Impact of the MIF -173G/C variant on cardiovascular disease risk: a meta-analysis of 9,047 participants
- 1Department of Biomedical Sciences, College of Health Sciences, QU Health, Qatar University, Doha, Qatar
- 2Human Nutrition Department, College of Health Sciences, QU Health, Qatar University, Doha, Qatar
- 3Pediatric Department, College of Medicine, Taibah University, King Faisal Specialist Hospital, Al-Madinah, Saudi Arabia
- 4Pediatric Department, Security Forces Medical Centre, Al-Madinah, Saudi Arabia
Introduction: Many factors contribute to the risk of cardiovascular disease (CVD), an umbrella term for several different heart diseases, including inflammation. Macrophage migration inhibitory factor (MIF) is an important immune modulator that has been shown to be involved in the pathogenesis of different heart diseases, so understanding pathogenic variants of the MIF gene is important for risk stratification. We therefore conducted a meta-analysis to investigate whether the MIF -173G/C (rs755622) polymorphism is associated with CVD.
Methods: The PubMed, Science Direct, and Embase databases were searched from inception to June 2023 for case-control studies of the MIF -173G/C polymorphism and its relationship to any type of CVD. Correlations between the MIF -173G/C polymorphism and CVD were estimated by pooling the odds ratios (ORs) with 95% confidence intervals in allelic, dominant, and recessive models using random-effects meta-analysis.
Results: A total of 9,047 participants (4141 CVD cases and 4906 healthy controls) from 11 relevant studies were included. In the total population, there was no significant association between the MIF -173G/C (rs755622) polymorphism and the risk of developing CVD in the three different models. In a stratified analysis by ethnicity, the allelic model (C vs G) was significantly associated with CVD in the Arab and Asian populations (OR = 0.56, CI 0.42 -0.75 and OR = 1.28, CI 1.12 -1.46, respectively); the dominant model (CC+CG vs GG) was significantly associated with CVD in the Arab population (OR = 0.42, CI 0.30 -0.61); while the recessive model (GG+GC vs CC) was associated with CVD susceptibility in the Arab population (OR = 3.84, CI 1.57 -9.41). There were no significant associations between the MIF -173 G/C polymorphism and CVD risk in the European population. Conclusion, the MIF -173G/C polymorphism is associated with CVD in some populations.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, PROSPERO (CRD42023441139).
1 Introduction
Macrophage migration inhibitory factor (MIF) is an immune cytokine with pro-inflammatory, enzymatic, and hormonal functions implicated the pathogenesis of inflammatory and neoplastic diseases. MIF has various functions including in leukocyte recruitment, regulation of immune responses, inflammation, counter-regulation of glucocorticoid activity, and cellular proliferation (1). It is expressed in several immune cell types including T cells, neutrophils, monocytes/macrophages, and eosinophils and also in pituitary cells, epithelial cells, smooth muscle cells, and cardiomyocytes (2, 3), suggesting that it can have diverse roles in various pathophysiological processes (4, 5). MIF is known to play a critical role in both innate and acquired immune responses and it upregulates the expression of pro-inflammatory cytokines (6, 7). In addition, MIF is implicated in cardiovascular diseases (CVD), acting as a reliable biomarker of disease severity and being readily detectable in the blood and at sites of inflammation (8). MIF may therefore have significant impact on the prognosis of CVD patients through its ability to modulate the disease phenotype.
CVD is a common and leading cause of mortality and morbidity worldwide (9, 10). Recognizing CVD as a serious concern for global health, the WHO launched the 25 × 25 Action Plan in 2013 to reduce premature mortality due to non-communicable diseases by 25% by 2025 (11). The 2015 Global Burden of Diseases study estimated that there are 422.7 million CVD cases and 17.59 million CVD deaths worldwide (10). Furthermore, its prevalence is increasing, mostly due to population growth and aging populations, with especially high prevalences in South and East Asia due to their large and rapidly growing populations. Conversely, CVD mortality rates decreased by ∼15% between 1990 and 2015 in some high-income and some middle-income countries (11), while mortality rates have plateaued in high-income regions such as Western Europe, North America, and Australia. Overall, middle-to-low-income countries appear to be disproportionately burdened by CVD mortality. Additionally, CVD occurs approximately 7–10 years later in females than in males, although it remains a major cause of death in females over 65 years. For instance, recent data from the National Health and Nutrition Examination revealed that the prevalence of MI has increased in females aged between 35 and 45 years over the past two decades while decreasing in similarly aged males (12). Deaths due to CVD are most amenable to rapid intervention, and preventing deaths from CVD requires reliable data on CVD risk factors to inform effective treatment and prevention. Individual predisposition to CVD is determined by both environmental and genetic risk factors, the most prevalent environmental factors being hypertension, hypercholesteremia, diabetes, obesity, smoking, stress, gender, ethnic origin, and a sedentary lifestyle (13–15).
CVD is an umbrella term for different heart diseases including coronary artery disease (CAD), myocardial infarction (MI), heart failure (HF), coronary artery abnormalities (CAA), acute coronary syndrome (ACS), and rheumatic heart disease (RHD) (Figure 1). CHD is defined as the narrowing of the coronary arteries leading to a reduced luminal diameter and hence a decrease in blood flow, and it is the most common cause of MI (16). Hypertension and hypercholesteremia accelerate atherosclerotic plaque development and formation due to endothelial injury, which increases endothelial permeability and allows plasma components to infiltrate (17). Plaque hemorrhage ultimately activates thrombus formation initiated by platelet aggregation. Furthermore, cholesterol and triglycerides contribute to plaque formation, both positively and negatively (18). Prolonged and silent atherosclerosis and plaque formation involve chronic inflammatory reactions that influence immune cell, platelet, and complement recruitment to the site of injury (18). Recent compelling evidence highlights a central role for macrophage proliferation within atherosclerotic lesions in driving disease progression. These macrophages, initially immune cells, undergo multiplication, contributing significantly to the pool of foam cells within arterial walls (19). As part of this process, myocardium-produced MIF is highly associated with the development of various CVDs (20), and there is increasing evidence that MIF plays a major role in atheroma formation and CVD progression.
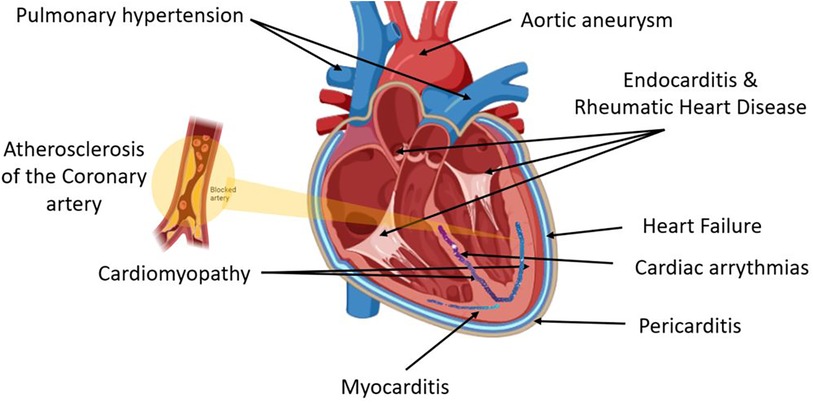
Figure 1. Cardiovascular disease (CVD) is an umbrella term for different heart diseases that include abnormalities of the pericardial layer (e.g., pericarditis and pericardial effusion), myocardial layer [e.g., myocarditis, cardiomyopathy, coronary artery disease (CAD), myocardial infarction (MI), heart failure (HF), coronary artery abnormalities (CAA), acute coronary syndrome (ACS)], the endocardial layer [e.g., valvular heart diseases including endocarditis and rheumatic heart disease (RHD)], abnormalities of the cardiac conductive system including all cardiac arrythmias, and lastly arterial abnormalities such as hypertension and aneurysm.
