- 1School of Allied Health and Community, University of Worcester, Worcester, United Kingdom
- 2Department of Nutrition, Food and Exercise Science, Florida State University, Tallahassee, FL, United States
- 3Moorfields Eye Hospital NHS Foundation Trust, London, United Kingdom
- 4Centre for Medical Education, Medical School, Cardiff University, Cardiff, United Kingdom
Aim: Young people with psychosis have higher rates of obesity, premature cardiovascular disease, and death compared to non-psychotic peers in the general population due to changes in metabolic regulation linked to antipsychotic medication and adverse health risk behaviors. The aim of this paper is to outline the development, implementation, and evaluation of a combined 12-week exercise and health behavior intervention delivered as part of an Early Intervention in Psychosis (EIP) routine service, within the UK.
Methods: Participants (n = 27) completed a 12-week combined intervention program, engaging in weekly, 90-min sessions comprising a healthy behavior education session (45 min), followed by a facilitated exercise session (45 min). Anthropometric data from participants (n = 26) were collected at baseline, 12 weeks, and 12 months post-intervention. Health behaviors and clinical measurements were assessed at baseline and 12 months.
Results: Mean baseline data suggests participants were at an increased health risk on entry to the program, with elevated values in mean body mass index (BMI; 70% overweight/obese), waist circumference, resting heart rate, and triglycerides. Fifty percent reported smoking daily, 64% ate < 5 fruits/vegetables per day, and 52% of participants were prescribed highly obesogenic antipsychotic medications (i.e., Olanzapine). At 12 weeks and 12 months, no changes were observed in mean BMI, waist circumference or any other clinical variable (p > 0.05). At 12 months, participants reported a positive impact on health behaviors including improved diet, increased physical activity levels, and cessation of substance use (n = 2), alcohol use (n = 2), and smoking (n = 4). Focus groups captured participant experiences, engagement with and satisfaction with the program, including challenges/barriers to program adherence.
Conclusions: The 12-week exercise and health behaviors program supported participants to attenuate their physical health risk which was sustained at 12-month follow-up. Self-reported positive health behavior changes are likely to have contributed to the prevention of excessive weight gain in this high-risk period. The evaluation was designed to have validity for a “real world EIP setting” and reflect the complexity of delivery to this participant group. Evaluation findings influenced subsequent commissioning of the physical health intervention as an ongoing element of routine EIP care within the participant site.
Introduction
Individuals with serious mental illness (SMI) have reduced life expectancy compared to non-psychotic peers primarily due to cardiometabolic disorders (1). Multiple etiological factors increase the risk of premature mortality in SMI including metabolic disturbance from second-generation antipsychotic medications (2, 3), adverse health behaviors (4, 5), and alterations in cardiometabolic, immune, and hepatic-pituitary-adrenal systems (6). Young people, with limited previous exposure to antipsychotic agents, appear to be particularly susceptible to rapid and pronounced weight gain when antipsychotic medication is initiated. Approximately, two-thirds of individuals with first episode psychosis (FEP) will experience clinically significant weight gain (by ≥ 7%) during the first 12 months of treatment (7). Propensity to cause weight gain differs between antipsychotics but none are weight neutral (2). Olanzapine is associated with the largest weight effects and higher doses are associated with greater cardiometabolic abnormalities (8). Weight gain is a common unwanted side effect of antipsychotic medication but can also be associated with other adverse lifestyle factors, including sedentary lifestyle, poor diet and unhealthy food habits, as well as an underlying genetic susceptibility to weight gain (9). Antipsychotic medications are also known to impair glucose metabolism, increase cholesterol and triglyceride levels and cause arterial hypertension, leading to metabolic syndrome and a higher risk of diabetes and cardiovascular disease (CVD) (10). Consequently, there has been a growing interest in interventions to control or attenuate weight gain and increased risk of metabolic syndrome to prevent diabetes and CVD in FEP.
Specific targets have been identified for prevention, screening, and treatment of diabetes and CVD in SMI, including programs to address obesity, smoking, hypertension, hyperlipidemia, and sedentary behavior (5, 11–14). Clinical Guidelines (CG155,178,185) and a Quality Standard (QS80) from the National Institute for Health and Care Excellence (15) recommend systematic physical health screening and monitoring for CVD risk, particularly for individuals prescribed antipsychotics, and recommend the use of combined health behavior interventions focused on healthy eating, physical activity (PA), and smoking cessation.
Research has shown a positive impact of physical health screening and intervention for participants with SMI and FEP on health risk behaviors, weight management, and physical health outcomes, findings which should decrease risk of CVD (16–25). Other intervention studies found no positive impact on weight and other long-term cardiovascular risk factors (13, 26, 27) including a large-scale study with schizophrenia and schizoaffective disorder which included FEP patients (28). Despite a growing body of evidence regarding the efficacy of physical health screening and interventions, Deenik et al. (29) suggested it is not sufficient to define policy aims targeting physical health, relying on the results of subsequent efficacy studies. Implementation research is needed to help practitioners develop, implement, and deliver effective real-world interventions in routine clinical practice which considers social determinants of health (funding, transport, social support networks) which may inhibit access to quality health care services (30).
Efforts to improve physical health in SMI patients have encountered patient and service-level barriers to implementation of routine physical healthcare screening, monitoring, and intervention. Patient-level barriers are both practical (money to buy clothing/equipment, transport challenges) and psychological, including psychotic symptoms, anxiety, low motivation, and self-efficacy (31, 32). Service-level barriers include lack of resources for equipment purchase, access to appropriate facilities, staff capacity, leadership engagement, organizational change, and financial policy strategies to support adoption and scaling up of successful intervention programs (33, 34). Gaughran et al. (26) concluded: “The search for effective, pragmatic physical health interventions deliverable in health care services remains”. A pragmatic approach, which considers barriers, social determinants of health and enablers of initial and ongoing participation, assumes particular importance when developing interventions for young people with psychosis (30–32). Further research is needed on effective strategies for program design and guidance for mental healthcare teams to support implementation and delivery of routine health behavior modification programs (35–37). This study comprised two key aims: (a) to document the context and factors influencing design, implementation, and delivery of the intervention, and (b) to evaluate the effectiveness of the intervention in improving clinical health markers and reducing adverse health behaviors that increase risk for cardiometabolic disease and premature mortality in an FEP population.
Materials and Methods
A team of healthcare professionals, researchers and service users were involved in design, implementation, and delivery of the “Supporting Health And Promoting Exercise” (SHAPE) program. Charitable funding from The Health Foundation funded intervention development, implementation, and evaluation. Evaluation employed an effectiveness-implementation hybrid design (35, 38) to assess the implementation of the SHAPE program in routine EIP clinical practice.
Intervention Design and Implementation
The program team, supported by an EIP service user reference group, met regularly to design the intervention and delivery strategy. Discussions were an iterative process to agree educational and exercise program content, location and mode of delivery, assessment of clinical data and health risk behaviors, staff and peer worker roles, branding/logo for the program, and recruitment of participants. It was agreed that an evidence-based approach would be to customize a successful FEP intervention model established within routine EIP care called the “Keeping the Body in Mind” (KBIM) intervention, developed and evaluated by the Bondi EIP service in Sydney, Australia (21, 22). Utilizing this as a framework, the team customized the program to address key factors and social determinants of health associated with implementation in UK EIP service setting.
Key program factors included the need for the intervention environment to foster a positive social identity and social identification (39). Social identity, defined as a person’s sense of who they are within a group or group membership, has been shown to be a key mechanism underpinning the effectiveness of group-based exercise and weight management programs (39). Social identification, a person identifying themselves as a group member, has been found to lead to greater subsequent effort in a group task (40). Participants were provided with a program t-shirt, drinks bottle, pedometer, and tape measure embossed with the SHAPE logo to foster social identity and identification with the group. The program was delivered at a university-community fitness center, instead of a clinical setting, to provide social engagement with other young people in an age-appropriate environment while still providing a clinical and research infra-structure (41).
Behavior modification, social support, and behavior shaping have all been shown to improve intervention effectiveness in SMI, so were embedded within the program design (11, 42). Social support has been shown to be a key component maximizing weight management program retention (42); therefore, we employed university students to support participants through group exercise sessions and to engage with participants in the fitness center outside of the formal SHAPE program on request. Two EIP peer support workers were recruited to support participant engagement and address and allay concerns about attendance and expectations. Peer support was also encouraged through group exercise sessions and team activities. To extend social support outside the program (42), a carers’ evening was incorporated into the program schedule to provide information about the SHAPE program, an opportunity to view the facilities, and an education session targeting family well-being and healthy eating followed by an optional 3-km walk using pedometers.
Access to transport and program accessibility, as well as suitable attire and footwear, were identified as potential barriers to participation (32). The program was delivered at a city center location easily accessible to buses and trains, and/or parking. Participants were encouraged to make their own travel arrangements; however, transport was provided by EIP staff or a community driver when required. Individuals unable to afford suitable exercise attire were supported financially by local charitable grant funding or personal welfare budget funding to reduce potential self-stigma and group marginalization. Mental health nurses from EIP oversaw program delivery and monitored mental and physical well-being and social functioning and fed back observations at weekly EIP team meetings. This close working afforded EIP teams the opportunity to identify and address individual barriers to attendance.
Maintenance of health behaviors and long-term sustainability were identified as key indicators of success. Components to effectively transition participants toward autonomous decision making about their physical health, including goal setting, practical strategies for food shopping, improving PA self-efficacy, and access to local health and fitness services were embedded within the program. Exercise program design was grounded using aspects of self-determination theory (43, 44), including key strategies structured to foster PA self-efficacy and improve perceived competence by offering a range of mastery experiences with education about monitoring exercise intensity and safe exercise progression (45). It was important to develop independent engagement with exercise during the program. To facilitate this, we offered an informal SHAPE session which allowed members a dedicated time to meet socially and exercise together. Toward the end of the program, care coordinators explored local provision to support transition to local fitness facilities for continuation of participants’ exercise regimens at program termination. EIP care planning included a review of program outcomes and how these could be maintained through continued SHAPE program attendance or using alternative local exercise options. SHAPE participants were offered a free gym membership for 12 months at the university fitness center.
