Five-year outcomes of double kissing mini-culotte stenting vs. mini-culotte stenting using drug-eluting stents for the treatment of true coronary bifurcation lesions
- 1Department of Cardiology, Bozhou People’s Hospital, Bozhou Hospital Affiliated to Anhui University of Science and Technology, Bozhou Hospital Affiliated to Anhui Medical University, Bozhou, Anhui, China
- 2Department of Cardiology, Union Hospital, Fujian Medical University, Fujian, China
- 3Department of Cardiology, Provincial Institute of Coronary Artery Disease, Fujian, China
- 4Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 5Department of Dermatology, Bozhou People’s Hospital, Bozhou Hospital Affiliated to Anhui University of Science and Technology, Bozhou Hospital Affiliated to Anhui Medical University, Bozhou, Anhui, China
Objective: This study aimed to compare the clinical outcomes of double kissing mini-culotte (DKMC) stenting with those of mini-culotte (MC) stenting in treating patients with true coronary bifurcation lesions (CBLs) in the clinical real world.
Methods: This retrospective observational cohort study included 180 consecutive patients with true CBLs (Medina type 1,1,1; 1,0,1; 0,1,1). All eligible patients underwent coronary angiography and percutaneous coronary intervention with two-stent techniques in our hospital; among them, 97 received DKMC treatment and 83 MC treatment. The primary clinical endpoints were the major adverse cardiovascular events (MACE), which included cardiac death, myocardial infarction, and target vessel/lesion revascularization (TVR/TLR). The secondary endpoints were stent thrombosis, in-stent restenosis, and individual components of MACE.
Results: Quantitative coronary angiography analysis (at 5 years) revealed that late lumen loss (0.25 ± 0.41 mm vs. 0.14 ± 0.32 mm, P = 0.032) and segmental diameter restenosis of the side branch (27.84 ± 12.34% vs. 19.23 ± 9.76%, P = 0.016) were lower in the DKMC treatment group than that in the MC treatment group. Notably, compared to that in the MC treatment group, the cumulative event rate of MACE at 5 years (22.8% vs. 8.3%, P = 0.007) and TVR/TLR (17.7% vs. 6.3%, P = 0.018) was higher in the DKMC treatment group, driven mainly by TVR/TLR. Especially, the DKMC group was related to a significant reduction in the primary and secondary endpoints in high-risk patients.
Conclusion: DKMC treatment was associated with less late lumen loss and restenosis in the side branch and a lower rate of cumulative MACE and TVR/TLR. DKMC treatment is more effective for treating true CBLs than MC treatment; however, these findings warrant further confirmation through a randomized clinical trial.
Introduction
Coronary bifurcation lesions (CBLs) account for about 15%–20% of lesions seen in patients requiring percutaneous coronary intervention (PCI) (1–3) and are challenging to treat. Compared with non-CBLs, CBL PCI technology is more difficult to handle, with lower success rates, higher incidences of complications, poorer long-term efficacy, and an increased risk of stent thrombosis (ST) (4–7).
PCI treatment of CBLs can be summarized as simple single-stent and complex double-stent strategies. The current expert consensus recommends CBLs with simple strategies, such as single stents or, if necessary, T stents. However, in treating CBLs with single stenting, the main vessel (MB) is undoubtedly well treated but may cause compression, dissection, acute occlusion, and even permanent loss of the side branch (SB). Therefore, the double-stent strategy, which avoids loss of intraoperative vital vascular occlusion and improves operation safety, is still an indispensable option for clinicians in treating severe true CBLs (8).
Popular used complex double-stent strategies including crush and culotte stenting. The latter is derived from several variants after several generations of improvement. The European Bifurcation Club (EBC) recommends mini-culotte (MC) stenting as a treatment approach for true CBLs (9). As a novel culotte technique, double kissing mini-culotte stenting (DKMC) has been associated with good clinical outcomes, while bench test data suggested that an additional kissing dilation approach facilitates the culotte technique (10–13). Nonetheless, no solid data confirm whether DKMC is superior to MC in treating true CBLs.
Therefore, we conducted an observational cohort study to evaluate the clinical efficacy of MC and DKMC in treating complex CBLs.
Methods
Study population
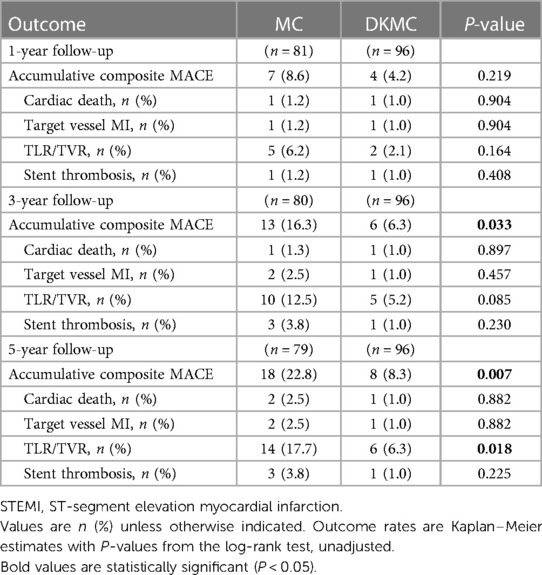
This observational cohort study included 180 consecutive “all comer” patients with true CBLs (≥50% diameter stenosis) diagnosed by angiography from 4,562 patients who were treated with PCI in the enrollment period and no less than 60 individuals in each group according to clinical results and statistical requirements, who were treated by either MC or DKMC with drug-eluting stents (DES) between January 2010 and March 2015. Patients were treated with the upfront or rescue two-stent strategy (MC group: 70 cases of the upfront two-stent strategy and 13 cases of the rescue two-stent strategy; DKMC group: 80 cases of the upfront two-stent strategy and 17 cases of the rescue two-stent strategy). The clinical flow charts of this mini-culotte stent technique are demonstrated in Figure 1.
