Functional hepatic deterioration determined by 13C-methacetin breath test is associated with impaired hemodynamics and late Fontan failure in adults
- 1Department of Congenital Heart Disease/Pediatric Cardiology, Deutsches Herzzentrum Berlin, Berlin, Germany
- 2Institute for Cardiovascular Computer-Assisted Medicine, Charité—Universitätsmedizin Berlin, Berlin, Germany
- 3Berlin Institute of Health, Charité-Universitätsmedizin Berlin, Berlin, Germany
- 4Charité Centre for Internal Medicine and Dermatology, Charité-Universitätsmedizin Berlin, Berlin, Germany
- 5Department of Gastroenterology and Hepatology, Charité—Universitätsmedizin Berlin, Berlin, Germany
- 6Department of General, Visceral and Vascular Surgery, Charité—Universitätsmedizin Berlin, Berlin, Germany
- 7Department of Pediatric Cardiology, Charité—Universitätsmedizin Berlin, Berlin, Germany
Background: Despite improved survival a substantial number of Fontan patients eventually develop late failure. Fontan-associated liver disease (FALD) is the most frequent end-organ dysfunction. Although impaired hemodynamics and Fontan failure correlate with FALD severity, no association between hepatic functional metabolic impairment and Fontan hemodynamics has been established.
Hypothesis: Metabolic liver function measured by liver maximum function capacity test (LiMAx®) correlates with Fontan hemodynamics and Fontan failure.
Methods: From 2020 to 2022, 58 adult Fontan patients [median age: 29.3 years, IQR (12.7), median follow-up time after Fontan operation: 23.2 years, IQR (8.7)] were analyzed in a cross-sectional study. Hemodynamic assessment included echocardiography, cardiopulmonary exercise testing and invasive hemodynamic evaluation. Fontan failure was defined based on commonly applied clinical criteria and our recently composed multimodal Fontan failure score.
Results: LiMAx® test revealed normal maximum liver function capacity in 40 patients (>315 μg/h*kg). In 18 patients a mild to moderate impairment was detected (140–314 μg/h*kg), no patient suffered from severe hepatic deterioration (≤ 139 μg/kg*h). Fontan failure was present in 15 patients. Metabolic liver function was significantly reduced in patients with increased pulmonary artery pressure (p = 0.041. r = −0.269) and ventricular end-diastolic pressure (p = 0.033, r = −0.325), respectively. In addition, maximum liver function capacity was significantly impaired in patients with late Fontan failure (289.0 ± 99.6 μg/kg*h vs. 384.5 ± 128.6 μg/kg*h, p = 0.007).
Conclusion: Maximum liver function capacity as determined by LiMAx® was significantly reduced in patients with late Fontan failure. In addition, elevated pulmonary artery pressure and end-diastolic ventricular pressure were associated with hepatic functional metabolic impairment.
Introduction
Despite its tremendous success in treating patients with univentricular anatomy, the Fontan operation remains a palliative procedure, which is characterized by abnormal hemodynamics (1, 2). In the long-term course, chronic venous congestion and low cardiac output lead to progressive clinical heart failure with limited treatment options (3, 4). The relative scarceness of effective pharmacological therapies and the limited applicability of mechanical circulatory support restrict end-stage therapeutic strategies to cardiac transplantation, which itself is associated with considerable morbidity and mortality (5, 6). The indications and optimal timing of cardiac transplantation in Fontan patients are still subject of ongoing debate. The urgency of addressing these issues is illustrated by the fact that within the next decades a significant increase in adult Fontan patients experiencing hemodynamic compromise and subsequently cardio-circulatory demise can be expected (7).
Liver-associated morbidity and mortality are well described in the adult Fontan population and constitute major risk factors significantly impacting survival rates after cardiac transplantation (8, 9). Additionally, the indication for a combined heart and liver transplantation is currently subject of ongoing debate. Therefore, reliable diagnostic modalities are required to monitor hepatic end-organ damage, determine the optimal timing for cardiac transplantation and define the indications for a combined heart-liver transplantation. The liver maximum capacity test (LiMAx®) has been developed to quantitatively determine metabolic liver function. Methacetin is exclusively metabolized by the cytochrome P4501A2 (CYP1A2) system, which exclusively exists in hepatocytes (10). Therefore, enzymatic cleavage of intravenously administrated 13C-methacetin into 13CO2, reliably correlates with hepatic parenchymal volume and metabolic function (10). Previously, we have demonstrated that structural hepatic alterations antecede functional hepatic impairment as assessed by LiMAx®, with maximum liver function capacity being well preserved in the majority of Fontan patients (11). However, the potential impacts of late Fontan failure and hemodynamics on metabolic liver function are unknown.