MIF is a short gene (<0.7 kb) at 22q11.2 composed of three short exons of 107, 172, and 66 base pairs (21). The MIF promoter harbors two polymorphisms that have a regulatory effect on gene transcription (22): the -974 CATT tetranucleotide repeat, which exists in 5–8 repeats (rs5844572), and the -173 G-to-C polymorphism (rs755622). The CATT5 repeat is associated with low MIF expression compared with the CATT6, CATT7, and CATT8 repeat alleles. By contrast, -173C allele is associated with high MIF gene expression (23). Both polymorphisms have been reported to be associated with different autoimmune and inflammatory diseases. A meta-analysis of 23 articles from different populations representing 5,559 cases and 7,335 controls reported an association between the -173G/C polymorphism and susceptibility to a wide range of different autoimmune diseases (24). Karakaya et al. reported an association between the MIF -173C allele and erythema nodosum in Löfgren syndrome patients but not sarcoidosis, indicating a role for MIF after the sarcoid inflammatory response has begun (25). Interestingly, MIF demonstrated a specific role in the recruitment and accumulation of inflammatory macrophages in an animal model of polymicrobial sepsis (26). Moreover, MIF was found to play an important role as a stress molecule counteracting the immunosuppressive effect of glucocorticoids in renal inflammation (27), and MIF deficiency suppressed apoptosis and protected the liver from ischemia-reperfusion injury (28). Conversely, MIF has been shown to play a protective role in Parkinson's disease (29).
Therefore, there is evidence that MIF is associated with CVD, with an association between the MIF -173C/G polymorphism and CVD reported in some but not all populations. To clarify this association, here we conducted a meta-analysis based on a systematic literature review to confirm whether MIF -173G/C (rs755622) is associated with the risk of developing CVD.
2 Materials and methods
2.1 Study design and objectives
This review followed Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Figure 2) (30) and was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42023441139) database. A PICO strategy was used to guide the study design: population, patients with CVD; intervention, association between the MIF -173G/C variant and CVD; and primary outcome, the association between MIF -173G/C and CVD.
2.2 Search strategy
The PubMed, Science Direct, and Embase databases were searched from inception of these databases to June 2023 using the following terms: macrophage migration inhibitory factor or MIF [TEXT WORD] and cardiovascular disease or coronary artery disease [TEXT WORD] or cardiomyopathy [TEXT WORD] or cardiac surgery [TEXT WORD] or HF [TEXT WORD] or rheumatic heart disease [TEXT WORD] or kawasaki disease [TEXT WORD], MI [TEXT WORD] or intracoronary thrombosis [TEXT WORD] or acute coronary syndrome [TEXT WORD] or sudden cardiac death [TEXT WORD]. In addition, the reference lists of compatible articles and conferences were reviewed. Two authors individually screened each article by title and abstract and then evaluated the full text to fully assess eligibility for inclusion.
2.3 Inclusion and exclusion criteria
The inclusion criteria were: (1) all conditions affecting the heart or blood vessels were included as CVD; (2) evaluated MIF -173 G/C polymorphisms and cardiovascular risk; (3) case-control or nested case-control design; (4) included the genotypes for the MIF -173G/C gene polymorphism in CVD cases and controls; and (5) the study reported that the distribution of genotypes among controls was in Hardy-Weinberg equilibrium (HWE). Exclusion criteria were: (1) failed to provide detailed data in the abstract and review; (2) the study was a duplicate; (3) failed to report the genotype frequency; and (4) the controls failed to meet HWE.
2.4 Data extraction and models
The author's details, date of publication, region of study, population ethnicity, number of genotypes analyzed, and the total number of cases and controls were recorded from each article. Two authors individually extracted the required data for each study article, and any disagreement was resolved by consensus or by consultation with a senior author.
Three different genetic models were used to assess the association between the genetic variant and the outcome: the dominant model (Model 1) was defined as the presence of the common allele (CC + CG vs. GG); the recessive genetic model (Model 2) was the presence of rare allele (GG + GC vs. CC); and the allelic model assessed the association between the alleles (C vs. G) and the outcome, regardless of whether it was dominant or recessive.
2.5 Quality assessment
Quality was assessed using the Newcastle-Ottawa quality assessment scale (NOS) for case-control studies. Data quality was judged based on comparability, selection, and outcome of interest for case-control study articles and was noted using a “star system”. To compare study quality, star counts were totaled (Table 1). Data validity was assessed by senior authors based on the provided criteria.
2.6 Statistical analysis
All statistical analyses were performed in STATA v17 (StataCorp, College Station, TX, United States). Heterogeneity between studies was assessed with the I2 statistic. The pooled odds ratio (OR) with 95% CI in the forest plot was analyzed using a random-effects model [restricted maximum likelihood (REML) method] with the subgroup option in Stata. Begg's funnel plot was used to qualitatively assess the risk of publication bias. All analyses were performed using Stata 18. A p-value <0.05 (two-sided) was considered statistically significant in all analyses.
3 Results
3.1 Study characteristics
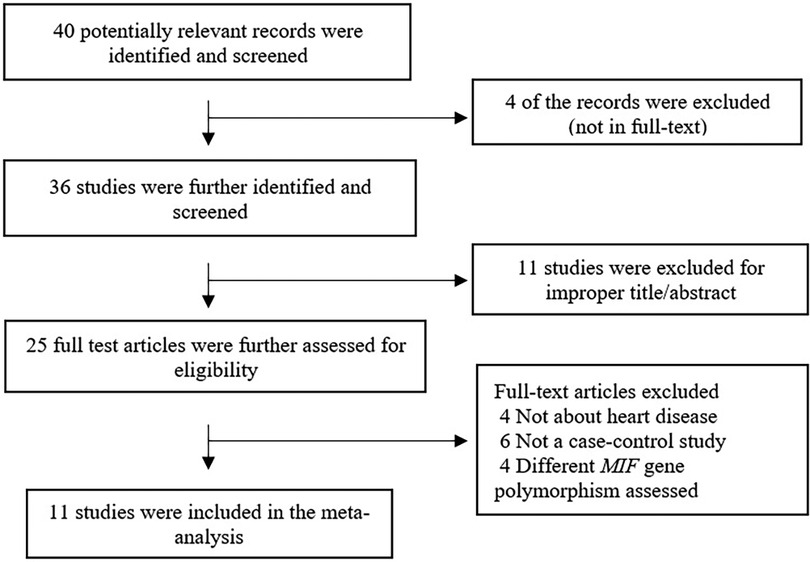
Figure 1 summarizes the search process. Forty articles were found in the initial search, 29 of which were excluded after applying exclusion criteria. Eleven articles met the inclusion criteria and were used in the meta-analysis, representing 4,906 controls and 4,141 cases.
