COVID-19 and Oral Fluids
- 1Department of Endodontics and Restorative Dentistry (RMeS U1229), Faculty of Dental Surgery, University of Nantes (CHU de Nantes), Nantes, France
- 2Department of Periodontology (RMeS U1229), Faculty of Dental Surgery, University of Nantes (CHU de Nantes), Nantes, France
- 3Faculty of Dentistry, McGill University, Montreal, QC, Canada
- 4Department of Endodontics and Restorative Dentistry (IRDL UMR 6027), Faculty of Dental Surgery, University of Brest (CHU de Brest), Brest, France
- 5Division of Restorative Dentistry and Periodontology, Dublin Dental University Hospital, Trinity College Dublin, Dublin, Ireland
- 6Department of Pedodontics (SPHERE UMR 1246), Faculty of Dental Surgery, University of Nantes (CHU de Nantes), Nantes, France
- 7Department of Dental Public Health (UPRES EA 3826), Faculty of Dental Surgery, University of Nantes (CHU de Nantes), Nantes, France
- 8Department of Oral Surgery (RMeS U1229), Faculty of Dental Surgery, University of Nantes (CHU de Nantes), Nantes, France
The coronavirus disease 2019 (COVID-19) is an acute infectious disease that has led to a global pandemic. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for this infection. This virus enters the body through the mucous membranes (oral, nasal, or conjunctival ocular) or the skin and replicates in the respiratory system (nasal cavity, throat, and the lung) but may cause damage to other systems and organs. Main transmission is through saliva droplets and oral fluids (OF) including saliva and non-salivary elements that contain infective viral loads of the virus. SARS-Cov-2 enters cells via receptor angiotensin-converting enzyme II (ACE2) and the action of an enzyme (furin) that cleaves the viral envelope and enhances the infection of the host cells. Although the origin, mechanism, and dynamics of SARS-CoV-2 in OF remain to be elucidated, its presence in OF is now established. Therefore, OF might be a diagnostic or monitoring tool for COVID-19 either alone or in combination with other tests in addition to the clinical examination. Moreover, the presence of SARS-CoV-2 in OF raises the question of the management of the infectious risk for dental practice. There is an urgent need to inform the dental community and ensure measures to protect dentists and dental staff from the new SARS-CoV-2. This comprehensive review of COVID-19 and oral health depicts the roles of OF in the transmission dynamics of SARS-CoV-2 and discusses the value of OF as a diagnostic tool and focuses on the management of risk transmission related to OF in dental practice.
Introduction
The term oral fluids (OF) or “whole saliva” refers to a hypotonic biofluid that includes saliva and non-salivary elements such as secretions (from naso-bronchial origin or from the gingival crevicular fluid), desquamated epithelial cells, cellular debris, serum, blood derivatives, microorganisms (virus, bacteria, parasites, and fungi), and exogenous substances (from food) (1–3) (Figure 1). The major salivary glands (parotid glands, submandibular glands, and sublingual glands) secrete 90% of the saliva, and the rest comes from minor salivary glands (i.e., labial and mucosal tissues) with a pH value of 6 to 7 (4). The wide variety of biomolecules that may be retrieved in the saliva (immunoglobulins, enzymes and enzyme inhibitors, growth factors and cytokines, mucins, hormones, and other glycoproteins) may serve as biomarkers (1). Therefore, saliva is often called the “mirror of the body” (2, 5).
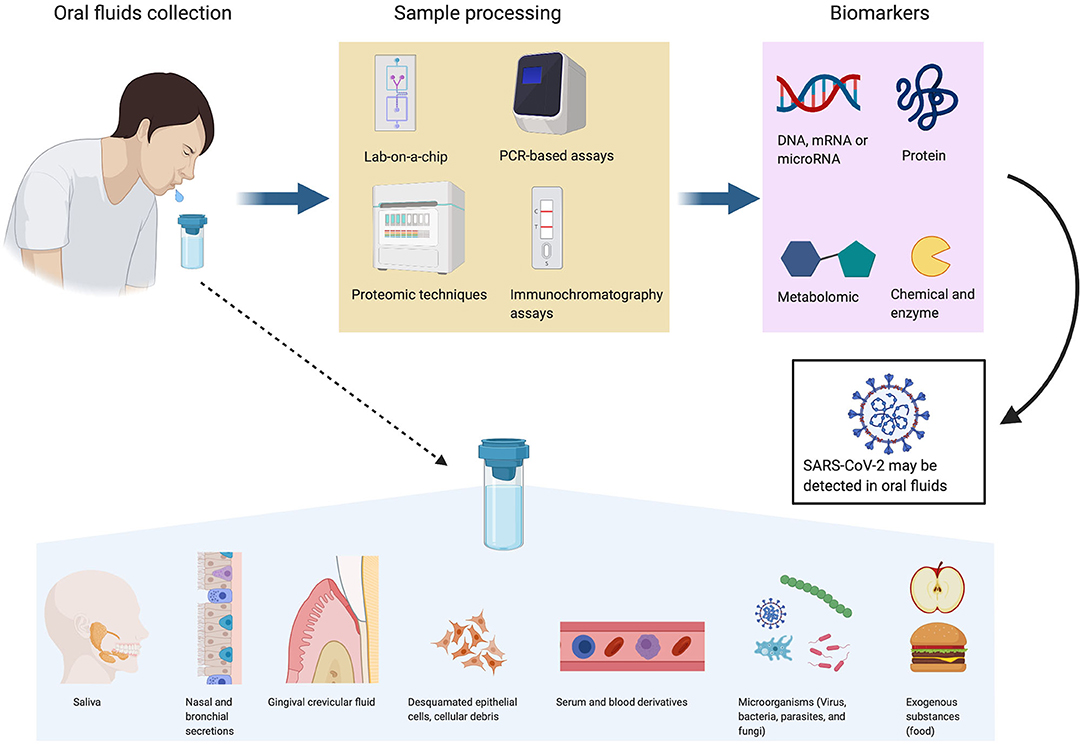
Figure 1. Oral fluids as a diagnostic and monitoring tool. Oral fluids (OF) are biofluids that contain saliva and non-salivary elements such as gingival crevicular fluid, nasal and bronchial secretions, serum and blood derivatives, microorganisms, desquamated epithelial cells, cellular debris, and exogenous substances. Biomarkers identified by different sample processes [lab-on-chip, polymerase chain reaction (PCR)-based assay, proteomic or immunochromatography assays] in OF may serve for diagnosis and monitoring of SARS-CoV-2 and also a significant number of diseases (cancer, autoimmune disease, viral, bacterial, cardiovascular, and metabolic diseases). Figures were created with BioRender.com.
