Women Specific Characteristics and 1-Year Outcome Among Patients Hospitalized for Peripheral Artery Disease: A Monocentric Cohort Analysis in a Tertiary Center
- 1Vascular Medicine Department, Hôpital Europeen Georges-Pompidou, Assistance Publique Hôpitaux de Paris, Université de Paris (APHP-CUP), Paris, France
- 2Université de Paris, Innovative Therapies in Haemostasis, INSERM, Paris, France
- 3Biosurgical Research Lab (Carpentier Foundation), Assistance Publique Hôpitaux de Paris, Centre-Université de Paris (APHP-CUP), Paris, France
- 4Université de Paris, Paris Research Cardiovascular Center (PARCC), INSERM U970, Paris, France
- 5Hematology Department, Hôpital Europeen Georges-Pompidou, Assistance Publique Hôpitaux de Paris, Université de Paris (APHP-CUP), Paris, France
- 6Interventional Radiology Department, Hôpital Europeen Georges-Pompidou, Assistance Publique Hôpitaux de Paris, Universit de Paris (APHP-CUP), Paris, France
- 7Vascular Surgery Department, Hôpital Europeen Georges-Pompidou, Assistance Publique Hôpitaux de Paris, Université de Paris (APHP-CUP), Paris, France
Although women have lower age-standardized cardiovascular disease incidence, prevalence, and death-related rates than men, there are also reports indicating that women with cardiovascular disease receive less care, fewer investigations, and have poorer outcomes after a coronary event. The aims of this study were to compare the characteristics of men and women hospitalized for peripheral artery disease (PAD), their cardiovascular and limb outcomes, and their 1-year mortality. The study is a prospective registry collecting data about all consecutive patients hospitalized for PAD within the vascular department of the tertiary center Georges-Pompidou European Hospital (Paris, France). Patients were required to have one of three inclusion criteria: previous revascularization of the lower limb or any lower limb artery occlusion due to an atherosclerotic vascular disease or hemodynamic evidence of PAD. Exclusion criteria were patients with lower extremity arterial occlusion due to another cause. All patients were followed-up for at least 12 months after the initial hospitalization. Among the 235 patients included, there were 61 women (26%), older than men with a median age of 75.6 and 68.3 years, respectively. Main cardiovascular risk factors and comorbidities were similar for men and women except more former or current smokers [145 (83.4%) vs. 33 (54.1%)] and more history of coronary heart disease [42 (24.1%) vs. 7 (11.5%)] in men. Most patients [138 (58.8%)] had critical limb ischemia and 97 (41.3%) had claudication, with no difference for sex. After discharge, 218 patients received an antithrombotic therapy (93.2%), 195 a lipid-lowering drug (83.3%), 185 an angiotensin converting enzyme inhibitor or angiotensin-receptor blocker (78.9%), similarly between sex. At 1-year, overall mortality, major adverse cardiovascular events, major adverse limb events did not differ with 23 (13.2%), 11 (6.3%) and 32 (18.4%) in men, and 8 (13.1%), 3 (4.9%), 15 (24.6%) in women, respectively, despite the difference in age. Overall mortality, cardiovascular outcomes, limb revascularization or amputation did not differ between men and women, 1-year after hospitalization for PAD although the latter were older, less smoker and had less coronary artery disease. Due to the small size of this cohort, larger studies and future research are needed to better understand sex-specific mechanisms in the pathophysiology and natural history of PAD.
Introduction
“Despite being responsible for causing 35% of deaths in women each year, cardiovascular disease in women remains understudied, under-recognized, and under-treated, with women under-represented in clinical trials” stated The Lancet women and cardiovascular disease Commission in May 2021 (1). Although women have lower age-standardized cardiovascular disease incidence, prevalence, and death rates than men (2) there are also reports indicating that women with cardiovascular disease receive less care, fewer investigations, and have poorer outcomes after a coronary event (3–6). The lower extremity arterial disease, also known as peripheral artery disease (PAD) in some extent, is the manifestation of the atherosclerotic cardiovascular disease (ASCVD) at the lower limbs level (7). Most patients presenting with asymptomatic PAD do not have a clinical history of cardiac or cerebral ischemic events, although they are at high risk for stroke, myocardial infarction and cardiovascular death (8, 9) with a 10-year cardiovascular mortality of 18.7% in men and 12.6% in women with a low ankle-brachial index (≤ 0.90) (10). Sex difference in symptomatic PAD has been scantly studied and women may be less likely to undergo revascularization than men and more likely under-treated (1, 6, 11–13).
The aims of this study were to compare the characteristics of patients hospitalized for PAD, their cardiovascular and limb outcomes, and their mortality at 1 year according to sex.
Materials and Methods
Study Design
The study is a prospective monocentric registry collecting exhaustive data about all patients consecutively hospitalized for PAD within the Georges-Pompidou European Hospital vascular department (APHP-Université de Paris). The methodology of the registry has already been published elsewhere (14). The protocol was approved by the local ethics committee (IRB CPP Sud Ouest et outre mer II 07 021 08) and patients provided written informed consent before enrollment. The data were retrieved from the patient's computerized record. We collected demographic, clinical, laboratory and imaging data as well as all the complications that had occurred for each patient followed.
Study Population
Eligible patients were at least 18 years of age and hospitalized for symptomatic lower extremity artery disease. Patients were required to have one of three inclusion criteria: previous revascularization of the lower limb or any lower limb artery occlusion due to an ASCVD or hemodynamic evidence of PAD as evidenced by an ankle-brachial index (ABI) of 0.90 or less or a toe-brachial index (TBI) of 0.60 or less, in accordance with current guidelines (7, 15). Exclusion criteria were patients with lower extremity arterial occlusion due to another cause than ASCVD.
