Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis
- 1The Affiliated Stomatological Hospital of Nanchang University, Nanchang, Jiangxi, China
- 2The Key Laboratory of Oral Biomedicine, Nanchang, Jiangxi, China
- 3Jiangxi Province Clinical Research Center for Oral Disease, Nanchang, Jiangxi, China
- 4The Second Clinical Medical College of Nanchang University, Nanchang, Jiangxi, China
- 5Department of Cardiology, Seventh People’s Hospital of Zhengzhou, Zhengzhou, China
- 6Department of Sports Rehabilitation, College of Human Kinesiology, Shenyang Sport University, Shenyang, China
Objectives: Studies have established a link between periodontal disease and cardiovascular disease (CVD), but it is unclear whether there is a sex difference in their association.
Methods: The PubMed, Embase, and Cochrane databases were searched until June, 21 2022. Cardiovascular outcomes included any CVD, myocardial infarction (MI), coronary heart disease (CHD), or stroke. Studies reported the prevalence of CVD in patients with periodontal disease and the relationship between periodontal disease and CVD. The study is registered with PROSPERO (CRD42022333663). The level of evidence and recommendations is assessed by the Grading of Recommendations for Assessment, Development and Evaluation (GRADE).
Results: Twenty-six studies were included. In patients with periodontal disease, the prevalence of CVD was 7.2% [9 studies; 95% confidence interval (CI): 2.7–13.6%], and prevalence for CHD, hypertension, stroke, and heart failure was 6.6, 25.3, 1, and 1.1%, respectively. There was a significant association between periodontal disease and CVD in men [odds ratio (OR) = 1.22; 95% CI: 1.12–1.34] and women (OR = 1.11; 95% CI: 1.05–1.17), with no significant sex difference (P > 0.05).
Conclusion: Cardiovascular disease is relatively common in patients with periodontal disease, and an increased risk of CVD is associated with periodontal disease independent of sex. Interventions targeting periodontal disease may be beneficial for CVD.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022333663.
Introduction
Cardiovascular disease (CVD) remains the leading cause of death, accounting for approximately one third of all deaths worldwide. The global incidence of CVDs is 10∼30%, showing a gradually increasing trend (1–3). China has the highest cardiovascular mortality rate, followed by India, the Russian Federation and the United States of America (4). However, periodontal disease is increasingly becoming one of the major obstacles to optimal outcomes for patients with cardiovascular disease (CVD) (5).
Periodontal disease is one of the most common inflammatory diseases in humans that destroys hard and soft tissues around the tooth, resulting in tooth loosening and loss (6). Severe periodontal disease affects 10.8% of the world’s population and is the sixth most common disease worldwide, with more than 700 million people suffering from severe periodontal disease (7). Periodontal disease can cause inflammation of periodontal tissue but also produce inflammatory mediators and can products that can cardiovascular health through blood circulation (8). In recent decades, longitudinal studies have revealed a firm link between periodontal tissue and an increased risk of CVD. However, no study has systematically studied the prevalence of CVD in patients with periodontal disease. Moreover, female sex is considered to have a protective effect on the incidence and development of CVD (9, 10). However, whether there is a sex difference in the relationship between periodontal disease and the risk of CVD remains unexplored. Hence, the present study aimed to (i) systematically evaluate the prevalence of CVD in patients with periodontal disease, and (ii) examine the sex-specific association of periodontal disease with CVD.
Materials and methods
Protocol registration
The study has been registered with PROSPERO (International Registry of Prospective Systems Review: https://www.crd.york.ac.uk/PROSPERO/ number: CRD42022333663). This meta-analysis was conducted in accordance with PRISMA 2021 guidelines for systems evaluation and meta-analysis 1 (11) (Supplementary Table 1).
Search strategy
The PubMed, Embase, and Cochrane Library online databases were searched up to June 25, 2022 with no language restriction. The MeSH search items and keywords were as follows: [“periodontal disease” (MeSH) OR “furcation defects” OR “gingival diseases” OR “peri-implantitis” OR “periapical diseases” OR “periodontal atrophy” OR “periodontal cyst” OR “periodontitis” OR “tooth migration” OR “periodontitis” OR “tooth mobility” OR “tooth loss”] AND [“cardiovascular diseases” (MeSH)]. The detailed search strategy is shown in Supplementary Table 2.
Study selection
After a database search, the retrieved studies were imported into Endnote X9 software (Thomson Reuters, New York, NY, USA). The title and abstract were selected by one author (YL) and verified by a second author (QH). Before data extraction and quality assessment, the whole article was qualified. The authors reached a consensus on the included studies, and the differences were resolved through in-depth discussion. Before the database search, a data extraction form was developed to identify key study information, including demographics, data sources, exclusion criteria, follow-up periods, diagnostic criteria, and outcome measures.
Strict eligibility criteria guided the search to ensure relevant study inclusion, reduce heterogeneity and increase the power of the results. The inclusion criteria for epidemiological studies were as follows: (1) adult’s patients with periodontal diseases (including periodontitis and gingivitis); (2) reported prevalence of CVD in patients with periodontal diseases; and (3) cross-sectional, retrospective, or prospective cohort and randomized controlled trials studies. Studies with sample size <10,000 were excluded.
Additionally, eligible criteria for studies of the relationship between periodontal disease and CVD were as follows according to the PICOS: (1) Types of participants: adults; (2) Exposure and comparator: patients with periodontal disease vs. without periodontal disease; and (3) Outcomes: sex-specific association between periodontal diseases and CVD. (4) Types of studies: retrospective or prospective cohort, case-control and randomized controlled trials studies. Studies were excluded from the review if they met any one of the following criteria: (1) Protocols, reviews, conference abstracts, or animal studies; (2) Studies with unavailable data even after contacting the corresponding author for further information.
Data extraction and quality assessment
Two authors (YL and QH) extracted relevant information from each study: (1) first author; (2) publication year; (3) country; (4) study design; (5) follow-up period; (6) basic characteristics of the population (sample size, mean age); (7) diagnosis for periodontal diseases; (8) outcomes; (9) adjustments; and (10) RR or OR with 95% CI in the adjusted model.
The Newcastle-Ottawa quality scale (NOS) was used to quantify the quality of cohort studies, with a score above six regarded as acceptable quality. We evaluated the quality and strength of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method, which evaluates each outcome according to the recommended rating assessment, development and evaluation. Two authors (QH and YL) evaluated the quality of evidence of each result, providing an evidence profile table by GRADE profiler software. The results were described in the outcome measure type section, whose footnotes were used to justify any decision to reduce or improve the quality of the evidence.
Statistical analysis
We treated RRs and HRs as equivalent to ORs, and pooled the summary ORs with corresponding 95% CIs using the inverse-variance method (12). Random effect meta-analysis was performed for the overall as well as separate CVD outcomes.
Heterogeneity was evaluated using the Higgins I-squared (I2) statistic (30, 50, and 75% represent low, moderate, and high heterogeneity, respectively) (13). Publication bias was addressed by the funnel plot, and Egger’s and Begg’s tests. To appraise the robustness and reliability of the primary study outcomes, we also carried out sensitivity analyses by omitting each study in turn. The statistical analysis was performed by RevMan software, version 5.4.1 (The Cochrane Collaboration, Nordic Cochrane Center, Copenhagen, Denmark) and Stata software, Version 16.0 (Stata Corp. LP, College Station, TX, USA). P < 0.05 double-sided was considered statistically significant.
