Recent advances and future prospective of topical and transdermal delivery systems
- 1Faculty of Pharmacy, Rhodes University, Grahamstown, South Africa
- 2Department of Pharmaceutics, School of Pharmaceutical Education and Research, Jamia Hamdard, India
- 3Therapeutics Research Group, The University of Queensland Diamantina Institute, Faculty of Medicine, University of Queensland, Brisbane, QLD, Australia
- 4UniSA Clinical and Health Sciences, University of South Australia, Basil Hetzel Institute for Translational Health Research, Adelaide, SA, Australia
Recent advances in topical and transdermal drug delivery systems have enabled targeted delivery of therapeutics to the site of action by enhancing drug permeation across the stratum corneum and increased bioavailability. Despite various technological advancements, some dermatoses still have limited treatment options due to potential adverse effects and challenges in formulation development. To address some of the limitations posed by conventional dermatotherapy, nano-based technologies have been developed and have demonstrated a significant improvement in dermatotherapy. Their distinct physicochemical properties demonstrate their overall superior therapeutic efficacy in providing sustained and effective targeted drug release, as well as improved solubility of hydrophobic actives with optimized drug formulations. These nanocarriers are commonly classified as polymeric, lipid-based, metallic, and vesicular nanocarriers, including nanoemulsions, nanofibers, and microneedles. This mini-review aims to address recent advances in nano-based technologies, providing a brief insight on some of the current and prospective technologies and approaches aimed at improving transdermal delivery.
1 Introduction
According to the global burden disease projects, skin diseases are considered the fourth leading cause of nonfatal disease burden (Seth et al., 2017; Flohr and Hay, 2021), with dermatitis being the highest contributor and cellulitis contributing the least in the global burden of skin diseases (Table 1). Out of 19,727 publications on skin diseases published between 2015 and 2020, acne ranked second on the global burden of skin disease but accounted for only 2.42% of the total publication in literature, demonstrating the need for more selective scientific research on skin diseases (Pulsipher et al., 2021). Furthermore, certain dermatoses are presented with limited treatment options, i.e., severe atopic dermatitis, despite current technological advancements owing to the presence of biological barriers, associated systemic adverse effects, and limitations in product formulation, i.e., drug solubility (Stefanov and Andonova, 2021). The skin is considered the largest organ of the body accounting for approximately 16% of an adult’s total body weight (McLafferty et al., 2012). Thus, it plays a significant role in maintaining homeostasis (Tortora and Derrickson, 2018), as well as acting as a chemical, physical, and biological barrier against exogenous environmental threats (Dehdashtian et al., 2018). While conventional topical and transdermal formulations such as creams and ointments are relatively sufficient in treating some dermatological diseases (Das Kurmi et al., 2017), the limitations and drawbacks regarding bioavailability and targeted drug delivery warrants the development and advancement in technologies to enhance drug permeation and increase drug delivery. This has necessitated the development of novel topical and transdermal drug delivery systems such as nano-based technologies that significantly improve dermatotherapy, address some of the formulation drawbacks, i.e., drug solubility (Krishnan and Mitragotri, 2020), as well as circumventing some of the subsequent challenges associated with oral drug delivery, i.e., hepatic first-pass effect, longer dosing frequencies, and patient compliance (Ramadon et al., 2022).
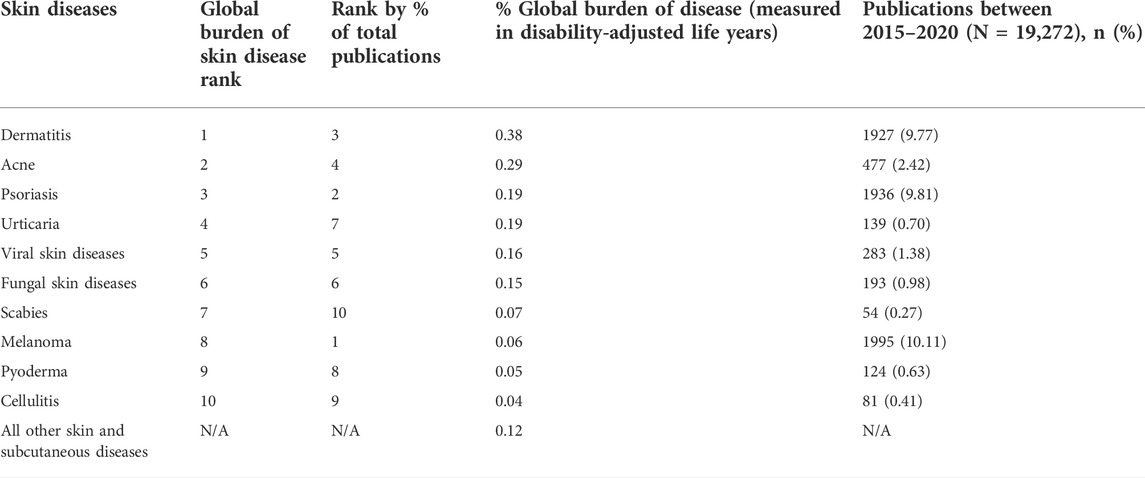
TABLE 1. Global burden of skin disease rankings and literature representation between 2015 and 2020 (adapted from Pulsipher et al., 2021) licensed under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
Due to their various benefits, the development and emergence of several nanocarriers employed in topical and transdermal delivery systems have gained huge attention of pharmaceutical scientists and dermatologists. Their distinct physicochemical properties (e.g., reduced size to a few nanometers) have demonstrated their overall superior therapeutic efficacy in providing sustained and effective targeted drug release (Mishra et al., 2018; Ghasemiyeh and Mohammadi-Samani, 2020). Nanocarriers are commonly classified as polymeric, lipid-based (liposomes, nanostructured lipid carriers, and solid-lipid nanoparticles), vesicular (niosomes, liposomes, transferosomes, ethosomes, and transethosomes), and metallic nanocarriers. With nanoemulsions, nanofibers and microneedles forming part of the classification (Krishnan and Mitragotri, 2020). Specific components such as surfactants and cosurfactants acting as permeation enhancers constitute some of the developed nano-formulations, with the potential ability to modify and alter the molecular and dynamic structure of the membranes by creating temporary pores and loosening the tight junctions between skin layers (Kapoor et al., 2018). Such molecular alterations lead to the reversible modification of the skin barriers, thus enhancing the ability of therapeutics to traverse the skin barrier via nanocarriers into the deeper layers of the skin (Ezealisiji and Okorie, 2018). While there are several advancements in topical and transdermal technologies, this mini-review was selectively aimed at addressing advancements in nano-based technologies, with a brief insight on some of the current and prospective technologies and approaches aimed at improving transdermal delivery.