All patients were followed-up for at least 12 months after the initial hospitalization. Patient care was provided according to the usual practice, without any change in management strategy. Phone contacts with the patients or their physicians have been performed when required. The primary end point was all cause mortality within 1 year. Registrar's offices have been consulted when required. Secondary end points were the occurrence of any event in the composite of cardiovascular death, myocardial infarction, or ischemic stroke defined as major adverse cardiovascular event (MACE), the occurrence of any event in the composite of lower limb major amputation or revascularization defined as major adverse limb event (MALE), or the occurrence of cancer.
Statistical Analysis
Discrete variables are presented as number and percentage, and continuous variables as median and interquartile range (IQR, 25th−75th percentile). Comparisons were made using chi-square test (or Fisher exact tests, when appropriate) for discrete variables, and Mann-Whitney test for continuous variables. All subsequent p-values are reported for 2-tailed tests with a 5% threshold. Overall survival, MACE and MALE were calculated using the Kaplan-Meier method, and the values were compared using the log-rank test. All analyses were performed using SPSS software vs. 13.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Cohort and Baseline Characteristics
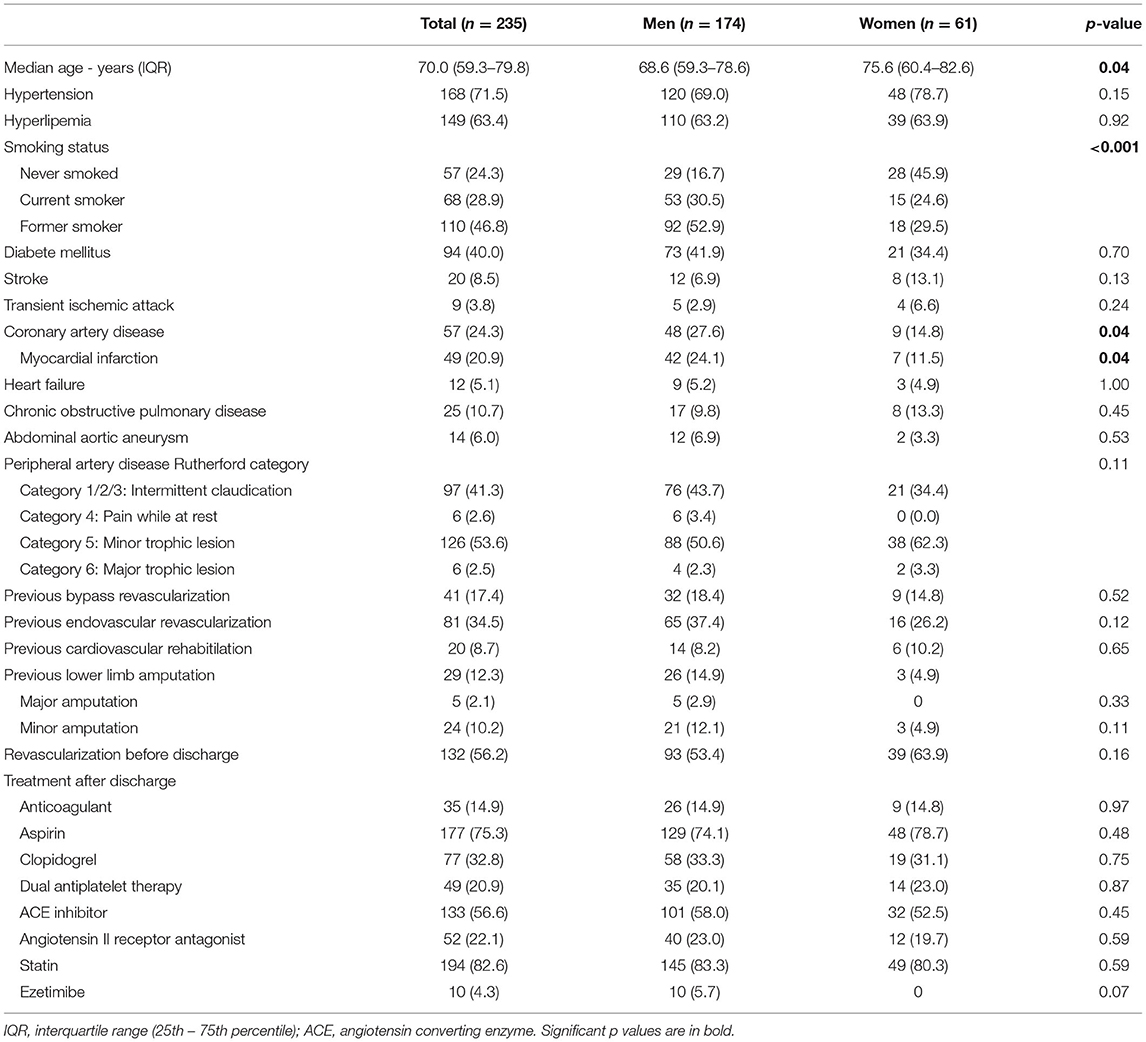
From January 2018 to January 2019 a total of 235 patients were included. Table 1 shows the characteristics of the patients. The median age was 70.0 (59.3–79.8) years. There were 61 (26%) women. They were older than men, with a median age of 75.6 (60.4–82.6) and 68.3 (59.3–78.6) years, respectively (p = 0.04). Main cardiovascular risk factors were similar in both sex, with a trend for more hypertension in women (78.7%, p = 0.15) and diabetes mellitus in men (41.9%, p = 0.70). However, tobacco use was significantly different with more former or current smokers in men (p < 0.001). In parallel, a history of coronary heart disease, and myocardial infarction in particular, was significantly higher in men [42 men (24.1%) vs. 7 women (11.5%), p = 0.04]. Other comorbidities reflected the clinical complexity of the patients with 12 (5.1%) patients with heart failure, 29 (12.3%) with stroke or transient ischemic attack, and 25 (10.7%) with chronic obstructive pulmonary disease. The majority of patients, 138 (58.8%) had critical limb ischemia defined as pain of the lower limb while at rest or tissue loss and 97 (41.3%) had claudication. Neither the PAD Rutherford category nor previous revascularizations differed between sex. Patients already had a history of vascular revascularization of the lower limbs with bypass for 41 (17.4%) patients and endovascular procedure for 81 (34.5%) patients. Already 29 (12.3%) patients had previous amputation. Half of the patients has undergone a revascularization before discharge. After discharge, 218 patients received an antithrombotic therapy (93.2%), 195 a lipid-lowering drug (83.3%), 185 an angiotensin converting enzyme inhibitor or angiotensin-receptor blocker (78.9%), similarly between sex.