The salivary glands have a high permeability that facilitates the exchange of biomolecules from blood due to the presence of abundant capillary networks. Hence, biomarkers circulating in blood can penetrate acini and finally be secreted in the saliva (6).
OF is thought to play an important role in personalized and future medicine. The presence of numerous biomarkers may help to diagnose or monitor a significant number of local or systemic diseases such as inflammatory disease, cancer, viral, bacterial, cardiovascular, and metabolic diseases (7). Sufficient quantities of OF can be collected repeatedly in a fast, easy, inexpensive, and non-invasive manner with minimum discomfort to the patient. In contrast with blood or serum, OF can be self-collected at home. These fluids do not require needle or special expertise, and OF is easy to store and ship since it does not clot. Despite these advantages, many expectations remain unfulfilled because of a lack of suitable cost-effective technologies (8, 9). Moreover, the development of highly sensitive technologies facilitates detection of very low concentrations of analytes (similar to blood). Therefore, the concept of salivaomics emerged in the 2000s and involved genomics, transcriptomics, proteomics, metabolomics, and microRNA (miRNA) analysis (10). OF are used routinely not only to detect and screen a variety of drugs including cannabis, marijuana, heroin, amphetamines, cocaine, and alcohol but also to detect virus such as human immunodeficiency virus (HIV) or hepatitis A, B, C, and D viruses (HAV, HBV, HCV, and HDV) (5, 11).
The pathologic role of OF is not fully understood and might be underestimated for dental/oral health professionals. Indeed, the oral cavity could constitute both a portal of entry and a reservoir for SARS-CoV-2. In the context of rapidly increasing knowledge about SARS-CoV-2 and its associated disease [coronavirus disease 2019 (COVID-19)], this overview focuses on the specific functions of OF in the COVID-19 outbreak and deals with their management in dentistry practice.
Coronavirus Disease (Covid-19)
SARS-CoV-2 Responsible for COVID-19
The World Health Organization (WHO) declared the COVID-19 a public health emergency on January 30, 2020. The number of confirmed cases and deaths from COVID-19 have increased exponentially (12).
COVID-19 is an acute infectious disease caused by a novel enveloped RNA betacoronavirus (2019-nCoV) (13). The name “coronavirus” comes from a heavily glycosylated, spike cell-surface protein (S protein). This S protein is thought to be responsible for the viral entry into cells. The S protein needs to be cleaved into two distinct functional domains (S1 and S2) via proteases found in the cell to fuse virus and cells. This cleavage is a critical step and is mediated by furin—a protein convertase (14) (Figure 2A).
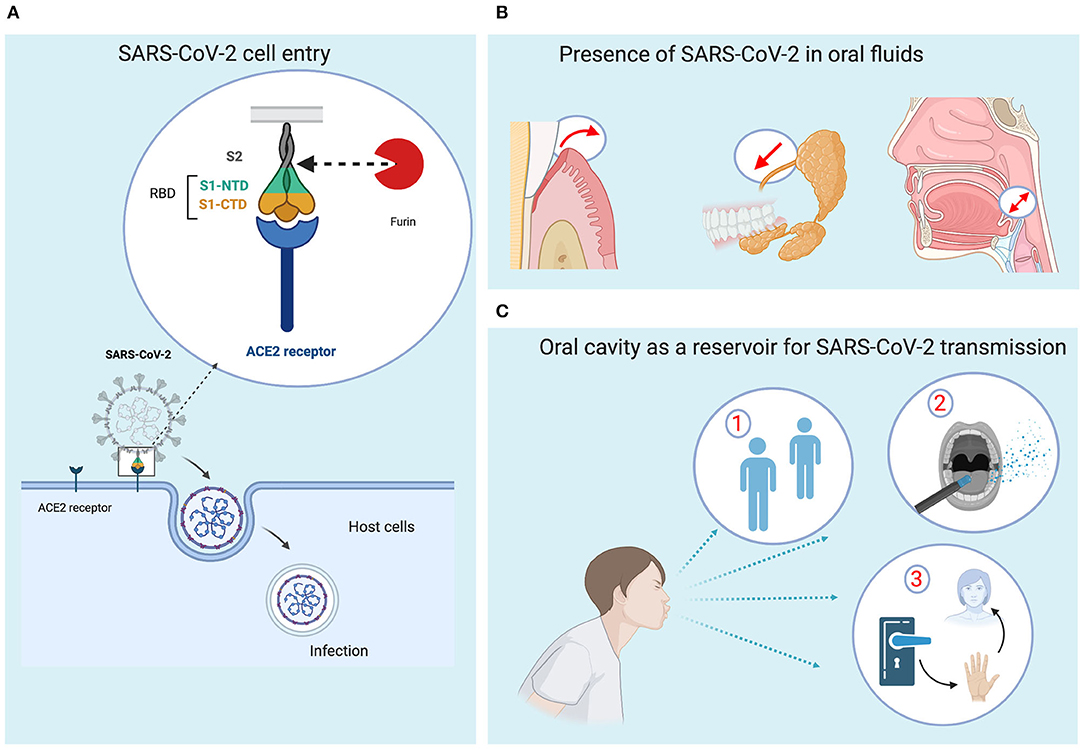
Figure 2. Roles of oral fluids in transmission dynamics of SARS-CoV-2. (A) The entry into the cells is mediated by the binding of the viral spike protein to the angiotensin-converting enzyme 2 (ACE2) receptor on the cells. There are two subunits, S1 and S2. S1 contains a receptor binding domain (RBD) with two terminal domains, N and C terminal domain (NTD and CTD), responsible for recognizing and binding with the cell surface receptor. The S2 subunit contains elements for the fusion to the membrane. The proteolytic cut at the S1/S2 site of the S glycoprotein by the host protease furin is required for the SARS-Cov-2 to enter into the cells. (B) SARS-CoV-2 may access the oral fluids through different routes (gingival crevicular fluid, salivary gland, and exchange with respiratory tract). (C) Transmission dynamics in dentistry practice. (1) Direct contact, (2) indirect contact, and (3) airborne aerosols may be responsible for SARS-CoV-2 transmission. Figures were created with BioRender.com.