Herein, we aimed to analyze the potential associations between maximum liver function capacity and (I) Fontan hemodynamics by clinical, echocardiographic and invasive assessments and (II) late Fontan failure.
Methods
Study design and patients
From 2019 to 2022 58 adult Fontan patients, who successively presented in our outpatient clinic for follow-up, were included in our cross-sectional observational study. All patients received measurement of maximal liver function capacity using LiMAx® test as well as a detailed hemodynamic and hepatic assessment. Exclusion criteria were intolerance to methacetin or paracetamol and/or patient age below 18 years. The institutional review board and ethics committee approved the study (decision number: EA2/127/18). All individual participants consented to participate in the study prior to inclusion.
Hemodynamic assessment
Hemodynamic assessment included clinical evaluation, echocardiography, cardiopulmonary exercise testing (CPET) and cardiac catheterization. Systolic ventricular function was measured by echocardiography based on the modified Simpson's method (12). Atrioventricular valve incompetence (AVVI) was classified as absent/mild, moderate or severe by visual assessment of the regurgitation jet dimensions in color Doppler sonography. CPET was performed following a standardized institutional protocol using a cycle ergometer. Peak oxygen uptake (VO2peak) was measured in ml/kg*min and normalized in % of age-, gender- and body dimension-adjusted reference values. Cardiac catheterization included measurements of mean pulmonary artery pressure (mPAP) and systemic ventricular end-diastolic pressure (SVEDP). Transpulmonary pressure gradient (TPG) was calculated as the difference between mPAP and pulmonary capillary wedge pressure. Cardiac output (CO) and pulmonary vascular resistance (PVR) were determined by Fick's principle using oximetry (13). For comparability, CO and PVR are indexed to body-surface area (Cardiac index, CI, l/min/m2; pulmonary vascular resistance index, PVRi, WU*m2). Fontan failure was defined as severe dysfunction of the Fontan circulation caused by impaired ventricular function, moderate to severe atrioventricular valve incompetence, increased pulmonary vascular resistance, recurrent arrhythmia or therapy-refractory protein-losing enteropathy based on commonly applied clinical criteria (14) and our previously described Fontan failure score (15). Briefly, the score includes a set of 15 clinical, echocardiographic, invasive hemodynamic and laboratory parameters and is calculated by assigning one score point for each score item beyond the defined threshold with a range from 0 to 15 points. A score ≥ 8 score points detects late Fontan failure with a sensitivity of 99.3 % and a specificity of 53.9 % (15).
Hepatic assessment
Hepatic assessment was performed based on our previously published institutional protocol (16) and consisted of laboratory analyses, hepatic ultrasound and liver stiffness measurement by transient elastography (TE). FibroTest® was computed on Biopredictive website (Paris, France; www.biopredictive.com).
Maximal liver function capacity
The LiMAx® test was performed following the standardized protocol of Stockmann et al. (10).
Briefly, a body weight-adjusted solution (2 mg/kg) of 13C-labeled methacetin was administered intravenously. The hepatozyte-specific CYP1A2 system metabolizes 13C-labeled methacetin into paracetamol and 13CO2, which is continuously measured in the exhaled air. The LiMAx test result is calculated based on the individually determined maximum delta-over-baseline ratio of 13CO2/12CO2 (10).
Statistical analysis
Data were collected from medical records of the German Heart Centre Berlin. Data are expressed as median and interquartile range, which were calculated as the 75th minus 25th percentile. Fontan follow-up duration was defined as the interval between Fontan operation and last follow-up. Correlations between maximum liver capacity, echocardiographic, hemodynamic and hepatic parameters as well as the Fontan failure score were assessed using Spearman's correlation and Mann-Whitney U test. Statistical analyses were performed using SPSS statistical software (version 23, IBM Corp., NY, USA). A p-value < 0.05 was considered statistically significant.
