Transcatheter aortic valve implantation (from inception to standard treatment): a single-center observational study
- 1Clinical Cardiovascular Research Group, Institute of Clinical Medicine, The Arctic University of Norway, Tromsø, Norway
- 2Department of Cardiothoracic Surgery, University Hospital of North Norway, Tromsø, Norway
- 3Department of Cardiology, University Hospital of North Norway, Tromsø, Norway
- 4Department of Gastrointestinal Surgery, University Hospital of North Norway, Tromsø, Norway
Background: Treatment of severe aortic stenosis with transcatheter aortic valve implantation (TAVI) was introduced in 2002. Since then, TAVI has become the primary treatment approach worldwide for advanced-age patients and younger patients with severe comorbidities. We aimed to evaluate the changes in patient demographics, complications, and mortality rates within 13 years.
Methods: This retrospective observational study included 867 patients who underwent TAVI at the University Hospital of North Norway in Tromsø from 2008 to 2021. The 13-year period was divided into period 1 (2008–2012), period 2 (2013–2017), and period 3 (2018–2021). The primary objective was to evaluate the changes in periprocedural (30 days), early (30–365 days), and late mortality rates (>365 days) between the periods. The secondary objective was to evaluate late mortality rates by sex and age groups: <70 years, 70–79 years, 80–89 years, and ≥90 years.
Results: The periprocedural mortality rates for periods 1, 2, and 3 were 10.3%, 2.9%, and 1.2%, respectively (P < 0.001). The early mortality rates were 5.6%, 5.8%, and 6.5%, respectively. No significant differences were observed in late mortality by sex or age group (<70, 70–79, and 80–89 years) with a median survival of 5.3–5.6 years. The median survival in patients aged ≥90 years was 4.0 years (P = 0.018).
Conclusion: Our findings indicate that most patients are octogenarians, and the burden of their comorbidities should be highly considered compared to their age when evaluating the procedural outcomes. As the incidence of most complications related to TAVI has decreased, the rates of permanent pacemaker implantation remain high. Important advancements in diagnostics, valve technology, and procedural techniques have improved the periprocedural mortality rates; however, early mortality remains unchanged and poses a clinical challenge that needs to be addressed in the future.
1 Introduction
In 2002, a French cardiologist and professor Alain Cribier performed the first transcatheter aortic valve implantation (TAVI) (1). Since then, TAVI has become the primary treatment modality for severe symptomatic aortic stenosis (AS). AS due to degenerative calcification is the predominant valvular heart disease in high-income countries (2), with a projected twofold increase in prevalence within the next five decades (3, 4). Previously, surgical aortic valve replacement (SAVR) was the only curative option prior to the introduction of TAVI. Currently, SAVR is primarily recommended for patients aged <70 years, with bicuspid valve anatomy, with concomitant multivessel coronary artery disease (CAD), with cardiac conditions requiring surgery, or deemed unsuitable for TAVI. In certain cases, patients may opt for a mechanical valve, despite the necessity for lifelong anticoagulation, owing to the lower rates of re-intervention compared with bioprosthetic valves. Previous randomized controlled trials have demonstrated comparable outcomes between TAVI and SAVR, with TAVI showing a favorable trend owing to its less invasive nature (5–12).
Current issues facing TAVI are the long-term durability of transcatheter heart valves (THVs), high rates of permanent pacemaker implantation (PPI), and the long-term impact of mild paravalvular leak (PVL) (13–15). As the indication of TAVI is expanded to younger age groups, the complexity of choosing the right intervention increases (16). Therefore, a multidisciplinary team is fundamental in ensuring that patients receive thorough information to enable effective shared decision-making (17, 18).
As the first university hospital in Norway, the University Hospital of Northern Norway (UNN) in Tromsø introduced its TAVI program in 2008. This study aimed to evaluate the changes in patient demographics, complications, and mortality rates associated with TAVI performed in a single center within a period of 13 years.
2 Materials and methods
2.1 Patient population and data collection
The study cohort included all patients with symptomatic severe AS who underwent TAVI at UNN Tromsø from September 2008 through December 2021. This 13-year timeframe was further divided into three periods according to the changes in the European guidelines for the treatment of valvular heart disease by the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery in 2012, 2017, and 2021 (19), respectively. The periods were chosen to examine guideline influences on patient selection and outcome, reduce selection bias and improve overall homogeneity. Periprocedural mortality, early mortality, late mortality, and complications were defined according to the Valve Academic Research Consortium 3 (VARC-3) criteria (20). The clinical characteristics, complications, and mortality data of all patients were retrospectively collected from the electronic medical records to obtain comprehensive information. The missing values were not imputed, and none of the patients were lost to follow-up, thus ensuring data integrity and completeness. The local data protection office approved the collection of data.
2.2 Preprocedural workup and procedural characteristics
All patients underwent clinical evaluation, transthoracic or transesophageal echocardiography (TEE), and computed tomography (CT) at our center to evaluate the disease severity and feasibility of TAVI. The heart team evaluated the suitability of each patient for TAVI. The contraindications for the procedure were a lifetime expectancy of less than 1 year, inability to provide informed consent, and low motivation, as expressed by the patient. The available version of the European System for Cardiac Operative Risk Evaluation (EuroSCORE) I/log/II was used for risk stratification.
All procedures were performed by a cardiovascular surgeon and interventional cardiologist. At their discretion, either a balloon-expanding valve (BEV) or a self-expanding valve (SEV) was used. The routes of access were as follows: trans-femoral (TF-TAVI), -apical (TA-TAVI), -aortic (TAo-TAVI), -carotid (TC-TAVI), or -subclavian/axillary (TSc-TAVI). The anesthesia used was either general or conscious sedation with localized anesthesia.
