- 1Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
- 2Department of Endocrinology and Metabolism, Peking University Third Hospital, Beijing, China
Several studies have reported the association between thyroid autoimmunity (TAI) and in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) outcomes. However, the findings remain controversial. We performed a large-scale retrospective cohort study to verify the effect of the presence of thyroid antibodies on IVF/ICSI outcomes and fetal growth and to evaluate the association between the types and titers of thyroid antibodies and adverse IVF/ICSI outcomes. A total of 16481 patients with infertility were referred to the Reproductive Center of Peking University Third Hospital for their first IVF/ICSI treatment between January 2018 and June 2019.Patients who sought IVF/ICSI treatment due to tubal or male factors infertility and who achieved fresh embryo transfer were included in our study. Finally, 778 patients with thyroid antibody positivity were selected as the TAI group, and 778 age-matched patients were included in the control group. The number of oocytes retrieved and high-graded embryos and the rates of clinical pregnancy, miscarriage, live birth, and preterm delivery were compared between the TAI and control groups. In addition, subgroup analysis was performed to demonstrate whether different types and titers of thyroid antibodies had different effects on IVF/ICSI outcomes. After adjusting for thyroid function, anti-Müllerian hormone levels, basal follicle stimulating hormone levels, basal estradiol levels and antral follicle count, the number of oocytes retrieved in the TAI group was significantly lower than that in the control group. No significant differences were observed between the two groups in the rates of clinical pregnancy, miscarriage, preterm delivery, live birth, and birth weight in singletons; however, the birth weight in twin pregnancy was significantly lower in the TAI group than in the control group. Subgroup analysis showed no association between the types or titers of thyroid antibodies and adverse IVF/ICSI outcomes. In conclusion, the presence of TAI in patients with infertility did not impair embryo quality or affect pregnancy outcomes, including clinical pregnancy, miscarriage, preterm delivery, and live birth. However, it decreased the number of oocytes retrieved and birth weight in twin pregnancy.
Introduction
Thyroid autoimmunity (TAI), diagnosed as the presence of thyroid antibody, is the most common autoimmune disorder among women of childbearing age. Accumulating evidence has demonstrated the association between thyroid antibody and pregnancy outcomes after in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) treatment, and studies based on the IVF/ICSI process have not only provided new models to investigate the association between thyroid antibodies and pregnancy outcomes but also enabled the analysis of the role of TAI in earlier gestational stages from oocyte fertilization to embryo implantation (1). For example, some studies showed that euthyroid women with TAI had a higher risk of adverse outcomes, such as miscarriage and preterm delivery, than healthy women without TAI, but other studies did not support the association. In addition, in a meta-analysis, including 12 studies, Andrea et al. performed a comprehensive analysis on the association between TAI and IVF/ICSI outcomes (2), and they concluded that the presence of thyroid antibodies exerted detrimental effects on pregnancy outcomes, including an increased risk of miscarriage and a decreased frequency of live birth. However, all the aforementioned studies failed to show any association between the presence of thyroid antibodies and the number of oocytes retrieved, fertilization rate, or implantation rate. Moreover, the sample sizes in the studies included in this meta-analysis were all small, and the conclusions in those studies were controversial.
Thyroglobulin and thyroid peroxidase function as key factors in the process of thyroid hormone (TH) biosynthesis and constitute the major thyroid autoantigens involved in the pathophysiology of TAI (3). The prevalence of thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TGAb) in patients with TAI is similar; however, the sensitivity and titers of TPOAb are always higher than those of TGAb (4). A large cross-sectional study reported that women with high titers of TPOAb or TGAb displayed impaired capacity of the thyroid gland adapting to enlarged demands during pregnancy, and the thyroid function was more prone to be affected in women with co-positive antibodies of TPOAb and TGAb than in those with isolated-positive antibody (5).
The present large retrospective cohort study aimed to investigate whether the presence of thyroid antibody is associated with IVF/ICSI outcomes, including the number of oocytes retrieved, embryo quality, clinical pregnancy rate, miscarriage rate, preterm delivery rate, live birth rate, and birth weight. In addition, subgroup analysis was performed to determine the association between the types and titers of thyroid antibody and pregnancy outcomes after IVF/ICSI.
Methods
Study Population
A total of 16481 patients with infertility were referred to the Reproductive Center of Peking University Third Hospital for their first IVF/ICSI treatment between January 2018 and June 2019. After excluding 89 patients with no oocyte retrieved, 92 patients without fertilization, 1924 patients with no embryo obtained, and 5154 patients with embryo cryopreservation, a total of 9222 patients who underwent standardized, controlled ovarian stimulation and achieved fresh embryo transfer were screened for eligibility. Patients were included in our study if they were 20 to 38 years old, had a regular menstrual period (21–35 days), were referred to the reproductive center for the first IVF/ICSI cycle due to tubal or male factors, and underwent fresh embryo transfer. Patients were excluded from the study if they had a history of other reproductive diseases, such as polycystic ovarian syndrome and endometriosis; a history of other thyroid diseases, such as hyperthyroidism or thyroid cancer; abnormal results on parental karyotyping; other endocrinological diseases, such as hyperprolactinemia and diabetes; or positive tests for the antinuclear antibody or lupus anticoagulants. Patients were also ineligible if they had abnormal thyroid function testing, except for thyroid antibody positivity, before IVF/ICSI treatment. A total of 778 patients with thyroid antibody positivity were selected as the TAI group. Women without thyroid antibody positivity were individually matched to a single patient with TAI at a ratio of 1:1 for age and 778 patients were selected in the control group for analysis (Figure 1).
