- 1Endocrinology Unit, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy
- 2Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
GH deficiency (GHD) in adult patients is a complex condition, mainly due to organic lesion of hypothalamic-pituitary region and often associated with multiple pituitary hormone deficiencies (MPHD). The relationships between the GH/IGF-I system and other hypothalamic-pituitary axes are complicated and not yet fully clarified. Many reports have shown a bidirectional interplay both at a central and at a peripheral level. Signs and symptoms of other pituitary deficiencies often overlap and confuse with those due to GH deficiency. Furthermore, a condition of untreated GHD may mask concomitant pituitary deficiencies, mainly central hypothyroidism and hypoadrenalism. In this setting, the diagnosis could be delayed and possible only after recombinant human Growth Hormone (rhGH) replacement. Since inappropriate replacement of other pituitary hormones may exacerbate many manifestations of GHD, a correct diagnosis is crucial. This paper will focus on the main studies aimed to clarify the effects of GHD and rhGH replacement on other pituitary axes. Elucidating the possible contexts in which GHD may develop and examining the proposed mechanisms at the basis of interactions between the GH/IGF-I system and other axes, we will focus on the importance of a correct diagnosis to avoid possible pitfalls.
Introduction
Growth hormone (GH) deficiency in adults (AGHD) is a complex condition characterized by a well-defined clinical phenotype including modified body composition (increased fat mass and loss of lean muscle mass), intermediate metabolism changes, reduced bone mass, compromised aerobic exercise capacity, impaired quality of life and increased cardiovascular risk profile (1–3).
Response to recombinant human growth hormone (rhGH) replacement therapy has a high inter-individual variability and, though several placebo-controlled and observational studies have provided information on its efficacy and safety, the results are still inconclusive, especially regarding quality of life (QoL) improvement and GH specific mortality reduction (2–5).
In adults, growth hormone deficiency (GHD) is often accompanied by other multiple pituitary hormone deficiencies (MPHD), mainly secondary to organic causes (pituitary tumour mass, surgery or radiation, traumatic brain injury, subarachnoid haemorrhage, hypophysitis, Sheehan’s syndrome, vascular damage, empty sella, hypothalamic infiltrative/inflammatory diseases or pituitary metastasis). Nonetheless, sometimes adult-onset GHD can be idiopathic, due to an impaired somatotroph function in the absence of an underlying pituitary lesion or defect. In this setting, the diagnosis can be extremely challenging due to its subtle manifestations and only an extended use of dynamic GH test may reveal such condition (6, 7).
Less frequently, adult GHD is of childhood origin, reconfirmed at adult height and after the transitional age. Childhood-onset GHD (CO GHD) is mostly occurring as an idiopathic isolated hormone deficiency, being additional MPHD rarely encountered (8, 9). However, there are cases of CO GHD reconfirmed in adulthood and the association with other MPHD represents an important predictive factor of persistent GHD, especially in the presence of organic lesions (i.e. craniopharyngiomas). Indeed, severe GHD tends to reconfirm in more than 90% of organic CO GHD and around 50% of idiopathic GHD (10). Moreover, among CO GHD associated with MPHD, it is worth mentioning congenital aetiologies due to mutations of the transcription factors involved in the embryologic development of the pituitary, namely PROP1, POU1F1 (PIT-1), HESX1, LHX3, LHX4 or SOX2 (11).
Clinical manifestations of MPHD are insidious and strictly dependent on the degree and severity of hormone deficiencies, the gender, the age of onset and the underlying comorbidities. In case organic cause, signs and symptoms related to mass effect can also be present.
The challenging management of MPHD is due to the complex and multifaceted interplay between the GH-IGF-I and other pituitary hormones axis, in which specific signs and symptoms of GHD often coincide with those of other deficits. Moreover, a condition of untreated GHD may mask other underlying pituitary deficiencies, mainly central hypothyroidism (CHT) and hypoadrenalism (CHA). In this setting, an appropriate diagnosis can be possible only after rhGH replacement. On the other hand, the concomitant reduction of other pituitary hormones can alter GH secretion and response to pharmacological stimuli, thus an appropriate replacement therapy is required in order to avoid GHD diagnosis pitfalls (1).
The impact of these interactions is more than theoretical: for instance, since rhGH start may increase cortisol metabolism in patients with MPHD, it is possible that GH treatment initiation could lead to acute adrenal insufficiency by “unmasking” a condition of unsubstituted CHA or require an adjustment of glucocorticoid replacement dosages.
Moreover, several androgens enhance GH effects in peripheral tissues (12) explaining why men are more responsive than young women to rhGH therapy and supporting a sexual dimorphism of rhGH effects at different end-points of the treatment (13).
As aforementioned, the clinical manifestations of AGHD may also be related to other underlying pituitary deficiencies or suboptimal replacement therapies. Thus, a correct diagnosis of hypopituitarism and the subsequent indication of appropriate replacement therapy can be crucial in the detection of the beneficial effects of GH therapy.
By elucidating the possible context in which GHD may develop and by examining the proposed mechanisms and the basis by which the GH/IGF-I system and other axes interact (Figure 1), we will enlighten the importance of reaching a correct diagnosis and establishing a correct management to avoid possible pitfalls.
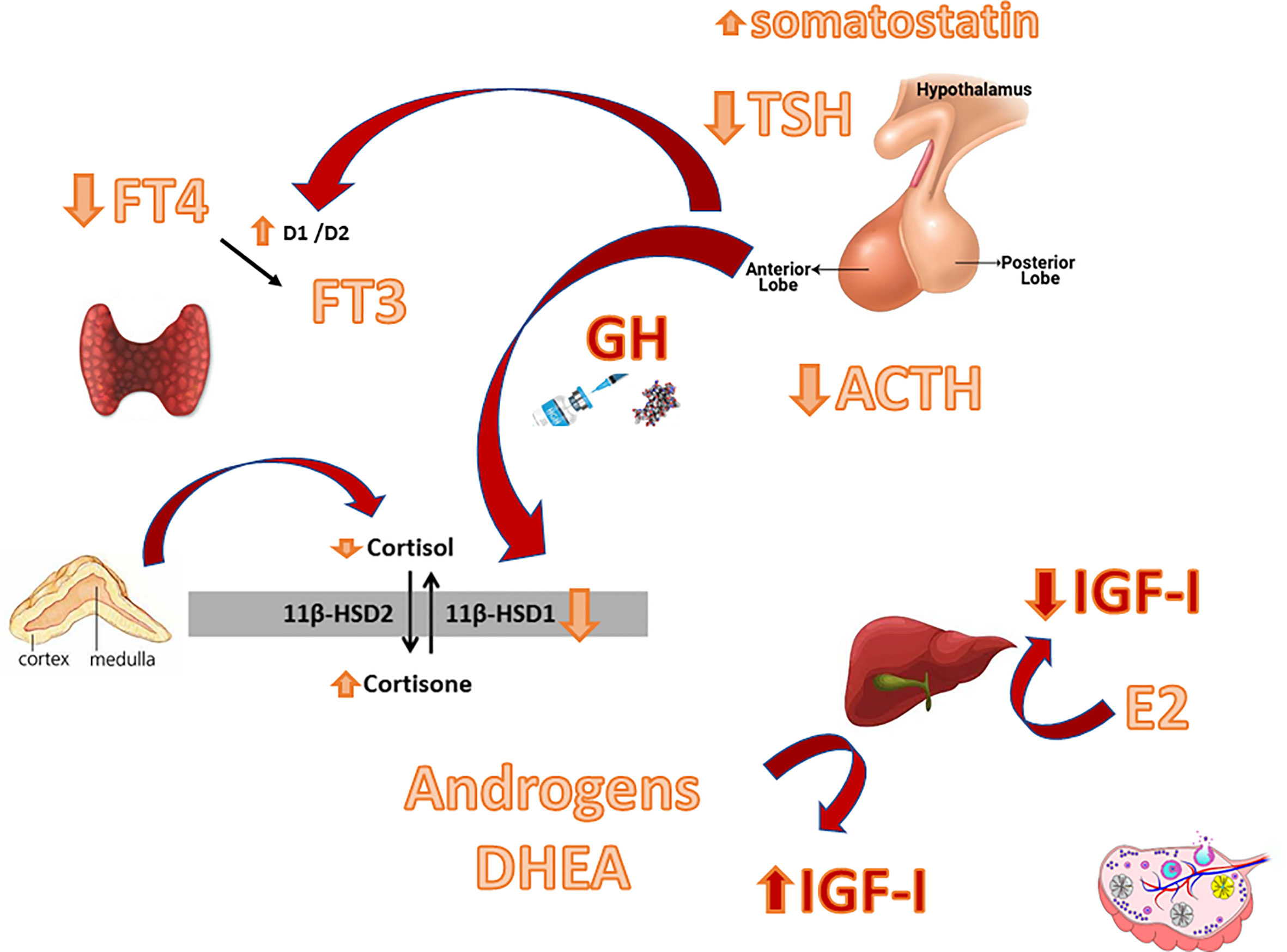
Figure 1 Main interactions between the GH-IGF-I system and other hypothalamic-pituitary axes. D1/D2: Deiodinase type 1/ Deiodinase type 2. 11ßHSD: 11ß-hydroxysteroid dehydrogenase.
