- 1Department of Medical Research and Education, Taipei Veterans General Hospital, Taipei, Taiwan
- 2School of Medicine, National Yang-Ming University, Taipei, Taiwan
- 3Faculty of Medicine, National Yang-Ming University, Taipei, Taiwan
- 4Institute of Physiology, National Yang-Ming University, Taipei, Taiwan
- 5Institute of Clinical Medicine, National Yang-Ming University, Taipei, Taiwan
- 6Division of Biostatistics and Bioinformatics, National Health Research Institutes, Taipei, Taiwan
- 7Cardiovascular Center, Taichung Veterans General Hospital, Taichung, Taiwan
- 8Division of Endocrinology and Metabolism, Tri-Service General Hospital, Taipei, Taiwan
- 9Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 10Graduate Institute of Clinical Medicine, National Taiwan University, Taipei, Taiwan
- 11Kuakini Medical Center, Honolulu, Hawaii
- 12Department of Public Health Sciences and Epidemiology, John A. Burns School of Medicine, University of Hawaii and Pacific Health Research Institute, Honolulu, Hawaii
- 13Division of Cardiovascular Medicine, Falk Cardiovascular Research Center, Stanford University, Stanford, CA, USA
- 14Information Service Center, Taipei Veterans General Hospital, Taipei, Taiwan
- 15Medical Genetics Institute, Cedars-Sinai Medical Center, University of California at Los Angeles, Los Angeles, CA, USA
Co-heritability of hypertension and insulin resistance (IR) within families not only implies genetic susceptibility may be responsible for these complex traits but also suggests a rational that biological candidate genes for hypertension may serve as markers for features of the metabolic syndrome (MetS). Thus we determined whether the T323C polymorphism (rs5333) of endothelin type A (ETA) receptor, a predominant receptor evoking potent vasoconstrictive action of endothelin-1, contributes to susceptibility to IR-associated hypertension in 1694 subjects of Chinese and Japanese origins. Blood pressures (BPs) and biochemistries were measured. Fasting insulin level, insulin-resistance homeostasis model assessment (HOMAIR) score, and area under curve of insulin concentration (AUCINS) were selected for assessing insulin sensitivity. Genotypes were obtained by methods of polymerase chain reaction-restriction fragment length polymorphism. Foremost findings were that minor allele frequency of the T323C polymorphism was noticeable lower in our overall Asian subjects compared to multi-national population reported in gene database; moreover both the genotypic and allelic frequencies of the polymorphism were significantly different between the two ethnic groups we studied. The genotype distributions at TT/TC/CC were 65, 31, 4% in Chinese and 51, 41, 8% in Japanese, respectively (p < 0.0001). Additionally, carriers of the C homozygote revealed characteristics of IR, namely significantly higher levels of fasting insulin, HOMAIR score, and AUCINS at 29.3, 35.3, and 39.3%, respectively, when compared to their counterparts with TT/TC genotypes in Chinese. Meanwhile, the CC genotype was associated with a higher level of high density lipoprotein cholesterol in Japanese. No association of the polymorphism with BP was observed. This study demonstrated for the first time that T323C polymorphism of ETA receptor gene was associated with an adverse insulin response in Chinese and a favorite atherogenic index in Japanese.
Introduction
Insulin resistance (IR) and hypertension are related traits. Individuals who are insulin resistant are glucose intolerant, hyperinsulinemic, have higher blood pressure (BP) as well as atherogenic dyslipidemia (1). The clustering of these cardiovascular disease (CVD) risks associated with IR displayed familial characteristics as well, often observed in diabetes and hypertension families (2–5). For example, path analysis showed co-heritability of BP and IR in Hispanic subjects with hypertension parents (6). Cosegregation of traits in family not only reveals genetic susceptibility but also implies genes contribute to one will predict other traits in the family members. Therefore, biological candidate genes for hypertension may serve as genetic markers for features of the metabolic syndrome (MetS) (7). Dysfunction of endothelin system (ETS) is one of ancillary clinical features of the MetS that could lead to abnormalities in cardiovascular structure and function.
Endothelins are a family of three peptides of 21 amino acids with strong vasoconstrictor effects (8, 9). Two receptors have been cloned, endothelin type A (ETA) and endothelin type B receptor (ETB) which bind the three endothelins with various affinities (10). Endothelin-1 (ET-1) and nitric oxide are the prototypes of endothelium-derived contracting factor and relaxing factor, respectively, and their effects on the cardiovascular system have been studied in depth (11–14). Experimental studies have provided evidence that ET-1 may exert a bidirectional vascular tonicity effect either by vasodilation through enhancing nitric oxide production via ETB receptors located in endothelial cells, or by vasoconstriction via activating of ETA receptors prevalently located in the vascular smooth muscle cells and/or blunting ETB receptors. Moreover, findings from both clinical and animal studies are in favor of a direct role of ET-1 in the pathogenesis of hypertension (15). In addition, we and others have previously reported in animal and cellular experiments showing that ET-1 and ETA receptor probably played a significant role in the pathogenesis of IR and its association with hypertension (16–19). However, the link between ETS and IR-associated hypertension in clinical settings remain to be elucidated.
The diverse expression pattern of the ETS components is associated with a complex pharmacology and its counteracting physiological actions. New modulators of the ETS have been described: retinoic acid (20), leptin (21), prostaglandins and hypoxia (22). Endothelins can be considered as regulators working in paracrine and autocrine fashion in a variety of organs in different cellular types. They affect the physiology and pathophysiology of the liver, muscle, skin, adipose tissue, and reproductive tract. In contrast, the genetic determinants of ETS and their association to the metabolic CVD risks have not been extensively studied. Previous findings (23–30) on associations of some genetic polymorphisms of endothelins and their receptors with BP were controversy; moreover, few features of MetS were ascertained in these studies (30).
The present study was investigated to address the genetic polymorphism of ETA receptor in a large Chinese and Japanese cohort with hypertensive proband. We hypothesized that ETA receptor, a component of the ETS shown to play essential roles in the maintenance of renal homeostasis and basal vascular tone, may confer susceptibility to IR-related hypertension due to its multiple expression sites and complex modulators. We demonstrated that ETA receptor exon 6 T323C polymorphism (rs5333) was associated with IR and hyperinsulinemia in Chinese, and was related with high density lipoprotein (HDL)-cholesterol and atherogenic index in Japanese.
Materials and Methods
Study Subjects
Subjects in this current study were those for the Stanford Asian-Pacific Program in Hypertension and Insulin Resistance (SAPPHIRe) genetic study that aimed at mapping genetic loci for hypertension and IR in a well-characterized population regarding IR and its related metabolic traits. The study design and subject recruitment for SAPPHIRe were described in detail previously (31). In brief, SAPPHIRe, a family based and sibling-paired design, incorporated both concordant sibling pairs (i.e., two or more hypertensive full siblings) and discordant sib-pairs (i.e., hypertensive and hypo-normotensive siblings). Probands were hypertensive individuals with Chinese or Japanese descent. The BP values at the 80th percentile or more were considered as hypertension, which was equivalent to systolic BP of at least 160 or diastolic BP of at least 95 mmHg or taking two or more medications for high BP. The hypo-normotensive sib controls was a combination of subjects whose BP values below the bottom 30th percentile (i.e., hypotensive) and whose BP not qualified for hyper- or hypotensive-status (i.e., normotensive). In this particular study, we included in the analysis only the non-diabetic subjects (fasting plasma glucose <126 mg/dl) to reduce the confounding effect of hyperglycemia on the quantitative measurements of glucose homeostasis. Consequently, the study population genotyped comprised of 929 hypertension and 765 hypo-normotension. Written informed consent from all participants was obtained and the study protocol was approved by the Institutional Review Board of each field center.
