Effect of statin add-on therapy on cardiovascular mortality
- 1California Cardiovascular Institute, Fresno, CA, United States
- 2Intermountain Heart Institute, Intermountain Health, Salt Lake, UT, United States
- 3Department of Physician Assistant Studies, College of Medical and Health Professional Science, Rocky Mountain University of Health Professions, Provo, UT, United States
- 4Arizona Center for Advanced Lipidology, Tucson, AZ, United States
Introduction: Cardiovascular (CV) disease remains a leading cause of mortality despite statin therapy. Statin add-on lipid-lowering therapies have been investigated for CV risk reduction, but their effect on CV mortality has not been reviewed.
Methods: This review describes CV outcomes trials of add-on therapies to statins, highlighting findings related to the primary composite CV endpoints and the more patient-centric endpoint of CV-related mortality.
Results: Add-on ezetimibe met its primary composite CV endpoint vs. statin alone (P = 0.016); however, the individual endpoint of death from CV causes did not differ between groups. Add-on therapy with proprotein convertase subtilisin/kexin type 9 inhibitors achieved the primary composite CV endpoints in the respective CV outcomes trials for alirocumab (P < 0.001) and evolocumab (P < 0.001); however, neither CV outcomes trial found a difference vs. placebo in CV-related mortality. In its CV outcomes trial, icosapent ethyl added to statin therapy significantly reduced the occurrence of the primary composite CV endpoint (P < 0.001) and the individual endpoint of risk of CV-related death (P = 0.03) vs. placebo. A CV outcomes trial of bempedoic acid monotherapy achieved its primary composite CV endpoint vs. placebo (P = 0.004) but not the endpoint of death from CV causes.
Discussion: Statin add-on therapies achieved their CV outcomes trial composite CV endpoints. Proprotein convertase subtilisin/kexin type 9 inhibitors and icosapent ethyl have approved indications for CV risk reduction. Only add-on therapy with icosapent ethyl demonstrated a significant reduction in CV mortality in the overall intent-to-treat population, possibly due to the unique pleiotropic mechanisms of eicosapentaenoic acid independent of lipid-lowering effects.
1 Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, accounting for 32% of deaths (1). Globally, 17.9 million CVD-related deaths occurred in 2019, most (85%) due to myocardial infarction (MI) and stroke (1). In the United States, heart disease was the leading cause of death in 2021, with an age-adjusted death rate of 173.8 per 100,000 persons, and stroke was the fifth leading cause of death with a rate of 41.1 per 100,000 persons (2).
For more than 3 decades, the lowering of low-density lipoprotein cholesterol (LDL-C) levels has been the primary treatment target for primary and secondary prevention of cardiovascular (CV) events, with statins at the forefront of CV risk reduction (3, 4). Despite achievement of LDL-C targets with high-dose statins, residual CV risk, including CV-related mortality risk, persists for many patients (5–7). Thus, adjunctive therapy to statins to achieve additional CV risk reduction is an area of clinical and research interest. Several novel, add-on lipid-lowering therapies have been approved in the post-statin era, including ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, icosapent ethyl (IPE), and bempedoic acid (8–12). The potential for CV risk reduction with these novel therapies has been investigated in landmark CV outcomes trials (13–17).
Cardiovascular outcomes trials typically assess efficacy using time-to-event analysis with the primary composite endpoint defined as time from randomization to time of first occurrence of any component of the composite endpoint (18). For example, a composite endpoint such as a major adverse cardiac event could include CV-related death or reinfarction, target vessel revascularization for ischemia, or stroke (18). From methodologic and regulatory standpoints, composite endpoints are pragmatic because they help ensure that sample sizes are not prohibitively high and durations of follow-up are not unreasonably long (18, 19). Use of composite endpoints increases the numbers of events that are captured and, consequently, increases statistical power and precision (19). A downside of using composite endpoints is that inclusion of components that may be less affected by the treatment can dilute or mask true treatment effects (18, 19); likewise, treatment effects on individual components of the composite endpoint may not be readily discerned. Although composite CV endpoints are useful in research, they are not particularly “patient-centric” and may not accurately reflect what patients deem to be the most important outcome(s) of an intervention (20, 21). We considered that the impact of add-on therapy on CV-related mortality [encompassing sudden cardiac death or death due to either acute MI, heart failure, stroke, CV procedure, CV hemorrhage, other CV causes (22)] may be a more patient-centric endpoint than a composite CV endpoint that encompasses CV-related mortality along with nonfatal CV events and CV procedures.