GH-IGF-I Axis and Hypothalamic-Pituitary-Adrenal Axis
Growth hormone and IGF-I, together with androgens, represent the main anabolic hormones and cortisol the main catabolic one, thus their actions are evidently linked. Several studies have reported a complex relationship between the GH/IGF-I and the hypothalamic-pituitary-adrenal (HPA) axis, both at a central and a peripheral level (14, 15).
At the hypothalamic-pituitary level, altered cortisol and ACTH secretion may affect GH release. Indeed, a substantial body of literature has described that a condition of eucortisolism is required to elicit a GH response to pharmacological testing (16). The clinical importance of this phenomenon is particularly evident in infants with severe ACTH deficiency, when, even in the presence of mutations of transcription factors not involved in GH axis regulation, a severe GHD can resolve with cortisol replacement (17).
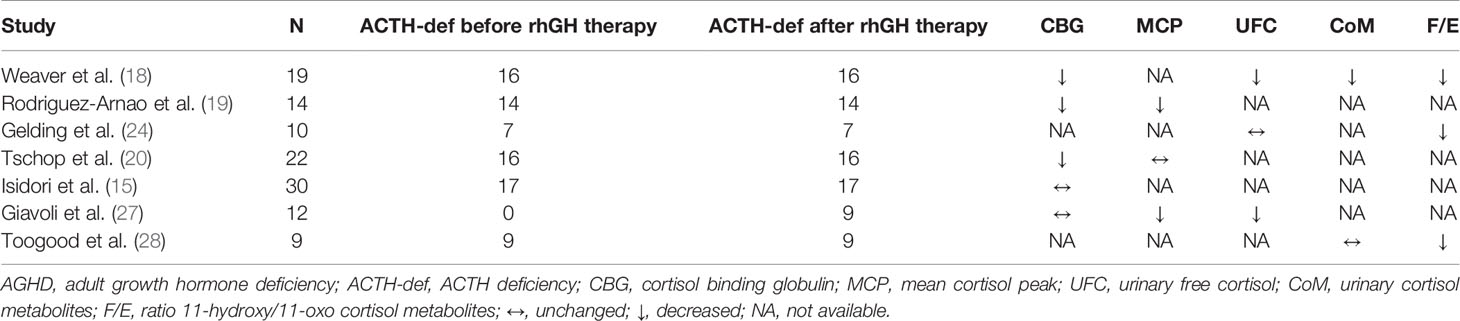
At the peripheral level, some studies reported a possible direct effect of GH therapy on cortisol-binding globulin (CBG) levels, but data are contradictory (15, 18–20).
Moreover, the GH-IGF-I axis interplay can act at a tissue level by modulating the activity of 11beta-hydroxysteroid dehydrogenase (11ßHSD): the well-known cortisol-cortisone shuttle (21). The type 1 isoenzyme (11ßHSD1) can be found in the liver, lung, adipose tissue, gonads, pituitary and central nervous system. It is a low affinity NADP(H)-dependent bi-directional enzyme which interconverts inactive cortisone to active cortisol (22). Conversely, Type 2 isoenzyme (11ßHSD2) is a unidirectional, NAD-dependant dehydrogenase, localized in the kidney, placenta, colon and in the salivary glands and has a dehydrogenase activity which converts active cortisol to cortisone (23). In this context, GH modulates cortisol metabolism mainly by inhibiting 11ßHSD1, thus leading to a reduced cortisone activation into cortisol (18, 24–28). The exact mechanism of modulation is far from being clear: in vitro studies indicate a dose-dependent inhibition of 11ßHSD1 activity induced by IGF-I, but not by GH (26). Whatsoever, the result is that in the lack of GH an increased amount of cortisol is locally generated. Indeed, it has been hypothesized that some of the phenotypic features of GHD can be explained by an alteration in 11ßHSD1 activity, especially in the liver and in the adipose tissue. In particular, the increased local 11ßHSD1 activity in adipose tissues (24, 29, 30), resulting in increased local cortisol exposure (29), could promote insulin resistance and visceral adiposity which tend to reduce after GH replacement (30, 31), possibly explaining its beneficial effects (32). An intriguing observation has been recently made by Agha and colleagues: the authors reported that 11ßHSD1 activity is regulated differently in patients with different aetiologies of hypopituitarism. In particular, they found that patients with a craniopharyngioma had higher 11ßHSD1 activity even during GH therapy compared to a matched group of patients with NFPAs/prolactinomas, with amplified cortisol production in adipose tissues and liver. The clinical significance of this observation remains unclear but the authors hypothesized that this condition may increase the risk of adverse metabolic outcomes (33). Indeed, regarding metabolic outcomes, patients with craniopharyngiomas seem to have a lower response to GH therapy than those with NFPA (34).
Our group investigated the effect of rhGH on the HPA axis in both adults and children with GHD. The former study was carried-out in 12 patients with adult-onset GHD due to surgically treated pituitary tumours and preserved HPA function, before and during rhGH therapy.
Urinary free cortisol, as well as basal and stimulated serum cortisol levels, were lower on therapy than before and a condition of CHA was unmasked in the majority of subjects. Since no change in CBG was found, the results were mainly ascribed to restoration of 11ßHSD1 activity inhibition induced by GH replacement (27). Nonetheless, in the setting of hypopituitarism, CBG levels need to be considered in women taking oral oestrogen replacement therapy since oestrogens increase CBG and consequently total cortisol levels, but not the unbound active fraction.
The above reported data suggest that in patients with organic hypopituitarism, GH deficiency may mask the presence of CHA. To confirm this observation, the study conducted in 10 children with idiopathic isolated GHD and normal pituitary MRI, showed no changes in the HPA axis during rhGH (35).
The major studies so far available on this topic are reported in Table 1.
All these observations, taken together, tend to support a strong interplay between the GH/IGF-I and the HPA axis. The clinical impact is particularly evident in patients with MPHD, who may experience a life-threatening adrenal crisis after rhGH initiation in the presence of an untreated CHA.
Thus, in patients with possible MPHD, the integrity of the HPA axis must be evaluated both before GH pharmacological stimulating tests (to avoid diagnostic pitfalls) and after rhGH start. Indeed, an underlying unsubstituted CHA might be unmasked by rhGH therapy, inducing a possible adrenal crisis. Moreover, in patients already under replacement for ACTH deficiency, steroid therapy should be adjusted, especially when using cortisone acetate (1, 36, 37).
GH-IGF-I Axis and Hypothalamic-Pituitary-Thyroid Axis
It is well ascertained that untreated hypothyroidism is associated with reduced IGF-I and IGF binding protein-3 (IGF-BP-3) and, indeed, even in subclinical hypothyroidism, these reduced levels of IGF-I increase with Levotiroxine (LT4) replacement therapy (38). This phenomenon is easily noticeable in hypothyroid children whose growth failure is reversible by the introduction of LT4 replacement therapy. Moreover, hypothyroidism induces a decrease in GH pulsatility and blunts GH responses to secretory stimuli, changes that are reversible after LT4 introduction, suggesting a possible underlying driven role of thyroid hormones (39).
Therefore, GH provocative tests as well as rhGH replacement therapy should be performed or administered only after the restoration of a condition of euthyroidism. Indeed, as LT4 accelerates cortisol clearance potentially triggering an Addisonian crisis in the presence of an underlying CHA, glucocorticoid replacement therapy should be started first. Thus, in patients with MPHD, hormone replacement therapies must be introduced following a well-defined order: first hydrocortisone, then LT4 (usually after a week), rhGH and, when indicated, sex hormones (36).
However, we have to consider that rhGH therapy can affect the regulation of the HPT axis and thyroid hormone concentrations by several different mechanisms.
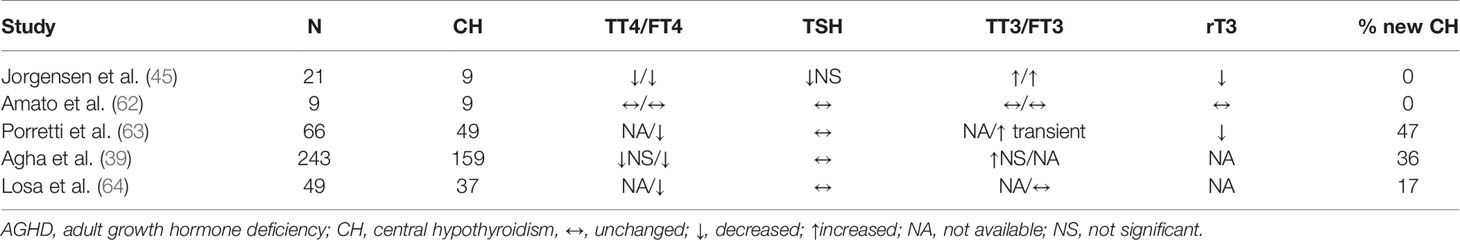
Firstly, at a peripheral level, GH induces the extra-thyroidal conversion of T4 to the active hormone triiodothyronine (T3), reducing the concentrations of the inactive form reverse-T3 (rT3) and increasing the T3/T4 ratio (40–45). The effect of GH on circulating T3 levels has been firstly described in animal models (46–48) where GH stimulates T4 conversion to T3. In untreated GHD patients, there is a decreased conversion of T4 to T3, with increased concentrations of rT3 (49). Even if the exact activation pathway under GH control is still unknown, an upregulation of type 2 iodothyroinine deiodinase by GH has been recently described in humans (50). In MPHD, the activity of type 2 deiodinase is usually increased to counterbalance, with a more efficient T3 production, the initial T4 reduction. This compensatory mechanism would be lacking in hypothyroid patients with GHD (50).
