Two large squirrels (Rodentia, Mammalia) from the Junggar Basin of northwestern China demonstrate early radiation among squirrels and suggest forested paleoenvironment in the late Eocene of Central Asia
- 1Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China
- 2CAS Center for Excellence in Life and Paleoenvironment, Beijing, China
- 3College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing, China
Fossil evidence is indispensable for studying the derivation, divergence, and dispersal of squirrels as they responded to global Cenozoic climatic and paleoenvironmental change. Among these fossil records, the earliest known definitive fossil squirrels in Eurasia occur after the Eocene/Oligocene Boundary and are slightly younger than the oldest records in North America. Here, we report the discovery of two new extinct large squirrel species from the late Eocene of the Junggar Basin in northwestern China. The dental morphologies of these new taxa represent tree and flying morphotypes, and their estimated body masses are approximately 1.2 kg and 2.6 kg, respectively. In addition, these extinct lineages push the age of the first appearance of Sciuridae in northern Asia into the late Eocene. Together with Douglassciurus and Oligospermophilus from North America, these two new squirrels from the Junggar Basin are the earliest records of sciurids, and analysis of their teeth clearly demonstrates that the three principle morphotypes of sciurids (flying, ground, and tree squirrels) had diverged from one another by the late Eocene. That proposed late Eocene divergence among the major groupings of sciurids is consistent with some molecular clock analyses and helps to document that macroevolutionary timing and pattern. Comparison with modern squirrel analogs for body masses over 1 kg points to these early Chinese species as having occupied forested habitats, and that hypothesis is congruent with published palynological studies. Furthermore, these two new squirrel taxa from Jeminay provide new data to evaluate the examination of the long-term aridification of Central Asia.
Introduction
Squirrels are a rodent group with small- to medium-sized bodies in the family Sciuridae, which is the fourth most diverse family of living mammals, consisting of 58 genera and ∼285 extant species (Thorington and Hoffmann, 2005; Thorington et al., 2012). The fossil squirrels have been allocated to more than 35 extinct genera (McKenna and Bell, 1997; Fossilworks Group, 2022). Previous work has revealed that the geographic distribution and diversification of both fossil and living sciurids were affected significantly by global climate change during the Cenozoic (Mercer and Roth, 2003) and that the evolution of arboreality possibly aided the divergence of the arboreal group from their hypothetical terrestrial/fossorial ancestors (Steppan et al., 2004). Known fossil records point to the late Eocene as a critical period for the derivation, early divergence, and radiation of sciurids (Goodwin, 2008; Fabre et al., 2012). This temporal interval coincides with the global climatic shift from greenhouse to icehouse climates during the Eocene–Oligocene Transition (EOT) (Zachos et al., 2001; Liu et al., 2009; Hren et al., 2013; Hutchinson et al., 2021), and that climate change possibly initiated or enhanced the aridification of the Asian continental interior in combination with the uplift of the Tibetan Plateau and retreat of Paratethys (e.g., Dupont-Nivet et al., 2007; Abels et al., 2011; Miao et al., 2012; Miao et al., 2013; Bosboom et al., 2014; Fang et al., 2015; Sun and Windley, 2015; Li et al., 2018). As a consequence, the Eocene–Oligocene climatic shift led to the large-scale extinction of marine invertebrates and terrestrial floristic and faunal turnover (e.g., Collinson et al., 1981; Prothero and Emry, 1994; Prothero et al., 2003; Hooker et al., 2004; Retallack et al., 2004), such as the Grande Coupure in Europe and the Mongolian Remodelling in North Asia (Stehlin, 1910; Meng and McKenna, 1998).
Sciurids have been considered to have originated in North America on the eve of the EOT, with the representatives Douglassciurus jeffersoni known at about 36.6 Ma (Douglass, 1901; Emry and Korth, 1996; Emry and Korth, 2001) or D. oaxacaensis at about 40 Ma (Ferrusquia-Villafranca et al., 2018). From that hypothetical origin, squirrels are thought to have dispersed into Europe and South Asia soon after the EOT, as documented by fossils of Oligopetes (Vianey-Liaud, 1974; Heissig, 1979; Vianey-Liaud, 1985; De Bruijn and Ünay, 1989; Welcomme et al., 2001). Squirrels appear to have immigrated rather late to North Africa in the Miocene and to South America in the Pleistocene (De Bruijn, 1999). The Eurasian Oligopetes has been presumed to be a Grande Coupure immigrant (Dawson, 2003; Heissig, 2003). However, a large temporal gap exists between the EOT and the first appearance of Sciuridae in the Mongolian Remodelling of North Asia (Meng and McKenna, 1998). In recent years, the Paleogene records of sciurids in North Asia have continued to grow, and they have demonstrated that squirrels were rather diverse in the early Oligocene (Minjin, 2004; Wang and Qiu, 2004; Wang and Dashzeveg, 2005; Maridet et al., 2014). In addition, fossils have suggested that sciurids also might date back to the late Eocene (Wang, 2008). In fact, we can confirm Eocene occurrence in northern Asia through the recognition of two new fossil species of large arboreal squirrels from late Eocene sediments of the Junggar Basin in northwestern China. These fossils and the taxa they represent greatly aid in improving our understanding of the derivation, early divergence, and spatiotemporal distribution of sciurids, and they support the occurrence of forested paleohabitat in the late Eocene of Central Asia.
All of the extant and extinct squirrel species comprise a monophyletic Sciuridae, which is the sister group to Aplodontidae (Huchon and Douzery, 2001; DeBry, 2003; Montgelard et al., 2008; Blanga-Kanfi et al., 2009; Fabre et al., 2012). Traditionally, Sciuridae were split into the two subfamilies which are the Pteromyinae (the flying squirrels with their gliding membrane) and Sciurinae (the non-flying tree and ground squirrels) (e.g., Simpson, 1945; Hoffmann et al., 1993; Thorington et al., 2002). Based on dental and mandibular morphologies, De Bruijn, (1999) recognized three morphotypes among the squirrels that include “ground,” “tree,” and “flying” squirrels, and he elevated Pteromyinae and Sciurinae to the family level, raising the Sciuridae to superfamily rank. Qiu (2019) summarized the known 32 genera of sciurid fossils in China and divided them into four subfamilies. In that taxonomy (Qiu, 2019), flying squirrels are still treated as an independent subfamily, Pteromyinae. However, molecular phylogenetic studies refined the higher level systematic arrangement of the Sciuridae and its extant lineages of five subfamilies: Ratufinae, Sciurillinae, Sciurinae, Callosciurinae, and Xerinae (Mercer and Roth, 2003; Steppan et al., 2004). In this treatment, the “flying squirrels” are monophyletic (Thorington, 1984; Oshida et al., 1996) and are treated as a tribe within the subfamily Sciurinae (Mercer and Roth, 2003; Steppan et al., 2004; Thorington and Hoffmann, 2005; Fabre et al., 2012). In addition to the five living subfamilies, there are two extinct subfamilies. One is the subfamily Cedromurinae proposed by Korth and Emry (1991) for the two extinct genera Cedromus Wilson (1949) and Oligospermophilus Korth (1987) from the late Eocene to late Oligocene of North America. This clade was subsequently accepted by some paleontologists (McKenna and Bell, 1997; Wang and Dashzeveg, 2005; Goodwin, 2008). The other is the subfamily Aepyosciurinae erected by Wang and Qiu (2003) for a specialized sciurid group with unilaterally hypsodont and lophodont cheek teeth. At present, it includes only genus Aepyosciurus and is restricted in the late Neogene and Quaternary (early Pliocene to early Pleistocene) of the Tibetan Plateau and North China (Wang and Qiu, 2003; Qiu et al., 2005; Cai et al., 2013; Wang et al., 2013; Li et al., 2014). Based on their dental morphologies, the two new species in this text from Jeminay, northwestern China, should be placed in the subfamily Sciurinae.
Materials and methods
Fossil sites
The new sciurid fossil specimens were discovered in Jeminay County in the northwestern part of the Xinjiang Uygur Autonomous Region of China. The new fossil sites are located in a gully west of the village of Xiaerhete, which is ∼10 km south of the Irtysh River and 6 km northwest of the county seat of Jeminay County, close to the China–Kazakhstan border. The Jeminay area has produced middle Eocene fossil plants including Taxodium sp., Ampelopsis sp., Populus sp., and Corylus sp. and mammals including Triplopus sp., Triplopus jeminaiensis, Lophialetes sp., and Hyaenodontidae gen. et sp. indet. (Wang F. Y., 1984; Jin, 2000). Jin (2000) considered the Cenozoic sediments in Jeminay area and along the Irtysh River as an eastern extension of the neighboring deposits in the Zaysan Basin of Kazakhstan. The squirrel fossils derive from two extremely close sites (XJ20140619LQ03 and XJ20140626NI01, N47°28′, E86°47′, elevation 869 m) and from almost the same layer (only a half-meter interval of two sites) consisting of ∼5 m of yellowish fluvial sandstone, grayish white siltstone, and sandy mudstone of the Keziletuogayi Formation (Figure 1). A large anseriform fossil (cf. Romainvilliinae) was previously discovered and reported from this same layer (Stidham and Ni, 2014). Lithologically, the squirrel fossil layer can be correlated to the Keziletuogayi Formation yielding an A3 mammalian zone fauna from the nearby Keziletuogayi section. Paleomagnetic dating of that interval provides an age range of 34.0–35.0 Ma (Sun et al., 2014). The associated mammals from the Keziletuogayi section include Brontotheriidae, Amynodontinae, Cadurcodon cf. C. ardynensis, and Ardynomys vinogradov, and that fauna also confirms its late Eocene age.