Combined Exercise and Health Behavior Intervention
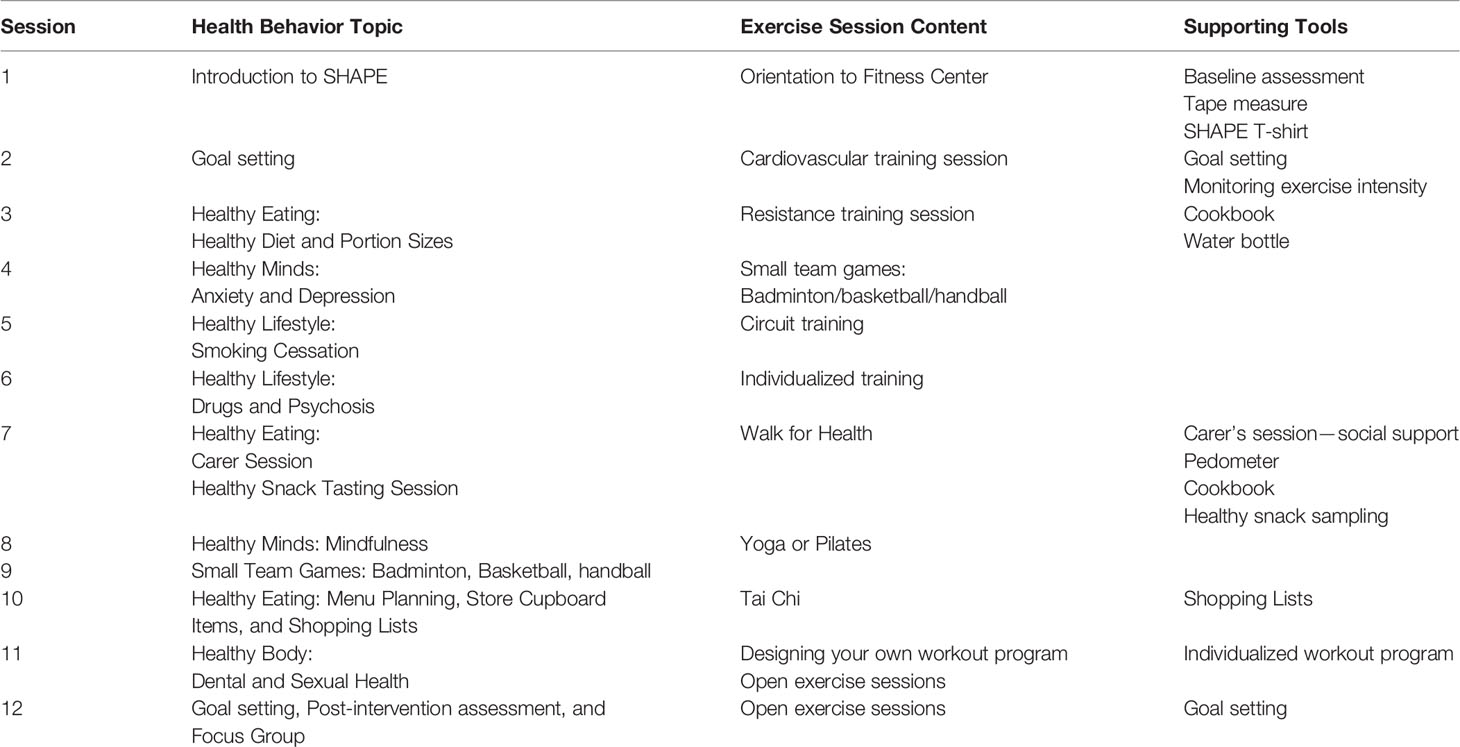
The SHAPE program was delivered as one component of the routine EIP care package. The program was a combined exercise and health behavior intervention delivered over a 12-week period in a weekly supervised group setting. Educational and supervised exercise sessions were designed and delivered to increase the participants’ understanding of how health behaviors impact short- and long-term physical health. Educational sessions included topics on: healthy eating (21, 37), substance avoidance (drugs and alcohol plus smoking cessation) (37, 46–48), mindfulness (49) and personal care (sleep management) (50, 51), sexual health (52), dental hygiene (53), and goal-setting (54). A trained nutritionist delivered three education sessions focused on healthy eating (5 fruit/vegetables per day, a balanced diet, portion sizes), menu planning, shopping lists, eating out and healthy snacking designed to enable participants to make healthier food and drink choices to help off-set weight gains. Specialist nutrition experts leading these sessions were shown to have the greatest impact on weight management and cardiometabolic risk reduction in people with SMI (55). Nutrition sessions were designed to be interactive and were supported with leaflets, a snack tasting session, and a free healthy eating cookbook. Participants were offered one-to-one nutrition support sessions following SHAPE program completion.
Following educational sessions, participants engaged in a 45-min exercise session led by qualified fitness experts and supported by university students. Exercise education included: how to use fitness equipment, how to self-monitor exercise intensity and progression, and appropriate exercise training techniques (56). Activities included: circuit training, cardiovascular exercise, weight training, Tai Chi, yoga, Pilates, power walking, and small team games (badminton, basketball and handball). Exercise sessions were designed to: a) educate participants on how to exercise safely and effectively; and b) improve PA self-efficacy and autonomous motivation by using a variety of exercises in different settings (fitness equipment, group training, small team games) (57). Utilizing a broad range of mastery experiences (opportunities to successfully achieve a goal), vicarious experiences (witnessing someone who is similar performing a task), and positive verbal persuasion (providing feedback on task performance) during exercise may improve self-efficacy and influence sustained behavior change (45, 58). Perceived variation of exercises with a range of tasks in different social contexts may improve individual’s felt experience and perception leading to increased autonomous motivation (59, 60). Table 1 highlights the SHAPE 12-week program schedule.
The program was delivered to five separate cohorts over a 12-month period. Delivery to multiple cohorts allowed for “real-time” participant feedback to modify the program. Focus groups were utilized to capture participant experiences, engagement and satisfaction with the program, including challenges/barriers to program adherence.
Participants
Individuals with FEP, from a countywide EIP service, comprising two teams covering separate geographical catchment areas, were invited to participate in the program. FEP is defined as the first time a person experiences a combination of symptoms known as psychosis. In the UK, EIP services provide support typically for up to the first three years after psychosis onset (61). Clinical diagnosis was determined by a team consultant psychiatrist during routine psychiatric assessment using International Classification of Diseases (ICD)-10 (62) criteria for a psychotic disorder. Participant eligibility was determined by a physical health lead nurse in consultation with case managers and individual service users, in the context of routine individualized care planning. Service users aged under 16 years old, or who were pregnant or lactating, or whose psychotic symptoms were not yet well-controlled on antipsychotic medication were unable to join the program. Service users who were unable to join, declined to attend, failed to engage, or dropped out from the program were given the opportunity to attend a later SHAPE cohort when their mental health had stabilized or other factors inhibiting attendance and participation had been resolved.
Participants provided voluntarily written consent for their routine clinical data to be used in the study. This study conformed to the principles of the Helsinski declaration and was granted ethical approval by the University of Worcester Research Ethics Committee (REC approval number: UWEC2014JS1).
Procedures
Assessment of Cardiometabolic Risk and Health Risk Behaviors
On acceptance onto EIP caseload, participants had a routine physical health assessment conducted by a mental health nurse. Clinical measures included: BMI, waist circumference, resting heart rate and blood pressure, glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), blood lipids, and prolactin. Blood lipids included measures of total cholesterol (TC), low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C), and triglycerides. Abdominal adiposity was assessed using waist circumference where measurements were made at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest (63). Blood tests to determine HbA1c and/or FPG, blood lipids, and prolactin were requested by the patients’ general practitioners and conducted by NHS pathology departments. All blood data were collected from electronic patient records. Physical health markers were assessed against NICE (15) criteria to determine risk levels for cardiovascular and metabolic disorders. All clinical measures were assessed at baseline and 12 months post-intervention as part of routine clinical care monitoring.
Health risk behaviors were assessed using self-reported measures for diet (eating > 5 fruit/vegetables per day; equivalent to 400 g a day based on 80 g portions) (64), tobacco use (current smoker or within last 6 months), alcohol use (Alcohol Use Disorders Identification Test) (65), substance use (yes/no response), and PA levels (Exercise Vital Sign (EVS) (66). Sedentary behaviors were determined as engaging in PA less than < 90 min of moderate PA per week (56). Anthropometric data were collected at week 1, immediately post-intervention (week 12), and 12 months post-intervention.
Program Evaluation
Attendance monitoring and semi-structured focus group discussions generated qualitative feedback as part of an iterative process to develop and refine the SHAPE program to meet user needs. Program adherence was monitored by recording attendance and individuals’ reasons for non-attendance and disengagement. Barriers to attendance were recorded and problem solved in SHAPE and EIP team meetings to support program uptake and adherence.
Focus group discussions were conducted after the last exercise session of the 12-week SHAPE program. The purpose of the focus groups was to provide participant observations and feedback on program participation to support evaluation and validate findings through use of participant quotes. Participants were invited, but not required to attend, and were assured it would not affect or influence program participation, continued use of the fitness facilities, or their EIP care or treatment. Focus groups were semi structured using open ended questions exploring participant reasons for joining SHAPE, experiences of the program, barriers and facilitators affecting participation, impact of the program on weight, PA levels, health behaviors and general functioning as well as suggestions for program change/improvement. Focus groups were conducted by the program lead, a clinical psychologist, who was not involved in the running of the SHAPE program but may have been known to some participants from involvement in their care within the EIP service. A facilitator, known to participants, managed recording equipment and their presence was designed to reduce participant anxiety by providing a familiar face. Focus groups lasted between 45 and 60 min and were audiotaped and transcribed verbatim to support coding and classifying to identify main themes and sub themes (67).
Data and Program Analysis
Baseline analyses were conducted for the total population and by gender. Repeated-measures analysis of variance was performed to determine differences over time for anthropometric data. Paired sample t-tests were used to determine significant differences from baseline to 12 months post-intervention for clinical markers. Data were analyzed using IBM SPSS Statistics for Windows, Version 25.0 (Armonk, NY: IBM Corp). Results are reported as the mean ± standard deviation (SD) and/or as a percent of the population sample. Probability values < 0.05 were considered significant.
Transcribed text from the focus group data were deductively analyzed drawing on pre-determined program factors including program location and environment, program design, program content, social support and impact (68). Transcripts were reviewed for content and coded for correspondence with key program factors and any new themes that emerged inductively from the data.
Key measures for program cost evaluation included: 1) percent change in body mass (69) and subsequent improvement in quality-adjusted life years (QALY), where the cost of Quality of Life Years has been shown to reduce if patients can (a) avoid 7% weight gain and (b) stop smoking (15); and 2) percent change in prevalence (based on BMI change) of CHD and Type 2 diabetes (70).