Results
Patient cohort
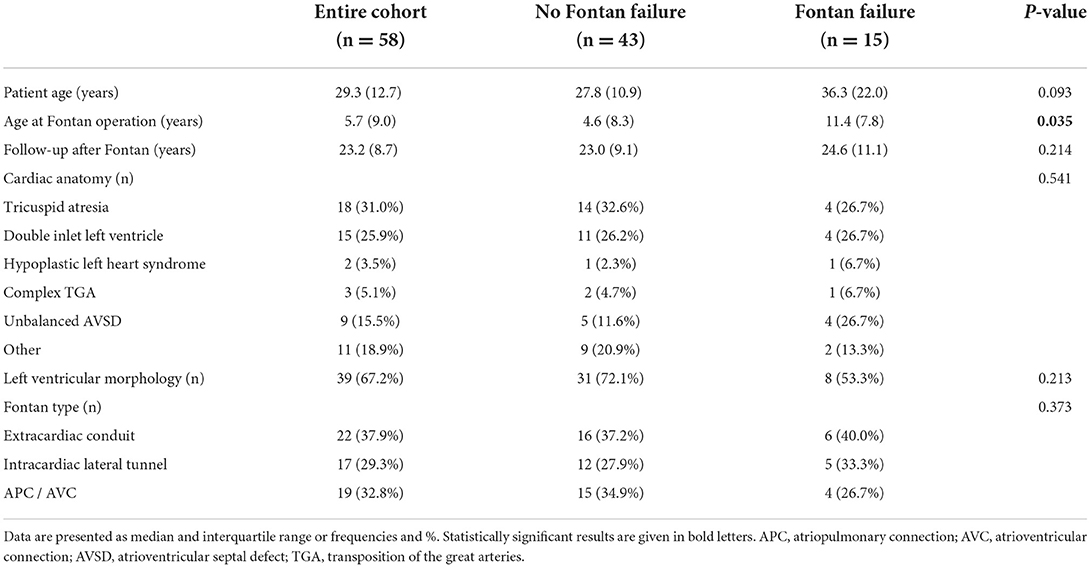
Patient characteristics of the entire cohort are provided in Table 1. Median patient age was 29.3 years (IQR 12.7) and median follow-up time after Fontan operation 23.2 (IQR 8.7). The most common underlying cardiac morphologies were tricuspid atresia (n = 18), double inlet left ventricle (n = 15) and unbalanced atrioventricular septal defect (n = 9). Fontan modifications consisted of extracardiac conduit in 22 patients, lateral tunnel in 17 patients and atriopulmonary/ atrioventricular connection (APC/AVC) in 19 patients. From the study cohort, 3 patients died during follow-up; 2 patients after cardiac transplantation and 1 patient on mechanical circulatory support. Two additional patients successfully underwent cardiac transplantation.
Hemodynamic assessment
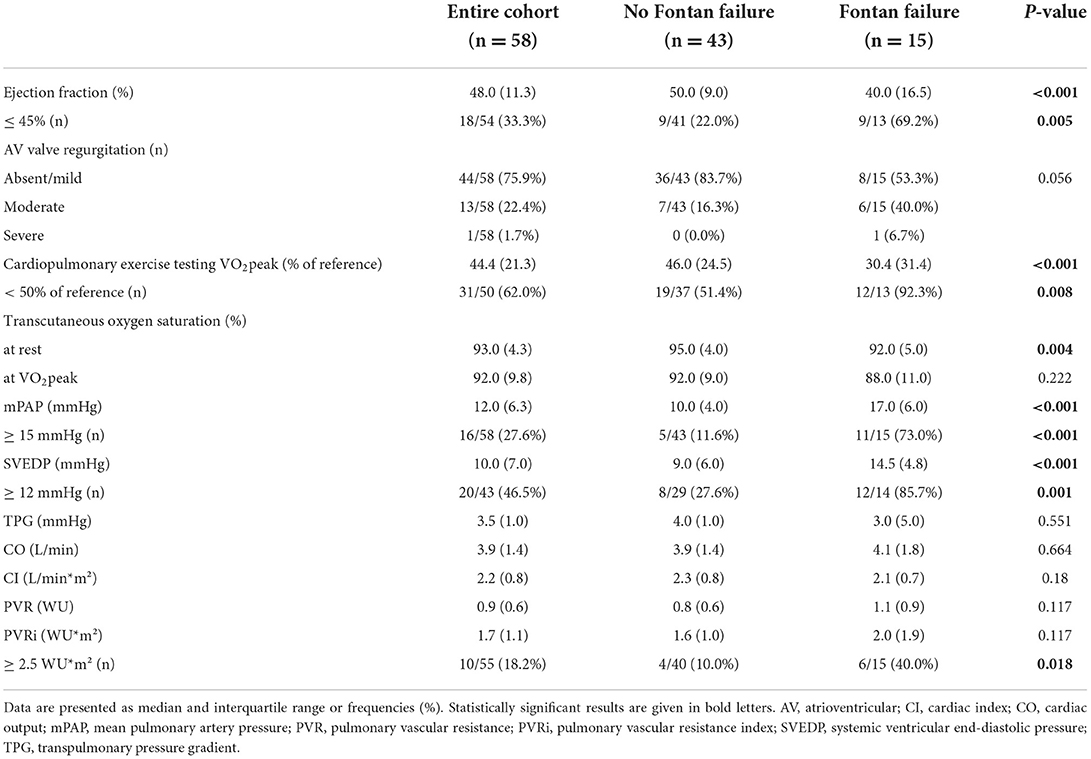
Results of hemodynamic assessment are presented in Table 2. Systolic ventricular function was preserved/ mildly impaired in 47 patients, moderately impaired in 9 patients and severely impaired in 2 patients. AVVI was classified as absent/mild in 44 patients, moderate in 13 patients and severe in 1 patient. Median percentage of reference VO2peak was 44.4 % (IQR 21.3) in the entire cohort. Invasive hemodynamic evaluation revealed mPAP, SVEDP and TPG to be within normal reference ranges (Table 1). Calculated median CI was 2.2 L/min/m2 (IQR 0.8) and median PVRi 2.3 WU*m2 (IQR 1.1). Late Fontan failure was diagnosed in 15 patients.