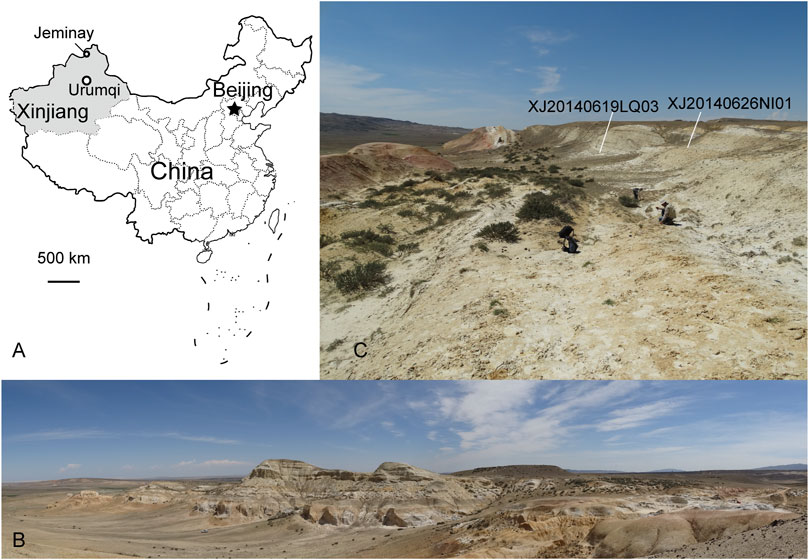
FIGURE 1. Late Eocene sciurid sites in Jeminay, northwestern Xinjiang, China. (A) Map showing the fossil sites located at Jeminay, Xijiang Uygur Autonomous Region, northwestern China; (B) panoramic view of the fossiliferous profile of the Keziletuogayi Formation that produced the additional material of fossil squirrels (photo taken by the first author QL on 20 June 2014); (C) a close-up view of the stratigraphy yielding the fossil squirrels.
Material
The fossil squirrel specimens include four enamel caps of one upper and three lower molars, collected by wet-sieving technique in 2014. The other rodent specimens are as yet unstudied. All specimens are housed at the Institute of Vertebrate Paleontology and Paleoanthropology (IVPP), Chinese Academy of Sciences, Beijing.
Comparative sample
For comparative purposes, we discuss all known late Eocene and early-late Oligocene sciurid genera (16 taxa). The following is a list of these genera.
Asian forms include Sciurus sp., Oligosciurus, Kherem, Marmotini gen. et sp. indet., and Plesiosciurus aff. sinensis. The specimens of Sciurus sp. reported by Bohlin (1946) include a right M3 (T. b. 202) and a right m2 (T. b. 593) from the late Oligocene Taben-buluk Basin of Gansu Province, China. Oligosciurus (Wang and Qiu, 2004) (type and only species: O. dangheensis) is based on the topotype jaw (IVPP V13556) from the lower Oligocene Paoniuquan Formation in the Danghe region of Gansu Province, China. The topotype belongs to an ontogenetically old individual with a heavily worn m1 and m2. Kherem (Minjin, 2004) comprises two members, K. hsandgoliensis (the type species) and K. asiatica, both of which were discovered in the Hsanda Gol Formation (early Oligocene) of the Valley of Lakes area, Mongolia (Minjin, 2004; Wang and Dashzeveg, 2005). Kherem was once assigned to the subfamily Cedromurinae recognized by Korth and Emry, (1991) (Wang and Dashzeveg, 2005). Recently, Maridet et al. (2014) treated K. asiatica as a synonym of K. hsandgoliensis, assigned Kherem to the subfamily Xerinae, and expanded the former temporal range from early Oligocene through early middle Miocene. We agree with Maridet et al. (2014) that K. asiatica is synonymous with K. hsandgoliensis. The material referred to as Marmotini gen. et sp. indet. described by Wang (2008) comprises a broken M1/2 (IVPP V 15003) from the Upper Eocene Houldjin Formation of Erenhot, Inner Mongolia, China. Its attribution to Sciuridae is doubtful. The specimens of Plesiosciurus aff. sinensis (Qiu and Lin, 1986) were discovered in Toglorhoi, Unkheltseg, Hotuliin Teeg, Ulann Tolgoi, and Loh (early Late Oligocene to early Middle Miocene) of the Valley of Lakes, Mongolia (Maridet et al., 2014, p. 274).
European forms include Palaeosciurus, Heteroxerus, and Oligopetes. Palaeosciurus Pomel, 1853 comprises five European species: P. feignouxi (type species), P. fissurae (Dehm, 1950), P. goti (Vianey-Liaud, 1974), P. sutteri (Ziegler and Fahlbusch, 1986), and P. ultimus (Mein and Ginsburg, 2002), and a Chinese species P. jiangi (Qiu, 2015). This genus ranges temporally from the earliest Oligocene to the early Miocene in Eurasia (de Bruijn, 1999; Qiu, 2015). Palaeosciurus goti from Mas de Got of France (MP22) was once regarded as the earliest sciurid in Europe (Vianey-Liaud, 1985). Heteroxerus Schaub (in Stehlin and Schaub, 1951, p. 200) (type species: H. hürzeleri) is a member of Xerini and ranges from the early Oligocene through the late Miocene in Europe (McKenna and Bell, 1997). Its holotype is a fragmentary lower dentition with m1-2 (Stehlin and Schaub, 1951, p. 201, figure 300). Oligopetes (Heissig, 1979) is the hitherto earliest known record of flying squirrels. It consists of three species, O. radialis (type species), O. lophulus, and O. obtusus, which all derive from the fissure fillings of Suevium (lower to middle Oligocene).
North American forms include Protospermophilus, Cedromus, Miospermophilus, Miosciurus, Protosciurus, Oligospermophilus, Nototamias, and Douglassciurus. Protospermophilus (Gazin, 1930) contains of a total of six species: P. quatalensis (type species), P. wortmani, P. kelloggi, P. angusticeps, P. oregonensis, and P. malheurensis (Cope, 1879; Gazin, 1930; Gazin, 1932; Matthew and Mook, 1933; Downs, 1956; Black, 1963). Temporally, they span from the early Arikareean to late Clarendonian NALMA (late Oligocene to late Miocene) (Goodwin, 2008). The genotype P. quatalensis is present in the Cuyama Basin (late Miocene) of California (USA). Cedromus (Wilson, 1949) consists of the type species Cedromys wardi and C. wilsoni (Korth and Emry, 1991). Cedromus ranges from the early Orellan to the late Whitneyan NALMA (early Oligocene to early Late Oligocene) (Goodwin, 2008). Miospermophilus (Black, 1963) comprises three species, namely, M. bryanti (type sepcies), M. wyomingensis, and the questionable M. lavertyi (Wilson, 1960; Black, 1963; Dalquest et al., 1996), and it spans from the early Arikareean to the late Clarendonian NALMA (late Oligocene to late Miocene) (Goodwin, 2008). Miosciurus (Black, 1963) contains one species, M. ballovianus (Cope, 1881). Miosciurus ballovianus is an early Arikareean NALMA (late Oligocene) sciurid from the United States, and thus far it has been found only in the “Diceratherium beds” (probably Turtle Cove Member, see Albright et al., 2008) of the John Day Formation of Oregon. Protosciurus (Black, 1963) includes P. condoni (type species), P. mengi, P. rachelae, and P. tecuyensis (Bryant, 1945). The genus ranges from early Orellan to early Hemingfordian NALMA (early Oligocene to late early Miocene), and it may have survived into the early Barstovian NALMA (early middle Miocene) (Goodwin, 2008). Oligospermophilus (Korth, 1987) (type and only species: O. douglassi), typical of the Orella Member of the Brule Formation (early Oligocene), Prairie Dog Creek of Nebraska (USA), was assigned originally to Protosciurus (Korth, 1981). Nototamias (Pratt and Morgan, 1989) is a chipmunk-sized sciurid with a Tamias-like upper dental pattern. Chronologically, it spans from the early Arikareean to the late Clarendonian NALMA (late Oligocene to late Miocene), similar to Protospermophilus (Goodwin, 2008). It comprises three species: N. hulberti (type species), N. quadratus, and N. ateles (Hall, 1930; Pratt and Morgan, 1989; Korth, 1992). Douglassciurus is a replacement name for Douglassia Emry and Korth (1996) (Emry and Korth, 2001). This genus consists of four species: D. jeffersoni (late Eocene), D. sapphirus (late Oligocene), D. bjorki (middle Oligocene), and D. oaxacaensis (late middle Eocene) (Douglass, 1901; Korth, 2009; Korth, 2014; Ferrusquia-Villafranca et al., 2018). The type species is D. jeffersoni from the Chadronian NALMA (late Eocene) Pipestone Springs Formation, Montana (USA), and it was originally referred to the genus Sciurus by Douglass (1901). Its generic and familial allocations were uncertain for a long time, attributed to Prosciurus (Matthew, 1903) of the Aplodontidae (Osborn and Matthew, 1909; Wood, 1937) or to Cedromus or Protosciurus of the Sciuridae (Wood, 1962; Black, 1963; Wood, 1980; Emry and Thorington, 1982).
Site and institutional abbreviations
IVPP, The Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing (China); MP, European Paleogene mammal faunal zones; XJ, prefix to Xinjiang of field localities of the IVPP; T.b., Taben-buluk area (Gansu Province, China) in Bohlin’s (1946) pioneering work.
Measurements and nomenclature
Specimens were measured using an Olympus SZX7 microscope with a precision of +/− 0.01 mm. The length is defined as the anteroposterior chord. The width is defined along the chord perpendicular to the length. The dental terminology (Figure 2) is mostly adopted from Qiu (1996) and Qiu, (2019) except for our usage of “anterobuccal cingulid” to replace their “anterobuccal cingulum” on m1/2 and additions of “anterobuccal sinusid” and “protocone crest.” The protocone crest is “a short crest extending anterobuccally from the protocone into the valley between the anterior cingulum and the protoloph” defined by Emry and Korth (1996), and it is equal to the “protostyl” used by Heissig (1979).