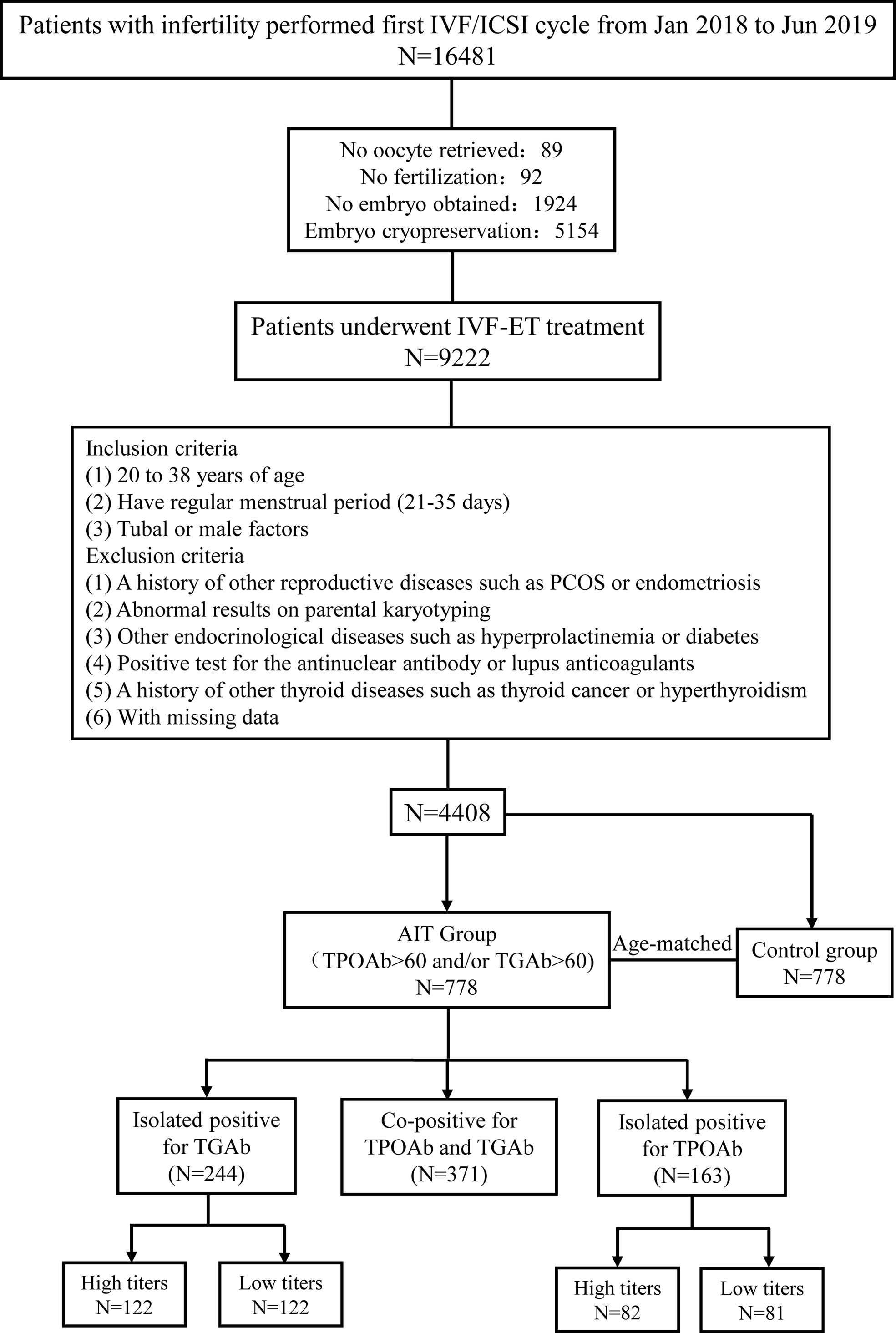
Figure 1 Flow chart of study cohort selection. IVF/ICSI, in vitro fertilization/intracytoplasmic sperm injection; TGAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody.
Assisted Reproductive Technology Procedure
All patients received a standardized, controlled ovarian stimulation (COS) regimen, oocyte retrieval, and fertilization, followed by fresh embryo transfer. Patients treated with ultralong-term and long-term protocols received a long-acting gonadotropin-releasing hormone (GnRH) agonist for downregulation. After downregulation was achieved, recombinant gonadotropins were administered for ovarian stimulation. Patients treated with the short-term protocol were simultaneously administered a short-acting GnRH agonist and recombinant gonadotropins for ovarian stimulation. In patients treated with the antagonist protocol, recombinant gonadotropins were initiated on day 2 of the menstrual cycle, and treatment with a GnRH antagonist was initiated when at least one follicle reached 12 mm in diameter and continued until the day when human chorionic gonadotropin (HCG) was administered. The individualized dose of gonadotropins was decided based on the patient’s age, body mass index (BMI), and anti-Müllerian hormone (AMH) levels. Recombinant HCG 250μg (Eiser, Serono, Germany)was administered to trigger oocyte maturation when at least two follicles reached 18 mm. Oocyte retrieval was performed 34 to 36 hours after HCG administration. Insemination was performed at 4 to 6 hours after oocyte retrieval by a conventional in vitro fertilization method or intracytoplasmic sperm injection according to the sperm quality. Up to two day-3 embryos or blastocysts were transferred 3 or 5 days after oocyte retrieval.
Study Outcomes
Clinical pregnancy was defined as at least one gestational sac in the uterus at 35 days after embryo transfer, as observed by ultrasonography. Miscarriage was defined as the loss of clinical pregnancy before 28 weeks of gestation. Live birth was defined as the delivery of at least one survived newborn, irrespective of gestation duration. Preterm delivery was defined as the delivery of a living fetus before 37 weeks of gestation.
Laboratory Testing
Blood samples for TH testing were taken within 6 months prior to the initiation of COS. Serum thyroid-stimulating hormone (TSH), free thyroxin (FT4), TPOAb, and TGAb levels were measured by a fully automatic chemiluminescence immunoassay analyzer (ADVIA Centaur XP, Siemens Healthcare Diagnostics). The reference values were 0.55–4.78 uIU/ml for TSH and 0.89–1.80 ng/dl for FT4. The level below 60 IU/ml was defined as negative for TPOAb or TGAb.
Categorization
Based on the different types of antibody positivity, patients in the TAI group were divided into three subgroups: group 1, co-positive for TPOAb and TGAb (TPOAb+ and TGAb+); group 2, isolated TGAb-positive (TPOAb- and TGAb+); group 3, isolated TPOAb-positive (TPOAb+ and TGAb-). Then, according to different antibody titers, patients with isolated TGAb positive or isolated TPOAb positive were further divided into the high and low titers groups based on sample size (Figure 1).
Statistics
Continuous data are shown as mean (standard deviation [SD]) for normally distributed data and median (interquartile range) for non-normally distributed data. Categorical data are presented as the number of cases (percentage). Continuous variables were compared using the Student’s t-test or one-way ANOVA, and categorical variables were compared using the chi-square test. Continuous variables without normal distributions were compared using the Mann-Whitney U test. Logistic regression analysis was conducted to calculate odds ratios (ORs) with 95% confidence intervals (CIs) after adjusting for relevant factors. Linear regression model was performed to analyze the association between the number of oocytes retrieved and relevant factors. All analyses were performed using SPSS 24 statistical software. Statistical significance was defined as two-sided P value < 0.05.