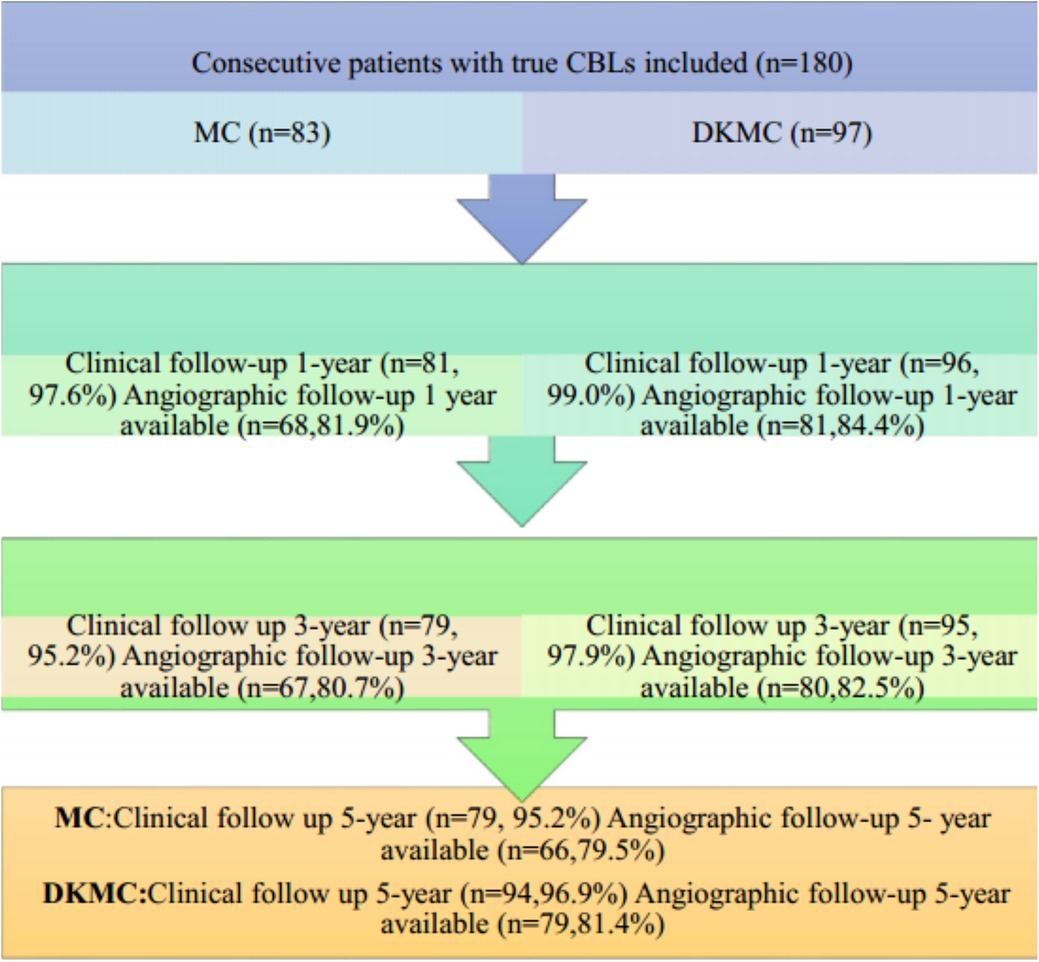
Figure 1. Clinical follow-up was scheduled at 1, 3, and 5 years after revascularization, and then annually thereafter. Routine angiographic follow-up was recommended at 1, 3, and 5 years after PCI.
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Medical Ethics Committee of Union Hospital, Fujian Medical University. The identity of patients remained anonymous, and the requirement for informed consent was waived due to the observational nature of the study (14, 15).
PCI was determined by an experienced heart team (interventional cardiologists) based on clinical risk, angiographic characteristics, and patients' preferences. All patients signed a written informed consent form.
The synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) scores was calculated for every patient to evaluate cardiac operative risk scores.
Procedures
All interventional procedures were performed as previously described and shown in Supplementary Material (Supplementary material S1 and Videos S1, S2) (10, 16, 17). The proximal optimizing technique (POT) was conducted in the two-stent strategy (18, 19). The stents were mainly selected from Resolute (Medtronic Cardiovascular, Santa Rosa, CA, USA), Xience V (Abbott Vascular, Santa Clara, CA, USA), Firebird-2 (Microport Co., Shanghai, China), and Excel (JW Medical System, Weihai, China). Bifurcation lesions were treated using either MC or DKMC (16). The stenting strategy was at the discretion of operators according to the lesions' characteristics and their experiences. Final kissing balloon dilation (fKBD) was performed with a non-compliant balloon according to the named time and pressure in the two-stent procedure. An intra-aortic balloon pump (IABP) was used in high-risk patients with severe heart failure. The use of intravascular ultrasound (IVUS) and the choice of a particular DES were at the discretion of the operators. Within 48 h after the procedure, cardiac troponin (cTn) values above 5 times the 99th percentile upper reference limit (URL) after PCI or 10 times after coronary artery bypass grafting (CABG) were used to define periprocedural PCI or CABG-related MI in patients with normal baseline cTn levels. “+” graft occlusion was defined according to the Academic Research Consortium (ARC)-like definition (for CABG). No patients undergoing CABG were enrolled.
All patients undergoing PCI were administered a loading dose of aspirin (300 mg) plus clopidogrel (300 or 600 mg) or ticagrelor (180 mg) before or during intervention procedures. Periprocedural anticoagulation followed the standard treatment.Patients received unfractionated heparin 100 IU/kg intravenously during the procedure, which was corrected to maintain an activated clotting time >300 s. Whether to use glycoprotein IIb/IIIa receptor inhibitors was left to the discretion of the operators. After discharge, all patients treated with PCI were prescribed a standard dual antiplatelet therapy regimen (aspirin 100 mg daily and clopidogrel 75 mg daily or ticagrelor 90 mg twice daily) for at least 12 months and were indefinitely continued with aspirin.