CT scanning and reconstruction
The specimens were CT-scanned using the 100 kv Micro–CT in the Key Laboratory of Vertebrate Evolution and Human Origins of the Chinese Academy of Sciences. The 3D virtual reconstruction was made with VGSTUDIO (Version 2.0, genuine authorized) software (Volume Graphics) installed in the computers of the laboratory following the standard procedure introduced by Ni et al. (2012).
Body mass estimation
Following Freudenthal and Martín-Suárez (2013), we first calculated the length of the lower tooth row using one of their regression equations (ln (LRsum)=0.51×ln (L×W of m1)+1.25) applied to the Sciuridae (Freudenthal and Martín-Suárez (2013), p.7). Then, we substituted the tooth row length into their other equation (ln (mass) = 3.023ln (row)−0.993) to obtain a body mass estimate (Freudenthal and Martín-Suárez (2013), p.8).
Systematic Paleontology
Order Rodentia Bowdich, (1821).
Family Sciuridae (Fischer de Waldheim, 1817)
Subfamily Sciurinae (Fischer de Waldheim, 1817)
Genus Junggarisciurus gen. nov.
Junggarisciurus jeminaiensis sp. nov.
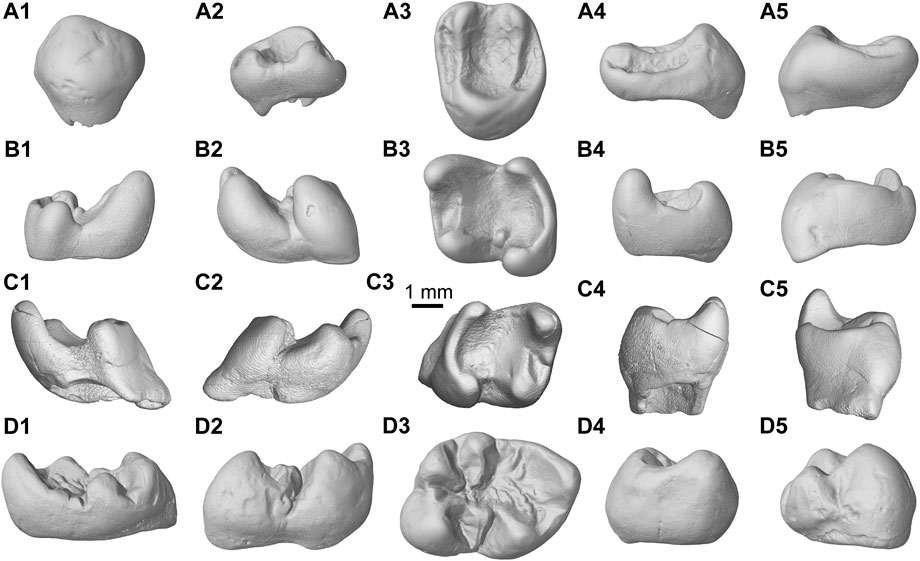
FIGURE 3. 3D virtual reconstruction of the molars of Junggarisciurus jeminaiensis gen. et. sp. nov. and Eopetes irtyshensis gen. et. sp. nov. (A1–A5) IVPP V23185, holotype of J. jeminaiensis, left M1/2; (B1–B5) IVPP V23186, left m1/2; (C1–C5) IVPP V23280, paratype, right m1/2; (D1–D5) IVPP V31378, holotype of E. irtyshensis, right m1/2.1, lingual view; 2, buccal view; 3, occlusal view; 4, anterior view; 5, posterior view. All in the same scale (bar equals 1 mm).
Etymology. “Junggar,” Mongolian, referring to the Junggar Basin, the provenance of this new taxon; “sciurus” is from Greek skiourus (squirrel); “Jeminay,” Mongolian, is the nearby county seat of the fossil sites.
Holotype. IVPP V23185, an isolated left M1/2 (Figures 3A1–5), collected from IVPP field site XJ20140619LQ03.
Paratype. IVPP V23280, a right m1/2 (Figures 3C1–5), collected from IVPP field site XJ20140619LQ03.
Referred specimen. IVPP V23186, a left m1/2 (Figures 3B1–5), collected from IVPP field site XJ20140626NI01.
Type Locality and Age. Jeminay, northwestern Xinjiang Uygur Autonomous Region, China, the Keziletuogayi Formation, late Eocene, 34.0–35.0 Ma.
Generic diagnosis
A large-sized sciurid. Molars are brachydont with incipient rugose floor in the trigon and talonid basins. M1/2 outline is quadrate; protocone is anteroposteriorly expanded; hypocone is absent; protoconule, metaconule, and mesotyle are indistinct; anterocone is reduced; protoloph and metaloph are complete and nearly parallel; metaloph is constricted at its junction with protocone; and protocone crest and ectoloph are absent. The m1/2 outline is more rectangular, metaconid is sharp, entoconid is well-delimited, mesoconid is developed or indistinct, hypoconulid and mesostylid are absent, trigonid basin is wide and enclosed by complete metalophid, talonid basin is incompletely enclosed with two notches near the center of the buccal and lingual sides, anterobuccal cingulid and entolophid are absent, anterobuccal sinusid at the junction of the protolophid is absent, ectolophid is weak and discontinuous, posterolophid is continuous and connected to entoconid. Slightly larger than Protosciurus condoni and remarkably larger than other known late Eocene–Oligocene sciurids. Furthermore, Junggarisciurus jeminaiensis differs from Kherem of Xerinae and Palaeosciurus, Oligopetes, Protospermophilus, Miospermophilus, Protosciurus, Nototamias, Douglassciurus, Plesiosciurus aff. sinensis, and Marmotini gen. et sp. of Sciurinae in its protoloph parallel to the metaloph on M1/2. Furthermore, it differs from Kherem and Heteroxerus of Xerinae and Oligopetes, Miospermophilus, and Miosciurus of Sciurinae in the absence of an anterobuccal sinusid on m1/2. It differs from Sciurus sp. (Sciurinae) in its less quadrate outline and well-developed metalophid, trigonid basin, and entoconid on m1/2. Compared to the taxa of Cedromurinae, it differs from Cedromus in its less developed or absent anteroconid, entolophid, and ectolophid on m1/2. It differs from Oligospermophilus by having more rounded corners, an absence of a hypocone and mesostyle on M1/2, and a less developed anteroconid, an entoconid merged into the posterolophid, and the absence of a mesostylid on m1/2. It differs from Oligosciurus in the presence of a mesostylid and mesoconid, a wide trigonid, a narrow buccal valley, and absence of an entolophid.
Specific diagnosis
Same as that of the generic diagnosis.
Measurements (in mm)
M1/2 (IVPP V23185): length × width = 3.63 × 4.75; m1/2 (IVPP V23186): 4.30 × 3.95; m1/2 (IVPP V23280): 4.15 × 3.90.
Description
The holotype M1/2 (Figures 3A1–5) has a quadrate outline that is wider than long. There are three marginal cusps and four lophs, which form a wide and deep middle and two narrow and shallow marginal depressions with an incipient rugose surface. The lingual main cusp is far higher than the buccal. Tooth anterior and buccal walls are less curved, while the posterior and lingual are rather rounded. The protocone is conspicuously large and anteroposteriorly expanded. The hypocone is absent. The paracone and metacone are small and nearly the same size. The anterocone is reduced and inconspicuous, merging with the anteroloph. Both the protoconule and metaconule are absent. No distinct parastyle, protoconule, or metaconule is present. The mesostyle is extremely small and inconspicuous. Both the protoloph and metaloph are complete, nearly parallel to each other, and extend slightly anterolingually toward the protocone. The protoloph is strong, and the metaloph is constricted distinctly at its junction with the protocone. The anteroloph and posteroloph are lower than the protoloph and metaloph. A protocone crest similar to that of Douglassciurus jeffersoni (see Emry and Korth, 1996) and an ectoloph resembling Oligopetes (Heissig, 1979) are not present. The trigon basin is deep and wide. The anterior valley is slightly narrower than the posterior. No roots are preserved.
The m1/2s (Figures 3B1–5, C1–5) have a nearly rectangular outline and are slightly anterobuccal-posterolingually compressed. There are four marginal cusps and three transverse lophs, surrounding a small, shallow anterior trigonid basin and a large, deep posterior talonid basin with a less rugose floor compared to Oligopetes and Palaeosciurus. The two anterior cusps project more than the two posterior cusps. The anterolingual metaconid is the highest and positioned slightly more anteriorly than the protoconid. The lingual entoconid is situated opposite of the buccal hypoconid, and it is well-delimited at lingoposterior corner of the tooth. The mesoconid is well developed on the referred specimen (IVPP V23186) but indistinct on the paratype (IVPP V23280). The anteroconid can be observed on the IVPP V23280 but merges with the anterolophid of the IVPP V23186. The hypoconulid and mesostylid are both absent. The metalophid is complete and encloses the trigonid basin. The ectolophid is weak and discontinuous. The posterolophid is strong and continuous, and it connects the hypoconid and entoconid. No entolophid (or hypolophid) is present. The buccal and lingual valleys are present as deep notches of external margin of the talonid basin. The anterobuccal cingulid is absent. There is no trace of an anterobuccal sinusid between anterolophid and protoconid. No roots are preserved.
Remarks
The Jeminay sample generally resembles that of living Sciurini, particularly Sciurus, by having a quadrate outline, a complete protoloph and metaloph, an absent metaconule on M1/2, a developed metalophid enclosing the trigonid basin, and a conspicuous entoconid on m1/2. It seems reasonable to refer the m1/2 to the tree squirrels (Qiu, 2019). However, it differs from Sciurus in its larger tooth size and having a transverse metalophid parallel to the anterolophid, a larger trigonid basin in contrast to that of Sciurus, and a V-shaped notch between the metaconid and entoconid on m1/2. For further comparative purposes, we discuss all known late Eocene and early-late Oligocene sciurid genera (16 taxa, above). The Jeminay sample differs from these taxa in both its dental size and morphology (see Generic diagnosis).
