In silico identification of potential inhibitors of acyl carrier protein reductase and acetyl CoA carboxylase of Plasmodium falciparum in antimalarial therapy
- 1West African Centre for Computational Research and Innovation, Accra, Ghana
- 2Department of Biochemistry and Forensic Sciences, School of Chemical and Biochemical Sciences, C. K. Tedam University of Science and Technology, Navrongo, Ghana
- 3West African Centre for Cell Biology of Infectious Pathogens (WACCBIP), Department of Biochemistry, Cell and Molecular Biology, University of Ghana, Navrongo, Ghana
- 4Department of Biological Sciences, Microbiology PhD. Program, College of Science, Technology, Engineering and Mathematics (C-STEM), Alabama State University, Montgomery, AL, United States
Malaria caused by Plasmodium falciparum, remains one of the most fatal parasitic diseases that has affected nearly a third of the world’s population. The major impediment to the treatment of malaria is the emergence of resistance of the P. falciparum parasite to current anti-malaria therapeutics such as Artemisinin (ART)-based combination therapy (ACT). This has resulted in countless efforts to develop novel therapeutics that will counter this resistance with the aim to control and eradicate the disease. The application of in silico modelling techniques has gained a lot of recognition in antimalarial research in recent times through the identification of biological components of the parasite for rational drug design. In this study we employed various in silico techniques such as the Virtual screening, molecular docking and molecular dynamic simulations to identify potential new inhibitors of biotin acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase, two enzyme targets that play a crucial role in fatty acid synthesis in the Plasmodium parasite. Initially, nine hit compounds were identified for each of the two enzymes from the ZINCPharmer database. Subsequently, all hit compounds bind favourably to the active sites of the two enzymes as well as show excellent pharmacokinetic properties. Three 3) of the hits for the biotin acetyl-coenzyme A (CoA) carboxylase and six 6) of the enoyl-acyl carrier reductase showed good toxicity properties. The compounds were further evaluated based on the Molecular Dynamics simulation that confirmed the binding stability of the compounds to the targeted proteins. Overall, the lead compounds ZINC38980461, ZINC05378039, and ZINC15772056, were identified for acetyl-coenzyme A (CoA) carboxylase whiles ZINC94085628, ZINC93656835, ZINC94080670, ZINC1774609, ZINC94821232 and ZINC94919772 were identified as lead compounds for enoyl-acyl carrier reductase. The identified compounds can be developed as a treatment option for the malaria disease although, experimental validation is suggested for further evaluation of the work.
Background
One of the most human deadly parasitic disease is malaria which affects more than a third of the global population with annual report of roughly 200 million cases in the past decade (World Health Organization, 2021a). In this regard, malaria has affected the livelihoods of large populations world-wide more than any other infectious disease. Another available data suggest an approximately half a billion individuals are infected with Plasmodium spp. Globally, with annual mortality ranging from 1.5 to 2.7 million, with children seriously affected (Garcia, 2010). Recently, an estimated 241 million malaria cases have been reported world-wide in 2020, wherein, roughly 627, 000, up to 69,000 have been estimated to have died from the disease within previous year under review (World Health Organization, 2021a). In view of the aforementioned alarming situation of the disease, there is urgent need to discover potent therapeutic targets for the development of new antimalarial drugs. This is because resistance to mainstream antimalarial drugs of Plasmodium falciparum impede the fight against malaria, which hamper the control and eradication strategies currently in place. Thus, the goal of eradicating the spread of malaria relies on finding therapeutic strategies against P. Falciparum.
The currently available antimalarial therapeutics as recommended by the World Health Organization include the Artemisinin (ART)-based combination therapy (ACT), antifolates, antimicrobials, Quinolines, etc (Tse et al., 2019), (World Health Organization, 2021b). ACT is a combination of two or more drugs that work against the malaria parasite in different ways and used as a first line treatment. Also, a combination therapy of artesunate (AS) and amodiaquine (AQ) is another antimalarial therapy that has been in use for several years (White, 2004). Despite the high potency and rapid action of these therapies in halting the spread of malaria, P. falciparum continues to find ways to resist being completely eradicated by acquiring resistance to the various available treatments (Dondorp et al., 2009). Also, there is a reported high rate of recrudescence specifically associated with ART monotherapy or ACT (Meshnick et al., 1996). The possible causes of recrudescence to these therapies is attributed to ART-induced ring-stage dormancy and recovery; although, little is known about the characteristics of dormant parasites. As such efforts are directed at finding new techniques to tackle the dormant state of the parasite to overcome this resistance (Kumar et al., 2018).
To date, most research works are geared towards understanding the biology of the parasite including studying its genome with the aim of identifying crucial drug targets to develop novel therapeutics (Vaughan et al., 2008), (Gunther et al., 2009). In spite of the numerous efforts made, only a few number of targets have been confirmed through in vivo investigations and thus provide reliable leads for malaria therapy. An experiment by Chen et al., reported that most metabolic pathways are downregulated in dihydroartemisinin (DHA)-induced dormant parasites. However, the fatty acid and pyruvate metabolic pathways remain the only active mechanisms in the dormant parasites (Chen et al., 2014). Thus the fatty acid synthetic pathway has been explored to be a crucial mechanism in the malaria parasite, hence possess a great potential for anti-malaria drug targets (Chen et al., 2014). The biotin acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase are two enzyme targets that play a crucial role in fatty acid synthesis in the Plasmodium parasite as reported by Chen et al. (2014). Particularly, these targets have been understood to interrupt recovery of the malaria parasites from ART-induced dormancy and reduces the rate of recrudescence after ART treatment (Chen et al., 2014). However, despite their potentials in inducing the recovery of P. falciparum parasites’ from dormancy, the available literature suggests inconsistent results. Also, the current known IC50 of inhibitors that causes recovery of dormant parasites is deemed too high for their use as medicines. Thus the surge for potential new inhibitors is on the rise.
The use of molecular modelling methods is widely employed in antimalarial research through the identification of biological components of the parasite that can be targeted to develop novel therapeutics (Biamonte et al., 2013). The discovery of these crucial enzyme targets (biotin acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase) in the fatty acid synthetic pathway therefore provides a means for the identification of potential new inhibitors with improved efficacy than existing inhibitors of these targets using in silico modelling techniques (Gornicki, 2003). Herein, we employed in silico techniques including the in-house Per ResidueEnergy Decomposition (PRED)-based pharmacophore modelling (Kumalo and Soliman, 2016), molecular docking, virtual screening and Molecular dynamic simulation to discover inhibitors of acyl-carrier protein and acyl-CoA carboxylase in P. falciparum. Additionally, the pharmacokinetic properties of all identified hit compounds were assessed which was followed by prediction of toxicity. We envision that findings from this study will form important basis for further experimental work to be carried out on these lead compounds to develop them into antimalarial agents.
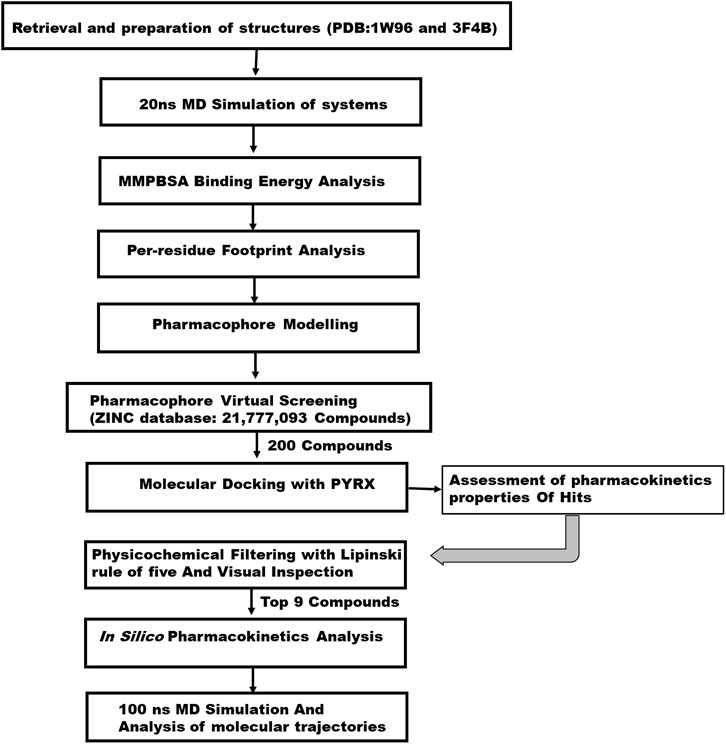
Computational methodology
Retrieval and preparation of protein and ligands.
