Low-density lipoprotein-cholesterol lowering effect of a nutraceutical regimen with or without ezetimibe in hypercholesterolaemic patients with statin intolerance
- 1Dobney Hypertension Centre, Medical School, University of Western Australia, Perth, WA, Australia
- 2School of Population Health, Curtin University, Perth, WA, Australia
- 3Medical School, University of Western Australia, Perth, WA, Australia
- 4Lipid Disorders Clinic, Department of Cardiology, Royal Perth Hospital, Perth, WA, Australia
Background: Statins are the most widely prescribed medication to lower low-density lipoprotein cholesterol (LDL-c). However, a significant portion of patients are unable to tolerate them due to side effects, most commonly muscle related. Nutraceuticals, natural plant derivatives with lipid-lowering properties, may provide an alternative to lower LDL-c in these patients.
Aims: To investigate whether a nutraceutical regimen, either alone or in combination with ezetimibe, can lower LDL-c in patients with hypercholesterolemia who are intolerant to statins.
Methods: Participants were recruited into a double-blind, randomized, placebo-controlled intervention study. Treatments were (i) placebo, (ii) nutraceutical (500 mg berberine, 200 mg red yeast rice (RYR), 2 g plant sterols)/daily, (iii) ezetimibe (10 mg)/daily, or (iv) the combination of nutraceutical and ezetimibe/daily. At baseline and week 8, all participants provide a fasting blood sample for assessment of lipid profile and safety bloods.
Results: Fifty participants were randomized, with 44 completing the treatment period. Following adjustment for baseline levels and compared with placebo, LDL-c was significantly reduced (all p < 0.0001) with ezetimibe (−1.02 mmol/L), nutraceutical (−1.15 mmol/L) and the nutraceutical and ezetimibe combination (−1.92 mmol/L). Non-HDL cholesterol was significantly reduced (all p < 0.0001) with ezetimibe (−1.29 mmol/L), nutraceutical (−1.37 mmol/L) and the nutraceutical and ezetimibe combination (−2.18 mmol/L). Remnant cholesterol and triglycerides was significantly reduced with the nutraceutical and ezetimibe combination (p = 0.018).
Conclusion: A nutraceutical regimen (berberine, RYR and plant sterols) and ezetimibe independently and additively lower LDL-c in patients with hypercholesterolemia who are intolerant to statins.
Introduction
Cardiovascular disease (CVD) is the leading cause of death. There is significant evidence to support lowering low-density lipoprotein cholesterol (LDL-c) to reduce atherosclerotic cardiovascular disease (ASCVD) (1). Meta-analysis highlights for every 1 mmol/L LDL-c reduction there is a significant 22% relative risk reduction in major vascular and coronary events (2). Statins are the most widely prescribed and evidence-based drugs for lowering LDL-c and reducing cardiovascular events (3). International guidelines recommend the use of statin therapy to reduce risk in a range of patient populations (clinical ASCVD, diabetes mellitus and hyperlipidemia), where the greater the LDL-c reduction (by >50%.), the greater the subsequent risk reduction (4, 5).
Statins competitively block the active site of HMG-CoA reductase, reducing hepatic cholesterol synthesis and increasing cell surface LDL receptor expression (6). This increases clearance of LDL-c from the bloodstream and reduces circulating levels by 20–55% (6). Combination therapy with ezetimibe has additional beneficial effects on both LDL-c and cardiovascular outcomes (7, 8). Statin toxicity or intolerance is most commonly reported as statin-associated muscle symptoms (SAMS), presenting as myalgia, myopathy, myositis with elevated CK, or at its most severe, rhabdomyolysis, with some people reporting additional joint and abdominal pain (9–11). Ezetimibe works by blocking the NPC1L1 transporter, inhibiting the absorption of cholesterol in the intestine, upregulating the LDL receptor and thereby lowering LDL-c. As demonstrated by the IMPROVE-IT study, it has incremental effects in lowering LDL-c and cardiovascular events in high risk populations when added to statin therapy (7, 8). Nutraceuticals are plant or food-based compounds or derivatives that provide health benefits (12, 13), including improving lipid profiles (14–16). Their use as adjuncts to standard pharmacotherapy is recommended by most international guidelines and in addition to their efficacy, their use may also be advantageous in terms of safety and tolerance, particularly in patients with documented SAMS (15). Berberine, red yeast rice (RYR), and plant sterols, have all been demonstrated to modestly reduce LDL-c via distinct yet complimentary mechanisms (16).
The aim of this pilot study was to investigate the efficacy of a nutraceutical regimen, either alone or in combination with ezetimibe, on LDL-c in hypercholesterolaemic patients with documented statin intolerance who are not currently achieving lipid targets. We hypothesized that the nutraceutical regimen, consisting of berberine, RYR and plant sterols, and ezetimibe would independently and additively lower plasma concentration of LDL-c in these patients.
