Effects of high-intensity interval exercise on arterial stiffness in individuals at risk for cardiovascular disease: a meta-analysis
- 1School of Physical Education, Wuhan Sport University, Wuhan, China
- 2School of Physical Education, Hunan University of Science and Technology, Xiangtan, China
Objective: The purpose of this meta-analysis was to investigate the effect of high-intensity interval training (HIIT) on arterial stiffness (AS) and vascular function in persons at high risk of cardiovascular disease (CVD).
Methods: We conducted a comprehensive search of randomized controlled trials (RCTs) published in electronic databases (PubMed, Web of Science, Cochrane, Embase, and Ebsco) since their inception through October 2023 to evaluate the effect of HIIT on AS and vascular function in persons at high risk for CVD. The weighted mean difference (WMD) and 95% confidence intervals (95% CI) were calculated, and heterogeneity was assessed using the I2 test.
Results: This study included 661 participants from 16 studies. HIIT significantly reduced pulse wave velocity (PWV) in persons at high risk for CVD [weighted mean difference (WMD), −0.62; 95% CI, −0.86–−0.38; P < 0.00001]. Subgroup analysis showed that the PWV improvement effect was better when the HIIT program was performed 2–3 times per week and the duration was controlled within 40 min [2–3 times, −0.67; 95% CI, −0.93–−0.41; P < 0.00001; time of duration, ≤40 min, −0.66; 95% CI, −0.91–−0.41; P < 0.00001]. HIIT significantly reduced systolic blood pressure (SBP, −5.43; 95% CI, −8.82–−2.04; P = 0.002), diastolic blood pressure (DPB, −2.96; 95% CI, −4.88–−1.04; P = 0.002), and resting heart rate (RHR, −4.35; 95% CI, −7.04–−1.66; P = 0.002), but had no significant effect on augmentation index (AIX, −2.14; 95% CI, −6.77–2.50; P = 0.37).
Conclusion: HIIT can improve PWV in high-risk individuals with CVD and reduce SBP, DBP, and RHR, but has no significant effect on AIX. HIIT can effectively improve AS and vascular function and can be recommended as an effective method to improve AS in high-risk persons with CVD.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42023471593.
1 Introduction
Cardiovascular disease CVD is one of the leading causes of mortality worldwide and a major risk factor for morbidity worldwide (1). Therefore, it is necessary to reduce the incidence and risk factors of CVD, as physical activity is known to reduce mortality from CVD (2). Changes in CVD risk factors, such as body weight, blood pressure, and lipids, explain 60% of the beneficial effects of exercise on major CVD outcomes, and the remaining 40% of the reduction in risk factors is associated with vascular hemodynamics (3, 4). Arterial stiffness (AS) is a strong independent predictor of all-cause mortality owing to cardiovascular events (5–8). Increased AS can lead to increased blood pressure, left ventricular hypertrophy, decreased ventricular diastolic function, coronary ischemic disease, and decreased sensitivity to arterial baroreflex (9–12). Therefore, preventing CVD by reducing the incidence of AS is essential.
Pulse wave velocity (PWV) is the gold standard for measuring AS, especially carotid-femoral pulse velocity (CF-PWV) (13). An increase of 1 m/s in PWV is associated with 14% and 15% increased risk of cardiovascular events and mortality, respectively (5). Therefore, finding effective interventions to reduce PWV is one of the main goals for preventing CVD and improving cardiovascular function (14, 15).
Exercise improves blood vessel function, and many meta-analyses on exercise and cardiovascular function have shown that appropriate exercise can effectively improve AS in different populations (16–20). Studies on the effect of exercise on AS mainly focus on sustained aerobic exercise. Previous meta-analyses have shown that continuous aerobic exercise is effective in reducing AS in hypertensive, elderly, and obese populations (16, 17, 21, 22). Although sustained aerobic exercise has many benefits in terms of improving blood vessel function, there are also some drawbacks; for example, participants tend to have an aversion to exercise due to the monotony of exercise forms, which reduces the beneficial effects of exercise (23). Conversely, HIIT has been shown to be equally or even better at stimulating health benefits than moderate-intensity continuous training (MICT) and is considered a time-saving aerobic exercise (24, 25). Additionally, HIIT has a higher level of compliance than MICT, and the benefits of exercise are even higher (25–28). Therefore, HIIT can be used as an effective alternative to continuous aerobic exercise (25–28). Previous studies have shown that resistance training has no harmful effects on AS in individuals at high risk of CVD (29). However, the effect of HIIT on AS in persons at a high risk of CVD or in those carrying high-risk factors for CVD is inconclusive.
Therefore, this study aimed to conduct a comprehensive systematic review and meta-review of randomized controlled trials (RCTs) to explore whether HIIT improves PWV, augmentation index (AIX), SBP, DBP, and RHR in persons at high risk for CVD. In addition, subgroup analyses were performed to examine whether HIIT intervention programs affected the effect of PWV improvement. The results of this study will provide theoretical references and suggestions for the exercise program of high-risk groups with CVD in the future.
2 Materials and methods
2.1 Trial registration
This systematic review and meta-analysis followed the PRISMA (Preferred Reporting Program for Systematic Reviews and Meta-Analysis) guidelines (30). The study topics and proposals were registered with PROSPERO (CRD42023471593).
2.2 Search strategy
Two experienced researchers (PL and RSW) applied the PICOS principles (subjects, interventions, comparisons, outcomes, and study design) to search electronic databases (PubMed, Web of Science, Cochrane, Embase, and Ebsco) from their inception until October 2023. For searches in PubMed/Cochrane, and Embase, terms from MeSH and Emtree were used, respectively. Search formula: (“High intensity interval training” OR “Interval training” OR “Aerobic interval training” OR “Combination training” OR “Intermittent training”) AND (“Arterial stiffness” OR “Vascular stiffness” OR “Aortic stiffness” OR “Pulse wave velocity” OR “Augmentation index”). Supplementary Table S1 provides specifics detail of the search method that was used for each database. If there was any disagreement between the two authors, a third author (WFG) participated in the discussion until a consensus was reached.
2.3 Eligibility criteria
Articles included in the analysis must meet the following criteria: (1) Randomized controlled trials of HIIT vs. no exercise, usual care, or sedentary; (2) HIIT program lasting at least 6 weeks or a combination of HIIT and other forms of training; (3) According to the American College of Sports Medicine (ACSM) guidelines,participants have CVD, have CVD risk factors, or are individuals with high levels of circulating pro-inflammatory factors; (4) Provide at least one central PWV or peripheral PWV outcome measure; and (5) The article is written in English. Interval exercise can be broadly defined as a short to moderate period (10 s to 5 min) of repetitive training sessions with an intensity above the anaerobic threshold, with intervals of low-intensity activity or rest between training sessions, usually without complete recovery (31, 32). According to the ACSM guidelines, high intensity is defined as a person's estimated >80%HR max (33), >65%VO2 peak, >60%HR reserve/VO2 reserve, or >14 Borg rating of perceived exertion (RPE), this work also includes sprint interval training, which is usually performed at “all out” extreme intensity for relatively short periods of time (34). According to the above criteria, the literature meeting the criteria is included in this paper.
2.4 Data extraction
Two researchers (PL and RSW) independently used the same standardized tables created in Microsoft Excel for data extraction, and any disagreements were resolved through discussion with another researcher (WFG). Information extracted from eligible articles included: (1) Document characteristics (author, publication year, country); (2) Participant characteristics (sample size, age, proportion of women, disease status/risk factors, and medication use); (3) Intervention characteristics (exercise type, exercise cycle, duration, exercise intensity, exercise frequency, total exercise change, and interval time); and (4) Outcome measures. If the data is missing, the researcher (PL) contacts the original author by e-mail to obtain the missing data.