Secondly, an interaction at a central level has also been postulated: increased somatostatinergic tone or T3 negative feedback within the thyrotropes, driven by increased T3 production from T4 deiodination, may inhibit TSH release (40–42, 44, 51, 52). In GH deficient adults on rhGH replacement, a significant blunting of the TSH nocturnal surge has indeed been reported (53, 54). However, other studies failed to find TSH variation during rhGH therapy (49). Moreover, whether the interaction between the GH/IGF-I and the HPT axis is directly mediated by GH or through IGF-I is still to be established. Some studies failed to support the influence of IGF-I administration on serum T3 (55). Furthermore, a much higher T3 increase has been described after GH than after IGF-I therapy in GHD patients suggesting that GH has a more direct potent effect on thyroid hormone metabolism (56).
Consistently, in GHD patients under LT4 replacement therapy, rhGH led to a dose-dependent increase in T4 to T3 conversion and a decrease in immunoreactive TSH levels, probably secondary to the increased free T3 in the thyrotropes or to the increased somatostatinergic tone (56, 57). These findings support the crucial role of GH in the HPT homeostasis. Moreover, in hypothyroid patients under LT4 replacement, another possible underlying mechanism is the GH-driven reduction of T4 half-life and the increase of T3 half-life (58) by affecting thyroxine clearance rate or inhibiting LT4 uptake from the gastrointestinal tract (59–61).
When considering GH deficient adults, the first reported results on the interaction with the HPT were controversial, due to the small sample sizes, different study protocols, biochemical analytic methods and criteria for GHD diagnosis, and the use of pituitary GH occasionally contaminated with TSH (45, 62). Nonetheless, subsequent available studies confirmed that, in GHD adults, as in children, rhGH therapy could unmask an underlying CHT. Indeed, a multicenter study evaluated a quite large cohort of patients with either adult or childhood onset severe GHD (17 euthyroid patients and 49 with central hypothyroidism) treated with different rhGH doses (3-12 mg/kg/day) and observed a significant reduction in FT4 and rT3 levels without changes in TSH, FT3 and TBG levels. Interestingly, the fall of FT4 levels was clinically relevant only in patients with organic hypopituitarism (63). A later study confirmed these assumptions in a group of 243 patients, in which the underlying presence of MPHD was found to be the major predictor for CHT development (40). Similar data have been confirmed in long-term follow-up (5 years of rhGH) (64). All in all, the GH-IGF-I and HPT axes interactions have possible tissue-specific effects: indeed, rhGH efficacy on energy expenditure, substrate use and metabolic plasticity can be attenuated by the fluctuations in thyroid hormone levels (65).
Table 2 summarizes the main studies on this topic.
More uncertainties exist over the effects of GH therapy on thyroid volume (TV) and morphology. Actually, TSH represents the major regulator of both thyroid hormone biosynthesis and thyroid growth. However, IGF-I itself has a proliferative role interacting with its own receptors, largely expressed in thyroid cells (66). Indeed, most acromegalic patients have goiter and IGF-I levels are positively correlated with TV, while hypopituitary patients tend to have reduced TV (67–70).
The finding of unchanged TV during rhGH in TSH- and GH-deficient children, adolescents and adults supports the idea that IGF-I has only a permissive role on the mitogenic action of TSH (71, 72). In fact, an increased TV in patients with congenital isolated GHD was found after 6 months of rhGH therapy (73). Finally, Curtò and collaborators, studying patients with childhood and adult onset GHD before and after 5 years of rhGH therapy, found smaller pretreatment TV in GHD patients than in healthy controls, with increased TV only in patients without concomitant CHT (74).
To summarize, organic GHD can frequently mask a state of CHT, thus it is mandatory to assess and carefully monitor thyroid function before and during rhGH administration, in order to start or adjust LT4 replacement when indicated (1, 37, 75). Indeed, while it is recommended to maintain FT4 in the mid-normal range in patients with CHT, in the presence of a concomitant untreated GH deficiency it would be sensible to aim for higher FT4 levels, given the underlying impairment of T4 to T3 conversion (76, 77). Moreover, most of FT4 variations occur within the first 6 months of therapy, thus the importance of an early revaluation of thyroid function after rhGH initiation (64, 78).
GH-IGF-I Axis and Hypothalamic-Pituitary-Gonadal Axis
In order to understand the complex interaction between the GH/IGF-I and the hypothalamic-pituitary-gonadal (HPG) axis it is crucial to take into consideration the sexual dimorphism of endogenous GH secretion. Indeed, during the pre-pubertal period, GH and IGF-I levels are similar between boys and girls (79) but in adults spontaneous 24-h GH secretion is approximately two-fold higher in women than in men, mostly due to increased pulse amplitude without a difference in pulse frequency (80). The first gender divergences, indeed, occur during puberty, when pulse GH amplitude in girls tend to precede the one in boys, according to the different timing of the pubertal growth spurt in the two sexes (81). Moreover, GH production declines more quickly with age in women than in men and during menopause this is usually associated with a significant gain in visceral fat mass (82).
Despite this important sexual dimorphism of GH levels, cross-sectional studies have found no difference in serum IGF-I concentrations between women and men (83), though in women a moderate raise of IGF-I levels related to increased GH secretion has been reported in the early follicular and periovulatory phase (82, 84).
The gender-independence of IGF-I levels in healthy adults, despite significantly higher GH concentrations in females, supports the presence of compensated GH resistance in women. This phenomenon is due to a direct inhibitory effect of oestrogen on hepatic but not peripheral IGF-I production. Underlying mechanisms that contribute to this liver sexual dimorphism are pituitary-independent and related to the interaction of oestrogens with their receptors. Namely, the induction of suppressor of cytokine signalling (SOCS)-2 and the inhibition of GHR-Janus kinase (JAK)-2-signal transducer and activator of transcription (STAT)-5 signalling pathway in the liver (85, 86) reduce IGF-I secretion from hepatocytes (87).
Moreover, oral administration of oestrogens introduces an open-loop feedback system during which the continuous and un-physiological suppression of hepatic IGF-I production and release, due to a first pass hepatic effect of oral oestrogen (82), is only partially compensated by increased pituitary GH secretion. In this context, oestrogen replacement discontinuation or omission tends to solve the resistance to GH administration. Serum IGF-I in the GH-deficient state is further lowered by oral oestrogen, but results unaffected by transdermal therapy (88, 89). This phenomenon can explain why IGF-I levels are lower in hypopituitary women than men, despite a similar degree of impaired GH secretion (90). Moreover, women with hypopituitarism tend to be more susceptible to the hepatic effects of oral oestrogens due to the lack of feedback in GH response. Cook et al., indeed, observed that GH requirements in men were not different from those in women not taking oestrogens, but that women taking oral oestrogens required at least a two-fold greater dosage of GH (91).
Interestingly, even in males, many reports have provided robust evidence that oestradiol, rather than testosterone itself, increases GH secretion via oestrogen receptor (92, 93) after aromatization from testosterone. In fact, recently, Birzniece and colleagues have shown that the stimulatory effect of testosterone on GH is completely hampered by oestrogen receptor antagonists and by aromatase inhibitors (94).
On the other hand, a study in males revealed that the association of hypogonadotropic hypogonadism (HH) and GHD has an additional lowering effect on testosterone, DHT and oestradiol levels versus that seen in isolated HH. This phenomenon supports a synergistic effect of GH/IGF1 on Leydig cell (LC) function (95). In this context, one would have expected an increase in testosterone levels with rhGH therapy. However, the literature available on this topic reported contrasting data. The only studies that showed an increase in testosterone levels included azoospermic (96) or hypogonadal patients (97) with GH and gonadotropin co-treatment. In contrast, in a double-blind placebo controlled trial performed in young males with childhood-onset GHD, Juul et al. (98) concluded that rhGH administration does not influence the HPG axis. Conversely, another study (99) carried out in males with idiopathic isolated GHD, showed that rhGH treatment displays an effect on LC function, increasing testosterone response to chorionic gonadotropin (CG). However, these studies included patients with either idiopathic or organic GHD or varied HPG axis status, being either normogonadic or hypogonadic under treatment with testosterone. Moreover, the high rhGH doses employed in these studies make it difficult to distinguish physiological and pharmacological rhGH effects. In another study on adult males with organic GHD and normal HPG axis we reported a significant decrease in serum testosterone levels strictly related to Sex Hormone Binding Globulin (SHBG) reduction. This suggests the importance of the evaluation of the HPG axis during rhGH treatment, utilizing free calculated testosterone, rather than total testosterone, in order to avoid unnecessary replacement therapy (100).
