Early Aortic Valve Replacement vs. Conservative Management in Asymptomatic Severe Aortic Stenosis Patients With Preserved Ejection Fraction: A Meta-Analysis
- 1Department of Cardiology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Jiaxing Key Laboratory of Cardiac Rehabilitation, Jiaxing, China
Background: Aortic stenosis (AS) is the most common valvular disease in developed countries. Until now, the specific timing of intervention for asymptomatic patients with severe aortic stenosis and preserved ejection fraction remains controversial.
Methods: A systematic search of four databases (Pubmed, Web of science, Cochrane library, Embase) was conducted. Studies of asymptomatic patients with severe AS or very severe AS and preserved left ventricular ejection fraction underwent early aortic valve replacement (AVR) or conservative care were included. The end points included all-cause mortality, cardiac mortality, and non-cardiac mortality.
Results: Four eligible studies were identified with a total of 1,249 participants. Compared to conservative management, patients who underwent early AVR were associated with lower all-cause mortality, cardiac mortality, and non-cardiac mortality rate (OR 0.16, 95% CI 0.09–0.31, P < 0.00001; OR 0.12, 95% CI 0.02–0.62, P = 0.01; OR 0.36, 95% CI 0.21–0.63, P = 0.0003, respectively).
Conclusions: Early AVR is preferable for asymptomatic severe AS patients with preserved ejection fraction.
Introduction
Aortic stenosis (AS) is the most common valvular disease in developed countries, which affects 5% of population >65 years and 3% of population over 75 (1–3). Degenerative process is the major etiology of AS, and ultimately leads to the valve remodeling and systemic blood flow restriction (4). The onset of symptoms (angina, dyspnea on exertion, and syncope) heralds a poor prognosis (5). For symptomatic AS patients, the annual mortality is close to 25% and the average survival time is only 2–3 years (6). Therefore, aortic valve replacement (AVR), either surgical or interventional, is strongly recommended, which is the current only feasible treatment for symptomatic AS (7, 8).
However, ~50% of patients with severe AS are asymptomatic at the time of diagnosis (3, 9). The mortality in asymptomatic patients without surgery ranges widely across the studies and the cumulative 5-year all-cause death incidences is up to 62% (10–13). However, the potential benefit of AVR for asymptomatic patients with severe AS may not outweigh the operative complications (14). It had been reported that operative mortality of isolated AVR for AS was 1–3% in patients <70 years old and 3–8% in senior patients (14). According to the current guidelines, AVR is recommended for asymptomatic AS patients with reduced left ventricular ejection fraction (LVEF) (7).
On the other hand, treatment for asymptomatic severe AS with preserved LVEF is still debatable. The recent guidelines suggest a “watching waiting” strategy for the remaining majority of asymptomatic patients (7). This recommendation was based on non-randomized studies (12, 15) with low evidence levels (16). Several recent studies reported that early AVR for severe asymptomatic AS was associated lower mortality and hospitalization for heart failure at 5-year of follow-up with improved long-term outcomes (13). To facilitate clinical decision-making, we conducted this meta-analysis to compare the outcomes of early AVR and conservative strategy in asymptomatic AS patients with preserved LVEF.
Patients and Methods
The review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement standards.
Searching Strategy
We searched four databases: Pubmed, Web of Science, Embase, and Cochrane library. The last search was performed on July 29, 2020. We used search terms as follows: “asymptomatic” and “aortic stenosis”. There were no language and year of publication constraints in literature search. The detailed searching strategy is provided in the online Supplementary File.
Eligibility Criteria and Study Selection
We included both randomized controlled trials (RCTs) and non-randomized studies if they met the following criteria: (1) patients with severe AS or very severe AS [severe AS was defined as an aortic valve area ≤1 cm2 or peak aortic velocity ≥4 m/s or mean transaortic pressure gradient ≥40 mmHg; very severe AS was defined as an aortic-valve area of ≤0.75 cm2 with either an aortic jet velocity of ≥4.5 m/s or a mean transaortic gradient of ≥50 mmHg]; (2) asymptomatic (without symptoms like exertional angina pectoris, syncope, exertional dyspnea, etc.); (3) LVEF ≥50% or preserved LVEF (LVEF was assessed by transthoracic echocardiography); and (4) included the outcomes of patients underwent early AVR procedure and patients received conservative care (early AVR procedure was defined as intervention before the onset of symptoms or declined LVEF). We excluded abstracts, reviews, case reports, meeting abstracts, and editorial material. Eligibility screening was conducted with a two-step strategy (title/abstract screening and full-text screening). Two independent reviewers screened all potentially eligible studies.