2.3 Statistical analysis
Continuous variables are presented as the mean ± standard deviation or the median with an interquartile range depending on the normality of distribution. Normally distributed variables were compared using Student's t-test or analysis of variance, while non-normally distributed variables were compared using the Wilcoxon rank-sum test. The Shapiro–Wilk test was used to assess the normality of the distribution. The categorical variables were presented as numbers with percentages and compared using the X2 test or Fisher's exact test if the expected event count was less than 5. Kaplan–Meier plots were used to present the time-to-event analysis, and the log-rank test was used to compare their differences. Potential predictors were assessed for clinical relevance and multicollinearity, and included in a backwards stepwise multivariable logistic regression analysis if the P-value ≤ 0.15. A two-tailed P-value of <0.05 was considered significant. STATA 17.0 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.was) was used for all analyses.
3 Results
3.1 Patients
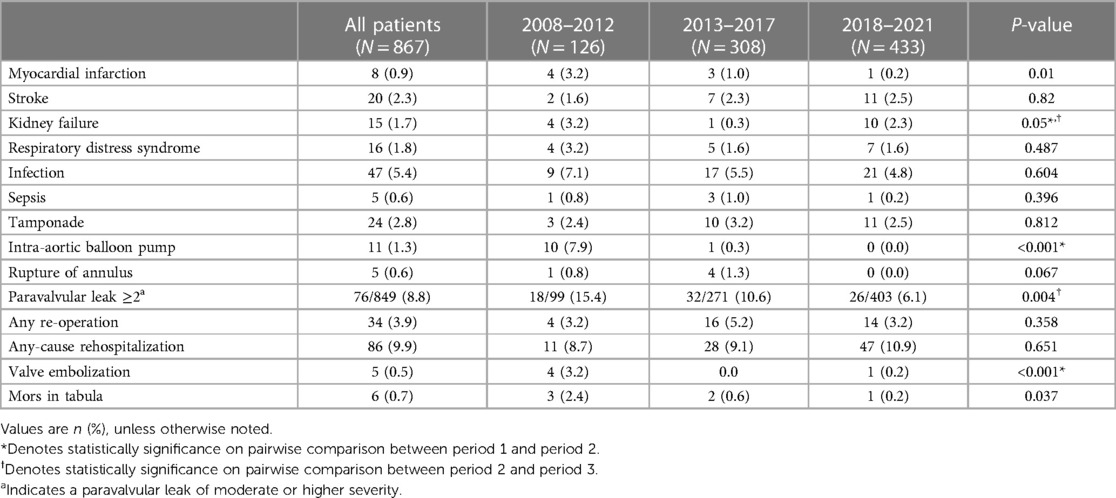
The baseline characteristics of the 867 patients who underwent TAVI between 2008 and 2021 are presented in Table 1. In general, period 1 exhibited a higher proportion of women and older patients. Additionally, there was a higher prevalence of NYHA class ≥3, previous myocardial infarction and higher mean pre-operative gradients. The estimated EuroSCORE II significantly decreased, transitioning from extreme to high risk in periods 1 and 2 to intermediate to low risk in period 3. From period 2 to period 3 the prevalence of CAD, peripheral