Genus Eopetes gen. nov.
Eopetes irtyshensis sp. nov.
Etymology. “Eo-,” Greek, means dawn; “petes” is derived from the Sanskrit “patara” meaning flying; “Irtysh,” Mongolian, refers the Irtysh River near the holotype locality.
Holotype. IVPP V31378, an isolated right m1/2 (Figures 3D1–5), collected from IVPP field site XJ20140619LQ03.
Type Locality and Age. Jeminay, northwestern Xinjiang Uygur Autonomous Region, China, the Keziletuogayi Formation, late Eocene, 34.0–35.0 Ma.
Generic diagnosis
A large-sized sciurid. Lower molar brachydont with rugose floor and extra ridges in the talonid basin. Entoconid, hypoconulid, mesoconid, and ectomesolophid well-developed. Entoconid relatively isolated. No anterobuccal cingulid or sinusid. Protolophid low and extending posterolingually. Metastylid and mesostylid well developed. Trigonid basin posteriorly open. Compared to taxa of flying squirrels, it differs from Oligopetes by the presence of well-developed entoconid and hypoconulid and the absence of anterobuccal cingulid and anterobuccal sinusid and having a crowded talonid basin. It differs from Parapetaurista, Miopetaurista, Hylopetodon, Yunopterus, Hylopetes, Pteromys, Petaurista, and Aeretes in the absence of an anterobuccal cingulid and sinusid, a limited talonid basin, and a robust hypoconulid. It differs from Belomys, Trogopterus, and the Pliopetaurista in lophs being conspicuously weaker than the cusps.
Species diagnosis
Same as that of the generic diagnosis.
Measurements (mm)
The holotype m1/2 (IVPP V31378), length × width = 5.8 × 4.6.
Description and comparison
The tooth is brachydont with heavily built cusps and extra ridges. It has a rhomboid occlusal outline with projecting anterolingual metaconid and posterobuccal hypoconid corners. The metaconid is the highest of the main cusps, and it is situated further anteriorly than the protoconid. Posterior to the lingual part of the metaconid, the metastylid is present and has a short transverse ridge. No anterobuccal cingulid is present. The anteroconid is situated between the metaconid and the protoconid, is much lower than them, and is connected to them through thin ridges. The protoconid is subtriangular in outline, and it is separated from the posterior mesoconid. The trigonid is open posteriorly. The protolophid is low and wavy, derives from the anterolingual part of the protoconid, and extends posterolingually into the talonid basin, failing to reach the entoconid. The mesoconid is prominent, subtriangular in shape, and as high as the entoconid. The ectomesolophid is derived from the mesoconid, tapers buccally, slopes ventrally, and interrupts the buccal valley (=hypoflexid) by an anterior narrow groove and a posterior enclosed fossa surrounded by the ectolophid, hypoconid, mesoconid, and ectomesolophid. The mesostylid is present on the lingual margin, and it is situated between the metastylid and entoconid. The entoconid is nearly isolated and opposite the mesoconid. The entolophid is short and bulging and is buccally contracted. The ectolophid is short and longitudinally links the mesoconid and the hypoconid. The hypoconid has a long anterobuccal crest and a short posterior arm with an extra cuspid. A notch exists between the posterior side of the hypoconid and hypoconulid. The hypoconulid is triangular in shape, lower than the hypoconid, higher than the entoconid, and as large as the entoconid. The hypoconulid is separated from the entoconid. The anterior part of the talonid basin has a wrinkled surface, but the posterior part is occupied by the heavily built entoconid and hypoconulid. The space demarcated by the entoconid, hypoconulid, and ectolophid is quite limited.
This m1/2 has a complicated occlusal structure, with a rugose talonid surface, extra ridges, and a prominent entoconid. It seems reasonable to refer the m1/2 to the grouping of flying squirrels (Qiu, 2019). The m1/2 has a heavily built entoconid, hypoconulid, and meseoconid, and it lacks an anterobuccal cingulid or sinusid. That morphology resembles that of some genera of flying squirrels, such as the extant Belomys and Trogopterus and the fossil Pliopetaurista, but the morphology differs from other flying squirrels in the absence of an anterobuccal cingulid and sinusid, a limited talonid basin, and a robust hypoconulid (Qiu, 2019). Furthermore, the morphology of this m1/2 is so unique as to distinguish it from other Oligocene flying squirrels such as Oligopetes or the living representatives through its lophs being conspicuously weaker than the cusps. Although this taxon is represented currently by only one lower tooth, it is worth erecting a new genus and species for it given its large size and unique morphology.
Results
Derivation and early divergence of sciurids
The family Sciuridae is generally thought to have derived from the Ischyromyidae (including Paramyinae) in North America (Matthew, 1910; Wilson, 1949; Wood, 1962; Black, 1963; Emry and Thorington, 1984; Korth, 1984; Emry and Korth, 1996). Korth and Emry (1991) proposed that the Sciuridae possibly shares with the Aplodontidae a common ancestor that arose from a Reithroparamys-like ischyromyid with the Aplodontidae. Their view was accepted by many researchers (Korth, 1994; Emry and Korth, 1996; Mercer and Roth, 2003; Goodwin, 2008) but is still questioned by a few (de Bruijn, 1999; Heissig, 2003). Junggarisciurus jeminaiensis from the Junggar Basin is easily distinguished from Reithroparamys or other ischyromyids, and it displays conspicuous dental features, such as the lack of a hypocone, protoconule, and metaconule on M1/2, the absence of an anterobuccal sinusid at the junction of the anteroconid and protoconid, an inconspicuous ectolophid, the absence of an entoconid crest, and a joining of the entoconid and posterolophid on m1/2 (Wood, 1962). It seems that there is no close affiliation between the sciurid J. jeminaiensis and the ischyromyid Reithroparamys. Junggarisciurus jeminaiensis shares some characters with the ischyromyid Hulgana ertnia, which was erected based on the material collected from the Upper Eocene “Ulan Gochu” beds at Jhama Obo, East Mesa, Inner Mongolia (China) (Dawson, 1968). The shared characteristics include large size, deep talonid basin relative to the main cusps, outline of the lower molar rhomboid, indistinct conules, and absent hypocone, style (id)s, and hypolophid. However, Hulgana ertnia’s P4-M2 have an anterolingual protrusion of the protocone with crests converging on the protocone and a concavity at the lingual-posterior of the protocone, and its lower molars have incompletely enclosed the trigonid and lack a mesoconid. Furthermore, on the buccal side the mandible of H. ertnia, the apex of the masseteric ridges is situated far posteriorly (posterior to m2), but that of the Sciuridae is placed anteriorly (m1 or posterior p4) (Qiu, 2019). Unfortunately, the specimen of Junggarisciurus jeminaiensis has no jaw, so the situation of the apex of the masseteric ridges of this species is unknown. Eopetes irtyshensis has a special dental morphology on m1/2 with crenulations and stylids, the presence of a robust entoconid, hypoconulid, and mesoconid, and the absence of an anterobuccal sinusid, resembling that of some flying squirrels, particularly the extant Belomys and Trogopterus and extinct Pliopetaurista (Qiu, 2019). Eopetes irtyshensis differs from Reithroparamys or other ischyromids in having a robust hypoconulid, a weaker metalophid, and an entolophid extending toward the mesoconid. It seems that the Sciuridae were not derived directly from any known ischyromids. In fact, there is still a large time gap between the records of latest ischyromids (early middle Eocene) and the earliest sciurids (late Eocene) (Tong and Li, 2019).
Mercer and Roth (2003) used the age of Douglassicurus (36 Ma) as the calibration of their molecular-clock tree of sciurids, which yielded an estimated divergence date of 50 Ma for the node joining Aplodontia and Sciuridae. It seems that we should explore for the origins of sciurids in the middle or even early Eocene (the interval of 50–36 Ma) of North America or Asia. Recent discovery of Douglassciurus oaxacaensis from late middle Eocene (about 40 Ma) Oaxaca, Mexico, seems to prove this judgment. Mercer and Roth (2003) also estimated that the divergence of the five major clades of squirrels had arisen penecontemporaneously with the EOT. These late Eocene squirrels from Jeminay lived in northwestern China at about 35–34 Ma in the late Eocene. The discovery of Junggarisciurus jeminaiensis and Eopetes irtyshensis provides a minimum age of calibration for the Sciurinae crown group fairly close to the molecular clock estimated late Eocene 36 Ma split. Based on the fossil-calibrated molecular-clock phylogeny of modern squirrel genera by Mercer and Roth (2003); Roth and Mercer (2008) further investigated the macroevolutionary processes among the linages since the origin of the Sciuridae. Roth and Mercer (2008) described the diversification of squirrels as taking place in three phases: initial (36–30 Ma); intervening (30–7 Ma); and recent (∼7 Ma to present) phases. Their results show that the initial phase of squirrel evolution was a rapid burst of diversification (Roth and Mercer, 2008). The two new taxa from Jeminay indicate that there were at least two genera of Sciurinae that occurred in late Eocene Asia.
