- General Surgery of Beijing Jishuitan Hospital, The Fourth Clinical Medical College of Peking University, Beijing, China
Background: The staging system for patients with anaplastic thyroid cancer (ATC) was updated in the 8th edition of the American Joint Committee on Cancer Staging Manual. A cut-off age of 55 years was stipulated as a prognostic factor for differentiated thyroid cancer; however, age was not considered for ATC patients. To this end, this study investigated the relationship between age at diagnosis and prognosis of ATC patients.
Methods: The clinical information on ATC patients was acquired from the Surveillance, Epidemiology, and End Results Program public database. Youden’s index and X-tile analyses were used to calculate the high-point age at diagnosis associated with prognosis. Cox proportional hazards models, Kaplan-Meier curves, and 1000-person-year were then used for verifying the accuracy of the high-point age.
Results: After inclusion/exclusion criteria was applied, 586 patients were included in this study. The high-point age was determined to be 70 years by both the Youden’s index and X-tile plot methods. The hazard ratio was 1.662 (95% confidence interval [CI]: 1.321-2.092), indicating that there was an increased risk of poor prognosis for patients > 70 years of age. The cancer-specific mortality rates per 1000-person-years for patients ≤ and > 70 years-old were 949.980 (95% CI: 827.323-1090.822) and 1546.667 (95% CI: 1333.114-1794.428), respectively. P-values were < 0.001 for the results shown above.
Conclusion: Our study found that age influenced the prognosis of ATC patients. Furthermore, we determined that the high-point age at diagnosis was 70 years and that > 70 years of age was associated with a poor prognosis. These results provide a useful addition to the staging manual and can improve the diagnosis, treatment strategies and prognosis of ATC patients.
Introduction
Thyroid cancer is the most common endocrine malignancy, and the incidence, is increasing at an alarming rate, especially in women (1–3). According to statistics, thyroid cancer has the fourth highest incidence rate of all malignant cancers (2, 4). The majority of thyroid tumors are differentiated thyroid cancers (DTCs), such as papillary and follicular tumors, which exhibit a good prognosis with a 5-year survival rate of 98% (3, 5). However, anaplastic thyroid cancers (ATC), a small subset of thyroid tumors, consists of undifferentiated cells with a median survival rate of 5 months and a 1-year-survival rate of < 20%, and account for 40–50% of thyroid cancer-specific mortality (6).
In the clinic, ATC usually presents as a neck mass that blocks the function of the esophagus and trachea and presents with symptoms of dysphagia, dysphonia or hoarseness, stridor, and/or dyspnea (7, 8). A previous multivariate analysis reported that age, presence of acute symptoms, leukocytosis, large tumor, and distant metastasis are independent factors for its prognosis (9). Another study also reported, surgical methods, chemotherapy, and gross residual disease to be amongst these factors (10).
It is common for patient age at diagnosis to be used as a prognostic factor for thyroid cancer patients (11). For DTC, age is included in the American Joint Committee on Cancer (AJCC) staging system (12, 13). Furthermore, in the most recent 8th edition, the cut-off point for age at diagnosis was increased from 45 to 55 years (14). Since a linear association between age and survival has been reported in previous studies, the high-point age at diagnosis is dispute (15).
The staging system was updated in the 8th edition of the AJCC manual (14); however, the age at diagnosis was not considered in the staging system for ATC patients (13). Thus, this study investigated the relationship between age at diagnosis and the prognosis of ATC patients to propose an accurate high-point age at diagnosis. Our results may provide additional information for the staging manual that may be of great value in the diagnosis, treatment, and prognosis of ATC patients.
Materials and Methods
Patients and Database
Data were acquired from the open access, authoritative database from the Surveillance, Epidemiology, and End Results (SEER) Program, launched in 1973 by the United States Centers for Disease Control and Prevention and National Cancer Institute. The SEER database includes information on patients with endocrine, respiratory, digestive system, and other tumors, and covers approximately 34.6% of the population in the United States. The data used in this study were obtained from a public anonymized database and ethics committee approval and informed consent were not required.
We enrolled 1286 patients with ATC from 2004 to 2017 using the ICD-0-3 SEER site/histology validation code 8021/3. The information entered for each patient included patient identification; race; age at diagnosis; sex; year of diagnosis; tumor (T)-stage; lymph node (N)-stage; metastasis (M)-stage; AJCC 7th edition staging; multifocality; tumor size; tumor extension; bone brain, liver, and lung metastasis; and surgical method. Moreover, we excluded patients who had missing information as follows: 1) patients whose AJCC staging information was missing (698 patients) and 2) patients whose months of survival were recorded as unknown (2 patients).
