Outcomes of COMBO therapy for severe mitral regurgitation compared with transcatheter edge-to-edge repair
- 1Department of Cardiology and Catheterization Laboratories, Shonan Kamakura General Hospital, Kamakura, Japan
- 2Heart Valve Center, Department of Cardiology, Cardiology I, Universitätsmedizin Mainz, Johannes Gutenberg-University Mainz, Mainz, Germany
Background: There are different types of transcatheter mitral valve repair (TMVr) currently in clinical use, including leaflet approximation, annular cinching, and restoration of the chordal apparatus of the mitral valve (MV). While the concomitant combination (COMBO) therapy of mitral transcatheter edge-to-edge repair (M-TEER) with another TMVr concept has been proven feasible, potentially offering patient-tailored treatment for severe mitral regurgitation (MR), a comparison with M-TEER alone has not been made.
Aims: To evaluate the procedural and clinical outcome of COMBO therapies compared with M-TEER alone.
Methods: We included consecutive patients undergoing COMBO and M-TEER between March 2015 and April 2018 at our Heart Valve Center, while excluding patients presenting a case of redo or with previous MV surgery. Procedural outcomes and all-cause mortality were compared between COMBO therapy vs. M-TEER alone.
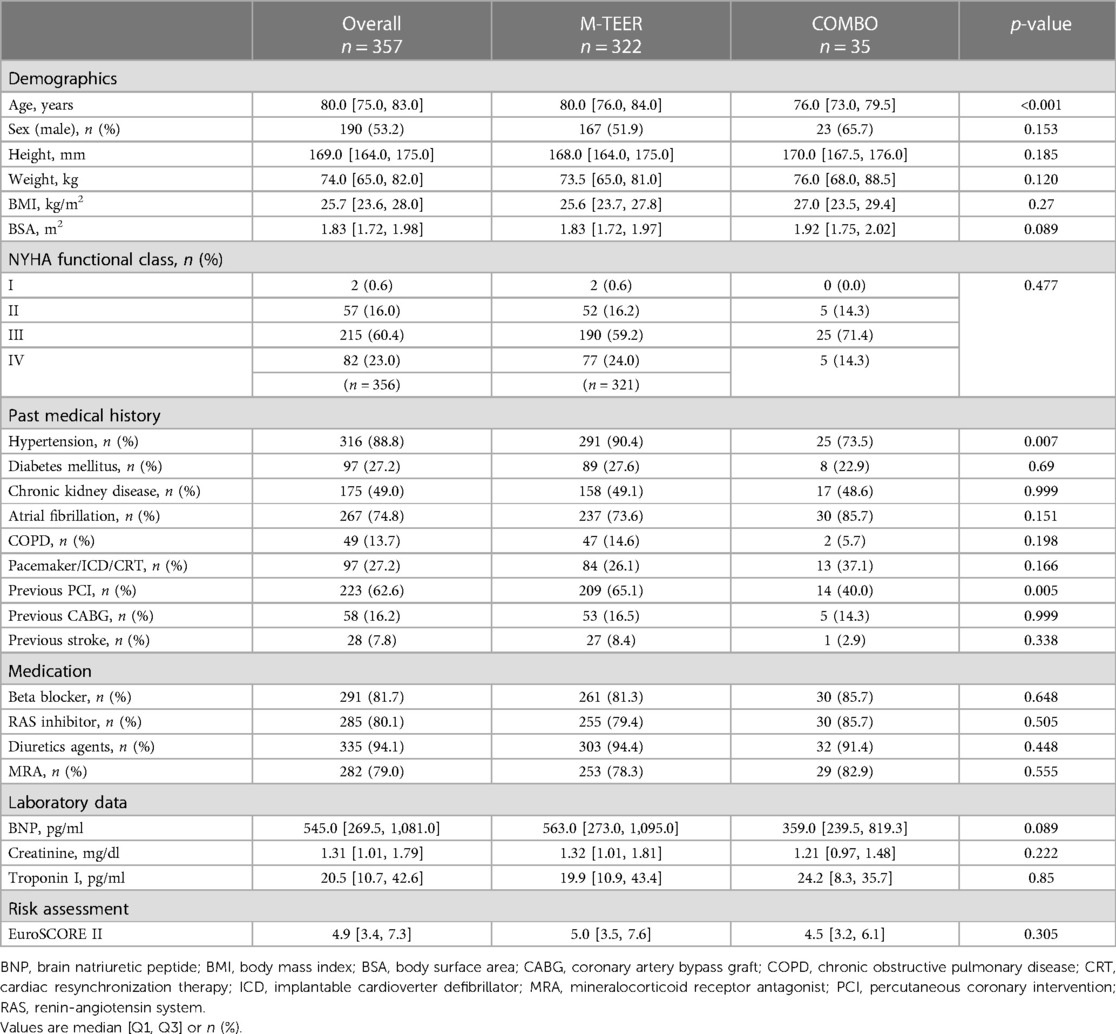
Results: A total of 357 patients (mean age 78.9 ± 7.0 years, 53.2% male, M-TEER n = 322, COMBO n = 35; COMBO: MitraClip and the Carillon mitral contour system n = 26, MitraClip and Cardioband n = 5, and MitraClip and NeoChord n = 4) were analyzed. Patients with COMBO therapy had larger left chamber sizes, a lower left ventricular systolic ejection fraction (LVEF; COMBO: 37.4 ± 13.8%, M-TEER: 47.9 ± 14.3%, p < 0.001), and a more severe MR grade (p < 0.001). There were no significant differences in the prevalence of residual MR ≧2+. However, the need for re-intervention, always employing M-TEER, was more common in the COMBO group. During a mean 3.6-year long-term follow-up, there was no significant difference of all-cause mortality between both groups (Log rank p = 0.921).
Conclusions: COMBO therapy may still be a beneficial therapy option for patients with severe MR who already have a more dilated left ventricle (LV), a more severe MR, and a more pronounced LV systolic dysfunction. The higher need for re-intervention in the COMBO group may signal more complex anatomies and possibly underlines the necessity of treating significant MR earlier. Future research is required to establish the COMBO approach as a toolbox-like treatment option, thus offering a patient-tailored approach depending on the individual anatomy and pathology.
Introduction
Mitral regurgitation (MR) is the most common among valvular heart diseases (VHDs) (1). It has been strongly associated with decreased quality of life, increased rate of heart failure (HF) hospitalization, and shortened survival (2, 3). Mitral regurgitation can be either primary or secondary in origin. Primary MR (PMR) is related to damage to any component of the MV apparatus, i.e., chordae, leaflets, and/or papillary muscles. Secondary MR (SMR) arises from annular dilatation and tethering of the leaflets caused by a dilated and dysfunctional left ventricle (LV; vSMR) or a dilated left atrium (LA, aSMR) (4–8).