The 3D crystal structure of the biotin acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase were retrieved from the RCSB Protein Data Bank (Burley et al., 2019) with respective PDB IDs: 1W96 (Shen et al., 2004) and 3F4B (Yu et al., 2008). The two structures were experimentally solved through the X-ray diffraction method with a resolution value of 1.80 Å and 2.49 Å for 1W96 and 3F4B respectively. The 1w96 structure was retrieved in complex with soraphen A, a known inhibitor of acetyl-coenzyme A carboxylase, hence this was used as a reference compound in the study. Similarly, the structure of 3F4B was retrieved in complex with Triclosan which is a validated inhibitor of enoyl-acyl carrier reductase enzyme. As such this was also employed as reference compound in identifying potential inhibitors of enoyl-acyl carrier reductase. The retrieved structures and the reference inhibitors were prepared for a 20 ns Molecular dynamic simulation to generate a Pharmacophore model at their stable states. Preparation for MD simulations was carried out on the graphical user interface of USCF chimera where all non-standard residues that are not relevant to the study were removed such as water, ions and other co-factors. In all, two systems were setup for a 20 ns MD simulation comprising acetyl-coenzyme A in complex with Soraphen A (1W96) and enoyl-acyl carrier reductase in complex with Triclosan (3F4B).
Pharmacophore model generation using per residue energy decomposition (PRED) based approach and virtual screening.
The main goal in the Identification of small molecule antagonists of biotin acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase is to model novel compounds that could also act in a similar or more potent inhibitory capacity towards the target protein as the identified reference inhibitors. Unlike other traditional pharmacophore modeling techniques, the validated in-house PRED method was used to outline the pharmacophoric features of the ligand-receptor in order to retrieve a more tailored potential hit/s (Cele et al., 2016)– (Issahaku et al., 2022). This pharmacophore model analyzes both the structural and chemical properties of proteins and ligands (Cele et al., 2016). To generate a PRED-based pharmacophore model, PRED decomposition was estimated via the MM/PBSA method for energy estimations after a 20ns MD simulations of the prepared complex systems. Pharmacophoric features based on the receptor-ligand interaction obtained from this short run MD simulation was selected. Residues Ile69, Lys73, Arg76, Ser77, Asn398, Val397, Gly396, Met393, Glu392 and Pro389 were found to be the highest energy contributing residues in the biotin acetyl-coenzyme A (CoA) carboxylase structure that interact with the ligand (Figure 1). Similarly, residues Ile333, Ala304, Ala305, Ile308, Val207, Gly204, Asn203, Ala202 and Tyr262 were identified as the highest energy contributing residues in the acyl carrier reductase complex (Figure 1). These identified moieties on each of the ligands that interacted with these residues were subsequently set as a query to generate a PRED-based pharmacophore models in ZINCPharma for the two targets of the fatty acid synthases pathways (Koes and Camacho, 2012). Subsequently, the zinc database was then screened for novel hits with similar features as the generated PRED-based pharmacophore models (Figure 1) (Huang et al., 2006). For hit screening, the filter was configured to query ZINC purchasable compounds with a molecular weight ≤ 500, with rotatable bonds set at ≤ 10. The “rule of five” proposed by Lipinski was also used as a cut-off (Pollastri, 2010). A total of 21,777,093 library of Compounds were generated from the zinc database for the generated pharmacophores. Nine hit compounds were selected for each of the queried models on the ZINCPharmer database further analysis (Koes and Camacho, 2012). The identified hits were downloaded in sdf file formats for further assessment.
Molecular docking of hit compounds
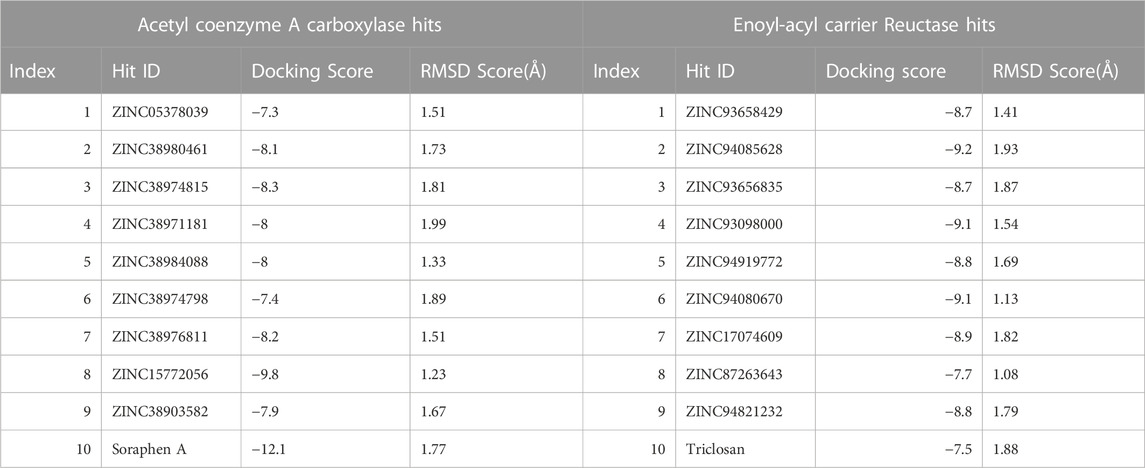
We conducted a molecular docking study on the identified hits to estimate the binding scores and also reveal the ligand-receptor best binding orientation between the hits and the acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase. Molecular docking is also necessary to rank the hits in order of strongest binding affinity to the targets as this will screen out the most active hit compounds and yield a stable complex. Prior to molecular docking the hits compounds were taken through energy minimization using Avogadro 1.2.0 software (Hanwell et al., 2012). The Avogadro software is incorporated with a UFF force field which optimizes the molecular geometries of the compounds and uses a steepest descent algorithm for structural minimization. The active site for each of the enzyme targets were determined using the grid positions of the native inhibitors (i.e., Soraphen A and Triclosan). The binding site based on the grid coordinates for acetyl-coenzyme A (CoA) carboxylase had the following coordinates; Centre (X = 12.93, Y = 26.86, Z = 117.21) and Dimensions (X = 25.99, Y = 20.32, Z = 21.16). The coordinates for the enoyl-acyl carrier reductase is as follow: Centre (X = −0.49, Y = −20.77, Z = −0.89) and Dimension (X = 21.68, Y = 20.32, Z = 15.22). Subsequently, molecular docking was carried out using AutoDock vina incorporated in PyRx for all hit compounds (Dallakyan and Olson, 2015), (Antón Vázquez et al., 2017). Output of docking was viewed on UCSF Chimera using the integrated ViewDock module after which the docking scores of the best pose for each complex were tabulated as illustrated in Table 1 saved for further analysis.
Assessment of pharmacokinetics properties of hits
In the computational drug design and development process, the early-stage assessment of pharmacokinetic parameters aids in the optimization of a molecular candidate to become an effective drug (Chaturvedi et al., 2001). As such the resulting compounds obtained was evaluated and analyzed based on their physicochemical properties such as absorption, distribution, metabolism and excretion (ADME). Using the online platform SwissADME (http://www.swissadme.ch/index.php) (Daina et al., 2017), which helps to predict and analyse pharmacokinetic and pharmacodynamics properties of selected compounds. This was necessary to evaluate the prospects of the identified hits to be developed for human use. Furthermore, In silico ADME studies are expected to reduce the risk of late-stage attrition of drug development and to optimize screening and testing by looking at only the promising compounds (Yamashita and Hashida, 2004). ADME properties were predicted based on the Lipinski’s rule of five (LRo5). LRo5 is a general standard for estimating the biological activity, good oral bioavailability coupled with the tendency of a drug molecule to penetrate various aqueous and lipophilic (membrane) barriers (Pollastri, 2010), (Owens and Lipinski, 2003). As all the identified hits were assessed for their drug-likeliness according the rule of five as shown in Table 2 and Table 3.
Prediction of toxicity
The prediction of chemical toxicity is a crucial step in the drug development process (Li et al., 2019). Not only are computational toxicity estimates faster in determining harmful levels in animals, but they can also assist to minimize the number of animal tests. Also, prioritizing compounds with a reduced risk of toxicity early in the drug development process should assist to reduce the high attrition rate in pharmaceutical R and D (Li et al., 2019). Chemists can be alerted if their suggested compounds are more likely to cause toxicity utilizing expert knowledge-based toxicity prediction. Toxicity prediction for the hit compounds was carried through ProTox-II (https://tox-new.charite.de/protox_II/) server (Banerjee et al., 2018). Toxicity endpoints such as mutagenicity, carcinogenicity, and other characteristics can be quantified both quantitatively and qualitatively to determine a chemical compound’s toxicity using this server (Segall and Barber, 2014).
Conformational stability analysis of top hits via molecular dynamic simulation
Molecular dynamic simulation was carried out for the top hits with good binding score, formed active interactions with targets, excellent pharmacokinetic properties and reduced toxicity effect. This is necessary to further analyse the impact of the selected hits on the stability and flexibility of the target enzymes. Receptor-Ligand structural assessment is crucial to the function of the receptor as any perturbation on the structural architecture of the protein/enzymes will ultimately alter its biological effect. All selected systems for MD simulation were prepared on UCSF Chimera comprising docked complexes of all nine 9) hits for each of the two targets, an unbound apo system for each of the targets and a reference system of soraphen A in complex with Acetyl CoA and triclosan in complex with Acyl carrier protein.