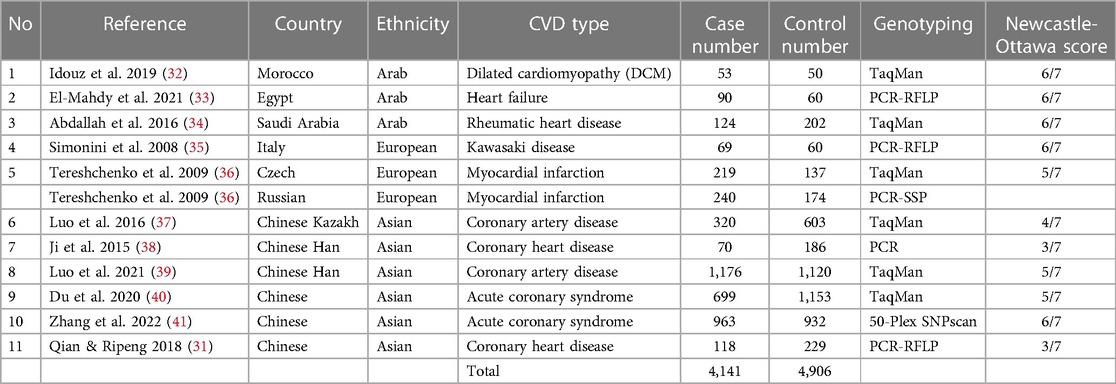
The characteristics of the included articles are summarized in Table 1. Of the included studies, three were performed in Arab populations, six in Asian populations, and two in European populations (31–41). All included studies were cross-sectional case-control studies that included the necessary data to calculate the possible association between the MIF -173G/C polymorphism and CVD. One study was published in Chinese, while the remaining studies were published in English.
The individual studies' quality was appraised utilizing the Newcastle-Ottawa Scale (NOS) scoring system. According to the NOS results, 73% of the included studies achieved a score of 5 or higher out of 7 on the NOS scale, indicating an overall good level of quality (Table 1).
3.2 Quantitative data synthesis
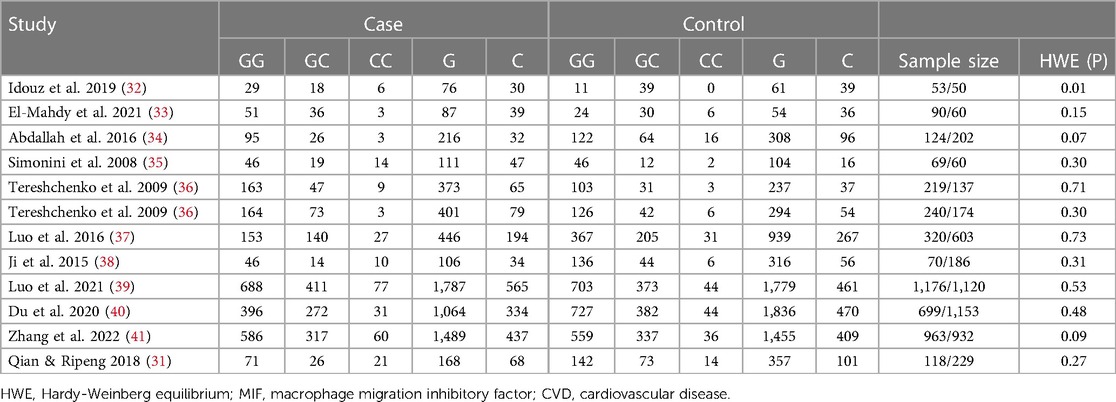
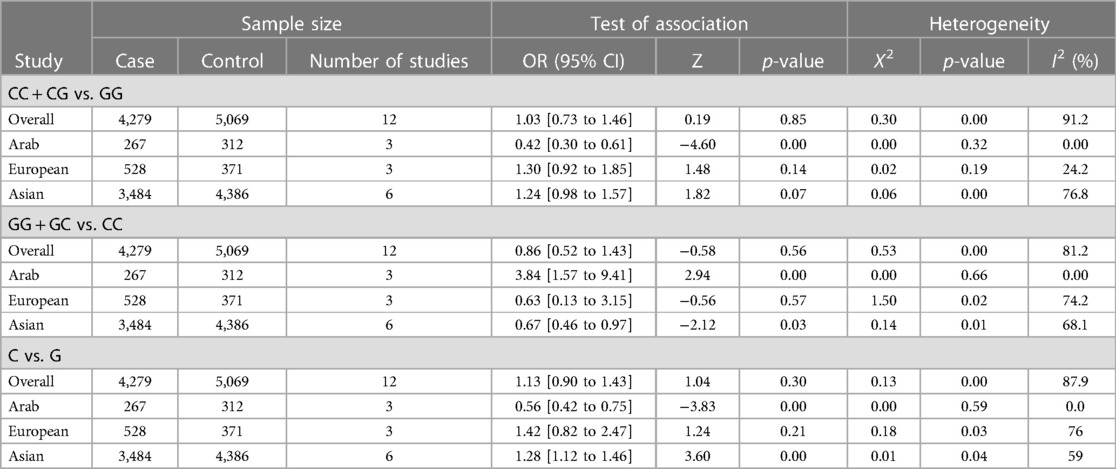
The distribution of the MIF -173 genotype in CVD is shown in Table 2, and the meta-analysis results are shown in Table 3. There was no significant impact of the MIF -173G/C polymorphism and the risk of CVD in the three models assessed: Model 1 (dominant): CC + CG vs. GG (Figure 3), Model 2 (recessive): GG + GC vs. CC (Figure 4), and Model 3 (allelic): C vs. G (Figure 5).