This brief review discusses CV outcomes trials of novel add-on therapies to statins, highlighting evidence for overall CV risk reduction and for CV-related mortality risk reduction.
2 Methods
A search of the contemporary literature (2013–2023) was conducted in PubMed/MEDLINE and clinicaltrials.gov to identify clinical trials that investigated the endpoints of CV risk reduction and CV-related mortality risk reduction among patients treated with add-on therapy to statins. Articles were limited to those in English. The keywords for the literature search were: cardiovascular outcomes, statin therapy, and cardiovascular mortality. A total of 12 clinical trials were identified between 2013 and 2023 and discussed in this review.
3 Cardiovascular outcomes trials of statin add-on therapy
3.1 Ezetimibe
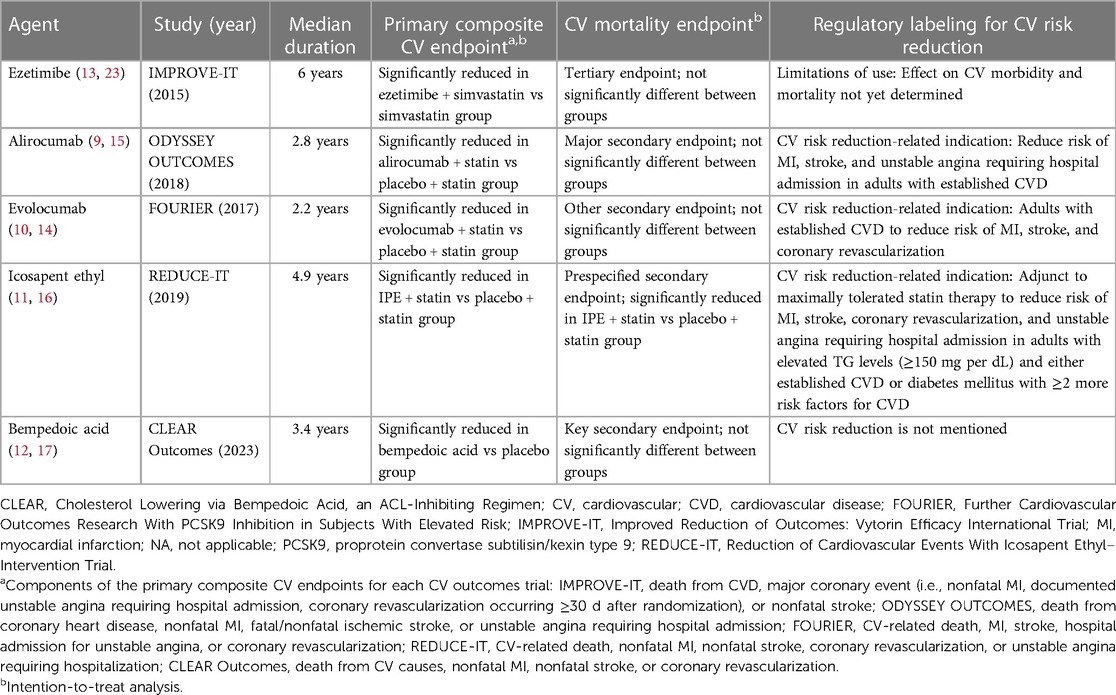
Ezetimibe, a selective inhibitor of intestinal cholesterol absorption, was first approved by the US Food and Drug Administration (FDA) in 2002 as a lipid-lowering agent, with indications that included adjunctive therapy to diet for reduction of elevated total cholesterol, LDL-C, and apolipoprotein B levels in patients with primary hyperlipidemia alone or in combination with a statin (23, 24). Subsequently, lipid-lowering indications were further expanded, including for reduction of non–high-density lipoprotein cholesterol levels alone or in combination with statins in patients with primary hyperlipidemia or in combination with fenofibrate in patients with mixed hyperlipidemia (23).