One-Year Outcomes
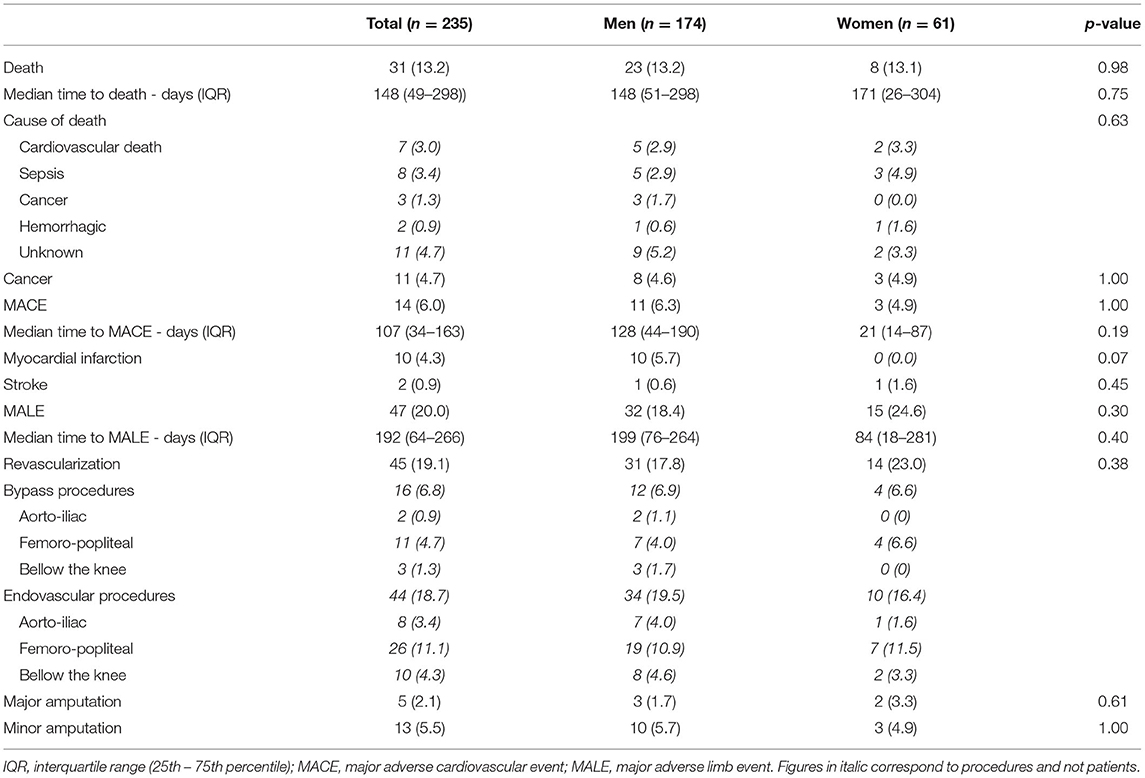
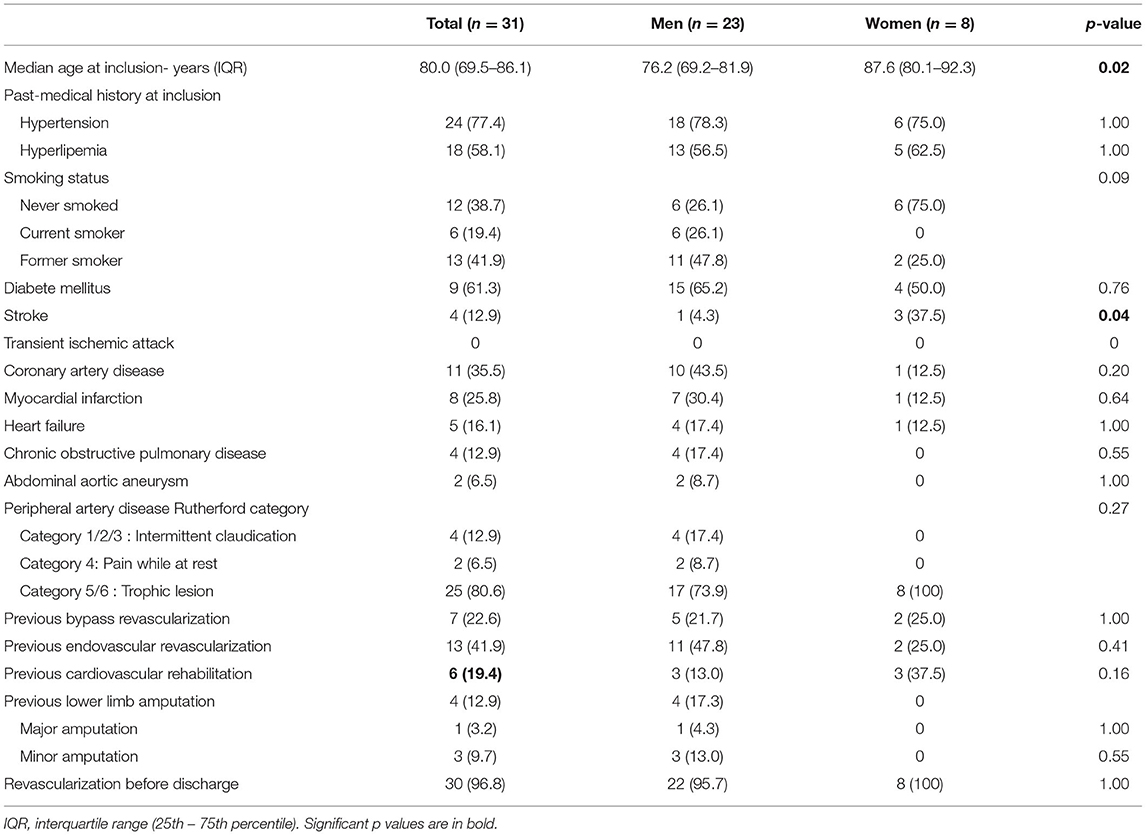
At 1 year, overall survival, MACE or MALE did not differ between sex, with 23 (13.2%), 11 (6.3%), and 32 (18.4%) in men, and 8 (13.1%), 3 (4.9%), 15 (24.6%) in women, respectively (Table 2). Sepsis and cardiovascular events were the leading cause of death. Cancer annual incidence was 5%. Although non-statistically significant, MACE affected more men [11 (6.3%) men vs. 3 (4.9%) women], especially myocardial infarction, whereas it was the contrary for MALE affecting more women [15 (24.6%) men vs. 32 (18.4%) women], especially within the first 90 days (Figure 1). In patients with intermittent claudication (Rutherford category 1, 2, or 3) or critical limb ischemia (Rutherford category 4, 5, or 6) overall survival, MACE and MALE were similar between sex (Figure 2). Distribution of the level of revascularization was similar between men and women. Major amputation occurred in 3 (1.7%) men and 2 (3.3%) women, and minor amputations in 10 (5.7%) men and 3 (4.9%) women. When focusing on characteristics at inclusion on patients who died within 1 year, women were older than men with a median age of 87.6 (80.1–92.3) and 76.2 years (69.2–81.9) respectively (p = 0.02) and had more frequently a stroke in their past-medical history 3 (37.5%) vs. 1 (4.3%) for men (p = 0.04) (Table 3).
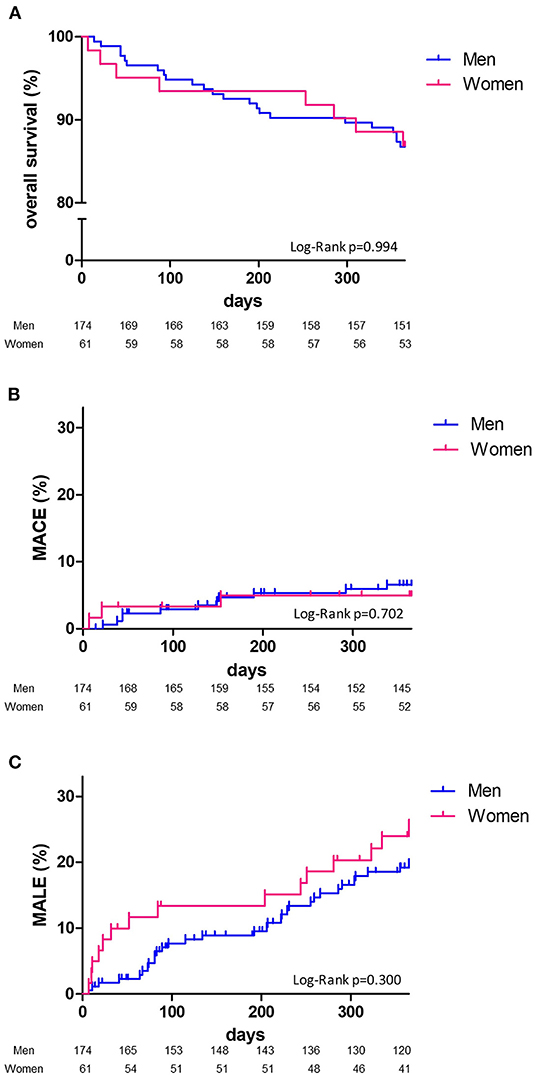
Figure 1. One-year Kaplan-Meier survival curves. Overall survival (A), major adverse cardiovascular events: MACE (B), major adverse limb events: MALE (C) and comparison according to sex (men, blue line; women, red line) with Log Rank test comparisons between men (blue) and women (red).