Statistical Analysis
We assessed the association between prognosis (mortality) and the age at diagnosis with Cox proportional hazards models. The results of the Cox analysis were adjusted for sex; race; year of diagnosis; tumor size; extension; multifocality; TNM-stage; bone, brain, liver, and lung metastasis; and surgical method. The optimal cut-off age was determined using the Youden’s index, which integrates sensitivity and specificity information with a value that ranges from 0-1, and X-tile plots, a tool for biomarker assessment and outcome-based cut-point optimization. Finally, Kaplan-Meier curves, Cox proportional hazards models, and mortality per 1000-person-years were used to determine the significance of the cut-off age.
Frequencies, proportions, and mean values ± standard deviations were used to present variables, as appropriate. P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS, version 22.0 (IBM Corp., Armonk, NY, USA), Stata/SE version 15 (Stata Corp, College Station, TX, USA), GraphPad Prism version 7 (GraphPad Software Inc., La Jolla, CA, USA), or X-tile 3.6.1 (Robert L Camp, M.D., Ph.D., Yale University, USA).
Results
General Characteristics of Study Population
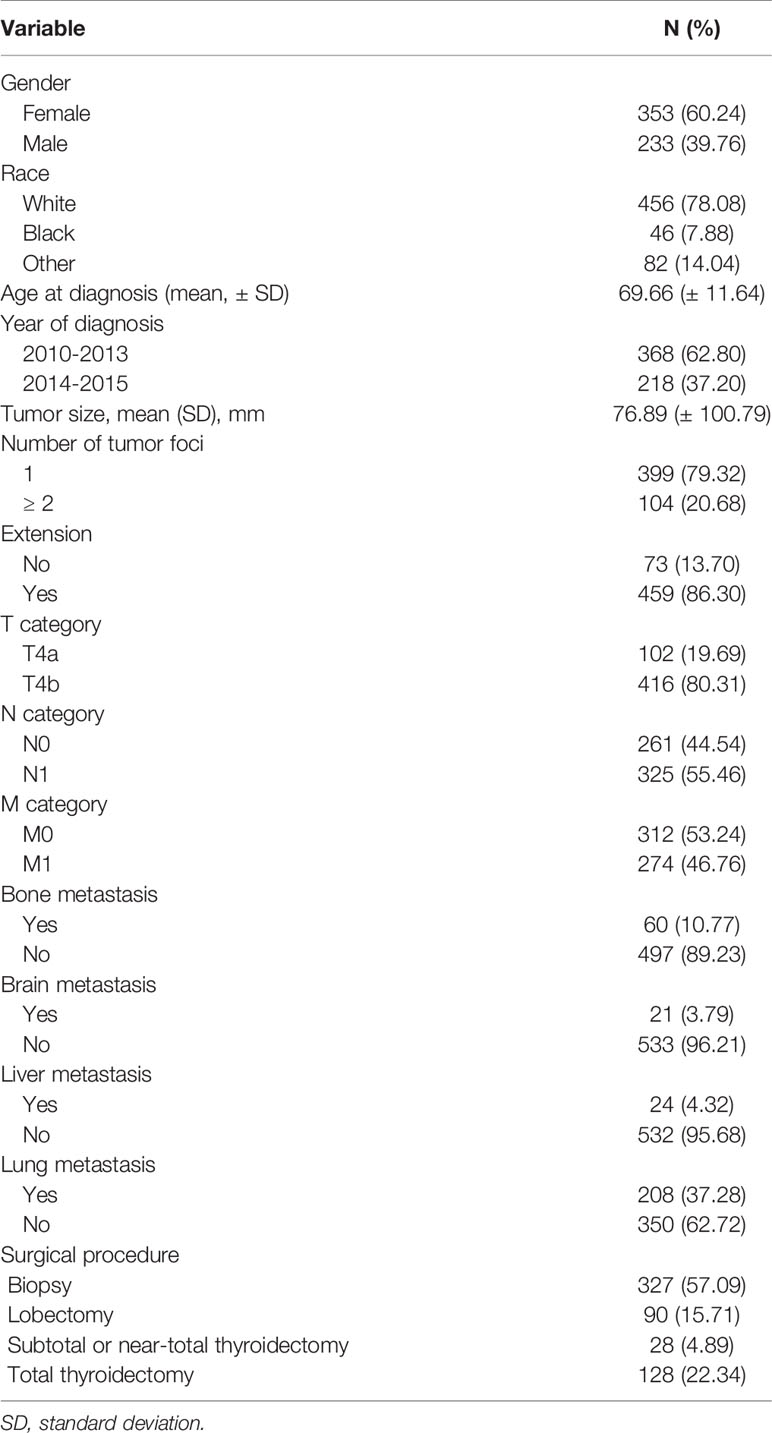
This study included 586 patients with ATC. Table 1 shows the demographic data, clinical characteristics, and treatment methods for patients with ATC. The mean age of the 586 patients was 69.66 ± 11.64 years-old, with a range of 26-85 years. Furthermore, 286 patients were > 70 years. Compared with patients whose age was ≤ 70 or > 70 years, the approximate ratio was 1:1. The female to male ratio was approximately 3: 2.