The novel coronavirus (2019-nCoV) shares similarity with other coronavirus species retrieved in bats. Pangolins are also suspected to be intermediate hosts of the 2019-nCoV (15). That is not surprising since the coronavirus family is zoonotic and can therefore spread between animals and humans. The coronavirus family includes severe acute respiratory syndrome coronavirus (SARS-CoV) that caused 774 deaths in 29 countries between November 2002 and July 2003, and the Middle East respiratory syndrome coronavirus (MERS-CoV) (861 deaths in 27 countries) first identified in 2012 (16, 17).
This 2019-nCoV does not share receptors such as aminopeptidase N and dipeptidyl peptidase with other coronaviruses. After first characterization, the spike surface protein exhibits higher affinity to angiotensin-converting enzyme II (ACE2)—its functional receptor (18). This feature makes it much easier to penetrate human cells than SARS-CoV and MERS-CoV (15, 19). These features allow this 2019-nCoV to spread easier than SARS and MERS. Based on the phylogenetic and taxonomic analysis, this novel virus was named SARS-CoV-2 by the International Committee on Taxonomy of Viruses (ICTV).
Clinical Symptoms and Complications of COVID-19
COVID-19 is a respiratory infection with clinical symptoms that may vary from no symptoms (asymptomatic) to several clinical signs including cough, fever, and shortness of breath to non-specific symptoms such as myalgia, fatigue, headache, xerostomia (dry mouth), and ageusia (loss of taste). Asymptomatic cases raise serious issues to control the outbreak since they may play a critical role in the transmission process (20). Patients of more than 60 years of age are at higher risk than children to be infected and to develop severe disease. However, recent studies reported that some children may develop severe disease and require prolonged intensive care support (21).
Normal or decreased leukocyte counts (in particular NK cells and NK and CD8+ T cells) (22, 23) and radiographic evidence of pneumonia are reported. Sudden and unexplained onset of hemoptysis, conjunctivitis, and diarrhea are also suggestive of COVID-19 (24–26). Complications such as respiratory distress syndrome, arrhythmia, and shock will appear in 15% of patients; SARS-CoV-2 is more likely to affect elderly people, and the risk of death is greater for older males (26–29).
Roles of Of in Transmission Dynamics of SARS-CoV-2
Transmission Routes of SARS-CoV-2
Human Expression of ACE2 and Furin for SARS-CoV-2 Cell Entry
The expression and distribution of the ACE2 in human body is essential to understand the potential infection routes of SARS-CoV-2 infection. ACE2 expression profile can be analyzed at single-cell resolution using the single-cell RNA sequencing (scRNA-Seq) technique and the single-cell transcriptome database. High ACE2 expression was identified in various cells from different organ systems such as respiratory tract (type II alveolar cells of lung), salivary glands, mucosa of oral cavity, esophagus upper and stratified epithelial cells, cholangiocytes, and absorptive enterocytes from ileum and colon, urinary tract, and also myocardial cells (30–34). High ACE2-expressing cells present in these different organs should be considered as potential portal of entry for SARS-CoV-2 infection (30).
Furin is a protease responsible for the proteolytic cleavage of envelope of different viruses enhancing infection with host cells by facilitating the viral fusion with host cell membranes (35). Furin is highly expressed in lung tissue (36, 37) and also in human tongue epithelia (38). Strategies based on inhibition of furin may be a promising approach to impede the viral activity of the SARS-CoV-2.
Oral Cavity as a Reservoir for SARS-CoV-2
When the patient is asymptomatic but highly contagious, the virus mainly accumulates in the nasal, oral, and pharyngeal mucosa and later will replicate in the lungs (39). The virus actively replicates in the throat for the first 5 days after the onset of symptoms (40). Following entry via endosomal pathway, SARS-CoV-2 releases its viral RNA into the host cytoplasm. This messenger RNA is then translated by host ribosomes, processed by many host proteins and enzymes to generate new RNA genomes. Assembly of virion takes place by interaction of viral RNA and protein at endoplasmic reticulum and Golgi complex. These virions are then released out of the cells by exocytosis (41). SARS-CoV-2 replication is a complex process that deserves attention since understanding of such mechanism is crucial to identify and develop therapeutic tools to inhibit SARS-CoV-2 action.