Regarding the treatment for severe MR with high surgical risk, various types of transcatheter mitral valve repair (TMVr) targeting the mitral annulus, the mitral valve (MV) chordae, as well as the MV leaflets have become feasible and safe alternatives to medical therapy and cardiac surgery (9). Especially, successful mitral transcatheter edge-to-edge repair (M-TEER) has shown to reduce mortality and HF hospitalization (10, 11). The current European Society of Cardiology (ESC) guidelines on VHD recommend M-TEER as class IIa therapy in SMR and class IIb in PMR for symptomatic severe MR patients with surgical high risk (12). There is a paucity of data on the combination of two TMVr strategies for annular and leaflet repair in one procedure only (COMBO therapy) to target the different pathophysiological components of MR (13–15). However, we recently demonstrated that COMBO therapy of TMVr is feasible and may support reverse remodeling of left cardiac chambers during 1 year after the procedure in a cohort of patients at high risk (16).
In this study, we compared the mortality and the need for re-intervention, as well as procedural and clinical outcomes between COMBO therapy and M-TEER alone for the treatment of severe symptomatic MR.
Methods
COMBO therapy was defined as a combination of M-TEER using MitraClip NT (Abbott Laboratories, Abbott Vascular, Santa Clara, CA, USA) (17) with any other TMVr-strategy. These were a combination with either indirect annuloplasty using the Carillon mitral contour system (CMCS; Cardiac Dimensions, Kirkland, WA, USA) (18, 19) or direct annuloplasty with the Cardioband (Edwards Lifesciences, Irvine, CA, USA) (20), to improve the mitral annular dilation to control SMR, or chordal repair with the transapical NeoChord DS 1000 (NeoChord Inc., St. Louis Park, MN, USA), designed to repair PMR caused by prolapse with artificial chords (21, 22).
Study population
Symptomatic consecutive patients presenting with severe MR and indication for transcatheter repair who underwent TMVr as single or COMBO therapeutic approach from March 2015 to April 2018 at our comprehensive Heart Valve Center were included. Redo cases, however, including both previous transcatheter and surgical interventions, as a heterogenous group were excluded for this analysis. Moreover, those cases with no comprehensive baseline echocardiography or those lost to follow-up were excluded from the cohort.
Procedures of transcatheter mitral valve repair
The Heart Team decided to recommend the transcatheter approach over a medical or surgical pathway in each patient. The Heart Team consisted of a cardiac surgeon, an interventional cardiologist, an interventional echocardiographer, and a cardiac anesthesiologist. The COMBO therapy was discussed as an option during the Heart Team deliberation in cases where treatment of the pathology by the dedicated device was deemed difficult, e.g., large mitral valve annulus in cases that were selected for transcatheter annuloplasty, or extended prolapse in cases selected for NeoChord implantation. This option was mainly put to discussion by both the interventional team, i.e., the interventional cardiologist and the interventional echocardiographer after the patient was considered unsuitable for cardiac surgery. The decision to employ COMBO therapy was ultimately made during the procedure at the discretion of the treating interventional cardiologist. In detail, during the procedures in the COMBO therapy group, the first procedure was performed as either CMCS (Figures 1A–E, red box) or Cardioband (Figures 1F–I, blue box) in patients suffering from SMR, or NeoChord (Figures 1J–N, green box) in patients with PMR caused by Prolapse and/or flail. Each procedure was then followed by M-TEER in the same session. The technical details of each procedure have been reported previously (17–22). All procedures were performed under general anesthesia using fluoroscopy and 3D transesophageal echocardiography (TEE) guidance in all cases.
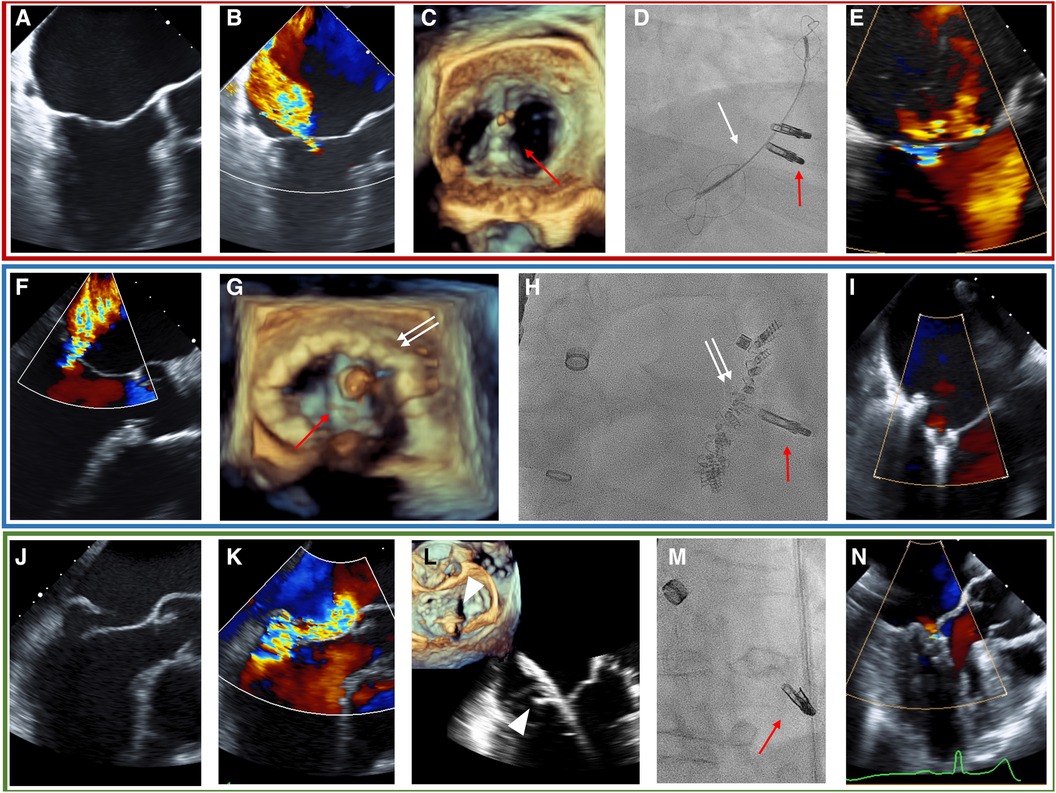
Figure 1. TEE and fluoroscopy (D, H, M) of the representative cases for each COMBO therapy using M-TEER (red arrow) with either the CMCS (white arrow) in SMR (A–E, red box), Cardioband (white double-arrow, blue box) in SMR (F–I), or NeoChord (white triangle, green box) in PMR due to posterior leaflet prolapse (J–N). (A) Tenting shows SMR. (B) Baseline MR. (C) 3D-echo imaging during procedure. (D) Fluoroscopic image during procedure. (E) Post-procedural MR. (F) Baseline MR in SMR. (G) 3D-echo imaging during procedure. (H) Fluoroscopic image during procedure. (I): Post-procedural MR. (J) Flail of posterior leaflet. (K) Baseline MR. (L) Grasping of posterior leaflet with NeoChord. (M) Fluoroscopic image during procedure. (N) Post-procedural image of MR with TEE.