Squirrels have been divided typically into three morphotypes (ground, tree, and flying) based on their dental and jaw morphologies (e.g., De Bruijn, 1999; Qiu, 2019). Junggarisciurus jeminaiensis belongs to the tree squirrel morphotype because it possesses a less rugose surface of the trigon and talonid basins, a complete metaloph, a nearly parallel protoloph and metaloph of M1/2, and a well-delimited entoconid of m1/2. The morphology of Eopetes irtyshensis is consistent with that of a typical flying squirrel because it exhibits a complicated dental pattern with a distinct rugose floor of the talonid basin, extra ridges and stylids, and a prominent entoconid. Of the known records, North America is possibly the birthplace of squirrels. Douglassciurus oaxacaensis from late middle Eocene (about 40 Ma) in Oaxaca, southeastern Mexico, is possibly the earliest squirrel (Ferrusquia-Villafranca et al., 2018). Due to the lack of upper molars, the morphotype of D. oaxacaensis is uncertain. Four late Eocene species, Douglassciurus jeffersoni and Oligospermophilus douglassi from North America and Junggarisciurus jeminaiensis and Eopetes irtyshensis from Central Asia, are the second earliest records of the Sciuridae (Figure 4). These fossil records demonstrate that by the late Eocene, the sciurids had diverged into the three principle morphotypes, represented by the earliest flying squirrel Eopetes irtyshensis from northwestern China (this text), the earliest ground squirrel Oligospermophilus douglassi from North America (Korth, 1987), and the earliest tree squirrels Douglassciurus jeffersoni from North America (Emry and Korth, 1996) and Junggarisciurus jeminaiensis from Jeminay, northwestern China (this text).

FIGURE 4. Chronologic ranges of Eocene to Oligocene sciurids from North America, Asia, and Europe. The fossil records of sciurids span the Eocene/Oligocene boundary in both North America and Asia but appear later in the early Oligocene of Europe. Douglassciurus oaxacaensis is the earliest member of sciurids in late middle Eocene, Junggarisciurus jeminaiensis, Eopetes irtyshensis, Douglassciurus jeffersoni, and Oligospermophilus douglassi form the second earliest confident records of sciurids in the late Eocene. Red dashed line, Grande Coupure; blue dashed line, Mongolian Remodelling; gray dot with dashed arrowed line, estimated date; asterisks, J. jeminaiensis and E. irtyshensis from Jeminay, northwestern Xinjiang, China. Abbreviations: P., Paleogene; m., middle Miocene.
After the Eocene/Oligocene boundary, members of Sciuridae rapidly spread into Europe and flourished across the Holarctic region during the Oligocene. That radiation is demonstrated by the occurrence of eight genera in the early Oligocene, represented by Heteroxerus (ground) and Palaeosciurus (tree) in Europe, Oligopetes (flying) in Europe and South Asia, Kherem (ground) and Oligosciurus (tree) in North Asia, and Oligospermophilus (ground), Cedromus (ground) and Protosciurus (tree) in North America (Figure 4). With the oldest sciurid records from Asia and North America predating the first occurrences in Europe, it would seem that sciurids are a component of the European Grand Coupure. Without a phylogenetic context, it is not presently possible to determine if the first sciurids in Europe came from Asia or North America. However, a hypothesis of an Asian origin for those dispersers would seem more likely given the preponderance of mammalian lineages known to have dispersed from Asia to Europe at the start of the Oligocene (coincident with climatic changes and the loss of seaway barriers). In parallel, the published bird (Anatidae: cf. Romainvilliinae) from the same bed as these new sciurid taxa represents an Asian record (late Eocene) predating the group’s first appearance in Europe (Oligocene) as another indicator of potential central-Asia-to-Europe dispersal (Stidham and Ni, 2014).
Body mass estimation and paleohabitat
The large size of these fossils is a striking feature of the two new fossil sciurid taxa from Jeminay, northwestern China. The smaller Junggarisciurus jeminaiensis is slightly larger than Protosciurus condoni and remarkably larger than the 22 other Eocene-to-Oligocene compared taxa in Table 1. To understand the rodents’ paleoecological roles, we estimated their body mass from these isolated cheek teeth using published methods (e.g., Legendre, 1989; Hopkins, 2008; Goodwin and Bullock, 2012; Freudenthal and Martín-Suárez, 2013). Following the regression equations of Freudenthal and Martín-Suárez (2013, p.7 and p. 8, see Body mass estimation in Materials and methods in this text), we calculated that the average body masses of Junggarisciurus jeminaiensis and Eopetes irtyshensis were ∼1.2 kg and 2.6 kg, respectively. Among living squirrels, the majority of taxa have average body weights less than 1.0 kg. Only a few are over 1.0 kg, including a tree squirrel Rheithrosciurus macrotis, several flying squirrels Ratufa, Petaurista, Petinomys crinitus, and Aeromys tephromelas, and most species of ground squirrels Cynomys and Marmota (Thorington and Heaney, 1981; Ernest, 2003; Hayssen, 2008; Jones et al., 2009; Thorington et al., 2012; Freudenthal and Martín-Suárez, 2013). Marmota has an extreme body mass ranging up to 8.0 kg, but Cynomys is normally less than 2 kg. Both of them are well adapted to their fossorial steppe environments. The other large living tree and flying squirrels (body mass concentrated in the range of 1–2 kg) all dwell in forests, ranging across tropical rainforest, subtropical deciduous, mixed deciduous and moist evergreen, coniferous and broadleaf, montane and riparian forests. Forests supply large tree or flying squirrels with diverse foods, including seeds, fruits, nuts, bark, insects, and even bird eggs (Thorington et al., 2012). Emry and Thorington (1984) considered that the large tree squirrels should have relatively few competitors, other than birds, for the rich source of nutrients in the forest.
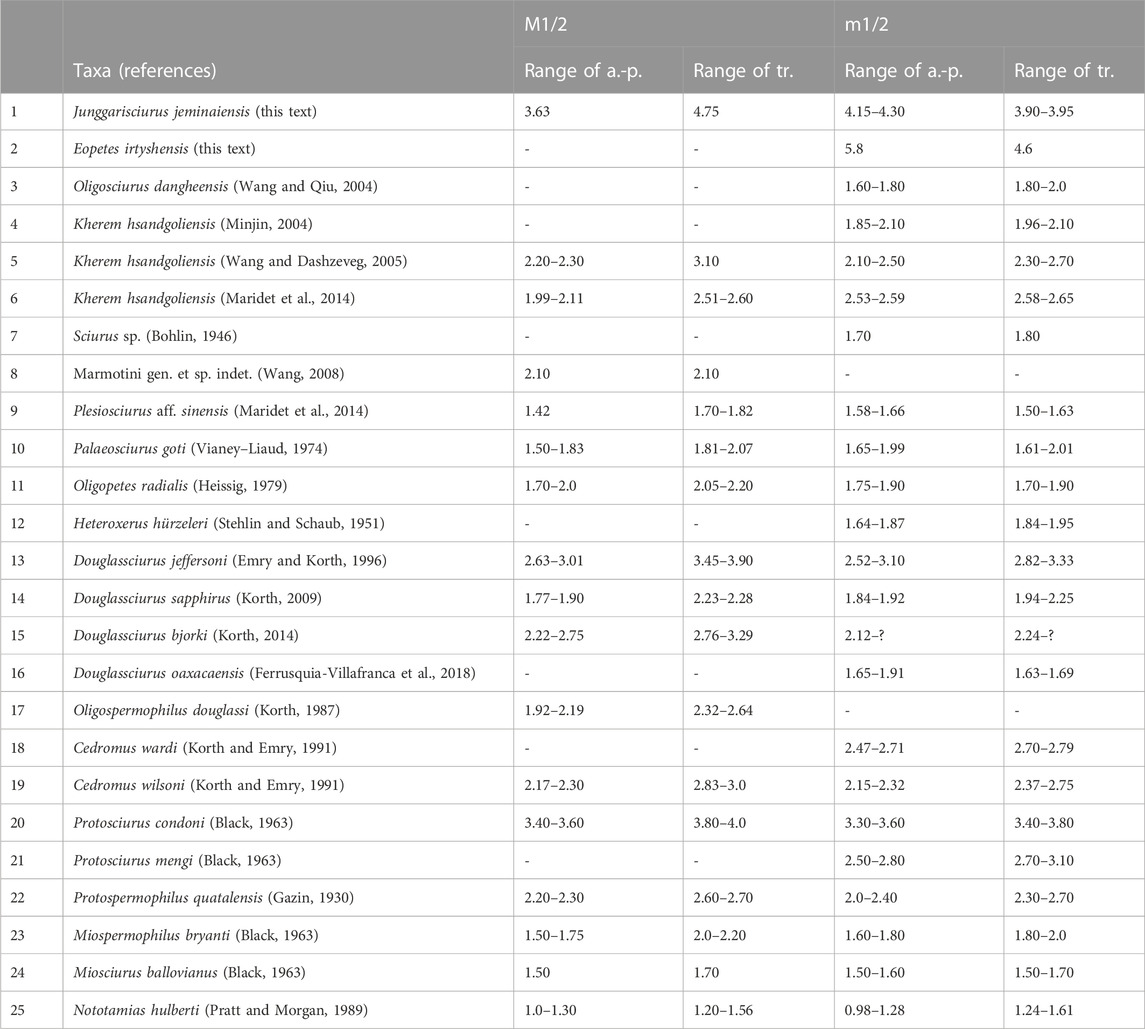
TABLE 1. Measurements (in mm) of M1/2s and m1/2s of late Eocene-Oligocene sciurids. a.-p., anteroposterior length; tr., maximum transverse width. Measurements of taxa 2–10 and 12–21 derive from the references listed in this table, and those of taxon 11 are estimated from the illustration by Stehlin and Schaub (1951, p. 201). All sources are in the References.
Compared to the monsoon-dominated climatic pattern after the Oligocene–Miocene Transition (OMT), the Paleogene climate of China is considered to have displayed a typical zonal pattern (Wang F. Y., 1984; Sun and Wang, 2005; Guo et al., 2008). During the Eocene and Oligocene, the Junggar Basin is hypothesized to have been situated in a warm-temperate zone (Wang P. X., 1984) or in a subtropical humid vegetation zone of northern China (Sun and Wang, 2005) and to have had a humid environment, contrasting with the arid or semi-arid belt of Guo et al. (2008). In the late Eocene Irtysh River area, fossil pollen assemblages are dominated by coniferous plants like Pinuspollenites, Piceaepollenites, Abiespollenites, and Podocarpidites, and broadleaved taxa such as Betulaepollenites, Quercoidites, Ulmipollenites, and Juglanspollenites, pointing to a mixed coniferous and broadleaved forest paleoenvironment (Sun et al., 2014). The occurrence of the large tree squirrel Junggarisciurus jeminaiensis and the flying squirrel Eopetes irtyshensis helps to support this palynological hypothesis of the existence of a forested paleoenvironment in the late Eocene Irtysh River area rather than arid conditions.