2 The skin as a barrier
Although the goal of this text is to provide insight into the new technologies and approaches in topical and transdermal drug delivery, a brief review on the skin as a barrier is necessary to facilitate the understanding of the main concepts behind the development of the new technologies. The skin surface area is about 2 m2 (Tortora and Derrickson, 2018), with the epidermis (the outermost surface of the skin) comprising of five layers: stratum corneum (SC), stratum lucidum, stratum granulosum, stratum spinosum and stratum basale, with the stratum corneum forming the outermost layer (Dehdashtian et al., 2018). The SC is a thick matrix of terminally differentiated keratinocytes dispersed within lipids, approximately 10 µm in thickness (Williams et al., 2018), and it is considered to be the rate-limiting step in percutaneous absorption (Higuchi, 1960). A healthy and intact SC is a barrier to both hydrophilic and large size molecules (Haq and Michniak-Kohn, 2018). The resistance of the SC to molecular diffusion limits transdermal drug delivery to compounds with a molecular weight of up to 500 Da (Bos and Meinardi, 2000). In addition, the permeation of the drug candidate by overcoming skin barrier could entirely depend on the condition and state of the skin (Haq and Michniak-Kohn, 2018). The SC covers the viable epidermis comprising of multiple skin layers made of viable keratinocytes, which is followed by the dermal layer composed of connective tissues (collagen and elastin), fibroblasts, and other extracellular components such as hair follicles and glands (Dehdashtian et al., 2018). Advanced nanotechnological derived nanocarriers can permeate into the epidermis and reach target sites for the treatment of skin-related disorders. These nanocarriers overcome the stratum corneum barrier by increasing drug solubility, and partitioning into the skin, which facilitates the delivery of the desired amount of the drug to the target site (Iqbal et al., 2018). While significantly important in transdermal and topical drug delivery, the discussion on percutaneous absorption is limited due to the objectives of this review; however, Roberts et al. and Benson et al. provide updated and comprehensive reviews on the history, progression, and advances in percutaneous absorption (Benson et al., 2019; Roberts et al., 2021).
3 Application of nanotechnology-based transdermal and topical delivery systems
There has been a rise in the development of transdermal and topical delivery systems for the effective treatment of skin-related disorders. The implementation of nanotechnology for the development of advanced therapeutic tools offers multiple advantages over conventional therapies, such as enhanced solubility of highly hydrophobic drugs and increased drug stability (Goyal et al., 2016). Such nanocarriers can be categorized into lipid-based, vesicle-based, polymeric, and metallic nanocarriers, nanoemulsions, nanofibers, as well as microneedle patches. Thus, this section will summarize several nanocarriers developed by advanced nanotechnologies.
3.1 Microneedles
This treatment technique has gained enormous attention from researchers owing to its various benefits, i.e., it avoids the degradation of therapeutics in the gastrointestinal tract, circumvents the first-pass metabolism in the liver (in the case of oral delivery), and prevents the invasive and painful approach via the intravenous delivery of drugs (Figure 3) (Fernando et al., 2018; Qu et al., 2022); hence, it is considered an effective approach in transdermal drug delivery. Microneedles are composed of an array of micron-sized needles that pierce the SC (10–20 um) and deliver the actives directly to the viable epidermis, bypassing the SC barrier and providing high drug bioavailability compared to creams and the transdermal patch (Figure 1) (Waghule et al., 2019; Avcil and Çelik, 2021; Ali et al., 2022; Qu et al., 2022). Due to these various advantages, microneedles and their patches have also been translated to clinical stages in the delivery of vaccines (Fernando et al., 2018); however, this strategy has some limitations, such as compatibility, loading capacity, dose restriction due to small size of microneedles, irritation, and inflammatory immune response (Avcil and Çelik, 2021). Nonetheless, their advantages outweigh the limitations and, therefore, microneedles (i.e., patches) have become a delivery system of choice for vaccines and therapeutics for a range of diseases (Fernando et al., 2018).