Moreover, some literature is available on the impact of rhGH treatment on infertility. Males with HH who failed to respond adequately to conventional infertility treatment showed increased testosterone secretion and improved fertility outcomes and sperm production after rhGH adjuvant therapy with gonadotropins (101). In addition, a prospective, open-label, non-randomized observational study of 14 men (26 to 35 years) with normogonadotropic idiopathic oligoasthenospermia found beneficial effects of six months of rhGH treatment on semen volume, count, and motility (102). On the contrary, in a small group of hypogonadotropic hypogonadal azoospermic patients, rhGH replacement therapy for six months, following a previous period of six months of gonadotropin treatment, while increasing testicular volume and testosterone levels, failed to induce the appearance of spermatozoa in the sperm (97). Undoubtedly, the interaction between the GH-IGF-I and the HPG axis plays a role in reproduction and fertility. However, data on the impact of rhGH therapy in non-GHD males are scanty and data on spermatogenesis and fertility in GHD adults, either treated or untreated, are missing.
Similarly, in females, the presence of GH receptors on oocytes suggests a direct action of GH at this level (103). Yet, IGF-I could mediate the reproductive effects of GH, being present in follicular fluid and involved in the cytoplasmic maturation, oocyte capability and granulosa cell function (104). Clinical studies evaluating female patients with suboptimal response to in vitro fertilization (IVF), have shown that the co-administration of GH with gonadotropin for controlled ovarian stimulation was associated with a reduction in the gonadotropin requirement, with a higher proportion of successful embryo transfer stage, higher pregnancy and live births rate (105, 106). These outcomes bring to light a possible role for rhGH treatment in oocyte and embryo quality improvement. However, in these patients, endogenous GH secretion was not investigated. When considering GHD, a study by De Boer and Coll reported decreased fertility even in patients without associated hypogonadism (107), suggesting the contribution of GHD to infertility. Giampietro et al. (108) presented four cases of infertility in women with isolated GHD and normal HPG function, in which initiation of rhGH led to efficacious conception and pregnancies. Similarly, in a recent case report by Albu et al, GH therapy contributed to IVF success by improving oocyte competence in a GHD patient. The author concluded that the influence of GH in enhancing oocyte quality should be taken into account in all infertile females with GHD, in order to improve treatment outcome especially when facing previous treatment failure (109). Nevertheless, the responsible mechanisms of GH action on fertility are not fully understood.
Differently from gonadal steroids, in females, DHEA influences the GH/IGF-I axes by increasing IGF-I response thus reducing GH requirement. On the other hand, no rhGH dose adjustment has been necessary in the male group taking testosterone replacement therapy (108, 109). The exact mechanism by which DHEA causes an increase in serum IGF-I levels is still unclear. Some authors have suggested a possible direct stimulatory effect of DHEA on IGF-I hepatic production or an inhibition of IGF-I clearance. On the other hand, DHEA could also enhance GH efficacy acting directly on GH receptors or through testosterone metabolism (110, 111).
To conclude, when treating hypopituitary patients, the gender differences in GH sensitivity and responsiveness are important aspects to take into consideration in clinical practice. In fact, GHD men are more responsive than young women to rhGH therapy, supporting a sexual dimorphism of rhGH effects in different end-points of the treatment. Female patients, indeed, usually require higher rhGH doses to normalize IGF-I levels, especially when receiving oral oestrogen. For this reason, in women with GHD and hypogonadotropic hypogonadism, a transdermal route of oestrogen replacement should be preferred for a cost-effective rhGH treatment.
On the contrary, in males, despite the GH-induced increase in circulating IGF-I by testosterone therapy may suggest the need of lower doses of rhGH, no clinical data have supported a dose reduction during testosterone treatment (112). Moreover, given the above mentioned studies, it is possible to conclude that rhGH treatment does not significantly change the hypothalamic-pituitary-testicular axis metabolism. In this contest, no adjustment of rhGH or testosterone therapy is needed.
Likewise, the reported preliminary data on the influence of the GH/IGF-I axis on fertility does not achieve at present sufficient consensus to be considered in clinical practice.
Conclusions
In conclusion, the experience developed during the last decades strengthens the view that rhGH replacement therapy is effective and safe in treating GHD in adulthood. Nonetheless, the adult with GHD is a complex patient, in whom the deficit is almost always part of a picture of MPHD. In this context, interactions between replacement therapies have to be taken into account, not only to tailor the best hormonal substitutions, but also to achieve a prompt and accurate diagnosis of hypopituitarism, that is of paramount importance in the management of these patients (Table 3). In particular, the state of untreated GHD may mask in a consistent manner a number of cases of central hypoadrenalism and/or hypothyroidism, whose diagnosis becomes possible only after rhGH replacement. Hence, the most recent Guidelines suggest the re-assessment of thyroid and adrenal function during rhGH therapy in patients with organic GHD. Similarly, in patients already under glucocorticoid and LT4 replacement, dosages should be adjusted and usually appropriately increased after rhGH start. In the same context, it is recommended using higher rhGH doses to normalize IGF-I levels in women receiving oral oestrogen and lower doses in women taking DHEA supplement. When possible, a transdermal route of oestrogen replacement should be preferred for a cost-effective rhGH treatment (1, 37).
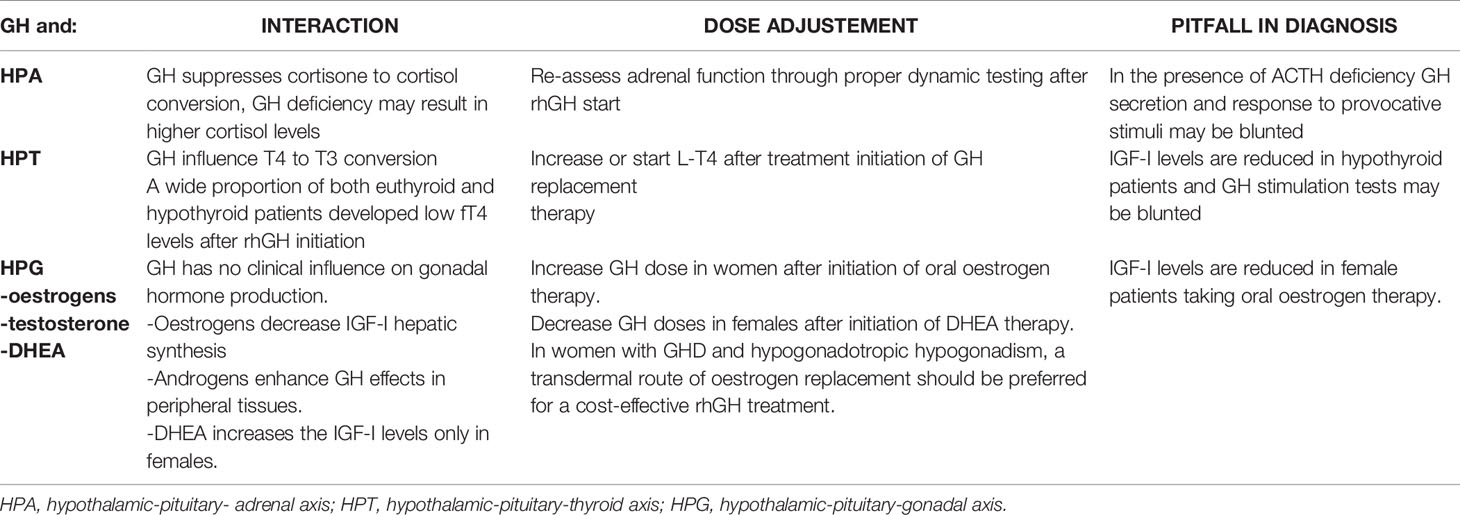
Table 3 GH deficiency and therapy in multiple pituitary hormone deficiency: interactions and dose adjustments.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation with the authors at time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, et al. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2016) 101(11):3888–921. doi: 10.1210/jc.2016-2118
2. Jørgensen JOL, Juul A. Growth Hormone Replacement Therapy in Adults: 30 Years of Personal Clinical Experience. Eur J Endocrinol (2018) 179(1):R47–56. doi: 10.1530/EJE-18-0306
3. Melmed S. Pathogenesis and Diagnosis of Growth Hormone Deficiency in Adults. N Engl J Med (2019) 380(26):2551–62. doi: 10.1056/NEJMra1817346
4. Stochholm K, Gravholt CH, Laursen T, Laurberg P, Andersen M, Kristensen LO, et al. Mortality and GH Deficiency: A Nationwide Study. Eur J Endocrinol (2007) 157(1):9–18. doi: 10.1530/EJE-07-0013