Data Extraction and Risk of Bias in Included Studies
Two independent authors extracted the relevant data. The extracted data included: (1) time, region and design; (2) study period; (3) follow-up period; (4) number of patients; (5) mean age and gender ratio of participants; and (6) LVEF, aortic valve area.
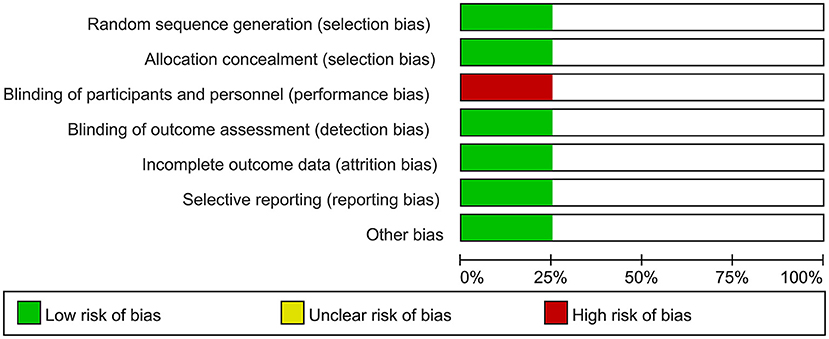
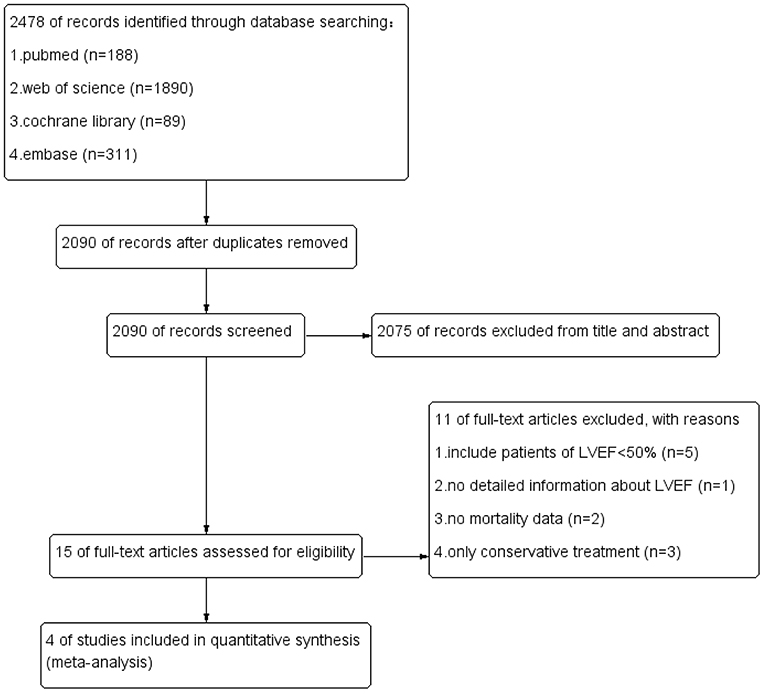
Two independent reviewers assessed the risk of bias among included RCT trials by Cochrane Risk of Bias tool. The risk of bias in included non-RCT studies was assessed by the Newcastle Ottawa scale. No disagreements arose in the quality assessment. Quality assessment of all included studies indicated low risk of bias. The overall risk of bias was graded as low risk. The results are enclosed as Table 1, Figure 1. Publication bias was assessed using funnel plots (Figure 2), which showed no evidence of publication bias.
Data Items
We sought data according to the following PICOS: P (Population), patients with asymptomatic severe asymptomatic AS (LVEF ≥50%); I (Intervention), early AVR; C (Comparison), conservative strategy; O (Outcome), all-cause mortality, cardiac mortality, and non-cardiac mortality; and S (Study type), RCT, and observational studies.
End Points
The primary end points were all-cause mortality and cardiac mortality. Secondary end point was non-cardiac mortality. This meta-analysis compared the prognosis of asymptomatic AS patients with preserved EF underwent early AVR procedure with those who received conservative care strategy.