The landmark Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) was a double-blind, event-driven, randomized study of 18,144 patients aged 50 years or older who had been hospitalized for acute coronary syndrome (acute MI; high-risk unstable angina) within the prior 10 days (13). Patients on long-term lipid-lowering therapy were required to have an LDL-C level between 50 mg per dL and 100 mg per dL and patients not receiving long-term lipid-lowering therapy were required to have an LDL-C level between 50 mg per dL and 125 mg per dL. Patients were randomized to receive ezetimibe 10 mg in combination with simvastatin 40 mg vs. simvastatin 40 mg and placebo to assess impact on CV outcomes over 7 years (13).
At 1 year, levels of LDL-C decreased from 93.8 mg per dL at baseline (both groups) to 53.2 mg per dL and 69.9 mg per dL in the ezetimibe plus simvastatin group and the simvastatin monotherapy groups, respectively (significant difference between groups; P < 0.001) (13). These LDL-C reductions were maintained over the course of the 7-year study.
The combination treatment met its primary composite endpoint, which was assessed from randomization to the first occurrence of death from CVD, major coronary event (i.e., nonfatal MI, documented unstable angina requiring hospital admission, coronary revascularization occurring ≥30 days after randomization), or nonfatal stroke (Table 1) (13). The event rate for the primary composite endpoint was 32.7% in the combination ezetimibe and simvastatin group vs. 34.7% in the simvastatin monotherapy group (HR 0.936; 95% CI, 0.89–0.99; P = 0.016) (13). Although ezetimibe met its primary composite endpoint of CV risk reduction, it is only approved by the US FDA for the reduction of lipid levels and is not approved for the reduction of CV risk (23).
Death from CV causes was a tertiary endpoint in IMPROVE-IT (13). When death from CV causes was separately considered from the primary composite endpoint, this component was not significantly different in the combination ezetimibe and simvastatin vs. statin monotherapy group (event rates of 6.9% vs. 6.8%, respectively; HR 1.0; 95% CI, 0.89–1.13; P = 1.00) (Figure 1) (13).
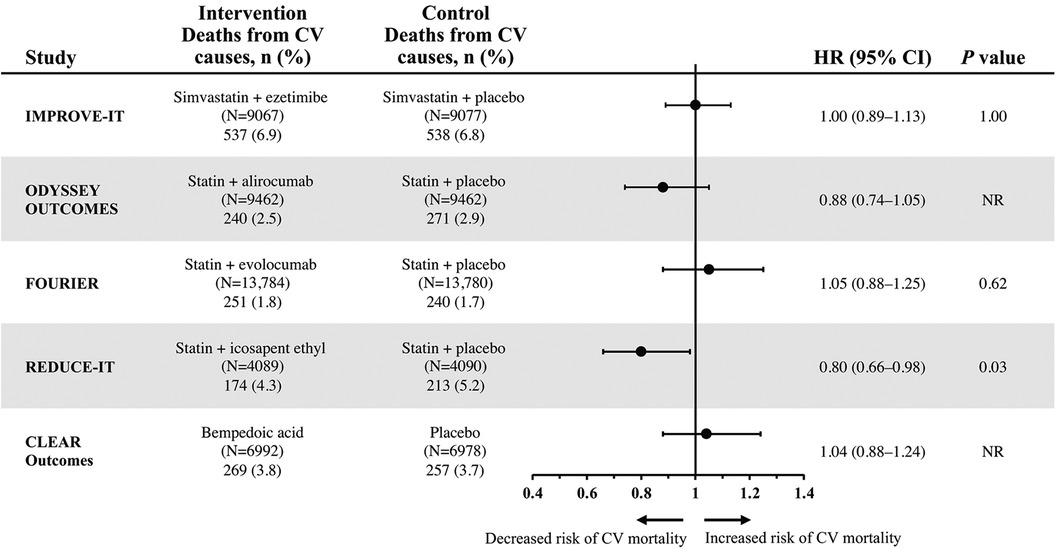
Figure 1. Hazard ratios of cardiovascular mortality for select lipid-lowering regimens from individual CV outcomes trials (13–17). CV, cardiovascular; NR, not reported.
