- Lab for Bone Metabolism, Xi'an Key Laboratory of Special Medicine and Health Engineering, Key Lab for Space Biosciences and Biotechnology, Research Center for Special Medicine and Health Systems Engineering, NPU-UAB Joint Laboratory for Bone Metabolism, School of Life Sciences, Northwestern Polytechnical University, Xi'an, China
Immune imbalance caused bone loss. Osteoimmunology is emerging as a new interdisciplinary field to explore the shared molecules and interactions between the skeletal and immune systems. In particular, T lymphocytes (T cells) play pivotal roles in the regulation of bone health. However, the roles and mechanisms of T cells in the treatment of osteoporosis are not fully understood. The present review aims to summarize the essential regulatory roles of T cells in the pathophysiology of various cases of osteoporosis and the development of T cell therapy for osteoporosis from osteoimmunology perspective. As T cell-mediated immunomodulation inhibition reduced bone loss, there is an increasing interest in T cell therapy in an attempt to treat osteoporosis. In summary, the T cell therapy may be further pursued as an immunomodulatory strategy for the treatment of osteoporosis, which can provide a novel perspective for drug development in the future.
Introductions
Osteoporosis is a prevailing metabolic bone disease in both men > 50 years and postmenopausal women, which increases bone fragility and may further result in bone fractures, thus significantly leading to serious health problems for patients (1). Worldwide, nearly 200 million people are diagnosed with osteoporosis annually, even leading to almost 9 million osteoporotic fractures (2). In the US, it was approximately 53.6 million of the adult population of years > 50 who suffered from osteoporosis and low bone mass (54% of the population) (3). In fact, osteoporosis patients not only suffer from the enormous pain and disability but also bring a huge economic burden for patients and their families. In the US, it has been estimated that the financial costs associated with bone fractures will reach $25.3 billion by the end of 2025 (4).
In traditional view, osteoporosis was considered as the imbalance of bone remodeling between osteoclasts and osteoblasts (5). Recently, the immune system was reported to regulate the bone system, which promoted the emergence of interdisciplinary field of osteoimmunology (6–9). The immune and bone systems share the same microenvironment. The immune system regulates osteocytes by the secretion of inflammatory factors and related ligand, which further affects bone formation and bone resorption (8, 10). T cells, B cells, and cytokines are important regulatory factors in the bone resorption. Among them, T cells play pivotal roles in the regulation of bone remodeling (11, 12). The osteoclast differentiation was enhanced, and the bone mineral density was decreased in the nude mice (T cell deficient), which was due to the immune imbalance of T cells promoting osteoclast differentiation and bone resorption (13, 14). In pathophysiological condition, activated T cells secreted multiple inflammatory factors and related ligands such as TNF-α, IL-1, IL-6, IL-17, and CD40L, which enhanced bone resorption and disrupted bone balance, resulting in bone loss (15, 16). Th17 cells are mainly involved in inducing bone resorption (osteoclastogenesis) (17), while Treg cells are major suppressors of bone loss (18, 19) by inhibiting differentiation of monocytes into osteoclasts (17, 20, 21). These reports indicated that immune imbalance promoted osteoclast differentiation, further leading to bone loss. However, the roles of T cells in osteoporosis and the underlying mechanism of T cells in the regulation of bone system are still unclear.
Recently, there is an increasing interest in immune therapies especially T cell therapies for the treatment of osteoporosis (22). For example, antiretroviral therapy worsens HIV-induced bone loss (23), which may be an important future approach to treat osteoporosis in human. That is because T cell reconstitution induces RANKL and TNFα production by B-cells and/or T-cells, which further enhancing bone resorption and bone loss. T cell therapy became the effective strategy for the treatment of osteoporosis. For example, RANKL/RANK inhibition may be an attractive approach for the treatment of postmenopausal osteoporosis (24). Sclareol is a natural product (initially isolated from the leaves and flowers of Salvia sclarea) with immune regulation and anti-inflammatory effects, and it prevents ovariectomy-induced bone loss in vivo and inhibits osteoclastogenesis in vitro via suppressing NF-κB and MAPK/ERK signaling pathways (25). Thus, it will be essential to develop T cell therapy that may be a huge potential for the treatment of osteoporosis in future clinical applications.
Herein, we briefly highlight the roles of T cells in various types of osteoporosis and uncover novel mechanisms of osteoimmunology, which provides new insight for clinical implications in the treatment of osteoporosis. Nonetheless, the underlying mechanisms of bone-immune interactions need to be further dissected, and an accumulative evidence continues to be made in favor of regulation roles of immune cells in osteoporosis. Most importantly, the T cell therapy may represent a suitable and potential approach to reinstate aberrant bone remodeling in the bone metabolism diseases.