Results
Literature search
As shown in Figure 1, an initial online database search resulted in 11,045 articles. After excluding 3,321 duplicated records and 7,643 irrelevant studies, 81 studies remained for full-text review. Fifty-five studies are excluded for the following reasons: (1) reviews or meta-analysis (n = 1); (2) without target data (n = 33); (3) without sufficient sample size (n = 20); (4) The type of research is inappropriate (n = 1). The excluded studies with detailed reasons were listed in Supplementary Table 3. Finally, 26 (14–39) studies were included in this meta-analysis, of which nine (14–22) were epidemiological studies and 20 (16, 17, 22–39) reported a sex-specific association between periodontal disease and CVD.
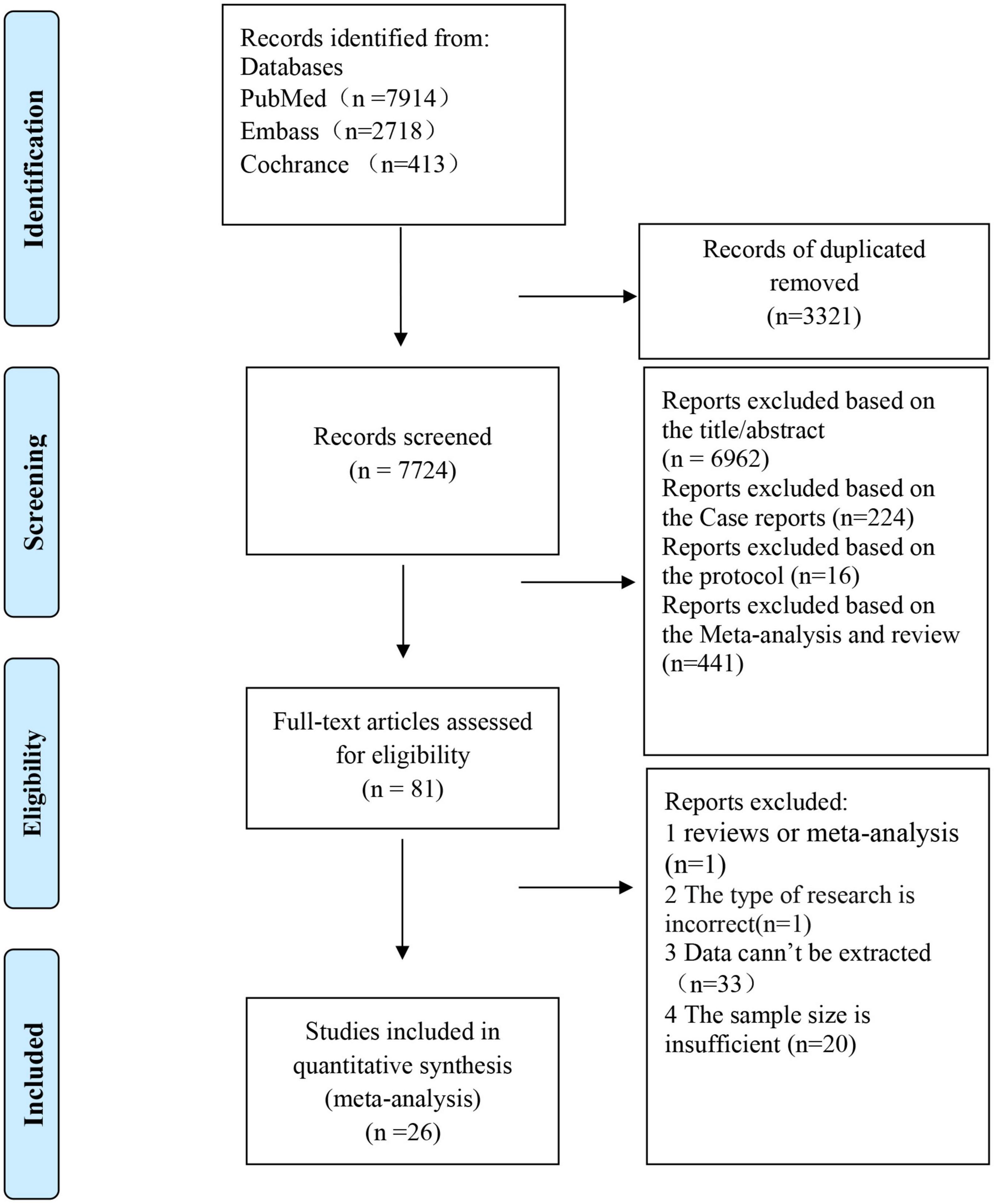
Figure 1. Flowchart of the study selection for the meta-analysis of an association between periodontal disease and cardiovascular disease [adapted from Moher et al. (67)].
Study characteristics and quality
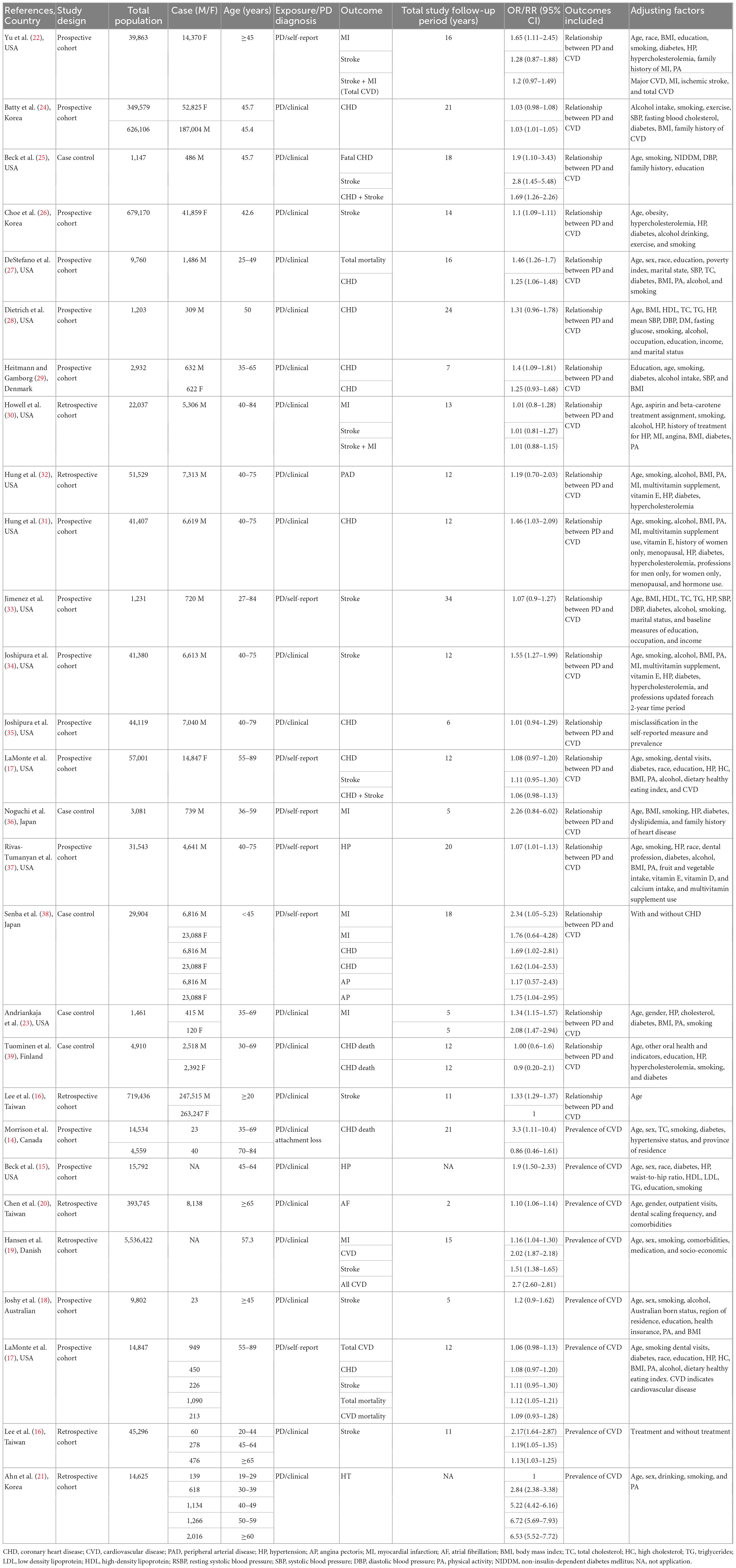
Table 1 summarizes the main characteristics of the included studies. The included studies were published between 1993 and 2019, and the sample sizes ranged from 1,231 to 626,106. Most studies were conducted in North America (n = 15), with seven and three being from Asia and Europe, respectively, while the remaining study was from Europe. Fifteen were prospective cohort studies, five were case-control studies and six were retrospective cohort studies. The follow-up time ranged from 2 years to 21 years. Sixteen (14, 16, 18–21, 23–27, 29, 33, 39–41) studies defined periodontal disease according to the Oral Hygiene Index-Simplified (OHI-S), and the remaining 10 (17, 22, 30–32, 34–38) studies were identified based on self-report. All studies defined CVD according to the International Classification of Diseases. Adjustments varied widely among the studies reporting a sex-specific association between periodontal disease and CVD, while the primary confounding factors were age, sex, smoking, alcohol consumption, hypertension (HP), diabetes, and heart failure (HF). Based on the NOS, twenty studies (16, 17, 22–39) reporting the association of periodontal disease and CVD were considered to be of moderate to high quality, with a score range of 6–9, and one (29) study had a score of 5 (Supplementary Table 4).