Conclusion and perspectives
Based on the large dimensions and unusual morphology of these isolated molar fossils, we erect two new fossil genera and species of squirrels from the late Eocene of the Jeminay area of the Junggar Basin in northwestern China. Junggarisciurus jeminaiensis displays molars with a “tree squirrel” morphotype, and Eopetes irtyshensis has a lower molar with a “flying squirrel” morphotype. These fossils and taxa are first two confirmed representatives of sciurids in the late Eocene of North Asia and they extend the first appearance of squirrels into the Eocene of Asia.
The impressive size of these two new species from the Junggar Basin leads to estimated body masses of Junggarisciurus jeminaiensis and Eopetes irtyshensis being ∼1.2 kg and 2.6 kg, respectively. All of the extant species of tree or flying morphotype squirrels over 1 kg inhabit forested habitats, and it would seem likely to regard J. jeminaiensis and E. irtyshensis as having lived in late Eocene forests of Central Asia, consistent with the palynologic analysis by Sun et al. (2014). That interpretation of a forested habitat in the late Eocene of Central Asia will feed into future work investigating the process and pattern of aridification of Central Asia.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
XN and QL led and CQ and HG participated in the fieldwork in the Jeminay area for correlating the stratigraphy and collecting fossils. QL digitized the fossil specimens. QL, XN, and TS wrote the manuscript draft. All authors discussed and revised the manuscript.
Funding
This work was supported by the Strategic Priority Research Program of Chinese Academy of Sciences, under Grant Nos XDB26000000 and XDA2007203.
Acknowledgments
The authors are indebted to the assistance of Lvzhou Li, Xiaoyu Lu, and Shubing Fu of the IVPP who undertook the fieldwork in the Jeminay area. The authors thank Dr. Yemao Hou of the IVPP for his help with CT scanning and Dr. Xiaoming Wang of the Natural History Museum of California for his advice and comments on the first draft of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abels, H. A., Dupont-Nivet, G., Xiao, G. Q., Bosboom, R., and Krijgsman, W. (2011). Step-wise change of asian interior climate preceding the Eocene–Oligocene Transition (EOT). Palaeogeogr. Palaeocl. 299, 399–412. doi:10.1016/j.palaeo.2010.11.028
Albright, L. B., Woodburne, M. O., Fremd, T. J., Swisher, C. C., MacFadden, B. J., and Scott, G. R. (2008). Revised chronostratigraphy and biostratigraphy of the John Day Formation (Turtle Cove and kimberly memmbers), Oregon, with implications for updated calibration of the Arikareean North American Land Mammal Age. J. Geol. 116, 211–237.
Black, C. C. (1963). A review of the North American tertiary Sciuridae. Bull. Mus. Comp. Zool. 130, 109–248.
Blanga-Kanfi, S., Miranda, H., Penn, O., Pupko, T., DeBry, R., and Huchon, D. (2009). Rodent phylogeny revised: Analysis of six nuclear genes from all major rodent clades. BMC Evol. Biol. 9, 71. doi:10.1186/1471-2148-9-71
Bohlin, B. (1946). The fossil mammals from the tertiary deposit of taben-buluk, western kansu. Part II: Simplicidentata, carinvora, artiodactyla, perissodactyla, and primates. Palaeont. Sin. N. Ser. C 8b, 1–259.
Bosboom, R., Dupont-Nivet, G., Grothe, A., Brinkhuis, H., Villa, G., Mandic, O., et al. (2014). Timing, cause and impact of the late Eocene stepwise sea retreat from the Tarim Basin (west China). Palaeogeogr. Palaeocl. 403, 101–118. doi:10.1016/j.palaeo.2014.03.035
Bowdich, T. E. (1821). An analysis of the natural classifications of mammalia, for the use of students and travellers. Paris: J. Smith.
Bryant, M. D. (1945). Phylogeny of nearctic Sciuridae. Am. Midl. Nat. 33, 257–390. doi:10.2307/2421337
Cai, B. Q., Zheng, S. H., Liddicoat, J. C., and Li, Q. (2013). “Chapter 8. Review of the litho-, bio–, and chronostratigraphy in the Nihewan Basin, Hebei, China,” in Fossil Mammals of Asia, Neogene Biostratigraphy and Chronology. Editors X. M. Wang, L. J. Flynn, and M. Fortelius (New York: Columbia University Press), 218–242.
Collinson, M. E., Fowler, K., and Boulter, M. C. (1981). Floristic changes indicate a cooling climate in the Eocene of southern England. Nature 291, 315–317. doi:10.1038/291315a0
Cope, E. D. (1881). On the Nimravidae and Canidae of the Miocene period of NorthNorth America. Bull. U.S. Geol. Geogr. Surv. Ter. 6, 165–181.
Cope, E. D. (1879). Second contribution to a knowledge of the Miocene fauna of Oregon. Paleontol. Bull. 31, 1–7.
Dalquest, W. W., Baskin, J. A., and Schultz, G. E. (1996). “Fossil mammals from a late Miocene (Clarendonian) site in Beaver County, Oklahoma,” in Contributions in mammalogy: A memorial volume honoring Dr. Editors J. Knox JonesJr., H. H. Genoways, and R. J. Baker (Lubbock: Museum of Texas Technical University), 107–137.
Dawson, M. R. (1968). Oligocene rodents (mammalia) from East Mesa, Inner Mongolia. Am. Mus. Novit. 2324, 1–12.
De Bruijn, H. (1999). “Superfamily Sciuroidea,” in The Miocene land mammals of Europe. Editors G. E. Rössner, and K. Heissig (München: Verlag Dr. Friedrich Pfeil), 271–280.
De Bruijn, H., and Ünay, E. (1989). Petauristinae (mammalia, Rodentia) from the Oligocene of Spain, Belgium, and Turkish thrace. Nat. Hist. Mus. L.A. Sci. Ser. 33, 139–145.
DeBry, R. (2003). Identifying conflicting signal in a multigene analysis reveals a highly resolved tree: The phylogeny of Rodentia (mammalia). Syst. Biol. 52, 604–617. doi:10.1080/10635150390235403
Dehm, R. (1950). Die Nagetiere aus dem Mittel-Miozän (Burdigalium) von Wintershof–West bei Eichstätt in Bayern. N. Jb. Min. Geol. Paläont. Abh. B 90, 321–428.
Douglass, E. (1901). Fossil mammalia of the White River beds of Montana. Trans. Am. Philos. Soc. 20, 237–279. doi:10.2307/1005478
Downs, T. (1956). The Mascall Fauna from the Montana territory. Univ. Calif. Publ. Geol. Sci. 31, 199–354.
Dupont-Nivet, G., Krijgsman, W., Langereis, C. G., Abels, H. A., Dai, S., and Fang, X. M. (2007). Tibetan Plateau aridification linked to global cooling at the Eocene–Oligocene transition. Nature 445, 635–638. doi:10.1038/nature05516
Emry, R. J., and Korth, W. W. (2001). Douglassciurus, new name for Douglassia Emry and Korth, 1996, not Douglassia bartsch, 1934. J. Vertebr. Paleontol. 21, 400. doi:10.1671/0272-4634(2001)021[0400:dnnfde]2.0.co;2
Emry, R. J., and Korth, W. W. (1996). The Chadronian squirrel “Sciurus” jeffersoni Douglass, 1901: A new generic name, new material, and its bearing on the early evolution of Sciuridae (Rodentia). J. Vertebr. Paleontol. 16, 775–780. doi:10.1080/02724634.1996.10011366
Emry, R. J., and Thorington, R. W. (1982). Descriptive and comparative osteology of the oldest fossil squirrel, Protosciurus (Rodentia: Sciuridae). Smithson. Contrib. Paleobiol. 47, 1–35. doi:10.5479/si.00810266.47.1
Emry, R. J., and Thorington, R. W. (1984). “The tree squirrel Sciurus as a living fossil,” in Living Fossils. Editors N. Eldredge, and S. Stanley (New York: Springer-Verlag), 23–31.
Ernest, S. K. M. (2003). Life history characteristics of placental nonvolant mammals. Ecology 84, 3402. doi:10.1890/02-9002
Fabre, P. H., Hautier, L., Dmitrov, D., and Douzery, E. J. P. (2012). A glimpse on the pattern of rodent diversification: A phylogenetic approach. BMC Evol. Biol. 12, 88–19. doi:10.1186/1471-2148-12-88
Fang, X. M., Zan, J. B., Appel, E., Lu, Y., Song, C. H., Dai, S., et al. (2015). An Eocene-Miocene continuous rock magnetic record from the sediments in the Xining Basin, NW China: Indication for cenozoic persistent drying driven by global cooling and Tibetan Plateau uplift. Geophys. J. Int. 201, 78–89. doi:10.1093/gji/ggv002
Ferrusquia-Villafranca, I., Flynn, L. J., Ruiz-Gonzalez, J. R., Torres-Hernandez, J. R., and Martinez-Hernandez, E. M. (2018). New Eocene rodents from northwestern Oaxaca, southeastern Mexico, and their paleobiological significance. J. Vert. Pal., e1514615. doi:10.1080/02724634.2018.1514615
Fossilworks Group (2022). Available at: http://www.fossilworks.org (Accessed June 25, 2022).
Freudenthal, M., and Martin-Suárez, E. (2013). Estimating body mass of fossil rodents. Scr. Geol. 145, 1–130.
