Excessive Iodine Promotes Pyroptosis of Thyroid Follicular Epithelial Cells in Hashimoto's Thyroiditis Through the ROS-NF-κB-NLRP3 Pathway
- Department of Molecular Medicine, Atomic Bomb Disease Institute, Nagasaki University, Nagasaki, Japan
A Commentary on
Excessive Iodine Promotes Pyroptosis of Thyroid Follicular Epithelial Cells in Hashimoto's Thyroiditis Through the ROS-NF-κB-NLRP3 Pathway
by Liu, J., Mao, C., Dong, L., Kang, P., Ding, C., Zheng, T., et al. (2019). Front. Endocrinol. 10:778. doi: 10.3389/fendo.2019.00778
It is well-known that iodine is an indispensable element for thyroid hormone synthesis, and both deficiency and excess in iodine cause thyroid dysfunction. The article mentioned above used a human normal thyroid cell line, Nthy-ori 3-1, to study the effect of excessive iodine on thyroid cell death (1), where cell death was induced by incubating the cells with extremely high concentrations of iodine (≥10−2 M). The same combination of Nthy-ori 3-1 cells and very high concentrations of iodine (up to 5 × 10−2 M) has also been previously used for studies on iodine-induced thyroid cell death, ER stress induction, etc. (2, 3).
I would like to raise two issues about this article. First is about the cell's capability of iodine uptake. The previous articles report that Nthy-ori 3-1 cells do not incorporate iodine (4, 5). Nthy-ori 3-1 cells were derived from HTori-3 cells which are immortalized human thyroid cells by transfection of simian virus 40 T-antigen (https://web.expasy.org/cellosaurus/CVCL_2659), and can grow without TSH. Thus, it is easy to assume that these cells are rather de-differentiated. In contrast, rat functional thyroid cell lines, FRTL5 and PCCL3, both of which are spontaneously immortalized cell lines and their growth is totally TSH-dependent, a characteristic of differentiated thyroid cells, take up iodine. We recently confirmed that PCCL3 cells incorporated 131I in a dose-dependent manner, reaching the peak at 30 min in the presence of TSH (2 mU/mL), which was completely abolished by 5 μM NaClO4 (perchlorate), a competitive inhibitor of sodium-iodine symporter (NIS), confirming NIS-mediated iodine uptake (6). Thus, I am afraid that the effect of iodine on Nthy-ori 3-1 cell death observed in (1) does not appear to be exerted by iodine incorporated into the cells.
Second is the concentration of iodine used. The physiological concentration of iodine in human sera is typically 2–6 × 10−8 M (5–15 μg/L) (7), and pretreatment with 10−6 M or more iodine suppresses subsequent iodine uptake and expression of NIS (i.e., Wolff-Chaikoff effect) in FRTL5 cells (8) and porcine thyroid cells in suspension culture (9). 10−3 M iodine is generally used as an excessive dose in FRTL5 and PCCL3 cells, which suppresses the expression of other thyroid differentiation genes such as thyrotropin receptor, thyroglobulin, and thyroid peroxidase (10–12), and induces pendrin expression/iodine efflux (13). Thus, the concentration of iodine used in (1) is 106 times higher than the physiological serum concentration, 104 times higher than that required for the Wolff-Chaikoff effect, and ≥10 times higher than the excess dose used in FRTL5 and PCCL3 cells.
Despite of a clear difference in the ability of iodine uptake between FRTL5 and Nthy-ori 3-1 as mentioned above, cell death can only be induced by very high doses of iodine (≥10−2 M) in both cells (1, 14). The ability of iodine uptake and cytotoxicity of iodine are summarized in the Table 1. In addition, human thyroid cells cultured in the presence of 1 mU/mL TSH are resistant to iodine-induced cell death with up to 3 × 10−2 M iodine for 24 h (15). Therefore, thyroid cells used in all the above reports are relatively resistant to a high dose of iodine, irrespective of their capability of iodine uptake. It is at present unknown as to how extremely high concentrations of iodine in the culture medium exert its cell killing effect in Nthy-ori 3-1 cells. Of interest, a recent paper has described the impairment of reproductive function in male rats by excess iodine, which is thought to be attributed to the aberrant expression of NIS in the testis (16).
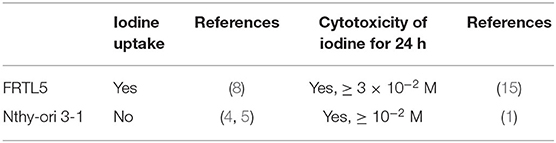
Table 1. Summary of iodine uptake and cytotoxicity of iodine in FRTL5/PCCL3 cells and Nthy-ori 3-1 cells.
Altogether, although it is understandable that one prefers to use the thyroid cells of human origin rather than of rodent origin, I would like to express my concern that Nthy-ori 3-1 cells may not be suitable for studies on the in vitro effect of iodine on thyroid cell function and/or survival.
Author Contributions
YN has written the manuscript.