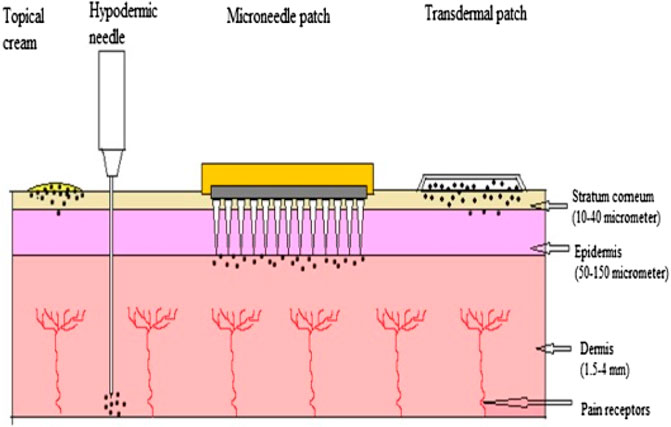
FIGURE 1. Comparison of topical cream, hypodermic needle, microneedle patch and transdermal patch adapted from Waghule et al., 2019, licensed under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
Caudill et al. designed microneedle arrays by utilizing a three dimensional (3D)-printing technique that induced the potent cellular immunity. The findings demonstrated that microneedle transdermal patch increased the vaccine component retention in the skin and enhanced the activation of immune cells as compared to the subcutaneous bolus injection. Overall, these 3D printed microneedle patches with vaccine components offer a successful and effective approach for a non-invasive and self-administered vaccination (Caudill et al., 2021). Further, Moon and associates developed an effective microneedle patch for successful delivery of inactivated polio and rotavirus vaccine (Moon et al., 2022).
3.2 Vesicular nanocarriers
Phospholipid nanocarriers have garnered much interest from scientists over the past years, particularly liposomes, and they are aimed at improving dermal targeting for the treatment of various skin-related diseases (Lai et al., 2020). Vesicular nanocarriers are made of actives enclosed in an aqueous core, with liposomes as the first developed vesicular nanocarrier (Mbah et al., 2014). The mechanisms of some of the vesicular nanocarriers are shown in Figure 2. Liposomes have demonstrated poor transdermal permeation (Wadher et al., 2018) and to improve their efficacy, optimization has been achieved by adding compounds to the classic phospholipid vesicles (which have poor skin permeation), to increase the effects of the active moiety at the target site (Wadher et al., 2018; Lai et al., 2020). One such example is the development of ethosomes, first introduced by Touitou et al., formed from introducing ethanol, which acts as a permeation enhancer, to the phospholipid vesicle (Touitou et al., 2000). Ethosomes are nano-vesicular carriers suitable for poorly soluble drugs shown to increase their drug permeation (Abouhussein, 2021; Apolinário et al., 2021). Powale et al. has recently demonstrated the improved stability and minimized degradation of clindamycin from an ethosome formulation (Powale et al., 2022). Other developed vesicular carriers include niosomes (comprised of non-ionic surfactants in lipid vesicles) (Ge et al., 2019), liposomes (comprised of phospholipids as the lipophilic phase and an aqueous core giving them their amphiphilic nature) (Fan et al., 2021), transfersomes (comprised of phospholipid-based vesicles with an edge activator) (Mahmood et al., 2021), as well as transethosomes (formed from the addition of a permeation enhancer to ethosomes) (Sudhakar et al., 2021).
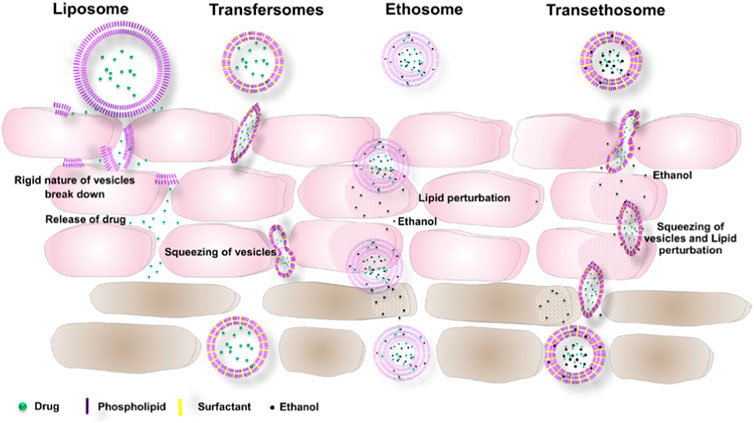
FIGURE 2. Mechanism of some vesicular nanocarriers across the stratum corneum adapted from Sudhakar et al., 2021, licensed under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
3.3 Polymeric nanocarriers
The solubility of drugs has posed a challenge in pharmaceutical formulation. Polymeric micelles have gained much interest as an innovative method to overcome the poor solubility and permeability of drugs across the skin (Ghezzi et al., 2021). Polymeric micelles are self-assembling nanocarriers consisting of amphiphilic, biocompatible, and biodegradable copolymers (natural and synthetic). Some of the commonly used natural polymers include gelatin and albumin polymers, whereas the synthetic polymers include polylactic acid and polyglycolic acid (Makhmalzade and Chavoshy, 2018; Mishra et al., 2018). They consist of a hydrophobic core and a hydrophilic shell formed from self-assembling in aqueous environment above critical micelle concentration (Makhmalzade and Chavoshy, 2018). They are nanocarriers that encapsulate hydrophilic, lipophilic, and charged compounds; decrease the potential for systemic side effects; and facilitate targeted drug delivery (Makhmalzade and Chavoshy, 2018; Yotsumoto et al., 2018). Yotsumoto and associates demonstrated the increased aqueous solubility of indomethacin and resveratrol (hydrophobic drugs) from polymeric micelle encapsulation with improved transdermal permeation (Yotsumoto et al., 2018). Compared to other nanocarriers, they are generally considered to have easier preparations and sterilization methods, are smaller in size, and exhibit better stability in different microenvironments (Mishra et al., 2018; Ghezzi et al., 2021).