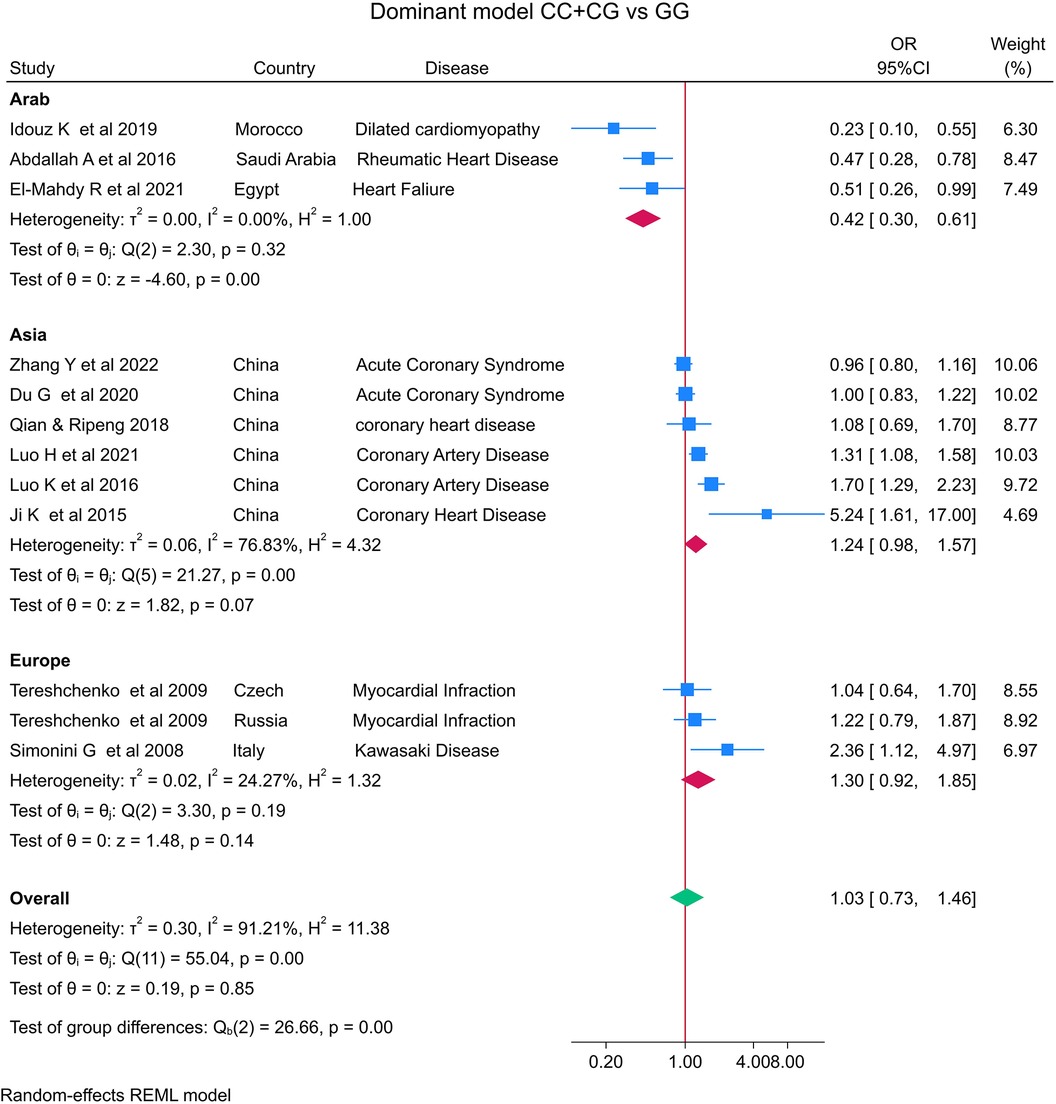
Figure 3. Forest plot of cardiovascular disease risk associated with the MIF -173 G/C polymorphism in Model 1 (CC + CG vs. GG) in Arab, Asian, and European ethnicities.
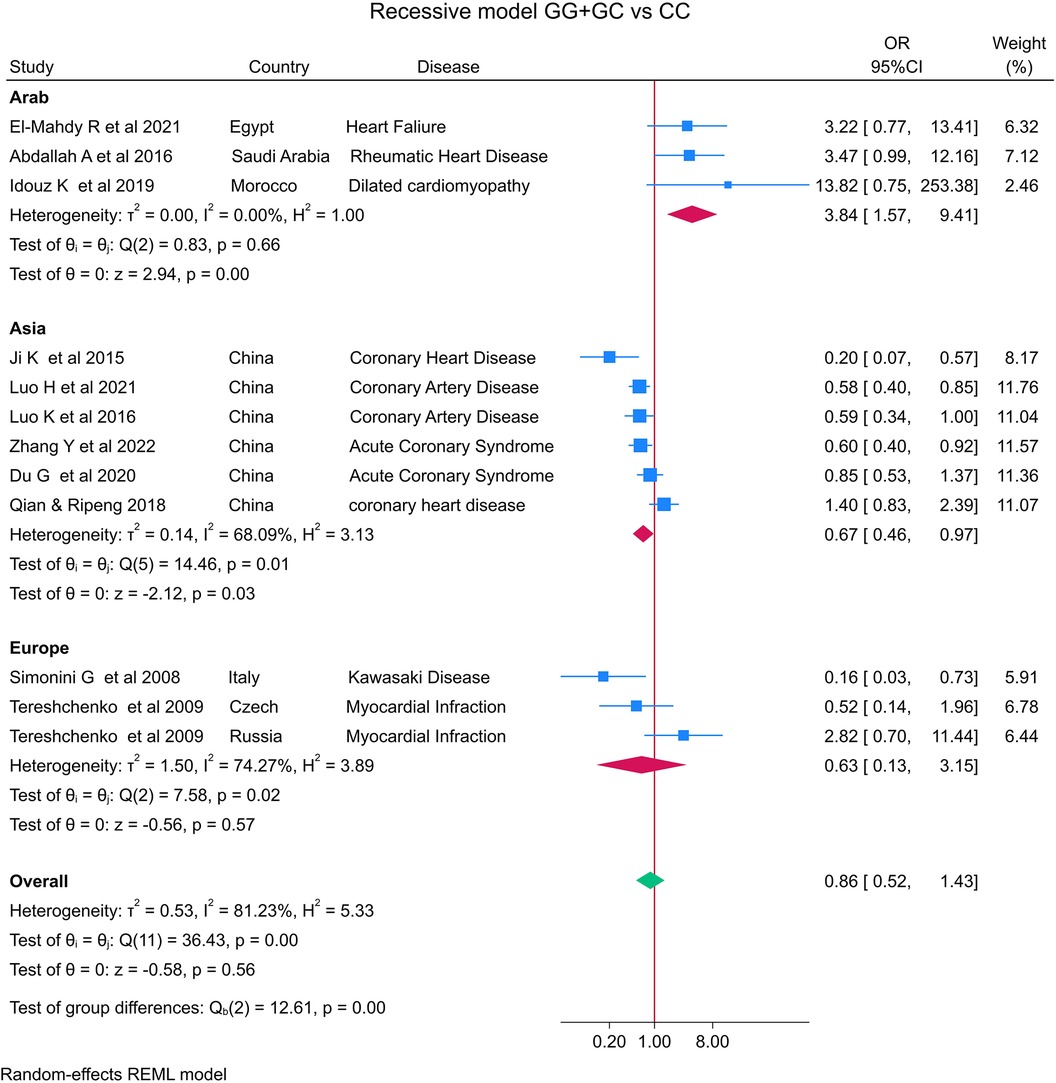
Figure 4. Forest plot of cardiovascular disease risk associated with the MIF -173 G/C polymorphism in model 2 (GG + GC vs. CC) in Arab, Asian, and European ethnicities.