Echocardiographic examinations
The ultrasound machines used were iE33 and Epiq7C (Philips, Andover, MA, USA), and GE Vivid E95 (GE Healthcare, Chicago, IL, USA). Images were acquired by the experienced senior cardiologists in the echocardiographic laboratory and were centrally evaluated by HY as external Corelab using IntelliSpace Cardiovascular and QLAB software (Philips). All echos analyzed were standard two-dimensional B-mode and Doppler TTE. All measurements were performed in accordance with the current recommendations of the American Society of Echocardiography (23, 24) and latest ESC VHD guidelines as well as MR and tricuspid regurgitation (TR) severity was graded according to current recommendations (12).
Study endpoint
The outcomes were compared between COMBO therapy and M-TEER. The primary outcome was defined as all-cause mortality, while the secondary outcome was defined as the composite events of all-cause mortality and re-intervention by surgery or transcatheter methods. All-cause mortality was ascertained from the entries in patients’ health records and a central data reconciliation with the bureau of vital statistics. The census date was 31 December 2021. Furthermore, we investigated the severity of residual MR, the pressure gradient (PG) of MV at discharge, and the prevalence of re-intervention during follow-up. The study fulfills the GCP (good clinical practice) and the Declaration of Helsinki requirements and was approved by the local ethics committee (Ref. 2019-14692).
Statistical analysis
All data endpoints were collected from records in our Heart Valve Center and the Rhineland-Palatinate bureau of vital statistics for outcome surveillance. Continuous as well as ordinally scaled variables are expressed as medians (Q1, Q3). Categorical variables are expressed as frequencies (%), using the χ2 test or Fisher's exact test to compare the groups. Kaplan–Meier analysis was performed using log rank test to compare the endpoint between both groups. Propensity score matching was based on age, left ventricular systolic ejection fraction (LVEF), and surgical risk as assessed by the EuroSCORE II score. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS statistics version 27 (IBM Corp., Chicago, IL, USA) and EZR version 1.55 (Saitama Medical Center, Japan).
Results
Baseline patient characteristics and echocardiographic data
Between March 2015 and April 2018, 451 patients underwent M-TEER for severe MR. Of them, 38 patients with a redo case or previous surgical MV intervention, 53 patients without baseline echocardiographic parameters, and 3 patients who were lost to follow-up were excluded (Supplementary Figure S1). The remaining 357 patients (mean 78.9 ± 7.0 years, 53.2% male, M-TEER n = 322, COMBO therapy n = 35) were analyzed. Table 1 shows baseline characteristics. Patients receiving COMBO therapy were younger, had a higher weight, were less likely suffering from arterial hypertension, and had less likely already been treated with percutaneous coronary intervention (PCI). There were no differences in medication and laboratory data. The calculated surgical risk was elevated for the whole cohort [EuroSCORE II 4.9 (Q1–Q3: 3.4, 7.3)], and similar in-between groups [M-TEER 5.0 (3.5, 7.6) vs. COMBO 4.5 (3.2, 6.1), p = 0.305].
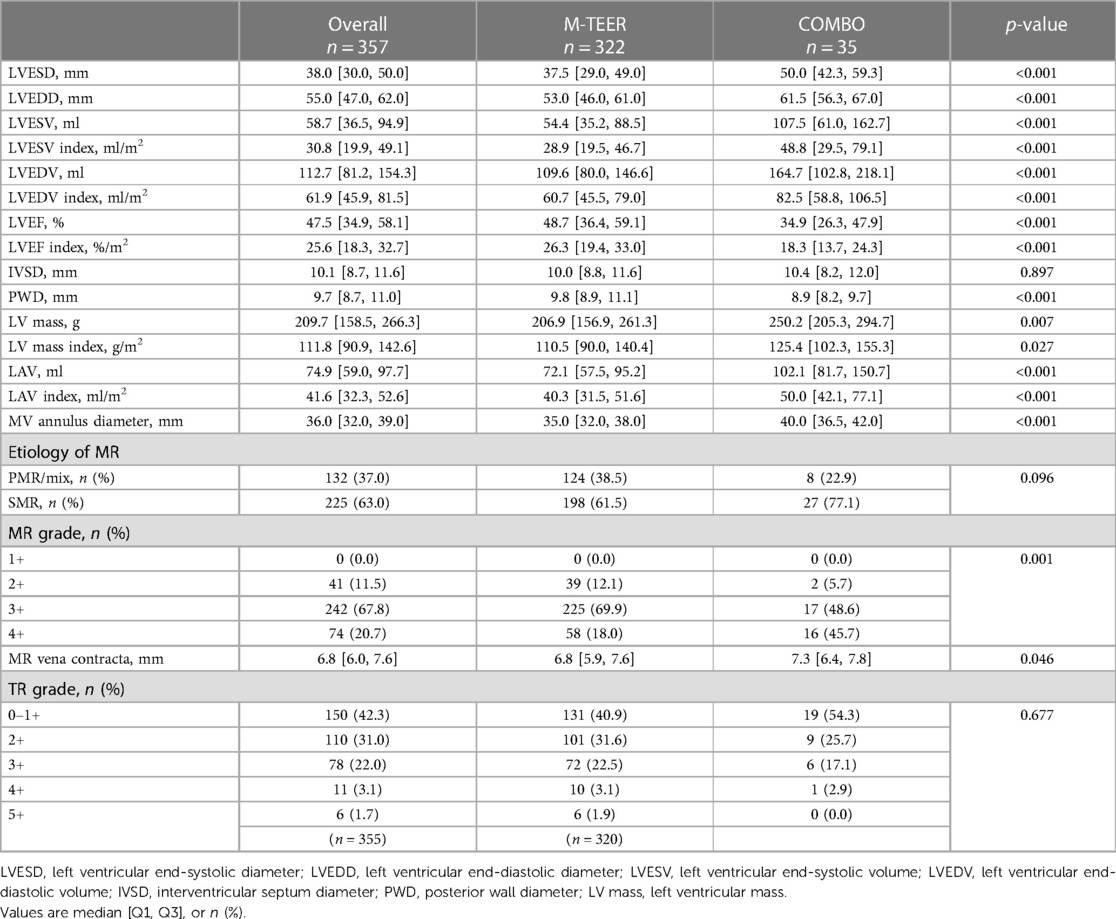
Baseline echocardiographic parameters are summarized in Table 2. Patients receiving COMBO therapy had a greater dilatation of the LA, as well as of the LV [LA volume (LAV)COMBO 118.6 ± 52.8 ml vs. LAVM−TEER 83.2 ± 60.0 ml (p = 0.001), LV end-systolic volume (LVESV)COMBO117.6 ± 80.9 ml vs. LVESVM−TEER 66.1 ± 43.9 ml (p < 0.001), LV end-diastolic volume (LVEDV)COMBO: 174.6 ± 95.8 ml vs. LVEDVM−TEER 118.8 ± 53.3 ml; p < 0.001]. Furthermore, LV dysfunction was also more severe in the COMBO group when compared with patients receiving M-TEER (LVEFCOMBO 37.4 ± 13.8% vs. LVEFM−TEER 47.9 ± 14.3%; p < 0.001). Moreover, patients in the COMBO therapy group had more severe MR grades [MR gradeCOMBO 3 (3, 4) vs. MR gradeM−TEER 3 (3, 3); distribution of MR grade 4+: COMBO 45.7% vs. M-TEER 18.0%; p = 0.001].