All molecular dynamics (MD) simulations were carried out using the graphic processing unit (GPU) version of the Particle Mesh Ewald Molecular Dynamics (PMEMD) of AMBER 18 software package (Case et al., 2018). Atomic partial charges for the hit compounds were generated by the ANTECHAMBER module by using the Restrained Electrostatic Potential (RESP) and the General Amber Force Field (GAFF) protocol (Sprenger et al., 2015). The receptors were parametized by the FF14SB (Maier et al., 2015) force field integrated in the Amber 18 suit. The Link Edit and parm (LEAP) module (Nikitin, 2014) of Amber 18 was then used to add hydrogens that are missing from the systems during preparation. Also, this module neutralizes the system by adding counter ions such as Na+ and Cl-after which the systems are solvated by suspending them in Transferable Intermolecular Potential with three Point (TIP3P) water box of size 8Å. A complexed coordinates and topology files of the receptor-ligand binding are generated for subsequent processing. The systems were minimized for 2000 energy steps. Initial minimization of 1,000 steps with steep descent were performed for all the systems with a restrain potential and then followed by another 1,000 steps minimization by conjugate gradient algorithm without restrain. The systems were then gradually heated from 0K to 300 K with a 5 kcal/mol. A harmonic restraint potential in NTP ensemble using Langevin thermostat of collision frequency of 1/ps. All the systems were then equilibrated at 300 K for 500 ps without restraint with a constant pressure at 1 bar using Berendsen barostat. SHAKE algorithm was used to restrain all hydrogen bonds (Gonnet and SHAKE, 2007). MD production of 100ns was then performed without restrain on the systems with target coupling of 2 ps and constant pressure at 1 bar. Analysing the trajectories and coordinates generated from the MD run was carried through the CPPTRAJ and PTRAJ modules (Roe and Cheatham, 2013) incorporated in Amber 18. The Root Mean Square Deviation (RMSD), and Root Mean Square Fluctuation (RMSF) were calculated for all the systems. Discovery Studio version v19.10.18289 (BIOVIA, 2017) and UCSF chimera were used to visualize the trajectories while Origin data version 6.0 tool (Seifert, 2014) was used to plot all graphs.
Binding free energy analysis via MM/GBSA method
The Molecular Mechanics/Generalized Born Surface Area (MM/GBSA) (Kollman et al., 2000; Massova and Kollman, 2000; Onufriev et al., 2000; Miller et al., 2012) method was employed in estimating the binding free energy for each of the inhibitor-bound systems. The binding free energy (ΔGbind) was calculated from the following equation:
Where ΔGbind is considered to be the summation of the gas phase and solvation energy terms less the entropy (TS) term
Egas is the sum of the AMBER force field internal energy terms Eint (bond, angle and torsion), the covalent van der Waals (Evdw) and the non-bonded electrostatic energy component (Eelec). The solvation energy is calculated from the following equation:
Where ΔGbind is taken to be the sum of the gas phase and solvation energy terms less the entropy (TΔS) term., Gcomplex represents energy of the receptor ligand complex. Whiles Greceptor and Gligand represents energies of receptor and ligand respectively. Egas denotes gas-phase energy; Eint signifies internal energy; and Eele and Evdw indicate the electrostatic and Van der Waals contributions, respectively. Egas is the gas phase, elevated directly from the FF14SB force terms. Gsol denotes solvation free energy, can be decomposed into polar and non-polar contribution states. The polar solvation contribution, GGB, is determined by solving the GB equation, whereas, GSA, the non-polar solvation contribution is estimated from the solvent accessible surface area (SASA) determined using a water probe radius of 1.4 Å. T and S correspond to temperature and total solute entropy, respectively. γ Is a constant (Sitkoff et al., 1994). Per-residue decomposition analyses were also carried out to estimate individual energy contribution of residues of the substrate pocket towards the affinity and stabilization of each target.
Results
Identification of hit compounds
A pharmacophore structure defines how the key molecular properties of a ligand-receptor interaction are organized. As shown in Figure 2, the top amino acids interacting with each of the targets are highlighted and subsequently, the pharmacophore model generated. The ZINCPharmer database, a subsidiary of the zinc database was utilized to identify potential hit compounds based on the modelled pharmacophore. From our findings nine hit compounds were identified for each of acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase from the ZINCPharmer query search (Figure 3). The hits were subjected to a molecular docking to assess their binding affinity to the target proteins.
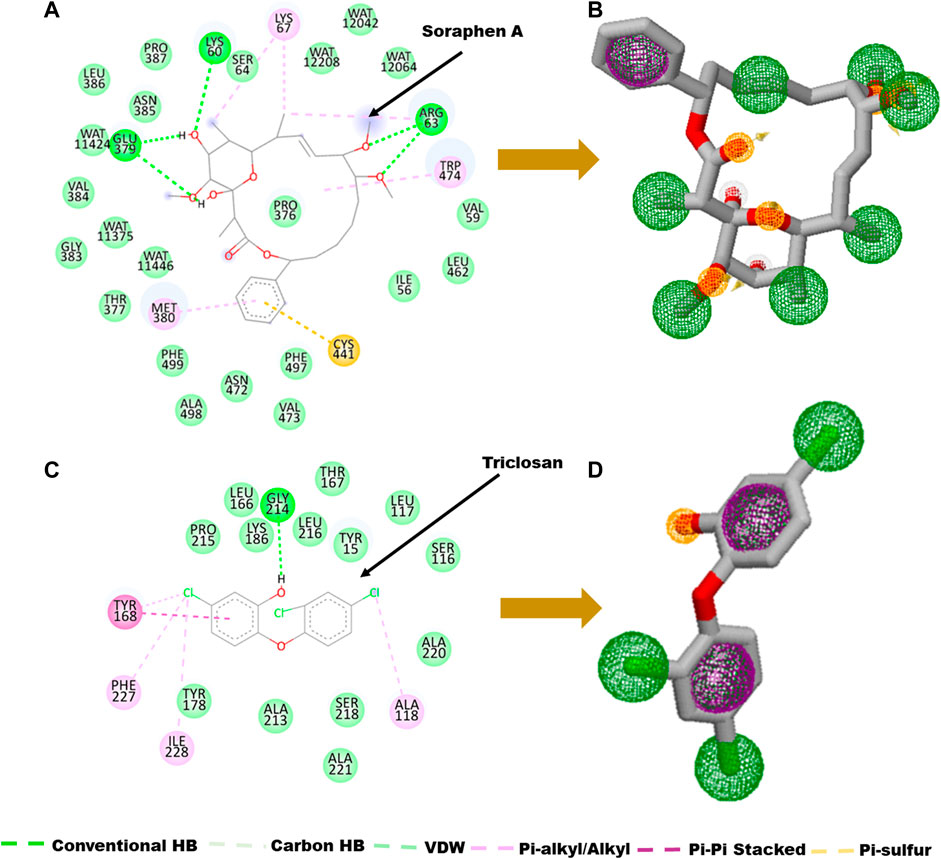
FIGURE 2. Ligand-residue interaction plot of (A) Soraphen A at the binding site of acetyl-coenzyme A (CoA) carboxylase enzyme and (C) Triclosan at the binding site of enoyl-acyl carrier reductase. (B–D) Generated pharmacophores showing aromatic (green) and hydrophobic moieties (Purple) and hydrogen donors (yellow).
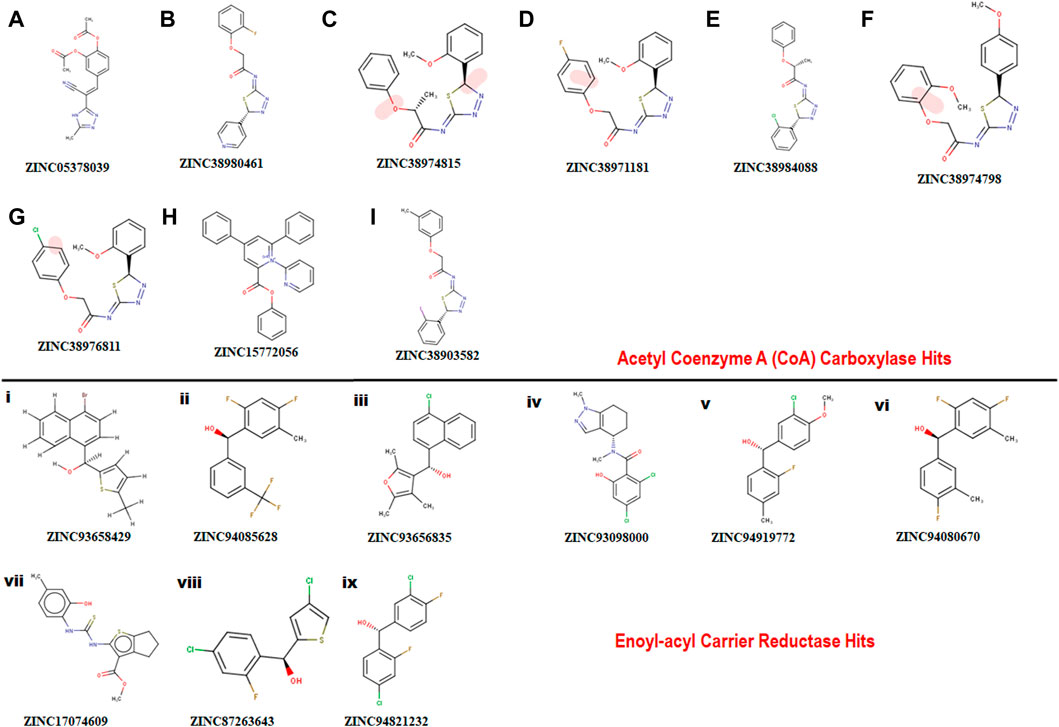
FIGURE 3. Showing 2D structures of identified hits. (A–I) comprising hits for Acetyl Coenzyme A (CoA) carboxylase and (i)-(ix) comprising hits for Enoyl-acyl carrier reductase.