Other post-procedure medication treatments such as statins, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), and beta-blockers were prescribed to patients undergoing PCI according to current clinical practice.
Follow-up
Clinical follow-up was scheduled at 1, 6, and 12 months (1 year), 3 years, and 5 years after revascularization, followed by annual visits thereafter. Routine angiographic follow-up was recommended at 1, 3, and 5 years after PCI. Angiography was performed beforehand if symptoms of angina recurred. Participants who did not adhere to the recommended follow-up processes were interviewed via telephone.
Study endpoints and definitions
The primary endpoint of this study was a patient-oriented composite of major adverse cardiac and cerebrovascular events (MACE) at 1-, 3-, and 5-year follow-ups, which included all-cause death, myocardial infarction (MI), and target vessel revascularization (TVR) or target lesion revascularization (TLR). In the analysis of cumulative endpoints, events were counted only once, whichever occurred first.
The secondary endpoints were a composite safety endpoint of all-cause death/MI/stroke, individual components of MACE, and ST.
Death was defined as post-procedure death from any cause and was classified as from either cardiac or non-cardiac causes, according to the ARC definition (20). Death was considered as cardiac origin unless a non-cardiac origin has definitely been proved. Cardiac death was defined as any death due to a cardiac cause (e.g., MI, low-output heart failure, fatal arrhythmias), procedure-related death, or death of unknown cause.
MI was defined according to the third universal definition of myocardial infarction (21). Evidence for MI mainly included elevated cTn with at least one value >99th percentile URL, symptoms of myocardial ischemia, electrocardiographic changes, and angiographic characteristics. Within 48 h after the procedure, cTn values >5 times the 99th percentile URL after PCI or 10 times after CABG were used to define periprocedural PCI or CABG-related MI in patients with normal baseline cTn levels (≤99th percentile URL). If the baseline values were elevated, stable, or falling, a rise of cTn values >20% was also considered evidence of periprocedural PCI-related MI. Q-wave MI was defined as MI together with a new pathologic Q-wave in no less than two contiguous leads after index treatment.
TVR was defined as any surgical or percutaneous repeat revascularization of any segment of the stented vessel (target lesion, upstream or downstream branches) within 1 year, including the left main, left anterior descending, and left circumflex coronary arteries. A planned staged PCI was not considered a TVR.
The occurrence of definite ST was defined according to the ARC definition (for PCI), and graft occlusion was defined according to the ARC-like definition (for CABG) (22, 23).
Statistical analysis
