Pulmonary vein capture is a predictor for long-term success of stand-alone pulmonary vein isolation with cryoballoon ablation in patients with persistent atrial fibrillation
- 1Department of Cardiovascular Medicine, Mayo Clinic Florida, Jacksonville, FL, United States
- 2School of Medicine, Emory University, Atlanta, GA, United States
Background: The mechanisms of AF development and progression are still not completely understood. Despite the relative efficacy of ablation, the risk of AF recurrence is substantial, particularly in patients with persistent AF (perAF). At present we do not have any reliable intra-procedural electrophysiologic predictors of long-term success of AF ablation other than pulmonary vein isolation. We evaluated selected intraprocedural pulmonary vein characteristics that may be helpful in future guidance of persistent AF ablation.
Methods: 390 consecutive procedures using cryoballoon for initial AF ablation were divided by clinical presentation (paroxysmal or persistent AF), and by pulmonary vein (PV) response to pacing after completion of ablation (discrete electrogram elicited with pacing—“PV capture” or not—“Control”). Patients were followed (median 20 months) for recurrent atrial arrhythmias as the primary end point of the study.
Results: PV capture was identified in 20.3% and 17.1% and patients with paroxysmal and persistent AF respectively (ns). In patients with persistent AF presence of PV capture was associated with significantly better outcomes compared to patients without PV capture (p < 0.001). In the group “persistent AF and PV capture”, an initial strategy of PV isolation and reisolation of the PVs (without additional lesions) for patients with recurrent atrial arrhythmias resulted in 20/23 (87%) patients in sinus rhythm off antiarrhythmic medications at study completion. In patients with paroxysmal AF, PV capture was not associated with outcome benefits. Specific electrophysiologic characteristics of PV (PV capture cycle length: PVCCL) did not have an impact on AF recurrence, although 25% shortening of PVCCL was observed after 60 s periods of pacing at short cycle lengths. No background demographic patient characteristic differences were identified between patients with vs. without PV capture.
Conclusion: The presence of PV capture was associated with better outcomes in patients with persistent AF. PV capture may identify those patients with persistent AF in whom cryoballoon PV isolation alone is sufficient as an initial ablation procedure and as the primary ablation strategy for recurrent AF.
Introduction
Randomized controlled trials in patients with atrial fibrillation have demonstrated that catheter ablation is superior to pharmacological therapy with reduced likelihood of recurrent AF and improved quality of life, and may confer a survival benefit in patients with accompanying congestive heart failure (1–4). Mechanisms underlying AF are complex and remain incompletely understood (1, 2). Despite relative efficacy of ablation, the risk of AF recurrence is still substantial, particularly in patients with persistent AF (1). Atrial substrate modification in addition to pulmonary vein isolation (PVI) has historically been thought to be necessary to achieve satisfactory success rates in patients with persistent atrial fibrillation (1). However, several randomized trials demonstrated that adjunctive RF ablation strategies did not result in higher freedom from arrhythmia than a PVI-only strategy but were associated with higher fluoroscopy and procedure times (4, 5). Recently, several studies have suggested that cryoballoon ablation of the PVs may be moderately effective for the initial treatment of patients with persistent AF (6–8). The presence of exit block from the pulmonary veins has been used as the traditional endpoint for effective pulmonary vein ablation (1). Evidence of exit block may be difficult to confirm and often its presence is implied by the presence of entrance block [GL (9)]. While successful production of exit block during cryoballoon ablation has been reported, we evaluated whether electrophysiologic properties of pulmonary veins (PV capture and PV cycle length) after cryoballoon ablation could identify patients with persistent atrial fibrillation might be successfully treated with strategies directed solely at PVI both at the index procedure and any necessary subsequent procedures (10).
Methods
Patient population and follow-up
Three hundred ninety consecutive initial PVI procedures using cryoballoon for symptomatic drug-refractory AF were retrospectively evaluated. Patients with self-limited episodes shorter than 7 days duration and no history of prior cardioversion (done for AF episodes less than 48 h duration) were defined as having paroxysmal AF (PAF), patients with AF who did not satisfy these criteria were placed to “persistent AF” group. Patients with a past medical history of atypical atrial flutter or prior ablation for atrial fibrillation were not included in the study. Antiarrhythmic drugs were stopped at least five half-lives prior to the ablation procedure (amiodarone stopped ≥1 month prior). Post-procedure follow-up was performed at a routine cardiology clinic visits at 1 month, 6 months, 12 months, and then annually or earlier as needed according to symptoms, including emergency department visits. All patients underwent ECG monitoring for 7–30 days with duration based on provider preference. Outcome analysis was started 3 months (“blanking period”) after the index ablation procedure. Antiarrhythmic medication was used based on the health provider's judgment during the first 3 months but was stopped after the blanking period.
The primary clinical outcome was recurrence of atrial arrhythmias detected by rhythm monitoring or symptoms reported by a patient at a follow-up visit with the need for additional therapy (cardioversion, re-ablation, drug therapy) or change to a rate control strategy. There were no patients lost to follow-up. Follow-up providers were blinded to electrophysiologic details of the ablation procedure. For patients who underwent a repeat ablation a second blanking period was not instituted.
