Blood pressure variability, nocturnal heart rate variability and endothelial function predict recurrent cerebro-cardiovascular events following ischemic stroke
- 1Sleep-Wake-Epilepsy Center, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 2Graduate School for Health Sciences, University of Bern, Bern, Switzerland
- 3Univ. Grenoble Alpes, Inserm, U1300, CHU Grenoble Alpes, Service Universitaire de Pneumologie Physiologie, Grenoble, France
- 4Department of Pulmonary Medicine and Allergology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 5Stroke Unit, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 6Department of Cardiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 7Ohio Sleep Medicine Institute, Dublin, OH, United States
Introduction: Cardiovascular parameters characterizing blood pressure (BP), heart rate (HR), endothelial function and arterial stiffness predict cerebro-cardiovascular events (CCVE) in the general population. Considering the paucity of data in stroke patients, we assessed these parameters as potential predictors of recurrent CCVE at acute stroke stroke.
Patients and methods: This is a secondary outcome analysis of a prospective observational longitudinal Sleep Deficiency & Stroke Outcome Study (ClinicalTrials.gov Identifier: NCT02559739). The study consecutively recruited acute ischemic stroke patients. Cardiovascular parameters (blood pressure variability [BPV], heart rate variability [HRV], endothelial function, and arterial stiffness) were assessed within the first week post-stroke. Future CCVE were recorded over a 3-year follow-up. Multivariate Cox regression analysis was used to investigate the prognostic value of 48 cardiovascular parameters regarding CCVE risk.
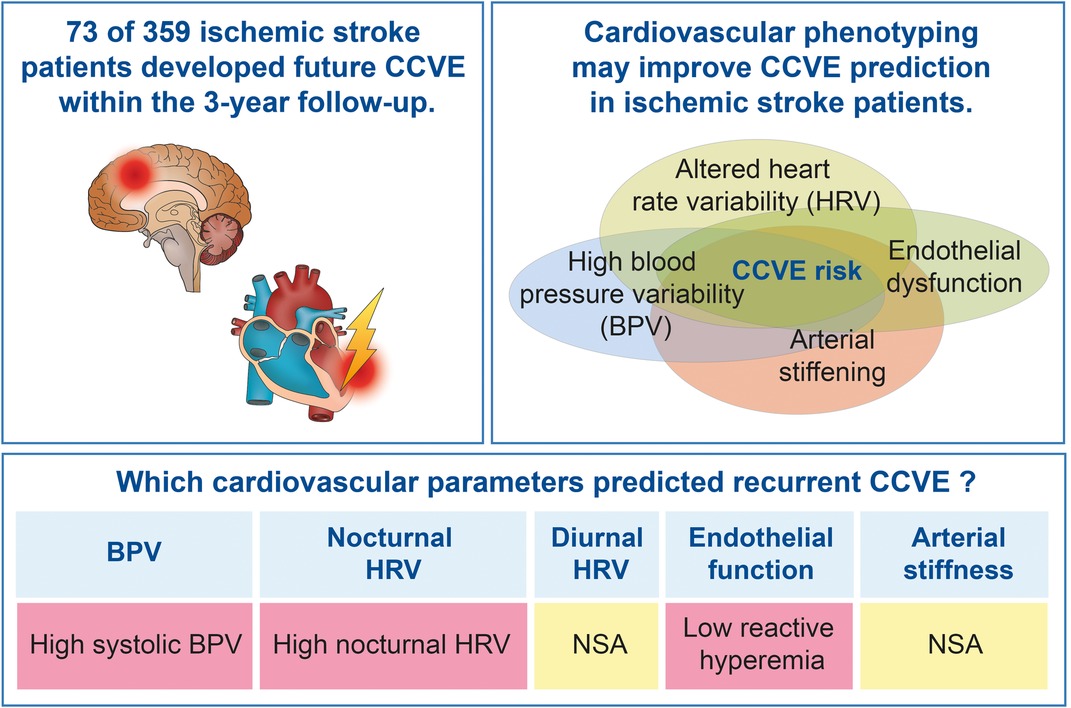
Results: Out of 447 recruited patients, 359 were included in this analysis. 20% of patients developed a future CCVE. A high variability of systolic BP (n = 333) and nocturnal HR (non-linear parameters; n = 187) at acute stroke predicted CCVE risk after adjustment for demographic parameters, cardiovascular risk factors and mean BP or HR, respectively. Endothelial dysfunction (n = 105) at acute stroke predicted CCVE risk after adjustment for age and sex, but not after adjustment for cardiovascular risk factors. Diurnal HR and arterial stiffness at acute stroke were not associated with CCVE risk.
Conclusion: High blood pressure variability, high nocturnal HRV and endothelial function contribute to the risk for future CCVE after stroke.
Introduction
The incidence of cerebro-cardiovascular events (CCVE) has decreased by nearly half in recent decades, largely due to modifications in cerebro-cardiovascular risk factors (1, 2). However, the risk for CCVE remains high in certain populations, such as ischemic stroke patients.
Ischemic stroke affects 94 per 100 000 people per year worldwide (3), and following a transient ischemic attack (TIA) or a minor ischemic stroke, 6.2% of patients are affected by recurrent CCVE within one year (4). This risk escalates to an estimated cumulative rate of 12.9% over five years (5).
Current approaches for the assessment of vascular risk are based on the clinical and laboratory characteristics (e.g., lipid profile) (6). Several studies confirmed the predictive value of high blood pressure variability (BPV) regarding future CCVE in ischemic stroke patients (7, 8), while little is known about the prognostic value of heart rate variability (HRV), endothelial function and arterial stiffness in this population (9). Moreover, the properties of these parameters in stroke patients, such as the associations with demographic and vascular risk factors, are not fully understood (10, 11).
We hypothesized that altered BPV, HRV, arterial stiffness and endothelial function represent a disequilibrium in cardiovascular homeostasis resulting in the increased risk for future CCVE after stroke. In this prospective study, our primary objective was to explore the ability of cardiovascular parameters at acute stroke to predict recurrent CCVE after stroke. The secondary objective was to characterize cardiovascular parameters in a population with acute stroke.
Methods
Study population
This is a secondary outcome analysis of the prospective longitudinal observational cohort study “Sleep Deficiency and Stroke Outcome” (SD&SO; clinicaltrials.gov: NCT02559739). The study was conducted at the Bern University Hospital and at the Ospedale Regionale di Lugano. However, the cardiovascular assessments included in this analysis were only performed in Bern.