3.4 Lipid-based nanocarriers
Lipid-based nanocarriers, i.e., solid-lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and liposomes have gained much attention due to their high bioavailability and ability to deliver macromolecules such as proteins. SLNs are the most common lipid-based nanocarrier developed as a substitute for liposomes, emulsions, and polymeric nanoparticles (Samimi et al., 2019). They are a solid matrix of colloidal lipid dispersions prepared using surfactants (stabilize the lipid dispersions), lipids (such as triglycerides, waxes and fatty acids), and water (Goyal et al., 2016). Furthermore, they offer many advantages including sustained and controlled release of drugs and physicochemical stability. They are also solid at both room and body temperature, and are biodegradable (Goyal et al., 2016; Samimi et al., 2019). Despite their safety and efficacy, some of the drawbacks include poor drug loading due to their crystalline structure, particle growth, and drug extrusion during storage (Samimi et al., 2019). Liposomes are spherical lipid vesicles surrounding an aqueous core to allow for encapsulation of both hydrophilic drugs (in the core) and lipophilic drugs (in the lipid bilayer) (Fan et al., 2021). Furthermore, they increase the drug circulation time in the body by reducing the interaction of the drug molecules with serum proteins and increase targeted drug delivery. They provide sustained release of drugs and can be prepared via several methods such as thin film hydration and solvent injection (Goyal et al., 2016). Nanostructure lipid carriers were developed to overcome the SLNs challenges. They are comprised of solid and liquid lipids to create a more disordered matrix that improves drug loading capacity. NLCs have greater stability and avoid the use of organic solvents (Samimi et al., 2019).
3.5 Metallic nanocarriers
The use of metallic nanocarriers has roused scientific interest due to their versatile application in science, increased therapeutic index, and site-specific drug targeting (Chandrakala et al., 2022). They are used as drug delivery carriers for various drugs such as nucleic acids, peptides, and chemotherapeutic drugs (Yih and Al‐Fandi, 2006; Mandal et al., 2009). Metallic nanoparticles such as gold, silver, platinum, zinc, and copper have enhanced tunable optical properties, high drug loading, and improved therapeutic efficacy. Their surfaces can be functionalized through hydrogen bonding, covalent bonding, and electrostatic interactions to conjugate active molecules. Furthermore, metallic nanoparticles offer several advantages, including increased aqueous solubility of hydrophobic drugs, increased drug circulation time in the blood, site-specific drug targeting, and controlled drug release (Chandrakala et al., 2022). The unique size of metallic nanoparticles enables diffusion through loose junctions to tumor sites, which increases their use in cancer treatment (Yih and Al‐Fandi, 2006). A study by Chelladurai et al. demonstrated the use of zinc oxide nanoparticles conjugated to mupirocin (a hydrophobic drug) in improved targeted drug delivery in human epidermoid cancer treatment (Chelladurai et al., 2022). Although efficacy has been demonstrated, metallic nanocarriers are associated with some limitations, such as higher toxicity and poor biocompatibility (Imran et al., 2022).
3.6 Nanoemulsions
To overcome some of the limitations in topical and transdermal drug delivery, nanoemulsions have been developed to improve penetration and absorption of active molecules, as well as to achieve controlled release (Sarheed et al., 2020; Souto et al., 2022). Nanoemulsions are lipid-based, colloidal oil in water dispersions of finely dispersed droplets (in the nm scale) with hydrophilic and lipophilic phases (Klang et al., 2012; Sarheed et al., 2020). They are biodegradable and offer increased solubility of both polar and non-polar compounds. Compared to conventional emulsions, they have greater thermodynamic and kinetic stability and an increased bioavailability of lipophilic compounds from the addition of surfactants/emulsifier (Rai et al., 2018; Yamada et al., 2018; Souto et al., 2022). The fluidic nature of nanoemulsions and surfactant interface promotes skin interaction (Rai et al., 2018). In addition, they have a high loading capacity of hydrophobic molecules and protect the active molecules from oxidation and hydrolysis, which improves bioavailability (Yamada et al., 2018). The development of a miconazole nanoemulsion was recently demonstrated by Farooq et al. to improve skin penetration compared to marketed products for antifungal treatment. The formulated nanoemulsion also showed stability, as well as good drug loading and in vitro drug release profiles. Overall, the nanoemulsion had improved permeation rates (Farooq et al., 2021). Furthermore, a clinical study by Yamada et al. demonstrated enhanced topical drug delivery of hydrophobic drugs in a tailorable nanoemulsion using elongated microparticles that could penetrate the dermal-epidermal junction and enhance drug permeability within the epidermis (Yamada et al., 2018).