The Effect of Age and the High Point
According to Table 2, after adjusting for the variables described above, the hazard ratio for prognosis and overall age at diagnosis was 1.022 (95% confidence interval [CI]: 1.012-1.032), P < 0.001.
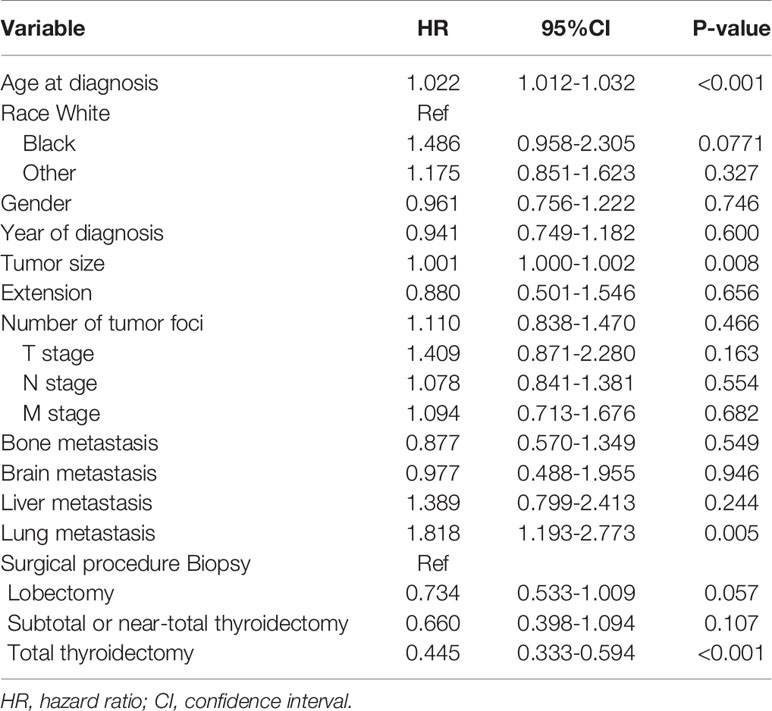
Table 2 Adjusted Cox proportional hazards analyses of cancer-specific mortality for patients with anaplastic thyroid cancer.
The cut-off year for the age at diagnosis was determined to be 70 years old, using both the Youden’s index and X-tile plots with data on cancer-specific and overall mortality. Results of sensitivity and 1-specificity are shown in Supplement Table 1; the maximum value was 0.075, corresponding to 70 years. The results of X-tile plots are shown for cancer-specific mortality (Figures 1A–C) and overall mortality (Figures 1D–F) for patients under and over 70 years of age. The survival curves indicated that patients under 70 years of age had less cancer-specific and overall mortality than patients over 70 years, with Kaplan-Meier curves showing greater cancer-specific and overall mortality for patients > 70 years than for those ≤ 70 years of age (P < 0.001).
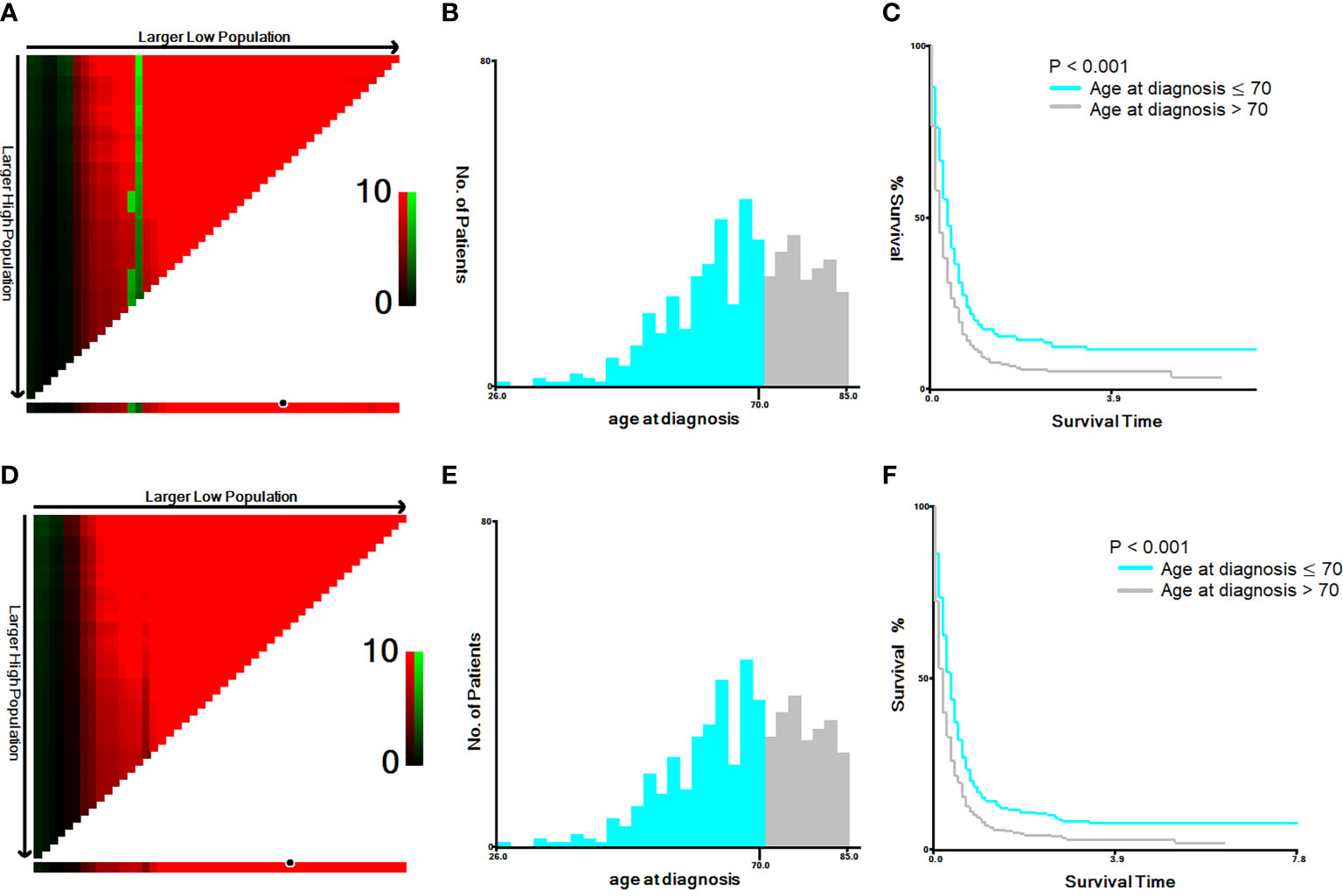