artery disease, systemic corticosteroid (SCT) usage, previous coronary artery bypass graft (CABG) surgery and previous cardiac surgery decreased. Across all periods the median aortic-valve area (AVA) increased, but rates of severe pulmonary hypertension declined.
3.2 Periprocedural, early, and late mortality by period
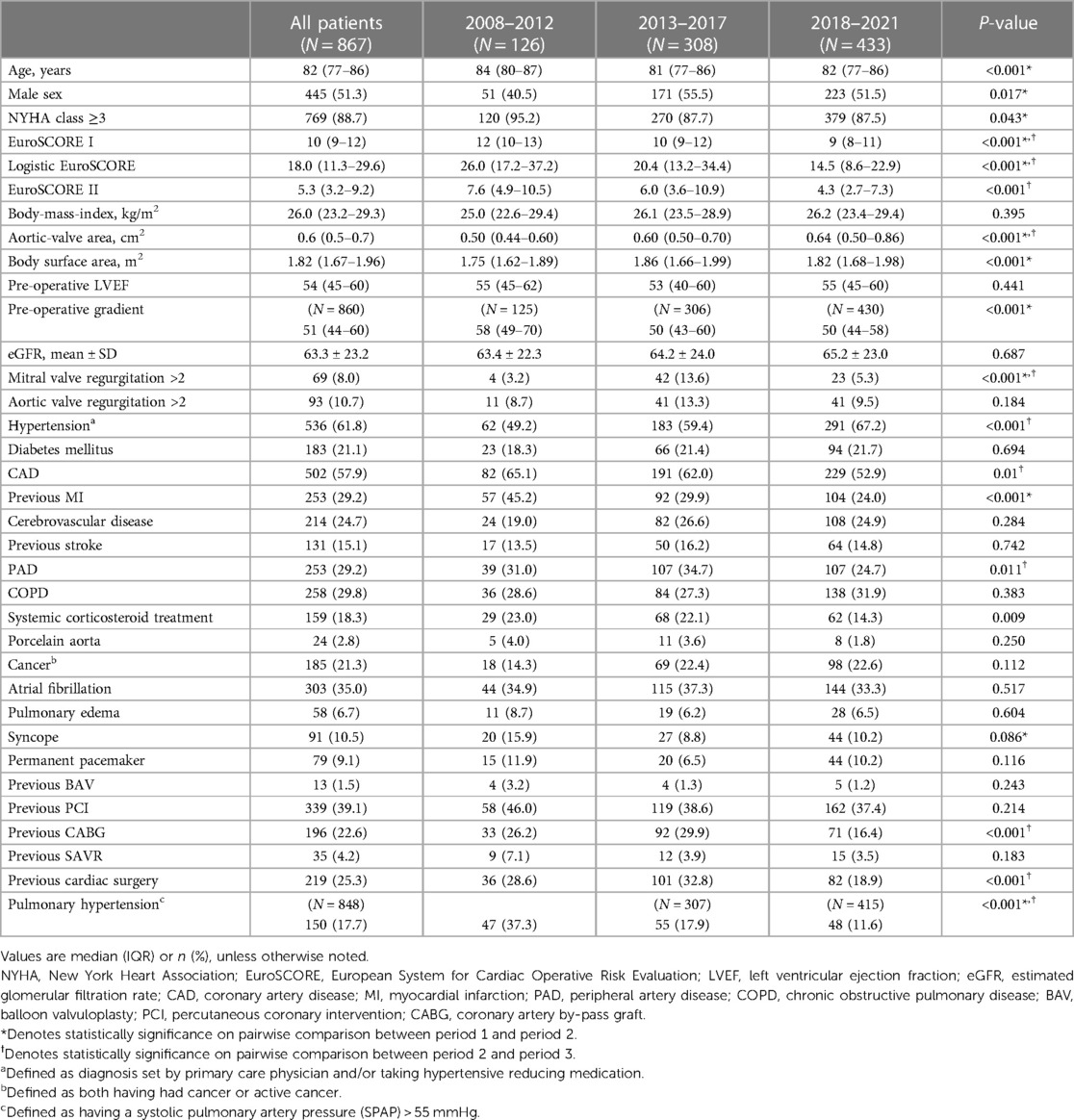
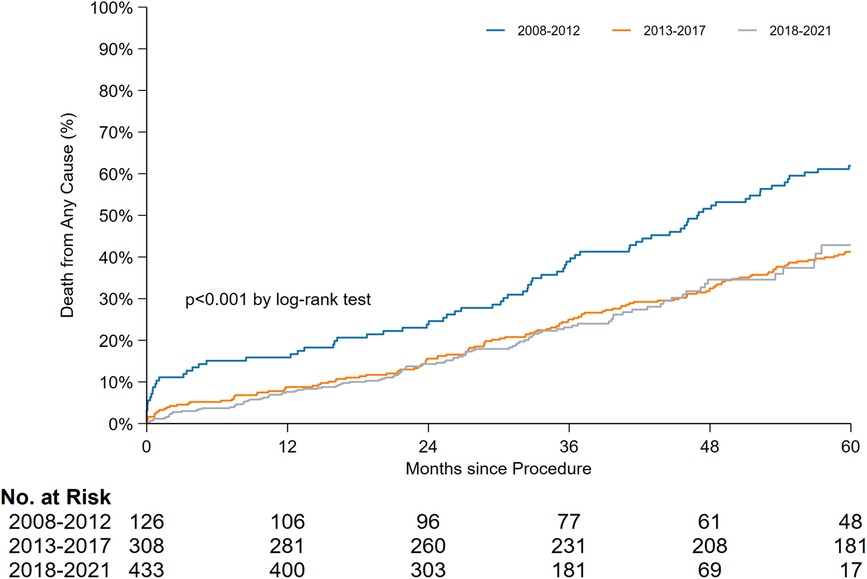
The periprocedural, early, and late mortality rates are shown in Figure 1. The periprocedural mortality rate significantly reduced from period 1 to period 2 (10.3% to 2.9% to 1.2%, P < 0.001). A pairwise comparison between period 2 and period 3 for periprocedural mortality (odds ratio [OR]: 0.39, 95% confidence interval [CI]: 0.13–1.17) and early mortality rates (OR: 1.11, 95% CI: 0.61–2.05) yielded no significant difference. Early mortality remained unchanged throughout the study period (5.6% to 5.8% to 6.5%, P = 0.904). Owing to the improvement in periprocedural mortality, the late mortality rate also improved. A landmark analysis of late mortality with the exclusion of periprocedural mortality using a log-rank test is presented in Figure 2, and the results are significant (P < 0.001).
3.3 Late mortality by sex and age groups
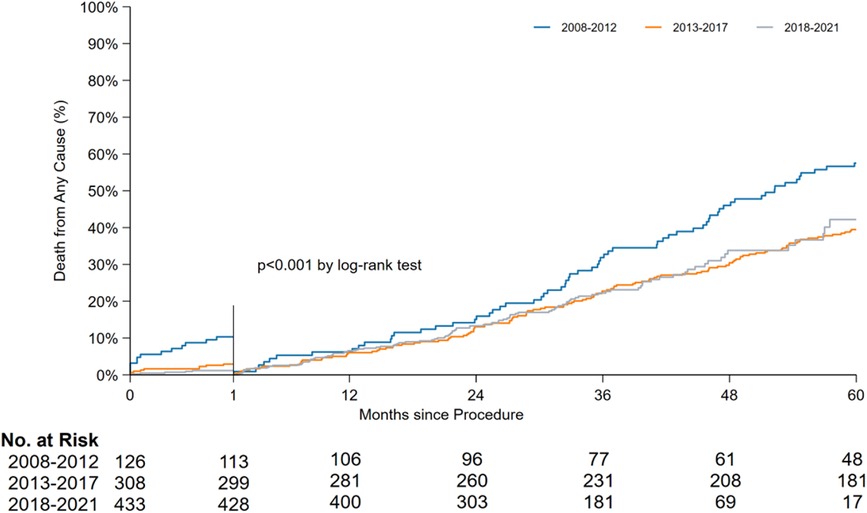
A comparison of the late mortality rate between sexes is shown in Figure 3. Of the 867 patients, 422 women and 445 men were included in the time-to-event comparison, and no significant difference was found in the 5-year overall mortality rate. The median survival in years along with the 95% CI were as follows: 5.3 years [4.8–5.8] for women and 5.5 years [4.9–6.1] for men (P = 0.47).