Phenotypic and Biochemical Characterization
SAPPHIRe study incorporated physical examinations and anthropometric measurements, routine clinical laboratory tests, an oral glucose tolerance test (OGTT), and administration of an informative questionnaire to obtain biochemical data and phenotypic information on subjects. Measurement protocols for BP and anthropometry were previously reported in detail (5, 32). Body mass index (BMI) was calculated as body weight in kilograms divided by body height square in meters. A standard dose of 75-g glucose monohydrate dissolved in water was administered to the study subjects after an overnight fast. Venous blood was sampled before and 1- and 2-h after oral glucose challenge, and plasma was prepared by immediate centrifugation. Plasma glucose was measured by a glucose oxidase method and plasma insulin was determined using an enzymatic immunoassay. Concentrations of fasting plasma glucose and insulin obtained from OGTT were applied to calculate insulin-resistance homeostasis model assessment (HOMAIR), a surrogate index for insulin sensitivity validated to the reference method of the euglycemic hyperinsulinemic clamp (33), using the formula as described by Matthews et al. (34): fasting insulin (μU/mL) × fasting plasma glucose (mmol/L)/22.5. High HOMAIR scores denoted low insulin sensitivity, i.e., more insulin resistant. Three surrogate indices, fasting insulin level, HOMAIR score, area under curve of insulin concentration (AUCINS), were selected for assessing insulin sensitivity. Clinical chemistries included fasting concentrations on total cholesterol, triglycerides, HDL cholesterol were determined by automated enzymatic methods using colorimetry as end point. Low density lipoprotein (LDL)- and very low density lipoprotein (VLDL)-cholesterol were calculated by Friedewald equation (35). All lipid determinations were standardized with the centers for disease control and prevention/national heart, lung, and blood institute (CDC/NHLBI) lipid standardization program and further assured by participating in the College of American pathologists (CAP) survey.
Genotyping of ETA Receptor Gene Polymorphism
Using polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) analysis, we determined the polymorphism of exon 6 codon 323 T → C in the ETA receptor gene. The DNA fragment containing the polymorphism site was amplified using the forward primer 5′-TTTCTCACTTTCCTTTAGCG-3′ and the reverse primer 5′-ACCTAAGTAATTCACATCGG-3′. PCR amplifications were carried out in a final volume of 30 μL by 30 cycles at 94, 50, and 72°C each for 30 s and followed by a final extension step at 72°C for 10 min. Blank controls containing no genomic DNA were included in each run of PCR amplification to monitor contamination. Agarose gel electrophoresis on randomly selected PCR products was performed routinely to check if amplification succeeded. For subsequent RFLP analysis, the PCR product was mixed with 1U AflII and 1 μg/mL BSA in the appropriate buffer (New England Biolabs, Beverly, MA, USA) and incubated at 37°C for 3 h to ensure the complete enzyme digestion. The digested products were separated by electrophoresis on 3% agarose gel, visualized by ethidium bromide staining and analyzed under UV light. The person who assessed the genotype was blinded to the clinical data of the subjects from whom the samples originated. Direct DNA sequencing using ABI PRISM377 machine together with BigDye™ Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Applied Biosystems, Foster City, CA, USA) on randomly selected PCR products from each genotype was also performed to validate the polymorphisms.
The restriction enzyme AflII is specific for the sequence C/TTAAG. Digestion of PCR product with AflII resulted in two fragments of different sizes at 67 and 87 bp for the existence of C allele. However, the restriction site is lost in the presence of T variant, and therefore digestion products shown a single band at 154 bp. Thus, three genotypes TT, TC, and CC at codon 323 in exon 6 of ETA receptor gene were defined according to numbers of fragments by RFLP, depending on the presence of C allele as a restriction site for AflII.
Statistical Analyses
Data were expressed as the mean and the standard deviation (mean ± SD), unless otherwise specified. The ETA receptor genotype distributions and allele frequencies between subjects with different ethnicity and BP status were compared by Chi-square analyses. The Hardy–Weinberg equilibrium was also examined using the Chi-square test. The association of the ETA receptor gene variant with the variables of interest was assessed using mixed model regression analyses as described previously (36), with familial effect controlled by adjusting for family clustering effect. The mean differences of metabolic variables between siblings discordant for the genotypes within families were further evaluated by the delta method in which the potential family random effect was eliminated (5, 36). Specifically, due to genetic dependence of siblings, differences in the phenotypic traits with regard to genotypes were compared by paired t tests, while pooling the T allele carriers together, before and after adjusting for possible confounding variables including age, gender, and BMI. All analyses were performed using the SAS statistical software package (SAS Institute Inc., Cary, NC, USA), and tests were two-sided throughout and a p-value of <0.05 was considered statistically significant.
Results
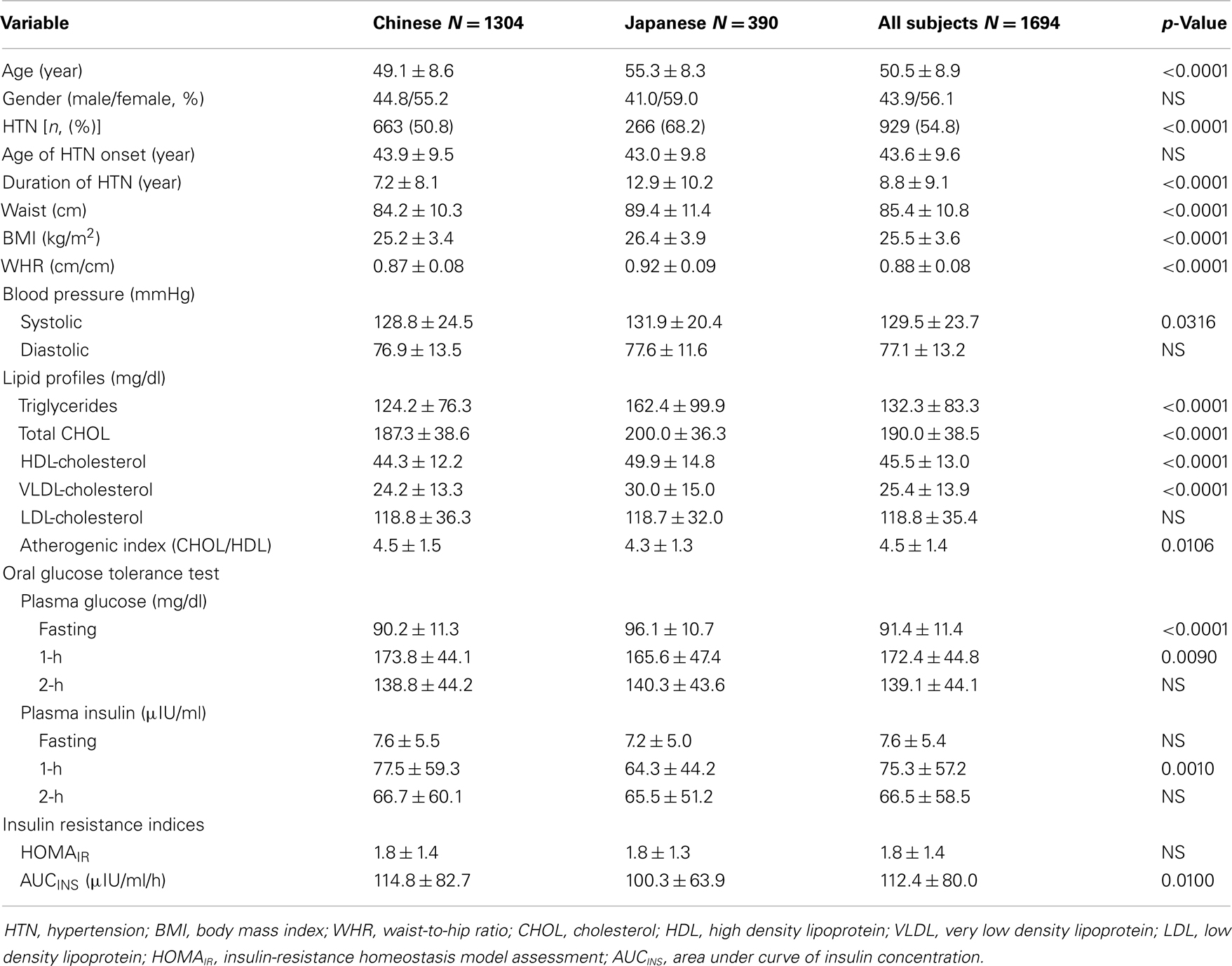
The general characteristics of the participants categorized by ethnicity are shown in Table 1. No significant differences were found in gender distribution and age onset for hypertension between the two ethnic groups. Chinese subjects were somewhat younger in age, had shorter duration in hypertension, and exhibited a smaller waist girth and lower BMI, and waist/hip ratio (WHR) than the Japanese. Japanese possessed elevated levels of blood lipids except for LDL-cholesterol and resulted in a decreased ratio of total- to HDL-cholesterol as compared to the Chinese. There were no consistent differences in the glucose homeostasis parameters such as fasting and postprandial glucose and insulin, and the surrogate insulin indices.