Figure 1 X-tile analysis of 586 anaplastic thyroid cancer patients based on cancer-specific mortality and overall mortality. ABBREVIATION: The training plots are shown in panel (A, D), with matched validation sets shown in panels (B, E) and (C, F). The optimal cut-point highlighted by the black circle in panel (A, D) is shown on a histogram of the entire cohort (B, E) and a Kaplan-Meier plot (C, F). The picture of (A–C) is for cancer-specific mortality; the picture (D–F) is for overall mortality. P values were determined by using the cut-point defined in the training set and applying it to the validation sets (P < 0.001).
Validation of Cut-Off Year
To confirm the accuracy of the cut-off age, we used Cox proportional hazards models with two groups, patients ≤ 70 years-old and > 70 years-old at diagnosis. After adjusting for the variables described above, the hazard ratio was 1.662 (95% CI: 1.321-2.092), with P < 0.001 (Table 3). The cancer-specific mortality rates were 949.980 (95% CI: 827.323-1090.822) and 1546.667 (95% CI: 1333.114-1794.428) 1000-person-years for patients who were diagnosed at ≤ 70 and > 70 years-old, respectively (Table 4). The overall mortality rates were 1082.316 (95% CI: 950.835-1231.978) and 1742.222 (95% CI: 1514.619-2004.027) 1000-person-years for the ≤ 70 years and > 70 years age groups, respectively (Table 4).