Results
Participant Recruitment and Program Adherence
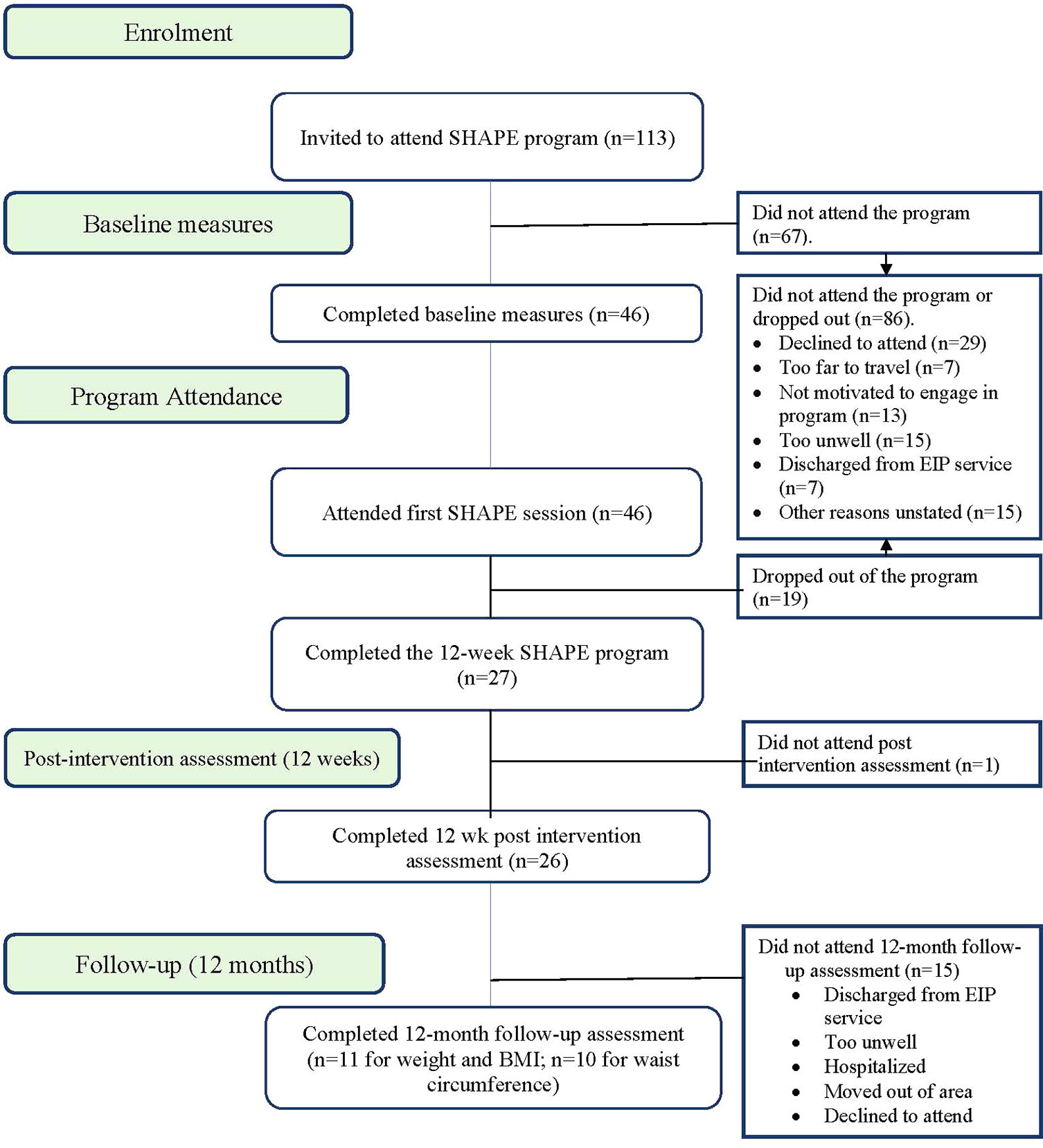
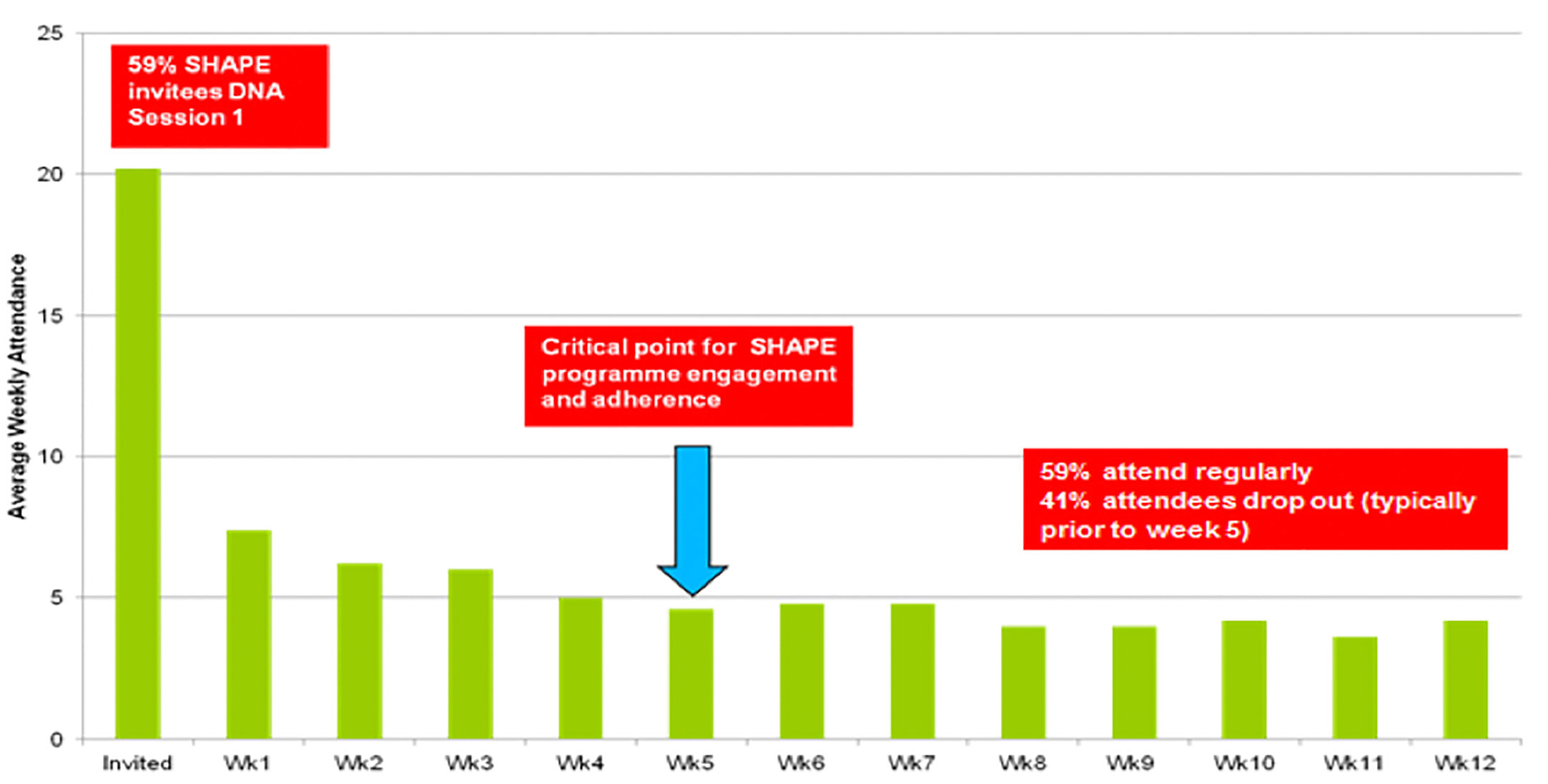
Figure 1 provides program enrollment, program attendance, and post-intervention attendance figures. Figure 2 shows 59% (n = 67) of invitees did not attend the program. Forty-one percent (n = 46) attended the first SHAPE program session. Week 5 was a critical point for program adherence, where participants attending up to this point were more likely to complete the whole program.
Participant Characteristics and Clinical Outcomes
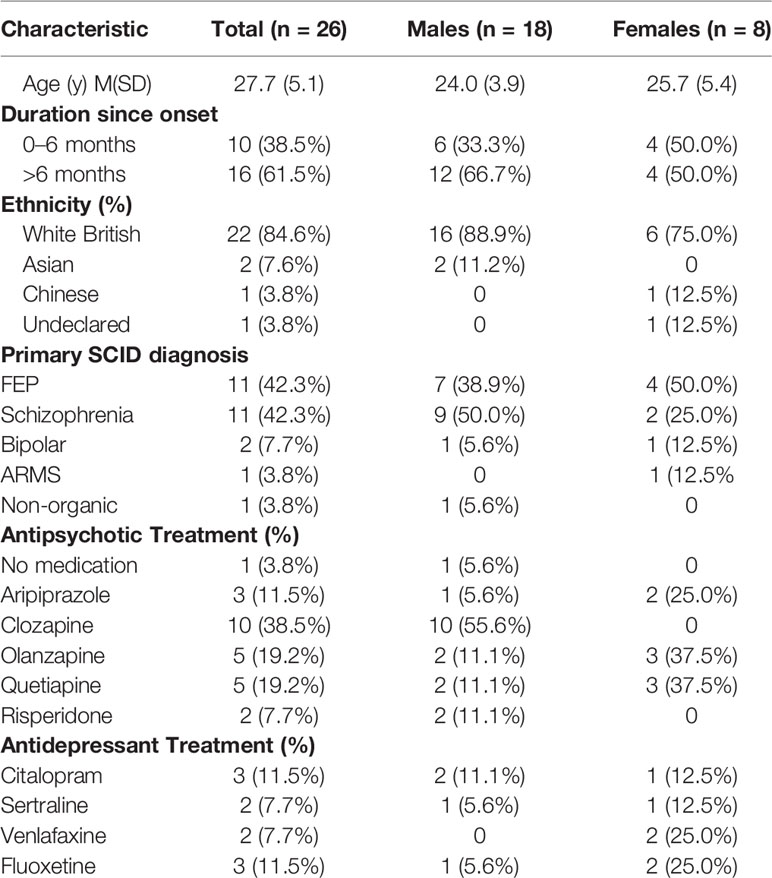
Twenty-seven participants aged 18–37 years completed the SHAPE program. Only 26 participants completed the 12-week post-intervention assessment. Completers were those participants who attended a minimum of five sessions. Table 2 provides demographics, diagnosis, and treatment characteristics by sex for these 26 participants.
