- 1Division of Nephrology, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Diabetes, Central Clinical School, Monash University, Melbourne, VIC, Australia
Background: To investigate the association between sex differences and end-stage kidney disease (ESKD) in patients with biopsy-confirmed diabetic kidney disease (DKD).
Method: We performed a retrospective cohort study. A total of 336 patients with biopsy-confirmed DKD who were followed up for at least 12 months were enrolled. Baseline clinical and pathological data at the time of biopsy were collected. ESKD was defined by an estimated glomerular filtration rate of <15 ml/min/1.73 m2 or initiation of renal replacement therapy. The association between sex differences and ESKD was assessed using the log-rank test and Cox regression.
Result: There were 239 (71%) male and 97 (29%) female patients in our cohort. Female patients had higher systolic blood pressure, total cholesterol and low-density lipoprotein cholesterol levels compared with male. There were a lower proportion of female patients in the very high risk grade according to the chronic kidney disease categories (37% of female vs. 44% of male). During a median follow-up time of 20 months, 101 (57.7%) male and 43 (44.3%) female entered into ESKD, with no significant difference by the log-rank test (P >0.05). Univariate [male: hazard ratio (HR) [95% confidence interval (CI)], 1.005, (0.702–1.439)] and multivariable ([male: HR (95%CI), 1.164, (0.675–2.007)]. Cox regression further showed that sex difference was not significantly associated with ESKD.
Conclusion: Female patients had the higher systolic blood pressure, total cholesterol, LDL-C, compared with male patients. However, there was no significant association observed between sex difference and ESKD in our study.
Introduction
Diabetic kidney disease (DKD) is one of the most common microvascular complications of diabetes. Despite improvements of management in basic research and clinical practice, DKD remains the leading cause of end-stage kidney disease (ESKD) worldwide (1, 2). In order to slow down the progression of DKD, recognizing patients with a high risk at an early stage is important. Sex differences have been taken into account in development or progression in several diseases such as diabetes (3), chronic kidney disease (CKD) (4), heart failure (5), and neuropsychiatric disorders (6). Recently, a study from the Chronic Renal Insufficiency Cohort that included 3,939 adults (half of them had diabetes) showed that male patients had the higher risk of CKD progression and death compared with female patients (4). Similarly, another large meta-analysis reported that males with CKD showed a more rapid decline in renal function than which in females, however, only patients with nondiabetic CKD were analyzed in that study (7).
The association between sex differences and the incidence or progression of DKD has been investigated in several studies, but not been well established with disparate conclusions (8). Different ethnic cohorts, age, type of diabetes and study designs can all cause the contradictory results. Moreover, most of the patients did not receive a kidney biopsy in these previous studies. Differences between DKD and nondiabetic kidney diseases greatly contribute to the challenges of understanding diabetic complications. Patients with nondiabetic kidney diseases might have confounded the results in previous study.
Therefore, in the current study, we aimed to investigate sex differences of clinical and pathological characteristics in patients with biopsy-confirmed DKD. We also aimed to evaluate the association between sex difference and ESKD.
Method
Study Design and Patients
We performed a retrospective cohort study. The study was approved by the ethics committee of West China Hospital of Sichuan University and all patients have signed a written informed consent form.
Patients with biopsy-confirmed DKD from January 2010 to December 2018 in our hospital were reviewed. Baseline data were collected from the hospital information system at the time patients received a kidney biopsy. The inclusion criteria were as follows: a. type 2 diabetes; b. biopsy-confirmed DKD; and c. follow-up for longer than 12 months (patients who developed ESKD in 12 months were also included). Type 2 diabetes was diagnosed in accordance with the 2018 American Diabetes Association criteria (9). Renal pathological classifications were based on the Renal Pathology Society in 2010 (10) by at least two professional pathologists. ESKD was defined as initiation of renal replacement therapy or eGFR less than 15 ml/min/1.73 m2.
Statistical Analysis
Continuous variables were described as mean ± standard deviation (SD) or median and quartiles on the basis of a normality test. Categorical variables were presented as counts with ratios. Differences of baseline data between male and female patients were evaluated appropriately by the Student’s t test or the Mann–Whitney test. The prognosis of the kidney was compared by the log-rank test and shown using the Kaplan–Meier curve. Univariate and multivariate Cox analysis were applied to determine the risk factors of ESKD. All analyses were conducted using SPSS software 22.0 and GraphPad Prism 7.0. A two-sided P-value of less than 0.05 was considered statistically significant.