3.7 Nanofibers/microfibers
These systems have seen an increase in wound healing for their ability to produce a local effect (Kamble et al., 2017; Kumar et al., 2021). Nanofibers offer the advantage of a high surface area, which enhances drug dissolution (Kamble et al., 2017). The drug molecules are entrapped in the polymer structure to provide a more controlled drug delivery system and an increased drug concentration in the carrier, which increases the flux of drug into the skin (Goyal et al., 2016; Kamble et al., 2017). One potential technique for producing nanofibers is electrospinning from various polymers to produce nanofibers with small diameter, large surface area and flexibility (Pant et al., 2019). Rancan and associates demonstrated the sustained local drug delivery from nanofiber mats of a poorly water-soluble antibiotic (ciprofloxacin) in wound healing. The nanofiber systems demonstrated efficiency in the treatment of pseudomonas aeruginosa in ex vivo human skin models (Rancan et al., 2019). Furthermore, Zhu et al. developed core-shell nanofibers for the treatment of melanoma. The nanofibers exhibited high drug encapsulation efficiency, and their study demonstrated the potential use of nanofibers in cancer treatment with targeted drug delivery (Zhu et al., 2019).
4 Prospective advancements in transdermal and topical delivery systems
Although nanotechnology has shown great potential in the advancement of topical and transdermal drug delivery, other emerging technologies and approaches have also shown conceivable prospective in improved drug delivery. One such example are biologics, which are becoming increasingly popular in transdermal delivery owing to the presence of biological barriers limiting drug permeation. Novel therapeutics such as anti-IL-13 inhibitor (tralokinumab), Janus kinase (JAK) inhibitors (baricitinib, tofacitinib), and anti-IL-4Rα antibody (dupilumab) are currently being investigated in the treatment of inflammatory diseases (Deleanu and Nedelea, 2019; Bieber, 2021). The Food and Drug Administration (FDA) has recently approved the first topical JAK inhibitor ruxolitinib cream for the treatment of mild to moderate atopic dermatitis not sufficiently treatable with prescription topical therapies (Chovatiya and Paller, 2021).
Hyaluronic-acid-based systems are also becoming increasing popular as they are widely used in the pharmaceutical industry for their enhanced permeability and biocompatibility. Hyaluronic acid is usually incorporated into nanoparticles, ethosomes, and liposomal transdermal systems, in the treatment of anti-inflammatory diseases such as atopic dermatitis and psoriasis (How et al., 2020).
Apart from microneedles, other physical penetration technologies in transdermal delivery such as sonophoresis (Nguyen and Banga, 2018; Park et al., 2019), iontophoresis (Park et al., 2019), and electroporation (Baveja, 2018) are being investigated. They are generally considered to be safe, efficient, and with high drug bioavailability. Furthermore, electroporation is an emerging method that delivers medication via small electrical impulses. This improves the permeation of hydrophilic drugs across the stratum corneum (Baveja, 2018). Sonophoresis involves the use of ultrasound waves at varying frequencies to disrupt the skin barrier and drive diffusion of drug across the skin, which enhances drug permeation. Low-frequency ultrasound enhances drug permeation to a greater extent compared to high-frequency ultrasound (Nguyen and Banga, 2018; Park et al., 2019), whereas in iontophoresis an electric field (the repulsive forces between similarly charged molecules) is used to push molecules into the skin. It offers fast drug release for the delivery of both charged and uncharged molecules (Park et al., 2019). However, the challenges that many of these prospective strategies face include cost and large-scale feasibility due to the patient-specific nature of the models. More intensive research and clinical studies are thus required to ascertain long-term effects of some of the techniques (Baveja, 2018).
5 Conclusion
An interesting aspect associated with transdermal drug delivery is the need to improve drug permeation across the skin. The limitations of conventional dermatotherapy are a continual driving force for the need to develop more enhanced and optimized topical and transdermal drug delivery systems. The implementation of nanotechnology for the development of advanced therapeutic tools is increasingly getting more scientific attention as it offers multiple advantages over conventional topical dermatotherapy. Although this review has demonstrated the great potential of nano-based carriers, it is important to consider prospective advancements in technology and approaches that improve targeted transdermal delivery to address some of the gaps and challenges transdermal delivery still faces.