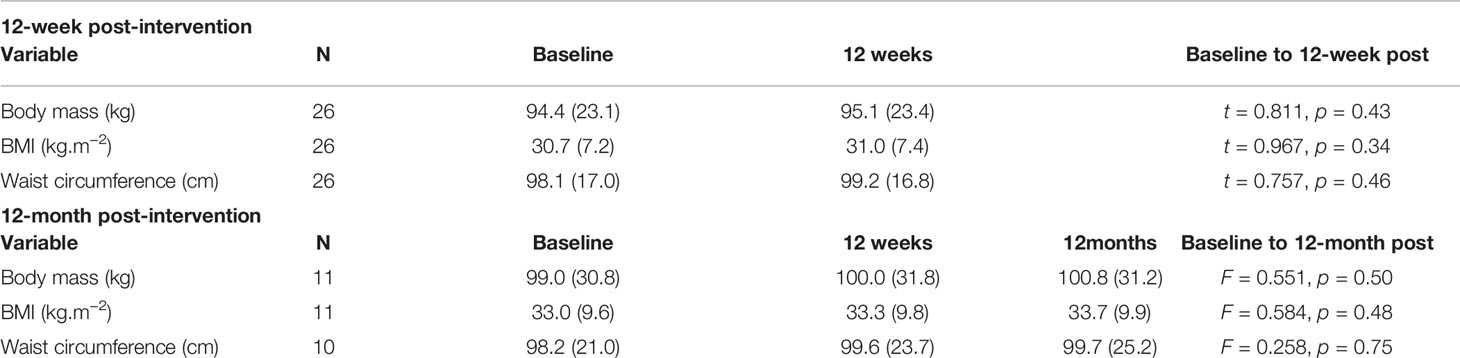
Tables 3 and 4 provide anthropometric and cardiometabolic data at 12-week and at 12-month follow-up. Mean baseline data (n = 26) suggest participants were already at an increased health risk due to elevated values in mean BMI (81% were overweight or obese), waist circumference (73% at increased risk), resting heart rate (64%), and blood pressure (58% pre-hypertensive; 27% hypertensive), and 50% met the criteria for dyslipidemia. Analysis of health behavior data showed that 39% had a sedentary behavior, 73% ate < 5 fruits/vegetables per day (~400 g equivalent), 46% reported smoking daily, 35% consumed alcohol, and 12% engaged in drug use. Fifty-two percent were prescribed the most obesogenic antipsychotic medications (Clozapine and Olanzapine). Over 85% had elevated resting blood pressure (> 120/80 mm Hg).
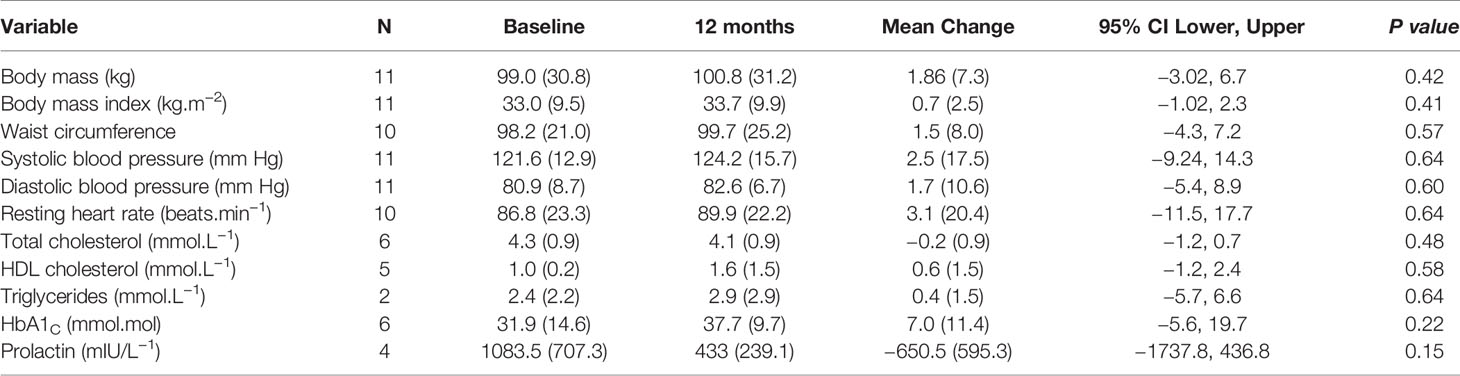
Table 4 Cardiometabolic risk markers and lifestyle behaviors at baseline and 12 months post-intervention [M(SD)].
At 12-week post-intervention (n = 26), there were no statistical changes in mean body mass, BMI, or waist circumference (p > 0.05). Seven participants maintained weight (±1 kg). Seven participants decreased weight (1.3–8.4 kg); four of whom lost > 5% of their body weight. Twelve participants increased weight (1.3–9.6 kg). Three participants (11.5%) exceeded the Lester UK guidelines of > 5 kg within 3 months (71), of whom, two (8.0%) exceeded the cut off for clinically meaningful weight gain > 7% of their bodyweight (16). Both participants were prescribed Olanzapine.
At 12-month post-intervention (n = 11), there was no statistical change in mean BMI, waist circumference, or any other clinical variable (p > 0.05). Two participants maintained weight (±1 kg). Three participants decreased weight (−2.2 to 6.6 kg); 2 of whom lost > 5% of their body weight. Six participants gained 1.3 to 3.4 kg. One individual (9.0%), taking Olanzapine, exceeded > 7% of their bodyweight and the Lester UK > 5-kg threshold. Multivariate analysis revealed no change over time for body mass (F =1.009; p = 0.403, ηp2 = 0.183), BMI (F = 0.792; p = 0.482; ηp2 = 0.150), or waist circumference (F= 0.165; p =0.851, ηp2 = 0.40).
At 12 months, positive impacts on health risk behaviors included: 5 (45%) participants eating > 5 fruit/vegetables daily (~400 g equivalent), 1 (9%) ceased substance use, 2 (18%) ceased alcohol use, 4 (36%) ceased smoking, and 4 (36%) were less sedentary.
Participant Feedback
Focus group quotes to illustrate main program factors are presented within Table 5. One main theme: “impact on wider functioning”, and two subthemes: “feeling better/sense of accomplishment”, and “program commitment”, were constructed inductively from the data during analysis.
Discussion
The current study sought to implement and evaluate a combined exercise and health behavior intervention within routine care practice in a UK EIP service. The SHAPE intervention represents the first UK evaluation of a combined exercise and health behavior intervention specifically targeting FEP to develop positive health behaviors and improve weight maintenance. The study showed that young people with psychosis are already at an increased risk for CVD and metabolic disorders at time of diagnosis, and early intervention to address their physical health may improve health behaviors and attenuate weight gain at 12 weeks with sustained benefits at 12-month follow-up. The combined exercise and health behavior program was offered as a core element of routine individual EIP care planning, but program participation was affected by patient choice, low motivation, geographical constraints, and intrusive mental health symptoms including social anxiety, low mood, and persistent psychotic symptoms.
Changes in Physical Health Outcomes
Participants in this study had elevated anthropometric and clinical markers at baseline indicative of increased CVD risk, likely to be linked to self-reported sedentary behavior, smoking, poor diet, high rates of obesity (4, 5), and antipsychotic medication (7). This aligns with previous research in the USA and Australia which has demonstrated a high prevalence of metabolic syndrome in people with schizophrenia at time of diagnosis and with illness progression (~35%–40%) (72–74); and as a consequence, an increased risk for CVD, impaired daily functioning, and premature mortality (72, 75–77).
As far as we are aware, there are only five small-scale studies internationally (16, 17, 21, 24, 41) which have specifically recruited from EIP services. Our findings concur with similar combined interventions in FEP in Australia, which also demonstrated attenuation of weight gain and lower BMI than controls at 3 months post-intervention (16, 21) as well as self-reported positive health behavior changes in PA, fitness, and diet (21). The SHAPE intervention achieved comparable outcomes and mitigated the expected rise in BMI and associated risks for type 2 diabetes and CHD typically observed in an FEP cohort. SHAPE is the first UK EIP intervention study UK to show that effects are maintained at 12-month follow-up, which, if sustained, would reduce associated longer-term health care and QALY costs. Without intervention, we would expect an FEP group to show additional weight gain over 12-month follow-up based on FEP weight gain projections over time (7).
Two other EIP intervention studies, in Canada (17) and the UK (24), involved an exercise training program only. Abdel-Baki et al. (17) employed a 14-week aerobic interval training (AIT) program and reported a decrease in waist circumference and improvements in cardiorespiratory fitness (p < 0.05). By comparison, SHAPE participants did not show a decrease in waist circumference but maintained mean waist circumference at 12-week and 12-month post-intervention. Firth et al. (24) involved a 10-week individualized supervised exercise program but failed to show any impact on body weight, BMI, or waist circumference although they did report improvements in negative symptoms and social functioning post-intervention. The latter study achieved a much higher program retention rate (81%) than other EIP intervention studies, including this study, but found adherence to unsupervised exercise was halved following program cessation. PA levels decreased significantly, and symptomatic improvements were only maintained for those who continued to exercise at 6-month follow-up (25). The remaining EIP intervention study in Denmark has only published a qualitative analysis to date (41). More research is needed to understand the sustained effectiveness of combined health behavior modification and exercise behavior change on weight maintenance and BMI in reducing cardiometabolic risk in FEP (21) and how changes in exercise and health risk behaviors may mediate these effects and can be successfully maintained in the longer term.
The SHAPE intervention appeared to be effective in attenuating antipsychotic induced weight gain for the majority (75%) of participants. Just under 8% of participants at 12 weeks and 9% at 12 months gained more than 7% of their bodyweight. Using the Lester UK guideline criterion (71), 12% participants were above the cut off at 12 weeks and 9% at 12 months. This is considerably lower than that observed in the Álvarez-Jiménez et al. (16) FEP intervention study where 39% of intervention participants had increased their baseline weight by more than 7% at 3 months. The importance of weight maintenance to reduce the risk of all-cause mortality was highlighted by Prospective Studies Collaboration et al. (78), stating that preventing weight gain from 28 to 32 kg.m−2 during early middle age would yield about 2 years of additional life expectancy. The cost of QALY has shown to be reduced if patients can avoid a 7% weight gain (15). Weight losses of as little as 5% of body weight in individuals at risk of metabolic syndromes can also result in clinically meaningful reductions in morbidity and risk of early mortality (79). In this study, 4 participants at 12 weeks and 2 participants at 12 months had decreased > 5% body mass. It may be plausible to expect that reductions in body weight for these participants could also result in corresponding reductions in morbidity and premature mortality.