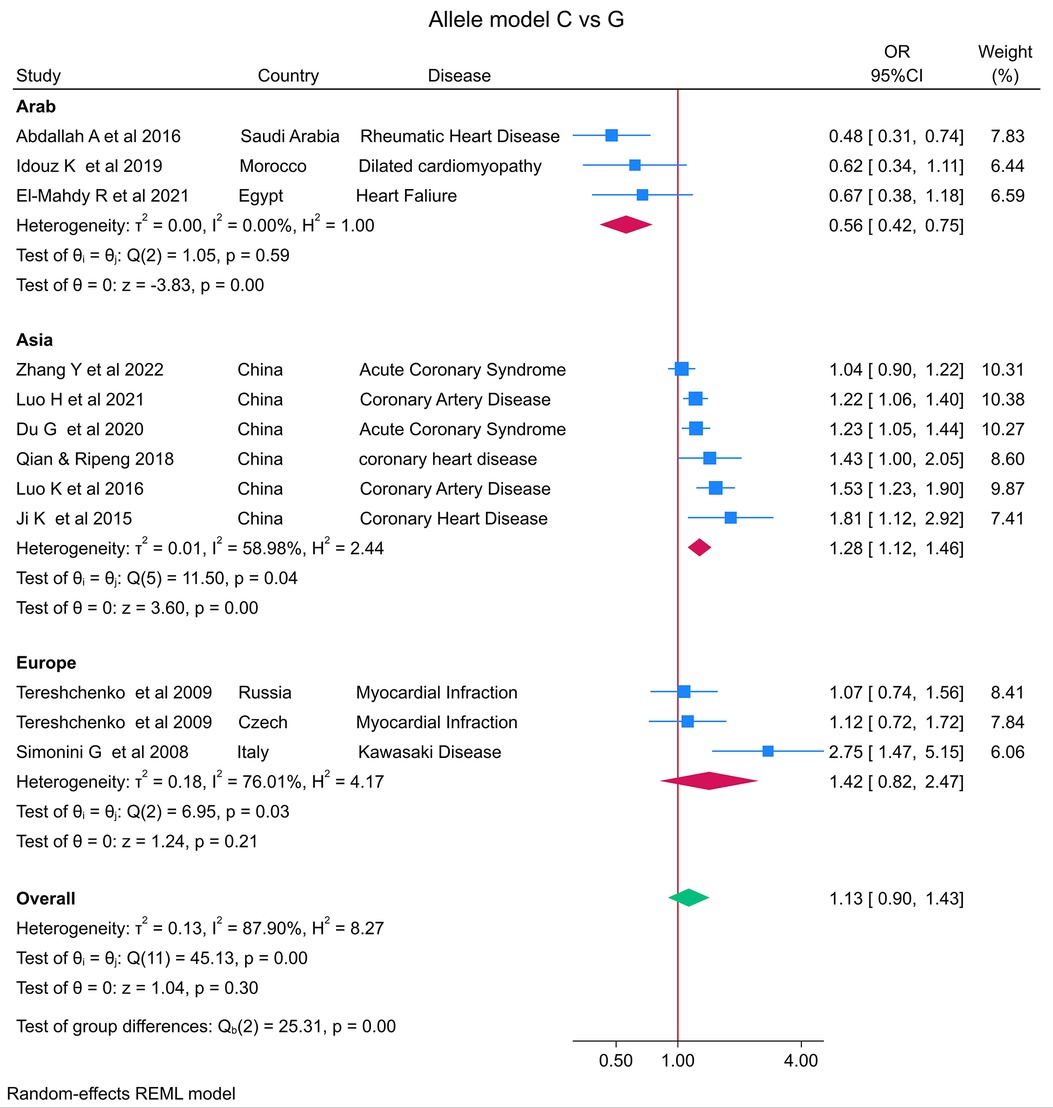
Figure 5. Forest plot of cardiovascular disease risk associated with the MIF -173 G/C polymorphism in model 3 (C vs. G) alleles in Arab, Asian, and European ethnicities.
In the stratified analysis by ethnicity, Model 1 (CC + CG vs. GG) and Model 2 (GG + GC vs. CC) demonstrated significant associations between the MIF -173G/C polymorphism and CVD in the Arab population (OR = 0.42, CI 0.30 to 0.61, p < 0.001 and OR = 3.84, CI 1.57 to 9.41, p < 0.001) (Figures 3, 4) but not in the European and Asian populations. Model 3 (C vs. G) also demonstrated a significant association between the MIF -173G/C polymorphism and the risk of CVD in the Arab population (OR = 0.56, CI 0.42 to 0.75, p < 0.001) (Figure 5) and in the Asian population (OR = 1.28, CI 1.12 to 1.46, p < 0.001) but not in the European population.
3.3 Publication bias analyses
Begg's funnel plot and Egger's test were performed to assess publication bias (Figure 6). For all three models, there was no obvious asymmetry and there was no evidence of publication bias for Model 1 (p = 0.851), Model 2 (p = 0.154), or Model 3 (p = 0.687).
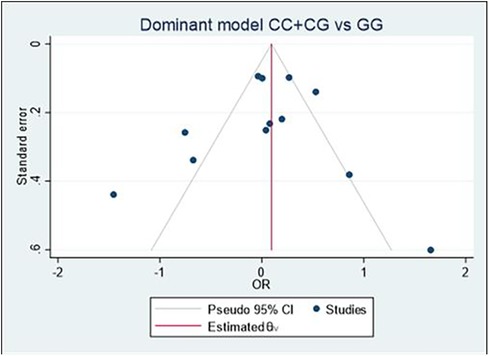
Figure 6. Begg's funnel plot of the MIF -173 polymorphism and CVD for all models; Model 1: CC + CG vs. GG, Model 2: GG + GC vs. CC, and Model 3: C vs. G. Egger's test was used to provide statistical evidence of funnel plot symmetry for the three models, and no evidence of publication bias was found in Model 1 (p = 0.851), Model 2 (p = 0.154), and Model 3 (p = 0.687).
4 Discussion
In this meta-analysis, we aimed to comprehensively review and quantify the literature to establish whether the MIF -173G/C (rs755622) polymorphism is associated with a risk of CVD development. By meta-analyzing eleven studies representing 4,279 cases and 5,069 controls, we found no significant association between the MIF -173G/C polymorphism and the risk of CVD in the overall study population in the three models assessed: CC + CG vs. GG (OR = 1.03), GG + GC vs. CC (OR = 0.86), and C vs. G allele (OR = 1.13). In addition, due to overall heterogeneity and variability in study outcomes between different studies, we conducted a subgroup analysis of the different ethnicities, which revealed that the MIF -173G/C polymorphism is significantly associated with a decreased risk of CVD in the Arab population but not the Asian or European populations in the CC + CG vs. GG model. In the second GG + GC vs. CC model, there was again a significant association between the MIF -173G/C polymorphism and the risk of CVD in the Arab population but not the Asian or European populations. Finally, for the C vs. G allele model, a significant association was observed in the Arab population (OR = 0.56) and the Asian population (OR = 1.28) but not the European population for the MIF -173G/C polymorphism and CVD risk. Our findings are similar to other meta-analyses of MIF, where the C allele was found to be more common within CAD patients (42) and those with chronic kidney diseases (43). However, in systemic lupus erythematosus, while serum MIF levels were associated with the disease, a meta-analysis found no association between the -173G/C polymorphism and the disease (44).