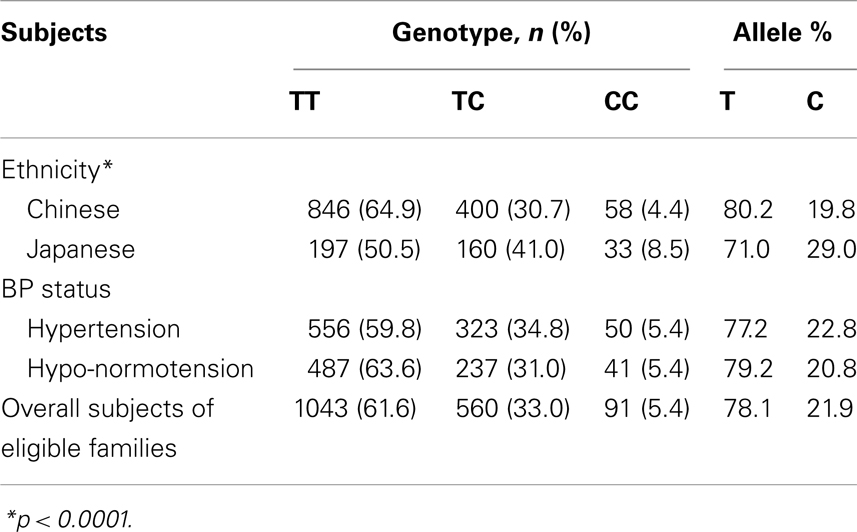
A total of 1694 biethnic sibling subjects (1304 Chinese and 390 Japanese) deriving from eligible families were analyzed. The genotype distributions in overall or each for Chinese and Japanese were all in compliance with the Hardy–Weinberg equilibrium (p = 0.166 for overall, p = 0.225 for Chinese, and p = 0.949 for Japanese). Significant differences (p < 0.0001) on genotype frequencies were observed between subjects with different ethnic backgrounds (Table 2). The C homozygote was present only in 4.4% of the Chinese but nearly doubled in 8.5% of the Japanese, whereas the more common T homozygote was at 64.9 and 50.5% for Chinese and Japanese, respectively. The relative frequencies of the T and C alleles of the ETA receptor gene in Chinese were 0.80 and 0.20, and in Japanese 0.71 and 0.29 (p < 0.0001). Neither genotype nor allele frequency was different with respect to gender. Moreover, there was no statistical significant difference in genotypes concerning the BP status in our studied subjects despite the anticipated role of ETA receptor in the regulation of cardiovascular complications.
Due to the significant ethnic differences, we next evaluated the association of genotypes of ETA receptor with the quantitative traits of the MetS in each individual ethnicity. Using a mixed model controlling for family effect, we observed association of ETA receptor genotypes with fasting and postprandial insulin concentrations, as well as HOMAIR and AUCINS indices in Chinese population before and after adjusting for confounding variables (Table 3). In the Japanese cohort, ETA receptor genotype was significantly associated with the VLDL- and HDL-cholesterol concentrations and consequently with the ratio of total to HDL cholesterol (Table 4). The adjustment for age, gender, and BMI did not affect the observations but strengthened the association seen in both Chinese and Japanese. To clearly illustrate the discrete nature of the association of ETA receptor genotype with MetS in Chinese and Japanese, comparisons on the mean values of the quantitative traits with significant differences were made and presented in Figure 1. Because the initial cohort was ascertained as a family study, the genetic association of ETA receptor alleles with variables of the MetS was further analyzed by delta method. The results were essentially unchanged and these analyses further confirmed the association of ETA receptor gene with the CVD risks of IR in Chinese (Data not shown).
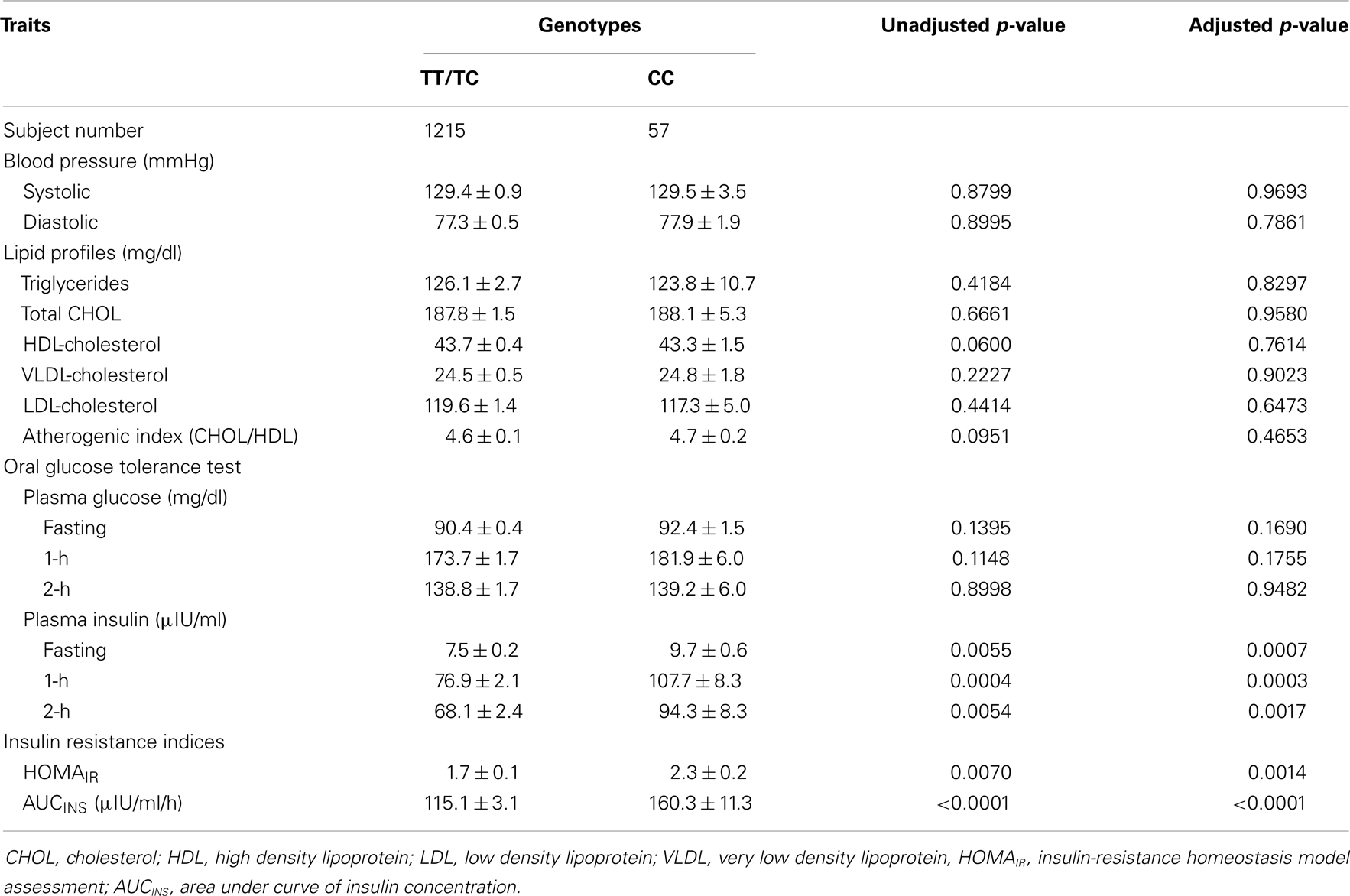
Table 3. Clinical characteristics of the Chinese siblings with discordant ETA receptor genotypes are compared with or without adjustment for age, gender, and BMI (Mean ± SD).