Results
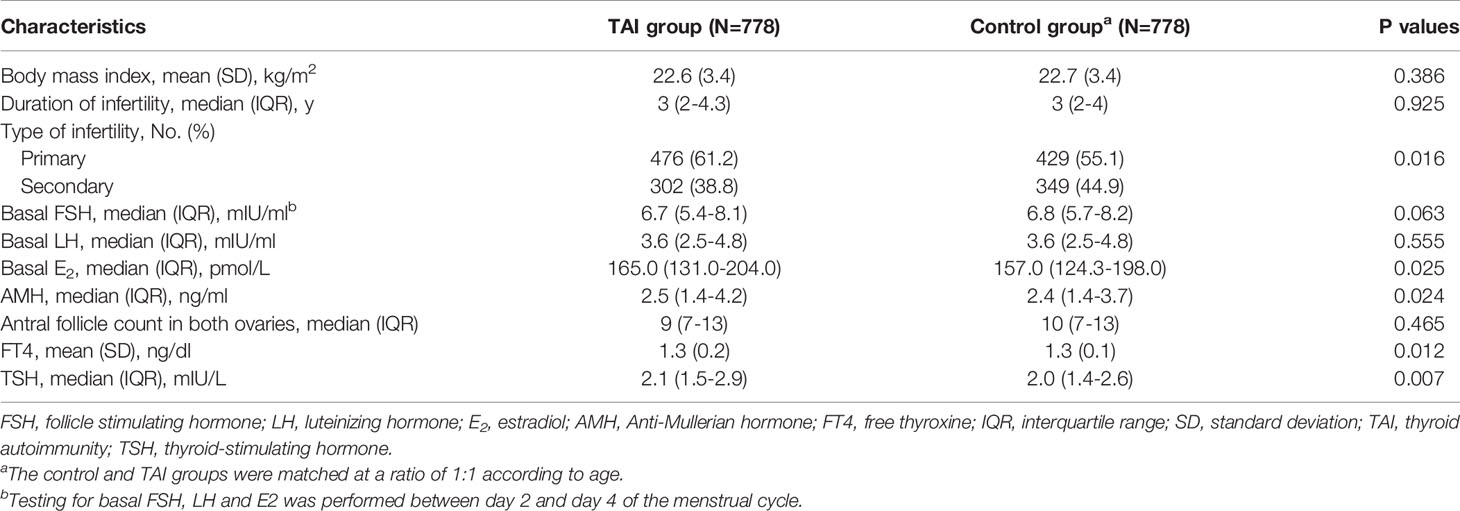
As shown in Table 1, there was no significant difference between the two groups in BMI or duration of infertility; however, significantly higher AMH levels (median [interquartile range]: 2.5 [1.4–4.2] vs. 2.4 [1.4–3.7], P = 0.024) and basal estradiol (E2) levels (median [interquartile range]: 165.0 [131.0–204.0] vs. 157.0 [124.3–198.0], P = 0.025) were observed in the TAI group than in the control group, indicating a different ovarian reserve between the two groups. The levels of basal follicle stimulating hormone (FSH) were lower in patients with TAI than those without TAI but the difference was not significant. No significant difference was observed in antral follicle count between two groups. A significant difference was observed in thyroid function between the TAI and control group. The levels of FT4 were significantly lower (mean [SD]: 1.3 [0.2] vs. 1.3 [0.1], P = 0.012), but the levels of TSH were significantly higher (median [interquartile range]: 2.1 [1.5–2.9] vs. 2.0 [1.4–2.6], P = 0.007) in patients with TAI than in controls.
Group characteristics regarding COS and fertilization procedures are shown in Table 2. There were no significant differences between the two groups in the protocols and gonadotropin dose of COS, hormone levels on the day of HCG trigger, endometrial thickness, fertilization procedures, timing of embryo transfer, the number of good-quality embryos or embryos transferred. In addition, no significant difference was found in the number of oocytes retrieved (median [interquartile range]: 10 (6–12) vs. 11 (7–13), P = 0.091); however, after adjusting for AMH levels, basal FSH levels, basal E2 levels, FT4 levels, TSH levels, and antral follicle count, the number of oocytes retrieved was significantly lower in patients with TAI than in controls (Regression Coefficient, -0.48; 95%CI, -0.06 to -0.90; P=0.025) (Table 3).
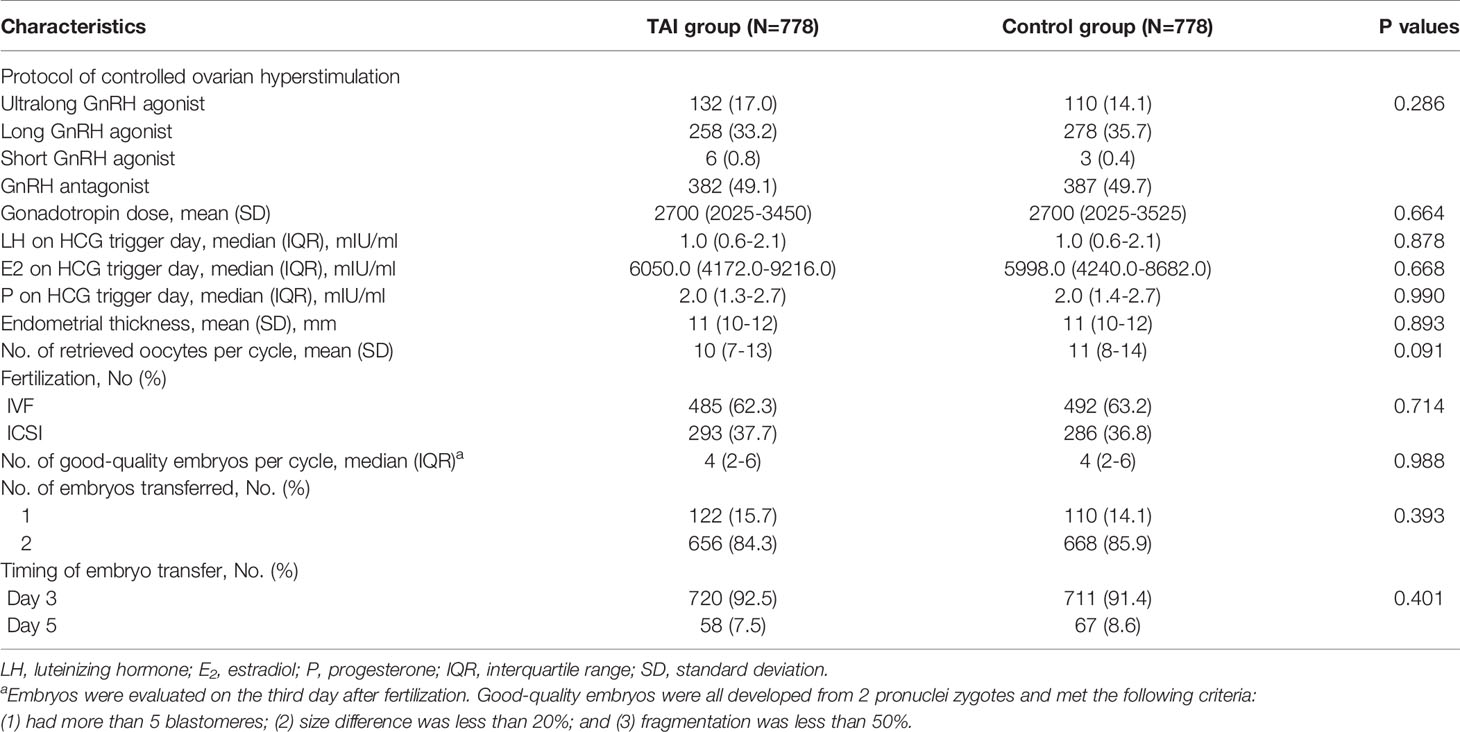
Table 2 Protocols of controlled ovarian stimulation and data of in vitro fertilization and embryo transfer.