Osteoimmunology and The Regulation of T Cell Cytokines in Osteoporosis
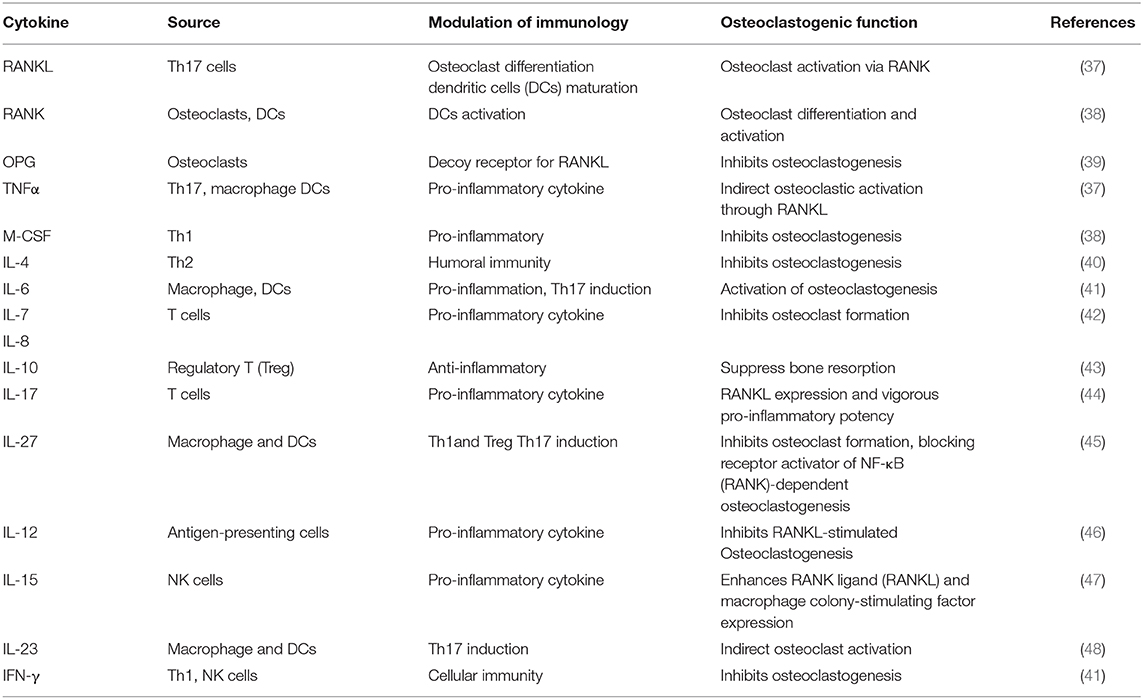
Osteoimmunology is the intricate interaction between the immune system and the bone system (6–9). The RANKL/RANK/OPG pathway is essential for the differentiation of bone-resorbing osteoclasts and immune regulation (26, 27). Activated T cells directly produce RANKL, which further stimulates osteoclast formation (28, 29). RANKL and RANK were identified as key factors in the mediation of bone remodeling, especially in the osteoclast formation (29, 30). Furthermore, the activated RANK facilitated the expression of tumor necrosis factor (TNF) receptor-associated factors (TRAFs), such as TRAF6, which leads to osteoclast differentiation (31, 32). In OVX mice, the low-dose RANKL of CD8+ Treg cells decreased the expression of inflammatory and osteoclastogenic cytokines, thus suppressing bone resorption (33). Multiple cytokines produced by T cell including interleukin (IL)-12, IL-17, IL-18, and TNF-α were involved in RANK signaling, and thus play essential roles in regulating osteoclastogenesis and osteoclast differentiation (34). In addition, activated T cells suppress osteoclast differentiation by the antiviral cytokine IFN-γ (35). Various inflammatory cytokines were necessary and sufficient for bone metabolism (11). IL-17A also upregulates the expression of RANK, thus promoting the osteoclastogenic activity of RANKL (36). All these studies indicated that T cell cytokines play essential roles in osteoporosis, which may be the potential targets for the treatment of osteoporosis. Various T cell cytokines are listed in Table 1.
The T Cells in The Regulation of Various Osteoporosis
T cells perform a dual role in the regulation of bone remodeling: resting T cells protect osteoclasts from bone resorption, and activated T cells actively regulate the osteoclasts generation. This review aims to summarize the regulatory roles of T cells in various types of osteoporosis such as chronic inflammation-induced osteoporosis, senile osteoporosis, estrogen deficiency-induced osteoporosis, parathyroid hormone (PTH)-induced osteoporosis, and glucocorticoid-induced osteoporosis (GIO).
The Regulatory Roles of T Cells in Chronic Inflammation-Induced Osteoporosis
Osteoporosis commonly occurred in various chronic inflammatory diseases, such as rheumatic arthritis (RA), gout, psoriatic disease, osteoarthritis, and axial spondylarthritis and even leads to functional disability and increased mortality (49–52). It is interesting to note that Tregs play pivotal roles in inflammation-induced bone loss by inhibiting the functions of Th17 cells (19, 53). In particular, Foxp3+ Treg cells play an indispensable role in bone and hematopoietic homeostasis acting on osteoclast development and function (54). In addition, in inflammation condition, the expression of nuclear factor of activated T cells cytoplasmic 1 (NFATc1), as well as by inflammatory cytokines such as TNFα, IL-1β, and IL-6 was induced and produced to promote osteoclast differentiation mediated by the RANKL-RANK and calcium signaling (8). INFγ, the main Th1 cytokine, can strongly inhibit osteoclast differentiation in vitro through the proteasomal degradation of TRAF6, indicating that T cells regulate osteoclastogenesis (28). The T cell subset, Tregs, also suppresses osteoclast formation and bone resorbing in vitro (53). CTLA-4 is the most essential regulator in the Treg-mediated inhibition of osteoclast differentiation, whereas the major cytokines of Tregs-TGFβ and IL-10 do not possess any essential roles (53). All these studies suggest that T cells and their related cytokine play pivotal roles in the regulation of osteoporosis, and they may be the potential therapeutic targets for bone loss.
Generally, chronic inflammatory diseases are associated with bone resorption. HIV-infected men had low CD4 T cells, which is inversely associated with bone loss (55). Some studies suggest that T cells are not associated with bone mineral density in HIV-infected patients treated with combination antiretroviral therapy (cART) (56). However, cART seems to influence bone mineral density (BMD) with the protective effect. Therefore, the regulatory roles for activated T cells in the pathogenesis of osteoporosis warrant further investigation. In RA patients, the enhanced osteoclast differentiation and activation lead to bone erosion and systematic osteoporosis (57). Indeed, inflammatory cytokines including RANKL, TNFα, IL-6, and IL-1 were elevated in RA patients, which promoted the osteoclast differentiation (58). Taken together, these studies suggest that the T cells may determine the osteoclast differentiation in the chronic inflammatory diseases, and the T cell regulatory therapy could potentially have significant impact on the drug development for osteoporosis. However, whether the T cell therapy is efficient for osteoporosis in clinical studies needs further investigation.
The Regulation Roles of T Cells in Senile Osteoporosis
Aging is always accompanied with the imbalance between bone formation and resorption, causing skeletal microarchitecture damage and bone loss (59). The production of naïve T cells is severely impaired due to a decreased output of lymphoid cells from the bone marrow and the deterioration of the thymus (60). Incidence and severity of osteoporosis are increased in the older population (61). The prevalence of low BMD is associated with immune activation and senescence induced by HIV infection (62). Total T cells were increased in the bone marrow (BM) with age, especially the highly differentiated CD8+ T cells without the expression of the co-stimulatory molecule CD28, while natural killer T (NKT) cells, monocytes, and naïve CD8+ T cells were decreased in the BM with age (63). It seems that the immune system abnormality plays important roles in the regulation of senile osteoporosis.
