP test will be performed at baseline and at 30 days post-randomization.
Secondary Endpoints
Feasibility
Adverse events, heart rate, systolic blood pressure, diastolic blood pressure, and oxyhemoglobin saturation of patients during training while in hospital will be recorded. In addition, the workloads of MIP and the Borg scores will be recorded after every day of training in the hospital. The satisfaction of patients in the IMT group will be evaluated using a questionnaire at discharge and again at 30 days post-randomization (Supplementary Appendix 1).
Pneumonia
Pneumonia will be defined as the presence of a new or progressive radiographic infiltrate plus at least two of three clinical features (fever > 38°C, white blood cell count [WBC] > 10 × 109/L or < 4 × 109/L and purulent secretions), based on American Thoracic Society guidelines (15). Events of pneumonia will be followed and recorded by experienced doctors when participants are in hospital. After discharge, participants will be followed by experienced researchers and if a diagnosis of pneumonia is considered possible, guidance will be provided for the patients to complete necessary examinations, until 30 days post-randomization.
Major Adverse Cardiovascular Events (MACE)
MACE will be defined as all-cause death, recurrence of myocardial infarction, or stroke at 30 days post-randomization.
Length of Stay (LOS)
Length of stay will be assessed by calculating the number of days the patient is in the hospital.
Pulmonary Function Tests
Pulmonary function tests will be assessed by the following spirometry parameters: forced expiratory volume in the first second of expiration (FEV1), forced vital capacity (FVC), FEV1/FVC and vital capacity (VC), and reported as a percentage of the predicted value. A spirometer (microQuark, COSMED) will be used for these measurements, which will be performed at baseline and at 30 days post-randomization.
Quality of Life
Questionnaires including the 36-item short-form (SF-36) (16), Modified Medical Research Council (MMRC) dyspnoea scale (17) and EQ-5D-3L (18) will be used for the assessment of quality of life in our participants. Both interviews will be performed twice, first at baseline and again at 30 days post-randomization.
Laboratory Testing
Routine clinical parameters, including laboratory tests such as electrolytes, troponin I/T, creatine kinase-MB (CK-MB), blood lipid, serum creatinine, and other clinical routine parameters examination will be performed within 12 h after admission. The eGFR will be assessed with the four-variable modification of diet in renal disease equation for Chinese patients (19). An additional 4 mL venous blood sample will be collected, and the isolated serum will be stored at −80°C for a future mechanism study.
Assessment and Reporting of Adverse Events
Adverse events including all-cause death, recurrence of myocardial infarction, stroke, stent thrombosis, angina, dyspnea, acute heart failure, arrhythmia, and other unforeseeable incidents will be recorded in detail during both the in-hospital and follow-up periods. The reporting and documentation of adverse events will be classified as IMT-related or non-IMT-related. This safety analysis will be conducted at the end of our research among patients who had at least 1 day of outpatient safety data.
Statistical Analysis
Sample Size Calculation
Our sample size calculation was based on a previous study (20). The mean of the MIP difference between the experimental group and the control group was estimated to be 15 cm H2O, the standard deviation was 12 cm H2O, the significance level was α = 0.05 and the test power was 1-β = 0.8. The resulting required sample size for each group was 11. Considering our projected 20% attrition rate, the sample size of each group should be increased to 14. To better study the secondary endpoints, a total of 60 cases will be included in the final analysis.
Data Analysis
Continuous data will be presented as mean ± standard deviation (SD) and we will use the Student's t-test or analysis of variance for comparisons between groups when the variables are normally distributed. Otherwise, we will present data as median and interquartile range and will use Wilcoxon rank-sum test for comparisons. Categorical data will be presented as percentages and χ2 test or Fisher's exact test will be selected for data comparison. For the study endpoints, pneumonia and MACE will be compared between the IMT group and the control group with the odds ratio (OR). Other endpoints will be compared between groups as appropriate. The SAS version 9.4 (SAS Institute, Cary, NC) will be used to perform our statistical analyses. P values will be 2-sided and P < 0.05 will be regarded as significant.