Author contributions
PT and IM prepared the first draft of the manuscript. YM and MR reviewed, supervised, and finalized the submission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abouhussein, D. M. (2021). Enhanced transdermal permeation of BCS class IV aprepitant using binary ethosome: Optimization, characterization and ex vivo permeation. J. Drug Deliv. Sci. Technol. 61, 102185. doi:10.1016/j.jddst.2020.102185
Ali, M., Namjoshi, S., Benson, H., Mohammed, Y., and Kumeria, T. (2022). Dissolvable polymer microneedles for drug delivery and diagnostics. J. Control. Release S01683659 (22), 561–589. doi:10.1016/j.jconrel.2022.04.043
Apolinário, A. C., Hauschke, L., Nunes, J. R., Lourenço, F. R., and Lopes, L. B. (2021). Design of multifunctional ethosomes for topical fenretinide delivery and breast cancer chemoprevention. Colloids Surfaces A Physicochem. Eng. Aspects 623, 126745. doi:10.1016/j.colsurfa.2021.126745
Avcil, M., and Çelik, A. (2021). Microneedles in drug delivery: Progress and challenges. Micromachines 12 (11), 1321. doi:10.3390/mi12111321
Baveja, S. (2018). Drug delivery: Steps toward replacing the pill. Berkeley Sci. J. 22 (2). doi:10.5070/bs3222039588
Benson, H. A., Grice, J. E., Mohammed, Y., Namjoshi, S., and Roberts, M. S. (2019). Topical and transdermal drug delivery: From simple potions to smart technologies. Curr. Drug Deliv. 16 (5), 444–460. doi:10.2174/1567201816666190201143457
Bieber, T. (2021). Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 21, 21–40. doi:10.1038/s41573-021-00266-6
Bos, J. D., and Meinardi, M. M. (2000). The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 9 (3), 165–169. doi:10.1034/j.1600-0625.2000.009003165.x
Caudill, C., Perry, J. L., Iliadis, K., Tessema, A. T., Lee, B. J., Mecham, B. S., et al. (2021). Transdermal vaccination via 3D-printed microneedles induces potent humoral and cellular immunity. Proc. Natl. Acad. Sci. U. S. A. 118 (39), e2102595118. doi:10.1073/pnas.2102595118
Chandrakala, V., Aruna, V., and Angajala, G. (2022). Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mat., 1–23. doi:10.1007/s42247-021-00335-x
Chelladurai, M., Margavelu, G., Vijayakumar, S., González-Sánchez, Z. I., Vijayan, K., Sahadevan, R., et al. (2022). Preparation and characterization of amine-functionalized mupirocin-loaded zinc oxide nanoparticles: A potent drug delivery agent in targeting human epidermoid carcinoma (A431) cells. J. Drug Deliv. Sci. Technol. 70, 103244. doi:10.1016/j.jddst.2022.103244
Chovatiya, R., and Paller, A. S. (2021). JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 148 (4), 927–940. doi:10.1016/j.jaci.2021.08.009
Das Kurmi, B., Tekchandani, P., Paliwal, R., and Rai Paliwal, S. (2017). Transdermal drug delivery: Opportunities and challenges for controlled delivery of therapeutic agents using nanocarriers. Curr. Drug Metab. 18 (5), 481–495. doi:10.2174/1389200218666170222150555
Dehdashtian, A., Stringer, T. P., Warren, A. J., Mu, E. W., Amirlak, B., and Shahabi, L. (2018). “Anatomy and physiology of the skin,” in Melanoma. Editor A. Riker (Cham, Switzerland: Springer), 15–26.
Deleanu, D., and Nedelea, I. (2019). Biological therapies for atopic dermatitis: An update. Exp. Ther. Med. 17 (2), 1061–1067. doi:10.3892/etm.2018.6989
Ezealisiji, K. M., and Okorie, H. N. (2018). Size-dependent skin penetration of silver nanoparticles: Effect of penetration enhancers. Appl. Nanosci. 8 (8), 2039–2046. doi:10.1007/s13204-018-0886-6
Fan, Y., Marioli, M., and Zhang, K. (2021). Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 192, 113642. doi:10.1016/j.jpba.2020.113642
Farooq, U., Rasul, A., Zafarullah, M., Abbas, G., Rasool, M., Ali, F., et al. (2021). Nanoemulsions as novel nanocarrieres for drug delivery across the skin: In-vitro, in-vivo evaluation of miconazole nanoemulsions for treatment of candidiasis albicans. Des. Monomers Polym. 24 (1), 240–258. doi:10.1080/15685551.2021.1965724
Fernando, G. J., Hickling, J., Flores, C. M. J., Griffin, P., Anderson, C. D., Skinner, S. R., et al. (2018). Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch™). Vaccine 36 (26), 3779–3788. doi:10.1016/j.vaccine.2018.05.053
Flohr, C., and Hay, R. (2021). Putting the burden of skin diseases on the global map. Br. J. Dermatol. 184 (2), 189–190. doi:10.1111/bjd.19704
Ge, X., Wei, M., He, S., and Yuan, W-E. (2019). Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 11 (2), 55. doi:10.3390/pharmaceutics11020055
Ghasemiyeh, P., and Mohammadi-Samani, S. (2020). Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Des. devel. Ther. 14, 3271–3289. doi:10.2147/DDDT.S264648
Ghezzi, M., Pescina, S., Padula, C., Santi, P., Del Favero, E., Cantù, L., et al. (2021). Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 332, 312–336. doi:10.1016/j.jconrel.2021.02.031
Goyal, R., Macri, L. K., Kaplan, H. M., and Kohn, J. (2016). Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 240, 77–92. doi:10.1016/j.jconrel.2015.10.049
Haq, A., and Michniak-Kohn, B. (2018). Effects of solvents and penetration enhancers on transdermal delivery of thymoquinone: Permeability and skin deposition study. Drug Deliv. 25 (1), 1943–1949. doi:10.1080/10717544.2018.1523256
Higuchi, T. (1960). Physical chemical analysis of percutaneous absorption process from creams and ointments. J. Soc. Cosmet. Chem. 11, 85–97.