Besides the respiratory tract cells, the oral cells and mucosa are also potential targets for SARS-CoV-2 due to their expression of ACE2 receptor and furin enzyme (34, 38, 42–44). The presence of SARS-CoV-2 in secretory saliva (45) may be due to different factors. First, the anatomical proximity between the respiratory tract and the oral cavity may explain frequent liquid droplet exchange (18). Second, SARS-CoV-2 may access oral cavity via gingival crevicular fluid that contains local proteins derived from serum and therefore viral elements (46). Finally, SARS-CoV-2 may occur in the oral cavity through secretion (Figure 2B). The first study on SARS-COV-1 in rhesus macaques demonstrated that the virus could rapidly infect salivary gland epithelial cells and therefore the salivary gland cells could play a crucial role in virus transmission (43).
Interestingly, ageusia (47) has been described as an early symptom of COVID-19 even before fever and other symptoms or nasal obstruction. Taste organs are widely distributed in oral tongue. Interestingly, ACE2 is more strongly expressed in the epithelial cells of the tongue than other buccal or gingival tissues (34).
A decrease in salivary flow and xerostomia is an associated clinical symptom of COVID-19. A cross-sectional survey in Wuhan showed that 46% of the patients reported xerostomia as one of their symptoms (42). These symptoms may be explained by dysfunction of tongue and/or salivary gland expressing ACE2 and furin. Moreover, irregular ulcers at the dorsum of the tongue have been suggested to be a potential inaugural clinical manifestation of COVID-19 that would appear prior to certain skin manifestations (48). Although further research is mandatory to elucidate mechanisms and oral symptoms of COVID-19, these data reveal that the mucosa, the tongue, and the salivary glands of the oral cavity may represent preferential sites of SARS-CoV-2 infection. Taken together, these data highlight the role of the oral cavity as a reservoir for SARS-CoV-2 and that OF may play a crucial role in the human-to-human transmission.
Role of Oral Cavity in SARS-CoV-2 Transmission
Similar to SARS, the median incubation period for SARS-CoV-2 is around 5 days and varies from 1 to 14 days. Peak transmission occurs 0.7 days before the onset of symptoms. Forty-four percent of transmissions occur in the pre-symptomatic phase, and infectivity is low beyond 7 days (39). Asymptomatic individuals have been tested positive for SARS-CoV-2, and virus transmission from asymptomatic carriers has been identified (28, 49, 50), suggesting that the virus spread can occur with mild or even in the absence of clinical symptoms (24, 28, 51). Virologic studies suggest that the viral load is highest in the first week of COVID-19 when the symptoms are generally mild (45, 47).
SARS-CoV-2 mainly spreads through the respiratory tract into a dynamic human-to-human transmission (52, 53). The two main routes known for SARS-CoV-2 transmission include (i) direct transmission through inhalation of droplets (Pflügge droplets) due to coughing or sneezing (within a radius of approximately 2 m) or (ii) direct contact between an area soiled with salivary secretions and nasal, oral, and ocular mucosa via handshakes, kisses, or hand-to-face contact (7) (Figure 2C). People usually touch their face 23–40 times per hour with 44% of these occurrences involving the mouth and/or nose (54). SARS-CoV-2 has been also detected in stool, gastrointestinal tract, urine samples, and semen (55). As a consequence, social distancing, frequent handwashing, and disinfection of objects have been promoted in order to minimize community spread of the disease.
Transmission Pattern in Dental Practice
Dental professionals are one part of the healthcare workers that face the greatest risk (56) because they may encounter patients with suspected or confirmed SARS-CoV-2 infection. Moreover, dental professionals have to provide care but also prevent nosocomial spread of infection with cross-contamination. SARS-CoV-2 transmission in dental settings may occur through three major routes. The first is direct exposure to projections of contaminated droplets, blood, saliva, or other patient materials during dental consultation (57).
The second possibility is indirect contact with contaminated surfaces and/or instruments (57). The coronaviruses probably persist for several hours on dry inert surfaces and up to 6 days in a humid environment (58). This duration is closely linked to temperature, residual humidity, starting inoculum, type of surface, as well as the presence of biological fluids.
The third possibility is inhalation of suspending airborne viruses. The recent awareness of this situation is due to potential dramatic consequences of the SARS-CoV-2 and because of the airborne spread during aerosol-generating dental procedures. Several procedures are susceptible to generate aerosols (ultrasonic instruments, high-speed handpieces, turbine, and air polisher); it is important to reduce production of droplets and aerosol during dental visits to zero (59). Moreover, particles from aerosols are theoretically small enough to stay airborne for an extended period before they settle on the environmental surfaces around the dental unit. Although the infectivity of the virus is not fully established, several authors have proposed that SARS-CoV-2 can be transmitted via aerosols (60–62) and would therefore be inhaled. Data regarding the average viral titer in the emitted aerosol particles as well as the minimum infectious dose in susceptible individuals are lacking.
Dental professionals are at a high risk for nosocomial infection and may become carriers of the disease. These risks are due to the features of dental visits potentially generating aerosols and contaminated droplets associated with the proximity of the dentist and dental staff to the patient.
Of Diagnostics in COVID-19
Early SARS-CoV-2 diagnosis is critical to treatment, avoiding complications, and decreasing the spread of the disease. The recommended diagnostic test is usually based on nasopharyngeal and oropharyngeal swabs. These tests present a risk of transmission of the virus to the healthcare workers due to contact with patients. However, these specimens can cause pain, discomfort, and bleeding and may cause complications in patients with thrombocytopenia (28) and make them inappropriate for serial monitoring of viral load. Sputum is a non-invasive lower respiratory tract specimen, but Huang et al. found that only 28% of patients with 2019-nCoV could produce sputum for diagnostic evaluation (24).
SARS-CoV-2 nucleic acid was detected in saliva in several studies (45, 63, 64). OF can be collected in three different ways: coughing out (deep throat saliva), saliva swabs (saliva in oral cavity), and directly from the salivary gland duct.
