Increased Uric Acid, Gamma-Glutamyl Transpeptidase and Alkaline Phosphatase in Early-Pregnancy Associated With the Development of Gestational Hypertension and Preeclampsia
- 1First Affiliated Hospital of Shantou University Medical College, Shantou, China
- 2Shantou University Medical College, Shantou, China
- 3Morsani College of Medicine, University of South Florida, Tampa, FL, United States
Background: Previous studies have reported that biomarkers of liver injury and renal dysfunction were associated with hypertensive disorders of pregnancy (HDP). However, the associations of these biomarkers in early pregnancy with the risk of HDP and longitudinal blood pressure pattern during pregnancy were rarely investigated in prospective cohort studies.
Methods: A total of 1,041 pregnant women were enrolled in this prospective cohort study. BP was assessed in four stages throughout pregnancy. The following biomarkers were measured at early pregnancy before 18 weeks gestation: lactate dehydrogenase (LDH), aspartate aminotransferase to alanine aminotransferase ratio (AST/ALT), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), uric acid (UA), and estimated glomerular filtration rate (eGFR). Linear mixed-effects and logistic regression models were used to examine the associations of these biomarkers with longitudinal BP pattern during pregnancy and HDP incidence, respectively.
Results: In unadjusted models, higher serum UA, GGT, ALP, and LDH levels, as well as lower eGFR and AST/ALT, were associated with higher BP levels during pregnancy and an increased risk of HDP. After adjustment for maternal age, pre-pregnancy BMI and other potential confounders, UA, GGT, ALP, and LDH remained positively associated with both BP and HDP. However, eGFR and AST/ALT were not associated with HDP after adjusting for potential confounders. When including all 6 biomarkers simultaneously in multivariable analyses, increased UA, GGT, and ALP significantly associated with gestational hypertension and preeclampsia.
Conclusion: This study suggests that increased UA, GGT, and ALP in early-pregnancy are independent risk factors of gestational hypertension and preeclampsia.
Introduction
Hypertensive disorders of pregnancy (HDP) affect up to 10% of pregnant women and remain major causes of maternal and perinatal morbidity and mortality worldwide (1–5). Identifying women at risk early in the course of such disorders, especially gestational hypertension and preeclampsia would facilitate intensive monitoring and intervention. Serum gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), aspartate aminotransferase to alanine aminotransferase ratio (AST/ALT) are common biomarkers of liver injury (6–8). Plasma creatinine (Cr), uric acid (UA), and estimated glomerular filtration rate (eGFR) are widely used as indicators for renal dysfunction (9, 10). Previous case-control studies have found that serum GGT, ALP, LDH, and UA levels are increased in women diagnosed with HDP (11–17). Recently, several studies have reported that lower AST/ALT was associated with insulin resistance, metabolic syndrome and cardiovascular disease (18–20). However, the associations of these biomarkers in early pregnancy with the risk of HDP were rarely investigated in prospective cohort studies.
Blood pressure (BP) changes progressively during pregnancy (21). In normal pregnancy, BP initially decreases until mid-pregnancy, and subsequently increases until delivery (21–23). A longitudinal study by Kac and colleagues recently reported that elevation of ALP, ALT, and Cr levels in the first trimester were associated with increased BP levels during pregnancy (24). As noted by the authors, the study involved a relatively small sample size and the study itself pertained to normotensive pregnant women. The associations reported prompt further investigation in large prospective cohort studies and the general population.
Accordingly, we investigated the associations of 6 hepatic and renal function biomarkers (GGT, ALP, AST/ALT, LDH, UA, and eGFR) in early pregnancy with longitudinal BP pattern during pregnancy and the risk of HDP in a large prospective cohort.
Materials and Methods
Study Population
This study was embedded in a population-based prospective cohort of pregnant women who received antenatal care in the First Affiliated Hospital of Shantou University Medical College in Shantou, China. Clinical characteristics of participants were assessed by questionnaire and physical examination at 7th−18th, 19th−27th, 28th−34th, and 35th−39th gestational weeks. Subjects were scheduled at enrollment for clinic visits during follow-up. Patients who missed their scheduled visits would be contacted for clinic visits, whereupon the patient's information would be collected. For patients who could not attend clinical visits, patients' information would be collected by telephone interviews with their families and electronic medical records. If patients could not be followed up through in-clinic visits, telephone interviews, or electronic medical records, such patients were recorded as a loss to follow-up. A total of 2,206 pregnant women were accumulatively recruited from March 2014 to April 2016 inclusive. Included were women who had baseline measurement of hepatic and renal biochemical function before 18 weeks gestation (N = 1,371). Exclusion criteria included in vitro fertilization or twin pregnancies (N = 16), miscarriage (N = 9), loss to follow-up (N = 221), or having been diagnosed with chronic hepatitis B (N = 74), chronic nephritis (N = 5), rheumatoid arthritis (N = 3), or systemic lupus erythematosus (N = 2). After exclusion, 1,041 participants were enrolled.
Blood Pressure Measurements
BP was assessed in four stages during pregnancy (7th−18th, 19th−27th, 28th−34th, and 35th−39th gestational weeks). At each visit, BP was measured thrice in the morning by qualified nurses using an Omron HEM-7052 automatic blood pressure monitor (Omron Healthcare Ltd., Dalian, China) according to the standard measurement procedure recommended by the American Heart Association (25). The mean of 3 readings was used in the analysis. Some subjects had missing BP measurements due to missed visits. In total, 3,274 blood pressure measurements were available for analysis, of which 31 subjects had one measurement, 225 subjects had two measurements, 347 subjects had 3 measurements, and 438 subjects had 4 measurements.
Hypertensive Disorders of Pregnancy
Maternal outcomes were obtained from medical records. A total of 60 pregnant women met the criteria of HDP, including 7 with chronic hypertension, 22 with gestational hypertension, and 31 experienced preeclampsia.
Chronic hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg before 20 weeks gestation. Gestational hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg without proteinuria, which had developed for the first time after 20 weeks gestation (26). Preeclampsia was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg with proteinuria (defined as ≥300 mg of protein in a 24-h urine specimen or ≥1+ in two random urine samples collected at least 4 h apart) (26).
Laboratory Analysis
Hepatic and renal function biochemical testing (comprising LDH, AST, ALT, ALP, GGT, UA, and Cr) was performed in the Department of Clinical Laboratory of the First Affiliated Hospital of Shantou University Medical College, using an automatic biochemical analyzer (Beckman counter AU5800, USA). Maternal overnight fasting blood samples were collected in the morning during early pregnancy (median 13.4 weeks, 95% range 7.1–18.0). The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (27).