The study assessed patients consecutively admitted to the Department of Neurology at Bern University Hospital (recruitment period: 7/2015–1/2018) with a diagnosis of acute ischemic stroke or transient ischemic attack (TIA, within 7 days from symptom onset). Eligible patients were 18–85 years of age and able to give informed consent and to follow the study procedures. The exclusion criteria were the following: primary hemorrhagic stroke, clinically unstable or life-threatening conditions (coma/stupor, severe heart failure, oxygen-dependent pulmonary disease or severe pulmonary complications, severe renal or liver insufficiency), pregnancy, drug or alcohol abuse.
This study followed the STROBE reporting guideline for cohort studies. The protocol was approved by the Institutional Review Boards and the cantonal ethics committees. The study was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice. All study participants provided written informed consent prior to participation.
Study procedures
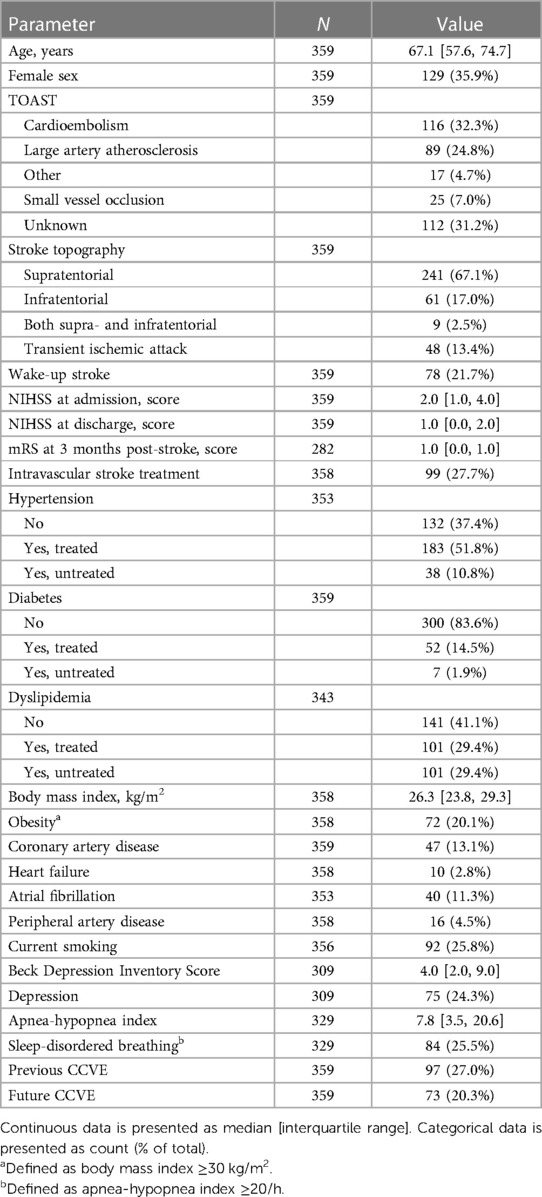
At hospital admission, demographics, anthropometric characteristics [body mass index (BMI)] and pre-stroke medical history were evaluated. Pre-stroke medical history included arterial hypertension (blood pressure ≥140/90 mmHg measured ≥3 times before stroke or patients under treatment for hypertension), diabetes (fasting glucose level ≥140 mg/dl or patients treated for diabetes), dyslipidemia (cholesterol level ≥250 mg/dl or previously under treatment), coronary artery disease, heart failure, smoking status, peripheral artery disease (PAD), atrial fibrillation and the symptoms of depression (according to Beck Depression Inventory, BDI).
The following stroke characteristics were assessed: stroke severity according to the National Institute of Health Stroke Scale (NIHSS; at admission and at discharge), stroke etiology according to TOAST stroke classification (12), stroke topography relative to tentorium (based on magnetic resonance imaging within clinical routine), recanalization therapy, wake-up stroke and post-stroke functional disability according to the modified Rankin Scale (mRS; at 3 months post-stroke).
The presence of sleep-disordered breathing was assessed using an overnight respiratory polygraphy (Nox T3®, Nox Medical, Reykjavík, Iceland) or a nocturnal Apnea link™ device (ResMed Bella Vista, Australia) within 7 days after stroke as part of the routine post-stroke work-up. Respiratory events were scored according to the American Academy of Sleep Medicine (AASM) criteria v2.4 (13).
Assessment of cardiovascular parameters
The following cardiovascular parameters were assessed within the first week from stroke onset: blood pressure, nocturnal and diurnal heart rate, endothelial function, and arterial stiffness. The physiological characteristics of the cardiovascular parameters according to the current literature are summarized in Supplementary Table S1. The assessment of cardiovascular parameters is detailed in Supplementary material S1.
Blood pressure
BP and BPV were assessed based on in-hospital and outpatient measurements. BPV characteristics estimated mean, the deviation from a central point (standard deviation [SD], coefficient of variation [CV], mean absolute deviation [MAD]), variance, successive deviation [successive variation, average real variability (ARV)] and range (14, 15).
Nocturnal heart rate
Mean nocturnal HR and HRV were assessed based on the available pulse rate data from the nocturnal respiratory polygraphy. Nocturnal HRV was calculated using HRVTool, an Open-Source Matlab Toolbox for Analyzing Heart Rate Variability based on pulse-wave form (16). HRV-parameters were represented in 3 main domains: the time domain was represented by HRV based on relative RR intervals (rrHRV), standard deviation of normal-to-normal beats (SDNN), standard deviation of successive differences (SDSD), triangular index (TI), root mean square of successive differences of successive RR intervals (RMSSD), and the percentage of adjacent NN intervals that differ from each other >50 ms (pNN50). The frequency domain was represented by normalized low frequency (nuLf) power, normalized high frequency (nuHF) power, nuLF/nuHf ratio, low frequency (LF) power, high frequency (HF) power and very low frequency (VLF) power. Lastly, non-linear measurements were represented by SD1 and SD2 from Poincaré plots, SD1/SD2 ratio, detrended fluctuation analysis α1 (DFA1), detrended fluctuation analysis α2 (DFA2) and approximate entropy (ApEn).
EndoPAT: diurnal heart rate, endothelial function and arterial stiffness
Approximately 20% of the patients underwent additional assessments of diurnal HRV, endothelial function and arterial stiffness with digital plethysmography (EndoPAT2000-device; Itamar Medical Ltd., Caesarea, Israel).