Deep Throat Saliva
Using viral culture, To et al. found that live viruses were present in deep throat saliva of infected individuals (45). Based on clinical examination and laboratory test, 12 patients with laboratory-confirmed SARS-CoV-2 infection were included. Eleven saliva specimens were positive for 2019-nCoV out of 12 patients (91.67%) using polymerase chain and testing the S gene of SARS-CoV-2. As a control, all saliva specimens from 33 patients whose nasopharyngeal specimens tested negative for SARS-CoV-2 also tested negative. In addition, serial saliva specimens were available for six patients. The viral load was highest in the earliest available specimens for five patients (83.3%), thus showing a downhill trend starting with hospitalization (45).
Another study from the same group analyzed the kinetics of SARS-CoV-2 load in saliva from COVID-19-tested SARS-CoV-2 RNA patients. Twenty cases (87.0%) showed detectable SARS-CoV-2 RNA in saliva among 23 COVID-19 patients included in this study. Salivary viral load changes over time: It is high during the first week after symptom onset and subsequently decreased with time. Low levels of SARS-CoV-2 RNA in saliva could still be quantified even after complete clinical recovery (63).
Saliva in Oral Cavity
Zhang et al. collected and compared different human samples (oral swabs, anal swabs, and blood samples) from positive patients. After medical treatments, half of the oral swabs (50%) were positive for SARS-CoV-2 RNA, four (26.7%) had positive anal swabs, six (40%) had positive blood test, and three (20%) were serum positive. The virus could be detected in anal swabs or the blood of patients, whereas oral swabs were negative, suggesting that oral swabs were likely better to indicate early infection than anal swabs (64).
Salivary Gland
Saliva specimens collected directly from the salivary gland duct are more likely to be detected at late stages of the disease, and SARS-CoV-2 nucleic acid positive tests in salivary-gland-originated saliva may indicate the severity of COVID-19 (42).
At-Home Collection of Saliva Specimens for Diagnostic Test
At-home collection of saliva is an important step for diagnostic testing. Besides the obvious comfort for the patient, at-home testing also decreases the risks to healthcare workers. On May 8, 2020, the FDA authorized a diagnostic test designed by Rutgers Clinical Genomics Laboratory using home-collected OF samples for COVID-19 testing. This test is prescription only and is currently the only authorized COVID-19 diagnostic test that uses saliva samples to test for SARS-CoV-2.
Other countries are validating these tests. A French consortium consisting of scientists from the CNRS laboratory Sys2Diag, the SkillCell biotechnology company, and Montpellier University Hospital (CHU de Montpellier) announced a clinical trial to evaluate the performance of the new EasyCov screening test beginning on April 11, 2020. In parallel, industrial development, production, and distribution chains are adapting their processes for rapid and mass deployment of the test to medical staff in May 2020.
As ACE2 receptors are highly expressed on the epithelial cells of oral mucosa and the base of the tongue, RT-PCR testing of a throat-wash sample may also be considered (65). In a recent study, Guo et al. found that the positive testing rate of throat washing was much higher than that of nasopharyngeal swabs. Although conducted only on 11 patients, this study highlights the feasibility and reliability of throat washing (66).
The early diagnosis of SARS-CoV-2 is crucial and still difficult; however, OF offers several advantages: non-invasive, more acceptable to patients, and more secure for healthcare workers. However, these tests must be accurate in addition to being useful.
Management of Of to Prevent Infection to SARS-CoV-2
To minimize the transmission risk of the disease, several measures may be undertaken in dentistry. These measures are based on general knowledge related to infection control in dental practice adapted to COVID-19. This proposal focuses on the management of OF and does not explore the entire transmission risk chain [i.e., schedules for managing suspected patient, dental rooms organization, handwashing, personal hygiene, personal protective equipment (PPE), airborne infection isolation rooms, bio-cleaning of potentially contaminated surfaces, and waste management]. Such information related to these general recommendations are detailed in several reports (67–71).
Preprocedural Mouthwashes
Preprocedural mouth rinses are recommended to decrease viral load in OF. The effects of chlorhexidine (commonly used as pre-procedural mouthwash in dental practice) may not be sufficient to clear SARS-CoV-2 (44). A study on inanimate surfaces like metal, glass, or plastic showed that the virus could be inactivated in 1 to 5 min by many disinfectants including 70% ethanol, 0.1% sodium hypochlorite, 1% povidone-iodine, and 0.5% hydrogen peroxide (58). Here, 0.02% chlorhexidine (CHX) was less effective than other biocidal agents; however, we advise caution with this concentration since 0.02% (CHX) is not the usual CHX concentration for mouthwashes.
Several reports have recommended oxidative agents containing mouthwashes with 1% hydrogen peroxide or 0.2% povidone-iodine (67, 68, 70). These recommendations are based on data obtained for other virus strains (SARS-CoV and MERS-CoV) (72). The efficiency of 1% hydrogen peroxide or 0.2% povidone-iodine on SARS-CoV-2 in OF is not yet fully demonstrated.
Radiographs and Dental Impressions
Although intraoral x-ray examination is necessary for numerous diagnostics in dentistry, it can stimulate saliva secretion and coughing (73); therefore, it should be taken with great care (44). To minimize the coughing or gagging reflex caused by contact of the operator's fingers, the patient may hold their own device. Extra-oral dental X-rays such as the panoramic X-ray or CBCT may be alternatively selected if indicated.
Since dental impressions may trigger coughing and increase flow rate, impressions and models should be carefully sanitized with cleaners such as 70% ethanol, phenolic and quaternary ammonium, 0.5% hydrogen peroxide, or sodium hypochlorite (1,000 ppm or 0.1%). Digital impressions are highly suggested.