2.5 Quality assessment and sensitivity analyses
The Physiotherapy Evidence Database tool (PEDro) was used to evaluate the study quality. Studies with scores ≥6 were considered high quality, 4–5 were average quality, and <4 were low quality. The rating scale contained 11 items as follows: (1) The inclusion conditions of the subjects were clear; (2) Random allocation; (3) Grouping blind; (4) The main prognostic indicators were consistent at baseline; (5) The subjects were blind; (6) Training blind; (7) Blind evaluators for at least one major outcome; (8) More than 85% of the subjects were measured for at least one major outcome; (9) Subjects received treatment or control conditions according to the assignment plan; (10) Report intergroup statistical results for at least one major outcome; (11) Provide point measurements and variation measurements for at least one major result. Two reviewers (PL and RSW) independently assessed methodological quality. We excluded blind entries from the original 11-item scale (group blinds, subject blinds, training blinds, and all evaluators of at least one major outcome blinds). This is because participants' control over blinding conditions cannot be guaranteed during the intervention. Evaluation disagreements among reviewers are resolved by discussing and reaching a consensus with the third author (WFG). Supplementary Table S1 provides specific quality assessment details. A sensitivity analysis was performed by excluding each study individually to determine whether the results changed.
2.6 Statistical analysis
Anterior and posterior mean and SD values for PWV, AIX, SBP, DBP, and RHR were extracted from each study, and we calculated the change value of the data before and after the intervention to achieve the combined effect to make the forest map. The Q statistic was used to determine the inter-study heterogeneity, and statistical significance was set at P < 0.1. I2 values were used to evaluate heterogeneity. I2 values of 0%, 25%, 50%, and 75% indicated no heterogeneity, mild heterogeneity, moderate heterogeneity, and high heterogeneity, respectively. When heterogeneity is low, the fixed effects model is used to combine the data, in any case, the random effects model is used. In the subgroup analysis, we conducted a subgroup analysis of subjects' characteristics (male-female ratio, body mass index, and medication situation), interval intervention characteristics (total exercise change, exercise cycle, exercise duration, and interval duration), and baseline characteristics to explore the effect of different conditions on PWV. Analysis results and forest maps were generated using Review Manager (RevMan) 5.3 (The Cochrane Collaboration, Copenhagen, Denmark, 2019) software. Overall, P < 0.05 was considered statistically significant. Stata17.0 (Stata Corporation, College Station, TX; US) software was used to generate funnel plots, visual tests of Begg's tests were performed to discuss publication bias, and statistical significance was set at P < 0.1.
3 Results
3.1 Study selection
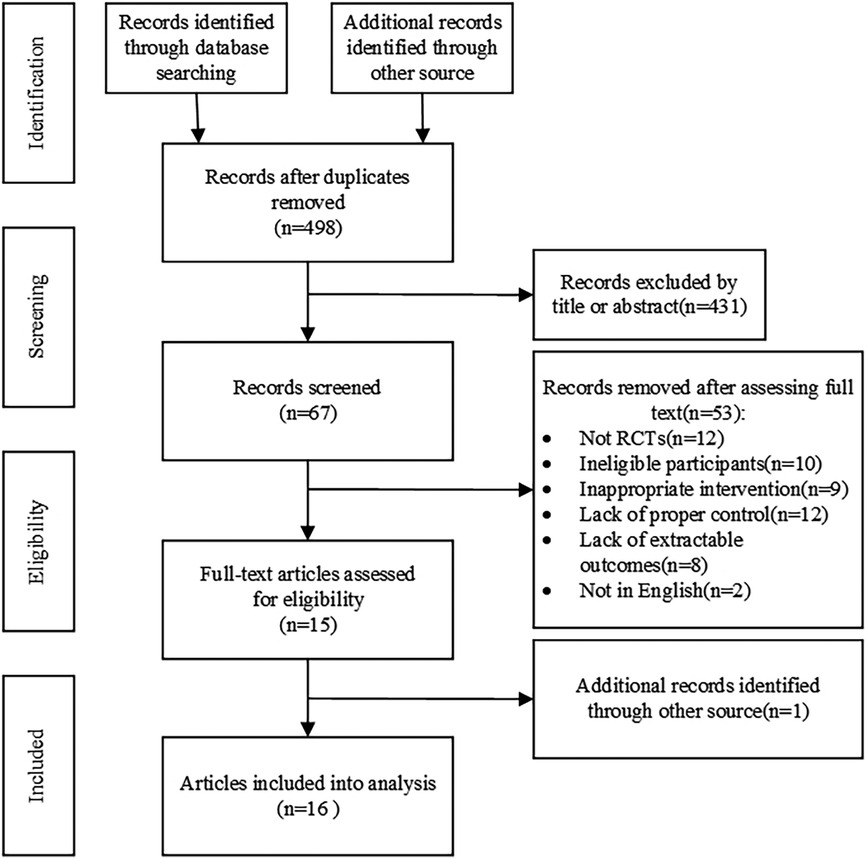
Figure 1 summarizes the research process for screening eligible conditions; 1,009 records were retrieved. After deleting duplicate literature, 67 records were found to be suitable by screening the titles and abstracts of the remaining literature; full-text screening was performed, and a total of 15 eligible references were obtained, and 1 was retrospectively obtained by other means. Sixteen studies were included in the meta-analysis. Fifty-three articles were excluded for the following reasons: not a randomized controlled trial (n = 12); subjects did not match (n = 10); the experimental intervention did not match (n = 9); the control group did not conform (n = 12); outcome index did not meet (n = 8); and language did not match (n = 2).
3.2 Characteristics of included studies
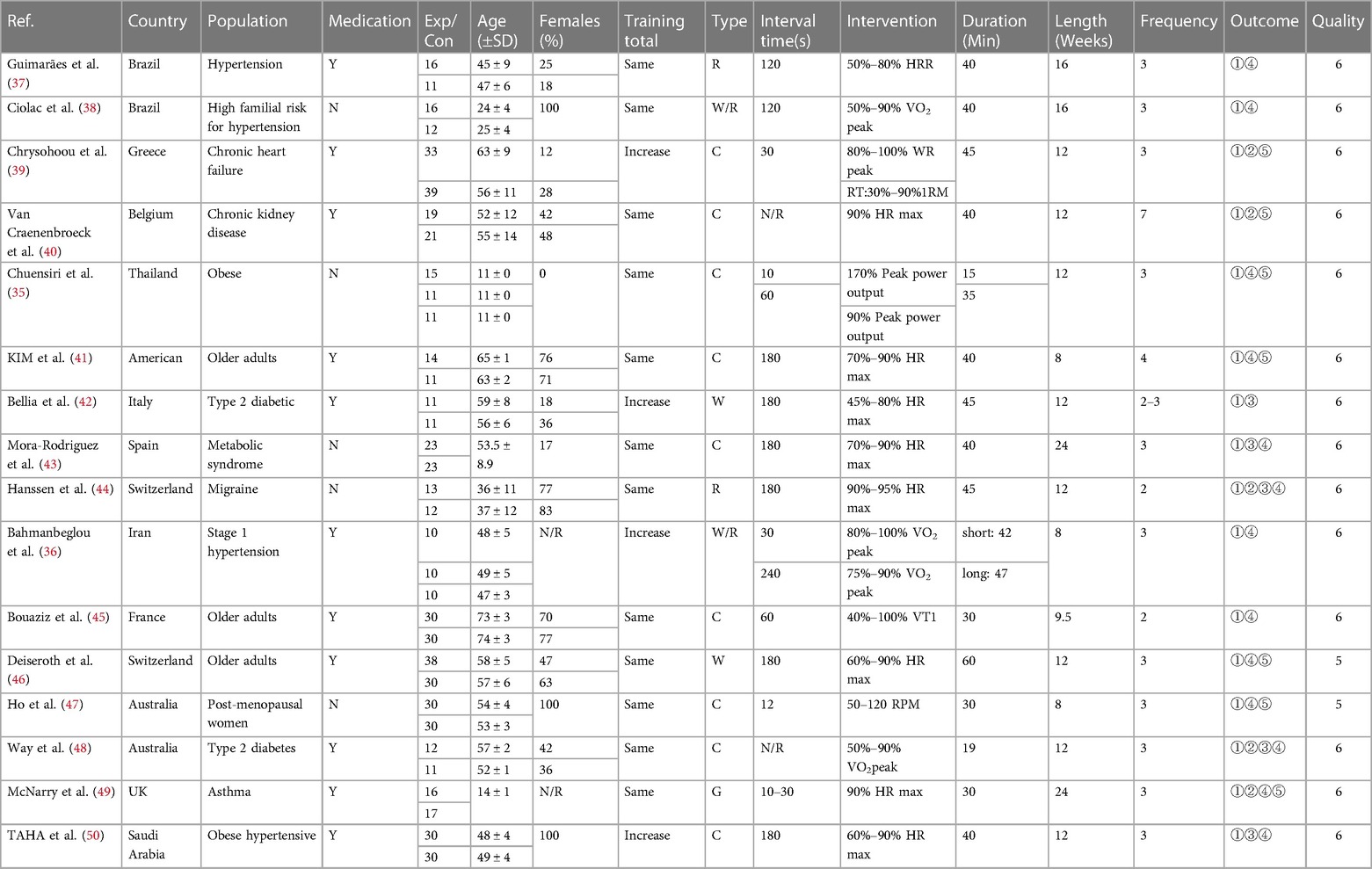
After screening, 16 studies and 18 research reports were included in this meta-analysis, among which two studies were extracted from the literature by Chuensiri et al. (35) and Bahmanbeglou et al. (36). The 18 research reports had a total sample size of 656, with 347 participants in the experimental group and 314 participants in the control group (Table 1).