Results
Baseline Clinical and Pathological Characteristics
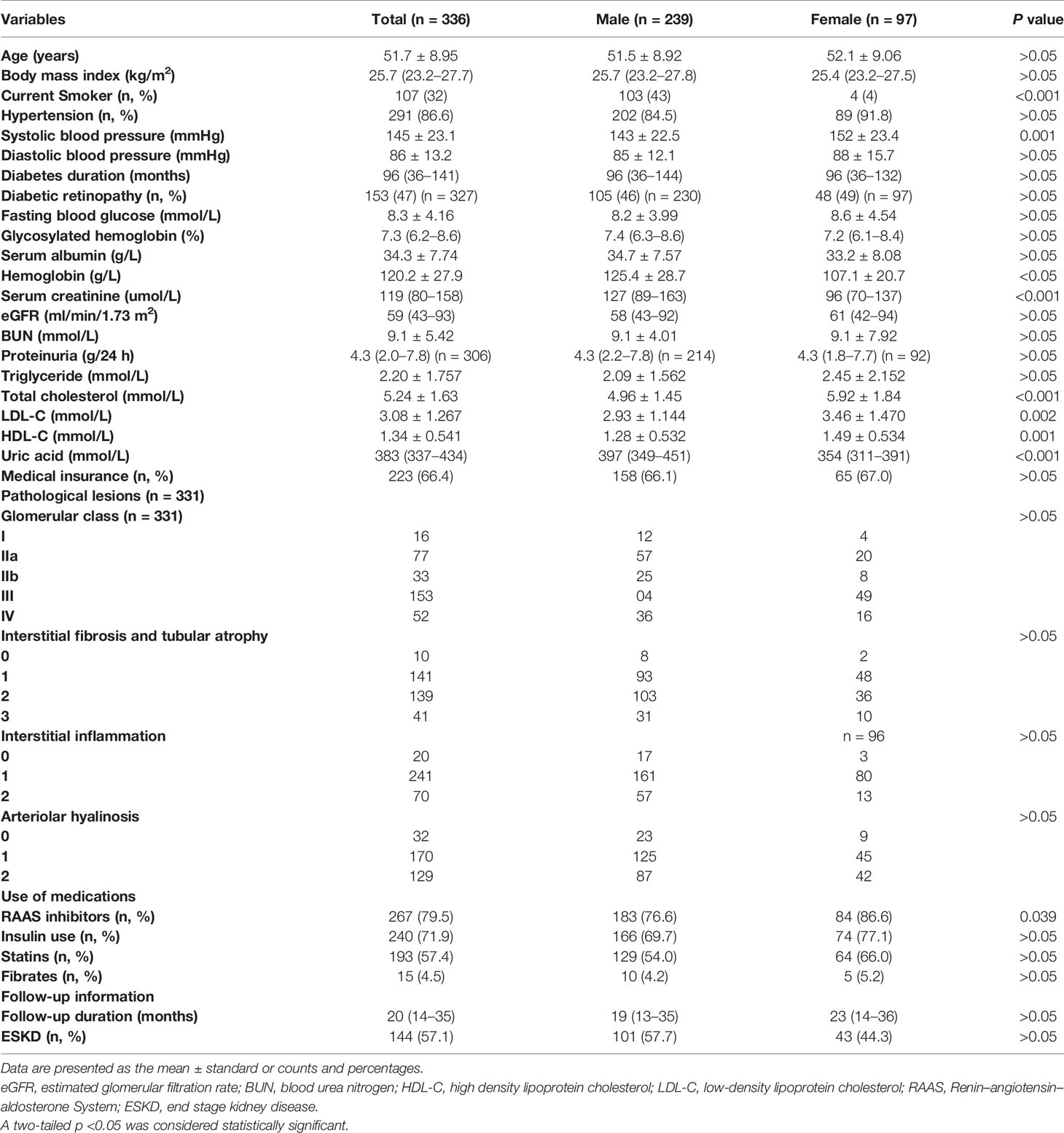
A total of 336 patients were enrolled in the study. Baseline clinical and pathological characteristics are shown in Table 1. Briefly, the mean age of patients was 51.7 ± 8.95 years old, 291 (86.6%) patients had hypertension, the median diabetic duration was 96 (36–141) months. The median eGFR was 59 (43–93) ml/min/1.73 m2 and the median proteinuria was 4.3 (2.0–7.8) g/24 h. There were 239 (71.1%) male and 97 (28.9%) female in our cohort; compared to male, female had the higher level of systolic blood pressure and lipid metabolism. Moreover, a significantly higher proportion of female patients received renin–angiotensin–aldosterone system (RAAS) inhibitors therapy. Male had the higher level of serum creatinine compared with female. There were no significantly differences in age, diastolic blood pressure, the duration of diabetes, the incidence of diabetic retinopathy, blood glucose, proteinuria, triglyceride, medical insurance, insulin use, statins and fibrates use. With regard to pathological lesions, 16 patients had glomerular class I, 77 had class IIa, 33 had class IIb, 153 had class III, and 52 had class IV. However, there were no significant differences in glomerular class, interstitial fibrosis and tubular atrophy (IFTA), interstitial inflammation and arteriolar hyalinosis between male and female patients.