Covariates
Maternal age, monthly per capita income, education level, nulliparous (yes or no), folic acid supplement intake during pregnancy (yes or no), family history of hypertension (yes or no), family history of diabetes mellitus (yes or no), smoking habit (yes or no), alcohol consumption (yes or no), pre-pregnancy weight and height were obtained by questionnaire on enrolment. Pre-pregnancy BMI was calculated as pre-pregnancy weight/height2 (kg/m2) (28).
Statistical Analysis
The hepatic and renal function biomarkers were categorized into tertiles and were analyzed as categorical variables (24). Linear mixed-effects regression models were used to evaluate the association of biomarkers with SBP and DBP change during pregnancy (22). This regression technique considers the correlation of repeated measurements in the same individual and allows incomplete outcome data, commonly applied to the analysis of repeated measurement data (29). Gestational age (linear and quadratic terms) was included in the linear mixed-effects regression models to fit the quadratic function of the association of blood pressure with time (24). Gestational age was included in the models as both random and fixed effects, whereas biomarkers and other covariates were analyzed as fixed effects. Maternal age, BMI, income, education, folic acid supplementation, family history of hypertension, family history of diabetes mellitus, as well as smoking and alcohol consumption have been reported to be associated with HDP (30–32). In order to adjust for these potential confounders, we applied 3 models: Model 1 was adjusted for gestational age (linear and quadratic terms); Model 2 was further adjusted for pre-pregnancy BMI, maternal age, monthly per capita income, education level, parity, folic acid supplementation during pregnancy, family history of hypertension, family history of diabetes mellitus, and smoking and alcohol consumption; Model 3 included all six biomarkers simultaneously and was adjusted for covariates as in Model 2. To further assess whether similar associations were also present in a normotensive population, we repeated the analyses in women who remained normotensive throughout pregnancy. The curve of longitudinal blood pressure change with gestational age was estimated using a crude linear mixed-effects regression model.
The associations of 6 early-pregnancy biomarkers with the risk of HDP were analyzed using three logistic regression models: Model 1 was unadjusted; Model 2 was adjusted for pre-pregnancy BMI, maternal age, monthly per capita income, education level, parity, folic acid supplement intake during pregnancy, family history of hypertension, family history of diabetes mellitus, smoking, alcohol consumption, and gestational age at sampling; Model 3 included all six biomarkers simultaneously and was adjusted for covariates as in Model 2.
In addition, among normal pregnancy, gestation hypertension, and preeclampsia, one way ANOVA was performed to analyze the difference of maternal age, pre-pregnancy BMI, gestational age at sampling weeks, UA, eGFR, GGT, ALP, and LDH. Chi-square was used to analyze the difference of monthly per capita income, education level, nulliparous, folic acid supplement intake during pregnancy, family history of hypertension, family history of diabetes mellitus, smoking habit, and alcohol consumption.
All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, Illinois, USA). Two-tailed P-values < 0.05 were considered statistically significant.
Results
Participant Characteristics
Baseline characteristics of the study population are shown in Table 1. Mean maternal age and pre-pregnancy BMI were 29.5 ± 4.3 years and 20.5 ± 2.9 kg/m2, respectively. Of all women included in the study, 44.3% was nulliparous and 19.9% had a family history of hypertension. At follow-up, 60 (5.8%) pregnant women had developed HDP.
Serum Biomarkers and Blood Pressure Pattern During Pregnancy
Figures 1, 2 show the longitudinal patterns of SBP and DBP change and early pregnancy biomarker level during different tertiles of pregnancy. The distribution of maternal blood pressure throughout pregnancy was shown in Supplementary Figures S1, S2. Women in the third tertile of LDH, GGT, ALP, and UA levels had higher SBP and DBP than those in the first tertile, whereas women in the third tertile of AST/ALT and eGFR had lower SBP and DBP during pregnancy than those in the first tertile.
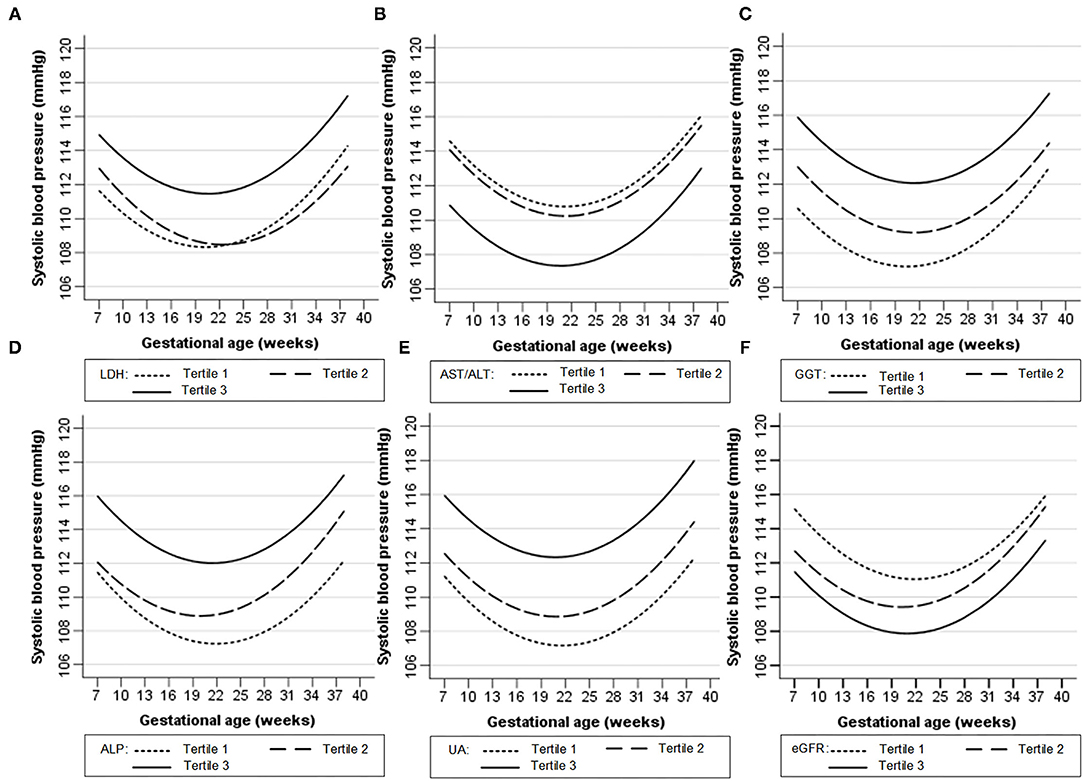
Figure 1. Longitudinal trend of systolic blood pressure change with gestational age vs. early-pregnancy biomarkers levels, predicted by linear mixed-effects regression models. (A) lactate dehydrogenase (LDH); (B) aspartate aminotransferase to alanine aminotransferase ratio (AST/ALT); (C) gamma-glutamyl transpeptidase (GGT); (D) alkaline phosphatase (ALP); (E) uric acid (UA); (F) estimated glomerular filtration rate (eGFR).