Recent discoveries suggest that T cell dysfunction induced the accumulation of cytokines, immunological mediators, and transcription factors, which affect osteoclast and osteoblast in the elderly (64). Cytokines such as IL-6, TNF-α, and IL-1 increased with age (65, 66). IL-1 and TNF-α activate the inducible NOS (iNOS) pathway, which inhibited osteoblast differentiation and enhanced osteoblast apoptosis in vitro (67). IL-12 derived from T cells, alone or combined with IL-18, was identified to inhibit osteoclast formation in vitro (68). IL-4 regulated osteoclast differentiation through the antagonism between STAT6 and NF-kB signaling (69). In addition, T cell mediated the bone balance by the inhibition of osteoclastogenesis through the crucial immunoregulatory control, mainly OPG expression and simultaneous production of cytokines (64). IFN-g, IL-12, and IL-18 inhibited the RANKL-induced maturation and activation of osteoclasts (64). Furthermore, senescent T cells impaired the production of IFN-γ, OPG, and osteoclast-inhibiting cytokines, which increased the incidence of aged osteoporosis. In addition, cytokines such as TGFβ and RANKL secreted by activated T cells can activate p38 MAPKs and further regulate bone development and remodeling. P38α MAPK mediates osteoclast proliferation and bone remodeling in an aging-dependent manner (70). Overall, T cells and their cytokines play important roles in the regulation of aged osteoporosis, which may be the novel targets for the treatment of osteoporosis, suggesting that T cell therapy could be used as immunotherapy and may be beneficial in counteracting immunosenescence in old population. Meanwhile, in females, osteoporosis occurrence is generally attributed to the decrease in estrogen, thus leading to estrogen deficiency-induced osteoporosis. The underlying mechanisms of T cells involved in the mediation of the postmenopausal osteoporosis were dissected in the next section, The Regulatory Roles of T Cells in Estrogen Deficiency-Induced Osteoporosis.
The Regulatory Roles of T Cells in Estrogen Deficiency-Induced Osteoporosis
The loss of estrogen initiates the inflammatory changes of bone-microenvironment state, inducing a rapid phase of bone loss leading to osteoporosis in half of postmenopausal women. In postmenopausal women, estrogen deficiency stimulates CD4+ T cell dysregulation and induces elevated circulating levels of inflammatory cytokines, especially TNFα, IFN-γ, IL-17, RANKL, and CD40L (71–74). These cytokines exert impressive regulatory effects on bone resorption. For example, TNF-α was overexpressed in the BM in postmenopausal osteoporosis, which promotes RANKL-induced osteoclast formation through the activation of NF-κB and PI3K/Akt signaling (74). Besides, TNF-α was identified to induce both autophagy and apoptosis in osteoblasts to enhance bone loss in postmenopausal women (75). Besides, estrogen deficiency increased the number of the costimulatory factors, CD40L, expressed on activated T cells, inducing the expressions of M-CSF and RANKL on stromal cells and downregulating the production of OPG, ultimately resulting in a remarkable increase in osteoclast numbers (76, 77). The pro-osteoclastic cytokines, such as IL-6, TNF-α, and IL-1, were increased significantly in estrogen deficiency-induced osteoporosis (78). All these studies indicated that the inflammatory cytokines and costimulatory factors of T cells changed significantly in estrogen deficiency-induced osteoporosis, which may provide the novel perspective for the treatment of bone loss in postmenopausal women.
Moreover, estrogen deficiency stimulates the IL-17 differentiation of Th17 cells (79) and augments the expression levels of pro-osteoclastogenic cytokines, such as TNF-a, IL-6, and RANKL, ultimately leading to bone loss. Nevertheless, IL-17 receptor deficiency induced more serious bone loss in OVX mice than that in control groups, implying that IL-17 may possess the bone protective effects (80). The pro-osteoclastogenic cytokine changes were reversed with the supplementary oral estrogen, indicating that estrogen may suppress Th17 differentiation and IL-17 production to protect bone health (81). In summary, in postmenopausal women, both aging and hormonal deficiency stimulate the deregulation of T cells contributing to the inflammatory, which increased bone resorption, resulting in a bone loss or osteoporosis. We believe that focusing on the potential biological mechanisms of T cells is of paramount importance for developing novel therapy strategies for the treatment of postmenopausal osteoporosis. However, further confirmation in phase I/II trials is needed to validate these strategies in a broader clinical evaluation.
The Regulatory Roles of T Cells in PTH-Induced Osteoporosis
PTH is a key calciotropic hormone and a critical regulator for postnatal skeletal development (82). The secretion of inflammatory or osteoclastogenic cytokines of T cells and bone cells was facilitated under long-term PTH administration, such as RANKL, TNF-α, and IL-17, which promoted the bone resorption (83). PTH induced bone loss via the expansion of intestinal TNF+ T and Th17 cells, and the increase in their S1P-receptor-1 mediated egress from the intestine and recruitment to the BM (84). So targeting the gut microbiota or T cell migration may represent novel therapeutic strategies for PTH-induced osteoporosis. In addition, PTH exploited CD4+ T cells to induce TNFα production that enhances the formation of IL-17A secreting Th17 T cells. Both TNFα and IL-17 further facilitated the development of an increased RANKL/OPG ratio favorable to osteoclastic bone resorption (85). Moreover, PTH boosted the production of TNF-α and RANKL in CD4+ T cells, which triggered osteoclastogenic generation and bone resorption activity (86). Clinical studies also showed that PTH treatment increased Th17 cell numbers and the IL-17 production in humans with primary hyperparathyroidism (34). IL-17 intensified PTH-induced bone loss through the stimulation of the RANKL production in osteoblast-lineage cells, which is parallel to the roles of IL-17 in estrogen deficiency-induced osteoporosis.