Study design and methods
Participants
Participants with documented statin intolerance and not currently achieving ideal lipid goals (fasting LDL-c > 1.8 mmol/L and < 7 mmol/L) were recruited from the Lipid Disorders Clinic, Department of Cardiology, Royal Perth Hospital. Statin intolerance was defined as an inability to tolerate two or more statins due to side effects (including myalgia) that started or increased during statin therapy and resolved on cessation, or severe intolerance to one statin at multiple doses, resulting in refusal to try additional statins. Participants who were taking ezetimibe or other cholesterol-lowering medication at screening (n = 20) underwent a 4-week washout prior to randomization. Exclusion criteria included recent acute coronary syndrome (<3 months), abnormal plasma biochemistry at screening suggesting an underlying cause of statin intolerance (e.g., abnormal thyroid function, concomitant medication use, hepatic or renal impairment), elevated creatine kinase (two times the upper limit of normal), pregnant or lactating women, < 30 or > 80 years of age, history of necrotizing auto-immune myositis or autoantibodies against HMG-CoA reductase, or inability to provide consent. The study was carried out in accordance with Good Clinical Practice and was approved by Royal Perth Hospital Human Research Ethics Committee (RGS0891). The trial was registered at www.anzctr.org.au as ACTRN12618000660280.
Study design and interventions
A randomized, double-blind, placebo-controlled study was carried out between May 2019 and January 2022. Participants underwent screening to assess suitability to take part in the study, which included assessment of their previous statin intolerance, a fasting blood sample (lipid profile, blood glucose, HbA1c, serum creatinine, renal function, liver function, vitamin D, full blood picture and thyroid function), measurement of height and weight, clinic BP, medical history, and medical assessment. All had received previous medical advice at first review in clinic on a fat-modified diet as well as the need to achieve a desirable body weight with diet and exercise. Following screening, participants were randomly allocated to a treatment group by computer generated random numbers. Treatment groups were (i) nutraceutical placebo + ezetimibe placebo; (ii) nutraceutical active + ezetimibe placebo; (iii) nutraceutical placebo + ezetimibe active; or (iv) nutraceutical active + ezetimibe active. The treatment period was 8 weeks.
Study treatments were prepared by Optima Ovest (Perth, Australia), a GMP licensed company and dispensed through Oxford Compounding Pharmacy in compliance with Therapeutic Goods Administration (TGA) requirements. The nutraceutical treatment regimen contained 500 mg berberine (berberine chloride), 200 mg RYR (equivalent to 3 mg monacolin K) and 2 g of plant sterols (vegetable oil plant sterol esters). Ezetimibe was given as a 10 mg dose, and the placebo contained avicel (microcrystalline cellulose). Active agents were encapsulated to ensure all medications were identical in appearance. Tablets were taken daily with food and compliance monitored through tablet count.
Lipid and lipoprotein analysis
Fasting plasma lipid profiles [total cholesterol, LDL-c, triglycerides, high-density lipoprotein cholesterol (HDL-c), non-HDL-c and remnant cholesterol (REM-c)] were assessed at weeks 0, 4 and 8. Total cholesterol, triglycerides and HDL-c were analyzed using enzymatic colorimetric assays (Abbot Architect c16000 Analyser) by PathWest Laboratories with reagents traceable to the National Reference System. LDL-c was calculated using the Friedewald equation, non-HDL-c was calculated by subtracting HDL-c from total cholesterol, and REM-c was calculated by subtracting HDL-c and LDL-c from total cholesterol.
Adverse events and participant reported quality of life outcomes
Safety biochemistry (creatine kinase, liver function, renal function, thyroid function and full blood picture) was performed at weeks 0 and 8 by PathWest Laboratories. All results flagged as being outside normal ranges were reviewed by the study clinician. All participants completed EQUAL EQ-5D quality of life questionnaire at weeks 0 and 8. In addition, regular contact was maintained with all participants to document any side effects and/or adverse events. Patient reported myalgia during the study treatment period was documented to include location and self-reported pain level, with review by the study clinician.
Statistical analyses
This was a pilot study investigating the safety and efficacy of nutraceuticals with or without ezetimibe in patients intolerant to statins. The primary outcome was reduction in LDL-c. Secondary outcomes were acceptability of the study treatments, including development of side effects and self-reported quality of life. Power calculations were based on 80 participants required to see a 0.8 mmol/L reduction in LDL-c with either the nutraceutical or ezetimibe intervention. Interim analysis of the results revealed significant improvements in LDL-c and as a result, the study was concluded early. Analysis of the results was carried out using the Statistical Packages for the Social Sciences (SPSS, Version 26). Analysis was carried out both as per protocol (all patients who completed the full 8-week treatment period) and on an intention-to-treat basis (patients who completed part of the 8-week treatment period), with results presented as mean ± SEM or percentage change from baseline. Analysis was carried out using a maximum-likelihood random effect mixed regression model for main effects, with intergroup comparisons performed using univariate analysis of variance (ANOVA) with Bonferroni post-hoc analysis to account for multiple comparisons. Categorical variables were analyzed using Chi-square. Statistical significance was considered if p < 0.05.