3.2 Proprotein convertase subtilisin/kexin type 9 inhibitors
3.2.1 Alirocumab
In 2015, the FDA approved the monoclonal antibody alirocumab, a PCSK9 inhibitor, as an adjunct to diet and maximally tolerated statin therapy for treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic CVD who require additional LDL-C lowering (9, 24). Subsequently, alirocumab received approval for CV risk reduction, specifically to reduce the risk of MI, stroke, and unstable angina requiring hospital admission in adults with established CVD (9). Approval of alirocumab for CV risk reduction was based on the pivotal, multicenter, event-driven, randomized, double-blind, placebo-controlled ODYSSEY OUTCOMES trial, which involved 18,924 patients aged 40 years or older hospitalized with acute coronary syndrome who were receiving maximally tolerated doses of either atorvastatin or rosuvastatin as well as add-on therapy with either alirocumab 75 mg or placebo every 2 weeks over a median of 2.8 years (15).
Mean LDL-C levels in the alirocumab add-on group were markedly reduced from 92 mg per dL at baseline to 40 mg per dL at four months, and LDL-C levels in the alirocumab group increased to 66 mg per dL by 48 months, remaining well below baseline levels; by contrast, LDL-C levels in the placebo add-on group increased to 103 mg per dL over the same time period (15). The ODYSSEY OUTCOMES trial met its primary composite endpoint, which included death from coronary heart disease, nonfatal MI, fatal or nonfatal ischemic stroke, or unstable angina requiring hospital admission (Table 1). Event rates for the primary composite endpoint were 9.5% in the alirocumab add-on group and 11.1% in the placebo add-on group (HR 0.85; 95% CI, 0.78–0.93; P < 0.001). Death from CV-related causes, a major secondary endpoint in ODYSSEY OUTCOMES, did not significantly differ between the treatment groups. Event rates were 2.5% in the alirocumab add-on group compared with 2.9% in the placebo add-on group (HR 0.88; 95% CI, 0.74–1.05) (Figure 1). No P value was reported for this CV mortality endpoint because hierarchical analysis was stopped after the first nonsignificant P value was observed for death from coronary heart disease (P = 0.38) (15).
3.2.2 Evolocumab
The monoclonal antibody evolocumab, a PCSK9 inhibitor, received FDA approval in 2015 (10). Initial indications included use as an adjunct to diet added to (a) maximally tolerated statin therapy for treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic CVD who require additional LDL-C lowering or (b) to other LDL-lowering therapies (e.g., statins, ezetimibe) in patients with homozygous familial hypercholesterolemia who require additional LDL-C lowering (10, 24). In 2021, an indication of CV risk reduction was added for evolocumab in adults with established CVD to reduce the risk of MI, stroke, and coronary revascularization (10). Findings from the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) were instrumental in helping evolocumab receive regulatory approval for CV risk reduction (10, 14).
FOURIER was a multinational, randomized, double-blind, placebo-controlled, event-driven CV outcomes trial that assessed add-on therapy with evolocumab (140 mg every 2 weeks or 420 mg monthly) vs. add-on placebo in 27,564 patients treated with statins who were aged between 40 and 85 years with clinically evident atherosclerotic CVD (i.e., diagnosis of MI or nonhemorrhagic stroke, symptomatic peripheral artery disease) as well as additional CV risk factors (e.g., age ≥65 years at randomization, current cigarette use, diabetes) for a median follow-up of approximately 2.2 years (14). Patients were required to be receiving an “optimized regimen” of lipid-lowering therapy (i.e., high-intensity statin with or without ezetimibe). In the evolocumab add-on group, median LDL-C levels decreased substantially from 92 mg per dL at baseline to 30 mg per dL within the first 12 weeks of treatment, with maintenance of this LDL-C reduction over time. Levels of LDL-C were relatively unchanged in the placebo add-on group. The primary endpoint, a composite of CV-related death, MI, stroke, hospital admission for unstable angina, or coronary revascularization, had a significantly lower event rate of 9.8% in patients in the evolocumab add-on group vs. 11.3% in patients in the placebo add-on group (HR 0.85; 95% CI, 0.79–0.92; P < 0.001) (Table 1) (14).
However, the individual component of CV-related death, which was one of several secondary endpoints in FOURIER, was not significantly reduced, with event rates of 1.8% in patients in the evolocumab add-on group vs. 1.7% in the placebo add-on group (HR 1.05; 95% CI, 0.88–1.25; P = 0.62) (Figure 1) (14).