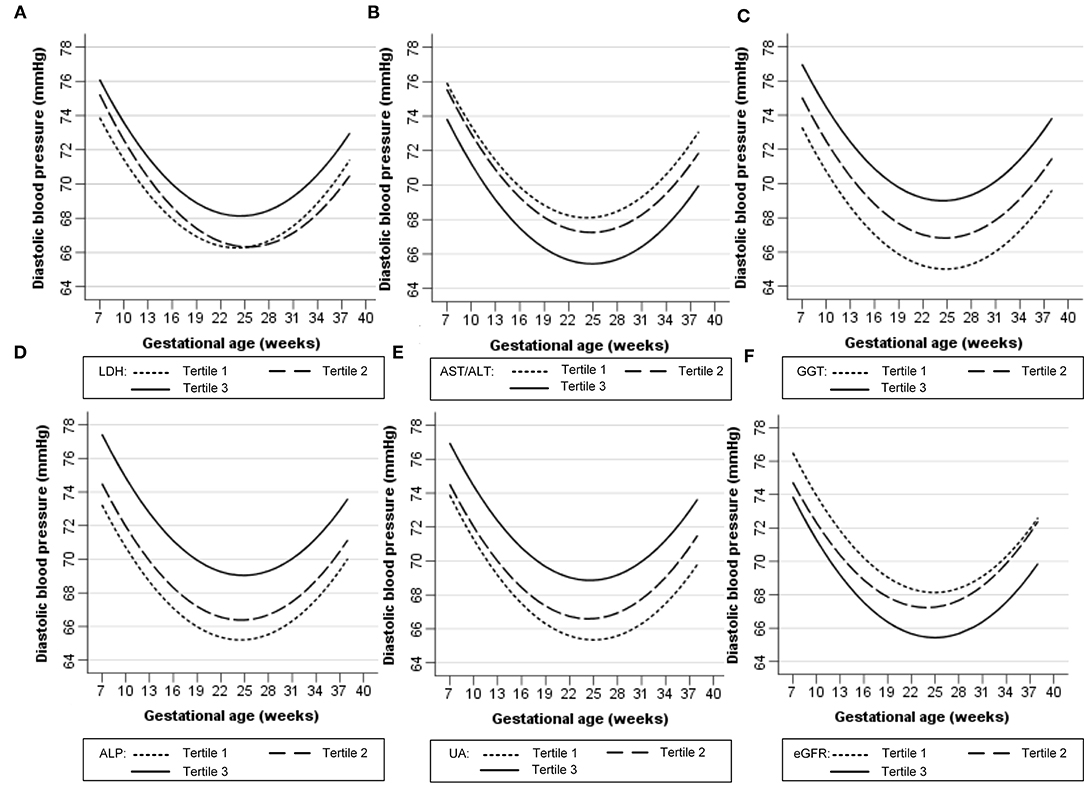
Figure 2. Longitudinal trend of diastolic blood pressure change with gestational age vs. early-pregnancy biomarkers levels, predicted by linear mixed-effects regression models. (A) lactate dehydrogenase (LDH); (B) aspartate aminotransferase to alanine aminotransferase ratio (AST/ALT); (C) gamma-glutamyl transpeptidase (GGT); (D) alkaline phosphatase (ALP); (E) uric acid (UA); (F) estimated glomerular filtration rate (eGFR).
Tables 2, 3 show the longitudinal associations of early-pregnancy biomarkers with SBP and DBP levels during pregnancy, respectively. In crude analyses (Model 1), higher UA, GGT, ALP, and LDH levels, as well as lower eGFR and AST/ALT, were associated with higher SBP and DBP levels during pregnancy. After adjustment for maternal age, pre-pregnancy BMI and other potential confounders (Model 2), higher UA, GGT, ALP, LDH, and AST/ALT remained associated with both increased SBP and DBP, whereas lower eGFR was only associated with increased DBP. When all 6 biomarkers were simultaneously included in multivariable analyses (Model 3), only increased UA, GGT, and ALP remained associated with increased BP. Similar results were found in pregnant women without HDP (Supplementary Tables S1, S2).
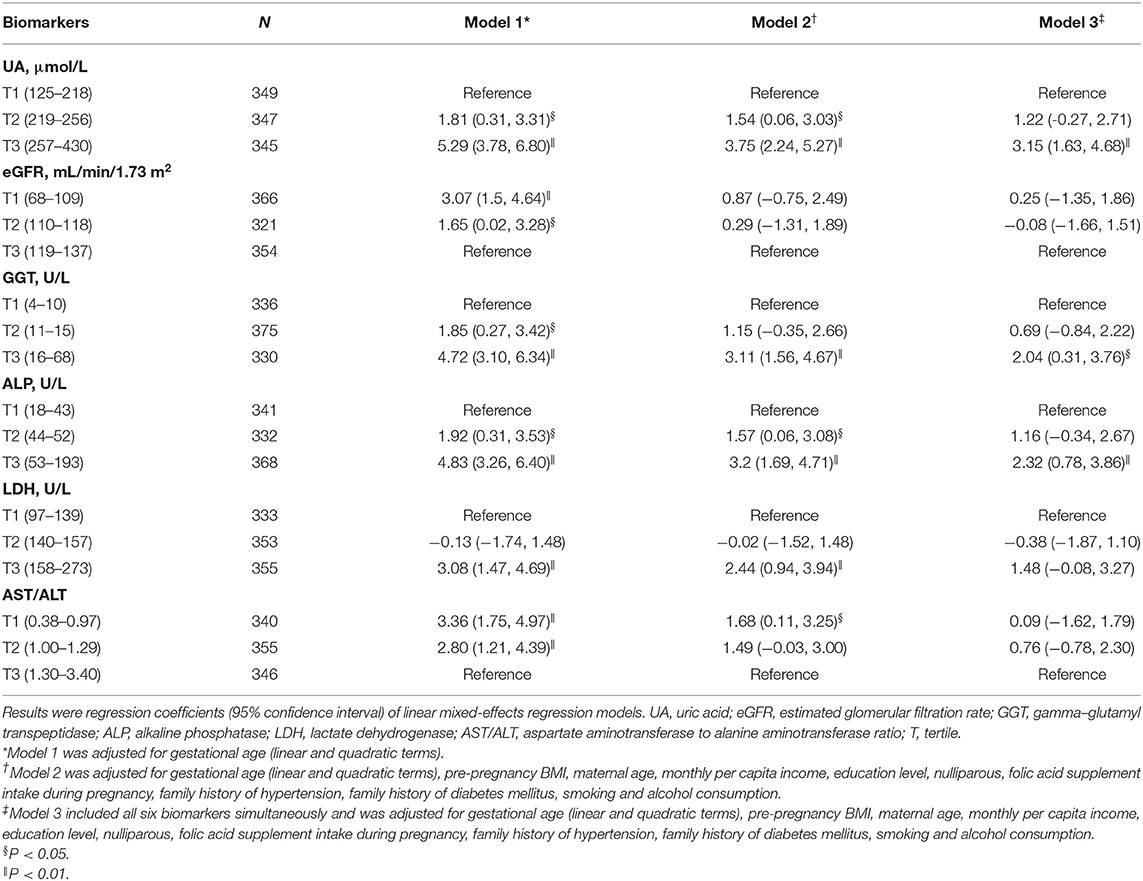
Table 2. Longitudinal associations of early-pregnancy biomarkers levels with systolic blood pressure levels during pregnancy.