Gazin, C. L. (1932). A Miocene mammalian fauna from southeastern Oregon. Carnegie Inst. Wash. Publ. 418, 36–86.
Gazin, C. L. (1930). A Tertiary Vertebrate Fauna from the Upper Cuyama Drainage Basin, 404. California: Carnegie Inst. Wash. Publ, 55–76.
Goodwin, T. H., and Bullock, K. M. (2012). Estimates of body mass for fossil giant ground squirrels, genus Paenemarmota. J. Mamm. 93, 1169–1177. doi:10.1644/11-mamm-a-312.3
Goodwin, T. H. (2008). “Chapter 21 Sciuridae,” in Evolution of tertiary mammals of North America, volume 2: Small mammals, xenarthrans, and marine mammals. Editors C. M. Janis, G. F. Gunnell, and M. D. Uhen (New York: Cambridge University Press), 355–376.
Guo, Z. T., Sun, B., Zhang, Z. S., Peng, S. Z., Xiao, G. Q., Ge, J. Y., et al. (2008). A major reorganization of Asian climate by the early Miocene. Clim. Past. 4, 153–174. doi:10.5194/cp-4-153-2008
Hall, E. R. (1930). Rodents and lagomorphs from the Barstow beds of southern California. Univ. Calif. Publ. Geol. Sci. 19, 313–318.
Hayssen, V. (2008). Patterns of body and tail length and body mass in Sciuridae. J. Mamm. 89, 852–873. doi:10.1644/07-mamm-a-217.1
Heissig, K. (1979). Die frühestern Flughörnchen und primitive Ailuravinae (Rodentia, Mammalia) aus dem suddeutschen Oligozän. Mitt. Bayer. Staatssamml. Paläontol. Hist. Geol. 19, 139–169.
Heissig, K. (2003). Origin and early dispersal of the squirrels and their relatives. Deinsea 10, 277–286.
Hoffmann, R. S., Anderson, C. G., Thorington, R. W., and Heaney, L. R. (1993). “Family Sciuridae,” in Mammal Species of the World. Editors D. E. Wilson, and D. M. Reeder (Washington: Smithsonian Institution Press), 419–465.
Hooker, J. J., Collinson, M. E., and Sille, N. P. (2004). Eocene–Oligocene mammalian faunal turnover in the hampshire basin, UK: Calibration to the global time scale and the major cooling event. J. Geol. Soc. 161, 161–172. doi:10.1144/0016-764903-091
Hopkins, S. B. (2008). Reassessing the mass of exceptionally large rodents using toothrow length and area as proxies for body mass. J. Mamm. 89, 232–243. doi:10.1644/06-mamm-a-306.1
Huchon, D., and Douzery, E. (2001). From the old world to the new world: A molecular chronicle of the phylogeny and biogeography of hystricognath rodents. Mol. Phylogenet. Evol. 20, 238–251. doi:10.1006/mpev.2001.0961
Hren, M. T., Sheldon, N. D., Grimes, S. T., Collinson, M. E., Hooker, J. J., Bugler, M., et al. (2013). Terrestrial cooling in Northern Europe during the Eocene-Oligocene Transition. PNAS 110, 7562–7567. doi:10.1073/pnas.1210930110
Hutchinson, D. K., Coxall, H. K., Lunt, D. L., Steinthorsdottir, M., de Boer, A. M., Baatsen, M., et al. (2021). The Eocene–Oligocene Transition: A review of marine and terrestrial proxy data, models and model–data comparisons. Clim. Past. 17, 269–315. doi:10.5194/cp-17-269-2021
Jones, K. E., Bielby, J., Cardillo, M., Fritz, S. A., O’Dell, J., Orme, D. L., et al. (2009). PanTHERIA: A species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648. doi:10.1890/08-1494.1
Korth, W. W. (1984). Earliest Tertiary evolution and radiation of rodents in North America. Bull. Carnegie Mus. Nat. Hist. 24, 1–71. doi:10.5962/p.228603
Korth, W. W., and Emry, R. J. (1991). The skull of Cerdormus and a review of the Cedromurinae (Rodentia, Sciuridae). J. Paleontol. 65, 984–994. doi:10.1017/s0022336000033291
Korth, W. W. (1992). Fossil small mammals from the Harrison Formation (late Arikareean: Earliest Miocene), Cherry County, Nebraska. Ann. Carnegie Mus. 61, 69–131. doi:10.5962/p.215172
Korth, W. W. (2009). Mammals from the Blue Ash local fauna (late Oligocene), south Dakota. Rodentia, Part 3: Family Sciuridae. Paludicola 7, 47–60.
Korth, W. W. (1981). New Oligocene rodents from Western North America. Ann. Carnegie Mus. 50, 289–318. doi:10.5962/p.214493
Korth, W. W. (2014). Rodents (mammalia) from the Whitneyan (middle Oligocene) Cedar Pass Fauna of south Dakota. Ann. Carnegie Mus. 82, 373–398. doi:10.2992/007.082.0404
Korth, W. W. (1987). Sciurid rodents (mammalia) from the Chadronian and Orellan (Oligocene) of Nebraska. J. Paleontol. 61, 1247–1255. doi:10.1017/s0022336000029620
Korth, W. W. (1994). The Tertiary Record of Rodents in North America. New York: Springer Science+Business Media.
Legendre, S. (1989). Les communautés de mammiféres du Paléogéne (eocéne supérieur et oligocéne) d’Europe occidentale: Structures, milieux et évolution. Münch. Geowiss. Abh. A16, 1–110.
Li, J. X., Yue, L. P., Roberts, A. P., Hirt, A. M., Pan, F., Guo, L., et al. (2018). Global cooling and enhanced Eocene Asian mid-latitude interior aridity. Nat. Commun. 9, 3026. doi:10.1038/s41467-018-05415-x
Li, Q., Xie, G. P., Takeuchi, G. T., Deng, T., Tseng, Z. J., Grohé, C., et al. (2014). Vertebrate fossils on the Roof of the World: Biostratigraphy and geochronology of highelevation Kunlun Pass Basin, northern Tibetan Plateau, and basin history as related to the Kunlun strike–slip fault. Palaeogeogr. Palaeocl. 411, 46–55. doi:10.1016/j.palaeo.2014.06.029
Linnaeus, C. (1758). Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tenth edition. Stockholm: Laurentii Salvii.
Liu, Z. H., Pagani, M., Zinniker, D., DeConto, R., Huber, M., Brinkhuis, H., et al. (2009). Global cooling during the Eocene–Oligocene climate transition. Science 323, 1187–1190. doi:10.1126/science.1166368
Maridet, O., Daxner-Höck, G., Badamgarav, D., and Göhlich, U. B. (2014). New discoveries of sciurids (rodents, Mammalia) from the Valley of Lakes (Central Mongolia). Ann. Naturhist. Mus. Wien Ser. A 116, 271–291.
Matthew, W. D. (1903). The fauna of the Titanotherium Beds at Pipestone Springs, Montana. Bull. Am. Mus. Nat. Hist. 19, 197–226.
Matthew, W. D., and Mook, C. C. (1933). New fossil mammals from the Deep River beds of Montana. Am. Mus. Novit. 601, 1–7.
Matthew, W. D. (1910). On the osteology and relationships of Paramys, and the affinities of the Ischyromyidae. Bull. Am. Nat. Hist. 21, 21–26.
McKenna, M. C., and Bell, S. K. (1997). Classification of Mammals above the Species Level. New York: Columbia University Press.
Mein, P., and Ginsburg, L. (2002). Sur l’âge relatif des différents dépôts karstiques Miocènes de La Grive-Saint-Alban (Isère). Mus. Hist. Nat. Lyon 2, 7–47. doi:10.3406/mhnly.2002.1328
Meng, J., and McKenna, M. C. (1998). Faunal turnovers of Paleogene mammals from the Mongolian Plateau. Nature 394, 364–367. doi:10.1038/28603
Mercer, J. M., and Roth, V. L. (2003). The effects of Cenozoic global change on squirrel phylogeny. Science 299, 1568–1572. doi:10.1126/science.1079705
Miao, Y. F., Fang, X. M., Wu, F. L., Cai, M. T., Song, C. H., Meng, Q. Q., et al. (2013). Late Cenozoic continuous aridification in the Western Qaidam Basin: Evidence from sporopollen records. Clim. Past. 9, 1863–1877. doi:10.5194/cp-9-1863-2013
Miao, Y. F., Herrmann, M., Wu, F. L., Yan, X. L., and Yang, S. L. (2012). What controlled mid-late Miocene long-term aridification in central Asia? - global cooling or Tibetan Plateau uplift: A review. Earth-Sci. Rev. 112, 155–172. doi:10.1016/j.earscirev.2012.02.003
Minjin, B. (2004). An Oligocene sciurid from the Hsanda Gol Formation, Mongolia. J. Vertebr. Paleontol. 24, 753–756. doi:10.1671/0272-4634(2004)024[0753:aosfth]2.0.co;2
Montgelard, C., Forty, E., Aranl, V., and Matthee, C. A. (2008). Suprafamilial relationships among Rodentia and the phylogenetic effect of removing fast–evolving nucleotides in mitochondrial, exon and intron fragments. BMC Evol. Biol. 8, 321. doi:10.1186/1471-2148-8-321
Ni, X. J., Flynn, J. J., and Wyss, A. R. (2012). Imaging the inner ear in fossil mammals: High-resolution CT scanning and 3–D virtual reconstructions. Palaeontol. Electron. 15 (2.18A), 1–10. doi:10.26879/288
Osborn, H. E., and Matthew, W. D. (1909). Cenozoic mammal horizons of Western North America, with faunal lists of the Tertiary Mammalia of the West. Bull. U.S. Geol. Surv. 361, 1–138.