Endothelial function, arterial stiffness and diurnal heart rate (mean and HRV) were represented as follows: (1) mean HR; (2) time domain of HRV—SDNN, RMSSD, pNN50 and TI; (3) frequency domain of HRV—LF power (LF), HF power (HF) and LF/HF; (4) endothelial function—reactive hyperemia index (RHI) and Framingham reactive hyperemia index (FRHI); (5) arterial stiffness—augmentation index and augmentation index corrected for a heart rate of 75 bpm (AI75).
Cerebro-cardiovascular events (CCVE)
The main outcome of the study was a composite of fatal and non-fatal future CCVE that included ischemic or hemorrhagic stroke, transient ischemic attack, myocardial infarction, unplanned hospitalization for unstable angina or heart failure and urgent revascularization. CCVE were assessed by a research fellow or a study nurse in a structured telephone interview over a 3-year (1095-day) period after study inclusion (Supplementary material S2).
Statistical analysis
The statistical analysis was exploratory and performed using R version 4.0.1 (cran.r-project.org/). Continuous variables were presented as median and interquartile range. Categorical variables were presented as count (% of total). Only the patients with available measurements of cardiovascular characteristics and available information about CCVE risk were included in the analysis (Figure 1A). Missing values were not imputed.
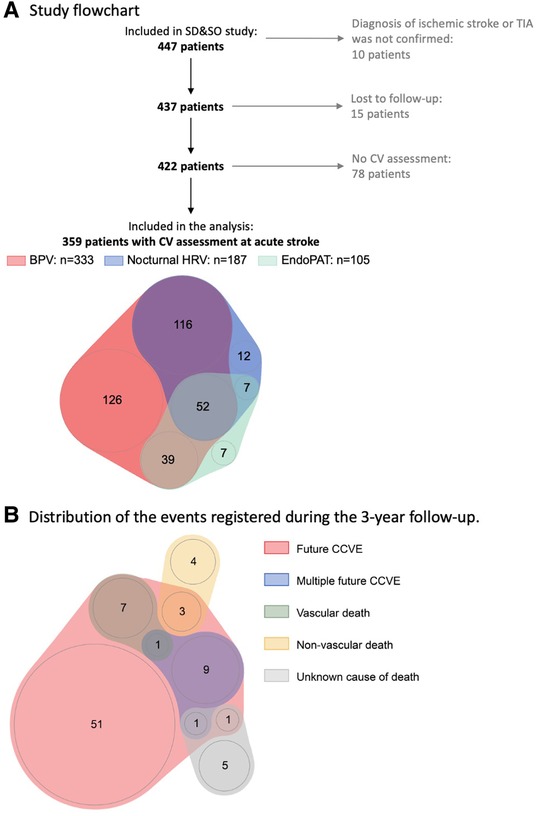
Figure 1. (A) Sleep Deficiency and Stroke Outcome study flowchart. (B) Distribution of the events registered during 3-year follow-up. 20% (73) patients developed 87 recurrent CCVE. EndoPAT assessments included diurnal HRV, endothelial function and arterial stiffness.
We performed a descriptive analysis, and, following inspection of the distribution of continuous variables, we log-transformed non-normally distributed variables (rrHRV, SDNN, SDSD, RMSSD, pNN50). We then used Spearman's rank correlation to investigate the associations between continuous variables: cardiovascular parameters, demographics, stroke characteristics and cardiovascular risk factors. The significant associations according to correlation analysis were further investigated using multiple linear regression adjusted for age and sex. Finally, we investigated the associations between cardiovascular parameters and future CCVE within 3 years post-stroke using univariate and multiple Cox regression models. We consecutively adjusted multiple Cox regression models as follows: (1) age + sex; (2) age + sex + cardiovascular risk factors. To delineate the potential impact of mean on variability (17), multiple Cox regression models that included systolic BPV, diastolic BPV, nocturnal HRV or diurnal HRV as independent variables were additionally adjusted for mean SBP, mean DBP, mean nocturnal HR or mean diurnal HR, respectively.
Cardiovascular risk factors were selected based on World Health Organization cardiovascular disease risk charts and Framingham Risk Score and included the presence of hypertension, diabetes and dyslipidemia as well as current smoking. These cardiovascular risk factors were coded as binary variables (treated or untreated vs. absent for hypertension, diabetes and dyslipidemia; presence or absence of current smoking). Since cardiovascular parameters are associated with atrial fibrillation (AF) (18–21), we additionally adjusted models for atrial fibrillation. We compared models using the Akaike Information Criterion (AIC). All statistical tests were two-sided and conducted at the 5% significance level.
Results
Study population
Of the 447 patients enrolled in the SD&SO study, 359 patients were included in the analysis (Figure 1A). Patients were 67.1 [57.6, 74.7] years old and 35.9% were female. The full study population, which included the patients with rather minor stroke, is shown in Table 1 and in Supplementary Tables S2, S3.
Associations between cardiovascular parameters at acute stroke
The results of correlation analysis are summarized in Supplementary Figure S1. In brief, high BPV (predominantly, diastolic BPV) was associated with high HRV, endothelial dysfunction and arterial stiffening. Furthermore, high nocturnal HRV was associated with high diurnal HRV, and endothelial dysfunction was associated with arterial stiffening. Only few associations between HRV, endothelial dysfunction and arterial stiffening were significant.
Association of cardiovascular parameters at acute stroke with demographic characteristics, stroke characteristics and comorbidities
At acute stroke, BP parameters were positively associated with age. Female sex was associated negatively with mean DBP and positively with coefficient of variation of DBP (DBP-CV). Both nocturnal and diurnal HRV at acute stroke were negatively associated with age, and arterial stiffness was positively associated with female sex. There were no other associations between demographic and cardiovascular parameters at acute stroke (Figure 2 and Supplementary Table S4).