5. He X, Barkan AL. Growth Hormone Therapy in Adults With Growth Hormone Deficiency: A Critical Assessment of the Literature. Pituitary (2020) 23(3):294–306. doi: 10.1007/s11102-020-01031-5
6. Melmed S. Idiopathic Adult Growth Hormone Deficiency. J Clin Endocrinol Metab (2013) 98(6):2187–97. doi: 10.1210/jc.2012-4012
7. Mancini A, Bruno C, Vergani E, Brunetti A, Palmisano G, Pontecorvi A. "Non-Classical" Indication for Provocative Testing of Growth Hormone: A Retrospective Cohort Study in Adult Patients Under Replacement Therapy. Endocr Metab Immune Disord Drug Targets (2021) 21(8):1406–12. doi: 10.2174/1871530320666200929141847
8. Binder G, Schnabel D, Reinehr T, Pfäffle R, Dörr HG, Bettendorf M, et al. Evolving Pituitary Hormone Deficits in Primarily Isolated GHD: A Review and Experts' Consensus. Mol Cell Pediatr (2020) 7(1):16. doi: 10.1186/s40348-020-00108-2
9. Brabant G, Poll EM, Jonsson P, Polydorou D, Kreitschmann-Andermahr I. Etiology, Baseline Characteristics, and Biochemical Diagnosis of GH Deficiency in the Adult: Are There Regional Variations? Eur J Endocrinol (2009) 161(suppl 1):S25–31. doi: 10.1530/EJE-09-0273
10. Aimaretti G, Baffoni C, Bellone S, DiVito L, Corneli G, Arvat E, et al. Retesting Young Adults With Childhood-Onset Growth Hormone (GH) Deficiency With GH-Releasing-Hormone-Plus-Arginine Test. J Clin Endocrinol Metab (2000) 85:3693–9. doi: 10.1210/jc.85.10.3693
11. Gregory LC, Dattani MT. The Molecular Basis of Congenital Hypopituitarism and Related Disorders. J Clin Endocrinol Metab (2020) 105(6):dgz184. doi: 10.1210/clinem/dgz184
12. Birzniece V, Ho KKY. Sex Steroids and the GH Axis: Implications for the Management of Hypopituitarism. Best Pract Res Clin Endocrinol Metab (2017) 31(1):59–69. doi: 10.1016/j.beem.2017.03.003
13. Burman P, Johansson AG, Siegbahn A, Vessby B, Karlsson FA. Growth Hormone (GH)-Deficient Men are More Responsive to GH Replacement Therapy Than Women. J Clin Endocrinol Metab (1997) 82(2):550–5. doi: 10.1210/jcem.82.2.3776
14. Le Roy C, Li JY, Stocco DM, Langlois D, Saez JM. Regulation by Adrenocorticotropin (ACTH), Angiotensin II, Transforming Growth Factor-1 and Insulin-Like Growth Factor I of Bovine Adrenal Cell Steroidogenic Capacity and Expression of ACTH Receptor, Steroidogenic Acute Regulatory Protein, Cytochrome P450c17 and 3-Hydroxysteroid Dehydrogenase. J Clin Endocrinol Metab (2000) 141:1599–607. doi: 10.1210/endo.141.5.7457
15. Isidori AM, Kaltsas GA, Perry L, Burrin JM, Besser GM, Monson JP. The Effect of Growth Hormone Replacement Therapy on Adrenal Androgen Secretion in Adult Onset Hypopituitarism. Clin Endocrinol (Oxf) (2003) 58:601–11. doi: 10.1046/j.1365-2265.2003.01759.x
16. Mazziotti G, Giustina A. Glucocorticoids and the Regulation of Growth Hormone Secretion. Nat Rev Endocrinol (2013) 9(5):265–76. doi: 10.1038/nrendo.2013.5
17. McEachern R, Drouin J, Metherell L, Huot C, Van Vliet G, Deal C. Severe Cortisol Deficiency Associated With Reversible Growth Hormone Deficiency in Two Infants: What is the Link? J Clin Endocrinol Metab (2011) 96(9):2670–4. doi: 10.1210/jc.2011-0129
18. Weaver JU, Theventhiran L, Noonan K, Burrin JM, Taylor NF, Norman MR, et al. The Effect of Growth Hormone Replacement on Cortisol Metabolism and Glucocorticoid Sensitivity in Hypopituitary Adults, Clin. Endocrinol (1994) 41:639–48. doi: 10.1111/j.1365-2265.1994.tb01830.x
19. Rodriguez-Arnao J, Perry L, Besser GM, Ross RJM. Growth Hormone Treatment in Hypopituitary GH Deficient Adults Reduces Circulating Cortisol Levels During Hydrocortisone Replacement Therapy. Clin Endocrinol (Oxf) (1996) 45:33–7. doi: 10.1111/j.1365-2265.1996.tb02057.x
20. Tschop M, Lahner H, Feldmeier H, Grasberger H, Morrison KM, Janssen OE, et al. Effects of Growth Hormone Replacement Therapy on Levels of Cortisol and Cortisol-Binding Globulin in Hypopituitary Adults. Eur J Endocrinol (2000) 143:769–73. doi: 10.1530/eje.0.1430769
21. Stewart PM, Toogood AA, Tomlinson JW. Horm Growth Hormone, Insulin-Like Growth Factor-I and the Cortisol-Cortisone Shuttle. Res (2001) 56(Suppl 1):1–6. doi: 10.1159/000048126
22. Jamieson P, Chapman KE, Edwards CR, Seckl JR. 11-Hydroxysteroid Dehydrogenase is an Exclusive 11-Reductase in Primary Cultures of Rat Hepatocytes: Effect of Physiochemical and Hormonal Manipulations. Endocrinology (1995) 136:4754–61. doi: 10.1210/endo.136.11.7588203
23. Seckl JR. 11 Beta-Hydroxysteroid Dehydrogenase Isoforms and Their Implications for Blood Pressure Regulation. Eur J Clin Invest (1993) 23(10):589–601. doi: 10.1111/j.1365-2362.1993.tb00720.x
24. Gelding SV, Taylor NF, Wood PJ, Noonan K, Weaver JU, Wood DF, et al. The Effect of GH Replacement on Cortisol-Cortisone Interconversion in Hypopituitary Adults: Evidence for GH Modulation of Extrarenal 11-HSD Activity. Clin Endocrinol (Oxf) (1998) 48:153–62. doi: 10.1046/j.1365-2265.1998.3641180.x
25. Walker BR, Andrew R, MacLeod KM, Padfield PL. Growth Hormone Replacement Inhibits Renal and Hepatic 11b Hydroxysteroid Dehydrogenases in ACTH Deficient Patients. Clin Endocrynol (1998) 49:257–63. doi: 10.1046/j.1365-2265.1998.00575.x
26. Moore JS, Monson JP, Kaltsas G, Putignano P, Wood PJ, Sheppard MC, et al. Modulation of 11b-Hydroxysteroid Dehydrogenase Isozymes by Growth Hormone and Insulin-Like Growth Factor: In Vivo and In Vitro Studies. J Clin Endocrinol Metab (1999) 84:4172–7. doi: 10.1210/jcem.84.11.6108
27. Giavoli C, Libe R, Corbetta S, Ferrante E, Lania A, Arosio M, et al. Effect of Recombinant Human Growth Hormone (GH) Replacement on the Hypothalamic-Pituitary-Adrenal Axis in Adult GH-Deficient Patients. J Clin Endocrinol Metab (2004) 89(11):5397–401. doi: 10.1210/jc.2004-1114
28. Toogood AA, Taylor NF, Shalet SM, Monson JP. Modulation of Cortisol Metabolism by Low-Dose Growth Hormone Replacement in Elderly Hypopituitary Patients. J Clin Endocrinol Metab (2007) 85:1727–30. doi: 10.1210/jcem.85.4.6505
29. Wake DJ, Rask E, Livingstone DE, Soderberg S, Olsson T, Walker BR. Local and Systemic Impact of Transcriptional Up-Regulation of 11beta-Hydroxysteroid Dehydrogenase Type 1 in Adipose Tissue in Human Obesity. J Clin Endocrinol Metab (2003) 88(8):3983–8. doi: 10.1210/jc.2003-030286
30. Paulsen SK, Pedersen SB, Jorgensen JO, Fisker S, Christiansen JS, Flyvbjerg A, et al. Growth Hormone (GH) Substitution in GH-Deficient Patients Inhibits 11beta-Hydroxysteroid Dehydrogenase Type 1 Messenger Ribonucleic Acid Expression in Adipose Tissue. J Clin Endocrinol Metab (2006) 91(3):1093–8. doi: 10.1210/jc.2005-1694
31. Swords FM, Carroll PV, Kisalu J, Wood PJ, Taylor NF, Monson JP. The Effects of Growth Hormone Deficiency and Replacement on Glucocorticoid Exposure in Hypopituitary Patients on Cortisone Acetate and Hydrocortisone Replacement. Clin Endocrinol (Oxf) (2003) 59(5):613–20. doi: 10.1046/j.1365-2265.2003.01894.x