Participants ranged in age from 11–75 years, with middle-aged and elderly studies accounting for the majority. 11 studies included medication use during the intervention period (36, 37, 39–42, 45, 46, 48–50), and 5 studies explicitly indicated no medication treatment during the intervention period (35, 38, 43, 44, 47). 6 studies included women with a female ratio of >70 percent (38, 41, 44, 45, 47, 50), three of which had all-female participants (38, 47, 50), and 2 studies did not report a sex ratio (36, 49). High-intensity interval training was used in all the included studies, which included 4 incremental exercises (36, 39, 42, 50), and the rest were equal exercises (35, 37, 38, 40, 41, 43–45, 47–49). 9 studies used bicycle intervention (35, 39–41, 43, 45, 47, 48, 50), 6 studies used treadmill intervention (36–38, 42, 44, 46), and 1 study used a game form (49). The intermittent time ranged from 10–240s, and most of the single intervention times were approximately 40 min. Except for KIM et al. (41) and Van Craenenbroeck et al. (40), 2 studies intervened 4 and 7 times per week, respectively, and the other studies intervened 2–3 times per week, with an intervention cycle greater than or equal to 8 weeks. PWV was tested in 16 studies,of which 10 used carotid-femoral PWV to assess AS (37–41, 43, 45, 46, 48, 49), and 1 used central aortic PWV to in review assess AS (44). 3 studies provided brachial-ankle PWV (35, 47, 50), 2 studies provided carotid-radial PWV (42, 45), and 1 study did not report specific AS assessment methods (36). In the subgroup analysis, we classified the carotid-femoral PWV and central aortic PWV as the central PWV for statistical analysis, while the brachial-ankle PWV and the carotid-radial PWV were divided into peripheral PWV for data analysis. 5 articles provided AIX (39, 40, 44, 48, 49) and 5 provided AIX-75 (42–44, 48, 50). Brachial arterial blood pressure was reported in 13 studies (35–38, 41, 42, 44–50). RHR before and after the intervention was reported in 7 studies (35, 39–41, 46, 47, 49).
3.3 Risk of bias
A funnel plot was constructed using Stata software to visually assess the risk offset (Figure 2). Since there were less than ten samples of RHR, a funnel plot was not drawn.
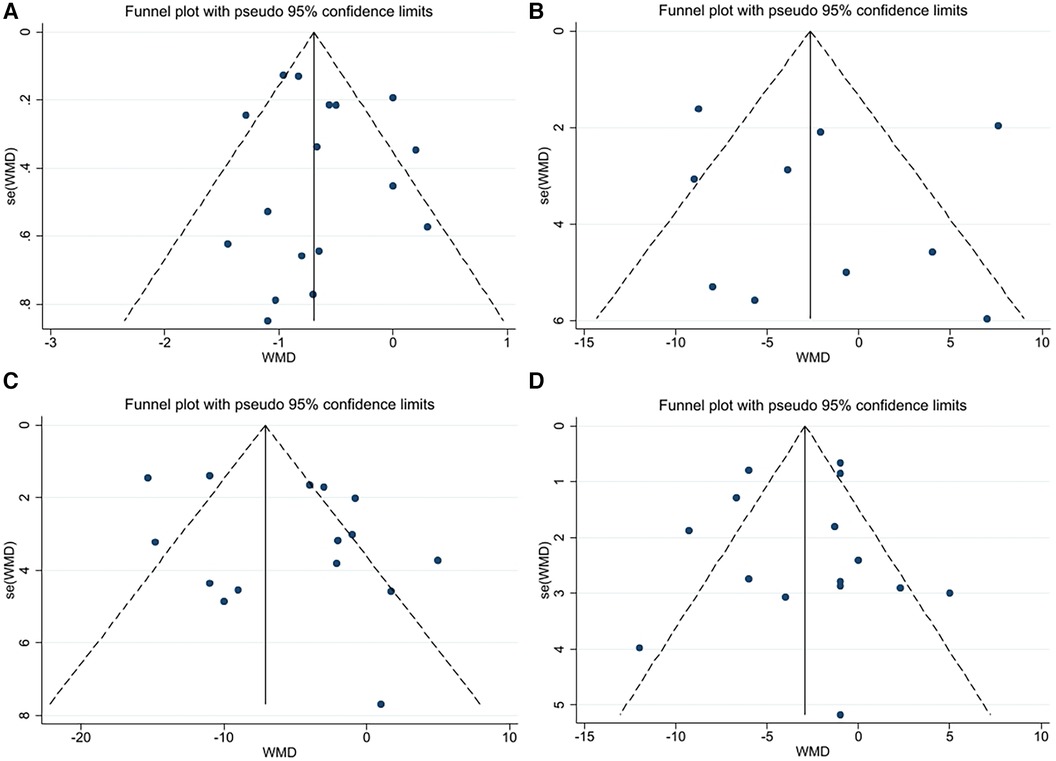
Figure 2. (A) Funnel plot for PWV. (B) Funnel plot for AIX. (C) Funnel plot for SBP. (D) Funnel plot for DBP.
3.4 Results of meta-analysis
3.4.1 Effect of high-intensity interval exercise on pulse wave velocity
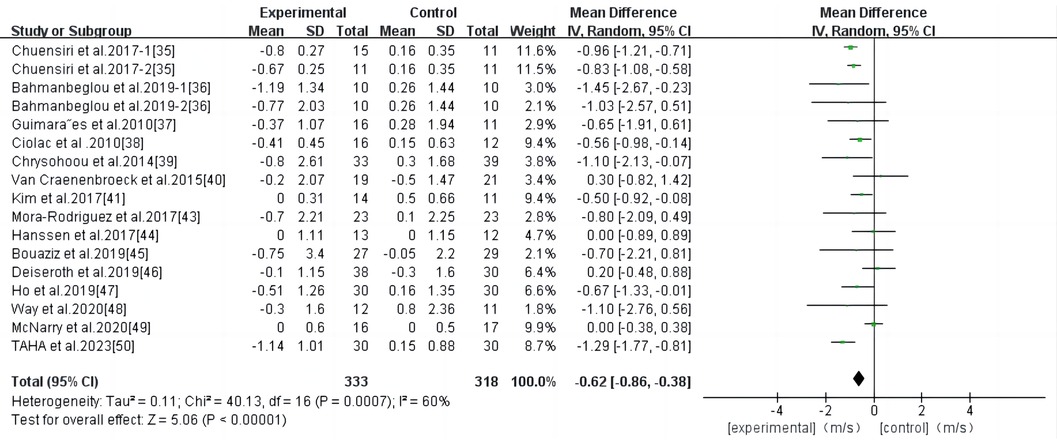
Eighteen studies were included in the analysis, and the results of the meta-analysis (Figure 3) showed a high degree of heterogeneity among the studies (I2 = 83%, P < 0.00001). Using the random effects model, the total effect size of HIIT significantly reduced PWV (WMD, −0.77; 95% CI, −1.11–−0.43]. Sensitivity analysis was used to exclude the included studies individually and assess the impact of each study on the PWV effect size. After the exclusion of the study by Bellia et al. (42), the heterogeneity was significantly reduced (I2 = 60), while the combined effect size was slightly reduced (WMD, −0.62; 95% CI, −0.86–−0.38), the results remained statistically significant (P < 0.00001); therefore, the combined analysis of this study was excluded. Begg and Egger's tests showed that there was no significant bias in the included studies (P > 0.1).