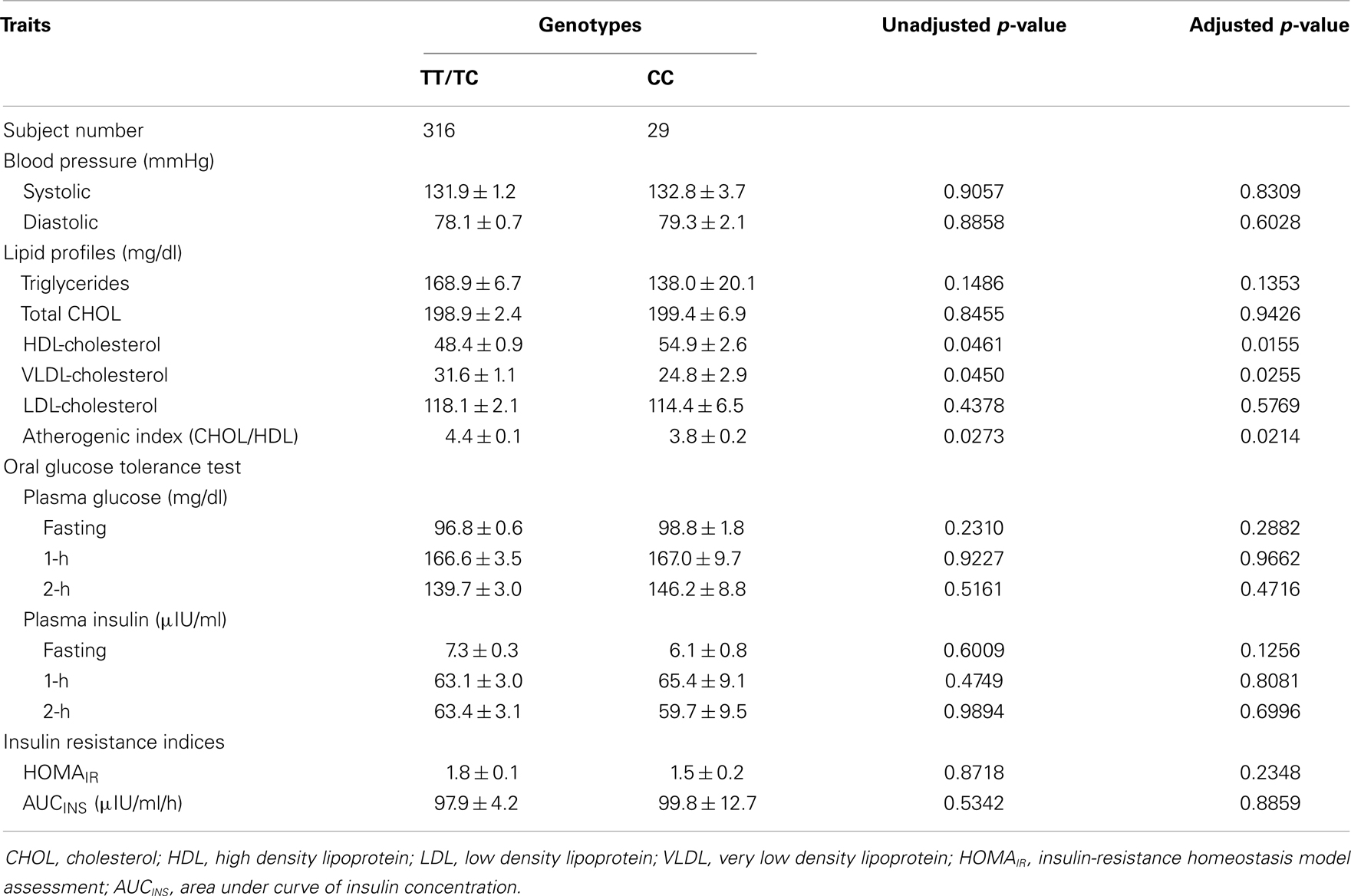
Table 4. Effect of the ETA receptor genotypes on clinical characteristics of Japanese discordant siblings before and after adjustment for age, gender, and BMI (Mean ± SD).
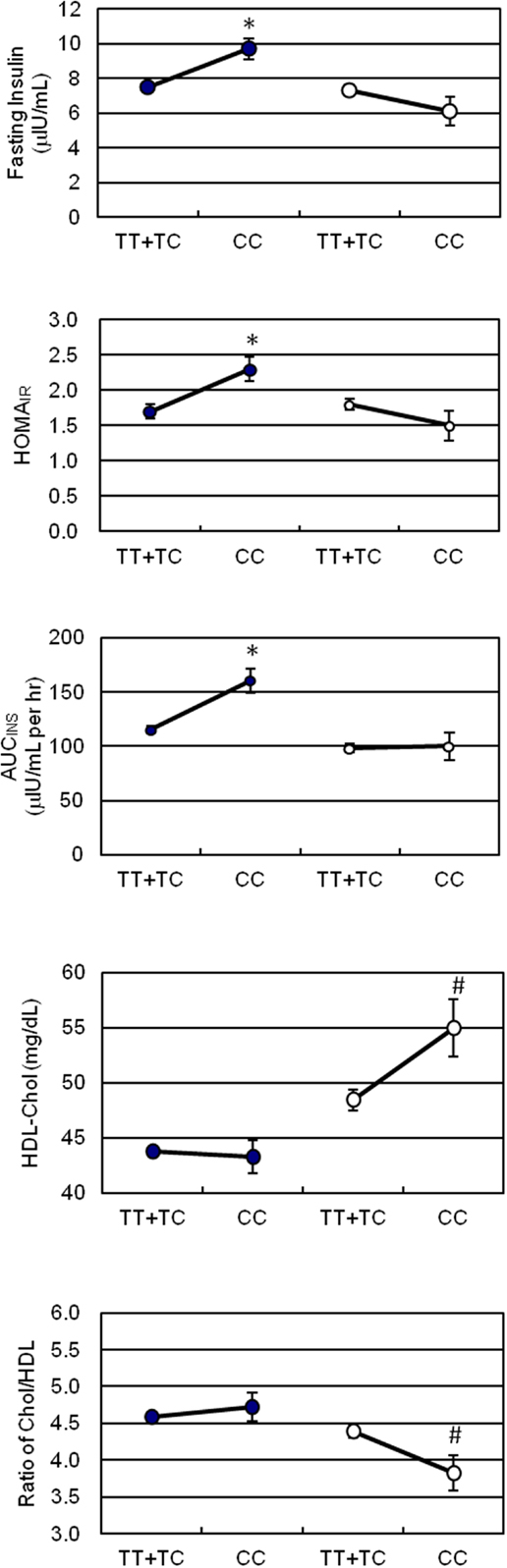
Figure 1. Effect of the ETA receptor genotypes on surrogate indices of insulin sensitivity, HDL-cholesterol, and atherogenic index. -∙- for Chinese, -∘- for Japanese. Vertical lines represent SD. Significant differences denote as *p < 0.01, #p < 0.05.