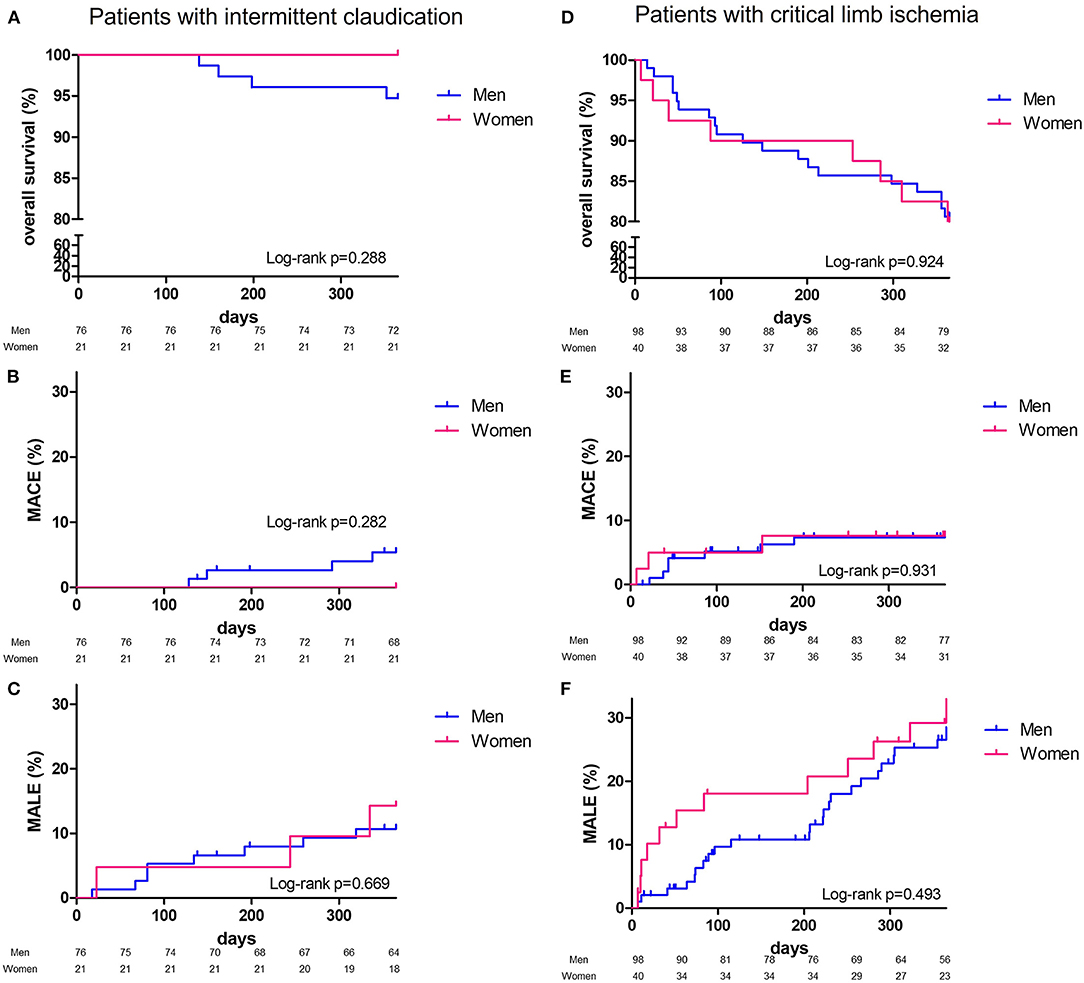
Figure 2. One-year Kaplan-Meier survival curves. Patients with intermittent claudicaion, overall survival (A), major adverse cardiovascular events: MACE (B), major adverse limb events: MALE (C); patients with critical limb ischemia, overall survival (D), major adverse cardiovascular events: MACE (E), major adverse limb events: MALE (F) and comparison according to sex (men, blue line; women, red line) with Log Rank test comparisons between men (blue) and women (red).
Discussion
In this study, we found that among patients hospitalized for PAD, women were older than men. However, there was no sex difference in overall mortality after 1 year of follow-up. Women smoked less and had less coronary artery disease, and we noticed a trend for fewer MACE and increased MALE vs. men. These data are consistent with the literature, where large studies observed that ASCVD outcomes were not higher in women compared with men (5).
Our study aimed at comparing characteristics and outcomes of patients hospitalized for PAD according to sex provides several key points. We did not find that women were under-treated or less likely to undergo revascularization than men (1, 6, 11, 12). Same rate of revascularization was observed in both men and women with even a trend to more procedures in women, but without any difference according to the level of limb revascularization (16, 17). Currently, the guidelines for symptomatic PAD recommend long-term treatments with at least antiplatelet agent and statin (7) to improve prognosis (18). Previous reports showed that this optimized therapy was less prescribed in women, suggesting a decrease in secondary prevention strategies in women compared to men (19). In our study, optimized therapy was approaching 80% of the patients whatever the sex. However, not all patients received statin at discharge. Forty patients (16.7%) patients either presented a previous intolerance or refused to take statin or ezetimibe. Our data are in accordance with large recent therapeutic trials. In the COMPASS trial (9), lipid-lowering therapy was present in 82.8 to 83.8% of the patients, and 73.0 to 73.7% in the EUCLID trial (8). No patient from this cohort had a PCSK9 inhibitor. This new lipid lowering class has been approved for reimbursement in France since 2020 only, when the LDL cholesterol goal is not achieved and in combination with a statin. The clinical presentation of the patients in this study was more severe than most of PAD cohorts (20–22) with almost 60% presenting with critical limb ischemia. Outpatients were not included in that study indeed, as a consequence, revascularizations for intermittent claudication were under-represented. Although intermittent claudication is considered a hallmark manifestation of PAD, women are more than twice as likely as men to report the presence of atypical exertional leg symptoms that sometimes began at rest or no symptoms at all (23, 24). Other associated comorbidities, such as osteoarthritis or osteoporosis, may also delay the diagnosis of PAD in women (25). Therefore, women have probably a long “latent phase” in which ASCVD and PAD progresses leading to more revascularization when hospitalized. This may explain why we observed that women presented with more tissue loss and less intermittent claudication as already reported (26). Interestingly, despite an increased age and the severe clinical presentation, the 1 year amputation-free survival was similar in both men and women (12, 27).