In atherosclerosis, macrophages play a pivotal role, undergoing proliferation and apoptosis. Macrophage proliferation contributes to plaque inflammation, while apoptosis, if excessive, may lead to plaque instability (45). MIF influences macrophage functions, promoting recruitment and inhibiting migration. This interplay is crucial in atherosclerotic plaque development. Within plaques, activated macrophages release pro-inflammatory signals and transform into foam cells by engulfing oxidized LDL cholesterol. The balance between macrophage dynamics and MIF's influence determines the progression and severity of atherosclerosis. Blockade of MIF reduces the aortic inflammatory response and is associated with reduction in aortic plaque and foam cell formation (46). In addition to its direct effects on inflammation and plaque stability, MIF interacts intricately with CXCL4L1, leading to the formation of prothrombotic and proinflammatory MIF-CXCL4L1 heterocomplexes (47, 48). These heterocomplexes have been implicated in promoting endothelial dysfunction, thrombosis, and exacerbation of inflammatory responses within the vascular environment (49). The presence of the -173 polymorphism in the MIF gene may modulate the formation or activity of these heterocomplexes, potentially influencing the progression and severity of CAD. Consequently, understanding the interplay between MIF protein levels, genetic variations, and the formation of MIF-CXCL4L1 heterocomplexes is crucial for deciphering the multifaceted molecular mechanisms underlying CAD and devising targeted therapeutic strategies aimed at disrupting these detrimental interactions (50).
Studies of patients with MI have demonstrated dual functions for the MIF polymorphism depending on disease severity and the patient's age. For instance, when cardiac ischemia is brief, MIF secreted by cardiomyocytes is cardioprotective through activation of AMP-activated protein kinase (AMPK) (51). Phosphorylation of AMPK stimulates glucose uptake through glucose transporter-4 (GLUT4). Conversely, when myocardial ischemia is prolonged, MIF activates immune cells, thereby increasing inflammation and cardiac remodeling by utilizing myofibroblasts to promote matrix protein synthesis (51). Similarly, Abdallah et al. (34) reported a similar dual function for MIF in RHD patients, with a lower frequency of the MIF -173C allele in RHD patients compared with controls and in those with later disease onset. Their findings suggested that MIF may help to clear pathogens and apoptotic cells during the early stages of RHD, perhaps protecting cardiomyocytes and delaying valvular damage. Conversely, after repetitive rheumatic insults, MIF may accelerate the recruitment of inflammatory cells and pro-inflammatory mediators, increasing regional inflammation and cardiac tissue damage. Similar studies on other diseases have also demonstrated that MIF is age-dependent. For instance, Das et al. (52) reported that adults expressing the low MIF (CATT5) allele were more susceptible to Gram-negative bacterial infections, while Lehmann et al. (53) found that adults expressing high levels of MIF polymorphisms were protected from sepsis mortality. In animal models, the protective role of MIF was lost in aged animals after ischemic heart injury, with low MIF expression impairing AMPK activation (54). These data suggest that it is important to consider the patient's age and disease stage when analyzing MIF polymorphisms.
Sex has also been reported to be associated with MIF polymorphisms. The MONICA/KORA Augsburg study concluded that female carriers of the MIF -173C polymorphism were at higher risk of coronary heart disease (55). This result was later confirmed in two studies of Chinese populations (37, 38). In inflammatory diseases, MIF -173 was found to be a disease severity marker for male multiple sclerosis patients (56), while the minor homozygous genotype for both the 974 CATT repeat and the -173G/C polymorphism were reported to protect female patients from major depressive disorder (57). However, another study showed that the MIF -173C allele is a susceptibility factor for depression in type 2 diabetes patients (58). These data suggest that these two polymorphisms are sex-specific disease modifiers.
There are several limitations to our meta-analysis. First, we identified relatively few studies for inclusion, and independent validation is now needed, especially for different ancestries. The number of studies for certain diseases and demographic subgroups was small, and the control group in one study was not in HWE. This precluded meaningful subgroup analysis with specific genotypes. In addition, several records without available original data were excluded from the final analysis. The chance of publication bias is high, as studies with statistically significant results are more likely to be published. The lack of representation of certain ethnicities leads to a reduction in the overall heterogeneity of the study samples, so the results require cautious interpretation. The influence of the MIF -173G/C variant on CVD may be affected by genetic, lifestyle, or environmental factors that were inconsistently measured across studies. This lack of consistent measurement may have led to underreporting of these phenomena in the context of MIF variants and CVD within the scope of this meta-analysis. Finally, variations in diagnostic methodology and criteria for CVD can contribute to inconsistencies, compromising the data integrity of published results. Consequently, these discrepancies may restrict the relevance of this meta-analysis.
In conclusion, our meta-analysis suggests that the MIF -173G/C polymorphism is not significantly associated with the risk of cardiovascular disease in the overall population. In subgroup analysis by ethnicity, the polymorphism was associated with a decreased risk of CVD in the Arab population. Future meta-analyses should consider the dual effect of MIF and the other promoter polymorphisms as well as disease status, sex, and patient age.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HF: Data curation, Methodology, Writing – original draft. WI: Formal Analysis, Investigation, Resources, Supervision, Validation, Writing – review & editing. ZS: Methodology, Formal Analysis, Writing – review & editing. FA: Validation, Visualization, Writing – review & editing. YA: Validation, Visualization, Writing – review & editing. AA: Validation, Writing – review & editing, Funding acquisition, Project administration, Resources. AA: Resources, Validation, Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This publication was supported by Qatar University, internal grant no. QUCP-CHS-2022-551. The findings achieved herein are solely the responsibility of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sumaiya K, Langford D, Natarajaseenivasan K, Shanmughapriya S. Macrophage migration inhibitory factor (MIF): a multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol Ther. (2022) 233:108024. doi: 10.1016/j.pharmthera.2021.108024
2. Kasama T, Ohtsuka K, Sato M, Takahashi R, Wakabayashi K, Kobayashi K. Macrophage migration inhibitory factor: a multifunctional cytokine in rheumatic diseases. Arthritis. (2010) 2010:1–10. doi: 10.1155/2010/106202
3. Daryadel A, Grifone RF, Simon H-U, Yousefi S. Apoptotic neutrophils release macrophage migration inhibitory factor upon stimulation with tumor necrosis factor-α. J Biol Chem. (2006) 281:27653–61. doi: 10.1074/jbc.M604051200
4. Mitchell RA, Metz CN, Peng T, Bucala R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). J Biol Chem. (1999) 274:18100–6. doi: 10.1074/jbc.274.25.18100
5. Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA. (2002) 99:345–50. doi: 10.1073/pnas.012511599
6. Rossi AG, Haslett C, Hirani N, Greening AP, Rahman I, Metz CN, et al. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J Clin Invest. (1998) 101:2869–74. doi: 10.1172/JCI1524
7. Morand EF, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov. (2006) 5:399–411. doi: 10.1038/nrd2029
8. Yu C-M, Lau C-P, Lai KW-H, Huang X-R, Chen W-H, Lan HY. Elevation of plasma level of macrophage migration inhibitory factor in patients with acute myocardial infarction. Am J Cardiol. (2001) 88:774–7. doi: 10.1016/S0002-9149(01)01850-1
9. Omran AR. The epidemiologic transition: a theory of the epidemiology of population change: the epidemiologic transition. Milbank Q. (2005) 83:731–57. doi: 10.1111/j.1468-0009.2005.00398.x
10. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. (2017) 70:1–25. doi: 10.1016/j.jacc.2017.04.052
11. World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva: World Health Organization (2013). Available online at: https://apps.who.int/iris/handle/10665/94384 (accessed May 21, 2023).
12. Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. (2019) 6:19. doi: 10.3390/jcdd6020019
13. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. (2017) 18:109–14. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_106_17
14. Abdallah AM, Abu-Madi M. The genetic control of the rheumatic heart: closing the genotype-phenotype gap. Front Med. (2021) 8:611036. doi: 10.3389/fmed.2021.611036
15. Abdallah AM, Alnuzha A, Al-Mazroea AH, Eldardear AE, AlSamman AY, Almohammadi Y, et al. IL10 promoter polymorphisms are associated with rheumatic heart disease in Saudi Arabian patients. Pediatr Cardiol. (2016) 37:99–105. doi: 10.1007/s00246-015-1245-y
16. Bräuninger H, Krüger S, Bacmeister L, Nyström A, Eyerich K, Westermann D, et al. Matrix metalloproteinases in coronary artery disease and myocardial infarction. Basic Res Cardiol. (2023) 118:18. doi: 10.1007/s00395-023-00987-2
17. Andreou I, Antoniadis AP, Shishido K, Papafaklis MI, Koskinas KC, Chatzizisis YS, et al. How do we prevent the vulnerable atherosclerotic plaque from rupturing? Insights from in vivo assessments of plaque, vascular remodeling, and local endothelial shear stress. J Cardiovasc Pharmacol Ther. (2015) 20:261–75. doi: 10.1177/1074248414555005
18. Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. (2021) 117:2525–36. doi: 10.1093/cvr/cvab303
19. Amin MN, Siddiqui SA, Ibrahim M, Hakim ML, Ahammed MS, Kabir A, et al. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. (2020) 8:2050312120965752. doi: 10.1177/2050312120965752
20. Tilstam PV, Qi D, Leng L, Young L, Bucala R. MIF family cytokines in cardiovascular diseases and prospects for precision-based therapeutics. Expert Opin Ther Targets. (2017) 21:671–83. doi: 10.1080/14728222.2017.1336227
21. Esumi N, Budarf M, Ciccarelli L, Sellinger B, Kozak CA, Wistow G. Conserved gene structure and genomic linkage for D-dopachrome tautomerase (DDT) and MIF. Mamm Genome. (1998) 9:753–7. doi: 10.1007/s003359900858
22. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. (2003) 3:791–800. doi: 10.1038/nri1200
23. Leng L, Siu E, Bucala R. Genotyping two promoter polymorphisms in the MIF gene: a -794 CATT5-8 microsatellite repeat and a -173 G/C SNP. Methods Mol Biol. (2020) 2080:67–84. doi: 10.1007/978-1-4939-9936-1_7
24. Du X, Li R, Song S, Ma L, Xue H. The role of MIF-173G/C gene polymorphism in the susceptibility of autoimmune diseases. Mediat Inflamm. (2020) 2020:7825072. doi: 10.1155/2020/7825072
25. Karakaya B, van Moorsel CHM, van der Helm-van Mil AHM, Huizinga TWJ, Ruven HJT, van der Vis JJ, et al. Macrophage migration inhibitory factor (MIF) -173 polymorphism is associated with clinical erythema nodosum in Löfgren’s syndrome. Cytokine. (2014) 69:272–6. doi: 10.1016/j.cyto.2014.05.020
26. Tilstam PV, Schulte W, Holowka T, Kim B-S, Nouws J, Sauler M, et al. MIF but not MIF-2 recruits inflammatory macrophages in an experimental polymicrobial sepsis model. J Clin Invest. (2021) 131:e127171. doi: 10.1172/JCI127171
27. Kong Y-Z, Chen Q, Lan H-Y. Macrophage migration inhibitory factor (MIF) as a stress molecule in renal inflammation. Int J Mol Sci. (2022) 23:4908. doi: 10.3390/ijms23094908
28. Chen S, Yu Q, Song Y, Cui Z, Li M, Mei C, et al. Inhibition of macrophage migration inhibitory factor (MIF) suppresses apoptosis signal-regulating kinase 1 to protect against liver ischemia/reperfusion injury. Front Pharmacol. (2022) 13:951906. doi: 10.3389/fphar.2022.951906
29. Basile MS, Battaglia G, Bruno V, Mangano K, Fagone P, Petralia MC, et al. The dichotomic role of macrophage migration inhibitory factor in neurodegeneration. Int J Mol Sci. (2020) 21:3023. doi: 10.3390/ijms21083023
30. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Br Med J. (2021) 372:n160. doi: 10.1136/bmj.n160
31. Qian L, Yin R. Macrophage migration inhibitory factor promoter polymorphisms (-173G/C) : relationship with coronary atherosclerotic disease subjects. J Med Res. (2018) (12):32–5.