Ablation procedure
Patients underwent ablation using a 28 mm cryoballoon (Medtronic Inc., Minneapolis, Minnesota). During procedures, patients were in deep analgosedation. Patients who presented in AF were electrically cardioverted to sinus rhythm at the start of the procedure. If patient rhythm could not be converted to sinus rhythm, the ablation procedure was started with the patient in AF, with additional cardioversion delivered as needed after defined milestones such as pulmonary vein encircling. Temperatures lower than −45°C were always sought with ablation of 240 s, shorter time (180 s) if lower temperatures were achieved (−50°C). Ablation stopped if temperature achieved −55°C. Diaphragmatic pacing techniques were used for identifying phrenic nerve injury during ablation of the right-sided pulmonary veins. Additional radiofrequency ablation of the cavotricuspid isthmus with confirmation of bidirectional block was performed for patients with previously clinically documented typical atrial flutter or if typical atrial flutter was induced or identified during the index procedure. Patients with inducible atypical atrial flutter underwent cardioversion or atrial pacing to resolve the atypical atrial flutter without additional mapping or ablation.
Evaluation of electrophysiologic properties of the PVs
After PV isolation a multipolar Lasso catheter (Johnson & Johnson Medical, New Brunswick, New Jersey) was placed in each PV, and all aspects of the PV were explored, and the presence of spontaneous PV activity was assessed. The lasso catheter allowed exploration of larger territory at the pulmonary vein os to account for PV anatomy variation, could be manipulated within the pulmonary vein to ensure tissue contact to all aspects of the pulmonary vein, and was less expensive in our experience since it can be reused. Since the lasso catheter was used for longer periods in the left atrium, guiding sheaths were flushed in the right atrium to decrease the potential risk of neurologic events. The PV was paced at multiple sites within the PV, beginning at a cycle length (CL) of 600 ms. PV capture was defined as a repetitive discrete EGM response within PV for more than 10 extrastimulus (10 mA) consecutively (Figure 1). The pacing cycle length was progressively decreased and electrogram behavior within the PV was recorded. When 2:1 PV capture was observed, the cycle length was increased to the point where 1:1 capture was reachieved and defined as the initial PV capture cycle length (PVCCL). After continuous pacing at a higher cycle length for 60 s, the pacing cycle length was then again progressively shortened and in most cases, 1:1 capture was observed at shorter cycle lengths that were previously associated with 2:1 block. The process was continued until no additional decrease in pacing cycle length with 1:1 capture was observed. The final PVCCL was defined as the minimum pacing cycle length in which 1:1 capture was observed. The ΔPVCCL was the difference between the initial PVCCL and final PVCCL. The decision on whether to use the adenosine to demonstrate “dormant” forms of PV-to-atrial conduction was left to the operator (11, 12).
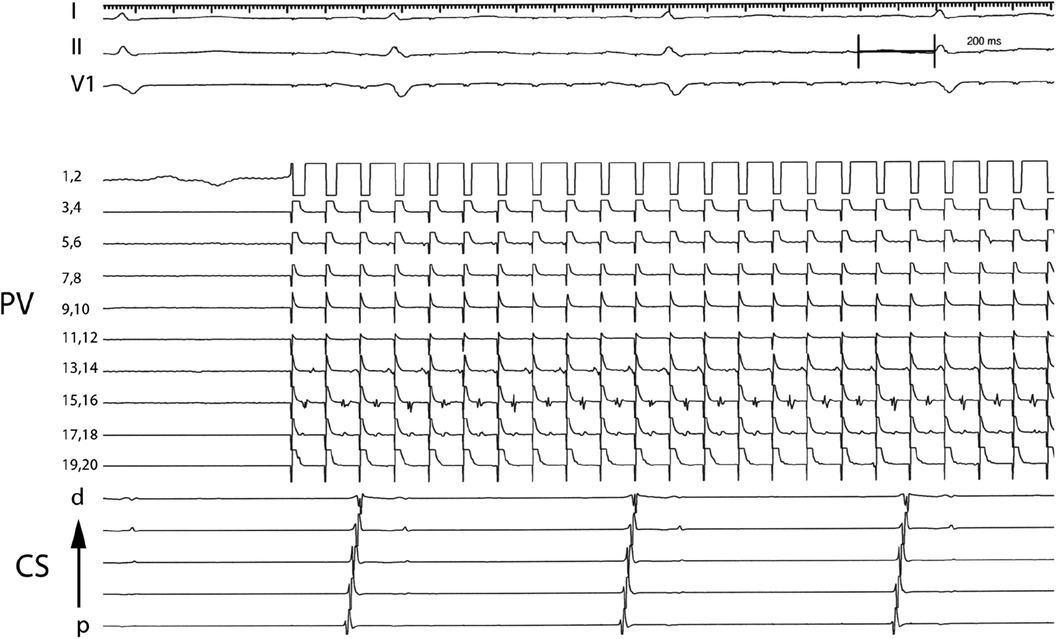
Figure 1. Electrograms demonstrating PV capture dissociated from atrial tissue. Pacing from electrode pair 1,2 at a cycle length of 100 ms results in 1:1 capture in PV tissue recorded by electrode pairs 17,18 and 15,16 and intermittent block in PV tissue recorded by electrode pairs 13,14 and 5,6. PV: Pulmonary vein; CS: Coronary sinus; p: proximal; d: distal.