Discussion
To the best of our knowledge, the proposed protocol is the first study to assess the safety and efficacy of IMT in patients with AMI undergoing primary PCI who are at a high risk of pneumonia during the acute period. Studies have rarely focused on the uncommonly high rate of pneumonia after AMI, so the potential benefits of IMT for these patients may have been neglected. We will focus on the improvement of inspiratory muscle strength after 30 days of training. In addition, we will record the clinical adverse events and the satisfaction of these patients and try to determine whether IMT has a potential benefit for decreasing incidence of pneumonia, MACE and length of stay (LOS), and also determine if IMT could benefit pulmonary function and quality of life.
For the consideration of pulmonary function test safety, the major evidence comes from one study, which reported the incidence of fatal cardiac events as 0.03%, and the incidence of other non-fatal events during testing as 1.49% and that symptom-limited protocols could increase the rate of major cardiac complications by 1.9 times when compared to submaximal tests (21). The incident risk was low, and the data were reported as a range; thus, the number of tests performed within 7 days after AMI was unknown. The previous guidelines suggested pulmonary function tests should not be performed within 30 days after AMI, whereas the new ERS guidelines regard AMI as a relative contraindication for pulmonary function testing (14, 22). Some scholars claimed that tests at 7 days after AMI deem to be safe (23). However, all of these suggestions were less evidence-based and further study should be performed. Although both focused inspiration and expiration could affect intrathoracic pressure, transmural pressure, and cardiac hemodynamics, whether they could result in clinical adverse events is unknown (24, 25). Two studies published in recent years have added to the safety data for use of pulmonary function tests in patients with AMI (8, 26) and the overall rates of mechanical complications are relatively lower in the primary PCI era (27), whereby fatal mechanical complications, such as ventricular rupture and left ventricular pseudoaneurysm, might require emergency surgery (28). In the present study, early transthoracic echocardiography will be performed to identify these patients. Adverse events, heart rate, systolic blood pressure, diastolic blood pressure, and oxyhemoglobin saturation of patients will be supervised. IMT has been demonstrated to be feasible and safe in patients who were undergoing CABG (7, 29–31), lung surgery (32), and those suffering from subacute stroke (33). By considering the above and rare IMT-related incidents that have been reported and excluding patients who might at high risk for adverse events due to pulmonary function testing, we believe that IMT for this cohort of patients with AMI might be relatively safe.
Previous studies have shown that IMT exceeding 2 weeks of therapy was effective for improving MIP (34), while the efficiency of implementing a shorter training time (< 10 days) still needs further investigation (29, 30, 35). As heart failure is common in patients with AMI, chronic heart failure would play an important role in the study cohort. The present recommendation for patients with heart failure participating in an IMT regimen is to begin with 30% MIP, then gradually increase to 60% MIP, 20–30 min per session with 3–5 exercise sessions per week (36). Some large-sample randomized trials have also confirmed the efficiency of these settings (31) and served as the basis of our IMT protocol design.
There are some limitations in our study. First, the present design is a single-center study, and the sample size is relatively small. Second, we selected guideline-based criteria for the diagnosis of pneumonia because no specific criteria have been developed for patients with AMI (15), and the criteria of the Centers for Disease Control and Prevention is not defined. AMI is associated with inflammatory reaction (37), and such a state might reduce the diagnostic power of most common clinical biomarkers. Although a new or progressive radiographic infiltrate and the presence of purulent secretions is the common diagnostic and prognostic indicator of pneumonia (31, 38–40), the significance of fever and white cell count may be limited by the inflammatory stage, which might lead to slightly overall excessive diagnoses (41–43). Third, the important aim of our study is to explore the role of IMT in preventing pneumonia. However, since no high-quality IMT study has been conducted in patients with AMI, we decided to calculate the sample size for our study using inspiratory muscle strength, and later extended this to better explore the effect of IMT on the secondary endpoints, especially pneumonia. Fourth, the model for calculating the risk of pneumonia was based on previous work in patients with ST-elevation myocardial infarction, without validation of its accuracy in our selected patient population (1, 2). Because there are not risk model for pneumonia in patients with AMI currently.