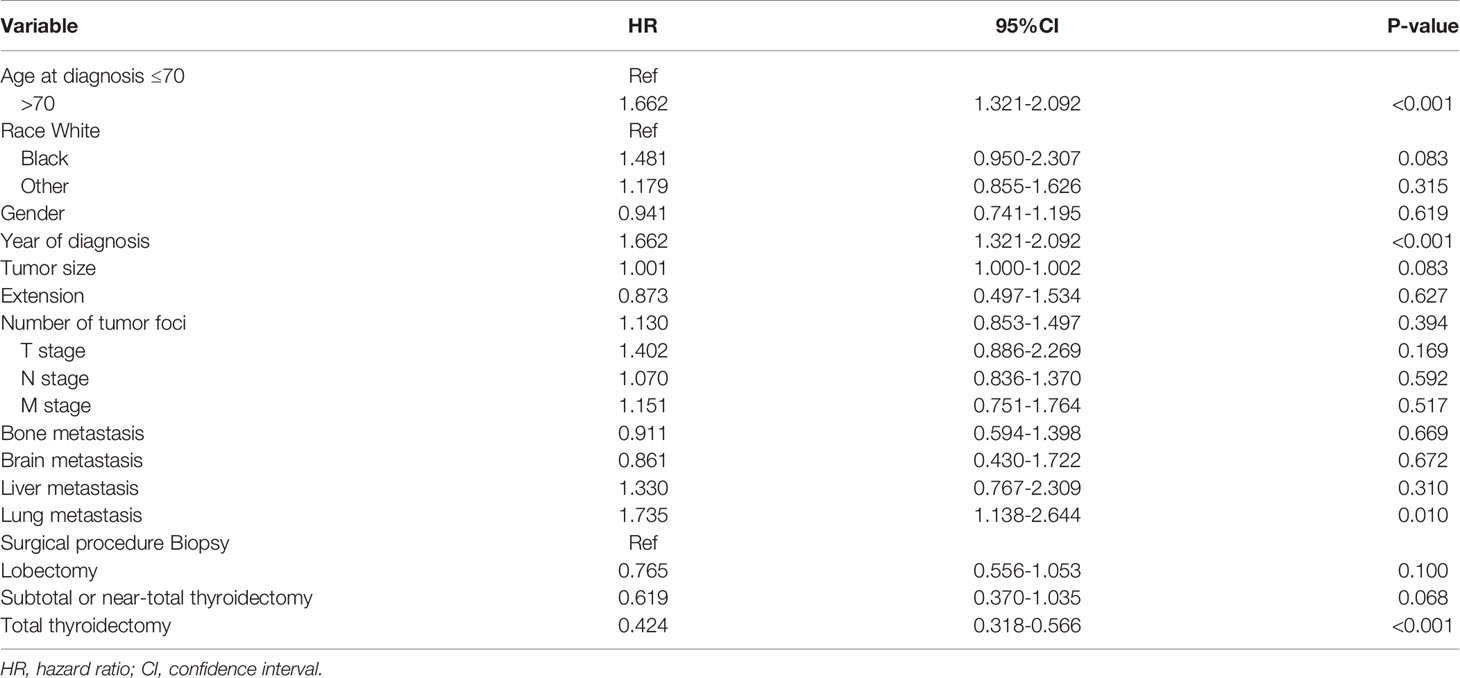
Table 3 Adjusted Cox proportional hazards analyses of cancer-specific mortality for patients with anaplastic thyroid cancer based on the cut-off age at diagnosis of 70 years.
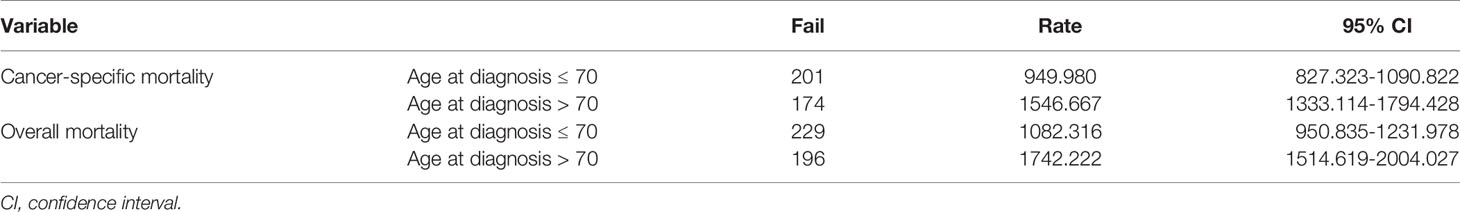
Table 4 Results of 1000-person-years for cancer-specific and overall mortality for anaplastic thyroid cancer patients with a dichotomous cut-off age at diagnosis of 70 years.
Table 5 shows the comparisons of clinicopathological features between patients who were ≤ and > 70 years of age. Sex, multifocality, tumor size, N-stage, M-stage, bone metastasis, and overall mortality were significantly different between the two groups of patients; however, there were no differences for the other characteristics.