Epidemiology of CVD in patients with periodontal disease
Nine studies (15–21, 24, 26) with 2,737,324 participants were included. The pooled prevalence of CVD in periodontal disease was 7.2% (95% CI: 2.7–13.6%), with high heterogeneity (I2 = 99.997%) (Figure 2).
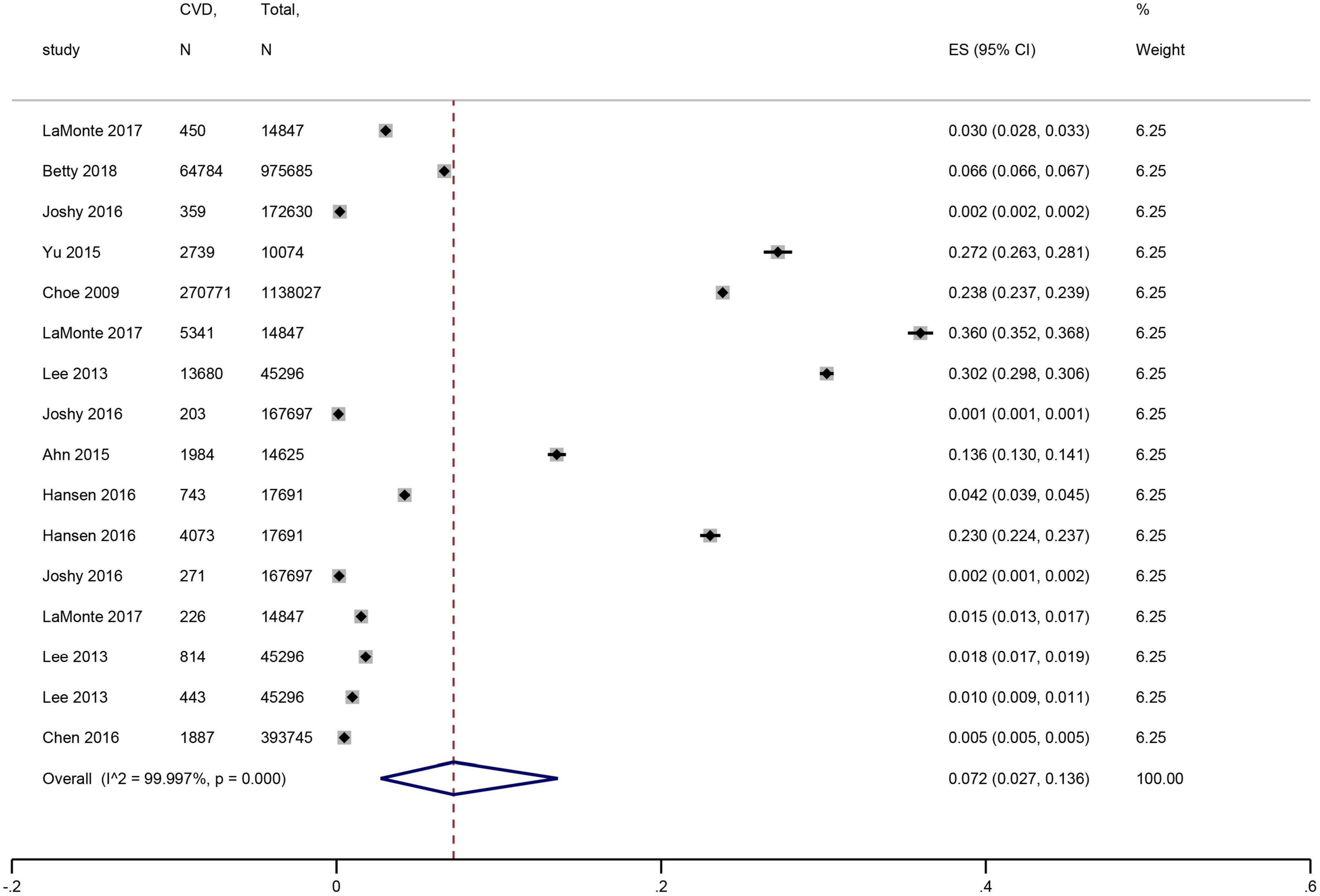
Figure 2. Forest plot of the prevalence of cardiovascular disease in patients with periodontal disease. In the forest plot, the diamond indicates the pooled estimate. Red boxes are relative to study size and the black vertical lines indicate 95% CIs around the effect size estimate.
In the subgroup analysis, younger patients (mean age <45 years) with periodontal disease showed a higher prevalence of CVD than older patients (mean age ≥45 years), whose effect size (ES) was 23.8 vs. 7.5% (P for subgroup difference < 0.001) (Figure 3A). Furthermore, no significant difference was shown according to the publication years (P = 1) (Figure 3B). Americans had the highest prevalence of CVD (ES: 13.3%), followed by Europeans (ES: 12%), Asians (ES: 8.2%), and Oceania (ES: 2%) (P for subgroup difference < 0.001) (Figure 3C). The pooled prevalence was 25.3% for HP, 6.6% for coronary heart disease (CHD), 1% for stroke, and 1.1% for HF (Supplementary Figure 1).