Changes in Health Risk Behaviors
Baseline self-report data identified a few adverse health risk behaviors among participants, notably: sedentary behavior, poor dietary intake, and smoking. Positive behavior changes in eating, PA, smoking, and substance use were reported by several participants at 12-month follow-up. These changes are important as they are among the top eight risk factors (alcohol use, tobacco use, high blood pressure, high body mass index, high cholesterol, high blood glucose, low fruit and vegetable intake, and physical inactivity) that account for 61% of cardiovascular deaths and combined, account for over three quarters of ischemic heart disease worldwide (80). The same WHO report identified that reducing these eight risk factors would increase life expectancy by almost five years.
Five participants reported positive changes in their eating habits reflected in eating 400 g equivalent of fruit/vegetables daily which is an important outcome in relation to tackling obesity and reducing the risk of CVD, diabetes, and premature mortality. Adequate consumption of fruit and vegetables reduces the risk for CVD, stomach cancer, and colorectal cancer (81, 82). Similarly, increases in PA are important in people with psychosis who have been shown to be more sedentary (83, 84) and, therefore, at greater risk of obesity and cardiometabolic diseases than their non-psychotic peers (85).
Four participants ceased smoking. Cessation of and reductions in smoking are also important as this has been identified as the most important risk factor for poor health and reduced life expectancy. It is the largest contributor to preventable illnesses including cardiovascular and respiratory diseases and explains much of the excess mortality and reduced life expectancy of people with mental health problems (46, 86). The benefits for smoking cessation and reduction are not just in relation to long term health risks but can also contribute to more immediate benefits in terms of lower stress levels, reduced financial burden associated with the costs of smoking, and improvements in mental health (87, 88).
Alcohol use has also been identified as one of the top five global risks for burden of disease where it is jointly responsible for one fifth of all Disability Adjusted Life Years (DALYs) (years of life lost due to premature death plus years of healthy life lost due to illness and disability), and one quarter of all deaths in the world (89). Two participants had ceased alcohol use at 12 months. Reducing use of alcohol would be expected to have a positive impact on life expectancy by nearly 5 years (89).
Focus group participants attributed weight, exercise, and health behavior changes to the SHAPE program and commented on wider beneficial impacts on their mental health including mood, motivation and functioning. Positive self-reported health behavior changes at 12 months are likely to have contributed to weight attenuation and other physical health benefits observed. Limitations of the study design preclude our ability to show definite associations between health behavior changes and changes in weight, BMI, and other cardiometabolic measures observed over time.
Program Engagement, Drop Out, and Adherence
Program recruitment was time consuming and required clinical team members to prioritize SHAPE involvement alongside other clinical roles. Young people with psychosis can experience many difficulties associated with their mental health including psychotic symptoms, social anxiety, and poor motivation (32). For some, the prospect of a group-delivered program was particularly daunting. Feedback from participants highlighted challenges associated with travel time and cost, group size, social anxiety, mood instability, difficulties coping, and maintaining motivation. Two thirds (59%) of invitees did not attend the first session due to lack of interest, poor motivation, poor mental health or travel concerns. Timing of program sessions was important where participant feedback stated that anything before 11 am was difficult, which led to scheduled sessions being moved to late morning to support attendance. Forty-one percent of participants dropped out of the program. This is not unique to SHAPE and appears to be a common observation in other EIP physical health intervention studies. STEPWISE self-management education program (Holt et al., 2019) also found program recruitment challenging, where 17% of recruits did not attend any sessions and only 23% attended all seven core and booster sessions. Abdel-Baki et al. (17) reported a drop out rate of 36% where only 64% of their participants completed the training program. Curtis et al. (21) reported attrition rates of 62% for the KBIM intervention and 52% for their standard care comparison group. In the COPUS intervention trial (41), 38% discontinued the intervention with the majority dropping out prior to the start or early in the program and only 23% of participants attended the final session of their 8-week program. Disengagement is a common challenge for EIP services and in FEP studies and has been linked to a number of factors including duration of untreated psychosis, symptom severity at baseline, insight, substance abuse, and lack of family involvement (90). Findings from the study identified the need for future program design to be cognizant of social determinants (finance, transport, support networks) influencing access to health care services and sustained program engagement.
Some participants were motivated and travelled considerable distances to attend weekly sessions, although, travel was an issue for several participants. The EIP service serves a rural catchment area (~630 square miles) which is challenging when identifying a suitable program location. Holt et al. (2019) supported program access using taxis which EIP staff found helpful but commented that participants were unlikely to have attended without this provision and doubted the financial sustainability in routine practice (34). When considering program sustainability, a single location may be an impediment to attendance, particularly for poorly motivated individuals; utilizing multiple locations may increase program accessibility.
Participant Experiences of and Feedback on the Program
Focus group feedback provided an iterative service improvement loop and enhanced program evaluation. Participants were positive about all aspects of the intervention and provided helpful feedback on program environment, location, timing, accessibility, and content and how these positively influenced motivation and program adherence. A couple talked about the value of the SHAPE t-shirts in creating a group identity. Several commented on the program delivery style encouraging self-determination and autonomy in relation to health choices, and confidence and mastery in relation to using gym exercise equipment. Participants commented on the different session components particularly appreciating the variety of educational session content and enjoyment of different group sports and particular items of gym equipment. They specifically talked about the value of goal setting, keeping an activity log, and the cookbook and step counter as helpful supporting tools. One participant suggested that step counters could have been introduced earlier in the program to encourage goal setting, monitor weekly activity levels and progress over the program course. Another suggested that an app, like MyFitness Pal, could assist with recording and monitoring activity. A number of health benefits from the program, beyond increasing PA or losing weight were identified. The provision of interactive nutrition sessions, including healthy food “sampling” sessions, encouraged participants to try new foods and consider accessible changes to their diet. The group-based delivery of the program reduced social isolation and encouraged group cohesion and contact between participants, both within and outside of the formal SHAPE sessions which was clearly valued by participants. The program also led to reported benefits in motivation, confidence, social anxiety, self-esteem, and smoking cessation and a broader impact on functioning, routine, and mood. Several participants talked about how the program had been a springboard enabling them to take control of and sustain health behavior changes. Participants valued family support and feedback on observed changes in weight, confidence and functioning. Focus group interviews also allowed exploration of factors affecting program adherence and barriers to participation, which were both logistical (cost, distance, and transport ease) as well as psychological (anxiety, low motivation, poor concentration, coping, and confidence related to mental health). These findings are consistent with several recent qualitative evaluations of health behavior programs with FEP groups which identified common features that were valued by participants and reported similar benefits from and barriers affecting participation and adherence (31, 32, 34, 41, 91, 92).
Program Design and Implementation
Deenik et al. (29) highlighted a need for effective physical health intervention studies with SMI to be translated into real-world settings, suggesting that research on program implementation in standard care practice is an essential step to translate effective health behavior interventions into routine mental health care. Unfortunately, there remains a “research to real-world” gap where research innovations are rarely effectively adopted and implemented in real-world health care settings (93), Both studies highlighted barriers to effective implementation including poor alignment between researchers, health care systems, and providers. They argued that moving research “from innovation to application” requires local input, using local options, and developed using a sustainable approach that enables frontline workers to continue implementation and delivery. Unlike many previous physical health interventions which started as a randomized clinical trial or research-based intervention, the SHAPE program was designed, implemented, and delivered in partnership with EIP service users, the EIP service team, and local researchers and health care professionals. All team members worked locally, were familiar with geography and infrastructure challenges of a large rural county catchment area, and aware of opportunities and local resources that could be harnessed to maximize intervention program design and delivery.
The customized intervention offered a coordinated, multi-professional, health and well-being intervention program which was designed to encourage participants to meet peer SHAPE participants and university student “exercise buddies” within a positive, youth friendly, socially inclusive setting. Other UK physical health intervention programs in FEP (24) did not include a group exercise component (42) or peer support opportunities (94), which have been identified as important factors in long term exercise program adherence and maintenance. In contrast to the Australian KBIM study (21), the SHAPE program was located at an accessible city center location within a university-exercise setting, which was deemed to be more normalizing than a clinical healthcare setting. Firth et al. (24) and Larsen et al. (41) similarly elected to locate their interventions in community fitness facilities. The delivery of exercise interventions in a clinical setting may engender health and safety concerns without having qualified instructors supervising exercise as well as equipment purchase and appropriate space concerns that might impinge on program scope and flexibility. Mental health care services may need to explore local resources and identify partners, such as exercise referral schemes or other community-based interventions, willing to collaborate to deliver lifestyle interventions outside the typical clinical health setting (37).
Similar to this study, participants in the COPUS Trial (41) stated that they enjoyed engaging in a “real-world” exercise setting and felt they were treated by exercise instructors as “normal”. Instructors and student interns engaged with clients during exercise providing instruction and motivational support; this also provided opportunities to monitor participant progress and engagement during the session. SHAPE participants could arrange to meet exercise buddies to use fitness facilities and follow their individualized exercise program outside of the formal SHAPE sessions. Involving University students in program delivery enhanced the “normalizing approach”, increased the mental health awareness of student instructors, and was evaluated positively by SHAPE participants, several of whom expressed an interest in becoming peer support workers on the SHAPE program or pursuing higher education study opportunities themselves. A combined exercise and health behavior group-based intervention appears to be most impactful for young people with FEP based on published studies to date (16, 17, 21, 24).
Program Challenges
Initially, attitudes toward the intervention within EIP service were mixed as several team members questioned whether this should be a treatment priority or an appropriate focus for the team, perceiving it as a primary care responsibility. Training sessions with EIP staff were provided by the program team about the importance of early physical health, their role in promoting good physical health (including comprehensive repeat physical health assessment and monitoring), and the need to increase referrals to the SHAPE program and to other intervention services including smoking cessation programs and sexual health services.
Changes to clinical practice were required to ensure standardized monitoring of physical health including: 1) improved physical health monitoring and intervention through comprehensive physical health checks, 2) monitoring, recording and evaluating physiological changes that increase risk of CVD and diabetes, 3) early identification of individuals at high risk for cardiometabolic disorders and referral for specialist intervention, and 4) increased communication with general practitioners to improve physical health care planning. These observations are not unique, Blythe and White (95) reported that London-based mental health nurses (n = 585) received limited educational support in their role to monitor physical health and poor communication between primary and secondary care services impacted their ability to address physical health needs of SMI patients.