3.4.2 Effect of high-intensity interval exercise on augmentation index
HIIT had no significant effect on the AIX in the high-risk individuals for CVD (WMD, −2.14; 95% CI, −6.77–2.50; P = 0.37) (Figure 4). There was a high heterogeneity among the studies (I2 = 83%). Begg and Egger's tests showed no significant bias in the included studies (P > 0.1).
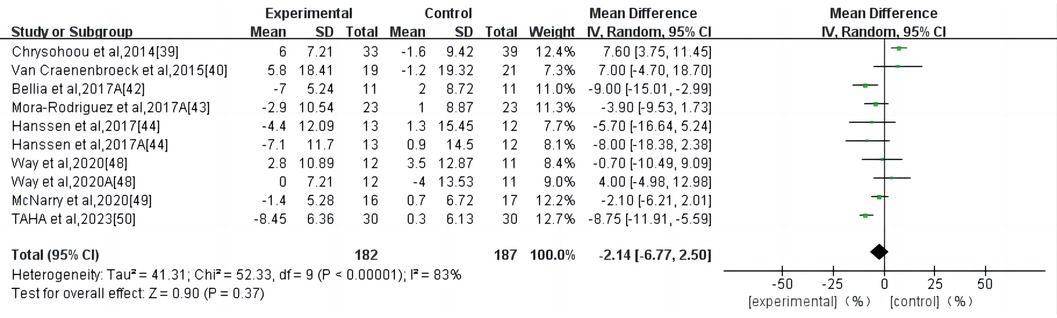
Figure 4. Meta-analysis results of high-intensity interval exercise on AIX. (A) AIX for 75 beats per minute.
3.4.3 Effect of high-intensity interval exercise on systolic blood pressure
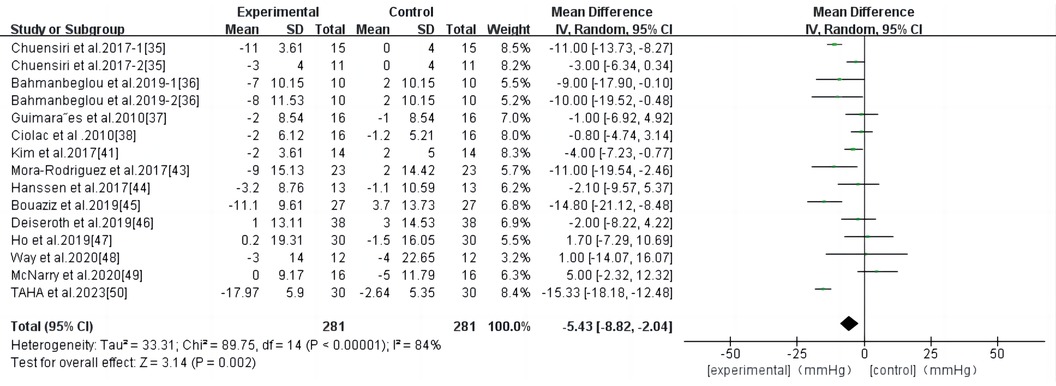
HIIT had a significant effect on SBP in persons with high-risk CVD (WMD, −5.43; 95% CI, −8.82–−2.04; P = 0.002) (Figure 5). There was a high heterogeneity among the studies (I2 = 84%). Begg and Egger's tests showed no significant bias in the included studies (P > 0.1).
3.4.4 Effect of high-intensity interval exercise on diastolic blood pressure
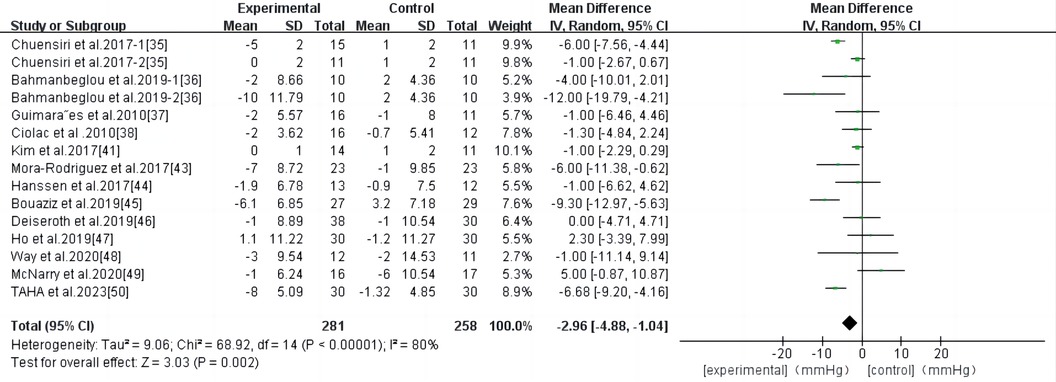
HIIT had a significant effect on DBP in persons with high-risk CVD (WMD, −2.96; 95% CI, −4.88–−1.04; P = 0.002) (Figure 6). There was significant heterogeneity among the studies (I2 = 80%). Begg and Egger's tests showed no significant bias in the included studies (P > 0.1).
3.4.5 Effect of high-intensity interval training on resting heart rate
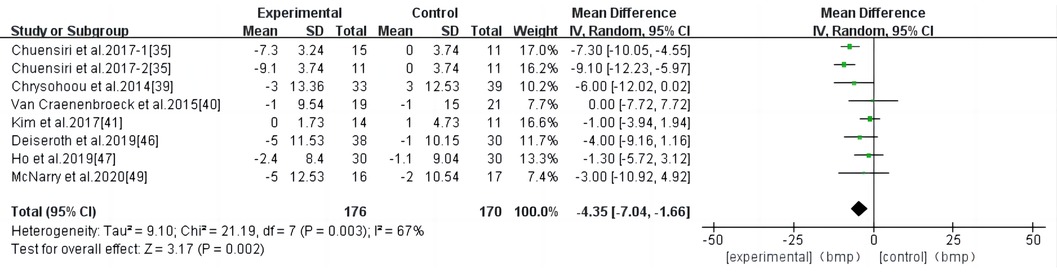
HIIT had a significant effect on RHR in individuals at a high risk of CVD (WMD, −4.35; 95% CI, −7.04–−1.66; P = 0.002) (Figure 7). There was a high heterogeneity among the studies (I2 = 67%). Begg and Egger's tests showed no significant bias in the included studies (P > 0.1).
3.4.6 Subgroup analysis
A subgroup analysis was performed based on the PWV measurement location, baseline blood pressure, participant characteristics (age, medication use, and body mass index), and exercise intervention characteristics (total exercise change, exercise cycle, exercise frequency, exercise duration, and interval time). Data from the study by Bellia et al. (42) were excluded and are summarized in Table 2.