Data management and statistical analysis
The measured values were checked for distribution type by the Shapiro–Wilk test. Continuous variables with non-normal distribution are represented as median [25th percentile, 75thpercentile], or as mean [ ± standard deviation]. Categorical variables were tested using Chi- square test. Arrhythmia recurrence was evaluated by the Kaplan–Meier method, differences in the freedom from arrhythmia were compared using the log-rank test. Statistical analysis was performed using SPSS version 22.0 (IBM, Armonk, New York, USA) and two-tailed p-values <0.05 were deemed to be statistically significant. The authors had full access to and take full responsibility for the integrity of the data. All the authors have read and agree to the manuscript as written.
Results
Baseline characteristics
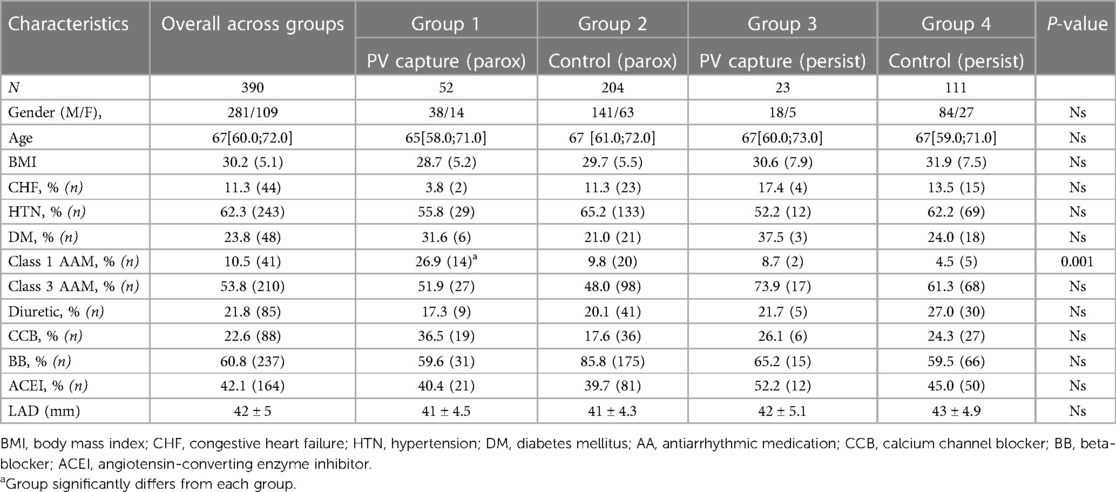
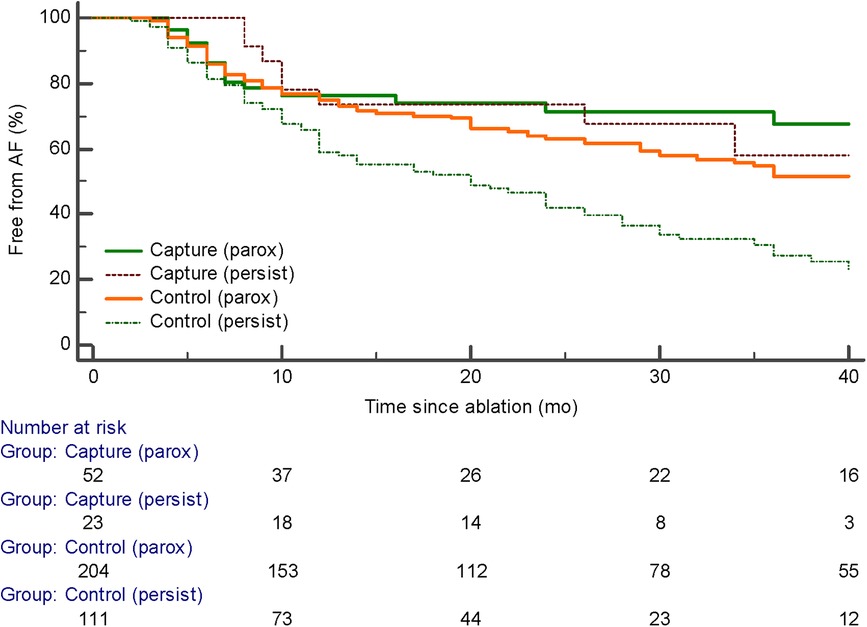
For purpose of our study patients undergoing AF ablation were divided into 4 groups depending on the type of AF (“persistent” vs. “paroxysmal”) and PV capture—whether discrete PV electrogram was identified during PV pacing or not (“PV capture” vs. “Control” group). Figure 1 shows an example of PV capture.