Continuous variables were presented as mean ± SD, and categorical variables were presented as number (%). Comparisons between the MC and DKMC groups in baseline characteristics were performed by the t-test or Wilcoxon rank-sum test for continuous data and using the chi-squared test or Fisher's exact test for categorical data, as appropriate. Cumulative event curves of the MC and DKMC groups were constructed by the Kaplan–Meier method and were compared using the log-rank test.
A P-value <0.05 was considered statistically significant, and all tests were two-tailed. All statistical analyses were performed with SPSS software (version 22.0, SPSS, IBM Corporation, Armonk, New York, USA).
Results
Baseline characteristics
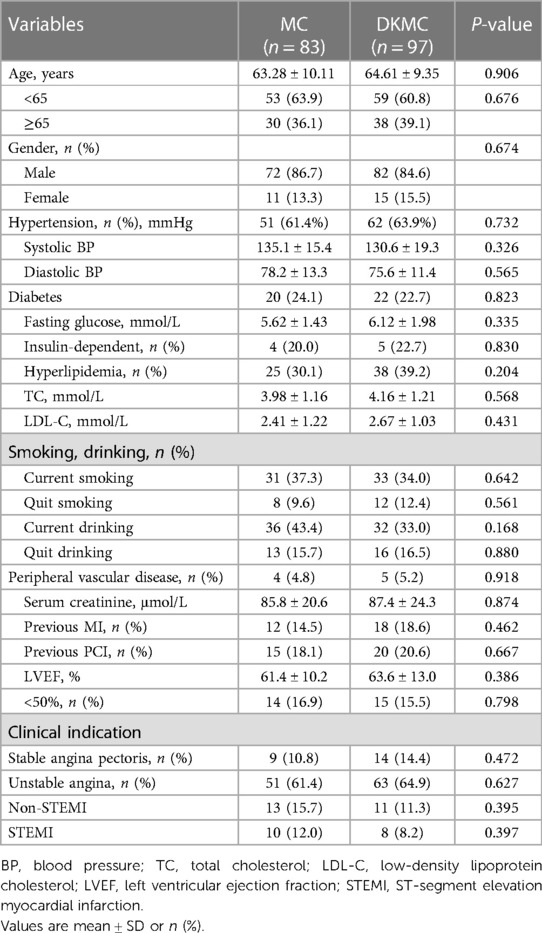
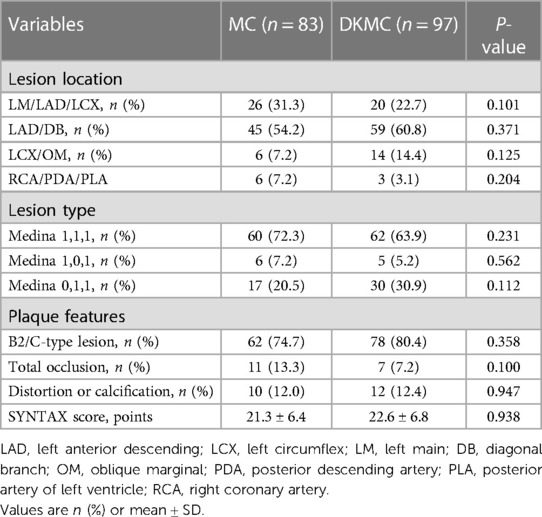
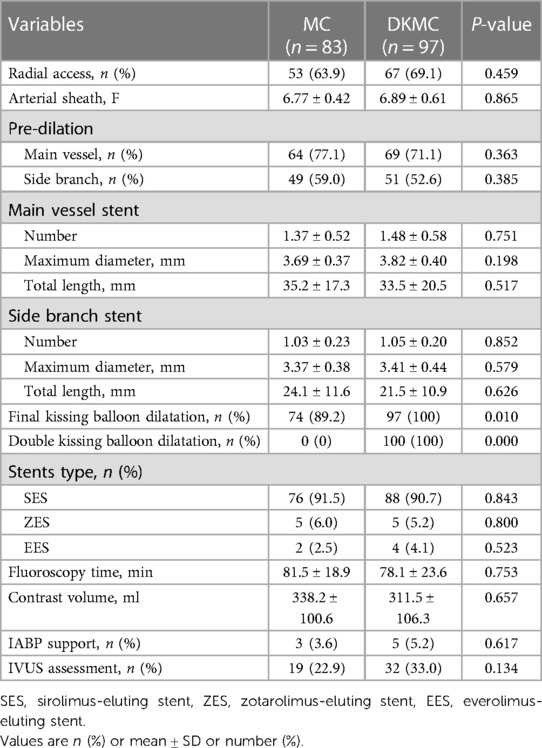
A total of 180 consecutive patients with true CBLs who received MC (n = 83) or DKMC (n = 97) treatment between January 2010 and March 2015 were included in the study. All these patients were completely followed up for 5 years (Figure 1). Baseline clinical characteristics, angiographic characteristics, and cardiac operative risk scores are given in Tables 1–3. The two groups exhibited no significant differences in other clinical characteristics, angiographic characteristics, cardiac operative risk scores, and procedural characteristics (all P’s > 0.05).
Clinical outcomes
Overall population
Of the 180 enrolled patients, 177 were followed up during 1 year, including 81 patients in the MC group (97.6% of the clinical follow-up) and 96 patients in the DKMC group (99.0% of the clinical follow-up). Quantitative coronary angiography (QCA) measurements of the two groups [68 patients (84.0%) in the MC treatment group and 81 patients (84.4%) in the DKMC treatment group] were followed up within 1 year after operation. At 3 years, the clinical follow-up was completed in 80 (96.4%) patients in the MC treatment group and 96 (99.0%) patients in the DKMC treatment group and the QCA follow-up was completed in 67 (80.7%) patients in the MC treatment group and 80 (82.5%) patients in the DKMC treatment group. At 5 years, the clinical follow-up was completed in 79 (95.2%) patients in the MC treatment group and 96 (99.0%) patients in the DKMC treatment group and the QCA follow-up was completed in 66 (79.5%) patients in the MC treatment group and 79 (81.4%) patients in the DKMC treatment group.
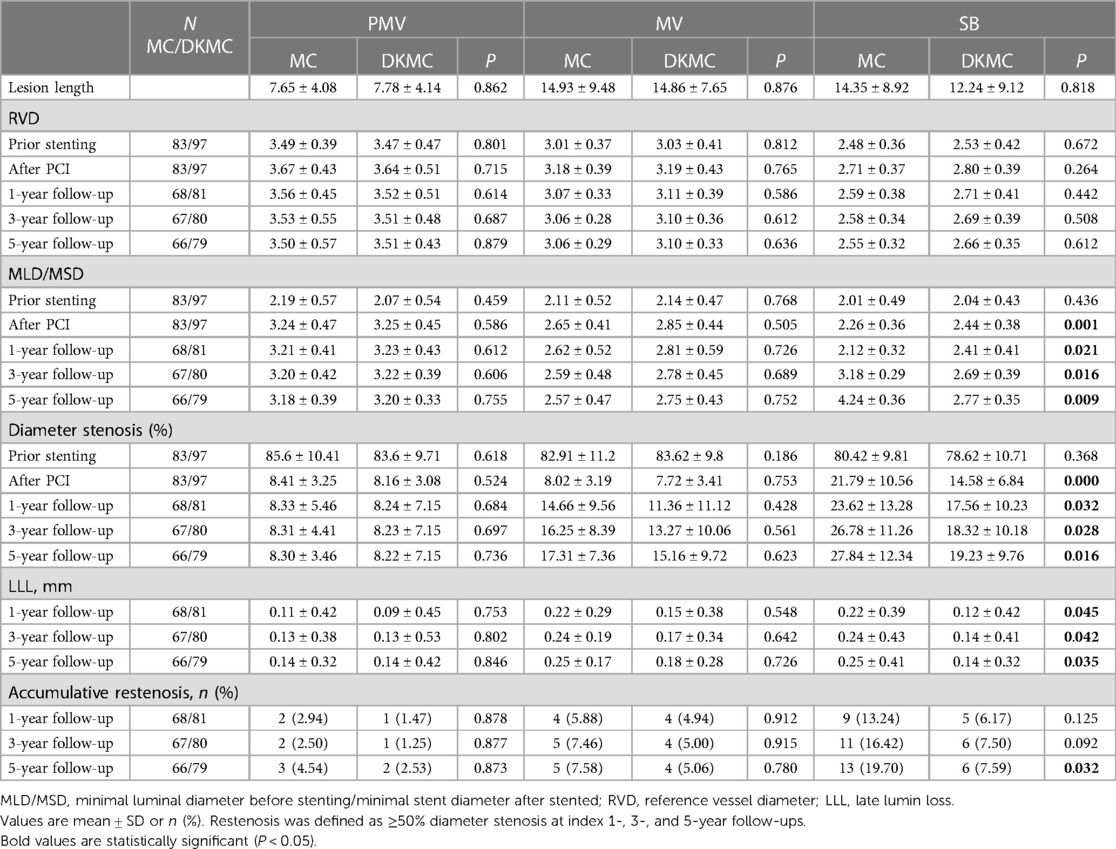
At 1 year, QCA analysis showed (Table 4) that late lumen loss (0.12 ± 0.42 mm vs. 0.22 ± 0.39 mm, P = 0.045) and segmental diameter restenosis of the side branch (17.56 ± 10.23% vs. 23.62 ± 13.28%, P = 0.032) were lower than in the MC group. There were similar rates of binary restenosis in the parent main vessel (1.2% vs. 2.9%) and the main branch (4.8% vs. 5.9%) in both groups, with a numerically lower rate of binary restenosis in the side branch in the DKMC group (6.2% vs. 13.2%, P = 0.141) irrespective of no significant differences.