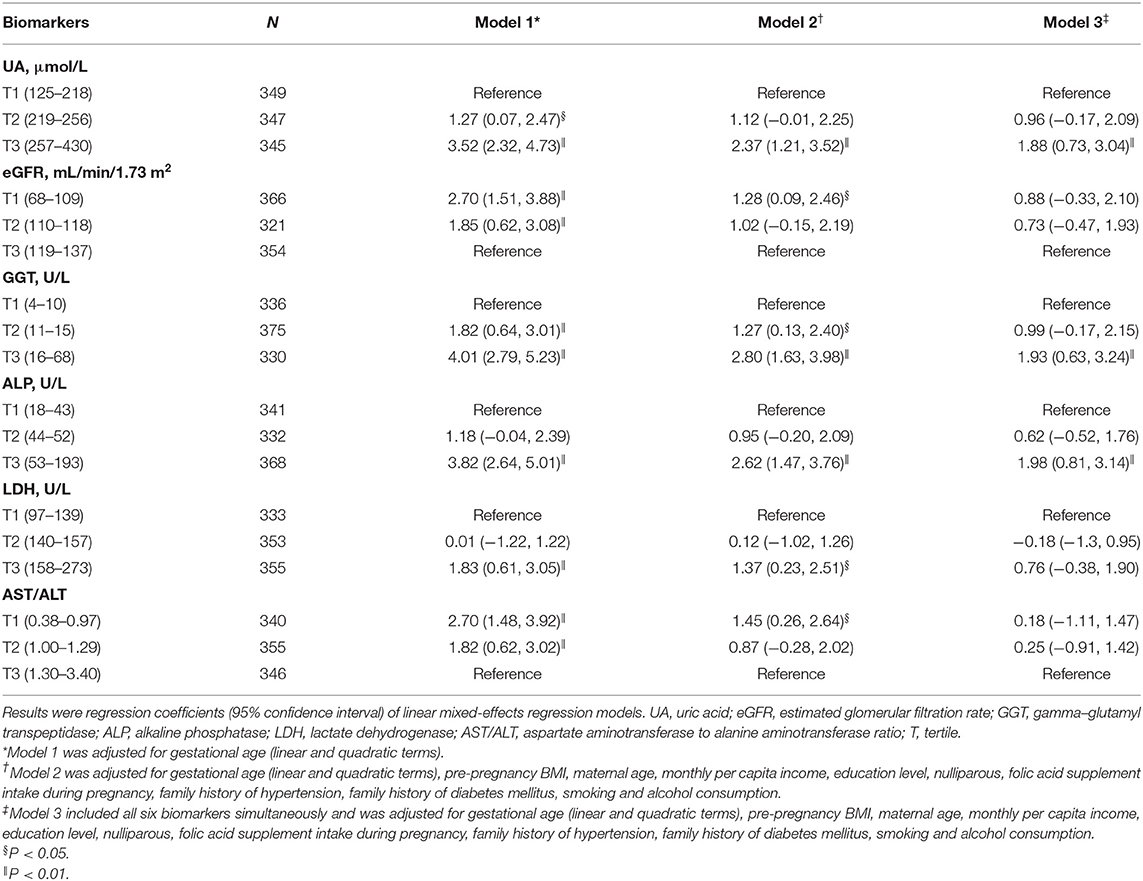
Table 3. Longitudinal associations of early-pregnancy biomarkers levels with diastolic blood pressure levels during pregnancy.
Serum Biomarkers and HDP
Table 4 presents the association of early-pregnancy biomarkers with the risk of HDP, using multivariable logistic regression models. In unadjusted analyses (Model 1), higher UA, GGT, ALP, and LDH levels, as well as lower eGFR and AST/ALT, were associated with increased risk of HDP. After adjustment for maternal age, pre-pregnancy BMI and other potential confounders (Model 2), only increased UA, GGT, ALP, and LDH remained associated with the risk of HDP. When all 6 biomarkers were simultaneously included in multivariable analyses (Model 3), the highest tertile of UA, GGT, and ALP were associated with a higher risk of HDP (odds ratio, 3.57 [95% confidence interval, 1.36–9.39]; odds ratio, 2.61 [95% confidence interval, 1.05–6.83]; odds ratio, 2.12 [95% confidence interval, 1.01–4.90], respectively). The associations of LDH, eGFR and AST/ALT with HDP were no longer statistically significant following multivariate adjustment for UA, GGT, and ALP (Model 3).