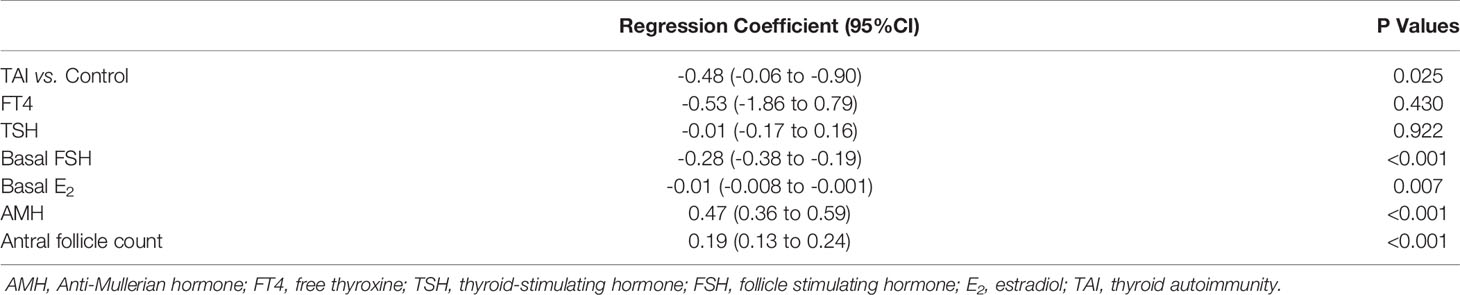
Table 3 Linear regression analysis of the number of oocytes retrieved in patients with TAI compared with controls.
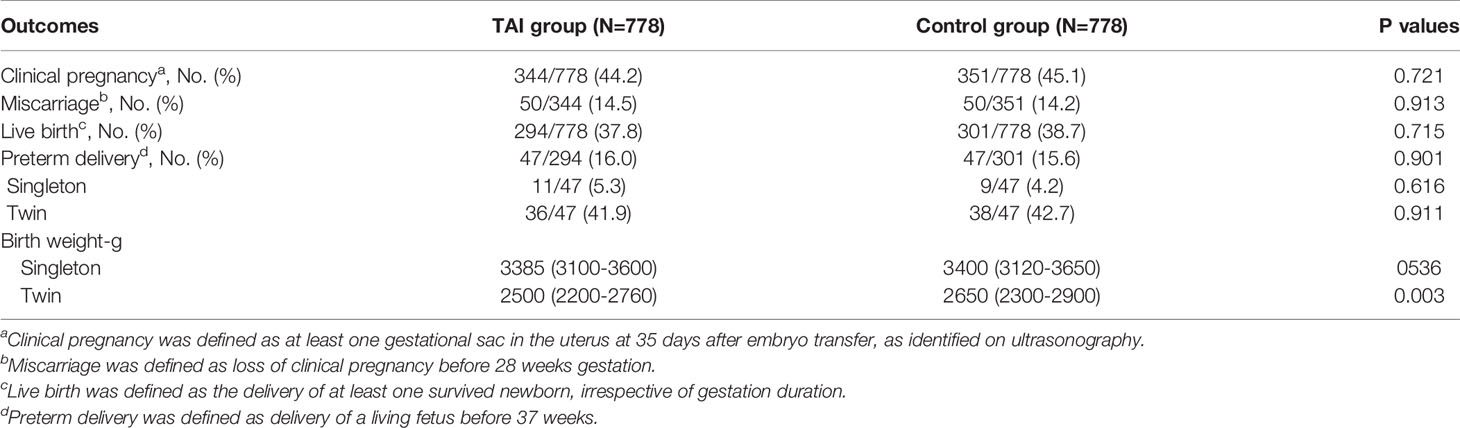
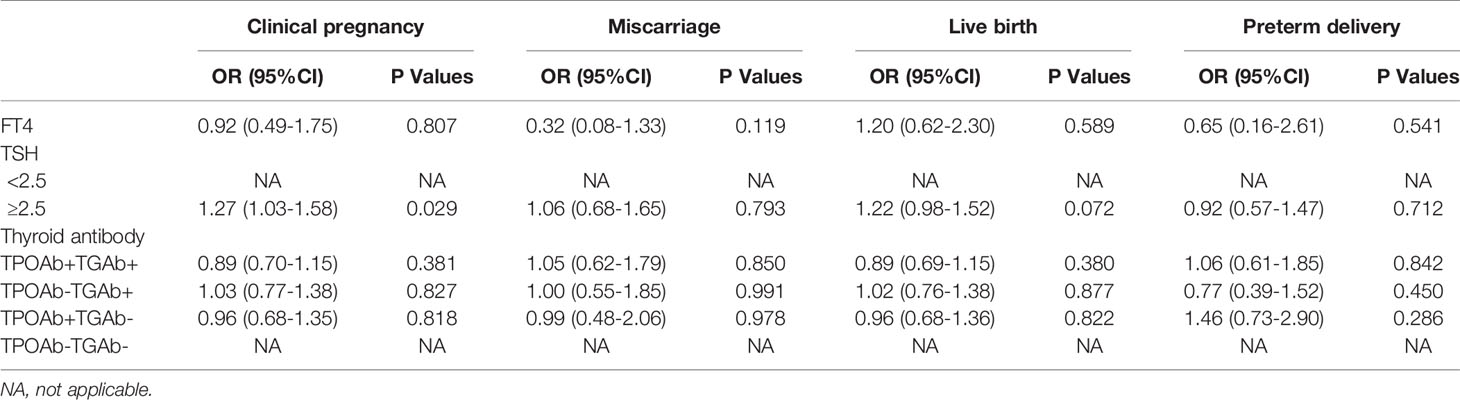
There were no significant differences between the TAI and control groups in the rates of clinical pregnancy (44.2% vs. 45.1%, P = 0.721), miscarriage (14.5% vs. 14.2%, P = 0.916), live birth (37.8% vs. 38.7%, P = 0.715), or preterm delivery (16.0% vs. 15.6%, P = 0.901), even after adjusting for FT4 and TSH levels, as well as the types of thyroid antibodies (Tables 4 and 5). Birth weight in singleton pregnancy was not significantly different between the two groups; however, birth weight in twin pregnancy was significantly lower in the TAI group than in the control group (median [interquartile range]: 2500 [2200–2760] vs. 2650 [2300–2900], P = 0.003) (Table 4).