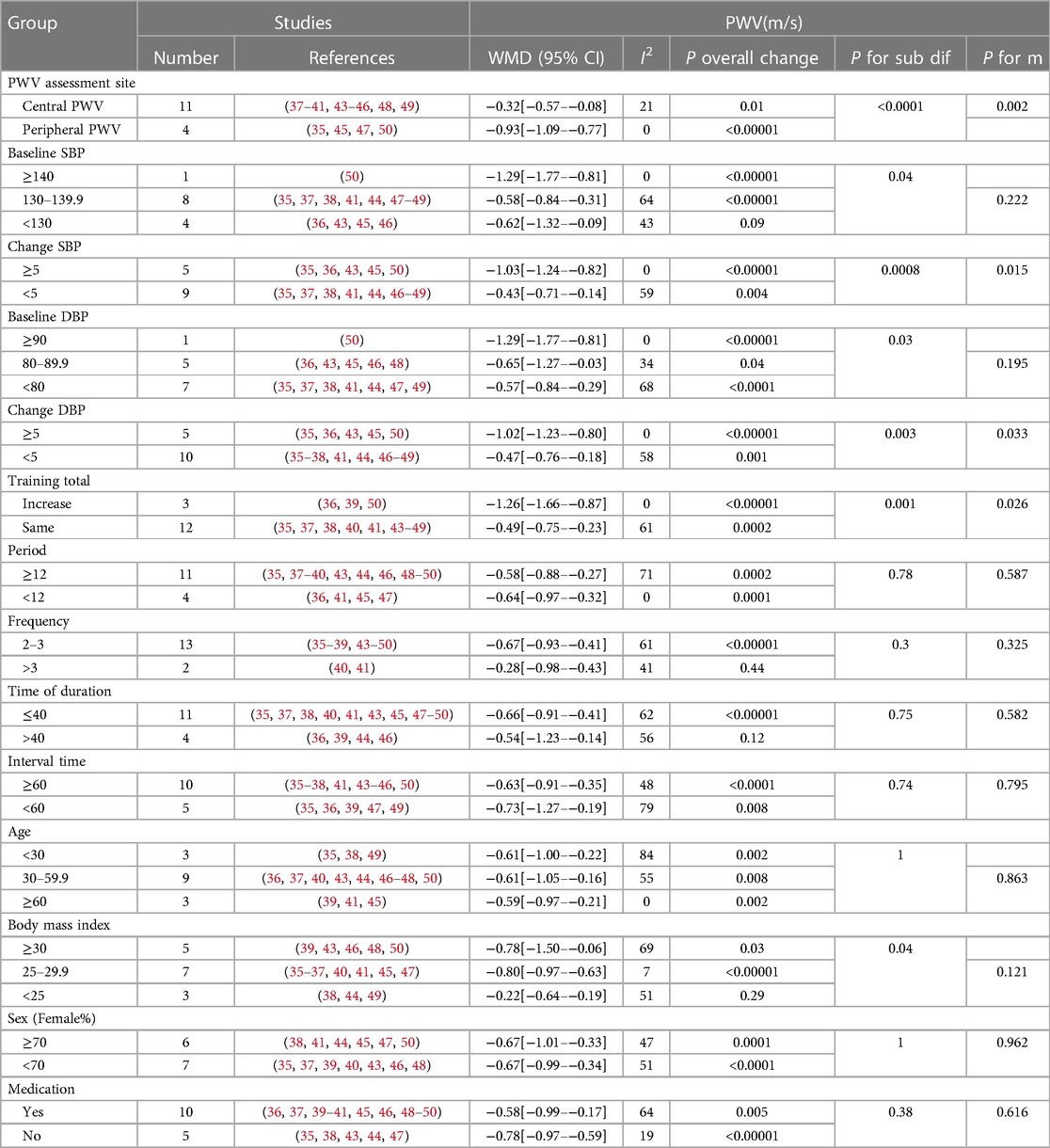
Table 2. Subgroup analyses assessing potential moderating factors for PWV in studies included in the meta-analysis.
4 Discussion
To our knowledge, this is the first meta-analysis of the effects of HIIT on AS and vascular function in persons with or carrying risk factors for CVD. This meta-analysis suggests that HIIT is effective in improving PWV in persons with CVD or risk factors for CVD. Performing HIIT 2–3 times/week for ≤40 min optimizes PWV improvement. HIIT also significantly improved SBP, DBP, and RHR in persons with CVD or risk factors for CVD but did not affect the AIX.
PWV is effective in assessing AS based on ultrasound measurements (51). The mechanisms leading to increased AS are complex, and oxidative stress and inflammation are the main causes of arteriosclerosis (52, 53). PWV is also associated with endothelial cells and smooth muscle function (54, 55). In addition, various substances and hormone levels in the body can affect AS, including nitric oxide (56–58), vasoconstrictors (59), advanced glycosylation end products (60), oxidized low-density lipoprotein (61), and aldosterone (62). Clinical studies have demonstrated that exercise can promote the production of nitric oxide and enhance its bioavailability to reduce AS (58). Published meta-analyses have shown a positive dose-response relationship between exercise intensity and AS improvement (18). This means that higher-intensity exercise may have a better effect on AS. Meanwhile, the physical activity guidelines issued by the ACSM recommend high-intensity exercise to maintain and improve cardiovascular health (63). Published meta-analyses have shown that aerobic and combined exercises (aerobic exercise combined with resistance) can effectively improve PWV in different populations (16–22). Consistent with previous findings, we showed that HIIT improved AS in persons with CVD or those at high risk for CVD (−0.62 m/s). According to the meta-analysis of Li et al. (17), long-term aerobic training can significantly reduce PWV (−0.75 m/s) in middle-aged and elderly people. A meta-analysis of adults aged ≥18 years by Ashor et al. (19) found that aerobic exercise significantly reduced PWV (−0.63 m/s). Zhang et al. (20) concluded that aerobic training significantly reduced cf-PWV in CVD population, and the result was similar to ours (−0.42 m/s vs. −0.32 m/s). The above evidence suggests that HIIT has a similar and more time-saving effect in improving PWV compared to MICT. While a small percentage of the interval literature we included would have been included in a meta-analysis of related aerobic exercise, we are the first to conduct a meta-analysis of HIIT with individuals who carry or have CVD risk factors, as well as a subgroup analysis of factors, such as intermittent exercise program and subject characteristics.
In the subgroup analysis of baseline blood pressure and pre and post intervention blood pressure changes, we observed that, while most groups achieved significant levels, the higher the baseline blood pressure or the greater the pre- and post-training blood pressure change, the more significant the PWV reduction. A published meta-analysis of aerobic exercise in hypertensive populations corroborates our results (17, 20). Montero et al. (64) concluded that aerobic training does not reduce AS in patients with (prehypertension) unless SBP is significantly reduced, or its duration is prolonged. This conclusion was confirmed by subgroup analysis of blood pressure changes (≥5) before and after HIIT intervention. Previous studies have also shown that PWV is highly correlated with blood pressure (65). A subgroup analysis of HIIT programs showed that HIIT performed 2–3 times per week for no more than 40 min resulted in a significant improvement in PWV. First, 90% of the studies were conducted two to three times a week and lasted ≤40 min. Therefore, it also weakens the strength of the evidence for this result. Second, although HIIT is better than MICT in terms of improving the heart, cardiovascular, and metabolic conditions, and HIIT can produce exercise enjoyment and exercise compliance similar to or better than MICT (66), it does not mean that longer or more frequent HIIT can achieve better exercise results, especially in CVD people. ACSM emphasizes that reasonable exercise intensity and frequency are more conducive to the recovery of the body, and also recommends HIIT once a week, and then increase the intensity and frequency of exercise when the body gradually to the intensity (33). This also reasonably explains why incremental training improved PWV better (−1.26 m/s vs. −0.49 m/s) than the same amount of training in the total training variation subgroup. We also found that PWV improved better in overweight or obese people who had or carried risk factors for cardiovascular disease. Studies have shown that excessive adipose tissue can produce a variety of the pro-inflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and plasminogen activator inhibitor, which leads to inflammation and deterioration of arterial stiffness (67). At the same time, inflammation and oxidative stress caused by obesity inhibit the maturation and secretion of adiponectin (68), and excess fat in the body reduces adiponectin levels, further exacerbating metabolic disorders (69, 70). Experimental studies have shown that adiponectin plays a decisive role in energy metabolism, anti-inflammatory, and cardiovascular health (71). Effective inhibition of adipose tissue during HIIT may be a reasonable explanation for this result.Numerous studies have shown that HIIT is effective in reducing body weight, visceral fat, body mass index, and subcutaneous adipose tissue in overweight and obese people (72–75). The above evidence explains why PWV improvements are better in overweight or obese people who have or carry cardiovascular disease risk factors. Finally, the positive effect of HIIT on PWV was independent of anti-hypertensive medication use, PWV assessment location, and male-to-female ratio. In future studies, it is necessary to expand the study of HIIT for a specific CVD population to find the best personalized program for different CVD populations, and provide a rich theoretical basis and practical examples for the prevention of arterial stiffness in common CVD populations.