Hepatic assessment
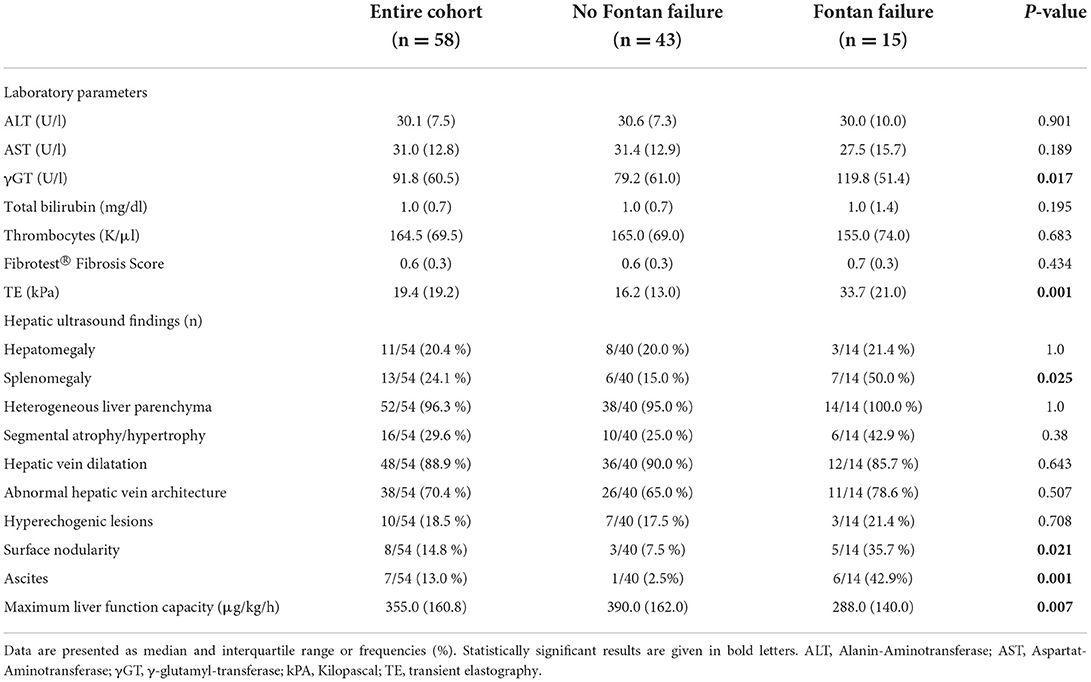
Results of hepatic assessment are listed Table 3. The laboratory parameters Alanin-Aminotransferase (ALT), Aspartat-Aminotransferase (AST), bilirubin and thrombocytes did not significantly differ between patients with and without Fontan failure, whereas γ-glutamyl-transferase (γGT) was significantly increased in patients with a failing Fontan circulation (p = 0.017). Results from hepatic ultrasound revealed that surface nodularity, ascites and segmental atrophy/ hypertrophy were more frequently detected in patients with Fontan failure (Table 3). Additionally, liver stiffness values measured by TE were significantly higher in patients with a failing Fontan circulation (p = 0.001), whereas Fibrotest® fibrosis score did not differ between patients with and without Fontan failure.