Statistical Analysis
We used odd ratios (ORs), hazard ratio (HR), and 95% confidence intervals (95% CIs) to serve as primary index statistics for dichotomous outcomes. OR, HR, and 95% CI were calculated for each end point using a random effects or fixed model effects according to I2 value (if I2 value of <50%, we used fixed model effects). Subjects underwent early AVR and conservative care were defined as AVR group and conservative group, respectively. An OR/HR <1 favors early AVR and an OR/HR > 1 supports conservative management. Statistical heterogeneity was assessed by I2 statistic. I2 value of <50% indicated no obvious heterogeneity and I2 value of more than 50% indicated obvious heterogeneity. P values <0.05 were considered statistically significant and all values were two-sided. The adjusted HR was extracted if available from observational studies. If the HR was not described in a study, it was calculated from a Kaplan–Meier curve. Survival data was extracted using the Engauge Digitizer 10.8. Statistical analysis was performed using the Review Manager 5.3 (Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) and survival analysis was performed with the IBM SPSS Statistics 26.
Results
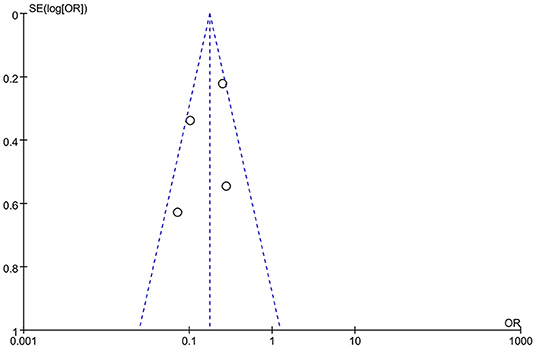
Identification of Studies and Quality Assessment and Baseline Characteristics of Included Studies
Our literature search yielded 2,478 studies. We acquired a total of 2,090 publications after the removal of duplicates. Fifteen articles were included for eligibility evaluation after careful screening of titles and abstracts. Eventually, four studies were included in this meta-analysis. The