Metabolic Characteristics Between Male and Female Patients
With regard to metabolic characteristics, the body mass index (male vs. female 25.7 (23.2–27.8) kg/m2 vs. 25.4 (23.2–27.5) kg/m2, P >0.05) and triglyceride (male vs. female 2.09 ± 1.562 mmol/L vs. 2.45 ± 2.152 mmol/L, P >0.05) were not significant different between male and female. However, compared with male patients, female patients had the significantly higher total cholesterol (male vs. female 4.96 ± 1.45 mmol/L vs. 5.92 ± 1.84 mmol/L, P <0.05), LDL-C (male vs. female 2.93 ± 1.144 mmol/L vs. 3.46 ± 1.470 mmol/L, P <0.05), HDL-C (male vs. female 1.28 ± 0.532 mmol/L vs. 1.49 ± 0.534 mmol/L, P <0.05), but lower uric acid (male vs. female 397 (349–451) mmol/L vs. 354 (311–391) mmol/L, P <0.05).
CKD Risk Categories
To evaluate the risk distribution between male and female patients, we used the CKD category heat map as recommended by the Kidney Disease Improving Global Outcomes (11). Patients were categorized into low risk (green), moderately increased risk (yellow), high risk (orange) and very high risk (red) grades by baseline proteinuria (24 hour-proteinuria of 306 patients were obtained) and eGFR. Those patients in the red category had the highest proteinuria and lowest GFR, and carried highest risk for events of cardiovascular disease, ESKD and mortality. A total of 9% (28/306) of patients were low risk, 21% (65/306) of patients had a moderately increased risk, 27% (84/306) were high risk, and 