At the 1-year follow-up, 2 (1.1%) patients suffered cardiac death, 2 (1.1%) patients suffered definite stent thrombosis, 7 (4.0%) patients received TVR and cumulative MACE occurred in 11 (6.2%) patients. At 3- and 5-year follow-ups, the accumulative incidences of MACE were 16.3% and 22.8% in the MC stenting group and 6.3% and 8.3% in the DKMC group, respectively (P < 0.05) (Table 5), mainly resulting from increased rates of TLR/TVR (12.5% vs. 5.2%; P = 0.085; 17.7% vs. 6.3%; P = 0.018) in the MC stenting group. The detailed cumulative clinical outcomes of the two groups are presented in Table 5 and Figure 2.
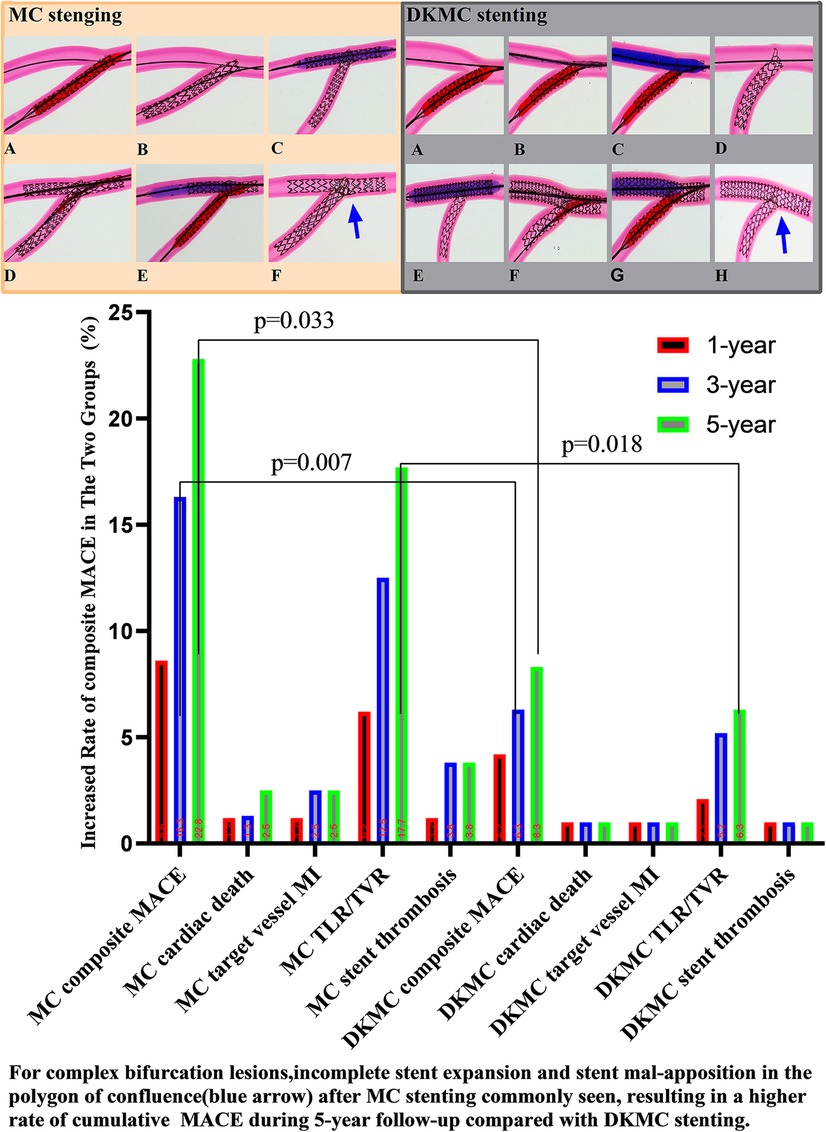
Figure 2. Top left: images of stents deployed in mock phantoms using the MC strategy. The detailed MC strategy process is shown in Supplementary Videos S1, S2 and pre-publication literature in Supplement material S1. In the culotte technique, the first stent is often deployed in the SB, usually across the SB to the main vessel (MV), with a protrusion about 3–5 mm (A, B). The MV then needs to be rewired through the strut of the first stent, and after predilatation with a non-compliant balloon (C), a second stent is then implanted (D). The procedure is completed after rewiring the SB by final kissing balloon dilatation of both branches (E). Then, with the proximal optimize technique (POT), a suitable non-compliance balloon is used to the proximal MB stent as necessary to end the procedure (F). Top right: images of stents deployed in mock phantoms using the DKMC strategy. The detailed DKMC strategy process is shown in Supplementary Videos S2 and pre-publication literature in Supplementary Appendix 1. DKMC strategy: Preimbed a balloon in the MB and position the side branch (SB) stent with mini-protrusion (1-2 mm) into the MV. The wire was then retrieved to the bifurcation and gently advanced through the struts of the stents (A), which was subsequently prodilated with a 1.5-mm balloon (if necessary in real world) (B); then with the NC-balloon in MB (match diameter of MB) and the NC-balloon ( match diameter of SB) for the first sequential intermediate kissing balloon dilation (siKBD) with 10 atmospheric pressure (ATM) (C), withdraw the two balloons and SB wire and insert the MB stent. After deploying the MB stent (D,E), withdraw the stent balloon. The wire was then retrieved to the SB and gently advanced through the struts (F), with a 1.5-mm balloon (if necessary) used for prodilation, followed by the final kissing balloon dilation (fKBD) procedure (G). The procedure concludes with POT (H). At 1-year follow-up, 2 (1.1%) patients suffered cardiac death, 2 (1.1%) patients suffered definite stent thrombosis, 7 (4.0%) patients received TVR, and cumulative MACE occurred in a total of 11 (6.2%) patients. At 3- and 5-year follow-ups, the accumulative incidences of MACE were 16.3% and 22.8% in the MC stenting group and 6.3% and 8.3% in the DKMC group, respectively (P < 0.05) (Table 5), mainly resulting from they increased rates of TLR/TVR (12.5% vs. 5.2%, P = 0.085; 17.7% vs. 6.3%, P = 0.018) in the MC stenting group.