Analysis of binding score of hits using molecular docking
Molecular docking was utilized extensively to predict the binding affinity and orientation of all screened hit compounds from the ZINCpharmer when they bind to their biological targets. Our findings illustrated in Table 1 show the respective binding scores of the hit compounds. Also, included are the assessment binding affinity scores for the reference compounds soraphen A and Triclosan used in the study. These reference native ligands Soraphen A and Triclosan were redocked into the active sites of their respective targets to ensure the chosen docking program was reliable. For most binding score analysis, the most negative value is usually indicative of a stronger binding and vice versa. In assessing the hits that target Acetyl Coenzyme A Carboxylase, the threshold of binding affinities was observed to be in the range −7.3 kcal/mol and −9.8 kcal/mol. Also, all hits bound to the active site with a low RMSD score below < 2 which indicates stability. Which is indicative of favorable binding of all hits to the active site of Acetyl Coenzyme A Carboxylase. However, ZINC15772056 had the highest binding score of −9.8 kcal/mol whiles ZINC05378039 had the lowest binding score among all the hits. The reference Soraphen compound showed to have the strongest binding score of −12.8 kcal/mol as predicted from the molecular docking analysis. Similarly, all hit compounds of Enoyl-acyl carrier reductase bound favourably to the active site of their target which is evidenced by the binding score threshold in the range −7.7 kcal/mol and −9.2 kcal/mol for all hit compounds. This finding shows that all screened hits bind favourably to Enoyl-acyl carrier reductase based on the molecular docking prediction as shown in Figure 4. However, ZINC94085628 had the highest binding score of −9.2 kcal/mol whiles ZINC87263643 had the lowest dock score of −7.7 kcal/mol. Overall, all hit compounds showed to have bound strongly in contrast to the reference Triclocan compound with low RMSD score correlating to a stable complex.
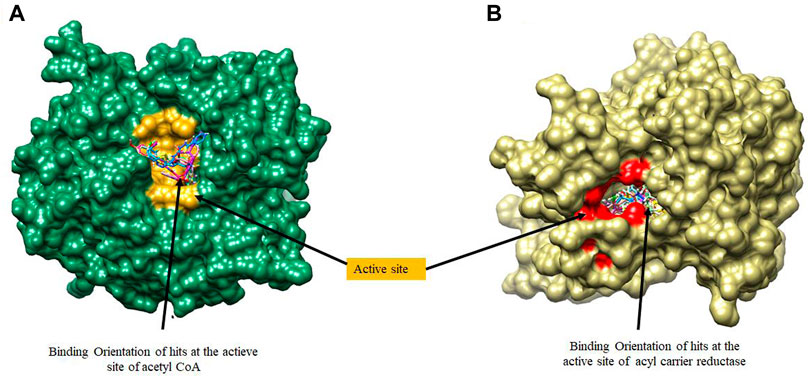
FIGURE 4. A surface view of the binding position of hits at the active sites of (A) Acetyl Coenzyme A Carboxylase and (B) Enoyl-acyl carrier reductase.
Assessment of ADME properties of hit compounds
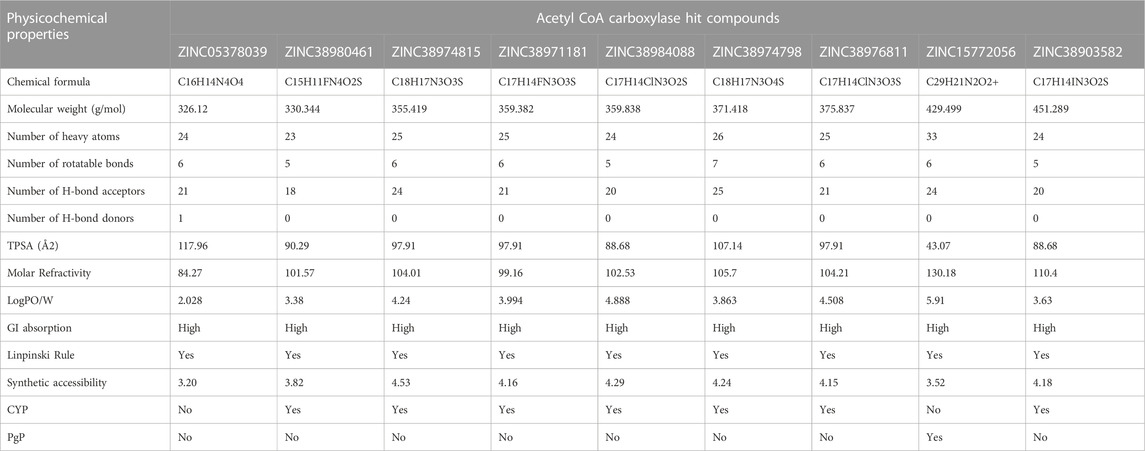
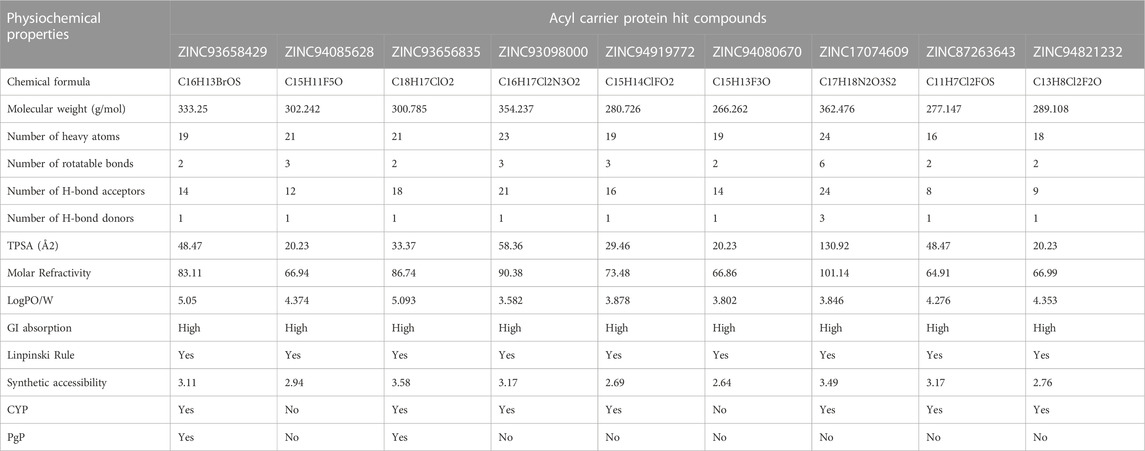
Beyond the experimental models, determining drug-likeness reveals pharmacokinetic and pharmacodynamics aspects that unavoidably impact metabolism, distribution, absorption, and excretion in human systems. In predicting the drug-likeliness of a chemical compound, the Lipinski’s rule of five (LRo5) is considered. LRo5 is a universal benchmark for determining a drug’s biological activity, excellent oral bioavailability, and ability to permeate different aqueous and lipophilic (membrane) barriers (Pollastri, 2010), (Lipinski et al., 2012). SwissADME (Daina et al., 2017) was used to predict physiochemical and pharmacokinetic properties of the screened compounds by following LRo5 thereby assessing their druggability. The descriptors, according to the LRo5 include molecular weight (MW) [≤ 500 Da], octanol-water partition coefficient [log p ≤ 5], Hydrogen bond donors (HBD) [≤ 5] and Hydrogen bond acceptors (HBA) [≤ 10]. As estimated from our findings in Table 2; Table 3 all hits across both targets have molecular weights below 500 Da threshold. A low molecular weight compound is mostly attributed to less toxicity and also an indication of a high tendency to be favoured for cellular uptake with little or no obstruction to its transport and distribution to target sites as opposed to compounds with larger MW. All other standards according to the LRo5 were matched by all identified hit compounds and hence passed the Linpinski’s assessment of druggability. Additionally, the synthetic accessibility of all identified hits was assessed on SwissADME as shown in Table 2. Generally, a score from one 1), indicates easy synthetic accessibility, to ten (10) indicating very difficult synthetic accessibility. All hits across both targets have a synthetic accessibility score below five 5), which indicates that they could be easily experimentally synthesized, according to the results predicted by SwissADME.