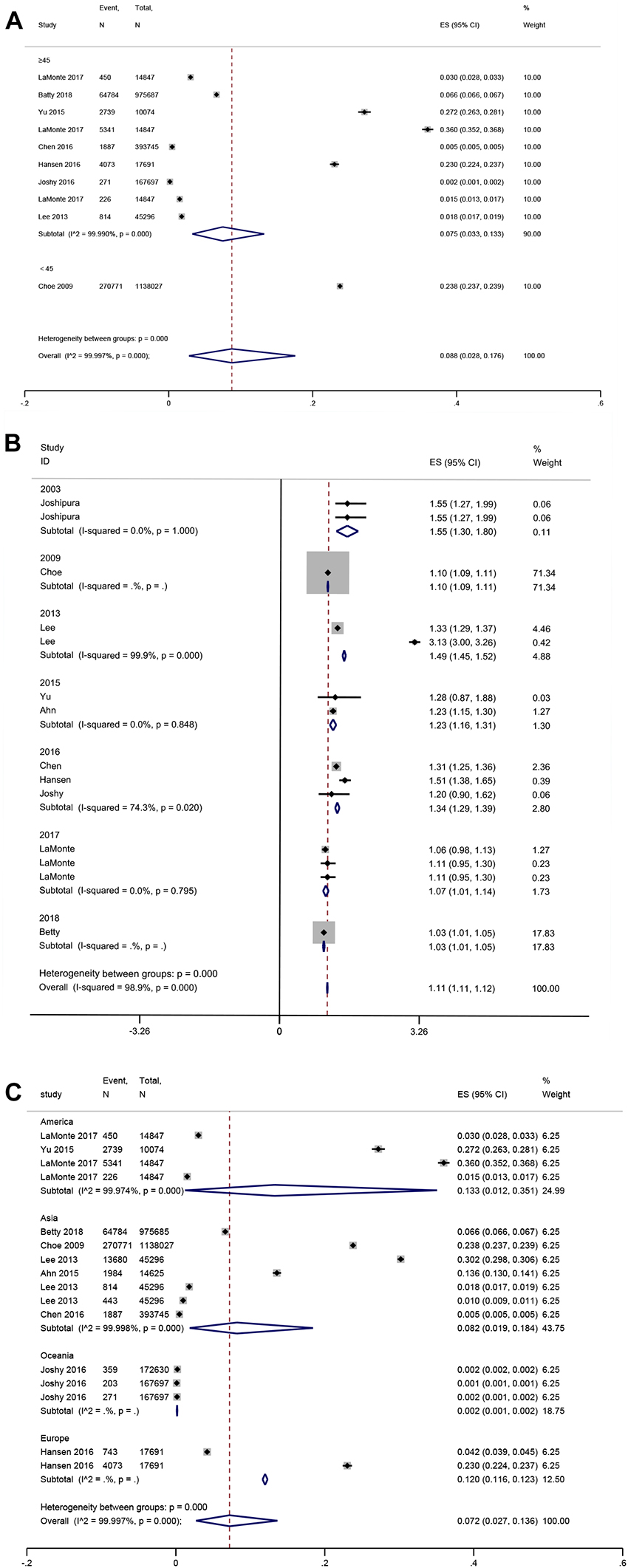
Figure 3. Forest plot of subgroup analysis of prevalence of cardiovascular disease in patients with periodontal disease [(A) Age, (B) Publication years, and (C) Region]. In the forest plot, the diamond indicates the pooled estimate. Red boxes are relative to study size and the black vertical lines indicate 95% CIs around the effect size estimate.
Sex-specific association between CVD and periodontal disease
Seventeen (16, 23–25, 27–39) studies with 1,308,625 men reported an association between periodontal disease and CVD (OR = 1.22; 95% CI: 1.12–1.34), with high heterogeneity (I2 = 93%; P < 0.001) (Figure 4A). Nine (17, 22–24, 26, 29, 38, 39) studies with 1,990,952 women reported an association between periodontal disease and CVD (OR = 1.11; 95% CI: 1.05–1.17; I2 = 85%; P = 0.0002) (Figure 4B), and there was no significant sex difference (P for interaction > 0.05).
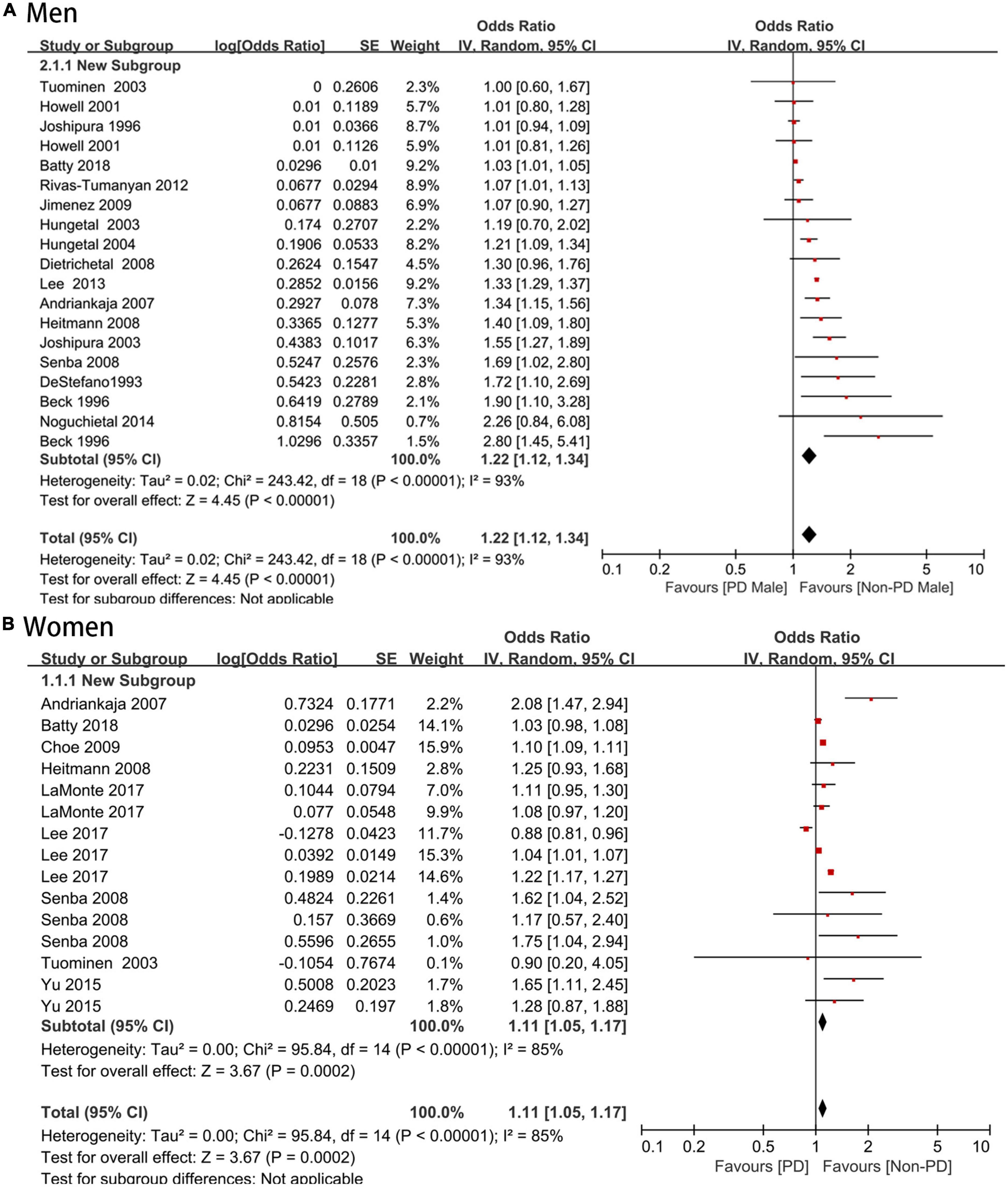
Figure 4. (A,B) Forest plot of the risk of cardiovascular disease in periodontal disease patients by sexes. In the forest plot, the diamond indicates the pooled estimate. Red boxes are relative to study size and the black vertical lines indicate 95% CIs around the effect size estimate.