Results
Participant characteristics
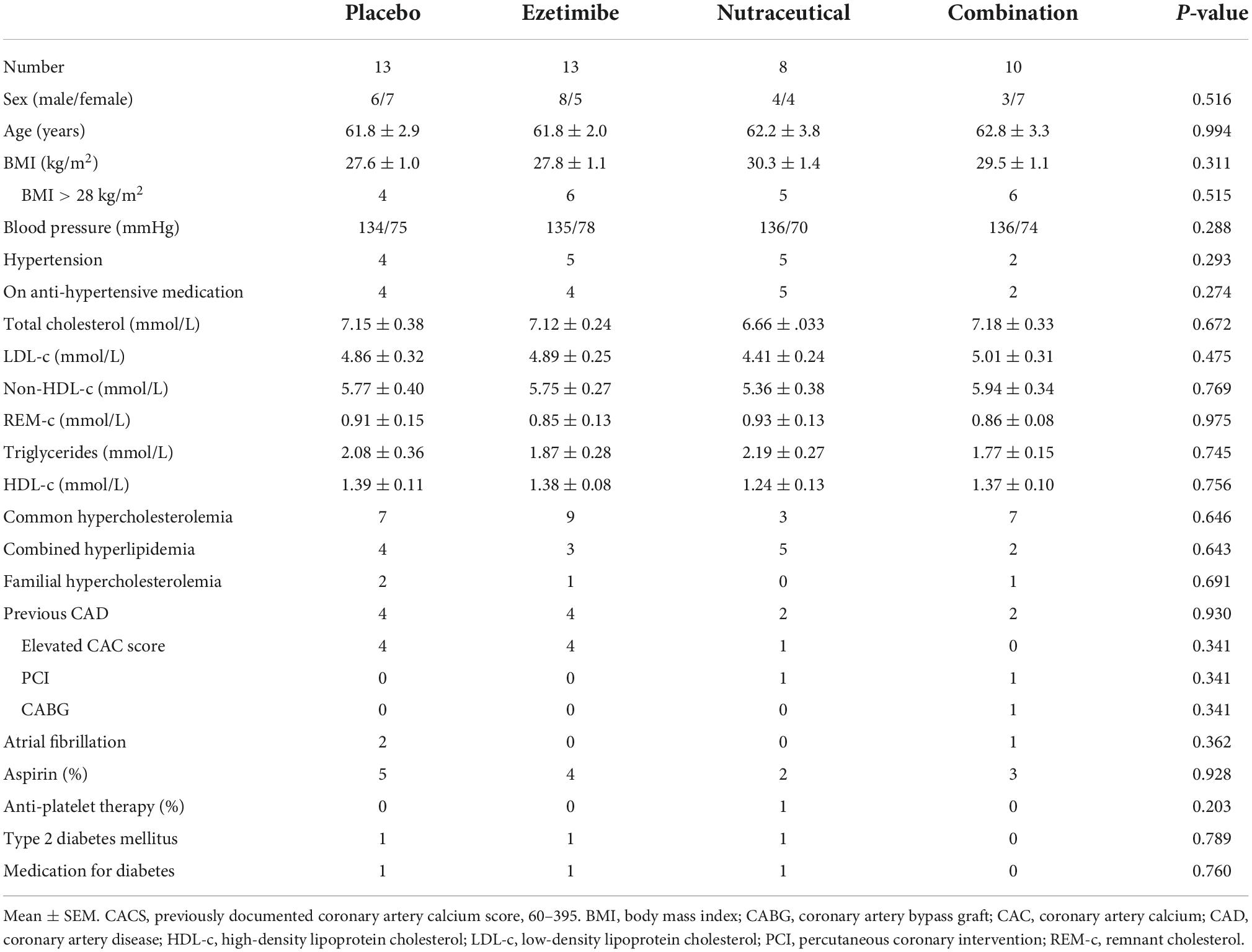
Ninety-nine participants from the Lipid Disorders Clinic were identified and screened. Of these, 49 were excluded or not interested in taking part, with the remaining 50 randomized to treatment. Previous documented statin intolerance revealed 3 participants had previously tried only 1 statin at multiple doses and experienced severe myalgia resulting in refusal to try additional statins. The remaining 47 participants had all tried = 2 statins at one or more doses and experienced myalgia. Additional self-reported side effects on statins included joint aches (n = 4), gastrointestinal issues (dyspepsia, bloating, nausea, n = 4) and/or neurological changes (brain fog, n = 7). The treatment groups were well matched at baseline, with no significant differences for age, gender, BMI, blood pressure or lipid profile. As expected, all participants had elevated total cholesterol, LDL-c and non-HDL-c (Table 1). Four (8%) patients had probable phenotypic familial hypercholesterolemia (FH, fasting LDL-c > 4.9 mmol/L and a Dutch Lipid Score = 5) and 14 (28%) patients had combined hyperlipidemia (fasting LDL-c > 4 mmol/L, triglycerides > 2 mmol/L, obesity). Twelve (24%) patients had evidence of coronary artery disease, with 9 having elevated coronary artery calcium score (CACS ranging from 60 to 395), with 2 having undergone previous percutaneous coronary intervention and 1 a coronary artery bypass graft. In addition, 16 (32%) had hypertension, with 15 on anti-hypertensive therapy and 3 (6%) patients had type 2 diabetes mellitus with all receiving treatment. Fourteen (28%) patients were on aspirin and 1 (2%) was on anti-platelet therapy (Table 1). Using the Australian Absolute Cardiovascular Risk Score calculator (excluding patients with probable FH and previous coronary revascularization), the average 5-year risk score at baseline was 11, placing the cohort at moderate-to-high risk (10–15%). Only 1 patient had had a previous coronary event (CABG). None of the patients met the Australian criteria for Pharmaceutical Benefits Schedule re-imbursement of a PCSK9 inhibitor.