The characteristics of the overall population evaluated were as follows: age 67 [60.0; 72.0] years, follow-up period was 20 [9; 36] months, 72% of patients were males, 53.8% of the patients were taking class III antiarrhythmic and 60.8% were taking beta-blockers before the index procedure, arterial hypertension was comorbidity in 62.3% and DM in 23.8% of cases. There were no significant differences between the groups studied besides of utilization of class I antiarrhythmics, which was significantly higher in patients with “paroxysmal AF and PV capture” vs. other groups (Tables 1 and 2). Left atrial diameter was not significantly different among patients the different groups.
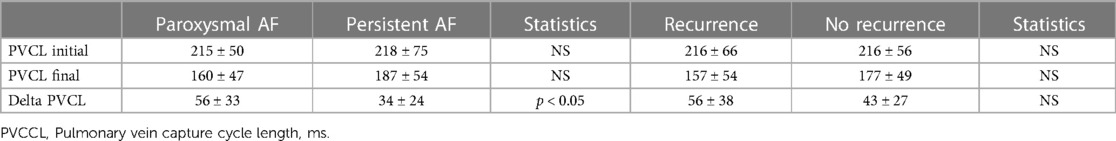
Table 2. Pv electrophysiologic characteristics in patients based on arrhythmia type or whether or not recurrent AF was identified.
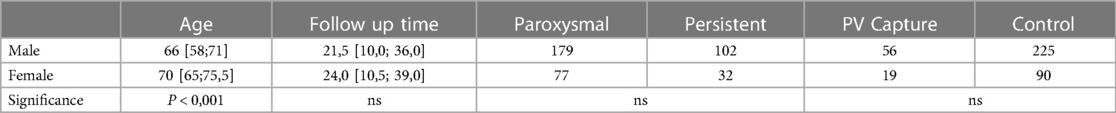
The predominance of male participants, and significantly older age of female participants (66 [58; 71] vs. 70 [65; 75,5] y.o. respectively) in our study mirrors the overall distribution of AF in population (Table 3). There were no significant cross-gender differences in the distribution of forms of AF and PV properties, as well as in the arrhythmia-free period after ablation.
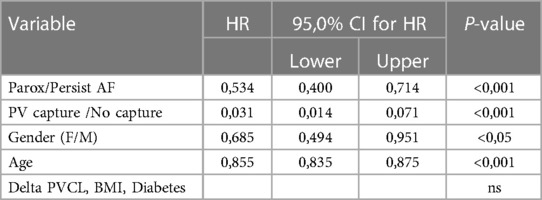
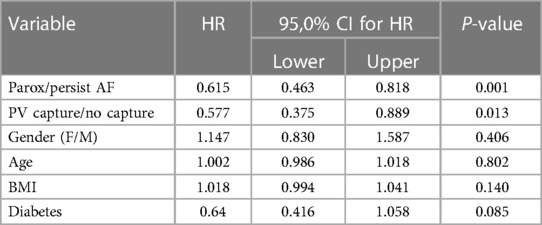
We analyzed the predictive value of baseline characteristics on arrhythmia-free survival by univariate and multivariate Cox proportional hazards regression (Tables 4 and 5). The type of arrhythmia and PV capture were the only predictors of the favorable outcome with both univariate and multivariate analysis. Other indicators evaluated such as age, BMI, usage of medications, and history of diabetes, failed to show significance with multivariate analysis.
Outcomes
PV capture was present in 52 of 256 (20.3%) of patients with paroxysmal AF and 23 of 134 (17.1%) of patients with persistent AF (ns). The development of recurrent atrial arrhythmias during follow-up was similar in patients with paroxysmal AF with or without PV capture (p = ns). The median follow-up was 20 months with no significant difference among the four groups. However, the presence of PV capture in persistent AF identified a group with favorable ablation outcomes similar to patients with paroxysmal AF. These patients had significantly fewer recurrent atrial arrhythmias than patients with persistent AF without PV capture (p < 0.001) (Figure 2). Of the 23 patients with persistent AF and pulmonary vein capture, 8 underwent repeat ablation >3 months after the index procedure. At repeat ablation, all patients had recurrent PV conduction and underwent reisolation of the pulmonary veins with no additional atrial lesions (Figure 3). At study completion, 20 of the 23 patients were in sinus rhythm without antiarrhythmic medications. Of the 111 patients with persistent AF and no evidence for pulmonary vein capture, 49 underwent repeat ablation, and 42 of 111 patients were in sinus rhythm without antiarrhythmic medications.