Primary and secondary outcomes
Based on the census date of 31 December 2021, the mean long-term follow-up duration in our cohort was 1,310 ± 711 days (COMBO; 1,307 ± 707 days, M-TEER; 1,332 ± 761 days, p = 0.847). Figure 2 shows the Kaplan–Meier curves about the survival and the composite events of survival and re-intervention as a comparison between COMBO therapy and M-TEER. Survival rate in all patients was 94.3% [95% confidence interval (CI): 79.0–98.5] at 30 days, 82.9% (95% CI: 65.8–91.9) after 1 year, 71.4% (95% CI: 53.4–83.5) after 2 years, and 62.9% (95% CI: 44.8–76.5) after 3 years (Figure 2A). There was no significant difference of survival and the composite endpoint of survival and re-intervention between both the groups (Log rank p = 0.921 and 0.543, respectively) (Figures 2A,B). Finally when using propensity score matching, neither M-TEER nor COMBO showed a difference in all-cause mortality (Log rank p = 0.567, Figure 2C) or the combined endpoint (Log rank p = 0.361, Figure 2D). The re-intervention was always M-TEER.
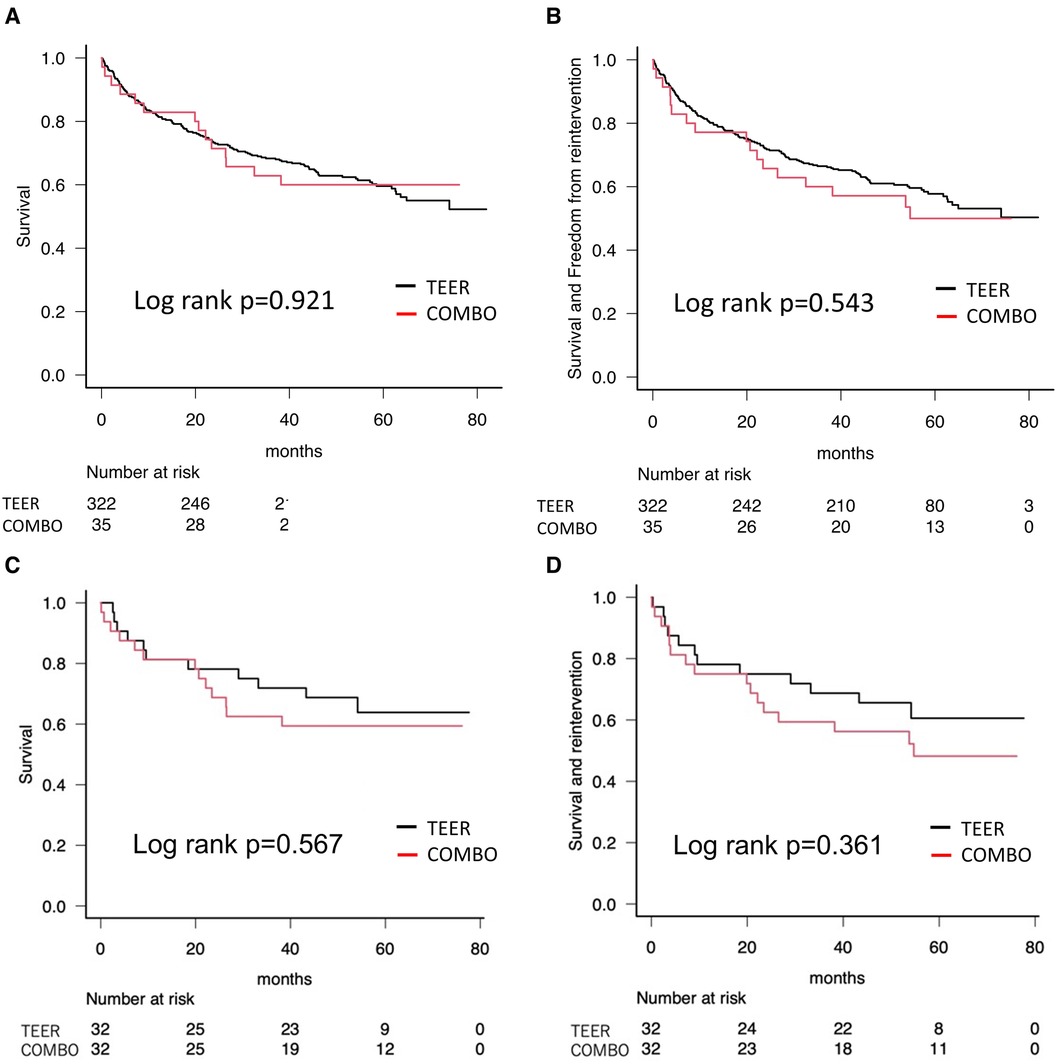
Figure 2. Kaplan–Meier curves showing survival. (A) All-cause mortality as a comparison between COMBO therapy and M-TEER. (B) All-cause mortality and re-intervention as a comparison between COMBO therapy and M-TEER. (C) Propensity score matched all-cause mortality. (D) Propensity score matched combined endpoint of all-cause mortality and re-intervention.
Supplementary Figure S2 reveals the Kaplan–Meier curves about the survival and the composite events of survival and re-intervention according to the etiology of MR, comparing both groups, with no significant difference in-between groups (Log rank p = 0.74, Log rank p = 0.643, respectively). Patients with COMBO therapy also had no significant difference in all-cause mortality and the composite events of survival and re-intervention according to the etiology of MR (Log rank p = 0.652 and 0.082, respectively; Supplementary Figure S3). As the rate of re-intervention seemed to be higher in patients for PMR using COMBO therapy (Supplementary Figure 2B), we conducted a separate analysis of each etiology. Here, there was also no difference in mortality when comparing the outcome of PMR (p = 0.409) vs. SMR (p = 0.443) (Supplementary Figure S4).
Procedure characteristics and outcomes
The procedure characteristics and follow-up in all COMBO therapy patients are demonstrated in Supplementary Table S1. Patients were treated with MitraClip and CMCS, MitraClip and Cardioband, and MitraClip and NeoChord (n = 26, n = 5, and n = 4, respectively). Twenty-five patients in M-TEER received another concomitant transcatheter valve intervention (aortic valve or tricuspid valve) and 15 patients received iatrogenic atrial septal defect closure at the end of the procedure for residual significant right-to-left shunt with deoxygenation, while no patient in COMBO therapy receiving any of the two additional treatments. There was no difference in the number of MitraClip NT used between both the groups (COMBO; 1.6 ± 0.7, M-TEER; 1.6 ± 0.6, p = 0.945). Short-term safety also showed no significant differences between groups (Supplementary Table S2). In detail, there were 7 (2%) overall in-hospital deaths [M-TEER n = 6 (1.9%) vs. COMBO n = 1 (2.9%), p = 0.517], 1 (0.3%) cardiac tamponade, and 3 (0.8%) strokes, each in the M-TEER group (p = 0.999, each). The median time from procedure to discharge was 5 (4, 7) days in all patients [M-TEER 5 (4, 6) vs. COMBO 5 (4, 9), p = 0.359].