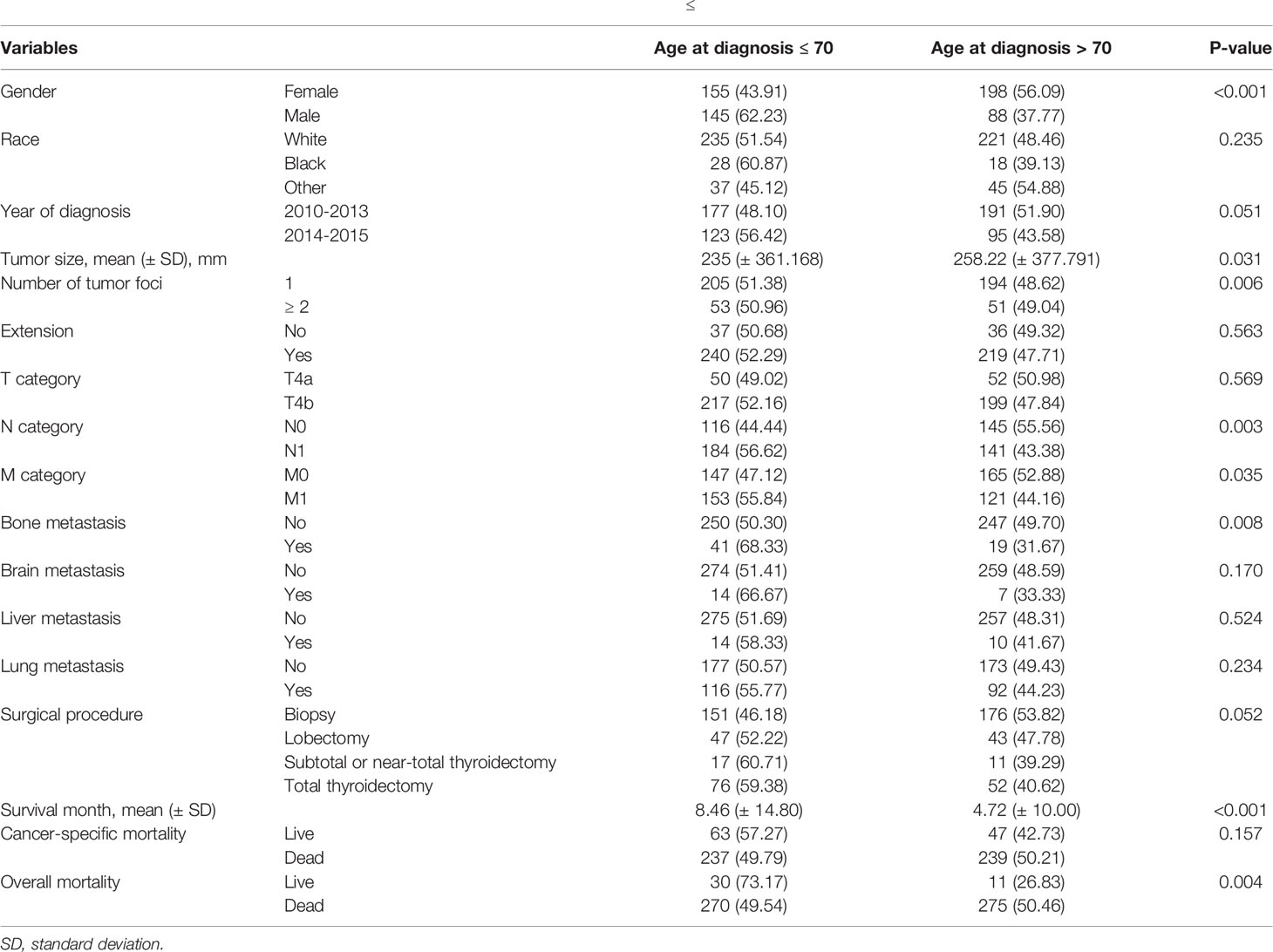
Table 5 Comparisons of clinicopathological characteristics between patients who were ≤ and > 70 years-old at the time of diagnosis of anaplastic thyroid cancer.
Discussion
Although there is a relationship between age and prognosis for different types of malignancies, thyroid cancer is unique because age is included as a staging variable (16). In 1983, the 2nd edition of the AJCC manual first settled on a dichotomous cut-off age of 45 years for DTC (17). The relationship between age and thyroid cancer has since been reevaluated. In 2018, a high-point age of 55 years was proposed because several recent studies suggested that mortality did not increase in patients with thyroid cancer before the age of 50 or 55 years at the time of diagnosis. The majority of deaths from thyroid cancer appeared to occur in those patients diagnosed after the age of 55 years (18, 19).
Besides patients diagnosed with DTC, the age at diagnosis should also be considered for other pathological types of thyroid cancer, such as ATC, for staging. Our study verified using Cox proportional hazard models that age influenced the prognosis of patients with ATC, and patients who were diagnosed at an older age had a worse prognosis. The Youden’s index and X-tile analyses showed that 70 years of age was the dichotomous cut-off for a worse prognosis for ATC patients.
It is reported that the largest group of patients with ATC were in their seventh and eighth decade of life (20). Although patients who were younger than 50 years-old have a better prognosis, such patients are few in number, and even rare under the age of 40 years (21). Thus, several studies have assessed the relationship between ATC severity and age at diagnosis and reported that age is an independent factor that was significantly associated with longer survival (9, 10).
Vladan’s et al. divided 150 ATC patients into 3 groups, <50 years-old, 51-70 years-old, and >70 years-old. They found that the chances of survival of the youngest age group were significantly better than that of the other two older age groups. In their study cohort, one-year survival of the youngest age group was observed in more than half the patients, whereas in the other two older groups one-year survival was 3-4 times less. Furthermore, during the first month from diagnosis of ATC, almost 30% of patients older than 70 years died, while less than 10% in the two younger age groups died (22).