Rubber Dam
A rubber dam is an effective physical barrier in the oral cavity to reduce the generation of aerosol and contaminated droplets. Rubber dams should be used in every possible clinical situation (67). Rubber dams effectively reduce the generation of aerosol and droplets 1 m from the procedure site by 70% (74). After correct placement, rubber dams can be disinfected with sodium hypochlorite. They should also cover the patient's nose (67).
Dental Devices and Procedures Susceptible to Produce Airborne Contamination
The sources of bio-aerosols in dental clinics are diverse: ultrasonic scalers, high-speed handpieces, air turbines, three-in-one syringes, air water syringes, air polishers, and air abrasion. Of these, scalers (ultrasonic and sonic) are considered the greatest source of aerosol contamination followed by air polishers, air–water syringes, and air turbines. Moreover, important contamination related to abrasive particles has also been demonstrated with air abrasion (75, 76). Therefore, in the COVID-19 context, dental devices and procedures susceptible to produce airborne contamination should be replaced with manual instruments when possible; otherwise, this should be limited to minimum (77). In these dental devices, high-volume evacuator combined with a second evacuator are recommended. Any dental handpieces that do not have an anti-retraction function should be avoided because such dental devices may aspirate and expel the contaminated debris and fluids by the SARS-CoV-2 during the dental procedures (44, 68). In addition, the virus may further penetrate and subsequently contaminate the air and water tubes within the dental unit; this can potentially lead to cross-contamination.
Discussion
In summary, SARS-CoV-2 transmission via saliva droplets is common because saliva contains the virus. Although SARS-CoV-2 is actively present in OF as well as nasal and bronchial secretion, it is still unclear whether it is present in the GCF. Similar to herpes simplex virus (HSV), Epstein–Barr virus (EBV), and human cytomegalovirus (CMV) (78, 79), GCF is of great interest to isolate and measure SARS-CoV-2; it can confirm the portal of entry into the oral cavity.
The gold standard for COVID-19 diagnosis may still be debatable due to the different clinical stages of the disease or the variable specificity and sensitivity of different diagnostic tests. Therefore, OF testing can be used as a complementary diagnostic tool (82). It could be done in addition to other methods for patients clinically suspected of COVID-19 such as detection of specific nucleic acids by real-time reverse transcription polymerase chain reaction (RT-PCR) [target gene as ORF1ab and N (26)] from throat swab samples or of serological IgG and IgM antibodies for SARS-CoV-2 or chest computed tomography. The advantages of OF to the patient and workers are numerous: affordable, convenient, low risk of virus transmission, and easy to manage. A better understanding of OF and their impact on the transmission of this virus and other viruses such as influenza may help dentists and healthcare professionals to improve their strategies for prevention as well as management of positive patients.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Lawrence HP. Salivary markers of systemic disease: noninvasive diagnosis of disease and monitoring of general health. J Can Dent Assoc. (2002) 68:170–4.
2. Kaczor-Urbanowicz KE, Martin Carreras-Presas C, Aro K, Tu M, Garcia-Godoy F, and Wong DTW. Saliva diagnostics – Current views and directions. Exp Biol Med. (2017) 242:459–72. doi: 10.1177/1535370216681550
3. Kaufman E, and Lamster IB. The diagnostic applications of saliva— A review. Crit Rev Oral Biol Med. (2002) 13:197–212. doi: 10.1177/154411130201300209
4. Spielmann N, and Wong DT. Saliva: diagnostics and therapeutic perspectives. Oral Dis. (2011) 17:345–54. doi: 10.1111/j.1601-0825.2010.01773.x
5. Segal A, and Wong DT. Salivary diagnostics: enhancing disease detection and making medicine better. Eur J Dent Educ. (2008) 12:22–9. doi: 10.1111/j.1600-0579.2007.00477.x
6. Zhang CZ, Cheng X-QQ, Li JY, Zhang P, Yi P, Xu X, et al. Saliva in the diagnosis of diseases. Int J Oral Sci. (2016) 8:133–7. doi: 10.1038/ijos.2016.38
7. Sabino-Silva R, Jardim ACG, Siqueira WL, Carolina A, Jardim G, Siqueira WL, et al. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Investig. (2020) 24:1619–21. doi: 10.1007/s00784-020-03248-x
8. Marsh PD, Do T, Beighton D, and Devine DA. Influence of saliva on the oral microbiota. Periodontol 2000. (2016) 70:80–92. doi: 10.1111/prd.12098
9. Khurshid Z, Zafar MS, Khan RS, Najeeb S, Slowey PD, and Rehman IU. Role of salivary biomarkers in oral cancer detection. Adv Clin Chem. (2018) 86:23–70. doi: 10.1016/bs.acc.2018.05.002
11. Mahboobi N, Porter SR, Karayiannis P, and Alavian SM. Oral fluid and hepatitis A, B and C: A literature review. J Oral Pathol Med. (2012) 41:505–16. doi: 10.1111/j.1600-0714.2011.01123.x
12. Dong E, Du H, and Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. (2020) 20:533–4. doi: 10.1016/S1473-3099(20)30120-1
13. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
14. Hoffmann M, Kleine-Weber H, and Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Cell Press. (2020). 78:779–784.e5 doi: 10.1016/j.molcel.2020.04.022
15. Kandeel M, Ibrahim A, Fayez M, and Al-Nazawi M. From SARS and MERS CoVs to SARS-CoV-2: moving toward more biased codon usage in viral structural and nonstructural genes. J Med Virol. (2020) 92:660–6. doi: 10.1002/jmv.25754
16. Park SE. Epidemiology, virology, and clinical features of severe acute respiratory syndrome -coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clin Exp Pediatr. (2020) 63:119–24. doi: 10.3345/cep.2020.00493