flow chart of literature search was shown in Figure 3. One eligible study was RCT, while the remaining three studies were prospective or retrospective cohort studies (Table 2). A total of 1,249 subjects with severe AS or very severe AS were included. Patients with asymptomatic severe and severe AS was assigned to the early AVR group (n = 588) or conservative strategy group (n = 661). The baseline characteristics of the four include articles were enclosed in Table 3. To assess the impact of patient selection on the pooled effect estimate, we performed a subgroup analysis, which exclusively included patients with very severe AS.
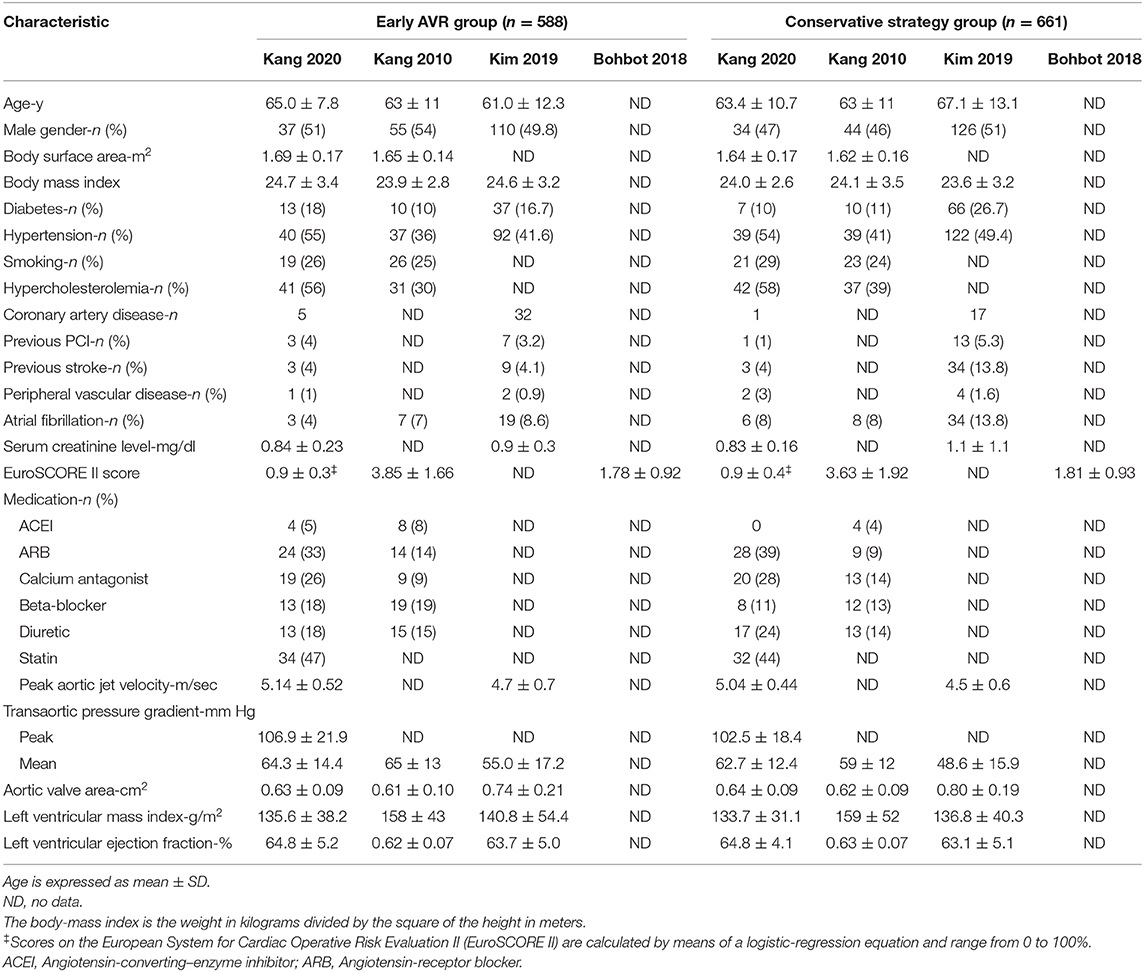
All-Cause Mortality
Four studies (1, 12, 17, 18) (1,249 patients: early AVR group = 588; conservative strategy group = 661) included data of all-cause mortality. Early AVR was associated with significantly lower mortality compared to conservative care (OR 0.16, 95% CI 0.09–0.31, P < 0.00001, I2 = 62%; HR 0.34, 95% CI 0.18–0.64, P = 0.0008, I2 = 62%) (Figures 4A,C). Two studies only included very severe asymptomatic AS patients. There was also a remarkable reduction in all-cause mortality among early AVR subjects (OR 0.15, 95% CI 0.04–0.56, P = 0.005, I2 = 63%) (Figure 4B).
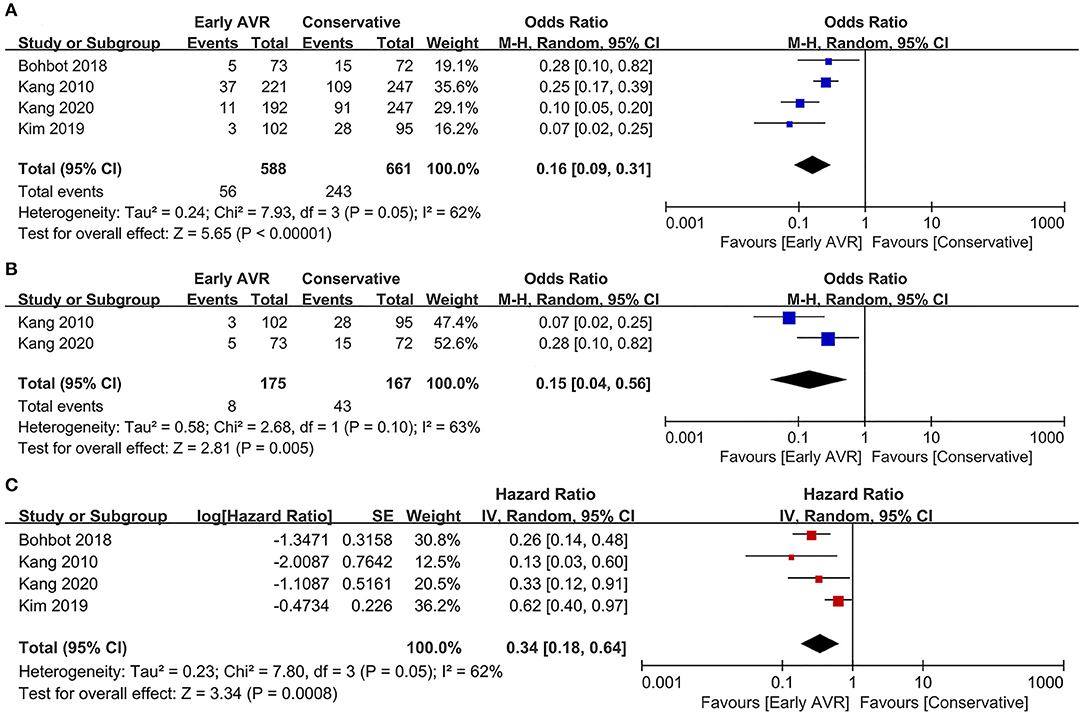
Figure 4. Assessment of the effect of early AVR on all-cause mortality. (A,C) Forrest plot of studies assessing the effect of early AVR and conservative strategy on all-cause mortality. (B) Forrest plot of studies assessing the effect of early AVR and conservative strategy in subjects with very severe AS on all-cause mortality.