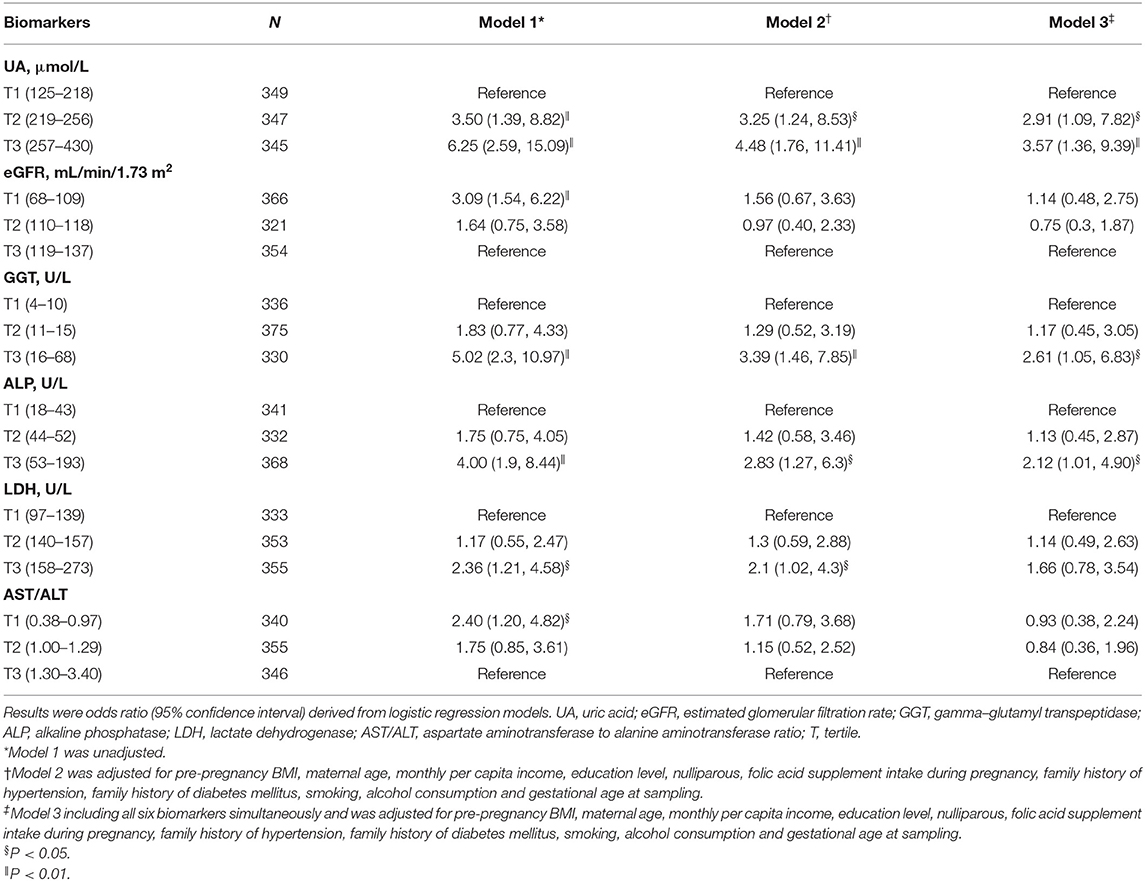
Table 4. Associations of early-pregnancy biomarkers levels with risk of hypertensive disorders of pregnancy.
UA, GGT, and ALP Levels in Normal Pregnancy and HDP
Table 5 presents baseline characteristics of normal pregnancy (group 1), chronic hypertension (group 2), gestation hypertension (group 3), and preeclampsia (group 4). Since the sample size of chronic hypertension is too small, statistical analysis was only performed in groups 1, 3, and 4 and presented in Figure 3. UA and ALP levels were significantly higher in gestational hypertension and preeclampsia as compared to normal pregnancy.
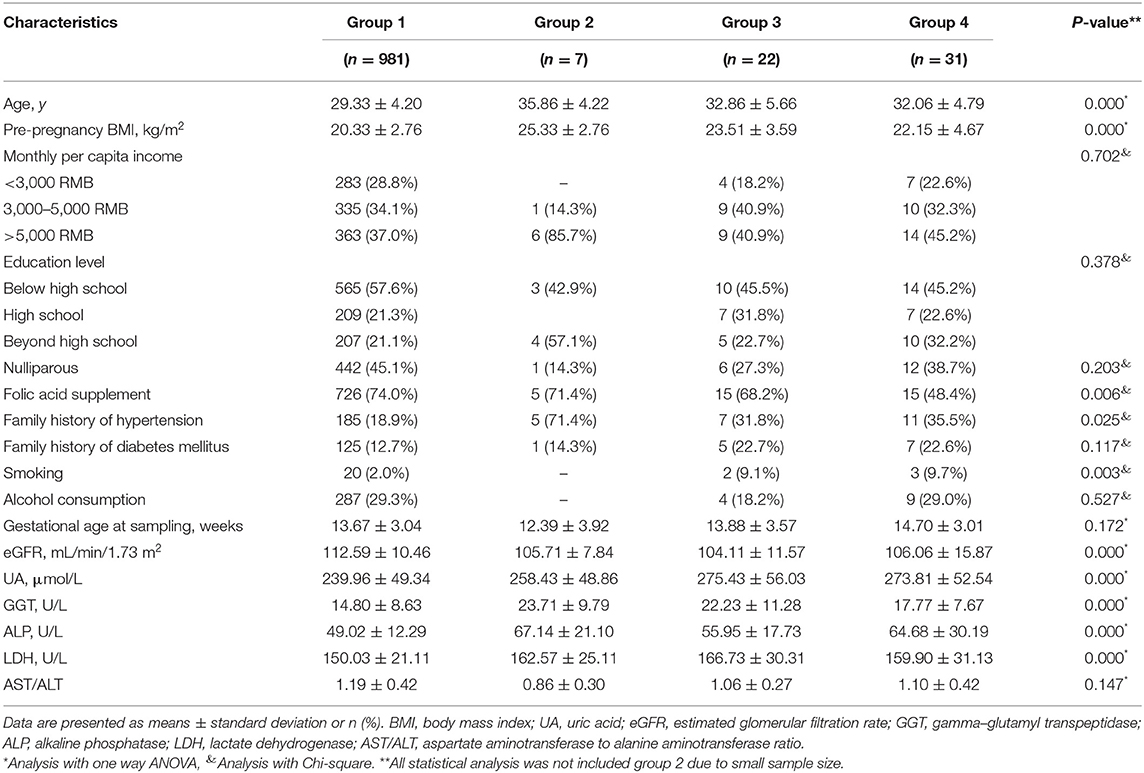
Table 5. Baseline characteristics of normal pregnancy (group 1), chronic hypertension (group 2), gestation hypertension (group 3), and preeclampsia (group 4).
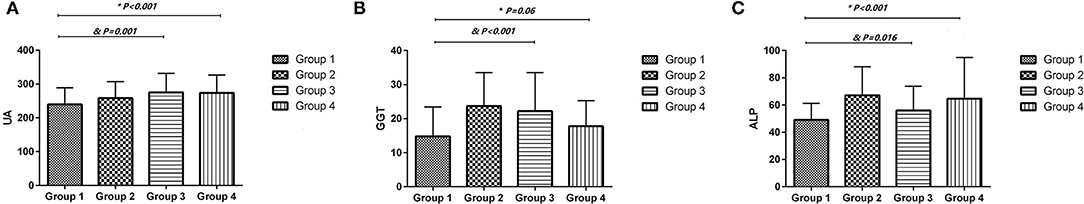
Figure 3. The uric acid, gamma-glutamyl transpeptidase and alkaline phosphatase levels were compared in normal pregnancy (group 1), gestation hypertension (group 3), and preeclampsia (group 4) by One way ANOVA. (A) uric acid (UA); (B) gamma-glutamyl transpeptidase (GGT); (C) alkaline phosphatase (ALP). Chronic hypertension (group 2) was not included for analysis due to small sample size. *Preeclampsia (group 4) compare to normal pregnancy (group1) by One way ANOVA. &Gestation hypertension (group 3) compare to normal pregnancy (group1) by One way ANOVA.