How, K. N., Yap, W. H., Lim, C. L. H., Goh, B. H., and Lai, Z. W. (2020). Hyaluronic acid-mediated drug delivery system targeting for inflammatory skin diseases: A mini review. Front. Pharmacol. 11, 1105. doi:10.3389/fphar.2020.01105
Imran, M., Paudel, K. R., Jha, S. K., Hansbro, P. M., Dua, K., and Mohammed, Y (2022). Dressing of multifunctional nanoparticles with natural cell-derived membranes for the superior chemotherapy. Nanomedicine 17 (10), 665-670. doi:10.2217/nnm-2022-0051
Iqbal, B., Ali, J., and Baboota, S. (2018). Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 57 (6), 646–660. doi:10.1111/ijd.13902
Kamble, P., Sadarani, B., Majumdar, A., and Bhullar, S. (2017). Nanofiber based drug delivery systems for skin: A promising therapeutic approach. J. Drug Deliv. Sci. Technol. 41, 124–133. doi:10.1016/j.jddst.2017.07.003
Kapoor, A., Mishra, S. K., Verma, D. K., and Pandey, P. (2018). Chemical penetration enhancers for transdermal drug delivery system. J. Drug Deliv. Ther. 8 (5), 62–66. doi:10.22270/jddt.v8i5-s.1952
Klang, V., Matsko, N. B., Valenta, C., and Hofer, F. (2012). Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 43 (2-3), 85–103. doi:10.1016/j.micron.2011.07.014
Krishnan, V., and Mitragotri, S. (2020). Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv. Drug Deliv. Rev. 153, 87–108. doi:10.1016/j.addr.2020.05.011
Kumar, L., Verma, S., Joshi, K., Utreja, P., and Sharma, S. (2021). Nanofiber as a novel vehicle for transdermal delivery of therapeutic agents: Challenges and opportunities. Futur. J. Pharm. Sci. 7 (1), 175. doi:10.1186/s43094-021-00324-1
Lai, F., Caddeo, C., Manca, M. L., Manconi, M., Sinico, C., Fadda, A. M., et al. (2020). What's new in the field of phospholipid vesicular nanocarriers for skin drug delivery. Int. J. Pharm. 583, 119398. doi:10.1016/j.ijpharm.2020.119398
Mahmood, S., Chatterjee, B., and Mandal, U. K. (2021). Pharmacokinetic evaluation of the synergistic effect of raloxifene loaded transfersomes for transdermal delivery. J. Drug Deliv. Sci. Technol. 63, 102545. doi:10.1016/j.jddst.2021.102545
Makhmalzade, B. S., and Chavoshy, F. (2018). Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 9 (1), 2–8. doi:10.4103/japtr.JAPTR_314_17
Mandal, D., Maran, A., Yaszemski, M. J., Bolander, M. E., and Sarkar, G. (2009). Cellular uptake of gold nanoparticles directly cross-linked with carrier peptides by osteosarcoma cells. J. Mat. Sci. Mat. Med. 20 (1), 347–350. doi:10.1007/s10856-008-3588-x
Mbah, C. C., Builders, P. F., and Attama, A. A. (2014). Nanovesicular carriers as alternative drug delivery systems: Ethosomes in focus. Expert Opin. Drug Deliv. 11 (1), 45–59. doi:10.1517/17425247.2013.860130
McLafferty, E., Hendry, C., and Farley, A. (2012). The integumentary system: Anatomy, physiology and function of skin. Nurs. Stand. 27 (3), 35–42. doi:10.7748/ns.27.3.35.s52
Mishra, D. K., Shandilya, R., and Mishra, P. K. (2018). Lipid based nanocarriers: A translational perspective. Nanomedicine 14 (7), 2023–2050. doi:10.1016/j.nano.2018.05.021
Moon, S-S., Richter-Roche, M., Resch, T. K., Wang, Y., Foytich, K. R., Wang, H., et al. (2022). Microneedle patch as a new platform to effectively deliver inactivated polio vaccine and inactivated rotavirus vaccine. NPJ vaccines 7 (1), 26. doi:10.1038/s41541-022-00443-7
Nguyen, H. X., and Banga, A. K. (2018). Electrically and ultrasonically enhanced transdermal delivery of methotrexate. Pharmaceutics 10 (3), 117. doi:10.3390/pharmaceutics10030117
Pant, B., Park, M., and Park, S-J. (2019). Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 11 (7), 305. doi:10.3390/pharmaceutics11070305
Park, J., Lee, H., Lim, G-S., Kim, N., Kim, D., Kim, Y-C., et al. (2019). Enhanced transdermal drug delivery by sonophoresis and simultaneous application of sonophoresis and iontophoresis. AAPS PharmSciTech 20 (3), 96. doi:10.1208/s12249-019-1309-z
Powale, S., Chandel, V. K., and Asati, S. (2022). Preparation and characterization of ethosomes for topical delivery of clindamycin. J. Drug Deliv. Ther. 12 (1), 109–113. doi:10.22270/jddt.v12i1.5190
Pulsipher, K. J., Szeto, M. D., Rundle, C. W., Presley, C. L., Laughter, M. R., Dellavalle, R. P., et al. (2021). Global burden of skin disease representation in the literature: Bibliometric analysis. JMIR Dermatol. 4 (2), e29282. doi:10.2196/29282
Qu, F., Geng, R., Liu, Y., and Zhu, J. (2022). Advanced nanocarrier-and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics 12 (7), 3372–3406. doi:10.7150/thno.69999
Rai, V. K., Mishra, N., Yadav, K. S., and Yadav, N. P. (2018). Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 270, 203–225. doi:10.1016/j.jconrel.2017.11.049