17. De Wit E, Van Doremalen N, Falzarano D, and Munster VJ. SARS and MERS: Recent insights into emerging coronaviruses. Nat Rev Microbiol. (2016) 14:523–34. doi: 10.1038/nrmicro.2016.81
18. Zhou P, Yang Lou X, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579:270–3. doi: 10.1038/s41586-020-2012-7
19. Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. (2020) 133:1015–24. doi: 10.1097/CM9.0000000000000722
20. Wu D, Wu T, Liu Q, and Yang Z. International Journal of Infectious Diseases The SARS-CoV-2 outbreak : what we know. Elsevier. (2020) 94:44–8. doi: 10.1016/j.ijid.2020.03.004
21. Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Heal. (2020) 4642:1–9. doi: 10.1016/s2352-4642(20)30177-2
22. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. (2020) 17:533–5. doi: 10.1038/s41423-020-0402-2
23. Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. (2020) 95: E131–34. doi: 10.1002/ajh.25774
24. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
25. Guan WJ, Ni ZY, Hu YHY, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
26. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
27. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
28. Chan JF, Yuan S, Kok K, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. (2020) 395:514–23. doi: 10.1016/S0140-6736(20)30154-9
29. Kui L, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. (2020) 133:1025–31. doi: 10.1097/CM9.0000000000000744
30. Zou X, Chen K, Zou J, Han P, Hao J, and Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. (2020) 14:185–92. doi: 10.1007/s11684-020-0754-0
31. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, and Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. (2020). doi: 10.1101/2020.01.26.919985
32. Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. (2020). doi: 10.1101/2020.01.30.927806
33. Letko M, Marzi A, and Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. (2020) 5:562–9. doi: 10.1038/s41564-020-0688-y
34. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. (2020) 12:1–5. doi: 10.1038/s41368-020-0074-x
35. Izaguirre G. The proteolytic regulation of virus cell entry by furin and other proprotein convertases. Viruses. (2019) 11:837. doi: 10.3390/v11090837
36. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E, et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. (2020) 176:104742. doi: 10.1016/j.antiviral.2020.104742
37. Mallapaty S. Why does the coronavirus spread so easily between people? Nature. (2020) 579:183. doi: 10.1038/d41586-020-00660-x
38. López De Cicco R, Watson JC, Bassi DE, Litwin S, and Klein-Szanto AJ. Simultaneous expression of furin and vascular endothelial growth factor in human oral tongue squamous cell carcinoma progression. Clin Cancer Res. (2004) 10:4480–8. doi: 10.1158/1078-0432.CCR-03-0670
39. Xu R, Cui B, Duan X, Zhang P, Zhou X, and Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. (2020) 12:11. doi: 10.1038/s41368-020-0080-z
40. He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. (2020) 26:672–5. doi: 10.1038/s41591-020-0869-5
41. Romano M, Ruggiero A, Squeglia F, Maga G, and Berisio R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells. (2020) 9:1267. doi: 10.3390/cells9051267
42. Chen L, Zhao J, Peng J, Li X, Deng X, Geng Z, et al. Detection of 2019- nCoV in Saliva and Characterization of Oral Symptoms in COVID-19 Patients (2020). Available online at SSRN: https://ssrn.com/abstract=3556665
43. Liu L, Wei Q, Alvarez X, Wang H, Du Y, Zhu H, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. (2011) 85:4025–30. doi: 10.1128/jvi.02292-10
44. Peng X, Xu X, Li Y, Cheng L, Zhou X, and Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. (2020) 12:1–6. doi: 10.1038/s41368-020-0075-9
45. To KK-W, Tsang OTY, Yip CC-Y, Chan KH, Wu TC, Chan JMCJ, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. (2020) 71:4–6. doi: 10.1093/cid/ciaa149
46. Lu B, Huang Y, Huang L, Li B, Zheng Z, Chen Z, et al. Effect of mucosal and systemic immunization with virus-like particles of severe acute respiratory syndrome coronavirus in mice. Immunology. (2010) 130:254–61. doi: 10.1111/j.1365-2567.2010.03231.x
47. Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Marcel A, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. (2020) 581:465–9. doi: 10.1038/s41586-020-2196-x
48. Chaux-Bodard A-G, Deneuve S, and Desoutter A. Letter to the Editor. Oral manifestation of Covid-19 as an inaugural symptom? J Oral Med Oral Surg. (2020) 26:18. doi: 10.1051/mbcb/2020011
49. Asadi S, Bouvier N, Wexler AS, and Ristenpart WD. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Sci Technol. (2020) 54:635–8. doi: 10.1080/02786826.2020.1749229
50. Chen J. Pathogenicity and transmissibility of 2019-nCoV—A quick overview and comparison with other emerging viruses. Microbes Infect. (2020) 22:69–71. doi: 10.1016/j.micinf.2020.01.004
51. Backer JA, Klinkenberg D, and Wallinga J. Incubation period of 2019 novel coronavirus (2019- nCoV) infections among travellers from Wuhan, China, 20 28 January 2020. Eurosurveillance. (2020) 25:1–6. doi: 10.2807/1560-7917.ES.2020.25.5.2000062
52. Kucharski AJ, Russell TW, Diamond C, Liu Y, Edmunds J, Funk S, et al. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis. (2020) 20:553–8. doi: 10.1016/S1473-3099(20)30144-4
53. Xu Y. Unveiling the origin and transmission of 2019-nCoV. Trends Microbiol. (2019) 28:239–40. doi: 10.1016/j.tim.2020.02.001
54. Kwok YLA, Gralton J, and McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. (2015) 43:112–4. doi: 10.1016/j.ajic.2014.10.015
55. Li D, Jin M, Bao P, Zhao W, and Zhang S. Clinical characteristics and results of semen tests among men with Coronavirus Disease 2019. JAMA Netw Open. (2020) 3:e208292. doi: 10.1001/jamanetworkopen.2020.8292