Funding
Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (19K09028).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Liu J, Mao C, Dong L, Kang P, Ding C, Zheng T, et al. Excessive iodine promotes pyroptosis of thyroid follicular epithelial cells in hashimoto's thyroiditis through the ROS-NF-κB-NLRP3 pathway. Front Endocrinol. (2019) 10:778. doi: 10.3389/fendo.2019.00778
2. Liu H, Zeng Q, Cui Y, Yu L, Zhao L, Hou C, et al. The effects and underlying mechanism of excessive iodide on excessive fluoride-induced thyroid cytotoxicity. Environ Toxicol Pharmacol. (2014) 38:332–40. doi: 10.1016/j.etap.2014.06.008
3. Liu H, Zeng Q, Cui Y, Zhao L, Zhang L, Fu G, et al. The role of the IRE1 pathway in excessive iodide- and/or fluoride-induced apoptosis in Nthy-ori 3-1 cells in vitro. Toxicol Lett. (2014) 224:341–8. doi: 10.1016/j.toxlet.2013.11.001
4. Tuncel M, Aydin D, Yaman E, Tazebay UH, Güç D, Dogan AL, et al. The comparative effects of gene modulators on thyroid-specific genes and radioiodine uptake. Cancer Biother Radiopharm. (2007) 22:443–9. doi: 10.1089/cbr.2006.319.A
5. Lemoine NR, Mayall ES, Jones T, Sheer D, McDermid S, Kendall-Taylor P, et al. Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br J Cancer. (1989) 60:897–903. doi: 10.1038/bjc.1989.387
6. Kurashige T, Shimamura M, Nagayama Y. N-Acetyl-L-cysteine protects thyroid cells against DNA damage induced by external and internal irradiation. Radiat Environ Biophys. (2017) 56:405-412. doi: 10.1007/s00411-017-0711-8
7. Iodine and Inorganic Iodides: Human Health Aspects (CICADS 72) (2009). Available online at: http://www.inchem.org/pages/cicads.html
8. Grollman EF, Smolar A, Ommaya A, Tombaccini D, Santisteban P. Iodine suppression of iodide uptake in FRTL-5 thyroid cells. Endocrinology. (1986) 118:2477–82. doi: 10.1210/endo-118-6-2477
9. Takasu N, Handa Y, Kawaoi A, Shimizu Y, Yamada T. Effects of iodide on thyroid follicle structure and electrophysiological potentials of cultured thyroid cells. Endocrinology. (1985) 117:71–6. doi: 10.1210/endo-117-1-71
10. Leoni SG, Galante PA, Ricarte-Filho JCM, Kimura ET. Differential gene expression analysis of iodide-treated rat thyroid follicular cell line PCCl3. Genomics. (2008) 91:356–66. doi: 10.1016/j.ygeno.2007.12.009
11. Serrano-Nascimento C, Calil-Silveira J, Goulart-Silva F, Nunes MT. New insights about the posttranscriptional mechanisms triggered by iodide excess on sodium/iodide symporter (NIS) expression in PCCl3 cells. Mol Cell Endocrinol. (2012) 349:154–61. doi: 10.1016/j.mce.2011.09.036
12. Eng PH, Cardona GR, Previti MC, Chin WW, Braverman LE. Regulation of the sodium iodide symporter by iodide in FRTL-5 cells. Eur J Endocrinol. (2001) 144:139–44. doi: 10.1530/eje.0.1440139
13. Calil-Silveira J, Serrano-Nascimento C, Kopp PA, Nunes MT. Iodide excess regulates its own efflux: a possible involvement of pendrin. Am J Physiol Cell Physiol. (2016) 310:C576–82. doi: 10.1152/ajpcell.00210.2015
14. Golstein J, Dumont JE. Cytotoxic effects of iodide on thyroid cells: difference between rat thyroid FRTL-5 cell and primary dog thyrocyte responsiveness. J Endocrinol Invest. (1996) 19:119–26. doi: 10.1007/bf03349847
15. Vitale M, Di Matola T, D'Ascoli F, Salzano S, Bogazzi F, Fenzi G, et al. Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinology. (2000) 141:598–605. doi: 10.1210/endo.141.2.7291
Keywords: thyroid cell, iodine uptake, Nthy-ori 3-1, PCCL3, sodium-iodine symporter
Citation: Nagayama Y (2020) Commentary: Excessive Iodine Promotes Pyroptosis of Thyroid Follicular Epithelial Cells in Hashimoto's Thyroiditis Through the ROS-NF-κB-NLRP3 Pathway. Front. Endocrinol. 11:581. doi: 10.3389/fendo.2020.00581
Received: 25 May 2020; Accepted: 16 July 2020;
Published: 28 August 2020.
Edited by:
Laurent M. Sachs, Muséum National d'Histoire Naturelle, FranceReviewed by:
Tommaso Aversa, University of Messina, ItalyCopyright © 2020 Nagayama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuji Nagayama, yujinagayama@gmail.com
 Yuji Nagayama
Yuji Nagayama