Sex-specific association between periodontal disease and coronary artery disease
An increased risk of coronary artery disease was found in male periodontal disease patients (OR = 1.19; 95% CI: 1.09–1.3) with moderate heterogeneity (I2 = 72%), which was consistent with the female group (OR = 1.18; 95% CI: 1.02–1.36; I2 = 83%), and no significant sex difference was shown (P > 0.05) (Figure 5A).
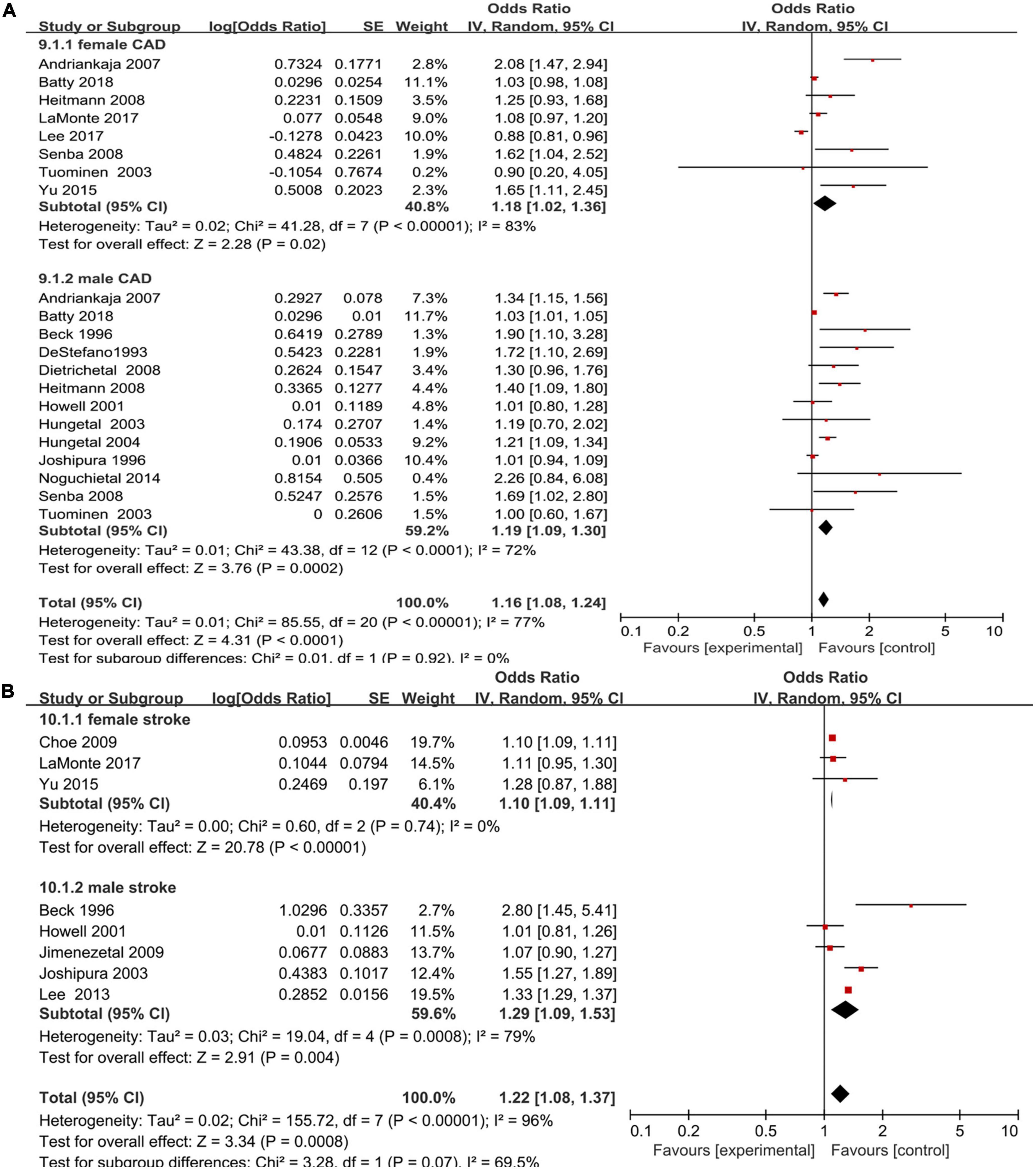
Figure 5. Forest plot of the risk of coronary artery disease and stroke in periodontal disease patients by sex [(A) Coronary artery disease and (B) stroke]. In the forest plot, the diamond indicates the pooled estimate. Red boxes are relative to study size and the black vertical lines indicate 95% CIs around the effect size estimate.
Sex-specific association between periodontal disease and stroke
For eight studies that reported an association between periodontal disease and stroke, there was a higher risk of stroke (16, 17, 22, 25, 26, 30, 33, 34) in both male and female periodontal disease patients (OR = 1.29, 95% CI: 1.09–1.53; I2 = 79% versus OR = 1.10, 95% CI: 1.09–1.11, I2 = 0%) with no significant sex difference (P > 0.05) (Figure 5B).
Publication bias and sensitivity analysis
As shown in Supplementary Figure 2, although some presence of bias was found by funnel plots, Egger’s test (P > 0.1), and Begg’s test (P > 0.1) showed no significant publication bias. Sensitivity analysis by omitting each study showed that our results were stable and reliable, with a range from 1.09 (95% CI: 1.03–1.15) to 1.14 (95% CI: 1.05–1.24) for the relationship between periodontal disease and CVD in females, and 1.24 (95% CI: 1.12–1.38) to 1.29 (95% CI: 1.15–1.43) for the relationship between periodontal disease and CVD in males (Supplementary Figure 3).
Quality assessment
The GRADE tool was used to evaluate the quality of evidence. All the included studies were observational studies; thus, so the initial level of evidence was low (42). Ultimately, the risk of CVD was evaluated as very low certainty (Supplementary Table 5).
Discussion
Major findings
The present meta-analysis showed that the pooled prevalence was 7.3% for CVD, 6.6% for CHD, 25.3% for HP, 1% for stroke, and 1.1% for HF among periodontal disease patients. Moreover, a significant association between periodontal disease and the risk of CVD was found independent of sex, with a summary OR of 1.22 (95% CI: 1.12–1.34) for females and 1.11 (95% CI: 1.05–1.17) for males. To the best of our knowledge, this article is the first to explore the sex-specific association of periodontal diseases and CVD.
Consistent with results of a previous meta-analysis (43), our study also showed that periodontal disease is associated with the risk of CVD. More importantly, our meta-analysis also showed that this association remains in both men and women, without sex differences. To ensure the rigor of our results, necessary analysis for potential publication bias was conducted, and the final results were reliable. CVD has always been shown to have a greater possibility of secondary oral problems. As Lazureanu et al. showed in their research on the prevalence of periodontal disease in patients with CVD (44), 77.5% of the 147 patients with CVD developed periodontal disease, implying a high incidence of periodontal disease among patients with CVD. However, few related studies have explored the incidence of CVD in patients with periodontal disease. Our study indicated that the prevalence of CVD in patients with periodontal disease reached 7.2%, suggesting that the occurrence of CVD is relatively common in patients with periodontal disease. As a result, we hypothesize that there may be a significant association between periodontal disease and CVD, which was also confirmed in our subsequent analysis about the association between periodontal disease and CVD.