Effects of study treatments on lipid profiles
Analysis of main effects revealed no significant interaction between the ezetimibe and nutraceutical treatments (Table 2). There were significant main effect reductions in LDL-c, total cholesterol and non-HDL-c with the nutraceuticals compared with placebo, as well as with ezetimibe compared with placebo, but no significant effect of either intervention on triglycerides, HDL-c or REM-c (Table 2). Comparative analysis of all four treatment groups individually revealed significant reductions in LDL-c of 22, 25, and 41% with the ezetimibe, nutraceutical and combination therapies, respectively, compared with placebo (Figure 1A). There were also significant reductions in total cholesterol and non-HDL-c with the ezetimibe, nutraceutical and combination therapies compared with placebo (Figures 1B,C). REM-c and triglycerides were significantly lower with the combination therapy compared with placebo (Figures 1D,E), while HDL-c was not altered by any of the treatments (Figure 1F). Within the whole cohort, only one patient attained an LDL-c of < 2.5 mmol/L at week 8 and this was with the nutraceutical and ezetimibe combination therapy.
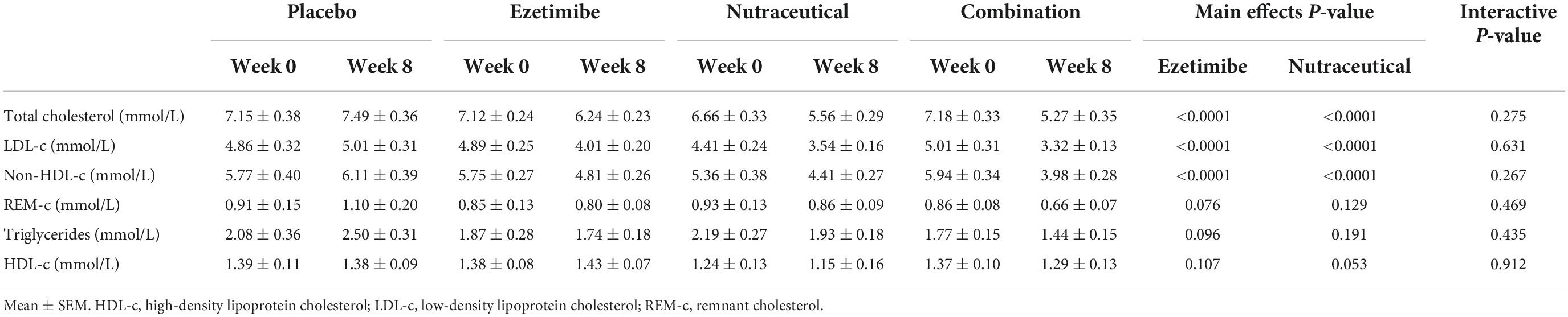
Table 2. Effect of the interventions on plasma total cholesterol, LDL-c, non-HDL-c, REM-c, triglycerides, and HDL-c over the 8-week treatment period.
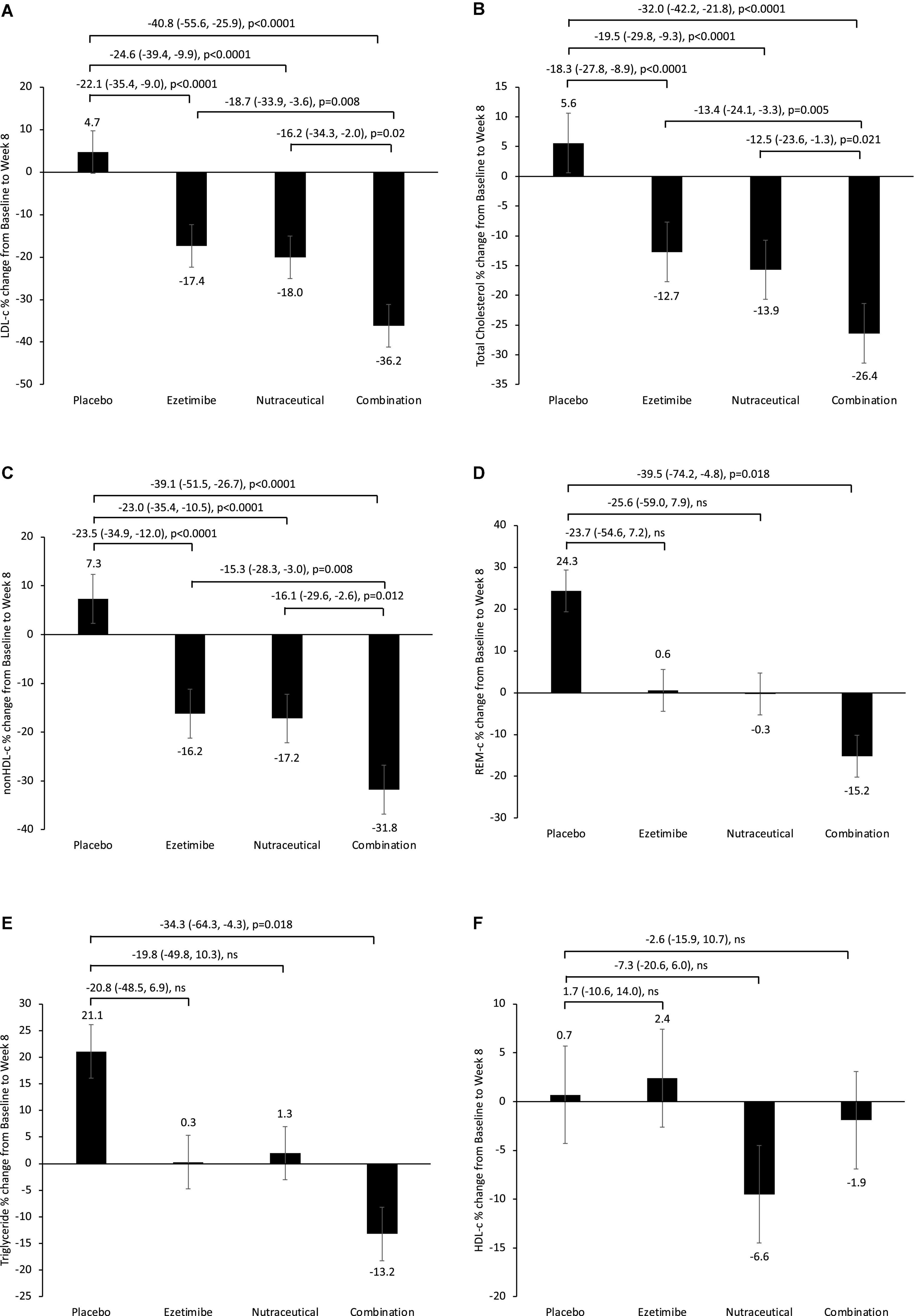
Figure 1. Percentage change from baseline in fasting plasma (A) LDL-c, (B) total cholesterol, (C) non-HDL-c, (D) REM-c, (E) triglycerides and (F) HDL-c, following 8-week treatment with placebo, ezetimibe, nutraceutical regimen, or combination. Intergroup comparisons via univariate ANOVA with Bonferroni post-host comparisons.