We also performed a meta-analysis of AIX, SBP, DBP, and RHR and found that HIIT has no significant effect on AIX but has a significant effect on SBP, DBP, and RHR. Different movement types are purely in dispute with AIX. For example, Ashor et al. (19) concluded that aerobic exercise can effectively reduce AIX in adults. Zhang et al. (20) concluded that long-term aerobic exercise can effectively reduce AIX of patients aged 50–60 years. These results were different from ours. However, a recently published meta-study by Li et al. (22) showed that regular aerobic exercise did not affect the AIX in obese and overweight older adults with or without comorbidities. Participants included in studies by Chrysohoou et al. (39) and Van Craenenbroeck et al. (40) fit the category of obese and overweight elderly persons. Chrysohoou et al. (39) also carried out related resistance exercises in the program, and many studies have shown that resistance exercises have adverse effects on AS (76–79). This may explain the reason why AIX was not significant in our study. Secondly, both articles used disease-related medications during the intervention period, and AIX increased significantly in the HIIT group after the intervention, while no significant change was observed in the control group. Perhaps there was an adverse reaction between some medications and HIIT, which resulted in the statistically insignificant effect of AIX in this study. In the future, more experimental studies are needed to prove whether there are adverse effects of medication use in combination with HIIT treatment. Finally, the small number of studies may also be one of the reasons why AIX is not significant in this article.
Many studies have shown that HIIT can positively affect blood pressure and is superior to MICT (25, 27, 28). We arrived at similar conclusions that HIIT significantly improved blood pressure and RHR in persons with CVD or risk factors for CVD. A decrease of 5 mmHg in SBP or 2 mmHg in DBP was associated with a 14% reduction in stroke mortality and a 9% reduction in coronary heart disease mortality (80, 81). We concluded that HIIT reduced SBP and DBP by 5.43 mmHg and 2.96 mmHg, respectively, with significant implications for reducing CVD mortality. Therefore, HIIT is a viable and effective treatment for lowering blood pressure. RHR is easy to obtain, non-invasive, inexpensive, and can be used as an independent indicator of risk factors and mortality in patients with CVD (82–84), which is more suitable for clinical application than other indicators (84). Reducing RHR may be a therapeutic target in the clinic, and an increase of 10 beats per min in RHR in patients with coronary heart disease is associated with an 8% increase in cardiovascular risk (85). We also conducted a meta-analysis of RHR and found that HIIT significantly reduced RHR by 4.35 beats/min, which reached a significant level. Therefore, HIIT has a positive effect on reducing cardiovascular risk by reducing RHR.
4.1 Limitations of the review
This study has some limitations. First, because the literature on HIIT's effect on AS in individuals at high risk for CVD is limited, we cannot consider the effect of HIIT on a particular high-risk population. Second, due to insufficient studies, we cannot conduct a more detailed subgroup analysis of the HIIT protocol, which limits our discussion on the effect of HIIT on arterial hardness in this population. Third, the included studies did not report on adverse events that occurred during the HIIT intervention, which weakened the application of HIIT in persons who were not adapted to high-intensity exercise. Finally, in order to ensure the comprehensiveness of the literature search, we did not strictly limit the population, which also weakened the standardization of the search items in this paper.
5 Conclusion
HIIT can improve PWV in persons with CVD or risk factors for CVD. Performing HIIT 2–3 times/week for ≤40 min optimizes PWV improvement. HIIT effectively reduces SBP, DBP, and RHR but does not affect AIX, offering a promising intervention for CVD risk reduction. Sports training is a complex project, and any parameter may have an impact on the exercise effect. Because of the particularity of HIIT exercise intensity, it is necessary to make exercise strategies according to the physical conditions of the participants. Future studies need to focus on designing targeted HIIT interventions for specific cardiovascular high-risk populations to optimize exercise regimens for AS reduction.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
PL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing. WG: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. WY: Writing – review & editing. RW: Writing – review & editing. YY: Formal Analysis, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The publication fee for this article was provided by Wuhan Sport University Young Teachers Youth Research Fund Project. Project number: 2021Z4 and Major Projects of Philosophy and Social Science Research in Higher Education Institutions in Hubei Province, 2023.
Acknowledgments
Thanks to all the persons involved in this study, whose contributions have had a crucial impact on the results of this study. This paper is a team effort.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1376861/full#supplementary-material
Abbreviations
HIIT, high-intensity interval training; AS, arterial stiffness; CVD, cardiovascular disease; PWV, pulse wave velocity; CF-PWV, carotid-femoral pulse velocity; AIX, augmentation index; SBP, systolic blood pressure; DPB, diastolic blood pressure; RHR, resting heart rate; MICT, moderate-intensity continuous training; RCTs, randomized controlled trials; ACSM, American College of Sports Medicine.
References
1. Lachat C, Otchere S, Roberfroid D, Abdulai A, Maria F, Seret A, et al. Diet and physical activity for the prevention of noncommunicable diseases in low- and middle-income countries: a systematic policy review. PLoS Med. (2013) 10(6):e1001465. doi: 10.1371/journal.pmed.1001465
2. Nocon M, Hiemann T, Mueller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. (2008) 15(3):239–46. doi: 10.1097/HJR.0b013e3282f55e09
3. Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events—potential mediating mechanisms. Circulation. (2007) 116(19):2110–8. doi: 10.1161/circulationaha.107.729939
4. Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol London. (2004) 561(1):1–25. doi: 10.1113/jphysiol.2004.068197
5. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness a systematic review and meta-analysis. J Am Coll Cardiol. (2010) 55(13):1318–27. doi: 10.1016/j.jacc.2009.10.061
6. Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. (2010) 31(15):1865–71. doi: 10.1093/eurheartj/ehq024.2
7. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. (2014) 63(7):636–46. doi: 10.1016/j.jacc.2013.09.063.2
8. Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events the framingham heart study. Circulation. (2010) 121(4):505–11. doi: 10.1161/circulationaha.109.886655.2
9. Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age-related and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation. (2001) 104(14):1627–32. doi: 10.1161/hc3901.096670.2
10. O'Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension (Dallas, Tex: 1979). (1990) 15(4):339–47. doi: 10.1161/01.Hyp.15.4.339.2
11. Liao DP. Arterial stiffness and the development of hypertension. Ann Med. (2000) 32(6):383–5. doi: 10.3109/07853890008995943.2
12. Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction a randomized, controlled, single-blind trial. J Am Coll Cardiol. (2013) 62(7):584–92. doi: 10.1016/j.jacc.2013.04.033.2
13. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. (2006) 27(21):2588–605. doi: 10.1093/eurheartj/ehl254
14. Szolnoky G, Gavaller H, Gonczy A, Bihari I, Kemeny L, Forster T, et al. The effects of below-knee medical compression stockings on pulse wave velocity of young healthy volunteers. J Strength Cond Res. (2021) 35(1):275–9. doi: 10.1519/jsc.0000000000002636.2
15. Cavalcante JL, Lima JAC, Redheuil A, Al-Mallah MH. Aortic stiffness current understanding and future directions. J Am Coll Cardiol. (2011) 57(14):1511–22. doi: 10.1016/j.jacc.2010.12.017