Also, the Cytochrome P450 (CYP) and P-glycoprotein (PgP) substrate was predicted as well. CYP is a complex and important component of drug metabolism. It is the root of many drug interactions due to inhibition, induction, and competition for common enzymatic pathways by different drugs. Table 2; Table 3 show the predicted CYP outcomes of all hits across the two enzymes. PgP activity of a compound determines the effectiveness of the drug in transporting a wide variety of substrates across extra- and intracellular membranes. The predicted PgP outcomes for all hits showed a variations in their effectiveness in transporting substrate across membranes as shown in Table 2 and Table 3.
Toxicity assessment
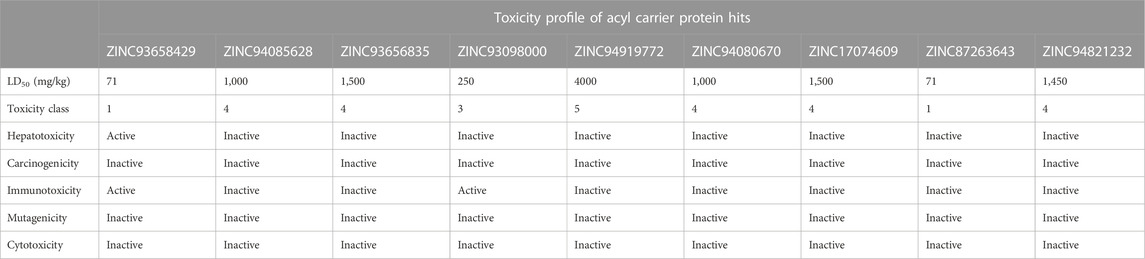
The study of a compound’s toxicity is an important part of the drug development process. As such the toxicity characteristics of a potential drug candidate must be identified before it may enter clinical trials. Toxicities are usually explored through expensive, time-consuming and life-threatening animal studies, thus in silico toxicity estimates are a good option. Compounds are divided into toxicity classes based on the severity of their effects. Also, through a single or short-term exposure, toxicity can disrupt the synthesis of important enzymes in an organism, leading to the failure of a key organ. Sometimes the chemicals developed as medication candidates are toxin-like and damaging to other organs, causing organ toxicity, immunotoxicity, mutagenicity, and cytotoxicity in humans and animals. The toxicity of the compounds was predicted using ProTox-II, an online chemical toxicity prediction platform that incorporates molecular similarity, fragment propensities, and machine learning to predict toxicities (Lipinski et al., 2012). The proTox-II server determines the toxic properties of compounds through the predicted median lethal dose (LD50) in mg/kg weight. Therefore, the toxicity of all identified hits were assessed in this study. Three 3) out of the nine 9) hits namely; ZINC05378039, ZINC38980461 and ZINC15772056 were identified to be in toxicity class IV for the acetyl coenzyme A carboxylase enzyme, indicative of non-toxicity and non-irritating. This finding highlights these three hits to be more favourable in terms of toxicity in contrast to the other hits.
Similarly, ZINC94085628, ZINC93656835, ZINC94080670, ZINC17074609, ZINC87263643, and ZINC94821232 for the enoyl—acyl carrier reductase enzyme, were in class IV, indicative of the excellent toxicity properties characterized by non-toxic and non-irritating properties. However, ZINC94919772 showed to be the most favourable in terms of toxicity and was identified to be in toxicity class V. Indicative of non-toxic, non-irritating, non-harmful when swallowed among others.
Hepatotoxicity induced by drugs is the most common cause of acute liver failure. It may be a result of direct toxicity from the administered drug or its metabolites. Complications of hepatotoxicity include: fever, diarrhoea, weight loss, headaches, nausea and most commonly hyperbilirubinemia and jaundice. In this experiment, all nine HIT compounds of acetyl co-enzyme A carboxylase enzyme shows activity for hepatotoxicity whiles eight of the hits for enoyl—acyl carrier reductase enzyme showed inactivity with only one 1) active hit for hepatotoxicity this implies that all compounds that show hepatotoxicity activity have the potential to cause damage to the liver, in contrast to the hits with an ‘inactive’ outcome.
The propensity of a substance to develop tumors is known as carcinogenicity. It is a multi-stage process that begins with the transformation of normal cells into tumor cells and involves numerous phases and intricate biological interactions that are influenced by elements like genetics, age, food, environment, and hormones. In the study, two of the nine compounds show activity for carcinogenicity in the acetyl co-enzyme A carboxylase hit compounds, this is indicative of an increased risk of the incidence of tumours. All hits of the enoyl—acyl carrier reductase enzyme were inactive towards carcinogenicity as shown Table 4. Immunogenicity is the potential of a compound to elicit an unwanted immune response against the compound. This experiment focused on chemical drugs, most of the hit compounds show inactivity for immunogenicity across both enzymes as shown in Figures 4, 5. Hence, the risk of an immune response and antibodies forming against the respective compounds is decreased. Mutagenicity is the potential of a compound to form mutations in proteins and cell structures in-vivo, hence altering the function of them. Cytotoxicity is the degree to which a substance can cause damage to a cell. This experiment scored well in cytotoxicity as all of the HIT compounds had predicted results of inactivity.
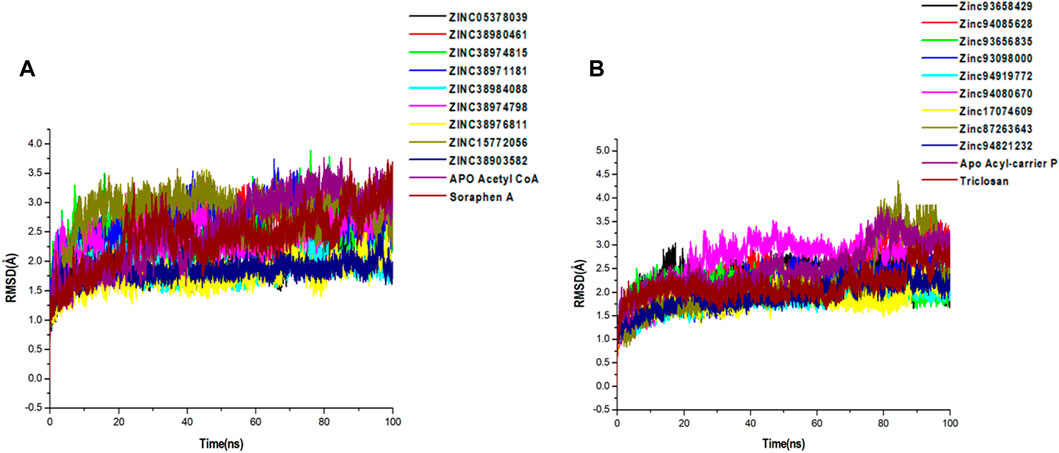
FIGURE 5. Comparative C-α RMSD plots showing the degree of stability and convergence of the hit compounds for (A) Acetyl coenzyme A carboxylase (B) Enoyl-acyl carrier reductase enzyme over the 100 ns MD simulation time.
Analysis of conformational dynamics of protein-ligand stability via MD simulation
An assessment of the conformational dynamics was carried out for the two fatty acid targets in complex with their respective hits to unveil insights into structural alterations via MD simulation. The MD simulation was also carried out to validate the findings from molecular docking and to elucidate the energetic contributions of binding free energy. We employed post-MD analyses protocols, including; Root-mean-square deviation (RMSD), and Root-mean-square fluctuation (RMSF), analysis to provide insights on the structural impact of the hit compounds on acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase. These post-MD protocols measure stability, and flexibility of the c-α atoms in the backbone of acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase during the 100ns simulation.
Structural stability
A 100-ns long MD trajectory was established to analyze the structural dynamics in the conformations of all systems. The overall protein convergence and stability of MD trajectories were determined based on RMSD, as shown in Figure 5. In the Acetyl-coenzyme A systems, convergence was attained early in the simulation after about 5 ns This was followed by steady atomic motions in all systems till the end of the simulation as shown in Figure 5A. None of the systems appeared to be unstable as shown by the plateau shape of atomic motions. Overall, the RMSD averages estimated for all the Acetyl CoA bound and unbound systems were 2.56Å, 2.09Å, 2.69Å, 2.42Å, 2.60Å, 1.88Å, 2.40Å, 1.77Å, 2.82Å, 1.85Å, and 2.43Å for apo, ZINC05378039, ZINC38980461, ZINC38974815, ZINC38971181, ZINC38984088, ZINC38974798, ZINC38976811, ZINC15772056, ZINC38903582, Soraphen A respectively.