Age is a vital risk factor for both CVD and periodontal disease. In subgroup analyses, younger patients with periodontal disease were shown to have an increased risk of CVD, with a prevalence of 23.8% in the younger group (age <45 years) versus 7.5% in older one (age ≥45 years). This was consistent with Joshipura et al. (34) who reported a stronger effect for younger men than for older men (≤55 years: RR = 2.17; 95% CI: 1.22–3.84 vs. >55 years: RR = 1.21; 95% CI: 0.92–1.59). Similarly, in a case-control study, four cases of clinical attachment loss of 4.5 to ≤6 mm were associated with a significantly increased risk of stroke (OR = 3.43; 95% CI: 1.39–8.50) in men ≤60 years, while there was no statistical significance in younger men (age >60 years) (OR = 1.71; 95% CI: 0.65–4.5). Men who were susceptible to periodontitis were shown to develop periodontal destruction earlier than those who were not. Thus, there is a stronger association between periodontitis and cerebrovascular disease in young men than in older men, which may further support the hypothesis of proinflammatory susceptibility.
Potential mechanism
Our results showed that periodontal disease is strongly associated with a high risk of CHD, MI, and CVD, suggesting that periodontal disease is significantly associated with CVD. Systemic inflammation may be the core mechanism to explain the association between periodontal disease and the increased risk of CVD. Considerable studies have shown that inflammation is a predisposing factor or cardiovascular disease (45, 46), and patients with periodontal disease have been confirmed to have a high CRP lever as well as other inflammatory markers in the circulation (47, 48), indicating that it may lead to systemic inflammation and thus induce the development of CVD. In addition, oral pathogens such as Porphyromonas gingivalis (P. gingivalis) can reach the blood by crossing the gingival epithelial-conjunctival barrier and vascular endothelial cells, thus aggravating the inflammation and immune response in the original atherosclerosis in blood vessels (49–52). In addition, one animal study had found that P. gingivalis can enhance the expression of high mobility group box 1 (HMGB1) in mice after myocardial infarction. HMGB1 is a nuclear protein that induces inflammation (53). Therefore, it can be inferred that the increased risk of myocardial infarction caused by periodontal disease may be related to HMGB-1. One study showed that P. gingivalis infection and invasion can accelerate programmed cell death and the role of myocardial matrix metalloproteinases 9, which is not conducive to the recovery process of myocardial infarction prognosis, and may eventually lead to cardiac rupture (54). The increased risk of stroke may be due to decreased vascular endothelial function in the gingival tissue after infection with periodontal disease. The damage of vascular function may be caused by the basic interaction between oxidative stress and nitric oxide induced by periodontal disease (55).
The effect of sex
Although our results showed that there was no difference between periodontal disease and CVD in different sexes, sex differences were shown in the coronary artery disease subgroup analysis, where men with periodontal disease had a higher risk of coronary artery disease than in women. In addition, a prospective cohort showed that the incidence of stroke in men was significantly higher than that in women in the total population and among the periodontal disease groups (56). This may arise from the expression of Y-encoded genes and the lack of cardiovascular protective effects of estrogen, leading to male-specific cardiovascular events. However, studies have also showed that female periodontal disease patients may have a higher risk of several CVDs. For example, an observational study showed that women with periodontal disease were 108% more likely to develop CVD (OR = 2.08, 95% CI: 1.47–2.94), which was higher than that of male periodontal disease patients (OR = 1.34, 95% CI: 1.15–1.57) (23). As a result, the sex-specific association between periodontal disease and CVD may be different in various types of CVDs. The mechanisms of how sex affects the relationship between periodontal disease and CVD are complex and still unclear, thus, further analysis is needed in the future. However, close monitoring of CVD in patients with periodontal disease is necessary in both men and women.
Clinical implications
Considering the proven association between CVD and periodontal disease, early examination and treatment for periodontal disease patients may play an important role in preventing the occurrence of CVD, which needs to be highly valued in daily clinical work. Additionally, treatment of periodontal disease may prevent the occurrence of CVD, and may also improve the status of CVD to some degree. As shown by Vidal et al. (57), periodontal therapy was able to attenuate arterial stiffness and reduce circulating inflammatory markers. Moreover, periodontal treatment with subunit-microbial doses of doxycycline could increase the level of serum apolipoprotein A and high-density lipoprotein, reduce total cholesterol levels, and further reduce the risk of cardiovascular events (58, 59). As a result, periodontal disease therapy may be a therapeutic target for reduce the risk of CVD, and further relevant research is needed. However, it is interesting to note that periodontal pathogens are found in the interdental spaces even in young people with healthy periodontitis. The interdental health is thought to be closely related to cardiovascular diseases (60–62), therefore, the oral health of the interdental space should be promoted during adolescence to prevent periodontal disease and thus cardiovascular disease. Traditional daily methods to maintain oral health between teeth by destroying biofilms are still not effective enough. Studies have shown that using calibrated interdental brushes to clean teeth every day can reduce interdental bleeding, inhibit periodontal pathogens, and re-establish symbiotic microflora. and reduce interdental inflammation (63, 64). These findings suggest that adherence to interdental cleanliness may be an effective way to help maintain optimal oral health, thereby preventing the emergence of periodontal disease and ultimately reducing the risk of cardiovascular disease. In addition, one study had shown that daily use of mouthwash can also help prevent periodontal disease (65).
Study limitations
There were still some limitations in present study. First, periodontal examination may be a more objective reflection of periodontal health than a self-reported diagnosis of periodontitis (66), but some of our included studies identified periodontal diseases by self-report. Second, some of the included studies were retrospective, which may have introduced recall bias, thus, further prospective studies are needed to confirm our results. Third, a high degree of heterogeneity was observed in our results due to the variation in the characteristics of the study population and study design. Finally, studies included in this meta-analysis are observational studies, however, observational studies cannot completely avoid some potential confusions, and the quality of evidence is not high.
Conclusion
The findings of this study suggest that CVD is common in patients with periodontal disease. Periodontal disease is associated with an increased risk of CVD independent of sex. Further trials are required to assess the effect of periodontal intervention on the CVD incidence.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
QD, YL, and QH contributed to the study concept and design and revised the draft. QL, XY, JC, ML, and ZY performed the search strategy and contributed to database research, acquisition of data, and statistical analyses. All authors participated in data analysis, reviewed, and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1114927/full#supplementary-material
References
1. Manolis AA, Manolis TA, Melita H, Manolis AS. Psoriasis and cardiovascular disease: the elusive link. Int Rev Immunol. (2019) 38:33–54. doi: 10.1080/08830185.2018.1539084
2. Colonia-García A, Gutiérrez-Vélez M, Duque-Duque A, de Andrade CR. Possible association of periodontal disease with oral cancer and oral potentially malignant disorders: a systematic review. Acta Odontol Scand. (2020) 78:553–9. doi: 10.1080/00016357.2020.1774076
3. Tezal M, Grossi SG, Genco RJ. Is periodontitis associated with oral neoplasms? J Periodontol. (2005) 76:406–10. doi: 10.1902/jop.2005.76.3.406
4. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021.