16. Cheng Y, Sun Z, Ya X, Zhou L, Wang M, Wang X, et al. Effect of exercise training on arterial stiffness in obese and overweight children: a meta-analysis. Eur J Pediatr. (2022) 181(7):2633–42. doi: 10.1007/s00431-022-04489-6
17. Li G, Lv Y, Su Q, You Q, Yu L. The effect of aerobic exercise on pulse wave velocity in middle-aged and elderly people: a systematic review and meta-analysis of randomized controlled trials. Front Cardiovasc Med. (2022) 9:960096. doi: 10.3389/fcvm.2022.960096.2
18. Li Y, Hanssen H, Cordes M, Rossmeissl A, Endes S, Schmidt-Trucksaess A. Aerobic, resistance and combined exercise training on arterial stiffness in normotensive and hypertensive adults: a review. Eur J Sport Sci. (2015) 15(5):443–57. doi: 10.1080/17461391.2014.955129.2
19. Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. Plos One. (2014) 9(10):e110034. doi: 10.1371/journal.pone.0110034.2
20. Zhang Y, Qi L, Xu L, Sun X, Liu W, Zhou S, et al. Effects of exercise modalities on central hemodynamics, arterial stiffness and cardiac function in cardiovascular disease: systematic review and meta-analysis of randomized controlled trials. PLoS One. (2018) 13(7):e0200829. doi: 10.1371/journal.pone.0200829
21. Lopes S, Afreixo V, Teixeira M, Garcia C, Leitao C, Gouveia M, et al. Exercise training reduces arterial stiffness in adults with hypertension: a systematic review and meta-analysis. J Hypertens. (2021) 39(2):214–22. doi: 10.1097/hjh.0000000000002619
22. Li P, Liu Z, Wan K, Wang K, Zheng C, Huang J. Effects of regular aerobic exercise on vascular function in overweight or obese older adults: a systematic review and meta-analysis. J Exerc Sci Fit. (2023) 21(4):313–25. doi: 10.1016/j.jesf.2023.06.002.2
23. Kiens B, Beyer N, Brage S, Hyldstrup L, Ottesen LS, Overgaard K, et al. Physical inactivity–consequences and correlations. Ugeskr Laeg. (2007) 169(25):2442–5.2. 17594841.17594841
24. Tucker WJ, Beaudry RI, Liang Y, Clark AM, Tomczak CR, Nelson MD, et al. Meta-Analysis of exercise training on left ventricular ejection fraction in heart failure with reduced ejection fraction: a 10-year update. Prog Cardiovasc Dis. (2019) 62(2):163–71. doi: 10.1016/j.pcad.2018.08.006.2
25. Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. (2015) 45(5):679–92. doi: 10.1007/s40279-015-0321-z.2
26. Cassidy S, Thoma C, Houghton D, Trenell MI. High-intensity interval training: a review of its impact on glucose control and cardiometabolic health. Diabetologia. (2017) 60(1):7–23. doi: 10.1007/s00125-016-4106-1.2
27. Leal JM, Galliano LM, Del Vecchio FB. Effectiveness of high-intensity interval training versus moderate-intensity continuous training in hypertensive patients: a systematic review and meta-analysis. Curr Hypertens Rep. (2020) 22(3):26. doi: 10.1007/s11906-020-1030-z.2
28. Way KL, Sultana RN, Sabag A, Baker MK, Johnson NA. The effect of high intensity interval training versus moderate intensity continuous training on arterial stiffness and 24 H blood pressure responses: a systematic review and meta-analysis. J Sci Med Sport. (2019) 22(4):385–91. doi: 10.1016/j.jsams.2018.09.228.2
29. Evans W, Willey Q, Hanson ED, Stoner L. Effects of resistance training on arterial stiffness in persons at risk for cardiovascular disease: a meta-analysis. Sports Med. (2018) 48(12):2785–95. doi: 10.1007/s40279-018-1001-6.2
30. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj Br Med J. (2009) 339:b2700. doi: 10.1136/bmj.b2700.2
31. Tschakert G, Hofmann P. High-Intensity intermittent exercise: methodological and physiological aspects. Int J Sports Physiol Perform. (2013) 8(6):600–10. doi: 10.1123/ijspp.8.6.600.2
32. Costa EC, Hay JL, Kehler DS, Boreskie KF, Arora RC, Umpierre D, et al. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with Pre- to established hypertension: a systematic review and meta-analysis of randomized trials. Sports Med. (2018) 48(9):2127–42. doi: 10.1007/s40279-018-0944-y.2
33. ACSM. High-Intensity Interval Training. Available online at: https://www.acsm.org/docs/default-source/files-for-resource-library/high-intensity-interval-training.pdf?sfvrsn=b0f72be6_2 (Accessed April 09, 2024).
34. Pescatello LS. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health (2014).
35. Chuensiri N, Suksom D, Tanaka H. Effects of high-intensity intermittent training on vascular function in obese preadolescent boys. Child Obes. (2018) 14(1):41–9. doi: 10.1089/chi.2017.002.2
36. Bahmanbeglou NA, Ebrahim K, Maleki M, Nikpajouh A, Ahmadizad S. Short-duration high-intensity interval exercise training is more effective than long duration for blood pressure and arterial stiffness but not for inflammatory markers and lipid profiles in patients with stage 1 hypertension. J Cardiopulm Rehabil Prev. (2019) 39(1):50–5. doi: 10.1097/hcr.0000000000000377
37. Guimaraes GV, Ciolac EG, Carvalho VO, D'Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. Interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. (2010) 33(6):627–32. doi: 10.1038/hr.2010.42.2
38. Ciolac EG, Bocchi EA, Bortolotto LA, Carvalho VO, Greve JMD, Guimaraes GV. Effects of high-intensity aerobic interval training vs. moderate exercise on hemodynamic, metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypertens Res. (2010) 33(8):836–43. doi: 10.1038/hr.2010.72.2
39. Chrysohoou C, Angelis A, Tsitsinakis G, Spetsioti S, Nasis I, Tsiachris D, et al. Cardiovascular effects of high-intensity interval aerobic training combined with strength exercise in patients with chronic heart failure. A randomized phase III clinical trial. Int J Cardiol. (2015) 179:269–74. doi: 10.1016/j.ijcard.2014.11.067.2
40. Van Craenenbroeck AH, Van Craenenbroeck EM, Van Ackeren K, Vrints CJ, Conraads VM, Verpooten GA, et al. Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in ckd stages 3–4: a randomized controlled trial. Am J Kidney Dis. (2015) 66(2):285–96. doi: 10.1053/j.ajkd.2015.03.015.2
41. Kim H-K, Hwang C-L, Yoo J-K, Hwang M-H, Handberg EM, Petersen JW, et al. All-extremity exercise training improves arterial stiffness in older adults. Med Sci Sports Exerc. (2017) 49(7):1404–11. doi: 10.1249/mss.0000000000001229.2
42. Bellia A, Iellamo F, De Carli E, Andreadi A, Padua E, Lombardo M, et al. Exercise individualized by trimpi method reduces arterial stiffness in early onset type 2 diabetic patients: a randomized controlled trial with aerobic interval training. Int J Cardiol. (2017) 248:314–9. doi: 10.1016/j.ijcard.2017.06.065.2
43. Mora-Rodriguez R, Ramirez-Jimenez M, Fernandez-Elias VE, Guio de Prada MV, Morales-Palomo F, Pallares JG, et al. Effects of aerobic interval training on arterial stiffness and microvascular function in patients with metabolic syndrome. J Clin Hypertens. (2018) 20(1):11–8. doi: 10.1111/jch.13130.2
44. Hanssen H, Minghetti A, Magon S, Rossmeissl A, Papadopoulou A, Klenk C, et al. Superior effects of high-intensity interval training vs. moderate continuous training on arterial stiffness in episodic migraine: a randomized controlled trial. Front Physiol. (2017) 8:1086. doi: 10.3389/fphys.2017.01086
45. Bouaziz W, Lang P-O, Schmitt E, Lepretre P-M, Lefebvre F, Momas C, et al. Effects of a short-term interval aerobic training program with recovery bouts on vascular function in sedentary aged 70 or over: a randomized controlled trial. Arch Gerontol Geriatr. (2019) 82:217–25. doi: 10.1016/j.archger.2019.02.017
46. Deiseroth A, Streese L, Kochli S, Wust RS, Infanger D, Schmidt-Trucksass A, et al. Exercise and arterial stiffness in the elderly: a combined cross-sectional and randomized controlled trial (examin age). Front Physiol. (2019) 10:1119. doi: 10.3389/fphys.2019.01119
47. Ho TY, Redmayne GP, Tran A, Liu D, Butlin M, Avolio A, et al. The effect of interval sprinting exercise on vascular function and aerobic fitness of post-menopausal women. Scand J Med Sci Sports. (2020) 30(2):312–21. doi: 10.1111/sms.13574
48. Way KL, Sabag A, Sultana RN, Baker MK, Keating SE, Lanting S, et al. The effect of low-volume high-intensity interval training on cardiovascular health outcomes in type 2 diabetes: a randomised controlled trial. Int J Cardiol. (2020) 320:148–54. doi: 10.1016/j.ijcard.2020.06.019