42% (129/306) were very high risk (Figure 1A). As for sex distribution, both approximately 30% of male and female patients had low and moderately increased risks, but more male had a higher risk than female (44% vs. 37%) (Figure 1B).
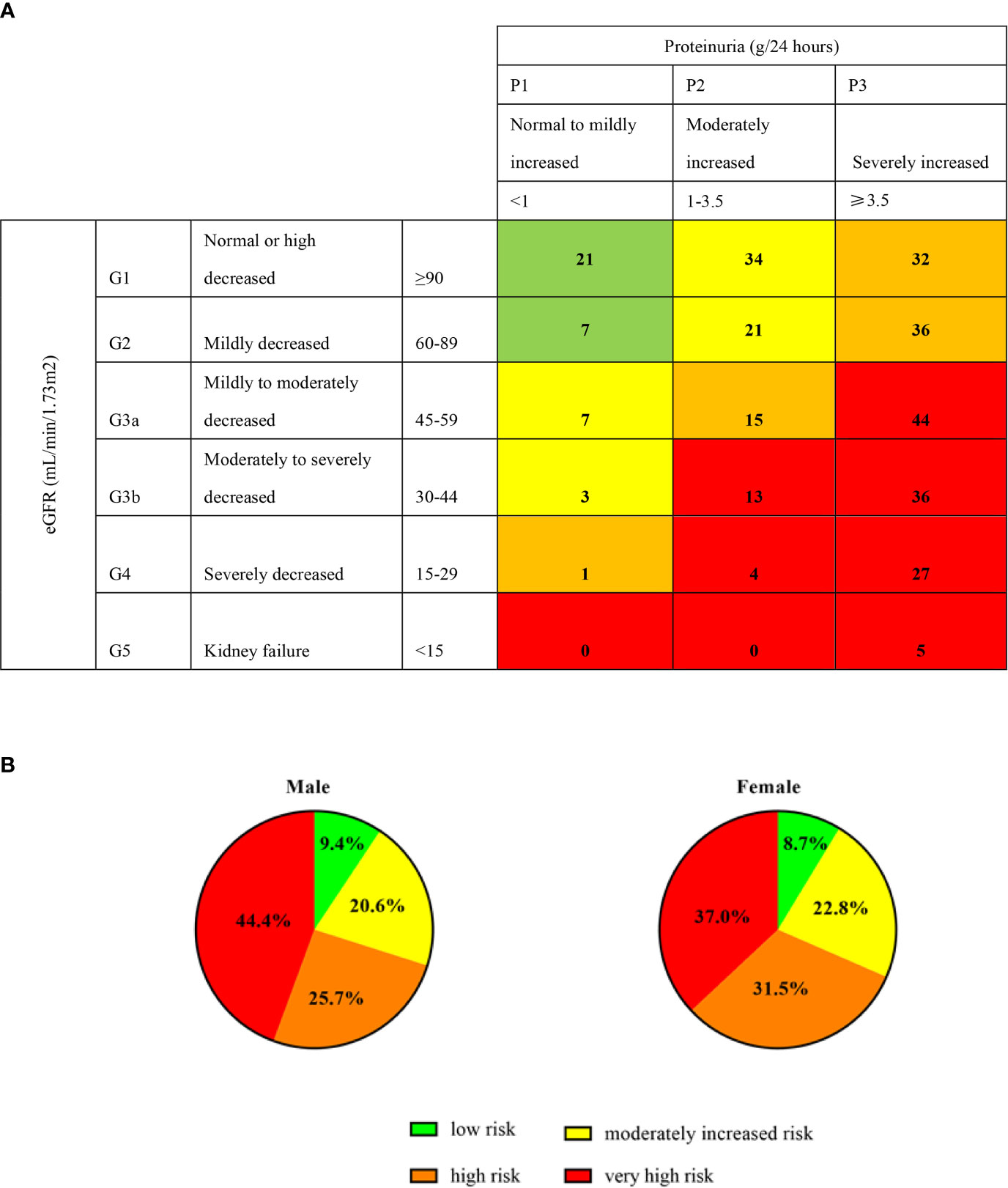
Figure 1 Prognosis of CKD categories and sex. Proteinuria (g/24 hours) of 306 patients were obtained at the baseline. Green, low risk (if no other markers of kidney disease, no CKD); Yellow: moderately increased risk; Orange: high risk; Red, very high risk. The digits in (A) cells represent the numbers of patients. (B) represent the percentage of male and female in different categories.
Sex Difference and ESKD
During a median follow-up period of 20 (14–35) months, a total of 144 (57.1%) patients developed ESKD. Specifically, there were 101 (57.7%) male, 18 (52.9%) premenopausal female, and 25 (39.7%) menopausal female suffered from ESKD during the follow-up time. There was no significant difference in kidney survival between male and female, and no difference between premenopausal and menopausal female (Figure 2).
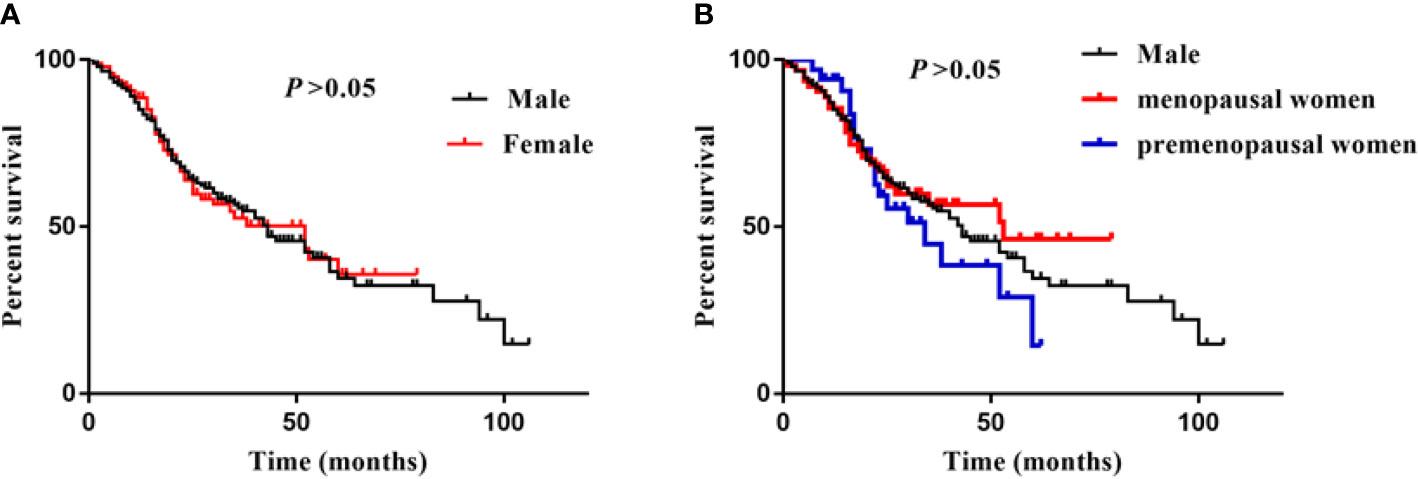
Figure 2 Sex difference and ESKD. (A) was showed survival curves of male and female, (B) was showed survival curves of male, menopausal/premenopausal women. Log-rank analysis was used to compared the percent survival between male and female. There was no significant difference between male, premenopusal and menopausal female.