Program Costs and Cost Effectiveness
Currently, there is a lack of cost effectiveness research examining supervised exercise programs in SMI, apart from two large scale unsuccessful intervention trials which reported no evidence for their cost effectiveness (28, 96). No EIP studies to date have reported on cost effectiveness. However, similar programs for diabetes, heart disease, and depression have shown exercise programs can produce large economic benefits (97).
Total setup and annual delivery costs of delivering a 12-week SHAPE program to 4 cohorts per year at 4 locations simultaneously within the County (total reach = 125–150 patients) were estimated at £40,000 pa (2015 prices) at an approximate cost of £260–£400 per person per annum. Implementation costs included staff training, recruitment of a program lead, SHAPE package and materials, venue costs, speakers for educational sessions and transport costs. This model allowed for sustainable delivery and for SHAPE to be delivered as one element in an integrated NHS mental and physical health care package.
Weight maintenance and reduction has been linked to improved life expectancy of 2–3 years and a reduced QALY cost if patients can avoid a 7% weight gain (15, 78). Equally, changes in eating, PA, smoking, and alcohol use reported by several participants at 12-month follow-up could potentially increase life expectancy by almost 5 years (89). This does assume participant engagement and program retention and for reported outcomes to be maintained long enough to benefit from the health states associated with them. Based on this, the estimated intervention costs and QALYs improvements linked to SHAPE outcomes would suggest that the SHAPE program is likely to be a cost-effective and value for money intervention. More research is needed on cost effectiveness of physical health intervention programs in FEP considering the unique barriers young people face when engaging in an exercise and health behavior intervention (31, 32) and their impact on successful program engagement, adherence, and likely outcomes.
Program Sustainability and Scale Up
Implementation and sustainability in a real-world setting eludes the majority of intervention studies to date and requires greater integration of an intervention program within the organization in which it is being implemented (coordination of resources, staffing) as well ongoing resourcing to sustain programs in the long term beyond pilot intervention trials (34, 98). Our program was successfully integrated into existing UK National Health Service (NHS) care to provide service users education and support to achieve sustainable health behavior change.
SHAPE data was used to make a case for investment and funding for the program to be scaled up and delivered in multiple sites countywide. SHAPE was formally commissioned and has since been rolled out across the County with SHAPE programs running weekly in six localities across the catchment area. The SHAPE model was included as a good practice case example on the NICE shared learning database (99) and adopted by other EIP teams regionally and nationally. The SHAPE physical health lead nurse role has been extended for the whole NHS Trust and a permanent exercise coach was employed to support the SHAPE program. Training on how, when and what to screen when undertaking routine physical health monitoring and the importance of a consistent, unified approach to collecting physiological measures, awareness of risk indicators, and what kinds of interventions are appropriate in response to risks identified has been rolled out for mental health professionals locally. Online lifestyle and nutrition education materials and a manual and toolkit were made available on a bespoke SHAPE program website.
Strengths and Limitations
A “real-world”, effectiveness-implementation hybrid approach to program evaluation was designed to enhance external validity and transfer to other “real-world” settings, which has been problematic with previous randomized controlled trials. Unlike other EIP physical health intervention studies which have employed a “waiting-list” control group (41) or a comparison “treatment as usual” FEP sample from the same service (24), the KBIM intervention was also embedded into routine EIP care (21) and similarly chose to draw on a comparison group recruited from another New South Wales EIP service who received “standard care”. Without a matched control group, we cannot definitively attribute changes reported to the SHAPE intervention, particularly when delivered as one element in a multi-component EIP routine care package, although participant focus group feedback and self-reported health behavior and exercise changes were attributed by participants to SHAPE program participation.
The participant sample is relatively small, although it is the largest intervention sample of all published EIP studies to date. Access to health records was limited to those participants who provided informed consent, so findings may not be reflective of all SHAPE participants or all people with early psychosis under the care of the EIP service. Current study findings are based on outcomes entirely from one EIP service in one geographical area and thus, a sample bias might exist. The area from which the sample was drawn is a mixed urban/rural county with varying levels of economic and social deprivation which might impact on engagement, adherence, and needs of participants. Moreover, it is possible that participants who declined assessments (and not included in the data sample) might have more risk factors for poor physical health linked to their circumstances and perhaps be less likely to report similar views to program participant peers. Equally, program participants might be more likely to engage with assessments and report benefits.
The results of the clinical data should be interpreted in the context of several important limitations. Measures employed in the study evaluation were restricted to those measures carried out at baseline and repeated annually as part of routine physical health assessment and review processes. Health risk behaviors were only routinely re-assessed as part of an annual physical health review and not available at 12-week post-intervention. Clinical measures from the two EIP teams were not always directly comparable with one another as there was no standardized approach in requesting clinical screening data at baseline or at 1 year into service. General practitioners made individual decisions regarding which blood tests were performed making it difficult to compare across teams and timepoints. To ensure a consistent, unified approach to collecting physiological measures (i.e., tests performed and timing) requires agreement across EIP teams in conjunction with primary care services. Similarly, there was some attrition in the 12-month follow-up data sample where health records were not accessible to extract 12-month follow-up physical health review outcomes. Data on antipsychotic exposure, adherence to antipsychotic medication, as well as any changes in antipsychotic medication type and dosage during the 12-month follow-up period were not captured and may be potential confounds influencing study findings.
A semi-structured focus group may have constrained participant responses by encouraging conformity and strategic biases and inhibiting participants from talking about issues that were perceived as particularly relevant to them, rather than a priori issues chosen by the program team. Some focus group participants may have been reluctant to talk about potentially sensitive topics in relation to their experience of other participants, negative consequences, or personal information in relation to impact on weight or health behavior in a group discussion setting. Participants separately consented to participate in the focus groups which not all participants chose to attend. Themes identified in the focus group transcript may be subject to bias and not necessarily reflect the experiences of all SHAPE participants or dropouts. Focus groups were conducted by the team members known to some participants within the EIP service. This may have led to an investigator bias in the way questions were asked and elaborated and a desirability bias on the part of participants affecting their responses. Transcripts were examined for main themes and may be subject to inherent investigator bias as the authors were involved in program design and evaluation.
Considerations and Learning for Future Physical Health Intervention Programs
Future intervention program design should consider combining exercise with lifestyle interventions that are grounded in behavior change theory to foster self-efficacy and improve perceived competence of program participants. Effective implementation requires close alignment between researchers, health care systems, and community providers to develop a sustainable approach and ensure continued implementation and delivery in routine healthcare. Intervention programs need to be of sufficient duration to provide practical skills for exercise, healthy eating, building self-efficacy and autonomy as well as an individualized transition strategy to sustain program outcomes. Consideration of the unique barriers, particularly funding concerns, that young people describe when engaging in an exercise and lifestyle intervention may be critical to successful program engagement and adherence.
Conclusion
At the time of diagnosis, SHAPE participants were already at an increased risk for cardiometabolic disorders due to elevated clinical markers and unhealthy health behaviors. The findings suggest SHAPE supported young people with FEP to attenuate their physical health risk following a 12-week combined exercise and health behavior intervention which was sustained at 12-month follow-up. Positive health behavior changes reported at 12-month follow-up may have mediated the sustained weight maintenance and improved physical health outcomes observed. Physical health interventions are needed early in the treatment process to address the increased risk for cardiometabolic disorders in individuals recently diagnosed with FEP. Using an effectiveness-implementation hybrid model, to design and evaluate a combined exercise and health behavior intervention for FEP patients in a real-world setting, may enhance program effectiveness and subsequent uptake of effective interventions into routine care.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Worcester Research Ethics Committee (REC approval number: UWEC2014JS1). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JS, LG, MB, BW, and EB devised the original protocol. JS, LG, MB, BW, EB, JB, RD, RH-S, and DH refined the protocol. JS was the chief investigator and oversaw the study. MB and RH-S recruited participants. MB and LG were responsible for study procedures. JS, BW, EB, JB, RD, DH, and RH-S provided feedback on the recruitment methods and helped refine the study procedures. MB, JB, RD, RH-S, and BW delivered the intervention. LG did the quantitative data analysis. JS did the qualitative data analysis. JS oversaw the data analysis. The writing team consisted of JS and LG who drafted and edited the report manuscript. DH and EB proofread the final manuscript. JS, LG, MB, BW, EB, JB, RD, RH-S, and DH critically reviewed drafts of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The Health Foundation: Shine Award 2014 and Spreading Improvement Award 2015; Postcode Anywhere, Diglis Basin, Worcester provided small grant funding for participant equipment (pedometers, water bottles, and tape measures).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The SHAPE program was supported by the Health Foundation, an independent charity working to improve the quality of healthcare in the UK. We would like to thank all of the stakeholders: Worcestershire Early Intervention Service, Worcestershire Health and Care NHS Trust, University of Worcester McClelland Centre, SHAPE participants and carers, health care professionals, University staff and students who took part in or supported the program. We would also like to thank Dr. David Shiers for his support and advice when establishing the SHAPE Program and his valuable comments and feedback on earlier manuscript drafts.
Abbreviations
CVD, Cardiovascular Disease; EIP, Early Intervention in Psychosis; FEP, First Episode of Psychosis; KBIM, Keeping the Body in Mind; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; PA, Physical Activity; PHE, Public Health England; SHAPE, Supporting Health and Promoting Exercise; SMI, Serious Mental Illness; QALY, Quality-Adjusted Life Years; UK, United Kingdom; WHO, World Health Organization.