Discussion
In this population-based prospective cohort study, we demonstrate that higher serum UA, GGT, ALP, and LDH levels in early pregnancy, as well as lower eGFR and AST/ALT, are associated with higher BP levels during pregnancy and an increased risk of HDP. When including all 6 biomarkers simultaneously in multivariable analyses adjusted for potential confounders, increased levels of UA, GGT, and ALP were significantly associated with gestational hypertension and preeclampsia. Our study suggests that elevated UA, GGT, and ALP in early pregnancy are independent risk factors for the development of gestational hypertension and preeclampsia.
UA
For UA, our results are consistent with previous cross-sectional and prospective studies demonstrating the positive association between UA and HDP (15, 33, 34). Furthermore, we find that increased serum UA in early pregnancy is independent factor associated with higher longitudinal BP progression during pregnancy. In contrast to our findings, Kac et al. reported UA in the first trimester was not associated with BP levels during pregnancy following adjustment for maternal BMI (24). This inconsistency may be due to differences in sample size and participant characteristics. The study, as stated by the authors, investigated a relatively small sample size of 225 and specifically studied normotensive pregnant women (24). However, when we repeated the analysis in pregnant women without HDP, increased UA remained associated with both higher SBP and DBP levels during pregnancy following adjustment for maternal BMI and other confounders (Supplementary Tables S1, S2).
Many experimental studies suggest that UA may play a casual role in the development of hypertension (14, 15). Hyperuricemia induced hypertension, which can be prevented by UA-lowering treatment (35). Hyperuricemia has been shown to stimulate the renin-angiotensin system, inhibit neuronal nitric oxide synthase and induce endothelial dysfunction (35, 36). In addition, UA has been reported to induce trophoblastic production of pro-inflammatory interleukin-1β through activation of inflammatory pathways (37). These underlying mechanisms may partly explain the role of UA in BP progression and the development of HDP.
GGT
Serum GGT is a known biomarker for liver injury and alcohol consumption (38). Several longitudinal studies have reported GGT as positively associated with BP progression and the risk of hypertension in non- pregnant persons (39–41). A study of almost 12,000 hypertensive adults found that higher baseline GGT levels were associated with higher follow-up BP and an increased risk of cardiovascular mortality (42). However, no prior longitudinal study has investigated the associations of serum GGT in early pregnancy with longitudinal BP during pregnancy and the risk of HDP. In this present study, serum GGT in early pregnancy is positively associated with BP levels during pregnancy and a risk for HDP having adjusted for various confounders. Although exact mechanisms that link GGT with BP progression and HDP are not fully elucidated, several possible explanations are posited: GGT plays a role in the generation of free radical species through its interaction with iron and other transition metals (43). Serum GGT has been positively associated with inflammatory markers such as fibrinogen, C-reactive protein (CRP), and F2-isoprostanes. Thus, elevated GGT could potentially act as an additional marker for oxidative stress and inflammation, which are, as demonstrated by Palei et al., important features of HDP (44).
ALP
In the current study, higher serum ALP in early pregnancy is also independently associated with elevated BP during pregnancy and increased HDP incidence. This finding confirms and extends the result of a previous study that has reported a positive association of ALP with BP during normal pregnancy (24). The relationship of ALP with BP and HDP may be partly explained by its correlation with vascular calcification. ALP is a hydrolase enzyme that catalyzes the hydrolysis of inorganic pyrophosphate, an inhibitor of vascular calcification (45). Increased levels of ALP have been found in vessels with medial calcification (46), and have been positively associated with higher risk of hypertension, peripheral arterial disease and cardiovascular diseases (47–49). In addition, many studies have reported that serum ALP positively correlated with the inflammatory marker, CRP (20, 50–52). High serum ALP levels may partially reflect the inflammatory process, which has been associated with the pathology of HDP (44). We further compared the levels of UA, GGT, and ALP between normal pregnancy and gestational hypertension and preeclampsia. UA, GGT, and ALP were associated preeclampsia (Figure 3).
In conclusion, our study provided evidence that higher UA, GGT, and ALP levels in early pregnancy are independent risk factors of gestational hypertension and preeclampsia. These findings suggest that UA, GGT, and ALP could be markers for the development of gestational hypertension and preeclampsia. However, it is not clear the elevations of UA, GGT, and ALP in early pregnancy is a casual factor or consequence of the development of gestational hypertension and preeclampsia. This would be an open and essential question for future studies that could provide new targets for treatment of gestational hypertension and preeclampsia. Our findings warrant further both clinical and experimental studies to identify the underlying mechanisms and clinical value in the early diagnosis, prevention and management of HDP.
Study Strengths and Limitations
The strengths of our study include the large prospective population-based cohort from early pregnancy onwards, standardized measurement of BP, the ability to adjust for multiple traditional confounders and the ability to simultaneously assess multiple biomarkers. However, our study has some limitations. First, this was a single-center study with a small number of HDP events. Second, the serum concentrations of hepatic and renal biomarkers were assayed solely in early pregnancy. Thus, the correlation of blood pressure and HDP with these biomarkers measured before pregnancy and after delivery cannot be evaluated. Third, since BP varies during the day according to a circadian rhythm (53) and our study did not include ambulatory blood pressure measurements, this may introduce some random measurement error in the analysis. Finally, the sample size of chronic hypertension in pregnancy was too small to perform statistical analysis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Research and Ethics Committee of First Affiliated Hospital of Shantou University Medical College. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XT, RL, and YC conceived and designed the study. WO, DL, SN, SC, and ML acquired the data. WO, XH, and JY analyzed the data. YC, WO, and MO'G prepared the manuscript. XT and YC reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by projects from Grant for Key Disciplinary Project of Clinical Medicine under the High-level University Development Program (2020), Innovation Team Project of Guangdong Universities (2019KCXTD003), Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG19B), Funding for Guangdong Medical Leading Talent (2019–2022), National Natural Science Foundation of China (82073659), and Dengfeng Project for the construction of high-level hospitals in Guangdong Province—the First Affiliated Hospital of Shantou University Medical College (202003-2).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the staffs and participants for their critical contributions to this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.756140/full#supplementary-material
Abbreviations
BP, blood pressure; HDP, hypertensive disorders of pregnancy; LDH, lactate dehydrogenase; AST/ALT, aspartate aminotransferase to alanine aminotransferase ratio; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; UA, uric acid; eGFR, estimated glomerular filtration rate; BMI, body mass index; T, tertile.