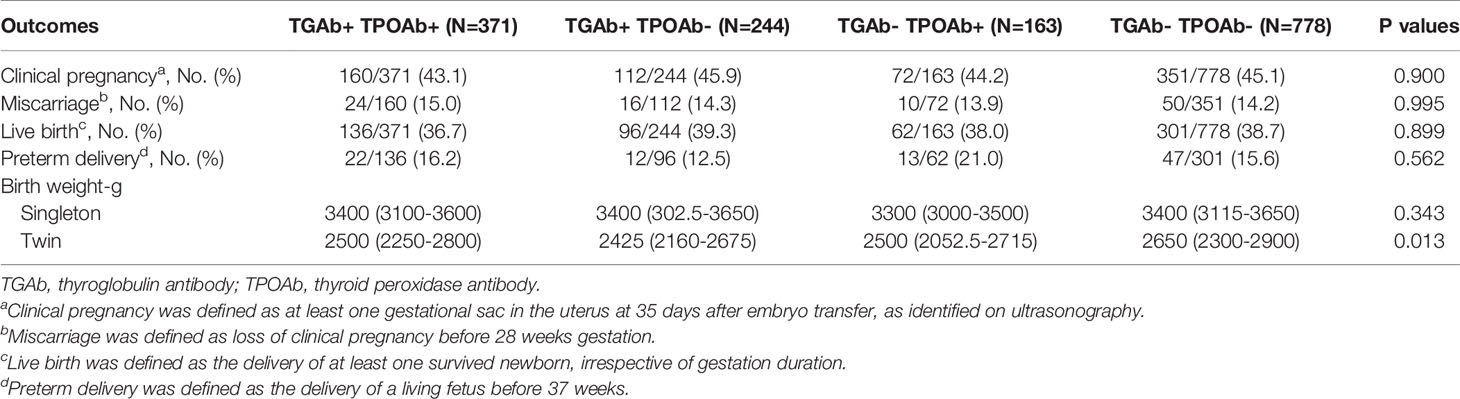
Based on the types of thyroid antibodies, the TAI group was divided into three subgroups: co-positive for TGAb and TPOAb, isolated positive for TGAb, and isolated positive for TPOAb. Subgroup analysis did not find any significant difference among these three groups and the control group in the rates of clinical pregnancy, miscarriage, live birth, and preterm delivery or live birth weight in singleton pregnancy (Table 6). The live birth weight in twin pregnancy was significantly higher in the control group than in each of the three subgroups in the TAI group; however, there was no significant difference among the three subgroups of the TAI group in this regard (Table 6).
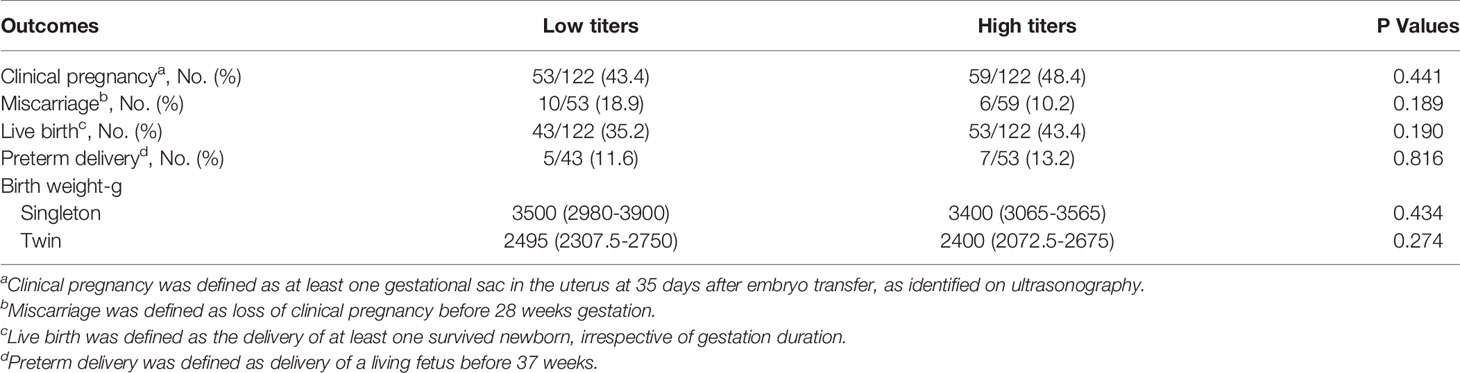
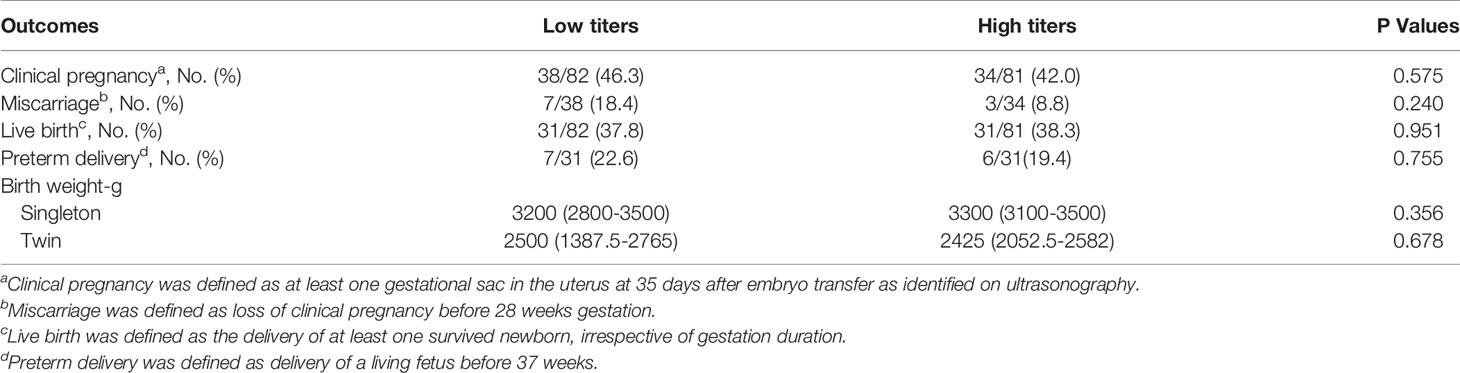
Based on TGAb titers, patients with isolated positive for TGAb were divided into the high (TGAb ≥ 133.2 IU/ml) and low (TGAb < 133.2 IU/ml) titer groups. There were no significant differences between the two groups in the rates of clinical pregnancy, miscarriage, live birth, and preterm delivery or birth weight in singleton or twin pregnancy (Table 7). Based on TPOAb titers, patients with isolated positive for TPOAb were divided into the high (TPOAb ≥ 165) and low (TPOAb < 165) titer groups. No significant difference was observed between the two groups in the rates of clinical pregnancy, miscarriage, live birth, and preterm delivery or birth weight in singleton or twin pregnancy (Table 8).
Discussion
The thyroid gland plays a critical role in regulating reproductive function and maintaining pregnancy balance. The mechanisms underlying the impact of TAI on IVF/ICSI outcomes from follicular growth to pregnancy remain unclear, but currently, there are two hypotheses. First, the presence of TAI triggers a subtle deficiency of thyroid reserve and consequently, leading to a reduced capacity of the thyroid gland to adapt to the augmented demand in the process of COS and early pregnancy. Second, TAI is an immune disorder, and thyroid antibodies with different titers indicates an immune imbalance that impairs the establishment of immune tolerance during pregnancy.
We compared ovarian reserve between the two groups based on the AMH level, antral follicle count, basal FSH levels and basal E2 levels. The AMH and E2 levels were significantly higher in patients with TAI than in age-matched controls, indicating a higher ovarian reserve in patients with TAI. Although a higher ovarian reserve was observed in patients with TAI, the number of oocytes retrieved was significantly lower in patients with TAI than in controls after adjusting for the levels of AMH, basal FSH, basal E2 and FT4, and TSH and antral follicle count. Immune imbalance and abnormal thyroid function during COS may affect follicle development in patients with TAI. In a recent study, Monteleone et al. revealed the expression of thyroid peroxidase in human granulosa cells by immunocytochemistry (14). Moreover, they collected the follicle fluid in women after oocyte retrieval and detected the presence of TPOAb and TGAb in the follicle fluid in women with TAI. The presence of thyroid antibodies in the follicular fluid suggests that antibody-mediated cytotoxicity impairs oocytes (6).