32. Morita J, Hakuno F, Hizuka N, Takahashi S, Takano K. Growth Hormone (GH) or Insulin-Like Growth Factor (IGF)-I Represses 11beta-Hydroxysteroid Dehydrogenase Type 1 (HSD1) mRNA Expression in 3T3-L1 Cells and its Activity in Their Homogenates. Endocr J (2009) 56(4):561–70. doi: 10.1507/endocrj.k08e-311
33. Agha A, Behan LA, Forde H, Taylor NF, Smith D, Thompson CJ, et al. Differential Regulation of 11 β -Hydroxysteroid Dehydrogenase Type 1 Activity in Patients With Differing Aetiologies of Hypopituitarism. Endocr Pract (2018) 24(10):875–81. doi: 10.4158/EP-2018-0154
34. Profka E, Giavoli C, Bergamaschi S, Ferrante E, Malchiodi E, Sala E, et al. Analysis of Short- and Long-Term Metabolic Effects of Growth Hormone Replacement Therapy in Adult Patients With Craniopharyngioma and non-Functioning Pituitary Adenoma. J Endocrinol Invest (2015) 38(4):413–20. doi: 10.1007/s40618-014-0196-0
35. Giavoli C, Bergamaschi S, Ferrante E, Ronchi CL, Lania AG, Rusconi R, et al. Effect of Growth Hormone Deficiency and Recombinant hGH (rhGH) Replacement on the Hypothalamic–Pituitary–Adrenal Axis in Children With Idiopathic Isolated GH Deficiency. Clin Endocrinol (2008) 68(2):247–51. doi: 10.1111/j.1365-2265.2007.03029.x
36. Alexandraki KI, Grossman A. Management of Hypopituitarism. J Clin Med (2019) 8(12):2153. doi: 10.3390/jcm8122153
37. Yuen KCJ, Biller BMK, Radovick S, Carmichael JD, Jasim S, Pantalone KM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Growth Hormone Deficiency in Adults and Patients Transitioning From Pediatric to Adult Care. Endocr Pract (2019) 25(11):1191–232. doi: 10.4158/GL-2019-0405
38. Akin F, Yaylali GF, Turgut S, Kaptanoglu B. Growth Hormone/Insulin-Like Growth Factor Axis in Patients With Subclinical Thyroid Dysfunction. Growth Horm IGF Res (2009) 19(3):252–5. doi: 10.1016/j.ghir.2008.11.003
39. Agha A, Walker D, Perry L, Drake WM, Chew SL, Jenkins PJ, et al. Unmasking of Central Hypothyroidism Following Growth Hormone Replacement in Adult Hypopituitary Patients. Clin Endocrinol (Oxf) (2007) 66(1):72–7. doi: 10.1111/j.1365-2265.2006.02688.x
40. Porter BA, Refetoff S, Rosenfeld RL, De Groat LJ, Lang US, Stark O. Abnormal Thyroxine Metabolism in Hyposomatotrophic Dwarfism and Inhibition of Responsiveness to TRH During GH Therapy. Pediatrics (1973) 51:668e74.
41. Sato T, Suzuki Y, Ishiguro K, Masuyama T. Enhanced Peripheral Conversion of Thyroxine to Triiodothyronine During hGH Therapy in GH Deficient Children. J Clin Endocrinol Metab (1977) 45:324–9. doi: 10.1210/jcem-45-2-324
42. Rezvani I, DiGeorge AM, Dowshen SA, Bourdony CJ. Action of Human Growth Hormone (hGH) on Extrathyroidal Conversion of Thyroxine (T4) to Triiodothironine (T3) in Children With Hypothyroidism. Pediatr Res (1981) 15:6–9. doi: 10.1203/00006450-198101000-00002
43. Grunfeld C, Sherman BM, Cavalieri RR. The Acute Effects of Human Growth Hormone Administration on Thyroid Function in Normal Men. J Clin Endocrinol Metab (1988) 67:1111–4. doi: 10.1210/jcem-67-5-1111
44. Wyatt DT, Gesundheit N, Sherman B. Changes in Thyroid Hormones Levels During Growth Hormone Therapy in Initially Euthyroid Patients: Lack of Need for Thyroxine Supplementation. J Clin Endocrinol Metab (1988) 83:3493–7. doi: 10.1210/jcem.83.10.5202
45. Jørgensen JO, Pedersen SA, Laurberg P, Weeke J, Skakkebaek NE, Christiansen JS. Effects of Growth Hormone Therapy on Thyroid Function of Growth Hormone Deficient Adults With and Without Concomitant Thyroxine-Substituted Central Hypothyroidism. J Clin Endocrinol Metab (1989) 69:1127–31. doi: 10.1210/jcem-69-6-1127
46. Kühn ER, Van Osselaer P, Siau O, Decuypere E, Moreels A. Thyroid Function in Newborn Lambs: Influence of Prolactin and Growth Hormone. J Endocrinol (1986) 109:215–9. doi: 10.1677/joe.0.1090215
47. Hannon K, Trenkle A. Relationship of Thyroid Status to Growth Hormone and Insulin-Like Growth Factor-I (IGF-I) in Plasma and IGF-I mRNA in Liver and Skeletal Muscle of Cattle. Domest Anim Endocrinol (1991) 8:595–600. doi: 10.1016/0739-7240(91)90029-j
48. Darras VM, Berghman LR, Vanderpooten A, Kühn ER. Growth Hormone Acutely Decreases Type III Iodothyronine Deiodinase in Chicken Liver. FEBS Lett (1992) 310:5–8. doi: 10.1016/0014-5793(92)81133-7
49. Portes ES, Oliveira JH, MacCagnan P, Abucham J. Changes in Serum Thyroid Hormones Levels and Their Mechanisms During Longterm Growth Hormone (GH) Replacement Therapy in GH Deficient Children. Clin Endocrinol (2000) 53:183–9. doi: 10.1046/j.1365-2265.2000.01071.x
50. Yamauchi I, Sakane Y, Yamashita T, Hirota K, Ueda Y, Kanai Y, et al. Effects of Growth Hormone on Thyroid Function are Mediated by Type 2 Iodothyronine Deiodinase in Humans. Endocrine (2018) 59(2):353–63. doi: 10.1007/s12020-017-1495-y
51. Oliner L, Ballantine JJ. Effect of Human Growth Hormone on Thyroidal Secretion, Radiothyroxine Turnover and Transport in Man. J Clin Endocrinol Metab (1968) 28:603–7. doi: 10.1210/jcem-28-5-603
52. Cobb WE, Reichlin S, Jackson IM. Growth Hormone Secretory Status is a Determinant of the Thyrotropin Response to Thyrotropin Releasing Hormone in Euthyroid Patients With Hypothalamic-Pituitary Disease. J Clin Endocrinol Metab (1981) 52:324–9. doi: 10.1210/jcem-52-2-324
53. Pirazzoli P, Cacciari E, Mandini M, Sganga T, Capelli M, Cicognani A, et al. Growth and Thyroid Function in Children Treated With Growth Hormone. J Pediatr (1992) 121:210–3. doi: 10.1016/s0022-3476(05)81190-4
54. Jørgensen JOL, Moeller J, Laursen T, Orskov H, Christiansen JS, Weeke J. Growth Hormone Administration Stimulates Energy Expenditure and Extrathyroidal Conversion of Thyroxine to Triiodothyronine in a Dose-Dependent Manner and Suppresses Circadian Thyrotropin Levels: Studies in GH Deficient Adults. Clin Endocrinol (1994) 41:609–14. doi: 10.1111/j.1365-2265.1994.tb01826.x
55. Klinger B, Ionesco A, Anin S, Laron Z. Effects of Insulin-Like Growth Factor I on the Thyroid Axis in Patients With Laron-Type Dwarfism and Healthy Subjects. Acta Endocrinol (Copenh) (1992) 127:515–9. doi: 10.1530/acta.0.1270515
56. Hussain MA, Schmitz O, Jørgensen JO, Christiansen JS, Weeke J, Schmid C, et al. Insulin-Like Growth Factor I Alters Peripheral Thyroid Hormone Metabolism in Humans: Comparison With Growth Hormone. Eur J Endocrinol (1996) 134:563–7. doi: 10.1530/eje.0.1340563
57. Oliveira JH, Barbosa ER, Kasamatsu T, Abucham J. Evidence for Thyroid Hormone as a Positive Regulator of Serum Thyrotropin Bioactivity. J Clin Endocrinol Metab (2007) 92(8):3108–13. doi: 10.1210/jc.2006-2217
58. Demura R, Yamaguchi H, Wakabayashi I, Demura H, Shizume K. The Effect of hGH on Hypothalamic-Pituitary-Thyroid Function in Patients With Pituitary Dwarfism. Acta Endocrinol (Copenh) (1980) 93:13–9. doi: 10.1530/acta.0.0930013
59. Giavoli C, Porretti S, Ferrante E, Cappiello V, Ronchi CL, Travaglini P, et al. Recombinant hGH Replacement Therapy and the Hypothalamus-Pituitary-Thyroid Axis in Children With GH Deficiency: When Should We be Concerned About the Occurrence of Central Hypothyroidism? Clin Endocrinol (2003) 59:806–10. doi: 10.1046/j.1365-2265.2003.01892.x