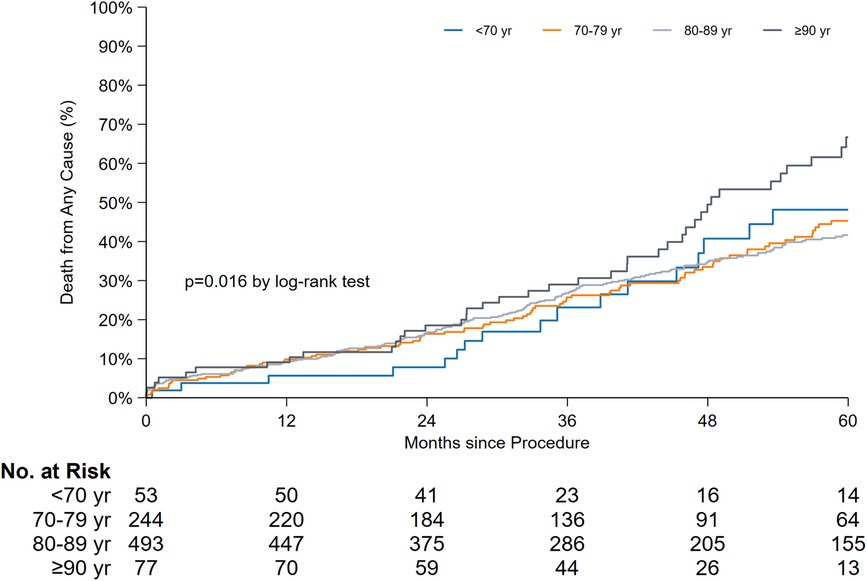
In terms of age-stratified late mortality comparison (Figure 4), a significant difference was observed. The median survival rates in years along with the 95% CI for each age group were as follows: < 70 years, 5.3 years [3.2–7.4]; 70–79 years, 5.5 years [4.6–6.4]; 80–89 years, 5.6 years [5.2–6.0]; and ≥90 years, 4.0 years [3.3–4.6] (P = 0.016). When the overall survival was compared with the exclusion of patients aged ≥90 years, the log-rank test results were not significant (P = 0.81).
3.4 Procedural and VARC-3 complications
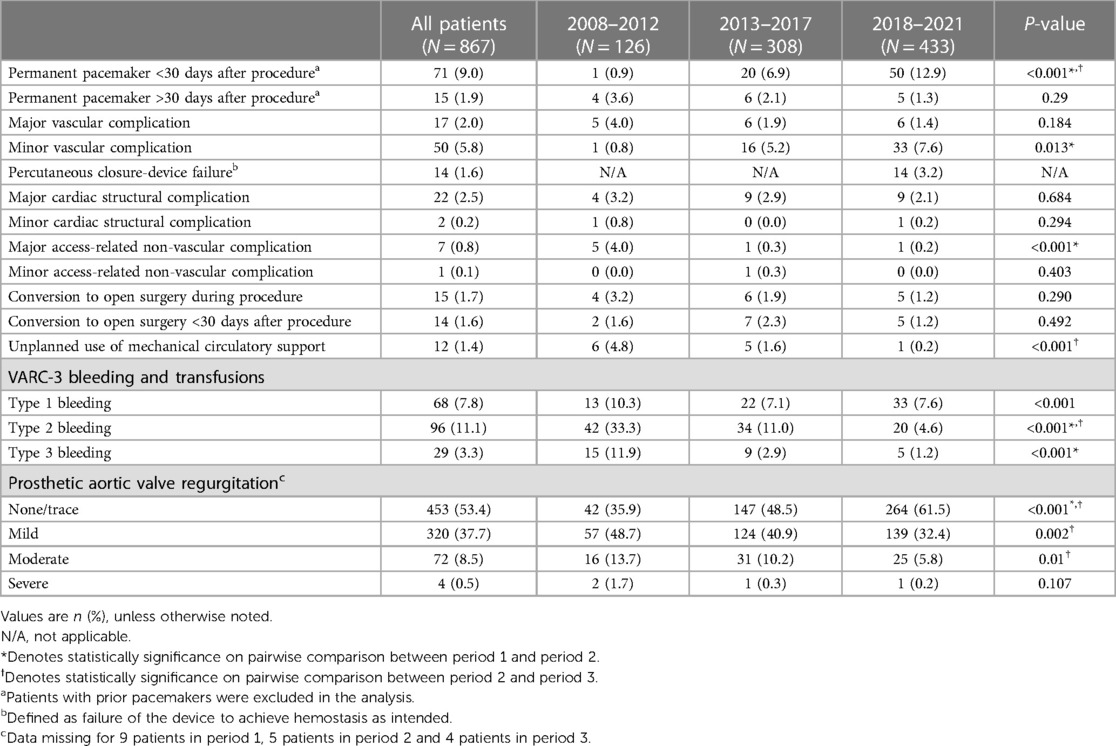
The procedural and VARC-3 complications are listed in Table 2 and Table 3. A significant reduction was observed in the prevalence of major access-related nonvascular complications and VARC-3 type 2–3 bleeding in period 1 compared with that in periods 2 and 3. The rate of early PPI increased between periods 1 and 2, and the PPI rate continued to increase from periods 2 to 3. An inverse relationship was observed between PPI and PVL, as a high proportion of patients had none/trace PVL in later periods. Percutaneous closure device failure was only observed in period 3 owing to the implementation of percutaneous TF-TAVI.