Maximal liver function capacity
Median maximum liver function capacity was 355.0 μg/kg*h (IQR 160.8), which corresponds to a normal hepatic function (≥ 315 μg/kg*h). In 18 patients maximum liver function capacity was moderately impaired (140–314 μg/kg*h), while no patient suffered from severe hepatic damage (≤ 139 μg/kg*h). No correlation was detected between systolic ventricular function or the extent of AVVI and maximum liver function capacity (p = 0.178, r = 0.186 and p = 0.873, r = −0.016, respectively). Additionally, no association was found between VO2peak and LiMAx® test results (p = 0.356, r = 0.133). No correlation was detected between maximal liver function capacity and resting or peak oxygen saturation (p1 = 0.202, r = 01.7; p2 = 0.056, r = −0.267). In patients with mPAP ≥ 15 mmHg maximal liver function capacity was significantly reduced compared to patients with mPAP <15 mmHg [294.0 μg/kg*h (IQR 126.0) vs. 388.5 μg/kg*h (IQR 177.0), p = 0.019, Figure 1A]. An SVEDP ≥ 12 mmHg was also associated with decreased maximum liver function capacity [331.5 μg/kg*h (IQR 136.5) vs. 401.0 μg/kg*h (IQR 173.0), p = 0.029; Figure 1B]. Finally, in patients with late Fontan failure, maximal liver function capacity was significantly impaired as compared to patients without evidence of Fontan failure [288.0 μg/kg/*h (IQR 140.0) vs. 390.0 μg/kg*h (IQR 162.0), p = 0.007; Figure 1C].
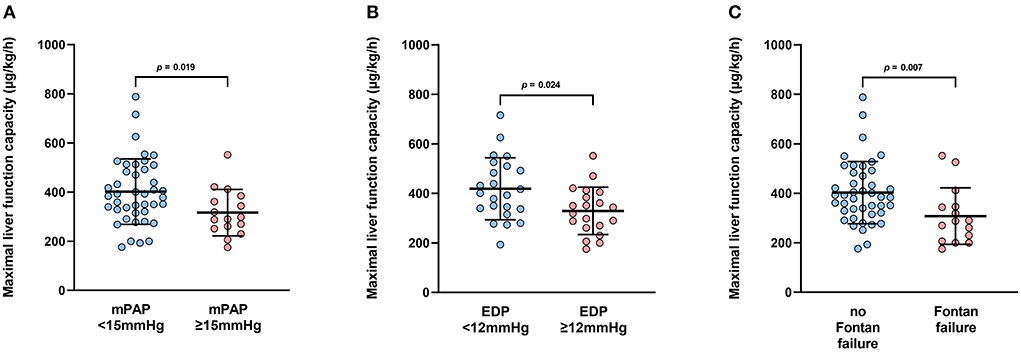
Figure 1. (A) Boxplots depict maximal liver function capacity according to mean pulmonary artery pressure (mPAP). mPAP <15 mmHg: n = 42; mPAP <15 mmHg: n = 15. (B) Boxplots depict maximal liver function capacity according to systemic ventricular end-diastolic pressure (SVEDP). SVEDP <12 mmHg: n = 23; SVEDP <12 mmHg: n = 19. (C) Boxplots depict maximum liver function capacity according to the presence of Fontan failure. Fontan failure; n = 15; no Fontan failure, n = 43.
Discussion
This is the first study to describe the association between Fontan hemodynamics, Fontan failure and maximum liver function capacity assessed by LiMAx®. Briefly, no correlation was detected between echocardiographic parameters or CPET results and metabolic liver function. Additionally, hemodynamic parameters such as CI and PVRi also showed no association with results from the LiMAx® test. However, maximum liver function capacity was significantly decreased in patients with increased mPAP (≥15 mmHg), and those with increased SVEDP (≥12 mmHg) as well as in patients with Fontan failure. In these patients, hepatic functional impairment was graded as moderate whereas no severe hepatic failure was detected.
The LiMAx® test was introduced to accurately quantify metabolic liver function based on the hepatocyte-specific cytochrome P4501A2 (CYP1A2) system and was evaluated in several clinical settings (17–19). We previously described the missing correlation between maximum liver function capacity and results from other diagnostic modalities such as laboratory parameters, TE and hepatic ultrasound (11). The major finding of our previous study was that metabolic liver function was preserved in the majority of adult Fontan patients even in those with clear evidence of advanced FALD.