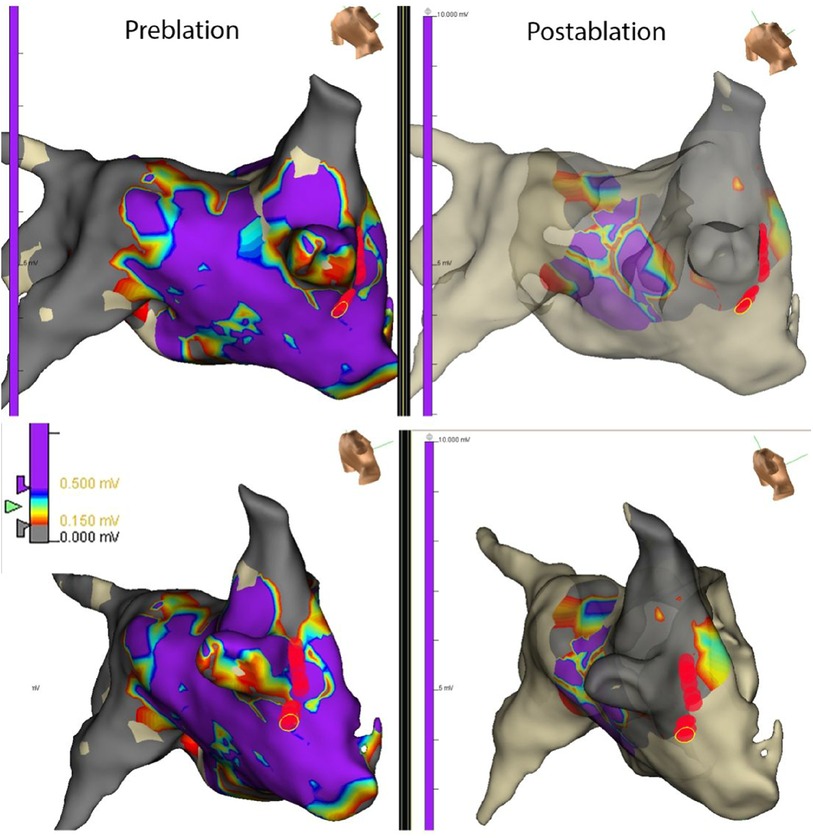
Figure 3. Map from a repeat ablation procedure showing electrograms measured from different regions of the left atrium and PV in a patient with persistent AF and PV capture identified at the index procedure. Recurrent conduction to the right-sided pulmonary veins was identified due to recurrent conduction in the septal and inferior regions of the right inferior pulmonary vein. Limited ablation with reisolation of the pulmonary veins (red circles) resulted in the elimination of recurrent AF at follow-up. The apparent changes in voltages noted in the posterior LA in the post-ablation maps are due to undercollection rather than a new scar.
PV electrophysiologic characteristics
For patients with PV capture, the electrophysiologic characteristics of the pulmonary vein tissue were evaluated (Table 2). The minimal PV capture was dynamic. With initial pacing, 2:1 block would be identified at the cycle length (CL) would vary, and with continued pacing the cycle length inevitably decreased. In several patients the pacing cycle length decreased to values less than 100 ms (Figure 1). In patients with PV capture CL (PVCCL) less than 150 ms, complex conduction patterns were also observed (Figure 4). The electrophysiologic properties of the PV did not differ between patients with paroxysmal or persistent AF and for the initial and final PVCCL, although a more significant reduction in PVCCL during pacing was noted in patients with paroxysmal AF. The presence of spontaneous dissociated PV potentials was more commonly observed in patients with paroxysmal AF when compared to patients with persistent AF (paroxysmal AF: 9.6% vs. persistent AF 4.3%; p < 0.001). PV capture was usually observed in only one PV (in almost all cases, the left superior PV or right superior PV), though 3 patients with paroxysmal AF had PV capture identified in two PVs. No electrophysiologic characteristics of the pulmonary vein were identified that had an impact on clinical outcomes for the entire group with PV capture or in patients with paroxysmal or persistent AF.
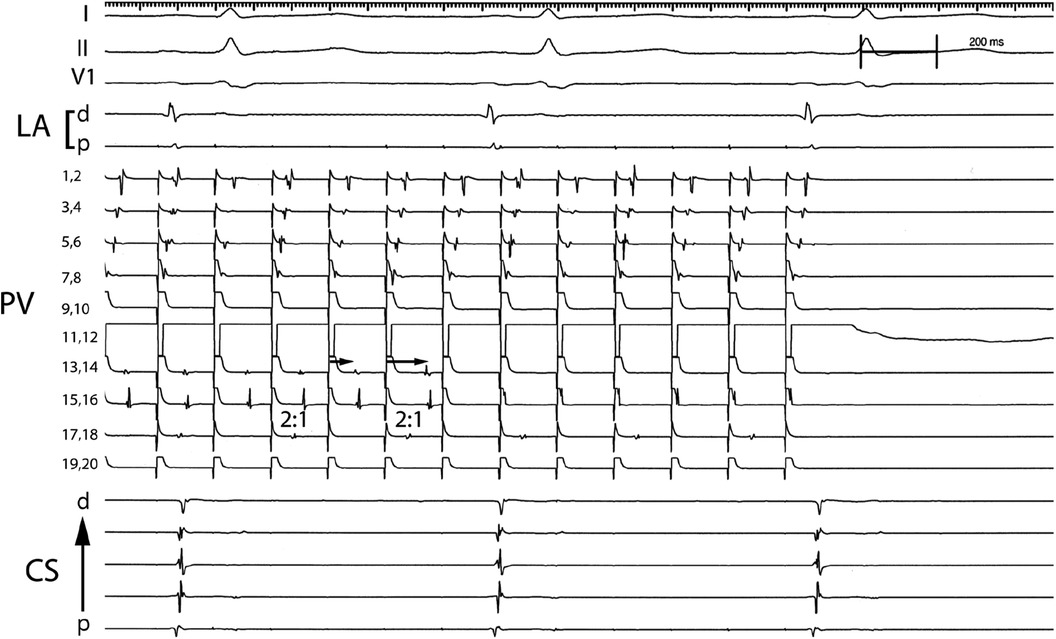
Figure 4. Complex conduction patterns within the PV observed with PV pacing. Pacing from electrode pair 11,12 in the PV results in 1:1 conduction that travels from electrode pairs 7,8 to 1,2 and 2:1 conduction in electrode pair 17,18 (2:1). Progressive delay in conduction is observed that appears to travel from electrode pair 13,14 to 15,16 (arrows) which after block is replaced with rapid 1:1 conduction observed in electrode pair 15,16 but absent activation in electrode pair 13,14. PV: Pulmonary vein; CS: Coronary sinus; p: proximal; d: distal.