Since phenotypic expression of IR-associated hypertension is diverse and bounded, we further performed correlation analysis by treating the indices of insulin sensitivity as dependent variables, and the potential contributors including the ETA receptor genotype, BMI, age, et cetera as independent variables. By this approach we could predict if any attribute, while controlling for other covariates, is independently associated with changes in the indices at all. Results on the levels of significance in the pooled studied subjects are present in Table 5. The correlations among interrelating IR traits for example BMI and waist circumference showed significance as would be expected. In particular, we found that ETA receptor T323C genotype was not only significantly (p = 0.0012) but also independently from BMI, waist circumference, triglycerides and HDL-cholesterol concentrations, and gender correlated with the index of AUCINS. Additionally, we observed that waist circumference turned into a more significant predictor than WHR to HOMAIR and AUCINS in our studied population.
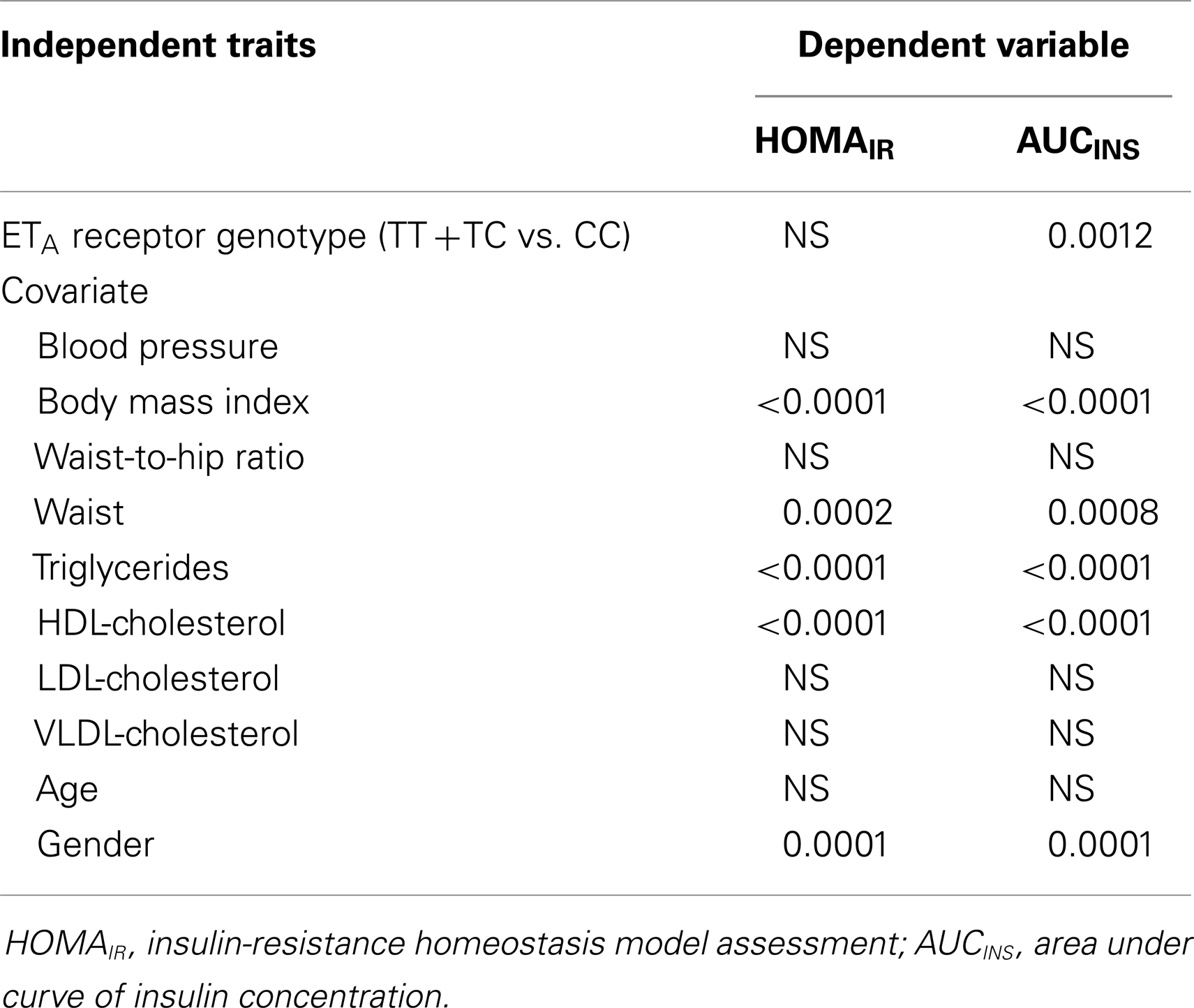
Table 5. Results on the p-values of correlation analysis using insulin sensitivity indices as dependent variables and insulin resistance related traits and genotypes as independent variables.
Discussion
Enhanced vascular gene expressions of ET-1 and ETA receptor, and an amelioration of hypertension via administration of selective ETA receptor antagonist observed previously in our fructose-induced hypertensive rats (18) and in comprehensive reviews (15, 37, 38) suggested that ETA receptor may play a role in the regulation of BP and consequently its antagonists may become one of the therapeutic strategies in clinical. To date, genetic variations and association studies of this putative hypertension gene (23–25, 29, 39) have been performed mostly in subjects of European ancestry but not in Asian origins excepta few literature regarding Japanese (26, 40). Therefore, we conducted this study to investigate whether the T323C polymorphism of the gene encoded for ETA receptor might be involved in the predisposition in IR-associated hypertension in a large Asian cohort ascertained via essential hypertension. The novel findings of this study are firstly the observation of noticeable lower minor allele frequency in our overall Asian subjects compared to multi-national population reported in gene database (http://www.genecards.org) at 0.22 and 0.35, respectively. Secondly, an ethnic difference between Chinese and Japanese we studied on the allele frequencies of the T323C variant was identified. Thirdly, the ETA receptor T323C polymorphism was associated with discrete features of the MetS in our two Asian populations.
Although significant differences on the genotypic and allelic frequencies of this T323C variant of the ETA receptor gene were observed between our subjects of Chinese and Japanese origin, no association of the T323C polymorphism with hypertension status was seen in either Asian ethnic group, i.e., neither systolic nor diastolic BP appeared to be influenced by the gene variant. Our findings supported and extended the prior observations in Caucasians (23, 24, 29) that there is no significant association of this solely gene polymorphism with BP. On the contrary, some other polymorphisms of ETS have been shown to be associated with BP either directly or in interaction with other genes/factors, for example C1363T in exon 8 of the ETA receptor on pulse pressure of normotension (24), G198T in exon 5 of the ET-1 gene together with BMI on BP (26), and A985G in 3′-untranslated region of the ET-2 gene on the severity of hypertension (41). Moreover, one association study reported a significant interaction of ETA receptor T323C and ET-1 138 insertion/deletion polymorphisms on diastolic BP in normotensive subjects (25).
Thus far, the predisposition of genes involved in ETS has been illustrated in the CVD including hypertension (23, 25, 26, 41), myocardial infarction (24), cardiomyopathy (39), and others (42). To our knowledge, no study has been conducted to determine the implication of the T323C polymorphism of ETA receptor gene in IR, an intermediate metabolic phenotype for hypertension (43). Thus, we further explored the association of the ETA receptor’s T323C polymorphism with the MetS related quantitative traits. A noteworthy finding in our study was that Chinese siblings who were carriers of the C homozygote, the rare allele of ETA receptor T323C polymorphism, manifested characteristics of IR, namely significantly higher levels of fasting insulin, HOMAIR score, and AUCINS at 29.3, 35.3, and 39.3%, respectively, when compared to their counterparts with TT/TC genotypes. Consistent with the observation, results from regression analysis revealed that the ETA receptor T323C genotype was a significant determinant for AUCINS independent from confounders in our pooled Asian population. Meanwhile, we observed that the CC genotype was significantly associated with an increased level of HDL cholesterol at 13.4% and consequently with a reduced ratio of Total/HDL cholesterol in Japanese. The current findings suggested a recessive effect of allele C in the ETA receptor T323C polymorphism on an adverse insulin response in Chinese and a favorite atherogenic index in Japanese.