Side effects, adverse events and self-reported quality of life
Six participants withdrew from the study within the first week following randomization (1 in the ezetimibe, 3 in the nutraceutical and 2 in the combination group) due to side effects (gastrointestinal, myalgia and joint aches) and were not included in the analysis. Three participants were unable to complete the full 8-week treatment period (1 in ezetimibe, 1 in nutraceutical and 1 in the combination group) due to unrelated events (admission for coronary artery bypass graft, flare up of ulcerative colitis and development of a rash following a spider bite) and were included in the intention-to-treat analysis. Minor gastrointestinal and myalgia side effects were reported equally across all four treatment groups. Serum creatine kinase levels, along with other safety blood parameters (liver and renal function) were not significantly different at weeks 0 and 8, nor were there any significant changes with any of the study treatments (Table 3). Analysis of the EQUAL EQ-5D questionnaires revealed no significant difference in perceived quality of life, including self-reported overall health rating from baseline to the end of the study treatment period, or between treatment groups (Supplementary Table 1).
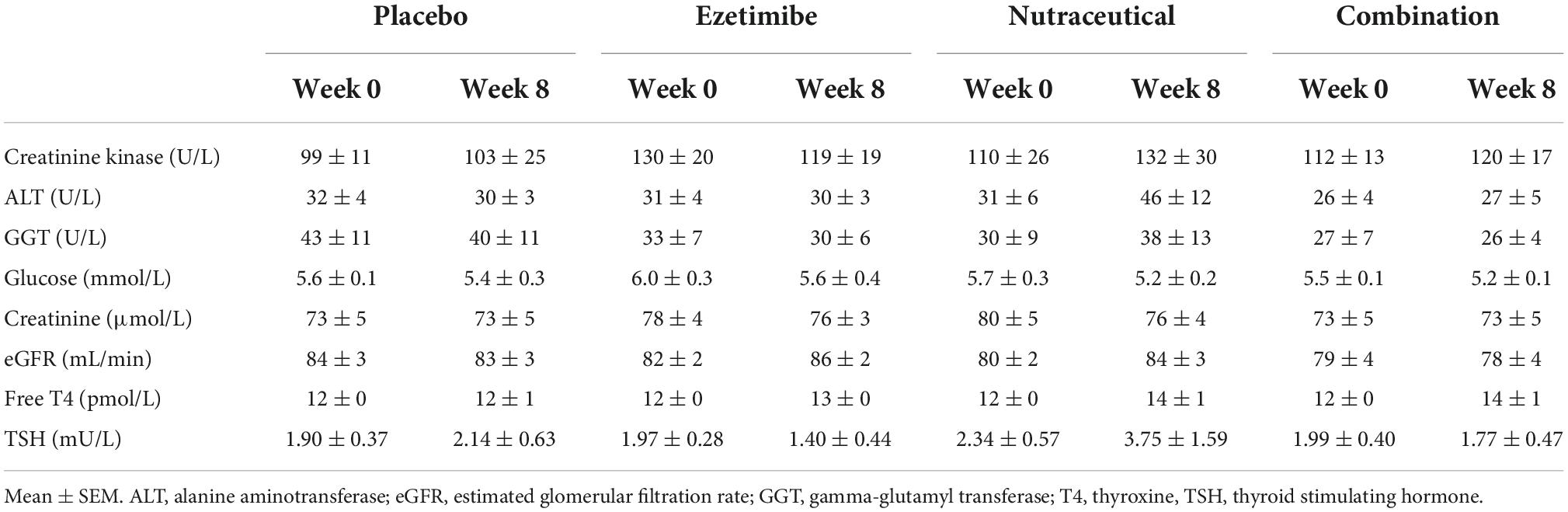
Table 3. Effect of the interventions on laboratory blood safety parameters including creatinine kinase, liver function, renal function, fasting glucose, and thyroid function over the 8-week treatment period.