Similarly, all systems in the Acyl-carrier protein setup reached a convergence early in the simulation and maintained steady atomic motions of c-α atoms to the 100 ns mark as shown in Figure 5B. All hit compounds including the unbound acyl carrier protein and the reference drug Triclosan appeared to be stable during the simulation. The estimated RMSD averages for all systems were 2.98Å, 2.28Å, 2.78Å, 2.10Å, 2.27Å, 1.95Å, 2.75Å, 2.23Å, 2.30Å, 2.08Å, and 2.45Å for Apo, ZINC93658429, ZINC94085628, ZINC93656835, ZINC93098000, ZINC94919772, ZINC94080670, ZINC17074609, ZINC87263643, ZINC94821232 and Triclosan respectively. These findings on structural stability of all systems highlight the reliability of our findings for further structural assessment.
Structural flexibility
We employed RMSF analysis to determine the change in motion of each residue as a measure of the flexibility of certain regions of the acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase structural architecture as shown in Figure 6. A greater RMSF value typically indicates a more flexible structure, while a lower average RMSF value generally indicates a less flexible or rigid conformation. The average RMSF values estimated for all the Acetyl CoA bound and unbound systems were 1.23Å, 1.06Å, 1.20Å, 1.10Å, 1.02Å, 1.06Å, 1.05Å, 1.03Å, 0.98Å, 1.04Å and 1.37Å for the apo, ZINC05378039, ZINC38980461, ZINC38974815, ZINC38971181, ZINC38984088, ZINC38974798, ZINC38976811, ZINC15772056, ZINC38903582, Soraphen A respectively (Figure 6A). The average RMSF estimated for the Acyl-carrier protein systems were 1.50Å, 1.18Å, 1.42Å, 1.03Å, 1.01Å, 1.06Å, 1.21Å, 1.05Å, 1.10Å, 1.18Å and 1.13Å for Apo, ZINC93658429, ZINC94085628, ZINC93656835, ZINC93098000, ZINC94919772, ZINC94080670, ZINC17074609, ZINC87263643, ZINC94821232 and Triclosan respectively (Figure 6B). The effect of the hits on both fatty acid targets was evident as shown from the difference in structural fluctuations in the initial complexed structures and the structures after MD simulation as shown in Figure 6A, B. Also shown in Figure 6, all the simulated systems including the unbound and an inhibitor bound complexes show a peak area of the protein at Met250, Pro450 and Ser482 for Acetyl coA and Asn78, Arg125 and Val278 for Acyl reductase, residual positions that fluctuate the most during the simulation. We can observe that during the simulation time, the amino acid residues of unbound targets have similar structural behaviour as that of the inhibitor bound systems of the fatty acid targets.
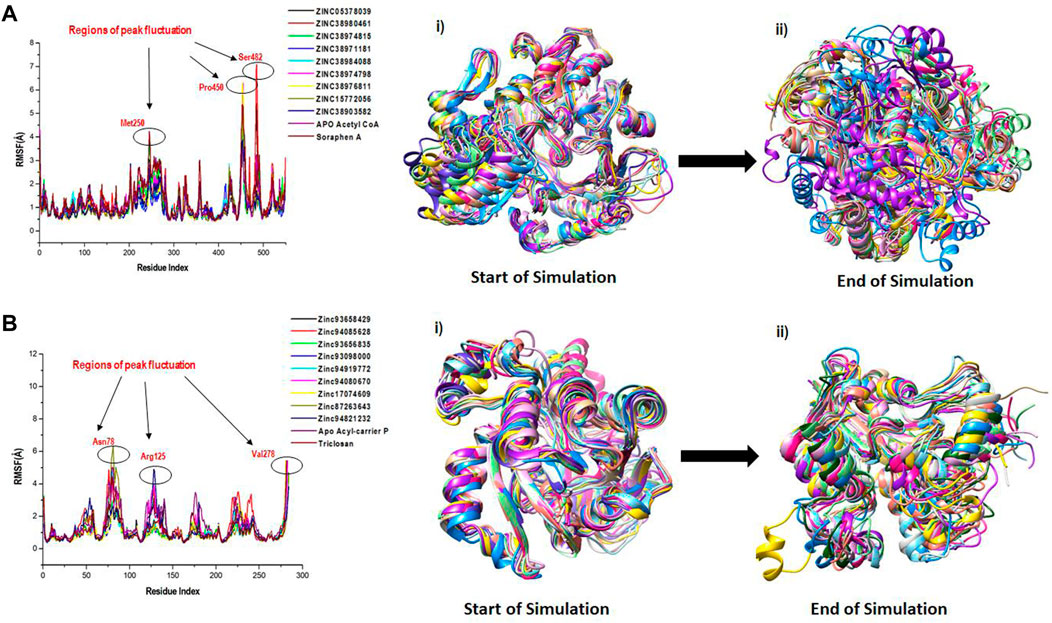
FIGURE 6. A plot of residual fluctuations in the unbound and bound (A) Acetyl co-enzyme A and (B) Acyl-carrier reductase enzyme. (i) Highlights the initial superimposed structures of all systems, and (ii) shows the various fluctuations that occurred at the end of the simulation.
Binding free energy assessment
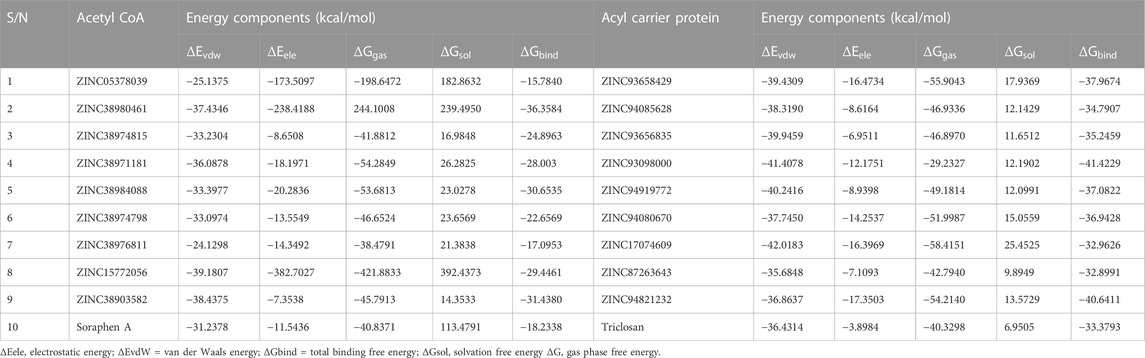
The mechanics/generalized-born surface area (MM/GBSA) method was employed to estimate the binding free energetics of the bound complexes of the all hits including the two reference compounds Soraphen A and Triclosan. The molecular mechanics generalized Born surface area (MM/GBSA) is very popular method for binding energy prediction and is known to be more accurate than most scoring functions in molecular docking and are computationally less demanding than alchemical free energy methods (Maier et al., 2015). The computed binding free energies of the acetyl-coenzyme A (CoA) carboxylase complexes ranged from −15.78 to −36.36 kcal/mol whiles that of the enoyl-acyl carrier reductase complexes ranged from −32.89 to −41.42 kcal/mol. Table 5 shows the energy terms that contribute to the binding free energy, the most favourable components being the Δ𝐸𝑒𝑙𝑒, Δ𝐸𝑣𝑑𝑤 and Δ𝐺gas, while ΔGsol was unfavourable. The energies presented by these compounds suggests the spontaneity, permeation and a measure of the reaction kinetics that characterize their complexing with the target proteins.
Discussion
Malaria still remains one of the mostly deadly parasitical disease in humans. A huge obstacle to the global efforts to control and eradicate malaria is the resistance of Plasmodium falciparum to conventional antimalarial therapies. As such several research studies have been geared towards identifying crucial therapeutics that will overcome this resistance. Advances in targeted therapy in antimalarial studies identified two crucial targets in the fatty acid synthetic pathway of the parasite. These new targets have been extensively investigated to interrupt recovery of parasites from ART-induced dormancy and to reduce the rate of recrudescence following ART treatment. The identification of these important targets have paved way for in-depth exploration into antimalarial therapy including the application in silico techniques in designing novel therapeutics that can pose an inhibitory effect to these targets.
In this study, we identified potential drug candidates against the acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase of the fatty acid synthesis pathways using in silico techniques. Soraphen A and Triclosan, known inhibitors of these targets, were used as reference compounds in screening for potential drugs from the ZINCPharmer database using the PRED Pharmacophore based virtual screening. This in silico technique allows the identification of moieties on the reference structures that form high affinity interactions with crucial amino acids residues on the target enzymes which form the basis for the generation of a pharmacophore. Subsequently, molecular docking was used to screen all hit compounds that were identified for each of the acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase targets. All the nine hit compounds of CoA showed favourable binding at the active site with good docking score and RMSD values < 2Å. Similarly, all the hit compounds in the acyl showed favourable binding as well with good binding score and lower RMSD score accounting for a stable binding. In most molecular docking studies an RMSD threshold <2Å is normally considered a good docking solution (Ramírez and Caballero, 2018). All our identified hits had RMSD less than the 2Å threshold.