Numerous studies provide convincing evidence that phenotypic aspects of MetS differ among ethnicities. Since MetS involves a constellation of highly intercorrelated quantitative phenotypes, factor analysis has been applied in attempts to simplify the risk factors and to discover the underlying pathophysiological mechanisms (44). Evaluation of the MetS in multi-ethnic groups including the SAPPHIRe participants has shown that the identified factor pattern was very similar in African Americans, Hispanics, Whites, and Japanese, but not entirely so in Chinese (45). The common factor, interpreted as obesity, explained the largest and comparable portion of the overall variance in both the Chinese and the Japanese though the factor loadings themselves were substantially different in magnitude between the two ethnic groups. On the other hand, insulin level was the major component of the second factor and in together with other variables contributed nearly 17% of the variance in Chinese, but insulin was merely an intermediate component for Japanese. Conversely, the lipid factor, characterized by a negative loading for HDL cholesterol, was more prominent in Japanese than in Chinese. These results suggest ethnic variability in susceptibility to the specific risk factors of the MetS, which strengthens our findings that the T323C polymorphism was associated with not only the distinct but the more prominent phenotype for each ethnic group. However, the mechanisms that this ETA receptor T323C polymorphism might exert its opposite influence on MetS in different racial people are not yet elucidated in this study.
Human genome contains a single copy of the ETA receptor gene which spans over 64 kb on chromosome 4q31.22 and comprises eight exons and seven intros. Though examining sole single nucleotide polymorphism might have limitations of interpretation, study on combination of four common haplotype blocks merely revealed mild effect on the risk of hypertension (29). It should be noted that this variant located within codon 323 in the sixth exon of ETA receptor gene is a silent polymorphism (CAT → CAC, His → His). As a silent polymorphism which nucleotide substitution on the third base of the codon does not alter the amino acid sequence of the receptor protein, it is unlikely that the variant itself is involved in the pathophysiology of MetS. One possibility for this silent polymorphism exerts its influence is that it is in linkage disequilibrium with a nearby causal variant which has not yet been identified. In addition, the wobble base pair is a fundamental unit for RNA secondary and tertiary structures and therefore may have functional consequences. Without further RNA studies, the possibility of unrecognized alleles, disrupting exonic splicing enhancers, and/or influencing gene transcription cannot be excluded. Although BP and IR have been shown to cosegregate in Hispanic families with a hypertensive proband (6), no correlation was observed between BP and insulin sensitivity index in our Asian subjects. The lack of relationship may partially be due to the fact that the measurement of BP of the hypertensive individuals in SAPPHIRe study was confounded by medication since majority of our hypertensive subjects were on antihypertensive treatment. In addition, our BP measurements feasible for large-scale study may not be sensitive enough as 24 h ambulatory recordings, a necessary tool to identify a relationship between BP and IR within the hypertensive range suggested by Nilsson et al. (46).
Beyond candidate genes in ETS for the predisposition of IR-associated hypertension, the notion of using biological candidate genes for interrelating traits as genetic markers for the features of MetS and/or diabetes has also been applied in other studies regardless successes or not. For example, Bayoglu et al. identified polymorphism rs10757278 on chromosome 9p21 which is risk locus for coronary artery disease may confer increased risk for MetS in Turkish people (47). Ned et al. merely found marginal association between inflammation-related genes and the susceptibility of albuminuria (48), and Moon et al. on the other hand, were unable to relate polymorphisms of pyruvate dehydrogenase kinase four gene, a mitochondrial kinase attributing to hyperglycemia, with MetS or diabetes in Korean (49). One example of linking candidate gene deletion in mice with a polymorphism association study in patients with hypertension has shown that rs10491334 of calcium/calmodulin-dependent kinase IV gene may play a pivotal role in hypertension via the regulation of endothelial nitric oxide synthase (50). Another example of candidate genotype polymorphism in a small sample sized population with a replication of another same ethnic population revealed a significant association of the platelet antigen variant of glycoprotein IIIa (PIA1/A2) with stroke in a sub-group of high risk hypertension patients (51). Moreover, recent genome-wide association studies (GWAS) have identified susceptibility loci for various component traits of MetS including lipid/lipoprotein profiles (52, 53) and obesity (54), or for MetS as an entity in European ancestry (52, 55) and Indian Asian (56). Given these research findings affirm the multifactorial aetiology and multigenic nature of MetS. However, these GWAS approach in combination with either bi- or multi-variate haplotypes done in Asian ethnic’s remains to be seen (55). A trans-ethnic GWAS with fine mapping of these genotypes with optimal sample size may find population specific associations (57) and a similar replication study conducted in a separate Chinese–Japanese population may be advised in further works (58).
In conclusion, we have successfully related the T323C polymorphism of ETA receptor gene to features of MetS in this study though no association between the gene variant and BP is reassuring in our hypertensive subjects of Chinese and Japanese origin. Our findings can be considered as the first time to be related directly to IR, and also the first in Asian populations despite its dissociated effects on different ethnicities. If reproducible in other independent ethnicities, our results reported herein suggest that the polymorphism of ETA receptor gene may serve as one of the independent determinants for manifested IR which is often a prelude to hypertension. Nevertheless, the functional significance of this particular gene variant, the inference on haplotype effects, and the gene–gene/gene-environment interactions remain to be elucidated. Determinants of CVD risk in diabetes beyond hyperglycemia has been an important issue to address lately, since intensive control of plasma sugar alone is often insufficient to reduce the incidence of major CVD in these patients (59). Components of MetS and many other CVD risk factors including those with pro-inflammatory, pro-thrombotic, and/or oxidative stress effects all need to be considered and tackled in addition to control of hyperglycemia. Among them, ET-1, the key member of ETS, by playing multiple roles relating IR, hypertension, as well as proliferative and pro-inflammatory effects may be a perspective target for drugs discovery and/or other intervention modalities useful not only for management but prevention of MetS, diabetes mellitus and related CVD (38).
Authors Contribution
Guarantor of integrity of the study and study concepts: Low-Tone Ho. Definition of intellectual content: Low-Tone Ho, John Grove, Thomas Quertermous. Literature research: Yung-Pei Hsu, Chi-Chung Juan, Low-Tone Ho. Clinical studies: Low-Tone Ho, Chih-Tai Ting, Kuang-Chung Shih, Lee-Ming Chuang, Kamal Masaki. Genotyping experiments: Yung-Pei Hsu, Chi-Chung Juan. Data acquisition: Chin-Fu Hsiao, Ming-Wei Lin, Yung-Pei Hsu, Chi-Chung Juan. Statistical analysis: Chin-Fu Hsiao, Ming-Wei Lin, Shu-Chiung Chiang. Manuscript preparation: Yung-Pei Hsu, Low-Tone Ho. Manuscript editing and revise: Low-Tone Ho, Yii-Der I. Chen.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by grants from the Taipei Veterans General Hospital (VGH890410-14, VGH 92-329) and the National Science Council ROC (NSC92-2314-B075-067, NSC93-2314-B075-039). We thank Ms. Ya-Hui Ko for technical support and Ms. Shu-Hsia Lin for help with the administration work. Gratitude is expressed to all study participants. We are also indebted to Prof. Jerome I. Rotter, University of California Los Angeles, for his inspiration and revision of the manuscript. The authors are members of the SAPPHIRe study group and like to appreciate multiple Grants’ supports including NIH of USA, NSC and NHRI of Taiwan, and the affiliate institutes.
Abbreviations
AUCINS, area under curve of insulin concentration; BMI, body mass index; BP, blood pressure; CAP, College of American pathologists; CDC/NHLBI, centers for disease control and prevention/national heart, lung, and blood institute; CVD, cardiovascular disease; ET-1, endothelin-1; ETA receptor, endothelin type A receptor; ETB receptor, endothelin type B receptor; ETS, endothelin system; GWAS, genome-wide association studies; HDL, high density lipoprotein; HOMAIR, insulin-resistance homeostasis model assessment; IR, insulin resistance; LDL, low density lipoprotein; MetS, metabolic syndrome; OGTT, oral glucose tolerance test; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism; SAPPHIRe, Stanford Asian-Pacific program in hypertension and insulin resistance; VLDL, very low density lipoprotein; WHR, waist/hip ratio.