Discussion
The present study has demonstrated the LDL-c lowering benefit of a nutraceutical regimen containing berberine, RYR and plant sterols, alone and in combination with ezetimibe, in patients intolerant to statins who on average were at moderate-to-high cardiovascular risk. The nutraceutical and ezetimibe combination lowered LDL-c by ∼2 mmol/L, and significantly lowered non-HDL-c, REM-c, triglycerides and total cholesterol. The observed reduction in LDL-c with the combination was greater than that seen with either treatment alone. These findings clearly demonstrate the LDL-c lowering efficacy of nutraceuticals in patients with hypercholesterolemia and mixed hyperlipidemia and further demonstrates their practical use as adjunct therapy to other lipid-lowering agents.
There are over 40 nutraceuticals suggested to have lipid-lowering properties and we and others have reported on the benefits of various nutraceuticals, either alone or in combination with ezetimibe (16–19). An International Lipid Expert Panel supports their use as alternative or adjunct therapies in statin intolerant patients due to their relative safety and potential LDL-c lowering properties (20, 21). Armolipid Plus, a proprietary formulation of 6 plant-based compounds has been the most widely studied, with clinical review and meta-analysis revealing significant reductions in LDL-c and triglycerides (22, 23). The nutraceutical regimen in our study consisted of berberine, RYR and plant sterols, which all have distinct yet complementary mechanisms for LDL-c reduction. Berberine acts as a natural PCSK9 inhibitor, the protein responsible for degradation of LDL receptors and thus can increase the number and activity of hepatic LDL receptors (15, 16). Fermentation of RYR by the fungus Monascus purpureus results in the formation of monacolin K (naturally occurring lovastatin), which can inhibit HMG-CoA reductase (15, 16). Several expert opinion position statements suggest that the use of RYR is both safe and effective for reducing LDL-c. While its use shouldn’t be in place of conventional lipid-lowering therapy, it may be considered in specific conditions, including statin intolerance (24, 25). Plant sterols are naturally occurring steroid like substances that are structurally similar to cholesterol, but poorly absorbed in the intestinal tract, thus decreasing the intestinal absorption of cholesterol by lowering cholesterol content in micelles (15, 16). The complementary actions of these three nutraceuticals in the present study resulted in reductions in LDL-c comparable to that seen with ezetimibe, which acts by inhibiting cholesterol absorption via the NPC1L1 transporter protein, and importantly, additive effects when taken in combination. Furthermore, this effect was observed without adversely affecting blood safety parameters or the development of self-reported side effects. The beneficial actions of these nutraceuticals may also offer alternative or adjunct therapies to the recently developed bempedoic acid and PCSK9 inhibitors, particularly in patients who are unable to tolerate statins and where use of these therapeutics is restricted. Bempedoic acid has been shown to be well-tolerated and to significantly reduce LDL-c when added to background therapy, including ezetimibe, in both statin-intolerant patients (26, 27), and in high-risk ASCVD and FH patients (28, 29). Analysis of the GAUSS-3 trial showed significant reductions in LDL-c levels in statin-intolerant patients treated with evolocumab compared to ezetimibe, with no significant muscle-related adverse events reported (30).
In spite of these observed positive effects on LDL-c, however, it should be noted that only one patient achieved a LDL-c of ≤ 2.5 mmol/L, the recommended target for moderate risk patients. This may be due in part to the presence of patients with very elevated levels of LDL-c, including 8% of the cohort being classified as having probable phenotypic FH and 28% as having combined hyperlipidemia. FH patients rarely achieve treatment goals with standard therapy. In patients with statin intolerance, treatment with bempedoic acid, bile acid sequestrants or PCSK9 inhibitors would be indicated. However, bempedoic acid is not registered in Australia, none met PCSK9 criteria and bile acid sequestrants are very poorly tolerated owing to gastrointestinal intolerance. As such, although significant reductions in LDL-c were observed, the present study demonstrates the need for further investigation into the benefits of different nutraceutical combinations, or the value of increasing the dose of individual nutraceutical components, particularly in moderate to high-risk and hard to treat patients.
Minor side effects of gastrointestinal upset and myalgia were reported equally across all four treatment groups, including placebo. This may be in part due to the “nocebo” effect; negative expectations about the effects of a treatment, resulting in higher than expected adverse event reporting (31–33). A recent double-blind, three-group, n-of-1 trial demonstrated that in patients who had discontinued statin therapy due to side effects, 90% of the symptom burden caused by a statin re-challenge was also caused by a placebo (34). Large-scale trials have also observed development of SAMS in statin intolerant patients randomized to either PCSK9 inhibitors and/or ezetimibe, drugs that operate via mechanisms distinct to statins (35, 36). The true prevalence of statin intolerance is unclear (9, 31, 37, 38), with a recent meta-analysis of randomized controlled trials and observational studies suggesting it is 9.1%, and even lower when using various international guidelines (39). Regardless, non-adherence or discontinuation of statin therapy significantly increases a person’s lifetime risk of major cardiovascular events and is thus a major clinical problem that needs to be addressed (40).
A limitation of the study is that 49% of the cohort were considered to be overweight or obese (BMI > 28 kg/m2) and we did not employ a dietary weight loss regimen to assess the incremental effect of weight loss on the primary endpoint of LDL-c reduction. Further, the sample size was relatively small and the period of intervention short. However, statistical power was increased by our factorial design. The formulations used in the study were not strictly practicable but were required to ensure adequate blinding. The strengths of the study were the double-blind, randomized placebo-controlled, factorial interventional design, with close monitoring of treatment related side effects.