Several studies have reported the presence of TH and TSH in the human follicular fluid, with similar levels in the serum (7, 8). TH may affect follicle development by promoting granulosa cell proliferation and triggering some alterations in the expression of genes involved in steroidogenesis (9–12). The change in thyroid function during COS has been widely investigated, and the elevated trend of TSH level has been reported in many studies, although the results for FT4 are inconsistent (13, 15). A prospective study conducted by Poppe et al. investigated different thyroid functions in women with or without TAI during COS and demonstrated a significant difference in TSH levels between the two groups (16). Different TH levels between women with and without TAI may provide a possible explanation for the decreased number of oocytes retrieved in patients with TAI. In our study, a significant difference was observed in FT4 and TSH levels between the two groups before COS, suggesting that the thyroid plays a possible role in affecting follicle development in the TAI group. However, we could not detect the difference in thyroid function between the two groups during COS due to the study’s retrospective design. Therefore, a further prospective study may be required to determine whether different levels of thyroid function affect follicle development and decrease the number of oocytes retrieved.
The association between TAI and IVF/ICSI outcomes has been reported in several studies, but the results remain controversial. Two studies revealed that the clinical pregnancy rate was significantly reduced in women with TAI (17, 18), whereas five studies failed to show this difference (19–23). Three studies reported that the presence of TAI decreased the birth or delivery rate (17, 18, 22), but five studies did not detect this effect (19, 20, 24–26). Two studies revealed that the miscarriage rate was increased in women with TAI (21, 22), but eight studies reported no difference in the miscarriage rate between women with and without TAI (17, 19, 20, 23–27). In our study, we found no significant differences between the two groups in the rates of clinical pregnancy, miscarriage, preterm delivery, or live birth, even after adjusting for FT4 levels, TSH levels, and thyroid antibody types. Subgroup analysis also failed to detect differences in pregnancy outcomes among patients with different types or titers of thyroid antibodies.