32. Idouz K, Drighil A, Naour O, Moundir C, Hakeem MA, Ali Bah R, et al. Protective role of macrophage migration inhibitory factor −173 G > C (rs755622) gene polymorphism in pediatric patients with dilated cardiomyopathy. Gene Rep. (2019) 16:100455. doi: 10.1016/j.genrep.2019.100455
33. El-Mahdy RI, Saleem TH, Essam OM, Algowhary M. Functional variants in the promoter region of macrophage migration inhibitory factor rs755622 gene (MIF G173C) among patients with heart failure: association with echocardiographic indices and disease severity. Heart Lung. (2021) 50:92–100. doi: 10.1016/j.hrtlng.2020.07.015
34. Abdallah AM, Al-Mazroea AH, Al-Harbi WN, Al-Harbi NA, Eldardear AE, Almohammadi Y, et al. Impact of MIF gene promoter variations on risk of rheumatic heart disease and its age of onset in Saudi Arabian patients. Front Immunol. (2016) 7:98. doi: 10.3389/fimmu.2016.00098
35. Simonini G, Corinaldesi E, Falcini F, Massai C, De Martino M, Cimaz R. Macrophage migration inhibitory factor gene polymorphisms in an Italian cohort of patients with Kawasaki disease. Pediatr Rheumatol. (2008) 6:1. doi: 10.1186/1546-0096-6-S1-P256
36. Tereshchenko IP, Petrkova J, Mrazek F, Lukl J, Maksimov VN, Romaschenko AG, et al. The macrophage migration inhibitory factor (MIF) gene polymorphism in Czech and Russian patients with myocardial infarction. Clin Chim Acta. (2009) 402:199–202. doi: 10.1016/j.cca.2008.12.034
37. Luo J-Y, Xu R, Li X-M, Zhou Y, Zhao Q, Liu F, et al. MIF Gene polymorphism rs755622 is associated with coronary artery disease and severity of coronary lesions in a Chinese Kazakh population: a case–control study. Medicine (Baltimore). (2016) 95:e2617. doi: 10.1097/MD.0000000000002617
38. Ji K, Wang X, Li J, Lu Q, Wang G, Xue Y, et al. Macrophage migration inhibitory factor polymorphism is associated with susceptibility to inflammatory coronary heart disease. BioMed Res Int. (2015) 2015:1–6. doi: 10.1155/2015/315174
39. Luo J-Y, Fang B-B, Du G-L, Liu F, Li Y-H, Tian T, et al. Association between MIF gene promoter rs755622 and susceptibility to coronary artery disease and inflammatory cytokines in the Chinese han population. Sci Rep. (2021) 11:8050. doi: 10.1038/s41598-021-87580-6
40. Du G-L, Luo J-Y, Wang D, Li Y-H, Fang B-B, Li X-M, et al. MIF gene rs755622 polymorphism positively associated with acute coronary syndrome in Chinese Han population: case–control study. Sci Rep. (2020) 10:140. doi: 10.1038/s41598-019-56949-z
41. Zhang J-Y, Zhao Q, Liu F, Li D-Y, Men L, Luo J-Y, et al. Genetic variation of migration inhibitory factor gene rs2070766 is associated with acute coronary syndromes in Chinese population. Front Genet. (2022) 12:750975. doi: 10.3389/fgene.2021.750975
42. Li D-Y, Zhang J-Y, Chen Q-J, Liu F, Zhao Q, Gao X-M, et al. MIF -173G/C (rs755622) polymorphism modulates coronary artery disease risk: evidence from a systematic meta-analysis. BMC Cardiovasc Disord. (2020) 20:300. doi: 10.1186/s12872-020-01564-4
43. Tziastoudi M, Pissas G, Raptis G, Cholevas C, Eleftheriadis T, Dounousi E, et al. A systematic review and meta-analysis of pharmacogenetic studies in patients with chronic kidney disease. IJMS. (2021) 22:4480. doi: 10.3390/ijms22094480
44. Bae S-C, Lee YH. Circulating macrophage migration inhibitory factor levels and its polymorphisms in systemic lupus erythematosus: a meta-analysis. Cell Mol Biol (Noisy-le-grand). (2017) 63:74–9. doi: 10.14715/cmb/2017.63.10.12
45. Schelemei P, Wagner E, Picard FSR, Winkels H. Macrophage mediators and mechanisms in cardiovascular disease. FASEB J. (2024) 38:e23424. doi: 10.1096/fj.202302001R
46. Burger-Kentischer A, Göbel H, Kleemann R, Zernecke A, Bucala R, Leng L, et al. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF). Atherosclerosis. (2006) 184:28–38. doi: 10.1016/j.atherosclerosis.2005.03.028
47. Yan Y, Thakur M, Van Der Vorst EPC, Weber C, Döring Y. Targeting the chemokine network in atherosclerosis. Atherosclerosis. (2021) 330:95–106. doi: 10.1016/j.atherosclerosis.2021.06.912
48. Leberzammer J, Von Hundelshausen P. Chemokines, molecular drivers of thromboinflammation and immunothrombosis. Front Immunol. (2023) 14:1276353. doi: 10.3389/fimmu.2023.1276353
49. Brandhofer M, Hoffmann A, Blanchet X, Siminkovitch E, Rohlfing A-K, El Bounkari O, et al. Heterocomplexes between the atypical chemokine MIF and the CXC-motif chemokine CXCL4L1 regulate inflammation and thrombus formation. Cell Mol Life Sci. (2022) 79:512. doi: 10.1007/s00018-022-04539-0
50. Li B, Li W, Li X, Zhou H. Inflammation: a novel therapeutic target/direction in atherosclerosis. CPD. (2017) 23:1216–27. doi: 10.2174/1381612822666161230142931
51. Dayawansa NH, Gao X-M, White DA, Dart AM, Du X-J. Role of MIF in myocardial ischaemia and infarction: insight from recent clinical and experimental findings. Clin Sci. (2014) 127:149–61. doi: 10.1042/CS20130828
52. Das R, Subrahmanyan L, Yang IV, van Duin D, Levy R, Piecychna M, et al. Functional polymorphisms in the gene encoding macrophage migration inhibitory factor are associated with gram-negative bacteremia in older adults. J Infect Dis. (2014) 209:764–8. doi: 10.1093/infdis/jit571
53. Lehmann LE, Book M, Hartmann W, Weber SU, Schewe J-C, Klaschik S, et al. A MIF haplotype is associated with the outcome of patients with severe sepsis: a case control study. J Transl Med. (2009) 7:100. doi: 10.1186/1479-5876-7-100
54. Ma H, Wang J, Thomas DP, Tong C, Leng L, Wang W, et al. Impaired macrophage migration inhibitory factor–AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation. (2010) 122:282–92. doi: 10.1161/CIRCULATIONAHA.110.953208
55. Herder C, Illig T, Baumert J, Müller M, Klopp N, Khuseyinova N, et al. Macrophage migration inhibitory factor (MIF) and risk for coronary heart disease: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Atherosclerosis. (2008) 200:380–8. doi: 10.1016/j.atherosclerosis.2007.12.025
56. Benedek G, Meza-Romero R, Jordan K, Zhang Y, Nguyen H, Kent G, et al. MIF and D-DT are potential disease severity modifiers in male MS subjects. Proc Natl Acad Sci USA. (2017) 114:E8421–9. doi: 10.1073/pnas.1712288114
57. Swoboda C, Deloch L, von Zimmermann C, Richter-Schmidinger T, Lenz B, Kornhuber J, et al. Macrophage migration inhibitory factor in major depressive disorder: a multilevel pilot study. Int J Mol Sci. (2022) 23:15460. doi: 10.3390/ijms232415460
Keywords: cardiovascular disease, macrophage migration inhibitory factor, polymorphism, meta-analysis, Arab, Asian, European
Citation: Fouda H, Ibrahim WN, Shi Z, Alahmadi F, Almohammadi Y, Al-Haidose A and Abdallah AM (2024) Impact of the MIF -173G/C variant on cardiovascular disease risk: a meta-analysis of 9,047 participants. Front. Cardiovasc. Med. 11:1323423. doi: 10.3389/fcvm.2024.1323423
Received: 17 October 2023; Accepted: 14 February 2024;
Published: 26 February 2024.
Edited by:
Peng Zhang, Institute of ENT and Shenzhen Key Laboratory of ENT, ChinaReviewed by:
Satyesh K Sinha, University of California, Los Angeles, United StatesGeorge Lisi, Brown University, United States
Shannon Moonah, University of Florida, United States
© 2024 Fouda, Ibrahim, Shi, Alahmadi, Almohammadi, Al-Haidose and Abdallah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atiyeh M. Abdallah aabdallah@qu.edu.qa
 Hamas Fouda1
Hamas Fouda1  Wisam N. Ibrahim
Wisam N. Ibrahim Zumin Shi
Zumin Shi Fahad Alahmadi
Fahad Alahmadi Yousef Almohammadi
Yousef Almohammadi Amal Al-Haidose
Amal Al-Haidose Atiyeh M. Abdallah
Atiyeh M. Abdallah