5. Carrizales-Sepúlveda EF, Ordaz-Farías A, Vera-Pineda R, Flores-Ramírez R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. (2018) 27:1327–34. doi: 10.1016/j.hlc.2018.05.102
6. Touil D, Oualha L, Douki N. Oral cancer: a major and growing public health problem towards a national policy of prevention and early detection in Tunisia. Pan Afr Med J. (2020) 37:291. doi: 10.11604/pamj.2020.37.291.25448
7. Tezal M, Sullivan MA, Reid ME, Marshall JR, Hyland A, Loree T, et al. Chronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg. (2007) 133:450–4. doi: 10.1001/archotol.133.5.450
8. Javed F, Warnakulasuriya S. Is there a relationship between periodontal disease and oral cancer? A systematic review of currently available evidence. Cri Rev Oncol Hematol. (2016) 97:197–205. doi: 10.1016/j.critrevonc.2015.08.018
9. Khan SS, Beach LB, Yancy CW. Sex-based differences in heart failure: JACC focus seminar 7/7. J Am Coll Cardiol. (2022) 79:1530–41. doi: 10.1016/j.jacc.2022.02.013
10. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. (2018) 138:198–205. doi: 10.1161/CIRCULATIONAHA.118.034271
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
12. Yan Z, Liu Y, Li W, Zhao X, Lin W, Zhang J, et al. Liver fibrosis scores and prognosis in patients with cardiovascular diseases: a systematic review and meta-analysis. Eur J Clin Invest. (2022) 52:e13855. doi: 10.1111/eci.13855
13. Liu X, Long C, Xiong Q, Chen C, Ma J, Su Y, et al. Association of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with risk of COVID-19, inflammation level, severity, and death in patients with COVID-19: a rapid systematic review and meta-analysis. Clin Cardiol. (2020). doi: 10.1002/clc.23421 [Epub ahead of print].
14. Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. (1999) 6:7–11. doi: 10.1177/204748739900600102
15. Beck JD, Eke P, Lin D, Madianos P, Couper D, Moss K, et al. Associations between IgG antibody to oral organisms and carotid intima-medial thickness in community-dwelling adults. Atherosclerosis. (2005) 183:342–8. doi: 10.1016/j.atherosclerosis.2005.03.017
16. Lee YL, Hu HY, Huang N, Hwang DK, Chou P, Chu D. Dental prophylaxis and periodontal treatment are protective factors to ischemic stroke. Stroke. (2013) 44:1026–30. doi: 10.1161/STROKEAHA.111.000076
17. LaMonte MJ, Genco RJ, Hovey KM, Wallace RB, Freudenheim JL, Michaud DS, et al. History of periodontitis diagnosis and edentulism as predictors of cardiovascular disease, stroke, and mortality in postmenopausal women. J Am Heart Assoc. (2017) 6:e004518. doi: 10.1161/JAHA.116.004518
18. Joshy G, Arora M, Korda RJ, Chalmers J, Banks E. Is poor oral health a risk marker for incident cardiovascular disease hospitalisation and all-cause mortality? Findings from 172 630 participants from the prospective 45 and up study. BMJ Open. (2016) 6:e012386. doi: 10.1136/bmjopen-2016-012386
19. Hansen GM, Egeberg A, Holmstrup P, Hansen PR. Relation of periodontitis to risk of cardiovascular and all-cause mortality (from a Danish nationwide cohort study). Am J Cardiol. (2016) 118:489–93. doi: 10.1016/j.amjcard.2016.05.036
20. Chen DY, Lin CH, Chen YM, Chen HH. Risk of atrial fibrillation or flutter associated with periodontitis: a nationwide, population-based, cohort study. PLoS One. (2016) 11:e0165601. doi: 10.1371/journal.pone.0165601
21. Ahn YB, Shin MS, Byun JS, Kim HD. The association of hypertension with periodontitis is highlighted in female adults: results from the Fourth Korea national health and nutrition examination survey. J Clin Periodontol. (2015) 42:998–1005. doi: 10.1111/jcpe.12471
22. Yu YH, Chasman DI, Buring JE, Rose L, Ridker PM. Cardiovascular risks associated with incident and prevalent periodontal disease. J Clinical Periodontol. (2015) 42:21–8. doi: 10.1111/jcpe.12335
23. Andriankaja OM, Genco RJ, Dorn J, Dmochowski J, Hovey K, Falkner KL, et al. Periodontal disease and risk of myocardial infarction: the role of gender and smoking. Eur J Epidemiol. (2007) 22:699–705. doi: 10.1007/s10654-007-9166-6
24. Batty GD, Jung KJ, Mok Y, Lee SJ, Back JH, Lee S, et al. Oral health and later coronary heart disease: cohort study of one million people. Eur J Prev Cardiol. (2018) 25:598–605. doi: 10.1177/2047487318759112
25. Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. (1996) 67:1123–37. doi: 10.1902/jop.1996.67.10s.1123
26. Choe H, Kim YH, Park JW, Kim SY, Lee SY, Jee SH. Tooth loss, hypertension and risk for stroke in a Korean population. Atherosclerosis. (2009) 203:550–6. doi: 10.1016/j.atherosclerosis.2008.07.017
27. DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. Br Med J. (1993) 306:688–91. doi: 10.1136/bmj.306.6879.688
28. Dietrich T, Jimenez M, Kaye EAK, Vokonas PS, Garcia RI. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. (2008) 117:1668–74.
29. Heitmann BL, Gamborg M. Remaining teeth, cardiovascular morbidity and death among adult Danes. Prev Med. (2008) 47:156–60. doi: 10.1016/j.ypmed.2008.04.007
30. Howell TH, Ridker PM, Ajani UA, Hennekens CH, Christen WG. Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians. J Am Coll Cardiol. (2001) 37:445–50. doi: 10.1016/S0735-1097(00)01130-X
31. Hung HC, Joshipura KJ, Colditz G, Manson JE, Rimm EB, Speizer FE, et al. The association between tooth loss and coronary heart disease in men and women. J Public Health Dent. (2004) 64:209–15. doi: 10.1111/j.1752-7325.2004.tb02755.x
32. Hung HC, Willett W, Merchant A, Rosner BA, Ascherio A, Joshipura KJ. Oral health and peripheral arterial disease. Circulation. (2003) 107:1152–7. doi: 10.1161/01.CIR.0000051456.68470.C8
33. Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol. (2009) 66:505–12. doi: 10.1002/ana.21742
34. Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. (2003) 34:47–52. doi: 10.1161/01.STR.0000052974.79428.0C
35. Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res. (1996) 75:1631–6. doi: 10.1177/00220345960750090301
36. Noguchi S, Toyokawa S, Miyoshi Y, Suyama Y, Inoue K, Kobayashi Y. Five-year follow-up study of the association between periodontal disease and myocardial infarction among Japanese male workers: my health up study. J Public Health. (2015) 37:605–11. doi: 10.1093/pubmed/fdu076
37. Rivas-Tumanyan S, Spiegelman D, Curhan GC, Forman JP, Joshipura KJ. Periodontal disease and incidence of hypertension in the health professionals follow-up study. Am J Hypertens. (2012) 25:770–6. doi: 10.1038/ajh.2012.32
38. Senba T, Kobayashi Y, Inoue K, Kaneto C, Inoue M, Toyokawa S, et al. The association between self-reported periodontitis and coronary heart disease–from my health up study. J Occup Health. (2008) 50:283–7. doi: 10.1539/joh.L7066