Oshida, T., Masuda, R., and Yoshida, M. C. (1996). Phylogenetic relationships among Japanese species of the family Sciuridae (Mammalia, Rodentia), inferred from nucleotide sequences of mitochondrial 12s ribosomal RNA genes. Zool. Sci. 13, 615–520. doi:10.2108/zsj.13.615
Pratt, A. E., and Morgan, G. S. (1989). New Sciuridae (Mammalia: Rodentia) from the early Miocene Thomas Farm local fauna, Florida. J. Vertebr. Paleont. 9, 89–100. doi:10.1080/02724634.1989.10011741
Prothero, D. R., and Emry, R. J. (1994). The Terrestrial Eocene-Oligocene Transition. New York: Columbia University Press.
Prothero, D. R., Ivany, L. C., and Nesbitt, E. A. (2003). From Greenhouse to Icehouse: The Marine Eocene-Oligocene Transition. New York: Columbia University Press.
Qiu, Z. D. (2019). “Family Sciuridae,” in Palaeoverterata Sinica Volum III Basal Synapsids and Mammals Fascicle 5 (1) (serial no. 18–1) Glires II: Rodentia I. Editors C. K. Li, and Z. D. Qiu (Beijing: Science Press), 70–160.
Qiu, Z. D., and Lin, Y. P. (1986). The Aragonian vertebrate fauna of Xiacaowan, Jiangsu - 5. Sciuridae (Rodentia, Mammalia). Vert. Palasiat. 24, 195–212.
Qiu, Z. D. (1996). Middle Miocene Micromammalian Fauna from Tunggur, Nei Mongol. Beijing: Science Press.
Qiu, Z. D. (2015). Revision and supplementary note on Miocene sciurid fauna of Sihong, China. Vert. Palasiat. 53, 219–237.
Qiu, Z. X., Deng, T., and Wang, B. Y. (2005). Early Pleistocene mammalian fauna from Longdan, Dongxiang, Gansu, China. Palaeont. Sin. N. Ser. C 27, 1–198.
Retallack, G. J., Orr, W. N., Prothero, D. R., Duncan, R. A., Kester, P. R., and Ambers, C. P. (2004). Eocene-Oligocene extinction and paleoclimatic change near Eugene, Oregon. GSA Bull. 116, 817–839. doi:10.1130/b25281.1
Roth, V. L., and Mercer, J. M. (2008). Differing rates of macroevlutionary diversification in arboreal squirrels. Cur. Sci. India 95, 857–861.
Simpson, G. G. (1945). The principles of classification and a classification of mammals. Bull. Am. Mus. Nat. Hist. 85, 1–350.
Stehlin, H. G. (1910). Remarques sur les faunules de Mammiféres des couches éocènes et oligocènes du Bassin de Paris. Bull. Soc. Géol. Fr. 9, 488–520.
Stehlin, H. G., and Schaub, S. (1951). Die Trigonodontie der simplicidentaten Nager. Schweiz. paläont. abh. 67, 1–385.
Steppan, S. J., Storz, B. L., and Hoffmann, R. S. (2004). Nuclear DNA phylogeny of the squirrels (Mammalia: Rodentia) and the evolution of arboreality from c-myc and RAG1. Mol. Phylogenet. Evol. 30, 703–719. doi:10.1016/s1055-7903(03)00204-5
Stidham, T. A., and Ni, X. J. (2014). Large anseriform (Aves: Anatidae: Rominvilliinae?) fossils from the late Eocene of Xinjiang, China. Vert. Palasiat. 52, 98–111.
Sun, J. M., Ni, X. J., Bi, S. D., Wu, W. Y., Ye, J., Meng, J., et al. (2014). Synchronous turnover of flora, fauna, and climate at the Eocene-Oligocene Boundary in Asia. Sci. Rep. 4, 7463. doi:10.1038/srep07463
Sun, J. M., and Windley, B. F. (2015). Onset of aridification by 34 Ma across the Eocene-Oligocene transition in central Asia. Geology 43, 1015–1018. doi:10.1130/g37165.1
Sun, X. J., and Wang, P. X. (2005). How old is the Asian monsoon system?—palaeobotanical records from China. Palaeogeogr. Palaeocl. 222, 181–222. doi:10.1016/j.palaeo.2005.03.005
Thorington, R. W. (1984). Flying squirrels are monophyletic. Science 225, 1048–1050. doi:10.1126/science.225.4666.1048
Thorington, R. W., and Heaney, L. R. (1981). Body proportions and gliding adaptations of flying squirrels (Petauristinae). J. Mamm. 62, 101–114. doi:10.2307/1380481
Thorington, R. W., and Hoffmann, R. S. (2005). “Family Sciuridae,” in Mammal species of the world. A taxonomic and geographic reference. Editors D. E. Wilson, and D. M Reeder. Third Edition Volume 2 (Baltimore: The Johns Hopkins University Press), 754–818.
Thorington, R. W., Koprowski, J. L., Steele, M. A., and Whatton, J. F. (2012). Squirrels of the World. Baltimore: The Johns Hopkins University Press.
Thorington, R. W., Pitassy, D., and Jansa, S. (2002). Phylogenies of flying squirrels (Pteromyinae). J. Mamm. Evol. 9, 99–135.
Tong, Y. S., and Li, Q. (2019). “Families Alagomyidae, Archetypomyidae, Ischyromyidae,” in Palaeoverterata Sinica Volum III Basal Synapsids and Mammals Fascicle 5 (1) (serial no. 18–1) Glires II: Rodentia I. Editors C. K. Li, and Z. D. Qiu (Beijing: Science Press), 20–42.
Vianey-Liaud, M. (1974). Palaeosciurus goti nov. sp., écureuil terrestre de l’Oligocène moyen du Quercy. Données nouvelles sur l’apparition des Sciuridés en Europe. Ann. Paléont. (Vert.) 60, 103–122.
Vianey-Liaud, M. (1985). “Possible evolutionary relationships among Eocene and lower Oligocene rodents of Asia, Europe and North America,” in Evolutionary relationships among rodents. Editors W. P. Luckett, and J. L. Hartenberger (New York: Plenum Press), 277–309.
Wang, B. Y. (2008). Additional rodent material from Houldjin Formation of Erenhot, Nei Mongol, China. Vert. Palasiat. 46, 21–30.
Wang, B. Y., and Dashzeveg, D. (2005). New Oligocene sciurids and aplodontids (Rodentia, Mammalia) from Mongolia. Vert. Palasiat. 43, 85–99.
Wang, B. Y., and Qiu, Z. X. (2003). Aepyosciurinae - a new subfamily of Sciuridae (Rodentia, Mammalia) from basal loess deposits at the northeastern border of Tibetan Plateau. Chin. Sci. Bull. 48, 691–695. doi:10.1007/bf03325657
Wang, B. Y., and Qiu, Z. X. (2004). Discovery of early Oligocene mammalian fossils from Danghe area, Gansu, China. Vert. Palasiat. 42, 130–143.
Wang, F. Y. (1984). Discovery of abundant early Tertiary plant fossils in Jeminay county, Xinjiang. Xijiang Geol. 2, 81–82.
Wang, P. X. (1984). “Progress in late Cenozoic palaeoclimatology of China: A brief review,” in The Evolution of the East Asian Environment. Editor R. O. Whyte (Hongkong: Hong Kong University), Vol. 1.
Wang, X. M., Li, Q., Xie, G. P., Saylor, J. E., Tseng, Z. J., Takeuchi, G. T., et al. (2013). Mio–Pleistocene Zanda Basin biostratigraphy and geochronology, pre–ice age fauna, and mammalian evolution in Western Himalaya. Palaeogeogr. Palaeoecl. 374, 81–95. doi:10.1016/j.palaeo.2013.01.007
Welcomme, J.-L., Benammi, M., Crochet, J.-Y., Marivaux, L., Métais, G., Antoine, P.-O., et al. (2001). Himalayan forelands: Palaeontological evidence for Oligocene detrital deposits in the Bugti hills (balochistan, Pakistan). Geol. Mag. 138, 397–405. doi:10.1017/s0016756801005428
Wilson, R. W. (1960). Early Miocene rodents and insectivores from northeastern Colorado. Univ. Kans. Paleont. Contrib. Vert. 7, 1–92.
Wood, A. E., and Jepsen, G. L. (1937). The mammalian fauna of the White River Oligocene. Part II. Rodentia. Trans. Am. Philos. Soc. 28, 155–269. doi:10.2307/1005501
Wood, A. E. (1962). The early tertiary rodents of the family Paramyidae. Am. Philos. Soc. 52, 3–261. doi:10.2307/1005914
Wood, A. E. (1980). The Oligocene rodents of North America. Am. Philos. Soc. 70, 1–68. doi:10.2307/1006314
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. (2001). Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693. doi:10.1126/science.1059412
Keywords: body mass, forest paleohabitat, Junggar Basin, late Eocene, morphotype, Sciuridae
Citation: Li Q, Ni X, Stidham TA, Qin C, Gong H and Zhang L (2023) Two large squirrels (Rodentia, Mammalia) from the Junggar Basin of northwestern China demonstrate early radiation among squirrels and suggest forested paleoenvironment in the late Eocene of Central Asia. Front. Earth Sci. 10:1004509. doi: 10.3389/feart.2022.1004509
Received: 27 July 2022; Accepted: 28 December 2022;
Published: 12 January 2023.
Edited by:
Grégoire Métais, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Lawrence John Flynn, Harvard University, United StatesWilma Wessels, Utrecht University, Netherlands
Copyright © 2023 Li, Ni, Stidham, Qin, Gong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Li, liqiang@ivpp.ac.cn; Xijun Ni, nixijun@ivpp.ac.cn
†These authors have contributed equally to this work
 Qiang Li
Qiang Li Xijun Ni
Xijun Ni Thomas A. Stidham
Thomas A. Stidham Chao Qin1,3
Chao Qin1,3  Limin Zhang
Limin Zhang