Due to the non-physiological hemodynamics, failure of the Fontan circulation is inevitable in the long term (20–22). Since therapeutic strategies are limited, cardiac transplantation remains the only viable end-stage treatment option but is associated with considerable mortality and morbidity (23). Additionally, guidelines for the timing of cardiac transplantation are missing and delayed listing may result in progressing secondary end-organ damage, which significantly contributes to post-transplant mortality (8, 9). Fontan-associated liver disease (FALD) is the most frequent end-organ dysfunction and encompasses all abnormalities in both liver structure and function with the end-stage being severe liver cirrhosis or hepatocellular carcinoma (24, 25). The indication for a combined heart and liver transplantation in failing Fontan patients is currently subject of ongoing debate. The decision whether a patient may benefit from single or multi-organ transplantation is challenging due to the lack of sound data to support or refute any given approach. Whereas successful isolated heart transplantation has been reported in the presence of hepatic cirrhosis (26), feasibility of combined heart and liver transplantation has also been demonstrated (27, 28). The most commonly encountered scenario in Fontan patients considered for cardiac transplantation is the inevitable presence of some degree of liver fibrosis with most patients demonstrating ‘cirrhotic' alterations on imaging. However, it has been shown that cirrhosis on biopsy is less common and often does not correlate with imaging modalities such as ultrasound, magnetic resonance imaging or computed tomography (29). These discrepancies between imaging and biopsy findings complicate the interpretation and classification of FALD and its clinical significance for therapeutic decision making.
The LiMAx® test may represent a valuable complementary diagnostic modality in the hepatic assessment of Fontan patients and provides a reproducible quantitative measurement of hepatocyte function. Since a deterioration of maximum liver function capacity seems to occur relatively late during the disease course, when a significant impairment of Fontan hemodynamics and Fontan failure is already evident, its occurrence may prove as a valuable indicator for the requirement of a timely evaluation for cardiac transplantation. In our cohort, maximum liver function capacity was moderately reduced in patients who received cardiac transplantation [247.0 μg/kg*h (IQR 148.8) vs. 369.5 μg/kg*h (IQR 182.5), p = 0.029), however, none of these patients fulfilled the criteria for a combined heart and liver transplantation such as hepatocellular carcinoma or severe liver cirrhosis. In patients who survived cardiac transplantation, improvements in morphological and laboratory FALD parameters were detected. This observation has also been reported by other institutions and might underline the remarkable hepatic potential for regeneration (11, 30, 31). Therefore, patients with mild to moderate impairment of metabolic liver function might be appropriate candidates for isolated cardiac transplantation, whereas a severely impaired maximum liver function capacity may indicate the necessity of a combined heart and liver transplantation. Since both, FALD and Fontan failure, are characterized by a slow progress and are often clinically disguised by patient‘s adaptation to their chronically reduced output state and clinical deterioration, it seems advisable to perform repeated measurement of maximum liver function capacity during long-term follow-up, however, based on the currently available data, no precise intervals can be recommended. In patients with severe hemodynamic and hepatic impairment a yearly evaluation might be required followed by the consultation of an experienced hepatologist.
However, further well-conducted research efforts are warranted to address these questions, including additional studies, which compare maximum liver function capacity before and after cardiac transplantation as well as explore correlations of hepatic metabolic function with histologic findings of liver biopsies.
Limitations
This study has several limitations. Since this is a cross-sectional single center study with a comparably small patient cohort, future multi-institutional studies are necessary to evaluate metabolic liver function in larger patient cohorts. Additionally, the longitudinal relationship between Fontan hemodynamics and hepatic function was not addressed by this study. Since the parameters SVEDP and PAP are included in the calculation of the Fontan failure score, the association between LiMAx and Fontan failure might be confounded. However, considering that SVEDP and PAP are only 2 of 15 parameters used for score calculations, the confounding effect seems negligible. Additionally, all of the 15 Fontan failure patients fulfill the clinical consensus definition criteria of Fontan failure (14). Parts of the data of our current study cohort (n = 38/58, 65%) have previously been published in a study with a different scope focusing on morphologic hepatic assessment (11).
Conclusions
We herein demonstrate that maximum liver function capacity measured by the LiMAx® test is impaired in patients with impaired Fontan hemodynamics and Fontan failure. Hence, the LiMAx® test represents a valuable complementary diagnostic modality for FALD and might be useful in evaluating the indication for combined heart and liver transplantation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethikkommission der Charité, Berlin. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization and formal analysis: AS, PK, and SO. Data collection: AS, NJ, MP, and PK. Investigation: AS, PK, FD, H-PM, TM, and HS. Supervision: FB and FT. Writing original draft: AS. Writing review and editing: PK and SO. All authors contributed to the article and approved the submitted version.
Funding
Parts of the study were funded by Kinderherzen e.V., who also finance the open access publication of the manuscript.