Women were older than men with almost 10 years apart when they presented with PAD in our study (28). One explanation may come from the fact that men were more current and former smokers than women, and would have develop PAD at younger age, as smoking, the most powerful contributor to PAD, increases the risk by 2 to 3 (29, 30). But another explanation may relate to the preponderant role that estrogens play in women, as reported decades ago in the Framingham study. Women in pre-menopause developed less coronary artery disease than women in post-menopause or women of same age with early menopause after ovariectomy indeed (31). Since, protective cardiovascular effects of estrogens have been demonstrated (32). They promote arterial vasodilation, decrease action of pro-inflammatory cytokines, lower low density lipoproteins and increase high density lipoproteins (32, 33). The drop in estrogen at menopause gives way to the development of ASCVD. But the impact of gonadotropins shall not be underestimated as secretion of follicle-stimulating hormone (FSH) from the pituitary gland begins to rise above normal levels before menopause, when estrogen levels are still normal, and become high after menopause responsible for a decrease of estrogen levels (34, 35). FSH levels were found to be correlated with the coronary calcium score and carotid intima-media thickness in women (36). Women with a lower increase in FSH during their transition to menopause may be less at risk of atherosclerosis than those with a medium or high increase in FSH at the same period (37, 38). Our team recently assessed the impact of gonadotropins in vitro on endothelial progenitor cells suggesting that gonadotropins blocking strategies could be a new interesting therapeutic approach in ASCVD (39).
Our study has some limitations. The limited number of patients included, and the short duration of follow-up make the power of this study insufficient to draw definitive conclusions. It may indeed underestimate the impact of sex on the characteristics, management, and prognosis of PAD patients. Also, our study was not powered for detecting differences in terms of hard cardiac or limb events between men and women. Our findings should be addressed with usual caution and would require studies within larger registries to be confirmed. International registries like the RECCORD registry (40), able to collect and sum-up data from multiple tertiary and non-tertiary centers would be very useful to provide a comprehensive dataset depicting the current real life practice and outcome of vascular care (40). We cannot provide details for intermittent claudication staging. We do not perform indeed a Strandness walking test for the hospitalized patients to provide these data. However, the patients requiring hospitalization in our tertiary center are severely impacted by their walking-distance and most report a walking distance below 200 m. However, the patients in our study shared the same cardiovascular risk factors and comorbidities than other PAD cohorts, where current smokers represented 16 to 39%, hypertension 63 to 81%, hyperlipidemia 57 to 67% and diabetes 26 to 44%. Besides PAD, patients had coronary artery disease in 24 to 52% and cerebrovascular disease in 13 to 23% (22, 41–45). Moreover, the accuracy of the data completion during follow-up was good as previously shown (14, 18, 41, 46).
Conclusion
We showed that overall mortality, cardiovascular outcomes, limb revascularization or amputation did not differ between men and women, 1 year after hospitalization for PAD in our tertiary center. Although the latter were older, less smoker and had less coronary artery disease. Due to the small size of this cohort, larger studies and future research are needed to better understand sex-specific mechanisms in the pathophysiology and natural history of PAD, including sex hormones and gonadotrophins changes over adult life.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by IRB CPP Sud Ouest et outre 79 mer II 07 021 08. The patients provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
TM, EM, and DS: conception and design. HM and EM: administrative support. GD, CC, AGa, GG, LK, JS, MS, PJ, and TM: provision of study materials or patients. AGu, NM, OS, and HM: collection and assembly of data. AGu, GD, TM, DS, NG, and EM: data analysis and interpretation. All authors: manuscript writing and final approval of manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, et al. The Lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet. (2021) 397:2385–438. doi: 10.1016/S0140-6736(21)00684-X
2. Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. (2017) 2:e000298. doi: 10.1136/bmjgh-2017-000298
3. Peters SAE, Woodward M, Jha V, Kennedy S, Norton R. Women's health: a new global agenda. BMJ Glob Health. (2016) 1:e000080. doi: 10.1136/bmjgh-2016-000080
4. Pagidipati NJ, Peterson ED. Acute coronary syndromes in women and men. Nat Rev Cardiol. (2016) 13:471–80. doi: 10.1038/nrcardio.2016.89