3.5 Procedural information
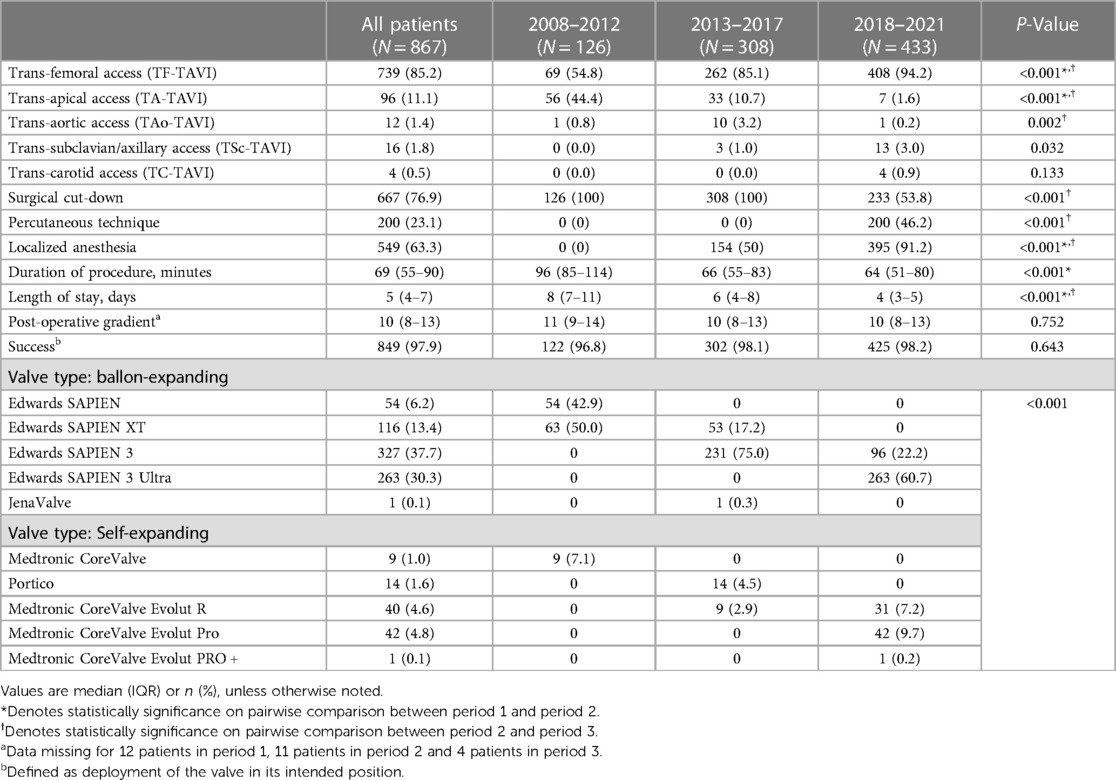
Table 4 presents the procedural information and valve characteristics. The implementation rate of TF-TAVI increased throughout the study period, and TF-TAVI accounted for 94.2% of all procedures performed in period 3. TA-TAVI, which accounted for almost half of the procedures performed in period 1, was rarely employed in period 3. The rate of performing TSc-TAVI increased in period 3. Percutaneous access was introduced in period 3 and was employed in half of the procedures. Local anesthesia was increasingly used and accounted for 91.2% of all procedures performed in period 3. The median procedural time (from 90 min to 60 min) and length of hospital stay (from 8 days to 4 days) significantly improved.
3.6 Multivariable regression analysis of early mortality
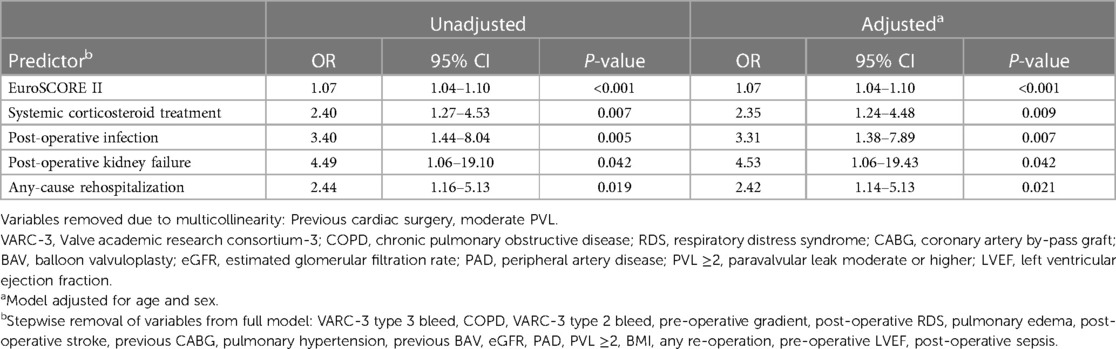
The results of the multivariable regression analysis are shown in Table 5. The variables included in the full model were selected via comparison of the 53 patients who died >30 to ≤365 days to the 787 survivors past 365 days. The 27 patients who died within the first 30 days were excluded (full analysis is available in Supplementary Material). We identified increasing EuroSCORE II, systemic corticosteroid treatment (SCT), post-operative infection, post-operative kidney failure and any-cause rehospitalization as independent predictors for early mortality.
4 Discussion
The main results of our study were as follows: (1) Periprocedural mortality declined, but early mortality remained stable over time. Due to improvements in periprocedural mortality, late mortality has also improved. (2) No significant difference was observed in late mortality stratified by sex. In relation to the age-stratified analysis, no significant differences were observed in the <70-, 70–79-, and 80–89-year age groups. A significant difference was observed when nonagenarians were included. (3) In terms of complications, an inverse relationship was found between PPI and PVL, with increasing PPI and decreasing PVL. (4) In terms of patient characteristics and comorbidities, the prevalence of most comorbidities decreased, and the risk profile tended to towards healthier patients. However, the median age of most TAVI recipients remained relatively unchanged at approximately 82 years.
When comparing our mortality data to other real-world data, a study from Germany reported periprocedural mortality rates of 6.3% in period 2 and 4.3% in period 3 (21), and other TAVI registries had comparable results to ours (22, 23). Although most registries depict improvements in periprocedural mortality and 1-year mortality, Arnold et al. demonstrated that between 2012 and 2018, the most important factors contributing to short-term outcomes were device technology and procedural factors (24). A study conducted by our group also found that TA-TAVI was a predictor of periprocedural mortality (25), comprising almost half of the procedures in period 1. In contrast with periprocedural mortality, early mortality did not improve during the study period. Our multivariable regression analysis found SCT to be the strongest predictive pre-operative dichotomous variable of early mortality. However, without having the cause of death for these patients extrapolating causal relationship is difficult. Nevertheless, we believe this to be a surrogate for long-standing chronic illness, which neither the EuroSCORE II nor the 2023 STS ACSD calculators incorporate. These insights demonstrate that identifying patients for whom TAVI is futile remains a clinical challenge. Potential reasons for this might include the low precision of the available risk score calculators (26) or lackluster assessment of frailty (27, 28). Inclusion of frailty and chronic illnesses to risk calculators can potentially aide heart teams to better identify futility, and individual pre- and rehabilitation needs. As illustrated in the landmark analysis, past 30-days the survival rate was similar, and advanced age and increased disease severity likely contributed to the higher rate of late mortality in period 1. With an unchanged early mortality rate, the biggest contributors to the improved late mortality rate are assumed to be factors that affect periprocedural mortality. However, our study's definitions of mortality were introduced by VARC-3 in 2021, making it difficult to provide direct comparisons. As TAVI has emerged as the leading modality for treating AS, a TAVI-specific risk assessment tool should be developed in future large international multicenter studies (26, 27, 29).