39. Tuominen R, Reunanen A, Paunio M, Paunio I, Aromaa A. Oral health indicators poorly predict coronary heart disease deaths. J Dent Res. (2003) 82:713–8. doi: 10.1177/154405910308200911
40. Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. (2008) 117:1668–74. doi: 10.1161/CIRCULATIONAHA.107.711507
41. Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. (2005) 76:2089–100. doi: 10.1902/jop.2005.76.11-S.2089
42. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
43. Larvin H, Kang J, Aggarwal VR, Pavitt S, Wu J. Risk of incident cardiovascular disease in people with periodontal disease: a systematic review and meta-analysis. Clin Exp Dent Res. (2021) 7:109–22. doi: 10.1002/cre2.336
44. Lazureanu PC, Popescu FG, Stef L, Focsa M, Vaida MA, Mihaila R. The Influence of periodontal disease on oral health quality of life in patients with cardiovascular disease: a cross-sectional observational single-center study. Medicina. (2022) 58:584. doi: 10.3390/medicina58050584
45. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. (2019) 2019:7092151. doi: 10.1155/2019/7092151
46. Ndrepepa G. Myeloperoxidase - A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta. (2019) 493:36–51. doi: 10.1016/j.cca.2019.02.022
47. Gu Y, Lee H-M, Sorsa T, Salminen A, Ryan ME, Slepian MJ, et al. Non-antibacterial tetracyclines modulate mediators of periodontitis and atherosclerotic cardiovascular disease: a mechanistic link between local and systemic inflammation. Pharmacol Res. (2011) 64:573–9. doi: 10.1016/j.phrs.2011.06.023
48. Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. (2005) 76:2106–15. doi: 10.1902/jop.2005.76.11-S.2106
49. Stewart R, West M. Increasing evidence for an association between periodontitis and cardiovascular disease. Circulation. (2016) 133:549–51. doi: 10.1161/CIRCULATIONAHA.115.020869
50. Aarabi G, Heydecke G, Seedorf U. Roles of oral infections in the pathomechanism of atherosclerosis. Int J Mol Sci. (2018) 19:1978. doi: 10.3390/ijms19071978
51. Chun YH, Chun KR, Olguin D, Wang HL. Biological foundation for periodontitis as a potential risk factor for atherosclerosis. J Periodontal Res. (2005) 40:87–95. doi: 10.1111/j.1600-0765.2004.00771.x
52. Khlgatian M, Nassar H, Chou HH, Gibson FC III, Genco CA. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells. Infect Immun. (2002) 70:257–67. doi: 10.1128/IAI.70.1.257-267.2002
53. Srisuwantha R, Shiheido Y, Aoyama N, Sato H, Kure K, Laosrisin N, et al. Porphyromonas gingivalis elevated high-mobility group box 1 levels after myocardial infarction in mice. Int Heart J. (2017) 58:762–8. doi: 10.1536/ihj.16-500
54. Shiheido Y, Maejima Y, Suzuki JI, Aoyama N, Kaneko M, Watanabe R, et al. Porphyromonas gingivalis, a periodontal pathogen, enhances myocardial vulnerability, thereby promoting post-infarct cardiac rupture. J Mol Cell Cardiol. (2016) 99:123–37. doi: 10.1016/j.yjmcc.2016.03.017
55. Funaki S, Tokutomi F, Wada-Takahashi S, Yoshino F, Yoshida A, Maehata Y, et al. Porphyromonas gingivalis infection modifies oral microcirculation and aortic vascular function in the stroke-prone spontaneously hypertensive rat (SHRSP). Microb Pathog. (2016) 92:36–42. doi: 10.1016/j.micpath.2015.12.009
56. Weissman S, Sinh P, Mehta TI, Thaker RK, Derman A, Heiberger C, et al. Atherosclerotic cardiovascular disease in inflammatory bowel disease: the role of chronic inflammation. World J Gastrointest Pathophysiol. (2020) 11:104–13. doi: 10.4291/wjgp.v11.i5.104
57. Vidal F, Cordovil I, Figueredo CM, Fischer RG. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J Clin Periodontol. (2013) 40:681–7. doi: 10.1111/jcpe.12110
58. Tüter G, Serdar M, Kurtiş B, Walker SG, Atak A, Toyman U, et al. Effects of scaling and root planning and subantimicrobial dose doxycycline on gingival crevicular fluid levels of matrix metalloproteinase-8, -13 and serum levels of HsCRP in patients with chronic periodontitis. J Periodontol. (2010) 81:1132–9. doi: 10.1902/jop.2010.090694
59. Tüter G, Kurtiş B, Serdar M, Aykan T, Okyay K, Yücel A, et al. Effects of scaling and root planning and sub-antimicrobial dose doxycycline on oral and systemic biomarkers of disease in patients with both chronic periodontitis and coronary artery disease. J Clin Periodontol. (2007) 34:673–81. doi: 10.1111/j.1600-051X.2007.01104.x
60. Teles R, Wang CY. Mechanisms involved in the association between periodontal diseases and cardiovascular disease. Oral Dis. (2011) 17:450–61. doi: 10.1111/j.1601-0825.2010.01784.x
61. Desvarieux M, Demmer RT, Jacobs DR, Papapanou PN, Sacco RL, Rundek T. Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: the oral infections and vascular disease epidemiology study. J Am Heart Assoc. (2013) 2:e000254. doi: 10.1161/JAHA.113.000254
62. Li C, Lv Z, Shi Z, Zhu Y, Wu Y, Li L, et al. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. (2014) 8:CD009197. doi: 10.1002/14651858.CD009197.pub2
63. Bourgeois D, Saliasi I, Llodra JC, Bravo M, Viennot S, Carrouel F. Efficacy of interdental calibrated brushes on bleeding reduction in adults: a 3-month randomized controlled clinical trial. Eur J Oral Sci. (2016) 124:566–71. doi: 10.1111/eos.12302
64. Bourgeois D, Bravo M, Llodra JC, Inquimbert C, Viennot S, Dussart C, et al. Calibrated interdental brushing for the prevention of periodontal pathogens infection in young adults - a randomized controlled clinical trial. Sci Rep. (2019) 9:15127. doi: 10.1038/s41598-019-51938-8
65. Saliasi I, Llodra JC, Bravo M, Tramini P, Dussart C, Viennot S, et al. Effect of a toothpaste/mouthwash containing carica papaya leaf extract on interdental gingival bleeding: a randomized controlled trial. Int J Environ Res Public Health. (2018) 15:2660. doi: 10.3390/ijerph15122660
66. LaMonte MJ, Hovey KM, Millen AE, Genco RJ, Wactawski-Wende J. Accuracy of self-reported periodontal disease in the women’s health initiative observational study. J Periodontol. (2014) 85:1006–18. doi: 10.1902/jop.2013.130488
Keywords: periodontal disease, cardiovascular disease, epidemiology, sex difference, prevalence
Citation: Leng Y, Hu Q, Ling Q, Yao X, Liu M, Chen J, Yan Z and Dai Q (2023) Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis. Front. Cardiovasc. Med. 10:1114927. doi: 10.3389/fcvm.2023.1114927
Received: 03 December 2022; Accepted: 06 February 2023;
Published: 27 February 2023.
Edited by:
Akbar Shafiee, Tehran University of Medical Sciences, IranReviewed by:
Oleh Andrukhov, Medical University of Vienna, AustriaFlorence Carrouel, Université Claude Bernard Lyon 1, France
Copyright © 2023 Leng, Hu, Ling, Yao, Liu, Chen, Yan and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Dai, 512968304@qq.com
 Yurong Leng
Yurong Leng Qinwen Hu4
Qinwen Hu4  Zhiwei Yan
Zhiwei Yan