Cardiac Mortality
Three studies (1, 17, 18) (810 patients: early AVR group = 396; conservative strategy group = 414) included data of cardiac mortality. Compared to conservative care group, early AVR was associated with significantly reduced cardiac mortality (OR 0.12, 95% CI 0.02–0.62, P = 0.01, I2 = 64%; HR 0.25, 95% CI 0.09–0.68, P = 0.007, I2 = 66%) (Figures 5A,C). For patients with very severe asymptomatic AS, there was also a significant reduction of cardiac mortality in early AVR group (OR 0.04, 95% CI 0.01–0.21, P = 0.0001, I2 = 0%) (Figure 5B).
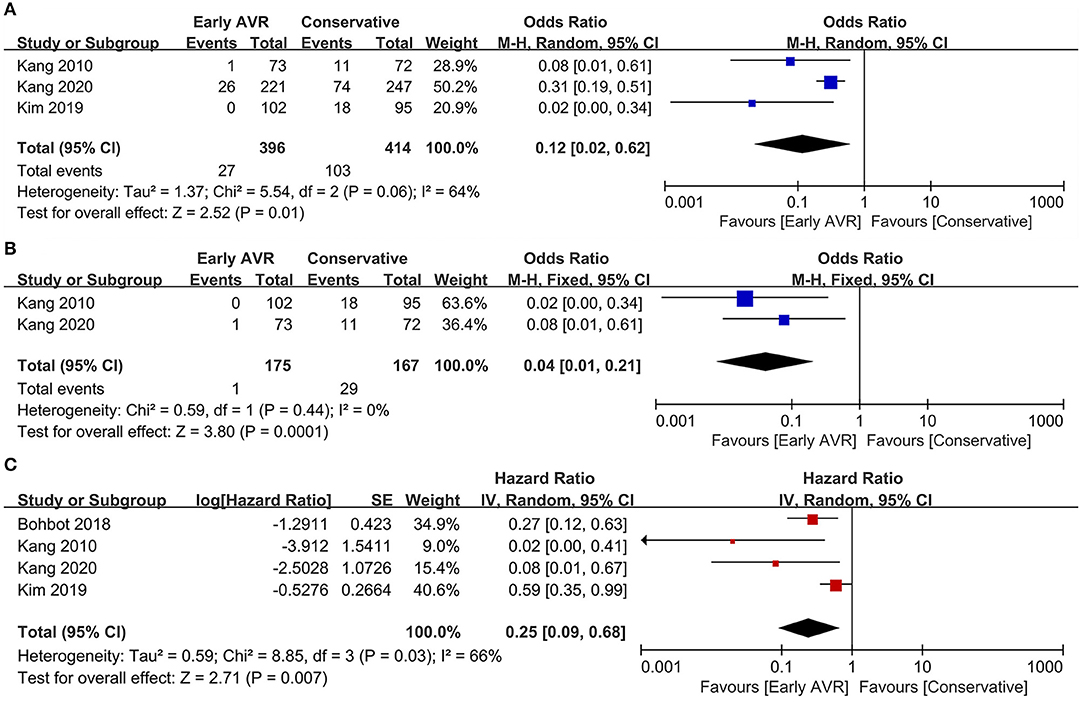
Figure 5. Assessment of the effect of early AVR on cardiac mortality. (A,C) Forrest plot of studies assessing the effect of early AVR and conservative strategy on cardiac mortality. (B) Forrest plot of studies assessing the effect of early AVR and conservative strategy in subjects with very severe AS on cardiac mortality.
Non-cardiac Mortality
Three studies (1, 17, 18) (810 patients: early AVR group = 396; conservative strategy group = 414) reported data of non-cardiac mortality. Compared to conservative care, early AVR significantly declined the non-cardiac mortality (OR 0.36, 95% CI 0.21–0.63, P = 0.0003, I2 = 13%) (Figure 6A). For patients with very severe asymptomatic AS, early AVR yielded no significant benefits over conservative care in non-cardiac mortality (OR 0.46, 95% CI 0.18–1.16, P = 0.10, I2 = 46%) (Figure 6B).