Notably, T cells also secreted PTH receptors involved in the regulation of trabecular bone development (87). For example, T cells promoted the signals of BMSC proliferation through the combination of CD40L on T cells and its receptor on BMSC, weakening the bone catabolic activity of cPTH, leading to a reduction of the RANKL to OPG ratio and osteoclastogenic activity (88). Several studies found that the intermittent PTH administration at low dosage increased bone formation and bone mass, thus attenuating bone loss (89, 90). The deletion of PTH receptor in BM mesenchymal progenitors results in a rapid increase in BM adipocyte accompanied with the reduction of bone mass. Given the essential regulatory roles of T cells for the PTH-induced bone loss, particular attention will be paid toward the combinations of intermittent PTH (iPTH) and T cell therapy for PTH-induced osteoporosis.
The Regulatory Roles of T Cells in GIO
Glucocorticoids (GCs) are extensively used for the treatment of immune and inflammatory disorders due to their powerful immunosuppressive and anti-inflammatory actions (91, 92). However, long-term exogenous GC therapy might cause rapid and pronounced bone loss and subsequently osteoporosis (93, 94). The pathogenesis of GIO was predominantly attributed to the fact that GCs impaired bone formation by reduction of osteoblast differentiation and activity via the expression of the osteoblast-specific transcription factor runt-related Runx2 (95–97). In addition, the long-term GC administration affects bone remodeling by whittling the insulin-like growth factor (IGF) in ossification (98). GCs enhanced the expression levels of RANKL in both osteoblasts and stromal cells, which triggered osteoclastogenesis and activated osteoclastic bone resorption by binding to the RANKL receptor RANK (99), thus resulting in the primary phase of rapid bone loss. On the other hand, GCs contributed to the apoptosis of certain T cell subsets, further augmented the secretion of RANKL, and directly induced osteoclast differentiation (100). Interestingly, different T cell subsets exhibit distinct sensitivity to GC-induced apoptosis. For example, Th17 cells, as an osteoclastogenesis-promoting factor, are resistant to GC-induced apoptosis and cytokine suppression mostly through the high production of IL-17 and RANKL (79). Therefore, GC therapy fails to inhibit the Th17 cell activation and the IL-17 and RANKL production. Excessive GCs could reduce the production of OPG, further promoting osteoclast differentiation and resulting in bone resorption. Given above, we assert that T cell therapy may be effective for the GC-induced osteoporosis.
T Cell Therapy for Osteoporosis
T cells and their secreted cytokines are responsible for bone resorption in various osteoporosis. T cell therapy may be a potentially therapeutic approach to osteoporosis. For example, anti-inflammatory therapies have shown good potential in an animal model, although they have not been widely used clinically to treat osteoporosis (101). Immune modulation therapy such as probiotics was considered as a novel strategy for bone loss (102–104). RANKL was considered as an activator of dendritic cell (DC) expression in T cells. Anti-RANKL therapeutic antibody drug, denosumab, has been successfully applied in the treatment of osteoporosis in clinics (105–107). In addition, a novel vaccine targeting RANKL by introducing a p-nitrophenylalanine at a single site in mRANKL immunization could prevent OVX-induced bone loss in mice (108). Notably, anti-RANKL antibody inhibited osteoporosis and bone destruction, but possesses no therapeutic effect on RA disease. Therefore, it is necessary to rethink about the underlying mechanisms of bone-related diseases.
Recently, extracts and natural products derived from traditional Chinese medicine (TCM) have great potential as well as advantages in the prevention and treatment of osteoporosis in terms of good therapeutic effect, low toxicity, and side effects (109, 110), and they have gained increasing attention from the medical community. For example, polysaccharides derived from persimmon leaves down-regulated RANKL-induced activation of mitogen-activated protein kinases (MAPKs) to suppress the nuclear factor of NFATc1 expression, thus possessing anti-osteoporotic effects in OVX-induced bone loss. The natural product cyperenoic acid is a terpenoid isolated from the medicinal plant Croton crassifolius, and it suppressed osteoclast differentiation by inhibiting the NF-κB pathway and suppressed RANKL expression (111). Baohuoside I is an active component of Herba Epimedii with the immune regulation functions of T cells and antioxidant activity, which serves as a candidate for treating postmenopausal osteoporosis (112). All these results indicated that drugs from TCM possess anti-osteoporosis effects by the regulation of T cells, and they may show great potential as therapeutic agents for osteoporosis. However, further experimental and clinical research remains to be specifically conducted to explore the cellular and molecular mechanisms of the drugs from TCM.
Conclusion and Perspective
The pathogen clearance of various types of osteoporosis would be impaired or would delay bone resorption due to the dysfunction of the T cells. Therefore, understanding the roles of T cells in the pathogenesis of osteoporosis and the mechanisms underlying these pathologies between the immune system and the bone system may lead to the development of new treatments for osteoporosis. However, further studies, especially clinical studies, are required to explore the safety of T cell therapy for bone loss.
Author Contributions
WZ designed, wrote and revised the whole manuscript. YH wrote the manuscript. AQ and KD helped to revise the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (No. 81901917), China's Postdoctoral Science Fund (No. 2017M623249), and the Key Research and Development Project of Shaanxi Province (No. 2018SF-363).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BMMs, Bone marrow macrophages; BMSCs, bone marrow stromal cells; Cbfa1, core-binding factor subunit alpha-1; DC, dendritic cell; GCs, glucocorticoids; GIO, glucocorticoid-induced osteoporosis; GSK-3β, glycogen synthase kinase 3β; IGF, insulin-like growth factor; IFN, interferon; M-CSF, macrophage-colony stimulating factor; MSCs, mesenchymal stem cells; NFATc1, nuclear factor of activated T cells cytoplasmic 1; NKT, natural killer T cells; iNOS, inducible NOS; RANKL, nuclear factor-kappa-B ligand; OVX, ovariectomized; OPG, osteoprotegerin; PTH, parathyroid hormone; T cells, T lymphocytes; TRAF6, TNF receptor associated factor 6; Runx2, Transcription Factor 2; RANK, receptor activator of NF-kB ligand; RA, rheumatoid arthritis.