In conclusion, we have considered the significance of this proposed study protocol that was designed to determine the feasibility and potential clinical benefits of IMT in patients with AMI undergoing primary PCI who are at high risk of pneumonia, using a randomized, controlled trial.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Guangdong Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
NT and YL contributed to the conception and design. YD, ZL, HZ, MZ, XC, and SZ searched the associated data. YD and YL drafted the manuscript. GZ, LX, CD, and JC provided the supervision support. LG, PH, and NT provided administrative support and performed data analysis. All authors contributed to the critical revisions and final approval of the manuscript.
Funding
This study was supported by a grant from the National Key R&D Program of China (2016YFC1301100), Science and Technology Special Funding of Guangdong Provincial people's Hospital (No. 2017bq03), the Science and Technology Planning Project of Guangzhou City (Grant No. 201906010089), Shuangqing Talent Program Project of Guangdong Provincial People's Hospital (No. KJ012019095), and the first clinical research training course seed fund of Guangdong Provincial People's Hospital (No. 2018lcpx04). The funders had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript. The work was not funded by any industry sponsors. All authors agreed to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.598054/full#supplementary-material
References
1. Piccaro de Oliveira P, Gonzales V, Lopes RD, Schmidt MM, Garofallo S, Santos RP, et al. Serious infections among unselected patients with ST-elevation myocardial infarction treated with contemporary primary percutaneous coronary intervention. Am Heart J. (2016) 181:52–9. doi: 10.1016/j.ahj.2016.08.005
2. Truffa AA, Granger CB, White KR, Newby LK, Mehta RH, Hochman JS, et al. Serious infection after acute myocardial infarction: incidence, clinical features, and outcomes. JACC Cardiovascular Intervent. (2012) 5:769–76. doi: 10.1016/j.jcin.2012.03.018
3. Nash MC, Strom JA, Pathak EB. Prevalence of major infections and adverse outcomes among hospitalized. ST-elevation myocardial infarction patients in Florida, 2006. BMC Cardiovascular Disord. (2011) 11:69. doi: 10.1186/1471-2261-11-69
4. Putot A, Chague F, Manckoundia P, Cottin Y, Zeller M. Post-infectious myocardial infarction: new insights for improved screening. J Clin Med. (2019) 8:827. doi: 10.3390/jcm8060827
5. ATS/ERS Statement on respiratory muscle testing. Am J Respirat Crit Care Med. (2002) 166:518–624. doi: 10.1164/rccm.166.4.518
6. Gomes Neto M, Martinez BP, Reis HF, Carvalho VO. Pre- and postoperative inspiratory muscle training in patients undergoing cardiac surgery: systematic review and meta-analysis. Clin Rehabil. (2017) 31:454–64. doi: 10.1177/0269215516648754
7. Hulzebos EH, Helders PJ, Favié NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. (2006) 296:1851–7. doi: 10.1001/jama.296.15.1851
8. Matos-Garcia BC, Rocco IS, Maiorano LD, Peixoto TCA, Moreira RSL, Carvalho ACC. A home-based walking program improves respiratory endurance in patients with acute myocardial infarction: a randomized controlled trial. Can J Cardiol. (2017) 33:785–91. doi: 10.1016/j.cjca.2016.12.004
9. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. (2010) 1:100–7. doi: 10.4103/0976-500X.72352
10. Liu Y, Dai Y, Chen J, Huang C, Duan C, Shao S, et al. Predictive value of the Canada acute coronary syndrome risk score for post-acute myocardial infarction infection. Eur J Int Med. (2020) 71:57–61. doi: 10.1016/j.ejim.2019.10.012
11. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. (1982) 14:377–81. doi: 10.1249/00005768-198205000-00012
12. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77.
13. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the american college of cardiology foundation/american heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. (2011) 124:e574–651. doi: 10.1161/CIR.0b013e31823a5596
14. Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respirat J. (2019) 53:1801214. doi: 10.1183/13993003.01214-2018
15. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respirat Crit Care Med. (2005) 171:388–416. doi: 10.1164/rccm.200405-644ST
16. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. (1992) 30:473–83. doi: 10.1097/00005650-199206000-00002
17. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. (1988) 93:580–6. doi: 10.1378/chest.93.3.580
18. McCartney PJ, Eteiba H, Maznyczka AM, McEntegart M, Greenwood JP, Muir DF, et al. Effect of low-dose intracoronary alteplase during primary percutaneous coronary intervention on microvascular obstruction in patients with acute myocardial infarction: a randomized clinical trial. JAMA. (2019) 321:56–68. doi: 10.1001/jama.2018.19802
19. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. (2006) 17:2937–44. doi: 10.1681/ASN.2006040368
20. Marco E, Ramirez-Sarmiento AL, Coloma A, Sartor M, Comin-Colet J, Vila J, et al. High-intensity vs. sham inspiratory muscle training in patients with chronic heart failure: a prospective randomized trial. Eur J Heart Failure. (2013) 15:892–901. doi: 10.1093/eurjhf/hft035
21. Hamm LF, Crow RS, Stull GA, Hannan P. Safety and characteristics of exercise testing early after acute myocardial infarction. Am J Cardiol. (1989) 63:1193–7. doi: 10.1016/0002-9149(89)90177-X
22. Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respirat J. (2005) 26:153–61. doi: 10.1183/09031936.05.00034505
23. Cooper BG. An update on contraindications for lung function testing. Thorax. (2011) 66:714–23. doi: 10.1136/thx.2010.139881
24. Buda AJ, Pinsky MR, Ingels NB, Daughters GT, Stinson EB, Alderman EL. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med. (1979) 301:453–9. doi: 10.1056/NEJM197908303010901
25. Denault AY, Gorcsan J, Pinsky MR. Dynamic effects of positive-pressure ventilation on canine left ventricular pressure-volume relations. J Appl Physiol. (2001) 91:298–308. doi: 10.1152/jappl.2001.91.1.298
26. Altree T, Bussell L, Nguyen P, Johnston S. Adverse cardiac outcomes after pulmonary function testing with recent myocardial infarction. Respirat Med. (2019) 155:49–50. doi: 10.1016/j.rmed.2019.07.003
27. Jones BM, Kapadia SR, Smedira NG, Robich M, Tuzcu EM, Menon V, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J. (2014) 35:2060–8. doi: 10.1093/eurheartj/ehu248
28. Durko AP, Budde RPJ, Geleijnse ML, Kappetein AP. Recognition, assessment and management of the mechanical complications of acute myocardial infarction. Heart. (2018) 104:1216–23. doi: 10.1136/heartjnl-2017-311473
29. Barros GF, Santos Cda S, Granado FB, Costa PT, Limaco RP, Gardenghi G. Respiratory muscle training in patients submitted to coronary arterial bypass graft. Revista Brasileira de Cirurgia Cardiovascular. (2010) 25:483–90.
30. Matheus GB, Dragosavac D, Trevisan P, Costa CE, Lopes MM, Ribeiro GC. Inspiratory muscle training improves tidal volume and vital capacity after CABG surgery. Revista brasileira de Cirurgia Cardiovascular. (2012) 27:362–9. doi: 10.5935/1678-9741.20120063
31. Hulzebos EH, van Meeteren NL, van den Buijs BJ, de Bie RA, Brutel de la Riviere A, Helders PJ. Feasibility of preoperative inspiratory muscle training in patients undergoing coronary artery bypass surgery with a high risk of postoperative pulmonary complications: a randomized controlled pilot study. Clin Rehabil. (2006) 20:949–59. doi: 10.1177/0269215506070691