Discussion
The present study evaluated whether the characteristics of PV response on pacing during cryoablation are related to long-term AF-freedom. Our results suggest that evidence of PVpotentials (PV capture) after PV isolation is present in 20.3% of patients with paroxysmal AF and 17.1% of patients with persistent AF. The presence of PV capture appears to identify a group of patients with persistent AF in whom PV isolation alone either at the index procedure or at any required follow-up procedure is associated with favorable outcomes. PV capture did not predict the clinical efficacy of PV isolation in paroxysmal AF. In a prior study of patients undergoing catheter ablation using r adiofrequency energy, the presence of dissociated PV potentials was identified in 19% of patients and their presence did not have an impact on outcomes (13). The majority of patients in this study had paroxysmal atrial fibrillation (74%), and similarly, we found that the presence of PV capture in patients with paroxysmal AF had no impact on the likelihood of recurrent AF. Another related intraprocedural study of 30 patients found that loss of PV capture was identified in 40% of patients acutely after PVI and found that this finding was associated with entrance block (14). Since, the mass of ablated tissue after cryoablation apopears to be larger than pulmonary vein ablation using radiofrequency energy, it may be that local PV capture after cryoablation may identify a subset of patients with persistent atrial fibrillation in whom greater PV muscle mass serves as an important arrhythmia mechanism (15). The difference in the impact of pulmonary vein capture between patients with paroxysmal atrial fibrillation and persistent atrial fibrillation provides indirect additional evidence for the importance of the pulmonary veins in patients with paroxysmal atrial fibrillation regardless of PV sleeve mass, and that patients with pulmonary vein “dependent” atrial fibrillation and larger PV sleeve mass may clinically present with persistent atrial fibrillation. Finally, though probably related, dissociated PV potentials were only observed in a small minority of patients with PV capture after cryoballoon ablation in the current study, and dissociated spontaneous PV potentials and PV capture may have different implications, particularly in the setting of different ablation energy sources (15).
The optimal lesion set for patients with persistent AF has not been established (1). Prior studies using cryoablation have suggested moderate success rates in patients with persistent AF (6–8). It is likely that persistent AF has multiple underlying electrophysiologic mechanisms and identifying the cause in an individual patient will be critical for designing a specific management or ablation strategy. The current study suggests that the presence of PV capture in patients with persistent AF identified a subset of patients in whom an initial approach of PV isolation is sufficient not only at initial ablation but also if subsequent ablation is required, even if atypical atrial flutter is induced with atrial pacing protocols. This study along with others emphasize the importance of identifying individual mechanisms that is particularly relevant for patients with persistent atrial fibrillation (16).
Prior studies have shown that heterogeneity of electrophysiologic characteristics of pulmonary vein tissue may be important in the underlying pathophysiology of atrial fibrillation (17, 18). Our study extends these findings and highlights the potential importance of electrophysiologic characteristics in atrial fibrillation. Our results suggest that the PVs have unique electrophysiologic properties such as very short paced PVCCL, in some cases < 100 ms that would develop with the progressive pacing. PVs also displayed rapid rate dependence with significant shortening of the PVCCL even after very short periods of tachycardia (60 s) and provides further evidence that “AF begets AF” (19). In addition, complex electrogram patterns suggestive of dynamic development of conduction block within different regions of the pulmonary vein that were dependent on the direction of the depolarization wavefront were identified similar to what has been described at the PV-left atrial junction and left atrium, but at even shorter cycle lengths (20, 21) However, even though these electrophysiologic characteristics from a mechanistic standpoint would appear to make AF more likely, similar to a previous study that used radiofrequency energy, in the current study the specific electrophysiologic characteristics of the PV did not have an identifiable impact on clinical outcomes in patients with paroxysmal or persistent AF (22). However, in patients with persistent atrial fibrillation, the presence of pulmonary vein capture after cryoballoon ablation may identify a group of patients in whom pulmonary vein isolation alone is sufficient both initially and at any required repeat procedures.