56. Gamio L. The Workers Who Face the Greatest Coronavirus Risk. The New York Times. New York, NY (2020).
57. WHO. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. (2020) 1–3. doi: 10.1056/NEJMoa2001316.5
58. Kampf G, Todt D, Pfaender S, and Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. (2020) 104:246–51. doi: 10.1016/j.jhin.2020.01.022
59. Cleveland JL, Gray SK, Harte JA, Robison VA, Moorman AC, and Gooch BF. Transmission of blood-borne pathogens in US dental health care settings: 2016 update. J Am Dent Assoc. (2016) 147:729–38. doi: 10.1016/j.adaj.2016.03.020
60. Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, et al. Detection of air and surface Contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in hospital rooms of infected patients. medRxiv. (2020) 125:20046557. doi: 10.1101/2020.03.29.20046557
61. Morawska L, and Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. (2020) 139:105730. doi: 10.1016/j.envint.2020.105730
62. Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. (2020) 86:557–60. doi: 10.1038/s41586-020-2271-3
63. To KKW, Tsang OTY, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. (2020) 20:565–74. doi: 10.1016/S1473-3099(20)30196-1
64. Zhang W, Du RH, Li B, Zheng X, Yang X-L, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. (2020) 9:386–9. doi: 10.1080/22221751.2020.1729071
65. Wang WK, Chen SY, Liu IJ, Chen YC, Chen HL, Yang CF, et al. Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg Infect Dis. (2004) 10:1213–9. doi: 10.3201/eid1007.031113
66. Guo W-LL, Jiang Q, Ye F, Li SQS-YYS-Q, Hong C, Chen L-YY, et al. Effect of throat washings on detection of 2019 novel coronavirus. Clin Infect Dis. (2020) ciaa416. doi: 10.1093/cid/ciaa416
67. Ather A, Patel B, Ruparel NB, Diogenes A, and Hargreaves KM. Coronavirus Disease 19 (COVID-19): implications for Clinical Dental Care. J Endod. (2020) 46:2020. doi: 10.1016/j.joen.2020.03.008
68. Fallahi HR, Keyhan SO, Zandian D, Kim S-G, and Cheshmi B. Being a front-line dentist during the Covid-19 pandemic: a literature review. Maxillofac Plast Reconstr Surg. (2020) 42:12. doi: 10.1186/s40902-020-00256-5
69. Meng L, Hua F, and Bian Z. Coronavirus Disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. (2020) 2019:481–7. doi: 10.1177/0022034520914246
70. Ren YF, Rasubala L, Malmstrom H, and Eliav E. Dental Care and oral health under the clouds of COVID-19. JDR Clin Transl Res. (2020) 5:202–10. doi: 10.1177/2380084420924385
71. French Society of Stomatology Maxillo-Facial Surgery and Oral Surgery (SFSCMFCO). Practitioners specialized in oral health and coronavirus disease 2019: professional guidelines from the French society of stomatology, maxillofacial surgery and oral surgery, to form a common front against the infectious risk. J Stomatol Oral Maxillofac Surg. (2020) 121:155–8. doi: 10.1016/j.jormas.2020.03.011
72. Eggers M, Koburger-Janssen T, Eickmann M, and Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect Dis Ther. (2018) 7:249–59. doi: 10.1007/s40121-018-0200-7
73. Vandenberghe B, Jacobs R, and Bosmans H. Modern dental imaging: a review of the current technology and clinical applications in dental practice. Eur Radiol. (2010) 20:2637–55. doi: 10.1007/s00330-010-1836-1
74. Samaranayake LP, Reid J, and Evans D. The efficacy of rubber dam isolation in reducing atmospheric bacterial contamination. ASDC J Dent Child. (1989) 56:442–4.
75. Harrel SK, and Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. (2004) 135:429–37. doi: 10.14219/jada.archive.2004.0207
76. Zemouri C, De Soet H, Crielaard W, and Laheij A. A scoping review on bio-Aerosols in healthcare & the dental environment. PLoS ONE. (2017) 12:e0178007. doi: 10.1371/journal.pone.0178007
77. Izzetti R, Nisi M, Gabriele M, and Graziani F. COVID-19 Transmission in dental practice: brief review of preventive measures in Italy. J Dent Res. (2020) 99:1030–38. doi: 10.1177/0022034520920580
78. Grenier G, Gagnon G, and Grenier D. Detection of herpetic viruses in gingival crevicular fluid of patients suffering from periodontal diseases: prevalence and effect of treatment. Oral Microbiol Immunol. (2009) 24:506–9. doi: 10.1111/j.1399-302X.2009.00542.x
Keywords: oral fluids, saliva, COVID-19, SARS-CoV-2, diagnostic, angiotensin-converting enzyme II, furin
Citation: Gaudin A, Badran Z, Chevalier V, Aubeux D, Prud'homme T, Amador del Valle G and Cloitre A (2020) COVID-19 and Oral Fluids. Front. Dent. Med. 1:8. doi: 10.3389/fdmed.2020.00008
Received: 04 June 2020; Accepted: 03 August 2020;
Published: 10 September 2020.
Edited by:
Johnah Galicia, Arthur A. Dugoni School of Dentistry, University of the Pacific, United StatesReviewed by:
Matthias Widbiller, University of Regensburg, GermanyXiaofei Zhu, Peking University, China
Copyright © 2020 Gaudin, Badran, Chevalier, Aubeux, Prud'homme, Amador del Valle and Cloitre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexis Gaudin, alexis.gaudin@univ-nantes.fr
 Alexis Gaudin
Alexis Gaudin Zahi Badran
Zahi Badran Valérie Chevalier4,5
Valérie Chevalier4,5  Davy Aubeux
Davy Aubeux Alexandra Cloitre
Alexandra Cloitre