Acknowledgments
We are grateful for the financial support by Kinderherzen e.V. and acknowledge Humedics for their generous donation of equipment for the LiMAx® test (methacetin, breathing masks).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, et al. 40-year follow up after the Fontan operation. Long-term outcomes of 1052 patients. J Am Coll Cardiol. (2015) 66:1700–10. doi: 10.1016/j.jacc.2015.07.065
2. Gewillig M, Brown SC, van de Bruaene A, Rychik J. Providing a framework of principles for conceptualising the Fontan circulation. Acta Paediatr. (2020)109:651–8. doi: 10.1111/apa.15098
3. Veldtman GR, Opotowsky AR, Wittekind SG, Rychik J, Penny DJ, Fogel M, et al. Cardiovascular adaptation to the Fontan circulation. Congenit Heart Dis. (2017) 12:699–710. doi: 10.1111/chd.12526
4. Mondesert B, Marcotte F, Mongeon FP, Dore A, Mercier LA, Ibrahim R, et al. Fontan circulation: success or failure? Can J Cardiol. (2013) 9:811–20. doi: 10.1016/j.cjca.2012.12.009
5. Ghanayem NS, Berger S, Tweddell JS. Medical management of the Failing Fontan. Pediatr Cardiol. (2007) 28:465–71. doi: 10.1007/s00246-007-9007-0
6. Miller JR, Lancaster TS, Callahan C, Abarbanell AM, Eghtesady P. An overview of mechanical circulatory support in single-ventricle patients. Transl Pediatr. (2018) 7:151–61. doi: 10.21037/tp.2018.03.03
7. Schilling C, Dalziel K, Nunn R, Du Plessis K, Shi WY, Celermajer D, et al. The Fontan epidemic: Population projections from Australia and New Zealand Fontan Registry. Int J Cardiol. (2016) 219:14–9. doi: 10.1016/j.ijcard.2016.05.035
8. Berg CJ, Bauer BS, Hageman A, Aboulhosn JA, Reardon LC. Mortality risk stratification in Fontan patients who underwent heart transplantation. Am J Cardiol. (2019) 119:1675–9. doi: 10.1016/j.amjcard.2017.02.005
9. Polyviou S, O'Sullivan J, Hasan A, Coats L. Mortality risk stratification in small patients cohorts: The post-Fontan heart transplantation paradigm. Am J Cardiol. (2018) 122:182–8. doi: 10.1016/j.amjcard.2018.03.021
10. Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. (2009) 250:119–25. doi: 10.1097/SLA.0b013e3181ad85b5
11. Schleiger A, Kramer P, Sallmon H, Jentsch N, Pileckaite M, Danne F, et al. Morphologic alteration precede functional impairement as determined by 13C-Methacetin breath test in adult Fontan patients. Front Cardiovasc Med. (2021) 8:764009. doi: 10.3389/fcvm.2021.764009 eCollection 2021
12. Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol. (2009) 104:419–28. doi: 10.1016/j.amjcard.2009.03.058
13. Egbe AC, Reddy YNV, Khan AR, Al-Otaibi M, Akintoye E, et al. Venous congestion and pulmonary vascular function in Fontan circulation: implications for prognosis and treatment. Int J Cardiol. (2018) 271:312–6. doi: 10.1016/j.ijcard.2018.05.039
14. Alsaied T, Rathod RH, Aboulhosn JA, Budts W, Anderson JB, Baumgartner H, et al. Reaching consensus for unified medical language in Fontan care. ESC Heart Fail. (2021) 8:3894–905. doi: 10.1002/ehf2.13294
15. Kramer P, Schleiger A, Schafstedde M, Danne F, Nordmeyer J, Berger F, Ovroutski S. A multimodal score accurately classifies Fontan failure and late mortality in adult Fontan patients. Front Cardiovasc Med. (2022) 7:767503. doi: 10.3389/fcvm.2022.767503
16. Schleiger A, Salzmann M, Kramer P, Danne F, Schubert S, Bassir C, et al. Severity of Fontan-associated liver disease correlates with Fontan hemodynamics. Pediatr Cardiol. (2020) 41:736–46. doi: 10.1007/s00246-020-02291-5
17. Stockmann M Lock JF Malinowski M Niehues SN Seehofer D and Neuhaus P. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB. (2010) 12:139–46. doi: 10.1111/j.1477-2574.2009.00151.x
18. Kaffarnik M, Lock JF, Vetter H, Ahmadi N, Lojewski C, Malinowski M, et al. Early diagnosis of sepsis-related hepatic dysfunction and its prognostic impact on survival: a prospective study with the LiMax test. Crit Care. (2013) 17:1–11. doi: 10.1186/cc13089