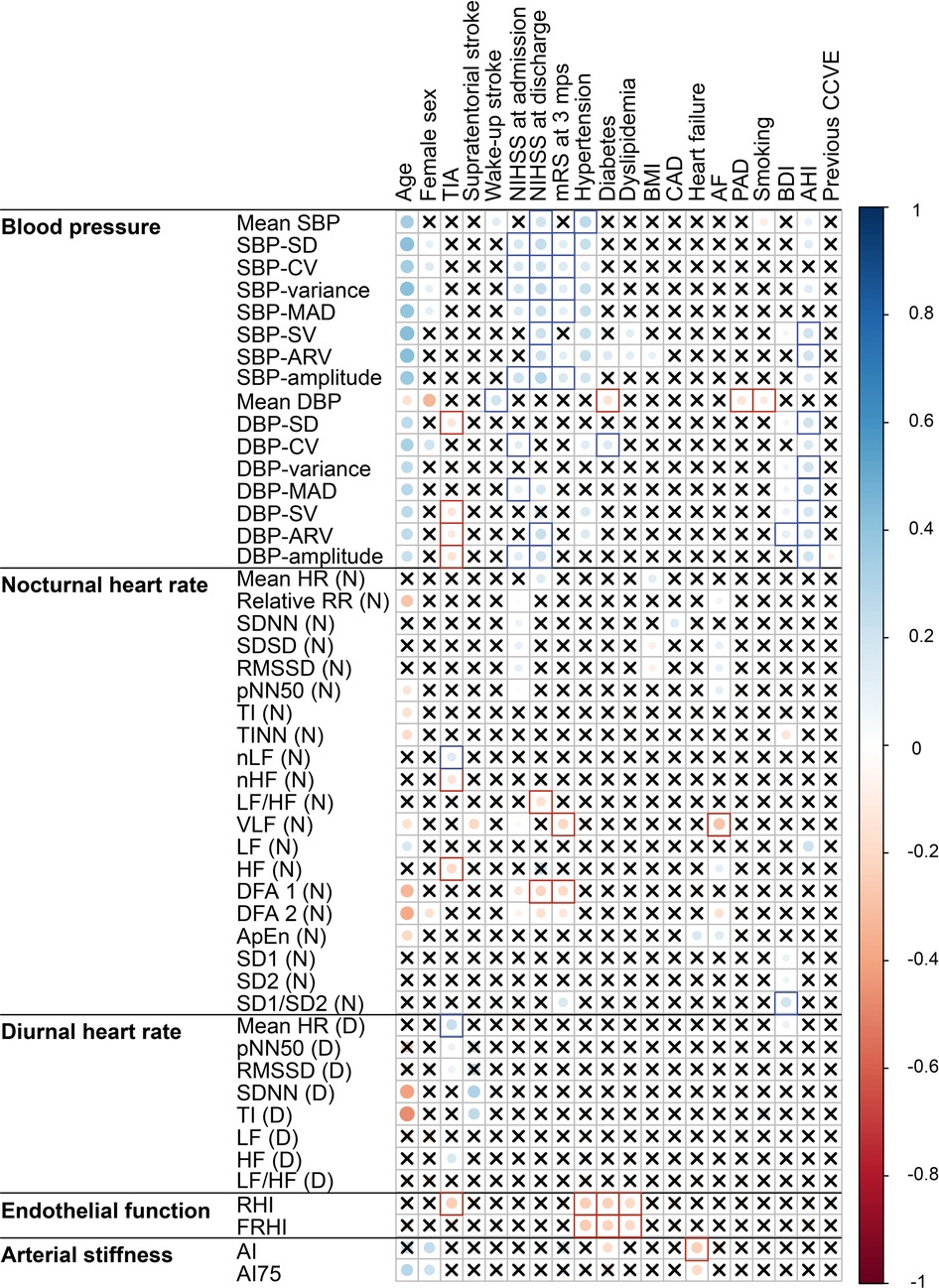
Figure 2. Correlation plot of the associations of cardiovascular parameters recorded at acute stroke with demographics, stroke characteristics and cardiovascular risk factors. Significant (p < 0.05) associations according to Spearman correlation test are shown as filled circles. Crosses mark the insignificant (p ≥ 0.05) associations. Blank squares mark the associations that remained significant in multiple regression models after adjustment for age and sex. The abbreviations for cardiovascular parameters are specified in the abbreviation list. AF, atrial fibrillation; bpm, beats per minute; BDI, Beck Depression Inventory; BMI, body mass index; CAD, coronary artery disease; TIA, transient ischemic attack.
High BP, BPV and nocturnal HRV (DFA1, LF/HF, VLF) at acute stroke were associated with severe neurological and functional disability at acute stroke and at 3 months post-stroke. Low diastolic BPV, HRV reflecting parasympathetic dominance (HF, nHF, LF) and good endothelial functioning were associated with TIA instead of stroke.
At acute stroke, mean systolic BP and BPV, nocturnal HRV as derived from frequency and non-linear domains, high diurnal HR, endothelial dysfunction and low arterial stiffness were associated with vascular risk factors (e.g., hypertension, diabetes and sleep-disordered breathing).
Association of cardiovascular characteristics at acute stroke with future cerebro-cardiovascular events
Seventy-three of 359 stroke patients (20%) developed 87 CCVE within the 3-year follow-up period (Figure 1B). Sixty-two patients (17%) developed a single CCVE, whereas 11 (3%) patients developed multiple CCVE. During the observation period, 14 of 359 stroke patients (4%) died from non-vascular causes. Regression models are presented in detail in Figures 3, 4 and Supplementary Table S5.