References
1. Sarafidis PA, Nilsson PM. The metabolic syndrome: a glance at its history. J Hypertens (2006) 24:621–6. doi: 10.1097/01.hjh.0000217840.26971.b6
2. Hunt KJ, Heiss G, Sholinsky PD, Province MA. Familial history of metabolic disorders and the multiple metabolic syndrome: the NHLBI family heart study. Genet Epidemiol (2000) 19:395–409. doi:10.1002/1098-2272(200012)19:4<395::AID-GEPI10>3.0.CO;2-3
3. Srinivasan SR, Frontini MG, Berenson GS. Longitudinal changes in risk variables of insulin resistance syndrome from childhood to young adulthood in offspring of parents with type 2 diabetes: the Bogalusa Heart Study. Metabolism (2003) 52:443–50. doi:10.1053/meta.2003.50065
4. Chen HS, Hwu CM, Kwok CF, Shih KC, Hsiao LC, Lee SH, et al. Insulin sensitivity in normotensive offspring of hypertensive parents. Horm Metab Res (2000) 32:110–4. doi:10.1055/s-2007-978601
5. Wu KD, Hsiao CF, Ho LT, Sheu WH, Pei D, Chuang LM, et al. Clustering and heritability of insulin resistance in Chinese and Japanese hypertensive families: a Stanford-Asia Pacific Program in Hypertension and Insulin Resistance Sibling Study. Hypertens Res (2002) 25:529–36. doi:10.1291/hypres.25.529
6. Xiang AH, Azen SP, Raffel LJ, Tan S, Cheng LS, Diaz J, et al. Evidence for joint genetic control of insulin sensitivity and systolic blood pressure in Hispanic families with a hypertensive proband. Circulation (2001) 103:78–83. doi:10.1161/01.CIR.103.1.78
7. Guo X, Cheng S, Taylor KD, Cui J, Hughes R, Quinones MJ, et al. Hypertension genes are genetic markers for insulin sensitivity and resistance. Hypertension (2005) 45:799–803. doi:10.1161/01.HYP.0000154786.17416.ea
8. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature (1988) 332:411–5.
9. Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A (1989) 86:2863–7.
10. Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev (1994) 46:325–415.
11. Perez del Villar C, Garcia Alonso CJ, Feldstein CA, Juncos LA, Romero JC. Role of endothelin in the pathogenesis of hypertension. Mayo Clin Proc (2005) 80:84–96. doi:10.4065/80.1.84
12. Penna C, Rastaldo R, Mancardi D, Cappello S, Pagliaro P, Westerhof N, et al. Effect of endothelins on the cardiovascular system. J Cardiovasc Med (2006) 7:645–52. doi:10.2459/01.JCM.0000242996.19077.ba
13. Rubio AR, Morales-Segura MA. Nitricoxide, an iceberg in cardiovascular physiology: far beyond vessel tone control. Arch Med Res (2004) 35:1–11. doi:10.1016/j.arcmed.2003.09.011
14. Esper RJ, Nordaby RA, Vilarino JO, Paragano A, Cacharron JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol (2006) 5:4. doi:10.1186/1475-2840-5-4
15. Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol (2005) 43:19–29. doi:10.1016/j.vph.2005.03.004
16. Chou YC, Perng JC, Juan CC, Jang SY, Kwok CF, Chen WL, et al. Endothelin-1 inhibits insulin-stimulated glucose uptake in isolated rat adipocytes. Biochem Biophys Res Commun (1994) 202:688–93.
17. Juan CC, Fang VS, Huang YJ, Kwok CF, Hsu YP, Ho LT. Endothelin-1 induces insulin resistance in conscious rats. Biochem Biophys Res Commun (1996) 227:694–9.
18. Juan CC, Fang VS, Hsu YP, Huang YJ, Hsia DB, Yu PC, et al. Overexpression of vascular endothelin-1 and endothelin-A receptors in a fructose-induced hypertensive rat model. J Hypertens (1998) 16:1775–82.
19. Juan CC, Fang VS, Kwok CF, Perng JC, Chou YC, Ho LT. Exogenous hyperinsulinemia causes insulin resistance, hyperendothelinemia, and subsequent hypertension in rats. Metabolism (1999) 48:465–71.
20. Yokota J, Kawana M, Hidai C, Aoka Y, Ichikawa K, Iguchi N, et al. Retinoic acid suppresses endothelin-1 gene expression at the transcription level in endothelial cells. Atherosclerosis (2001) 159:491–6. doi:10.1016/S0021-9150(01)00530-5
21. Savoia C, Schiffrin EL. Significance of recently identified peptides in hypertension: endothelin, natriuretic peptides, adrenomedullin, leptin. Med Clin North Am (2004) 88:39–62. doi:10.1016/S0025-7125(03)00122-6
22. Gupte SA, Zias EA, Sarabu MR, Wolin MS. Role of prostaglandins in mediating differences in human internal mammary and radial artery relaxation elicited by hypoxia. J Pharmacol Exp Ther (2004) 311:510–8. doi:10.1124/jpet.104.070995
23. Stevens PA, Brown MJ. Genetic variability of ET-1 and ETA receptor genes in essential hypertension. J Cardiovasc Pharmacol (1995) 26:S9–12.
24. Nicaud V, Poirier O, Behague I, Herrmann SM, Mallet C, Troesch A, et al. Polymorphisms of the Endothelin-A and -B receptor genes in relation to blood pressure and myocardial infarction: the Etude Cas-Temoins sur l’Infarctus du Myocarde (ECTIM) Study. Am J Hypertens (1999) 12:304–10.
25. Brown MJ, Sharma P, Stevens PA. Association between diastolic blood pressure and variants of the endothelin-1 and endothelin-2 genes. J Cardiovasc Pharmacol (2000) 35:S41–3. doi:10.1097/00005344-200000002-00010
26. Jin JJ, Nakura J, Wu Z, Yamamoto M, Abe M, Tabara Y, et al. Association of endothelin-1 gene variant with hypertension. Hypertension (2003) 41:163–7. doi:10.1161/01.HYP.0000043680.75107.CF
27. Ormezzano O, Poirier O, Mallion JM, Nicaud V, Amar J, Chamontin B, et al. A polymorphism in the endothelin-A receptor gene is linked to baroreflex sensitivity. J Hypertens (2005) 23:2019–26. doi:10.1097/01.hjh.0000184748.49189.36
28. Benjafield AV, Katyk K, Morris BJ. Association of EDNRA, but not WNK4 or FKBP1B, polymorphisms with essential hypertension. Clin Genet (2003) 64:433–8. doi:10.1034/j.1399-0004.2003.00148.x
29. Rahman T, Baker M, Hall DH, Avery PJ, Keavney B. Common genetic variation in the type A endothelin-1 receptor is associated with ambulatory blood pressure: a family study. J Hum Hypertens (2008) 22:282–8. doi:10.1038/sj.jhh.1002322
30. Wiltshire S, Powell BL, Jennens M, McCaskie PA, Carter KW, Palmer LJ, et al. Investigating the association between K198N coding polymorphism in EDN1 and hypertension, lipoprotein levels, the metabolic syndrome and cardiovascular disease. Hum Genet (2008) 123:307–13. doi:10.1007/s00439-008-0481-0
31. Ranade K, Hsuing AC, Wu KD, Chang MS, Chen YT, Hebert J, et al. Lack of evidence for an association between alpha-adducin and blood pressure regulation in Asian population. Am J Hypertens (2000) 13:704–9. doi:10.1016/S0895-7061(00)00238-7
32. Hwu CM, Hsaio CF, Sheu WH, Pei D, Tai TY, Quertermous T, et al. Sagittal abdominal diameter is associated with insulin sensitivity in Chinese hypertensive patients and their siblings. J Hum Hypertens (2003) 17:193–8. doi:10.1038/sj.jhh.1001532
33. Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care (2000) 23:57–63. doi:10.2337/diacare.23.1.57
34. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28:412–9.
35. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem (1972) 18:499–502.
36. Chuang LM, Hsiung CA, Chen YD, Ho LT, Shen WH, Pei D, et al. Sibling-based associated study of the PPARgamma 2 Pro12Ala polymorphism and metabolic variables in Chinese and Japanese hypertension families: a SAPPHIRe study. J Mol Med (2001) 79:656–64. doi:10.1007/s001090100255
37. Motte S, McEntee K, Naeije R. Endothelin receptor antagonists. Pharmacol Ther (2006) 110:386–414. doi:10.1016/j.pharmthera.2005.08.012
38. Ram CV. Possible therapeutic role of endothelin antagonists in cardiovascular disease. Am J Ther (2003) 10:396–400. doi:10.1097/00045391-200311000-00004
39. Herrmann S, Schmidt-Petersen K, Pfeifer J, Perrot A, Bit-Avragim N, Eichhorn C, et al. A polymorphism in the endothelin-A receptor gene predicts survival in patients with idiopathic dilated cardiomyopathy. Eur Heart J (2001) 22:1948–53. doi:10.1053/euhj.2001.2626
40. Ishikawa K, Funayama T, Ohtake Y, Kimura I, Ideta H, Nakamoto K, et al. Association between glaucoma and gene polymorphism of endothelin type A receptor. Mol Vis (2005) 11:431–7.
41. Sharma P, Hingorani A, Jia H, Hopper R, Brown MJ. Quantitative association between a newly identified molecular variant in the endothelin-2 gene and human essential hypertension. J Hypertens (1999) 17:1281–7.
42. Ellis KL, Pilbrow AP, Potter HC, Frampton CM, Doughty RN, Whalley GA, et al. Association between endothelin type A receptor haplotypes and mortality in coronary heart disease. Pers Med (2012) 9:341–9. doi:10.2217/pme.12.10
43. Wang WC, Chang IS, Chang CH, Curb D, Ho LT, Hsiung CA. Incorporating endophenotypes into allele-sharing based linkage tests. Genet Epidemiol (2006) 30:133–42. doi:10.1002/gepi.20133
44. Lawlor DA, Ebrahim S, May M, Smith GD. (Mis)use of factor analysis in the study of insulin resistance syndrome. Am J Epidemiol (2004) 159:1013–8. doi:10.1093/aje/kwh150
45. Kraja AT, Rao DC, Weder AB, Mosley TH, Tumer ST, Hsiung CA, et al. An evaluation of the metabolic syndrome in a large multi-ethnic study: the Family Blood Pressure Program. Nutr Metab (2005) 2:17. doi:10.1186/1743-7075-2-17
46. Nilsson PM, Lind L, Andresson PE, Hanni A, Berne C, Baron J, et al. On the use of ambulatory blood pressure recordings and insulin sensitivity in support of the insulin-hypertension hypothesis. J Hypertens (1994) 12:965–9.
47. Bayoglu B, Cakmak HA, Yuksel H, Can G, Karadag B, Ulutin T, et al. Chromosome 9p21 rs10757278 polymorphism is associated with the risk of metabolic syndrome. Mol Cell Biochem (2013) 379:77–85. doi:10.1007/s11010-013-1629-3
48. Ned RM, Yesupriya A, Imperatore G, Smelser DT, Moonesinghe R, Chang M, et al. Inflammation gene variants and susceptibility to albuminuria in the U.S. population: analysis in the Third National Health and Nutrition Examination Survey (NHANES III), 1991-1994. BMC Med Genet (2010) 11:155. doi:10.1186/1471-2350-11-155
49. Moon SS, Lee JE, Lee YS, Kim SW, Jeoung NH, Lee IK, et al. Association of pyruvate dehydrogenase kinase 4 gene polymorphisms with type 2 diabetes and metabolic syndrome. Diabetes Res Clin Pract (2012) 95:230–6. doi:10.1016/j.diabres.2011.09.035
50. Santulli G, Cipolletta E, Sorriento D, Del Giudice C, Anastasio A, Monaco S, et al. CaMK4 gene deletion induces hypertension. J Am Heart Assoc (2012) 1:e001081. doi:10.1161/JAHA.112.001081
51. Lanni F, Santulli G, Izzo R, Rubattu S, Zanda B, Volpe M, et al. The PlA1/A2 polymorphism of glycoprotein IIIa and cerebrovascular events in hypertension: increased risk of ischemic stroke in high-risk patients. J Hypertens (2007) 25:551–6. doi:10.1097/HJH.0b013e328013cd67
52. Kristiansson K, Perola M, Tikkanen E, Kettunen J, Surakka I, Havulinna AS, et al. Genome-wide screen for metabolic syndrome susceptibility loci reveals strong lipid gene contribution but no evidence for common genetic basis for clustering of metabolic syndrome traits. Circ Cardiovasc Genet (2012) 5:242–9. doi:10.1161/CIRCGENETICS.111.961482
53. Chasman DI, Paré G, Mora S, Hopewell JC, Peloso G, Clarke R, et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet (2009) 5:e1000730. doi:10.1371/journal.pgen.1000730
54. Fall T, Ingelsson E. Genome-wide association studies of obesity and metabolic syndrome. Mol Cell Endocrinol (2012). doi:10.1016/j.mce.2012.08.018
55. Kraja AT, Vaidya D, Pankow JS, Goodarzi MO, Assimes TL, Kullo IJ, et al. A bivariate genome-wide approach to metabolic syndrome. Diabetes (2011) 60:1329–39. doi:10.2337/db10-1011
56. Zabaneh D, Balding DJ. A genome-wide association study of the metabolic syndrome in Indian Asian men. PLoS One (2010) 5:e11961. doi:10.1371/journal.pone.0011961
57. Wu Y, Waite LL, Jackson AU, Sheu WHH, Buyske S, Absher D, et al. Trans-ethnic fine-mapping of lipid loci identifies population-specific signals and allelic heterogeneity that increases the trait variance explained. PLoS Genet (2013) 9:e1003379. doi:10.1371/journal.pgen.1003379
Keywords: ETA receptor, T323C polymorphism, metabolic syndrome, hypertension
Citation: Ho L-T, Hsu Y-P, Hsiao C-F, Ting C-T, Shih K-C, Chuang L-M, Masaki K, Grove J, Quertermous T, Juan C-C, Lin M-W, Chiang S-C and Chen Y-DI (2013) Endothelin type A receptor genotype is a determinant of quantitative traits of metabolic syndrome in Asian hypertensive families: a SAPPHIRe study. Front. Endocrinol. 4:172. doi: 10.3389/fendo.2013.00172
Received: 14 September 2013; Paper pending published: 30 September 2013;
Accepted: 25 October 2013; Published online: 28 November 2013.
Edited by:
Gaetano Santulli, Columbia University, USAReviewed by:
Undurti Narasimha Das, UND Life Sciences, USACarmine Del Giudice, University of Naples Federico II, Italy
Copyright: © 2013 Ho, Hsu, Hsiao, Ting, Shih, Chuang, Masaki, Grove, Quertermous, Juan, Lin, Chiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Low-Tone Ho, Department of Medical Research and Education, Taipei Veterans General Hospital, No. 201 Shih-Pai Road Section 2, Taipei 11217, Taiwan e-mail: ltho@vghtpe.gov.tw
 Low-Tone Ho
Low-Tone Ho Yung-Pei Hsu
Yung-Pei Hsu Chin-Fu Hsiao6
Chin-Fu Hsiao6 Lee-Ming Chuang
Lee-Ming Chuang