Older patients were more likely to show a worse prognosis in thyroid cancer, which might have been affected by various factors. A study has pointed out that radioactive iodine (RAI), thyroid-stimulating hormone (TSH) levels, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) homology, immune system decline, and genetic variation are associated with poor prognosis in thyroid cancer patients of advanced age (23).
ATC is a carcinoma with a high mortality rate. With increasing age, the selection of treatments for ATC patients is limited. In a previous study, researchers reported that subgroup analysis showed significance for radiotherapy, while multivariate analysis did not (7). In additional, they also pointed out that radiotherapy can provide benefit in terms of on loco-regional control but is not associated with an increase in survival (7).
For ATC patients, surgery had a greater impact on the overall survival (24). Furthermore, different surgical methods also impacted the survival rate. The prognosis of patients undergoing total thyroidectomy was significantly better than that of those undergoing partial thyroidectomy/lobectomy/subtotal thyroidectomy/subtotal thyroidectomy. The median survival time was also significantly improved in those who underwent total thyroidectomy (25). However, in addition to tumor size and location, the age of the patient also plays a crucial role in the choice of surgical method, and thus, the following adjuvant therapy.
There were limitations in this study. Because of the lack of serology-related indicators and molecular markers in the SEER database, we could not analyze the relationship between serology-related indicators and/or molecular markers and age at diagnosis of ATC. Furthermore, our study cohort had no limits of means to download the chemotherapy information, on external irradiation of ATC patients from the SEER database. Thus, we have not taken chemotherapy or external irradiation into consideration, which might influence the prognosis of patients. However, we will continue to pay attention to this issue in our future work. This study, evaluated cancer-specific and overall mortality in different age groups of ATC patients. Further, studies will be needed to investigate the associations between ATC prognosis and serology-related indicators and/or molecular markers.
In conclusion, we confirmed that the age at diagnosis influences ATC patients’ prognosis and calculated that the high-point of age at diagnosis was 70 years-old. We believe that these data should be considered in the next edition of the AJCC Staging Manual, and will aid in accurately diagnosing patients with ATC, provide for more specific treatments, and improve the prognosis prediction of these patients. We hope that future studies will be conducted to confirm our results and further study the relationship between age and patients with ATC.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: SEER database.
Author Contributions
All authors contributed to the article and approved the submitted version. NK, QX, and NB collected the data, and assured the integrity and accuracy of these data. NK and AC performed the data analysis and interpretation. NK prepared the figures. NB, ST, and ZZ provided administrative support. NK wrote the first draft of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.704596/full#supplementary-material
References
1. Kitahara CM, Sosa JA. The Changing Incidence of Thyroid Cancer. Nat Rev Endocrinol (2016) 12(11):646–53. doi: 10.1038/nrendo.2016.110
2. Morris LG, Tuttle RM, Davies L. Changing Trends in the Incidence of Thyroid Cancer in the United States. JAMA Otolaryngol– Head Neck Surg (2016) 142(7):709–11. doi: 10.1001/jamaoto.2016.0230
3. Cabanillas ME, McFadden DG, Durante C. Thyroid Cancer. Lancet (London England) (2016) 388(10061):2783–95. doi: 10.1016/s0140-6736(16)30172-6
4. Lin B, Ma H, Ma M, Zhang Z, Sun Z, Hsieh IY, et al. The Incidence and Survival Analysis for Anaplastic Thyroid Cancer: A SEER Database Analysis. Am J Trans Res (2019) 11(9):5888–96.
5. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
6. Saini S, Tulla K, Maker AV, Burman KD, Prabhakar BS. Therapeutic Advances in Anaplastic Thyroid Cancer: A Current Perspective. Mol Cancer (2018) 17(1):154. doi: 10.1186/s12943-018-0903-0
7. Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, et al. Anaplastic Thyroid Carcinoma: From Clinicopathology to Genetics and Advanced Therapies. Nat Rev Endocrinol (2017) 13(11):644–60. doi: 10.1038/nrendo.2017.76
8. Sun C, Li Q, Hu Z, He J, Li C, Li G, et al. Treatment and Prognosis of Anaplastic Thyroid Carcinoma: Experience From a Single Institution in China. PloS One (2013) 8(11):e80011. doi: 10.1371/journal.pone.0080011
9. Sugitani I, Miyauchi A, Sugino K, Okamoto T, Yoshida A, Suzuki S. Prognostic Factors and Treatment Outcomes for Anaplastic Thyroid Carcinoma: ATC Research Consortium of Japan Cohort Study of 677 Patients. World J Surg (2012) 36(6):1247–54. doi: 10.1007/s00268-012-1437-z
10. Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, et al. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid: Off J Am Thyroid Assoc (2020) 30(10):1505–17. doi: 10.1089/thy.2020.0086
11. Shah S, Boucai L. Effect of Age on Response to Therapy and Mortality in Patients With Thyroid Cancer at High Risk of Recurrence. J Clin Endocrinol Metab (2018) 103(2):689–97. doi: 10.1210/jc.2017-02255