References
1. Dai Z, Zhang Y, Lu N, Felson DT, Kiel DP, Sahni S. Association between dietary fiber intake and bone loss in the Framingham Offspring Study. J Bone Miner Res. (2018) 33:241–9. doi: 10.1002/jbmr.3308
2. Yaacobi E, Sanchez D, Maniar H, Horwitz DS. Surgical treatment of osteoporotic fractures: an update on the principles of management. Injury. (2017) 48:S34–40. doi: 10.1016/j.injury.2017.08.036
3. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. (2016) 29:2520–6. doi: 10.1002/jbmr.2269
4. Yu B, Wang C-Y. Osteoporosis: the result of an ‘aged’ bone microenvironment. Trends Mol Med. (2016) 22:641–4. doi: 10.1016/j.molmed.2016.06.002
5. Raisz LG, Seeman E. Causes of age-related bone loss and bone fragility: an alternative view. Eur J Bone Miner. Res. (2002) 16:1948–52. doi: 10.1359/jbmr.2001.16.11.1948
6. Okamoto K, Nakashima T, Shinohara M, Negishi-Koga T, Komatsu N, Terashima A, et al. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. (2017) 97:1295–349. doi: 10.1152/physrev.00036.2016
7. Srivastava RK. Osteoimmunology: the nexus between bone and immune system. Front Biosci. (2018) 23:464–92. doi: 10.2741/4600
8. Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. (2009) 5:667–76. doi: 10.1038/nrrheum.2009.217
9. Takayanagi H. Osteoimmunology in 2014: two-faced immunology-from osteogenesis to bone resorption. Nat Rev Rheumatol. (2015) 11:74–6. doi: 10.1038/nrrheum.2014.219
10. Okamoto K, Takayanagi H. Osteoimmunology. Cold Spring Harb Perspect Med. (2018) 9:a031245. doi: 10.1101/cshperspect.a031245
11. Srivastava RK, Dar HY, Mishra PK. Immunoporosis: immunology of osteoporosis-role of t cells. Front. Immunol. (2018) 9:657. doi: 10.3389/fimmu.2018.00657
12. Kalyan S. It may seem inflammatory, but some T cells are innately healing to the bone. J Bone Miner Res. (2016) 31:1997–2000. doi: 10.1002/jbmr.2875
13. Harmer D, Falank C, Reagan MR. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front Endocrinol. (2019) 9:788. doi: 10.3389/fendo.2018.00788
14. Li Y, Toraldo G, Li A, Yang X, Weitzmann MN. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. (2007) 109:3839–48. doi: 10.1182/blood-2006-07-037994
15. Weitzmann MN, Ofotokun I. Physiological and pathophysiological bone turnover-role of the immune system. Nat Rev Endocrinol. (2016) 12:518–32. doi: 10.1038/nrendo.2016.91
16. Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinol. (2002) 143:1108–18. doi: 10.1210/endo.143.3.8701
17. Dar HY, Shukla P, Mishra PK, Anupam R, Mondal RK, Tomar GB, et al. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. (2018) 8:46–56. doi: 10.1016/j.bonr.2018.02.001
18. Buchwald ZS, Kiesel JR, Yang C, DiPaolo R, Novack DV, Aurora R. Osteoclast-induced Foxp3+ CD8 T-cells limit bone loss in mice. Bone. (2013) 56:163–73. doi: 10.1016/j.bone.2013.05.024
19. Glowacki AJ, Yoshizawa S, Jhunjhunwala S, Vieira AE, Garlet GP, Sfeir C, et al. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc Natl Acad Sci USA. (2013) 110:18525–30. doi: 10.1073/pnas.1302829110
20. Luo C, Wang L, Sun C, Li D. Estrogen enhances the functions of CD4+CD25+Foxp3+ regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell Mol Immunol. (2011) 8:50–8. doi: 10.1038/cmi.2010.54
21. Yuan FL, Li X, Lu W-G, Xu R-S, Zhao Y-Q, Li C-W, et al. Regulatory T cells as a potent target for controlling bone loss. Biochem Biophys Res Commun. (2010) 402:173–6. doi: 10.1016/j.bbrc.2010.09.120
22. Tanaka Y. Clinical immunity in bone and joints. J Bone Miner Metab. (2018) 37:2–8. doi: 10.1007/s00774-018-0965-5
23. Ofotokun I, Titanji K, Vikulina T, Roser-Page S, Yamaguchi M, Zayzafoon M, et al. Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun. (2016) 6:8282. doi: 10.1038/ncomms9282
24. Santana-Davila R, Show LQ. The use of combination immunotherapies as frontline therapy for non-small cell lung cancer. Future Oncol. (2018) 14:191–4. doi: 10.2217/fon-2017-0124
25. Jin HM, Shao ZX, Wang QQ, Miao JS, Bai XQ, Liu Q, et al. Sclareol prevents ovariectomy-induced bone loss in vivo and inhibits osteoclastogenesis in vitro via suppressing NF-κB and MAPK/ERK signaling pathways. Food Funct. (2019) 10:6556. doi: 10.1039/C9FO00206E
26. Bozec A, Zaiss MM. T regulatory cells in bone remodelling. Curr Osteoporos Rep. (2017) 15:121–5. doi: 10.1007/s11914-017-0356-1
27. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. (2014) 5:511. doi: 10.3389/fimmu.2014.00511
28. Takayanagi H, Ogasawara K, Hida S, Chiba T, Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. (2000) 408:600–5. doi: 10.1038/35046102
29. Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. (2002) 20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753
30. Ginaldi L, Benedetto MCD, Martinis MD. Osteoporosis, inflammation and ageing. Immun Ageing. (2005) 2:1–5. doi: 10.1186/1742-4933-2-14
31. Schett G, David JP. The multiple faces of autoimmune-mediated bone loss. Nat Rev Endocrinol. (2010) 6:698–706. doi: 10.1038/nrendo.2010.190
33. Pacifici R. T cells, osteoblasts, and osteocytes: interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann N Y Acad Sci. (2016) 1364:11–24. doi: 10.1111/nyas.12969
34. Li JY, D'Amelio P, Robinson J, Walker LD, Vaccaro C, Luo T, et al. IL-17A is increased in humans with primary hyperparathyroidism and mediates PTH-induced bone loss in mice. Cell Metab. (2015) 22:799–810. doi: 10.1016/j.cmet.2015.09.012
35. Kotake S, Nanke Y, Mogi M, Kawamoto M, Furuya T, Yago T, et al. IFN-γ-producing human T cells directly induce osteoclastogenesis from human monocytes via the expression of RANKL. Eur J Immunol. (2005) 35:3353–63. doi: 10.1002/eji.200526141
36. Adamopoulos IE, Chao CC, Geissler R, Laface D, Bowman EP. Interleukin17A upregulates receptor activator of NF-κB on osteoclast precursors. Arthritis Res Ther. (2010) 12:1–11. doi: 10.1186/ar2936
37. Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. (2008) 473:139–46. doi: 10.1016/j.abb.2008.03.018
38. Adamopoulos IE, Bowman EP. Immune regulation of bone loss by Th17 cells in oestrogen-deficient osteoporosis. Eur J Clin Invest. (2013) 43:1195–203. doi: 10.1111/eci.12158
39. Harrington L. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. (2006) 18:349–56. doi: 10.1016/j.coi.2006.03.017
40. Mangashetti LS, Khapli SM, Wani MR. IL-4 inhibits bone-resorbing activity of mature osteoclasts by affecting NF-KB and Ca2+ Signaling. J Immunol. (2005) 175:917–25. doi: 10.4049/jimmunol.175.2.917
41. Yun TJ, Chaudhary PM, Shu GL, Frazer JK, Ewings MK, Schwartz SM, et al. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol. (1998) 161:6113–21.