Additionally, the pharmacokinetics was assessed by evaluating the ADME properties based on the Lipinski’s rule of five (RO5). The RO5 is used to demonstrate the drug-like properties of all selected compounds and serves as a justification for molecules that agrees with the rule. All the nine selected hits for both targets show good pharmacokinetic properties as well as passing the Linpinski’s test of “druggability”. Subsequently, the toxicity of all hit compounds was evaluated to unravel any harmful effects of the selected compounds on humans or animals. Three of the potential drug candidates for the Acetyl-coenzyme A target comprising ZINC05378039, ZINC38980461 and ZINC15772056 show the best toxicity properties characterized by being identified in the toxicity class iv indicative of no or less toxicity. They also show to be inactive towards Carcinogenicity, Immunotoxicity, Mutagenicity and Cytotoxicity effects. In the enoyl-acyl carrier reductase hit compounds, five 5) of the hits (ZINC94085628, ZINC93656835, ZINC94080670, ZINC1774609 AND ZINC94821232) were in class iv, indicating no or less toxic, however ZINC94919772 had the best overall toxicity properties which includes being in a toxicity class V correlating to no toxicity effects as well inactive towards Carcinogenicity, Immunotoxicity, Mutagenicity and Cytotoxicity.
Furthermore, molecular dynamic simulations was used to unveil the stability and flexibility of the selected ligands against the two fatty acid targets (Salifu et al., 2022). The cα atoms of the protein-ligand complexes were used to calculate the RMSD of the system that confirm low deviation of the system (Salifu et al., 2022), (Abdullahi et al., 2018). Generally, the acceptable threshold for an average change in RMSD of the protein-ligand complex 1-3Å (Ramírez and Caballero, 2018). As such any RMSD value larger than the 1-3Å threshold indicates a vast conformational change in the protein structure hence unacceptable (Ramírez and Caballero, 2018). The RMSD averages for all the selected hit compounds in this study was within the 1–3 Å threshold which is indicative of good stability. The stability of the simulated systems highlights the reliability of our findings and further show the impact of the hit compounds on the targets. The fluctuation of the protein targets was also determined based on the RMSF value that also confirm averagely low fluctuations in all the inhibitor bound systems correlating to a less flexible protein structure.
In several drug design studies, the Molecular Mechanics Generalized Born Surface Area (MM-GBSA) approach has been used to accurately predict binding free energies (Genheden et al., 2012), (Genheden and Ryde, 2015). The estimated binding free energies of the Acetyl-coenzyme A in complex with all hit compounds ranged from −15.78 to −36.36 kcal/mol. Table 6 shows the energy terms that contributes to the total binding free energy, the findings show the major driving/favorable components to be Δ𝐸𝑒𝑙𝑒𝑐, Δ𝐸𝑣𝑑𝑤 and Δ𝐺gas, while Δ𝐺sol was unfavourable. Similarly, in the enoyl-acyl carrier reductase complexes, the binding free energies were in the range −32.89 to −41.42 kcal/mol. These energies were similarly driven by the Δ𝐸𝑒𝑙𝑒𝑐, Δ𝐸𝑣𝑑𝑤 and Δ𝐺gas energy components whiles the Δ𝐺sol term remain unfavourable. ZINC38980461 had the most favourable binding energy (−36.36 kcal/mol) among the acetyl-coenzyme A hits whiles ZINC93098000 had the highest binding free energy (−41.42 kcal/mol) in the enoyl-acyl reductase hit compounds. Overall, all hit compounds for both targets displayed favourable binding free energies towards the respective target which highlights their potential as inhibitors of these target proteins.
Conclusively, based on our analysis of all hit compounds screened via the PRED-pharmacophore method for the acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase of the fatty acid synthesis pathways, we identified ZINC38980461, ZINC05378039, and ZINC15772056 as the lead compounds that can be developed further into potential drug candidates of acetyl-coenzyme A, whiles ZINC94085628, ZINC93656835, ZINC94080670, ZINC1774609, ZINC94821232 and ZINC94919772 were identified as the compounds with best properties against the enoyl-acyl reductase enzyme. The evaluations of the selected lead compounds were based on consistency in showing favourable results across different parameters including good docking score, excellent pharmacokinetic properties, no toxicity tendencies, ability to stabilize target enzyme with minimal fluctuations and a favourable binding free energy. Also, leads were identified to be ZINC purchasable and also possess the potential of being easily synthesized via experimental assays. The common vendors where these leads can be purchased as listed in the ZINC database include: Enamine BBmake-on-demand (BBV-43139487), Molport make-on-demand (MolPort-026–268-957), Chemspace BB premium (CSC001515773), Chemspace Building Blocks (CSC001515773) and Selena Building Blocks (SEL11975463). These lead compounds can therefore be developed further into drug candidates for inhibiting acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase of the fatty acid synthesis pathways in malaria therapy.
Conclusion
The discovery of inhibitory molecules for a specific target protein is increasingly gaining attention in drug design due to the efficiency and speed that comes with the process. Using a computer-aided drug design methodology, we show in this study how novel acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase inhibitors were quickly and effectively identified (CADD). Three lead compounds ZINC38980461, ZINC05378039, and ZINC15772056, were identified for acetyl-coenzyme A (CoA) carboxylase whiles ZINC94085628, ZINC93656835, ZINC94080670, ZINC1774609, ZINC94821232 and ZINC94919772 were identified as lead compounds for enoyl-acyl carrier reductase, through the use of PRED based Pharmacophore method, virtual screening, molecular docking, ADMET, Toxicity assessment and MD simulation techniques in the CADD. These compounds may be able to inhibit the respective activities of acetyl-coenzyme A (CoA) carboxylase and enoyl-acyl carrier reductase and thereby interrupt the recovery of the falciparum parasite in host cell.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ES: Conceptualized the work, Contributed in the writing and editing of manuscript. IR: Assisted in setting up systems for MD simulations and proof reading of Manuscript. FO: Contributed to writing of manuscript. JA: Supervised the work, assisted in writing and editing of work and final proof reading. JAA: Proof read manuscript.
Acknowledgments
The authors acknowledge, The Centre of High-Performance Computing (CHPC, www.chpc.ac.za), Cape Town, South Africa, for their resources in carrying out this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullahi, M., Olotu, F. A., and Soliman, M. E. (2018). Allosteric inhibition abrogates dysregulated LFA-1 activation: Structural insight into mechanisms of diminished immunologic disease. Comput. Biol. Chem. 73, 49–56. doi:10.1016/j.compbiolchem.2018.02.002
Antón Vázquez, V., Pascual, L., Corominas, H., and Giménez Torrecilla, I. (2017). Protein-ligand docking with PyRx. Reumatol. Clínica 13 (5), 294–296. doi:10.1016/j.reuma.2016.04.008
Banerjee, P., Eckert, A. O., Schrey, A. K., and Preissner, R. (2018). ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 46 (W1), W257–W263. doi:10.1093/nar/gky318
Biamonte, M. A., Wanner, J., and Le Roch, K. G. (2013). Recent advances in malaria drug discovery. Bioorg. Med. Chem. Lett. 23, 2829–2843. doi:10.1016/j.bmcl.2013.03.067
Burley, S. K., Berma, H. M., Bhikadiya, C., Bi, C., Chen, L., Di Costanzo, L., et al. (2019). RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology, and energy. Nucleic Acids Res. 47 (D1), D464–D474.
Case, D. A., I. Y., B.-S., Brozell, S. R., Cerutti, D. S., Cheatham, T. E., Cruzeiro, W. D. V., et al. (2018). AMBER. San Francisco: University of California 18.