3.3 Icosapent ethyl
Icosapent ethyl is a highly purified form of the omega-3 fatty acid eicosapentaenoic acid (EPA) ethyl ester that initially received FDA approval in 2012 as an adjunct to diet to reduce triglyceride (TG) levels in adults with severe hypertriglyceridemia (11, 24). Following the results of the Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial (REDUCE-IT) (16) in 2019, IPE received FDA approval for an indication related to CV risk reduction: as an adjunct to maximally tolerated statin therapy to reduce the risk of MI, stroke, coronary revascularization, and unstable angina requiring hospital admission in adults with elevated TG levels (≥150 mg per dL) and either established CVD or diabetes mellitus with at least 2 additional risk factors for CVD (11, 16).
REDUCE-IT was a phase 3b, multicenter, event-driven, randomized, double-blind, placebo-control led trial that compared add-on therapy with IPE 4 g vs. addition of placebo in 8179 patients treated with statins aged 45 years or older with established CVD (secondary prevention) or aged 50 or older years with diabetes and at least one additional risk factor (primary prevention) over a median of 4.9 years (16). Eligible patients had fasting TG levels 150 to 499 mg per dL and LDL-C levels 41 to 100 mg per dL while receiving a stable dose of statin for at least 4 weeks. The median LDL-C level at baseline was 75 mg per dL. Overall, 71% of the patient population had established CVD and 58% had type 2 diabetes mellitus (16).
The primary composite endpoint was a time-to-event analysis, including CV-related death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina (16). Add-on therapy with IPE significantly reduced the occurrence of the primary composite endpoint vs. addition of placebo, with event rates of 17.2% vs. 22% (HR 0.75; 95% CI, 0.68–0.83; P < 0.001) (Table 1). Notably, IPE also significantly reduced the risk of CV-related death, a prespecified secondary endpoint, with event rates of 4.3% in the IPE add-on group vs. 5.2% in the placebo group (HR 0.80; 95% CI, 0.66–0.98; P = 0.03) (Figure 1) (16).
3.4 Bempedoic acid
In 2020, the FDA approved the adenosine triphosphate citrate lyase inhibitor bempedoic acid as an adjunct to diet and maximally tolerated statin therapy for treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic CVD who require additional lowering of LDL-C levels (12, 25). Approval was based on results from 2 pivotal phase 3, randomized, double-blind, placebo-controlled clinical trials, namely Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen [CLEAR] Harmony (26) and CLEAR Wisdom (27). CLEAR Harmony and CLEAR Wisdom included 2,230 and 779 patients, respectively, with atherosclerotic CVD, heterozygous familial hypercholesterolemia, or both, who were receiving stable doses of maximally tolerated lipid-lowering therapy (26, 27). While these were lipid-lowering studies, both reported rates of CV-related deaths among their adverse events. No significant differences were found in CV-related mortality between the add-on bempedoic acid and placebo groups in either study (26, 27).
Recently, a CV outcomes trial was conducted to evaluate whether bempedoic acid may reduce CV risk. In the 2023, event-driven, CLEAR Outcomes trial, 13,970 patients intolerant to statins were randomized to receive bempedoic acid 180 mg or placebo daily over a median of 40.6 months (17). After 6 months of treatment, LDL-C levels were reduced from the mean baseline level of 139 mg per dL to 107 mg per dL in the bempedoic acid group, while LDL-C level was relatively unchanged at 136 mg per dL in the placebo group. The primary composite endpoint included death from CV causes, nonfatal MI, nonfatal stroke, or coronary revascularization. The CLEAR Outcomes achieved its primary composite efficacy endpoint, with significantly lower event rates of 11.7% in the bempedoic acid group vs. 13.3% of patients in the placebo group (HR 0.87; 95% CI, 0.79–0.96; P = 0.004) (Table 1). By contrast, death from CV causes, a key secondary endpoint, was not significantly different between groups, with event rates of 3.8% and 3.7% in the bempedoic acid and placebo groups, respectively (HR 1.04; 95% CI, 0.88–1.24) (Figure 1). No P value was reported for this CV mortality endpoint because hierarchical analysis was stopped after the first nonsignificant P value was observed for fatal or nonfatal stroke (P = 0.16) (17).