There are several limitations to the current study. The most important limitation is that the results are derived from a single center with relatively small number of participants with persistent AF and PV capture. However, despite the limited number of patients, this design allowed this analysis to be performed, since the ablation strategies for management of patients with paroxysmal AF and persistent AF were consistent and similar. In addition, there are likely many factors that lead to recurrent atrial fibrillation in persistent AF (23). It is notable that a strategy only targeting pulmonary vein isolation at initial study and for repeat ablation resulted in a high likelihood of maintaining sinus rhythm without antiarrhythmic medications in this selected group of patients with persistent AF. Although left atrial diameters were similar among the different study groups, another limitation is the absence of left atrial voltage data, particularly for those patients with persistent atrial fibrillation. A recent meta-analysis suggests that low voltage area-guided substrate modification may have a beneficial effect on outcomes though a randomized controlled trial found that addition ablation at scarred regions identified by MRI and mapping was not associated with improved outcomes (24). However, identifying potential patients with persistent atrial fibrillation in whom pulmonary vein isolation alone could be satisfactory may be a useful strategy to minimize the potential of collateral damage resulting from more extensive ablation in the left atrium.
Conclusion
Currently, no specific optimal approach for ablation of persistent AF has been identified, and this likely represents the significant heterogeneity in underlying mechanisms in these patients which may partially explain the relative lack of success of trials that have evaluated the use of anatomic ablation strategies. At present, we do not have any reliable intra-procedural electrophysiologic predictors of long-term success of AF ablation in patients with persistent atrial fibrillation. However, the presence of PV capture may identify a group of patients in which PV isolation using a cryoballoon approach is sufficient at the index procedure and if recurrent atrial fibrillation (AF) with evident reconnection of a pulmonary vein, directing efforts toward PV reisolation without additional ablation may be optimal.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Mayo Clinic Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AB: Writing – original draft, Formal analysis. CL: Data curation, CK: Resources. RM: Visualization. KV: Methodology, Resources. FK: Conceptualization, Project administration, Supervision, Review & editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2017) 14(10):e275–444. doi: 10.1016/j.hrthm.2017.05.012
2. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. (2019) 321(13):1261–74. doi: 10.1001/jama.2019.0693
3. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. (2018) 378(5):417–27. doi: 10.1056/NEJMoa1707855
4. Verma V, Jiang C-Y, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. (2015) 372(19):1812–22. doi: 10.1056/NEJMoa1408288
5. Vogler J, Willems S, Sultan A, Schreiber D, Lüker J, Servatius H, et al. Pulmonary vein isolation versus defragmentation: the CHASE-AF clinical trial. J Am Coll Cardiol. (2015) 66(24):2743–52. doi: 10.1016/j.jacc.2015.09.088
6. Su WW, Reddy VY, Bhasin K, Champagne J, Sangrigoli RM, Braegelmann KM, et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the multicenter STOP persistent AF trial. Heart Rhythm. (2020) 17(11):1841–7. doi: 10.1016/j.hrthm.2020.06.020
7. Boveda S, Metzner A, Nguyen DQ, Julian Chun KR, Goehl K, Noelker G, et al. Single-Procedure outcomes and quality-of-life improvement 12 months post-cryoballoon ablation in persistent atrial fibrillation: results from the multicenter CRYO4PERSISTENT AF trial. JACC Clin Electrophysiol. (2018) 4(11):1440–7. doi: 10.1016/j.jacep.2018.07.007
8. Sawhney V, Schilling RJ, Providencia R, Cadd M, Perera D, Chatha S, et al. Cryoablation for persistent and longstanding persistent atrial fibrillation: results from a multicentre European registry. Europace. (2020) 22(3):375–81. doi: 10.1093/europace/euz313
9. Duytschaever M, De Meyer G, Acena M, El-Haddad M, De Greef Y, Van Heuverswyn F, et al. Lessons from dissociated pulmonary vein potentials: entry block implies exit block. Europace. (2013) 15(6):805–12. doi: 10.1093/europace/eus353
10. Boveda S, Providência R, Albenque JP, Combes N, Combes S, Hireche H, et al. Real-time assessment of pulmonary vein disconnection during cryoablation of atrial fibrillation: can it be ‘achieved’ in almost all cases? Europace. (2014) 16(6):826–33. doi: 10.1093/europace/eut366