In conclusion, the present study has demonstrated the significant beneficial effect of a nutraceutical regimen containing berberine, RYR and plant sterols, alone and in combination with ezetimibe, in lowering LDL-c in patients intolerant to statins. The observed ∼2 mmol/L reduction in LDL-c with the combination therapy is likely to be clinically significant and if sustained, could result in reduction in CVD events (2). Although minor side-effects were reported across all patient groups, the nutraceuticals, either alone or in combination with ezetimibe, had no adverse effects on blood safety parameters or self-reported quality of life. Further research on the formulation of various nutraceutical combinations and doses to improve effectiveness and palatability, whilst still being available at an acceptable cost, is warranted, as well as their use as adjuncts to other treatments, including PCSK9 inhibitors and bempedoic acid.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by the Royal Perth Hospital Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
NW conducted the study, initial data analysis, and first draft of the manuscript, with subsequent input from CR and GW. All authors contributed to the study design and funding acquisition, reviewed, and edited the manuscript prior to submission.
Funding
This study was funded by a National Heart Foundation of Australia Vanguard Grant (ID101861).
Acknowledgments
We thank the participants who took part in the study as well as the nurses in the Lipid Disorders Clinic, Department of Cardiology, Royal Perth Hospital.
Conflict of interest
Author GW received honoraria for advisory boards and lectures or research grants from Amgen, Arrowhead, Astra Zeneca, Esperion, Novartis, Pfizer, Regeneron and Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1060252/full#supplementary-material
References
1. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. (2017) 38:2459–72. doi: 10.1093/eurheartj/ehx144
2. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. (2016) 316:1289–97. doi: 10.1001/jama.2016.13985
3. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. (2005) 366:1267–78. doi: 10.1016/S0140-6736(05)67394-1
4. Grundy NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2018) 139:e1082–143. doi: 10.1161/CIR.0000000000000624
5. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2019) 41:111–88. doi: 10.1093/eurheartj/ehz826
6. Buhaescu I, Izzedine H. Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem. (2007) 40:575–84. doi: 10.1016/j.clinbiochem.2007.03.016
7. Cannon CP, Blazing MA, Giugliano RP, McCagg A, white ja, theroux p, et al. ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. (2015) 372:2387–97. doi: 10.1056/NEJMoa1410489
8. Murphy SA, Cannon CP, Blazing MA, Giugliano RP, White JA, Lokhnygina Y, et al. Reduction in total cardiovascular events with ezetimibe/simvastatin post-acute coronary syndrome: the IMPROVE-IT trial. J Am Coll Cardiol. (2016) 67:353–61. doi: 10.1016/j.jacc.2015.10.077
9. Keen HI, Krishnarajah J, Bates TR, Watts GF. Statin myopathy: the fly in the ointment for the prevention of cardiovascular disease in the 21st century? Exp Opin Drug Safety. (2014) 13:1227–39. doi: 10.1517/14740338.2014.937422
10. Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernandez I, Trallero-Araguas E, Milisenda JC, Martinez MA, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Exp Rev Clin Immunol. (2018) 14:215–24. doi: 10.1080/1744666X.2018.1440206
11. Saxon DR, Eckel RH. Statin intolerance: a literature review and management strategies. Prog Cardiovas Dis. (2016) 59:153–64. doi: 10.1016/j.pcad.2016.07.009
12. Barbagallo CM, Cefalu AB, Noto D, Averna MR. Role of nutraceuticals in hypolipidemic therapy. Front Cardiovas Med. (2015) 2:22. doi: 10.3389/fcvm.2015.00022
13. Mannarino MR, Ministrini S, Pirro M. Nutraceuticals for the treatment of hypercholesterolemia. Eur J Int Med. (2014) 25:592–9. doi: 10.1016/j.ejim.2014.06.008
14. Johnston TP, Korolenko TA, Pirro M, Sahebkar A. Preventing cardiovascular heart disease: promising nutraceutical and non-nutraceutical treatments for cholesterol management. Pharmacol Res. (2017) 120:219–25. doi: 10.1016/j.phrs.2017.04.008
15. Sahebkar A, Serban MC, Gluba-Brzozka A, Mikhailidis DP, Cicero AF, Rysz J, et al. Lipid-modifying effects of nutraceuticals: an evidence-based approach. Nutrition. (2016) 32:1179–92. doi: 10.1016/j.nut.2016.04.007
16. Ward N, Sahebkar A, Banach M, Watts G. Recent perspectives on the role of nutraceuticals as cholesterol-lowering agents. Curr Opin Lipidol. (2017) 28:495–501. doi: 10.1097/MOL.0000000000000455
17. Ward NC, Pang J, Ryan JDM, Watts GF. Nutraceuticals in the management of patients with statin-associated muscle symptoms, with a note on real-world experience. Clin Cardiol. (2018) 41:159–65. doi: 10.1002/clc.22862
18. Backes JM, Ruisinger JF, Gibson CA, Moriarty PM. Statin-associated muscle symptoms-managing the highly intolerant. J Clin Lipidol. (2017) 11:24–33. doi: 10.1016/j.jacl.2017.01.006