At our center, the late mortality was similar between sexes and age groups, excluding patients aged >90 years, throughout the study period. Although this was an unadjusted analysis, the findings imply that the comorbidities have more significant contributions to survival than the chronological age, which is supported by the existing literature (30–32). A recent study by Masiero et al. investigating sex-specific considerations in TAVI found that female-specific anatomical and pathophysiological factors require a tailored approach to minimize periprocedural risks and improve postoperative outcomes (33). With the aging of the population and the increasing application of TAVI to younger patients who generally have more comorbidities, age should not be used as a primary criterion for deciding the type of treatment in patients with AS. Instead, anatomical, and procedural factors, the burden of comorbidities, and outcome expectations should guide the shared decision-making between the patient and the heart-team.
An inverse relationship was observed between the rates of PPI and PVL after TAVI. First, the reduction in the PVL rate can be attributed to the improved diagnostic workup using multidetector CT (MDCT) rather than using TEE to reduce the prosthesis/annulus sizing mismatch (34–36). Second, technical improvements in the latest generations of THVs with outer-sealing skirts have reduced the incidence of more than moderate PVL to less than 1% (8, 11). Furthermore, procedural improvements owing to enhanced operator expertise have led to more favorable THV deployments. In relation to the increasing rates of PPI, De Torres-Alba et al. found that the 3rd generation SAPIEN 3, with its increased strut height and outer sealing skirt, led to deeper implantations than the 2nd generation SAPIEN XT (37). As 3rd and 4th generation THV exerts more pressure on the membranous septum length, it may explain the increased risk of conduction disturbances (38). Considering this, the rates of PPI for 3rd generation and 4th generation (SAPIEN 3 Ultra) BEV are similar (39), but our data shows an increase in PPI from period 2 to period 3. This finding implies there are procedural factors or changes in the patient population that could explain the increased PPI rate. Our current hypotheses to the increasing PPI rate from period 2 to period 3 are: First, the increasing use of SEVs might have contributed as SEVs are found to have higher rates of PPI than BEVs (38). Second, the possibility of slippage during the rapid pacing during BEV deployment, potentially due to the higher procedural volume (less time available per procedure) or impatience of operators in training. Third, operators facilitating for a potential valve-in-valve procedure leading to deeper placements to mitigate the risk of future coronary obstruction. In conclusion, identifying the causal explanation to period 3's increased PPI rate requires analysis of pre-operative arrhythmias, CT measurements of the membranous septum and its relation to the implantation depth (ID). As demonstrated by Sammour and colleagues, reducing the SAPIEN-3 ID by 50% led to an equal reduction in PPI and new onset left bundle branch block (40). Adopting techniques which reduce PPI, as well as improving valve design to minimize radial forces exerted onto the conduction system will be important to reduce TAVI morbidity and mortality.
A patient demographic that trends towards lower-risk patients, but with little or no change in the age comparable to the findings of similar studies (21–23, 41). This was expected, as TAVI was initially only for those deemed too high risk for SAVR. Currently, TAVI is preferred over SAVR in 75% of patients aged 65–80 years (42). These observations are supported by the results of the landmark TAVI vs. SAVR trials, which favor TAVI owing to its less invasive nature, shorter hospital stay, and quicker recovery (7–9). Meanwhile, care should be taken when interpreting the changes in comorbidities owing to the small sample in our study. However, the established risk factors for SAVR, such as previous cardiac surgery or CABG (42), have been markedly reduced, implying that more patients in the low-risk strata are being offered TAVI. Although the indications for TAVI are expanding to low-risk patients, this emphasizes the need to collaborate with a heart-team, determine the patient's preference, and establish a shared decision-making approach to develop a more tailored AS treatment.
5 Study limitations
The study has multiple limitations. First, the single-center retrospective observational design of the study might not be generalizable to other hospitals or populations. A multi-center study could provide more robust findings. The retrospective data-collection implies more missing data compared to a retrospective study design. Additionally, UNN is the regional hospital for a large geographic area comparable to the United Kingdom. Therefore, several unmeasured confounders due to remoteness are present, related to delayed diagnostics and variabilities in frequency and quality of follow-up care. Second, the relatively small sample size increases the margin of error in our predictive models, as is evident by the broad confidence intervals. Also, low statistical power and susceptibility of outliers are common problems related to small sample sizes. This is especially important with regards to our finding that early mortality has remained unchanged. These observations should be investigated in larger studies to both validate the results and properly identify areas of improvement. Third, there are several factors that limit the external validity of the study: The STS risk calculator was not used. There were no control groups, such as patients undergoing SAVR, to directly compare outcomes which could strengthen findings. Patients were selected for TAVI by the heart team based on multiple factors. This selection process could introduce bias that may impact results. However, throughout the study period there were few changes of the operators performing the procedures. That could imply that a larger part of the results and its interpretations are because of changes to device-technology and patient demographics contrary to variations of operator skills. Moreover, since our study is consistent with those of similar, larger registry-based observational studies, the volume of patients is sufficient to attain a high international standard. Lastly, our study is subject to data dredging, but adherence to the definitions and outcomes proposed by VARC-3 minimizes this risk.
6 Conclusion
As TAVI has revolutionized the treatment of severe AS in the last two decades, our findings indicate that most patients are octogenarians and that the burden of their comorbidities should be highly considered rather than their age when assessing for procedural outcomes. As most early complications of TAVI have been resolved, the rates of PPIs are high, and remains the Achilles' heel of TAVI. Important advancements in diagnostics, valve technology, and procedural techniques have improved the periprocedural mortality rates; however, early mortality remains unchanged and poses a clinical challenge that needs to be addressed in the future.
Data availability statement
The datasets presented in this article are not readily available because the data used in our study cannot be shared, even upon request. This is because they contain sensitive medical information which can compromise patient anonymity. Access to and the distribution of such data are strictly regulated by law. Requests to access the datasets should be directed to martin.p.hoydahl@uit.no.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
MPH: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. RB: Conceptualization, Data curation, Methodology, Writing – review & editing. AR: Conceptualization, Methodology, Writing – review & editing. DK: Conceptualization, Data curation, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The Arctic University of Norway (UiT) funded the publication fees.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
RB has received speakers fee from Abbott and has previously been a proctor for Edwards Lifesciences.