To evaluate risk factors of ESKD in patients with DKD, we performed univariate and multivariable Cox regression analyses (Table 2). Specifically, the hazard ratio (HR) and 95% confidence interval (CI) of male was 1.005 (0.702–1.439, P = 0.978), which indicated there was no association between sex and ESKD. The higher levels of systolic blood pressure, proteinuria, total cholesterol, LDL-C, HDL-C, advanced class of glomerular lesion, IFTA, interstitial inflammation, arteriolar hyalinosis, incidence of diabetic retinopathy, and the lower levels of serum albumin and eGFR were associated with ESKD. Moreover, when we adjusted for essential clinical and pathological indices, sex was still not associated with ESKD (HR and 95% CI, 1.164, 0.675–2.007, P = 0.584). However, a higher levels of interstitial inflammation (HR and 95% CI, 1.705, 1.041–2.791, P = 0.034), and the lower serum albumin (HR and 95% CI, 0.895, 0.858–0.932, P < 0.001) and eGFR (HR and 95% CI, 0.969, 0.959–0.979, P < 0.001) were independently associated with ESKD.
Discussion
DKD has become the leading cause of ESKD, which has led to a heavy economic burden on individuals and countries (2). Therefore, recognizing risk factors of ESKD would be beneficial for to slowing the progression of DKD. The association between sex difference and ESKD in patients with DKD has not been well established. In the current study, the proportion of male was higher than that of female. We also found that male patients had relatively good control of lipid metabolism. However, more male patients were in the high very risk grade of CKD categories at baseline compared with female. However, there was no association between sex difference and ESKD in our study.
Increasing studies have investigated the effect of sex differences on DKD development and progression, however, but different cohorts have reported conflicting findings. In studies that enrolled patients with type 2 diabetes, it seems that more results indicated female has greater risk of DKD progression (8). A study from Japan (12) (247 male and 97 female) showed that the mean annual decline in the eGFR was 3.5% in female and 2.0% per year in male. However, this study only enrolled patients with diabetes or those at the early stage of CKD (mean eGFR >90 ml/min/1.73 m2, only 28.5% of patients had proteinuria). Similarly, several studies showed that African American, Hispanic and Pima Indian female had a higher risk of DKD and disease progression (13–15). Nevertheless, another prospective observational study (227 male and 60 female) enrolled patients with type 2 diabetes and persistent macroalbuminuria (≥300 mg/24 h) and showed that sex difference had no association with DKD progression (16). This previous finding is similar to our results. In our study, the ratio of male and female (approximately 2.5) was consistent with previous studies, but patients with the lower eGFR and greater proteinuria. Moreover, studies have found that the effect of sex is less apparent in DKD than in non-DKD (17, 18). Our patients with DKD were all diagnosed by a kidney biopsy, which excluded non-DKD, and this could explain the results.
The recognition of underlying mechanism of sex differences in diseases remains limited. Sex hormones are considered to be the main driver of sex disparities in the incidence and progression of CKD. A meta-analysis that included 11,345 patients clearly indicated that male was associated with a faster progression of nondiabetic CKD (7). However, this renoprotective effect was only evident in premenopausal female (19, 20). Once patients suffer from diabetes, the renoprotective effect of female is generally considered lost, even in premenopausal female (21). Accumulating evidence suggests that patients with diabetes have unbalanced levels of sex hormones, where expression of estradiol is decreased, but testosterone is increased, in female with diabetes (22, 23). Moreover estrogen replacement alleviates pathological lesions in animal DKD models (24–26), and can even attenuate proteinuria and improve creatinine clearance in postmenopausal female with diabetes (27). In our study, most of female were during perimenopause which worsened the imbalance of hormones. This could also explain why there was no significantly difference in kidney survival among premenopausal, menopausal female and male in our cohort.