References
1. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. (2014) 2:e323–33. doi: 10.1016/S2214-109X(14)70227-X
2. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. (2021) 398:341–54. doi: 10.1016/S0140-6736(20)32335-7
3. Liu M. Spotlight on the relationship between heart disease and mental stress. Heart and Mind. (2021) 5:1–3. doi: 10.4103/hm.hm_12_21
4. Jiang W. Neuropsychocardiology-Evolution and advancement of the heart-mind field. Heart and Mind. (2017) 1:59–64. doi: 10.4103/hm.hm_13_17
5. Eizaguirre N, Rementeria GP, González-Torrez MÁ, Gaviria M. Updates in vascular dementia. Heart and Mind. (2017) 1:22–35. doi: 10.4103/hm.hm_4_16
6. Mutanen A, Lohi J, Merras-Salmio L, Koivusalo A, Pakarinen MP. Prediction, identification and progression of histopathological liver disease activity in children with intestinal failure. J Hepatol. (2021) 74:593–602. doi: 10.1016/j.jhep.2020.09.023
7. Li MX, Zhao H, Bi XY, Li ZY, Yao XS, Li H, et al. Lactate dehydrogenase is a prognostic indicator in patients with hepatocellular carcinoma treated by sorafenib: results from the real life practice in HBV endemic area. Oncotarget. (2016) 7:86630–47. doi: 10.18632/oncotarget.13428
8. Su CW, Chan CC, Hung HH, Huo TI, Huang YH, Li CP, et al. Predictive value of aspartate aminotransferase to alanine aminotransferase ratio for hepatic fibrosis and clinical adverse outcomes in patients with primary biliary cirrhosis. J Clin Gastroenterol. (2009) 43:876–83. doi: 10.1097/MCG.0b013e31818980ac
9. Takae K, Nagata M, Hata J, Mukai N, Hirakawa Y, Yoshida D, et al. Serum uric acid as a risk factor for chronic kidney disease in a japanese community- the hisayama study. Circ J. (2016) 80:1857–62. doi: 10.1253/circj.CJ-16-0030
10. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. (2017) 389:1238–52. doi: 10.1016/S0140-6736(16)32064-5
11. Wu J, Zhou W, Li Q, Yuan R, Li H, Cui S. Combined use of serum gamma glutamyl transferase level and ultrasonography improves prediction of perinatal outcomes associated with preeclamptic pregnancy. Clin Chim Acta. (2017) 475:97–101. doi: 10.1016/j.cca.2017.09.018
12. Portelinha A, Cerdeira AS, Belo L, Tejera E, Pinto F, Pinto A, et al. Altered alanine aminotransferase and gamma-glutamyl transpeptidase in women with history of preeclampsia: association with waist-to-hip ratio and body mass index. Eur J Gastroenterol Hepatol. (2009) 21:196–200. doi: 10.1097/MEG.0b013e32831d81a7
13. Dacaj R, Izetbegovic S, Stojkanovic G, Dreshaj S. Elevated liver enzymes in cases of preeclampsia and intrauterine growth restriction. Med Arch. (2016) 70:44–7. doi: 10.5455/medarh.2016.70.44-47
14. Makuyana D, Mahomed K, Shukusho FD, Majoko F. Liver and kidney function tests in normal and pre-eclamptic gestation–a comparison with non-gestational reference values. Cent Afr J Med. (2002) 48:55–9.
15. Padma Y, Aparna VB, Kalpana B, Ritika V, Sudhakar PR. Renal markers in normal and hypertensive disorders of pregnancy in Indian women: a pilot study. Int J Reprod Contracept Obstet Gynecol. (2013) 2:514–20. doi: 10.5455/2320-1770.ijrcog20131205
16. Udenze I, Amadi C, Awolola N, Makwe CC. The role of cytokines as inflammatory mediators in preeclampsia. Pan Afr Med J. (2015) 20:219. doi: 10.11604/pamj.2015.20.219.5317
17. Hunter RJ, Pinkerton JH, Johnston H. Serum placental alkaline phosphatase in normal pregnancy and preeclampsia. Obstet Gynecol. (1970) 36:536–46.
18. Kawamoto R, Kohara K, Kusunoki T, Tabara Y, Abe M, Miki T. Alanine aminotransferase/aspartate aminotransferase ratio is the best surrogate marker for insulin resistance in non-obese Japanese adults. Cardiovasc Diabetol. (2012) 11:117. doi: 10.1186/1475-2840-11-117
19. Labayen I, Ruiz JR, Ortega FB, Davis CL, Rodriguez G, Gonzalez-Gross M, et al. Liver enzymes and clustering cardiometabolic risk factors in European adolescents: the HELENA study. Pediatr Obes. (2015) 10:361–70. doi: 10.1111/ijpo.273
20. Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. (2005) 54:3140–7. doi: 10.2337/diabetes.54.11.3140
21. Grindheim G, Estensen ME, Langesaeter E, Rosseland LA, Toska K. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens. (2012) 30:342–50. doi: 10.1097/HJH.0b013e32834f0b1c
22. Farias DR, Franco-Sena AB, Rebelo F, Schlussel MM, Salles GF, Kac G. Total cholesterol and leptin concentrations are associated with prospective changes in systemic blood pressure in healthy pregnant women. J Hypertens. (2014) 32:127–34. doi: 10.1097/HJH.0000000000000037
23. Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Established preeclampsia risk factors are related to patterns of blood pressure change in normal term pregnancy: findings from the Avon Longitudinal Study of Parents and Children. J Hypertens. (2011) 29:1703–11. doi: 10.1097/HJH.0b013e328349eec6
24. Kac G, Mendes RH, Farias DR, Eshriqui I, Rebelo F, Benaim C, et al. Hepatic, renal and inflammatory biomarkers are positively associated with blood pressure changes in healthy pregnant women: a prospective cohort. Medicine. (2015) 94:e683. doi: 10.1097/MD.0000000000000683
25. Palatini P, Asmar R, O'Brien E, Padwal R, Parati G, Sarkis J, et al. Recommendations for blood pressure measurement in large arms in research and clinical practice: position paper of the European society of hypertension working group on blood pressure monitoring and cardiovascular variability. J Hypertens. (2020) 38:1244–50. doi: 10.1097/HJH.0000000000002399
26. American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. (2013) 122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88
27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
28. Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. JAMA. (2018) 319:2419–29. doi: 10.1001/jama.2018.7270
29. Quene H, van den Bergh H. Examples of mixed-effects modeling with crossed random effects and with binomial data. J Memory Lang. (2008) 59:413–25. doi: 10.1016/j.jml.2008.02.002
30. Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS ONE. (2014) 9:e100180. doi: 10.1371/journal.pone.0100180
31. Li Z, Ye R, Zhang L, Li H, Liu J, Ren A. Folic acid supplementation during early pregnancy and the risk of gestational hypertension and preeclampsia. Hypertension. (2013) 61:873–9. doi: 10.1161/HYPERTENSIONAHA.111.00230
32. Engel SM, Scher E, Wallenstein S, Savitz DA, Alsaker ER, Trogstad L, et al. Maternal active and passive smoking and hypertensive disorders of pregnancy: risk with trimester-specific exposures. Epidemiology. (2013) 24:379–86. doi: 10.1097/EDE.0b013e3182873a73
33. Vyakaranam S, Bhongir AV, Patlolla D, Chintapally R. Study of serum uric acid and creatinine in hypertensive disorders of pregnancy. Int J Med Sci Public Health. (2015) 4:1424–8. doi: 10.5455/ijmsph.2015.15042015294
34. Wolak T, Sergienko R, Wiznitzer A, Paran E, Sheiner E. High uric acid level during the first 20 weeks of pregnancy is associated with higher risk for gestational diabetes mellitus and mild preeclampsia. Hypertens Pregnancy. (2012) 31:307–15. doi: 10.3109/10641955.2010.507848
35. Liu CW, Ke SR, Tseng GS, Wu YW, Hwang JJ. Elevated serum uric acid is associated with incident hypertension in the health according to various contemporary blood pressure guidelines. Nutr Metab Cardiovasc Dis. (2021) 31:1209–18. doi: 10.1016/j.numecd.2021.01.003
36. Ponticelli C, Podesta MA, Moroni G. Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. (2020) 98:1149–59. doi: 10.1016/j.kint.2020.05.056
37. Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, et al. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol. (2011) 65:542–8. doi: 10.1111/j.1600-0897.2010.00960.x
38. Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. (2001) 38:263–355. doi: 10.1080/20014091084227
39. Cheung BM, Ong KL, Tso AW, Cherny SS, Sham PC, Lam TH, et al. Gamma-glutamyl transferase level predicts the development of hypertension in Hong Kong Chinese. Clin Chim Acta. (2011) 412:1326–31. doi: 10.1016/j.cca.2011.03.030
40. Onat A, Can G, Örnek E, Çiçek G, Ayhan E, Dogan Y. Serum γ-glutamyltransferase: independent predictor of risk of diabetes, hypertension, metabolic syndrome, and coronary disease. Obesity. (2012) 20:842–8. doi: 10.1038/oby.2011.136
41. Zatu MC, Van Rooyen JM, Kruger A, Schutte AE. Alcohol intake, hypertension development and mortality in black South Africans. Eur J Prev Cardiol. (2016) 23:308–15. doi: 10.1177/2047487314563447
42. McCallum L, Panniyammakal J, Hastie CE, Hewitt J, Patel R, Jones GC, et al. Longitudinal blood pressure control, long-term mortality, and predictive utility of serum liver enzymes and bilirubin in hypertensive patients. Hypertension. (2015) 66:37–43. doi: 10.1161/HYPERTENSIONAHA.114.04915
43. Lee DH, Blomhoff R, Jacobs DR Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. (2004) 38:535–9. doi: 10.1080/10715760410001694026
44. Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf). (2013) 208:224–33. doi: 10.1111/apha.12106
45. Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int. (2008) 73:989–91. doi: 10.1038/ki.2008.104
46. Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. (1999) 100:2168–76. doi: 10.1161/01.CIR.100.21.2168
47. Webber M, Krishnan A, Thomas NG, Cheung BM. Association between serum alkaline phosphatase and C-reactive protein in the United States National Health and Nutrition Examination Survey 2005-2006. Clin Chem Lab Med. (2010) 48:167–73. doi: 10.1515/CCLM.2010.052
48. Cheung BM, Ong KL, Wong LY. Elevated serum alkaline phosphatase and peripheral arterial disease in the United States National Health and Nutrition Examination Survey 1999-2004. Int J Cardiol. (2009) 135:156–61. doi: 10.1016/j.ijcard.2008.03.039
49. Tonelli M, Curhan G, Pfeffer M, Sacks F, Thadhani R, Melamed ML, et al. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation. (2009) 120:1784–92. doi: 10.1161/CIRCULATIONAHA.109.851873
50. Cheung BM, Ong KL, Cheung RV, Wong LY, Wat NM, Tam S, et al. Association between plasma alkaline phosphatase and C-reactive protein in Hong Kong Chinese. Clin Chem Lab Med. (2008) 46:523–7. doi: 10.1515/CCLM.2008.111
51. Kunutsor SK, Bakker SJ, Kootstra-Ros JE, Gansevoort RT, Gregson J, Dullaart RP. Serum alkaline phosphatase and risk of incident cardiovascular disease: interrelationship with high sensitivity c-reactive protein. PLoS ONE. (2015) 10:e0132822. doi: 10.1371/journal.pone.0132822
52. Kuroda R, Nogawa K, Watanabe Y, Morimoto H, Sakata K, Suwazono Y. Association between high-sensitive c-reactive protein and the development of liver damage in japanese male workers. Int J Environ Res Public Health. (2021) 18:2985–96. doi: 10.3390/ijerph18062985
Keywords: biomarker, blood pressure, hypertensive disorders of pregnancy, longitudinal cohort study, pregnancy
Citation: Chen Y, Ou W, Lin D, Lin M, Huang X, Ni S, Chen S, Yong J, O'Gara MC, Tan X and Liu R (2021) Increased Uric Acid, Gamma-Glutamyl Transpeptidase and Alkaline Phosphatase in Early-Pregnancy Associated With the Development of Gestational Hypertension and Preeclampsia. Front. Cardiovasc. Med. 8:756140. doi: 10.3389/fcvm.2021.756140
Received: 10 August 2021; Accepted: 21 September 2021;
Published: 15 October 2021.
Edited by:
Zhen Yang, The First Affiliated Hospital of Sun Yat-Sen University, ChinaReviewed by:
Lingfang Zeng, King's College London, United KingdomWanling Xuan, Augusta University, United States
Copyright © 2021 Chen, Ou, Lin, Lin, Huang, Ni, Chen, Yong, O'Gara, Tan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuerui Tan, doctortxr@126.com
†These authors have contributed equally to this work and share first authorship
 Yequn Chen
Yequn Chen Weichao Ou1,2†
Weichao Ou1,2†  Xuerui Tan
Xuerui Tan Ruisheng Liu
Ruisheng Liu