5. Walli-Attaei M, Joseph P, Rosengren A, Chow CK, Rangarajan S, Lear SA, et al. Variations between women and men in risk factors, treatments, cardiovascular disease incidence, and death in 27 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. (2020) 396:97–109. doi: 10.1016/S0140-6736(20)30543-2
6. Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, et al. A call to action: women and peripheral artery disease: a scientific statement from the American heart association. Circulation. (2012) 125:1449–72. doi: 10.1161/CIR.0b013e31824c39ba
7. Aboyans V, Ricco JB, Bartelink MLEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS) Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European stroke organization (ESO) the task force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (ESC) and of the European society for vascular surgery (ESVS). Eur Heart J. (2018) 39:763–816. doi: 10.1093/eurheartj/ehx095
8. Hiatt WR, Fowkes FGR, Heizer G, Berger JS, Baumgartner I, Held P, et al. Ticagrelor vs. Clopidogrel in Symptomatic Peripheral Artery Disease. N Engl J Med. (2017) 376:32–40. doi: 10.1056/NEJMoa1611688
9. Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. (2018) 391:219–29. doi: 10.1016/S0140-6736(17)32409-1
10. Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality: a meta-analysis. JAMA. (2008) 300:197–208. doi: 10.1001/jama.300.2.197
11. Amaranto DJ, Abbas F, Krantz S, Pearce WH, Wang E, Kibbe MR. An evaluation of gender and racial disparity in the decision to treat surgically arterial disease. J Vasc Surg. (2009) 50:1340–7. doi: 10.1016/j.jvs.2009.07.089
12. Feinglass J, McDermott MM, Foroohar M, Pearce WH. Gender differences in interventional management of peripheral vascular disease: evidence from a blood flow laboratory population. Ann Vasc Surg. (1994) 8:343–9. doi: 10.1007/BF02132995
13. Desai R, Singh S, Fong HK, Goyal H, Gupta S, Zalavadia D, et al. Racial and sex disparities in resource utilization and outcomes of multi-vessel percutaneous coronary interventions (a 5-year nationwide evaluation in the United States). Cardiovasc Diagn Ther. (2019) 9:18–29. doi: 10.21037/cdt.2018.09.02
14. Cambou JP, Aboyans V, Constans J, Lacroix P, Dentans C, Bura A. Characteristics and outcome of patients hospitalised for lower extremity peripheral artery disease in France: the COPART registry. Eur J Vasc Endovasc Surg. (2010) 39:577–85. doi: 10.1016/j.ejvs.2010.02.009
15. Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2017) 135:e686–725. doi: 10.1161/CIR.0000000000000470
16. Rieß HC, Debus ES, Heidemann F, Stoberock K, Grundmann RT, Behrendt CA. Gender differences in endovascular treatment of infrainguinal peripheral artery disease. Vasa. (2017) 46:296–303. doi: 10.1024/0301-1526/a000634
17. Ortmann J, Nüesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg. (2012) 55:98–104. doi: 10.1016/j.jvs.2011.07.074
18. Pros N, Cambou JP, Aboyans V, Malloizel Delaunay J, Constans J, Lacroix P, et al. Hospital discharge risk score for 1-year all-cause mortality or non-fatal cardiovascular events in patients with lower-extremity peripheral artery disease, with and without revascularisation. Eur J Vasc Endovasc Surg. (2013) 45:488–96. doi: 10.1016/j.ejvs.2013.01.034
19. Pâquet M, Pilon D, Tétrault JP, Carrier N. Protective vascular treatment of patients with peripheral arterial disease: guideline adherence according to year, age and gender. Can J Public Health. (2010) 101:96–100. doi: 10.1007/BF03405572
20. Feringa HHH, Karagiannis SE, van Waning VH, Boersma E, Schouten O, Bax JJ, et al. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. (2007) 45:936–43. doi: 10.1016/j.jvs.2007.01.024
21. Cacoub P, Cambou JP, Kownator S, Belliard JP, Beregi JP, Branchereau A, et al. Prevalence of peripheral arterial disease in high-risk patients using ankle-brachial index in general practice: a cross-sectional study. Int J Clin Pract. (2009) 63:63–70. doi: 10.1111/j.1742-1241.2008.01953.x
22. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. (2006) 295:180–9. doi: 10.1001/jama.295.2.180
23. McDermott MM, Greenland P, Liu K, Criqui MH, Guralnik JM, Celic L, et al. Sex differences in peripheral arterial disease: leg symptoms and physical functioning. J Am Geriatr Soc. (2003) 51:222–8. doi: 10.1046/j.1532-5415.2003.51061.x
24. Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, et al. population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. (2007) 45:1185–91. doi: 10.1016/j.jvs.2007.02.004
25. Vouyouka AG, Kent KC. Arterial vascular disease in women. J Vasc Surg. (2007) 46:1295–302. doi: 10.1016/j.jvs.2007.07.057
26. Jackson EA, Munir K, Schreiber T, Rubin JR, Cuff R, Gallagher KA, et al. Impact of sex on morbidity and mortality rates after lower extremity interventions for peripheral arterial disease. J Am Coll Cardiol. (2014) 63:2525–30. doi: 10.1016/j.jacc.2014.03.036
27. Malmstedt J, Leander K, Wahlberg E, Karlström L, Alfredsson L, Swedenborg J. Outcome after leg bypass surgery for critical limb ischemia is poor in patients with diabetes: a population-based cohort study. Diabetes Care. (2008) 31:887–92. doi: 10.2337/dc07-2424