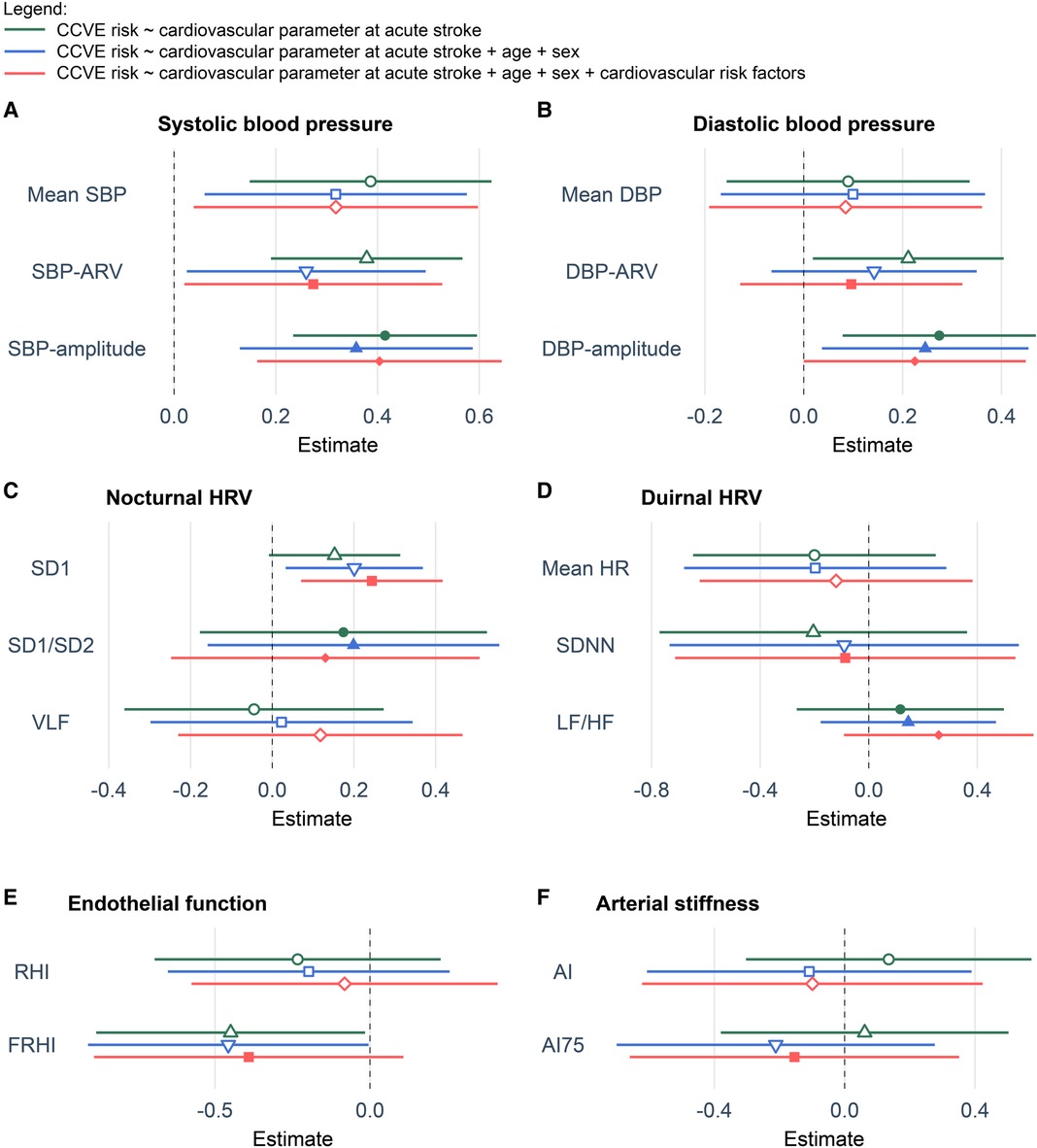
Figure 3. Coefficient plots of Cox regression models showing the association of cardiovascular parameters at acute stroke with the risk for future CCVE. (A) Systolic blood pressure. (B) Diastolic blood pressure. (C) Nocturnal heart rate. (D) Diurnal heart rate. (E) Endothelial function. (F) Arterial stiffness. All multivariate models including blood pressure variability or heart rate variability were respectively adjusted for mean SBP, mean DBP, mean nocturnal HR or mean diurnal HR. All values are presented as z-scores. Cardiovascular risk factors included hypertension, diabetes, dyslipidemia, current smoking and atrial fibrillation. The abbreviations for cardiovascular parameters are specified in the abbreviation list.
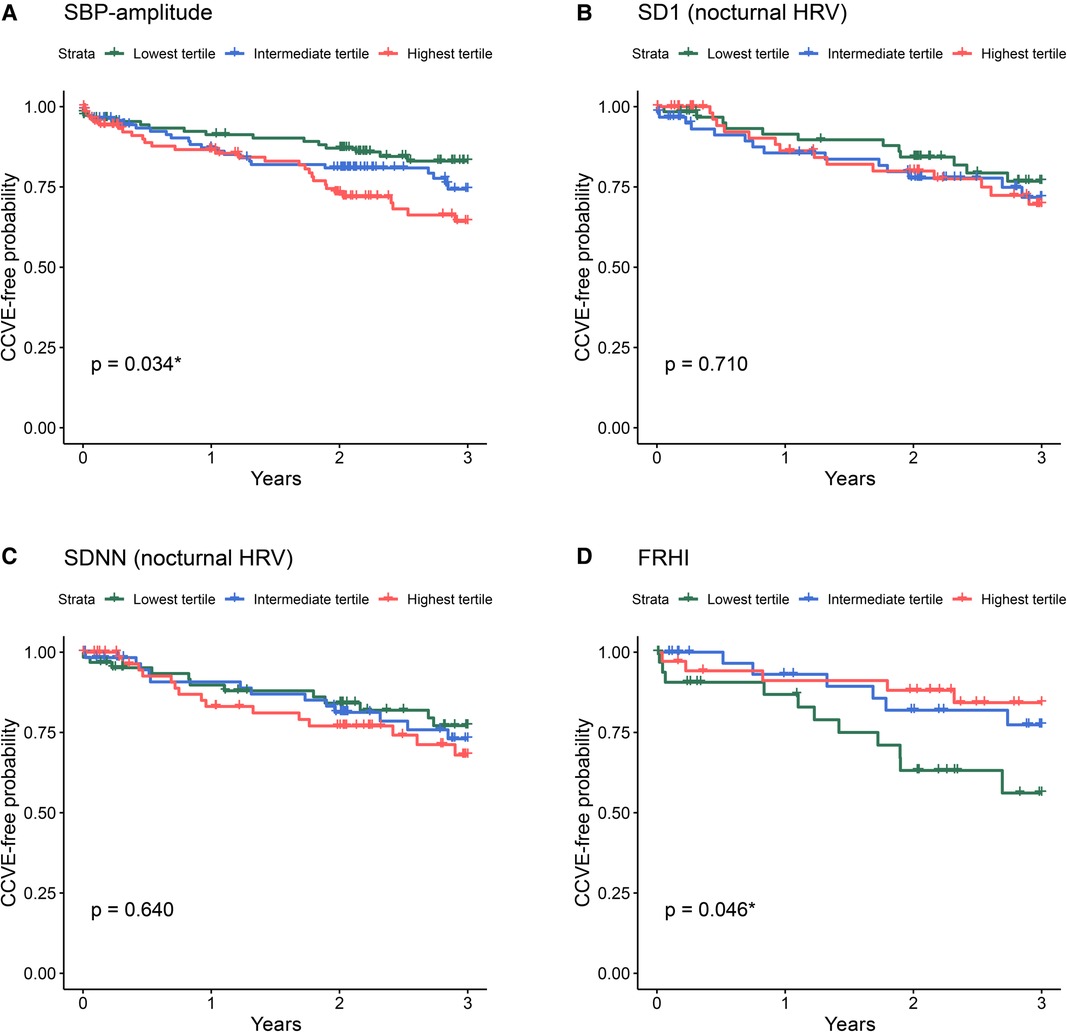
Figure 4. Kaplan-Meier survival curves for the risk for future CCVE according to the tertiles of selected cardiovascular parameters at acute stroke: SBP-amplitude (A), SD1 of nocturnal HRV (B), SDNN of nocturnal HRV (C), and FRHI (D). The abbreviations for cardiovascular parameters are specified in the abbreviation list.