Figure 6. Assessment of the effect of early AVR on non-cardiac mortality. (A) Forrest plot of studies assessing the effect of early AVR and conservative strategy on non-cardiac mortality. (B) Forrest plot of studies assessing the effect of early AVR and conservative strategy in subjects with very severe AS on non-cardiac mortality.
Survival Analysis
The cumulative overall survival rate, calculated with life table analysis, was 91% at 5 years in the early AVR group compared with 68% in the conservative-care group (P < 0.001) (Figure 7A). The 5 year survival rates (survival free of cardiac death) were 96 and 80% in the early AVR group and conservative-care group, respectively (P < 0.001) (Figure 7B).
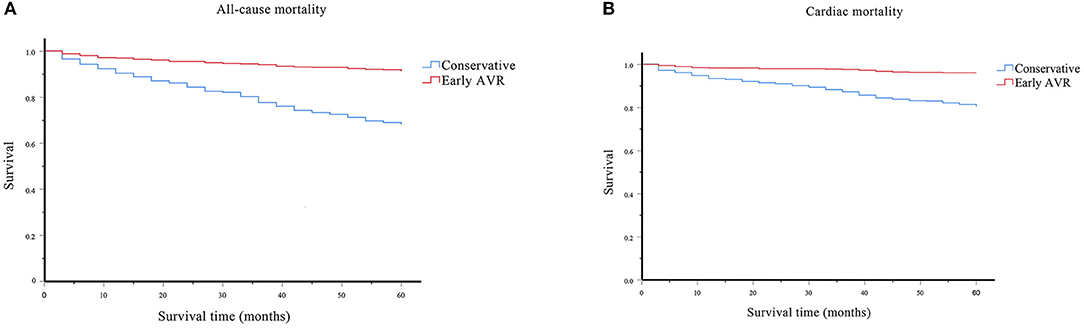
Figure 7. Survival assessment of the effect of early AVR on all-cause and cardiac mortality. (A) Survival analysis of all-cause mortality on subjects underwent early AVR or conservative strategy. (B) Survival analysis of cardiac mortality on subjects underwent early AVR or conservative strategy.
Comment
To the best of our knowledge, this is the first meta-analysis that compares the outcomes of early AVR procedure and conservative care approach in asymptomatic severe AS patients with preserved LVEF. Our analysis consists of 1,249 asymptomatic severe AS patients (LVEF ≥ 50%). In this meta-analysis, we conclude that early surgery is associated with a lower risk of all-cause mortality, cardiac mortality, and non-cardiac mortality.
A key predicament is whether the risk of AVR outweighs the risk of conservative treatment in patients of asymptomatic severe AS. Previous meta-analysis confirms an overall mortality reduction in AS patients after early AVR (19–21). These studies included patients with reduced LVEF, and did not exclude subjects who developed symptoms but deferred AVR procedure (19). Thus, we conducted this meta-analysis and demonstrate that asymptomatic severe AS patients with preserved LVEF could also benefit from early surgery. For patients with very severe asymptomatic AS, we draw the same conclusions considering its potential in reducing all-cause mortality and cardiac mortality. However, there is no significant difference in non-cardiac mortality. Moreover, we pooled the survival data from the studies. The pooled data indicate that early AVR reduces all-cause mortality in asymptomatic severe AS patients with preserved LVEF.
The risk stratification of patients with asymptomatic severe AS is controversial. Valvuloarterial impedance and left ventricular global longitudinal strain were deemed as sensitive markers to identify early AVR candidates (LVEF ≥ 50%) (22). Serum B-type natriuretic peptide (BNP) level, with a cut-off value of 100 pg/mL, was related to AS-related adverse events in asymptomatic patients (LVEF ≥ 50%) (23). Patients with BNP <100 pg/mL might benefit from watchful waiting strategy (23). However, the natriuretic peptides stratification had not been adapted in current clinical guidelines due to the lack of clinical evidence (24). In patients with asymptomatic AS (LVEF ≥ 50%), hs-TnT >10 ng/L was related with higher risk of events within 12 months (25). However, these findings were derived from observational studies and need to be confirmed in RCTs.