There are other possible mechanisms contribute to sex differences. Studies have suggested that more adolescent female with diabetes had hyperfiltration in the early stage of DKD (28, 29). Additionally, the higher baseline total cholesterol and LDL-C of our female patients, which was consistent with a cohort from Australia (30), also increased the risk of hyperfiltration. Hyperfiltration traditionally indicates a poor kidney prognosis, but a recent study from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) followed up 446 patients with type 1 diabetes for longer than 20 years found that early hyperfiltration was not associated with decreased renal dysfunction (31). Therefore, although female patients with diabetes are more likely to have hyperfiltration, this does not affect kidney prognosis. The expression and mechanism of several therapeutic targets had been found different between male and female. Specifically, some studies have observed that male had the higher expression of ANG II (32, 33), and ANG II is recognized to mediate renal inflammation (34). Additionally, the expressions of sodium-glucose co-transporters (SGLT) 1 and SGLT2 have been found higher in female rats than in male rats (35, 36). A recent meta-analysis also showed that a reduction in major adverse cardiac events with SGLT2 inhibitors was less in female with diabetes compared with male with diabetes (37). The underlying mechanisms of these differences remain unclear, but it is worthy to be further investigated to provide individual therapy and improve prognosis of patients with diabetes.
There were several limitations should be addressed. First, this was a retrospective cohort study, and we only observed the relationship between sex differences and kidney prognosis. therefore, prospective studies are warranted to determine the underlying causative relationship. Second, our study only included Chinese patients, various genetic backgrounds might have affected our results. Third, we had no opportunity to evaluate the levels of sex hormones at baseline owing to the study design. Fourth, the sample size was limited and patients were in a relatively severe disease stage because we only enrolled patients with biopsy-confirmed DKD. Therefore, further prospective and large sample size DKD cohorts are required to investigate the issue.
Conclusion
In patients with biopsy-confirmed DKD, female patients had the higher systolic blood pressure, total cholesterol, LDL-C levels, compared with male patients. However, there was no significant association was observed between sex difference and ESKD in our study.
Perspectives And Significance
Sex differences play an important role in many diseases including cancers or chronic diseases. However, the association between sex differences and the incidence or progression of DKD has not been well established with disparate conclusions. Therefore, we investigate the issue in patients with biopsy-confirmed DKD. We found that female patients had the higher systolic blood pressure, total cholesterol, LDL-C levels. However, there was no association between sex difference and ESKD in our study. The study provides relatively strong evidence to illustrate the associations.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by ethics committee of West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YTW, JZ, and FL planed, analyzed and wrote the manuscript. JLZ, YCW, RZ, and HR collected data, check analysis and gave some suggestions. MC revised the manuscript and gave lot of suggestions. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Grant 8197031494 from the National Natural Science Foundation of China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
1. This manuscript has been released as a pre-print at Research square (38). https://doi.org/10.21203/rs.3.rs-61662/v1.
2. We thank Ellen Knapp, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
References
1. Levin A, Tonelli M, Bonventre J, Coresh J, Donner J, Fogo AB, et al. Global Kidney Health 2017 and Beyond: A Roadmap for Closing Gaps in Care, Research, and Policy. Lancet (2017) 390(10105):1888–917. doi: 10.1016/S0140-6736(17)30788-2
2. Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Barnighausen T, et al. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care (2018) 41(5):963–70. doi: 10.2337/dc17-1962