Discussion
These results suggest that DKMC and MC stenting techniques are safe and effective in treating complex CBLs. The incidence of MACE at the 1-year follow-up was similar in both groups, but DKMC was associated with less late lumen loss and restenosis in the side branch, with a numerically lower rate of cumulative MACE and TVR/TLR. In addition, MC stenting for CBLs was associated with significantly increased rates of accumulative incidences of MACE over 5 years of follow-up. DKMC stenting is more effective in treating true CBLs than MC stenting.
Expert consensus has suggested that provisional T-stenting is superior to systematic double-stent techniques and should be used as the initial treatment strategy for most CBLs (class I recommendations). The single-stent strategy is mainly used for SB with mild-to-moderate localized stenosis. A double-stent strategy is conducted for large SB with a high risk of occlusion. In such cases, MB is stented while the ostium of SB is squeezed to occlusion, after which SB (IIA class) can be implanted. Clinically, the biggest technical challenge of remedial implantation of SB stents is the precise positioning of the stent in the SB ostium. Therefore, current recommendations for EBC as a provisional strategy are considered the standard of care where two stenting techniques are reserved for unsuccessful side branch results. Therefore, MC can be used to further save the side branch vessels and for re-revascularization. As DKMC stenting is associated with good clinical outcomes, we evaluated the MC and DKMC stenting bifurcation techniques in this retrospective clinical practice.
Based on the results of several recently randomized clinical trials, there was no sufficient evidence to support an absolute advantage in the clinical efficacy of many CBLs (4, 11, 24). In the EBC TWO study, 200 patients with CBL and significant stenosis were randomized to either a provisional T-stenting or a culotte stenting group. In the provisional group, 60% received T-stenting. At 12 months, the MACE rate was 7.7% for the provisional group and 10.3% for the culotte group (25). Zhang et al. compared provisional stenting (PS) with culotte stenting and found equal MACE rates at 9 months (26). The BBK II trial, involving 300 patients randomized to T-stenting and small protrusion technique (TAP) stenting or culotte stenting, reported restenosis rates of 17% for TAP and 6.5% for culotte (P = 0.006), with 1-year TLR rates of 12.0% and 6.0%, respectively (P = 0.069) (27). Culotte stenting showed lower angiographic restenosis than TAP. Regarding the DK mini-culotte technique vs. T-provisional stenting, Fan et al. reported lower MACE rates (4.55% vs. 13.6%) and TLR/TVR rates (1.52% vs. 12.12%, P = 0.033) in the DK mini-culotte group. SB restenosis was also lower in the DK mini-culotte group (5.6% vs. 22.4%, P = 0.014) (10). However, this study's results, which were based on complex coronary bifurcation lesions while the standard MC stenting was used as a control, suggested that DKMC's technical improvements are successful and effective.
The main difference between MC and DKMC is that DKMC is based on MC and increases the sequential intermediate balloon kissing (siKBD), so the difference between MC and DKMC is likely due to siKBD. Bench test and intravascular ultrasound findings reported that a “napkin,” gap, or metallic ridge is usually seen at the ostial SB after classic culotte stenting, leading to incomplete coverage of the ostial SB and resulting in increased TLR. While MC stenting originates from classic culotte stenting and features fewer struts of SB stretching into the MB lumen, there are still inherent shortcomings associated with MC (28). In addition, clinic trials revealed that an increased number of connectors improve device-related outcomes among a range of contemporary very thin stent models. In contrast, DKMC introduces one time of kissing balloon inflation (siKBD) before stenting the MB. Finishing the procedure with a final kissing balloon inflation enables the full coverage of the ostial SB and reduces restenosis in the ostial SB, which may result in less TVR/TLR (29).
Although antiplatelet therapy was left at the physician's discretion, patients with acute coronary syndrome were enrolled. The duration of DAPT may be associated with different outcomes according to admission diagnosis. Even in complex PCI scenarios such as bifurcation lesions, an extended DAPT strategy was associated with reducing MACE in acute coronary syndromes (ACS) in this cohort. Although there were no convincing data after stenting bifurcation lesions, a complex stenting approach resulted as an independent factor for TVR/TLR. A similar TVR rate (6.3%) after DKMC stenting in the present study was slightly lower than the DK-Crush III previously reported (30). It is possible that both DK crush and DKMC techniques are safe for true CBLs and that optimizing the expansion of the SB stent, as done with the DK crush technique, could lead to a lower risk of TVR/TLR. In addition, in the present study, restenotic lesions were most commonly localized in the SB, which is consistent with the previous results. Thus, we argue that DKMC stenting was superior to MC stenting in the current study.