3.5 Ongoing CV outcomes trials
Clinical trials evaluating CV outcomes are currently underway for several novel add-on lipid-lowering agents. The ORION-4 and VICTORION-2 Prevent trials are ongoing to determine whether treatment with inclisiran, a small interfering ribonucleic acid that inhibits hepatic PCSK9 production, decreases the risk of CV events (28). The PREVAIL trial is currently investigating whether obicetrapib, a cholesteryl ester transfer protein inhibitor, lowers the risk of CV events in patients with CVD on maximally tolerated lipid-modifying therapy (29, 30). The impact of a reduction in lipoprotein (a) on CV outcomes is being investigated in two clinical trials: Lp(a) Horizon assesses pelacarsen, an antisense oligonucleotide, among patients with established CVD, and OCEAN(a) investigates olpasiran, a small interfering ribonucleic acid, among patients with prior MI or percutaneous coronary intervention (31–33).
4 Discussion
For clinicians caring for patients with established atherosclerotic CVD or with CV risk factors, knowledge that one of their patients has succumbed to a CV event brings into focus the finality of death. This stark reminder serves as a motivator to continue to strive for prevention of CV-related death. A comprehensive approach is essential and should not only optimize CV risk factors such as lipid levels, glucose levels, and blood pressure, but it should also address residual CV risk using add-on therapies.
This review found that ezetimibe, evolocumab, alirocumab, and IPE are effective, add-on, lipid-modulating therapies that can address the residual CV risk that persists in patients treated with statins. Additionally, bempedoic acid reduces CV risk in patients intolerant to statins. Each of these therapies has demonstrated significant reduction in CV risk in their respective CV outcomes trials, as measured by primary composite CV endpoints (13–17); however, when they are separately examined, the more patient-centric outcome of CV-related mortality was only significantly reduced by IPE (Figure 1) (13–17). The impact of IPE in total CVD event reduction in REDUCE-IT was substantial; for every 1,000 patients treated with IPE over 5 years, approximately 159 total primary endpoints could be prevented, including 12 CV deaths, 42 MIs, 14 strokes, 76 coronary revascularizations, and 16 hospitalizations for unstable angina (34). Unlike other add-on therapies that primarily exert LDL-C-lowering effects, it is thought that the mechanisms underlying CV risk reduction with IPE are due to pleiotropic effects of EPA that extend beyond cholesterol and TG lowering. These pleiotropic effects of EPA have been extensively reviewed and include anti-inflammatory, anti-oxidation, anti-arrhythmic, anti-thrombotic, and anti-platelet mechanisms and cell membrane stability/signaling effects (35, 36). Their importance is supported by the finding in REDUCE-IT that changes in serum EPA levels were associated with most of the observed CV risk reduction, with only minimal contribution by changes in TG, LDL-C, high-density lipoprotein cholesterol (HDL-C), non-HDL-C, apolipoprotein B, high-sensitivity C-reactive protein, and remnant lipoprotein cholesterol (35, 37, 38). Risk reductions with IPE were also similar across baseline LDL-C level categories, confirming LDL-C independent pathways are involved in its mechanism of action, in contrast to the other statin add-on agents described in this review. Of note, IPE is the only omega-3 fatty acid drug approved by the FDA to reduce CV risk, with consistent CV benefit and trends observed across studies, including REDUCE-IT (11, 16), the recent Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy–Statin and Eicosapentaenoic Acid (RESPECT-EPA) (39), and in an earlier EPA trial, the Japan EPA Lipid Intervention Study (40). Utilizing REDUCE-IT US event rates, FDA eligibility criteria, and National Health and Nutrition Examination Survey data, an estimated 4.6 million US adults would be eligible for IPE and 27,377 CV deaths would be prevented over 4.9 years (41, 42).
Author contributions
JRN: Writing – review & editing. VL: Writing – review & editing. JLA: Writing – review & editing. NC: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This review was sponsored by Amarin Pharma, Inc.
Acknowledgments
Medical writing and editorial support were provided by Kulvinder Singh, PharmD, Noud van Helmond, MD, PhD, and Sherri Damlo, MS, ELS at Peloton Advantage, LLC, an OPEN Health company (Parsippany, NJ), and were funded by Amarin Pharma, Inc.
Conflict of interest
JRN—Speakers bureau for Amarin and Amgen; stock shareholder for Amgen; advisory board for Amarin. VL—Research grant paid to department from Janssen; consultant for Novartis. JLA—Site investigator for the ongoing ORION studies of inclisiran and the HORIZON study of pelacarsen, sponsored by Novartis; past investigator for trials for Milestone and SomaLogic; member of the Data and Safety Monitoring Board for a Faraday Pharma trial (receives no personal income). NC—Consultant for Novartis and Amarin; speaker for Amgen; speaker consultant for Regeneron.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cardiovascular diseases (CVDs): World Health Organization (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (December 20, 2023).
2. Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2021. NCHS Data Brief, no 456. Hyattsville, MD, USA: National Center for Health Statistics (2022).
3. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. (2017) 38:2459–72. doi: 10.1093/eurheartj/ehx144
4. Harrington RA. Statins-Almost 30 years of use in the United States and still not quite there. JAMA Cardiol. (2017) 2:66. doi: 10.1001/jamacardio.2016.4709
5. Wong ND, Zhao Y, Quek RGW, Blumenthal RS, Budoff MJ, Cushman M, et al. Residual atherosclerotic cardiovascular disease risk in statin-treated adults: the multi-ethnic study of atherosclerosis. J Clin Lipidol. (2017) 11:1223–33. doi: 10.1016/j.jacl.2017.06.015
6. Fruchart JC, Davignon J, Hermans MP, Al-Rubeaan K, Amarenco P, Assmann G, et al. Residual macrovascular risk in 2013: what have we learned? Cardiovasc Diabetol. (2014) 13:26. doi: 10.1186/1475-2840-13-26
7. Stone NJ. Reducing residual risk in secondary prevention of cardiovascular disease. Circulation. (2012) 125:1958–60. doi: 10.1161/CIRCULATIONAHA.112.101782
8. Rizzo M, Wierzbicki AS. New lipid modulating drugs: the role of microsomal transport protein inhibitors. Curr Pharm Des. (2011) 17:943–9. doi: 10.2174/138161211795428768
13. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. (2015) 372:2387–97. doi: 10.1056/NEJMoa1410489
14. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. (2017) 376:1713–22. doi: 10.1056/NEJMoa1615664
15. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. (2018) 379:2097–107. doi: 10.1056/NEJMoa1801174
16. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. (2019) 380:11–22. doi: 10.1056/NEJMoa1812792
17. Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. (2023) 388:1353–64. doi: 10.1056/NEJMoa2215024
18. Gómez G, Gómez-Mateu M, Dafni U. Informed choice of composite end points in cardiovascular trials. Circ Cardiovasc Qual Outcomes. (2014) 7:170–8. doi: 10.1161/CIRCOUTCOMES.113.000149
19. Marx N, McGuire DK, Perkovic V, Woerle HJ, Broedl UC, von Eynatten M, et al. Composite primary End points in cardiovascular outcomes trials involving type 2 diabetes patients: should unstable angina be included in the primary end point? Diabetes Care. (2017) 40:1144–51. doi: 10.2337/dc17-0068
20. Fanaroff AC, Cyr D, Neely ML, Bakal J, White HD, Fox KAA, et al. Days alive and out of hospital: exploring a patient-centered, pragmatic outcome in a clinical trial of patients with acute coronary syndromes. Circ Cardiovasc Qual Outcomes. (2018) 11:e004755. doi: 10.1161/circoutcomes.11.suppl_1.3
21. Stolker JM, Spertus JA, Cohen DJ, Jones PG, Jain KK, Bamberger E, et al. Rethinking composite end points in clinical trials: insights from patients and trialists. Circulation. (2014) 130:1254–61. doi: 10.1161/CIRCULATIONAHA.113.006588
22. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. (2018) 137:961–72. doi: 10.1161/CIRCULATIONAHA.117.033502
24. Drugs@FDA: FDA-Approved Drugs: U.S. Food and Drug Administration (2023). Available online at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm (accessed February 20, 2024).
25. Cicero AFG, Fogacci F, Cincione I. Evaluating pharmacokinetics of bempedoic acid in the treatment of hypercholesterolemia. Expert Opin Drug Metab Toxicol. (2021) 17:1031–8. doi: 10.1080/17425255.2021.1951222
26. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. (2019) 380:1022–32. doi: 10.1056/NEJMoa1803917
27. Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA. (2019) 322:1780–8. doi: 10.1001/jama.2019.16585
28. Ray KK, Raal FJ, Kallend DG, Jaros MJ, Koenig W, Leiter LA, et al. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. (2023) 44:129–38. doi: 10.1093/eurheartj/ehac594
29. Cardiovascular Outcome Study to Evaluate the Effect of Obicetrapib in PatientsWith Cardiovascular Disease (PREVAIL) NCT05202509: ClinicalTrials.gov (2023). Available online at: https://clinicaltrials.gov/study/NCT05202509?cond=nct05202509&rank=1 (accessed February 20, 2024).