Our study had several limitations. First, the meta-analysis consists of only four studies, including three non-randomized trials that were subjected to possible selection bias. Moreover, two articles were from the same group. Second, not every end-point was reported in the four included studies. Third, there was not enough data about operative mortality. Consequently, we could not compare operative mortality. Fourth, we failed to include asymptomatic patients who underwent transcatheter aortic valve replacement (TAVR) due to limited data, which may be safer for high risk surgery candidates. Finally, we failed to obtain original survival data.
In summary, our meta-analysis suggests that early AVR is associated with reduced all-cause mortality, cardiac mortality, and non-cardiac mortality compared to conservative management in asymptomatic severe AS patients with preserved LVEF. More randomized controlled trials are currently underway (26–30). Based on the current findings in our meta-analysis, we tend to suggest clinicians to take an early interventional strategy for asymptomatic AS patients with preserved LVEF.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
TY and YL collected data and performed analysis. TY and ZC wrote the manuscript. YL, CB, and ZC made revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by funding from the National Natural Science Foundation of China (No. 81970396 to ZC and No. 81900416 to YL), and the Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholars (No. LR20H020002 to ZC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.621149/full#supplementary-material
References
1. Kang DH, Park SJ, Lee SA, Lee S, Kim DH, Kim HK, et al. Early surgery or conservative care for asymptomatic aortic stenosis. N Engl J Med. (2020) 382:111–9. doi: 10.1056/NEJMoa1912846
2. Généreux P, Stone GW, O'Gara PT, Marquis-Gravel G, Redfors B, Giustino G, et al. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. (2016) 67:2263–88. doi: 10.1016/j.jacc.2016.02.057
3. Pineda AM, Kiefer TL. Asymptomatic severe aortic valve stenosis-when to intervene: a review of the literature, current trials, and guidelines. Curr Cardiol Rep. (2018) 20:129. doi: 10.1007/s11886-018-1072-x
4. Lindman BR, Clavel MA, Mathieu P, Lung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nat Rev Dis Primers. (2016) 2:16006. doi: 10.1038/nrdp.2016.6
5. Lindman BR, Dweck MR, Lancellotti P, Généreux P, Piérard LA, O'Gara PT, et al. Management of asymptomatic severe aortic stenosis. JACC Cardiovasc Imaging. (2020) 13:481–93. doi: 10.1016/j.jcmg.2019.01.036
6. Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD Jr, Gualano SK, et al. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes. (2009) 2:533–9. doi: 10.1161/CIRCOUTCOMES.109.848259
7. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Thorac Cardiovasc Surg. (2014) 148: e1–132. doi: 10.1161/CIR.0000000000000029
8. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2017) 70:252–89. doi: 10.1016/j.jacc.2017.03.011
9. Campo J, Tsoris A, Kruse J, Karim A, Andrei AC, Liu MH, et al. Prognosis of severe asymptomatic aortic stenosis with and without surgery. Ann Thorac Surg. (2019) 108:74–9. doi: 10.1016/j.athoracsur.2019.01.031
10. Marechaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. (2010) 31:1390–7. doi: 10.1093/eurheartj/ehq076
11. Pai RG, Kapoor N, Bansal RC, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. (2006) 82:2116–22. doi: 10.1016/j.athoracsur.2006.07.043
12. Bohbot Y, Pasquet A, Rusinaru D, Delabre J, Delpierre Q, Altes A, et al. Asymptomatic severe aortic stenosis with preserved ejection fraction: early surgery versus conservative management. J Am Coll Cardiol. (2018) 72:2938–9. doi: 10.1016/j.jacc.2018.09.049
13. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. (2015) 66:2827–38. doi: 10.1016/j.jacc.2015.10.001
14. Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. (2009) 137:82–90. doi: 10.1016/j.jtcvs.2008.08.015
15. Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. (1997) 95:2262–70. doi: 10.1161/01.CIR.95.9.2262