Limitations
There are several limitations in the present study. First, this was an observational single-center study with a relatively small sample size. Moreover, the 5-year follow-up was inadequate to compare the treatment outcomes after PCI with MC vs. DKMC; thus, the durability of these results on a long-term basis remains unknown.
Conclusion
Our data suggested that DKMC stenting for CBLs medical treatment is associated with less late lumen loss and restenosis in the side branch, with a numerically lower cumulative MACE and TVR/TLR than standard MC stenting; yet these findings need to be further confirmed by a randomized clinical trial.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Union Hospital, Fujian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ST: Writing – original draft. LZ: Data curation, Methodology, Software, Writing – review & editing. QT: Data curation, Visualization, Writing – review & editing. FH: Formal analysis, Investigation, Resources, Writing – review & editing. YW: Data curation, Project administration, Writing – review & editing. LC: Funding acquisition, Investigation, Methodology, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the National Natural Science Foundation of China (grant no. 82170333), the Key Projects of Natural Science Foundation of Anhui Province Universities, China (no. KJ2020A0336), the Key Project of the Natural Science Foundation of Fujian Province, China (no. 2018J01300), the Medical Science and Technology Research Project of Henan Province (grant no. SBGJ202102153), Henan Provincial Science and Technology Research Projects (grant nos. 212102310799 and 222102310577), and the Bozhou City Key R&D Program of Anhui Province of China (nos. bzzc2023060 and bzzc2021050).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1336750/full#supplementary-material
References
1. Burzotta F, Lassen JF, Louvard Y, Lefevre T, Banning AP, Daremont O, et al. European Bifurcation Club white paper on stenting techniques for patients with bifurcated coronary artery lesions. Catheter Cardiovasc Interv. (2020) 96(5):1067–79. doi: 10.1002/ccd.29071
2. Chen X, Li X, Zhang JJ, Han Y, Kan J, Chen L, et al. 3-year outcomes of the DKCRUSH-V trial comparing DK crush with provisional stenting for left main bifurcation lesions. JACC Cardiovasc Interv. (2019) 12(19):1927–37. doi: 10.1016/j.jcin.2019.04.056
3. Chiabrando JG, Lombardi M, Vescovo GM, Wohlford GF, Koenig RA, Abbate A, et al. Stenting techniques for coronary bifurcation lesions: evidence from a network meta-analysis of randomized clinical trials. Catheter Cardiovasc Interv. (2021) 97(3):E306–18. doi: 10.1002/ccd.29097
4. Hitora Y, Teraoka T, Tanaka A, Uemura Y, Tobe A, Sakakibara K, et al. Clinical outcomes following percutaneous coronary intervention for bifurcation lesions: kissing balloon inflation vs. sequential dilation. Cardiovasc Interv Ther. (2021) 36(4):436–43. doi: 10.1007/s12928-020-00728-5
5. Su CS, Chang KH, Lai CH, Chen YW, Lin TH, Pan HC, et al. Acute angiographic and intermediate-term clinical results of patients with non-left main coronary bifurcation lesions treated with BVS by jailed semi-inflated balloon technique and provisional side-branch stenting strategy. J Interv Cardiol. (2019) 2019:9896267. doi: 10.1155/2019/9896267
6. Perfetti M, Fulgenzi F, Radico F, Toro A, Procopio A, Maddestra N, et al. Calcific lesion preparation for coronary bifurcation stenting. Cardiol J. (2019) 26(5):429–37. doi: 10.5603/CJ.a2019.0094
7. Omeh DJ, Shlofmitz E. Restenosis of Stented Coronary Arteries. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2024). PMID: 31424723.
8. Tan S, Ramzy J, Burgess S, Zaman S. Percutaneous coronary intervention for coronary bifurcation lesions: latest evidence. Curr Treat Options Cardiovasc Med. (2020) 22(2):6. doi: 10.1007/s11936-020-0806-4
9. Burzotta F, Lassen JF, Lefevre T, Banning AP, Chatzizisis YS, Johnson TW, et al. Percutaneous coronary intervention for bifurcation coronary lesions: the 15(th) consensus document from the European Bifurcation Club. EuroIntervention. (2021) 16(16):1307–17. doi: 10.4244/EIJ-D-20-00169
10. Fan L, Chen L, Luo Y, Zhang L, Zhong W, Lin C, et al. DK mini-culotte stenting in the treatment of true coronary bifurcation lesions: a propensity score matching comparison with T-provisional stenting. Heart Vessels. (2016) 31(3):308–21. doi: 10.1007/s00380-014-0611-7
11. Raungaard B, Christiansen EH, Botker HE, Hansen HS, Ravkilde J, Thuesen L, et al. Comparison of durable-polymer zotarolimus-eluting and biodegradable-polymer biolimus-eluting coronary stents in patients with coronary artery disease: 3-year clinical outcomes in the randomized SORT OUT VI trial. JACC Cardiovasc Interv. (2017) 10(3):255–64. doi: 10.1016/j.jcin.2016.11.007
12. Adriaenssens T, Byrne RA, Dibra A, Iijima R, Mehilli J, Bruskina O, et al. Culotte stenting technique in coronary bifurcation disease: angiographic follow-up using dedicated quantitative coronary angiographic analysis and 12-month clinical outcomes. Eur Heart J. (2008) 29(23):2868–76. doi: 10.1093/eurheartj/ehn512
13. Toth GG, Sasi V, Franco D, Prassl AJ, Di Serafino L, Ng JCK, et al. Double-kissing culotte technique for coronary bifurcation stenting. EuroIntervention. (2020) 16(9):e724–33. doi: 10.4244/EIJ-D-20-00130