Univariate Cox regression analysis (Figure 3, green lines) showed the significant association of CCVE risk with several BP parameters. There was a significant association of CCVE risk with high SDNN and a trend for a potential association of CCVE risk with high SD1 and SD2. Endothelial dysfunction (FRHI) was significantly associated with CCVE risk. This univariate analysis is partially illustrated in Kaplan-Meier survival curves for the risk for future CCVE according to the tertiles of selected cardiovascular parameters (Figure 4).
In a second step, we used multiple Cox regression models adjusted for age, sex and mean value of cardiovascular parameter (whenever applicable; Figure 3, blue lines). In these models, the associations of most BP parameters and endothelial dysfunction (FRHI) with CCVE risk remained significant. Moreover, the association of high SD1 and SD2 with CCVE risk became significant.
In a third step, we further adjusted the multiple Cox regression models by adding vascular risk factors (Figure 3, red lines). In these models, the association of SBP parameters and non-linear HRV parameters (SD1 and SD2) remained significant, while the associations of DBP parameters and FRHI with CCVE risk lost their significance. The Akaike Information Criterion (AIC) values of these models were lower than the AIC values of multiple Cox regression models adjusted only for age, sex and mean BP or heart rate, indicating better model properties. The AIC values of these models were also lower than the AIC value of the similar multiple Cox regression model that included only demographic parameters and vascular risk factors (AIC: 753.19; Supplementary Figure S2).
Discussion
In this study, we comprehensively describe cardiovascular parameters at acute stroke and explore their ability to predict recurrent CCVE over 3 years (Figure 5). We found that high BPV, high nocturnal HRV and low reactive hyperemia are associated with increased risk of future CCVE in acute stroke patients. Overall, our findings align with established associations between cardiovascular parameters and known vascular risk factors, such as high BPV with sleep-disordered breathing (22) and endothelial dysfunction with hypertension, diabetes and dyslipidemia (23). Prior work demonstrated a reduction in HRV during the nighttime in non-dipping patients with hypertension (24). Similarly, our study revealed a strong positive correlation between high BPV and high nocturnal HRV.

Figure 5. Schematic representation of the associations of cardiovascular parameters at acute stroke with the risk for future CCVE (cerebro-cadriovascular events). NSA, no significant associations.
Additionally, we demonstrated the associations between cardiovascular parameters (high BP, BPV, nocturnal HRV) and stroke severity and functional disability. However, it remains uncertain whether these cardiovascular parameters are merely associated with severe stroke or if severe stroke leads to alterations in these parameters. Our study population was skewed towards patients with minor strokes, limiting our ability to predict the actual improvement in stroke outcome associated with these cardiovascular parameters due to floor effects.
Finally, we observed that mean systolic BP, systolic BPV, nocturnal HRV and endothelial dysfunction at acute stroke reflect the risk for future recurrent CCVE in stroke patients, even after adjustment for demographic and cardiovascular risk factors. Indeed, high systolic BP and BPV parameters during the acute stroke phase predict recurrent CCVE, in line with prior research (25). In our cohort, SBP-amplitude was the most indicative parameter with the largest effect size. Although diastolic BP and the other BPV parameters did predict recurrent CCVE in univariate analysis, these associations lost significance after adjustment for demographic and cardiovascular risk factors.
In general, high HRV is typically considered indicative of healthy cardiovascular autonomic function, while low HRV is commonly viewed as a cardiovascular risk factor in the general population without prior cardiovascular events (26). Although most studies concentrated on diurnal HRV (26), Binici et al. (27) demonstrated that low nocturnal HRV, as assessed from time-domain measurements (SDNN), was associated with stroke risk in the general population (n = 653). However, within our study population, non-linear HRV-based variables reflecting unpredictable HRV [SD1 and SD2 (27, 28)] during the acute stroke phase were indicative of CCVE risk (28, 29). Our findings accord with the data from HypnoLaus study showing the association of CCVE risk in general population with non-linear HRV measurements reflecting altered acceleration capacity, deceleration capacity and heart rate fragmentation (30). The contrasting patterns of association between nocturnal and diurnal HRV and CCVE risk may be explained by sleep-wake disorders, which are recognized as an independent cardiovascular risk factor (31). For instance, sleep-disordered breathing is common after a stroke and may be accompanied by decreased diurnal HRV and increased nocturnal HRV (32, 33). In turn, the diminished physiological circadian fluctuation of autonomic functions, as reflected by time-domain HRV, was associated with an unfavorable cardiovascular and metabolic profile (24).
Our study did not show an association between diurnal HRV and CCVE risk in stroke patients. While an association of diurnal HRV with CCVE risk was reported in general population (7), our results support a previously reported lack of the association of diurnal HRV with CCVE risk in acute stroke patients (n = 308) over 1-year follow-up (9). We assume that this discrepancy might be related to altered mechanisms of resting heart rate regulation in acute stroke.
Furthermore, our study did not confirm the association between arterial stiffness and CCVE risk. Notably, a prior study had reported a link between arterial stiffness and the need for urgent revascularization in patients with a high cardiovascular risk (34). This discrepancy might be related to the difference in outcome measures between the studies. However, we show an association of endothelial dysfunction at acute stroke with future CCVE risk supporting previous findings in patients at high cardiovascular risk (35, 36).
Strengths and limitations
The strengths of the study include the prospective design with a broad cardiovascular phenotyping. Additionally, we followed stroke patients for CCVEs during a period of 3-years and compared the fit of Cox regression models predicting CCVE risk.
The present study has several limitations. First, sample size and number of events are small for a robust evaluation of a large set of parameters. Second, the large number of exploratory analyses, without control for multiplicity, limits the confidence around key findings, and larger cohort studies are needed to evaluate their applicability.