16. Singh A, Greenwood JP, Berry C, Dawson DK, Kelly DJ, et al. Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: the prognostic importance of microvascular dysfunction in aortic stenosis (PRIMID AS) study. Eur Heart J. (2017) 38:1222–9. doi: 10.1093/eurheartj/ehx001
17. Kim HJ, Kim JB, Kim HR, Ju MH, Kang DY, Lee SA, et al. Impact of valve replacement on long-term survival in asymptomatic patients with severe aortic stenosis. Am J Cardiol. (2019) 123:1321–8. doi: 10.1016/j.amjcard.2019.01.035
18. Kang DH, Park SJ, Rim JH, Yun SC, Kim DH, Song JM, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. (2010) 121:1502–9. doi: 10.1161/CIRCULATIONAHA.109.909903
19. Lim WY, Ramasamy A, Lloyd G, Bhattacharyya S. Meta-analysis of the impact of intervention versus symptom-driven management in asymptomatic severe aortic stenosis. Heart. (2017) 103:268–72. doi: 10.1136/heartjnl-2016-309830
20. Sá MPBO, Cavalcanti LRP, Escorel Neto ACA, Perazzo AM, Simonato M, Clavel MA, et al. Early aortic valve replacement versus watchful waiting in asymptomatic severe aortic stenosis: a study-level meta-analysis. Struct Heart. (2019) 3:483–90. doi: 10.1080/24748706.2019.1652946
21. Genereux P, Stone G, O'Gara P, Guillaume GM, Bjorn R, Gennaro G, et al. Early aortic valve replacement versus a conservative strategy for asymptomatic severe aortic stenosis: meta-analysis of observational studies. J Am Coll Cardiol. (2016) 67:2210. doi: 10.1016/S0735-1097(16)32211-2
22. Huded CP, Masri A, Kusunose K, Goodman AL, Grimm RA, Gillinov AM, et al. Outcomes in asymptomatic severe aortic stenosis with preserved ejection fraction undergoing rest and treadmill stress echocardiography. J Am Heart Assoc. (2018) 7:e007880. doi: 10.1161/JAHA.117.007880
23. Nakatsuma K, Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, et al. B-type natriuretic peptide in patients with asymptomatic severe aortic stenosis. Heart. (2019) 105:384–90. doi: 10.1136/heartjnl-2018-313746
24. Martinez-Selles M, Bayes-Genis A. Asymptomatic severe aortic stenosis: biomarkers are welcome. Heart. (2019) 105:355–6. doi: 10.1136/heartjnl-2018-314122
25. Ferrer-Sistach E, Lupón J, Cediel G, Teis A, Gual F, Serrano S, et al. High-sensitivity troponin T in asymptomatic severe aortic stenosis. Biomarkers. (2019) 24:334–40. doi: 10.1080/1354750X.2019.1567818
26. NCT. Danish National Randomized Study on Early Aortic Valve Replacement in Patients with Asymptomatic Severe Aortic Stenosis. Available online at: https://clinicaltrials.gov/show/NCT03972644 (accessed June 3, 2019).
27. NCT. The Early Valve Replacement in Severe Asymptomatic Aortic Stenosis Study. Available online at: https://clinicaltrials.gov/show/NCT04204915 (accessed December 19, 2019).
28. NCT. Early Valve Replacement Guided by Biomarkers of LV Decompensation in Asymptomatic Patients with Severe AS. Available online at: https://clinicaltrials.gov/show/NCT03094143 (accessed March 29, 2017).
29. NCT. Evaluation of Transcatheter Aortic Valve Replacement Compared to Surveillance for Patients with Asymptomatic Severe Aortic Stenosis. Available online at https://clinicaltrials.gov/show/NCT03042104 (accessed February 3, 2017).
30. NCT. Aortic Valve Replacement Versus Conservative Treatment In Asymptomatic Severe Aortic Stenosis. Available online at: https://clinicaltrials.gov/show/NCT02436655 (accessed May 7, 2015).
Keywords: asymptomatic, aortic stenosis, aortic valve replacement, conservative treatment, preserved ejection fraction
Citation: Yuan T, Lu Y, Bian C and Cai Z (2021) Early Aortic Valve Replacement vs. Conservative Management in Asymptomatic Severe Aortic Stenosis Patients With Preserved Ejection Fraction: A Meta-Analysis. Front. Cardiovasc. Med. 7:621149. doi: 10.3389/fcvm.2020.621149
Received: 25 October 2020; Accepted: 29 December 2020;
Published: 03 February 2021.
Edited by:
Felix Jansen, University Hospital Bonn, GermanyReviewed by:
Francesco Pollari, Nürnberg Hospital, GermanyFlorian Kahles, University Hospital RWTH Aachen, Germany
Copyright © 2021 Yuan, Lu, Bian and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhejun Cai, caizhejun@zju.edu.cn; Chang Bian, bianchang@zju.edu.cn
†These authors have contributed equally to this work
 Tan Yuan
Tan Yuan Yi Lu1†
Yi Lu1†  Zhejun Cai
Zhejun Cai