Although the birth weight in singleton pregnancy was not different between the TAI and control groups in our study, the birth weight in twin pregnancy was significantly lower in patients with TAI than in controls. Decreased birth weight might result from abnormal thyroid function during the pregnancy period. Adequate TH availability during pregnancy is crucial for fetal growth and development. Fetal demand for TH during early pregnancy predominantly depends on the placental transfer of maternal TH because the fetal thyroid gland is non-functional until 18–20 weeks of pregnancy. Elevated production of thyroid-binding globulin and expression of deiodinase 3 in the placenta further increase the demand for TH availability. HCG, produced specifically in early pregnancy, can stimulate the thyroid gland via binding to the TSH receptor, thus triggering an elevation in serum FT4 levels and a subsequent feedback suppression of TSH secretion. The HCG-mediated thyroid response is important for maintaining adequate TH during early pregnancy (28). A recent study reported that compared with singleton pregnancy, twin pregnancy was associated with a lower TSH levels and a higher FT4 levels during early pregnancy, which were due to increased stimulation of the thyroid gland because of higher HCG levels (29). Thus, a stronger thyroidal response to HCG is needed in twin pregnancy to maintain higher TH. However, the presence of thyroid antibodies has been proved to be a risk factor associated with impairment of the thyroid response to HCG. Notably, an association between HCG and FT4 or TSH was impaired in women with positive thyroid antibodies, compared with in those with negative thyroid antibody (5, 30). In addition, Zhang et al. stated that impaired thyroid response to HCG in the first trimester led to fetal growth restriction and lower crown-rump length (31). Thus, an impaired thyroidal response might lead to lower weight in twin pregnancy in our study.
There are some limitations in our study. First, our study was a retrospective study; thus, the presence of biases involved in retrospective data collection cannot be excluded. Second, this study only included the participants seeking IVF/ICSI treatment due to tubal or male factors; hence, our study can only explain the association of TAI and pregnancy outcomes in patients without other reproductive disorders, and the impact of TAI on IVF/ICSI outcomes in patients with combined reproductive and endocrine diseases needs to be further investigated. Lastly, our study did not investigate the use of levothyroxine because of the retrospective design; however, our previous study has shown that the use of levothyroxine did not affect IVF/ICSI outcomes (32).
In summary, the study revealed that the presence of TAI did not affect embryo quality and pregnancy outcomes but decreased the number of oocytes received and birth weight in twin pregnancy. There was no association between the types and titers of thyroid antibodies and adverse IVF/ICSI outcomes. Further prospective studies are needed to illustrate the mechanisms underlying the impact of TAI on follicle development and fetal growth.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Peking University Third Hospital Medical Science Research Ethics Committee. The ethics committee waived the requirement of written informed consent for participation. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
NH, JQ, and HC developed the conception of the study and all authors contributed to the research discussion. NH, LC, YL, HW, and RL took part in patients follow-up and contributed to the data analysis. NH wrote the initial draft of the paper and all authors contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 81871212) and Science Foundation of Peking University Third Hospital (BYSYLXHG2019003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Poppe K, Autin C, Veltri F, Sitoris G, Kleynen P, Praet JP, et al. Thyroid Disorders and In Vitro Outcomes of Assisted Reproductive Technology: An Unfortunate Combination? Thyroid (2020) 30:1177–85. doi: 10.1089/thy.2019.0567
2. Busnelli A, Paffoni A, Fedele L, Somigliana E. The Impact of Thyroid Autoimmunity on IVF/ICSI Outcome: A Systematic Review and Meta-Analysis. Hum Reprod Update (2016) 22:793–4. doi: 10.1093/humupd/dmw034
3. Carvalho DP, Dupuy C. Thyroid Hormone Biosynthesis and Release. Mol Cell Endocrinol (2017) 458:6–15. doi: 10.1016/j.mce.2017.01.038
4. McLachlan SM, Rapoport B. Why Measure Thyroglobulin Autoantibodies Rather Than Thyroid Peroxidase Autoantibodies? Thyroid (2004) 14:510–20. doi: 10.1089/1050725041517057
5. Hou Y, Liu A, Li J, Wang H, Yang Y, Li Y, et al. Different Thyroidal Responses to Human Chorionic Gonadotropin Under Different Thyroid Peroxidase Antibody and/or Thyroglobulin Antibody Positivity Conditions During the First Half of Pregnancy. Thyroid (2019) 29:577–85. doi: 10.1089/thy.2018.0097
6. Monteleone P, Parrini D, Faviana P, Carletti E, Casarosa E, Uccelli A, et al. Female Infertility Related to Thyroid Autoimmunity: The Ovarian Follicle Hypothesis. Am J Reprod Immunol (2011) 66:108–14. doi: 10.1111/j.1600-0897.2010.00961.x
7. Wakim AN, Polizotto SL, Buffo MJ, Marrero MA, Burholt DR. Thyroid Hormones in Human Follicular Fluid and Thyroid Hormone Receptors in Human Granulosa Cells. Fertil Steril (1993) 59:1187–90. doi: 10.1016/s0015-0282(16)55974-3
8. De Silva M. Detection and Measurement of Thyroid Stimulating Hormone in Human Follicular Fluid. J Reprod Med (1994) 39:679–80.