60. Behan LA, Monson JP, Agha A. The Interaction Between Growth Hormone and the Thyroid Axis in Hypopituitary Patients. Clin Endocrinol (Oxf) (2011) 74(3):281–8. doi: 10.1111/j.1365-2265.2010.03815.x
61. Giavoli C, Profka E, Rodari G, Lania A, Beck-Peccoz P. Focus on GH Deficiency and Thyroid Function. Best Pract Res Clin Endocrinol Metab (2017) 31(1):71–8. doi: 10.1016/j.beem.2017.02.003
62. Amato G, Izzo G, Salzano I, Bellastella A. Recombinant Human Growth Hormone Treatment at Low Doses Does Not Significantly Change Thyroid Function in Growth Hormone Deficient Adults. J Endocrinol Investig (1996) 19:563–6. doi: 10.1007/BF03349017
63. Porretti S, Giavoli C, Ronchi C, Lombardi G, Zaccaria M, Valle D, et al. Recombinant Human GH Replacement Therapy and Thyroid Function in a Large Group of Adult GH-Deficient Patients: When Does L-T4 Therapy Become Mandatory? J Clin Endocrinol Metab (2002) 87:2042–5. doi: 10.1210/jcem.87.5.8479
64. Losa M, Scavini M, Gatti E, Rossini A, Madaschi S, Formenti I, et al. Long-Term Effects of Growth Hormone Replacement Therapy on Thyroid Function in Adults With Growth Hormone Deficiency. Thyroid (2008) 18:1249–54. doi: 10.1089/thy.2008.0266
65. Glynn N, Kenny H, Salim T, Halsall DJ, Smith D, Tun TK, et al. Alterations In Thyroid Hormone Levels Following Growth Hormone Replacement Exert Complex Biological Effects. Endocr Pract (2018) 24(4):342–50. doi: 10.4158/EP-2017-0223
66. Van der Lan BF, Freeman JL, Asa SL. Expression of Growth Factors and Growth Factors Receptors in Normal and Tumorous Human Thyroid Tissues. Thyroid (1995) 5:67–73. doi: 10.1089/thy.1995.5.67
67. Cannavò S, Squadrito S, Finocchiaro MD, Curtò L, Almoto B, Vieni A, et al. Goiter and Impairment of Thyroid Function in Acromegalic Patients: Basale Evaluation and Follow-Up. Hormone Metab Res (2000) 32:290–195. doi: 10.1055/s-2007-978620
68. Cheung NW, Boyages SC. The Thyroid Gland in Acromegaly: Un Ultrasonographic Study. Clin Endocrinol (1997) 46:545–9. doi: 10.1046/j.1365-2265.1997.1680985.x
69. Gasperi M, Martino E, Manetti L, Arosio M, Porretti S, Faglia G, et al. Prevalence of Thyroid Diseases in Patients With Acromegaly: Results of an Italian Multi-Center Study. J Endocrinol Invest (2002) 25(3):240–5. doi: 10.1007/BF03343997
70. Natchev E, Vandeva S, Kovatcheva R, Kirilov G, Kalinov K, Zacharieva S. Thyroid Gland Changes in Patients With Acromegaly. Arch Endocrinol Metab (2020) 64(3):269–75. doi: 10.20945/2359-3997000000247
71. Keskin M, Bayramoglu E, Aycan Z. Effects of 1-Year Growth Hormone Replacement Therapy on Thyroid Volume and Function of the Children and Adolescents With Idiopathic Growth Hormone Deficiency. J Pediatr Endocrinol Metab (2017) 30(11):1187–90. doi: 10.1515/jpem-2017-0210
72. Cheung NW, Lou JC, Boyages SC. Growth Hormone Does Not Increase Thyroid Size in the Absence of Thyrotropin: A Study in Adults With Hypopituitarism. J Clin Endocrinol Metab (1996) 81:1179–83. doi: 10.1210/jcem.81.3.8772597
73. Leite NT, Salvatori R, Alcântara MR, Alcântara PR, Oliveira CR, Oliveira JL, et al. Effects of Depot Growth Hormone Replacement on Thyroid Function and Volume in Adults With Congenital Isolated Growth Hormone Deficiency. J Clin Investig (2012) 35:265–8. doi: 10.3275/7608
74. Curtò L, Giovinazzo S, Alibrandi A, Campennì A, Trimarchi F, Cannavò S, et al. Effects of GH Replacement Therapy on Thyroid Volume and Nodule Development in GH Deficient Adults: A Retrospective Color Study. Eur J Endocrinol (2015) 172:543–52. doi: 10.1530/EJE-14-0966
75. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML, et al. Evaluation and Treatment of Adult Growth Hormone Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2011) 96:1587–609. doi: 10.1210/jc.2005-2227
76. Martins MR, Doin FC, Komatsu WR, Barros-Neto TL, Moises VA, Abucham J. Growth Hormone Replacement Improves Thyroxine Biological Effects: Implications for Management of Central Hypothyroidism. J Clin Endocrinol Metab (2007) 92(11):4144–53. doi: 10.1210/jc.2007-0941
77. Doin FC, Rosa-Borges M, Martins MR, Moisés VA, Abucham J. Diagnosis of Subclinical Central Hypothyroidism in Patients With Hypothalamic-Pituitary Disease by Doppler Echocardiography. Eur J Endocrinol (2012) 166(4):631–40. doi: 10.1530/EJE-11-0907
78. Fierro G, Hoffman AR. Treatment of the Adult Growth Hormone Deficiency Syndrome With Growth Hormone: What are the Implications for Other Hormone Replacement Therapies for Hypopituitarism? Growth Horm IGF Res (2020) 52:101316. doi: 10.1016/j.ghir.2020.101316
79. Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jørgensen K, et al. Serum Insulin-Like Growth Factor-I in 1030 Healthy Children, Adolescents, and Adults: Relation to Age, Sex, Stage of Puberty, Testicular Size, and Body Mass Index. J Clin Endocrinol Metab (1994) 78(3):744–52. doi: 10.1210/jcem.78.3.8126152
80. Ho KV, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, et al. Effects of Sex and Age on the 24-Hour Profile of Growth Hormone Secretion in Man: Importance of Endogenous Oestradiol Concentrations. J Clin Endocrinol Metab (1987) 64:51–8. doi: 10.1210/jcem-64-1-51
81. Rose SR, Municchi G, Barnes KM, Kamp GA, Uriarte MM, Ross JL, et al. Spontaneous Growth Hormone Secretion Increases During Puberty in Normal Girls and Boys. J Clin Endocrinol Metab (1991) 73(2):428–35. doi: 10.1210/jcem-73-2-428
82. Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen Regulation of Growth Hormone Action. Endocrine Rev (2004) 25:693–721. doi: 10.1210/er.2003-0035
83. Fisker S, Jørgensen JOL, Vahl N, Orskov H, Christiansen JS. Impact of Gender and Androgen Status an IGF-I Levels in Normal and GH Deficient Adults. Eur J Endocrinol (1999) 141:601–8. doi: 10.1530/eje.0.1410601
84. Ovesen P, Vahl N, Fisker S, Veldhuis JD, Christiansen JS, Jørgensen JOL. Increased Pulsatile, But Not Basal, Growth Hormone Secretion Rates and Plasma Insulin-Like Growth Factor 1 Levels During the Perlovulatory Interval in Normal Women. J Clin Endocrinol Metab (1998) 83:1662–7. doi: 10.1210/jcem.83.5.4761
85. Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, et al. Requirement of STAT5b for Sexual Dimorphism of Body Growth Rates and Liver Gene Expression. Proc Natl Acad Sci U S A (1997) 94:7239–44.27. doi: 10.1073/pnas.94.14.7239
86. Fernández-Pérez L, Guerra B, Díaz-Chico JC, Flores-Morales A. Estrogens Regulate the Hepatic Effects of Growth Hormone, a Hormonal Interplay With Multiple Fates. Front Endocrinol (Lausanne) (2013) 4:66. doi: 10.3389/fendo.2013.00066
87. Tenuta M, Carlomagno F, Cangiano B, Kanakis G, Pozza C, Sbardella E, et al. Somatotropic-Testicular Axis: A Crosstalk Between GH/IGF-I and Gonadal Hormones During Development, Transition, and Adult Age. Andrology (2021) 9(1):168–84. doi: 10.1111/andr.12918
88. Wolthers T, Hoffman DM, Nugent AG, Duncan MW, Umpleby M, Ho KK. Oral Estrogen Antagonizes the Metabolic Actions of Growth Hormone in Growth Hormone-Deficient Women. Am J Physiol Endocrinol Metab (2001) 281:E1191–6. doi: 10.1152/ajpendo.2001.281.6.E1191