Cele, F. N., Ramesh, M., and Soliman, M. E. S. (2016). Per-residue energy decomposition pharmacophore model to enhance virtual screening in drug discovery: A study for identification of reverse transcriptase inhibitors as potential anti-HIV agents. Drug Des. devel. Ther. 10, 1365–1377. doi:10.2147/DDDT.S95533
Chaturvedi, P. R., Decker, C. J., and Odinecs, A. (2001). Prediction of pharmacokinetic properties using experimental approaches during early drug discovery. Curr. Opin. Chem. Biol. 5, 452–463. doi:10.1016/S1367-5931(00)00228-3
Chen, N., LaCrue, A. N., Teuscher, F., Waters, N. C., Gatton, M. L., Kyle, D. E., et al. (2014). Fatty acid synthesis and pyruvate metabolism pathways remain active in dihydroartemisinin-induced dormant ring stages of plasmodium falciparum. Antimicrob. Agents Chemother. 58 (8), 4773–4781. doi:10.1128/AAC.02647-14
Daina, A., Michielin, O., and Zoete, V. (2017). SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7, 42717. doi:10.1038/srep42717
Dallakyan, S., and Olson, A. J. (2015). Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 1263, 243–250. doi:10.1007/978-1-4939-2269-7_19
Dondorp, A. M., Nosten, F., Yi, P., Das, D., Phyo, A. P., Tarning, J., et al. (2009). Artemisinin resistance inPlasmodium falciparummalaria. Drug Ther. (NY) 361 (5), 455–467. doi:10.1056/nejmoa0808859
Genheden, S., Kuhn, O., Mikulskis, P., and Ryde, U. (2012). The normal-mode entropy in the MM/GBSA method: E ff ect of system truncation, bu ff er region, and dielectric constant. J. Chem. Inf. Model. 52 (8), 2079–2088. doi:10.1021/ci3001919
Genheden, S., and Ryde, U. (2015). The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 10, 449–461. doi:10.1517/17460441.2015.1032936
Gonnet, P., and Shake, P. (2007). P-SHAKE: A quadratically convergent SHAKE in. J. Comput. Phys. 220 (2), 740–750. doi:10.1016/j.jcp.2006.05.032
Gornicki, P. (2003). Apicoplast fatty acid biosynthesis as a target for medical intervention in apicomplexan parasites. Int. J. Parasitol. 33, 885–896. doi:10.1016/S0020-7519(03)00133-4
Gunther, S., Matuschewski, K., and Mu, S. (2009). Knockout studies reveal an important role of plasmodium lipoic acid protein ligase A1 for asexual blood stage. Parasite Surviv. 4 (5). doi:10.1371/journal.pone.0005510
Hanwell, M. D., Curtis, D. E., Lonie, D. C., Vandermeersch, T., Zurek, E., and Hutchison, G. R. (2012). Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 4 (1), 17. doi:10.1186/1758-2946-4-17
Huang, N., Shoichet, B. K., and Irwin, J. J. (2006). Benchmarking sets for molecular docking. J. Med. Chem. 49, 6789–6801. doi:10.1021/jm0608356
Issahaku, A. R., Mukelabai, N., Agoni, C., Rudrapal, M., Aldosari, S. M., Almalki, S. G., et al. (2022). Characterization of the binding of MRTX1133 as an avenue for the discovery of potential KRASG12D inhibitors for cancer therapy. Sci. Rep. 12 (1), 17796. doi:10.1038/s41598-022-22668-1
Koes, D. R., and Camacho, C. J. (2012). ZINCPharmer: Pharmacophore search of the ZINC database. Nucleic Acids Res. 40 (W1), 409–414. doi:10.1093/nar/gks378
Kollman, P. A., Massova, I., Reyes, C., Kuhn, B., Huo, S., Chong, L., et al. (2000). Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Comb. Mol. Mech. Continuum Models 33 (12), 889–897. doi:10.1021/ar000033j
Kumalo, H. M., and Soliman, M. E. (2016). Per-residue energy footprints-based pharmacophore modeling as an enhanced in silico approach in drug discovery: A case study on the identification of novel β-secretase1 (BACE1) inhibitors as anti-alzheimer agents. Cell. Mol. Bioeng. 9, 175–189. doi:10.1007/s12195-015-0421-8
Kumar, S., Bhardwaj, T. R., Prasad, D. N., and Singh, R. K. (2018). Drug targets for resistant malaria: Historic to future perspectives. Biomed. Pharmacother. 104, 8–27. doi:10.1016/j.biopha.2018.05.009
Li, C., Wang, J., Wang, Y., Gao, H., Wei, G., Huang, Y., et al. (2019). Recent progress in drug delivery. Acta Pharm. Sin. B 9, 1145–1162. doi:10.1016/j.apsb.2019.08.003
Lipinski, C. A., Lombardo, F., Dominy, B. W., and Feeney, P. J. (2012). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 64, 4–17. doi:10.1016/j.addr.2012.09.019
Maier, J. A., Martinez, C., Kasavajhala, K., Wickstrom, L., Hauser, K. E., and Simmerling, C. (2015). ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11 (8), 3696–3713. doi:10.1021/acs.jctc.5b00255
Massova, I., and Kollman, P. A. (2000). Combined molecular mechanical and continuum solvent approach (MM-PBSA/GBSA) to predict ligand binding. Perspect. Drug Discov. Des. 18, 113–135. doi:10.1023/a:1008763014207
Meshnick, S. R., Taylor, T. E., and Kamchonwongpaisan, S. (1996). Artemisinin and the antimalarial endoperoxides: From herbal remedy to targeted chemotherapy. Microbiol. Rev. 60 (2), 301–315. doi:10.1128/mr.60.2.301-315.1996
Miller, B. R., Mcgee, T. D., Swails, J. M., Homeyer, N., Gohlke, H., and Roitberg, A. E. (2012). MMPBSA. py: An E ffi cient program for end-state free energy calculations. J. Chem. theory Comput. 8 (9), 3314–3321. doi:10.1021/ct300418h
Onufriev, A., Bashford, D., and Case, D. A. (2000). Modification of the generalized born model suitable for macromolecules. J. Phys. Chem. 104 (15), 3712–3720. doi:10.1021/jp994072s
Owens, J., and Lipinski, C. A. (2003). Chris Lipinski discusses life and chemistry after the rule of five. Drug Discov. Today 8, 12–16. doi:10.1016/S1359-6446(02)02556-4
Pollastri, M. P. (2010). Overview on the rule of five. Curr. Protoc. Pharmacol. 49, 9–12. doi:10.1002/0471141755.ph0912s49
Ramírez, D., and Caballero, J. (2018). Is it reliable to take the molecular docking top scoring position as the best solution without considering available structural data? Molecules 23 (5), 1038. doi:10.3390/molecules23051038
Roe, D. R., and Cheatham, T. E. (2013). PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9 (7), 3084–3095. doi:10.1021/ct400341p
Salifu, E. Y., Rashid, I. A., and Soliman, M. E. S. (2022). Impact of compound mutations I1171N + F1174I and I1171N + L1198H on the structure of ALK in NSCLC pathogenesis: Atomistic insights. J. Biomol. Struct. Dyn. 0 (0), 1–9. doi:10.1080/07391102.2022.2072390
Segall, M. D., and Barber, C. (2014). Addressing toxicity risk when designing and selecting compounds in early drug discovery. Drug Discov. Today 19 (5), 688–693. doi:10.1016/j.drudis.2014.01.006
Seifert, E. (2014). OriginPro 9.1: Scientific data analysis and graphing software – software review. J. Chem. Inf. Model 54, 1552. doi:10.1021/ci500161d
Shen, Y., Volrath, S. L., Weatherly, S. C., Elich, T. D., and Tong, L. (2004). A mechanism for the potent inhibition of eukaryotic acetyl-coenzyme A carboxylase by soraphen A, a macrocyclic polyketide natural product. Mol. Cell 16 (6), 881–891. doi:10.1016/j.molcel.2004.11.034
Sitkoff, D., Sharp, K. A., and Honig, B. (1994). Accurate calculation of hydration free energies using macroscopic solvent models. J. Phys. Chem. 98 (7), 1978–1988. doi:10.1021/j100058a043
Sprenger, K. G., Jaeger, V. W., and Pfaendtner, J. (2015). The general AMBER force field (GAFF) can accurately predict thermodynamic and transport properties of many ionic liquids. J. Phys. Chem. B 119 (18), 5882–5895. doi:10.1021/acs.jpcb.5b00689
Tse, E. G., Korsik, M., and Todd, M. H. (2019). The past, present and future of anti-malarial medicines. Malar. J. 18 (1), 93–21. doi:10.1186/s12936-019-2724-z
Vaughan, A. M., O'Neill, M. T., Tarun, A. S., Camargo, N., Phuong, T. M., Aly, A. S. I., et al. (2008). Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. December 11, 506–520. doi:10.1111/j.1462-5822.2008.01270.x
White, N. J. (2004). Antimalarial drug resistance. Rev. Ser. Antimalar. drug Resist. 113 (8), 1084–1092. doi:10.1172/jci21682
Yamashita, F., and Hashida, M. (2004). Silico approaches for predicting ADME properties of drugs. Drug metabolism Pharmacokinet. 19 (5), 327–338.
Keywords: malaria, in silico, artemisinin, zincpharmer, virtual screening
Citation: Salifu EY, Abugri J, Rashid IA, Osei F and Ayariga JA (2023) In silico identification of potential inhibitors of acyl carrier protein reductase and acetyl CoA carboxylase of Plasmodium falciparum in antimalarial therapy. Front. Drug. Discov. 3:1087008. doi: 10.3389/fddsv.2023.1087008
Received: 01 November 2022; Accepted: 10 January 2023;
Published: 20 January 2023.
Edited by:
Fidele Ntie-Kang, University of Buea, CameroonReviewed by:
Pritika Ramharack, South African Medical Research Council, South AfricaAna Guimaraes, Oswaldo Cruz Foundation (Fiocruz), Brazil
Eugene Etchri Edjem Jean Megnassan, University of Nangui Abrogoua, Côte d'Ivoire
Copyright © 2023 Salifu, Abugri, Rashid, Osei and Ayariga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Abugri, jabugri@cktutas.edu.gh
 Elliasu Y. Salifu1
Elliasu Y. Salifu1  James Abugri
James Abugri Issahaku A. Rashid
Issahaku A. Rashid Joseph Atia Ayariga
Joseph Atia Ayariga