42. Sun-Kyeong L, Kalinowski JF, Jastrzebski SL, Lynn P, Lorenzo JA. Interleukin-7 is a direct inhibitor of in vitro osteoclastogenesis. Endocrinology. (2003) 144:3524–31. doi: 10.1210/en.2002-221057
43. Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. (2011) 32:428–33. doi: 10.1016/j.it.2011.06.002
44. Arboleya L, Castañeda S. Osteoimmunology: the study of the relationship between the immune system and bone tissue. Reumatol Clin. (2013) 9:303–15. doi: 10.1016/j.reumae.2013.02.004
45. Woodward J. Regulation of haematopoietic progenitor cell proliferation and survival The involvement of the osteoblast. Cell Adh Migr. (2010) 4:4–6. doi: 10.4161/cam.4.1.10106
46. Nagata N. Inhibition of RANKL-induced osteoclast formation in mouse bone marrow cells IL-12 : involvement of IFN-γ possibly induced from non-T cell population. Bone. (2003) 33:721–32. doi: 10.1016/S8756-3282(03)00213-8
47. Hayday AC. γδ T Cells and the lymphoid stress-surveillance response. Immunity. (2009) 31:184–96. doi: 10.1016/j.immuni.2009.08.006
48. Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. (2006) 203:2673–82. doi: 10.1084/jem.20061775
49. Clayton ES, Hochberg MC. Osteoporosis and osteoarthritis, rheumatoid arthritis and spondylarthropathies. Curr Osteoporos Rep. (2013) 11:257–62. doi: 10.1007/s11914-013-0172-1
50. Gulati AM, Michelsen B, Diamantopoulos A, Grandaunet B, Salvesen Ø, Kavanaugh A, et al. Osteoporosis in psoriatic arthritis: a cross-sectional study of an outpatient clinic population. RMD Open. (2018) 4:e000631. doi: 10.1136/rmdopen-2017-000631
51. Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. (2012) 8:656–64. doi: 10.1038/nrrheum.2012.153
52. Ødegård S, Landewé R, van der Heijde D, Kvien TK, Mowinckel P, Uhlig T. Association of early radiographic damage with impaired physical function in rheumatoid arthritis: a ten-year, longitudinal observational study in 238 patients. Arthritis Rheum. (2006) 54:68–75. doi: 10.1002/art.21548
53. Zaiss MM, Axmann R, Zwerina J, Polzer K, Gückel E, Skapenko A, et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. (2007) 56:4104–12. doi: 10.1002/art.23138
54. Fischer L, Herkner C, Kitte R, Dohnke S, Riewaldt J, Kretschmer K, et al. Foxp3+ regulatory T cells in bone and hematopoietic homeostasis. Front Endocrinol. (2019) 10:578. doi: 10.3389/fendo.2019.00578
55. Kwak MK, Lee EJ, Park JW, Park SY, Kim BJ, Kim TH, et al. CD4 cell count is inversly associated with lumbar spine bone mass in HIV-infected men under the age of 50 years. Oateoporosis Int. (2019) 30:1501–30. doi: 10.1007/s00198-019-05115-2
56. Krikke M, Klomberg RCW, Veer EVD, Tesselaar K, Verhaar HJJ, Hoepelman AIM, et al. Osteoporosis and osteopenia are not associated with T-cell activation in older cART-treated HIV-infected patients. Neth J Med. (2017) 75:138–44.
57. Jung YK, Kang YM, Han S. Osteoclasts in the inflammatory arthritis: implications for pathologic osteolysis. Immune Netw. (2019) 19:1–13. doi: 10.4110/in.2019.19.e2
58. Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther. (2011) 13:1–11. doi: 10.1186/ar3380
59. Fei D, Zhang Y, Wu J, Zhang H, Liu A, He X, et al. Cav 1.2 regulates osteogenesis of bone marrow-derived mesenchymal stem cells via canonical Wnt pathway in age-related osteoporosis. Aging Cell. (2019) 18:e12967. doi: 10.1111/acel.12967
60. Pangrazzi L, Weinberger B. T cells, aging and senescence. Exp Gerontol. (2020) 22:110887. doi: 10.1016/j.exger.2020.110887
61. Borrelli J, Anglen JO (editors). Arthroplasty for the treatment of fractures in the older patient (indications and current techniques) || The relationship of peak bone mass, aging, and bone loss to osteoporosis and fragility fractures. In: Arthroplasty for the Treatment of Fractures in the Older Patient Chapter. New York, NY: Springer (2018). p. 3–17.