Ramadon, D., McCrudden, M. T., Courtenay, A. J., and Donnelly, R. F. (2022). Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 12 (4), 758–791. doi:10.1007/s13346-021-00909-6
Rancan, F., Contardi, M., Jurisch, J., Blume-Peytavi, U., Vogt, A., Bayer, I. S., et al. (2019). Evaluation of drug delivery and efficacy of ciprofloxacin-loaded povidone foils and nanofiber mats in a wound-infection model based on ex vivo human skin. Pharmaceutics 11 (10), 527. doi:10.3390/pharmaceutics11100527
Roberts, M. S., Cheruvu, H. S., Mangion, S. E., Alinaghi, A., Benson, H. A., Mohammed, Y., et al. (2021). Topical drug delivery: History, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 177, 113929. doi:10.1016/j.addr.2021.113929
Samimi, S., Maghsoudnia, N., Eftekhari, R. B., and Dorkoosh, F. (2019). “Lipid-based nanoparticles for drug delivery systems,” in Characterization and biology of nanomaterials for drug delivery. Editors S. Mohapatra, S. Ranjan, N. Dasgupta, R. Mishra, and S. Thomas (London: Elsevier), 47–76. doi:10.1016/B978-0-12-814031-4.00003-9
Sarheed, O., Shouqair, D., Ramesh, K., Khaleel, T., Amin, M., Boateng, J., et al. (2020). Formation of stable nanoemulsions by ultrasound-assisted two-step emulsification process for topical drug delivery: Effect of oil phase composition and surfactant concentration and loratadine as ripening inhibitor. Int. J. Pharm. 576, 118952. doi:10.1016/j.ijpharm.2019.118952
Seth, D., Cheldize, K., Brown, D., and Freeman, E. E. (2017). Global burden of skin disease: Inequities and innovations. Curr. Dermatol. Rep. 6 (3), 204–210. doi:10.1007/s13671-017-0192-7
Souto, E. B., Cano, A., Martins-Gomes, C., Coutinho, T. E., Zielińska, A., Silva, A. M., et al. (2022). Microemulsions and nanoemulsions in skin drug delivery. Bioengineering 9 (4), 158. doi:10.3390/bioengineering9040158
Stefanov, S. R., and Andonova, V. Y. (2021). Lipid nanoparticulate drug delivery systems: Recent advances in the treatment of skin disorders. Pharmaceuticals 14 (11), 1083. doi:10.3390/ph14111083
Sudhakar, K., Fuloria, S., Subramaniyan, V., Sathasivam, K. V., Azad, A. K., Swain, S. S., et al. (2021). Ultraflexible liposome nanocargo as a dermal and transdermal drug delivery system. Nanomaterials 11 (10), 2557. doi:10.3390/nano11102557
Tortora, G. J., and Derrickson, B. H. (2018). Principles of anatomy and physiology. 15th ed. Hoboken: John Wiley & Sons.
Touitou, E., Dayan, N., Bergelson, L., Godin, B., and Eliaz, M. (2000). Ethosomes—novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 65 (3), 403–418. doi:10.1016/s0168-3659(99)00222-9
Wadher, K., Pounikar, S. D., Trivedi, S., and Umekar, M. (2018). Ethosome: A novel vesicular carrier. Int. J. Innov. Res. Adv. Stud. 5, 13–20. doi:10.2174/1567201815666180116091604
Waghule, T., Singhvi, G., Dubey, S. K., Pandey, M. M., Gupta, G., Singh, M., et al. (2019). Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 109, 1249–1258. doi:10.1016/j.biopha.2018.10.078
Williams, A. (2018). “Topical and transdermal drug delivery,”. Editors M. Aulton, and K. Taylor. 5th ed. (London: Elsevier).
Yamada, M., Tayeb, H., Wang, H., Dang, N., Mohammed, Y. H., Osseiran, S., et al. (2018). Using elongated microparticles to enhance tailorable nanoemulsion delivery in excised human skin and volunteers. J. Control. Release 288, 264–276. doi:10.1016/j.jconrel.2018.09.012
Yih, T., and Al‐Fandi, M. (2006). Engineered nanoparticles as precise drug delivery systems. J. Cell. Biochem. 97 (6), 1184–1190. doi:10.1002/jcb.20796
Yotsumoto, K., Ishii, K., Kokubo, M., and Yasuoka, S. (2018). Improvement of the skin penetration of hydrophobic drugs by polymeric micelles. Int. J. Pharm. 553 (1-2), 132–140. doi:10.1016/j.ijpharm.2018.10.039
Keywords: new technologies, nanoparticles, nanocarriers, topical and transdermal delivery, drug/active, future technologies
Citation: Tapfumaneyi P, Imran M, Mohammed Y and Roberts MS (2022) Recent advances and future prospective of topical and transdermal delivery systems. Front. Drug. Deliv. 2:957732. doi: 10.3389/fddev.2022.957732
Received: 31 May 2022; Accepted: 07 July 2022;
Published: 05 September 2022.
Edited by:
Nina Dragicevic, Singidunum University, SerbiaReviewed by:
Sarbari Acharya, KIIT University, IndiaCopyright © 2022 Tapfumaneyi, Imran, Mohammed and Roberts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yousuf Mohammed, y.mohammed@uq.edu.au; Michael S Roberts, m.roberts@uq.edu.au
 Pronalis Tapfumaneyi
Pronalis Tapfumaneyi Mohammad Imran
Mohammad Imran Yousuf Mohammed
Yousuf Mohammed Michael S. Roberts
Michael S. Roberts