32. Rosero ID, Ramirez-Velez R, Lucia A, Martinez-Velilla N, Santos-Lozano A, Valenzuela PL, et al. Systematic review and meta-analysis of randomized, controlled trials on preoperative physical exercise interventions in patients with non-small-cell lung cancer. Cancers. (2019) 11:944. doi: 10.3390/cancers11070944
33. Messaggi-Sartor M, Guillen-Sola A, Depolo M, Duarte E, Rodriguez DA, Barrera MC, et al. Inspiratory and expiratory muscle training in subacute stroke: a randomized clinical trial. Neurology. (2015) 85:564–72. doi: 10.1212/WNL.0000000000001827
34. Kendall F, Oliveira J, Peleteiro B, Pinho P, Bastos PT. Inspiratory muscle training is effective to reduce postoperative pulmonary complications and length of hospital stay: a systematic review and meta-analysis. Disabil Rehabil. (2018) 40:864–82. doi: 10.1080/09638288.2016.1277396
35. Chen X, Hou L, Zhang Y, Liu X, Shao B, Yuan B, et al. The effects of five days of intensive preoperative inspiratory muscle training on postoperative complications and outcome in patients having cardiac surgery: a randomized controlled trial. Clin Rehabil. (2019) 33:913–22. doi: 10.1177/0269215519828212
36. Bjarnason-Wehrens B, Predel HG. Inspiratory muscle training - an inspiration for more effective cardiac rehabilitation in heart failure patients? Eur J Prev Cardiol. (2018) 25:1687–90. doi: 10.1177/2047487318798917
37. Libby P, Pasterkamp G, Crea F, Jang IK. Reassessing the mechanisms of acute coronary syndromes. Circ Res. (2019) 124:150–60. doi: 10.1161/CIRCRESAHA.118.311098
38. Huang J, Lai Y, Zhou X, Li S, Su J, Yang M, et al. Short-term high-intensity rehabilitation in radically treated lung cancer: a three-armed randomized controlled trial. J Thoracic Dis. (2017) 9:1919–29. doi: 10.21037/jtd.2017.06.15
39. Lai Y, Huang J, Yang M, Su J, Liu J, Che G. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. J Surg Res. (2017) 209:30–6. doi: 10.1016/j.jss.2016.09.033
40. Chumillas S, Ponce JL, Delgado F, Viciano V, Mateu M. Prevention of postoperative pulmonary complications through respiratory rehabilitation: a controlled clinical study. Arch Phys Med Rehabil. (1998) 79:5–9. doi: 10.1016/S0003-9993(98)90198-8
41. Cho HO, Nam CW, Lee HM, Shin HW, Cho YK, Yoon HJ, et al. Fever after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction is associated with adverse outcomes. Int J Cardiol. (2014) 170:376–80. doi: 10.1016/j.ijcard.2013.11.017
42. Kawashima C, Matsuzawa Y, Akiyama E, Konishi M, Suzuki H, Hashiba K, et al. Prolonged fever after ST-segment elevation myocardial infarction and long-term cardiac outcomes. J Am Heart Associat. (2017) 6:e005463. doi: 10.1161/JAHA.116.005463
Keywords: pneumonia, inspiratory muscle training, acute myocardial infarction, percutaneous coronary intervention, intervention
Citation: Liu Y, Dai Y, Liu Z, Zhan H, Zhu M, Chen X, Zhang S, Zhang G, Xue L, Duan C, Chen J, Guo L, He P and Tan N (2021) The Safety and Efficacy of Inspiratory Muscle Training for Patients With Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention: Study Protocol for a Randomized Controlled Trial. Front. Cardiovasc. Med. 7:598054. doi: 10.3389/fcvm.2020.598054
Received: 23 August 2020; Accepted: 03 December 2020;
Published: 12 January 2021.
Edited by:
Yoshihiro Fukumoto, Kurume University, JapanReviewed by:
Erik Hulzebos, University Medical Center Utrecht, NetherlandsGuilherme Augusto De Freitas Fregonezi, Federal University of Rio Grande, Brazil
Copyright © 2021 Liu, Dai, Liu, Zhan, Zhu, Chen, Zhang, Zhang, Xue, Duan, Chen, Guo, He and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Tan, gdtanning@126.com; Lan Guo, GdLanGuo@126.com; PengCheng He, gdhpc100@126.com
†These authors have contributed equally to this work
 YuanHui Liu
YuanHui Liu YiNing Dai1†
YiNing Dai1†  ChongYang Duan
ChongYang Duan JiYan Chen
JiYan Chen PengCheng He
PengCheng He Ning Tan
Ning Tan