19. Malinowski M, Jara M, Lüttgert K, Orr J, Lock JF, Schott E, et al. Enzymatic liver function correlates with disease severity of patients with liver cirrhosis: a study with the LiMAx test. Dig Dis Sci. (2014) 12:2983–9291. doi: 10.1007/s10620-014-3250-z
20. Goldberg DJ, Shaddy RE, Ravishankar C, Rychik J. The failing Fontan: etiology, diagnosis and management. Expert Rev Cardiovasc Ther. (2011) 9:785–93. doi: 10.1586/erc.11.75
21. Book WM, Gerardin J, Saraf A, Marie Valente A, Rodriguez F 3rd. Clinical phenotypes of Fontan failure: implications for management. Congenit Heart Dis. (2016) 11:296–308. doi: 10.1111/chd.12368
22. Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, et al. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation. (2019) 140:e234–e284. doi: 10.1161/CIR.0000000000000696
23. Tabarsi N, Guan M, Simmonds J, Toma M, Kiess M, Tsang V, et al. Meta-analysis of the effectiveness of heart transplantation in patients with a failing Fontan. Am J Cardiol. (2017) 119:1269–74. doi: 10.1016/j.amjcard.2017.01.001
24. Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward-Sadler H, et al. Hepatic changes in the failing Fontan circulation. Heart. (2007) 93:594–584. doi: 10.1136/hrt.2006.094516
25. Gaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma and hepatocellular carcinoma. J Thorac Cardiovasc Surg. (2007) 129:1348–52. doi: 10.1016/j.jtcvs.2004.10.005
26. Simpson KE, Esmaeeli A, Khanna G, White F, Turnmelle Y, Eghtesady P, et al. Liver cirrhosis in Fontan patients does not affect 1-year post-heart transplant mortality or markers of liver function. J Heart Lung Heart Transplant. (2014) 33:170–7. doi: 10.1016/j.healun.2013.10.033
27. D'Souza BA, Fuller S, Gleason LP, Hornsby N, Wald J, Krok K, et al. Single-center outcomes of combined heart and liver transplantation in the failing Fontan. Clin Transplant. (2017).
28. Bryant 3rd R, Rizwan R, Zafar F, Shah SA, Chin C, Tweddell JS, Morales DL. Contemporary outcomes of combined heart-liver transplant in patients with congenital heart disease. Transplantation. (2018) 102:e67–73. doi: 10.1097/TP.0000000000001978
29. Munstermann ID, Duijinhouver AL, Kendall TJ, Bronkhorst CM, Ronot M, van Wettere M, et al. The clinical spectrum of Fontan-associated liver disease: results from a prospective multimodality screening cohort. Eur Heart J. (2018) 00:1–12. doi: 10.1093/eurheartj/ehy620
30. Bouchardy J, Meyer P, Yerly P, Blanche C, Hullin R, Giostra E, et al. Regression of advanced liver fibrosis after heart transplantation in a patient with prior Fontan surgery for complex congenital heart disease. Circ Heart Fail. (2018) 11:e003754. doi: 10.1161/CIRCHEARTFAILURE.117.003754
Keywords: late Fontan failure, Fontan-associated liver disease, end-organ dysfunction, Fontan hemodynamics, metabolic liver function
Citation: Schleiger A, Kramer P, Sallmon H, Jentsch N, Pileckaite M, Danne F, Schafstedde M, Müller H-P, Müller T, Tacke F, Jara M, Stockmann M, Berger F and Ovroutski S (2022) Functional hepatic deterioration determined by 13C-methacetin breath test is associated with impaired hemodynamics and late Fontan failure in adults. Front. Cardiovasc. Med. 9:952080. doi: 10.3389/fcvm.2022.952080
Received: 24 May 2022; Accepted: 15 August 2022;
Published: 07 September 2022.
Edited by:
Petru Liuba, Lund University, SwedenReviewed by:
Rachael Cordina, Sydney Local Health District, AustraliaOmar R. J. Tamimi, King Fahd Medical City, Saudi Arabia
Copyright © 2022 Schleiger, Kramer, Sallmon, Jentsch, Pileckaite, Danne, Schafstedde, Müller, Müller, Tacke, Jara, Stockmann, Berger and Ovroutski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anastasia Schleiger, schleiger@dhzb.de
 Anastasia Schleiger
Anastasia Schleiger Peter Kramer
Peter Kramer Hannes Sallmon
Hannes Sallmon Niklas Jentsch
Niklas Jentsch Marta Pileckaite1
Marta Pileckaite1  Friederike Danne
Friederike Danne Marie Schafstedde
Marie Schafstedde Hans-Peter Müller
Hans-Peter Müller Frank Tacke
Frank Tacke Felix Berger
Felix Berger Stanislav Ovroutski
Stanislav Ovroutski