28. Srivaratharajah K, Abramson BL. Women and peripheral arterial disease: a review of sex differences in epidemiology, clinical manifestations, and outcomes. Can J Cardiol. (2018) 34:356–61. doi: 10.1016/j.cjca.2018.01.009
29. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. (2015) 116:1509–26. doi: 10.1161/CIRCRESAHA.116.303849
30. Fowkes FGR, Aboyans V, Fowkes FJI, McDermott MM, Sampson UKA, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. (2017) 14:156–70. doi: 10.1038/nrcardio.2016.179
31. Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. (1976) 85:447–52. doi: 10.7326/0003-4819-85-4-447
32. Nguyen L, Liles DR, Lin PH, Bush RL. Hormone replacement therapy and peripheral vascular disease in women. Vasc Endovasc Surg. (2004) 38:547–56. doi: 10.1177/153857440403800609
33. Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome. Arch Intern Med. (2008) 168:1568–75. doi: 10.1001/archinte.168.14.1568
34. Klein NA, Soules MR. Endocrine changes of the perimenopause. Clin Obstet Gynecol. (1998) 41:912–20. doi: 10.1097/00003081-199812000-00017
35. Sohrabji F, Okoreeh A, Panta A. Sex hormones and stroke: beyond estrogens. Horm Behav. (2019) 111:87–95. doi: 10.1016/j.yhbeh.2018.10.010
36. Munir JA, Wu H, Bauer K, Bindeman J, Byrd C, Feuerstein IM, et al. The perimenopausal atherosclerosis transition: relationships between calcified and non-calcified coronary, aortic, and carotid atherosclerosis and risk factors and hormone levels. Menopause. (2012) 19:10–5. doi: 10.1097/gme.0b013e318221bc8d
37. El Khoudary SR, Santoro N, Chen HY, Tepper PG, Brooks MM, Thurston RC, et al. Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. Eur J Prev Cardiol. (2016) 23:694–703. doi: 10.1177/2047487315607044
38. Zhu D, Li X, Macrae VE, Simoncini T, Fu X. Extragonadal effects of follicle-stimulating hormone on osteoporosis and cardiovascular disease in women during menopausal transition. Trends Endocrinol Metab. (2018) 29:571–80. doi: 10.1016/j.tem.2018.06.001
39. Détriché G, Gendron N, Philippe A, Gruest M, Billoir P, Rossi E, et al. Gonadotropins as novel active partners in vascular diseases: insight from angiogenic properties and thrombotic potential of endothelial colony-forming cells. J Thromb Haemost. (2022) 20:230-237. doi: 10.1111/jth.15549
40. M Malyar N, Stausberg J, Ito WD, Kölble H, Langhoff R, Lawall H, et al. Rationale and design of the recording courses of vascular diseases registry (RECCORD registry). VASA Z Gefasskrankheiten. (2017) 46:262–267. doi: 10.1024/0301-1526/a000631
41. Mirault T, Galloula A, Cambou JP, Lacroix P, Aboyans V, Boulon C, et al. Impact of betablockers on general and local outcome in patients hospitalized for lower extremity peripheral artery disease: the COPART registry. Medicine. (2017) 96:e5916. doi: 10.1097/MD.0000000000005916
42. Steg PG, Bhatt DL, Wilson PWF, D'Agostino R, Ohman EM, Röther J, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. (2007) 297:1197–206. doi: 10.1001/jama.297.11.1197
43. Blacher J, Cacoub P, Luizy F, Mourad JJ, Levesque H, Benelbaz J, et al. Peripheral arterial disease vs. other localizations of vascular disease: the ATTEST study. J Vasc Surg. (2006) 44:314–8. doi: 10.1016/j.jvs.2006.04.002
44. Diehm C. Association of low ankle brachial index with high mortality in primary care. Eur Heart J. (2006) 27:1743–9. doi: 10.1093/eurheartj/ehl092
45. The PERART/ARTPER study group, Alzamora MT, Forés R, Baena-Díez JM, Pera G, Toran P, et al. The peripheral arterial disease study (PERART/ARTPER): prevalence and risk factors in the general population. BMC Public Health. (2010) 10:38. doi: 10.1186/1471-2458-10-38
Keywords: peripheral arterial disease, cardiovascular disease, sex differences, vascular surgical procedure, lower extremity arterial disease
Citation: Détriché G, Guédon A, Mohamedi N, Sellami O, Cheng C, Galloula A, Goudot G, Khider L, Mortelette H, Sitruk J, Gendron N, Sapoval M, Julia P, Smadja DM, Mirault T and Messas E (2022) Women Specific Characteristics and 1-Year Outcome Among Patients Hospitalized for Peripheral Artery Disease: A Monocentric Cohort Analysis in a Tertiary Center. Front. Cardiovasc. Med. 9:824466. doi: 10.3389/fcvm.2022.824466
Received: 29 November 2021; Accepted: 17 January 2022;
Published: 07 February 2022.
Edited by:
Grigorios Korosoglou, GRN Klinik Weinheim, GermanyReviewed by:
Mariya Kronlage, Heidelberg University Hospital, GermanySorin Giusca, GRN Klinik Weinheim, Germany
Copyright © 2022 Détriché, Guédon, Mohamedi, Sellami, Cheng, Galloula, Goudot, Khider, Mortelette, Sitruk, Gendron, Sapoval, Julia, Smadja, Mirault and Messas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grégoire Détriché, gregoire.detriche@aphp.fr
†These authors have contributed equally to this work
 Grégoire Détriché
Grégoire Détriché Alexis Guédon
Alexis Guédon Nassim Mohamedi1
Nassim Mohamedi1  Olfa Sellami
Olfa Sellami Lina Khider
Lina Khider Nicolas Gendron
Nicolas Gendron David M. Smadja
David M. Smadja Tristan Mirault
Tristan Mirault