References
1. Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry (2017) 16:163–80. doi: 10.1002/wps.20420
2. Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PloS One (2014) 9(4):e94112. doi: 10.1371/journal.pone.0094112
3. Balõtšev R, Haring L, Koido K, Leping V, Kriisa K, Zilmer M, et al. Antipsychotic treatment is associated with inflammatory and metabolic biomarkers alterations among first-episode psychosis patients: A 7-month follow-up study. Early Interv Psychiatry (2019) 13(1):101–09. doi: 10.1111/eip.12457
4. Bushe C, Taylor M, Haukka J. Mortality in schizophrenia: a clinical endpoint. J Psychopharmacol (2010) 24:17–25. doi: 10.1177/1359786810382468
5. Galletly CA, Foley DL, Waterreus A, Watts GF, Castle DJ, McGrath JJ, et al. Cardiometabolic risk factors in people with psychotic disorders: the second Australian national survey of psychosis. Aust NZ J Psychiat (2012) 46(8):753–61. doi: 10.1177/0004867412453089
6. Pillinger T, D’Ambrosio E, McCutcheon R, Howes OD. Is psychosis a multisystem disorder? A meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Mol Psychiatry (2019) 24(6):776–94. doi: 10.1038/s41380-018-0058-9
7. Álvarez-Jiménez M, González-Blanch C, Crespo-Facorro B, Hetrick S, Rodríguez-Sánchez JM, Pérez-Iglesias R, et al. Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs (2008) 22(7):547–62. doi: 10.2165/00023210-200822070-00002
8. Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-Time use in children and adolescents. JAMA (2009) 302(16):1765–73. doi: 10.1001/jama.2009.1549
9. Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsych Dis Treat (2017) 13:2231–41. doi: 10.2147/NDT.S113099
10. De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol (2011) 8(2):114–26. doi: 10.1038/nrendo.2011.156
11. Richardson C, Faulkner G, McDevitt J, Skrinar G, Hutchinson D, Piette J. Integrating physical activity into mental health services for persons with serious mental illness. Psychiatr Serv (2005) 56:324–31. doi: 10.1176/appi.ps.56.3.324
12. Latoo J, Omodunbi O, Hindley D, Derbyshire A, Kane R. Physical health of people with severe mental illness: Don’t just screen… intervene. Age (2015) 14(36):15–36. doi: 10.1177/1755738018823800
13. Osborn D, Burton A, Hunter R, Marston L, Atkins L, Barnes T, et al. Clinical and cost-effectiveness of an intervention for reducing cholesterol and cardiovascular risk for people with severe mental illness in English primary care: a cluster randomised controlled trial. Lancet Psychiatry (2018) 5(2):145–54. doi: 10.1016/S2215-0366(18)30007-5
14. Gilbody S, Peckham E, Bailey D, Arundel C, Heron P, Crosland S, et al. Smoking cessation for people with severe mental illness (SCIMITAR+): a pragmatic randomised controlled trial. Lancet Psychiatry (2019) 6:379–90. doi: 10.1016/S2215-0366(19)30047-1
15. National Institute for Health and Care Excellence. Clinical Guideline [CG178]. Psychosis and schizophrenia in adults: prevention and management. London: National Institute for Health and Care Excellence (2014).
16. Álvarez-Jiménez M, González-Blanch C, Vázquez-Barquero JL, Pérez-Iglesias R, Martínez-García O, Pérez-Pardal T, et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: A randomized controlled trial. J Clin Psychiatry (2006) 67:1253–60. doi: 10.4088/JCP.v67n0812
17. Abdel-Baki A, Brazzini-Poisson V, Marois F, Letendre É, Karelis AD. Effects of aerobic interval training on metabolic complications and cardiorespiratory fitness in young adults with psychotic disorders: a pilot study. Schizophr Res (2013) 149:112–15. doi: 10.1016/j.schres.2013.06.040
18. Scheewe TW, Backx FJG, Takken T, Jörg F, van Strater ACP, Kroes RS, et al. Exercise therapy improves mental and physical health in schizophrenia: A randomised controlled trial. Acta Psychiatr Scand (2013) 127:464–73. doi: 10.1111/acps.12029
19. Bruins J, Jorg F, Bruggeman R, Sloof C, Corpeleijn E, Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: A meta-analysis. PloS One (2014) 9(12):e112276. doi: 10.1371/journal.pone.0112276
20. Naslund JA, Whiteman KL, McHugo GJ, Aschbrenner KA, Marsch LA, Bartels SJ. Lifestyle interventions for weight loss among overweight and obese adults with serious mental illness: a systematic review and meta-analysis. Gen Hosp Psychiatry (2017) 47:83–102. doi: 10.1016/j.genhosppsych.2017.04.003
21. Curtis J, Watkins A, Rosenbaum S. Keeping the Body in Mind: An individualised lifestyle and life-skills intervention to prevent antipsychotic-induced weight gain in first episode psychosis. Early Interv Psychiatry (2016) 10(3):267–76. doi: 10.1111/eip.12230
22. Curtis J, Watkins A, Teasdale S, Lederman O, Kalucy M, Lappin J, et al. 2-year follow-up: Still keeping the body in mind. Aust NZ J Psychiatry (2018) 52(6):602–3. doi: 10.1177/0004867417753553
23. Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med (2015) 45(7):1343–61. doi: 10.1017/S0033291714003110
24. Firth J, Carney R, Elliott R, French P, Parker S, McIntyre R, et al. Exercise as an intervention for first-episode psychosis: a feasibility study. Early Interv Psychiatry (2018a) 12(3):307–15. doi: 10.1111/eip.12329
25. Firth J, Carney R, French P, Elliott R, Cotter J, Yung A. Long-term maintenance and effects of exercise in early psychosis: a six-month follow up to an intervention study. Early Interv Psychiatry (2018b) 12(4):578–85. doi: 10.1111/eip.12365
26. Gaughran F, Stahl D, Ismail K, Greenwood K, Atakan Z, Gardner-Sood P, et al. Randomised control trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT). BMC Psychiatry (2017) 17:413. doi: 10.1186/s12888-017-1571-0
27. Speyer H, Norgaard HC, Birk M, Karlsen M, Storch Jakobsen A, Pedersen K, et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry (2016) 15:155–65. doi: 10.1002/wps.20318
28. Holt R, Gossage-Worrall R, Hind D, Bradburn MJ, Mccrone P, Morris T, et al. Structured lifestyle education for people with schizophrenia, schizoaffective disorder and first-episode psychosis (STEPWISE): randomised controlled trial. Brit J Psychiatr (2019) 214(02):63–73. doi: 10.1192/bjp.2018.167
29. Deenik J, Czosnek L, Teasdale SB, Stubbs B, Firth J, Schuch FB, et al. From impact factors to real impact: translating evidence on lifestyle interventions into routine mental health care. Transl Behav Med (2019), 10(4):1070–73. doi: 10.1093/tbm/ibz067
30. Adler NE, Glymour MM, Fielding J. Addressing social determinants of health and health inequalities. JAMA (2016) 316(16):1641–42. doi: 10.1001/jama.2016.14058
31. Firth J, Rosenbaum S, Stubbs B, Gorczynski P, Yung AR, Vancampfort D. Motivating factors and barriers towards exercise in severe mental illness: A systematic review and meta-analysis. Psychol Med (2016b) 46:2869–81. doi: 10.1017/S0033291716001732
32. Brooke LE, Gucciardi DF, Ntoumanis N, Lin A. Qualitative investigation of perceived barriers to and enablers of sport participation for young people with first episode psychosis. Early Interv Psychiatry (2020) 14:293–306. doi: 10.1111/eip.12854
33. De Hert M, Cohen D, Bobes J, Cetkovich-Bakmas M, Leucht S, Ndetei DM, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry (2011) 10(2):138–51. doi: 10.1002/j.2051-5545.2011.tb00036.x
34. Gossage-Worrall R, Hind D, Barnard-Kelly KD, Shiers D, Etherington A, Swaby L, et al. STructured lifestyle education for people WIth SchizophrEnia (STEPWISE): mixed methods process evaluation of a group-based lifestyle education programme to support weight loss in people with schizophrenia. BMC Psychiatry (2019) 19:358. doi: 10.1186/s12888-019-2282-5
35. Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. Brit Med J (2013) 347:f6753. doi: 10.1136/bmj.f6753
36. Lederman O, Suetani S, Stanton R, Chapman J, Korman N, Rosenbaum S, et al. Embedding exercise interventions as routine mental health care: implementation strategies in residential, inpatient and community settings. Australas Psychiatry (2017) 25(5):451–55. doi: 10.1177/1039856217711054
37. Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry (2019) 6(8):675–712. doi: 10.1016/S2215-0366(19)30387-6
38. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care (2012) 50(3):217. doi: 10.1097/MLR.0b013e3182408812
39. Stevens M, Rees T, Coffee P, Steffens NK, Haslam SA, Polman R. A social identity approach to understanding and promoting physical activity. Sports Med (2017) 47:1911–18. doi: 10.1007/s40279-017-0720-4
40. Høigaard R, Boen F, De Cuyper B, Peters DM. Team identification reduces social loafing and promotes social laboring in cycling. Int J Appl Sports Sci (2013) 25:33–40. doi: 10.24985/ijass.2013.25.1.33
41. Larsen LQ, Schnor H, Tersbøl BP, Ebdrup BH, Nordsborg NB, Midtgaard J. The impact of exercise training complementary to early intervention in patients with first-episode psychosis: a qualitative sub-study from a randomized controlled feasibility trial. BMC Psychiatry (2019) 19:192. doi: 10.1186/s12888-019-2179-3
42. Public Health England. Uptake and retention in group-based weight-management services Literature review and behavioural analysis. Final report. London: Public Health England (2018).
43. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol (2000) 55:68–78. doi: 10.1037/0003-066X.55.1.68
44. Hagger MS. Chatzisarantis NLD. Self-determination theory and the psychology of exercise. Int Rev Sport Exerc Psychol (2008) 1:79–103. doi: 10.1080/17509840701827437
45. Artinian NT, Fletcher GF, Mozaffarian D, Kris-Etherton P, Van Horn L, Lichtenstein AH, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circ (2010) 122(4):406–41. doi: 10.1161/CIR.0b013e3181e8edf1
46. Campion J, Shiers D, Britton J, Gilbody S, Bradshaw T. Primary care guidance on smoking and mental disorders—update 2014. London: Royal College of General Practitioners and Royal College of Psychiatrists (2014).
47. Gilbody S, Peckham E, Man MS, Mitchell N, Li J, Becque T, et al. Bespoke smoking cessation for people with severe mental ill health (SCIMITAR): a pilot randomised controlled trial. Lancet Psychiatry (2015) 2(5):395–402. doi: 10.1016/S2215-0366(15)00091-7
48. Wisdom JP, Manuel JI, Drake RE. Substance use disorder among people with first-episode psychosis: a systematic review of course and treatment. Psychiat Service Serv (2011) 62(9):1007–12. doi: 10.1176/ps.62.9.pss6209_1007
49. Carrière K, Khoury B, Günak MM, Knäuper B. Mindfulness-based interventions for weight loss: a systematic review and meta-analysis. Obes Rev (2018) 19(2):164–77. doi: 10.1111/obr.12623
50. St-Onge MP. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev (2017) 18:34–9. doi: 10.1111/obr.12499
51. Tubbs AS, Khader W, Fernandez F, Grandner MA. The common denominators of sleep, obesity, and psychopathology. Curr Opin Psychol (2020) 34:84–8. doi: 10.1016/j.copsyc.2019.11.003
52. Adan Sanchez AY, McMillan E, Bhaduri A, Pehlivan N, Monson K, Badcock P, et al. High-risk sexual behaviour in young people with mental health disorders. Early Interv Psychiatry (2019) 13(4):867–73. doi: 10.1111/eip.12688
53. Kisely S. No mental health without oral health. Can J Psychiatry (2016) 61(5):277–82. doi: 10.1177/0706743716632523
54. Avery A, Langley-Evans SC, Harrington M, Swift JA. Setting targets leads to greater long-term weight losses and ‘unrealistic’ targets increase the effect in a large community-based commercial weight management group. J Hum Nutr Diet (2016) 29(6):687–96. doi: 10.1111/jhn.12390
55. Teasdale S, Ward P, Rosenbaum S, Samaras K, Stubbs B. Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness. Br J Psychiatry (2017) 210(2):110–8. doi: 10.1192/bjp.bp.115.177139
56. American College of Sports Medicine, Riebe D, Ehrman JK, Liguori G, Magal M. ACSM"s Guidelines for Exercise Testing and Prescription. 10th Ed. Philadelphia: Wolters Kluwer (2018).
57. Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M, et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry (2018) 54:124–44. doi: 10.1016/j.eurpsy.2018.07.004
59. Dimmock J, Jackson B, Podlong L, Magaraggia C. The effect of variety expectations on interest, enjoyment, and locus of causality in exercise. Motiv Emot (2012) 37:146–53. doi: 10.1007/s11031-012-9294-5
60. Sylvester BD, Standage M, Ark T, Sweet SN, Crocker PRE, Zumbo BD, et al. Is variety a spice of (an active) life?. Perceived variety, exercise behavior, and the mediating role of autonomous motivation. J Sport Exerc Psychol (2014) 36(5):516–27. doi: 10.1123/jsep.2014-0102
61. National Collaborating Centre for Mental Health. Implementing the early intervention in psychosis access and waiting time standard: Guidance. London: National Institute for Clinical Excellence (2016).
62. World Health Organization. International classification of diseases (ICD-10) (2014). Available at: http://www.who.int/classifications/icd/en/ (Accessed July 12, 2020).
63. World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva. Geneva: World Health Organization (2011) p. 8–11.
64. NHS Digital. Health Survey for England. Fruit and Vegetable Consumption . Available at: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2018 (Accessed July 12, 2020).
65. O’Hare T, Sherrer MV, LaButti A, Emrick K. Validating the alcohol use disorders identification test with persons who have a serious mental illness. Res Soc Work Pract (2004) 14:36–42. doi: 10.1177/1049731503257667
66. Coleman KJ, Ngor E, Reynolds K. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc (2012) 44:2071–76. doi: 10.1249/MSS.0b013e3182630ec1
67. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psych (2006) 3:77–101. doi: 10.1191/1478088706qp063oa
68. Hamilton A, Finley E. Qualitative methods in implementation research: an introduction. Psych Res (2019) 280:112516. doi: 10.1016/j.psychres.2019.112516
69. McQuigg M, Brown JE, Broom J, Laws RA, Reckless JP, Noble PA, et al. The counterweight programme: prevalence of CVD risk factors by body mass index and the impact of 10% weight change. Obes Res Clin Pract (2008) 2:15–27. doi: 10.1016/j.orcp.2008.01.002
70. Lewis L, Taylor M, Broom J, Johnston KL. The cost-effectiveness of the LighterLife weight management programme as an intervention for obesity in England. Clin Obes (2014) 4(3):180–8. doi: 10.1111/cob.12060
71. Shiers DE, Rafi I, Cooper SJ, Holt RIG. Positive Cardiometabolic Health Resource: an intervention framework for patients with psychosis and schizophrenia. 2014 update. London: Royal College of Psychiatrists (2014).
72. Meyer JM, Nasrallah HA, McEvoy JP, Goff DC, Davis SM, Chakos M, et al. The Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE) Schizophrenia Trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res (2005) 80:9–18. doi: 10.1016/j.schres.2005.07.015
73. Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry (2015) 14(3):339–47. doi: 10.1002/wps.20252
74. Hahn LA, Mackinnon A, Foley DL, Morgan VA, Waterreus A, Watts GF, et al. Counting up the risks: How common are risk factors for morbidity and mortality in young people with psychosis? Early Interv Psychiatry (2018) 12(6):1045–51. doi: 10.1111/eip.12406
75. McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res (2005) 80:19–32. doi: 10.1016/j.schres.2005.07.014
76. Meyer JM, Davis VG, Goff DC, McEvoy JP, Nasrallah HA, Davis SM, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res (2008) 101(1–3):273–86. doi: 10.1016/j.schres.2007.12.487
77. Thornicroft G. The scandal of premature mortality. Br J Psychiatry (2011) 199(6):441–2. doi: 10.1192/bjp.bp.111.092718
78. Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet (2009) 373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4
79. nstitute of Medicine (US) Committee to Develop Criteria for Evaluating the Outcomes of Approaches to Prevent and Treat Obesity. Weighing the Options: Criteria for Evaluating Weight-Management Programs. Thomas PR, editor. Washington DC: National Academies Press (1995).
80. World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization (2009).
81. Aune D. Plant foods, antioxidant biomarkers, and the risk of cardiovascular disease, cancer, and mortality: A review of the evidence. Adv Nutr (2019) 10(4):S404–21. doi: 10.1093/advances/nmz042
82. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol (2017) 46(3):1029–56. doi: 10.1093/ije/dyw319
83. Soundy A, Wampers M, Probst M, De Hert M, Stubbs B, Vancampfort D. Physical activity and sedentary behaviour in outpatients with schizophrenia: a systematic review and meta-analysis. Int J Ther Rehabil (2013) 20:588–96. doi: 10.12968/ijtr.2013.20.12.588
84. Stubbs B, Williams J, Gaughran F, Craig T. How sedentary are people with psychosis? A systematic review and meta-analysis. Schizophr Res (2016) 117:103–9. doi: 10.1016/j.schres.2016.01.034
85. Vancampfort D, Knapen J, Probst M, Scheewe T, Remans S, De Hert M. A systematic review of correlates of physical activity in patients with schizophrenia. Acta Psychiat Scand (2012) 125:352–62. doi: 10.1111/j.1600-0447.2011.01814.x
86. National Institute for Health and Care Excellence. Smoking: acute, maternity and mental health services (2013). Available at: https://www.nice.org.uk/guidance/ph48 (Accessed on July 12, 2020).
87. Mendelsohn CP, Kirby DP, Castle DJ. Smoking and mental illness. An update for psychiatrists. Australas Psychiatry (2015) 23:37–43. doi: 10.1177/1039856214562076
88. Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. Brit Med J (2014) 348:g1151. doi: 10.1136/bmj.g1151
89. World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization (2009).
90. Doyle R, Turner N, Fanning F, Brennan D, Renwick L, Lawlor E, et al. First-episode psychosis and disengagement from treatment: a systematic review. Psych Serv (2014) 65:603–11. doi: 10.1176/appi.ps.201200570
91. Firth J, Carney R, Jerome L, Elliott R, French P, Yung AR. The effects and determinants of exercise participation in first-episode psychosis: a qualitative study. BMC Psychiatry (2016a) 16:36. doi: 10.1186/s12888-016-0751-7
92. Watkins A, Denney-Wilson E, Curtis J, Teasdale S, Rosenbaum S, Ward PB, et al. Keeping the body in mind: a qualitative analysis of the experiences of people experiencing first-episode psychosis participating in a lifestyle intervention programme. Int J Ment Health Nurs (2020) 29(2):278–89. doi: 10.1111/inm.12683
93. Kilbourne AM, Braganza MZ, Bowersox NW, Goodrich DE, Miake-Lye I, Floyd N, et al. Research lifecycle to increase the substantial real-world impact of research: accelerating innovations to application. Med Care (2019) 57(10):S206. doi: 10.1097/MLR.0000000000001146
94. Martire LM, Helgeson VS. Close relationships and the management of chronic illness: associations and interventions. Am Psychol (2017) 72(6):601–12. doi: 10.1037/amp0000066
95. Blythe J, White J. Role of the mental health nurse towards physical health care in serious mental illness: an integrative review of 10 years of UK literature. J Ment Health (2012) 21(3):193–201. doi: 10.1111/j.1447-0349.2011.00792.x
96. Heslin M, Patel A, Stahl D, Gardner-Sood P, Mushore M, Smith S, et al. Randomised controlled trial to improve health and reduce substance use in established psychosis (IMPaCT): cost-effectiveness of integrated psychosocial health promotion. BMC Psychiatry (2017) 17:407. doi: 10.1186/s12888-017-1570-1
97. Deloitte Access Economics. Value of Accredited Exercise Physiologists in Australia Report for Exercise and Sports Science Australia. Sydney: Deloitte Access Economics (2015).
98. McGinty EE, Gudzune KA, Dalcin A, Jerome GJ, Dickerson F, Gennusa J, et al. Bringing an effective behavioral weight loss intervention for people with serious mental illness to scale. Front Psychiatry (2018) 9:604. doi: 10.3389/fpsyt.2018.00604
99. National Institute for Health and Care Excellence. Shared Learning Database. SHAPE: supporting health and promoting exercise in young people with psychosis (2017). Available at: https://www.nice.org.uk/sharedlearning/shape-supporting-health-and-promoting-exercise-in-young-people-with-psychosis (Accessed July 12, 2020).
Keywords: early psychosis, health risk behaviors, exercise, cardiometabolic risk, combined exercise and dietary intervention, implementation research
Citation: Smith J, Griffiths LA, Band M, Hird-Smith R, Williams B, Bold J, Bradley E, Dilworth R and Horne D (2020) Early Intervention in Psychosis: Effectiveness and Implementation of a Combined Exercise and Health Behavior Intervention Within Routine Care. Front. Endocrinol. 11:577691. doi: 10.3389/fendo.2020.577691
Received: 29 June 2020; Accepted: 30 September 2020;
Published: 26 October 2020.
Edited by:
Jackie Curtis, University of New South Wales, AustraliaReviewed by:
Simon Rosenbaum, University of New South Wales, AustraliaScott B. Teasdale, University of New South Wales, Australia
Copyright © 2020 Smith, Griffiths, Band, Hird-Smith, Williams, Bold, Bradley, Dilworth and Horne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jo Smith, jo.smith2@worc.ac.uk
 Jo Smith
Jo Smith Lisa A. Griffiths
Lisa A. Griffiths Marie Band3
Marie Band3 Briony Williams
Briony Williams Justine Bold
Justine Bold Eleanor Bradley
Eleanor Bradley Richard Dilworth
Richard Dilworth Dominic Horne
Dominic Horne