There was no difference in the post-interventional MV PG at discharge [COMBO 3.1 ± 1.5 mmHg (34/35), M-TEER 3.5 ± 1.6 mmHg (308/322), p = 0.250]. While not statistically significant, there was a trend toward higher prevalence of residual MR ≧2+ at discharge in the COMBO group [COMBO 34.3% (12/35) vs. M-TEER 20.6% (65/315), p = 0.083]. During follow-up, four patients were referred for redo M-TEER in the COMBO group, significantly more than in the M-TEER group (COMBO 11.4%, M-TEER 2.5%, p = 0.022).
Clinically, we observed a shift toward better New York Heart Association (NYHA) functional classes in each group. While the NYHA functional class was statistically not significant in-between groups at baseline, at 30-day follow-up (p = 0.004), as well as at 1-year follow-up or later (p = 0.056), the groups differed with an apparent greater gain for the COMBO group (Figure 3A). Similarly, all groups saw a decrease in MR over time, while the differences in baseline MR grade favoring the M-TEER group were sustained (Figure 3B).
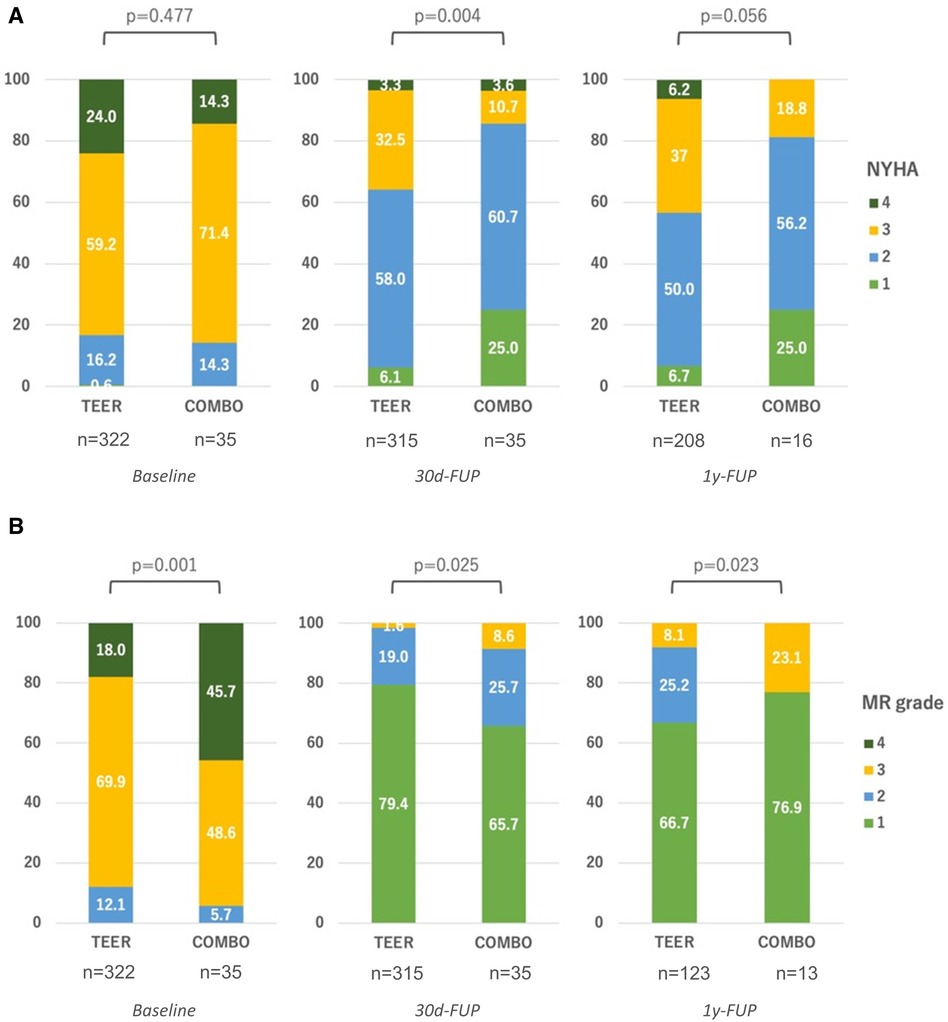
Figure 3. Clinical results during follow-ups. (A) Development of NYHA functional class between groups; (B) Development of residual MR between groups. FUP, follow-up.
Discussion
This study demonstrates that COMBO therapy appears as feasible in a patient collective more severely affected by MR grade, chamber dilatation, and systolic dysfunction as in patients treated with M-TEER alone: we found no significant difference in short-term safety parameters, in all-cause mortality, and in the composite events of all-cause mortality and re-intervention compared with M-TEER, independent of the etiology of MR. This is further highlighted by the long follow-up with a mean of 3.6 years.
There was a higher need for re-intervention in the COMBO group, and a tendency to higher residual MR grades. This comes as no surprise, as the baseline MR grades were higher in the COMBO therapy group, as well as the dimensions and volumes of the left-sided heart chambers. This suggests a disease stage in the COMBO treatment group less amendable to MV repair, as enlarged left-sided heart dimensions, as well as increased severity of MR grade, have been shown to be predictors of unsuccessful MV repair (25). This is further underlined by the finding that this difference in MR grade was sustained over time.
Also, general differences in baseline characteristics suggest a sicker patient population. Despite these differences, the survival rate was comparable between the groups. Aside from the obvious limitations of this study discussed in what follows, one reason might be that the population of the COMBO therapy group was younger, also having a lower likelihood of arterial hypertension and previous PCI. The LVEF was significantly lower by ≥10%, arising from more dilated LVs in systole and diastole. In HF populations, dilation of the LV has earlier been identified as an independent contributor to a poor prognosis in patients with or without myocardial infarction (26, 27). MV interventions for SMR may not lead to better outcomes in HF patients with enlarged LV dimensions (28, 29). In surgical annuloplasty for SMR, the outcome is poor when the baseline LV end-diastolic diameter exceeds 65 mm (28). Furthermore, not just LV dimensions but also the relationship between LV dimensions and MR severity should be considered. The relatively novel framework of proportionality in SMR patients was devised from the insights gained from the MitraFR and COAPT trials (11, 29, 30). In MitraFR, M-TEER did not lead to preferable clinical and functional outcomes with larger LV volumes and lesser MR grade [proportionate MR: LVEDV index 135 ± 35 ml/m2, effective regurgitant orifice area (EROA) 31 ± 10 mm2] (31). Conversely, in COAPT, with smaller LV volumes and more MR (disproportionate MR: LVEDV index 101 ± 34 ml/m2, EROA 41 ± 15 mm2), M-TEER had a significant effect on the clinical and survival outcomes starting at 1 year, and remaining stable up to 5 years (30). This indicates that treatment of SMR may not be effective when the left ventricle is too large (32). These anatomic and functional parameters can characterize the determinants of successful SMR treatment to help the decision-making in clinical practice (31, 33). In this context, COMBO therapy itself in our study may show the need for more intensive therapy in a population that “came in late” on a temporal and disease progression scale. Yet, if these patients would have had even less favorable results when treated with M-TEER only remains a speculation.