49. McNarry MA, Lester L, Ellins EA, Halcox JP, Davies G, Winn CON, et al. Asthma and high-intensity interval training have no effect on clustered cardiometabolic risk or arterial stiffness in adolescents. Eur J Appl Physiol. (2021) 121(7):1967–78. doi: 10.1007/s00421-020-04590-4
50. Taha MM, Aneis YM, Hasanin ME, Felaya EE, Aldhahi MI, Abdeen HAA. Effect of high intensity interval training on arterial stiffness in obese hypertensive women: a randomized controlled trial. Eur Rev Med Pharmacol Sci. (2023) 27(9):4069–79. doi: 10.26355/eurrev_202305_32314
51. Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. (2003) 23(4):554–66. doi: 10.1161/01.Atv.0000060460.52916.D6
52. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. (2012) 53(2):258–61. doi: 10.3349/ymj.2012.53.2.258
53. Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, et al. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. (2011) 218(1):90–5. doi: 10.1016/j.atherosclerosis.2011.04.033
54. Santos-Parker JR, LaRocca TJ, Seals DR. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ. (2014) 38(4):296–307. doi: 10.1152/advan.00088.2014
55. Wang X, Keith JC Jr, Struthers AD, Feuerstein GZ. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther. (2008) 26(3):214–23. doi: 10.1111/j.1755-5922.2008.00051.x
56. Teixeira-Lemos E, Nunes S, Teixeira F, Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. (2011) 10:12. doi: 10.1186/1475-2840-10-12
57. Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee D-c, et al. Exercise and the cardiovascular system clinical science and cardiovascular outcomes. Circ Res. (2015) 117(2):207–19. doi: 10.1161/circresaha.117.305205
58. Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens. (2013) 26(9):1093–102. doi: 10.1093/ajh/hpt080
59. Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res. (2005) 66(2):393–401. doi: 10.1016/j.cardiores.2004.10.023
60. Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation—a mini-review. Gerontology. (2012) 58(3):227–37. doi: 10.1159/000334668
61. Lee SY, Burns SF, Ng KKC, Stensel DJ, Zhong L, Tan FHY, et al. Pulse wave velocity is associated with increased plasma Oxldl in ageing but not with Fgf21 and habitual exercise. Antioxidants. (2020) 9(3). doi: 10.3390/antiox9030221
62. Mahmud A. Arterial stiffness and the renin-anglotensin-aldosterone system. J Renin Angiotensin Aldosterone Syst. (2004) 5(3):102–8. doi: 10.3317/jraas.2004.025
63. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health—updated recommendation for adults from the American college of sports medicine and the American heart association. Circulation. (2007) 116(9):1081–93. doi: 10.1161/circulationaha.107.185649
64. Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. (2014) 173(3):361–8. doi: 10.1016/j.ijcard.2014.03.072
65. Paini A, Aggiusti C, Bertacchini F, Stassaldi D, Capellini S, de Ciuceis C, et al. Relationship between arterial stiffness and unattended or attended blood pressure values. J Hypertens. (2020) 38(2):243–8. doi: 10.1097/hjh.0000000000002232
66. Marriott CFS, Petrella AFM, Marriott ECS, Boa Sorte Silva NC, Petrella RJ. High-intensity interval training in older adults: a scoping review. Sports Med Open. (2021) 7(1):49. doi: 10.1186/s40798-021-00344-4
67. Jennersjö P, Ludvigsson J, Länne T, Nystrom FH, Ernerudh J, Östgren CJ. Pedometer-determined physical activity is linked to low systemic inflammation and low arterial stiffness in type 2 diabetes. Diabet Med. (2012) 29(9):1119–25. doi: 10.1111/j.1464-5491.2012.03621.x
68. Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. (2008) 34(1):2–11. doi: 10.1016/j.diabet.2007.09.004
69. Becic T, Studenik C, Hoffmann G. Exercise increases adiponectin and reduces leptin levels in prediabetic and diabetic individuals: systematic review and meta-analysis of randomized controlled trials. Med Sci (Basel). (2018) 6(4). doi: 10.3390/medsci6040097
70. Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. (2003) 26(8):2442–50. doi: 10.2337/diacare.26.8.2442
71. Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology. (2004) 145(12):5589–97. doi: 10.1210/en.2004-0503
72. Meng C, Yucheng T, Shu L, Yu Z. Effects of school-based high-intensity interval training on body composition, cardiorespiratory fitness and cardiometabolic markers in adolescent boys with obesity: a randomized controlled trial. BMC Pediatr. (2022) 22(1):112. doi: 10.1186/s12887-021-03079-z
73. Valsdottir TD, Øvrebø B, Falck TM, Litleskare S, Johansen EI, Henriksen C, et al. Low-Carbohydrate high-fat diet and exercise: effect of a 10-week intervention on body composition and CVD risk factors in overweight and obese women-A randomized controlled trial. Nutrients. (2020) 13(1). doi: 10.3390/nu13010110
74. Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Elnegamy TE, Soliman GS, et al. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: a comparative randomized controlled trial. Medicine (Baltimore). (2020) 99(10):e19471. doi: 10.1097/md.0000000000019471
75. Chen X, He H, Xie K, Zhang L, Cao C. Effects of various exercise types on visceral adipose tissue in individuals with overweight and obesity: a systematic review and network meta-analysis of 84 randomized controlled trials. Obes Rev. (2024) 25(3):e13666. doi: 10.1111/obr.13666
76. Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, et al. Unfavorable effects of resistance training on central arterial compliance—a randomized intervention study. Circulation. (2004) 110(18):2858–63. doi: 10.1161/01.Cir.0000146380.08401.99
77. Okamoto T, Masuhara M, Ikuta K. Effects of eccentric and concentric resistance training on arterial stiffness. J Hum Hypertens. (2006) 20(5):348–54. doi: 10.1038/sj.jhh.1001979
78. Okamoto T, Masuhara M, Ikuta K. Effects of muscle contraction timing during resistance training on vascular function. J Hum Hypertens. (2009) 23(7):470–8. doi: 10.1038/jhh.2008.152
79. Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med. (2013) 47(6):393. doi: 10.1136/bjsports-2012-090488
80. Whelton SP, Chin A, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized controlled trials. Circulation. (2001) 103(9):1369. doi: 10.1161/circ.103.suppl_1.9998-97
81. Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. (1995) 155(7):701–9. doi: 10.1001/archinte.155.7.701
82. Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. (2005) 26(10):967–74. doi: 10.1093/eurheartj/ehi190
83. Cooney MT, Vartiainen E, Laakitainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. (2010) 159(4):612–U134. doi: 10.1016/j.ahj.2009.12.029
84. Okin PM, Kjeldsen SE, Julius S, Hille DA, Dahlof B, Edelman JM, et al. All-cause and cardiovascular mortality in relation to changing heart rate during treatment of hypertensive patients with electrocardiographic left ventricular hypertrophy. Eur Heart J. (2010) 31(18):2271–9. doi: 10.1093/eurheartj/ehq225
85. Ho JE, Bittner V, DeMicco DA, Breazna A, Deedwania PC, Waters DD. Usefulness of heart rate at rest as a predictor of mortality, hospitalization for heart failure, myocardial infarction, and stroke in patients with stable coronary heart disease (data from the treating to new targets tnt trial). Am J Cardiol. (2010) 105(7):905–11. doi: 10.1016/j.amjcard.2009.11.035
Keywords: high-intensity interval exercise, arterial stiffness, cardiovascular disease, pulse wave velocity, resting heart rate, augmentation index, systolic blood pressure, diastolic blood pressure
Citation: Luo P, Wu R, Gao W, Yan W, Wang R and Ye Y (2024) Effects of high-intensity interval exercise on arterial stiffness in individuals at risk for cardiovascular disease: a meta-analysis. Front. Cardiovasc. Med. 11:1376861. doi: 10.3389/fcvm.2024.1376861
Received: 26 January 2024; Accepted: 4 April 2024;
Published: 17 April 2024.
Edited by:
Roberto Vazquez-Padron, University of Miami, United StatesReviewed by:
Natsuki Hasegawa, Ritsumeikan University, JapanAsako Zempo-Miyaki, Ryutsu Keizai University, Japan
© 2024 Luo, Wu, Gao, Yan, Wang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifeng Gao 2012027@whsu.edu.cn
 Ping Luo
Ping Luo Ruoshan Wu
Ruoshan Wu Weifeng Gao1*
Weifeng Gao1*  Yufang Ye
Yufang Ye