11. Tokuda M, Matsuo S, Isogai R, Uno G, Tokutake K, Yokoyama K, et al. Adenosine testing during cryoballoon ablation and radiofrequency ablation of atrial fibrillation: a propensity score-matched analysis. Heart Rhythm. (2016) 13(11):2128–34. doi: 10.1016/j.hrthm.2016.08.018
12. Andrade JG, Deyell MW, Nattel S, Khairy P, Dubuc M, Champagne J, et al. Prevalence and clinical impact of spontaneous and adenosine-induced pulmonary vein reconduction in the contact-force vs. Cryoballoon atrial fibrillation ablation (CIRCA-DOSE) study. Heart Rhythm. (2020) 17(6):897–904. doi: 10.1016/j.hrthm.2020.01.017
13. Lee G, Kalman JM, Vohra JK, Teh A, Medi C, Ling LH, et al. Dissociated pulmonary vein potentials following antral pulmonary vein isolation for atrial fibrillation: impact on long-term outcome. Heart. (2011) 97(7):579–84. doi: 10.1136/hrt.2010.211367
14. Lee G, Kalman JM, Vohra JK, Teh A, Medi C, Ling L-H, et al. Loss of local capture of the pulmonary vein myocardium after antral isolation: prevalence and clinical significance. J Cardiovasc Electrophysiol. (2015) 26(3):242–50. doi: 10.1111/jce.12585
15. Kurose J, Kiuchi K, Fukuzawa K, Takami M, Mori S, Suehiro H, et al. Lesion characteristics between cryoballoon ablation and radiofrequency ablation with a contact force-sensing catheter: late-gadolinium enhancement magnetic resonance imaging assessment. J Cardiovasc Electrophysiol. (2020) 31(10):2572–81. doi: 10.1111/jce.14664
16. Dhillon GS, Honarbakhsh S, Graham A, Ahluwalia N, Abbas H, Welch S, et al. Driver characteristics associated with structurally and electrically remodeled atria in persistent atrial fibrillation. Heart Rhythm O2. (2022) 3(6Part A):631–8. doi: 10.1016/j.hroo.2022.09.016
17. Kawai S, Mukai Y, Inoue S, Yakabe D, Nagaoka K, Sakamoto K, et al. Location and coupling interval of an ectopic excitation determine the initiation of atrial fibrillation from the pulmonary veins. J Cardiovasc Electrophysiol. (2022) 33(4):629–37. doi: 10.1111/jce.15371
18. Kumagai K, Ogawa M, Noguchi H, Yasuda T, Nakashima H, Saku K. Electrophysiologic properties of pulmonary veins assessed using a multielectrode basket catheter. J Am Coll Cardiol. (2004) 43(12):2281–9. doi: 10.1016/j.jacc.2004.01.051
19. Allessie MA. Atrial electrophysiologic remodeling: another vicious circle? J Cardiovasc Electrophysiol. (1998) 9(12):1378–93. doi: 10.1111/j.1540-8167.1998.tb00114.x
20. Wong GR, Nalliah CJ, Lee G, Voskoboinik A, Prabhu S, Parameswaran R, et al. Dynamic atrial substrate during high-density mapping of paroxysmal and persistent AF: implications for substrate ablation. JACC Clin Electrophysiol. (2019) 5(11):1265–77. doi: 10.1016/j.jacep.2019.06.002
21. Lee G, Spence S, Teh A, Goldblatt J, Larobina M, Atkinson V, et al. High-density epicardial mapping of the pulmonary vein-left atrial junction in humans: insights into mechanisms of pulmonary vein arrhythmogenesis. Heart Rhythm. (2012) 9(2):258–64. doi: 10.1016/j.hrthm.2011.09.010
22. Prabhu S, Kalla M, Peck KY, Voskoboinik A, McLellan AJA, Pathik B, et al. Pulmonary vein activity does not predict the outcome of catheter ablation for persistent atrial fibrillation: a long-term multicenter prospective study. Heart Rhythm. (2018) 15(7):980–6. doi: 10.1016/j.hrthm.2018.02.029
23. Bavishi AA, Kaplan RM, Peigh G, Diaz CL, Baman JR, Trivedi A, et al. Patient characteristics as predictors of recurrence of atrial fibrillation following cryoballoon ablation. Pacing Clin Electrophysiol. (2019) 42(6):694–704. doi: 10.1111/pace.13669
Keywords: atrial fibrillation, cryoballoon, pulmonary vein, persistent atrial fibrillation, ablation, electrophysiology
Citation: Babak A, Kauffman CB, Lynady C, McClellan R, Venkatachalam K and Kusumoto F (2024) Pulmonary vein capture is a predictor for long-term success of stand-alone pulmonary vein isolation with cryoballoon ablation in patients with persistent atrial fibrillation. Front. Cardiovasc. Med. 10:1150378. doi: 10.3389/fcvm.2023.1150378
Received: 24 January 2023; Accepted: 29 November 2023;
Published: 12 February 2024.
Edited by:
Rui Providência, Institute of Health Informatics Research—University College London, United KingdomReviewed by:
Hikmet Yorgun, Hacettepe University, TürkiyeDaehoon Kim, Severance Cardiovascular Hospital, Republic of Korea
© 2024 Babak, Kauffman, Lynady, McClellan, Venkatachalam and Kusumoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexey Babak a.babakmd@gmail.com
 Alexey Babak
Alexey Babak Christine Bienvenue Kauffman1
Christine Bienvenue Kauffman1  Fred Kusumoto
Fred Kusumoto