Three-quarters of the patients in the COMBO therapy group were treated with the CMCS combined with M-TEER. With respect to the CMCS, Anker et al. reported that even patients with severely enlarged LV diameters experienced LV reverse remodeling and reduced hospitalization rates for HF 1 year after the procedure (32). Hence, one might draw the clinical implication from our findings that COMBO therapy—especially with M-TEER and CMCS—might be effective in patients even with enlarged LV volumes not amenable for an M-TEER alone strategy. We cannot know whether these patients could have achieved similar results with one therapy alone, as Cardioband, CMCS, and NeoChord show improvements of MR, and there are vast data demonstrating effectiveness of M-TEER. However, since the baseline characteristics demonstrate that the COMBO group had larger LA and LV volumes, larger mitral annulus diameters, lower LVEF, and a more severe MR, all predictors of worse outcome, COMBO therapy shows effectiveness even in these difficult cases.
The M-TEER devices were those available during 2015–2018. Since then, newer iterations have been developed, with the MitraClip XTW of the fourth generation being larger and wider than the MitraClip NT that was used most often in this study. One might argue that fewer patients would therefore require COMBO therapy, with larger M-TEER devices possibly being more effective. Conversely, COMBO therapy could also be used in more patients that were considered not treatable before, and furthermore, COMBO therapy itself could be more effective using the larger M-TEER devices, especially since Cardioband and CMCS already offer a wide range of device sizes to fit different, i.e., larger anatomies, while a COMBO therapy with NeoChord and larger M-TEER devices in PMR could be used to address cases with especially pronounced leaflet redundancy.
The survival rate after M-TEER was 73.2% at 5 years in the operable EVEREST trial with PMR and SMR, 57.2% at 3 years in the COAPT trial with SMR only, and 66.1% at 2 years in the MitraFR trial with advanced progression SMR patients (10, 11, 29). Looking at the 5-year COAPT data, the initial result favoring treatment with M-TEER remained stable with all-cause mortality at 42.7% in the device group and 32.8% in the control group (30). The survival rate after CMCS implantation was 67.9% at 3 years (n = 74) (34). With respect to Cardioband, the survival rate was reported as being 87% at 1 year (n = 60) in SMR, and with respect to NeoChord, 94.0% at 3 years (n = 203, in pure PMR) (35, 36).
COMBO therapy can likely increase procedure risks in a surgical high-risk population. Our findings indicate that patients with COMBO therapy that had no direct procedure-related complications seem to have a favorable short-term and long-term mortality compared with the patients treated with M-TEER.
The gains over time in NYHA functional class were consistent throughout both groups, as can be expected with any therapy addressing symptomatic MR. The differences found between groups at the two follow-up intervals, apparently favoring the COMBO therapies, must be carefully interpreted. On the one hand, the patients in the COMBO group seemed sicker, as mentioned. Therefore, although there was no difference in NYHA functional class at baseline, this group might have had “more to gain” clinically, especially as MR seemed more severe in this group while almost all patients of both the groups were in NYHA functional class III or even IV, anyway. On the other hand, this interpretation is subject to the obvious limitations of this study.
Our study demonstrates that in patients with more enlarged LV, more profound systolic impairment, and more severe MR grades, COMBO therapy can provide meaningful results. Therefore, by paying attention to the functional and anatomic parameters of the individual patient characteristics, our treatment toolbox is enhanced.
Limitations
There are several limitations to this study: Its design is retrospective and observational from a single center, so hidden confounders could be present. Furthermore, the number of patients treated with COMBO therapy is small. Therefore, especially small differences between the study groups might have been missed. The procedures were carried out in a dedicated, large volume Heart Valve Center, making results possibly not generalizable. We excluded three foreign patients that were lost to follow-up, who were not listed in the bureau of vital statistics. This could possibly cause a selection bias, when the clinical outcome was death. Yet, because the patients were all from the M-TEER alone group, the impact would most likely be small.
Then, while the COMBO approach was suggested as an option during the Heart Team deliberations, the ultimate decision was made by the interventionalist during the procedure. This most likely caused a significant selection bias as was discussed previously: the patients in the COMBO group suffered from more pronounced disease, but other confounders might also be present.
The follow-up rate of 38% for M-TEER, and 45% for COMBO, at 1 year was only moderate, calling the validity of the data somewhat into question. Then, it might be argued that the effectiveness of the different strategies might be better analyzed using propensity matching based on anatomical parameters, such as mitral pathology, indices of left atrial and left ventricular size and function. However, with the limitations already mentioned, especially the retrospective design, only moderate follow-up rate and without a core lab to offer a uniform echocardiographic analysis, a propensity score matching would not be feasible. However, while this study is certainly not powered to detect fine significant differences in mortality, and performance of propensity score matching in small sample sizes shows reduced performance (37), the analysis based on age, LVEF, and surgical risk still adds information to demonstrate the feasibility of the COMBO approach in selected patients. Furthermore, since the data on mortality was gathered from census, this somewhat compensates for the only moderate follow-up adherence.
Changes of medical drug therapy treatment for heart failure after the procedure were not documented like in more rigorous randomized clinical trials. This could have an impact on the results of this study. Also, SGLT-2 inhibitors were not administered for HF in this group, because approval for this medication to treat patients suffering from heart failure with reduced ejection fraction (HFrEF) was granted in late 2020.
Because the field of interventions in structural heart disease is evolving quickly, extrapolation of our findings to the latest generation of devices, e.g., M-TEER (Edwards Pascal P5, or Abbott MitraClip fourth generation), or future generations, e.g., annuloplasty devices, may not be possible.
This is not a randomized study. The improvement in outcomes seen for patients with more severe MR and larger chamber sizes in the COMBO group shows that COMBO therapy seems effective and safe, but not superior to M-TEER. In fact, the insights of this study are intended as “proof of concept,” demonstrating that physicians may need to use a toolbox to individualize patient treatment, rather than to be confined to a “one size fits all” solution.
This study did not focus on PMR or SMR alone. Moreover, the COMBO therapy group consisted of three different devices combined with M-TEER for the sample volume leading to some heterogeneity. However, the patients with COMBO therapy with two devices other than M-TEER, for example the CMCS and NeoChord, were excluded from this study, and the human experience with the COMBO therapy so far is limited in registries or publications.
To fully appreciate the potential of COMBO therapy, and to make a deeper impact on clinical practice, prospective—and preferably randomized—clinical research is required in the future, each focusing on one COMBO approach. Since the patients included in this study showed severe comorbidities and challenging anatomies and/or pathologies, a reasonable choice could be to include symptomatic patients suffering from severe MR that show already significant dilatation of the mitral annulus. When investigating NeoChord, the patients suffering from PMR caused by extensive prolapse and flail of one leaflet would be considered, whereas in SMR, these patients, also showing significant dilatation of the LV and pronounced tethering of the leaflets, would most likely be logical choices for treatment with either CMCS or Cardioband.