89. Isotton AL, Wender MC, Casagrande A, Rollin G, Czepielewski MA. Effects of Oral and Transdermal Estrogen on IGF1, IGFBP3, IGFBP1, Serum Lipids, and Glucose in Patients With Hypopituitarism During GH Treatment: A Randomized Study. Eur J Endocrinol (2012) 166(2):207–13. doi: 10.1530/EJE-11-0560
90. Svensson J, Johannsson G, Bengtsson BA. Insulin-Like Growth Factor-I in Growth Hormone-Deficient Adults: Relationship to Population-Based Normal Values, Body Composition and Insulin Tolerance Test. Clin Endocrinol (Oxf) (1997) 46:579–86. doi: 10.1046/j.1365-2265.1997.1851001.x
91. Cook DM, Ludlam WH, Cook MB. Route of Estrogen Administration Helps to Determine Growth Hormone (GH) Replacement Dose in GH-Deficient Adults. J Clin Endocrinol Metab (1999) 84:3956–60. doi: 10.1210/jcem.84.11.6113
92. Veldhuis JD, Mielke KL, Cosma M, Soares-Welch C, Paulo R, Miles JM, et al. Aromatase and 5alpha-Reductase Inhibition During an Exogenous Testosterone Clamp Unveils Selective Sex Steroid Modulation of Somatostatin and Growth Hormone Secretagogue Actions in Healthy Older Men. J Clin Endocrinol Metab (2009) 94(3):973–81. doi: 10.1210/jc.2008-2108
93. Roelfsema F, Yang RJ, Takahashi PY, Erickson D, Bowers CY, Veldhuis JD. Aromatized Estrogens Amplify Nocturnal Growth Hormone Secretion in Testosterone-Replaced Older Hypogonadal Men. J Clin Endocrinol Metab (2018) 103(12):4419–27. doi: 10.1210/jc.2018-00755
94. Birzniece V, McLean M, Reddy N, Ho KKY. Disparate Effect of Aromatization on the Central Regulation of GH Secretion by Estrogens in Men and Postmenopausal Women. J Clin Endocrinol Metab (2019) 104(7):2978–84. doi: 10.1210/jc.2019-00265
95. Giton F, Trabado S, Maione L, Sarfati J, Le Bouc Y, Brailly-Tabard S, et al. Sex Steroids, Precursors, and Metabolite Deficiencies in Men With Isolated Hypogonadotropic Hypogonadism and Panhypopituitarism: A GCMS-Based Comparative Study. J Clin Endocrinol Metab (2015) 100(2):E292–296. doi: 10.1210/jc.2014-2658
96. Balducci R, Toscano V, Mangiantini A, Bianchi P, Guglielmi R, Boscherini B. The Effect of Growth Hormone Administration on Testicular Response During Gonadotropin Therapy in Subjects With Combined Gonadotropin and Growth Hormone Deficiencies. Acta Endocrinol (Copenh) (1993) 128(1):19–23. doi: 10.1530/acta.0.1280019
97. Giagulli VA. Absence of Effect of Recombinant Growth Hormone to Classic Gonadotropin Treatment on Spermatogenesis of Patients With Severe Hypogonadotropic Hypogonadism. Arch Androl (1999) 43(1):47–53. doi: 10.1080/014850199262724
98. Juul A, Andersson AM, Pedersen SA, Jørgensen JOL, Christiansen JS, Groome NP, et al. Effects of Growth Hormone Replacement Therapy on IGF-Related Parameters and on the Pituitary-Gonadal Axis in GH-Deficient Males. Hormone Res (1998) 49:269–78. doi: 10.1159/000023186
99. Carani C, Granata ARM, De Rosa M, Garau C, Zarrilli S, Paesano L, et al. The Effect of Chronic Treatment With GH on Gonadal Function in Men With Isolated GH Deficiency. Eur J Endocrinol (1999) 140:224–30. doi: 10.1530/eje.0.1400224
100. Giavoli C, Ferrante E, Ermetici F, Bergamaschi S, Ronchi CL, Lania AG, et al. Effect of Recombinant hGH (rhGH) Replacement on Gonadal Function in Male Patients With Organic Adult-Onset GH Deficiency. Clin Endocrinol (Oxf) (2006) 65(6):717–21. doi: 10.1111/j.1365-2265.2006.02655.x
101. Shoham Z, Conway GS, Ostergaard H, Lahlou N, Bouchard P, Jacobs HS. Cotreatment With Growth Hormone for Induction of Spermatogenesis in Patients With Hypogonadotropic Hypogonadism. Fertil Steril (1992) 57(5):1044–51. doi: 10.1016/S0015-0282(16)55023-7
102. Kalra S, Kalra B, Sharma A. Growth Hormone Improves Semen Volume, Sperm Count and Motility in Men With Idiopathic Normogonadotropic Infertility. Endocr Abstr (2008) 16:P613.
103. Weall BM, Al-Samerria S, Conceicao J, Yovich JL, Almahbobi G. A Direct Action for GH in Improvement of Oocyte Quality in Poor-Responder Patients. Reproduction (2015) 149(2):147–54. doi: 10.1530/REP-14-0494
104. Hull KL, Harvey S. Growth Hormone and Reproduction: A Review of Endocrine and Autocrine/Paracrine Interactions. Int J Endocrinol (2014) 2014:234014. doi: 10.1155/2014/234014
105. Kolibianakis EM, Venetis CA, Diedrich K, Tarlatzis BC, Griesinger G. Addition of Growth Hormone to Gonadotrophins in Ovarian Stimulation of Poor Responders Treated by in-Vitro Fertilization: A Systematic Review and Meta-Analysis. Hum Reprod Update (2009) 15(6):613–22. doi: 10.1093/humupd/dmp026
106. Yovich JL, Stanger JD. Growth Hormone Supplementation Improves Implantation and Pregnancy Productivity Rates for Poor-Prognosis Patients Undertaking IVF. Reprod BioMed Online (2010) 21(1):37–49. doi: 10.1016/j.rbmo.2010.03.013
107. de Boer JA, Schoemaker J, van der Veen EA. Impaired Reproductive Function in Women Treated for Growth Hormone Deficiency During Childhood. Clin Endocrinol (Oxf) (1997) 46(6):681–9. doi: 10.1046/j.1365-2265.1997.1800999.x
108. Giampietro A, Milardi D, Bianchi A, Fusco A, Cimino V, Valle D, et al. The Effect of Treatment With Growth Hormone on Fertility Outcome in Eugonadal Women With Growth Hormone Deficiency: Report of Four Cases and Review of the Literature. Fertil Steril (2009) 91:930.e7–11. doi: 10.1016/j.fertnstert.2008.09.065
109. Albu D, Albu A. Is Growth Hormone Administration Essential for In Vitro Fertilization Treatment of Female Patients With Growth Hormone Deficiency? Syst Biol Reprod Med (2019) 65(1):71–4. doi: 10.1080/19396368.2018.1492044
110. Brooke AM, Kalingag LA, Miraki-Moud F, Camacho-Hübner C, Maher KT, Walker DM, et al. Dehydroepiandrosterone (DHEA) Replacement Reduces Growth Hormone (GH) Dose Requirement in Female Hypopituitary Patients on GH Replacement. Clin Endocrinol (Oxf) (2006) 65(5):673–80. doi: 10.1111/j.1365-2265.2006.02648.x
111. Van Thiel SW, Romijn JA, Pereira AM, Biermasz NR, Roelfsema F, van Hernert A, et al. Effects of DHEA, Superimposed on Growth Hormone Substitution, on Quality of Life and IGF-I in Patients With Secondary Adrenal Insufficiency: A Randomised, Placebo Controlled, Crossover Trial. J Clin Endocrinol Metab (2005) 90(6):3295–303. doi: 10.1210/jc.2004-1802
Keywords: growth hormone deficiency, hypopituitarism, central hypoadrenalism, central hypothyroidism, hypogonadotropic hypogonadism
Citation: Profka E, Rodari G, Giacchetti F and Giavoli C (2021) GH Deficiency and Replacement Therapy in Hypopituitarism: Insight Into the Relationships With Other Hypothalamic-Pituitary Axes. Front. Endocrinol. 12:678778. doi: 10.3389/fendo.2021.678778
Received: 10 March 2021; Accepted: 27 September 2021;
Published: 19 October 2021.
Edited by:
Luca Persani, Istituto Auxologico Italiano (IRCCS), ItalyReviewed by:
Roberto Lanes, Hospital de Clinicas Caracas, VenezuelaAntonio Mancini, Catholic University of the Sacred Heart, Rome, Italy
Copyright © 2021 Profka, Rodari, Giacchetti and Giavoli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulia Rodari, rodarigiulia@gmail.com, giulia.rodari@unimi.it
†These authors have contributed equally to this work
 Eriselda Profka
Eriselda Profka Giulia Rodari
Giulia Rodari Federico Giacchetti
Federico Giacchetti Claudia Giavoli
Claudia Giavoli