62. Jiménez B, Sainz T, Díaz L, Mellado MJ, Navarro ML, Rojo P. Low bone mineral density in vertically HIV-infected children and adolescents: risk factors and the role of T-cell activation and senescence. Pediatr Infect Dis J. (2017) 36:578–83. doi: 10.1097/INF.0000000000001506
63. Naismith E, Pangrazzi L, Grasse M, Keller M, Miggitsch C, Weinberger B, et al. Peripheral antibody concentrations are associated with highly differentiated T cells and inflammatory processes in the human bone marrow. Immun Ageing. (2019) 16:21. doi: 10.1186/s12979-019-0161-z
64. Martinis MD, Benedetto MCD, Mengoli LP, Ginaldi L. Senile osteoporosis: is it an immune-mediated disease? Inflamm Res. (2006) 55:399–404. doi: 10.1007/s00011-006-6034-x
65. Mundy GR. Osteoporosis and inflammation. Nutr Rev. (2007) 65:S147–51. doi: 10.1111/j.1753-4887.2007.tb00353.x
66. Deng L, Hu G, Jin L, Wang C, Niu H. Involvement of microRNA-23b in TNF-α-reduced BMSC osteogenic differentiation via targeting runx2. J Bone Miner Metab. (2018) 36:648–60. doi: 10.1007/s00774-017-0886-8
67. Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. (2005) 115:282–90. doi: 10.1172/JCI200523394
68. Horwood NJ, Elliott J, Martin TJ, Gillespie MT. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol. (2001) 166:4915–21. doi: 10.4049/jimmunol.166.8.4915
69. Abu-Amer Y. IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-κB. J Clin Invest. (2001) 107:1375–85. doi: 10.1172/JCI10530
70. Cong Q, Jia H, Li P, Qiu S, Yeh J, Wang Y, et al. p38α MAPK regulates proliferation and differentiation of osteoclast progenitors and bone remodeling in an aging-dependent manner. Sci Rep. (2017) 7:45964. doi: 10.1038/srep45964
71. Zhang J, Fu Q, Ren Z, Wang Y, Wang C, Shen T, et al. Changes of serum cytokines-related Th1/Th2/Th17 concentration in patients with postmenopausal osteoporosis. Gynecol Endocrinol. (2015) 31:183–90. doi: 10.3109/09513590.2014.975683
72. Giorgio M, Patrizia DA, Roberta F, Giacomina B. Bone-immune cell crosstalk: bone diseases. J Immunol Res. (2015) 2015:1–11. doi: 10.1155/2015/108451
73. Sang C, Zhang J, Zhang Y, Chen F, Cao X, Guo L. TNF-α promotes osteoclastogenesis through JNK signaling-dependent induction of Semaphorin3D expression in estrogen-deficiency induced osteoporosis. J Cell Physiol. (2017) 232:3396–408. doi: 10.1002/jcp.25784
74. Zha L, He L, Liang Y, Qin H, Yu B, Chang L, et al. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed Pharmacother. (2018) 102:369–74. doi: 10.1016/j.biopha.2018.03.080
75. Du D, Zhou Z, Zhu L, Hu X, Lu J, Shi C, et al. TNF-α suppresses osteogenic differentiation of MSCs by accelerating P2Y2 receptor in estrogen-deficiency induced osteoporosis. Bone. (2018) 117:161–70. doi: 10.1016/j.bone.2018.09.012
76. Douin-Echinard V, Laffont S, Seillet C, Delpy L, Krust A, Chambon P, et al. Estrogen receptor alpha, but not beta, is required for optimal dendritic cell differentiation and CD40-induced cytokine production. J Immunol. (2008) 180:3661–9. doi: 10.4049/jimmunol.180.6.3661
77. Zheng L, Wang W, Ni J, Mao X, Song D, Liu T, et al. Role of autophagy in tumor necrosis factor-α-induced apoptosis of osteoblast cells. J Investig Med. (2017) 65:1014–20. doi: 10.1136/jim-2017-000426
78. Moffett SP, Zmuda JM, Oakley JI, Beck TJ, Cauley JA, Stone KL, et al. Tumor necrosis factor-α polymorphism, bone strength phenotypes, and the risk of fracture in older women. J Clin Endocrinol Metab. (2005) 90:3491–7. doi: 10.1210/jc.2004-2235
79. Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D, et al. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS ONE. (2012) 7:e44552. doi: 10.1371/journal.pone.0044552
80. Xu F, Dong Y, Huang X, Chen P, Huang S. Pioglitazone affects the OPG/RANKL/RANK system and increase osteoclastogenesis. Mol Med Rep. (2016) 14:2289–96. doi: 10.3892/mmr.2016.5515
81. Pacifici, R. (2007). T cells and post menopausal osteoporosis in murine models. Arthritis Res Ther. 9:1–4. doi: 10.1186/ar2126
82. Wein MN, Kronenberg HM. Regulation of bone remodeling by parathyroid hormone. Cold Spring Harb Perspect Med. (2018) 8:a031237. doi: 10.1101/cshperspect.a031237
83. Roberto P. The Role of IL-17 and TH17 cells in the bone catabolic activity of PTH. Front Immunol. (2016) 7:57. doi: 10.3389/fimmu.2016.00057
84. Yu M, Tyagi AM, Li JY, Adams J, Denning TL, Weitzmann MN, et al. PTH induces bone loss via microbial-dependent expansion of intestinal TNF + T Cells and Th17 cells. Nat Commun. (2020) 11:468. doi: 10.1038/s41467-019-14148-4
85. Neale Weitzmann M, Pacifici R. Parathyroid diseases and T cells. Curr Osteoporosis Rep. (2017) 15:135–41. doi: 10.1007/s11914-017-0359-y
86. Pacifici R. T cells: critical bone regulators in health and disease. Bone. (2010) 47:461–71. doi: 10.1016/j.bone.2010.04.611
87. Li JY, Walker LD, Tyagi AM, Adams J, Weitzmann MN, Pacifici R. The sclerostin-independent bone anabolic activity of intermittent PTH treatment is mediated by T-Cell–produced Wnt10b. J Bone Miner Res. (2014) 29:43–54. doi: 10.1002/jbmr.2044
88. Hesham T, Brahmchetna B, Jau-Yi L, Jonathan A, Tatsuya K, Neale WM, et al. Disruption of PTH receptor 1 in t cells protects against pth-induced bone loss. PLoS ONE. (2010) 5:e12290. doi: 10.1371/journal.pone.0012290