We did not analyze the cost-effectiveness of the different treatment strategies. Therefore, COMBO therapy might not be as feasible as desired, especially when local reimbursement is limited or restricted.
In consequence, this study's findings should be interpreted with caution and as “hypothesis generating.”
Conclusions
This study showed that, compared with M-TEER alone, there were no significant differences in safety outcomes. Yet, patients treated with COMBO therapy suffered from more impaired LVEF, more severe MR, and larger ventricles at baseline. Although the re-intervention rate was higher in this higher-risk patient population, promising results could be obtained. This demonstrates that COMBO therapy is feasible and safe, possibly offering a toolbox to individualize patient treatment. The higher need for re-intervention in the COMBO group possibly underlines the need to treat severe MR earlier in smaller ventricles. However, future research is required to establish the COMBO approach as such a toolbox-like treatment option.
Impact on daily practice
Combination (COMBO) therapy, which refers to the therapeutic strategy with more than two TMVr devices, is rarely used as a treatment for patients with severe MR. However, we compared this procedure with M-TEER, which is now a most common TMVr for severe MR, and found that COMBO therapy might be beneficial even in patients who already had a more dilated LV and a more pronounce LV systolic dysfunction. Now various TMVr devices are available for the treatment of severe MR in clinical use, offering a toolbox with its uses determined by the background and the anatomy of the patient.
Data availability statement
The datasets presented in this article are not readily available because of local data security restrictions (Datenschutzbeauftragter, University Medicine Mainz). Requests to access the datasets should be directed to tobias.ruf@unimedizin-mainz.de.
Ethics statement
The studies involving humans were approved by Ethikkommission der Landesärztekammer Rheinland-Pfalz, Körperschaft des öffentlichen Rechts, Deutschhausplatz 3, 55116 Mainz, Fon 06131 28822-0, Mail kammer@laek-rlp.de. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study design is a retrospective analysis.
Author contributions
HY: Data collection, interpretation of data, and writing of manuscript. TR: Data collection, writing of manuscript, interpretation of data, conception and design of the work, and drafting the work. TG: Revising it critically for important intellectual content. MG: Revising it critically for important intellectual content. JZ: Data collection and interpretation of data. BS: Data collection and interpretation of data. TM: Revising it critically for important intellectual content and provide approval for publication of the content. RB: Revising it critically for important intellectual content and provide approval for publication of the content. All authors contributed to the article and approved the submitted version.
Acknowledgments
HY was invited to this analysis on a research and travel grant to Heart Valve Center Mainz, Germany, from Shonan Kamakura General Hospital, Kamakura, Japan in 2021 to 2022. This work contains parts of the doctoral theses of Julia Zirbs and Bend Schwidtal.
Conflict of interest
HY is Speaker and Japanese training faculty member for Abbott Laboratories. TR is Speaker, proctor, and/or preceptor for Abbott, Cardiac Dimensions, Edwards Lifesciences, and NeoChord. RvB is in the Advisory board and speaker for Abbott Vascular, BMS, Edwards Lifesciences, JenaValve, Medtronic, and NeoChord.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1223588/full#supplementary-material
Supplementary Figure S1
Patient flow chart. MR, mitral regurgitation; M-TEER, mitral transcatheter edge-to-edge repair.
Supplementary Figure S2
Kaplan–Meier curves according to etiology of MR. (A) All-cause mortality as a comparison between COMBO therapy and M-TEER. (A) All-cause mortality and re-intervention as a comparison between COMBO therapy and M-TEER. MR, mitral regurgitation; PMR, primary MR; SMR, secondary MR; M-TEER, mitral transcatheter edge-to-edge repair.
Supplementary Figure S3
The Kaplan–Meier curves in COMBO therapy according to etiology of MR. (A) All-cause mortality. (A) All-cause mortality and re-intervention. MR, mitral regurgitation; PMR, primary MR; SMR, secondary MR; M-TEER, mitral transcatheter edge-to-edge repair.
Supplementary Figure S4
The Kaplan–Meier curves in each MR etiology. (A) SMR. (B) PMR. M-TEER, mitral transcatheter edge-to-edge repair.
Supplementary Table S1
Procedural characteristics and outcomes.
Supplementary Table S2
In-hospital safety.
References
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. (2006) 368:1005–11. doi: 10.1016/S0140-6736(06)69208-8
2. Grigioni F, Tribouilloy C, Avierinos JF, Barbieri A, Ferlito M, Trojette F, et al. Outcomes in mitral regurgitation due to flail leaflets: a multicenter European study. JACC Cardiovasc Imaging. (2008) 1:133–41. doi: 10.1016/j.jcmg.2007.12.005
3. Sannino A, Smith RL 2nd, Schiattarella GG, Trimarco B, Esposito G, Grayburn PA. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a systematic review and meta-analysis. JAMA Cardiol. (2017) 2:1130–9. doi: 10.1001/jamacardio.2017.2976
4. Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J. (2012) 164:163–76. doi: 10.1016/j.ahj.2012.05.014
5. El Sabbagh A, Reddy YNV, Nishimura RA. Mitral valve regurgitation in the contemporary era: insights into diagnosis, management, and future directions. JACC Cardiovasc Imaging. (2018) 11:628–43. doi: 10.1016/j.jcmg.2018.01.009
6. Deferm S, Bertrand PB, Verbrugge FH, Verhaert D, Rega F, Thomas JD, et al. Atrial functional mitral regurgitation: JACC review topic of the week. J Am Coll Cardiol. (2019) 73:2465–76. doi: 10.1016/j.jacc.2019.02.061
7. Mesi O, Gad MM, Crane AD, Ramchand J, Puri R, Layoun H, et al. Severe atrial functional mitral regurgitation: clinical and echocardiographic characteristics, management and outcomes. JACC Cardiovasc Imaging. (2021) 14:797–808. doi: 10.1016/j.jcmg.2021.02.008
8. Doldi P, Stolz L, Orban M, Karam N, Praz F, Kalbacher D, et al. Transcatheter mitral valve repair in patients with atrial functional mitral regurgitation. JACC Cardiovasc Imaging. (2022) 15:1843–51. doi: 10.1016/j.jcmg.2022.05.009
9. De Backer O, Wong I, Taramasso M, Maisano F, Franzen O, Sondergaard L. Transcatheter mitral valve repair: an overview of current and future devices. Open Heart. (2021) 8(1):e001564. doi: 10.1136/openhrt-2020-001564
10. Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. (2015) 66:2844–54. doi: 10.1016/j.jacc.2015.10.018
11. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. (2018) 379:2307–18. doi: 10.1056/NEJMoa1806640
12. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. (2021) 60:727–800. doi: 10.1093/ejcts/ezab389
13. Latib A, Ancona MB, Ferri L, Montorfano M, Mangieri A, Regazzoli D, et al. Percutaneous direct annuloplasty with Cardioband to treat recurrent mitral regurgitation after MitraClip implantation. JACC Cardiovasc Interv. (2016) 9:e191–2. doi: 10.1016/j.jcin.2016.06.028
14. von Bardeleben RS, Colli A, Schulz E, Ruf T, Wrobel K, Vahl CF, et al. First in human transcatheter COMBO mitral valve repair with direct ring annuloplasty and NeoChord leaflet implantation to treat degenerative mitral regurgitation: feasibility of the simultaneous toolbox concept guided by 3D echo and computed tomography fusion imaging. Eur Heart J. (2018) 39:1314–5. doi: 10.1093/eurheartj/ehx595
15. Rogers JH, Boyd WD, Smith TWR, Ebner AA, Bolling SF. Combined MitraClip edge-to-edge repair with millipede IRIS mitral annuloplasty. JACC Cardiovasc Interv. (2018) 11:323–4. doi: 10.1016/j.jcin.2017.11.007
16. Yokoyama H, Ruf TF, Geyer M, Tamm AR, Da Rocha ESJG, Gossler TAM, et al. Reverse cardiac remodeling in patients undergoing combination therapy of transcatheter mitral valve repair. Front Cardiovasc Med. (2023) 10:1029103. doi: 10.3389/fcvm.2023.1029103
17. Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (endovascular valve edge-to-edge REpair study) cohort. J Am Coll Cardiol. (2009) 54:686–94. doi: 10.1016/j.jacc.2009.03.077
18. Schofer J, Siminiak T, Haude M, Herrman JP, Vainer J, Wu JC, et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON mitral annuloplasty device European union study. Circulation. (2009) 120:326–33. doi: 10.1161/CIRCULATIONAHA.109.849885
19. Witte KK, Lipiecki J, Siminiak T, Meredith IT, Malkin CJ, Goldberg SL, et al. The REDUCE FMR trial: a randomized sham-controlled study of percutaneous mitral annuloplasty in functional mitral regurgitation. JACC Heart Fail. (2019) 7(11):945–55. doi: 10.1016/j.jchf.2019.06.011
20. Maisano F, Taramasso M, Guidotti A, Nietlispach F. The Cardioband: strategies for optimal patient selection and optimised results. EuroIntervention. (2016) 12:Y61–3. doi: 10.4244/EIJV12SYA15
21. Seeburger J, Rinaldi M, Nielsen SL, Salizzoni S, Lange R, Schoenburg M, et al. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: the TACT trial (transapical artificial chordae tendinae) proof of concept. J Am Coll Cardiol. (2014) 63:914–9. doi: 10.1016/j.jacc.2013.07.090
22. Colli A, Adams D, Fiocco A, Pradegan N, Longinotti L, Nadali M, et al. Transapical NeoChord mitral valve repair. Ann Cardiothorac Surg. (2018) 7:812–20. doi: 10.21037/acs.2018.11.04
23. Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. (2012) 13:1–46. doi: 10.1093/ehjci/jer316
24. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. (2017) 30:303–71. doi: 10.1016/j.echo.2017.01.007
25. Kongsaerepong V, Shiota M, Gillinov AM, Song JM, Fukuda S, McCarthy PM, et al. Echocardiographic predictors of successful versus unsuccessful mitral valve repair in ischemic mitral regurgitation. Am J Cardiol. (2006) 98:504–8. doi: 10.1016/j.amjcard.2006.02.056
26. White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. (1987) 76:44–51. doi: 10.1161/01.CIR.76.1.44
27. Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. (1997) 336:1350–5. doi: 10.1056/NEJM199705083361903
28. Braun J, van de Veire NR, Klautz RJ, Versteegh MI, Holman ER, Westenberg JJ, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg. (2008) 85:430–6; discussion 436–7. doi: 10.1016/j.athoracsur.2007.08.040
29. Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. (2018) 379:2297–306. doi: 10.1056/NEJMoa1805374
30. Stone GW, Abraham WT, Lindenfeld J, Kar S, Grayburn PA, Lim DS, et al. Five-year follow-up after transcatheter repair of secondary mitral regurgitation. N Engl J Med. (2023) 388:2037–48. doi: 10.1056/NEJMoa2300213
31. Pibarot P, Delgado V, Bax JJ. MITRA-FR vs. COAPT: lessons from two trials with diametrically opposed results. Eur Heart J Cardiovasc Imaging. (2019) 20:620–4. doi: 10.1093/ehjci/jez073
32. Anker SD, Starling RC, Khan MS, Friede T, Filippatos G, Lindenfeld J, et al. Percutaneous mitral valve annuloplasty in patients with secondary mitral regurgitation and severe left ventricular enlargement. JACC Heart Fail. (2021) 9:453–62. doi: 10.1016/j.jchf.2021.03.002
33. Packer M, Grayburn PA. Contrasting effects of pharmacological, procedural, and surgical interventions on proportionate and disproportionate functional mitral regurgitation in chronic heart failure. Circulation. (2019) 140:779–89. doi: 10.1161/CIRCULATIONAHA.119.039612
34. Lipiecki J, Kaye DM, Witte KK, Haude M, Kapadia S, Sievert H, et al. Long-term survival following transcatheter mitral valve repair: pooled analysis of prospective trials with the carillon device. Cardiovasc Revasc Med. (2020) 21:712–6. doi: 10.1016/j.carrev.2020.02.012
35. Messika-Zeitoun D, Nickenig G, Latib A, Kuck KH, Baldus S, Schueler R, et al. Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur Heart J. (2019) 40:466–72. doi: 10.1093/eurheartj/ehy424
36. Gerosa G, Nadali M, Longinotti L, Ponzoni M, Caraffa R, Fiocco A, et al. Transapical off-pump echo-guided mitral valve repair with neochordae implantation mid-term outcomes. Ann Cardiothorac Surg. (2021) 10:131–40. doi: 10.21037/acs-2020-mv-86
Keywords: mitral regurgitation, transcatheter mitral valve repair (TMVr), mitral transcatheter edge-to-edge therapy, M-TEER, COMBO therapy
Citation: Yokoyama H, Ruf TF, Gößler TAM, Geyer M, Zirbs J, Schwidtal BL, Münzel T and von Bardeleben RS (2024) Outcomes of COMBO therapy for severe mitral regurgitation compared with transcatheter edge-to-edge repair. Front. Cardiovasc. Med. 11:1223588. doi: 10.3389/fcvm.2024.1223588
Received: 16 May 2023; Accepted: 8 February 2024;
Published: 26 February 2024.
Edited by:
Simon H. Sündermann, Charité University Medicine Berlin, GermanyReviewed by:
Tetsu Tanaka, University Hospital Bonn, GermanyYthan H. Goldberg, Lenox Hill Hospital, United States
© 2024 Yokoyama, Ruf, Gößler, Geyer, Zirbs, Schwidtal, Münzel and von Bardeleben. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tobias Friedrich Ruf tobias.ruf@unimedizin-Mainz.de
†These authors have contributed equally to this work and share first authorship
 Hiroaki Yokoyama
Hiroaki Yokoyama Tobias Friedrich Ruf
Tobias Friedrich Ruf Theresa Ann Maria Gößler2
Theresa Ann Maria Gößler2  Martin Geyer
Martin Geyer Thomas Münzel
Thomas Münzel Ralph Stephan von Bardeleben
Ralph Stephan von Bardeleben