19. Toth PP, Patti AM, Giglio RV, Nikolic D, Castellino G, Rizzo M, et al. Management of statin intolerance in 2018: still more questions than answers. Am J Cardiovasc Drugs. (2018) 18:157–73. doi: 10.1007/s40256-017-0259-7
20. Banach M, Patti AM, Giglio RV, Cicero AFG, Atanasov AG, Bajraktari G, et al. The role of nutraceuticals in statin intolerant patients. J Am Coll Cardiol. (2018) 72:96–118. doi: 10.1016/j.jacc.2018.04.040
21. Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, et al. Lipid-lowering nutraceuticals in clinical practice: position paper from an international lipid expert panel. Nutr Rev. (2017) 75:731–67. doi: 10.1093/nutrit/nux047
22. Millan J, Cicero AF, Torres F, Anguera A. Effects of a nutraceutical combination containing berberine (BRB), policosanol, and red yeast rice (RYR), on lipid profile in hypercholesterolemic patients: a meta-analysis of randomised controlled trials. Clin Invest Arterioscl Publi Ofic Soc Espanola De Arterioscl. (2016) 28:178–87. doi: 10.1016/j.arteri.2016.03.002
23. Barrios V, Escobar C, Cicero AF, Burke D, Fasching P, Banach M, et al. A nutraceutical approach (armolipid Plus) to reduce total and LDL cholesterol in individuals with mild to moderate dyslipidemia: review of the clinical evidence. Atheroscl Suppl. (2017) 24:1–15. doi: 10.1016/j.atherosclerosissup.2016.10.003
24. Banach M, Catapano AL, Cicero AFG, Escobar C, Foger B, Katsiki N, et al. Red yeast rice for dyslipidaemias and cardiovascular risk reduction: a position paper of the international lipid expert panel. Pharmacol Res. (2022) 183:106370. doi: 10.1016/j.phrs.2022.106370
25. Banach M, Bruckert E, Descamps OS, Ellegard L, Ezhov M, Foger B, et al. The role of red yeast rice (RYR) supplementation in plasma cholesterol control: a review and expert opinion. Atherosclerosis Supplements. (2019) 39:e1–8. doi: 10.1016/j.atherosclerosissup.2019.08.023
26. Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. (2018) 277:195–203. doi: 10.1016/j.atherosclerosis.2018.06.002
27. Laufs U, Ballantyne CM, Banach M, Bays H, Catapano AL, Duell PB, et al. Efficacy and safety of bempedoic acid in patients not receiving statins in phase 3 clinical trials. J Clin Lipidol. (2022) 16:286–97. doi: 10.1016/j.jacl.2022.03.001
28. Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prevent Cardiol. (2020) 27:593–603. doi: 10.1177/2047487319864671
29. Ballantyne CM, Banach M, Bays HE, Catapano AL, Laufs U, Stroes ESG, et al. Long-term safety and efficacy of bempedoic acid in patients with atherosclerotic cardiovascular disease and/or heterozygous familial hypercholesterolemia (from the CLEAR harmony open-label extension study). Am J Cardiol. (2022) 174:1–11. doi: 10.1016/j.amjcard.2022.03.020
30. Nissen SE, Stroes E, Dent-Acosta RE, Rosenson RS, Lehman SJ, Sattar N, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. (2016) 315:1580–90. doi: 10.1001/jama.2016.3608
31. Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K, et al. Statin intolerance - an attempt at a unified definition. Position paper from an international lipid expert panel. Arch Med Sci. (2015) 11:1–23.
32. Mach F, Ray KK, Wiklund O, Corsini A, Catapano AL, Bruckert E, et al. Adverse effects of statin therapy: perception vs. the evidence - focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. (2018) 39:2526–39. doi: 10.1093/eurheartj/ehy182
33. Khan S, Holbrook A, Shah BR. Does googling lead to statin intolerance? Int J Cardiol. (2018) 262:25–7. doi: 10.1016/j.ijcard.2018.02.085
34. Wood FA, Howard JP, Finegold JA, Nowbar AN, Thompson DM, Arnold AD, et al. N-of-1 trial of a statin, placebo, or no treatment to assess side effects. N Engl J Med. (2020) 383:2182–4. doi: 10.1056/NEJMc2031173
35. Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. (2012) 308:2497–506. doi: 10.1001/jama.2012.25790
36. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. (2015) 9:758–69. doi: 10.1016/j.jacl.2015.08.006
37. Tobert JA, Newman CB. Statin tolerability: in defence of placebo-controlled trials. Eur J Prevent Cardiol. (2016) 23:891–6. doi: 10.1177/2047487315602861
38. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. (2019) 124:328–50. doi: 10.1161/CIRCRESAHA.118.312782
39. Bytyci I, Penson PE, Mikhailidis DP, Wong ND, Hernandez AV, Sahebkar A, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. (2022) 43:3213–23. doi: 10.1093/eurheartj/ehac015
Keywords: statin intolerance, nutraceuticals, ezetimibe, low-density lipoprotein (LDL), lipids
Citation: Ward NC, Reid CM and Watts GF (2022) Low-density lipoprotein-cholesterol lowering effect of a nutraceutical regimen with or without ezetimibe in hypercholesterolaemic patients with statin intolerance. Front. Cardiovasc. Med. 9:1060252. doi: 10.3389/fcvm.2022.1060252
Received: 03 October 2022; Accepted: 11 November 2022;
Published: 25 November 2022.
Edited by:
Menno Hoekstra, Leiden University, NetherlandsReviewed by:
Antonina Giammanco, University of Palermo, ItalyŽeljko Reiner, University Hospital Centre Zagreb, Croatia
Copyright © 2022 Ward, Reid and Watts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalie C. Ward, natalie.ward@uwa.edu.au
 Natalie C. Ward
Natalie C. Ward Christopher M. Reid2
Christopher M. Reid2  Gerald F. Watts
Gerald F. Watts