12. Edge SB, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th Edition. Chicago, IL: Springer (2010).
13. Edge SB, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, et al. AJCC Cancer Staging Manual. 8th Edition. Chicago, IL: Springer (2017). doi: 10.1007/978-3-319-40618-3
14. Berg AN, Seethala RR. The American Joint Committee on Cancer Eighth Edition: Changes in Thyroid Carcinoma Staging and an Update on Reporting. AJSP: Rev Rep (2018) 23(3):145–8. doi: 10.1097/pcr.0000000000000244
15. Kazaure HS, Roman SA, Sosa JA. The Impact of Age on Thyroid Cancer Staging. Curr Opin Endocrinol Diabetes Obes (2018) 25(5):330–4. doi: 10.1097/med.0000000000000430
16. McLeod DS, Jonklaas J, Brierley JD, Ain KB, Cooper DS, Fein HG, et al. Reassessing the NTCTCS Staging Systems for Differentiated Thyroid Cancer, Including Age at Diagnosis. Thyroid: Off J Am Thyroid Assoc (2015) 25(10):1097–105. doi: 10.1089/thy.2015.0148
17. Ellis FH Jr, Watkins E Jr, Krasna MJ, Heatley GJ, Balogh K. Staging of Carcinoma of the Esophagus and Cardia: A Comparison of Different Staging Criteria. J Surg Oncol (1993) 52(4):231–5. doi: 10.1002/jso.2930520406
18. Bischoff LA, Curry J, Ahmed I, Pribitkin E, Miller JL. Is Above Age 45 Appropriate for Upstaging Well-Differentiated Papillary Thyroid Cancer? Endocr Pract (2013) 19(6):995–7. doi: 10.4158/ep13029.Or
19. Mazurat A, Torroni A, Hendrickson-Rebizant J, Benning H, Nason RW, Pathak KA. The Age Factor in Survival of a Population Cohort of Well-Differentiated Thyroid Cancer. Endocr Connect (2013) 2(3):154–60. doi: 10.1530/ec-13-0056
20. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. Prognostic Factors and Therapeutic Strategy for Anaplastic Carcinoma of the Thyroid. World J Surg (2001) 25(5):617–22. doi: 10.1007/s002680020166
21. Pichardo-Lowden A, Durvesh S, Douglas S, Todd W, Bruno M, Goldenberg D. Anaplastic Thyroid Carcinoma in a Young Woman: A Rare Case of Survival. Thyroid: Off J Am Thyroid Assoc (2009) 19(7):775–9. doi: 10.1089/thy.2009.0025
22. Zivaljevic V, Tausanovic K, Paunovic I, Diklic A, Kalezic N, Zoric G, et al. Age as a Prognostic Factor in Anaplastic Thyroid Cancer. Int J Endocrinol (2014) 2014:240513. doi: 10.1155/2014/240513
23. Haymart MR. Understanding the Relationship Between Age and Thyroid Cancer. Oncologist (2009) 14(3):216–21. doi: 10.1634/theoncologist.2008-0194
24. Chen J, Tward JD, Shrieve DC, Hitchcock YJ. Surgery and Radiotherapy Improves Survival in Patients With Anaplastic Thyroid Carcinoma: Analysis of the Surveillance, Epidemiology, and End Results 1983-2002. Am J Clin Oncol (2008) 31(5):460–4. doi: 10.1097/COC.0b013e31816a61f3
Keywords: anaplastic thyroid cancer, age at diagnosis, staging system, AJCC, SEER
Citation: Kong N, Xu Q, Zhang Z, Cui A, Tan S and Bai N (2021) Age Influences the Prognosis of Anaplastic Thyroid Cancer Patients. Front. Endocrinol. 12:704596. doi: 10.3389/fendo.2021.704596
Received: 03 May 2021; Accepted: 09 July 2021;
Published: 27 July 2021.
Edited by:
Natalia Simona Pellegata, Helmholtz-Gemeinschaft Deutscher Forschungszentren (HZ), GermanyReviewed by:
Carl Christofer Juhlin, Karolinska Institutet (KI), SwedenAlessandro Prete, University of Pisa, Italy
Copyright © 2021 Kong, Xu, Zhang, Cui, Tan and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Bai, bnbn6112@126.com
 Na Kong
Na Kong Nan Bai
Nan Bai