30. Nelson AJ, Sniderman AD, Ditmarsch M, Dicklin MR, Nicholls SJ, Davidson MH, et al. Cholesteryl ester transfer protein inhibition reduces Major adverse cardiovascular events by lowering apolipoprotein B levels. Int J Mol Sci. (2022) 23(16):9417. doi: 10.3390/ijms23169417
31. Assessing the Impact of Lipoprotein (a) Lowering With Pelacarsen (TQJ230) on Major Cardiovascular Events in Patients With CVD (Lp(a)HORIZON) NCT04023552: ClinicalTrials.gov (2023). Available online at: https://clinicaltrials.gov/ct2/show/NCT04023552 (accessed February 20, 2024).
32. Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. (2022) 42:e48–60. doi: 10.1161/ATV.0000000000000147
33. Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction (OCEAN(a))—Outcomes Trial NCT05581303: ClinicalTrials.gov (2024). Available online at: https://clinicaltrials.gov/ct2/show/NCT05581303 (accessed February 20, 2024).
34. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. (2019) 73:2791–802. doi: 10.1016/j.jacc.2019.02.032
35. Nelson JR, Budoff MJ, Wani OR, Le V, Patel DK, Nelson A, et al. EPA’s pleiotropic mechanisms of action: a narrative review. Postgrad Med. (2021) 133:651–64. doi: 10.1080/00325481.2021.1921491
36. Trivedi K, Le V, Nelson JR. The case for adding eicosapentaenoic acid (icosapent ethyl) to the ABCs of cardiovascular disease prevention. Postgrad Med. (2021) 133:28–41. doi: 10.1080/00325481.2020.1783937
37. Bhatt DL, Miller M, Steg G, Brinton EA, Jacobson TA, Ketchum SB, et al., editors. EPA levels and cardiovascular outcomes in the reduction of cardiovascular events with icosapent ethyl-intervention trial [oral presentation]. Annual Scientific Session of the American College of Cardiology (2020) March 28-30, 2020; Chicago, IL.
38. Szarek M, Bhatt D, Miller M, Steg PG, Brinton EA, Jacobson TA, et al. Eicosapentaenoic acid, arachidonic acid, and triglyceride levels mediate most of the benefit of icosapent ethyl in REDUCE-IT [abstract]. Eur Heart J. (2023) 44(suppl 2):1309.
39. Daida H, Nishizaki Y, Iwata H, Inoue T, Hirayama A, Kimura K, et al., editors. Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy—Statin and Eicosapentaenoic Acid (RESPECT-EPA) [oral presentation]. Annual Scientific Sessions of the American Heart Association (2022). November 5-7, 2022; Chicago, IL.
40. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. (2007) 369:1090–8. doi: 10.1016/S0140-6736(07)60527-3
41. Bhatt DL, Miller M, Brinton EA, Jacobson TA, Steg G, Ketchum SB, et al. REDUCE-IT USA: results from the 3,146 patients randomized in the United States. Circulation. (2020) 141:367–75. doi: 10.1161/CIRCULATIONAHA.119.044440
Keywords: cardiovascular mortality, bempedoic acid, eicosapentaenoic acid, ezetimibe, icosapent ethyl, proprotein convertase subtilisin/kexin type 9 inhibitors
Citation: Nelson JR, Le V, Anderson JL and Ciffone N (2024) Effect of statin add-on therapy on cardiovascular mortality. Front. Cardiovasc. Med. 11:1308695. doi: 10.3389/fcvm.2024.1308695
Received: 6 October 2023; Accepted: 27 February 2024;
Published: 13 March 2024.
Edited by:
Matthew Budoff, Lundquist Institute for Biomedical Innovation, United StatesReviewed by:
Federica Fogacci, University of Bologna, ItalyFlorentina Ligia Furtunescu, Carol Davila University of Medicine and Pharmacy, Romania
© 2024 Nelson, Le, Anderson and Ciffone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John R. Nelson jr4nelson@yahoo.com
 John R. Nelson
John R. Nelson Viet Le
Viet Le Jeffrey L. Anderson2
Jeffrey L. Anderson2