Conclusions
In summary, we comprehensively describe cardiovascular parameters (BPV, nocturnal and diurnal HRV, endothelial function and arterial stiffness) in patients with a minor stroke. The risk for future CCVE was associated with high systolic BPV, high and unpredictable non-linear nocturnal HRV and low reactive hyperemia at acute stroke. We propose that phenotyping based on cardiovascular parameters could expand the current knowledge about the prediction of cardiovascular risk.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Boards (Inselspital Bern, Ospedale Regionale di Lugano) and the cantonal ethics committees (Bern, Ticino). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
IF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – review & editing. NM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Validation. MD: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review & editing. SB: Conceptualization, Writing – review & editing. SBD: Conceptualization, Data curation, Writing – review & editing, Supervision. AB: Conceptualization, Data curation, Supervision, Writing – review & editing. TH: Conceptualization, Writing – review & editing, Data curation. MH: Conceptualization, Data curation, Writing – review & editing. ER: Conceptualization, Writing – review & editing. CB: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing, Data curation, Formal Analysis. CLAB: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. MHS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Swiss National Science Foundation grant #320030_149752.
Acknowledgments
The authors would like to thank Nicole Duttweiler, Saskia Salzmann, Nadja Steiner and Dr. med. Stefan Bauer for their contributions to data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1288109/full#supplementary-material
References
1. Takashima N, Arima H, Turin TC, Nakamura Y, Sugihara H, Morita Y, et al. The 21-year trend of stroke incidence in a general Japanese population: results from the takashima stroke registry, 1990–2010. Cerebrovasc Dis. (2022) 5(51):570–6. doi: 10.1159/000521643
2. Camacho X, Nedkoff L, Wright FL, Nghiem N, Buajitti E, Goldacre R, et al. Relative contribution of trends in myocardial infarction event rates and case fatality to declines in mortality: an international comparative study of 1.95 million events in 80.4 million people in four countries. Lancet Public Health. (2022) 7(3):e229–39. doi: 10.1016/S2468-2667(22)00006-8
3. World Stroke Organization. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. (2022). p. 1–14.
4. Amarenco P, Lavallée PC, Labreuche J, Albers GW, Bornstein NM, Canhão P, et al. One-Year risk of stroke after transient ischemic attack or Minor stroke. N Engl J Med. (2016) 374(16):1533–42. doi: 10.1056/NEJMoa1412981
5. Amarenco P, Lavallée PC, Monteiro Tavares L, Labreuche J, Albers GW, Abboud H, et al. Five-year risk of stroke after TIA or Minor ischemic stroke. N Engl J Med. (2018) 378(23):2182–90. doi: 10.1056/NEJMoa1802712
6. van t Klooster CC, Bhatt DL, Steg PG, Massaro JM, Dorresteijn JAN, Westerink J, et al. Predicting 10-year risk of recurrent cardiovascular events andcardiovascular interventions in patients with established cardiovascular disease: results from UCC-SMART and REACH. Int J Cardiol. (2021) 325:140–8. doi: 10.1016/j.ijcard.2020.09.053
7. Lees T, Shad-Kaneez F, Simpson AM, Nassif NT, Lin Y, Lal S. Heart rate variability as a biomarker for predicting stroke, post-stroke complications and functionality. Biomark Insights. (2018) 13:1–13. doi: 10.1177/1177271918786931
8. de Havenon A, Stoddard G, Saini M, Wong KH, Tirschwell D, Bath P. Increased blood pressure variability after acute ischemic stroke increases the risk of death: a secondary analysis of the virtual international stroke trial archive. JRSM Cardiovasc Dis. (2019) 8:204800401985649. doi: 10.1177/2048004019856496
9. von Rennenberg R, Krause T, Herm J, Hellwig S, Scheitz JF, Endres M, et al. Heart rate variability and recurrent stroke and myocardial infarction in patients with acute mild to moderate stroke. Front Neurol. (2021) 12:1–7. doi: 10.3389/fneur.2021.772674
10. Cassidy JM, Cramer SC. Spontaneous & therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res. (2017) 8(1):33–46. doi: 10.1007/s12975-016-0467-5
11. Tobaldini E, Proserpio P, Oppo V, Figorilli M, Fiorelli EM, Manconi M, et al. Cardiac autonomic dynamics during sleep are lost in patients with TIA and stroke. J Sleep Res. (2020) 29(3):1–9. doi: 10.1111/jsr.12878
12. Love BB, Bendixen BH. Classification of subtype of acute ischemic stroke definitions for use in a multicenter clinical trial. Stroke. (1993) 24(1):35–41. doi: 10.1161/01.STR.24.1.35
13. Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Version 2.4. Darien, IL: American Academy of Sleep Medicine (2017).
14. Mistry EA, Mehta T, Mistry A, Arora N, Starosciak AK, De Los Rios La Rosa F, et al. Blood pressure variability and neurologic outcome after endovascular thrombectomy: a secondary analysis of the BEST study. Stroke. (2020) 2(51):511–8. doi: 10.1161/STROKEAHA.119.027549
15. Geary RC. The ratio of the mean deviation to the standard deviation as a test of normality. Biometrika. (1935) 27(3/4):310. doi: 10.2307/2332693
16. Vollmer M. HRVTool - an open-source matlab toolbox for analyzing heart rate variability. 2019 Computing in Cardiology Conference (CinC). (2019) 45:3–6.
17. Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens. (2018) 20(7):1133–7. doi: 10.1111/jch.13304
18. Kim SH, Lim KR, Seo JH, Ryu DR, Lee BK, Cho BR, et al. Higher heart rate variability as a predictor of atrial fibrillation in patients with hypertension. Sci Rep. (2022) 12(1):1–8. doi: 10.1038/s41598-021-99269-x
19. Lee SR, Choi YJ, Choi EK, Do HK, Lee E, Cha MJ, et al. Blood pressure variability and incidence of new-onset atrial fibrillation: a nationwide population-based study. Hypertension. (2020):309–15. doi: 10.1161/HYPERTENSIONAHA.119.13708
20. Khan AA, Thomas GN, Lip GYH, Shantsila A. Endothelial function in patients with atrial fibrillation. Ann Med. (2020) 52(1–2):1–11. doi: 10.1080/07853890.2019.1711158
21. Lage JGB, Bortolotto AL, Scanavacca MI, Bortolotto LA, da C Darrieux FC. Arterial stiffness and atrial fibrillation: a review. Clinics. (2022) 77:100014. doi: 10.1016/j.clinsp.2022.100014
22. Lettau F, Schwarz EI, Stradling JR, Kohler M. Blood pressure variability in obstructive sleep apnoea: data from 4 randomised controlled CPAP withdrawal trials. Respiration. (2017) 93(5):311–8. doi: 10.1159/000465528
23. Deedwania PC. Mechanisms of endothelial dysfunction in the metabolic syndrome. Curr Diab Rep. (2003) 3(4):289–92. doi: 10.1007/s11892-003-0019-8
24. Di Raimondo D, Miceli G, Casuccio A, Tuttolomondo A, Buttà C, Zappulla V, et al. Does sympathetic overactivation feature all hypertensives? Differences of sympathovagal balance according to night/day blood pressure ratio in patients with essential hypertension. Hypertens Res. (2016) 39(6):440–8. doi: 10.1038/hr.2016.6
25. Webb AJS, Mazzucco S, Li L, Rothwell PM. Prognostic signifcance of blood pressure variability on beat-to-beat monitoring after transient ischemic attack and stroke. Stroke. (2018) 49(1):62–7. doi: 10.1161/STROKEAHA.117.019107
26. Hillebrand S, Gast KB, De Mutsert R, Swenne CA, Jukema JW, Middeldorp S, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. (2013) 15(5):742–9. doi: 10.1093/europace/eus341
27. Binici Z, Mouridsen MR, Køber L, Sajadieh A. Decreased nighttime heart rate variability is associated with increased stroke risk. Stroke. (2011) 42(11):3196–201. doi: 10.1161/STROKEAHA.110.607697
28. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:1–17. doi: 10.3389/fpubh.2017.00258
29. Hardstone R, Poil SS, Schiavone G, Jansen R, Nikulin VV, Mansvelder HD, et al. Detrended fluctuation analysis: a scale-free view on neuronal oscillations. Front Physiol. (2012) 3:1–13. doi: 10.3389/fphys.2012.00450
30. Berger M, Solelhac G, Marques-Vidal P, Haba-Rubio J, Vollenweider P, Waeber G, et al. Association between nocturnal heart rate variability and incident cardiovascular disease events: the HypnoLaus population-based study. Heart Rhythm. (2022) 19(4):632–9. doi: 10.1016/j.hrthm.2021.11.033
31. Bassetti CLA, Randerath W, Vignatelli L, Ferini-Strambi L, Brill AK, Bonsignore MR, et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur Respir J. (2020) 55(4):1901104. doi: 10.1183/13993003.01104-2019
32. Sequeira VCC, Bandeira PM, Azevedo JCM. Heart rate variability in adults with obstructive sleep apnea: a systematic review. Sleep Science. (2019) 12(3):214–21. doi: 10.5935/1984-0063.20190082
33. Nam EC, Chun KJ, Won JY, Kim JW, Lee WH. The differences between daytime and nighttime heart rate variability may usefully predict the apnea-hypopnea index in patients with obstructive sleep apnea. J Clin Sleep Med. (2022) 18(6):1557–63. doi: 10.5664/jcsm.9912
34. Choi JH, Kim SY, Joo SJ, Kim KS. Augmentation index is associated with coronary revascularization in patients with high Framingham risk scores: a hospital-based observational study. BMC Cardiovasc Disord. (2015) 15(1):1–7. doi: 10.1186/1471-2261-15-1
35. Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, et al. Peripheral endothelial function and cardiovascular events. J Am Heart Assoc. (2013) 2:1–21. doi: 10.1161/JAHA.113.000426
36. Liu W, Meng M, Chen J, Wang L, Sun Z, Li X, et al. Reactive hyperemia index in patients on maintenance hemodialysis: cross-sectional data from a cohort study. Sci Rep. (2017) 7:1–7. doi: 10.1038/s41598-016-0028-x
Glossary
ApEn, approximate entropy; AI, augmentation index; AI75, augmentation index normalized for 75 bpm; AIC, Akaike Information Criterion; ARV, average real variability; AS, arterial stiffness; BPV, blood pressure variability; CCVE, cerebro-cardiovascular events; CV, coefficient of variation; D, diurnal; DBP, diastolic blood pressure; DFA1, detrended fluctuation analysis component α1; DFA2, detrended fluctuation analysis component α2; EF, endothelial function; FRHI, Framingham Reactive Hyperemia Index; HFnu, normalized high frequency power; HF, high frequency power; HR, heart rate; HRV, heart rate variability; LFnu, normalized low frequency power; HFnu, normalized high frequency power; LF, low frequency power; MAD, mean absolute deviation; mps, months post-stroke; N, nocturnal; pNN50, percentage of adjacent NN intervals that differ from each other by more than 50 ms; RHI, reactive hyperemia index; RMSSD, root mean square of successive differences of successive RR intervals; SBP, systolic blood pressure; SD, standard deviation; SD1, SD1 from Poincaré plot; SD2, SD2 from Poincaré plot; SDNN, standard deviation of normal-to-normal beats; SDSD, standard deviation of successive RR interval differences; SV, successive variation; TI, triangular index; TIA, transient ischemic attack; TINN, triangular interpolation of NN interval histogram; VLF, very low frequency power.
Keywords: blood pressure variability, heart rate variability, arterial stiffness, endothelial dysfunction, cerebro-cardiovascular risk, ischemic stroke, cardiovascular outcome, EndoPAT
Citation: Filchenko I, Mürner N, Dekkers MPJ, Baillieul S, Duss SB, Brill A-K, Horvath T, Heldner MR, Rexhaj E, Bernasconi C, Bassetti CLA and Schmidt MH (2023) Blood pressure variability, nocturnal heart rate variability and endothelial function predict recurrent cerebro-cardiovascular events following ischemic stroke. Front. Cardiovasc. Med. 10:1288109. doi: 10.3389/fcvm.2023.1288109
Received: 3 September 2023; Accepted: 13 October 2023;
Published: 16 November 2023.
Edited by:
Luigi Sironi, University of Milan, ItalyReviewed by:
Simone Beretta, San Gerardo Hospital, ItalyGiuseppe Miceli, University of Palermo, Italy
© 2023 Filchenko, Mürner, Dekkers, Baillieul, Duss, Brill, Horvarth, Heldner, Rexhaj, Bernasconi, Bassetti and Schmidt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Markus H. Schmidt Markus.Schmidt@insel.ch
 Irina Filchenko
Irina Filchenko Nicolas Mürner1
Nicolas Mürner1  Sebastien Baillieul
Sebastien Baillieul Mirjam R. Heldner
Mirjam R. Heldner Emrush Rexhaj
Emrush Rexhaj Corrado Bernasconi
Corrado Bernasconi