3. Narayan KMV, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime Risk for Diabetes Mellitus in the United States. United States: Am Med Assoc (2003) 290:1884–90. doi: 10.1001/jama.290.14.1884
4. Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, et al. Sex-Related Disparities in CKD Progression. J Am Soc Nephrol (2019) 30(1):137–46. doi: 10.1681/ASN.2018030296
5. Kadkhodayan A, Lin CH, Coggan AR, Kisrieva-Ware Z, Schechtman KB, Novak E, et al. Sex Affects Myocardial Blood Flow and Fatty Acid Substrate Metabolism in Humans With Nonischemic Heart Failure. J Nucl Cardiol (2017) 24(4):1226–35. doi: 10.1007/s12350-016-0467-6
6. Thibaut F. The Role of Sex and Gender in Neuropsychiatric Disorders. France: Les Laboratoires Servier (2016) 18:351–2. doi: 10.31887/DCNS.2016.18.4/fthibaut
7. Neugarten J, Acharya A, Silbiger SR. Effect of Gender on the Progression of Nondiabetic Renal Disease: A Meta-Analysis. United States (2000) 11:319. doi: 10.1681/ASN.V112319
8. Maric-Bilkan C. Sex Differences in Diabetic Kidney Disease. Mayo Clinic Proc (2020) 95(3):587–99. doi: 10.1016/j.mayocp.2019.08.026
9. Association AD. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes 2018. Diabetes Care (2018) 41(Suppl 1):13–27. doi: 10.2337/dc18-S002
10. Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic Classification of Diabetic Nephropathy. J Am Soc Nephrol (2010) 21(4):556–63. doi: 10.1681/ASN.2010010010
11. Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: Behind the Scenes, Need for Guidance, and a Framework for Moving Forward. Kidney Int (2014) 85(1):49–61. doi: 10.1038/ki.2013.444
12. Kajiwara A, Kita A, Saruwatari J, Miyazaki H, Kawata Y, Morita K, et al. Sex Differences in the Renal Function Decline of Patients With Type 2 Diabetes. J Diabetes Res (2016) 2016:1–8. doi: 10.1155/2016/4626382
13. Crook ED, Patel SR. Diabetic Nephropathy in African-American Patients. Philadelphia: Curr Med Group (2004) 4:455–61. doi: 10.1007/s11892-004-0056-y
14. Looker HC, Krakoff J, Funahashi T, Matsuzawa Y, Tanaka S, Nelson RG, et al. Adiponectin Concentrations are Influenced by Renal Function and Diabetes Duration in Pima Indians With Type 2 Diabetes. J Clin Endocrinol Metab (2004) 89(8):4010–7. doi: 10.1210/jc.2003-031916
15. Young BA, Maynard C, Boyko EJ. Racial Differences in Diabetic Nephropathy, Cardiovascular Disease, and Mortality in a National Population of Veterans. United States: Am Diabetes Assoc (2003) 26:2392–9. doi: 10.2337/diacare.26.8.2392
16. Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving H. Progression of Nephropathy in Type 2 Diabetic Patients. Kidney Int (2004) 66(4):1596–605. doi: 10.1111/j.1523-1755.2004.00925.x
17. Maric C. Sex, Diabetes and the Kidney. Am J Physiol-Renal Physiol (2009) 296(4):F680–8. doi: 10.1152/ajprenal.90505.2008
18. Shen Y, Cai R, Sun J, Dong X, Huang R, Tian S, et al. Diabetes Mellitus as a Risk Factor for Incident Chronic Kidney Disease and End-Stage Renal Disease in Women Compared With Men: A Systematic Review and Meta-Analysis. Endocrine (2017) 55(1):66–76. doi: 10.1007/s12020-016-1014-6
19. Simon P, Ramée M, Autuly V, Laruelle E, Charasse C, Cam G, et al. Epidemiology of Primary Glomerular Diseases in a French Region. Variations According to Period Age. United States: Elsevier Inc (1994) 46:1192–8. doi: 10.1038/ki.1994.384
20. Coggins CH, Breyer Lewis J, Caggiula AW, Castaldo LS, Klahr S, Wang SR. Differences Between Women and Men With Chronic Renal Disease. England (1998) 13:1430–7. doi: 10.1093/ndt/13.6.1430
21. Jones CA, Krolewski AS, Rogus J, Xue JL, Collins A, Warram JH. Epidemic of End-Stage Renal Disease in People With Diabetes in the United States Population: Do We Know the Cause? Kidney Int (2005) 67(5):1684–91. doi: 10.1111/j.1523-1755.2005.00265.x
22. Matsushita M, Tamura K, Osada S, Kogo H. Effect of Troglitazone on the Excess Testosterone and LH Secretion in Thyroidectomized, Insulin-Resistant, Type 2 Diabetic Goto-Kakizaki Rats. Endocrine (2005) 27(3):301–5. doi: 10.1385/ENDO:27:3:301
23. Salonia A, Lanzi R, Scavini M, Pontillo M, Gatti E, Petrella G, et al. Sexual Function and Endocrine Profile in Fertile Women With Type 1 Diabetes. United States: Am Diabetes Assoc (2006) 29:312–6. doi: 10.2337/diacare.29.02.06.dc05-1067
24. Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol Replacement Improves Renal Function and Pathology Associated With Diabetic Nephropathy. Am J Physiol Renal Physiol (2005) 288(2):F399–405. doi: 10.1152/ajprenal.00195.2004
25. Potier M, Karl M, Zheng F, Elliot SJ, Striker GE, Striker LJ. Estrogen-Related Abnormalities in Glomerulosclerosis-Prone Mice: Reduced Mesangial Cell Estrogen Receptor Expression and Prosclerotic Response to Estrogens. Am J Pathol (2002) 160(5):1877–85. doi: 10.1016/S0002-9440(10)61134-0
26. Catanuto P, Doublier S, Lupia E, Fornoni A, Berho M, Karl M, et al. 17 Beta-Estradiol and Tamoxifen Upregulate Estrogen Receptor Beta Expression and Control Podocyte Signaling Pathways in a Model of Type 2 Diabetes. Kidney Int (2009) 75(11):1194–201. doi: 10.1038/ki.2009.69
27. Szekacs B, Vajo Z, Varbiro S, Kakucs R, Vaslaki L, Acs N, et al. Postmenopausal Hormone Replacement Improves Proteinuria and Impaired Creatinine Clearance in Type 2 Diabetes Mellitus and Hypertension. BJOG (2000) 107(8):1017–21. doi: 10.1111/j.1471-0528.2000.tb10406.x
28. Bjornstad P, Nehus E, El GL, Bacha F, Libman IM, McKay S, et al. Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes: An Observational Analysis of Data From the TODAY Clinical Trial. Am J Kidney Dis (2018) 71(1):65–74. doi: 10.1053/j.ajkd.2017.07.015
29. Lovshin JA, Škrtić M, Bjornstad P, Moineddin R, Daneman D, Dunger D, et al. Hyperfiltration, Urinary Albumin Excretion, and Ambulatory Blood Pressure in Adolescents With Type 1 Diabetes Mellitus. Am J Physiol-Renal Physiol (2018) 314(4):F667–74. doi: 10.1152/ajprenal.00400.2017
30. Kautzky-Willer A, Stich K, Hintersteiner J, Kautzky A, Kamyar MR, Saukel J, et al. Sex-Specific-Differences in Cardiometabolic Risk in Type 1 Diabetes: A Cross-Sectional Study. England: BioMed Cent (2013) 12:78. doi: 10.1186/1475-2840-12-78
31. Molitch ME, Gao X, Bebu I, de Boer IH, Lachin J, Paterson , et al. Early Glomerular Hyperfiltration and Long-Term Kidney Outcomes in Type 1 Diabetes: The DCCT/EDIC Experience. Clin J Am Soc Nephrol (2019) 14(6):854–61. doi: 10.2215/CJN.14831218
32. Brewster UC, Perazella MA. The Renin-Angiotensin-Aldosterone System and the Kidney: Effects on Kidney Disease. Am J Med (2004) 116(4):263–72. doi: 10.1016/j.amjmed.2003.09.034
33. Clotet S, Riera M, Pascual J, Soler MJ. RAS and Sex Differences in Diabetic Nephropathy. Am J Physiol-Renal Physiol (2016) 310(10):F945–57. doi: 10.1152/ajprenal.00292.2015
34. Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, Sánchez-López E, Carvajal G, et al. Angiotensin II: A Key Factor in the Inflammatory and Fibrotic Response in Kidney Diseases. England (2006) 21:16–20. doi: 10.1093/ndt/gfi265
35. Balen D, Ljubojević M, Breljak D, Brzica H, lender VŽ, Koepsell H, et al. Revised Immunolocalization of the Na+-d-Glucose Cotransporter SGLT1 in Rat Organs With an Improved Antibody. Am J Physiol-Cell Physiol (2008) 295(2):C475–89. doi: 10.1152/ajpcell.00180.2008
36. Sabolić I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, et al. Expression of Na+-d-glucose Cotransporter SGLT2 in Rodents Is Kidney-Specific and Exhibits Sex and Species Differences. Am J Physiol-Cell Physiol (2012) 302(8):C1174–88. doi: 10.1152/ajpcell.00450.2011
37. Singh AK, Singh R. Gender Difference in Cardiovascular Outcomes With SGLT-2 Inhibitors and GLP-1 Receptor Agonist in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardio-Vascular Outcome Trials. Diabetes Metab Syndrome: Clin Res Rev (2020) 14(3):181–7. doi: 10.1016/j.dsx.2020.02.012
Keywords: sex differences, diabetic kidney disease, end stage kidney disease, risk factors, type 2 diabetes
Citation: Wang Y, Zhang J, Zhang J, Wu Y, Zhang R, Ren H, Cooper ME and Liu F (2021) Sex Differences in Biopsy-Confirmed Diabetic Kidney Disease. Front. Endocrinol. 12:670674. doi: 10.3389/fendo.2021.670674
Received: 22 February 2021; Accepted: 05 July 2021;
Published: 29 July 2021.
Edited by:
Charumathi Sabanayagam, Singapore Eye Research Institute (SERI), SingaporeReviewed by:
Min Pan, Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, ChinaToru Aizawa, Aizawa Hospital, Japan
Copyright © 2021 Wang, Zhang, Zhang, Wu, Zhang, Ren, Cooper and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Liu, liufangfh@163.com
 Yiting Wang
Yiting Wang Jue Zhang
Jue Zhang Junlin Zhang1
Junlin Zhang1 Yucheng Wu
Yucheng Wu Mark E. Cooper
Mark E. Cooper Fang Liu
Fang Liu