9. Canipari R, Mangialardo C, Di Paolo V, Alfei F, Ucci S, Russi V, et al. Thyroid Hormones Act as Mitogenic and Pro Survival Factors in Rat Ovarian Follicles. J Endocrinol Invest (2019) 42:271–82. doi: 10.1007/s40618-018-0912-2
10. Verga Falzacappa C, Timperi E, Bucci B, Amendola D, Piergrossi P, D’Amico D, et al. T(3) Preserves Ovarian Granulosa Cells From Chemotherapy-Induced Apoptosis. J Endocrinol (2012) 215:281–9. doi: 10.1530/JOE-12-0153
11. Verga Falzacappa C, Mangialardo C, Patriarca V, Bucci B, Amendola D, Raffa S, et al. Thyroid Hormones Induce Cell Proliferation and Survival in Ovarian Granulosa Cells COV434. J Cell Physiol (2009) 221:242–53. doi: 10.1002/jcp.21849
12. Hapon MB, Gamarra-Luques C, Jahn GA. Short Term Hypothyroidism Affects Ovarian Function in the Cycling Rat. Reprod Biol Endocrinol (2010) 8:14. doi: 10.1186/1477-7827-8-14
13. Muller AF, Verhoeff A, Mantel MJ, De Jong FH, Berghout A. Decrease of Free Thyroxine Levels After Controlled Ovarian Hyperstimulation. J Clin Endocrinol Metab (2000) 85:545–8. doi: 10.1210/jcem.85.2.6374
14. Monteleone P, Faviana P, Artini PG. Thyroid Peroxidase Identified in Human Granulosa Cells: Another Piece to the Thyroid-Ovary Puzzle? Gynecol Endocrinol (2017) 33:574–6. doi: 10.1080/09513590.2017.1296424
15. Poppe K, Glinoer D, Tournaye H, Schiettecatte J, Haentjens P, Velkeniers B. Thyroid Function After Assisted Reproductive Technology in Women Free of Thyroid Disease. Fertil Steril (2005) 83:1753–7. doi: 10.1016/j.fertnstert.2004.12.036
16. Poppe K, Glinoer D, Tournaye H, Schiettecatte J, Devroey P, van Steirteghem A, et al. Impact of Ovarian Hyperstimulation on Thyroid Function in Women With and Without Thyroid Autoimmunity. J Clin Endocrinol Metab (2004) 89:3808–12. doi: 10.1210/jc.2004-0105
17. Litwicka K, Arrivi C, Varricchio MT, Mencacci C, Greco E. In Women With Thyroid Autoimmunity, Does Low-Dose Prednisolone Administration, Compared With No Adjuvant Therapy, Improve In Vitro Fertilization Clinical Results? J Obstet Gynaecol Res (2015) 41:722–8. doi: 10.1111/jog.12615
18. Kilic S, Tasdemir N, Yilmaz N, Yuksel B, Gul A, Batioglu S. The Effect of Anti-Thyroid Antibodies on Endometrial Volume, Embryo Grade and IVF Outcome. Gynecol Endocrinol (2008) 24:649–55. doi: 10.1080/09513590802531112
19. Łukaszuk K, Kunicki M, Kulwikowska P, Liss J, Pastuszek E, Jaszczołt M, et al. The Impact of the Presence of Antithyroid Antibodies on Pregnancy Outcome Following Intracytoplasmatic Sperm Injection-ICSI and Embryo Transfer in Women With Normal Thyreotropine Levels. J Endocrinol Invest (2015) 38:1335–43. doi: 10.1007/s40618-015-0377-5
20. Tan S, Dieterle S, Pechlavanis S, Janssen OE, Fuhrer D. Thyroid Autoantibodies Per Se do Not Impair Intracytoplasmic Sperm Injection Outcome in Euthyroid Healthy Women. Eur J Endocrinol (2014) 170:495–500. doi: 10.1530/EJE-13-0790
21. Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, et al. Levothyroxine Treatment in Thyroid Peroxidase Antibody-Positive Women Undergoing Assisted Reproduction Technologies: A Prospective Study. Hum Reprod (2005) 20:1529–33. doi: 10.1093/humrep/deh843
22. Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, et al. Assisted Reproduction and Thyroid Autoimmunity: An Unfortunate Combination? J Clin Endocrinol Metab (2003) 88:4149–52. doi: 10.1210/jc.2003-030268
23. Muller AF, Verhoeff A, Mantel MJ, Berghout A. Thyroid Autoimmunity and Abortion: A Prospective Study in Women Undergoing In Vitro Fertilization. Fertil Steril (1999) 71:30–4. doi: 10.1016/s0015-0282(98)00394-x
24. Chai J, Yeung WT, Lee CV, Li HR, Ho P, Ng HE. Live Birth Rates Following In Vitro Fertilization in Women With Thyroid Autoimmunity and/or Subclinical Hypothyroidism. Clin Endocrinol (2014) 80:122–7. doi: 10.1111/cen.12220
25. Mintziori G, Goulis DG, Gialamas E, Dosopoulos K, Zouzoulas D, Gitas G, et al. Association of TSH Concentrations and Thyroid Autoimmunity With IVF Outcome in Women With TSH Concentrations Within Normal Adult Range. Gynecol Obstet Investig (2014) 77:84–8. doi: 10.1159/000357193
26. Negro R, Formoso G, Coppola L, Presicce G, Mangieri T, Pezzarossa A, et al. Euthyroid Women With Autoimmune Disease Undergoing Assisted Reproduction Technologies: The Role of Autoimmunity and Thyroid Function. J Endocrinol Invest (2007) 30:3–8. doi: 10.1007/BF03347388
27. Karacan M, Alwaeely F, Cebi Z, Berberoglugil M, Batukan M, Ulug M, et al. Effect of Antithyroid Antibodies on ICSI Outcome in Antiphospholipid Antibody-Negative Euthyroid Women. Reprod BioMed Online (2013) 27:376–80. doi: 10.1016/j.rbmo.2013.07.002
28. Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid Disease in Pregnancy: New Insights in Diagnosis and Clinical Management. Nat Rev Endocrinol (2017) 13:610–22. doi: 10.1038/nrendo.2017.93
29. Chen Z, Yang X, Zhang C, Ding Z, Zhang Y, Korevaar TIM, et al. Thyroid Function Test Abnormalities in Twin Pregnancies. Thyroid (2020) 31:572–9. doi: 10.1089/thy.2020.0348
30. Korevaar TI, Steegers EA, Pop VJ, Broeren MA, Chaker L, de Rijke YB, et al. Thyroid Autoimmunity Impairs the Thyroidal Response to Human Chorionic Gonadotropin: Two Population-Based Prospective Cohort Studies. J Clin Endocrinol Metab (2017) 102:69–77. doi: 10.1210/jc.2016-2942
31. Zhang Y, Zhang C, Yang X, Yang S, Meng Y, Liu Z, et al. Association of Maternal Thyroid Function and Thyroidal Response to Human Chorionic Gonadotropin With Early Fetal Growth. Thyroid (2019) 29:586–94. doi: 10.1089/thy.2018.0556
Keywords: thyroid autoimmunity, in vitro fertilization/intracytoplasmic sperm injection, thyroid antibodies, pregnancy outcomes, fetal weight
Citation: Huang N, Chen L, Lian Y, Wang H, Li R, Qiao J and Chi H (2021) Impact of Thyroid Autoimmunity on In Vitro Fertilization/Intracytoplasmic Sperm Injection Outcomes and Fetal Weight. Front. Endocrinol. 12:698579. doi: 10.3389/fendo.2021.698579
Received: 21 April 2021; Accepted: 21 June 2021;
Published: 08 July 2021.
Edited by:
Yang Xu, Peking University First Hospital, ChinaCopyright © 2021 Huang, Chen, Lian, Wang, Li, Qiao and Chi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Qiao, jie.qiao@263.net; Hongbin Chi, chihb@163.com
 Ning Huang
Ning Huang Lixue Chen
Lixue Chen Ying Lian
Ying Lian Haining Wang
Haining Wang Rong Li
Rong Li Jie Qiao
Jie Qiao Hongbin Chi
Hongbin Chi