The remaining authors declare that the research was conducted in the absence of commercial or financial relationships that could be construed as potential conflicts of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1298346/full#supplementary-material
References
1. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. (2002) 106(24):3006–8. doi: 10.1161/01.cir.0000047200.36165.b8
2. Coffey S, Roberts-Thomson R, Brown A, Carapetis J, Chen M, Enriquez-Sarano M, et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. (2021) 18(12):853–64. doi: 10.1038/s41569-021-00570-z
3. Iung B, Vahanian A. Degenerative calcific aortic stenosis: a natural history. Heart. (2012) 98(Suppl 4):iv7–13. doi: 10.1136/heartjnl-2012-302395
4. Ramos J, Monteagudo JM, Gonzalez-Alujas T, Fuentes ME, Sitges M, Pena ML, et al. Large-Scale assessment of aortic stenosis: facing the next cardiac epidemic? Eur Heart J Cardiovasc Imaging. (2018) 19(10):1142–8. doi: 10.1093/ehjci/jex223
5. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, et al. 5-year Outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (partner 1): a randomised controlled trial. Lancet. (2015) 385(9986):2477–84. doi: 10.1016/S0140-6736(15)60308-7
6. Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, et al. 5-year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol. (2018) 72(22):2687–96. doi: 10.1016/j.jacc.2018.08.2146
7. Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, et al. Five-Year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. (2020) 382(9):799–809. doi: 10.1056/NEJMoa1910555
8. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. (2021) 77(9):1149–61. doi: 10.1016/j.jacc.2020.12.052
9. Jorgensen TH, Thyregod HGH, Ihlemann N, Nissen H, Petursson P, Kjeldsen BJ, et al. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur Heart J. (2021) 42(30):2912–9. doi: 10.1093/eurheartj/ehab375
10. Van Mieghem NM, Deeb GM, Sondergaard L, Grube E, Windecker S, Gada H, et al. Self-Expanding transcatheter vs surgical aortic valve replacement in intermediate-risk patients: 5-year outcomes of the surtavi randomized clinical trial. JAMA Cardiol. (2022) 7(10):1000–8. doi: 10.1001/jamacardio.2022.2695
11. Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 3-year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. (2023) 81(17):1663–74. doi: 10.1016/j.jacc.2023.02.017
12. Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, et al. Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med. (2023) 389(21):1949–60. doi: 10.1056/NEJMoa2307447
13. Marengo G, Elia E, Bruno F, Franchin L, Piroli F, De Filippo O, et al. Durability of transcatheter aortic valves, current evidence and future prospective. Vessel Plus. (2021) 2021:12. doi: 10.20517/2574-1209.2020.58
14. Zito A, Princi G, Lombardi M, D'Amario D, Vergallo R, Aurigemma C, et al. Long-term clinical impact of permanent pacemaker implantation in patients undergoing transcatheter aortic valve implantation: a systematic review and meta-analysis. Europace. (2022) 24(7):1127–36. doi: 10.1093/europace/euac008
15. Sa MP, Jacquemyn X, Van den Eynde J, Tasoudis P, Erten O, Sicouri S, et al. Impact of paravalvular leak on outcomes after transcatheter aortic valve implantation: meta-analysis of kaplan-meier-derived individual patient data. Struct Heart. (2023) 7(2):100118. doi: 10.1016/j.shj.2022.100118
16. Basman C, Pirelli L, Singh VP, Reimers CD, Hemli J, Brinster DR, et al. Lifetime management for aortic stenosis: planning for future therapies. J Cardiol. (2022) 80(3):185–9. doi: 10.1016/j.jjcc.2021.12.010
17. Khanji MY, Ricci F, Galusko V, Sekar B, Chahal CAA, Ceriello L, et al. Management of aortic stenosis: a systematic review of clinical practice guidelines and recommendations. Eur Heart J Qual Care Clin Outcomes. (2021) 7(4):340–53. doi: 10.1093/ehjqcco/qcab016
18. Webb JG, Blanke P, Meier D, Sathananthan J, Lauck S, Chatfield AG, et al. Tavi in 2022: remaining issues and future direction. Arch Cardiovasc Dis (2022) 115(4):235–42. doi: 10.1016/j.acvd.2022.04.001
19. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 esc/eacts guidelines for the management of valvular heart disease. Eur Heart J (2022) 43(7):561–632. doi: 10.1093/eurheartj/ehab395
20. Genereux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. (2021) 42(19):1825–57. doi: 10.1093/eurheartj/ehaa799
21. Demal TJ, Weimann J, Ojeda FM, Bhadra OD, Linder M, Ludwig S, et al. Temporal changes of patient characteristics over 12 years in a single-center transcatheter aortic valve implantation cohort. Clin Res Cardiol. (2023) 112(5):691–701. doi: 10.1007/s00392-023-02166-8
22. Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, et al. Sts-acc tvt registry of transcatheter aortic valve replacement. J Am Coll Cardiol. (2020) 76(21):2492–516. doi: 10.1016/j.jacc.2020.09.595
23. Mauri V, Abdel-Wahab M, Bleiziffer S, Veulemans V, Sedaghat A, Adam M, et al. Temporal trends of tavi treatment characteristics in high volume centers in Germany 2013-2020. Clin Res Cardiol. (2022) 111(8):881–8. doi: 10.1007/s00392-021-01963-3
24. Arnold SV, Manandhar P, Vemulapalli S, Vekstein AM, Kosinski AS, Carroll JD, et al. Mediators of improvement in tavr outcomes over time: insights from the sts-acc tvt registry. Circ Cardiovasc Interv. (2023) 16(7):e013080. doi: 10.1161/CIRCINTERVENTIONS.123.013080