14. Ahn JM, Kang DY, Yun SC, Ho Hur S, Park HJ, Tresukosol D, et al. Everolimus-eluting stents or bypass surgery for multivessel coronary artery disease: extended follow-up outcomes of multicenter randomized controlled BEST trial. Circulation. (2022) 146(21):1581–90. doi: 10.1161/CIRCULATIONAHA.122.062188
15. Filion KB, Azoulay L, Platt RW, Dahl M, Dormuth CR, Clemens KK, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med. (2016) 374(12):1145–54. doi: 10.1056/NEJMoa1506115
16. Hu F, Tu S, Cai W, Jiang Z, Zheng H, Xiao L, et al. Double kissing mini-culotte versus mini-culotte stenting: insights from micro-computed tomographic imaging of bench testing. EuroIntervention. (2019) 15(5):465–72. doi: 10.4244/EIJ-D-18-00688
17. Chen E, Cai W, Chen LL. Crush versus culotte stenting techniques for coronary bifurcation lesions: a systematic review and meta-analysis of clinical trials with long-term follow-up. Medicine (Baltimore). (2019) 98(14):e14865. doi: 10.1097/MD.0000000000014865
18. Nakao F. Optimization of proximal optimizing technique and re-proximal optimizing technique: let us re-heat the POT! Int J Cardiol. (2019) 292:98–9. doi: 10.1016/j.ijcard.2019.06.019
19. Cai W, Chen L, Zhang L, Tu S, Fan L, Chen Z, et al. Branch ostial optimization treatment and optimized provisional t-stenting with polymeric bioresorbable scaffolds: ex-vivo morphologic and hemodynamic examination. Medicine (Baltimore). (2018) 97(43):e12972. doi: 10.1097/MD.0000000000012972
20. Baumbach A, Bourantas CV, Serruys PW, Wijns W. The year in cardiology: coronary interventions. Eur Heart J. (2020) 41(3):394–405. doi: 10.1093/eurheartj/ehz947
21. Zeymer U. Diagnosis and initial management of acute myocardial infarction. MMW Fortschr Med. (2019) 161(4):34–6. doi: 10.1007/s15006-019-0223-3
22. Lee MS, Tadwalkar RV, Fearon WF, Kirtane AJ, Patel AJ, Patel CB, et al. Cardiac allograft vasculopathy: a review. Catheter Cardiovasc Interv. (2018) 92(7):E527–536. doi: 10.1002/ccd.27893
23. Ullrich H, Munzel T, Gori T. Coronary stent thrombosis—predictors and prevention. Dtsch Arztebl Int. (2020) 117(18):320–6. doi: 10.3238/arztebl.2020.0320
24. Ormiston JA, Webster MW, Webber B, Stewart JT, Ruygrok PN, Hatrick RI. The “crush” technique for coronary artery bifurcation stenting: insights from micro-computed tomographic imaging of bench deployments. JACC Cardiovasc Interv. (2008) 1(4):351–7. doi: 10.1016/j.jcin.2008.06.003
25. Hildick-Smith D, Behan MW, Lassen JF, Chieffo A, Lefevre T, Stankovic G, et al. The EBC TWO study (European bifurcation coronary TWO): a randomized comparison of provisional T-stenting versus a systematic 2 stent culotte strategy in large caliber true bifurcations. Circ Cardiovasc Interv. (2016) 9(9):e003643. doi: 10.1161/CIRCINTERVENTIONS.115.003643
26. Zhang L, Zhong W, Luo Y, Chen L. A pilot study on culottes versus crossover single stenting for true coronary bifurcation lesions. Acta Cardiol Sin. (2016) 32(4):450–9. doi: 10.6515/acs20151112a
27. Ferenc M, Gick M, Comberg T, Rothe J, Valina C, Toma A, et al. Culotte stenting vs. TAP stenting for treatment of de-novo coronary bifurcation lesions with the need for side-branch stenting: the Bifurcations Bad Krozingen (BBK) II angiographic trial. Eur Heart J. (2016) 37(45):3399–405. doi: 10.1093/eurheartj/ehw345
28. Foin N, Torii R, Mortier P, De Beule M, Viceconte N, Chan PH, et al. Kissing balloon or sequential dilation of the side branch and main vessel for provisional stenting of bifurcations: lessons from micro-computed tomography and computational simulations. JACC Cardiovasc Interv. (2012) 5(1):47–56. doi: 10.1016/j.jcin.2011.08.019
29. Raphael CE, O'Kane PD. Contemporary approaches to bifurcation stenting. JRSM Cardiovasc Dis. (2021) 10:2048004021992190. doi: 10.1177/2048004021992190
30. Zhang YJ, Zhu H, Shi SY, Muramatsu T, Pan DR, Ye F, et al. Comparison between two-dimensional and three-dimensional quantitative coronary angiography for the prediction of functional severity in true bifurcation lesions: insights from the randomized DK-CRUSH II, III, and IV trials. Catheter Cardiovasc Interv. (2016) 87(Suppl 1):589–98. doi: 10.1002/ccd.26405
Keywords: percutaneous coronary intervention, coronary bifurcation lesions, culotte stenting, stent thrombosis, DK mini-culotte stenting
Citation: Tu S, Zhang L, Tian Q, Hu F, Wang Y and Chen L (2024) Five-year outcomes of double kissing mini-culotte stenting vs. mini-culotte stenting using drug-eluting stents for the treatment of true coronary bifurcation lesions. Front. Cardiovasc. Med. 11:1336750. doi: 10.3389/fcvm.2024.1336750
Received: 11 November 2023; Accepted: 20 March 2024;
Published: 9 April 2024.
Edited by:
Mauro Malvè, Public University of Navarre, SpainReviewed by:
Abhinav Grover, Medical College of Wisconsin, United StatesWolfgang Rottbauer, Ulm University Medical Center, Germany
© 2024 Tu, Zhang, Tian, Hu, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianglong Chen lianglongchenuh@126.com
†These authors have contributed equally to this work
 Sheng Tu1,2,3,4,†
Sheng Tu1,2,3,4,†  Qingqing Tian
Qingqing Tian Lianglong Chen
Lianglong Chen