89. Fan Y, Hanai J-I, Le PT, Bi R, Maridas D, DeMambro V, et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. (2017) 25:661–72. doi: 10.1016/j.cmet.2017.01.001
90. Sang WK, Pajevic PD, Selig M, Barry KJ, Yang JY, Chan SS, et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. (2012) 27:2075–84. doi: 10.1002/jbmr.1665
91. Geurtzen K, Vernet A, Freidin A, Rauner M, Hofbauer LC, Schneider JE, et al. Immune suppressive and bone inhibitory effects of prednisolone in growing and regenerating zebrafish tissues. J Bone Miner Res. (2017) 32:2476–88. doi: 10.1002/jbmr.3231
92. Sato AY, Peacock M, Bellido T. Glucocorticoid excess in bone and muscle. Clin Rev Bone Miner Metab. (2018) 16:1–15. doi: 10.1007/s12018-018-9242-3
93. Compston J. Glucocorticoid-induced osteoporosis: an update. Endocrine. (2018) 61:7–16. doi: 10.1007/s12020-018-1588-2
94. Wang N, Zhang J, Yang JX. Growth factor progranulin blocks tumor necrosis factor-α-mediated inhibition of osteoblast differentiation. Genet Mol Res. (2016) 15. doi: 10.4238/gmr.15038126
95. Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. (2010) 11:517–31. doi: 10.1016/j.cmet.2010.05.005
96. Reid IR. Glucocorticoid-induced osteoporosis. New Engl J Med. (2010) 14:279–98. doi: 10.1053/beem.2000.0074
97. Weinstein RS. Glucocorticoid-induced bone disease. N Engl J Med. (2011) 365:62–70. doi: 10.1056/NEJMcp1012926
98. Beattie J, Al-Khafaji H, Noer PR, Alkharobi HE, Alhodhodi A, Meade J, et al. (2018). Insulin-like growth factor-binding protein action in bone tissue: a key role for pregnancy- associated plasma protein-A. Front Endocrinol. 9:31. doi: 10.3389/fendo.2018.00510
99. Wehmeyer C, Pap T, Buckley CD, Naylor AJ. The role of stromal cells in inflammatory bone loss. Clin Exp Immunol. (2017) 189:1–11. doi: 10.1111/cei.12979
100. Banuelos J, Lu NZ. A gradient of glucocorticoid sensitivity among helper T cell cytokines. Cytokine Growth Factor Rev. (2016) 31:27–35. doi: 10.1016/j.cytogfr.2016.05.002
101. Xie Y, Zhang LC, Xiong Q, Gao YP, Ge W, Tang PF. Bench-to-bedside strategies for osteoporotic fracture: from osteoimmunology to mechanosensation. Bone Res. (2019) 7:259–71. doi: 10.1038/s41413-019-0066-7
102. Dar HY, Singh A, Shukla P, Anupam R, Mondal RK, Mishra PK, et al. High dietary salt intake correlates with modulated Th17-Treg cell balance resulting in enhanced bone loss and impaired bone-microarchitecture in male mice. Sci Rep. (2018) 8:2503. doi: 10.1038/s41598-018-20896-y
103. Kennel KA, Drake MT. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc. (2009) 84:632–8. doi: 10.1016/S0025-6196(11)60752-0
104. Sharma D, Singh J. Synthesis and characterization of fatty acid grafted chitosan polymer and their nanomicelles for non-viral gene delivery applications. Bioconjug Chem. (2017) 28:2772–83. doi: 10.1021/acs.bioconjchem.7b00505
105. Bone H, Brandi M, Brown J, Chapurlat R, Cummings S, Czerwinski E, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. (2017) 5:513–23. doi: 10.1016/S2213-8587(17)30138-9
106. Juji T, Hertz M, Aoki K, Horie D, Ohya K, Gautam A, et al. A novel therapeutic vaccine approach, targeting RANKL, prevents bone destruction in bone related disorders. J Bone Miner Metabol. (2002) 20:266–8. doi: 10.1007/s007740200038
107. Zaheer S, LeBoff M, Lewiecki EM. Denosumab for the treatment of osteoporosis. Expert Opin Drug Metab Toxicol. (2015) 11:461–70. doi: 10.1517/17425255.2015.1000860
108. Li F, Li H, Zhai Q, Li FY, Wu TL, Sha X, et al. A new vaccine targeting RANKL, prepared by incorporation of an unnatural amino acid into RANKL, prevents OVX-induced bone loss in mice. Biochem Biophys Res Commun. (2018) 499:648–54. doi: 10.1016/j.bbrc.2018.03.205
110. Yang YH, Li B, Zheng XF, Chen JW, Chen K, Jiang SD, et al. Oxidative damage to osteoblasts can be alleviated by early autophagy through the endoplasmic reticulum stress pathway-implications for the treatment of osteoporosis. Free Radic Biol Med. (2014) 77:10–20. doi: 10.1016/j.freeradbiomed.2014.08.028
111. Chawalitpong S, Chokchaisiri R, Suksamrarn A, Katayama S, Mitani T, Nakamura T. Cyperenoic acid suppresses osteoclast differentiation and delays bone loss in a senile osteoporosis mouse model by inhibiting non-canonical NF-κB pathway. Sci Rep. (2018) 8:5625. doi: 10.1038/s41598-018-23912-3
Keywords: osteoimmunology, T lymphocytes, osteoporosis, bone formation, bone resorption
Citation: Zhang W, Dang K, Huai Y and Qian A (2020) Osteoimmunology: The Regulatory Roles of T Lymphocytes in Osteoporosis. Front. Endocrinol. 11:465. doi: 10.3389/fendo.2020.00465
Received: 31 December 2019; Accepted: 15 June 2020;
Published: 11 August 2020.
Edited by:
Lilian Irene Plotkin, Indiana University Bloomington, United StatesReviewed by:
Rajeev Aurora, Saint Louis University, United StatesAnna Teti, University of L'Aquila, Italy
Copyright © 2020 Zhang, Dang, Huai and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Airong Qian, qianair@nwpu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Wenjuan Zhang
Wenjuan Zhang Kai Dang
Kai Dang Ying Huai
Ying Huai Airong Qian
Airong Qian