25. Kjonas D, Dahle G, Schirmer H, Malm S, Eidet J, Aaberge L, et al. Predictors of early mortality after transcatheter aortic valve implantation. Open Heart. (2019) 6(1):e000936. doi: 10.1136/openhrt-2018-000936
26. Kjonas D, Dahle G, Schirmer H, Malm S, Eidet J, Aaberge L, et al. Risk scores for prediction of 30-day mortality after transcatheter aortic valve implantation: results from a two-center study in Norway. Health Sci Rep. (2021) 4(2):e283. doi: 10.1002/hsr2.283
27. Puri R, Iung B, Cohen DJ, Rodes-Cabau J. Tavi or No tavi: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J. (2016) 37(28):2217–25. doi: 10.1093/eurheartj/ehv756
28. Afilalo J, Lauck S, Kim DH, Lefevre T, Piazza N, Lachapelle K, et al. Frailty in older adults undergoing aortic valve replacement: the frailty-avr study. J Am Coll Cardiol. (2017) 70(6):689–700. doi: 10.1016/j.jacc.2017.06.024
29. Al-Azizi K, Shih E, DiMaio JM, Squiers JJ, Moubarak G, Kluis A, et al. Assessment of tvt and sts risk score performances in patients undergoing transcatheter aortic valve replacement. J Soc Cardiovasc Angiogr Interv. (2023) 2(3):100600. doi: 10.1016/j.jscai.2023.100600
30. van der Kley F, van Rosendael PJ, Katsanos S, Kamperidis V, Marsan NA, Karalis I, et al. Impact of age on transcatheter aortic valve implantation outcomes: a comparison of patients aged ≤80 years versus patients >80 years. J Geriatr Cardiol. (2016) 13(1):31–6. doi: 10.11909/j.issn.1671-5411.2016.01.004
31. Lantelme P, Aubry M, Peng JC, Riche B, Souteyrand G, Jaafar P, et al. Comorbidities may offset expected improved survival after transcatheter aortic valve replacement. Eur Heart J Open. (2022) 2(3):oeac029. doi: 10.1093/ehjopen/oeac029
32. van den Brink FS, Wijtsma I, Amrane H, Vossenberg TNE, Haenen J, Porta F, et al. Outcome of transcatheter aortic valve replacement in patients over 85 years of age versus patients aged 85 and younger. Neth Heart J. (2022) 30(10):473–8. doi: 10.1007/s12471-022-01693-9
33. Masiero G, Paradies V, Franzone A, Bellini B, De Biase C, Karam N, et al. Sex-Specific considerations in degenerative aortic stenosis for female-tailored transfemoral aortic valve implantation management. J Am Heart Assoc. (2022) 11(19):e025944. doi: 10.1161/JAHA.121.025944
34. Leipsic J, Gurvitch R, Labounty TM, Min JK, Wood D, Johnson M, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging. (2011) 4(4):416–29. doi: 10.1016/j.jcmg.2011.01.014
35. Sakrana AA, Nasr MM, Ashamallah GA, Abuelatta RA, Naeim HA, Tahlawi ME. Paravalvular leak after transcatheter aortic valve implantation: is it anatomically predictable or procedurally determined? Mdct study. Clin Radiol. (2016) 71(11):1095–103. doi: 10.1016/j.crad.2016.07.016
36. Mylotte D, Dorfmeister M, Elhmidi Y, Mazzitelli D, Bleiziffer S, Wagner A, et al. Erroneous measurement of the aortic annular diameter using 2-dimensional echocardiography resulting in inappropriate corevalve size selection: a retrospective comparison with multislice computed tomography. JACC Cardiovasc Interv. (2014) 7(6):652–61. doi: 10.1016/j.jcin.2014.02.010
37. De-Torres-Alba F, Kaleschke G, Vormbrock J, Orwat S, Radke R, Feurle M, et al. Delayed pacemaker requirement after transcatheter aortic valve implantation with a new-generation balloon expandable valve: should we monitor longer? Int J Cardiol. (2018) 273:56–62. doi: 10.1016/j.ijcard.2018.07.131
38. Sammour Y, Krishnaswamy A, Kumar A, Puri R, Tarakji KG, Bazarbashi N, et al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2021) 14(2):115–34. doi: 10.1016/j.jcin.2020.09.063
39. Nazif TM, Cahill TJ, Daniels D, McCabe JM, Reisman M, Chakravarty T, et al. Real-world experience with the sapien 3 ultra transcatheter heart valve: a propensity-matched analysis from the United States. Circ Cardiovasc Interv. (2021) 14(9):e010543. doi: 10.1161/CIRCINTERVENTIONS.121.010543
40. Sammour Y, Banerjee K, Kumar A, Lak H, Chawla S, Incognito C, et al. Systematic approach to high implantation of sapien-3 valve achieves a lower rate of conduction abnormalities including pacemaker implantation. Circ Cardiovasc Interv. (2021) 14(1):e009407. doi: 10.1161/CIRCINTERVENTIONS.120.009407
41. Graversen PL, Butt JH, Ostergaard L, Jensen AD, Warming PE, Strange JE, et al. Changes in aortic valve replacement procedures in Denmark from 2008 to 2020. Heart. (2023) 109(7):557–63. doi: 10.1136/heartjnl-2022-321594
Keywords: transcatheter aortic valve implantation, transcatheter aortic valve replacement, aortic stenosis, mortality, complications
Citation: Høydahl MP, Busund R, Rösner A and Kjønås D (2024) Transcatheter aortic valve implantation (from inception to standard treatment): a single-center observational study. Front. Cardiovasc. Med. 11:1298346. doi: 10.3389/fcvm.2024.1298346
Received: 21 September 2023; Accepted: 4 January 2024;
Published: 15 January 2024.
Edited by:
Masahiko Asami, Mitsui Memorial Hospital, JapanReviewed by:
Mohanad Hamandi, University of Pittsburgh Medical Center, United StatesAndreas Mitsis, Nicosia General Hospital, Cyprus
© 2024 Høydahl, Busund, Rösner and Kjønås. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Petter Høydahl martin.p.hoydahl@uit.no
 Martin Petter Høydahl
Martin Petter Høydahl Rolf Busund1,2
Rolf Busund1,2  Didrik Kjønås
Didrik Kjønås